- 1Division of Pathology, Exploratory Oncology Research and Clinical Trial Center, National Cancer Center, Kashiwa, Japan
- 2Division of Surgical Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 3Advanced Clinical Research of Cancer, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 4Division of Surgical Oncology, The University of Tokyo, Tokyo, Japan
- 5Division of Surgical Oncology, Tokyo Medical and Dental University, Tokyo, Japan
A new histological classification of neuroendocrine tumors (NETs) was established in WHO 2010. ENET and NCCN proposed treatment algorithms for colorectal NET. Retrospective study of NET of the large intestine (colorectal and appendiceal NET) was performed among institutions allied with the Japanese Society for Cancer of the Colon and Rectum, and 760 neuroendocrine tumors from 2001 to 2011 were re-assessed using WHO 2010 criteria to elucidate the clinicopathological features of NET in the large intestine. Next, the clinicopathological relationship with lymph node metastasis was analyzed to predict lymph node metastasis in locally resected rectal NET. The primary site was rectum in 718/760 cases (94.5%), colon in 30/760 cases (3.9%), and appendix in 12/760 cases (1.6%). Patients were predominantly men (61.6%) with a mean age of 58.7 years. Tumor size was <10 mm in 65.4% of cases. Proportions of NET G1, G2, G3, and mixed adeno-neuroendocrine carcinoma (MANEC) were 88.4, 6.3, 3.9, and 1.3%, respectively. Of the 760 tumors, 468 were locally resected, and 292 were surgically resected with lymph node dissection. Rectal NET showed a higher proportion of NET G1, and colonic and appendiceal NET was more commonly G3 and MANEC. Of the 292 surgically resected cases, 233 NET G1 and G2 located in the rectum were used for the prediction of lymph node metastasis. Lymphatic and blood vessel invasion were independent predictive factors of lymph node metastasis. NET G2 cases showed more frequent lymph node metastasis than that seen in NET G1 cases, but this was not an independent predictor of lymph node metastasis. Of the 98 surgically resected cases <10 mm in size, we found 9 cases with lymph node metastasis (9.2%). All cases were NET G1, and eight of the nine cases were positive either for lymphatic invasion or blood vessel invasion. Using the WHO classification, we found NET in the large intestine showed a tumor-site-dependent variety of histological and clinicopathological features. Risk of lymph node metastasis in rectal NET was confirmed even in lesions smaller than 10 mm. Concordant assessment of vascular invasion will be required to estimate lymph node metastasis in small lesions.
Introduction
A new histological classification of neuroendocrine tumors (NETs) was determined in the WHO 2010 classification, and the UICC TNM classification seventh edition or ENET classification proposed the staging of gastrointestinal NET (1–3). NETs arise from the whole organ, and gastrointestinal NET was traditionally divided by the embryological origin of the foregut, midgut, and hindgut. Midgut and hindgut NET arise in the large intestine and the variable histologic features of NET in this organ could complicate identification even when using identical WHO 2010 classifications. Furthermore, large clinicopathological data sets of NET based on the WHO 2010 classification are not available, and the utility of the WHO 2010 classification as a prognostic factor is not well established in NET of the large intestine. Recently, clinical guidelines for gastrointestinal NET were also proposed by NCCN and ENET (4, 5). However, the indication for local excision or endoscopic resection in small lesions is not fully determined (6). Similar to early colorectal adenocarcinoma, the clinicopathological association between colorectal NET and lymph node status in surgically resected cases has been studied to predict lymph node metastasis (7, 8). In this study, retrospective assessment of NET of the large intestine (colorectal and appendiceal NET) was performed among the 68 Japanese institutions allied with the Japanese Society for Cancer of the Colon and Rectum (JSCCR) to clarify the clinicopathological features of NET in the large intestine using WHO 2010 criteria. Next, using surgically resected rectal NET G1 and G2, the clinicopathological relationship with lymph node metastasis was analyzed to assess the current therapeutic algorithm.
