The Therapeutic Potential of Monocyte/Macrophage Manipulation in the Treatment of Chemotherapy-Induced Painful Neuropathy
- Wolfson Centre for Age-Related Diseases, Guy’s Hospital Campus, King’s College London, London, United Kingdom
In cancer treatments a dose-limiting side-effect of chemotherapeutic agents is the development of neuropathic pain, which is poorly managed by clinically available drugs at present. Chemotherapy-induced painful neuropathy (CIPN) is a major cause of premature cessation of treatment and so a greater understanding of the underlying mechanisms and the development of novel, more effective therapies, is greatly needed. In some cases, only a weak correlation between chemotherapy-induced pain and neuronal damage is observed both clinically and preclinically. As such, a critical role for non-neuronal cells, such as immune cells, and their communication with neurons in CIPN has recently been appreciated. In this mini-review, we will discuss preclinical evidence for the role of monocytes/macrophages in the periphery in CIPN, with a focus on that which is associated with the chemotherapeutic agents vincristine and paclitaxel. In addition we will discuss the potential mechanisms that regulate monocyte/macrophage–neuron crosstalk in this context. Informed by preclinical data, we will also consider the value of monocytes/macrophages as therapeutic targets for the treatment of CIPN clinically. Approaches that manipulate the signaling pathways discussed in this review show both promise and potential pitfalls. Nonetheless, they are emerging as innovative therapeutic targets with CX3CL1/R1-regulation of monocyte/macrophage–neuron communication currently emerging as a promising front-runner.
Introduction
Chemotherapy-induced painful neuropathy (CIPN) is a dose-limiting side-effect of chemotherapeutic agents including taxanes, vinca alkaloids, and platinum-based compounds (Seretny et al., 2014). Currently available analgesics such as Gabapentin shows limited efficacy for CIPN and begets numerous undesirable side-effects themselves such as dizziness and nausea (Bloodworth, 2005; Dougherty et al., 2007). Consequently, chemotherapy is often prematurely terminated, which can jeopardize treatment success. The development of novel, more effective analgesics, which requires advances in our understanding of the underlying mechanisms of CIPN and thus identification of innovative targets, is therefore greatly needed. CIPN was initially considered to depend entirely on the responses of neurons injured by chemotherapeutic agents. Preclinical evidence, however, has uncovered a critical role of immune cell communication with neurons in the underlying mechanisms of CIPN. Indeed, a key observation indicating that neurons alone do not regulate CIPN is that pain can still arise in the absence of extensive neuronal damage, which is the case for the vinca alkaloid vincristine (Old et al., 2014). It is well-established that chemotherapeutic agents stimulate the immune system (Zitvogel et al., 2008) and so it is unsurprising that recent investigation has given consideration to immune cells in CIPN. Indeed, microglial communication with neurons in the spinal cord dorsal horn, through alterations in their expression and release of cytokines and chemokines, has been shown to be pivotal in several chronic pain states including CIPN (Malcangio, 2016; Montague and Malcangio, 2017). In some preclinical models of CIPN, however, immune cells in the central nervous system do not appear to orchestrate pain and immune cell signaling in the periphery, at the site of injury, plays a more pertinent role (Old et al., 2014).
Most chemotherapeutic agents do not penetrate the blood–brain barrier (BBB) but can cross the blood–nerve barrier (BNB) where they accumulate in dorsal root ganglia (DRG) and peripheral nerves, exerting toxicity that is exacerbated by the absence of a lymphatic system in the endoneural compartment (Weimer, 2003; Balayssac et al., 2005; Cavaletti et al., 2008). Penetration of the BNB can be attributed to relatively low levels of P-glycoprotein transporter activity, which limits the efficiency of toxin removal (Balayssac et al., 2005). As well as the intrusion of toxins, immune cells also infiltrate into peripheral nervous tissue. The penetration of toxins and immune cells through the BNB is exacerbated as a consequence of BNB breakdown by matrix metalloproteinases (MMPs), some of which are upregulated by chemotherapeutic agents (Peters et al., 2007). The peripheral nervous system is therefore considerably more susceptible than the central nervous system to chemotherapy-associated toxicity.
In this mini-review, we consider the role of immune cells in the periphery, specifically monocytes/macrophages, and consider the therapeutic potential of their manipulation for the prevention and/or treatment of pain in CIPN. Neuropathic pain that is associated with different chemotherapeutic agents is likely to be regulated by distinct underlying mechanisms. Here, we will focus predominantly on preclinical models of vincristine and paclitaxel neuropathic pain.
