General Commentary to the “Management of Biochemical Recurrence after Primary Localized Therapy for Prostate Cancer” by Darwish O. M. and Raj G. V.
- Department of Urology, The University of Texas Southwestern Medical Center at Dallas, Dallas, TX, USA
Clinically localized prostate cancer is typically managed by well established therapies like radical prostatectomy, brachytherapy, and external beam radiation therapy. While many patients can be cured with definitive local therapy, some will have biochemical recurrence (BCR) of disease detected by a rising serum prostate-specific antigen (PSA). Management of these patients is nuanced and controversial. The natural history indicates that a majority of patients with BCR will not die from prostate cancer but from other causes. Despite this, a vast majority of patients with BCR are empirically treated with non-curable systemic androgen deprivation therapy (ADT), with its myriad of real and potential side effects. In this review article, we examined the very definition of BCR after definitive local therapy, the current status of imaging studies in its evaluation, the need for additional therapies, and the factors involved in the decision making in the choice of additional therapies. This review aims to help clinicians with the management of patients with BCR. The assessment of prognostic factors including absolute PSA level, time to recurrence, PSA kinetics, multivariable nomograms, imaging, and biopsy of the prostatic bed may help stratify the patients into localized or systemic recurrence. Patients with low-risk of systemic disease may be cured by a salvage local therapy, while those with higher risk of systemic disease may be offered the option of ADT or a clinical trial. An algorithm incorporating these factors is presented.
Introduction
The most common primary local therapies used for clinically localized prostate cancer are radical prostatectomy (RP) and radiation therapy (RT), including external beam radiation therapy (EBRT). While these primary therapies are associated with a high cancer control rates for localized disease, up to a third of patients undergoing these therapies will have a biochemical recurrence (BCR) after local therapy (Djavan et al., 2003; Khan et al., 2003).
Biochemical recurrence is a clinical state characterized by a rising serum prostate-specific antigen (PSA), with or without clinical or radiographic metastasis. Patients with BCR have a variable clinical course: some will have indolent course with no adverse long-term effect on their survival; others may have a rapid clinical progression, with metastasis to bone and increased risk of Prostate Cancer Specific Mortality (PCSM). Comparison of outcomes reveal that patients with BCR have a 88% 10-year overall survival rate in contrast to the 93% 10-year overall survival rate in men without BCR (Jhaveri et al., 1999). In a landmark paper evaluating BCR following RP, the median time from BCR to clinical progression was noted to be 8 years and from metastasis to PCSM was 5 years (Pound et al., 1999) indicating that median survival from BCR was 13 years. While recent studies have demonstrated even longer median survival after BCR (up to 16 years), a subset of men with aggressive prostate cancer die much sooner after PSA recurrence (Freedland et al., 2005).
This review will discuss the natural history of BCR, review current management options for patients with BCR, and evaluate management options after primary definitive treatment for localized prostate cancer.
BCR Definition
Prostate-specific antigens are typically evaluated every 3 months after surgery. Serum PSA should reach undetectable levels in 4 weeks in a majority of cases of patients undergoing RP, as the half-life of PSA is 2.5–3 days (Partin and Oesterling, 1994). A detectable PSA may reflect presence of benign prostatic tissue left after RP which may still produce PSA (Djavan et al., 2005). Often, serial evaluation of PSAs can help evaluate the clinical significance of a detectable PSA. For example, a man with a detectable and low PSA level of 0.05 ng/ml after RP may have a persistently detectable PSA without significant change for a long time. Such a patient is unlikely to progress and suffer PCSM. Thus, a detectable PSA after RP alone may not mandate salvage intervention. In contrast, a patient with a detectable and serially rising PSA of 0.5 ng/ml after RP is more indicative of residual prostate cancer and may benefit from salvage intervention.
Since a detectable PSA after RP alone does not indicate BCR, several studies have evaluated if specific PSA cutoffs could define BCR (Amling et al., 2001; Stephenson et al., 2006). EAU guidelines define BCR with both a post-RP PSA cutoff of 0.2 ng/ml, and two sequential PSA values ≥0.2 ng/ml (Aus et al., 2005). Indeed, only half of men with a detectable PSA in the 0.2–0.29 ng/ml range had a subsequent PSA progression and could be defined as having BCR (Amling et al. 2001). A PSA level ≥0.4 ng/ml correlated with a 79% risk of PSA progression (Amling et al., 2001). The PSA Working Group defined BCR with a PSA cutoff ≥0.4 ng/ml with a subsequent elevated level (Scher et al., 2004). A retrospective evaluation of BCR criteria showed that PSA cutoff ≥0.4 ng/ml had the highest correlation with the risk of clinical progression (Stephenson et al., 2006).
