The Opioid System in Temporal Lobe Epilepsy: Functional Role and Therapeutic Potential
- Department of Pharmacology, Medical University of Innsbruck, Innsbruck, Austria
Temporal lobe epilepsy is considered to be one of the most common and severe forms of focal epilepsies. Patients often develop cognitive deficits and emotional blunting along the progression of the disease. The high incidence of resistance to antiepileptic drugs and a frequent lack of admissibility to surgery poses an unmet medical challenge. In the urgent quest of novel treatment strategies, neuropeptides are interesting candidates, however, their therapeutic potential has not yet been exploited. This review focuses on the functional role of the endogenous opioid system with respect to temporal lobe epilepsy, specifically in the hippocampus. The role of dynorphins and kappa opioid receptors (KOPr) as modulators of neuronal excitability is well understood: both the reduced release of glutamate as well of postsynaptic hyperpolarization were shown in glutamatergic neurons. In line with this, low levels of dynorphin in humans and mice increase the risk of epilepsy development. The role of enkephalins is not understood so well. On one hand, some agonists of the delta opioid receptors (DOPr) display pro-convulsant properties probably through inhibition of GABAergic interneurons. On the other hand, enkephalins play a neuro-protective role under hypoxic or anoxic conditions, most probably through positive effects on mitochondrial function. Despite the supposed absence of endorphins in the hippocampus, exogenous activation of the mu opioid receptors (MOPr) induces pro-convulsant effects. Recently-expanded knowledge of the complex ways opioid receptors ligands elicit their effects (including biased agonism, mixed binding, and opioid receptor heteromers), opens up exciting new therapeutic potentials with regards to seizures and epilepsy. Potential adverse side effects of KOPr agonists may be minimized through functional selectivity. Preclinical data suggest a high potential of such compounds to control seizures, with a strong predictive validity toward human patients. The discovery of DOPr-agonists without proconvulsant potential stimulates the research on the therapeutic use of neuroprotective potential of the enkephalin/DOPr system.
Introduction
With a prevalence of 1–2% worldwide, epilepsy is one of the most frequent neurological diseases affecting people of all ages (Thurman et al., 2011; WHO, 2015). Of the 870 million people living in the European Region, over 5 million suffer from epilepsy. Epilepsy has major adverse effects on both social and psychological well-being, including social isolation, stigmatization, or disability, thus, resulting in lower educational achievement and worse employment outcomes (WHO, 2015). In line with this, the International League Against Epilepsy (ILAE) defined epilepsy as “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures and by the neurobiologic, cognitive, psychological, and social consequences of this condition.”
The term epilepsy comprises of a group of chronic neurological diseases that can be characterized by epileptic seizures, as a result of excessive electrical discharges in a group of brain cells (Fisher et al., 2005, 2014). Epileptic seizures are episodes that vary from brief and nearly undetectable to prolonged convulsions, and may involve a part of the brain (partial) or multiple brain centers or the entire brain (generalized), and are sometimes accompanied with a loss of consciousness and control of bowel or bladder functions. The cause of epilepsy is mostly unknown (ca. 60%), although several patients have a history of brain injury, stroke, brain tumor, and substance use disorders (Berkovic et al., 2006; Thurman et al., 2011; WHO, 2015). Genetic, congenital, or developmental epilepsies are more common among younger people, while brain tumors and stroke are more common causes in older people.
About 70% of all epilepsy patients suffer from focal seizures arising from a distinct brain region, the temporal lobe. Mesial temporal lobe epilepsy (mTLE, with the hippocampus as epileptogenic focus) is considered as one of the most frequent types of epilepsy (Blumcke et al., 2012; Goldberg and Coulter, 2013). mTLE with hippocampal sclerosis represents one of the most refractory forms of human epilepsy (Asadi-Pooya et al., 2017). One main factor responsible for neuronal losses and seizure induction is excessive glutamate release (Meldrum, 2002), which may result from impaired inhibitory signaling. The mainstay treatment of epilepsy relies on antiepileptic drugs (AEDs), mostly for the person's entire life. Notably, 30–50% of the patients are refractory to the presently available pharmacological treatments (Laxer et al., 2014). Moreover, the current pharmacotherapies of epilepsy causes a number of side effects (e.g., sedation, nausea, depression, headache, ataxia) in 10 to 90% of people (Eadie, 2012; Perucca and Gilliam, 2012). In 2008, the FDA issued a black-box warning that several AEDs increased the risk of suicidal thoughts and behavior among the users (Mula and Sander, 2013). In patients whose seizures cannot be efficiently controlled by AEDs or neuro-stimulation, surgical resection of the epileptogenic focus remains the ultimate solution (Duncan, 2007; Bergey, 2013). Besides, only about 50–80% reach seizure freedom for at least 1 year (Spencer and Huh, 2008).
In the quest for alternative treatment options, neuropeptides have received an increasing attention. Neuropeptide systems have been demonstrated to play crucial roles in the modulation of neuronal excitability. Several neuropeptides, such as neuropeptide Y, galanin, somatostatin, ghrelin, and dynorphin, have been reported to have direct antiepileptic and antiepileptogenic effects, and they represent promising potential drug targets (Kovac and Walker, 2013). The aim of this review is to reflect upon the opioid system's function in epileptogenesis and temporal lobe epilepsy as well as their therapeutic potentials.
The Endogenous Opioid System in the Hippocampus: General Aspects
The three classical opioid receptors, the kappa opioid receptor (KOPr), delta opioid receptor (DOPr), and mu opioid receptor (MOPr), are Gi/o-coupled 7-transmembrane domain proteins, and they share highly homologous protein sequences (60% amino acid sequence identity) including a common opioid receptor binding pocket within the helical transmembrane core. The extracellular domains are crucial for selectivity, however, the opioid system is fairly promiscuous and the affinities of dynorphins, endorphins, and enkephalins to KOPr, MOPr, and DOPr vary only in the range of one potency (Schwarzer, 2009).
The opioid receptor-like (ORL)-1 receptor was also classified as an opioid receptor due to its genetic sequence homology to other opioid receptors. It displays, however, distinct pharmacological properties, and therefore, is not considered to be a classical opioid receptor. Although ORL-1 and its endogenous ligand nociceptin might be involved in seizures and epilepsy (Bregola et al., 2002a,b; Binaschi et al., 2003; Aparicio et al., 2004; Rocha et al., 2007, 2009), it will not be discussed in this review.
The Dynorphin/KOPr System
Although there is only one prodynorphin (pDyn) gene known in mammals, different splice variants (Horikawa et al., 1983; Telkov et al., 1998; Nikoshkov et al., 2005), and a variety of different mature peptides, were reported from brain (Yakovleva et al., 2006). In vitro studies of different pDyn-derived peptides applied to KOPr suggested a rank order of potency with Dyn A1-17 > (10–20 times) BigDyn = Dyn B = Dyn B 1-29 = α-neo-endorphin > (10–20 times) Dyn A 1-8 = β-neo-endorphin (James et al., 1984). Regional regulation of the trafficking and processing of pDyn at synapses may be important for the fine-tuning of synaptic transmission (Yakovleva et al., 2006).
High pDyn mRNA expression was observed in the amygdala, entorhinal cortex, dentate gyrus, nucleus accumbens, dorsomedial hypothalamus, and premammillary nucleus in humans (Hurd, 1996; Nikoshkov et al., 2005) and rodents (Morris et al., 1986; Merchenthaler et al., 1997; Lin et al., 2006). In the limbic system, strong expression of pDyn mRNA was noted in human (Hurd, 1996), while entorhinal cortex lacks expression in rat (Merchenthaler et al., 1997) or mouse (Lin et al., 2006). Neurons of the central amygdala contain the highest amounts of pDyn in rodents, in human brain, cortical subnuclei express higher amounts of pDyn (Capper-Loup and Kaelin-Lang, 2008).
Immunoreactivy and mRNA distribution display little mismatch (Khachaturian et al., 1982; Vincent et al., 1982b; Fallon and Leslie, 1986). Immunoreactive fibers, such as hippocampal mossy fibers or local circuits in the cortex and amygdala were found (Vincent et al., 1982a; Weber and Barchas, 1983; Code and Fallon, 1986; Fallon and Leslie, 1986). Ultrastructural evidence suggests the presence of Dyn also in dendrites (Van Bockstaele et al., 1995; Hara et al., 2006). In fact, beside the N-type calcium channel-mediated Dyn release at axon terminals, L-type channel-dependent somatodendritic Dyn release was proposed to play an important functional role (Simmons et al., 1995). The distribution of KOPr suggests axonal and dendritic auto- and/or heteroreceptors. How this complex situation may influence neuronal excitability is best demonstrated in the hippocampal granule cells. KOPr on the dendrites of hippocampal granule cells (Mathieu-Kia et al., 2001) may be activated by the Dyn originating from perforant path fiber in humans (Hurd, 1996), or from granule cell dendrites in guinea pigs (Simmons et al., 1995). This is supported by potentially presynaptic KOPr mediated inhibition of perforant path terminals upon the stimulation of hippocampal granule cells (Wagner et al., 1993; Drake et al., 1994). Besides guinea pig, other rodents too display such effects, closely depending on the presence of kappa opioid receptors on the perforant path terminals (Salin et al., 1995). The KOPr present in axo-axonal synapses of mossy fibers mediate heterosynaptic inhibition of neighboring mossy fibers (Weisskopf et al., 1993). CA3 pyramidal neurons may by hyperpolarized through postsynaptic KOPr. Dyn acting on KOPr placed on CA1 pyramidal cells may be derived from perforant path fibers in humans. Functionally, KOPr were shown to be involved in the modulation of hippocampal transmission and LTP (Wagner et al., 1993; Salin et al., 1995; Terman et al., 2000; Huge et al., 2009). Beside glutamatergic neurons, some groups of GABAergic interneurons also express KOPr (Racz and Halasy, 2002). The potential inhibition of inhibitory neurons suggests some excitatory effects under certain conditions.