Monocyte/Macrophages and Pain-Like Behavior In Preclinical Cipn
Monocytes are heterogenous, plastic blood cells that monitor environmental changes and alter their phenotype accordingly, differentiating into either inflammatory or anti-inflammatory subsets (Ingersoll et al., 2011; Yang et al., 2014). The primary role of monocytes was initially considered to be most prominent under steady-state conditions, with monocytes infiltrating into tissue and differentiating into tissue-resident macrophages, which serve the function of clearing cellular debris (Mueller et al., 2003). Monocyte phenotype and function, however, are now known to be more extensive, with specific subtypes possessing distinct pathophysiological functions (Yang et al., 2014). “Classic” inflammatory monocytes, for example, express a specific subset of toll-like receptors (TLRs) as well as the chemokine receptor CCR2, which regulates recruitment of monocytes to sites of injury/inflammation (Kurihara et al., 1997; Ginhoux and Jung, 2014). At present, monocytes are most commonly identified according to their expression of the inflammatory marker lymphocyte antigen 6 complex C (Ly6C). Ly6C-positive (+) monocytes are considered to possess an inflammatory phenotype and express high levels of CCR2 while “patrolling,” Ly6C-negative (-) monocytes are conventionally negative for CCR2 but instead express an alternative chemokine receptor – CX3CR1, which is exclusively activated by CX3CL1 (Si et al., 2010; Clark and Malcangio, 2014). At a steady state, Ly6C+ monocytes differentiate into a Ly6C-CX3 phenotype in the circulation, which patrol the endothelium (Carlin et al., 2013). Under adverse conditions, however, CX3CR1 signaling in monocytes mediates their rapid infiltration through the endothelium and into tissue where they differentiate into macrophages (Auffray et al., 2007).
Infiltration of monocytes into the DRG and sciatic nerve, where they differentiate into inflammatory macrophages, has been observed in several preclinical models of CIPN and corresponds with model-associated pain. In rats treated with the taxane paclitaxel, for example, the number of macrophages in the DRG is significantly elevated concurrent with the development of cold hyperalgesia and mechanical hypersensitivity (Peters et al., 2007). The association between monocyte/macrophage infiltration and some preclinical models of CIPN has been reinforced pharmacologically. Minocycline, for example, which alongside other actions, inhibits monocyte/macrophage infiltration (Liu et al., 2010), has been shown to alleviate oxaliplatin-induced pain (Boyette-Davis and Dougherty, 2011). Furthermore, depletion of macrophages using liposome-encapsulated clodronate (LCL) reduces paclitaxel-associated mechanical hypersensitivity. LCL concurrently lowers the paclitaxel-induced increase in macrophages in the DRG as well as the expression of the proinflammatory cytokine tumor necrosis factor alpha (TNFα), suggesting that macrophages comprise a feedback mechanism that increases monocyte/macrophage infiltration and proinflammatory cytokine expression (Zhang et al., 2016). This association between monocyte/macrophage infiltration and pain also applies to preclinical CIPN induced by vinca alkaloids. In a vincristine model of CIPN for instance, mechanical hypersensitivity and the elevation of macrophages in the DRG and sciatic nerve occur concomitantly within 24 h of the first vincristine dose and remain elevated during, and a few weeks after, treatment completion (Old et al., 2014). Furthermore, when mechanical hypersensitivity is no longer present a few weeks after treatment cessation, the number of macrophages in the DRG and sciatic nerve is also no longer elevated, suggesting that macrophage elevation in the DRG and sciatic nerve is functionally linked to pain-like behavior. Indeed, transient depletion of macrophages using LCL significantly delays the onset of vincristine-induced mechanical hypersensitivity (Old et al., 2014).
The strong association between increased monocyte/macrophage infiltration into the DRG and sciatic nerve with pain-like behavior in several preclinical models of CIPN suggests that manipulating monocytes/macrophages in the periphery has prophylactic and therapeutic potential for CIPN associated with some chemotherapeutic agents and could form the basis of innovative therapies. In order to identify the most efficacious approach, an understanding of how monocytes/macrophages communicate with neurons in response to chemotherapy treatment is essential. An established means by which macrophages communicate with neurons is chemokine signaling. Indeed, evidence for the role of chemokine-mediated macrophage–neuron communication as well as monocyte–endothelium crosstalk in some preclinical models of CIPN has strengthened considerably in the last few years.
Chemokine-Mediated Monocyte/Macrophage–Neuron Communication
CX3CL1/R1 Signaling in CIPN
At present, the chemokine that perhaps appears to have the most authentic role in mediating monocyte/macrophage–neuron crosstalk in the periphery in some models of CIPN is CX3CL1. CX3CL1 (fractalkine) is the only member of the CX3C family of chemokines that was first described 20 years ago (Bazan et al., 1997). CX3CL1 exists as both membrane-tethered and soluble forms and in the periphery is expressed by endothelial cells (Imaizumi et al., 2004). Soluble, peripheral CX3CL1 is generated constitutively by cleavage mediated by the endothelial-derived metalloprotease ADAM10, while ADAM17 regulates cleavage during adverse conditions (Hundhausen et al., 2003; Hurst et al., 2009). Unlike other chemokines, for which signaling is promiscuous (Bennett et al., 2011), CX3CL1 exclusively activates CX3CR1, which is expressed by patrolling monocytes (Jung et al., 2000). Endothelial CX3CL1 activation of CX3CR1 in monocytes plays a role in, although is not essential for, monocyte crawling along the endothelium (Carlin et al., 2013), while soluble CX3CL1 activation of CX3CR1 promotes their transendothelial migration (Schwarz et al., 2010).
