Systems approach to the study of brain damage in the very preterm newborn
- 1Neuroepidemiology Unit, Boston Children’s Hospital, Boston, MA, USA
- 2Department of Neurology, Harvard Medical School, Boston, MA, USA
- 3Inserm, U1141, Paris, France
- 4Department of Perinatal Imaging and Health, Department of Division of Imaging Sciences and Biomedical Engineering, King’s College London, King’s Health Partners, St. Thomas’ Hospital, London, UK
- 5Department of Systems Biology and Bioinformatics, University of Rostock, Rostock, Germany
- 6Stellenbosch Institute for Advanced Study (STIAS), Stellenbosch, South Africa
- 7Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, MA, USA
- 8Perinatal Epidemiology Unit, Department of Gynecology and Obstetrics, Hannover Medical School, Hannover, Germany
Background: A systems approach to the study of brain damage in very preterm newborns has been lacking.
Methods: In this perspective piece, we offer encephalopathy of prematurity as an example of the complexity and interrelatedness of brain-damaging molecular processes that can be initiated inflammatory phenomena.
Results: Using three transcription factors, nuclear factor-kappa B (NF-κB), Notch-1, and nuclear factor erythroid 2 related factor 2 (NRF2), we show the inter-connectedness of signaling pathways activated by some antecedents of encephalopathy of prematurity.
Conclusions: We hope that as biomarkers of exposures and processes leading to brain damage in the most immature newborns become more readily available, those who apply a systems approach to the study of neuroscience can be persuaded to study the pathogenesis of brain disorders in the very preterm newborn.
Introduction
Very preterm newborns are vulnerable to forms of brain damage that differ from those that occur in infants born near term (Volpe, 2011). Subsumed under the name of “encephalopathy of prematurity,” they include injuries/dysfunctions of cerebral white matter, as well as neuronal/axonal/synaptic disturbances. Increasingly, inflammation is recognized as a contributor to or correlate of the processes leading to these forms of damage (Adén et al., 2010; Van Steenwinckel et al., 2014), especially if prolonged or recurrent (Dammann and Leviton, 2014). We prepared this perspective piece to bring to the attention of the wider readership that this is potentially fruitful area of study by systems approaches.
This essay is divided into three parts. The first summarizes what is currently known about the antecedents and characteristics associated with brain damage in the very low gestational age newborn (ELGAN). The second part exemplifies the systems approach by describing the connectedness among pathways involved in inflammation ascendance and resolution, and among pathways involved with brain damage promotion and repair. Sometimes, the pathways and their components have pleiotropic properties, resulting in diverse effects including not only inflammation and inflammation resolution, but also brain damage as well as brain repair. The final part is our hopeful view of the future.
Part 1: Why the Immature Brain is Vulnerable
We begin our illustration of systems epidemiology with the antecedents of perinatal brain damage in the very preterm newborn and with characteristics of the mother, placenta, and newborn that influence brain damage risk. The very preterm newborn is thought to be at heightened risk because of the combination of endogenous vulnerability and exposures.
Our linking endogenous vulnerability and these exposures to molecular pathways and networks achieves four goals. First, the exercise hints at the complexity of the processes involved. Second it identifies candidates for modulating the destructive processes, enhancing inflammation resolution and promoting brain repair needed to restore normal brain structure. Third, it brings systems epidemiology to the attention of those not familiar with it. Fourth, it points in a direction we hope others will consider traveling.
Endogenous Vulnerability
By and large, the lower the gestational age, the higher the risk of brain damage (Locatelli et al., 2005; Himpens et al., 2008; Glinianaia et al., 2011; Sannia et al., 2013). Although low gestational age appears to be the best overall indicator of immaturity, not all infants born at 25 weeks of gestation are identically immature. Early indicators of physiologic instability, as identified with the Score for Neonatal Acute Physiology (SNAP) might provide supplemental information (and therefore more precision) about immaturity (Dammann et al., 2010).
The infant born too soon is not yet able to provide growth factors needed for normal growth (Sanders and Harvey, 2008), let alone for protection against perturbations (Dammann and Leviton, 1999). The influence of systemic physiologic instability remains uncertain (Bakewell-Sachs et al., 2009). The very preterm infant is born during the time her brain undergoes many developmental processes include those involved in laying the ground work for normal myelination, as well as those involved in the migration of subplate neurons from the germinal matrix to the thalamus and cortex, and synaptogenesis. These brain developmental processes in full swing at very low gestational ages appear to be especially vulnerable to disturbances (Back et al., 2007; Leviton and Gressens, 2007; McCarran and Goldberg, 2007; Verney et al., 2012).
The very preterm newborn seems more likely than the term newborn to exhibit intermittent or sustained systemic inflammation (ISSI; Dammann and Leviton, 2014). Such systemic inflammation in the very preterm newborn has repeatedly been an antecedent of brain damage (Nelson et al., 1998; Hansen-Pupp et al., 2008; Leviton et al., 2011a,b; O’Shea et al., 2012).
The preterm newborn appears to have limited ability to synthesize proteins with anti-inflammatory characteristics (Chheda et al., 1996; Jones et al., 1996; Blahnik et al., 2001). So does the immature rat brain (Brochu et al., 2011). In addition, components of the fetal systemic inflammatory response appear to be considerably more vigorous in very preterm newborns than in gestationally-older newborns (Rebuck et al., 1995; Berner et al., 1998; Nanthakumar et al., 2000; Rozycki et al., 2002; Schultz et al., 2002; Yoon et al., 2003; Athayde et al., 2005; Tatad et al., 2008). The result is that preterm newborns tend to have a pro-inflammatory imbalance in both the blood and brain.
An extremely low birth weight for gestational age appears to be a very good indicator of intra-uterine stimuli that promote epigenetic phenomena (Grissom and Reyes, 2013; Sookoian et al., 2013). This leads to the situation where an apparent indicator of endogenous vulnerability is really a reflection of processes that led to the intra-uterine growth restriction and the consequences of those processes. For example, growth-restricted newborns are more likely than others to be exposed post-natally to barotrauma, (Bose et al., 2009) which can lead to systemic inflammation (Bose et al., 2013). Whether because of epigenetic phenomena or other processes, severely growth-restricted newborns display a heightened inflammatory response, only part of which is explained by an increased need for assisted ventilation (McElrath et al., 2013). One possible explanation for this might be that pre-eclampsia, a known antecedent of intra-uterine growth restriction, contributes additionally to the adversities of very preterm newborn (Morsing and Maršál, 2014) but this remains controversial (Love et al., 2012). Another possible explanation is that hyperoxia-induced lung injury in animals involves inflammatory phenomena, (Weichelt et al., 2013; Martin et al., 2014), just as some of hyperoxia-induced cerebral white matter damage appears to be a consequence of inflammation, (Nold et al., 2013; Pham et al., 2014; Schmitz et al., 2014) or exacerbated by inflammation (Brehmer et al., 2012).
Some children born to obese women tend to score lower on measures of intelligence than their peers born to women who are neither overweight nor obese (Neggers et al., 2003; Helderman et al., 2012; Basatemur et al., 2013; Tanda et al., 2013). More studies have shown what appears to be a maternal obesity effect, than have failed to do so (Van Lieshout et al., 2011; Brion, 2013; Van Lieshout, 2013). Among the explanations invoked to explain the obesity-impaired development link are the neonatal inflammation associated with mother’s obesity (van der Burg et al., 2013), and epigenetic phenomena (Liu et al., 2014).
One example of a two-hit model begins with a sub-injurious noxious dose, which is followed by a fully-injurious dose. If these occur within a narrow time range, then the injury is greater than would occur without the earlier stimulus, resulting in what the authors identify as sensitization (Eklind et al., 2005; Mallard, 2012). Yet, with different time spans, the first of the two hits can appear to be protective (Mallard, 2012). This reduced probability and extent of damage in this case is called preconditioning (Hagberg et al., 2004) or tolerance (Lin et al., 2010).
Exogenous Vulnerability
Many of the exposures associated with increased risk of brain damage are linked to systemic inflammation. We classify exposures by the time of their onset, distinguishing mainly between antenatal and postnatal exposures.
In Utero Exposures
Maternal characteristics, including pre-pregnancy body mass index, (Van Lieshout et al., 2011; Love et al., 2012; Basatemur et al., 2013; Casas et al., 2013; Hinkle et al., 2013; Kerstjens et al., 2013; Tanda et al., 2013), pregnancy weight gain, (Huang et al., 2014), and diet (Ojha et al., 2015), or their surrogates influence the child’s neurodevelopmental function. In addition, microbial invasion of the amniotic cavity (Romero et al., 2007; Fichorova et al., 2011) and histologic inflammation of the placenta and umbilical vessels (Hecht et al., 2011) have been associated with increased concentrations of inflammatory proteins in cord blood or blood obtained shortly after birth. Genito-urinary infections have also been associated with systemic inflammation in the newborn (Fichorova et al., 2015).
Early Postnatal Exposures
Bacteremia. Preterm newborns also have fragile skin, limited synthesis of complement components, antimicrobial proteins and peptides, and T(H)17-polarizing cytokine production, and multiple indwelling tubes, catheters, and lines, and all of which likely contribute to susceptibility to infection (Adkins, 2013; Cuenca et al., 2013). The frequently-occurring consequence of bacteremia can readily be followed/accompanied by systemic inflammation (Leviton et al., 2012).
Necrotizing enterocolitis and isolated intestinal perforation. Ligation of receptors of the innate immune system, including Toll-like receptors and the intracellular pathogen recognition receptor NOD2/CARD15 appear to be involved in the initiation of enterocyte apoptosis, which can destroy intestinal barriers and lead to necrotizing enterocolitis (Siggers and Hackam, 2011). Without destroying intestinal barriers, ligation of toll-like receptors might also lead to the inflammation that characterizes necrotizing enterocolitis (Afrazi et al., 2014; Lu et al., 2014). Thus, it is possible that necrotizing enterocolitis is a marker/consequence of inflammation, although it is probably more likely that necrotizing enterocolitis contributes to systemic inflammation (Martin et al., 2013).
Ventilation. Mechanical (pressure-limited/targeted) ventilation leads to systemic inflammation in very preterm newborns (Capoluongo et al., 2005; Turunen et al., 2006, 2011; Sarafidis et al., 2011; Bose et al., 2013). Although replacing pressure-limited ventilation equipment with volume-targeted equipment has the potential to reduce ventilation-induced lung injury and inflammation and their consequences (Peng et al., 2014), the avoidance of invasive ventilation is increasingly recognized as perhaps the optimal strategy (Strueby and Thébaud, 2014).
