Dm5-HT2B: Pharmacological Characterization of the Fifth Serotonin Receptor Subtype of Drosophila melanogaster
- 1Cologne Biocenter and Zoological Institute, University of Cologne, Cologne, Germany
- 2Department of NMR-Supported Structural Biology, Leibniz-Institut für Molekulare Pharmakologie, Berlin, Germany
- 3Institute of Complex Systems – Cellular Biophysics (ICS-4), Forschungszentrum Jülich, Jülich, Germany
- 4Behavioral Physiology and Sociobiology (Zoology II), Biocenter, University of Würzburg, Würzburg, Germany
Serotonin (5-hydroxytryptamine, 5-HT) is an important regulator of physiological and behavioral processes in both protostomes (e.g., insects) and deuterostomes (e.g., mammals). In insects, serotonin has been found to modulate the heart rate and to control secretory processes, development, circadian rhythms, aggressive behavior, as well as to contribute to learning and memory. Serotonin exerts its activity by binding to and activating specific membrane receptors. The clear majority of these receptors belong to the superfamily of G-protein-coupled receptors. In Drosophila melanogaster, a total of five genes have been identified coding for 5-HT receptors. From this family of proteins, four have been pharmacologically examined in greater detail, so far. While Dm5-HT1A, Dm5-HT1B, and Dm5-HT7 couple to cAMP signaling cascades, the Dm5-HT2A receptor leads to Ca2+ signaling in an inositol-1,4,5-trisphosphate-dependent manner. Based on sequence similarity to homologous genes in other insects, a fifth D. melanogaster gene was uncovered coding for a Dm5-HT2B receptor. Knowledge about this receptor’s pharmacological properties is very limited. This is quite surprising because Dm5-HT2B has been attributed to distinct physiological functions based on genetic interference with its gene expression. Mutations were described reducing the response of the larval heart to 5-HT, and specific knockdown of Dm5-HT2B mRNA in hemocytes resulted in a higher susceptibility of the flies to bacterial infection. To gain deeper understanding of Dm5-HT2B’s pharmacology, we evaluated the receptor’s response to a series of established 5-HT receptor agonists and antagonists in a functional cell-based assay. Metoclopramide and mianserin were identified as two potent antagonists that may allow pharmacological interference with Dm5-HT2B signaling in vitro and in vivo.
Introduction
The biogenic amine serotonin (5-hydroxytryptamine, 5-HT) is an ancient neuroactive substance and present throughout the animal kingdom. Serotonin plays a key role in regulating and modulating many physiological and behavioral processes in both protostomes and deuterostomes. In humans, malfunction of the serotonergic system has been associated with several impairments and diseases, such as schizophrenia, migraine, depression, suicidal behavior, infantile autism, eating disorders, and obsessive-compulsive disorder (for reviews, Green, 2006; Geyer and Vollenweider, 2008; Berger et al., 2009).
To gain insight into serotonergic function(s), insects are highly attractive models. In comparison with vertebrates and especially mammals, they allow assessing the anatomical distribution, development, and neurophysiological properties of serotonergic neurons with unprecedented inter-individual reproducibility and precision. Applying this experimental strategy, the activity of serotonergic neurons has been related to physiological functions and changes in behavior (for reviews, Walz et al., 2006; Blenau and Thamm, 2011; Ellen and Mercer, 2012; Nall and Sehgal, 2014; Vleugels et al., 2015). In fruit flies (Drosophila melanogaster), certain behavioral effects have been ascribed to the serotonergic system. In D. melanogaster larvae, serotonin modulates the heart rate (Dasari and Cooper, 2006) and is involved in olfactory processing (Python and Stocker, 2002), feeding behavior (Neckameyer et al., 2007; Neckameyer, 2010), locomotion (Majeed et al., 2016), and responses to light (Rodriguez Moncalvo and Campos, 2009). In adult flies, serotonergic neurons participate in the regulation of nutrient balance (Vargas et al., 2010; Ro et al., 2016), insulin signaling and organismal growth (Kaplan et al., 2008; Luo et al., 2012, 2014), locomotion (Neckameyer et al., 2007; Majeed et al., 2016), olfactory processing (Dacks et al., 2009), aggression (Dierick and Greenspan, 2007; Alekseyenko et al., 2010, 2014; Alekseyenko and Kravitz, 2014), circadian rhythm (Yuan et al., 2005), sleep (Yuan et al., 2006), courtship and mating behavior (Becnel et al., 2011), and learning (Sitaraman et al., 2008, 2012; Lee et al., 2011).
The diverse cellular and behavioral effects of serotonin in insects are mediated by a family of G protein-coupled receptors (GPCRs). So far, four 5-HT receptor subtypes have been pharmacologically characterized in D. melanogaster. These are Dm5-HT1A and Dm5-HT1B (CG16720 and CG15113; Saudou et al., 1992), Dm5-HT2A (CG1056; Colas et al., 1995), and Dm5-HT7 (CG12073; Witz et al., 1990). These GPCRs are assumed to be orthologs of mammalian 5-HT1A, 5-HT2, and 5-HT7 receptors. Due to sequence homology with a 5-HT2 receptor in the spiny lobster, Panulirus interruptus, a second 5-HT2 receptor gene (CG42796) has been postulated in D. melanogaster (Clark et al., 2004). This observation was corroborated by independent bioinformatics studies (Hauser et al., 2006; Blenau and Thamm, 2011) and was substantiated experimentally by molecular cloning (Gasque et al., 2013). The receptor was named Dm5-HT2B. Although orthologous receptors have been characterized in other insects as well, e.g., the honeybee Apis mellifera (Thamm et al., 2013) and the kissing bug Rhodnius prolixus (Paluzzi et al., 2015), knowledge about the pharmacological properties of Dm5-HT2B is rather limited. This is quite surprising because Dm5-HT2B has been attributed to distinct physiological functions. For example, Dm5-HT2B receptor mutations reduce the response of the larval heart to 5-HT (Majeed et al., 2014). Furthermore, knockdown of Dm5-HT2B gene expression by RNAi in hemocytes caused reduced phagocytotic clearance and thus resulted in a higher susceptibility of the flies to bacterial infection (Qi et al., 2016). At the behavioral level, it has been uncovered that reducing the level of Dm5-HT2B expression by either RNAi or transposon insertion into the gene locus leads to a decrease in anxiety-like behavior (Mohammad et al., 2016).
The aim of the current study was to focus on the pharmacological properties of the Dm5-HT2B receptor. The cDNA encoding Dm5-HT2B was amplified on mRNA extracted from D. melanogaster heads. A cell line was established constitutively expressing Dm5-HT2B. Since 5-HT2B receptors are known to cause inositol-1,4,5-trisphosphate (IP3)-mediated Ca2+ release from intracellular stores, we examined Dm5-HT2B functionality by Ca2+ fluorimetry. The receptor’s pharmacological profile was established after applying concentration series of various agonists and antagonists. In addition to serotonin as the native ligand, 5-methoxytryptamine and 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) were very potent agonists. Receptor activity was efficiently blocked by metoclopramide and mianserin. Thus, this study provides important new data regarding the pharmacological characteristics of the fifth 5-HT receptor of the fruit fly.
Materials and Methods
Cloning of the Dm5-ht2b cDNA
Poly(A)+ RNA was prepared from 180 heads of male flies (D. melanogaster, w1118) by using the Micro-Fast TrackTM 2.0 Kit (Invitrogen, Karlsruhe, Germany). Synthesis of cDNA employed the AccuScriptTM High Fidelity First Strand cDNA Synthesis Kit (Stratagene, Amsterdam, Netherlands). For amplification of the entire coding region of Dm5-ht2b, specific primers were designed based on available sequence information (Brody and Cravchik, 2000; Clark et al., 2004; Hauser et al., 2006; Gasque et al., 2013): sense primer 5′-CAGAGTAGAGCGCACAATAGG-3′ (position -35 to -15); antisense primer 5′-GTTTGCCCGGTTTAACG-3′ (position 2724 to 2740; TIB Molbiol, Berlin, Germany). The polymerase chain reaction (PCR) was carried out for 30 s at 98°C (1 cycle) followed by 35 cycles of 10 s at 98°C, 30 s at 62°C, 90 s at 72°C, and a final extension of 10 min at 72°C. The reaction was performed with Phusion® High Fidelity DNA Polymerase (New England Biolabs, Frankfurt am Main, Germany). PCR products were cloned into pGEM-T vector (Promega, Mannheim, Germany) and subsequently sequenced (GATC Biotech AG, Konstanz, Germany).