CX3CR1-expressing monocytes appear to orchestrate the development of pain in a preclinical vincristine model of CIPN. Specifically, in CX3CR1 knock-out (KO) mice, there is a significant delay in the induction of mechanical hypersensitivity by vincristine that resembles the delay observed when macrophages are transiently depleted with LCL (Old et al., 2014). Concurrent with delayed mechanical hypersensitivity, a delay in monocyte infiltration into the sciatic nerve is also observed in CX3CR1 KO mice. Whereas the number of cells expressing the macrophage marker F4/80 is elevated within 1 day of the first vincristine dose in control mice, CX3CR1 KO mice do not display a significant increase in F4/80+ cells in the sciatic nerve until day 5 – the same time point at which mechanical hypersensitivity appears in these mice. Intriguingly, injury-associated monocyte/macrophage infiltration into the sciatic nerve does not appear to be affected in CX3CR1 KO mice following partial sciatic nerve ligation (Staniland et al., 2010), suggesting that the involvement of CX3CR1 signaling in monocytes/macrophages is model-specific. Although vincristine does not appear to increase endothelial expression of CX3CL1, it does increase endothelial expression of adhesion molecules, which could promote recruitment of CX3CR1-expressing monocytes and subsequent infiltration into the sciatic nerve. Here, macrophages generate reactive oxygen species (ROS) in response to vincristine in a CX3CR1-dependent manner, which in turn activate TRPA1 channels thus evoking pain (Figure 1) (Old et al., 2014).
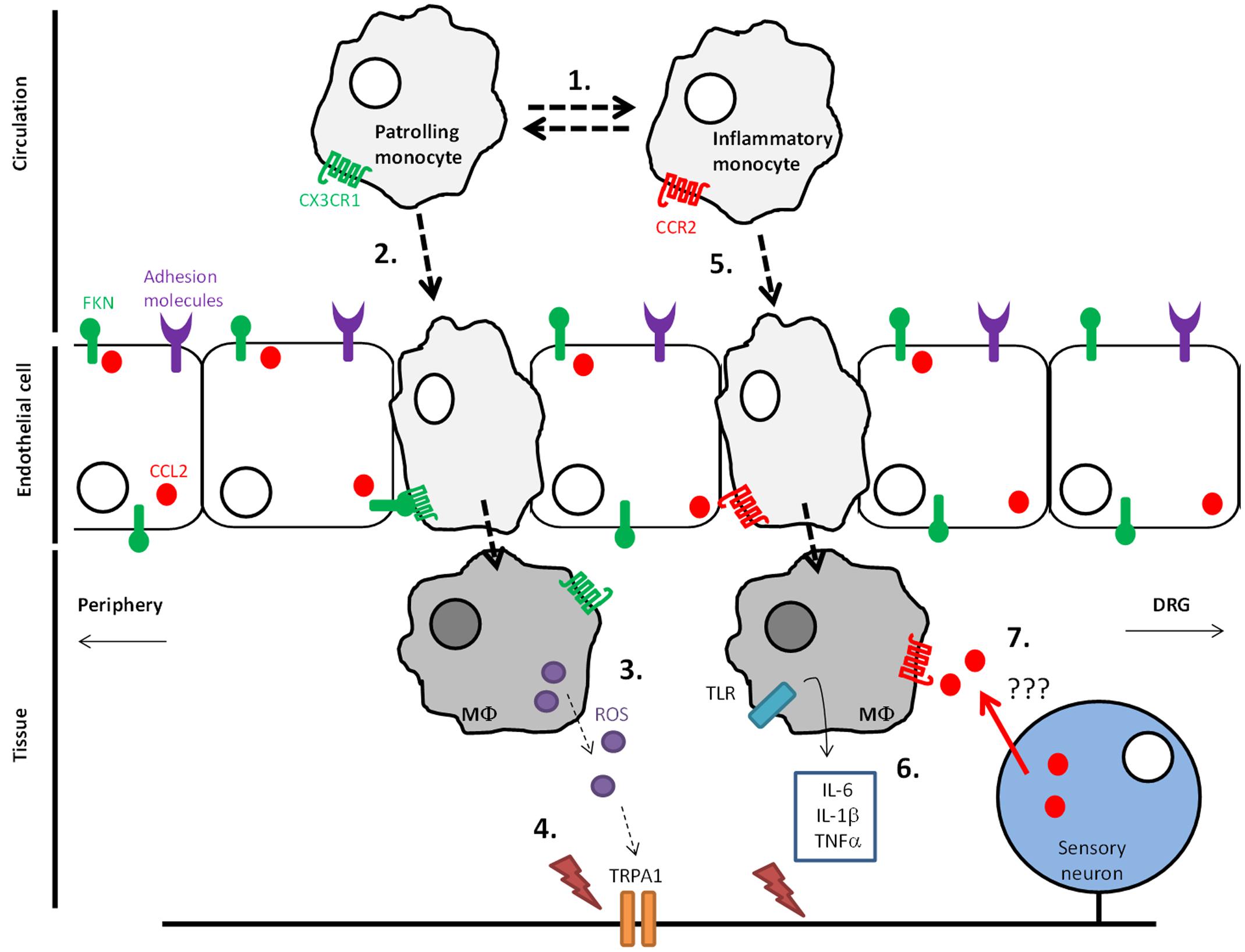
FIGURE 1. Monocyte infiltration into DRG and peripheral nervous tissue in vincristine-induced neuropathic pain. (1) Under steady-state conditions Ly6C-CX3 monocytes, which patrol the endothelium, dominate over inflammatory Ly6C+CX3 monocytes in the circulation. (2) During adverse conditions such as those that occur following chemotherapy exposure, CX3 monocytes infiltrate through the endothelium and into nervous tissue in a soluble CX3CL1-dependent manner, which is produced by cleavage mediated by ADAM17 in adverse conditions (3). (4) CX3 monocytes differentiate into macrophages in nervous tissue where they express reactive oxygen species (ROS). ROS activation of TRPA1 channels in peripheral nerve fibers evokes pain. (5) During adverse conditions, Ly6C+ monocytes also infiltrate into peripheral nervous tissue in a CCL2-dependent manner. (6) macrophages in tissue generate cytokines, which activate nociceptive fibers. (7) Indirect evidence suggests the presence of CCL2 in the periphery can serve as a positive feedback mechanism by which infiltration of monocytes is increased further.
