- 1Department of Radiation Oncology, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA
- 2Department of Cardiothoracic Surgery, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA
Purpose: Locally recurrent non-small cell lung cancer (LR-NSCLC) remains challenging to treat, particularly in patients having received prior radiotherapy. Heterogeneous populations and varied treatment intent in existing literature result in significant limitations in evaluating efficacy of lung re-irradiation. In order to better establish the impact of re-irradiation in patients with LR-NSCLC following high-dose radiotherapy, we report outcomes for patients treated with prior sublobar resection and brachytherapy that subsequently underwent stereotactic body radiotherapy (SBRT).
Methods: A retrospective review of patients initially treated with sublobar resection and I125 vicryl mesh brachytherapy, who later developed LR-NSCLC along the suture line, was performed. Patients received salvage SBRT with curative intent. Dose and fractionation were based on tumor location and size, with a median prescription dose of 48 Gy in 4 fractions (range 20–60 Gy in 1–4 fractions).
Results: Thirteen consecutive patients were identified with median follow-up of 2.1 years (range 0.7–5.6 years). Two in-field local failures occurred at 7.5 and 11.1 months, resulting in 2-year local control of 83.9% (95% CI, 63.5–100.0%). Two-year disease-free survival and overall survival estimates were 38.5% (95% CI, 0.0–65.0%) and 65.8% (95% CI, 38.2–93.4%). Four patients (31%) remained disease-free at last follow-up. All but one patient who experienced disease recurrence developed isolated or synchronous distant metastases. Only one patient (7.7%) developed grade ≥3 toxicity, consisting of grade 3 esophageal stricture following a centrally located recurrence previously treated with radiofrequency ablation.
Conclusion: Despite high-local radiation doses delivered to lung parenchyma previously with I125 brachytherapy, re-irradiation with SBRT for LR-NSCLC results in excellent local control with limited morbidity, allowing for potential disease cure in a subset of patients.
Introduction
Improved access to computed tomography (CT) and adoption of screening with low-dose CT, which has been proven to reduce lung cancer mortality, has led to greater detection of earlier stage lung cancers in a high-risk population (1, 2). Despite this improvement in screening, approximately 25% of patients with early stage non-small cell lung cancer (NSCLC) have poor pulmonary function, limiting their ability to tolerate lobectomy (3). To avoid the survival detriment seen with untreated NSCLC, potentially curative alternatives for this medically high-risk population include stereotactic body radiotherapy (SBRT), hypofractionated conventional radiotherapy, radiofrequency ablation, and sublobar resection (4–8). Historical data suggested sublobar resection resulted in inferior local control as compared to lobectomy, leading to integration of I125 vicryl mesh brachytherapy to reduce this risk (9, 10).
Recently published results from a randomized trial demonstrate a low rate of local relapse altogether, resulting in no demonstrated benefit to vicryl mesh brachytherapy following sublobar resection (11). Nonetheless, local relapse for patients treated with prior brachytherapy or high-dose radiotherapy such as SBRT has limited salvage options following locally recurrent disease due to concerns of toxicity with lung re-irradiation coupled with poor pulmonary reserve. Without effective salvage therapy, locoregional recurrence often results in death (12, 13). Re-irradiation with SBRT or EBRT has been previously evaluated with varying results regarding both toxicity and clinical outcomes (14–22).
No published data exist regarding treatment of patients following vicryl mesh brachytherapy, where greater concern for necrosis and pneumonitis theoretically may exist due to high-local doses. Here, we report outcomes and toxicities from a subset of patients with locally recurrent NSCLC following sublobar resection and I125 vicryl mesh brachytherapy treated with salvage SBRT.
Materials and Methods
Following Institutional Review Board approval, a retrospective review was conducted for patients with NSCLC treated with SBRT at the University of Pittsburgh Cancer Institute. Patients included previously received sublobar resection with I125 vicryl mesh brachytherapy for a primary NSCLC, later developing local recurrence adjacent to the brachytherapy mesh. All patients received re-irradiation using SBRT with varying fractionation regimens, based on the proximity of critical structures and at discretion of the treating physician. Re-irradiation was defined by the relation of the planning target volume (PTV) to the vicryl mesh, such that the PTV was within 1 cm from the mesh.
