- 1Department of Social and Environmental Health Research, London School of Hygiene and Tropical Medicine, London, UK
- 2INRS-Institut Armand-Frappier, Laval, QC, Canada
- 3Centre de recherche du Centre hospitalier de l’Université de Montréal (CRCHUM), Montréal, QC, Canada
- 4Département de médecine sociale et préventive, Université de Montréal, Montréal, QC, Canada
While diets rich in fruit and vegetables appear to reduce lung cancer risk, the evidence for individual carotenoid and vitamin intakes has been judged too limited to reach firm conclusions. Data from a case–control study of lung cancer (Montreal, QC, Canada, 1996–2002) were used to investigate the role of dietary intakes of β-carotene, α-carotene, β-cryptoxanthin, lutein/zeaxanthin, lycopene, and vitamin C in lung cancer risk. In-person interviews elicited dietary information from 1,105 incident cases and 1,449 population controls. Usual frequency of consumption of 49 fruits and vegetables 2 years prior to diagnosis/interview was collected. Odds ratios (ORs) and 95% confidence intervals (CIs) between intake variables and lung cancer were estimated using logistic or polytomous regression, adjusting for potential confounding factors including a detailed smoking history. ORs associated with upper versus lower tertiles of intake were 0.66 (95% CI = 0.51–0.84) for β-carotene, 0.70 (95% CI = 0.55–0.90) for α-carotene, 0.65 (95% CI = 0.51–0.84) for β-cryptoxanthin, 0.75 (95% CI = 0.59–0.95) for lycopene, and 0.74 (95% CI = 0.58–0.96) for vitamin C. ORs suggestive of a protective effect were found for elevated intakes of β-carotene, α-carotene, β-cryptoxanthin, and lycopene in male heavy smokers and of vitamin C in female heavy smokers. Selected antioxidants were also associated with a lower risk of lung cancer in female moderate smokers, and of squamous cell carcinoma, adenocarcinoma, and small cell carcinoma. These results suggest that several dietary antioxidants found in common food sources may protect against lung cancer, even among heavy smokers.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide. Global statistics show that in 2012 alone, lung cancer was responsible for an estimated 1.6 million deaths or 19.4% of all cancer-related deaths (1). Since survival remains low, the main hope for reducing the burden of this disease lies in prevention. Cigarette smoking is the foremost risk factor for lung cancer, accounting for up to 90% of all cases (2). Large-scale smoking prevention and cessation efforts have been attempted (3–5), and efforts in that direction must be pursued. However, other modifiable risk factors must be identified so that all possible lung cancer prevention strategies can be implemented. The recognized multifactorial etiology of lung cancer suggests that factors other than smoking, such as diet, can influence its occurrence (6).
Results from a large systematic review published by the World Cancer Research Fund (WCRF) in 2007, as well as the 2016 update of this report, suggested a protective role of diets rich in fruit and vegetables (7, 8). These foods are thought to protect against lung cancer due to their content in micronutrients that have chemopreventive properties such as antioxidant activity. Antioxidants found in the highest concentrations in both the human diet and serum samples include vitamin C and the specific carotenoids β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene (9). In its assessment, the WCRF stated that while the evidence suggests that foods rich in carotenoids probably decrease lung cancer risk, no firm conclusion could yet be reached about the role of individual carotenoids or vitamin C as the evidence for these was too limited or inconsistent, respectively (7).
Although several studies have examined associations between individual carotenoids and vitamin C and lung cancer, including case–control studies (10–17), cohort studies (18–31), pooled analyses (32, 33), and meta-analyses (34–36), results remain equivocal. This may be attributable to a combination of small sample sizes in some studies (10, 12–19, 21–24, 28) and inadequate control of smoking in others, usually because they were not able to control for time since quitting in addition to other dimensions of smoking (25, 28, 30). Most studies focused exclusively on one sex or the other (11, 12, 14–19, 21, 23, 24, 26–28, 31), and if there was effect modification by sex, this would lead to heterogeneity of results. A few studies have suggested that antioxidants may relate differently to lung cancer risk depending on sex (10, 20, 25), smoking intensity (20, 22, 27, 31, 37) and histological subtype of the tumor (13, 16, 26, 36), and heterogeneity of risk among such subgroups may have contributed to inconsistencies in the literature.
This prompted us to examine the relationship between intake of selected antioxidants and the risk of lung cancer in a large population-based case–control study, using updated food composition databases and detailed control for confounding by several different dimensions of smoking. We examined potential effect modification by sex and by smoking intensity on the antioxidant–lung cancer relationship, and examined the association between these micronutrients and four tumor histological subtypes.
Materials and Methods
Study Population and Data Collection
Data from a population-based case–control study conducted in Montreal, Québec (QC), Canada, were used. Eligible subjects were men and women, aged 35–75 years and living in the Montreal area. Cases were ascertained from 18 hospitals in the Montreal Metropolitan region, providing almost complete coverage of lung cancer diagnoses in the area (~98%). Potential cases were identified through active monitoring of hospital pathological reports and included histologically confirmed primary lung cancer cases diagnosed between January 1996 and December 1997. Concurrently, controls were randomly selected from the general population through electoral lists. In Canada, these lists include the names and addresses of practically all Canadian citizens residing in the country and are updated through active population enumeration. Controls were frequency matched to cases by age (5-year group), sex, and electoral district (comprising some 40,000 electors). The study and protocol were approved by the following Research Ethics Committees: the Institut National de la Recherche Scientifique, Hôpital Notre-Dame, Hôpital St-Luc, Hôtel-Dieu de Montréal, Hôpital Maisonneuve-Rosemont, Hôpital Jean-Talon, Hôpital Charles-Lemoyne, Hôpital Fleury, Hôpital du Sacré-Coeur de Montréal, Hôpital Lachine, Hôpital Santa Cabrini, Jewish General Hospital, Royal-Victoria Hospital, Montreal General Hospital, St-Mary’s Hospital, Lakeshore General Hospital, Hôpital de Lasalle, Montreal Chest Hospital, Hôpital de Verdun, and Hôpital Pierre-Boucher. The study was carried out in accordance with their recommendations. All subjects provided written informed consent in accordance with the Declaration of Helsinki. A total of 1,434 cases and 2,182 controls were invited to participate in the study: 1,203 (83.9%) cases and 1,513 (70.6%) controls accepted. Trained interviewers conducted in-person interviews with the subject or a proxy respondent (if the subject was deceased or too ill to respond). Information was collected on a wide range of factors including subjects’ socioeconomic background, detailed occupational history, smoking history (smoking status, changes in smoking intensity levels and interruptions, cigarette-years, time since cessation), lifetime intake of alcoholic beverages (wine, beer, spirits), and dietary intake.