Manipulating CX3CL1/R1 signaling in monocytes/ macrophages in the sciatic nerve could thus provide a prophylactic treatment for the development of pain in CIPN. A caveat is that CX3CR1 KO is global and may not represent targeted pharmacological inhibition. To strengthen the prophylactic potential of CX3CL1/R1 clinically, pharmacological studies must support transgenic studies. Indeed, pharmacological inhibition of CX3CL1/R1 signaling in monocytes/macrophages shows promise in the paclitaxel preclinical model of CIPN, with intrathecal pre-treatment of rats with a CX3CR1 neutralizing antibody significantly reducing paclitaxel-associated mechanical hypersensitivity (Huang et al., 2014). Furthermore, paclitaxel-associated monocyte infiltration into the DRG is also reduced by prophylactic administration of the antibody as is macrophage activation, as demonstrated by decreased p38 phosphorylation. The study also reports reduction in neuronal apoptosis demonstrated by reduced caspase 3 expression in the presence of the antibody (Huang et al., 2014).
Pharmacological inhibition of CX3CR1 signaling in monocytes/macrophages therefore appears to constitute a potential prophylactic treatment for CIPN associated with vincristine and paclitaxel treatment. A valuable feature of CX3CL1/R1 signaling is its high fidelity, which limits the likelihood of unexpected side-effects. It is important to appreciate, however, that, unlike other chemokines, CX3CL1 is constitutively expressed and thus targeting CX3CL1/R1 signaling could disrupt critical homeostatic processes. Moreover, the eventual development of pain in CX3CR1 KO mice suggests that the role of CX3CL1 signaling in monocytes/macrophages changes over time and mechanisms underlying vincristine-induced neuropathic pain, for example, are dynamic. Targeting CX3CR1 signaling in monocytes/macrophages could therefore provide a prophylactic treatment, which could form part of a tailored, combination therapy. Alternative mediators of monocyte/macrophage communication with neurons should therefore be identified in order to uncover additional potential targets for treating vincristine pain in patients at different stages of chemotherapy and indeed CIPN associated with other chemotherapeutic agents. Indeed, monocytes/macrophages in the periphery express other chemokines and their receptors, which have been implicated in several preclinical models of chronic pain.
Alternative Macrophage-Derived Chemokines and Chronic Pain
Chemokine (C-C motif) ligand 4 (CCL4), otherwise known as macrophage inflammatory protein 1b is an alternative chemokine that is expressed by macrophages (Chong et al., 2002). CCL4 signals via the CCR5 receptor that is also expressed by macrophages, however, unlike CX3CL1/R1 signaling, the CCL4/R5 partnership does not display fidelity (Jones et al., 2011). CCL4 activation of CCR5 in macrophages has been associated with chronic pain induced by surgical damage. For instance, following partial sciatic nerve ligation, CCL4 mRNA is significantly elevated in macrophages alongside pain-like behavior, and the inhibition of CCL4 using local application of a neutralizing antibody in the sciatic nerve alleviates surgical-induced pain (Saika et al., 2012). CCL4/R5 signaling in peripheral macrophages has not been specifically investigated in the context of preclinical CIPN, however, recent evidence has indicated that the expression of CCL4 increases centrally following paclitaxel treatment, yet intriguingly, decreases in the DRG (Makker et al., 2017) making it only a weak candidate for a peripheral mediator of paclitaxel-induced painful neuropathy.
Monocytes under inflammatory conditions express CCR2 (Yang et al., 2014). Indeed, existing evidence implicates neuronal CCL2/R2 signaling in several chronic pain models, including CIPN. For instance, neuronal CCL2/R2 signaling in the DRG has been strongly implicated in chemotherapy pain and CCL2/R2 signaling is known to mediate neuron–macrophage communication (Kwon et al., 2015). In a preclinical paclitaxel pain model for instance, expression of both CCL2 and CCR2 increases in DRG neurons alongside the development of mechanical hypersensitivity (Zhang et al., 2013). The increase in macrophages in the DRG in this model is well-established and it is therefore plausible that elevated CCL2 could also activate CCR2 expressed by macrophages in addition to DRG neurons (White et al., 2005). What remains to be validated, however, is whether or not neurons release the CCL2 that they have been shown to express. Nonetheless, increased CCL2 in the DRG could constitute a feed-forward mechanism by which CCL2 stimulates further monocyte/macrophage infiltration (Groh et al., 2010). Indeed, intrathecal administration of an anti-CCL2 antibody not only blocks paclitaxel-associated pain behavior, but also reduces the associated monocyte/macrophage infiltration into the DRG, although whether this effect is direct or indirect, as well as the precise site of action, has yet to be established (Zhang et al., 2016).