At the time of recurrence, patients underwent either CT or 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/CT (PET/CT) for re-staging and/or radiation treatment planning. Patients with biopsy-confirmed or radiographic nodal or distant metastases were excluded. Determination of recurrent NSCLC was either histologically proven or radiographically defined based on morphology and/or serial imaging.
Patients received SBRT through various platforms: CyberKnife™ (Accuray, Inc., Sunnyvale, CA, USA), Trilogy™ (Varian Medical Systems, Palo Alto, CA, USA), or TrueBeam™ (Varian Medical Systems). Treatment simulation consisted of a four-dimensional high-resolution CT scan (4DCT) with intravenous contrast if medically feasible. A custom BodyFIX™ vacuum bag (Elekta AB, Stockholm, Sweden) was used for immobilization. Respiratory gating was then utilized for TrueBeam™ or Trilogy™ treatment based on tumor motion, with a cut-off of >5 mm in any dimension on raw phase images to indicate the need for gating. The Synchrony™ Respiratory Tracking System (Accuray, Inc.) was utilized for real-time tracking with CyberKnife™, in conjunction with pre-placed fiducials.
Treatment planning in either MultiPLAN™ (Accuray, Inc.) or Eclipse (Varian Medical Systems) was completed, identifying the gross tumor volume (GTV) on end-exhalation or free breathing CT simulation scans based on the need for gating. Tumors treated on the CyberKnife™ platform had PTV expansions of 1 cm in the craniocaudal direction and 0.5 cm radially similar to that in Radiation Therapy Oncology Group (RTOG) 0236 (4). For TrueBeam™ or Trilogy™ treatment, a minimum expansion of 5 mm was added for a PTV, incorporating an additional margin for tumor motion assessed on 4DCT. Typically, an incorporated internal target volume (ITV) involved adding the extent of motion within the gated window to the minimum PTV margin in the direction of movement (23). Given variations in fractionation regimens, dosimetric constraints varied although at least 95% of the PTV was expected to be covered by the prescription dose. Treatment was delivered every other day.
Follow-up imaging consisted of CT or PET/CT at intervals based on physician discretion, initially starting 8–12 weeks from completion of SBRT. Criteria for local failure were based on the RTOG 0236 definition: ≥20% increase in greatest dimension per CT and evidence of tumor viability via FDG-avidity or histologic confirmation (4). Regional failure included hilar, mediastinal, and/or supraclavicular nodal failure. All other failures, including the contralateral lung, were coded as distant metastases unless a new solitary lung lesion was present, suggestive of a new primary lung cancer. Common Terminology Criteria for Adverse Events (version 4.03) was used to record toxicity.
Statistical analysis was conducted using SPSS version 22 (IBM Corp., Armonk, NY, USA). Kaplan–Meier methods were used to assess local control, distant-metastasis free survival, disease-free survival, and overall survival. Log-rank test was conducted to assess factors associated with the various treatment outcomes. Biological effective doses (BEDs) were calculated using the linear-quadratic equation with an α/β value of 10 for tumor. For descriptive purposes, BED values were converted to equivalent dose at 2 Gy (EQD2) when discussing toxicity.