One explanation for the systemic inflammation accompanying lung injury invokes correlates of autophagy, a lysosomal degradation pathway that can eliminate (usually damaged) cytoplasmic components, including intracellular remnants of invasive microorganisms (Mizumura et al., 2012). Autophagy can also be induced by pro-inflammatory inducers or promoters (Levine et al., 2011), and inhibited by proteins that have anti-inflammatory characteristics (IL-4, IL-5, IL-6 and IL-10). Compared to their peers, mice with impaired autophagy have less lung injury after ventilation, as well as much less activation of the canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway (López-Alonso et al., 2013). Here we have an example of how understanding of molecular processes appears to hold the promise of explaining what might account for what epidemiologists report. On the other hand, what applies to the lung might not apply to the brain (François et al., 2014).
Nutrition. Optimizing protein and energy intake and balance in the neonatal period primarily influences cognition, while relative energy deficiency appears to result in smaller total brain volumes (Keunen et al., 2015). Nevertheless “meta-analyzes of trial data have not provided convincing evidence for supplementation with specific nutrients to improve developmental outcomes.
Low socio-economic state. Low socio-economic state might contribute to diminished/ impaired function in ELGANs through multiple means and times along the course of development (Miller et al., 2009; Lucassen et al., 2013; Surén et al., 2013). Among very preterm newborns, those born to women who have characteristics of low social class tend to do less well than their peers born to women of higher socioeconomic status (Wang et al., 2008; Huhtala et al., 2012; Morinis et al., 2013). Indeed, regardless of gestational age at the time of birth, children reared in settings characterized by insecurity, stress, and diminished stimulation tend to do less well than their peers raised in more secure and stimulating environments (Bradley and Corwyn, 2002). Among the measurable effects are smaller brain volumes (Hanson et al., 2013) and lower scores on measures of overall language as well as receptive and expressive language skills (Wild et al., 2013).
Some of the limitations of children reared in disadvantaged homes have been attributed to the plethora of diminished opportunities for learning (Evans and Kim, 2010). More recently, epigenetic phenomena have been invoked as explanations for the association between social disadvantage early in life and later dysfunction (Ehlert, 2013; Tung and Gilad, 2013; Saban et al., 2014).
Part 2. Molecular Consequences of Exposure to Inflammatory Stimuli
This section should be viewed as our attempt to show the interrelatedness among diverse pathways linking inflammation with brain damage in the very preterm newborn. We focus on three pathways nuclear factor-kappaB (NF-κB), Notch, and nuclear factor erythroid 2 related factor 2 (NRF2), whose connectedness will illustrate some of the interrelationships/complexities we consider most important. Each influences the other two. Each is closely linked to inflammation, and each has nervous system effects that go beyond inflammation.
Innate immune mechanisms include the inflammatory reactions of neutrophils and monocytes, usually triggered by organisms, their components or products. An inflammatory stimulus influences the expression of thousands genes (Zak and Aderem, 2009; Orozco et al., 2012). Perhaps that is why after severe trauma and burn injury, the circulating leukocyte transcriptome provides evidence of a “genomic storm” (Xiao et al., 2011).
The inflammatory response is characterized by a set of complex, cascading non-linear processes mediated by a large array of immune cells and inflammatory cytokines (Rankin, 2004).
Coordination of this complex response is achieved with transcription factors, which are proteins that bind to DNA and regulate gene expression (Medzhitov and Horng, 2009). Operating at multiple levels and differently in different tissues, they influence each cell’s sensitivity to inflammatory stimuli and response capabilities, as well as regulating signaling pathways and gene expression. Roughly 8% of genes in the human genome encode transcription factors.
The innate immune system is activated when receptors on local macrophages, such as Toll-like receptors (TLRs), recognize pathogen-associated molecular patterns (PAMPs), usually bacterial or viral components such as lipopolysaccharide, or endogenous damage-associated molecular patterns (DAMPs) (including those released by injured cells) (Kong and Le, 2011; Lin et al., 2011). Toll-like receptors also recognize thrombin (Babu et al., 2012) as well as other extravascular blood components (Wang, 2010), perhaps explaining why the occurrence of blood components in the extravascular space in developing white matter might contribute to local damage (Adler et al., 2010).
The ligation of Toll-like receptors (TLRs) activates NF-κB, AP1, CCAAT/enhancer binding protein delta (CREB), c/EBP, and IRF transcription factors (Newton and Dixit, 2012), as well as the mitogen-activated protein kinase (MAPK) pathway (Arthur and Ley, 2013). The response to this activation includes the production and release of inflammatory mediators, such as cytokines and chemokines. These, in turn, activate endothelial cells of local blood vessels resulting in the synthesis and release of adhesion molecules and the ability to recruit circulating leukocytes to the area, and allow them access to the inflamed tissue. Newly-arrived leukocytes are activated by cytokines released by the local inflammatory cells and thereby become able to eliminate the invaders or damaged tissue.
Transcription factors often function together to regulate components of the inflammatory response. For example, three transcription factors, NF-κB1 (an initiator), C/EBPdelta (an amplifier) and ATF3 (an attenuator) form a regulatory circuit that discriminates between transient and persistent Toll-like receptor 4-induced signals (Litvak et al., 2009).
An overly simplistic view of these three pathways is that the anti-inflammatory properties of NRF2 have the potential to modulate the pro-inflammatory tendencies of NF-κB. All three pathways can be viewed as pleiotropic. Nevertheless the authors of one paper felt compelled to write, “One of the greatest challenges in studying Notch signaling is the inability to predict the outcome of Notch activation, owing to its multiple roles” (Ables et al., 2011), while others wrote, “Although, this pathway is remarkably short, with no second messenger involved, it regulates expression of more than hundred target genes in a tissue-specific manner” (Borggrefe and Liefke, 2012). In addition to the contribution of context and cross-talk, some of the multiplicity and diversity of the pleiotropic functions of transcription factors have been attributed to epigenetic tendencies (Sarnico et al., 2012).
We provide just a hint of the complexity of these three pathways and their interconnections in the Figure 1. Not shown in the Figure 1 are the relationships between adult brain diseases and NF-κB (Cai, 2009; Ridder and Schwaninger, 2009; Harari and Liao, 2010; Nogueira et al., 2011; Nestler, 2012; Sako et al., 2012; Crampton and O’keeffe, 2013; Hoesel and Schmid, 2013; Mc Guire et al., 2013; Zhou and Hu, 2013; Alvira, 2014; Gupta and Sharma, 2014; Laprairie et al., 2014; Snow et al., 2014; Tu et al., 2014; Uekawa et al., 2014; Xiao et al., 2014; Zhou and Zhou, 2014; Zhao et al., 2015), NRF2 (van Muiswinkel and Kuiperij, 2005; Kumar et al., 2012; Sandberg et al., 2013; Arnold et al., 2014; Ding et al., 2014; Chen et al., 2015b; Djordjevic et al., 2015; Zhao et al., 2015), and Notch (John et al., 2002; Minter et al., 2005; Arumugam et al., 2006; Ban et al., 2006; Nagarsheth et al., 2006; Elyaman et al., 2007; Jurynczyk et al., 2008; Yuan and Yu, 2010; Tsugane et al., 2012), as well as between epigenetic phenomena and NF-κB (Arzate-Mejía et al., 2011; Sarnico et al., 2012; Narayan et al., 2015), NRF2 (Martinez et al., 2009; Arzate-Mejía et al., 2011; Gao et al., 2015), and Notch (Arzate-Mejía et al., 2011; Cama et al., 2013; Sun et al., 2014; Schwanbeck, 2015).
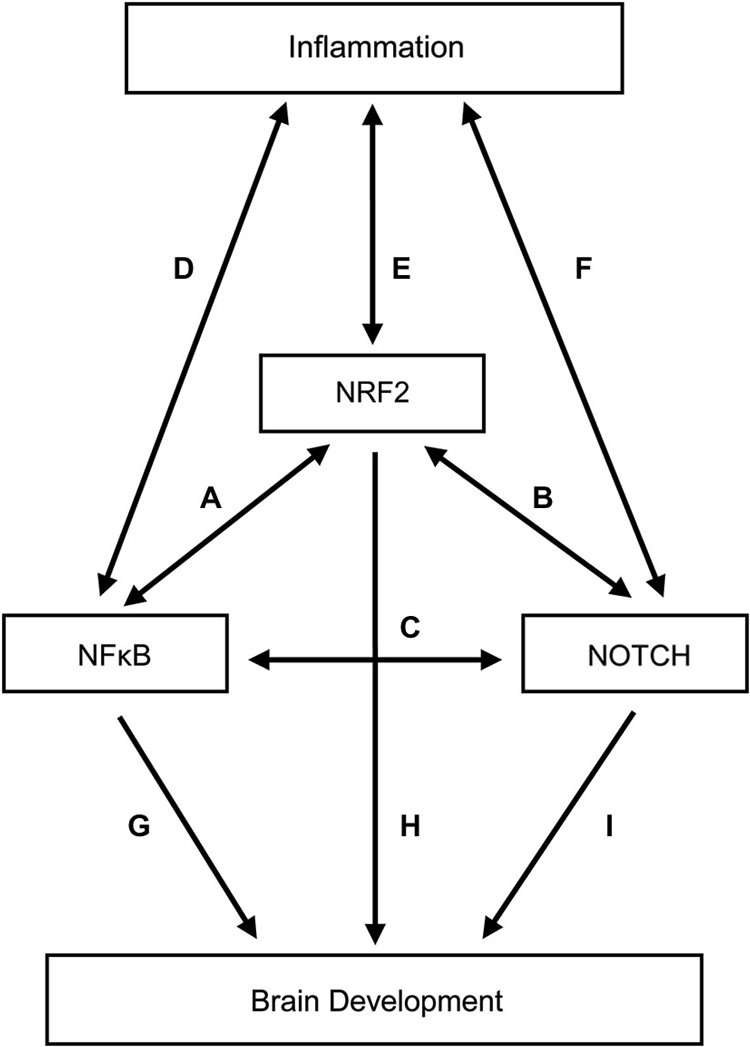
Figure 1. Relationships among inflammation, three transcription factors (NF-κB, NRF-2, and Notch), and brain development. To minimize complexity, the contributions of NF-κB, NRF-2, and Notch to brain damage in adults are not included in this figure. The letters adjacent to the lines identify a selected set of citations that support the existence of a relationship indicated by the line. (A) (Liu et al., 2008; Buelna-Chontal and Zazueta, 2013; Ganesh Yerra et al., 2013; Sandberg et al., 2013; Yang et al., 2013a,b; Djordjevic et al., 2015). (B) (Yang et al., 2013a; Kim et al., 2014; Wakabayashi et al., 2014). (C) (Ang and Tergaonkar, 2007; Hurlbut et al., 2007; Scholzke and Schwaninger, 2007; Osipo et al., 2008; Poellinger and Lendahl, 2008; Yang et al., 2013a; Yao et al., 2013b; Li et al., 2014; Yu et al., 2014). (D) (Covert et al., 2005; Werner et al., 2005; Liu et al., 2008; O’Dea and Hoffmann, 2009; Kim et al., 2011; Ruland, 2011; Bartuzi et al., 2013; Bakunina et al., 2015; Zhao et al., 2015). (E) (Kumar et al., 2012; Brune et al., 2013; Ruiz et al., 2013; Sandberg et al., 2013; Bakunina et al., 2015; Zhao et al., 2015). (F) (Hoyne et al., 2001; Arumugam et al., 2006; Fung et al., 2007; Fernandez et al., 2008; Palaga et al., 2008; Ito et al., 2012; Wongchana and Palaga, 2012; Zhang et al., 2012; Radtke et al., 2013; Yao et al., 2013b; Cheng et al., 2015). (G) (O’Dea and Hoffmann, 2009; Crampton and O’keeffe, 2013; Snow et al., 2014). (H) (Gupta et al., 2013). (I) (Wang et al., 1998; Oya et al., 2009; Imayoshi et al., 2013; Yao et al., 2013a; Kotasová et al., 2014; Schwanbeck, 2015).