Currently, tangible evidence for a role of CCL2/R2 signaling in monocytes/macrophages in the periphery in some preclinical models of CIPN has not been obtained. However, the involvement of CCL2/R2 signaling in chronic pain and the expression of CCR2 in inflammatory macrophages make CCL2/R2 signaling in macrophages an intuitive candidate for the regulation of CIPN, particularly at later stages when the role of CX3CL1/R1-mediated monocyte/macrophage signaling appears to be less pertinent.
Cytokine Production By Macrophages In Peripheral Tissue
In addition to chemokines, macrophages in the periphery express and release proinflammatory cytokines, which have well-established pronociceptive effects (Sommer and Kress, 2004). Cytokines are diverse glycoproteins that are predominantly secreted by immune cells such as macrophages. Interleukin-6 (IL-6), IL-1β, and TNFα are the most consistently elevated cytokines in response to damage and inflammation.
Interleukin-6 (IL-6)
The proinflammatory cytokine IL-6 is secreted predominantly by macrophages in adverse conditions. IL-6 signals classically via membrane-bound IL-6R, which is expressed by neurons (Erta et al., 2012). IL-6R can also exist as a soluble form following cleavage by either ADAM10 or ADAM17 and signaling via the soluble receptor, which is referred to as IL-6 trans-signaling, is associated with monocytes of a proinflammatory phenotype (Scheller et al., 2011).
The expression of IL-6 by macrophages in peripheral tissue has been associated with vincristine-induced pain behavior preclinically. Following one cycle of vincristine treatment, the development of pain behavior in mice is accompanied by an elevation of macrophages in the DRG and sciatic nerve, which also display positive immunoreactivity for IL-6 (Kiguchi et al., 2008b). Furthermore, inhibition of IL-6 by local injection of a neutralizing antibody in the vicinity of the sciatic nerve results in a significant alleviation of mechanical hypersensitivity (Kiguchi et al., 2008b). IL-6, however, has also been suggested to potentially possess anti-inflammatory properties (Scheller et al., 2011) and so the side-effect profile associated with its inhibition could be problematic. Nonetheless, its production by macrophages and subsequent activation of neurons could provide an additional mechanism for monocyte/macrophage–neuron communication in vincristine-induced neuropathic pain.
Interleukin 1β (IL-1β)
Interleukin 1β is a proinflammatory cytokine, which signals via the IL-1 receptor 1. As is the case with IL-6, IL-1β signaling also has the capacity to trigger macrophage differentiation (Schenk et al., 2014). Due to its ability to rapidly excite nociceptive fibers, IL-1β was one of the first cytokines to be associated with chronic peripheral pain conditions, with IL-1β KO mice demonstrating resistance to surgery-induced pain (Kleibeuker et al., 2008). IL-1β is expressed by bone marrow-derived macrophages in response to a variety of chemotherapeutic agents. Agents such as vincristine, cisplatin, paclitaxel, melphalan, and methotrexate, for example, have all been shown to stimulate IL-1β production in LPS-primed bone marrow-derived macrophages (Wong et al., 2014). The contribution of such production to chemotherapy-induced pain, however, as yet to be determined.
Tumor Necrosis Factor Alpha (TNFα)
Tumor necrosis factor alpha is also produced and secreted by a number of cell types, however, in the context of chronic pain, elevation of TNFα occurs predominantly in macrophages (Sommer and Schäfers, 1998). As is the case with ILs, TNFα also rapidly and directly stimulates and sensitizes A- and C-fibers (Schäfers and Sorkin, 2008), providing a potential pathway by which monocyte/macrophage–neuron communication could occur. Alterations in TNFα expression both peripherally and centrally have been observed in certain models of preclinical CIPN. Specifically, increases in expression of TNFα in the sciatic nerve and spinal cord have been found to occur alongside pain-like behavior in vincristine rat and mouse models, respectively (Kiguchi et al., 2008a; Muthuraman et al., 2011). Not only are macrophages a major source of TNFα, but they are also responsive to it, with TNFα stimulation of macrophages resulting in increased cytokine production (Parameswaran and Patial, 2010). TNFα signaling in macrophages could therefore constitute a feed-forward mechanism, which maintains cytokine production and chronic communication with neurons.
Toll-Like Receptor Activation of Macrophages
As well as considering monocyte/macrophage signaling, one must also consider their activation when identifying approaches for the manipulation of monocytes/macrophages. One of the mechanisms by which the release of inflammatory mediators from macrophages is triggered is via the activation of TLRs expressed at their cell surface. Macrophages express an array of TLRs, which can stimulate cytokine release. Activation of TLR4, for example, results in the release of both TNFα and IL-1β, while stimulation of TLRs 3, 9, and 7 stimulate the release of IL-1α and 1β (Nicotra et al., 2012). TLR activation of macrophages has been associated with the regulation of chronic pain as it constitutes a feed-forward mechanism, with activation of macrophages via TLRs 3, 7, and 9 signaling resulting in an consequential upregulation of TRPV1 expression in DRG neurons (Diogenes et al., 2011).
TLR4-mediated activation of macrophages in the DRG has been shown to be involved in preclinical paclitaxel-associated painful neuropathy. In this model, in which macrophages are elevated in the DRG alongside the occurrence of mechanical hypersensitivity, administration of a TLR4 antagonist, LPS-RS, alongside paclitaxel, significantly reduces paclitaxel-associated pain behavior as well as monocyte/macrophage infiltration into the DRG (Zhang et al., 2016).