Results
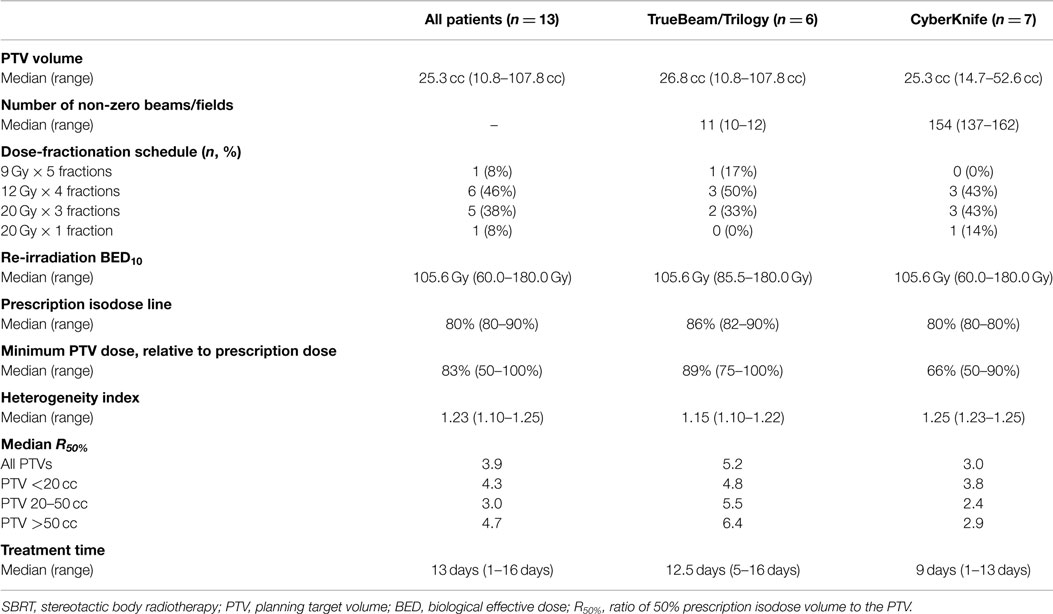
Thirteen patients were identified with recurrent NSCLC along the brachytherapy mesh, of which nine patients (69%) had histologic confirmation (Table 1). Recurrence occurred at a median of 3.8 years from initial diagnosis (range 0.9–9.5 years). Despite a median age of 71 years, the median Karnofsky performance status score was 90% (range 60–100%). The right upper (38%) and left upper (31%) lobes were the most common locations, with most recurrences located >2 cm from the central bronchial tree (69%). Patients were treated using either TrueBeam/Trilogy (46%) or CyberKnife (54%). The most common fractionation schemes were 48 Gy in 4 fractions (46%) or 60 Gy in 3 fractions (38%), resulting in a median BED10 of 105.6 Gy (Table 2).
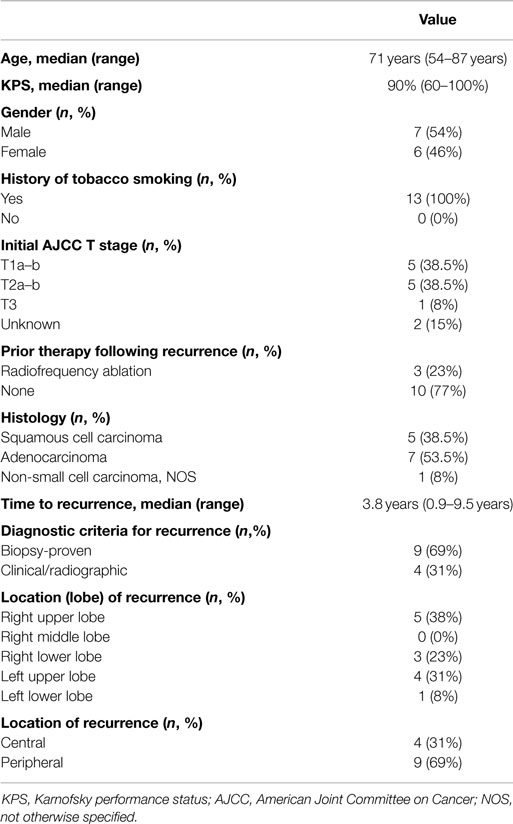
Table 1. Patient and disease-related characteristics at the time of stereotactic body re-irradiation.