To add to the complexity, epigenetic phenomena influence the balance between inflammatory and anti-inflammatory capabilities (Correa et al., 2011; Sarnico et al., 2012; Aithal and Rajeswari, 2013; Cai et al., 2013; Su et al., 2013). As might be expected, systems biologists are paying attention to epigenetic phenomena (Sookoian et al., 2013; Dekker, 2014; Maze et al., 2014; Schadt et al., 2014; Klin et al., 2015; Thakur et al., 2015). Preconditioning/tolerance, the diminished damage that follows a damaging exposure when it is preceded by a sub-injurious exposure (Hagberg et al., 2004), has also attracted the attention of systems biologists (Voit, 2009; Jusko, 2013; León et al., 2013; Gong et al., 2014).
Part 3. Biomarkers of Exposures and Processes Leading to Brain Damage
Most of the biomarkers of injury to the immature brain are proteins measured in peripheral blood (including, S100B, activin A, adrenomedullin, neuron-specific enolase, oxidative stress markers, glial fibrillary acidic protein, and hemeoxygenase-1) (Douglas-Escobar and Weiss, 2012; Serpero et al., 2013). They give some information about the networks that might have been injured.
Biomarkers of the newborn’s response to potentially adverse exposures, include indicators of systemic inflammation (Leviton et al., 2012; Machado et al., 2014) and other (usually inflammatory) processes associated with injury to the immature brain (Dammann and O’shea, 2008; Malaeb and Dammann, 2009). Markers associated with oxidative stress, including advanced oxidation protein products and total hydroperoxides, malondialdehyde, ascorbate, allantoin (the oxidation product of uric acid) and carbonyl proteins, as well indicators of antioxidant capacity, (vitamins A, E and C) (Negi et al., 2012; Perrone et al., 2012) have the potential to add to our knowledge of the relationship between oxidative stress and encephalopathy of prematurity (Kakita et al., 2009).
In addition, new imaging techniques appear to be increasingly available to identify early structural changes in the brain, (Melbourne et al., 2013; Counsell et al., 2014) just as amplitude EEG is becoming used more widely to assess the maturation and well being of the very preterm newborn (Sohn et al., 2013; Welch et al., 2013; Natalucci et al., 2014). In light of such information about putative-damaging exposures/processes and brain function and structure of very preterm newborns, systems biologists should be able model phenomena related to brain damage in immature humans.
Part 4. The Future
As those who study systems biology and systems medicine have concentrated on finding a small number of nodes that might serve as targets for therapies and prophylaxis, epidemiologists have concentrated on identifying the exposures that lead to activation of these nodes. For example, in light of our own work, which identified “prolonged” ventilation as a source of postnatal systemic inflammation (Bose et al., 2013), we encourage those responsible for the well-being of very preterm newborns to find and use ventilation strategies that minimize barotrauma and tracheitis (Vendettuoli et al., 2014).
We also identified intermittent and/or sustained systemic inflammation as an antecedent of perinatal brain damage in very preterm newborns (Leviton et al., 2011a,b; O’Shea et al., 2012). With the involvement of elevated concentrations of cytokines, chemokines, adhesion molecules, matrix metalloproteinases, and inflammation-associated growth factors, a therapy that alters the equilibrium at a single, or even a small number of nodes seems incapable of reducing the damage associated with inflammation, let alone contributing to inflammation resolution, and initiating and promoting brain repair. Although inhibitors of a single pro-inflammatory cytokine have diminished the severity of rheumatoid arthritis and inflammatory bowel disease, the most effective appear to have broad effects (Macdonald, 2010). This recognition that a single, highly-specific drug is unlikely to be especially effective has prompted the use of a “cocktail” of multiple drugs targeting multiple inflammatory signaling pathways (Kwon et al., 2013) or a broad spectrum single drug (Belur et al., 2013; Sánchez-Aguilar et al., 2013). Nevertheless, a single, narrow-spectrum inhibitor/modulator might prove effective (Girard et al., 2012; Kight and McCarthy, 2014; Chen et al., 2015a; Zhang et al., 2015). How relevant these efforts are to minimizing brain damage in preterm newborns remains to be seen.
We agree that the best approach is to minimize exposure to brain-damaging insults. Second best is to utilize drugs that target the nodes most likely to be involved (Deboy et al., 2006; Saliba et al., 2007).
Thus, the more information about interrelationships systems biologists and others can provide, the brighter the future for reducing disease burdens on society.
We had four goals in preparing this perspective. First, to show how the antecedents of brain damage can be linked to molecular perturbations. Second, to show how complex and interrelated are the molecular changes that follow exposures that precede the onset of brain damage. Third, to show how a systems approach might expand our understanding, and thereby lead to therapeutic options for encephalopathy of prematurity. Fourth, to call attention to the benefits systems biologists might receive by their study of disturbances to normal brain development, which might provide genetic, proteomic, metabolomic, epigenomic, and microbiomic data needed for models of disease in humans (Nicholson, 2006; Barabási et al., 2011; Espinosa-Jeffrey et al., 2013; Li, 2013; Wolkenhauer, 2013; Wolkenhauer et al., 2013).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Preparation of this manuscript was supported by the National Institutes of Health (5U01NS040069, 1R01EY021820, and 5P30HD018655-28). Inserm, Paris Diderot University, and Fondation Grace de Monaco. The sponsors did not see this manuscript prior to submission.
References
Ables, J. L., Breunig, J. J., Eisch, A. J., and Rakic, P. (2011). Not(ch) just development: notch signalling in the adult brain. Nat. Rev. Neurosci. 12, 269–283. doi: 10.1038/nrn3024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Adén, U., Favrais, G., Plaisant, F., Winerdal, M., Felderhoff-Mueser, U., Lampa, J., et al. (2010). Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: key role of TNFalpha pathway and protection by etanercept. Brain Behav. Immun. 24, 747–758. doi: 10.1016/j.bbi.2009.10.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Adkins, B. (2013). Neonatal immunology: responses to pathogenic microorganisms and epigenetics reveal an “immunodiverse” developmental state. Immunol. Res. 57, 246–257. doi: 10.1007/s12026-013-8439-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Adler, I., Batton, D., Betz, B., Bezinque, S., Ecklund, K., Junewick, J., et al. (2010). Mechanisms of injury to white matter adjacent to a large intraventricular hemorrhage in the preterm brain. J. Clin. Ultrasound 38, 254–258. doi: 10.1002/jcu.20683
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Afrazi, A., Branca, M. F., Sodhi, C. P., Good, M., Yamaguchi, Y., Egan, C. E., et al. (2014). Toll like receptor 4-mediated Endoplasmic reticulum stress in intestinal crypts induces Necrotizing Enterocolitis. J. Biol. Chem. 289, 9584–9599. doi: 10.1074/jbc.m113.526517
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Aithal, M. G., and Rajeswari, N. (2013). Role of Notch signalling pathway in cancer and its association with DNA methylation. J. Genet. 92, 667–675. doi: 10.1007/s12041-013-0284-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alvira, C. M. (2014). Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions. Birth Defects Res. A Clin. Mol. Teratol. 100, 202–216. doi: 10.1002/bdra.23233
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ang, H. L., and Tergaonkar, V. (2007). Notch and NFkappaB signaling pathways: do they collaborate in normal vertebrate brain development and function? Bioessays 29, 1039–1047. doi: 10.1002/bies.20647
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arnold, P., Mojumder, D., Detoledo, J., Lucius, R., and Wilms, H. (2014). Pathophysiological processes in multiple sclerosis: focus on nuclear factor erythroid-2-related factor 2 and emerging pathways. Clin. Pharmacol. 6, 35–42. doi: 10.2147/cpaa.s35033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arthur, J. S., and Ley, S. C. (2013). Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692. doi: 10.1038/nri3495
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arumugam, T. V., Chan, S. L., Jo, D. G., Yilmaz, G., Tang, S. C., Cheng, A., et al. (2006). Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat. Med. 12, 621–623. doi: 10.1038/nm1403
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arzate-Mejía, R. G., Valle-Garcia, D., and Recillas-Targa, F. (2011). Signaling epigenetics: novel insights on Cell. Signal.ing and epigenetic regulation. IUBMB Life 63, 881–895. doi: 10.1002/iub.557
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Athayde, N., Wang, J., Wang, X., and Trudinger, B. (2005). Fetuses delivered following preterm prelabor rupture of the membranes are capable of stimulating a proinflammatory response in endothelial cells. J. Soc. Gynecol. Investig. 12, 118–122. doi: 10.1016/j.jsgi.2004.10.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Babu, R., Bagley, J. H., Di, C., Friedman, A. H., and Adamson, C. (2012). Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg. Focus 32, E8, 1–12. doi: 10.3171/2012.1.focus11366
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Back, S. A., Riddle, A., and McClure, M. M. (2007). Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke 38, 724–730. doi: 10.1161/01.str.0000254729.27386.05
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bakewell-Sachs, S., Medoff-Cooper, B., Escobar, G. J., Silber, J. H., and Lorch, S. A. (2009). Infant functional status: the timing of physiologic maturation of premature infants. Pediatrics 123, e878–886. doi: 10.1542/peds.2008-2568
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bakunina, N., Pariante, C. M., and Zunszain, P. A. (2015). Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology doi: 10.1111/imm.12443. [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ban, M., Booth, D., Heard, R., Stewart, G., Goris, A., Vandenbroeck, K., et al. (2006). Linkage disequilibrium screening for multiple sclerosis implicates JAG1 and POU2AF1 as susceptibility genes in Europeans. J. Neuroimmunol. 179, 108–116. doi: 10.1016/j.jneuroim.2006.06.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barabási, A. L., Gulbahce, N., and Loscalzo, J. (2011). Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 12, 56–68. doi: 10.1038/nrg2918
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bartuzi, P., Hofker, M. H., and Van De Sluis, B. (2013). Tuning NF-kappaB activity: a touch of COMMD proteins. Biochim. Biophys. Acta 1832, 2315–2321. doi: 10.1016/j.bbadis.2013.09.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Basatemur, E., Gardiner, J., Williams, C., Melhuish, E., Barnes, J., and Sutcliffe, A. (2013). Maternal prepregnancy BMI and child cognition: a longitudinal cohort study. Pediatrics 131, 56–63. doi: 10.1542/peds.2012-0788d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Belur, P. K., Chang, J. J., He, S., Emanuel, B. A., and Mack, W. J. (2013). Emerging experimental therapies for intracerebral hemorrhage: targeting mechanisms of secondary brain injury. Neurosurg. Focus 34, E9, 1–8. doi: 10.3171/2013.2.focus1317