Definition of BCR following RT is also controversial. Following RT, PSA levels may not decrease to undetectable levels and may settle at a stable detectable level., Further, PSA fluctuations (the so called “PSA bounce”) are common in the first 2 years after RT (Hanlon et al., 2001; Rosser et al., 2002; Sengoz et al., 2003). Indeed, the median time to PSA nadir is 18 months (Zagars, 1992). Finally, concomitant use of androgen deprivation therapy (ADT) either prior to or along with RT complicates the interpretation of BCR. Thus, unlike after RP, a single PSA cutoff for BCR post-RT cannot be defined. To address this issue, in 1997, the American Society for Therapeutic Radiology and Oncology (ASTRO) defined BCR criteria as three consecutive rises in the PSA level above nadir (American Society for Therapeutic Radiology and Oncology Consensus Panel, 1997). Since the ASTRO criteria did not specify a PSA cutoff, a man whose post-RT PSA rose from a nadir of 0.05 ng/ml to 0.06, 0.07, and 0.08 ng/ml on subsequent evaluations could be classified as having BCR. To address this issue, in 2005, the Phoenix criteria for post-RT BCR were defined as PSA increase ≥2 ng/ml above nadir (Roach et al., 2006). Currently, ASTRO criteria are typically used in RT-only treated patients and Phoenix criteria in both RT-only or RT + ADT treated patients.
Natural History of BCR
Up to a third of the 90,000 patients treated annually for clinically localized prostate cancer will experience BCR. Among these patients with BCR, one-third will eventually have clinical progression to metastases (Pound et al., 1999; Ward et al., 2003). A vast majority of patients with BCR will die of other causes (DOC); only those who progress to metastasis may suffer PCSM. Figure 1 illustrates the disease states of prostate cancer patients and their likelihood of mortality from either PCSM or DOC.
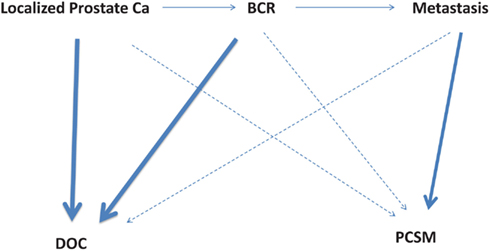
Figure 1. Disease states of localized prostate cancer patients and their likelihood of mortality from either other causes (DOC, death of other causes) or prostate cancer (PCSM, prostate cancer specific mortality). The bold lines indicate a higher risk of mortality, while the dotted lines indicate a lower risk of mortality. BCR, biochemical recurrence; PCSM, prostate cancer specific mortality; DOC, death of other causes.
Evaluation of Patients with BCR
Biochemical recurrence may signify local or metastatic recurrence. Patients with localized recurrence may be managed with localized salvage therapies. In contrast, patients with metastatic recurrence require systemic salvage therapies. Thus, the evaluation of patients with BCR is primarily to attempt stratification into local or metastatic recurrence, using strategies described below.
Absolute PSA Level
The absolute serum PSA level after initial local therapy is an indicator of the disease volume. Higher the PSA level, greater the burden of recurrence, and higher the risk of metastatic disease. A serum PSA level greater than 40 ng/ml is strongly associated with metastatic recurrence (Zagars, 1992). Further, a serum PSA level above 1 ng/ml indicates a higher risk of failure of localized salvage therapy (Stephenson et al., 2004a).
Time to Recurrence
The time period from initial local therapy to BCR has been shown to correlate with the site of recurrence. A shorter time to BCR after initial local therapy is associated with a higher risk of metastatic recurrence. In contrast, a longer time to BCR after initial local therapy is associated with a higher risk of localized recurrence (Pound et al., 1999; Freedland et al., 2005). While a true cutoff of the time to BCR has not been established, a time to BCR ≤2 years after RP strongly implicates a distant or metastatic recurrence while a time period of >2 years suggests a local recurrence (Pound et al., 1999).