Materials and Methods
Pathological re-evaluations of NET according to the WHO 2010 criteria were performed in cases with NET of the large intestine removed from 2001 to 2011 in the 68 institutions of the JSCCR. The collected clinicopathological information was patient age, sex, tumor size, tumor location, pathological diagnosis based on WHO 2010 criteria, depth of tumor invasion, mitotic index [<2/10 high power fields (HPF), 2–20/10 HPF, or >20/10 HPF], Ki-67 labeling index (≤2, 3–20, or >20%), and resection method (surgical, endoscopic, or other). Information of lymph node metastasis (present or absent) was collected in all surgically resected cases, and cases underwent radical surgery after the local resection were included in resected cases. These data were registered into a Web system developed by the JSCCR. Clinical outcome or prognosis was not investigated in this study. Clinicopathological features of NET in the large intestine were evaluated, and compared among rectal, colonic, and appendiceal NET, and the association between clinicopathological features and lymph node metastasis was assessed using surgically resected rectal NET G1 and G2 to estimate risk factors of lymph node metastasis in locally resected tumors. This study was approved by the review board in the JSCCR and National Cancer Center (2014-332).
Statistical Analysis
All continuous data were described as mean and SD in this study. As therapeutic strategy of NET in large intestine is divided by tumor size of 10 and 20 mm, case number and frequency of the tumors <10, ≥10 and <20, and ≥20 mm were calculated. Comparison of clinicopathological features among rectal, colonic, and appendiceal NET was performed using the χ2-test and Student’s t-test.
Clinicopathological factors associated with lymph node metastasis in rectal NET G1 and G2 were assessed using χ2-test and Student’s t-test, and multiple logistic regression analyses were performed to identify independent predictive factors of synchronous lymph node metastasis. A probability value of <0.01 was regarded as statistically significant in this study.
Results
In this study, 760 cases from 68 institutions were entered and clinicopathological features were evaluated (Table 1). Of the 760 tumors, 718 (94.5%) were located in the rectum, 30 (3.9%) were in the colon, and 12 (1.6%) were in the appendix. In total, 461 (60.7%) patients were men and 299 (39.3%) were women, with a mean age of 58.7 years. The frequencies of NET G1, G2, NEC (G3), and mixed adeno-neuroendocrine carcinoma (MANEC) were 672 (88.4%), 48 (6.3%), 30 (3.9%), and 10 (1.3%), respectively. Of the 760 NET, 468 were locally resected, and 292 were surgically resected with lymph node dissection, which included cases who underwent radial surgery after the local resection. As for tumor size, the mean size was 11.7 mm, and 497 tumors (65.4%) were smaller than 10 mm. Most tumors were limited to the mucosa or submucosa (657 cases, 86.4%), showed mitosis in <2/10 HPF (694 cases, 91.3%), and had a Ki-67 index of ≤2% (675 cases, 88.8%). Lymphatic vessel invasion and blood vessel invasion were found in 136 (17.9%) and 173 (22.8%) cases, respectively. Lymph node metastasis was found in 108 cases of 292 surgically resected cases (37.0%). As for clinicopathologic differences among colonic, rectal, and appendiceal NETs, patient ages were different among them, significantly (P < 0.01), and were in the order of colonic (68.6 ± 11.9 years), rectal (58.6 ± 12.4 years), and appendiceal NET (44.7 ± 22.6 years). Distribution of histological grade was also different between rectal NET and others. Compared with colonic and appendiceal NET, rectal NET was predominantly NET G1 (656 cases, 91.4%). However, colonic NET included more NET G3/NEC (14 cases, 46.7%) and MANEC (6 cases, 20.0%), and appendiceal NET included more NET G3/NEC (7 cases, 58.3%). Most of the local resections were performed in rectal NET (467/468 cases, 99.8%). Tumor sizes were 9.7 ± 12.3 mm for rectal NET, 51.3 ± 37.8 mm for colonic NET, and 28.8 ± 36.2 mm for appendiceal NET; rectal NETs were significantly smaller than colonic and appendiceal NET (P < 0.01). The distribution of tumor depth is also different between rectal NET and others, and tumor depth in rectal NET was limited to the mucosa or submucosa in 684 cases (95.3%), while over half of colonic and appendiceal NET invaded the muscular layer or deeper (P < 0.01). Concordant with WHO 2010 criteria, cases with low mitotic and Ki-67 indices were seen more often in rectal NET than in others. Lymphatic vessel and blood vessel invasions were more frequently seen in colonic NET than in rectal NET. Also, lymph node metastasis in surgically resected cases was seen more frequently in colonic NET than in others, significantly (P < 0.01).