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Berner, R., Niemeyer, C. M., Leititis, J. U., Funke, A., Schwab, C., Rau, U., et al. (1998). Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8 and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr. Res. 44, 469–477. doi: 10.1203/00006450-199810000-00002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blahnik, M. J., Ramanathan, R., Riley, C. R., and Minoo, P. (2001). Lipopolysaccharide-induced tumor necrosis factor-alpha and IL-10 production by lung macrophages from preterm and term neonates. Pediatr. Res. 50, 726–731. doi: 10.1203/00006450-200112000-00016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Borggrefe, T., and Liefke, R. (2012). Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle 11, 264–276. doi: 10.4161/cc.11.2.18995
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bose, C. L., Laughon, M. M., Allred, E. N., O’shea, T. M., Van Marter, L. J., Ehrenkranz, R. A., et al. (2013). Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine 61, 315–322. doi: 10.1016/j.cyto.2012.10.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bose, C., Van Marter, L. J., Laughon, M., O’shea, T. M., Allred, E. N., Karna, P., et al. (2009). Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 124, e450–e456. doi: 10.1542/peds.2008-3249
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bradley, R. H., and Corwyn, R. F. (2002). Socioeconomic status and child development. Annu. Rev. Psychol. 53, 371–399. doi: 10.4324/9781410607027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brehmer, F., Bendix, I., Prager, S., Van De Looij, Y., Reinboth, B. S., Zimmermanns, J., et al. (2012). Interaction of inflammation and hyperoxia in a rat model of neonatal white matter damage. PLoS One 7:e49023. doi: 10.1371/journal.pone.0049023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brion, M. J. (2013). Commentary: can maternal-paternal comparisons contribute to our understanding of maternal pre-pregnancy obesity and its association with offspring cognitive outcomes? Int. J. Epidemiol. 42, 518–519. doi: 10.1093/ije/dyt041
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brochu, M. E., Girard, S., Lavoie, K., and Sébire, G. (2011). Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: an experimental study. J. Neuroinflammation 8:55. doi: 10.1186/1742-2094-8-55
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brune, B., Dehne, N., Grossmann, N., Jung, M., Namgaladze, D., Schmid, T., et al. (2013). Redox control of inflammation in macrophages. Antioxid. Redox Signal. 19, 595–637. doi: 10.1089/ars.2012.4785
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Buelna-Chontal, M., and Zazueta, C. (2013). Redox activation of Nrf2 and NF-kappaB: a double end sword? Cell. Signal. 25, 2548–2557. doi: 10.1016/j.cellsig.2013.08.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cai, D. (2009). NFkappaB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle 8, 2542–2548. doi: 10.4161/cc.8.16.9386
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cai, Z., Fan, L. W., Kaizaki, A., Tien, L. T., Ma, T., Pang, Y., et al. (2013). Neonatal systemic exposure to lipopolysaccharide enhances susceptibility of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Dev. Neurosci. 35, 155–171. doi: 10.1159/000346156
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cama, A., Verginelli, F., Lotti, L. V., Napolitano, F., Morgano, A., D’orazio, A., et al. (2013). Integrative genetic, epigenetic and pathological analysis of paraganglioma reveals complex dysregulation of NOTCH signaling. Acta Neuropathol. 126, 575–594. doi: 10.1007/s00401-013-1165-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Capoluongo, E., Vento, G., Santonocito, C., Matassa, P. G., Vaccarella, C., Giardina, B., et al. (2005). Comparison of serum levels of seven cytokines in premature newborns undergoing different ventilatory procedures: high frequency oscillatory ventilation or synchronized intermittent mandatory ventilation. Eur. Cytokine Netw. 16, 199–205.
Casas, M., Chatzi, L., Carsin, A. E., Amiano, P., Guxens, M., Kogevinas, M., et al. (2013). Maternal pre-pregnancy overweight and obesity and child neuropsychological development: two Southern European birth cohort studies. Int. J. Epidemiol. 42, 506–517. doi: 10.1093/ije/dyt002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, Z., Mao, X., Liu, A., Gao, X., Chen, X., Ye, M., et al. (2015b). Osthole, a natural coumarin improves cognitive impairments and BBB dysfunction after transient global brain ischemia in C57 BL/6J mice: involvement of Nrf2 pathway. Neurochem. Res. 40, 186–194. doi: 10.1007/s11064-014-1483-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, X., Sadowska, G. B., Zhang, J., Kim, J. E., Cummings, E. E., Bodge, C. A., et al. (2015a). Neutralizing anti-interleukin-1beta antibodies modulate fetal blood-brain barrier function after ischemia. Neurobiol. Dis. 73, 118–129. doi: 10.1016/j.nbd.2014.09.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheng, Y. L., Choi, Y., Sobey, C. G., Arumugam, T. V., and Jo, D. G. (2015). Emerging roles of the gamma-secretase-notch axis in inflammation. Pharmacol. Ther. 147C, 80–90. doi: 10.1016/j.pharmthera.2014.11.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chheda, S., Palkowetz, K. H., Garofalo, R., Rassin, D. K., and Goldman, A. S. (1996). Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatr. Res. 40, 475–483. doi: 10.1203/00006450-199609000-00018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Correa, F., Mallard, C., Nilsson, M., and Sandberg, M. (2011). Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3beta. Neurobiol. Dis. 44, 142–151. doi: 10.1016/j.nbd.2011.06.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Counsell, S. J., Ball, G., and Edwards, A. D. (2014). New imaging approaches to evaluate newborn brain injury and their role in predicting developmental disorders. Curr. Opin. Neurol. 27, 168–175. doi: 10.1097/wco.0000000000000073
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Covert, M. W., Leung, T. H., Gaston, J. E., and Baltimore, D. (2005). Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science 309, 1854–1857. doi: 10.1126/science.1112304
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Crampton, S. J., and O’keeffe, G. W. (2013). NF-kappaB: emerging roles in hippocampal development and function. Int. J. Biochem. Cell Biol. 45, 1821–1824. doi: 10.1016/j.biocel.2013.05.037
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cuenca, A. G., Wynn, J. L., Moldawer, L. L., and Levy, O. (2013). Role of innate immunity in neonatal infection. Am. J. Perinatol. 30, 105–112. doi: 10.1055/s-0032-1333412
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dammann, O., and Leviton, A. (1999). Brain damage in preterm newborns: might enhancement of developmentally-regulated endogenous protection open a door for prevention? Pediatrics 104, 541–550. doi: 10.1542/peds.104.3.541
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dammann, O., and Leviton, A. (2014). Intermittent or sustained systemic inflammation and the preterm brain. Pediatr. Res. 75, 376–380. doi: 10.1038/pr.2013.238
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dammann, O., Naples, M., Bednarek, F., Shah, B., Kuban, K. C., O’shea, T. M., et al. (2010). SNAP-II and SNAPPE-II and the risk of structural and functional brain disorders in extremely low gestational age newborns: the ELGAN study. Neonatology 97, 71–82. doi: 10.1159/000232588
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dammann, O., and O’shea, T. M. (2008). Cytokines and perinatal brain damage. Clin. Perinatol. 35, 643–663. doi: 10.1016/j.clp.2008.07.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Deboy, C. A., Xin, J., Byram, S. C., Serpe, C. J., Sanders, V. M., and Jones, K. J. (2006). Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp. Neurol. 201, 212–224. doi: 10.1016/j.expneurol.2006.04.028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dekker, J. (2014). Two ways to fold the genome during the cell cycle: insights obtained with chromosome conformation capture. Epigenetics Chromatin 7:25. doi: 10.1186/1756-8935-7-25
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ding, Y., Chen, M., Wang, M., Zhang, T., Park, J., Zhu, Y., et al. (2014). Neuroprotection by acetyl-11-keto-beta-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci. Rep. 4:7002. doi: 10.1038/srep07002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Djordjevic, J., Djordjevic, A., Adzic, M., Mitic, M., Lukic, I., and Radojcic, M. B. (2015). Alterations in the Nrf2-Keap1 signaling pathway and its downstream target genes in rat brain under stress. Brain Res. 1602, 20–31. doi: 10.1016/j.brainres.2015.01.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Douglas-Escobar, M., and Weiss, M. D. (2012). Biomarkers of brain injury in the premature infant. Front. Neurol. 3:185. doi: 10.3389/fneur.2012.00185
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ehlert, U. (2013). Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology 38, 1850–1857. doi: 10.1016/j.psyneuen.2013.06.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eklind, S., Mallard, C., Arvidsson, P., and Hagberg, H. (2005). Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr. Res. 58, 112–116. doi: 10.1203/01.pdr.0000163513.03619.8d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Elyaman, W., Bradshaw, E. M., Wang, Y., Oukka, M., Kivisakk, P., Chiba, S., et al. (2007). JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J. Immunol. 179, 5990–5998. doi: 10.4049/jimmunol.179.9.5990
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Espinosa-Jeffrey, A., Barajas, S. A., Arrazola, A. R., Taniguchi, A., Zhao, P. M., Bokhoor, P., et al. (2013). White matter loss in a mouse model of periventricular leukomalacia is rescued by trophic factors. Brain Sci. 3, 1461–1482. doi: 10.3390/brainsci3041461
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Evans, G. W., and Kim, P. (2010). Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann. N Y Acad. Sci. 1186, 174–189. doi: 10.1111/j.1749-6632.2009.05336.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fernandez, L., Rodriguez, S., Huang, H., Chora, A., Fernandes, J., Mumaw, C., et al. (2008). Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp. Hematol. 36, 545–558. doi: 10.1016/j.exphem.2007.12.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fichorova, R. N., Beatty, N., Sassi, R. R., Yamamoto, H., Allred, E. N., and Leviton, A. (2015). Systemic inflammation in the extremely low gestational age newborn following maternal genito-urinary infections. Am. J. Reprod. Immunol. 73, 162–174. doi: 10.1111/aji.12313
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fichorova, R. N., Onderdonk, A. B., Yamamoto, H. M. L. D., Dubois, A. M., Allred, E., Leviton, A., et al. (2011). Microbe-specific modulation of inflammatory response in extremely low gestational age newborns. MBio 2, e00280–e00310. doi: 10.1128/mBio.00280-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