Multiple Sequence Alignments and Phylogenetic Analysis
Amino-acid sequences used for phylogenetic analysis were identified by protein-protein Basic Local Alignment Search Tool (BLAST) searches of the National Center for Biotechnology Information (NCBI) database with the deduced amino acid sequence of Dm5-ht2b (Dm5-HT2B) as “bait.” Values for identity (ID) and similarity (S) were calculated by using the BLOSUM62 substitution matrix in BioEdit 7.1.9. Phylogenetic analysis was conducted as described by Reim et al. (2017) using Bayesian analysis (MrBayes v.3.2.6; Ronquist et al., 2012) with the substitution model LG +G, determined by Protest 3.4.2 (Darriba et al., 2011). Human rhodopsin (HsRHOD) and D. melanogaster FMRFamide receptor (DmFMRFaR) sequences were used to root the phylogenetic tree.
Construction of Expression Vectors
An expression-ready construct of Dm5-ht2b was generated in pcDNA3.1 vector (Invitrogen/ThermoFisher Scientific, Darmstadt, Germany). PCR was performed with specific primers (sense primer: 5′-AATAAGCTTCCACCATGGAAGAGGATGTGTATGCC-3′; antisense primer first-round PCR: 5′-TGGGACGTCGTATGGGTATCTGCTCGGTCGCCAGG-3′; antisense primer second-round PCR: 5′-TTTTCTAGACTCGAGTTAAGCGTAGTCTGGGACGTCGTATGGGTA-3′). PCR products were digested with HindIII and XhoI, and subcloned into pcDNA3.1(+) vector (Invitrogen). Thus, the resulting construct contained the Kozak consensus motif (CCACC, Kozak, 1984) immediately 5′ to the ATG-codon and a hemagglutinin A (HA) epitope tag (amino acid sequence: YPYDVPDYA) at the 3′ end of the Dm5-ht2b cDNA and was named pcDm5-ht2b-HA. The insert fragment was checked by DNA sequencing.
Functional Expression in Mammalian Cell Lines
Approximately 8 μg of pcDm5-ht2b-HA was transfected into exponentially growing HEK 293 cells (∼4 × 105 cells per 5-cm Petri dish) by a modified calcium phosphate method (Chen and Okayama, 1987). Stably transfected cells were selected in the presence of the antibiotic G418 (0.8 mg/ml). Isolated foci were propagated and analyzed for the expression of Dm5-HT2B-HA receptor either by immunocytochemistry, Western blotting or by functional Ca2+ imaging upon receptor activation.
Functional and Pharmacological Characterization of Dm5-HT2B
The ability of the Dm5-HT2B-HA receptor (hereafter referred to as Dm5-HT2B) to activate Gq proteins was assessed by monitoring changes in [Ca2+]i with the Ca2+-sensitive fluorescent dye Fluo-4 (Invitrogen). Non-transfected HEK 293 cells and cells expressing Dm5-HT2B were grown in minimal essential medium (MEM + GlutaMAXTMI (Gibco/ThermoFisher Scientific, Darmstadt, Germany) containing 2% (w/v) UtroserTM G (Pall, Dreieich, Germany), 1 × non-essential amino acids and 1 × antibiotics/antimycotics) in 96-well plates to a density of ∼3 × 104 cells per well. In this format, each vertical row (=8 wells) of the 96-well plate is incubated with the same ligand concentration. Cells were loaded at room temperature with Fluo-4 as described earlier (Thamm et al., 2013; Blankenburg et al., 2015) in extracellular solution [ES = in mM: 120 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 10 Glucose, pH 7.4 (NaOH)]. Plates were transferred into a fluorescence reader (FLUOstar Galaxy/Optima; BMG Labtech, Offenburg, Germany) to monitor Fluo-4 fluorescence. The excitation wavelength was 485 nm, and fluorescence emission was detected at 520 nm. Various concentrations of biogenic amines and synthetic receptor ligands were added, once Fluo-4 fluorescence had reached a stable value in each well. The changes in Fluo-4 fluorescence were recorded automatically. Concentration-response curves for putative agonists/antagonists were established in at least two independent experiments with octuplicate determinations (s.a.) per data point. Data were analyzed and displayed by using PRISM 5.0.4 software (GraphPad, San Diego, CA, USA).
Results
Cloning of Dm5-ht2b cDNA and Structural Properties of Dm5-HT2B
The sequence of a second potential 5-HT2 receptor from D. melanogaster had been annotated in previous studies (Brody and Cravchik, 2000; Clark et al., 2004; Hauser et al., 2006). Later, Dm5-ht2b (CG42796) was experimentally proven to encode a functional 5-HT receptor (Gasque et al., 2013). Here, we used the available sequence information and applied a PCR-based strategy to amplify the full-length Dm5-ht2b cDNA for subsequent detailed pharmacological characterization of this receptor. The Dm5-ht2b cDNA contains an open reading frame (ORF) of 2,715 bp and encodes a protein of 904 amino-acid residues (Dm5-HT2B) with a calculated molecular mass of 99.5 kDa. The hydrophobicity profile according to Kyte and Doolittle (1982) and prediction of transmembrane helices using TMHMM Server v. 2.0 (Krogh et al., 2001) suggest seven trans-membrane (TM) domains (Figures 1A,B), which is a characteristic feature of GPCRs. The TM segments are flanked by an extracellular N-terminus of 74 residues and an intracellular C-terminus of 26 residues. The Dm5-HT2B receptor contains an extremely long third cytoplasmic loop (CPL3) of 563 residues. We submitted the Dm5-HT2B sequence to Phyre2 (Kelley et al., 2015) and obtained a three dimensional-model of the receptor (Figure 1C).
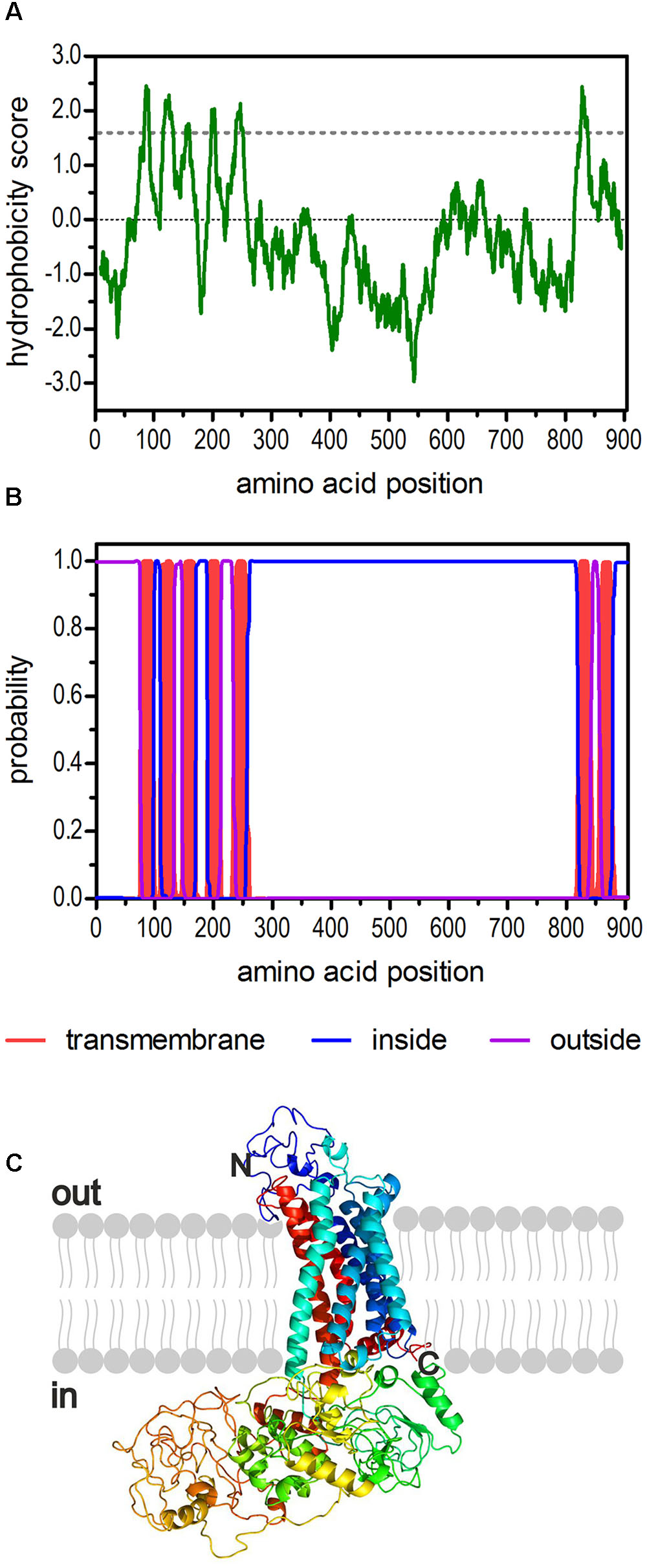
FIGURE 1. Structural characteristics of the deduced amino acid sequence of Dm5-HT2B. (A) Hydrophobicity profile of Dm5-HT2B. The profile was calculated according to the algorithm of Kyte and Doolittle (1982) using a window size of 19 amino acids. Peaks with scores greater than 1.6 (dashed line) indicate possible transmembrane regions. (B) Prediction of transmembrane domains with TMHMM server v. 2.0 (Krogh et al., 2001). Putative transmembrane domains are indicated in red. Extracellular regions are shown as purple line, intracellular regions as blue line. (C) The primary sequence of Dm5-HT2B was submitted to Phyre2 (Kelley et al., 2015). The 3D model of the receptor is color-coded (rainbow). The extracellular N-terminus and the intracellular C-terminus are labeled.