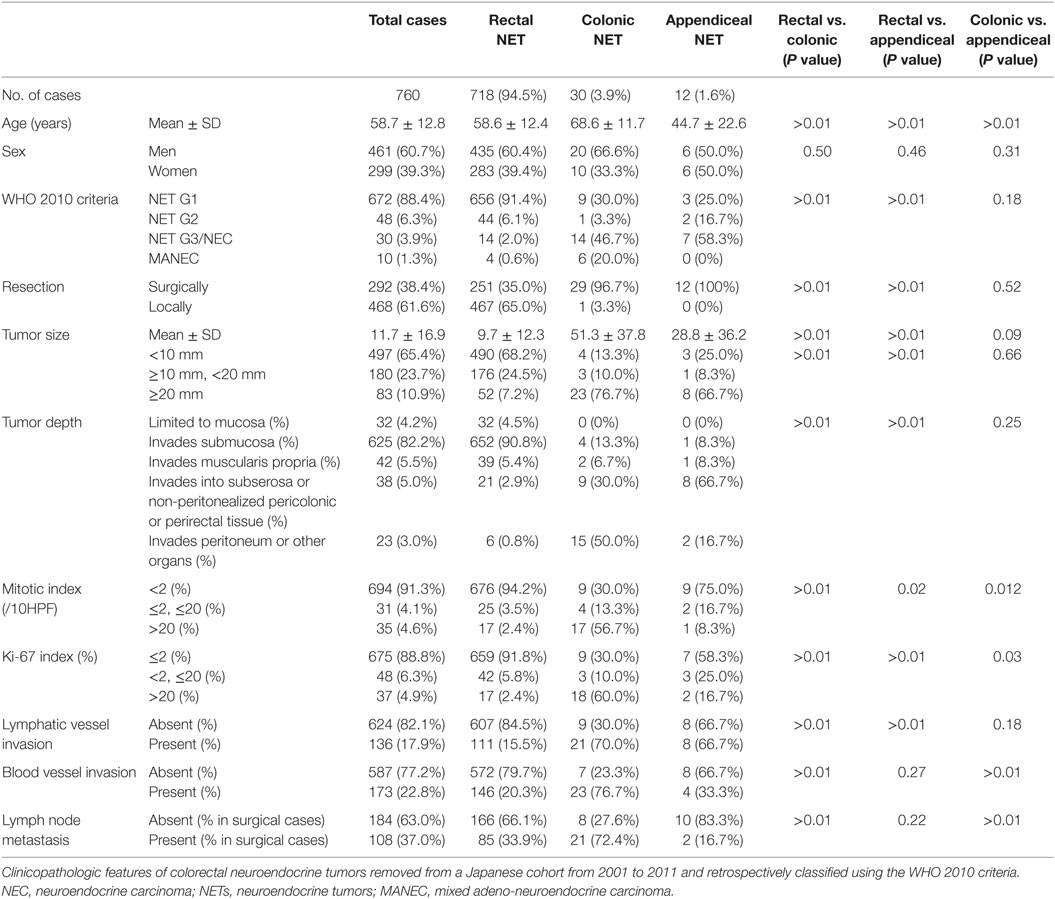
Table 1. Clinicopathological features of colorectal neuroendocrine tumor based on WHO 2010 criteria.