Dietary Intake
Diet was assessed using a semiquantitative food frequency questionnaire (FFQ) adapted from the instrument developed by the Canadian Cancer Registries Epidemiology Research Group (38), which was based on two extensively validated questionnaires: the National Cancer Institute’s Block Questionnaire (39) and the Nurses’ Health Study FFQ (40). Modifications were made to reflect the diet of the study population and to capture major dietary sources of carotenoids and vitamin C among adults living in Québec, Canada. The FFQ was pre-tested for face validity in a subgroup of the target population to ensure that questions were well understood. The questionnaire covered 77 food items, including 49 fruits and vegetables grouped into 25 individual statements. Frequency of intake 2 years prior to diagnosis or interview, in terms of a typical portion size specified for each question, was reported as “7 or more times per week,” “4 to 6 times per week,” “1 to 3 times per week,” “1 to 3 times per month,” and “never or less than once per month.” The nutrient content of foods was extracted from the Canadian Nutrient File (version 2007b) (41). For categories with closed frequency ranges, the mid-point value was used to assign a weekly frequency of intake of each food (42, 43). The following values were assigned to each category: 7, 5, 2, 0.5, or 0 times per week. Individual daily intakes of β-carotene, α-carotene, β-cryptoxanthin, lutein/zeaxanthin, lycopene, and vitamin C were then calculated by multiplying the weekly frequency of intake of each food by the nutrient content of the specified portion size and then summing the contributions from all foods and dividing by 7. The food sources of each nutrient are presented in Table S1 in Supplementary Material. Nutrient intakes were adjusted for total energy with the residual method using the predicted nutrient intake for the median daily energy intake among women and men in the sample. Adjusting nutrient intakes for energy reduces measurement error for specific nutrients and removes extraneous variation, allowing the direct evaluation of variation due to dietary composition rather than absolute nutrient intakes (42). Energy-adjusted nutrient values were categorized into tertile levels of intake based on the frequency distribution among controls. In all, 162 subjects (6%) were excluded from the dietary analyses either because they had not filled in the dietary section or because they had answered less than 50% of the dietary questions. It has indeed been observed previously that it is reasonable to exclude subjects for whom 50% or more of items on a FFQ are left unanswered, if missing values are randomly distributed across the questionnaire (44). The final sample for analysis thus consisted of 2,554 subjects, i.e., 1,105 lung cancer cases and 1,449 population controls.
Statistical Analyses
Logistic regression models were used to estimate the risk of lung cancer associated with micronutrient intakes. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated comparing second and third tertile levels of intake (referred to as medium and high intakes) to the first tertile level of intake (low intake) for each micronutrient. Polytomous regression models were applied to estimate the association between micronutrient intakes and risk of squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and large cell carcinoma. Covariates included in regression models were age (continuous), sex (woman or man), respondent status (self or proxy), ethnic origin (French ancestry, English/Irish/Scottish ancestry, other), education level (elementary, secondary, post-secondary), body mass index (BMI) 2 years prior to interview (continuous), total daily energy intake (in kilocalories, continuous), and cigarette smoking. Cigarette smoking was modeled using three variables as suggested by Leffondré et al. (45), i.e., (i) ever smoked (yes or no); (ii) natural logarithm of cigarette-years (number of cigarettes smoked per day multiplied by smoking duration in years, continuous); and (iii) time since cessation (in years, continuous). Potential confounding by other variables, including income, cumulative intake of alcoholic beverages, and exposure to asbestos at work (46), was evaluated by a 10% change-in-estimate criterion, and none of those covariates were empirical confounders in this dataset. Effect modification by sex and by lifetime smoking intensity was examined by including relevant cross-products in the multivariate models. Tests for linear trend across intake tertiles were carried out by modeling nutrient intake tertiles as continuous variables. Statistical analyses were performed using SPSS version 19.0 (47).
Results
Sample Description
A description of study participants is provided in Table 1. Cases and controls were similar with respect to age but differed significantly on sociodemographic characteristics, their reliance on proxy respondents, BMI, history of cigarette smoking, and lifetime alcohol intake. As compared to controls, cases were more likely to be of French ancestry and to be less educated. Proxy respondents provided information for a significantly larger proportion of cases than controls. Compared to controls, cases were more likely to have ever smoked and to have greater values for cigarette-years, and were less likely to be former smokers. The distribution of histological subtypes differed by sex. Among males, a similar proportion of cases had been diagnosed with squamous cell carcinoma and adenocarcinoma, while adenocarcinoma was the most prevalent tumor histology among women.
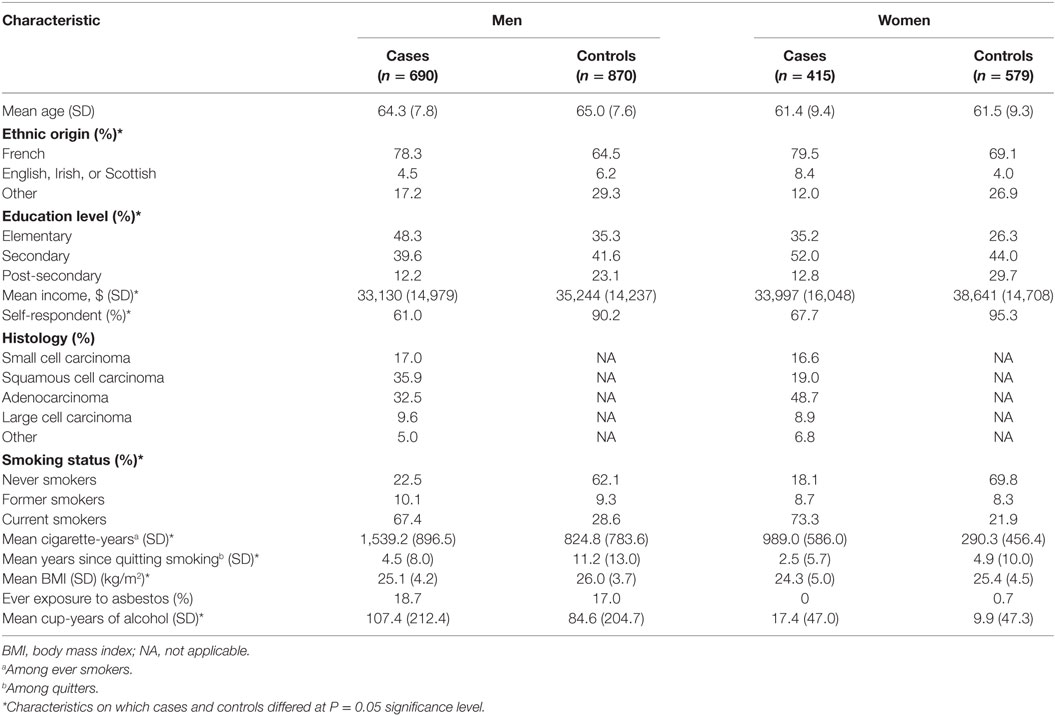
Table 1. Selected characteristics of cases and population controls, by sex, Montreal, QC, Canada, 1996–2002.
Median intake values of selected antioxidants among cases and controls are presented in Table 2. Among both men and women, and for all antioxidants studied, cases had lower median daily intakes than controls.