PSA Kinetics
Generally, a shorter PSA doubling time (PSA-DT) indicates a rapidly growing tumor, a higher risk of clinical progression to metastatic disease and a higher risk of PCSM (Trapasso et al., 1994; D’Amico et al., 2005). In one study, patients post-RP with a longer PSA-DT (mean 11.7 months) had a higher risk of localized prostate cancer and a lower risk of clinical progression than patients with a shorter PSA-DT (4.3 months; Trapasso et al., 1994). Patients with PSA-DT of <3 months represented a minority (10–15%) of men with BCR but had the highest risk of systemic recurrence (D’Amico et al., 2005). Similarly in men post-RT, systemic recurrences were associated with higher PSA nadir and shorter PSA-DT (Crook et al., 1998). Patients with a PSA-DT <3 months had the greatest risk of PCSM, with a median survival of 6 years (D’Amico et al., 2003).
Multivariable Prediction Tools
Multivariable prediction tools such as nomograms were developed using clinico-pathologic and biochemical risk factors such as PSA-DT, time to BCR, and Gleason score to predict clinical progression after BCR (Pound et al., 1999). Parameters that magnified the risk of systemic relapse included a PSA-DT ≤3 months, time to BCR ≤3 years, and Gleason score ≥7 (Freedland et al., 2005).
Imaging
Traditional imaging to evaluate BCR involves either a bone scan or computed tomography scan (CT scan) or Magnetic Resonance Imaging (MRI) studies (Nguyen et al., 2007). These techniques are suboptimal for the evaluation of the patient with BCR with serum PSA levels below 10 ng/ml (Cher et al., 1998; Novo et al., 2006). The role of endorectal MRI is limited in evaluation of the patients with BCR because of the low signal intensity of T2 weighted images in radiated tissue (Sugimura et al., 1990; Nudell et al., 2000). Magnetic Resonance Spectroscopy (MRS) measures elevation in choline or decrease in citrate in prostate cancer tissue (Coakley et al., 2004) with a reported sensitivity of 77% compared to 68% with MRI only. Newer MRI techniques under investigation include Dynamic Contrast Enhanced MRI, which measures early gadolinium washout in prostate cancer (Rouviere et al., 2004) and Diffusion MRI imaging that measure degree of cellular crowding (Kim et al., 2009).
Radiolabeled imaging such as the ProstaScint scan or the traditional FDG PET scan has not lived up to the initial promise in evaluating the extent of the recurrent prostate cancer (Thomas et al., 2003). Recently, investigational PET tracers may have utility in evaluating the extent of the recurrent disease in the BCR state. For patients with BCR and PSA levels <2.5 ng/ml, 11C-choline PET in one study was reported to have a sensitivity of 89% and a positive predictive value of 72% (Rinnab et al., 2009). Similarly, 18F-choline PET sensitivity and specificity in detecting bone metastases from prostate cancer was reported to be 79 and 97%, respectively (Beheshti et al., 2010). Currently, there is no uniformly accepted imaging modality that can distinguish local versus systemic recurrence.
Biopsy
Prostate biopsy can be used to confirm local relapse in cases of palpable disease on the DRE after initial local therapy. The routine use of such biopsies is not warranted (Koppie et al., 2001). Further, the interpretation of the biopsy after RT is associated with high false-positive results and requires experienced pathologists (Molinie et al., 2008).
Options for Salvage Therapy
Patients with systemic recurrence are not candidates for localized salvage therapies, such as salvage RP, salvage RT, or salvage cryotherapy. Systemic therapies such as ADT may be appropriate for patients with systemic recurrences. The management of patients with recurrences is nuanced and should include an assessment of the risk-benefit ratio of intervention and patient preferences. Critical assessment using life expectancy tables (Cowen et al., 2006) and clinical progression nomograms (Pound et al., 1999; D’Amico et al., 2005; Freedland et al., 2005) may help decide on the need for observation or intervention with salvage therapy. Since every salvage local therapy or systemic therapy for patients with BCR is associated with a significant risk of complications, these interventions must be judiciously used. A thorough evaluation of patients prior to initiation of salvage local therapies to rule out systemic disease and thus avoid the unwanted side effects of salvage local therapies is an absolute must.