Targeting TLR signaling, however, is likely to be complicated. Not only do macrophages express numerous TLRs, but neurons in the DRG also express TLRs 1, 2, 3, 4, 5, 6, and 9 (Ochoa-Cortes et al., 2010). The analgesic effects of targeting TLRs could therefore be equally attributed to inhibition of neuronal TLR activation.
Conclusion: the Therapeutic Potential of Monocyte/Macrophage Manipulation
Currently available analgesics show a limited efficacy at treating CIPN. The underlying mechanisms are poorly understood and are likely to vary with different chemotherapeutic agents. We are beginning to uncover and understand the importance of monocyte/macrophage–neuron communication in the mediation CIPN and accumulating preclinical evidence is indicative of its promising potential as an innovative prophylactic and therapeutic strategy.
Although monocyte/macrophage manipulation for the treatment of CIPN remains preclinical to date, the approach has entered clinical trials in the context of other pathological conditions. A monoclonal antibody against colony stimulating factor 1 (CSF-1), also known as macrophage stimulating factor, which regulates the differentiation of macrophages, has been used in clinical trials for treating solid tumors and appears to be well-tolerated (Panni et al., 2013). Most notably, a humanized monoclonal antibody against CX3CL1 has also been found to be safe and well-tolerated in the clinic when used in Rheumatoid Arthritis and Crohn’s Disease patients (Imai and Yasuda, 2016). The application of monocyte/macrophage manipulation to CIPN patients is therefore feasible in light of patients’ tolerability to such an approach in other contexts.
Novel pain therapies should not themselves jeopardize the success of chemotherapy and should have minimal side-effects in order to avoid reducing the patient’s quality of life further. CX3CL1/R1 displays a high fidelity signaling relationship, which is likely to limit the occurrence of unexpected side-effects. Furthermore, the role of CX3CL1/R1-mediated monocyte/macrophage–neuron communication in the periphery in vincristine- and paclitaxel-associated painful neuropathy specifically is arguably supported by the most tangible evidence at present and the humanized monoclonal antibody against CX3CL1 is well-tolerated clinically. The manipulation of monocytes/macrophages via manipulation of CX3CL1/R1 signaling therefore appears to be the current front-runner for prophylactic treatment of neuropathic pain in patients treated with vincristine and paclitaxel. Whether this also applies to other chemotherapeutic agents, however, remains unknown. The next step of this exciting journey is to identify other approaches for monocyte/macrophage manipulation that will compliment CX3CL1/R1 inhibition, allowing us to develop tailored therapies that can be used to treat patients at various stages of chemotherapy treatment.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
KM was funded by the Medical Research Council grant no. MR/M023893/1.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Auffray, C., Fogg, D., Garfa, M., Elain, G., Join-Lambert, O., Kayal, S., et al. (2007). Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670. doi: 10.1126/science.1142883
Balayssac, D., Cayre, A., Authier, N., Bourdu, S., Penault-Llorca, F., Gillet, J. P., et al. (2005). Patterns of P-glycoprotein activity in the nervous system during vincristine-induced neuropathy in rats. J. Peripher. Nerv. Syst. 10, 301–310. doi: 10.1111/j.1085-9489.2005.10308.x
Bazan, J. F., Bacon, K. B., Hardiman, G., Wang, W., Soo, K., Rossi, D., et al. (1997). A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644. doi: 10.1038/385640a0
Bennett, L. D., Fox, J. M., and Signoret, N. (2011). Mechanisms regulating chemokine receptor activity. Immunology 134, 246–256. doi: 10.1111/j.1365-2567.2011.03485.x
Bloodworth, D. (2005). Issues in opioid management. Am. J. Phys. Med. Rehabil. 84(Suppl. 3), S42–S55.
Boyette-Davis, J., and Dougherty, P. M. (2011). Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp. Neurol. 229, 353–357. doi: 10.1016/j.expneurol.2011.02.019
Carlin, L. M., Stamatiades, E. G., Auffray, C., Hanna, R. N., Glover, L., Vizcay-Barrena, G., et al. (2013). Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375. doi: 10.1016/j.cell.2013.03.010
Cavaletti, G., Nicolini, G., and Marmiroli, P. (2008). Neurotoxic effects of antineoplastic drugs: the lesson of pre-clinical studies. Front. Biosci. 13:3506–3524. doi: 10.2741/2945
Chong, I. W., Lin, S. R., Hwang, J. J., Huang, M. S., Wang, T. H., Hung, J. Y., et al. (2002). Expression and regulation of the macrophage inflammatory protein-1 alpha gene by nicotine in rat alveolar macrophages. Eur. Cytokine Netw. 13, 242–249.