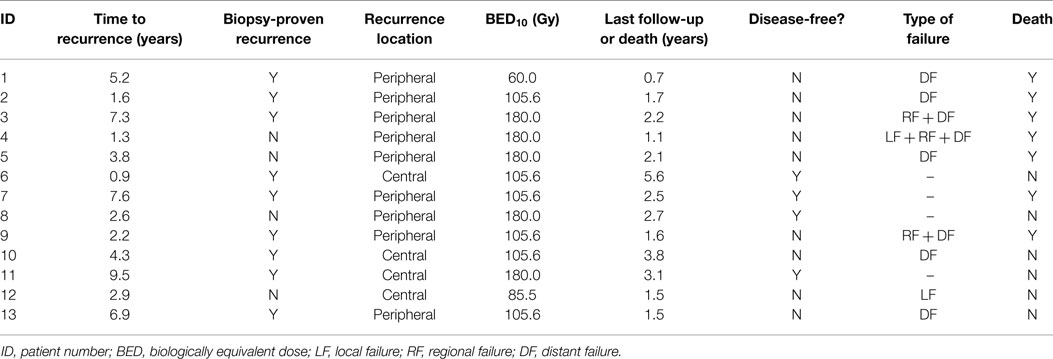
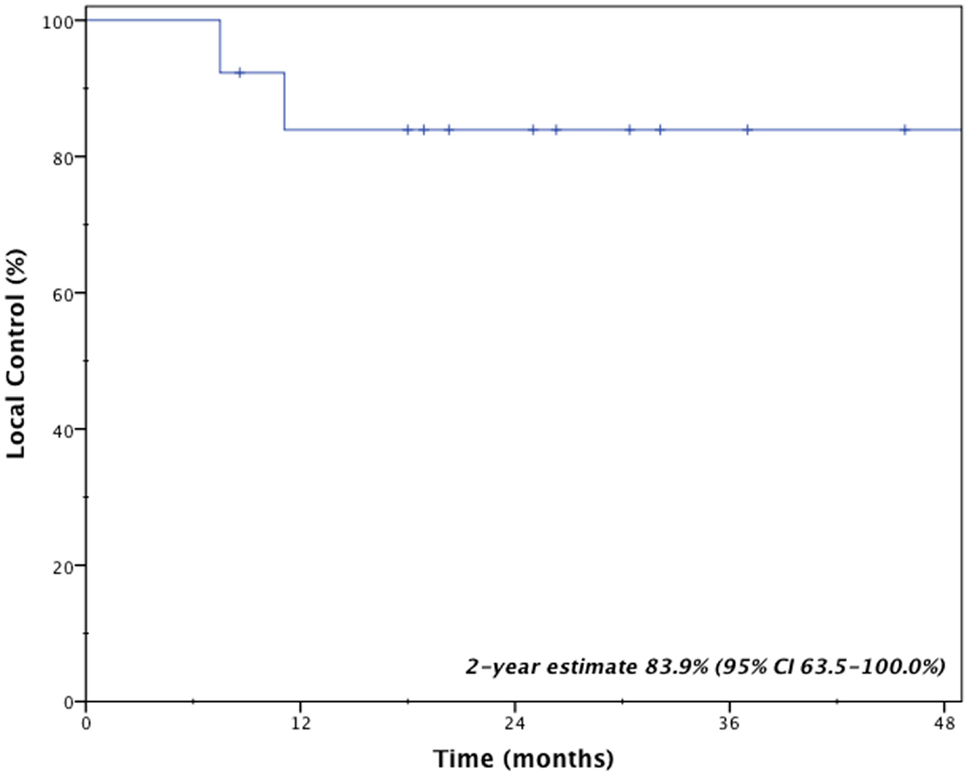
Clinical outcomes are indicated in Table 3. With a median follow-up time of 2.1 years (range 0.7–5.6 years), two patients (15.4%) developed local failure, one with isolated local failure and the other patient with simultaneous local, regional, and distant failure. Both local recurrences occurred within the planning target volumes at 7.5 and 11.1 months after receiving a BED10 of 85.5 and 180.0 Gy, respectively. Re-irradiation planning treatment volumes for these two patients were 16.6 and 25.3 cc. The 2-year Kaplan–Meier estimated local control rate was 83.9% (95% CI, 63.5–100.0%; Figure 1). No factors were found to be associated with local control, including PTV volume, BED10, time to recurrence or tumor location.