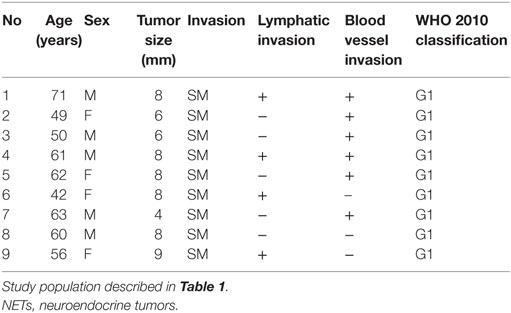
Next, we investigated the risk factor of synchronous lymph node metastasis, especially in small rectal NET G1 and G2. At first, we evaluated the association between clinicopathological features and lymph node metastasis using all 233 surgically resected rectal NET G1 and G2. Clinicopatholgoical features are shown in Table 2, and lymph node metastasis was found in 70 cases (30.0%) in these cases. Table 3 shows the association between clinicopathological features and lymph node metastasis. NET G2, large tumor size, tumor invasion into the muscularis propria or deeper, high mitotic index, high Ki-67 index, lymphatic vessel invasion, and blood vessel invasion were associated with lymph node metastasis in univariate analysis. Furthermore, lymphatic and blood vessel invasion were independent predictors of lymph node metastasis in multivariate analysis. Although NET G2 cases showed more lymph node metastasis than that seen in NET G1 cases, that was not an independent predictor of lymph node metastasis in rectal NET. Table 4 (A) shows the association between clinicopathological features and lymph node metastasis in surgically resected rectal NET G1 and G2 <20 mm. Tumor size, lymphatic invasion, and blood vessel invasion were independent predictors of lymph node metastasis. NET G2 cases tended to show more frequent lymph node metastasis, but this difference was not statistically significant. Of 489 rectal NET G1 and G2 <10 mm, 98 were surgically resected, and 391 were locally resected. The differences between them are shown in Table 5. Surgically resected cases were larger and showed more frequent lymphatic vessel and blood vessel invasion than that seen with locally resected cases, and lymph node metastasis was seen in 9 cases (9.2%) of 98 surgically resected cases. A clinicopathological comparison between cases with and without lymph node metastasis is shown in Table 4 (B). Of the clinicopathological features, although not an independent risk factor in multivariate analysis, blood vessel invasion was found more frequently in cases with lymph node metastasis in the univariate analysis. The clinicopathological features of nine cases with lymph node metastasis are shown in Table 6. All nine cases were NET G1, and the tumor depth was limited to the mucosa or submucosa. Importantly, eight of the nine cases showed either lymphatic or blood vessel invasion.
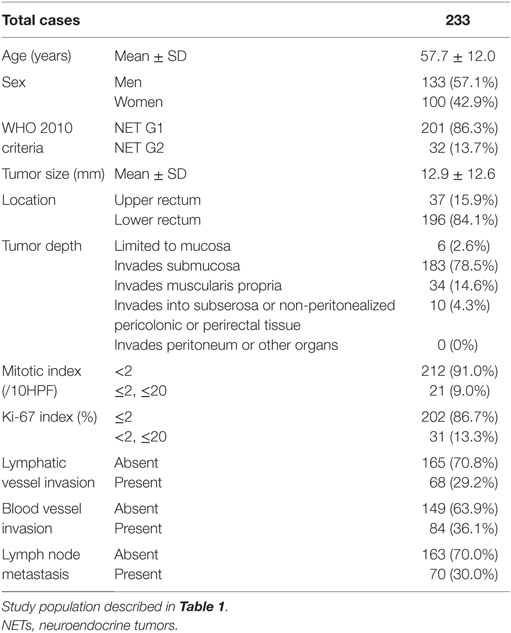
Table 2. Clinicopathological features of surgically resected rectal NET G1 and G2 based on WHO 2010 criteria.
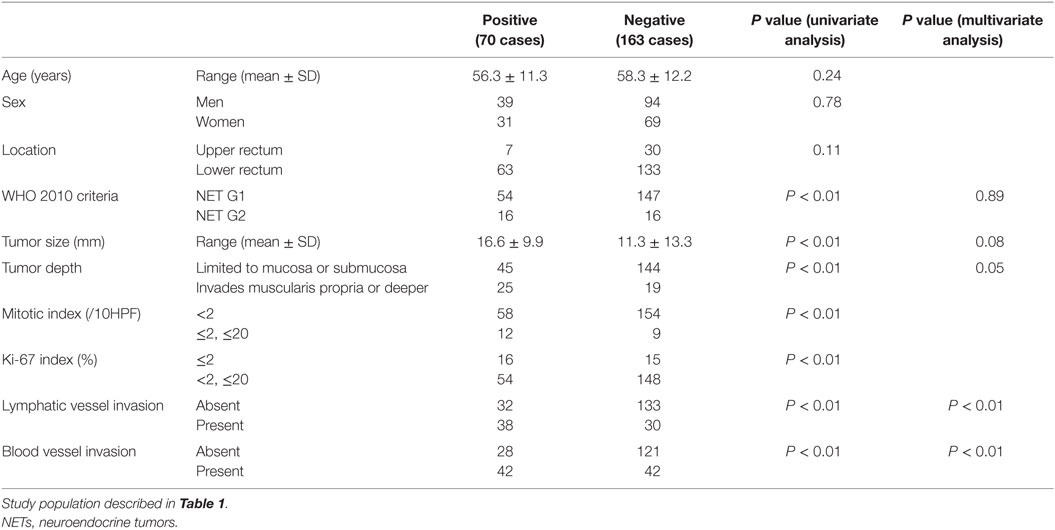
Table 3. Association between clinicopathological features and lymph node metastasis in surgically resected rectal NET G1 and G2 Lymph node metastasis.