Clark, A. K., and Malcangio, M. (2014). Fractalkine/CX3CR1 signaling during neuropathic pain. Front. Cell. Neurosci. 8:121. doi: 10.3389/fncel.2014.00121
Diogenes, A., Ferraz, C. C., Akopian, A. N., Henry, M. A., and Hargreaves, K. M. (2011). LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res. 90, 759–764. doi: 10.1177/0022034511400225
Dougherty, P. M., Cata, J. P., Burton, A. W., Vu, K., and Weng, H. R. (2007). Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J. Pain Symptom Manage 33, 166–179. doi: 10.1016/j.jpainsymman.2006.08.006
Erta, M., Quintana, A., and Hidalgo, J. (2012). Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 8, 1254–1266. doi: 10.7150/ijbs.4679
Ginhoux, F., and Jung, S. (2014). Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404. doi: 10.1038/nri3671
Groh, J., Heinl, K., Kohl, B., Wessig, C., Greeske, J., Fischer, S., et al. (2010). Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1X. Hum. Mol. Genet. 19, 3530–3543. doi: 10.1093/hmg/ddq269
Huang, Z. Z., Li, D., Liu, C. C., Cui, Y., Zhu, H. Q., Zhang, W. W., et al. (2014). CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav. Immun. 40, 155–165. doi: 10.1016/j.bbi.2014.03.014
Hundhausen, C., Misztela, D., Berkhout, T. A., Broadway, N., Saftig, P., Reiss, K., et al. (2003). The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 102, 1186–1195. doi: 10.1182/blood-2002-12-3775
Hurst, L. A., Bunning, R. A., Couraud, P. O., Romero, I. A., Weksler, B. B., Sharrack, B., et al. (2009). Expression of ADAM-17, TIMP-3 and fractalkine in the human adult brain endothelial cell line, hCMEC/D3, following pro-inflammatory cytokine treatment. J. Neuroimmunol. 210, 108–112. doi: 10.1016/j.jneuroim.2009.02.008
Imai, T., and Yasuda, N. (2016). Therapeutic intervention of inflammatory/immune diseases by inhibition of the fractalkine (CX3CL1)-CX3CR1 pathway. Inflamm. Regen. 36, 9. doi: 10.1186/s41232-016-0017-2
Imaizumi, T., Yoshida, H., and Satoh, K. (2004). Regulation of CX3CL1/fractalkine expression in endothelial cells. J. Atheroscler. Thromb. 11, 15–21. doi: 10.5551/jat.11.15
Ingersoll, M. A., Platt, A. M., Potteaux, S., and Randolph, G. J. (2011). Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 32, 470–477. doi: 10.1016/j.it.2011.05.001
Jones, K. L., Maguire, J. J., and Davenport, A. P. (2011). Chemokine receptor CCR5: from AIDS to atherosclerosis. Br. J. Pharmacol. 162, 1453–1469. doi: 10.1111/j.1476-5381.2010.01147
Jung, S., Aliberti, J., Graemmel, P., Sunshine, M. J., Kreutzberg, G. W., Sher, A., et al. (2000). Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114. doi: 10.1128/MCB.20.11.4106-4114.2000
Kiguchi, N., Maeda, T., Kobayashi, Y., and Kishioka, S. (2008a). Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neurosci. Lett. 445, 140–143. doi: 10.1016/j.neulet.2008.09.009
Kiguchi, N., Maeda, T., Kobayashi, Y., Kondo, T., Ozaki, M., and Kishioka, S. (2008b). The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur. J. Pharmacol. 592, 87–92. doi: 10.1016/j.ejphar.2008.07.008
Kleibeuker, W., Gabay, E., Kavelaars, A., Zijlstra, J., Wolf, G., Ziv, N., et al. (2008). IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav. Immun. 22, 200–208. doi: 10.1016/j.bbi.2007.07.009
Kurihara, T., Warr, G., Loy, J., and Bravo, R. (1997). Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186, 1757–1762. doi: 10.1084/jem.186.10.1757
Kwon, M. J., Shin, H. Y., Cui, Y., Kim, H., Thi, A. H., Choi, J. Y., et al. (2015). CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J. Neurosci. 35, 15934–15947. doi: 10.1523/JNEUROSCI.1924-15
Liu, C. C., Lu, N., Cui, Y., Yang, T., Zhao, Z. Q., Xin, W. J., et al. (2010). Prevention of paclitaxel-induced allodynia by minocycline: effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol. Pain 6:76. doi: 10.1186/1744-8069-6-76
Makker, P. G., Duffy, S. S., Lees, J. G., Perera, C. J., Tonkin, R. S., Butovsky, O., et al. (2017). Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLOS ONE 12:e0170814. doi: 10.1371/journal.pone.0170814
Montague, K., and Malcangio, M. (2017). The therapeutic potential of targeting chemokine signalling in the treatment of chronic pain. J. Neurochem. 141, 520–531. doi: 10.1111/jnc.13927
Mueller, M., Leonhard, C., Wacker, K., Ringelstein, E. B., Okabe, M., Hickey, W. F., et al. (2003). Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab. Invest. 83, 175–185. doi: 10.1097/01.LAB.0000056993.28149.BF
Muthuraman, A., Singh, N., and Jaggi, A. S. (2011). Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem. Toxicol. 49, 2557–2563. doi: 10.1016/j.fct.2011.06.069
Nicotra, L., Loram, L. C., Watkins, L. R., and Hutchinson, M. R. (2012). Toll-like receptors in chronic pain. Exp. Neurol. 234, 316–329. doi: 10.1016/j.expneurol.2011.09.038