Salvage RT
If every patient with BCR after primary RP underwent salvage RT, less than half of these patients would benefit. Salvage RT after primary RP has been associated with a 4-year progression-free probability of 45% (Stephenson et al., 2004a). Patients with Gleason score 8–10, pre-RT PSA >2.0 ng/ml, positive surgical margins, seminal vesicle involvement (SVI) and/or PSA-DT ≤10 months had a higher risk of recurrence after salvage RT. A salvage RT nomogram that calculates the 6-year BCR-free probability may help identify patients who may best benefit from salvage RT (Stephenson et al., 2007). Patients at high-risk for recurrence on the salvage RT nomogram should be spared from the toxicity of salvage RT, which includes bladder irritation, radiation cystitis, radiation proctitis, impotence, and the long-term risk of secondary malignancies.
For patients undergoing salvage RT, the extension of the radiation field from prostatic fossa-alone to include the full pelvis including obturator nodes may further decrease recurrence rates after salvage RT (Kim et al., 2004; Spiotto et al., 2007). High-risk patients receiving both ADT and RT had a recurrence rate of 52.7% if the whole pelvis was radiated compared to 18.2% for patients with prostate fossa-alone radiation (Spiotto et al., 2007). Additionally, the concomitant use of ADT to salvage RT may also decrease recurrence rates (Bolla, 2005). The subset of patients with SVI on the RP specimen had the greatest benefit from the addition of ADT to salvage RT (King et al., 2004).
Salvage RP
Salvage RP may be offered to patients with biopsy proven prostate-only recurrence after primary RT. Salvage RP may be associated with a significant rate of post-operative incontinence (44–77%), bladder neck contractures (22–41%), and rectal injury (2–10%; Ward et al., 2005; Sanderson et al., 2006). In experienced hands, salvage RP can be safely performed with minimal complications (Eastham). The 5-year disease free survival has been reported to be 61% (Amling et al., 1999) with a 17–36% PCSM (Cheng et al., 1998; Bianco et al., 2005; Sanderson et al., 2006). Surgical margin status at the time of salvage RP is an important factor in predicting biochemical recurrence-free survival (BRFS). Negative margins correlated with 80% BRFS compared to 44% in patients with positive margins (Garzotto and Wajsman, 1998). The use of nerve-sparing approach helped in preservation of erectile function in some patients (Stephenson et al., 2004b). Newer reports of robotic-assisted salvage prostatectomy are showing promising results (Boris et al., 2009).
Salvage Cryotherapy
This therapy has recently emerged as an alternative less invasive procedure in the treatment of localized BCR with a 5-year BRFS of 42% (Izawa et al., 2002). The oncologic efficacy of salvage cryotherapy was more pronounced in low-risk patients (BRFS = 73%) than in intermediate (BRFS = 45%) or high-risk patients (BRFS = 11%; Ismail et al., 2007). Salvage cryotherapy is associated with a significant complications, including up to 73% of urinary incontinence and 72% of erectile dysfunction (Pisters et al., 1997).
Androgen Deprivation Therapy
Systemic options are centered around the use of ADT as monotherapy (Cooperberg et al., 2003). ADT is associated with an initial dramatic shrinkage of tumors, long-term control of serum PSA and significant relief of symptoms for metastatic disease. A majority of patients with BCR are managed by ADT, including 60% of patients who underwent primary RP and 94% of patients who received primary RT (Agarwal et al., 2008). However, the use of ADT for non-metastatic BCR-only disease is controversial, as it is neither benign nor associated with an advantage in overall survival. The side effects from prolonged ADT is associated with potentially significant hot flashes, increased bone turnover, osteoporosis, loss of muscle mass, and increased fracture risk, sexual dysfunction, and loss of libido, memory loss, increased fat deposition, altered lipid profiles, and a significantly increased risk of cardiovascular events and cardiovascular morbidity (Saigal et al., 2007; Tsai et al., 2007). These side effects have prompted evaluation of either delaying initiation of ADT until either metastases or a pre-defined serum PSA level could both offer the benefits of ADT and minimize the potential side effects. Evaluation of early versus delayed ADT in 1352 men with BCR showed a delay in clinical progression with early ADT only in men with high-risk features (Gleason ≥8 or PSA-DT ≤10 months; Moul et al., 2004). Alternatively, the use of intermittent ADT in men with BCR may offer both PSA control and minimize side effects, with ADT initiated or reinitiated at pre-defined PSA levels. In order to prevent the potentially crippling skeletal complications associated with ADT (Saad et al., 2004), Zoledronic acid (Zometa), and Denosumab (Xgeva) may be used in patients with metastatic disease, while Denosumab (Prolia) may be used in patients with non-metastatic BCR.