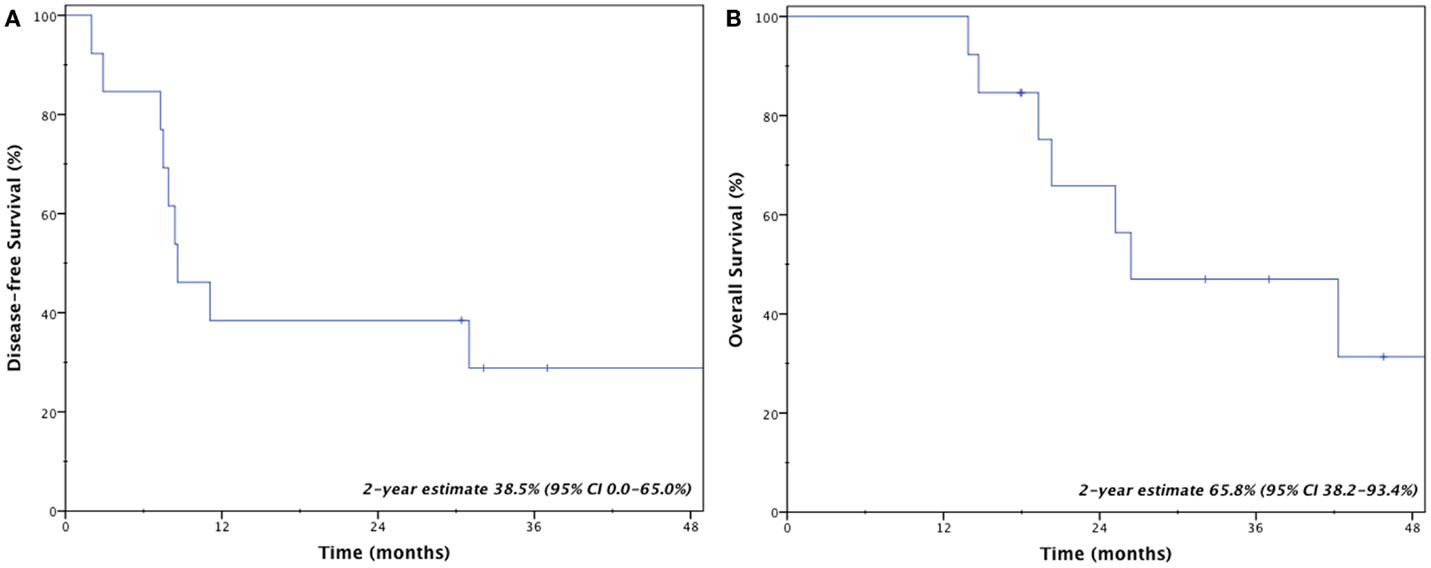
Four patients (31%) remain disease-free at last follow-up; three patients (23%) are both alive and disease-free. Crude rates of disease recurrence were as follows: isolated local (n = 1, 7.7%); synchronous local, regional, and distant (n = 1, 7.7%); synchronous regional and distant (n = 2, 15.4%); and isolated distant (n = 5, 38.5%). Two-year estimates for disease-free survival and overall survival were 38.5% (95% CI, 0.0–65.0%) and 65.8% (95% CI, 38.2–93.4%), respectively (Figure 2). Median overall survival was 26.4 months.
No patients developed grade 3 or greater pulmonary toxicity, including lung fibrosis and pneumonitis. However, two patients (15.4%) did develop grade 2 fibrosis and grade 2 dyspnea at 9.4 and 10.1 months after treatment. No grade 2 esophageal toxicities were seen. One patient (7.7%) developed a grade 3 esophageal stricture at 3.1 months after treatment requiring endoscopic dilatation. Of particular note, this patient had a central tumor recurrence treated with radiofrequency ablation prior to SBRT. Given proximity to central structures, the patient received 45 Gy in 5 fractions, although the maximal point dose to the esophagus was 38.8 Gy (7.8 Gy/fraction), resulting in an EQD2 of 83.8 Gy.
Discussion
Management of locally recurrent non-small cell lung cancer (LR-NSCLC) remains challenging due to limitations from prior therapy and presence of medical comorbidities that often preclude aggressive therapy. For this reason, less invasive therapies with limited risk of morbidity are often ideal. Stereotactic body radiotherapy provides such benefits and enables the ability to deliver conformal and high doses to tumors. However, for patients who received prior high-dose radiotherapy, concern always exists regarding added toxicity from re-irradiation. Results presented here suggest that even with previous high-local doses to normal lung from brachytherapy, salvage SBRT resulted in limited toxicity and provided an efficacious salvage option for locally recurrent lung cancer. Specifically, the 2-year local control rate remained high at 83.9% with a median survival of 26.4 months.
Clinical outcomes following re-irradiation of LR-NSCLCs have been difficult to interpret due to heterogeneous populations and loose definitions of re-irradiation. For example, among 11 studies reviewed by Jeremic et al., only 3 trials delivered external beam re-irradiation using curative doses (median dose ≥50 Gy) (15). Nonetheless, local control remains limited with external beam re-irradiation, ranging from 16.7 to 42.0% in these trials (14, 24, 25). In a recently published larger cohort of 102 patients, McAvoy et al. identified 41 patients with locoregional recurrence within the prior radiotherapy field, among which 46% local control was achieved after re-irradiation using various modalities (26). Although many of these series included more advanced lung cancer at recurrence, re-irradiation with conventional fractionation appears to result in, at best, modest rates of local control.