Taken together, the Dyn/KOPr system is ideally positioned to modulate synaptic transmission at all excitatory synapses of the hippocampus. Most prominent is the control of the granule cells, which are considered a main input filter of the limbic system.
The Enkephalin/DOPr System
Met- and Leu-enkephalin (Enk) are pentapetides encoded in the proenkephalin gene. Moreover, Met-Enk can be processed from proopiomelanocortin (POMC) and Leu-Enk from pDyn. The strongest expression of Enk is found in the basal ganglia, with comparably low expression levels in the hippocampus (Miller and Pickel, 1980). In the rat hippocampus, Enk immunoreactivity was observed in the lateral perforant path and a small number of morphologically characteristic granule cells (Gall et al., 1981; Stengaardpedersen, 1983). Immunoreactivity was also observed in mossy fibers and pyramidal cells in the area CA4 of rats (Stengaardpedersen, 1983), however at a very low level. Low levels of immunoreactivity in rodents are in line with low levels of Met-Enk mRNA (Bing et al., 1997; Schwarzer and Sperk, 1998). In human hippocampi, Enk immunoreactivity was observed in numerous granule cells, interneurons in the molecular layer, as well as pyramidal cells in the hippocampus proper and subiculum (Kulmala, 1985). However, no Enk was observed in the primate perforant path fibers (Gall, 1988). Species differences in Enk immunoreactivity in the hippocampus proper appear to depend on the expression of Enk in the perforant path.
Enks preferentially bind to DOPr, but with only 10-fold lower affinity to MOPr (Clarke et al., 2003). Also, DOPr has a relatively high affinity for β-endorphin (Hughes et al., 1975). The action of DOPr and also that of MOPr in the hippocampus is mainly disinhibitory (Zieglgansberger et al., 1979): MOPr and DOPr activation reduce GABAergic input, thus causing disinhibition (Neumaier et al., 1988) and thereby facilitating synaptic plasticity and seizure susceptibility (Cohen et al., 1992; Morris and Johnston, 1995). The location of Enk in the hippocampus supports the notion of the Enk/DOPr's role in the modulation of inhibitory transmission; Leu-Enk-immunoreactive terminals are often close to GABAergic terminals, perikarya, and dendrites (Commons and Milner, 1996). In mouse hippocampi, DOPr are mainly located presynaptically on inhibitory GABAergic interneurons with some intracellularly located receptors in the pyramidal and granule cells (Rezai et al., 2012). Whether these intracellular receptors represent a pool of spare receptors or serve specific function is unclear. Activation of DOPr in the hippocampus inhibits spontaneous GABA release (Lupica, 1995) and results in net excitatory potential (Drake et al., 2007). Generally, DOPr activation inhibits intracellular cAMP formation and exerts modulatory effects on Ca2+ and K+ channels and other such 2nd messenger actions (Quock et al., 1999).
It is important to consider that the Enk/DOPr system is very dynamic; owing to agonist-induced internalization and “cross-talk” with other neurotransmitter-systems. Thus, there are several lines of evidence suggesting MOPr/DOPr crosstalk and heterodimerization (for review see Peppin and Raffa, 2015). Thus, MOPr-dependent effects on migration of intracellularly localized DOPr to the membrane surface (Cahill et al., 2003; Morinville et al., 2004) were described through inflammation. Dynamic receptor synthesis and degradation (reviewed by van Rijn et al., 2013), as well as the fast turnover rates of Enk (Hughes et al., 1975; Simantov and Snyder, 1976) are important components of this flexibility.
Due to the location of Enk/DOPr it's regulatory role is considered mostly on modulation of inhibitory signaling. Inhibition of GABAergic interneurons on one hand facilitates excitatory signaling, on the other hand may loosen synchronization and control.
The Endorphin/MOPr System
α-, β-, γ- and δ-endorphins are processed from proopiomelanocortin (POMC), which is expressed by interneurons of the dentate gyrus (Niikura et al., 2013). β-endorphin has been reported to be present in the hippocampus (Zakarian and Smyth, 1979, 1982), but these findings were not reproduced in later reports (Chavkin et al., 1985; Drake et al., 2007). α- and β-neoendorphin can be processed from pDyn, which is expressed in granule cells and contained in perforant path fibers (see above), but also Dyn and Enk, which may stimulate MOPr with considerable potency. MOPr receptors were detected by autoradiography in all sub-regions of the hippocampus (Slamberova et al., 2003). These MOPr are localized perisomatically, dendritically, and presynaptically on different classes of GABAergic interneurons (Drake and Milner, 2002). Activation of MOPr, like the activation of all other opioid receptors, causes a reduction of Ca2+ currents through P/Q-, N- and L-type channels and activation of Kir3 K+ channels through direct interaction of the βγ subunits of the G-protein with the channels (Al-Hasani and Bruchas, 2011). Moreover, MOPr couple to Gαi, inhibiting cAMP formation upon activation. Activation of MOPr was shown to alter synaptic plasticity in CA1, and thereby, impair spatial memory (Mansouri et al., 1997, 1999; Pourmotabbed et al., 1998). Moreover, the activation of MOPr disrupts synchronization of CA1 neuronal activity (Faulkner et al., 1998).
At present it is difficult to judge the role of MOPr in the hippocampus. Further studies on interaction with the other opioid systems are required to understand their functional role.
The Endogenous Opioid System in the Hippocampus: Alterations in Epilepsy
mTLE is associated with a number of functional, morphological, and neuropathological alterations, which impact upon the endogenous opioid system. The resulting alterations in the opioid system may contribute to or counteract seizure susceptibility, by modulation of glutamatergic and GABAergic transmission (Table 1).
The Dynorphin/KOPr System
Dyn is expressed in large quantities in mossy fibers of rodents (McGinty et al., 1983) and humans (Houser et al., 1990; Houser, 1992). This pool of Dyn is depleted during seizures due to the long-lasting, high frequency stimulation, inducing the release of large dense core vesicles. This was observed in animal models of temporal lobe epilepsy. Thus, kainic acid injection reduced Dyn levels for several hours when injected intrastriatally (Kanamatsu et al., 1986b), or several days, when given systemically to rodents (Gall, 1988; Douglass et al., 1991; Lason et al., 1992b). Electroconvulsive shocks depleted the Dyn pool for about 6 h when applied once, but up to 2 weeks upon repetitive treatment (Kanamatsu et al., 1986a; Xie et al., 1989b). This is paralleled by a transient increase in mRNA expression, ranging from 200 to 1,300% in distinct models (Xie et al., 1989b; Douglass et al., 1991; Lason et al., 1992a,b; Hong et al., 1993; Schwarzer and Sperk, 1998). This causes a transient recovery of the depleted Dyn pools. Nevertheless, Dyn levels appear subsequently decreased for a period of at least 28 days (Rocha and Maidment, 2003).
Similar reductions were reported from several kindling models of epileptogenesis (Iadarola et al., 1986; McGinty et al., 1986; Morris et al., 1987; Lee et al., 1989; Xie et al., 1989a; Rosen et al., 1992; Harrison et al., 1995). Functionally important data came from a microdialysis study, reporting significantly-reduced extracellular opioid peptide levels during the interictal period in fully kindled rats (Rocha et al., 1997). In surgically removed hippocampal tissue of mesial temporal lobe epilepsy patients, dynorphin immunoreactivity is also reduced (de Lanerolle et al., 1997), despite elevated mRNA levels in the granule cells of the hippocampus, if the patient experienced seizures within 48 h before surgery (Pirker et al., 2009).
Ca2+ may play a dual role in the complex regulation of expression of pDyn. Ca2+ activates CREB. CREB bound to CRE sites increases the activity of the pDyn promoter. On the other hand, Ca2+ also enhances the expression of DREAM (downstream regulatory element antagonizing modulator), which counteracts CREB by binding to the DRE (downstream regulatory element) sequence in the promoter (Cheng et al., 2002). Pronounced seizure-induced DREAM expression was shown in the mouse hippocampus (Matsu-ura et al., 2002).
Besides the regulation of pDyn expression, the Dyn/KOPr system in the hippocampus is also affected by pathological and morphological changes. Partial loss of KOPr expressing somatostatin-immunoreactive interneurons (Racz and Halasy, 2002), and pyramidal neurons are characteristic features of temporal lobe epilepsy. By contrast, mossy fibers sprout to the supergranular layer (for review see (Ben-Ari, 2001) and innervate the basal dendrites of granule cells.
Dyn immunoreactivity in tissue of patients suffering from mesial temporal lobe epilepsy differs between epilepsies with or without mossy fiber sprouting (Houser et al., 1990; de Lanerolle et al., 1997, 2003). pDyn mRNA (de Lanerolle et al., 1992) and peptide (Gall, 1988; de Lanerolle et al., 1997) were observed in hilar interneurons and CA3 pyramidal neurons in mesial temporal lobe epilepsy. This was neither observed in healthy brain nor in epilepsies without hippocampal sclerosis and mossy fiber sprouting, such as mass-associated or paradoxical temporal lobe epilepsy (Hurd, 1996). Reduced tissue levels of Dyn-immunoreactivity in mTLE (de Lanerolle et al., 1997) may be due to neuronal loss, excessive release during seizures (Sperk et al., 1986; Marksteiner et al., 1989; McDermott and Schrader, 2011), or Dyn down-regulation.
Like for Dyn–immunoreactivity, the hippocampi of patients suffering from mass-associated or paradoxical temporal lobe epilepsy displayed similar [3H]U69,593 binding as post-mortem controls. Reduced binding in area CA1 in mTLE patients appears to be dependent on neuronal loss, as the subiculum is spared from both (de Lanerolle et al., 1997).