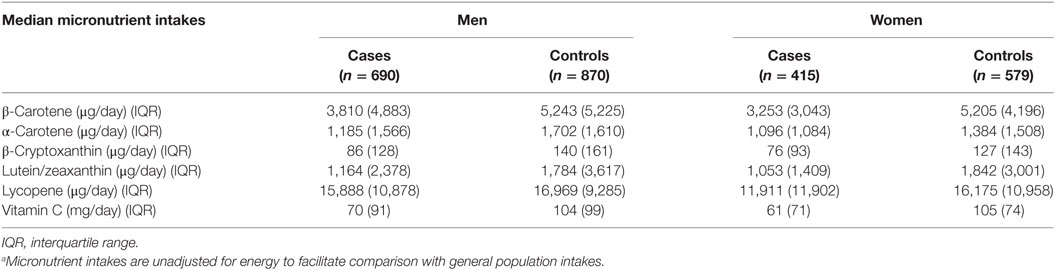
Table 2. Median micronutrient intakesa and interquartile ranges for cases and population controls, by sex, Montreal, QC, Canada, 1996–2002.
Correlation between Individual Micronutrients
As expected, most individual micronutrients were highly correlated with one another (Table S2 in Supplementary Material). One notable exception was lycopene, which showed correlation coefficients with other micronutrients ranging from 0.34 to 0.50, whereas the correlation between the other micronutrients ranged from 0.52 to 0.94. This high level of collinearity impeded our ability to estimate the independent effect of each micronutrient on lung cancer risk, and this should be kept in mind when interpreting the results.
Main Effects
Table 3 presents adjusted ORs for lung cancer associated with medium and high, versus low intake levels of energy-adjusted micronutrients. Sex did not emerge as an effect modifier of the association between micronutrients and lung cancer, with P values for the interaction terms ranging from 0.389 for α-carotene to 0.925 for β-cryptoxanthin in the fully adjusted models; therefore, results based on the entire sample are presented, after adjustment for sex. When compared to those with low levels of intake, subjects in the highest intake level of β-carotene, α-carotene, β-cryptoxanthin, lycopene, and vitamin C had a statistically significant lower risk of lung cancer. For all these micronutrients except vitamin C, significant dose–response trends were observed.
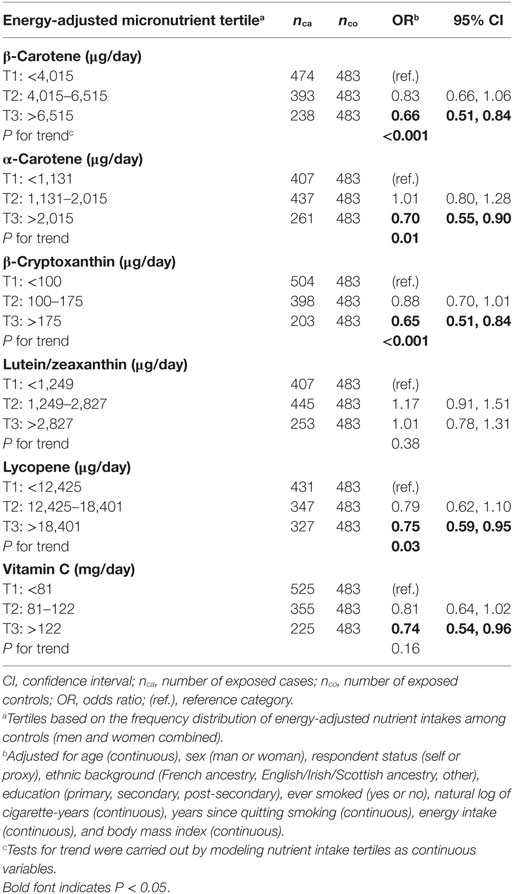
Table 3. Adjusted ORs and tests for trend for lung cancer risk according to dietary intakes of specific carotenoids and vitamin C in tertiles, Montreal, QC, Canada, 1996–2002.
Associations by Cumulative Smoking Intensity
Tables 4 and 5 show associations between micronutrient intakes and lung cancer risk by cumulative smoking intensity, in men and women, respectively. Results are presented separately for men and women since cumulative smoking intensity varied substantially between sexes, and the sample included a substantial group of never smoking women for whom potential confounding by smoking would not be an issue. As well, although most nutrient-smoking intensity interaction terms did not reach statistical significance, with P values in the fully adjusted models ranging between 0.046 for β-cryptoxanthin in women and 0.910 for vitamin C in men, stratified results allow for comparison with recent studies (31). As very few men had never smoked, never and light smokers were combined into a single category. In almost all strata, the point estimates were lower in the high micronutrient category than in the low micronutrient category, although not all of these were statistically significantly lower. In general, the strongest protective effects in men were observed among heavy intensity smokers, whereas in women, they occurred among intermediate intensity smokers. Among men who were never and light smokers, the only statistically significant association was observed among those in the second intake level of lutein/zeaxanthin, who were at greater risk of lung cancer compared to those in the lowest intake level. There were no significant associations among men who smoked at intermediate intensity levels. Heavy smoking men were at lesser risk of lung cancer when consuming high intakes of all micronutrients, although a statistically significant inverse association was found only for β-carotene, α-carotene, β-cryptoxanthin, and lycopene. There were no significant associations among women who never smoked, although it should be noted that numbers of subjects were small. Among women in the intermediate smoking intensity category, intakes in the third tertile of β-carotene and second tertiles of β-cryptoxanthin, lycopene, and vitamin C were associated with a lower lung cancer risk, while both medium and high intakes of vitamin C showed an inverse relationship in heavy smoking women. Two-sided tests for trend assessing the dose–response relationship were significant at the 0.05 level for β-carotene, α-carotene, β-cryptoxanthin, and lycopene in heavy smoking men, as well as for vitamin C in heavy smoking women.
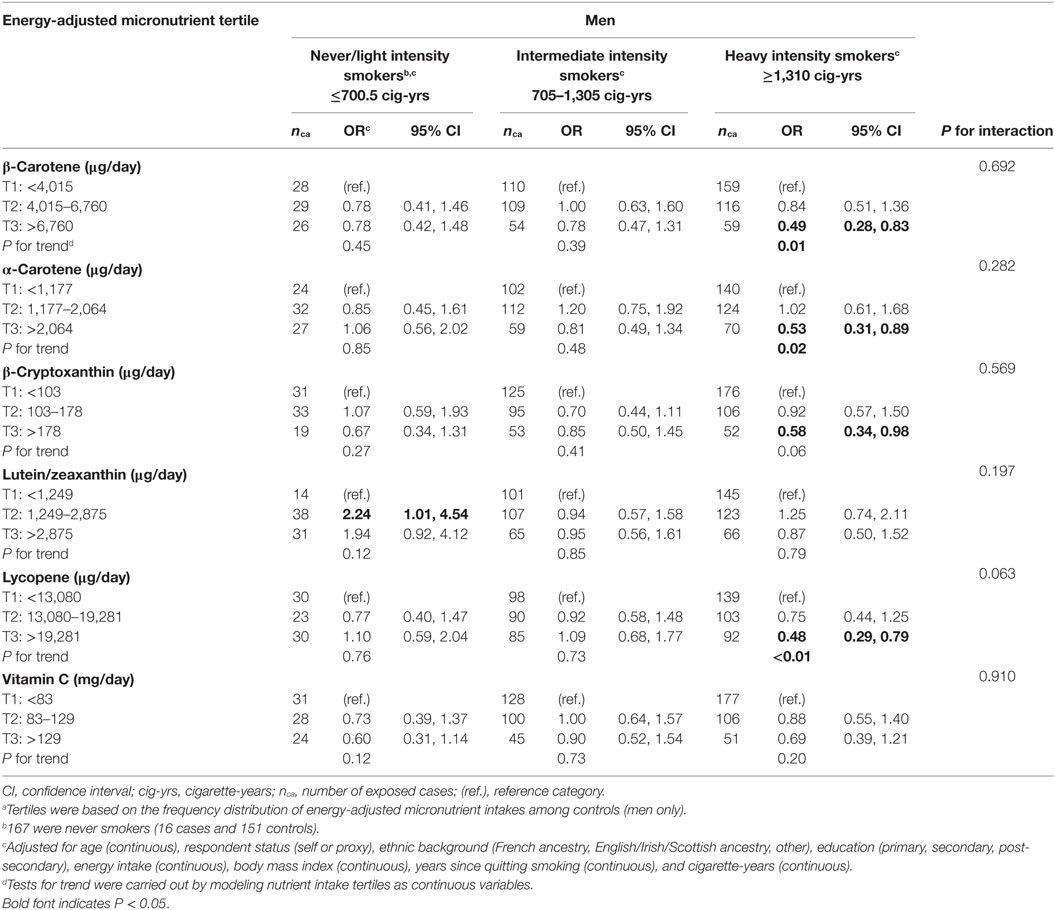
Table 4. Adjusted ORs and tests for trend for lung cancer risk according to dietary intakes of specific carotenoids and vitamin C in tertilesa in men, stratified by cumulative smoking intensity, Montreal, QC, Canada, 1996–2002.