Several publications have addressed feasibility and toxicity using SBRT re-irradiation for lung tumors. Many of these series are limited again by mixed treatment intent, varying definitions of re-irradiation and diverse histology and disease stage (16, 17, 19–22). Adequate estimation of long-term clinical outcomes for patients with LR-NSCLC alone within the prior radiotherapy field is therefore difficult to determine. Only two of these studies either reported separate outcomes or included only LR-NSCLC with SBRT re-irradiation defined as overlap with the prior treatment field (17, 19). Hearn et al. reported 10 patients treated with salvage SBRT, resulting in crude local control and overall survival rates of 60 and 30% (17). Parks et al. identified 29 patients treated with repeat SBRT, where 13 patients underwent re-irradiation of in-field recurrences leading to a 2-year locoregional relapse-free survival rate of 58% (19). These two studies suggest that despite a high-equivalent dose delivered using SBRT, locoregional control appears only slightly improved, if not comparable, to other radiotherapy methods. Conversely, in the present study, 2-year local control remained excellent at 83.9%. Such a finding may reflect rigorously selected patients, where many underwent PET/CT re-staging with identification of isolated local disease. Other explanations include comparably long re-treatment intervals (median time to re-irradiation 3.8 years), which may attest to disease biology and initial disease stage. Multivariate analysis of the prior study by McAvoy et al. illustrated improved local control and survival with a re-treatment interval >6 months and lower initial T stage (26). Lastly, in patients with prior brachytherapy, cell-kill mechanisms may be different from that delivered through SBRT. Thus, patients treated with prior brachytherapy may be responsive to re-irradiation using high doses per fraction (i.e., SBRT). Nonetheless, these findings, among a much more homogenous population, should indicate that in properly selected patients, re-irradiation with SBRT for locally recurrent NSCLC can provide improved local control in a shorter treatment course.
Re-irradiation, particularly using high-dose regimens such as that seen with SBRT, comes with added concerns of toxicity. In re-irradiation series using external beam radiotherapy, rates of grade 3 or greater pneumonitis and esophagitis range from 5 to 21 and 4 to 6%, respectively (15). Here, we reported selectively on patients with recurrence near brachytherapy mesh to illustrate that despite prior high-radiation doses, severe pulmonary toxicity rates remain exceedingly low in a carefully planned and well-executed schema of stereotactic radiotherapy, a more conformal technique. Lung parenchyma functions as a parallel organ and thus volume of functional lung irradiated plays a larger factor than maximum point dose. Such findings have been confirmed using external beam radiotherapy, showing that volume of lung irradiated, even at low doses, correlates with risk of pneumonitis and atelectasis (27–30). Similar dose–volume parameters have been established for stereotactic body radiotherapy (31, 32). Utilizing SBRT for re-irradiation of lung lesions limits the volume of normal lung receiving dose greater than that seen with conventional methods, resulting in low rates of severe pneumonitis as confirmed here.
In the setting of SBRT re-irradiation for lung tumors, tumor volume and central structure tolerance should have a greater impact on management decisions as opposed to concerns over high-local doses to lung parenchyma. In our study, we identified one patient who developed late grade 3 esophagitis after receiving adjacent radiofrequency ablation and a maximal point dose of 38.8 Gy (EQD2 83.8 Gy). Studies using external beam radiotherapy for re-irradiation have shown low rates of grade 3 esophagitis (4–6%), although this may be a function of tumor location (15). High doses with SBRT may be less forgiving to central mediastinal structures, as evidenced in both prospective and retrospective series (16, 21, 33). In the setting of re-irradiation, Peulen et al. noted all grade 4–5 toxicities occurred in centrally located lesions (16). Three patients developed grade 5 complications due to hemorrhage. Kilburn et al. noted one patient death due to development of an aortoesophageal fistula (21). Thus, the approach of re-irradiation using SBRT should be taken cautiously for centrally located lesions.