Sequence motifs which are essential for three-dimensional structure, ligand binding, and signal transduction of the receptor are well conserved between the various 5-HT2B receptors (Figure 2) and are also present in Dm5-HT2B. Three consensus motifs for potential N-glycosylation (N-X-S/T) are located in the extracellular N-terminus of Dm5-HT2B (Figure 2). A cysteine residue in the C-terminus (Cys892) is a possible site for post-translational palmitoylation. Twenty phosphorylation sites for protein kinase A (PKA), 38 phosphorylation sites for protein kinase C (PKC) and nine phosphorylation sites for protein kinase G (PKG) are present within intracellular domains of Dm5-HT2B (Figure 2). N-glycosylation sites were predicted by NetNGlyc 1.0 Server1 and putative palmitoylation sites were predicted using GPS-Lipid2. Putative phosphorylation sites were predicted by NetPhos 3.1 Server3 (Blom et al., 2004).
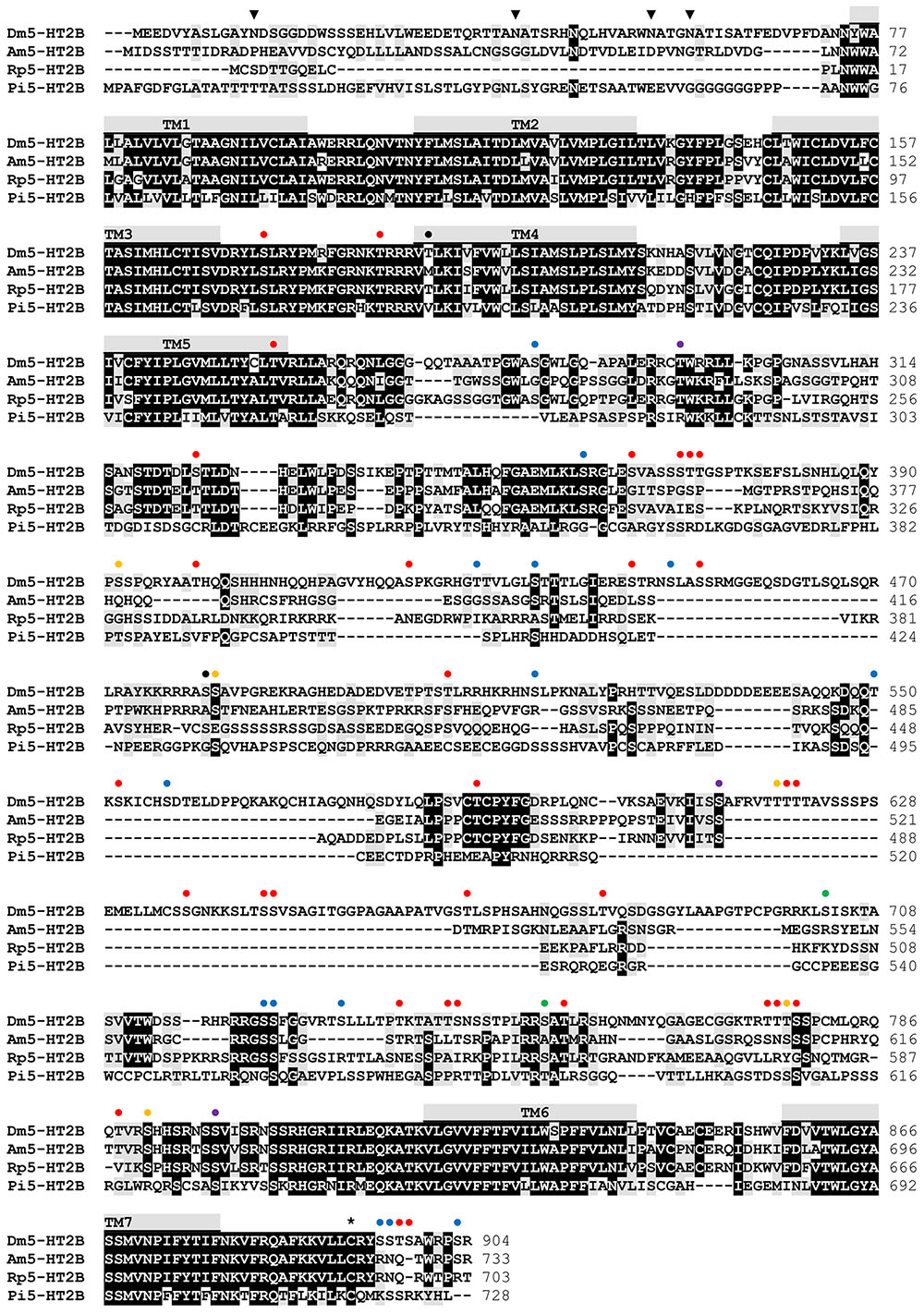
FIGURE 2. Amino acid sequence alignment of Dm5-HT2B and orthologous receptors from Apis mellifera (Am5-HT2B, CBX90121), Rhodnius prolixus (Rp5-HT2B, AKQ13312), and Panulirus interruptus (Pi5-HT2B, AAS57919). Identical residues (≥75%) are shown as white letters against black, whereas conservatively substituted residues are shaded. Putative transmembrane domains (TM1–TM7) are indicated by gray bars. Potential N-glycosylation sites ( ), PKA phosphorylation sites (
), PKA phosphorylation sites ( ), PKC phosphorylation sites (
), PKC phosphorylation sites ( ), phosphorylation sites for both PKA and PKC (
), phosphorylation sites for both PKA and PKC ( ), PKG phosphorylation sites (
), PKG phosphorylation sites ( ), phosphorylation sites for both PKA and PKG (
), phosphorylation sites for both PKA and PKG ( ), phosphorylation sites for all three kinases (
), phosphorylation sites for all three kinases ( ), and putative palmitoylation sites (∗) of Dm5-HT2B are indicated. The amino acid position is given on the right.
), and putative palmitoylation sites (∗) of Dm5-HT2B are indicated. The amino acid position is given on the right.
A comparison of the Dm5-HT2B amino-acid sequence with NCBI databases identified several orthologous protostomian and deuterostomian 5-HT2 receptors. The highest amino acid identity (ID) and similarity (S) was found with the 5-HT2B receptor of A. mellifera (Am5-HT2B; Thamm et al., 2013; ID 48.5%, S 58.0%). Homology was also pronounced to 5-HT2B receptors from the kissing bug R. prolixus (Rp5-HT2B; Paluzzi et al., 2015; ID 44.6%, S 54.1%), and the crustaceans P. interruptus (Pi5-HT2B; Clark et al., 2004; ID 33.3%, S 45.9%), Procambarus clarkii (Pc5-HT2B; Spitzer et al., 2008; ID 34.1%, S 46.0%), and Macrobrachium rosenbergii (Mr5-HT2B; Vázquez-Acevedo et al., 2009; ID 32.8%, S 46.0%). In phylogenetic tree analyses (Figure 3), Dm5-HT2B forms a highly supported cluster with other protostomian 5-HT2B receptors. This protostomian 5-HT2B cluster represents the sister group to deuterostomian 5-HT2 receptors within a monophyletic 5-HT2 receptor group. However, the basal branching of 5-HT-receptor subgroups is not stable and thus has to be subject of future studies.