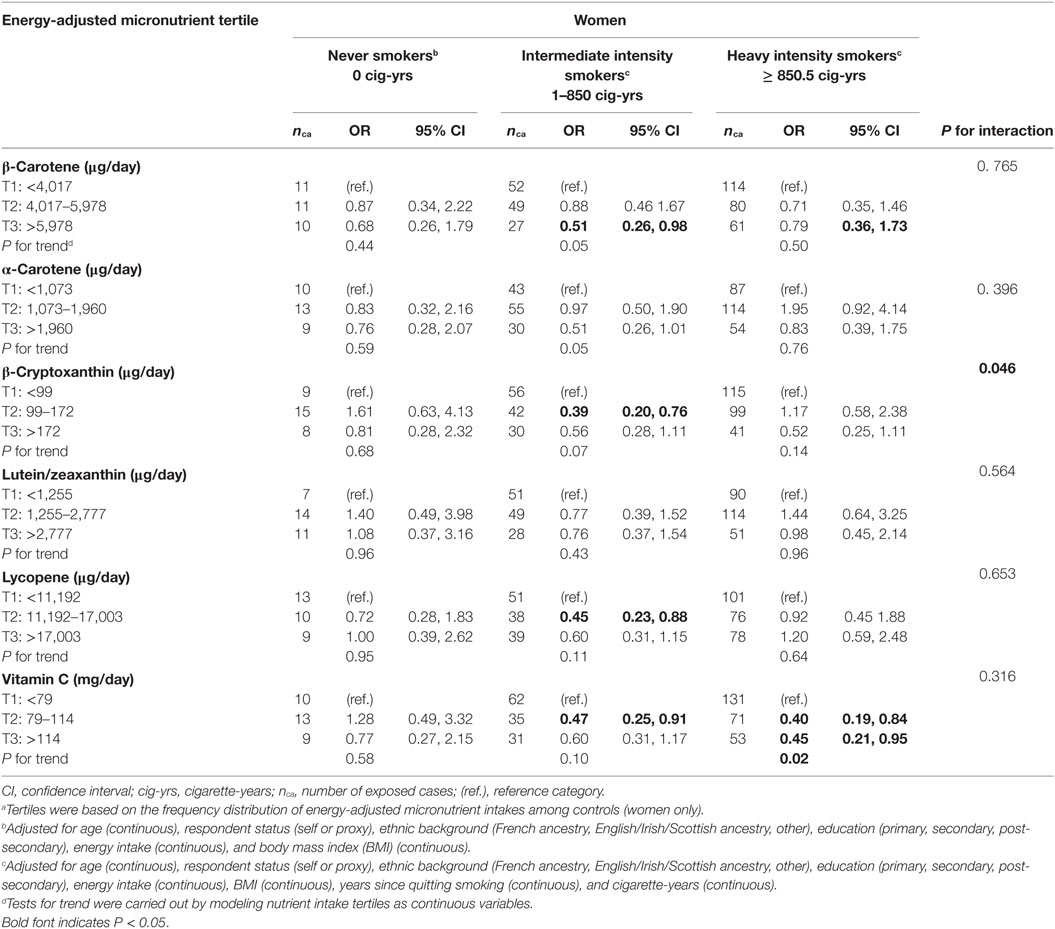
Table 5. Adjusted ORs and test for trend for lung cancer risk according to dietary intakes of specific carotenoids and vitamin C in tertilesa in women, stratified by cumulative smoking intensity, Montreal, QC, Canada, 1996–2002.
Associations by Histological Subtype of the Tumor
Odds ratios for the association between micronutrient intakes and each of the four most common histological subtypes of lung cancer are presented in Table 6. There were no clear differences in the patterns of results between squamous cell carcinoma, adenocarcinoma, and small cell carcinoma. While there was some variation in the point estimates, the confidence limits for any given micronutrient overlapped considerably between the histologic subtypes. When compared to subjects in the lowest tertile level of intake, those with the highest intake levels of β-carotene, α-carotene, lycopene, and vitamin C had a statistically significant lower risk of squamous cell carcinoma, while both medium and high intakes of β-cryptoxanthin suggested a protective effect for this histological subtype. High intakes of β-carotene and α-carotene were associated with a reduced risk of adenocarcinoma, while both medium and high intakes of β-cryptoxanthin and lycopene suggested a protective effect for small cell carcinoma. High intakes of all other antioxidants were associated with a non-significant decrease in risk of squamous cell carcinoma, adenocarcinoma, and small cell carcinoma, save for lutein/zeaxanthin which was related to a slightly increased risk of small cell carcinoma. High intakes of β-carotene, β-cryptoxanthin, lutein/zeaxanthin, lycopene, and vitamin C were similarly associated with statistically non-significant increases in risk of large cell carcinoma, although it should be noted that these results were based on relatively few cases. Statistically significant trends (two-sided, P < 0.05) for a lower risk of squamous cell carcinoma were observed with increased levels of β-carotene, α-carotene, β-cryptoxanthin, lycopene, and vitamin C, and similarly between adenocarcinoma and β-carotene and α-carotene, as well as between small cell carcinoma and both β-cryptoxanthin and lycopene.