The loss of Dyn, probably resulting in a lack of inhibition of voltage-gated Ca2+ currents in the hippocampal granule cells (Jeub et al., 1999), may be functionally important. Increased Ca2+ currents lead to augmented glutamate release, thereby facilitating the generation of seizures. Of note is the fact, that the loss of inhibition of voltage-gated Ca2+ currents was closely associated with mossy fiber sprouting and hippocampal sclerosis.
Overall, alterations in the Dyn/KOPr system in epilepsy suggests a loss of inhibition on glutamatergic neurons (Table 1). This may contribute to the progression of disease development and severity. However, the depletion of Dyn, whilst conservation of the KOPr offers the possibility of pharmacological intervention.
The Enkephalin/DOPr System and MOPr
The expression of Enk in the hippocampi of naive rodents is mostly restricted to interneurons. However, Enk expression is upregulated in granule cells subsequent to seizures (Hong et al., 1980; Yoshikawa et al., 1985). In the model of unilateral injection of kainic acid into the dorsal hippocampus of mice, the upregulation of pEnk mRNA appeared to be independent of seizures, which is distinct of the regulation of other neuropeptides like pDyn or pNPY. The promoter of the Enk gene contains 2 CRE sites, that regulate the cAMP and phorbol-ester-inducible expression of pEnk in conjuction with a downstream AP-2 site (Comb et al., 1986, 1988; Grove et al., 1989). Moreover, PKA was shown to influence the activity of the human pEnk promoter (Huggenvik et al., 1991). In human patients, β-endorphin appears to be elevated in the CSF postictally (Pitkanen et al., 1987). Judging from the plasma levels of the patients, Marek et al. (2010) suggest that β-endorphin concentrations are related to the frequency of seizures and the duration of the disease, while leu-enkephalin concentrations are related primarily to the duration of the disease.
Under physiological conditions, DOPr and MOPr are distributed in diffuse patterns in the hippocampus, as shown by receptor autoradiography (Mansour et al., 1988), with MOPr being more prominent, however, they are relatively distant from synapses (Drake and Milner, 1999). Upon seizures, both MOPr and DOPr have been reported to change their distribution patterns and function in accordance with both morphological and pathological alterations (Bausch and Chavkin, 1997; Skyers et al., 2003). The number of DOPr or MOPr immunopositive neurons in the hippocampus appears to be reduced in both, the hilus and granule cell layer. By contrast, diffuse immunoreactivity for DOPr and MOPr appeared increased in the inner molecular layer in the pilocarpine model of TLE (Bausch and Chavkin, 1997). The increase in MOPr in the inner molecular layer may be associated with a variety of fibers originating from granule cells or surviving GABAergic interneurons, as well as septal or supramamillary projections (Skyers et al., 2003). Receptor binding of MOPr also increases upon seizures in human mTLE, however, this effect seems to be restricted mainly to the temporal cortex (Frost et al., 1988; Rocha et al., 2009). Specific MOPr splice variants were observed in some forms of intractable epilepsy (Fricchione et al., 2008), however, their function remains unclear.
In epilepsy numerous GABAergic interneurons die, suggesting a large reduction of neurons expressing DOPr or MOPr. However, survival of interneurons in animal models of TLE differ quite significantly from human conditions, thus translatability of rodent studies has to be considered carefully (Table 1).
The Endogenous Opioid System in the Hippocampus: Implications in Epilepsy
Opioids, and, in particular, dynorphin, have been implicated in the modulation of neuronal excitability in-vitro (Henriksen et al., 1982; Siggins et al., 1986). In-vivo, opioid receptors, with their ligands form neuromodulatory systems, playing major roles not only in nociceptive pathways, in affective behavior, neuroendocrine physiology, and autonomic functions (Kieffer and Evans, 2009), but also in epilepsy, and importantly, through their action in the hippocampus. That the opioid systems modulate seizure activity has been shown by opiates (Hong et al., 1993), and that opioid receptors adapt following spontaneous seizures has been demonstrated by using a non-subtype selective opioid receptor PET radioligand, showing a brain-region specific upregulation of opioid receptor availability (Hammers et al., 2007).
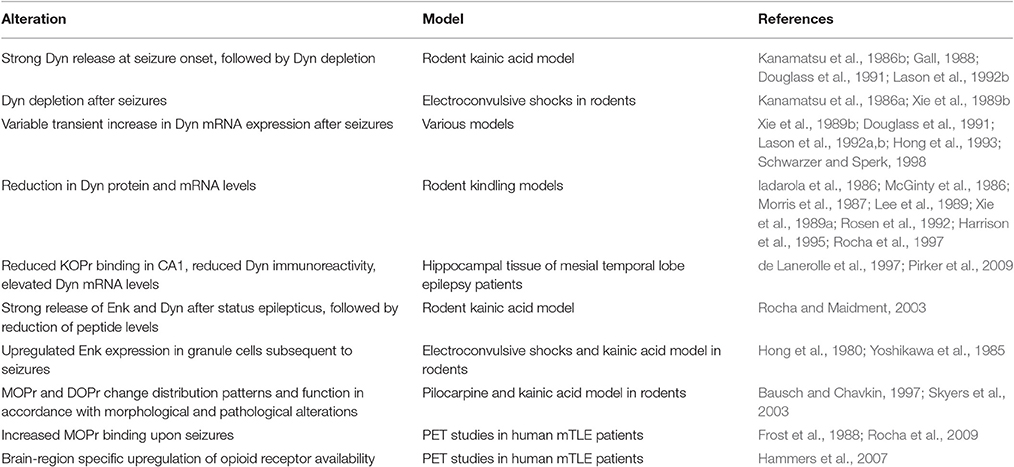
Dyn and Enk, despite the similar molecular actions of their preferential receptors (KOPr and DOPr/MOPr) exert very different effects on seizure-induction, most likely due to their differential localization within the hippocampus (see Figure 1). However, though KOPr is predominantly expressed on glutamatergic neurons and their activation yields a net inhibitory effect, DOPr and MOPr are often located on neurons inhibiting glutamatergic principal neurons, thus, their activation may results in net disinhibitory effects (Table 2).
Figure 1. Simplified scheme of hippocampal dentate gyrus network control by opioid receptors. Blue represents KOPr, red DOPr and yellow MOPr for more detailed descriptions of the individual opioid systems' functions in the hippocampus, see Drake and Milner (1999), Rezai et al. (2012), and Schwarzer (2009). DOPr are often located on GABAergic neurons containing neuropeptide Y (NPY) and somatostatin (SOM) (Commons and Milner, 1996), MOPr are often on GABAergic neurons containing parvalbumin (PARV) (Drake and Milner, 1999). Note that especially DOPr and MOPr could also be active as heterodimers. Activation of both, MOPr and DOPr has predominantly disinhibitory effects on granule cells.
Table 2. Implications of the hippocampal endogenous opioid system on excitability, epilepsy, and neuroprotective potentials.
The Dynorphin/KOPr System
The dominant effect of the endogenous dynorphin is anticonvulsant (Tortella et al., 1986, 1989, 1990; Przewlocka et al., 1995; Solbrig et al., 2006), antiepileptogenic, and is mediated via the kappa opioid receptor (Loacker et al., 2007). Deletion of the coding region of the prodynorphin gene in mice resulted in an increased seizure susceptibility and affected neurodegeneration during epileptogenesis. In line with this, low prodynorphin levels, due to mutations in the promoter regions in humans (Stogmann et al., 2002; Gambardella et al., 2003), result in an increased vulnerability toward epilepsy.
The Enkephalin/DOPr System and MOPr
Mazarati et al. (1999) showed that DOPr inhibition prevents, and DOPr-activation facilitates self-sustained status epilepticus in a model of perforant path stimulation. These effects are, however, strongly dependent on the applied agonists (Saitoh et al., 2011; Clynen et al., 2014; Chung et al., 2015), and might differ across species. For example, the DOPr agonist SNC80 appears to produce stronger convulsions in the rat (Broom et al., 2002; Jutkiewicz et al., 2006), than in the rhesus monkey (Negus et al., 1998; Danielsson et al., 2006). Induction of seizures, furthermore, might be predominantly depending on the DOPr activation on forebrain GABAergic neurons, as Chung et al. (2015) demonstrated using SNC80 on mice with DOPr knocked out specifically in those neurons.
Due to the mainly inhibitory net effects of MOPr in the hippocampus, microinjections of β-endorphin into the hippocampus result in generalized convulsions, however, administration into the ventricle strongly reduces this effect (Cain et al., 1990). Systemic administration of MOPr agonists occasionally even result in anti-convulsant effects, maybe due to the differential actions in other brain regions (reviewed in Simmons and Chavkin, 1996). Still, seizure development appears to be more dependent on MOPr activation than on DOPr activation, as the former, but not the latter, causes convulsions (Lee et al., 1989; Hong et al., 1993).
Interestingly, agonists at DOPr can block certain effects of MOPr agonists and vice versa (ONeill et al., 1997), potentially reflecting the competition of the different agonists at the receptor subtypes or heterodimerization.
The Endogenous Opioid System in the Hippocampus: Potentials in Epilepsy Therapy
The patterns of regulations during epileptogenesis differ strongly between Dyn and Enk, which is demonstrated for mRNAs in response to status epilepticus (Hong et al., 1993). While the increase of pDyn mRNA was transient, followed by a reduction, pEnk expression appeared to be lastingly increased. Whether such continuous Enk mRNA upregulation plays a role in promoting epileptogenesis or in counteracting effects requires further investigation, and that has been discussed below (Table 2). With respect to the described anti-convulsant properties of Dyn, reduced mRNA levels suggest that the application of exogenous KOPr-agonists in these periods may be beneficial.
