- 1Department of Radiation Oncology, Klinikum rechts der Isar, Technische Universität München, München, Germany
- 2Department of Radiation Sciences (DRS), Institute of Innovative Radiotherapy (iRT), Helmholtz Zentrum München, Neuherberg, Germany
Hyperthermia (HT) is one of the hot topics that have been discussed over decades. However, it never made its way into primetime. The basic biological rationale of heat to enhance the effect of radiation, chemotherapeutic agents, and immunotherapy is evident. Preclinical work has confirmed this effect. HT may trigger changes in perfusion and oxygenation as well as inhibition of DNA repair mechanisms. Moreover, there is evidence for immune stimulation and the induction of systemic immune responses. Despite the increasing number of solid clinical studies, only few centers have included this adjuvant treatment into their repertoire. Over the years, abundant prospective and randomized clinical data have emerged demonstrating a clear benefit of combined HT and radiotherapy for multiple entities such as superficial breast cancer recurrences, cervix carcinoma, or cancers of the head and neck. Regarding less investigated indications, the existing data are promising and more clinical trials are currently recruiting patients. How do we proceed from here? Preclinical evidence is present. Multiple indications benefit from additional HT in the clinical setting. This article summarizes the present evidence and develops ideas for future research.
Introduction
Hyperthermia (HT) is defined as an exogenous, supraphysiological elevation of tissue/body temperature. The beginning of modern HT dates back to the 1700s when remissions of malignant tumors were repeatedly associated with concomitant bacterial infections. This effect was first systematically investigated at the break of the 19th century by Coley (1). Patients with unresectable sarcomas received injections of bacterial vaccines for fever induction. In total, a cure rate of 20% was achieved (2). It took several decades of technological developments for local/locoregional heat application until HT alone became available for clinical application.
Nowadays, HT is either administered independently or, more often, in combination with radiotherapy (RT) or chemotherapy (CT). HT alone is being used for direct ablation of single tumor lesions with temperatures exceeding 50°C. Multiple techniques are being used to obtain necessary temperature coverage such as high-intensity focused ultrasound and radiofrequency-, microwave-, or infrared laser-based heating via ablation catheters directly inserted into the tumor (3).
In bimodal treatment schemes such as thermoradiotherapy (RTHT) and chemoradiotherapy (RTCT) as well as in trimodal thermochemoradiotherapy (RTHTCT), HT is utilized for augmentation of treatment effects of the concomitant oncological therapy. Necessary tissue temperatures are significantly lower ranging from 39 to 43°C (4, 5).
In this literature-based review, a brief introduction to HT physiology, cell biology, and immune response is given to examine the underlying modes of action of HT. Currently used HT techniques for heat delivery and temperature control are described. The clinical evidence of combining RT with HT is summarized and sorted per tumor entity. To this end, a PubMed search was conducted searching for the term “hyperthermia” in combination with tumor entities treatable by RT, and terms describing technical aspects such as “biology,” “physiology,” “chemotherapy,” and “radiation therapy.” Special emphasis is given to recent meta-analyses and published prospective trials.
Preclinical Evidence
Changes in Perfusion and Oxygenation
Data and the respective interpretation of HT-induced changes in perfusion and oxygenation remain controversial and are briefly described in the following. A comprehensive review of this topic has been published by Vaupel and Kelleher (6). There is evidence that mild HT can increase blood perfusion of the heated tissue, preferentially at the beginning of tumor heating (7, 8). It has been reported that this can lead to increased oxygen delivery via an improvement of microcirculation (9). This is especially true in cases when the oxygen demand of the tissue is reduced. It has been proposed that direct heat-dependent cell killing and loss of mitochondrial membrane potential contribute to this phenomenon (10, 11). On the contrary, other studies showed increased oxygen consumption at elevated tissue temperature (van’t Hoff’s law!) counteracting the oxygenating effect of increased perfusion (12). An increase in oxygen availability may favor oxygenation of hypoxic cells (7). The effect appears to be preferentially in diffusion-limited, chronic hypoxia (13, 14). Whether the radiosensitizing effect outlasts the time frame of increased perfusion remains so far unclear. Some studies have reported increased perfusion extending over 24 h after HT, which would benefit following RT/CT sessions (15, 16). Other studies could not reproduce this result (17). As hypoxia is a central causative factor for radioresistance, a decrease in hypoxia by HT may be responsible for the observed radiosensitization.
Induction of Cell Death
Hyperthermia has been shown to confer cell death by apoptosis or mitotic catastrophe (18, 19). It has been reported that HT triggers unfolding of especially heat-labile non-histone nuclear proteins leading to aggregation, due to exposition of hydrophobic groups, with surrounding proteins and subsequent association with the nuclear matrix. As consequence, basic nuclear matrix-dependent functions such as transcription, replication, or DNA repair are impaired (20, 21). Malfunction of DNA replication finally causes chromosome aberrations, genome instability, and cell death by mitotic catastrophe (22). Apoptosis may be mediated by cell death membrane receptor activation and subsequent caspase 3 activation (23). The extent of apoptosis appears to differ among different tumor types (24). In addition, the permeability of the cellular and mitochondrial membranes is altered leading to cellular Ca2+-spikes as well as mitochondrial depolarization with resulting bursts of reactive oxygen species. Both mechanisms may further enhance protein instability and apoptosis (25–27).