François, A., Terro, F., Quellard, N., Fernandez, B., Chassaing, D., Janet, T., et al. (2014). Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Mol. Brain 7:56. doi: 10.1186/s13041-014-0056-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fung, E., Tang, S. M., Canner, J. P., Morishige, K., Arboleda-Velasquez, J. F., Cardoso, A. A., et al. (2007). Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation 115, 2948–2956. doi: 10.1161/circulationaha.106.675462
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ganesh Yerra, V., Negi, G., Sharma, S. S., and Kumar, A. (2013). Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 1, 394–397. doi: 10.1016/j.redox.2013.07.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gao, Y., Yan, Y., and Huang, T. (2015). Human agerelated cataracts: epigenetic suppression of the nuclear factor erythroid 2related factor 2mediated antioxidant system. Mol. Med. Rep. 11, 1442–1447. doi: 10.3892/mmr.2014.2849
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Girard, S., Sebire, H., Brochu, M. E., Briota, S., Sarret, P., and Sebire, G. (2012). Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries. Brain Behav. Immun. 26, 1331–1339. doi: 10.1016/j.bbi.2012.09.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Glinianaia, S. V., Rankin, J., and Colver, A. (2011). Cerebral palsy rates by birth weight, gestation and severity in North of England, 1991–2000 singleton births. Arch. Dis. Child. 96, 180–185. doi: 10.1136/adc.2010.183939
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gong, G., Bai, S., Wu, W., Hu, L., Liu, Y., Niu, J., et al. (2014). Lrg participates in lipopolysaccharide preconditioning-induced brain ischemia injury via TLR4 signaling pathway. J. Mol. Neurosci. 54, 20–26. doi: 10.1007/s12031-014-0240-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grissom, N. M., and Reyes, T. M. (2013). Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int. J. Dev. Neurosci. 31, 406–414. doi: 10.1016/j.ijdevneu.2012.11.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, K., Chandran, S., and Hardingham, G. E. (2013). Human stem cell-derived astrocytes and their application to studying Nrf2-mediated neuroprotective pathways and therapeutics in neurodegeneration. Br. J. Clin. Pharmacol. 75, 907–918. doi: 10.1111/bcp.12022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, S., and Sharma, B. (2014). Pharmacological benefit of I1-imidazoline receptors activation and nuclear factor kappa-B (NF-kappaB) modulation in experimental Huntington’s disease. Brain Res. Bull. 102, 57–68. doi: 10.1016/j.brainresbull.2014.02.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hagberg, H., Dammann, O., Mallard, C., and Leviton, A. (2004). Preconditioning and the developing brain. Semin. Perinatol. 28, 389–395. doi: 10.1053/j.semperi.2004.10.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hansen-Pupp, I., Hallin, A. L., Hellström-Westas, L., Cilio, C., Berg, A. C., Stjernqvist, K., et al. (2008). Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr. Res. 64, 183–188. doi: 10.1203/pdr.0b013e318176144d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hanson, J. L., Hair, N., Shen, D. G., Shi, F., Gilmore, J. H., Wolfe, B. L., et al. (2013). Family poverty affects the rate of human infant brain growth. PLoS One 8:e80954. doi: 10.1371/journal.pone.0080954
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Harari, O. A., and Liao, J. K. (2010). NF-kappaB and innate immunity in ischemic stroke. Ann. N Y Acad. Sci. 1207, 32–40. doi: 10.1111/j.1749-6632.2010.05735.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hecht, J. L., Fichorova, R. N., Tang, V. F., Allred, E. N., McElrath, T. F., Leviton, A., et al. (2011). Relationship between neonatal blood protein profiles and placenta histologic characteristics in ELGANs. Pediatr. Res. 69, 68–73. doi: 10.1203/pdr.0b013e3181fed334
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Helderman, J. B., O’shea, T. M., Kuban, K. C. K., Allred, E. N., Hecht, J. L., Dammann, O., et al. (2012). Antenatal antecedents of low scores on the Bayley scales of infant development at 24 months among children born before the 28th post-menstrual week. The ELGAN study. Pediatrics 129, 494–502. doi: 10.1542/peds.2011-1796
Himpens, E., Van Den Broeck, C., Oostra, A., Calders, P., and Vanhaesebrouck, P. (2008). Prevalence, type, distribution and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev. Med. Child Neurol. 50, 334–340. doi: 10.1111/j.1469-8749.2008.02047.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hinkle, S. N., Sharma, A. J., Kim, S. Y., and Schieve, L. A. (2013). Maternal prepregnancy weight status and associations with children’s development and disabilities at kindergarten. Int. J. Obes. (Lond) 37, 1344–1351. doi: 10.1038/ijo.2013.128
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hoesel, B., and Schmid, J. A. (2013). The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 12:86. doi: 10.1186/1476-4598-12-86
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hoyne, G. F., Dallman, M. J., Champion, B. R., and Lamb, J. R. (2001). Notch signalling in the regulation of peripheral immunity. Immunol. Rev. 182, 215–227. doi: 10.1034/j.1600-065x.2001.1820118.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huang, L., Yu, X., Keim, S., Li, L., Zhang, L., and Zhang, J. (2014). Maternal prepregnancy obesity and child neurodevelopment in the collaborative perinatal project. Int. J. Epidemiol. 43, 783–792. doi: 10.1093/ije/dyu030
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huhtala, M., Korja, R., Lehtonen, L., Haataja, L., Lapinleimu, H., Rautava, P., et al. (2012). Parental psychological well-being and behavioral outcome of very low birth weight infants at 3 years. Pediatrics 129, e937–e944. doi: 10.1542/peds.2011-2411d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hurlbut, G. D., Kankel, M. W., Lake, R. J., and Artavanis-Tsakonas, S. (2007). Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 19, 166–175. doi: 10.1016/j.ceb.2007.02.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Imayoshi, I., Shimojo, H., Sakamoto, M., Ohtsuka, T., and Kageyama, R. (2013). Genetic visualization of notch signaling in mammalian neurogenesis. Cell. Mol. Life Sci. 70, 2045–2057. doi: 10.1007/s00018-012-1151-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ito, T., Connett, J. M., Kunkel, S. L., and Matsukawa, A. (2012). Notch system in the linkage of innate and adaptive immunity. J. Leukoc. Biol. 92, 59–65. doi: 10.1189/jlb.1011529
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
John, G. R., Shankar, S. L., Shafit-Zagardo, B., Massimi, A., Lee, S. C., Raine, C. S., et al. (2002). Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat. Med. 8, 1115–1121. doi: 10.1038/nm781
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, C. A., Cayabyab, R. G., Kwong, K. Y., Stotts, C., Wong, B., Hamdan, H., et al. (1996). Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr. Res. 39, 966–975. doi: 10.1203/00006450-199606000-00007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jurynczyk, M., Jurewicz, A., Raine, C. S., and Selmaj, K. (2008). Notch3 inhibition in myelin-reactive T cells down-regulates protein kinase C theta and attenuates experimental autoimmune encephalomyelitis. J. Immunol. 180, 2634–2640. doi: 10.4049/jimmunol.180.4.2634
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jusko, W. J. (2013). Moving from basic toward systems pharmacodynamic models. J. Pharm. Sci. 102, 2930–2940. doi: 10.1002/jps.23590
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kakita, H., Hussein, M. H., Yamada, Y., Henmi, H., Kato, S., Kobayashi, S., et al. (2009). High postnatal oxidative stress in neonatal cystic periventricular leukomalacia. Brain Dev. 31, 641–648. doi: 10.1016/j.braindev.2008.10.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kerstjens, J. M., de Winter, A. F., Sollie, K. M., Bocca-Tjeertes, I. F., Potijk, M. R., Reijneveld, S. A., et al. (2013). Maternal and pregnancy-related factors associated with developmental delay in moderately preterm-born children. Obstet. Gynecol. 121, 727–733. doi: 10.1097/aog.0b013e3182860c52
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keunen, K., Van Elburg, R. M., Van Bel, F., and Benders, M. J. (2015). Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr. Res. 77, 148–155. doi: 10.1038/pr.2014.171
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kight, K. E., and McCarthy, M. M. (2014). Using sex differences in the developing brain to identify nodes of influence for seizure susceptibility and epileptogenesis. Neurobiol. Dis. 72(Pt. B), 136–143. doi: 10.1016/j.nbd.2014.05.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, K. H., Lyu, J. H., Koo, S. T., Oh, S. R., Lee, H. K., Ahn, K. S., et al. (2011). MyD88 is a mediator for the activation of Nrf2. Biochem. Biophys. Res. Commun. 404, 46–51. doi: 10.1016/j.bbrc.2010.11.051
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, J. H., Thimmulappa, R. K., Kumar, V., Cui, W., Kumar, S., Kombairaju, P., et al. (2014). NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J. Clin. Invest. 124, 730–741. doi: 10.1172/jci70812
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Klin, A., Shultz, S., and Jones, W. (2015). Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci. Biobehav. Rev. 50C, 189–203. doi: 10.1016/j.neubiorev.2014.10.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kong, Y., and Le, Y. (2011). Toll-like receptors in inflammation of the central nervous system. Int. Immunopharmacol. 11, 1407–1414. doi: 10.1016/j.intimp.2011.04.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kotasová, H., Procházková, J., and Pacherník, J. (2014). Interaction of notch and gp130 signaling in the maintenance of neural stem and progenitor cells. Cell. Mol. Neurobiol. 34, 1–15. doi: 10.1007/s10571-013-9996-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kumar, H., Koppula, S., Kim, I. S., More, S. V., Kim, B. W., and Choi, D. K. (2012). Nuclear factor erythroid 2-related factor 2 signaling in Parkinson disease: a promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS Neurol. Disord. Drug Targets 11, 1015–1029. doi: 10.2174/1871527311211080012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kwon, Y. S., Pineda, E., Auvin, S., Shin, D., Mazarati, A., and Sankar, R. (2013). Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J. Neuroinflammation 10:30. doi: 10.1186/1742-2094-10-30
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Laprairie, R. B., Warford, J. R., Hutchings, S., Robertson, G. S., Kelly, M. E., and Denovan-Wright, E. M. (2014). The cytokine and endocannabinoid systems are co-regulated by NF-kappaB p65/RelA in cell culture and transgenic mouse models of Huntington’s disease and in striatal tissue from Huntington’s disease patients. J. Neuroimmunol. 267, 61–72. doi: 10.1016/j.jneuroim.2013.12.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
León, K., García-Martínez, K., and Carmenate, T. (2013). Mathematical models of the impact of IL2 modulation therapies on T cell dynamics. Front. Immunol. 4:439. doi: 10.3389/fimmu.2013.00439
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Levine, B., Mizushima, N., and Virgin, H. W. (2011). Autophagy in immunity and inflammation. Nature 469, 323–335. doi: 10.1038/nature09782