Our study, like many others evaluating re-irradiation, is limited by both the retrospective nature of review and small sample size. We intentionally identified a select population in order to provide a clear analysis of a comparable patient cohort as opposed to that done in a number of re-irradiation studies. Although varying fractionation regimens make direct interpretation challenging, a majority received more commonly utilized regimens (48 Gy in 4 fractions or 60 Gy in 3 fractions). Additionally, with a median follow-up time of 2.1 years, whether these favorable local control rates would persist over time remains unknown. Despite these limitations, these results should provide re-assurance that in properly selected patients with locally recurrent NSCLC, even in heavily irradiated regions, stereotactic body radiotherapy can provide excellent local control with limited morbidity, resulting in cure among a small subset of patients. In the future, better tolerated and/or targeted systemic therapy may aid in decreasing the high rate of distant metastases in this population, which remained the predominant mode of failure.
Conclusion
Stereotactic body radiotherapy for locally recurrent NSCLC following prior radiotherapy is an effective salvage therapy with limited morbidity, even despite high doses of prior radiotherapy with I125 vicryl mesh brachytherapy. Severe pulmonary parenchymal toxicity remains low with re-irradiation using SBRT, likely related to limited dose to large lung volumes. Centrally located tumors should be cautiously selected for re-irradiation using SBRT. Although a proportion of patients may achieve cure, for most patients, optimization of systemic therapy is critical to offset the risk of distant metastases.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med (2011) 365(5):395–409. doi: 10.1056/NEJMoa1102873
2. Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, et al. CT screening for lung cancer brings forward early disease. The randomised Danish lung cancer screening trial: status after five annual screening rounds with low-dose CT. Thorax (2012) 67(4):296–301. doi:10.1136/thoraxjnl-2011-200736
3. Mentzer SJ, Swanson SJ. Treatment of patients with lung cancer and severe emphysema. Chest (1999) 116(6 Suppl):477S–9S. doi:10.1378/chest.116.suppl_3.477S
4. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA (2010) 303(11):1070–6. doi:10.1001/jama.2010.261
5. Soliman H, Cheung P, Yeung L, Poon I, Balogh J, Barbera L, et al. Accelerated hypofractionated radiotherapy for early-stage non-small-cell lung cancer: long-term results. Int J Radiat Oncol Biol Phys (2011) 79(2):459–65. doi:10.1016/j.ijrobp.2009.11.003
6. Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol (2008) 9(7):621–8. doi:10.1016/S1470-2045(08)70155-4
7. Pennathur A, Luketich JD, Abbas G, Chen M, Fernando HC, Gooding WE, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg (2007) 134(4):857–64. doi:10.1016/j.jtcvs.2007.04.060
8. Landreneau RJ, Normolle DP, Christie NA, Awais O, Wizorek JJ, Abbas G, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol (2014) 32(23):2449–55. doi:10.1200/JCO.2013.50.8762
9. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung cancer study group. Ann Thorac Surg (1995) 60(3):615–22. doi:10.1016/0003-4975(95)00537-U
10. Fernando HC, Santos RS, Benfield JR, Grannis FW, Keenan RJ, Luketich JD, et al. Lobar and sublobar resection with and without brachytherapy for small stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg (2005) 129(2):261–7. doi:10.1016/j.jtcvs.2004.09.025
11. Fernando HC, Landreneau RJ, Mandrekar SJ, Nichols FC, Hillman SL, Heron DE, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol (2014) 32(23):2456–62. doi:10.1200/JCO.2013.53.4115
12. Hung JJ, Hsu WH, Hsieh CC, Huang BS, Huang MH, Liu JS, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax (2009) 64:192–6. doi:10.1136/thx.2007.094912
13. Noble J, Ellis PM, Mackay JA, Evans WK; Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol (2006) 1:1042–58. doi:10.1097/01243894-200611000-00021
14. Okamoto Y, Murakami M, Yoden E, Sasaki R, Okuno Y, Nakajima T, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys (2002) 52(2):390–6. doi:10.1016/S0360-3016(01)02644-X