Ochoa-Cortes, F., Ramos-Lomas, T., Miranda-Morales, M., Spreadbury, I., Ibeakanma, C., Barajas-Lopez, C., et al. (2010). Bacterial cell products signal to mouse colonic nociceptive dorsal root ganglia neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G723–G732. doi: 10.1152/ajpgi.00494.2009
Old, E. A., Nadkarni, S., Grist, J., Gentry, C., Bevan, S., Kim, K. W., et al. (2014). Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J. Clin. Invest. 124, 2023–2036. doi: 10.1172/JCI71389
Panni, R. Z., Linehan, D. C., and DeNardo, D. G. (2013). Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy 5, 1075–1087. doi: 10.2217/imt.13.102
Parameswaran, N., and Patial, S. (2010). Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 20, 87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.10
Peters, C. M., Jimenez-Andrade, J. M., Jonas, B. M., Sevcik, M. A., Koewler, N. J., Ghilardi, J. R., et al. (2007). Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp. Neurol. 203, 42–54. doi: 10.1016/j.expneurol.2006.07.022
Saika, F., Kiguchi, N., Kobayashi, Y., Fukazawa, Y., and Kishioka, S. (2012). CC-chemokine ligand 4/macrophage inflammatory protein-1β participates in the induction of neuropathic pain after peripheral nerve injury. Eur. J. Pain 16, 1271–1280. doi: 10.1002/j.1532-2149.2012.00146
Schäfers, M., and Sorkin, L. (2008). Effect of cytokines on neuronal excitability. Neurosci. Lett. 437, 188–193. doi: 10.1016/j.neulet.2008.03.052
Scheller, J., Chalaris, A., Schmidt-Arras, D., and Rose-John, S. (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888. doi: 10.1016/j.bbamcr.2011.01.034
Schenk, M., Fabri, M., Krutzik, S. R., Lee, D. J., Vu, D. M., Sieling, P. A., et al. (2014). Interleukin-1b triggers the differentiation of macrophages with enhanced capacity to present mycobacterial antigen to T cells. Immunology 141, 174–180. doi: 10.1111/imm.12167
Schwarz, N., Pruessmeyer, J., Hess, F. M., Dreymueller, D., Pantaler, E., Koelsch, A., et al. (2010). Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1. Cell Mol. Life Sci. 67, 4233–4248. doi: 10.1007/s00018-010-0433-4
Seretny, M., Currie, G. L., Sena, E. S., Ramnarine, S., Grant, R., MacLeod, M. R., et al. (2014). Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155, 2461–2470. doi: 10.1016/j.pain.2014.09.020
Si, Y., Tsou, C. L., Croft, K., and Charo, I. F. (2010). CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J. Clin. Invest. 120, 1192–1203. doi: 10.1172/JCI40310
Sommer, C., and Kress, M. (2004). Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 361, 184–187. doi: 10.1016/j.neulet.2003.12.007
Sommer, C., and Schäfers, M. (1998). Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 784, 154–162. doi: 10.1016/S0006-8993(97)01327-9
Staniland, A. A., Clark, A. K., Wodarski, R., Sasso, O., Maione, F., D’Acquisto, F., et al. (2010). Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J. Neurochem. 114, 1143–1157. doi: 10.1111/j.1471-4159.2010.06837
Weimer, L. H. (2003). Medication-induced peripheral neuropathy. Curr. Neurol. Neurosci. Rep. 3, 86–92. doi: 10.1007/s11910-003-0043-8
White, F. A., Sun, J., Waters, S. M., Ma, C., Ren, D., Ripsch, M., et al. (2005). Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl. Acad. Sci. U.S.A. 102, 14092–14097. doi: 10.1073/pnas.0503496102
Wong, J., Tran, L. T., Magun, E. A., Magun, B. E., and Wood, L. J. (2014). Production of IL-1β by bone marrow-derived macrophages in response to chemotherapeutic drugs: synergistic effects of doxorubicin and vincristine. Cancer Biol. Ther. 15, 1395–1403. doi: 10.4161/cbt.29922
Yang, J., Zhang, L., Yu, C., Yang, X. F., and Wang, H. (2014). Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2:1. doi: 10.1186/2050-7771-2-1
Zhang, H., Boyette-Davis, J. A., Kosturakis, A. K., Li, Y., Yoon, S. Y., Walters, E. T., et al. (2013). Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J. Pain 14, 1031–1044. doi: 10.1016/j.jpain.2013.03.012
Zhang, H., Li, Y., de Carvalho-Barbosa, M., Kavelaars, A., Heijnen, C. J., Albrecht, P. J., et al. (2016). Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J. Pain 17, 775–786. doi: 10.1016/j.jpain.2016.02.011
Keywords: monocyte, macrophage, chemotherapy-induced painful neuropathy (CIPN), chemokine, cytokine, therapy
Citation: Montague K and Malcangio M (2017) The Therapeutic Potential of Monocyte/Macrophage Manipulation in the Treatment of Chemotherapy-Induced Painful Neuropathy. Front. Mol. Neurosci. 10:397. doi: 10.3389/fnmol.2017.00397
Received: 04 April 2017; Accepted: 13 November 2017;
Published: 27 November 2017.
Edited by:
Ildikó Rácz, Universitätsklinikum Bonn, GermanyReviewed by:
Sung Jun Jung, Hanyang University, South KoreaSangsu Bang, Duke University, United States
Copyright © 2017 Montague and Malcangio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karli Montague, karli.montague@kcl.ac.uk Marzia Malcangio, marzia.malcangio@kcl.ac.uk
 Karli Montague
Karli Montague Marzia Malcangio
Marzia Malcangio