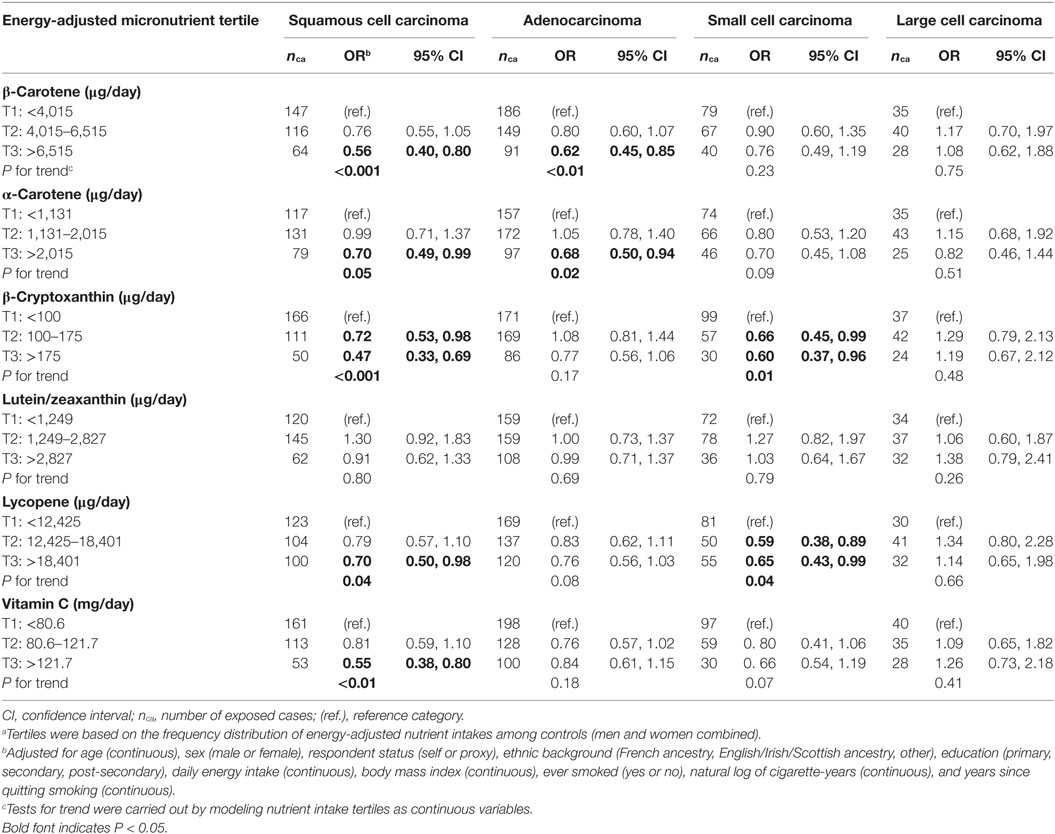
Table 6. Adjusted ORs and tests for trend for risk of four histologic subtypes of lung cancer according to dietary intakes of specific carotenoids and vitamin C in tertilesa, Montreal, QC, Canada, 1996–2002.
Associations for Selected Fruit and Vegetable Groups
To facilitate comparisons with other studies and describe the relevant food sources in our population, we also evaluated whether specific fruit and vegetable groups high in carotenoids and vitamin C were associated with lung cancer risk. We observed that medium and high weekly intakes of fruits, vegetables, cruciferous vegetables, and citrus fruits, as well as medium intake levels of tomato products and high intakes of carrot products were inversely related to lung cancer risk (Table S3 in Supplementary Material).
Discussion
In our study, high dietary intakes of β-carotene, α-carotene, β-cryptoxanthin, lycopene, and vitamin C were associated with a decreased risk of lung cancer in main effects analyses. Findings for β-carotene concord with those of a recent meta-analysis in which a pooled relative risk of 0.77 (95% CI: 0.68–0.87) was reported (36). Our results also give credence to a protective role of several other dietary carotenoids on lung cancer and for which evidence was judged to be too limited to draw firm conclusions by the WCRF (7). We found that high intakes of lycopene, a carotenoid which is derived from food sources that tend to differ from those of other carotenoids and vitamin C, lowered the risk of lung cancer. To our knowledge, only one previous study also reported such an association (27). We also found an inverse association between high intakes of vitamin C and lung cancer risk, a finding in line with two studies (33, 35) but at odds with another (31). We did not observe that high intakes of lutein/zeaxanthin significantly lowered the risk of lung cancer, a finding that contrasts with several observational studies (12, 15, 23, 25, 26). A defining characteristic of these studies is that, unlike ours, most were conducted in men exclusively. Nonetheless, there was no indication of effect modification by sex in our study.
Our study is one of the few to have investigated the role of individual antioxidants in separate strata of smoking intensity (20, 22, 27, 31), and the first to simultaneously control for lifetime intensity and time since quitting smoking, two important dimensions of smoking history for lung cancer (48). Although the interactions between micronutrients and smoking intensity were not statistically significant, stratification by cumulative smoking intensity is an improvement over previous studies in which analyses were stratified by smoking status only (i.e., never/former/current smoking) (49), since the former approach is likely to result in more homogenous groups of smokers than the latter. We found that high intakes of β-carotene, α-carotene, β-cryptoxanthin, and lycopene were associated with a reduction in lung cancer risk among male heavy smokers, while vitamin C reduced the risk of lung cancer among female heavy smokers. This suggests that heavy intensity smokers, who suffer from high oxidative stress due to smoking and are at a disproportionate risk of developing lung cancer, can benefit from high intakes of selected antioxidants. Our findings are in line with a number of previous studies. In one study, the associations between antioxidants and lung cancer were stronger among heavy smokers than among never or light smokers, although there was no significant interaction with smoking (31). In another, an inverse association between serum carotenoid levels and lung cancer death was observed among smokers, but not among never/former smokers (50). Our findings for β-carotene are at odds with those from the CARET (51) and the ATBC (52) randomized controlled trials in which β-carotene supplementation in smokers was associated with an increase in lung cancer incidence. However, these associations disappeared when subsequent analyses considered a longer follow-up post-trial (53, 54). Our results regarding never smoking women concord with previous studies (16, 28), although based on small numbers. Furthermore, our data suggest a preventive effect of vitamin C against lung cancer in women who have never smoked, an association which, to our knowledge, has not been reported previously. We also found that women whose cumulative smoking history ranged from 1 to 850 cigarette-years benefited from intakes in the third tertile level of β-carotene and in the second tertile level of β-cryptoxanthin, lycopene, and vitamin C, findings not reported before.
In analyses stratified by histological subtype, we found similar patterns of results by the three most common tumor subtypes, with selected antioxidants being inversely related to risk. We observed that high intakes of β-carotene, α-carotene, β-cryptoxanthin, lycopene, and vitamin C were associated with a reduced risk of squamous cell carcinoma, while high intakes of β-carotene and α-carotene lowered the risk of adenocarcinoma, and both medium and high intakes of β-cryptoxanthin and lycopene reduced the risk of small cell carcinoma. Our results pertaining to risk of squamous cell carcinoma and β-carotene (36), β-cryptoxanthin (26, 32), and vitamin C intakes (20, 26, 33) are comparable to those observed in earlier studies. We found statistically non-significant associations between high intakes of β-carotene, β-cryptoxanthin, lutein/zeaxanthin, lycopene, and vitamin C and an increased risk of large cell carcinoma. To our knowledge, our study is the first to report on dietary antioxidant intakes and risk of large cell carcinoma, a rarer histological subtype (2).