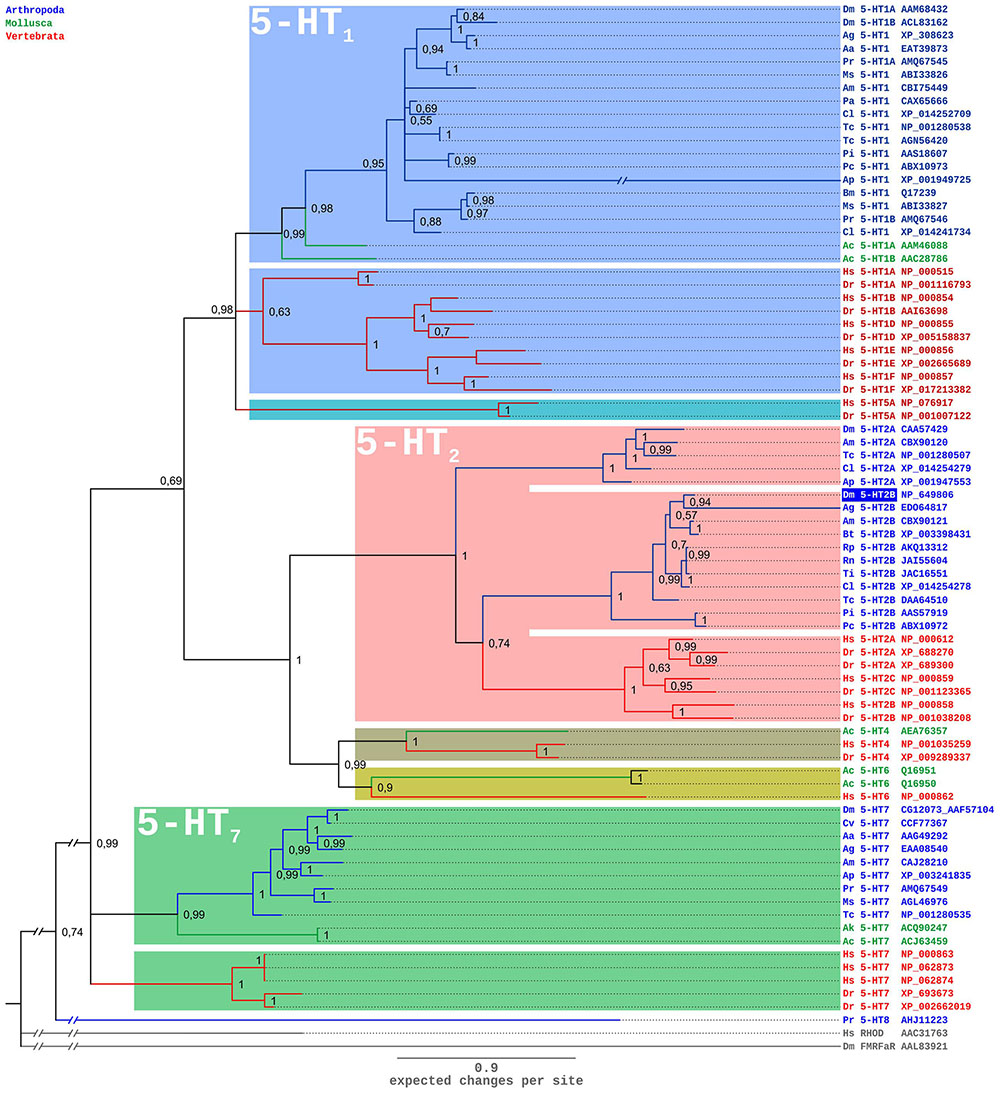
FIGURE 3. Bayesian phylogeny of 5-HT receptors. Alignments were performed using Clustal W (Thompson et al., 1994) by using the core amino-acid sequences lacking the variable regions of the N- and C-terminus and the third cytoplasmic loop. Human rhodopsin (Hs RHOD) and D. melanogaster FMRFamide receptor (Dm FMRFaR) were used to root the tree. Numbers at branches represent the posterior probabilities. Receptor subclasses are highlighted by distinct colors. Abbreviations of species in alphabetical order: Aa Aedes aegypti, Ac Aplysia californica, Ag Anopheles gambiae, Ak Aplysia kurodai, Am Apis mellifera, Ap Acyrthosiphon pisum, Bm Bombyx mori, Bt Bombus terrestris, Cv Cimex lectularius, Cv Calliphora vicina, Dm Drosophila melanogaster, Dr Danio rerio, Hs Homo sapiens, Ms Manduca sexta, Pa Periplaneta americana, Pc Procambarus clarkii, Pi Panulirus interruptus, Pr Pieris rapae, Rn Rhodnius neglectus, Tc Tribolium castaneum, Ti Triatoma infestans.
Functional and Pharmacological Properties of Dm5-HT2B
In a first set of experiments, Dm5-HT2B-expressing cells and non-transfected HEK 293 cells were incubated with the biogenic amines dopamine, histamine, octopamine, serotonin, and tyramine (1 μM each, Figure 4A). The application of serotonin led to an increase in the fluorescence signal in Dm5-HT2B-expressing but not in non-transfected cells. Neither dopamine, octopamine nor tyramine evoked responses in transfected or non-transfected cells. Histamine, however, caused a rise in Ca2+-dependent Fluo-4 fluorescence in both, Dm5-HT2B-expressing and non-transfected HEK 293 cells. This effect is due to endogenously expressed histamine (H1) receptors in the HEK 293 cell line used in this study (Meisenberg et al., 2015).
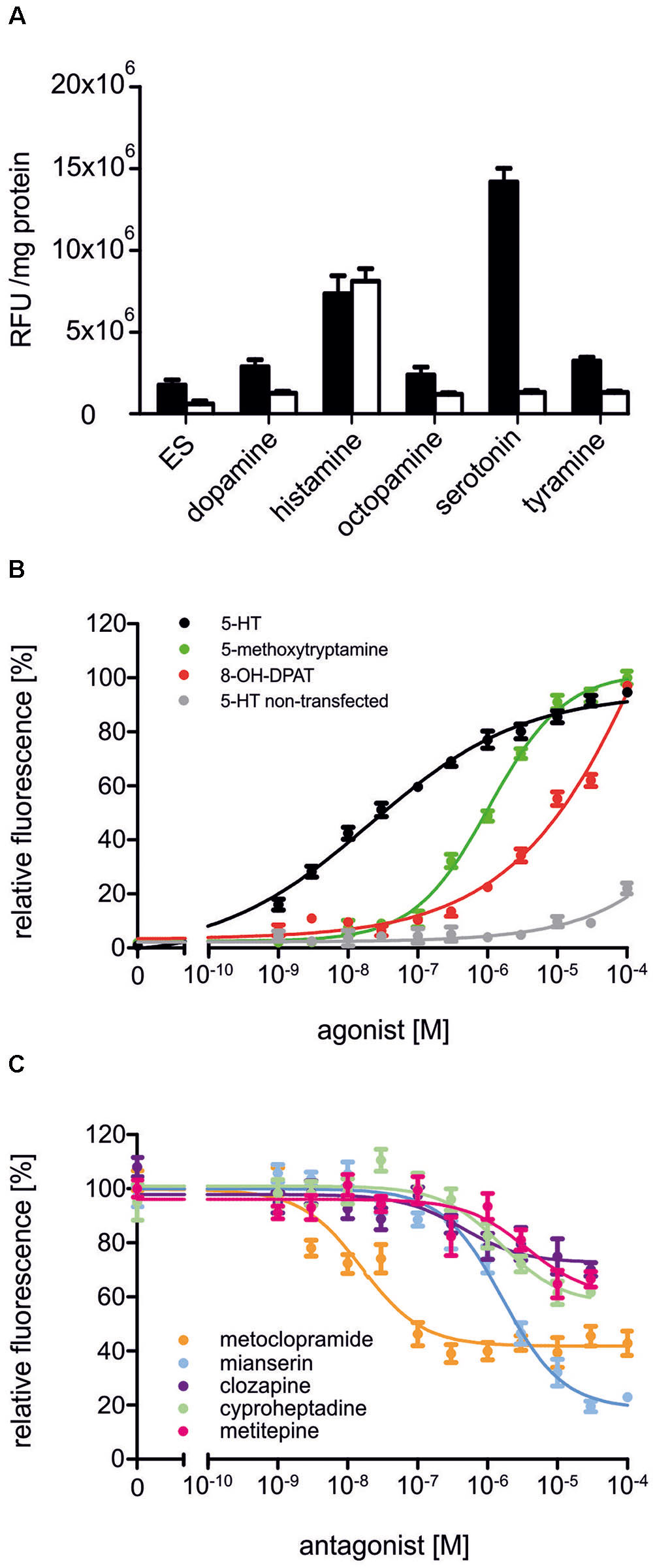
FIGURE 4. Pharmacological properties of Dm5-HT2B. (A) Effect of different biogenic amines on Ca2+-dependent Fluo-4 fluorescence in Dm5-HT2B-expressing and non-transfected HEK 293 cells. Bars represent changes in relative fluorescence units (RFU/mg protein) in Dm5-HT2B-expressing (black bars) and non-transfected HEK 293 cells (white bars). Biogenic amines were applied in a concentration of 10-6 M. Mean values ± SD were calculated from octuplicate determinations. ES, extracellular solution. (B) Concentration-dependent effects of serotonin on Dm5-HT2B-expressing (black) and non-transfected HEK 293 cells (gray) as well as of 5-methoxytryptamine (green) and 8-OH-DPAT (red) on Dm5-HT2B-expressing cells. Data from representative experiments are shown. (Each data point represents the mean ± SD of an octuplicate determination. The relative fluorescence signal (%) for measurements with serotonin was normalized to the value measured in the presence of 10-4 M serotonin in Dm5-HT2B-expressing cells (=100%). The relative fluorescence signals (%) for measurements with 5-methoxytryptamine and 8-OH-DPAT were normalized to the value measured in the presence of 10-4 M of the respective ligand (=100%). (C) Concentration-dependent effects of potential antagonists on serotonin-stimulated Dm5-HT2B-evoked Ca2+ signals. Increasing concentrations (10-9 M to 10-4 M) of receptor antagonists were added to the receptor-expressing cell line. The Ca2+-dependent Fluo-4 signals were registered and normalized to the fluorescence evoked with 10-7 M serotonin (=100%). Data from representative experiments are shown. Each data point represents the mean ± SD of an octuplicate determination.)