Conclusion
The management of patients with BCR after definitive local therapy to the prostate is still not well defined and complex. Most men with BCR will DOCs than their disease. Currently, the majority of these patients are treated with ADT, despite its significant detrimental side effects. Critical evaluation of these patients and the risk-benefit ratio of each intervention must be performed to ascertain their optimal individualized management.
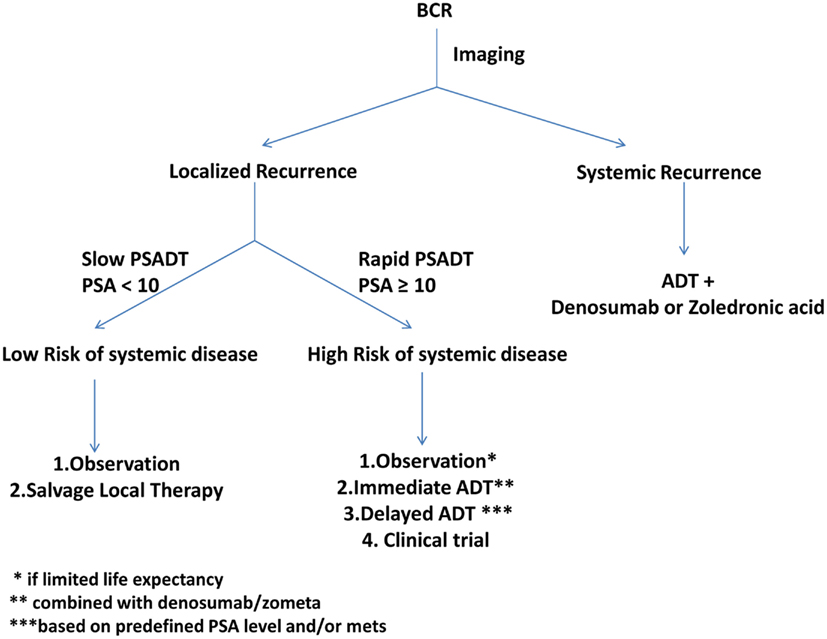
An algorithm describing our approach to patients with BCR is detailed (Figure 2). Patients with radiographically localized BCR and low-risk of failure of salvage therapies should be identified using available nomograms and offered the choice of either observation or a potentially curable salvage local therapy. Patients at high-risk for failure of salvage therapies should be offered either immediate ADT or delayed ADT upon either a predefined PSA value or metastasis. Alternatively, these patients should be offered a clinical trial.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agarwal, P. K., Sadetsky, N., Konety, B. R., Resnick, M. I., Carroll, P. R., and Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). (2008). Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 112, 307–314.
American Society for Therapeutic Radiology and Oncology Consensus Panel Consensus statement: guidelines for PSA following radiation therapy. (1997). Int. J. Radiat. Oncol. Biol. Phys. 37, 1035–1041.
Amling, C. L., Bergstralh, E. J., Blute, M. L., Slezak, J. M., and Zincke, H. (2001). Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J. Urol. 165, 1146–1151.
Amling, C. L., Lerner, S. E., Martin, S. K., Slezak, J. M., Blute, M. L., and Zincke, H. (1999). Deoxyribonucleic acid ploidy and serum prostate specific antigen predict outcome following salvage prostatectomy for radiation refractory prostate cancer. J. Urol. 161, 857–862; discussion 862–863.
Aus, G., Heidenreich, A., Bolla, M., Joniau, S., Matveev, V. B., Schmid, H. P., and Zattoni, F. (2005). EAU guidelines on prostate cancer. Eur. Urol. 48, 546–551.
Beheshti, M., Vali, R., Waldenberger, P., Fitz, F., Nader, M., Hammer, J., Loidl, W., Pirich, C., Fogelman, I., and Langsteger, W. (2010). The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol. Imaging Biol. 12, 98–107.
Bianco, F. J. Jr., Scardino, P. T., Stephenson, A. J., Diblasio, C. J., Fearn, P. A., and Eastham, J. A. (2005). Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 62, 448–453.
Bolla, M. (2005). Does adjuvant androgen suppression after radiotherapy for prostate cancer improve long-term outcomes? Nat. Clin. Pract. Urol. 2, 536–537.
Boris, R. S., Bhandari, A., Krane, L. S., Eun, D., Kaul, S., and Peabody, J. O. (2009). Salvage robotic-assisted radical prostatectomy: initial results and early report of outcomes. BJU Int. 103, 952–956.