In addition to their antioxidant activity, carotenoids and vitamin C may act to prevent lung carcinogenesis through different mechanisms. For instance, the pro-vitamin A carotenoids such as β-carotene, α-carotene, and β-cryptoxanthin can be metabolized into vitamin A and play a role in cell differentiation (9). β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene may also inhibit cancerous cell proliferation and contribute to cell-to-cell communication (55–58). In addition, β-carotene and vitamin C have been shown to stimulate apoptosis (57, 59), while α-carotene is thought to inhibit the promotion of carcinogenesis (55, 60). It is therefore conceivable that exposure to carotenoids and vitamin C could play a role in preventing carcinogenesis at different points during the lifetime, which makes them prime candidates for lung cancer prevention.
Fruits and vegetables containing carotenoids and vitamin C are also rich in other nutrients and phytochemicals which could be responsible for their observed protective role against lung cancer. For example, dithiolthiones and isothiocyanates found in cruciferous vegetables and vegetables from the Allium genus increase the activity of enzymes involved in carcinogen detoxification (7, 61, 62). Selenium, an essential element, acts as a co-factor for glutathione peroxidase, an enzyme known to protect membranes from oxidation and which also plays a role in carcinogen detoxification (63, 64). Flavonoids are also found in fruits and vegetables, and in our study population, we previously observed a reduced lung cancer risk with intake of some flavonoid subclasses (65). Our results regarding selected groups of fruits and vegetables high in a diversity of chemopreventive micronutrients support their protective role against lung cancer. This is somewhat at odds with results from a recent case–control study conducted in Spain, which found no statistically significant reduction in lung cancer risk associated with consumption of selected fruit and vegetable groups (66) but concords with findings from large meta-analyses (7, 8, 49, 67). One should therefore keep in mind that although dietary intake of carotenoids and vitamin C may help prevent lung cancer, they could also be surrogates for other protective compounds found in selected fruit and vegetables.
Important strengths of our study include the large sample size which allowed for stratification by smoking intensity, for studying specific sources of antioxidants, for examining the impact of diet on different tumor histological subtypes, and for comprehensive control of potential confounding by smoking. Good coverage of the case population in the area and the use of incident, histologically confirmed primary lung cancer cases are also key strengths of our study. Advantages specific to our FFQ are that it contained an extensive food list and was specifically designed to cover major carotenoid and vitamin C sources in the study population. We also used updated local nutrient databases to carry out the conversion from food to nutrient intake.
This study also has some limitations. It would have been of interest to study associations in light of disease aggressiveness, but this information was not available. While the case and control participation rates were high, it is still possible that participating controls were more health conscious than the general population, thus biasing risk estimates away from the null. As well, proxy respondents provided answers for almost 40% of lung cancer cases which could have resulted in measurement error. However, sensitivity analyses indicated that associations between antioxidants and lung cancer did not differ considerably whether we considered self-respondents only or proxy respondents as well. Unmeasured changes in dietary patterns over time are another possible source of bias, although diet has been shown to be relatively stable over time (44). The study was presented to potential and actual participants as a study on environmental factors and the collection of food intake data was one of many themes covered during the interview. These precautions should have reduced the risk that subjects would self-select into the study or report intakes based on their perception of the role of antioxidants on their health status. Finally, although the diet questionnaire focused on the period 2 years prior to diagnosis, cases might have been influenced by their illness when reporting their dietary habits.
Measurement error in dietary intakes inevitably occurred in the study. The FFQ used here was adapted from widely used validated questionnaires (39, 44), which served to develop other FFQ validated and used in various Canadian and Québec populations (38, 68, 69). However, it was not comprehensively tested for reliability and validity in the present study population. Measurement error also likely stemmed from the grouping of more than one food into a single statement, from the lack of information on the exact quantities of foods consumed and on whether foods were consumed cooked or not, and from the relatively broad categories from which subjects could choose to report their intake frequency. We elected to assign mid-point values to each closed category; true intake values might have been close or far from these. Nutrient values attributed to each statement represented the average nutrient content of a food item selected from the statement and under a certain form such as raw or cooked, a choice based on Canadian and Québec food consumption statistics (70, 71). While each of these limitations may have contributed to exposure misclassification, it was likely non-differential with respect to case–control status, thereby attenuating associations. Non-differential misclassification of an exposure with more than two categories may theoretically lead to bias away from the null, but this requires misclassification between two non-adjacent categories, an extreme situation that is unlikely to be present in our data (72). Furthermore, it is highly unlikely that a trend would be reversed by such non-differential misclassification (73). The possibility also remains that the dietary data collected with the FFQ better reflected more recent intakes than remote ones. It could be hypothesized that this latter exposure would be more etiologically relevant with respect to lung cancer development which occurs over many years. Nonetheless, antioxidants are suspected to play a protective role at different times in carcinogenesis. Indeed, antioxidants can inhibit both initiation and promotion stages of tumorigenesis by protecting cells against oxidative damage (74, 75). Therefore, despite the fact that our FFQ assessed more recent antioxidant intakes, it remains a useful measurement tool to capture an effect at the promotion stage.
Finally, although careful attention was given to the control for smoking by using an algorithm shown to be superior to many others in this specific study population (45), residual confounding due to smoking cannot be excluded. Such confounding, if present, could have produced bias toward the null in the estimated associations between nutrient intake and lung cancer risk. However, such a bias could not explain the associations, albeit non-significant ones, observed among the stratum of non-smoking women. We also tested a range of other potential confounding variables such as income, lifetime alcohol intake, and exposure to asbestos, which ultimately were not included in our models because of a lack of effect on the associations.
Overall, our findings contribute both new knowledge and confirmatory data to the as yet inconclusive body of evidence on the hypothesized associations between high intakes of individual carotenoids and vitamin C, and lung cancer risk. They suggest a protective effect of selected antioxidants against lung cancer in general, for different smoking intensity groups and particularly heavy smokers, and for the squamous cell, adenocarcinoma, and small cell histological tumor subtypes. Even though smoking remains the strongest predictor of lung cancer risk, it appears desirable, in light of these findings, to further promote consumption of fruits and vegetables rich in carotenoids and vitamin C to reduce the lung cancer burden among both smokers and non-smokers.
Ethics Statement
This study was carried out in accordance with the recommendations of McGill University’s Institutional Review Board, the Ethics Committee of the Research Center of Hôtel-Dieu de Montréal and of all individual ethics committees listed in the Material and Methods section, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the McGill University’s institutional Review Board (A12-E06-99) and the Ethics Committee of the Research Center of Hôtel-Dieu de Montréal (HDM-941213-18).
Author Contributions
This manuscript was at the core of MS’s Master’s dissertation. MS conceptualized the research question and was responsible for reviewing the literature, cleaning the data, conducting the analyses, interpreting the results, and writing the manuscript. M-EP and M-CR were MS’s dissertation supervisors and provided overall guidance at all stages of the project and manuscript preparation. AK provided constructive feedback on the manuscript from its first version to the final submitted one. JS and M-EP were co-principal investigators of the original study, provided access to the data, and contributed invaluable feedback as part of manuscript revisions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the following people. Lesley Richardson supervised the fieldwork. Louise Nadon, Jerome Asselin, and Javier Pintos worked on database management and data cleanup. Marie Desy provided help with statistical analyses.