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leviton, A., and Gressens, P. (2007). Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 30, 473–478. doi: 10.1016/j.tins.2007.05.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leviton, A., Kuban, K. C., Allred, E. N., Fichorova, R. N., O’Shea, T. M., Paneth, N., et al. (2011b). Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum. Dev. 87, 325–330. doi: 10.1016/j.earlhumdev.2011.01.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leviton, A., Kuban, K., O’Shea, T. M., Paneth, N., Fichorova, R., Allred, E. N., et al. (2011a). The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr. 158, 897–903.e5. doi: 10.1016/j.jpeds.2010.11.059
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leviton, A., O’Shea, T. M., Bednarek, F. J., Allred, E. N., Fichorova, R. N., Dammann, O., et al. (2012). Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr. 101, 355–359. doi: 10.1111/j.1651-2227.2011.02527.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, H. (2013). Systems biology approaches to epidemiological studies of complex diseases. Wiley Interdiscip. Rev. Syst. Biol. Med. 5, 677–686. doi: 10.1002/wsbm.1242
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, L., Zhao, F., Lu, J., Li, T., Yang, H., Wu, C., et al. (2014). Notch-1 signaling promotes the malignant features of human breast cancer through NF-kappaB activation. PLoS One 9:e95912. doi: 10.1371/journal.pone.0095912
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lin, Q., Li, M., Fang, D., Fang, J., and Su, S. B. (2011). The essential roles of toll-like receptor signaling pathways in sterile inflammatory diseases. Int. Immunopharmacol. 11, 1422–1432. doi: 10.1016/j.intimp.2011.04.026
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lin, H. Y., Wu, C. L., and Huang, C. C. (2010). The Akt-endothelial nitric oxide synthase pathway in lipopolysaccharide preconditioning-induced hypoxic-ischemic tolerance in the neonatal rat brain. Stroke 41, 1543–1551. doi: 10.1161/strokeaha.109.574004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Litvak, V., Ramsey, S. A., Rust, A. G., Zak, D. E., Kennedy, K. A., Lampano, A. E., et al. (2009). Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat. Immunol. 10, 437–443. doi: 10.1038/ni.1721
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, X., Chen, Q., Tsai, H. J., Wang, G., Hong, X., Zhou, Y., et al. (2014). Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ. Mol. Mutagen. 55, 223–230. doi: 10.1002/em.21827
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, G. H., Qu, J., and Shen, X. (2008). NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 1783, 713–727. doi: 10.1016/j.bbamcr.2008.01.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Locatelli, A., Ghidini, A., Paterlini, G., Patanè, L., Doria, V., Zorloni, C., et al. (2005). Gestational age at preterm premature rupture of membranes: a risk factor for neonatal white matter damage. Am. J. Obstet. Gynecol. 193, 947–951. doi: 10.1016/j.ajog.2005.06.039
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
López-Alonso, I., Aguirre, A., González-López, A., Fernández, Á. F., Amado-Rodríguez, L., Astudillo, A., et al. (2013). Impairment of autophagy decreases ventilator-induced lung injury by blockade of the NF-kappaB pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L844–L852. doi: 10.1152/ajplung.00422.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Love, E. R., Crum, J., and Bhattacharya, S. (2012). Independent effects of pregnancy induced hypertension on childhood development: a retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 165, 219–224. doi: 10.1016/j.ejogrb.2012.08.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lu, P., Sodhi, C. P., and Hackam, D. J. (2014). Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 21, 81–93. doi: 10.1016/j.pathophys.2013.11.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lucassen, P. J., Naninck, E. F., van Goudoever, J. B., Fitzsimons, C., Joels, M., and Korosi, A. (2013). Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 36, 621–631. doi: 10.1016/j.tins.2013.08.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Macdonald, T. T. (2010). Inside the microbial and immune labyrinth: totally gutted. Nat. Med. 16, 1194–1195. doi: 10.1038/nm1110-1194
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Machado, J. R., Soave, D. F., da Silva, M. V., de Menezes, L. B., Etchebehere, R. M., Monteiro, M. L., et al. (2014). Neonatal sepsis and inflammatory mediators. Mediators Inflamm. 2014:269681. doi: 10.1155/2014/269681
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Malaeb, S., and Dammann, O. (2009). Fetal inflammatory response and brain injury in the preterm newborn. J. Child Neurol. 24, 1119–1126. doi: 10.1177/0883073809338066
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mallard, C. (2012). Innate immune regulation by toll-like receptors in the brain. ISRN Neurol. 2012:701950. doi: 10.5402/2012/701950
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martin, C. R., Bellomy, M., Allred, E. N., Fichorova, R. N., and Leviton, A. (2013). Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal Pediatr. Pathol. 32, 222–234. doi: 10.3109/15513815.2012.721477
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martin, C. R., Zaman, M. M., Gilkey, C., Salguero, M. V., Hasturk, H., Kantarci, A., et al. (2014). Resolvin D1 and lipoxin A4 improve alveolarization and normalize septal wall thickness in a neonatal murine model of hyperoxia-induced lung injury. PLoS One 9:e98773. doi: 10.1371/journal.pone.0098773
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martinez, A. M., Schuettengruber, B., Sakr, S., Janic, A., Gonzalez, C., and Cavalli, G. (2009). Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling. Nat. Genet. 41, 1076–1082. doi: 10.1038/ng.414
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maze, I., Shen, L., Zhang, B., Garcia, B. A., Shao, N., Mitchell, A., et al. (2014). Analytical tools and current challenges in the modern era of neuroepigenomics. Nat. Neurosci. 17, 1476–1490. doi: 10.1038/nn.3816
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McCarran, W. J., and Goldberg, M. P. (2007). White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J. Neurosci. 27, 4220–4229. doi: 10.1523/jneurosci.5542-06.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McElrath, T. F., Allred, E. N., Van Marter, L., Fichorova, R. N., Leviton, A., and ELGAN Study Investigators (2013). Perinatal systemic inflammatory responses of growth-restricted preterm newborns. Acta Paediatr. 102, e439–e442. doi: 10.1111/apa.12339
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mc Guire, C., Prinz, M., Beyaert, R., and van Loo, G. (2013). Nuclear factor kappa B (NF-kappaB) in multiple sclerosis pathology. Trends Mol. Med. 19, 604–613. doi: 10.1016/j.molmed.2013.08.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Medzhitov, R., and Horng, T. (2009). Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9, 692–703. doi: 10.1038/nri2634
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Melbourne, A., Eaton-Rosen, Z., Bainbridge, A., Kendall, G. S., Cardoso, M. J., Robertson, N. J., et al. (2013). Measurement of myelin in the preterm brain: multi-compartment diffusion imaging and multi-component T2 relaxometry. Med. Image Comput. Comput. Assist. Interv. 16, 336–344.
Miller, G. E., Chen, E., Fok, A. K., Walker, H., Lim, A., Nicholls, E. F., et al. (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U S A 106, 14716–14721. doi: 10.1073/pnas.0902971106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Minter, L. M., Turley, D. M., Das, P., Shin, H. M., Joshi, I., Lawlor, R. G., et al. (2005). Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 6, 680–688. doi: 10.1038/ni1209
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mizumura, K., Cloonan, S. M., Haspel, J. A., and Choi, A. M. (2012). The emerging importance of autophagy in pulmonary diseases. Chest 142, 1289–1299. doi: 10.1378/chest.12-0809
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Morinis, J., Carson, C., and Quigley, M. A. (2013). Effect of teenage motherhood on cognitive outcomes in children: a population-based cohort study. Arch. Dis. Child. 98, 959–964. doi: 10.1136/archdischild-2012-302525
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Morsing, E., and Maršál, K. (2014). Pre-eclampsia- an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum. Dev. 90, 99–101. doi: 10.1016/j.earlhumdev.2013.12.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nagarsheth, M. H., Viehman, A., Lippa, S. M., and Lippa, C. F. (2006). Notch-1 immunoexpression is increased in Alzheimer’s and Pick’s disease. J. Neurol. Sci. 244, 111–116. doi: 10.1016/j.jns.2006.01.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nanthakumar, N. N., Fusunyan, R. D., Sanderson, I., and Walker, W. A. (2000). Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc. Natl. Acad. Sci. U S A 97, 6043–6048. doi: 10.1073/pnas.97.11.6043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Narayan, V., Ravindra, K. C., Liao, C., Kaushal, N., Carlson, B. A., and Prabhu, K. S. (2015). Epigenetic regulation of inflammatory gene expression in macrophages by selenium. J. Nutr. Biochem. 26, 138–145. doi: 10.1016/j.jnutbio.2014.09.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Natalucci, G., Hagmann, C., Bernet, V., Bucher, H. U., Rousson, V., and Latal, B. (2014). Impact of perinatal factors on continuous early monitoring of brain electrocortical activity in very preterm newborns by amplitude-integrated EEG. Pediatr. Res. 75, 774–780. doi: 10.1038/pr.2014.32
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neggers, Y. H., Goldenberg, R. L., Ramey, S. L., and Cliver, S. P. (2003). Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstet. Gynecol. Scand. 82, 235–240. doi: 10.1034/j.1600-0412.2003.00090.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Negi, R., Pande, D., Kumar, A., Khanna, R. S., and Khanna, H. D. (2012). Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth weight neonates. J. Matern. Fetal Neonatal Med. 25, 1338–1341. doi: 10.3109/14767058.2011.633672
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nelson, K. B., Dambrosia, J. M., Grether, J. K., and Phillips, T. M. (1998). Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann. Neurol. 44, 665–675. doi: 10.1002/ana.410440413
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nestler, E. J. (2012). Transcriptional mechanisms of drug addiction. Clin. Psychopharmacol. Neurosci. 10, 136–143. doi: 10.9758/cpn.2012.10.3.136
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Newton, K., and Dixit, V. M. (2012). Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4:a006049. doi: 10.1101/cshperspect.a006049
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nicholson, J. K. (2006). Global systems biology, personalized medicine and molecular epidemiology. Mol. Syst. Biol. 2:52. doi: 10.1038/msb4100095
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nogueira, L., Ruiz-Ontañon, P., Vazquez-Barquero, A., Moris, F., and Fernandez-Luna, J. L. (2011). The NFκB pathway: a therapeutic target in glioblastoma. Oncotarget 2, 646–653.