To further investigate the pharmacological properties of Dm5-HT2B, concentration-response curves on Dm5-HT2B-expressing and non-transfected HEK 293 cells were established for serotonin. A series of serotonin concentrations was applied ranging from 10-9 M to 10-4 M. The concentration-response curve for Dm5-HT2B was sigmoid and saturated at a serotonin concentration of 3 × 10-5 M (Figure 4B). Half-maximal activation of Dm5-HT2B (EC50) was at 2.11 × 10-8 M. In non-transfected HEK 293 cells, a slight increase in the fluorescence signal was observed at the highest ligand concentration applied (10-4 M).
Two potential agonists were tested for their activity on Dm5-HT2B-expressing cells. For 5-methoxytryptamine and 8-OH-DPAT, concentration series ranging from 10-9 M to 10-4 M were applied and Ca2+-dependent Fluo-4 fluorescence was monitored (Figure 4B). Both ligands caused specific responses. The EC50 for 5-methoxytryptamine was 1.05 × 10-6 M. In contrast to serotonin and 5-methoxytryptamine, the concentration-response curve for 8-OH-DPAT did not saturate and, therefore, the deduced EC50 of ≅6.5 × 10-4 M might be taken with some caution.
Next, we examined the ability of potential receptor antagonists for impairing Dm5-HT2B activity. Measurements were performed with increasing concentrations of the antagonists clozapine, cyproheptadine, ketanserin, metitepine (also known as methiothepin), methysergide, metoclopramide, mianserin, prazosin, SB 242084, and spiperone on a background of 10-7 M serotonin.
In Dm5-HT2B-expressing cells, many of the antagonists caused a decrease of the serotonin-induced Ca2+-dependent fluorescence signals. Representative data are shown in Figure 4C. Ligand concentrations that led to half-maximal inhibition of Dm5-HT2B-induced responses (IC50) were determined from the concentration-response curves and are summarized in Table 1. The most effective antagonist on serotonin-stimulated Dm5-HT2B was metoclopramide with an IC50 of 1.78 × 10-8 M. The order of antagonist efficiency (IC50) on the Dm5-HT2B receptor was: metoclopramide > clozapine > cyproheptadine > mianserin > metitepine (Table 1). Two ligands, prazosin and spiperone, also caused a reduction of the cellular response. However, the signals did not reach saturation and, due to solubility problems higher concentrations could not be tested (see Supplementary Figure S1). Therefore, IC50 values were not calculated from these concentration-response curves. For three ligands, i.e., ketanserin, methysergide, and SB 242084, we did not observe any effect on serotonin-stimulated Dm5-HT2B-expressing cells.
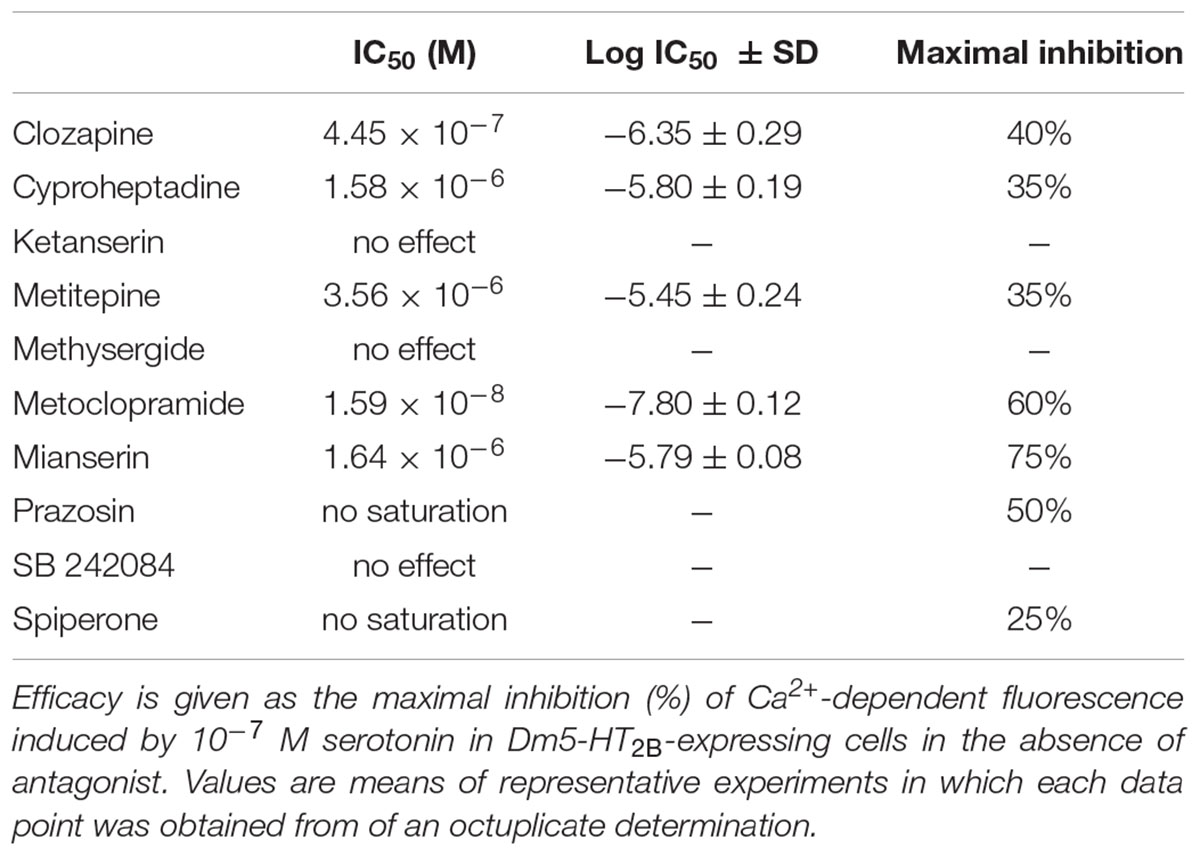
TABLE 1. IC50 values (potency) and relative efficacy were calculated from concentration-response curves for each drug.
Discussion
There is ongoing interest to precisely understand the physiological and behavioral roles of serotonergic signaling. To meet this challenge, important steps are to determine the molecular and functional-pharmacological properties of 5-HT receptor subtypes and to address their distribution within the CNS. Based on a rich body of data, a picture emerges that, e.g., insects and mammals share similar modes of drug action as well as cellular and behavioral responses to serotonergic neurotransmission. Using model insects such as D. melanogaster might accelerate the gain of knowledge. Here, we have focused on elucidating the pharmacological properties of a D. melanogaster 5-HT receptor, Dm5-HT2B. The pharmacological profile can be used for designing rational in vitro and in vivo studies to uncover the contribution of Dm5-HT2B to the animal’s development, physiology, and behavior.
Molecular Features of the Dm5-HT2B Receptor
Four genes encoding 5-HT receptor subtypes were already cloned from D. melanogaster in the 90′s of the last century. These were Dm5-HT1A and Dm5-HT1B (CG16720 and CG15113; Saudou et al., 1992), Dm5-HT2A (CG1056; Colas et al., 1995), and Dm5-HT7 (CG12073; Witz et al., 1990). These GPCRs share cognate properties with mammalian 5-HT1A, 5-HT2, and 5-HT7 receptors. Resulting from bioinformatics screening and gene annotation, another GPCR gene (CG42796; Brody and Cravchik, 2000; Hauser et al., 2006; Blenau and Thamm, 2011) was uncovered encoding a protein with pronounced similarity to a 5-HT2 receptor in the spiny lobster, P. interruptus (Clark et al., 2004). The receptor was named Dm5-HT2B. In a recent study in which D. melanogaster larvae were used to screen for drugs that mediate food intake, the 5-HT receptor antagonist metitepine was identified as a potent anorectic drug (Gasque et al., 2013). Using cell-based assays, the authors could show that metitepine is an antagonist of all five D. melanogaster 5-HT receptors including Dm5-HT2B (Gasque et al., 2013). While Gasque et al. (2013) could identify Dm5-HT2A as the sole molecular target for feeding inhibition by metitepine, they did not establish a full pharmacological profile for Dm5-HT2B. Here, we provide additional information on the molecular and pharmacological properties of this fifth 5-HT receptor subtype of the fruit fly.