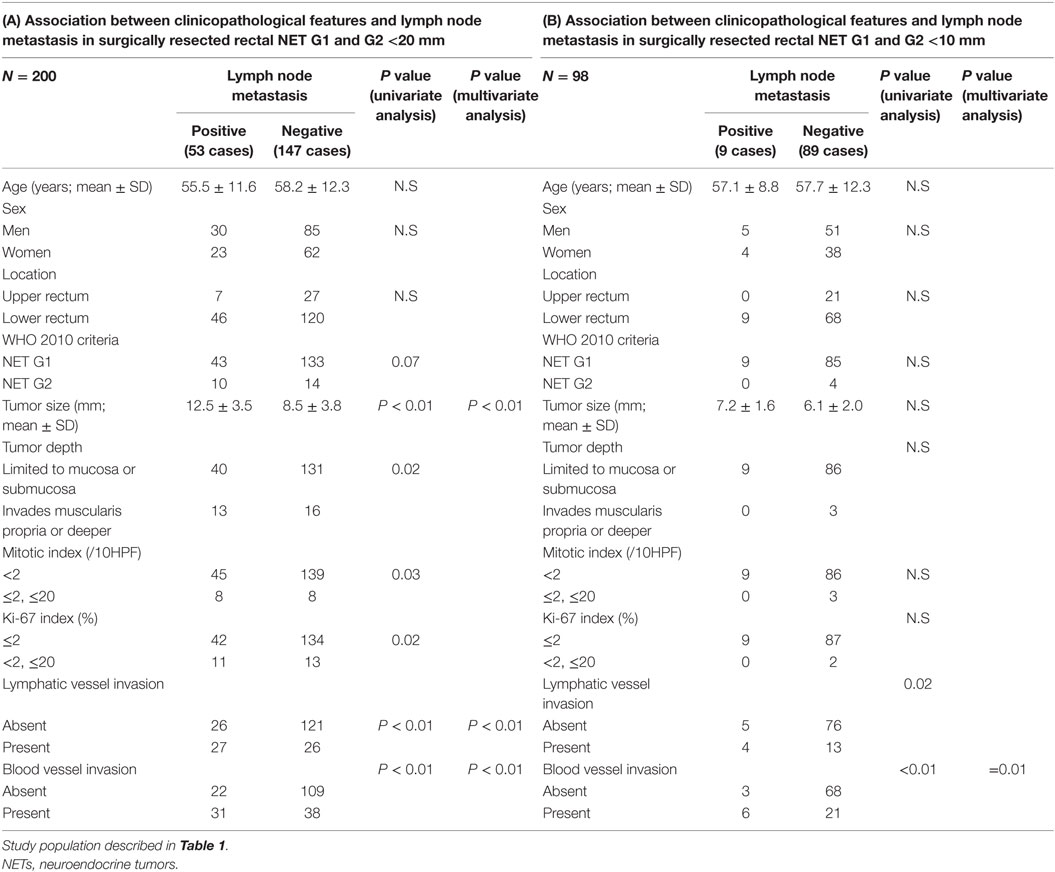
Table 4. Association between clinicopathological features and lymph node metastasis in surgically resected rectal NET G1 and G2 <20 and <10 mm.