Cheng, L., Sebo, T. J., Slezak, J., Pisansky, T. M., Bergstralh, E. J., Neumann, R. M., Iczkowski, K. A., Zincke, H., and Bostwick, D. G. (1998). Predictors of survival for prostate carcinoma patients treated with salvage radical prostatectomy after radiation therapy. Cancer 83, 2164–2171.
Cher, M. L., Bianco, F. J. Jr., Lam, J. S., Davis, L. P., Grignon, D. J., Sakr, W. A., Banerjee, M., Pontes, J. E., and Wood, D. P. Jr. (1998). Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J. Urol. 160, 1387–1391.
Coakley, F. V., Teh, H. S., Qayyum, A., Swanson, M. G., Lu, Y., Roach, M. III, Pickett, B., Shinohara, K., Vigneron, D. B., and Kurhanewicz, J. (2004). Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology 233, 441–448.
Cooperberg, M. R., Grossfeld, G. D., Lubeck, D. P., and Carroll, P. R. (2003). National practice patterns and time trends in androgen ablation for localized prostate cancer. J. Natl. Cancer Inst. 95, 981–989.
Cowen, M. E., Halasyamani, L. K., and Kattan, M. W. (2006). Predicting life expectancy in men with clinically localized prostate cancer. J. Urol. 175, 99–103.
Crook, J. M., Choan, E., Perry, G. A., Robertson, S., and Esche, B. A. (1998). Serum prostate-specific antigen profile following radiotherapy for prostate cancer: implications for patterns of failure and definition of cure. Urology 51, 566–572.
D’Amico, A. V., Chen, M. H., Roehl, K. A., and Catalona, W. J. (2005). Identifying patients at risk for significant versus clinically insignificant postoperative prostate-specific antigen failure. J. Clin. Oncol. 23, 4975–4979.
D’Amico, A. V., Moul, J. W., Carroll, P. R., Sun, L., Lubeck, D., and Chen, M.-H. (2003). Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J. Natl. Cancer Inst. 95, 1376–1383.
Djavan, B., Milani, S., and Fong, Y. K. (2005). Benign positive margins after radical prostatectomy means a poor prognosis – pro. Urology 65, 218–220.
Djavan, B., Moul, J. W., Zlotta, A., Remzi, M., and Ravery, V. (2003). PSA progression following radical prostatectomy and radiation therapy: new standards in the new millennium. Eur. Urol. 43, 12–27.
Freedland, S. J., Humphreys, E. B., Mangold, L. A., Eisenberger, M., Dorey, F. J., Walsh, P. C., and Partin, A. W. (2005). Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 294, 433–439.
Garzotto, M., and Wajsman, Z. (1998). Androgen deprivation with salvage surgery for radiorecurrent prostate cancer: results at 5-year followup. J. Urol. 159, 950–954; discussion 954–955.
Hanlon, A. L., Pinover, W. H., Horwitz, E. M., and Hanks, G. E. (2001). Patterns and fate of PSA bouncing following 3D-CRT. Int. J. Radiat. Oncol. Biol. Phys. 50, 845–849.
Ismail, M., Ahmed, S., Kastner, C., and Davies, J. (2007). Salvage cryotherapy for recurrent prostate cancer after radiation failure: a prospective case series of the first 100 patients. BJU Int. 100, 760–764.
Izawa, J. I., Madsen, L. T., Scott, S. M., Tran, J. P., McGuire, E. J., Von Eschenbach, A. C., and Pisters, L. L. (2002). Salvage cryotherapy for recurrent prostate cancer after radiotherapy: variables affecting patient outcome. J. Clin. Oncol. 20, 2664–2671.
Jhaveri, F. M., Zippe, C. D., Klein, E. A., and Kupelian, P. A. (1999). Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology 54, 884–890.
Khan, M. A., Han, M., Partin, A. W., Epstein, J. I., and Walsh, P. C. (2003). Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology 62, 86–91; discussion 91–92.
Kim, B. S., Lashkari, A., Vongtama, R., Lee, S. P., and Parker, R. G. (2004). Effect of pelvic lymph node irradiation in salvage therapy for patients with prostate cancer with a biochemical relapse following radical prostatectomy. Clin. Prostate Cancer 3, 93–97.