With 904 amino acid residues and a calculated molecular weight of 99.5 kDa, the Dm5-HT2B protein is rather large. More than half of the residues (563 amino acids) are present in the third cytoplasmic loop. Interestingly, the Dm5-HT2A receptor is of similar size and contains 869 amino acid residues (Colas et al., 1995). This receptor also harbors a long third cytoplasmic loop of 321 residues but, in addition, Dm5-HT2A has a long N-terminal loop which consists of 286 residues. For this receptor, two variants have been described. Either a point mutation changing Pro52 to Ser in the N-terminus (Schaerlinger et al., 2007) or the complete deletion of the N-terminal domain leads to a significant gain of the receptor’s affinity for serotonin (Colas et al., 1997) compared to the wild type protein. Orthologous receptors to Dm5-HT2A and Dm5-HT2B have been characterized from other insects as well. The Am5-HT2B receptor from the honeybee also contains a large third cytoplasmic loop consisting of 399 residues (Thamm et al., 2013). However, with 80.7 kDa (733 amino acid residues) the protein is smaller than Dm5-HT2B. With 653 residues, the honeybee Am5-HT2A receptor is the smallest protein of this foursome. For both honeybee 5-HT2 receptor subtypes, several splice variants were molecularly cloned (Thamm et al., 2013). None of these variants gave rise to functional receptors upon heterologous expression of the constructs. This finding, however, does not rule out that full-length and modified variants may assemble in native tissues and thereby potentially expand the repertoire of serotonin binding partners in the honeybee.
Although Dm5-HT2B is set apart by the length of its primary structure from other GPCRs, the protein shares most of the cognate features characterizing this huge gene family. The N-terminus of Dm5-HT2B contains several consensus motifs for post-translational glycosylation (Figure 2). A large number of phosphorylation sites to common protein kinases are spread throughout the intracellular loops (Figure 2). Which of these sites participate in receptor desensitization and/or internalization (Lefkowitz and Shenoy, 2005; Kelly et al., 2008) upon serotonin stimulation awaits independent experimental testing. In addition to site-directed mutagenesis of single or multiple phosphorylation sites, a deletion strategy might be applied to successively reduce the size of the third cytoplasmic loop connecting transmembrane regions (TM) five and six (Figure 2). After heterologous expression of these receptor variants, their signaling properties can be examined and quantified by Ca2+ fluorimetry. Finally, residues in the binding site for serotonin that is formed by the transmembrane segments of Dm5-HT2B are well conserved. Notably, the aspartic acid residue (D153; D3.32; nomenclature to Ballesteros and Weinstein, 1995) in TM3 is a potential binding partner of the protonated amino group of serotonin. A serine residue (S237; S5.43) in TM5 could bind to the 5-hydroxy group of serotonin’s phenyl moiety. Phenylalanine and/or tryptophan residues in TM6 and TM7 (Figure 2) might contribute to π-π interaction with delocalized electrons in serotonin and stabilize the receptor ligand interaction.
Although we haven’t experimentally addressed the expression pattern of the Dm5-ht2b gene in this study, compelling evidence is available from previous studies supporting the general finding that 5-HT receptors are widely expressed in the CNS throughout development of D. melanogaster (Yuan et al., 2005, 2006; Nichols, 2007). Since we and others (Gasque et al., 2013) have cloned the cDNA encoding Dm5-HT2B from adult tissue, the previous statement also holds for Dm5-HT2B. Within the brain of adult flies, Dm5-HT2B is expressed in the pars intercerebralis, the ellipsoid body, and photoreceptor cells (Gnerer et al., 2015). Whether the receptor participates in the regulation of heart function in D. melanogaster as suggested by recent experiments (Majeed et al., 2014) or is differentially expressed in male and female nervous tissue (Goldman and Arbeitman, 2007), awaits further testing.
Pharmacological Properties of Dm5-HT2B
The Dm5-HT2B receptor was functionally expressed in HEK 293 cells. Coupling of Dm5-HT2B to intracellular signaling cascades was examined via cell-endogenous G-proteins. Like its protostomian and deuterostomian orthologs, Dm5-HT2B caused intracellular Ca2+ release after stimulation with serotonin or synthetic agonists like 5-methoxytryptamine or 8-OH-DPAT. With an EC50 of 2 × 10-8 M, activation of the receptor was much more sensitive to serotonin compared to 5-methoxytryptamine (EC50 ≅1 × 10-6 M) or 8-OH-DPAT (EC50 ≅6.5 × 10-4 M). Since the concentration-response curve with 8-OH-DPAT did not saturate, this latter value should be taken with caution. More recently, two 5-HT2 receptors from the honeybee, Am5-HT2A and Am5-HT2B, have been molecularly and pharmacologically characterized using the same heterologous expression system (Thamm et al., 2013). With EC50 values of 2.57 × 10-8 M and 3.25 × 10-8 M both receptors share similar potencies for serotonin as Dm5-HT2B and the Cv5-HT2A receptor from Calliphora vicina (2.4 × 10-8 M; Röser et al., 2012), which was also expressed in HEK 293 cells. With an EC50 of 2.01 × 10-7 M an orthologous receptor cloned from R. prolixus (Rp5-HT2B; Paluzzi et al., 2015) was an order of magnitude less sensitive to serotonin. It should be mentioned here, that Rp5-HT2B was not expressed in HEK 293 cells but in a recombinant Chinese hamster ovary cell line (CHOK1-aeq) and that ligand affinity may be influenced by the expression system used. In contrast to Dm5-HT2B, where half-maximal stimulation with 5-methoxytryptamine was at ≅1 × 10-6 M, both honeybee 5-HT2 receptors and the C. vicina receptor displayed EC50 values in the nanomolar range [Am5-HT2A, 7 × 10-8 M; Am5-HT2B, 6.04 × 10-8 M (Thamm et al., 2013); Cv5-HT2A, 6.7 × 10-8 M (Röser et al., 2012)]. Similar to the results obtained for 5-methoxytryptamine, Dm5-HT2B receptor activation by 8-OH-DPAT (EC50 ≅6.5 × 10-4 M) was less efficacious than that of Am5-HT2A (EC50 = 5.59 × 10-5 M) and Am5-HT2B receptors (EC50 = 5.6 × 10-7 M; Thamm et al., 2013) or the Cv5-HT2A receptor (EC50 = 6.2 × 10-5 M; Röser et al., 2012). Thus, although active on Dm5-HT2B, both 5-methoxytryptamine and 8-OH-DPAT may not serve as alternatives to serotonin in specifically stimulating the receptor since both are likely to activate additional receptor subtypes at concentrations required for in vivo application in D. melanogaster.