The Dynorphin/KOPr System
The approach to activate KOPr in the periods of low Dyn has indeed been shown to have a potential to suppress seizures (Tortella, 1988; Takahashi et al., 1990; Solbrig et al., 2006; Loacker et al., 2007), and it increases the survival of neurons in the hippocampus and amygdala after unilateral injection of kainic acid into the hippocampus of mice (Schunk et al., 2011). Clinical trials, using the full KOPr agonists spiradoline or enadoline, have failed due to dysphoric side-effects in the 1990s (Barber and Gottschlich, 1997; Schwarzer, 2009). As a consequence, industrial research has been essentially discontinued. Recently, we reported that by using biased KOPr-agonists, the anticonvulsant/antiseizure effects can be separated from the dysphoric effects (Zangrandi et al., 2016), opening new therapeutical potentials.
A promising approach to target the opioid system and counteract seizures in a disease modifying way, is adeno-associated virus (AAV) gene therapy. AAV-gene therapy is a promising tool to target a broad array of neurological diseases (Weinberg et al., 2013). Several pre-clinical studies on AAV-mediated gene-delivery of neuropeptides are already available (for a review see Kovac and Walker, 2013). Gene-therapy for Dyn might be an interesting approach to activate KOPr and achieve anti-convulsant effects through replenishing Dyn in different phases of depletion of endogenous Dyn. Due to local restriction of the therapy, side effects known from systemic application of KOPr agonists may be avoided.
The Enkephalin/DOPr System
The described pro-convulsant properties of some DOPr agonists and the initial continuous upregulation of Enk mRNA during epileptogensis suggest Enk to act as a potential driving force of epileptogenesis. Interestingly, however, the activation of DOPr has also been implicated in neuroprotection, suggesting their potential dual role in epilepsy; upregulation during epileptogenesis might be beneficial, even though the net effect of DOPr activation seems to be pro-convulsant. Neuroprotection of DOPr has been reported specifically for hypoxia/ischemia (Mayfield and D'Alecy, 1994; Zhang et al., 2000; Yang et al., 2009), glutamate-induced excitotoxic injury (Zhang et al., 2000), and oxidative stress (Narita et al., 2006), potentially via their positive effects on mitochondrial function (Zhu et al., 2009, 2011). Leu-Enk peptide has been shown to be upregulated in hippocampus and cortex after hypoxic preconditioning (Gao et al., 2012). Also, DOPr is upregulated through hypoxic preconditioning, and a decrease of Leu-Enk during severe hypoxia is inhibited, counteracting the increased p38 MAPK activity, cytochrome c release and apoptosis, induced by severe hypoxia (Ma et al., 2005).
Furthermore, the differential effects of different MOPr agonists, the complexity and dynamics of the Enk/MOPr system, and the dubiety whether the hippocampal Enk/MOPr system is required for MOPr induced seizures warrant further elucidation of this system's involvement, especially in epileptogenesis.
Importantly, the convulsive properties of some DOPr agonists represent a major drawback in their great pharmacological potentials for chronic pain (reviewed by Gaveriaux-Ruff and Kieffer, 2011) and mood disorders (reviewed by Chung and Kieffer, 2013); DOPr are importantly involved in the control of emotional responses, such as anxiety and depression-like behaviors (Filliol et al., 2000; Pradhan et al., 2011). An anxiogenic phenotype has been reported for both, DOPr- (Filliol et al., 2000) and Enkephalin-knockout (Konig et al., 1996) mice, which is not the case in mice deficient for KOPr or MOPr. Accordingly, pharmacological tools have been used to target many of these behavioral effects of the DOPr/Enk system (Pradhan et al., 2011). In general, DOPr agonists tested in clinical trials for other applications (like ADL5859 and 5747, AZD5258 and AZD2327), appeared to have elicited no severe adverse effects, and were, overall, well-tolerated (Charles and Pradhan, 2016; Richards et al., 2016). Therefore, DOPr agonists (without proconvulsive potential) represent promising drug-candidates, despite all of the mentioned examples failing to reach their primary clinical endpoints for their respective purposes.
The availability of MOPr in the hippocampus and its involvement in seizure susceptibility makes it a potential target in epilepsy and seizures. This system is understudied in epilepsy research, however, owing probably also to a lack of primary endogenous ligands to study regulations in epilepsy.
Conclusion
In conclusion, a better understanding of the complex opioid system in the hippocampus, including functionally selective agonists and di-/oligomerizations of opioid receptors and neuroprotective effects (in particular of DOPr), is giving rise to new therapeutic concepts, and will drive research on new medical applications of the opioid systems. Specifically, the Dyn/KOPr system bears great potentials to target epilepsy and epileptogenesis, also for the fashioning of disease-modifying treatments, with the possibility of the elimination of side-effects through design, and the selection of relevant agonists and attractive delivery methods, such as gene therapy approaches. Pro-convulsant properties of DOPr activation can be avoided by the selection of adequate agonists with the desired functional selectivity to specifically exploit the neuroprotective potential of DOPr activation. Yet, the neuroprotective effects of DOPr activation require further investigations. Providing the identification of DORr agonists, which conserve the neuroprotective but lack proconvulsant effects, may be of great interest in the course of epilepsy therapy.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
CS has a patent application pending for the use of preprodynorphin viral vectors in gene therapy for focal epilepsy.
The other author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work of JB and CS related to this review was in part financed by the Austrian Science Fund (I-977, W-1206, P16123, P18471).
References
Al-Hasani, R., and Bruchas, M. R. (2011). Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115, 1363–1381. doi: 10.1097/ALN.0b013e318238bba6
Aparicio, L. C., Candeletti, S., Binaschi, A., Mazzuferi, M., Mantovani, S., Di, B., et al. (2004). Kainate seizures increase nociceptin/orphanin FQ release in the rat hippocampus and thalamus: a microdialysis study. J. Neurochem. 91, 30–37. doi: 10.1111/j.1471-4159.2004.02633.x
Asadi-Pooya, A. A., Stewart, G. R., Abrams, D. J., and Sharan, A. (2017). Prevalence and incidence of drug-resistant mesial temporal lobe epilepsy in the United States. World Neurosurg. 99, 662–666. doi: 10.1016/j.wneu.2016.12.074
Barber, A., and Gottschlich, R. (1997). Novel developments with selective, non-peptidic kappa-opioid receptor agonists. Exp. Opin. Investig. Drugs 6, 1351–1368. doi: 10.1517/13543784.6.10.1351
Bausch, S. B., and Chavkin, C. (1997). Changes in hippocampal circuitry after pilocarpine-induced seizures as revealed by opioid receptor distribution and activation. J. Neurosci. 17, 477–492.
Ben-Ari, Y. (2001). Cell death and synaptic reorganizations produced by seizures. Epilepsia 42(Suppl. 3), 5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x
Bergey, G. K. (2013). Neurostimulation in the treatment of epilepsy. Exp. Neurol. 244, 87–95. doi: 10.1016/j.expneurol.2013.04.004
Berkovic, S. F., Mulley, J. C., Scheffer, I. E., and Petrou, S. (2006). Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 29, 391–397. doi: 10.1016/j.tins.2006.05.009
Binaschi, A., Zucchini, S., Bregola, G., Rodi, D., Mazzuferi, M., Reinscheid, R. K., et al. (2003). Delayed epileptogenesis in nociceptin/orphanin FQ-deficient mice. Neuroreport 14, 825–827. doi: 10.1097/00001756-200305060-00009
Bing, G. Y., Wilson, B., Hudson, P., Jin, L., Feng, Z. H., Zhang, W. Q., et al. (1997). A single dose of kainic acid elevates the levels of enkephalins and activator protein-1 transcription factors in the hippocampus for up to 1 year. Proc. Natl. Acad. Sci. U.S.A. 94, 9422–9427. doi: 10.1073/pnas.94.17.9422
Blumcke, I., Coras, R., Miyata, H., and Ozkara, C. (2012). Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathol. 22, 402–411. doi: 10.1111/j.1750-3639.2012.00583.x
Bregola, G., Zucchini, S., Frigati, L., Candeletti, S., Romualdi, P., Reinscheid, R., et al. (2002a). Involvement of the neuropeptide orphanin FQ/nociceptin in kainate and kindling seizures and epileptogenesis. Epilepsia 43, 18–19. doi: 10.1046/j.1528-1157.43.s.5.43.x
Bregola, G., Zucchini, S., Rodi, D., Binaschi, A., D'Addario, C., Landuzzi, D., et al. (2002b). Involvement of the neuropeptide nociceptin/orphanin FQ in kainate seizures. J. Neurosci. 22, 10030–10038.
Broom, D. C., Nitsche, J. F., Pintar, J. E., Rice, K. C., Woods, J. H., and Traynor, J. R. (2002). Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J. Pharmacol. Exp. Ther. 303, 723–729. doi: 10.1124/jpet.102.036525
Cahill, C. M., Morinville, A., Hoffert, C., O'Donnell, D., and Beaudet, A. (2003). Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain 101, 199–208. doi: 10.1016/S0304-3959(02)00333-0
Cain, D. P., Boon, F., and Corcoran, M. E. (1990). Involvement of multiple piate receptors in opioid kindlingI. Brain Res. 517, 236–244. doi: 10.1016/0006-8993(90)91032-C
Capper-Loup, C., and Kaelin-Lang, A. (2008). Lateralization of dynorphin gene expression in the rat striatum. Neurosci. Lett. 447, 106–108. doi: 10.1016/j.neulet.2008.09.071
Charles, A., and Pradhan, A. A. (2016). Delta-opioid receptors as targets for migraine therapy. Curr. Opin. Neurol. 29, 314–319. doi: 10.1097/WCO.0000000000000311
Chavkin, C., Shoemaker, W. J., McGinty, J. F., Bayon, A., and Bloom, F. E. (1985). Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J. Neurosci. 5, 808–816.