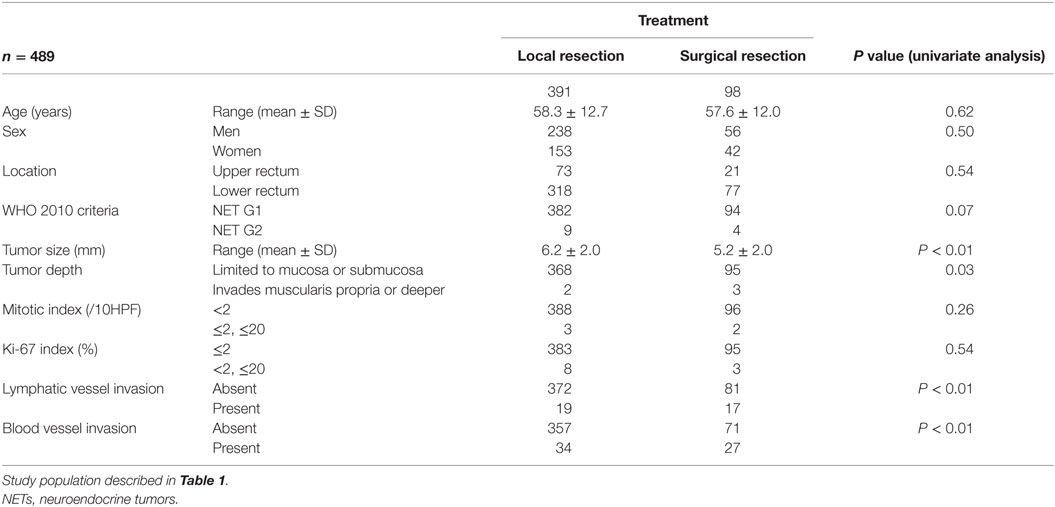
Table 5. Clinicopathological difference between locally resected and surgically resected rectal NET G1 and G2 <10 mm.
Discussion
In this study, we examined the clinicopathological association with synchronous lymph node metastasis in surgically resected rectal NET G1 and G2. Gastrointestinal NET without lymph node metastasis can be cured by local resection. Although many primary NETs themselves can be removed endoscopically, lymph node metastases can remain. Therefore, prediction of lymph node metastasis using the primary tumor is important to determine a patient’s need for surgical resection with lymph node dissection. Tumor size and tumor depth have been reported to be important for the prediction of lymph node metastasis in rectal NET (9, 10). T stage in current ENET TNM and AJCC/UICC TNM classification was determined by tumor size and depth. ENET and NCCN guidelines recommended local resection in tumors <20 mm and without muscular invasion which corresponding to pT1a and 1b (4, 5). However, our results from surgical specimens of rectal NET G1 and G2 revealed that lymphatic vessel and blood vessel invasion was the independent risk factors of lymph node metastasis. Then, 26.5% of lymph node metastasis was found in tumors smaller than 20 mm. Furthermore, 9.2% of lymph node metastases were found in tumors smaller than 10 mm, and most of these cases were positive for vascular invasion. Similar results were reported previously by Konishi et al. (11). They reported a 7% rate of lymph node metastasis in tumors smaller than 10 mm, and all cases had lymphatic vessel invasion. The JSCCR and Japan Neuroendocrine Tumor Society recommend, based on this result, local resection only for tumors smaller than 10 mm, and without muscular invasion or vascular invasion (12). There were no distinct guidelines about the indication of local resection during this study period of 2001 and 2011. Despite this, many clinicians in local institutions recommended surgical resection in tumors near 10 mm in size or cases with vascular invasion. Such a decision by local clinicians is thought to be reflected in the difference between surgically resected and locally resected cases in rectal NET <10 mm, where 9.2% of cases were positive for lymph node metastasis. Therefore, clinicians should remember that some cases <20 mm or even <10 mm in size may show lymph node metastasis in rectal NET. However, good clinical results after endoscopic resection of NET have also been reported (13). Therefore, further study with follow-up data is required to determine the optimal treatment algorithm in small rectal NET. All institutions in this study have also entered into a pathology survey, and their assessment statuses were investigated (14). In this survey, 88.6% of these institutions routinely performed immunohistochemical and histochemical staining in the diagnosis of NET, and 90.3% of these institutions routinely performed immunohistochemical and histochemical staining to assess vascular invasion. Detailed pathology evaluations were also performed by institutions entered in this study. Inter-observer differences in the assessment of vascular invasion have been reported. However, this difference seemed to be affected by the tumor size. Many NETs were found to be smaller than 10 mm in this study. Therefore, vascular invasion in rectal NET with a small size can be concordantly assessed (15, 16). Pathological analyses used in treatment algorithms should be designed to ensure objectivity in the assessment of vascular invasion in NET. In addition to the utility of describing histological features of NET from different organs, NET classification can be a predictive or prognostic factor. Our result revealed that G2 classified tumors in the WHO 2010, although not an independent risk factor did show more frequent lymph node metastasis. However, this study did not investigate clinical outcomes and recurrence, and these associations with NET classification should be investigated in the future. Similarly, this study investigated only resectable tumors and distant metastasis was not investigated. Further clinicopathological analysis will be required to investigate risk factor of metachronal distant metastasis.