15. Jeremic B, Videtic GM. Chest reirradiation with external beam radiotherapy for locally recurrent non-small-cell lung cancer: a review. Int J Radiat Oncol Biol Phys (2011) 80(4):969–77. doi:10.1016/j.ijrobp.2011.01.069
16. Peulen H, Karlsson K, Lindberg K, Tullgren O, Baumann P, Lax I, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol (2011) 101(2):260–6. doi:10.1016/j.radonc.2011.09.012
17. Hearn JW, Videtic GM, Djemil T, Stephans KL. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys (2014) 90(2):402–6. doi:10.1016/j.ijrobp.2014.05.048
18. Trakul N, Harris JP, Le QT, Hara WY, Maxim PG, Loo BW Jr, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol (2012) 7(9):1462–5. doi:10.1097/JTO.0b013e31825f22ce
19. Parks J, Kloecker G, Woo S, Dunlap NE. Stereotactic body radiation therapy as salvage for intrathoracic recurrence in patients with previously irradiated locally advanced non-small cell lung cancer. Am J Clin Oncol (2014). doi:10.1097/COC.0000000000000039
20. Seung SK, Solhjem M. Salvage SBRT for previously irradiated lung cancer. J Cancer Ther (2011) 2:190–5. doi:10.4236/jct.2011.22024
21. Kilburn JM, Kuremsky JG, Blackstock AW, Munley MT, Kearns WT, Hinson WH, et al. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol (2014) 110:505–10. doi:10.1016/j.radonc.2013.11.017
22. Kelly P, Balter PA, Rebueno N, Sharp HJ, Liao Z, Komaki R, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys (2010) 78(5):1387–93. doi:10.1016/j.ijrobp.2009.09.070
23. Zhao B, Yang Y, Li T, Li X, Heron DE, Huq MS. Image-guided respiratory-gated lung stereotactic body radiotherapy: which target definition is optimal? Med Phys (2009) 36(6):2248–57. doi:10.1118/1.3129161
24. Wu KL, Jiang GL, Qian H, Wang LJ, Yang HJ, Fu XL, et al. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. Int J Radiat Oncol Biol Phys (2003) 57(5):1345–50. doi:10.1016/S0360-3016(03)00768-5
25. Tada T, Fukuda H, Matsui K, Hirashima T, Hosono M, Takada Y, et al. Non-small-cell lung cancer: reirradiation for loco-regional relapse previously treated with radiation therapy. Int J Clin Oncol (2005) 10(4):247–50. doi:10.1007/s10147-005-0526-5
26. McAvoy S, Ciura K, Wei C, Rineer J, Liao Z, Chang JY, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys (2014) 90(4):819–27. doi:10.1016/j.ijrobp.2014.07.030
27. Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys (1999) 45(2):323–9. doi:10.1016/S0360-3016(99)00183-2
28. Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys (1998) 42(1):1–9. doi:10.1016/S0360-3016(98)00196-5
29. Lee HK, Vaporciyan AA, Cox JD, Tucker SL, Putnam JB Jr, Ajani JA, et al. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys (2003) 57(5):1317–22. doi:10.1016/S0360-3016(03)01373-7
30. Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS, Wei X, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys (2007) 68(1):94–102. doi:10.1016/j.ijrobp.2006.12.031
31. Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol (2010) 97(1):65–70. doi:10.1016/j.radonc.2010.04.027
32. Matsuo Y, Shibuya K, Nakamura M, Narabayashi M, Sakanaka K, Ueki N, et al. Dose – volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys (2012) 83(4):e545–9. doi:10.1016/j.ijrobp.2012.01.018
Keywords: SBRT, radiosurgery, re-irradiation, lung cancer, brachytherapy, non-small cell lung cancer, recurrent
Citation: Gill BS, Clump DA, Burton SA, Christie NA, Schuchert MJ and Heron DE (2015) Salvage stereotactic body radiotherapy for locally recurrent non-small cell lung cancer after sublobar resection and I125 vicryl mesh brachytherapy. Front. Oncol. 5:109. doi: 10.3389/fonc.2015.00109
Received: 10 January 2015; Accepted: 26 April 2015;
Published: 11 May 2015
Edited by:
Brian Timothy Collins, Georgetown Hospital, USAReviewed by:
Andre Konski, University of Pennsylvania, USAWenyin Shi, Thomas Jefferson University, USA
Sunyoung Jang, Princeton Radiation Oncology, USA
Charles B. Simone, University of Pennsylvania, USA
Copyright: © 2015 Gill, Clump, Burton, Christie, Schuchert and Heron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dwight E. Heron, Department of Radiation Oncology, University of Pittsburgh Cancer Institute, 5230 Centre Avenue, Pittsburgh, PA 15232, USA, herond2@upmc.edu
 Beant S. Gill
Beant S. Gill David A. Clump
David A. Clump Steven A. Burton
Steven A. Burton Neil A. Christie2
Neil A. Christie2 Dwight E. Heron
Dwight E. Heron