Cheng, H. Y., Pitcher, G. M., Laviolette, S. R., Whishaw, I. Q., Tong, K. I., Kockeritz, L. K., et al. (2002). DREAM is a critical transcriptional repressor for pain modulation. Cell 108, 31–43. doi: 10.1016/S0092-8674(01)00629-8
Chung, P. C. S., Boehrer, A., Stephan, A., Matifas, A., Scherrer, G., Darcq, E., et al. (2015). Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav. Brain Res. 278, 429–434. doi: 10.1016/j.bbr.2014.10.029
Chung, P. C. S., and Kieffer, B. L. (2013). Delta opioid receptors in brain function and diseases. Pharmacol. Ther. 140, 112–120. doi: 10.1016/j.pharmthera.2013.06.003
Clarke, S., Zimmer, A., Zimmer, A. M., Hill, R. G., and Kitchen, I. (2003). Region selective up-regulation of micro-, delta- and kappa-opioid receptors but not opioid receptor-like 1 receptors in the brains of enkephalin and dynorphin knockout mice. Neuroscience 122, 479–489. doi: 10.1016/j.neuroscience.2003.07.011
Clynen, E., Swijsen, A., Raijmakers, M., Hoogland, G., and Rigo, J. M. (2014). Neuropeptides as targets for the development of anticonvulsant drugs. Mol. Neurobiol. 50, 626–646. doi: 10.1007/s12035-014-8669-x
Code, R. A., and Fallon, J. H. (1986). Some projections of dynorphin-immunoreactive neurons in the rat central nervous system. Neuropeptides 8, 165–172. doi: 10.1016/0143-4179(86)90043-0
Cohen, G. A., Doze, V. A., and Madison, D. V. (1992). Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron 9, 325–335. doi: 10.1016/0896-6273(92)90171-9
Comb, M., Birnberg, N. C., Seasholtz, A., Herbert, E., and Goodman, H. M. (1986). Acyclic AMP- and phorbol ester-inducibleDNA element. Nature 323, 353–356. doi: 10.1038/323353a0
Comb, M., Mermod, N., Hyman, S. E., Pearlsberg, J., Ross, M. E., and Goodman, H. M. (1988). Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. Embo J. 7, 3793–3805.
Commons, K. G., and Milner, T. A. (1996). Cellular and subcellular localization of delta opioid receptor immunoreactivity in the rat dentate gyrus. Brain Res. 738, 181–195. doi: 10.1016/S0006-8993(96)00774-3
Danielsson, I., Gasior, M., Stevenson, G. W., Folk, J. E., Rice, K. C., and Negus, S. S. (2006). Electroencephalographic and convulsant effects of the delta opioid agonist SNC80 in rhesus monkeys. Pharmacol. Biochem. Behav. 85, 428–434. doi: 10.1016/j.pbb.2006.09.012
de Lanerolle, N. C., Brines, M., Williamson, A., Kim, J. H., and Spencer, D. D. (1992). Neurotransmitters and their receptors in human temporal lobe epilepsy. Epilepsy Res. Suppl. 7, 235–250.
de Lanerolle, N. C., Kim, J. H., Williamson, A., Spencer, S. S., Zaveri, H. P., Eid, T., et al. (2003). A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia 44, 677–687. doi: 10.1046/j.1528-1157.2003.32701.x
de Lanerolle, N. C., Williamson, A., Meredith, C., Kim, J. H., Tabuteau, H., Spencer, D. D., et al. (1997). Dynorphin and the kappa 1 ligand [3H]U69,593 binding in the human epileptogenic hippocampus. Epilepsy Res. 28, 189–205. doi: 10.1016/S0920-1211(97)00044-2
Douglass, J., Grimes, L., Shook, J., Lee, P. H., and Hong, J. S. (1991). Systemic administration of kainic acid differentially regulates the levels of prodynorphin and proenkephalin mRNA and peptides in the rat hippocampus. Brain Res. Mol. Brain Res. 9, 79–86. doi: 10.1016/0169-328X(91)90132-H
Drake, C. T., Chavkin, C., and Milner, T. A. (2007). Opioid systems in the dentate gyrus. Prog. Brain Res. 163, 245–263. doi: 10.1016/S0079-6123(07)63015-5
Drake, C. T., and Milner, T. A. (1999). Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 849, 203–215. doi: 10.1016/S0006-8993(99)01910-1
Drake, C. T., and Milner, T. A. (2002). Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus 12, 119–136. doi: 10.1002/hipo.1107
Drake, C. T., Terman, G. W., Simmons, M. L., Milner, T. A., Kunkel, D. D., Schwartzkroin, P. A., et al. (1994). Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J. Neurosci. 14, 3736–3750.
Eadie, M. J. (2012). Shortcomings in the current treatment of epilepsy. Exp. Rev. Neurother. 12, 1419–1427. doi: 10.1586/ern.12.129
Fallon, J. H., and Leslie, F. M. (1986). Distribution of dynorphin and enkephalin peptides in the rat brain. J. Comp. Neurol. 249, 293–336. doi: 10.1002/cne.902490302
Faulkner, H. J., Traub, R. D., and Whittington, M. A. (1998). Disruption of synchronous gamma oscillations in the rat hippocampal slice: a common mechanism of anaesthetic drug action. Br. J. Pharmacol. 125, 483–492. doi: 10.1038/sj.bjp.0702113
Filliol, D., Ghozland, S., Chluba, J., Martin, M., Matthes, H. W., Simonin, F., et al. (2000). Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 25, 195–200. doi: 10.1038/76061
Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., et al. (2014). ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55, 475–482. doi: 10.1111/epi.12550
Fisher, R. S., van Emde Boas, W., Blume, W., Elger, C., Genton, P., Lee, P., et al. (2005). Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46, 470–472. doi: 10.1111/j.0013-9580.2005.66104.x
Fricchione, G., Zhu, W., Cadet, P., Mantione, K. J., Bromfield, E., Madsen, J., et al. (2008). Identification of endogenous morphine and a mu 3-like opiate alkaloid receptor in human brain tissue taken from a patient with intractable complex partial epilepsy. Med. Sci. Monitor 14, CS45–CS49.
Frost, J. J., Mayberg, H. S., Fisher, R. S., Douglass, K. H., Dannals, R. F., Links, J. M., et al. (1988). Mu-opiate receptors measured by positron emission tomography are increased in temporal-lobe epilepsy. Ann. Neurol. 23, 231–237. doi: 10.1002/ana.410230304
Gall, C. (1988). Seizures induce dramatic and distinctly different changes in enkephalin, dynorphin, and CCK immunoreactivities in mouse hippocampal mossy fibers. J. Neurosci. 8, 1852–1862.
Gall, C., Brecha, N., Karten, H. J., and Chang, K. J. (1981). Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J. Comp. Neurol. 198, 335–350. doi: 10.1002/cne.901980211
Gambardella, A., Manna, I., Labate, A., Chifari, R., Serra, P., La Russa, A., et al. (2003). Prodynorphin gene promoter polymorphism and temporal lobe epilepsy. Epilepsia 44, 1255–1256. doi: 10.1046/j.1528-1157.2003.18003.x
Gao, C. J., Niu, L., Ren, P. C., Wang, W., Zhu, C., Li, Y. Q., et al. (2012). Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience 202, 352–362. doi: 10.1016/j.neuroscience.2011.11.060
Gaveriaux-Ruff, C., and Kieffer, B. L. (2011). Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav. Pharmacol. 22, 405–414. doi: 10.1097/FBP.0b013e32834a1f2c
Goldberg, E. M., and Coulter, D. A. (2013). Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat. Rev. Neurosci. 14, 337–349. doi: 10.1038/nrn3482
Grove, J. R., Deutsch, P. J., Price, D. J., Habener, J. F., and Avruch, J. (1989). Plasmids encoding PKI(1-31), a specific inhibitor of cAMP-stimulated gene-expression, inhibit the basal transcriptional activity of some but not all cAMP regulated DNA response elements in JEG-3 cellsT. J. Biol. Chem. 264, 19506–19513.
Hammers, A., Asselin, M.-C., Hinz, R., Kitchen, I., Brooks, D. J., Duncan, J. S., et al. (2007). Upregulation of opioid receptor binding following spontaneous epileptic seizures. Brain 130, 1009–1016. doi: 10.1093/brain/awm012
Hara, Y., Yakovleva, T., Bakalkin, G., and Pickel, V. M. (2006). Dopamine D1 receptors have subcellular distributions conducive to interactions with prodynorphin in the rat nucleus accumbens shell. Synapse 60, 1–19. doi: 10.1002/syn.20273
Harrison, M. B., Shumate, M. D., and Lothman, E. W. (1995). Opioid peptide expression in models of chronic temporal lobe epilepsy. Neuroscience 65, 785–795. doi: 10.1016/0306-4522(94)00529-E
Henriksen, S. J., Chouvet, G., McGinty, J., and Bloom, F. E. (1982). Opioid peptides in the hippocampus: anatomical and physiological considerations. Ann. N. Y. Acad. Sci. 398, 207–220. doi: 10.1111/j.1749-6632.1982.tb39495.x
Hong, J. S., McGinty, J. F., Lee, P. H., Xie, C. W., and Mitchell, C. L. (1993). Relationship between hippocampal opioid peptides and seizures. Prog. Neurobiol. 40, 507–528. doi: 10.1016/0301-0082(93)90020-S
Hong, J. S., Wood, P. L., Gillin, J. C., Yang, H. Y. T., and Costa, E. (1980). Changes of hippocampal Met-enkephalin content after recurrent motor seizures. Nature 285, 231–232. doi: 10.1038/285231a0
Horikawa, S., Takai, T., Toyosato, M., Takahashi, H., Noda, M., Kakidani, H., et al. (1983). Isolation and structural organization of the human preproenkephalin B gene. Nature 306, 611–614. doi: 10.1038/306611a0
Houser, C. R. (1992). Morphological changes in the dentate gyrus in human temporal lobe epilepsy. Epilepsy Res. Suppl. 7, 223–234.