Kim, C. K., Park, B. K., and Lee, H. M. (2009). Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion-weighted MRI. J. Magn. Reson. Imaging 29, 391–397.
King, C. R., Presti, J. C. Jr., Gill, H., Brooks, J., and Hancock, S. L. (2004). Radiotherapy after radical prostatectomy: does transient androgen suppression improve outcomes? Int. J. Radiat. Oncol. Biol. Phys. 59, 341–347.
Koppie, T. M., Grossfeld, G. D., Nudell, D. M., Weinberg, V. K., and Carroll, P. R. (2001). Is anastomotic biopsy necessary before radiotherapy after radical prostatectomy? J. Urol. 166, 111–115.
Molinie, V., Mahjoub, W. K., and Balaton, A. (2008). Histological modifications observed in prostate after preserving treatments for prostate cancer and their impact on Gleason score interpretation. Ann. Pathol. 28, 363–373.
Moul, J. W., Wu, H., Sun, L., McLeod, D. G., Amling, C., Donahue, T., Kusuda, L., Sexton, W., O’Reilly, K., Hernandez, J., Chung, A., and Soderdahl, D. (2004). Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J. Urol. 171, 1141–1147.
Nguyen, P. L., D’Amico, A. V., Lee, A. K., and Suh, W. W. (2007). Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer 110, 1417–1428.
Novo, J. F., López, S. P., Aguiló, F. L., and Miranda, E. F. (2006). Diagnostic methodology for the biochemical recurrence of prostate cancer after brachytherapy. Arch. Esp. Urol. 59, 1063–1067.
Nudell, D. M., Wefer, A. E., Hricak, H., and Carroll, P. R. (2000). Imaging for recurrent prostate cancer. Radiol. Clin. North Am. 38, 213–229.
Partin, A. W., and Oesterling, J. E. (1994). The clinical usefulness of prostate specific antigen: update 1994. J. Urol. 152(Pt 1), 1358–1368.
Pisters, L. L., von Eschenbach, A. C., Scott, S. M., Swanson, D. A., Dinney, C. P., Pettaway, C. A., and Babaian, R. J. (1997). The efficacy and complications of salvage cryotherapy of the prostate. J. Urol. 157, 921–925.
Pound, C. R., Partin, A. W., Eisenberger, M. A., Chan, D. W., Pearson, J. D., and Walsh, P. C. (1999). Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597.
Rinnab, L., Simon, J., Hautmann, R. E., Cronauer, M. V., Hohl, K., Buck, A. K., Reske, S. N., and Mottaghy, F. M. (2009). [(11)C]choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J. Urol. 27, 619–625.
Roach, M. III, Hanks, G., Thames, H. Jr., Schellhammer, P., Shipley, W. U., Sokol, G. H., and Sandler, H. (2006). Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 65, 965–974.
Rosser, C. J., Kuban, D. A., Levy, L. B., Chichakli, R., Pollack, A., Lee, A. K., and Pisters, L. L. (2002). Prostate specific antigen bounce phenomenon after external beam radiation for clinically localized prostate cancer. J. Urol. 168, 2001–2005.
Rouviere, O., Valette, O., Grivolat, S., Colin-Pangaud, C., Bouvier, R., Chapelon, J. Y., Gelet, A., and Lyonnet, D. (2004). Recurrent prostate cancer after external beam radiotherapy: value of contrast-enhanced dynamic MRI in localizing intraprostatic tumor – correlation with biopsy findings. Urology 63, 922–927.
Saad, F., Olsson, C., and Schulman, C. C. (2004). Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur. Urol. 46, 731–739; discussion 739–740.
Saigal, C. S., Gore, J. L., Krupski, T. L., Hanley, J., Schonlau, M., Litwin, M. S., and Urologic Diseases in America Project. (2007). Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer 110, 1493–500.
Sanderson, K. M., Penson, D. F., Cai, J., Groshen, S., Stein, J. P., Lieskovsky, G., and Skinner, D. G. (2006). Salvage radical prostatectomy: quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J. Urol. 176, 2025–2031; discussion 2031–2032.
Scher, H. I., Eisenberger, M., D’Amico, A. V., Halabi, S., Small, E. J., Morris, M., Kattan, M. W., Roach, M., Kantoff, P., Pienta, K. J., Carducci, M. A., Agus, D., Slovin, S. F., Heller, G., Kelly, W. K., Lange, P. H., Petrylak, D., Berg, W., Higano, C., Wilding, G., Moul, J. W., Partin, A. N., Logothetis, C., and Soule, H. R. (2004). Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J. Clin. Oncol. 22, 537–556.