This multi-institutional study also provided extensive clinicopathological information about NET of the large intestine in Japan according to NET classifications. Rectal NET has been reported to occur with higher frequency among Asian/Pacific islanders (16). In this study, we also found a very high frequency of rectal NET in the Japanese population, and tumor distributions in the large intestine are thought to be much different from that seen in Western countries (17). Next, we also found site-dependent histological variation within the large intestine in the Japanese cohort (18). Over 90% of rectal carcinoids were NET G1, and the ratio was higher than that found in the colon or appendix. This predominant incidence of G1 in rectal NET is also higher than the 40–70% range of NET in other gastrointestinal organs reported previously (19–23). Therefore, this report revealed that in addition to the tumor size or invasion, the distribution of histological grade and type is also variable among rectal, colonic, and appendiceal NET in Asian populations. Furthermore, we first reported the incidence of MANEC in the colon and rectum; MANEC were found in 1.3% of total cases, and were more frequently seen in colonic NET. Further study will be required to establish a therapeutic strategy for MANEC. We succeeded to elucidate anatomical site-dependent histological variety of NET in large intestine using uniform classification. WHO 2010 criteria can be used to understand the histological and biological variety of NET within whole body.
In conclusion, the clinicopathological features of NET were variable among the rectum, colon, and appendix, and histological variability among them clearly emerged using the NET classification. In rectal NET, our study revealed lymph node metastases even in tumors smaller than 10 mm, demonstrating that indications for surgical resection should be re-examined, especially in small NET with vascular invasion.
Author Contributions
Conception, design of the work: MK, AO, NSaito, MI, TW, and KS. Data acquisition, analysis, and interpretation: MK, KI, NSakuyama, KK, and SK. Drafting: MK. Agreement and final approval: all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
We received two grants for this study, which were Grant from Japanese Society for Cancer of the Colon and Rectum, and Grant in Aid for National Cancer Center, Japan. Grant Number: 26-A-7 and 19.
References
1. Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. New York: Wiley-Blackwell (2009).
2. Rindi G, Kloppel G, Couvelard A, Komminoth P, Korner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch (2007) 451:757–62. doi:10.1007/s00428-007-0452-1
3. Bosman TCF, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System. Lyon: IARC (2010).
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Neuroendocrine Tumors, Version 1. (2015). Available from: http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf
5. Ramage JK, De Herder WW, Fave GD, Ferolla P, Ferone D, Ito T, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology (2016) 103:139–43. doi:10.1159/000443166
6. Scherubl H, Jensen RT, Cadiot G, Stolzel U, Kloppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastroint Endosc (2011) 3:133–9. doi:10.4253/wjge.v3.i7.133
7. Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol (2015) 28:872–9. doi:10.1038/modpathol.2015.36
8. Kojima M, Shiokawa A, Ohike N, Ohta Y, Kato H, Iwaku K, et al. Clinical significance of nuclear morphometry at the invasive front of T1 colorectal cancer and relation to expression of VEGF-A and VEGF-C. Oncology (2005) 68:230–8. doi:10.1159/000086779
9. Untch BR, Bonner KP, Roggin KK, Reidy-Lagunes D, Klimstra DS, Schattner MA, et al. Pathologic grade and tumor size are associated with recurrence-free survival in patients with duodenal neuroendocrine tumors. J Gastrointest Surg (2014) 18:457–62; discussion 462–3. doi:10.1007/s11605-014-2456-x
10. Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC II. A proposed staging system for rectal carcinoid tumors based on an analysis of 4701 patients. Surgery (2008) 144:460–6. doi:10.1016/j.surg.2008.05.005
11. Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H, et al. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut (2007) 56:863–8. doi:10.1136/gut.2006.109157
12. Japan Neuroendocrine Tumor Society. Clinical Guideline for Pancreatic and Gastrointestinal Neuroendocrine Tumors. 1st ed. Tokyo: Kanehara (2015). (in Japanese).