Houser, C. R., Miyashiro, J. E., Swartz, B. E., Walsh, G. O., Rich, J. R., and Delgado-Escueta, A. V. (1990). Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J. Neurosci. 10, 267–282.
Huge, V., Rammes, G., Beyer, A., Zieglgansberger, W., and Azad, S. C. (2009). Activation of kappa opioid receptors decreases synaptic transmission and inhibits long-term potentiation in the basolateral amygdala of the mouse. Eur. J. Pain 13, 124–129. doi: 10.1016/j.ejpain.2008.03.010
Huggenvik, J. I., Collard, M. W., Stofko, R. E., Seasholtz, A. F., and Uhler, M. D. (1991). Regulation of the human enkephalin promoter by 2 isoforms of the catalytic subunit of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase. Mol. Endocrinol. 5, 921–930. doi: 10.1210/mend-5-7-921
Hughes, J., Smith, T., Morgan, B., and Fothergill, L. (1975). Purification and properties of enkephalin - possible endogenous ligand for morphine receptor. Life Sci. 16, 1753–1758. doi: 10.1016/0024-3205(75)90268-4
Hurd, Y. L. (1996). Differential messenger RNA expression of prodynorphin and proenkephalin in the human brain. Neuroscience 72, 767–783. doi: 10.1016/0306-4522(96)00002-4
Iadarola, M. J., Shin, C., McNamara, J. O., and Yang, H. Y. (1986). Changes in dynorphin, enkephalin and cholecystokinin content of hippocampus and substantia nigra after amygdala kindling. Brain Res. 365, 185–191. doi: 10.1016/0006-8993(86)90738-9
James, I. F., Fischli, W., and Goldstein, A. (1984). Opioid receptor selectivity of dynorphin gene products. J. Pharmacol. Exp. Ther. 228, 88–93.
Jeub, M., Lie, A., Blumcke, I., Elger, C. E., and Beck, H. (1999). Loss of dynorphin-mediated inhibition of voltage-dependent Ca2+ currents in hippocampal granule cells isolated from epilepsy patients is associated with mossy fiber sprouting. Neuroscience 94, 465–471. doi: 10.1016/S0306-4522(99)00249-3
Jutkiewicz, E. M., Baladi, M. G., Folk, J. E., Rice, K. C., and Woods, J. H. (2006). The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J. Pharmacol. Exp. Ther. 317, 1337–1348. doi: 10.1124/jpet.105.095810
Kanamatsu, T., McGinty, J. F., Mitchell, C. L., and Hong, J. S. (1986a). Dynorphin- and enkephalin-like immunoreactivity is altered in limbic-basal ganglia regions of rat brain after repeated electroconvulsive shock. J. Neurosci. 6, 644–649.
Kanamatsu, T., Obie, J., Grimes, L., McGinty, J. F., Yoshikawa, K., Sabol, S., et al. (1986b). Kainic acid alters the metabolism of Met5-enkephalin and the level of dynorphin A in the rat hippocampus. J. Neurosci. 6, 3094–3102.
Khachaturian, H., Watson, S. J., Lewis, M. E., Coy, D., Goldstein, A., and Akil, H. (1982). Dynorphin immunocytochemistry in the rat central nervous system. Peptides 3, 941–954. doi: 10.1016/0196-9781(82)90063-8
Kieffer, B. L., and Evans, C. J. (2009). Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 56, 205–212. doi: 10.1016/j.neuropharm.2008.07.033
Konig, M., Zimmer, A. M., Steiner, H., Holmes, P. V., Crawley, J. N., Brownstein, M. J., et al. (1996). Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 383, 535–538. doi: 10.1038/383535a0
Kovac, S., and Walker, M. C. (2013). Neuropeptides in epilepsy. Neuropeptides 47, 467–475. doi: 10.1016/j.npep.2013.10.015
Kulmala, H. K. (1985). Immunocytochemical localization of enkephalin-like immunoreactivity in neurons of human hippocampal-formation - effects of aging and Alzheimers-disease. Neuropathol. Appl. Neurobiol. 11, 105–115. doi: 10.1111/j.1365-2990.1985.tb00008.x
Lason, W., Przewlocka, B., and Przewlocki, R. (1992a). The effects of excitatory amino acids on proenkephalin and prodynorphin mRNA levels in the hippocampal dentate gyrus of the rat; an in situ hybridization study. Brain Res. Mol. Brain Res. 12, 243–247. doi: 10.1016/0169-328X(92)90090-X
Lason, W., Przewlocka, B., and Przewlocki, R. (1992b). The prodynorphin system in the rat hippocampus is differentially influenced by kainic acid and pentetrazole. Neuroscience 51, 357–362. doi: 10.1016/0306-4522(92)90320-2
Laxer, K. D., Trinka, E., Hirsch, L. J., Cendes, F., Langfitt, J., Delanty, N., et al. (2014). The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 37, 59–70. doi: 10.1016/j.yebeh.2014.05.031
Lee, P. H., Zhao, D., Xie, C. W., McGinty, J. F., Mitchell, C. L., and Hong, J. S. (1989). Changes of proenkephalin and prodynorphin mRNAs and related peptides in rat brain during the development of deep prepyriform cortex kindling. Brain Res. Mol. Brain Res. 6, 263–273. doi: 10.1016/0169-328X(89)90072-7
Lin, S., Boey, D., Lee, N., Schwarzer, C., Sainsbury, A., and Herzog, H. (2006). Distribution of prodynorphin mRNA and its interaction with the NPY system in the mouse brain. Neuropeptides 40, 115–123. doi: 10.1016/j.npep.2005.11.006
Loacker, S., Sayyah, M., Wittmann, W., Herzog, H., and Schwarzer, C. (2007). Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa opioid receptors. Brain 130, 1017–1028. doi: 10.1093/brain/awl384
Lupica, C. R. (1995). Delta-enkephalin and Mu-enkephalin inhibit spontaneous GABA-mediated IPSCs via cyclic-AMP-independent mechanism in th erat hippocampus. J. Neurosci. 15, 737–749.
Ma, M. C., Qian, H., Ghassemi, F., Zhao, P., and Xia, Y. (2005). Oxygen-sensitive delta-opioid receptor-regulated survival and death signals - Novel insights into neuronal preconditioning and protection. J. Biol. Chem. 280, 16208–16218. doi: 10.1074/jbc.M408055200
Mansour, A., Khatchaturian, H., Lewis, M. E., Akil, H., and Watson, S. J. (1988). Anatomy of CNS opioid receptors. Trends Neurosci. 11, 308–314. doi: 10.1016/0166-2236(88)90093-8
Mansouri, F. A., Motamedi, F., and Fathollahi, Y. (1999). Chronic in vivo morphine administration facilitates primed-bursts-induced long-term potentiation of Schaffer collateral-CA1 synapses in hippocampal slices in vitro. Brain Res. 815, 419–423. doi: 10.1016/S0006-8993(98)01148-2
Mansouri, F. A., Motamedi, F., Fathollahi, Y., Atapour, N., and Semnanian, S. (1997). Augmentation of LTP induced by Primed-Bursts tetanic stimulation in hippocampal CA1 area of morphine dependent rats. Brain Res. 769, 119–124. doi: 10.1016/S0006-8993(97)00608-2
Marek, B., Kajdaniuk, D., Kos-Kudla, B., Kapustecki, J., Swietochowska, E., Ostrowska, Z., et al. (2010). Mean daily plasma concentrations of beta-endorphin, leu-enkephalin, ACTH, cortisol, and DHEAS in epileptic patients with complex partial seizures evolving to generalized tonic-clonic seizures. Endokrynol. Pol. 61, 103–110.
Marksteiner, J., Sperk, G., and Maas, D. (1989). Differential increases in brain levels of neuropeptide Y and vasoactive intestinal polypeptide after kainic acid-induced seizures in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 339, 173–177.
Mathieu-Kia, A. M., Fan, L. Q., Kreek, M. J., Simon, E. J., and Hiller, J. M. (2001). Mu-, delta- and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer's disease patients. Brain Res. 893, 121–134. doi: 10.1016/S0006-8993(00)03302-3
Matsu-ura, T., Konishi, Y., Aoki, T., Naranjo, J. R., Mikoshiba, K., and Tamura, T. A. (2002). Seizure-mediated neuronal activation induces DREAM gene expression in the mouse brain. Brain Res. Mol. Brain Res. 109, 198–206. doi: 10.1016/S0169-328X(02)00562-4
Mayfield, K. P., and D'Alecy, L. G. (1994). Delta-1 opioid agonist acutely increases hypoxic tolerance. J. Pharmacol. Exp. Ther. 268, 683–688.