Nold, M. F., Mangan, N. E., Rudloff, I., Cho, S. X., Shariatian, N., Samarasinghe, T. D., et al. (2013). Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc. Natl. Acad. Sci. U S A 110, 14384–14389. doi: 10.1073/pnas.1306859110
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
O’Dea, E., and Hoffmann, A. (2009). NF-κB signaling. Wiley Interdiscip. Rev. Syst. Biol. Med. 1, 107–115. doi: 10.1002/wsbm.30
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ojha, S., Fainberg, H. P., Sebert, S., Budge, H., and Symonds, M. E. (2015). Maternal health and eating habits: metabolic consequences and impact on child health. Trends Mol. Med. 21, 126–133. doi: 10.1016/j.molmed.2014.12.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Orozco, L. D., Bennett, B. J., Farber, C. R., Ghazalpour, A., Pan, C., Che, N., et al. (2012). Unraveling inflammatory responses using systems genetics and gene-environment interactions in macrophages. Cell 151, 658–670. doi: 10.1016/j.cell.2012.08.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
O’Shea, T. M., Allred, E. N., Kuban, K., Dammann, O., Paneth, N., Fichorova, R., et al. (2012). Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at two years in extremely premature infants. J. Pediatr. 160, 395–401.e4. doi: 10.1016/j.jpeds.2011.08.069
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Osipo, C., Golde, T. E., Osborne, B. A., and Miele, L. A. (2008). Off the beaten pathway: the complex cross talk between Notch and NF-κB. Lab. Invest. 88, 11–17. doi: 10.1038/labinvest.3700700
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oya, S., Yoshikawa, G., Takai, K., Tanaka, J. I., Higashiyama, S., Saito, N., et al. (2009). Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience 158, 683–692. doi: 10.1016/j.neuroscience.2008.10.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Palaga, T., Buranaruk, C., Rengpipat, S., Fauq, A. H., Golde, T. E., Kaufmann, S. H., et al. (2008). Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur. J. Immunol. 38, 174–183. doi: 10.1002/eji.200636999
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peng, W., Zhu, H., Shi, H., and Liu, E. (2014). Volume-targeted ventilation is more suitable than pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 99, F158–F165. doi: 10.1136/archdischild-2013-304613
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Perrone, S., Tataranno, M. L., Negro, S., Cornacchione, S., Longini, M., Proietti, F., et al. (2012). May oxidative stress biomarkers in cord blood predict the occurrence of necrotizing enterocolitis in preterm infants? J. Matern. Fetal Neonatal Med. 25(Suppl. 1), 128–131. doi: 10.3109/14767058.2012.663197
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pham, H., Vottier, G., Pansiot, J., Duong-Quy, S., Bollen, B., Dalous, J., et al. (2014). Inhaled NO prevents hyperoxia-induced white matter damage in neonatal rats. Exp. Neurol. 252, 114–123. doi: 10.1016/j.expneurol.2013.11.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Poellinger, L., and Lendahl, U. (2008). Modulating Notch signaling by pathway-intrinsic and pathway-extrinsic mechanisms. Curr. Opin. Genet. Dev. 18, 449–454. doi: 10.1016/j.gde.2008.07.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Radtke, F., MacDonald, H. R., and Tacchini-Cottier, F. (2013). Regulation of innate and adaptive immunity by Notch. Nat. Rev. Immunol. 13, 427–437. doi: 10.1038/nri3445
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rankin, J. A. (2004). Biological mediators of acute inflammation. AACN Clin. Issues 15, 3–17. doi: 10.1097/00044067-200401000-00002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rebuck, N., Gibson, A., and Finn, A. (1995). Neutrophil adhesion molecules in term and premature infants: normal or enhanced leucocyte integrins but defective L-selectin expression and shedding. Clin. Exp. Immunol. 101, 183–189. doi: 10.1111/j.1365-2249.1995.tb02296.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ridder, D. A., and Schwaninger, M. (2009). NF-κB signaling in cerebral ischemia. Neuroscience 158, 995–1006. doi: 10.1016/j.neuroscience.2008.07.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Romero, R., Gotsch, F., Pineles, B., and Kusanovic, J. P. (2007). Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications and fetal injury. Nutr. Rev. 65, S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rozycki, H. J., Comber, P. G., and Huff, T. F. (2002). Cytokines and oxygen radicals after hyperoxia in preterm and term alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L1222–L1228. doi: 10.1152/ajplung.00230.2001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ruiz, S., Pergola, P. E., Zager, R. A., and Vaziri, N. D. (2013). Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 83, 1029–1041. doi: 10.1038/ki.2012.439
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ruland, J. (2011). Return to homeostasis: downregulation of NF-κB responses. Nat. Immunol. 12, 709–714. doi: 10.1038/ni.2055
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Saban, K. L., Mathews, H. L., Devon, H. A., and Janusek, L. W. (2014). Epigenetics and social context: implications for disparity in cardiovascular disease. Aging Dis. 5, 346–355. doi: 10.14336/AD.2014.0500346
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sako, W., Ito, H., Yoshida, M., Koizumi, H., Kamada, M., Fujita, K., et al. (2012). Nuclear factor κ B expression in patients with sporadic amyotrophic lateral sclerosis and hereditary amyotrophic lateral sclerosis with optineurin mutations. Clin. Neuropathol. 31, 418–423. doi: 10.5414/NP300493
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Saliba, E., Favrais, G., and Gressens, P. (2007). Neuroprotection of the newborn: from bench to cribside. Semin. Fetal Neonatal Med. 12, 239–240. doi: 10.1016/j.siny.2007.01.024
Sánchez-Aguilar, M., Tapia-Perez, J. H., Sanchez-Rodriguez, J. J., Vinas-Rios, J. M., Martinez-Perez, P., De La Cruz-Mendoza, E., et al. (2013). Effect of rosuvastatin on cytokines after traumatic head injury. J. Neurosurg. 118, 669–675. doi: 10.3171/2012.12.jns121084
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sandberg, M., Patil, J., D’angelo, B., Weber, S. G., and Mallard, C. (2013). NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79, 298–306. doi: 10.1016/j.neuropharm.2013.11.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sanders, E. J., and Harvey, S. (2008). Peptide hormones as developmental growth and differentiation factors. Dev. Dyn. 237, 1537–1552. doi: 10.1002/dvdy.21573
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sannia, A., Natalizia, A. R., Parodi, A., Malova, M., Fumagalli, M., Rossi, A., et al. (2013). Different gestational ages and changing vulnerability of the premature brain. J. Matern. Fetal Neonatal Med. doi: 10.3109/14767058.2013.796166. [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sarafidis, K., Stathopoulou, T., Agakidou, E., Taparkou, A., Soubasi, V., Diamanti, E., et al. (2011). Comparable effect of conventional ventilation versus early high-frequency oscillation on serum CC16 and IL-6 levels in preterm neonates. J. Perinatol. 31, 104–111. doi: 10.1038/jp.2010.78
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sarnico, I., Branca, C., Lanzillotta, A., Porrini, V., Benarese, M., Spano, P. F., et al. (2012). NF-kappaB and epigenetic mechanisms as integrative regulators of brain resilience to anoxic stress. Brain Res. 1476, 203–210. doi: 10.1016/j.brainres.2012.04.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schadt, E. E., Buchanan, S., Brennand, K. J., and Merchant, K. M. (2014). Evolving toward a human-cell based and multiscale approach to drug discovery for CNS disorders. Front. Pharmacol. 5:252. doi: 10.3389/fphar.2014.00252
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schmitz, T., Krabbe, G., Weikert, G., Scheuer, T., Matheus, F., Wang, Y., et al. (2014). Minocycline protects the immature white matter against hyperoxia. Exp. Neurol. 254, 153–165. doi: 10.1016/j.expneurol.2014.01.017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scholzke, M. N., and Schwaninger, M. (2007). Transcriptional regulation of neurogenesis: potential mechanisms in cerebral ischemia. J. Mol. Med. (Berl) 85, 577–588. doi: 10.1007/s00109-007-0196-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schultz, C., Rott, C., Temming, P., Schlenke, P., Moller, J. C., and Bucsky, P. (2002). Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr. Res. 51, 317–322. doi: 10.1203/00006450-200203000-00009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schwanbeck, R. (2015). The role of epigenetic mechanisms in notch signaling during development. J. Cell. Physiol. 230, 969–981. doi: 10.1002/jcp.24851
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Serpero, L. D., Bellissima, V., Colivicchi, M., Sabatini, M., Frigiola, A., Ricotti, A., et al. (2013). Next generation biomarkers for brain injury. J. Matern. Fetal Neonatal Med. 26(Suppl. 2), 44–49. doi: 10.3109/14767058.2013.829688
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Siggers, R. H., and Hackam, D. J. (2011). The role of innate immune-stimulated epithelial apoptosis during gastrointestinal inflammatory diseases. Cell. Mol. Life Sci. 68, 3623–3634. doi: 10.1007/s00018-011-0821-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Snow, W. M., Stoesz, B. M., Kelly, D. M., and Albensi, B. C. (2014). Roles for NF-kappaB and gene targets of NF-kappaB in synaptic plasticity, memory and navigation. Mol. Neurobiol. 49, 757–770. doi: 10.1007/s12035-013-8555-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sohn, J. A., Kim, H. S., Lee, E. H., Lee, J., Lee, J. A., Choi, C. W., et al. (2013). Developmental change of amplitude-integrated electroencephalographic activity in preterm infants with intraventricular hemorrhage. Early Hum. Dev. 89, 961–966. doi: 10.1016/j.earlhumdev.2013.09.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sookoian, S., Gianotti, T. F., Burgueno, A. L., and Pirola, C. J. (2013). Fetal metabolic programming and epigenetic modifications: a systems biology approach. Pediatr. Res. 73, 531–542. doi: 10.1038/pr.2013.2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Strueby, L., and Thébaud, B. (2014). Advances in bronchopulmonary dysplasia. Expert Rev. Respir. Med. 8, 327–338. doi: 10.1586/17476348.2014.899907
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Su, Z. Y., Khor, T. O., Shu, L., Lee, J. H., Saw, C. L., Wu, T. Y., et al. (2013). Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chem. Res. Toxicol. 26, 477–485. doi: 10.1021/tx300524p
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, F., Wan, M., Xu, X., Gao, B., Zhou, Y., Sun, J., et al. (2014). Crosstalk between miR-34a and Notch signaling promotes differentiation in Apical Papilla stem cells (SCAPs). J. Dent. Res. 93, 589–595. doi: 10.1177/0022034514531146
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Surén, P., Roth, C., Bresnahan, M., Haugen, M., Hornig, M., Hirtz, D., et al. (2013). Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309, 570–577. doi: 10.1097/01.ogx.0000431313.84585.bd
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tanda, R., Salsberry, P. J., Reagan, P. B., and Fang, M. Z. (2013). The impact of prepregnancy obesity on children’s cognitive test scores. Matern. Child Health J. 17, 222–229. doi: 10.1007/s10995-012-0964-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tatad, A. M., Nesin, M., Peoples, J., Cheung, S., Lin, H., Sison, C., et al. (2008). Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology 94, 8–15. doi: 10.1159/000112541