Inhibition of receptor-mediated Ca2+ signaling in the cell line constitutively expressing Dm5-HT2B was examined with a series of synthetic antagonists. In addition to substances that completely lacked inhibitory potential on the receptor (i.e., ketanserin, methysergide, and SB 242084), we observed three distinct types of inhibition profiles on Dm5-HT2B. Two antagonists caused saturating responses and reduced serotonin-evoked Ca2+-dependent fluorescence to values ≤ 40% of control measurements. With an IC50 of 1.59 × 10-8 M, metoclopramide was more potent than mianserin (IC50 = 1.64 × 10-6 M). Serotonin-evoked cellular Ca2+ responses were reduced to 40 and 25% of control measurements without antagonists by metoclopramide and mianserin, respectively. Responses to clozapine (IC50 = 4.45 × 10-7 M), cyproheptadine (IC50 = 1.58 × 10-6 M), and metitepine (IC50 = 3.56 × 10-6 M) also saturated but all three substances were much less potent inhibitors at the receptor than metoclopramide or mianserin (Figure 4C). A maximal reduction to 60% of the serotonin-evoked signal was achieved with clozapine (Table 1). Finally, prazosin and spiperone also reduced serotonin-induced Ca2+-dependent fluorescence in the cell line but the responses did not saturate. From the whole series of antagonists used in the current study, only metitepine has been tested in an earlier study by Gasque et al. (2013), who expressed Dm5-HT2B in HEK 293T cells to investigate the pharmacology of this drug on D. melanogaster 5-HT receptors. Using Ca2+ fluorimetry on individual cells expressing Dm5-HT2B, the authors reported an IC50 of 2 × 10-6 M which is very similar to the value determined in the current study. Interestingly, metitepine has been uncovered as a potent anorectic drug in D. melanogaster larvae (Gasque et al., 2013). Although active on all five 5-HT receptor subtypes of the fruit fly, metitepine exhibited its anti-feeding activity only by interfering with Dm5-HT2A signaling (Gasque et al., 2013). Some of the antagonists tested on Dm5-HT2B in the current study had been examined previously on honeybee, C. vicina, and R. prolixus 5-HT receptors, too. Clozapine, cyproheptadine, metitepine, and mianserin inhibited Am5-HT2A receptors in the micromolar range and reduced serotonin-induced Ca2+-dependent fluorescence by 44, 36, 39, and 49%, respectively (Thamm et al., 2013). Interestingly, at the Am5-HT2B receptor metitepine did not have any activity at all. In contrast, clozapine, cyproheptadine, and mianserin blocked Ca2+-dependent fluorescence to 5, 23, and 24%, respectively, with IC50 values in the low micromolar range (Thamm et al., 2013). Efficient inhibitors acting on the blowfly Cv5-HT2A receptor were metitepine and clozapine which reduced serotonin-induced Ca2+ signals to 15 and 25% of control measurements with IC50 values of 1.2 × 10-6 M and 15 × 10-6 M, respectively (Röser et al., 2012). Cyproheptadine, ketanserin, and mianserin reduced activity of the R. prolixus Rp5-HT2B receptor by ≥ 50% at the highest from three concentrations tested, i.e., 10-7, 10-6, and 10-5 M (Paluzzi et al., 2015).
In the current study, we identified metoclopramide as the most potent antagonist at the Dm5-HT2B receptor. This was surprising, since metoclopramide is an established dopamine D2 receptor antagonist in vertebrates, where it also inhibits serotonin-gated ion channels (5-HT3 receptors) and activates 5-HT4 receptors (Tonini et al., 1995). The latter effects have been therapeutically used to interfere with emesis. Until now, no information is available regarding the pharmacology of metoclopramide on the remaining four D. melanogaster 5-HT receptors. Future studies must show whether metoclopramide is really a Dm5-HT2B-specific or a rather non-selective antagonist of 5-HT receptors in the fruit fly. Metoclopramide has also been shown to bind to the tyramine receptor TyrR (CG7431; Arakawa et al., 1990; Ki = 4.6 × 10-6 M) and to block the β-adrenergic-like octopamine receptor Octβ3R (CG42244; Maqueira et al., 2005) in D. melanogaster, although only a high concentration of 10-5 M was tested in the latter study. Due to its pronounced sensitivity to Dm5-HT2B (IC50 = 1.59 × 10-8 M), however, the concentration required for in vivo experimentation to specifically target this receptor subtype might be kept rather low. In summary, our data may facilitate future behavioral pharmacological studies on the role of Dm5-HT2B in the fruit fly. Such studies would be desirable, since current knowledge on the role of this 5-HT receptor subtype solely depend on the investigation of flies that have been genetically manipulated.
Author Contributions
WB designed and evaluated experiments, wrote the paper; DS conducted experiments and evaluated data; SB conducted experiments and evaluated data; MT conducted experiments; AB designed experiments and wrote the paper.
Funding
This study was supported by a grant from the German Research Foundation (BL 469/7-1).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fnsys.2017.00028/full#supplementary-material
Footnotes
- ^http://www.cbs.dtu.dk/services/NetNGlyc/
- ^http://lipid.biocuckoo.org/
- ^http://www.cbs.dtu.dk/services/NetPhos/
References
Alekseyenko, O. V., Chan, Y. B., Fernandez, M. P., Bülow, T., Pankratz, M. J., and Kravitz, E. A. (2014). Single serotonergic neurons that modulate aggression in Drosophila. Curr. Biol. 24, 2700–2707. doi: 10.1016/j.cub.2014.09.051
Alekseyenko, O. V., and Kravitz, E. A. (2014). Serotonin and the search for the anatomical substrate of aggression. Fly 8, 200–205. doi: 10.1080/19336934.2015.1045171
Alekseyenko, O. V., Lee, C., and Kravitz, E. A. (2010). Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5:e10806. doi: 10.1371/journal.pone.0010806
Arakawa, S., Gocayne, J. D., McCombie, W. R., Urquhart, D. A., Hall, L. M., Fraser, C. M., et al. (1990). Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron 4, 343–354.
Ballesteros, J., and Weinstein, H. (1995). Integrated methods for the construction of three-dimensional models of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428.
Becnel, J., Johnson, O., Luo, J., Nässel, D. R., and Nichols, C. D. (2011). The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE 6:e20800. doi: 10.1371/journal.pone.0020800
Berger, M., Gray, J. A., and Roth, B. L. (2009). The expanded biology of serotonin. Annu. Rev. Med. 60, 355–366. doi: 10.1146/annurev.med.60.042307.110802
Blankenburg, S., Balfanz, S., Hayashi, Y., Shigenobu, S., Miura, T., Baumann, O., et al. (2015). Cockroach GABAB receptor subtypes: molecular characterization, pharmacological properties and tissue distribution. Neuropharmacology 88, 134–144. doi: 10.1016/j.neuropharm.2014.08.022
Blenau, W., and Thamm, M. (2011). Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies: lessons from Drosophila melanogaster and Apis mellifera. Arthropod Struct. Dev. 40, 381–394. doi: 10.1016/j.asd.2011.01.004
Blom, N., Sicheritz-Pontén, T., Gupta, R., Gammeltoft, S., and Brunak, S. (2004). Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649.
Brody, T., and Cravchik, A. (2000). Drosophila melanogaster G protein-coupled receptors. J. Cell Biol. 150, F83–F88.
Chen, C., and Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745–2752.
Clark, M. C., Dever, T. E., Dever, J. J., Xu, P., Rehder, V., Sosa, M. A., et al. (2004). Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J. Neurosci. 24, 3421–3435.
Colas, J. F., Choi, D. S., Launay, J. M., and Maroteaux, L. (1997). Evolutionary conservation of the 5-HT2B receptors. Ann. N. Y. Acad. Sci. 812, 149–153. doi: 10.1111/j.1749-6632.1997.tb48157.x
Colas, J. F., Launay, J. M., Kellermann, O., Rosay, P., and Maroteaux, L. (1995). Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc. Natl. Acad. Sci. U.S.A. 92, 5441–5445.
Dacks, A. M., Green, D. S., Root, C. M., Nighorn, A. J., and Wang, J. W. (2009). Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J. Neurogenet. 23, 366–377. doi: 10.3109/01677060903085722
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27, 1164–1165. doi: 10.1093/bioinformatics/btr088
Dasari, S., and Cooper, R. L. (2006). Direct influence of serotonin on the larval heart of Drosophila melanogaster. J. Comp. Physiol. B 176, 349–357.
Dierick, H. A., and Greenspan, R. J. (2007). Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678–682.
Ellen, C. W., and Mercer, A. R. (2012). Modulatory actions of dopamine and serotonin on insect antennal lobe neurons: insights from studies in vitro. J. Mol. Histol. 43, 401–404. doi: 10.1007/s10735-012-9401-7
Gasque, G., Conway, S., Huang, J., Rao, Y., and Vosshall, L. B. (2013). Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci. Rep. 3:sre02120. doi: 10.1038/srep02120
Geyer, M. A., and Vollenweider, F. X. (2008). Serotonin research: contributions to understanding psychoses. Trends Pharmacol. Sci. 29, 445–453. doi: 10.1016/j.tips.2008.06.006
Gnerer, J. P., Venken, K. J., and Dierick, H. A. (2015). Gene-specific cell labeling using MiMIC transposons. Nucleic Acids Res. 43:e56. doi: 10.1093/nar/gkv113
Goldman, T. D., and Arbeitman, M. N. (2007). Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet. 3:e216.
Hauser, F., Cazzamali, G., Williamson, M., Blenau, W., and Grimmelikhuijzen, C. J. (2006). A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog. Neurobiol. 80, 1–19.
Kaplan, D. D., Zimmermann, G., Suyama, K., Meyer, T., and Scott, M. P. (2008). A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes Dev. 22, 1877–1893. doi: 10.1101/gad.1670508
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., and Sternberg, M. J. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858. doi: 10.1038/nprot.2015.053
Kelly, E., Bailey, C. P., and Henderson, G. (2008). Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 153, S379–S388.
Kozak, M. (1984). Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 12, 857–872.
Krogh, A., Larsson, B., von Heijne, G., and Sonnhammer, E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580.