Mazarati, A., Liu, H., and Wasterlain, C. (1999). Opioid peptide pharmacology and immunocytochemistry in an animal model of self-sustaining status epilepticus. Neuroscience 89, 167–173. doi: 10.1016/S0306-4522(98)00320-0
McDermott, C. M., and Schrader, L. A. (2011). Activation of kappa opioid receptors increases intrinsic excitability of dentate gyrus granule cells. J. Physiol. London 589, 3517–3532. doi: 10.1113/jphysiol.2011.211623
McGinty, J. F., Henriksen, S. J., Goldstein, A., Terenius, L., and Bloom, F. E. (1983). Dynorphin is contained within hippocampal mossy fibers: immunochemical alterations after kainic acid administration and colchicine-induced neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 80, 589–593. doi: 10.1073/pnas.80.2.589
McGinty, J. F., Kanamatsu, T., Obie, J., Dyer, R. S., Mitchell, C. L., and Hong, J. S. (1986). Amygdaloid kindling increases enkephalin-like immunoreactivity but decreases dynorphin-A-like immunoreactivity in rat hippocampus. Neurosci. Lett. 71, 31–36. doi: 10.1016/0304-3940(86)90252-1
Meldrum, B. S. (2002). Concept of activity-induced cell death in epilepsy: historical and contemporary perspectives. Prog. Brain Res. 135, 3–11. doi: 10.1016/S0079-6123(02)35003-9
Merchenthaler, I., Maderdrut, J. L., Cianchetta, P., Shughrue, P., and Bronstein, D. (1997). In situ hybridization histochemical localization of prodynorphin messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 384, 211–232. doi: 10.1002/(SICI)1096-9861(19970728)384:2<211::AID-CNE4>3.0.CO;2-4
Miller, R. J., and Pickel, V. M. (1980). The distribution and function of teh enkephalins. J. Histochem. Cytochem. 28, 903–917. doi: 10.1177/28.8.7003009
Morinville, A., Cahill, C. M., Aibak, H., Rymar, V. V., Pradhan, A., Hoffert, C., et al. (2004). Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J. Neurosci. 24, 5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004
Morris, B. J., Haarmann, I., Kempter, B., Hollt, V., and Herz, A. (1986). Localization of prodynorphin messenger rna in rat brain by in situ hybridization using a synthetic oligonucleotide probe. Neurosci. Lett. 69, 104–108. doi: 10.1016/0304-3940(86)90423-4
Morris, B. J., and Johnston, H. M. (1995). A role for hippocampal opioids in long-term functional plasticity. Trends Neurosci. 18, 350–355. doi: 10.1016/0166-2236(95)93927-P
Morris, B. J., Moneta, M. E., ten Bruggencate, G., and Hollt, V. (1987). Levels of prodynorphin mRNA in rat dentate gyrus are decreased during hippocampal kindling. Neurosci. Lett. 80, 298–302. doi: 10.1016/0304-3940(87)90471-X
Mula, M., and Sander, J. W. (2013). Suicide risk in people with epilepsy taking antiepileptic drugs. Bipolar. Disord. 15, 622–627. doi: 10.1111/bdi.12091
Narita, M., Kaneko, C., Miyoshi, K., Nagumo, Y., Kuzumaki, N., Nakajima, M., et al. (2006). Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31, 739–750. doi: 10.1038/sj.npp.1300858
Negus, S. S., Gatch, M. B., Mello, N. K., Zhang, X. Y., and Rice, K. (1998). Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J. Pharmacol. Exp. Ther. 286, 362–375.
Neumaier, J. F., Mailheau, S., and Chavkin, C. (1988). Opioid receptor-mediated responses in the dentate gyrus and CA1 region of the rat hippocampus. J. Pharmacol. Exp. Ther. 244, 564–570.
Niikura, K., Zhou, Y., Ho, A., and Kreek, M. J. (2013). Proopiomelanocortin (POMC) expression adn conditioned place aversion during protracted withdrawal from chronic intermittent escalating-dose heroin in POMC-EGFP promoter transgenic mice. Neuroscience 236, 220–232. doi: 10.1016/j.neuroscience.2012.12.071
Nikoshkov, A., Hurd, Y. L., Yakovleva, T., Bazov, I., Marinova, Z., Cebers, G., et al. (2005). Prodynorphin transcripts and proteins differentially expressed and regulated in the adult human brain. FASEB J. 19, 1543–1545. doi: 10.1096/fj.05-3743fje
ONeill, S. J., Collins, M. A., Pettit, H. O., McNutt, R. W., and Chang, K. J. (1997). Antagonistic modulation between the delta opioid agonist BW373U86 and the mu opioid agonist fentanyl in mice. J. Pharmacol. Exp. Ther. 282, 271–277.
Peppin, J. F., and Raffa, R. B. (2015). Delta opioid agonists: a concise update on potential therapeutic applications. J. Clin. Pharm. Ther. 40, 155–166. doi: 10.1111/jcpt.12244
Perucca, P., and Gilliam, F. G. (2012). Adverse effects of antiepileptic drugs. Lancet Neurol. 11, 792–802. doi: 10.1016/S1474-4422(12)70153-9
Pirker, S., Gasser, E., Czech, T., Baumgartner, C., Schuh, E., Feucht, M., et al. (2009). Dynamic up-regulation of prodynorphin transcription in temporal lobe epilepsy. Hippocampus 19, 1051–1054. doi: 10.1002/hipo.20633
Pitkanen, A., Jolkkonen, J., and Riekkinen, P. (1987). Beta-endorphin, somatostatin, and prolactin levels in cerebrospinal-fluid of epielptic patients after generalized convulsion. J. Neurol. Neurosurg. Psychiatry 50, 1294–1297. doi: 10.1136/jnnp.50.10.1294
Pourmotabbed, A., Motamedi, F., Fathollahi, Y., Mansouri, F. A., and Semnanian, S. (1998). Involvement of NMDA receptors and voltage-dependent calcium channels on augmentation of long-term potentiation in hippocampal CA1 area of morphine dependent rats. Brain Res. 804, 125–134. doi: 10.1016/S0006-8993(98)00676-3
Pradhan, A. A., Befort, K., Nozaki, C., Gaveriaux-Ruff, C., and Kieffer, B. L. (2011). The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol. Sci. 32, 581–590. doi: 10.1016/j.tips.2011.06.008
Przewlocka, B., Lason, W., Machelska, H., van Luijtelaar, G., Coenen, A., and Przewlocki, R. (1995). Kappa opioid receptor agonists suppress absence seizures in WAG/Rij rats. Neurosci. Lett. 186, 131–134. doi: 10.1016/0304-3940(95)11303-E
Quock, R. M., Burkey, T. H., Varga, E., Hosohata, Y., Hosohata, K., Cowell, S. M., et al. (1999). The delta-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol. Rev. 51, 503–532.
Racz, B., and Halasy, K. (2002). Kappa opioid receptor is expressed by somatostatin- and neuropeptide Y-containing interneurons in the rat hippocampus. Brain Res. 931, 50–55. doi: 10.1016/S0006-8993(02)02259-X
Rezai, X., Faget, L., Bednarek, E., Schwab, Y., Kieffer, B. L., and Massotte, D. (2012). Mouse delta opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells. Cell. Mol. Neurobiol. 32, 509–516. doi: 10.1007/s10571-011-9791-1
Richards, E. M., Mathews, D. C., Luckenbaugh, D. A., Ionescu, D. F., Machado-Vieira, R., Niciu, M. J., et al. (2016). A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology 233, 1119–1130. doi: 10.1007/s00213-015-4195-4
Rocha, L., Cuellar-Herrera, M., Velasco, M., Velasco, F., Velasco, A.-L., Jimenez, F., et al. (2007). Opioid receptor binding in parahippocampus of patients with temporal lobe epilepsy: its association with the antiepileptic effects of subacute electrical stimulation. Seizure 16, 645–652. doi: 10.1016/j.seizure.2007.05.007
Rocha, L. L., Evans, C. J., and Maidment, N. T. (1997). Amygdala kindling modifies extracellular opioid peptide content in rat hippocampus measured by microdialysis. J. Neurochem. 68, 616–624. doi: 10.1046/j.1471-4159.1997.68020616.x
Rocha, L., and Maidment, N. T. (2003). Opioid peptide release in the rat hippocampus after kainic acid-induced status epilepticus. Hippocampus 13, 472–480. doi: 10.1002/hipo.10078
Rocha, L., Orozco-Suarez, S., Alonso-Vanegas, M., Villeda-Hernandez, J., Gaona, A., Paldy, E., et al. (2009). Temporal lobe epilepsy causes selective changes in mu opioid and nociceptin receptor binding and functional coupling to G-proteins in human temporal neocortex. Neurobiol. Dis. 35, 466–473. doi: 10.1016/j.nbd.2009.06.008
Rosen, J. B., Cain, C. J., Weiss, S. R., and Post, R. M. (1992). Alterations in mRNA of enkephalin, dynorphin and thyrotropin releasing hormone during amygdala kindling: an in situ hybridization study. Brain Res. Mol. Brain Res. 15, 247–255. doi: 10.1016/0169-328X(92)90115-R
Saitoh, A., Sugiyama, A., Nemoto, T., Fujii, H., Wada, K., Oka, J.-I., et al. (2011). The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav. Brain Res. 223, 271–279. doi: 10.1016/j.bbr.2011.04.041
Salin, P. A., Weisskopf, M. G., and Nicoll, R. A. (1995). A comparison of the role of dynorphin in the hippocampal mossy fiber pathway in guinea pig and rat. J. Neurosci. 15, 6939–6945.
Schunk, E., Aigner, C., Stefanova, N., Wenning, G., Herzog, H., and Schwarzer, C. (2011). Kappa opioid receptor activation blocks progressive neurodegeneration after kainic acid injection. Hippocampus 21, 1010–1020. doi: 10.1002/hipo.20813
Schwarzer, C. (2009). 30 years of dynorphins–new insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 123, 353–370. doi: 10.1016/j.pharmthera.2009.05.006
Schwarzer, C., and Sperk, G. (1998). Glutamate-stimulated neuropeptide Y mRNA expression in the rat dentate gyrus: a prominent role of metabotropic glutamate receptors. Hippocampus 8, 274–288.
Siggins, G. R., Henriksen, S. J., Chavkin, C., and Gruol, D. (1986). Opioid peptides and epileptogenesis in the limbic system: cellular mechanisms. Adv. Neurol. 44, 501–512.
Simantov, R., and Snyder, S. H. (1976). Isolation and structure identification of a morphine-like peptide enkepahalin in bovine brain. Life Sci. 18, 781–788. doi: 10.1016/0024-3205(76)90002-3
Simmons, M. L., and Chavkin, C. (1996). k-Opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol. Pharmacol. 50, 80–85.