13. Nakamura K, Osada M, Goto A, Iwasa T, Takahashi S, Takizawa N, et al. Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol (2016) 51:448–55. doi:10.3109/00365521.2015.1107752
14. Ikeda K, Kojima M, Saito N, Sakuyama N, Koushi K, Watanabe T, et al. Current status of the histopathological assessment, diagnosis, and reporting of colorectal neuroendocrine tumors: a web survey from the Japanese Society for Cancer of Colon and Rectum. Pathol Int (2016) 66:94–101. doi:10.1111/pin.12388
15. Kojima M, Puppa G, Kirsch R, Basturk O, Frankel WL, Vieth M, et al. Blood and lymphatic vessel invasion in pT1 colorectal cancer: an international concordance study. J Clin Pathol (2015) 68:628–32. doi:10.1136/jclinpath-2014-202805
16. Kojima M, Shimazaki H, Iwaya K, Kage M, Akiba J, Ohkura Y, et al. Pathological diagnostic criterion of blood and lymphatic vessel invasion in colorectal cancer: a framework for developing an objective pathological diagnostic system using the Delphi method, from the Pathology Working Group of the Japanese Society for Cancer of the Colon and Rectum. J Clin Pathol (2013) 66:551–8. doi:10.1136/jclinpath-2012-201076
17. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol (2008) 26:3063–72. doi:10.1200/JCO.2007.15.4377
18. Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer (2010) 17:909–18. doi:10.1677/ERC-10-0152
19. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer (2003) 97:934–59. doi:10.1002/cncr.11105
20. Yang M, Tian B, Zhang Y, Su A-P, Yue P-J, Xu S, et al. Evaluation of the World Health Organization 2010 grading system in surgical outcome and prognosis of pancreatic neuroendocrine tumors. Pancreas (2014) 43:1003–8. doi:10.1097/MPA.0000000000000153
21. Russolillo N, Vigano L, Razzore P, Langella S, Motta M, Bertuzzo F, et al. Survival prognostic factors of gastro-enteric-pancreatic neuroendocrine tumors after primary tumor resection in a single tertiary center: comparison of gastro-enteric and pancreatic locations. Eur J Surg Oncol (2015) 41:751–7. doi:10.1016/j.ejso.2015.02.011
22. Brunner SM, Weber F, Werner JM, Agha A, Farkas SA, Schlitt HJ, et al. Neuroendocrine tumors of the pancreas: a retrospective single-center analysis using the ENETS TNM-classification and immunohistochemical markers for risk stratification. BMC Surg (2015) 15:49. doi:10.1186/s12893-015-0033-1
23. Pasaoglu E, Dursun N, Ozyalvacli G, Hacihasanoglu E, Behzatoglu K, Calay O. Comparison of World Health Organization 2000/2004 and World Health Organization 2010 classifications for gastrointestinal and pancreatic neuroendocrine tumors. Ann Diagn Pathol (2015) 19:81–7. doi:10.1016/j.anndiagpath.2015.01.001
Keywords: neuroendocrine tumor, carcinoid tumor, WHO classification 2010, lymph node metastasis, rectum, colon
Citation: Kojima M, Ikeda K, Saito N, Sakuyama N, Koushi K, Kawano S, Watanabe T, Sugihara K, Ito M and Ochiai A (2016) Neuroendocrine Tumors of the Large Intestine: Clinicopathological Features and Predictive Factors of Lymph Node Metastasis. Front. Oncol. 6:173. doi: 10.3389/fonc.2016.00173
Received: 11 April 2016; Accepted: 06 July 2016;
Published: 18 July 2016
Edited by:
Michael J. Wargovich, University of Texas Health Science Center at San Antonio, USAReviewed by:
Akira Shimatsu, National Hospital Organization Kyoto Medical Center, JapanCarlos Teixeira Brandt, Federal University of Pernambuco, Brazil
Copyright: © 2016 Kojima, Ikeda, Saito, Sakuyama, Koushi, Kawano, Watanabe, Sugihara, Ito and Ochiai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atsushi Ochiai, aochiai@east.ncc.go.jp
 Motohiro Kojima
Motohiro Kojima Koji Ikeda
Koji Ikeda Norio Saito
Norio Saito Naoki Sakuyama2,3
Naoki Sakuyama2,3