Kyte, J., and Doolittle, R. F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132.
Lee, P. T., Lin, H. W., Chang, Y. H., Fu, T. F., Dubnau, J., Hirsh, J., et al. (2011). Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 108, 13794–13799. doi: 10.1073/pnas.1019483108
Lefkowitz, R. J., and Shenoy, S. K. (2005). Transduction of receptor signals by β–arrestins. Science 308, 512–517.
Luo, J., Becnel, J., Nichols, C. D., and Nässel, D. R. (2012). Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor. Cell. Mol. Life Sci. 69, 471–484. doi: 10.1007/s00018-011-0789-0
Luo, J., Lushchak, O. V., Goergen, P., Williams, M. J., and Nässel, D. R. (2014). Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior. PLoS ONE 9:e99732. doi: 10.1371/journal.pone.0099732
Majeed, Z. R., Abdeljaber, E., Soveland, R., Cornwell, K., Bankemper, A., Koch, F., et al. (2016). Modulatory action by the serotonergic system: behavior and neurophysiology in Drosophila melanogaster. Neural Plast. 2016:7291438. doi: 10.1155/2016/7291438
Majeed, Z. R., Satcy, A., and Cooper, R. L. (2014). Pharmacological and genetic identification of serotonin receptor subtypes on Drosophila larval heart and aorta. J. Comp. Physiol. B 184, 205–219. doi: 10.1007/s00360-013-0795-7
Maqueira, B., Chatwin, H., and Evans, P. D. (2005). Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 94, 547–560.
Meisenberg, A., Kaschuba, D., Balfanz, S., Jordan, N., and Baumann, A. (2015). Molecular and functional profiling of histamine receptor-mediated calcium ion signals in different cell lines. Anal. Biochem. 486, 96–101. doi: 10.1016/j.ab.2015.06.037
Mohammad, F., Aryal, S., Ho, J., Stewart, J. C., Norman, N. A., Tan, T. L., et al. (2016). Ancient anxiety pathways influence Drosophila defense behaviors. Curr. Biol. 26, 981–986. doi: 10.1016/j.cub.2016.02.031
Nall, A., and Sehgal, A. (2014). Monoamines and sleep in Drosophila. Behav. Neurosci. 128, 264–272. doi: 10.1037/a0036209
Neckameyer, W. S. (2010). A trophic role for serotonin in the development of a simple feeding circuit. Dev. Neurosci. 32, 217–237. doi: 10.1159/000304888
Neckameyer, W. S., Coleman, C. M., Eadie, S., and Goodwin, S. F. (2007). Compartmentalization of neuronal and peripheral serotonin synthesis in Drosophila melanogaster. Genes Brain Behav. 6, 756–769.
Nichols, C. D. (2007). 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Dev. Neurobiol. 67, 752–763.
Paluzzi, J. P., Bhatt, G., Wang, C. H., Zandawala, M., Lange, A. B., and Orchard, I. (2015). Identification, functional characterization, and pharmacological profile of a serotonin type-2b receptor in the medically important insect, Rhodnius prolixus. Front. Neurosci. 9:175. doi: 10.3389/fnins.2015.00175
Python, F., and Stocker, R. F. (2002). Immunoreactivity against choline acetyltransferase, gamma-aminobutyric acid, histamine, octopamine, and serotonin in the larval chemosensory system of Drosophila melanogaster. J. Comp. Neurol. 453, 157–167.
Qi, Y. X., Huang, J., Li, M. Q., Wu, Y. S., Xia, R. Y., and Ye, G. Y. (2016). Serotonin modulates insect hemocyte phagocytosis via two different serotonin receptors. Elife 5:e12241. doi: 10.7554/eLife.12241
Reim, T., Balfanz, S., Baumann, A., Blenau, W., Thamm, M., and Scheiner, R. (2017). AmTAR2: functional characterization of a honeybee tyramine receptor stimulating adenylyl cyclase activity. Insect Biochem. Mol. Biol. 80, 91–100. doi: 10.1016/j.ibmb.2016.12.004
Ro, J., Pak, G., Malec, P. A., Lyu, Y., Allison, D. B., Kennedy, R. T., et al. (2016). Serotonin signaling mediates protein valuation and aging. Elife 5:e16843. doi: 10.7554/eLife.16843
Rodriguez Moncalvo, V. G., and Campos, A. R. (2009). Role of serotonergic neurons in the Drosophila larval response to light. BMC Neurosci. 10:66. doi: 10.1186/1471-2202-10-66
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Röser, C., Jordan, N., Balfanz, S., Baumann, A., Walz, B., Baumann, O., et al. (2012). Molecular and pharmacological characterization of serotonin 5-HT2α and 5-HT7 receptors in the salivary glands of the blowfly Calliphora vicina. PLoS ONE 7:e49459. doi: 10.1371/journal.pone.0049459
Saudou, F., Boschert, U., Amlaiky, N., Plassat, J. L., and Hen, R. (1992). A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 11, 7–17.
Schaerlinger, B., Launay, J. M., Vonesch, J. L., and Maroteaux, L. (2007). Gain of affinity point mutation in the serotonin receptor gene 5-HT2Dro accelerates germband extension movements during Drosophila gastrulation. Dev. Dyn. 236, 991–999.
Sitaraman, D., LaFerriere, H., Birman, S., and Zars, T. (2012). Serotonin is critical for rewarded olfactory short-term memory in Drosophila. J. Neurogenet. 26, 238–244. doi: 10.3109/01677063.2012.666298
Sitaraman, D., Zars, M., Laferriere, H., Chen, Y. C., Sable-Smith, A., Kitamoto, T., et al. (2008). Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 105, 5579–5584. doi: 10.1073/pnas.0710168105
Spitzer, N., Edwards, D. H., and Baro, D. J. (2008). Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii. J. Exp. Biol. 211, 92–105.
Thamm, M., Rolke, D., Jordan, N., Balfanz, S., Schiffer, C., Baumann, A., et al. (2013). Function and distribution of 5-HT2 receptors in the honeybee (Apis mellifera). PLoS ONE 8:e82407. doi: 10.1371/journal.pone.0082407
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680.
Tonini, M., Candura, S. M., Messori, E., and Rizzi, C. A. (1995). Therapeutic potential of drugs with mixed 5-HT4 agonist/5-HT3 antagonist action in the control of emesis. Pharmacol. Res. 31, 257–260.
Vargas, M. A., Luo, N., Yamaguchi, A., and Kapahi, P. (2010). A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 20, 1006–1011. doi: 10.1016/j.cub.2010.04.009
Vázquez-Acevedo, N., Reyes-Colón, D., Ruíz-Rodríguez, E. A., Rivera, N. M., Rosenthal, J., Kohn, A. B., et al. (2009). Cloning and immunoreactivity of the 5-HT1Mac and 5-HT2Mac receptors in the central nervous system of the freshwater prawn Macrobrachium rosenbergii. J. Comp. Neurol. 513, 399–416. doi: 10.1002/cne.21979
Vleugels, R., Verlinden, H., and Vanden Broeck, J. (2015). Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2:e314. doi: 10.14800/nt.314
Walz, B., Baumann, O., Krach, C., Baumann, A., and Blenau, W. (2006). The aminergic control of cockroach salivary glands. Arch. Insect Biochem. Physiol. 62, 141–152.
Witz, P., Amlaiky, N., Plassat, J. L., Maroteaux, L., Borrelli, E., and Hen, R. (1990). Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 87, 8940–8944.
Yuan, Q., Joiner, W. J., and Sehgal, A. (2006). A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol. 16, 1051–1062.
Keywords: biogenic amine, Ca2+, cAMP, cellular signaling, insect, G protein-coupled receptor, inositol-1,4,5-trisphosphate, second messenger
Citation: Blenau W, Stöppler D, Balfanz S, Thamm M and Baumann A (2017) Dm5-HT2B: Pharmacological Characterization of the Fifth Serotonin Receptor Subtype of Drosophila melanogaster. Front. Syst. Neurosci. 11:28. doi: 10.3389/fnsys.2017.00028
Received: 28 February 2017; Accepted: 25 April 2017;
Published: 11 May 2017.
Edited by:
Irina T. Sinakevitch, Arizona State University, USAReviewed by:
Jean-Paul V. Paluzzi, York University, CanadaDeborah Baro, Georgia State University, USA
Copyright © 2017 Blenau, Stöppler, Balfanz, Thamm and Baumann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnd Baumann, a.baumann@fz-juelich.de
 Wolfgang Blenau
Wolfgang Blenau Daniel Stöppler
Daniel Stöppler Sabine Balfanz
Sabine Balfanz Markus Thamm
Markus Thamm Arnd Baumann
Arnd Baumann