Funding
This study was supported by The National Cancer Institute of Canada (grant # 010736 Oc 86565), The National Health Research and Development Program (grant # 6605-4730-800), The Medical Research Council of Canada (grant # 37673 MT 14704), and the Canadian Institutes of Health Research (grant # MOP 14704). MS held Master’s training awards from the Fonds de Recherche en Santé du Québec (FRSQ) and from CIHR. M-CR, AK, and M-EP held Career Awards from CIHR and/or the FRQS. JS holds the Guzzo-Cancer Research Society Chair in Environment and Cancer.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fonc.2017.00023/full#supplementary-material.
References
1. International Agency for Research on Cancer (IARC). GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Lyon, France: IARC (2013).
2. Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest (2003) 123(1 Suppl):21S–49S. doi: 10.1378/chest.123.1_suppl.21S
3. IARC, editor. Tobacco Smoke and Involuntary Smoking. Lyon, France: International Agency for Research on Cancer (2004).
4. Health Canada. Strong Foundation, Renewed Focus – An Overview of Canada’s Federal Tobacco Control Strategy 2012–17 (2012). Available from: https://www.canada.ca/en/health-canada/services/publications/healthy-living/strong-foundation-renewed-focus-overview-canada-federal-tobacco-control-strategy-2012-17.html
5. World Health Organization. Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva: World Health Organization (2008).
6. Epstein KR. The role of carotenoids on the risk of lung cancer. Semin Oncol (2003) 30(1):86–93. doi:10.1053/sonc.2003.50020
7. World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research (2007).
8. Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro-Rosenblatt D, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol (2016) 27(1):81–96. doi:10.1093/annonc/mdv381
9. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med (2005) 26(6):459–516. doi:10.1016/j.mam.2005.10.001
10. Le Marchand L, Wilkens LR, Kolonel LN. Ethnic differences in the lung cancer risk associated with smoking. Cancer Epidemiol Biomarkers Prev (1992) 1(2):103–7.
11. Ziegler RG, Mayne ST, Swanson CA. Nutrition and lung cancer. Cancer Causes Control (1996) 7(1):157–77.
12. De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Carzoglio JC, Ronco A, et al. Dietary antioxidants and lung cancer risk: a case-control study in Uruguay. Nutr Cancer (1999) 34(1):100–10. doi:10.1207/S15327914NC340114
13. Brennan P, Butler J, Agudo A, Benhamou S, Darby S, Fortes C, et al. Joint effect of diet and environmental tobacco smoke on risk of lung cancer among nonsmokers. J Natl Cancer Inst (2000) 92(5):426–7. doi:10.1093/jnci/92.5.426
14. De Stefani E, Brennan P, Boffetta P, Mendilaharsu M, Deneo-Pellegrini H, Ronco A, et al. Diet and adenocarcinoma of the lung: a case-control study in Uruguay. Lung Cancer (2002) 35(1):43–51. doi:10.1016/S0169-5002(01)00281-1
15. Marchand JL, Luce D, Goldberg P, Bugel I, Salomon C, Goldberg M. Dietary factors and the risk of lung cancer in New Caledonia (South Pacific). Nutr Cancer (2002) 42(1):18–24. doi:10.1207/S15327914NC421_3
16. Wright ME, Mayne ST, Swanson CA, Sinha R, Alavanja MC. Dietary carotenoids, vegetables, and lung cancer risk in women: the Missouri women’s health study (United States). Cancer Causes Control (2003) 14(1):85–96. doi:10.1023/A:1022565601937
17. Lagiou P, Samoli E, Lagiou A, Katsouyanni K, Peterson J, Dwyer J, et al. Flavonoid intake in relation to lung cancer risk: case-control study among women in Greece. Nutr Cancer (2004) 49(2):139–43. doi:10.1207/s15327914nc4902_4
18. Chow WH, Schuman LM, McLaughlin JK, Bjelke E, Gridley G, Wacholder S, et al. A cohort study of tobacco use, diet, occupation, and lung cancer mortality. Cancer Causes Control (1992) 3(3):247–54.
19. Shibata A, Paganini-Hill A, Ross RK, Yu MC, Henderson BE. Dietary beta-carotene, cigarette smoking, and lung cancer in men. Cancer Causes Control (1992) 3(3):207–14.
20. Bandera EV, Freudenheim JL, Marshall JR, Zielezny M, Priore RL, Brasure J, et al. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States). Cancer Causes Control (1997) 8(6):828–40.
21. Ocke MC, Bueno-de-Mesquita HB, Feskens EJ, van Staveren WA, Kromhout D. Repeated measurements of vegetables, fruits, beta-carotene, and vitamins C and E in relation to lung cancer. The Zutphen Study. Am J Epidemiol (1997) 145(4):358–65.
22. Yong LC, Brown CC, Schatzkin A, Dresser CM, Slesinski MJ, Cox CS, et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am J Epidemiol (1997) 146(3):231–43.
23. Knekt P, Jarvinen R, Teppo L, Aromaa A, Seppanen R. Role of various carotenoids in lung cancer prevention. J Natl Cancer Inst (1999) 91(2):182–4.
24. Speizer FE, Colditz GA, Hunter DJ, Rosner B, Hennekens C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes Control (1999) 10(5):475–82.
25. Michaud DS, Feskanich D, Rimm EB, Colditz GA, Speizer FE, Willett WC, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr (2000) 72(4):990–7.
26. Voorrips LE, Goldbohm RA, Brants HA, van Poppel GA, Sturmans F, Hermus RJ, et al. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol Biomarkers Prev (2000) 9(4):357–65.
27. Holick CN, Michaud DS, Stolzenberg-Solomon R, Mayne ST, Pietinen P, Taylor PR, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol (2002) 156(6):536–47. doi:10.1093/aje/kwf072
28. Rohan TE, Jain M, Howe GR, Miller AB. A cohort study of dietary carotenoids and lung cancer risk in women (Canada). Cancer Causes Control (2002) 13:231. doi:10.1023/A:1015048619413
29. Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomarkers Prev (2003) 12(4):350–8.
30. Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev (2003) 12(9):890–8.
31. Takata Y, Xiang YB, Yang G, Li H, Gao J, Cai H, et al. Intakes of fruits, vegetables, and related vitamins and lung cancer risk: results from the Shanghai Men’s Health Study (2002-2009). Nutr Cancer (2013) 65(1):51–61. doi:10.1080/01635581.2013.741757
32. Mannisto S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev (2004) 13(1):40–8. doi:10.1158/1055-9965.EPI-038-3
33. Cho E, Hunter DJ, Spiegelman D, Albanes D, Beeson WL, van den Brandt PA, et al. Intakes of vitamins A, C and E and folate and multivitamins and lung cancer: a pooled analysis of 8 prospective studies. Int J Cancer (2006) 118(4):970–8. doi:10.1002/ijc.21441
34. Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr (2008) 88(2):372–83.
35. Luo J, Shen L, Zheng D. Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Sci Rep (2014) 4:6161. doi:10.1038/srep06161
36. Yu N, Su X, Wang Z, Dai B, Kang J. Association of dietary vitamin A and beta-carotene intake with the risk of lung cancer: a meta-analysis of 19 publications. Nutrients (2015) 7(11):9309–24. doi:10.3390/nu7115463
37. Ruano-Ravina A, Figueiras A, Freire-Garabal M, Barros-Dios JM. Antioxidant vitamins and risk of lung cancer. Curr Pharm Des (2006) 12(5):599–613. doi:10.2174/138161206775474396
38. Pan SY, Ugnat A-M, Mao Y, Wen SW, Johnson KC; The Canadian Cancer Registries Epidemiology Research Group. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev (2004) 13(9):1521–7.
39. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol (1986) 124(3):453–69.
40. Hu FB, Satija A, Rimm EB, Spiegelman D, Sampson L, Rosner B, et al. Diet assessment methods in the nurses’ health studies and contribution to evidence-based nutritional policies and guidelines. Am J Public Health (2016) 106(9):1567–72. doi:10.2105/ajph.2016.303348
41. Fichier canadien sur les éléments nutritifs [Internet]. (2007). Available from: http://www.santecanada.ca/fcen
42. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr (1997) 65(4 Suppl):1220S–8S; discussion 9S–31S.
43. Sauvageot N, Alkerwi A, Adelin A, Guillaume M. Validation of the food frequency questionnaire used to assess the association between dietary habits and cardiovascular risk factors in the NESCAV study. J Nutr Food Sci (2013):3(3):208. doi:10.4172/2155-9600.1000208
45. Leffondré K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol (2002) 156(9):813–23. doi:10.1093/aje/kwf122
46. Pintos J, Parent ME, Rousseau MC, Case BW, Siemiatycki J. Occupational exposure to asbestos and man-made vitreous fibers, and risk of lung cancer: evidence from two case-control studies in Montreal, Canada. J Occup Environ Med (2008) 50(11):1273–81. doi:10.1097/JOM.0b013e31818345bb
48. Rachet B, Siemiatycki J, Abrahamowicz M, Leffondre K. A flexible modeling approach to estimating the component effects of smoking behavior on lung cancer. J Clin Epidemiol (2004) 57(10):1076–85. doi:10.1016/j.jclinepi.2004.02.014
49. Sun Y, Li Z, Li J, Li Z, Han JA. Healthy dietary pattern reduces lung cancer risk: a systematic review and meta-analysis. Nutrients (2016) 8(3):134. doi:10.3390/nu8030134
50. Min KB, Min JY. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci (2014) 105(6):736–43. doi:10.1111/cas.12405
51. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med (1996) 334(18):1150–5.
52. The ATBC Study Group. Incidence of cancer and mortality following {alpha}-tocopherol and {beta}-carotene supplementation: a postintervention follow-up. JAMA (2003) 290(4):476–85. doi:10.1001/jama.290.4.476
53. Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL Jr, Omenn GS, et al. The beta-carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst (2004) 96(23):1743–50. doi:10.1093/jnci/djh320
54. Virtamo J, Taylor PR, Kontto J, Männistö S, Utriainen M, Weinstein SJ, et al. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-year post-intervention follow-up of the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study. Int J Cancer (2014) 135(1):178–85. doi:10.1002/ijc.28641
55. Murakoshi M, Nishino H, Satomi Y, Takayasu J, Hasegawa T, Tokuda H, et al. Potent preventive action of alpha-carotene against carcinogenesis: spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by alpha-carotene than by beta-carotene. Cancer Res (1992) 52(23):6583–7.
56. Kim DJ, Takasuka N, Kim JM, Sekine K, Ota T, Asamoto M, et al. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MNU and DMH. Cancer Lett (1997) 120(1):15–22.
57. Sharoni Y, Danilenko M, Levy J. Anticancer activity of carotenoids: from human studies to cellular processes and gene regulation. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in Health and Disease. New York, USA: Marcel Dekker (2004). 567 p.
58. Lian F, Hu KQ, Russell RM, Wang XD. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int J Cancer (2006) 119(9):2084–9. doi:10.1002/ijc.22111
59. Flagg EW, Coates RJ, Greenberg RS. Epidemiologic studies of antioxidants and cancer in humans. J Am Coll Nutr (1995) 14(5):419–27.
60. Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature (1981) 290(5803):201–8.
61. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc (1996) 96(10):1027–39. doi:10.1016/S0002-8223(96)00273-8
62. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer (2003) 3(10):768–80. doi:10.1038/nrc1189
63. Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev (2004) 13(5):771–8.
64. Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc (2005) 64(4):527–42. doi:10.1079/PNS2005467
65. Christensen KY, Naidu A, Parent ME, Pintos J, Abrahamowicz M, Siemiatycki J, et al. The risk of lung cancer related to dietary intake of flavonoids. Nutr Cancer (2012) 64(7):964–74. doi:10.1080/01635581.2012.717677
66. Tarrazo-Antelo AM, Ruano-Ravina A, Abal Arca J, Barros-Dios JM. Fruit and vegetable consumption and lung cancer risk: a case-control study in Galicia, Spain. Nutr Cancer (2014) 66(6):1030–7. doi:10.1080/01635581.2014.936951
67. Wang M, Qin S, Zhang T, Song X, Zhang S. The effect of fruit and vegetable intake on the development of lung cancer: a meta-analysis of 32 publications and 20,414 cases. Eur J Clin Nutr (2015) 69(11):1184–92. doi:10.1038/ejcn.2015.64
68. Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr (2006) 9(1):84–93. doi:10.1079/PHN2005763
69. Shatenstein B, Nadon S, Godin C, Ferland G. Development and validation of a food frequency questionnaire. Can J Diet Pract Res (2005) 66(2):67–75. doi:10.3148/66.2.2005.67
70. Statistique Canada. In: Division de l’agriculture, editor. Consommation des aliments au Canada – Partie I. Ottawa: Ministre de l’industrie (2002).
71. Bertrand L. Les Québécoises et les Québécois mangent-ils mieux? Rapport de l’Enquête québécoise sur la nutrition, 1990. Montréal: Ministère de la Santé et des Services sociaux, gouvernement du Québec (1995).
72. Dosemeci M, Wacholder S, Lubin JH. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol (1990) 132(4):746–8.
73. Weinberg CR, Umbach DM, Greenland S. When will nondifferential misclassification of an exposure preserve the direction of a trend? Am J Epidemiol (1994) 140(6):565–71.
74. Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med (1990) 8(6):583–99.
Keywords: antioxidant, ascorbic acid, carotenoid, case–control study, lung neoplasm, vitamin C
Citation: Shareck M, Rousseau M-C, Koushik A, Siemiatycki J and Parent M-E (2017) Inverse Association between Dietary Intake of Selected Carotenoids and Vitamin C and Risk of Lung Cancer. Front. Oncol. 7:23. doi: 10.3389/fonc.2017.00023
Received: 04 November 2016; Accepted: 07 February 2017;
Published: 28 February 2017
Edited by:
Stella Koutros, National Cancer Institute, USAReviewed by:
Wagner Ricardo Montor, Faculdade de Ciências Médicas da Santa Casa de São Paulo, BrazilJerry Polesel, Centro di Riferimento Oncologico (IRCCS), Italy
Copyright: © 2017 Shareck, Rousseau, Koushik, Siemiatycki and Parent. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Elise Parent, marie-elise.parent@iaf.inrs.ca
 Martine Shareck
Martine Shareck Marie-Claude Rousseau
Marie-Claude Rousseau Anita Koushik3,4
Anita Koushik3,4 Marie-Elise Parent
Marie-Elise Parent