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thakur, G. S., Daigle, B. J. Jr., Dean, K. R., Zhang, Y., Rodriguez-Fernandez, M., Hammamieh, R., et al. (2015). Systems biology approach to understanding post-traumatic stress disorder. Mol. Biosyst. 11, 980–993. doi: 10.1039/c4mb00404c
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tsugane, S., Takizawa, S., Kaneyama, T., Ichikawa, M., Yagita, H., Kim, B. S., et al. (2012). Therapeutic effects of anti-Delta1 mAb on Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J. Neuroimmunol. 252, 66–74. doi: 10.1016/j.jneuroim.2012.08.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tu, X. K., Yang, W. Z., Chen, J. P., Chen, Y., Ouyang, L. Q., Xu, Y. C., et al. (2014). Curcumin inhibits TLR2/4-NF-kappaB signaling pathway and attenuates brain damage in permanent focal cerebral ischemia in rats. Inflammation 37, 1544–1551. doi: 10.1007/s10753-014-9881-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tung, J., and Gilad, Y. (2013). Social environmental effects on gene regulation. Cell. Mol. Life Sci. 70, 4323–4339. doi: 10.1007/s00018-013-1357-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Turunen, R., Andersson, S., Laivuori, H., Kajantie, E., Siitonen, S., Repo, H., et al. (2011). Increased postnatal inflammation in mechanically ventilated preterm infants born to mothers with early-onset preeclampsia. Neonatology 100, 241–247. doi: 10.1159/000325159
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Turunen, R., Nupponen, I., Siitonen, S., Repo, H., and Andersson, S. (2006). Onset of mechanical ventilation is associated with rapid activation of circulating phagocytes in preterm infants. Pediatrics 117, 448–454. doi: 10.1542/peds.2005-0123
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Uekawa, K., Hasegawa, Y., Ma, M., Nakagawa, T., Katayama, T., Sueta, D., et al. (2014). Rosuvastatin ameliorates early brain injury after subarachnoid hemorrhage via suppression of superoxide formation and nuclear factor-kappa B activation in rats. J. Stroke Cerebrovasc. Dis. 23, 1429–1439. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van der Burg, J. W., Allred, E. N., Mcelrath, T. F., Fichorova, R. N., Kuban, K., O’shea, T. M., et al. (2013). Is maternal obesity associated with sustained inflammation in extremely low gestational age newborns? Early Hum. Dev. 89, 949–955. doi: 10.1016/j.earlhumdev.2013.09.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Lieshout, R. J. (2013). Role of maternal adiposity prior to and during pregnancy in cognitive and psychiatric problems in offspring. Nutr. Rev. 71(Suppl. 1), S95–S101. doi: 10.1111/nure.12059
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Lieshout, R. J., Taylor, V. H., and Boyle, M. H. (2011). Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes. Rev. 12, e548–e559. doi: 10.1111/j.1467-789x.2010.00850.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Muiswinkel, F. L., and Kuiperij, H. B. (2005). The Nrf2-ARE signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr. Drug Targets CNS Neurol Disord. 4, 267–281. doi: 10.2174/1568007054038238
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Steenwinckel, J., Schang, A. L., Sigaut, S., Chhor, V., Degos, V., Hagberg, H., et al. (2014). Brain damage of the preterm infant: new insights into the role of inflammation. Biochem. Soc. Trans. 42, 557–563. doi: 10.1042/bst20130284
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vendettuoli, V., Bellù, R., Zanini, R., Mosca, F., Gagliardi, L., Italian Neonatal Network, et al. (2014). Changes in ventilator strategies and outcomes in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 99, F321–F324. doi: 10.1136/archdischild-2013-305165
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Verney, C., Pogledic, I., Biran, V., Adle-Biassette, H., Fallet-Bianco, C., and Gressens, P. (2012). Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J. Neuropathol. Exp. Neurol. 71, 251–264. doi: 10.1097/nen.0b013e3182496429
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Voit, E. O. (2009). A systems-theoretical framework for health and disease: inflammation and preconditioning from an abstract modeling point of view. Math. Biosci. 217, 11–18. doi: 10.1016/j.mbs.2008.09.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Volpe, J. J. (2011). Systemic inflammation, oligodendroglial maturation and the encephalopathy of prematurity. Ann. Neurol. 70, 525–529. doi: 10.1002/ana.22533
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wakabayashi, N., Skoko, J. J., Chartoumpekis, D. V., Kimura, S., Slocum, S. L., Noda, K., et al. (2014). Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell. Biol. 34, 653–663. doi: 10.1128/mcb.01408-13
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, J. (2010). Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 92, 463–477. doi: 10.1016/j.pneurobio.2010.08.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, S., Sdrulla, A. D., disibio, G., Bush, G., Nofziger, D., Hicks, C., et al. (1998). Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75. doi: 10.1016/s0896-6273(00)80515-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, L. W., Wang, S. T., and Huang, C. C. (2008). Preterm infants of educated mothers have better outcome. Acta Paediatr. 97, 568–573. doi: 10.1111/j.1651-2227.2008.00738.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Weichelt, U., Cay, R., Schmitz, T., Strauss, E., Sifringer, M., Bührer, C., et al. (2013). Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 41, 966–973. doi: 10.1183/09031936.00012412
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Welch, C., Helderman, J., Williamson, E., and O’shea, T. M. (2013). Brain wave maturation and neurodevelopmental outcome in extremely low gestational age neonates. J. Perinatol. 33, 867–871. doi: 10.1038/jp.2013.79
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Werner, S. L., Barken, D., and Hoffmann, A. (2005). Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science 309, 1857–1861. doi: 10.1126/science.1113319
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wild, K. T., Betancourt, L. M., Brodsky, N. L., and Hurt, H. (2013). The effect of socioeconomic status on the language outcome of preterm infants at toddler age. Early Hum. Dev. 89, 743–746. doi: 10.1016/j.earlhumdev.2013.05.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wolkenhauer, O. (2013). The role of theory and modeling in medical research. Front. Physiol. 4:377. doi: 10.3389/fphys.2013.00377
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wolkenhauer, O., Auffray, C., Jaster, R., Steinhoff, G., and Dammann, O. (2013). The road from systems biology to systems medicine. Pediatr. Res. 73, 502–507. doi: 10.1038/pr.2013.4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wongchana, W., and Palaga, T. (2012). Direct regulation of interleukin-6 expression by Notch signaling in macrophages. Cell. Mol. Immunol. 9, 155–162. doi: 10.1038/cmi.2011.36
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xiao, L., Liu, Y., and Wang, N. (2014). New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 306, H317–H325. doi: 10.1152/ajpheart.00182.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xiao, W., Mindrinos, M. N., Seok, J., Cuschieri, J., Cuenca, A. G., Gao, H., et al. (2011). A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590. doi: 10.1084/jem.20111354
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang, Y. C., Lii, C. K., Wei, Y. L., Li, C. C., Lu, C. Y., Liu, K. L., et al. (2013b). Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. J. Nutr. Biochem. 24, 204–212. doi: 10.1016/j.jnutbio.2012.05.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang, J. J., Tao, H., Huang, C., and Li, J. (2013a). Nuclear erythroid 2-related factor 2: a novel potential therapeutic target for liver fibrosis. Food Chem. Toxicol. 59, 421–427. doi: 10.1016/j.fct.2013.06.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yao, L., Cao, Q., Wu, C., Kaur, C., Hao, A., and Ling, E. A. (2013a). Notch signaling in the central nervous system with special reference to its expression in microglia. CNS Neurol. Disord. Drug Targets 12, 807–814. doi: 10.2174/18715273113126660172
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yao, L., Kan, E. M., Kaur, C., Dheen, S. T., Hao, A., Lu, J., et al. (2013b). Notch-1 signaling regulates microglia activation via NF-kappaB pathway after hypoxic exposure in vivo and in vitro. PLoS One 8:e78439. doi: 10.1371/journal.pone.0078439
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoon, B. H., Romero, R., Moon, J., Chaiworapongsa, T., Espinoza, J., Kim, Y. M., et al. (2003). Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J. Matern. Fetal Neonatal Med. 13, 32–38. doi: 10.1080/jmf.13.1.32.38
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yu, Y., Wang, L., Tang, W., Zhang, D., and Shang, T. (2014). RNA interference-mediated knockdown of Notch-1 inhibits migration and invasion, down-regulates matrix metalloproteinases and suppresses NF-kappaB signaling pathway in trophoblast cells. Acta Histochem. 116, 911–919. doi: 10.1016/j.acthis.2014.03.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yuan, T. M., and Yu, H. M. (2010). Notch signaling: key role in intrauterine infection/inflammation, embryonic development and white matter damage? J. Neurosci. Res. 88, 461–468. doi: 10.1002/jnr.22229
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zak, D. E., and Aderem, A. (2009). Systems biology of innate immunity. Immunol. Rev. 227, 264–282. doi: 10.1111/j.1600-065X.2008.00721.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, J., Sadowska, G. B., Chen, X., Park, S. Y., Kim, J. E., Bodge, C. A., et al. (2015). Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J. doi: 10.1096/fj.14-258822. [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Q., Wang, C., Liu, Z., Liu, X., Han, C., Cao, X., et al. (2012). Notch signal suppresses toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor κB activation. J. Biol. Chem. 287, 6208–6217. doi: 10.1074/jbc.m111.310375
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, X., Gonzales, N., and Aronowski, J. (2015). Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR and NF-κB. CNS Neurosci. Ther. 21, 357–366. doi: 10.1111/cns.12350
Zhou, W., and Hu, W. (2013). Anti-neuroinflammatory agents for the treatment of Alzheimer’s disease. Future Med. Chem. 5, 1559–1571. doi: 10.4155/fmc.13.125
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhou, J., and Zhou, S. (2014). Inflammation: therapeutic targets for diabetic neuropathy. Mol. Neurobiol. 49, 536–546. doi: 10.1007/s12035-013-8537-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: nuclear factor-kappa B (NF-κB), Notch-1, nuclear factor erythroid 2 related factor 2 (NRF2), inflammation, prematurity, brain, epigenetic, systems biology
Citation: Leviton A, Gressens P, Wolkenhauer O and Dammann O (2015) Systems approach to the study of brain damage in the very preterm newborn. Front. Syst. Neurosci. 9:58. doi: 10.3389/fnsys.2015.00058
Received: 12 February 2015; Accepted: 26 March 2015;
Published online: 14 April 2015.
Edited by:
Mikhail Lebedev, Duke University, USAReviewed by:
Jon F. Watchko, University of Pittsburgh School of Medicine, USATalkad S. Raghuveer, University of Kansas School of Medicine at Wichita, USA
Copyright © 2015 Leviton, Gressens, Wolkenhauer and Dammann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan Leviton, Neuroepidemiology Unit, Boston Children’s Hospital, Au-414, 300 Longwood Avenue, Boston, MA 02115-5724, USA alan.leviton@childrens.harvard.edu
 Alan Leviton
Alan Leviton Pierre Gressens
Pierre Gressens Olaf Wolkenhauer
Olaf Wolkenhauer Olaf Dammann
Olaf Dammann