Sengoz, M., Abacioglu, U., Cetin, I., and Turkeri, L. (2003). PSA bouncing after external beam radiation for prostate cancer with or without hormonal treatment. Eur. Urol. 43, 473–477.
Spiotto, M. T., Hancock, S. L., and King, C. R. (2007). Radiotherapy after prostatectomy: improved biochemical relapse-free survival with whole pelvic compared with prostate bed only for high-risk patients. Int. J. Radiat. Oncol. Biol. Phys. 69, 54–61.
Stephenson, A. J., Scardino, P. T., Kattan, M. W., Pisansky, T. M., Slawin, K. M., Klein, E. A., Anscher, M. S., Michalski, J. M., Sandler, H. M., Lin, D. W., Forman, J. D., Zelefsky, M. J., Kestin, L. L., Roehrborn, C. G., Catton, C. N., DeWeese, T. L., Liauw, S. L., Valicenti, R. K., Kuban, D. A., and Pollack, A. (2007). Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 25, 2035–2041.
Stephenson, A. J., Shariat, S. F., Zelefsky, M. J., Kattan, M. W., Butler, E. B., Teh, B. S., Klein, E. A., Kupelian, P. A., Roehrborn, C. G., Pistenmaa, D. A., Pacholke, H. D., Liauw, S. L., Katz, M. S., Leibel, S. A., Scardino, P. T., and Slawin, K. M. (2004a). Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 291, 1325–1332.
Stephenson, A. J., Scardino, P. T., Bianco, F. J. Jr., and Eastham, J. A. (2004b). Salvage therapy for locally recurrent prostate cancer after external beam radiotherapy. Curr. Treat. Options Oncol. 5, 357–365.
Stephenson, A. J., Stephenson, A. J., Kattan, M. W., Eastham, J. A., Dotan, Z. A., Bianco, F. J. Jr., Lilja, H., and Scardino, P. T. (2006). Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J. Clin. Oncol. 24, 3973–3978.
Sugimura, K., Carrington, B. M., Quivey, J. M., and Hricak, H. (1990). Postirradiation changes in the pelvis: assessment with MR imaging. Radiology 175, 805–813.
Thomas, C. T., Bradshaw, P. T., Pollock, B. H., Montie, J. E., Taylor, J. M., Thames, H. D., McLaughlin, P. W., DeBiose, D. A., Hussey, D. H., and Wahl, R. L. (2003). Indium-111-capromab ‘pendetide radioimmunoscintigraphy and prognosis for durable biochemical response to salvage radiation therapy in men after failed prostatectomy. J. Clin. Oncol. 21, 1715–1721.
Trapasso, J. G., deKernion, J. B., Smith, R. B., and Dorey, F. (1994). The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J. Urol. 152(Pt 2), 1821–1825.
Tsai, H. K., D’Amico, A. V., Sadetsky, N., Chen, M.-H., and Carroll, P. R. (2007). Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J. Natl. Cancer Inst. 99, 1516–1524.
Ward, J. F., Blute, M. L., Slezak, J., Bergstralh, E. J., and Zincke, H. (2003). The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J. Urol. 170, 1872–1876.
Ward, J. F., Sebo, T. J., Blute, M. L., and Zincke, H. (2005). Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J. Urol. 173, 1156–1160.
Keywords: prostate cancer, radical prostatectomy, radiation therapy, biochemical recurrence, PSA recurrence, salvage
Citation: Darwish OM and Raj GV (2012) Management of biochemical recurrence after primary localized therapy for prostate cancer. Front. Oncol. 2:48. doi: 10.3389/fonc.2012.00048
Received: 19 March 2012; Accepted: 30 April 2012;
Published online: 23 May 2012.
Edited by:
Gennady Bratslavsky, SUNY Upstate Medical University, USAReviewed by:
Jonathan Silberstein, Memorial Sloan-Kettering Cancer Center, USAUlka N. Vaishampayan, Karmanos Cancer Institute/Wayne State University, USA
Copyright: © 2012 Darwish and Raj. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ganesh V. Raj, Department of Urology, The University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd., Dallas, TX 75390-9110, USA. e-mail: ganesh.raj@utsouthwestern.edu
 Oussama M. Darwish
Oussama M. Darwish