Simmons, M. L., Terman, G. W., Gibbs, S. M., and Chavkin, C. (1995). L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron 14, 1265–1272. doi: 10.1016/0896-6273(95)90273-2
Skyers, P. S., Einheber, S., Pierce, J. P., and Milner, T. A. (2003). Increased mu-opioid receptor labeling is found on inner molecular layer terminals of the dentate gyrus following seizures. Exp. Neurol. 179, 200–209. doi: 10.1016/S0014-4886(02)00018-3
Slamberova, R., Rimanoczy, A., Bar, N., Schindler, C. J., and Vathy, I. (2003). Density of mu-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus 13, 461–471. doi: 10.1002/hipo.10076
Solbrig, M. V., Adrian, R., Baratta, J., Lauterborn, J. C., and Koob, G. F. (2006). Kappa opioid control of seizures produced by a virus in an animal model. Brain 129, 642–654. doi: 10.1093/brain/awl008
Spencer, S., and Huh, L. (2008). Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 7, 525–537. doi: 10.1016/S1474-4422(08)70109-1
Sperk, G., Wieser, R., Widmann, R., and Singer, E. A. (1986). Kainic acid-induced seizures - changes in somatostatin, substance-P and neurotensin. Neuroscience 17, 1117–1126. doi: 10.1016/0306-4522(86)90081-3
Stengaardpedersen, K. (1983). Comparative mapping of opioid receptors and enkephalin immunoreactive nerve-terminals in the rat hippocampus - a radiohistochemical and immunohistochemical study. Histochemistry 79, 311–333. doi: 10.1007/BF00491768
Stogmann, E., Zimprich, A., Baumgartner, C., Aull-Watschinger, S., Hollt, V., and Zimprich, F. (2002). A functional polymorphism in the prodynorphin gene promotor is associated with temporal lobe epilepsy. Ann. Neurol. 51, 260–263. doi: 10.1002/ana.10108
Takahashi, M., Senda, T., Tokuyama, S., and Kaneto, H. (1990). Further evidence for the implication of a kappa-opioid receptor mechanism in the production of psychological stress-induced analgesia. Jpn. J. Pharmacol. 53, 487–494. doi: 10.1254/jjp.53.487
Telkov, M., Geijer, T., and Terenius, L. (1998). Human prodynorphin gene generates several tissue-specific transcripts. Brain Res. 804, 284–295. doi: 10.1016/S0006-8993(98)00706-9
Terman, G. W., Drake, C. T., Simmons, M. L., Milner, T. A., and Chavkin, C. (2000). Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J. Neurosci. 20, 4379–4388.
Thurman, D. J., Beghi, E., Begley, C. E., Berg, A. T., Buchhalter, J. R., Ding, D., et al. (2011). Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 52(Suppl. 7), 2–26. doi: 10.1111/j.1528-1167.2011.03121.x
Tortella, F. C. (1988). Endogenous opioid peptides and epilepsy: quieting the seizing brain? Trends Pharmacol. Sci. 9, 366–372. doi: 10.1016/0165-6147(88)90256-8
Tortella, F. C., Echevarria, E., Lipkowski, A. W., Takemori, A. E., Portoghese, P. S., and Holaday, J. W. (1989). Selective kappa antagonist properties of nor-binaltorphimine in the rat MES seizure model. Life Sci. 44, 661–665. doi: 10.1016/0024-3205(89)90470-0
Tortella, F. C., Robles, L., Echevarria, E., Hunter, J. C., and Hughes, J. (1990). PD117302, a selective non-peptide opioid kappa agonist, protects against NMDA and maximal electroshock convulsions in rats. Life Sci. 46, PL1–PL7. doi: 10.1016/0024-3205(90)90501-H
Tortella, F. C., Robles, L., and Holaday, J. W. (1986). U50,488, a highly selective kappa opioid: anticonvulsant profile in rats. J. Pharmacol. Exp. Ther. 237, 49–53.
Van Bockstaele, E. J., Gracy, K. N., and Pickel, V. M. (1995). Dynorphin-immunoreactive neurons in the rat nucleus accumbens: ultrastructure and synaptic input from terminals containing substance P and/or dynorphin. J. Comp. Neurol. 351, 117–133. doi: 10.1002/cne.903510111
van Rijn, R. M., DeFriel, J. N., and Whistler, J. L. (2013). Pharmacological traits of delta opioid receptors: pitfalls or opportunities? Psychopharmacology 228, 1–18. doi: 10.1007/s00213-013-3129-2
Vincent, S., Hokfelt, T., Christensson, I., and Terenius, L. (1982a). Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur. J. Pharmacol. 85, 251–252. doi: 10.1016/0014-2999(82)90477-0
Vincent, S. R., Hokfelt, T., Christensson, I., and Terenius, L. (1982b). Dynorphin-immunoreactive neurons in the central nervous system of the rat. Neurosci. Lett. 33, 185–190. doi: 10.1016/0304-3940(82)90249-X
Wagner, J. J., Terman, G. W., and Chavkin, C. (1993). Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature 363, 451–454. doi: 10.1038/363451a0
WHO (2015). Fact Sheet 999: Epilepsy. Available online at: http://www.who.int/mediacentre/factsheets/fs999/en/
Weber, E., and Barchas, J. D. (1983). Immunohistochemical distribution of dynorphin B in rat brain: relation to dynorphin A and alpha-neo-endorphin systems. Proc. Natl. Acad. Sci. U.S.A. 80, 1125–1129. doi: 10.1073/pnas.80.4.1125
Weinberg, M. S., Samulski, R. J., and McCown, T. J. (2013). Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology 69, 82–88. doi: 10.1016/j.neuropharm.2012.03.004
Weisskopf, M. G., Zalutsky, R. A., and Nicoll, R. A. (1993). The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature 362, 423–427. doi: 10.1038/362423a0
Xie, C. W., Lee, P. H., Douglass, J., Crain, B., and Hong, J. S. (1989a). Deep prepyriform cortex kindling differentially alters the levels of prodynorphin mRNA in rat hippocampus and striatum. Brain Res. 495, 156–160. doi: 10.1016/0006-8993(89)91230-4
Xie, C. W., Lee, P. H., Takeuchi, K., Owyang, V., Li, S. J., Douglass, J., et al. (1989b). Single or repeated electroconvulsive shocks alter the levels of prodynorphin and proenkephalin mRNAs in rat brain. Brain Res. Mol. Brain Res. 6, 11–19. doi: 10.1016/0169-328X(89)90023-5
Yakovleva, T., Bazov, I., Cebers, G., Marinova, Z., Hara, Y., Ahmed, A., et al. (2006). Prodynorphin storage and processing in axon terminals and dendrites. FASEB J. 20, 2124–2126. doi: 10.1096/fj.06-6174fje
Yang, Y. L., Xia, X. W., Zhang, Y., Wang, Q., Li, L., Luo, G. H., et al. (2009). Delta-opioid receptor activation attenuates oxidative injury in the ischemic rat brain. J. Neurol. Sci. 285, S82–S82. doi: 10.1016/S0022-510X(09)70347-8
Yoshikawa, K., Hong, J. S., and Sabol, S. L. (1985). Electroconvulsive shock increases preproenkephalin messenger-RNA abundance in rat hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 82, 589–593. doi: 10.1073/pnas.82.2.589
Zakarian, S., and Smyth, D. (1979). Distribution of active and inactive forms of endorphins in rat pituitary and brain. Proc. Natl. Acad. Sci. U.S.A. 76, 5972–5976. doi: 10.1073/pnas.76.11.5972
Zakarian, S., and Smyth, D. G. (1982). Distribution of beta-endorphin-related peptides in rat pituitary and brain. Biochem. J. 202, 561. doi: 10.1042/bj2020561
Zangrandi, L., Burtscher, J., MacKay, J. P., Colmers, W. F., and Schwarzer, C. (2016). The G-protein biased partial kappa opioid receptor agonist 6'-GNTI blocks hippocampal paroxysmal discharges without inducing aversion. Br. J. Pharmacol. 173, 1756–1767. doi: 10.1111/bph.13474
Zhang, J., Haddad, G. G., and Xia, Y. (2000). delta-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 885, 143–153. doi: 10.1016/S0006-8993(00)02906-1
Zhu, M., Li, M., Tian, X., Ou, X., Zhu, C., and Guo, J. (2009). Neuroprotective role of delta-opioid receptors against mitochondrial respiratory chain injury. Brain Res. 1252, 183–191. doi: 10.1016/j.brainres.2008.11.030
Zhu, M., Li, M., Yang, F., Ou, X., Ren, Q., Gao, H., et al. (2011). Mitochondrial ERK plays a key role in delta-opioid receptor neuroprotection against acute mitochondrial dysfunction. Neurochem. Int. 59, 739–748. doi: 10.1016/j.neuint.2011.08.005
Keywords: hippocampus, dynorphin, enkephalin, endorphin, seizures, kappa-opioid receptor, mu-opioid receptor, delta-opioid receptor
Citation: Burtscher J and Schwarzer C (2017) The Opioid System in Temporal Lobe Epilepsy: Functional Role and Therapeutic Potential. Front. Mol. Neurosci. 10:245. doi: 10.3389/fnmol.2017.00245
Received: 22 May 2017; Accepted: 24 July 2017;
Published: 07 August 2017.
Edited by:
Detlev Boison, Legacy Health, United StatesReviewed by:
Munjal M. Acharya, University of California, Irvine School of Medicine, United StatesPhilip Forsyth Copenhaver, Oregon Health & Science University, United States
Copyright © 2017 Burtscher and Schwarzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christoph Schwarzer, schwarzer.christoph@i-med.ac.at
 Johannes Burtscher
Johannes Burtscher Christoph Schwarzer
Christoph Schwarzer