Inhibition of DNA Repair Mechanisms
As mentioned above, there is sufficient evidence showing inhibition of DNA repair mechanisms upon HT. Krawczyk et al. have demonstrated inhibition of homologous recombination at clinically achievable mild HT temperatures (41–42.5°C) associated with BRCA2 degradation and its reduced accumulation at double-strand break sites (28). Further on, HT impairs the function of the Ku heterodimer by reducing its DNA-binding capacity and preventing the initiation of non-homologous end joining at DNA double-strand breaks sites (29). In addition, base excision after cell radiation has been shown to be reduced upon heat administration (30). In summary, HT acts on multiple levels including excision repair, non-homologous end joining, and homologous recombination influencing the repair of DNA lesions as well as single-strand and double-strand breaks (29–31). As a consequence, the effects of DNA damaging treatments such as CT or RT are enhanced. A more detailed review was recently published discussing existing evidence (32).
Immune Stimulation
Besides direct effects on cell metabolism, HT appears to trigger multiple immune responses on local and systemic levels. Toraya-Brown and Fiering published a thorough review covering this aspect (33). In summary, HT increases expression of immunogenic surface receptors such as MICA and MHC-I enhancing effectiveness and function of natural killer (NK) cells and of CD8+ cells, respectively (34, 35). The expression of heat shock proteins (HSPs) such as HSP70 is increased. After binding intracellular proteins, HSPs get secreted stimulating the activity of NK cell- and antigen-presenting dendritic cells (36, 37). Presentation of these tumor antigens can cause specific antitumor immune responses effected by CD8+ cells (38). Tumor antigens are also provided by increased release of exosomes (39). Direct enhancement of immunogenic activity of leukocytes is mediated by increased lysis acitivy of NK cells, activation of macrophages, maturation of dendritic cells, and increased IFNy production as well as cytotoxicity of CD8+ cells (34, 40–42). In addition, immune cell trafficking is enhanced by increased perfusion and permeability (43). Following elevated intratumoral IL-6 signaling, it may further be facilitated by increased cell adhesion molecule expression such as ICAM-I (44).
Heat Delivery and Temperature Control
Heating Techniques
Heating techniques can be divided by the size, penetration depth, and region of energy deposition. Local or regional HT is mostly used to enhance local therapy such as RT or CT. Alternatively, hyperthermic isolated limb perfusion to administer CT agents is performed. Whole-body hyperthermia (WBHT) has been applied either alone or in combination with CT for the treatment of metastatic disease. Different approaches including capacitive, radiative, infrared-A, or ultrasound have been used for clinical HT treatments (45). The clinically most relevant methods are described in the following.
Capacitive Heating Systems
Capacitive heating systems work with two electrodes positioned on both sites of the body with direct body contact using a water bolus. Heat is induced by the resulting currents and is directed toward the smallest electrode (46). Capacitive heating tends to create high power densities around the bolus’ edges but good heat coverage of targets inside of the fat layer (47). On the contrary, in obese patients, therapy-limiting local hot spots can occur causing painful subcutaneous burns (48).
Radiative Heating Systems
Radiative heating systems work with frequencies ranging from 75 to 915 MHz (spectrum of radiowaves and microwaves) and use a water bolus for electromagnetic coupling. Compared to capacitive heating, radiative systems appear to yield better power disposition and temperature distribution leading to better target coverage (47). The applicable temperature is sometimes limited due to local temperature hot spots. The accuracy of such systems depends on construction details such as the number, positioning and design of antennas or properties of the water bolus (49). In recent years, increasingly complex systems have been introduced comprising multiple antennas such as the commercially available Sigma applicators, build in a circular arrangement, or the AMC-8 phased array HT system (50, 51). Site-specific systems such as the HYPERcollar3D system, which was developed for the treatment of carcinomas of the head and neck, take into account local characteristics of target areas to optimize temperature coverage (52). Alternatively, antennas can be interstitially implanted or used for endocavitary HT in direct approximation to tumors (45). In order to perform superficial or interstitial HT, heating systems are used applying higher frequencies such as 915 MHz (53).
Walter-Filtered Infrared-A (wIRA)-Based Systems
Walter-filtered infrared-A-based systems have successfully been used for the treatment of superficial tumors (54, 55). Infrared-A radiation is generated by a halogen lamp, passing through a water filter. The range of therapeutically relevant temperatures is limited to a depth of 15–20 mm [with good therapeutic temperature coverage (56)]. Due to the technical setup a very short interval between HT and RT combined with heat isolation procedures enables quasi-simultaneous RTHT, optimizing the synergistic effect (see below).
Pretherapeutic hyperthermia treatment planning can be used to optimize tumor temperatures. It uses dielectric models created on the basis of segmented CT or MRI data assigning literature-based dielectric properties to distinct tissue types. So, the specific absorption rate (SAR) of the respective tissue can be calculated and used for a finite element-based prediction of temperature distributions. In clinical studies, calculated SAR values correlated well with measured SAR values, relative temperature increase and clinical data regarding hot spots in patients with pelvic tumors (57–59).
Treatment Temperatures
Preclinical experiments were initially performed with relatively high temperatures ranging from 43 to 45°C focusing on direct cytotoxic effects. However, in the clinical setting, temperatures above 42.5°C were only achieved in small tumor subvolumes. While trying to reach targeted temperatures, therapy-limiting hotspots occurred causing substantial side effects. In these cases, this led to a reduction of target temperatures or early termination of HT (60, 61). This was regarded as failure of delivering adequate thermal doses, which lead to a rapid decline in HT-usage in the mid-to-late 1990s. It took several more years until the beneficial effects of mild HT (39.5–43°C), as described above, became known. Nowadays, mild HT has become the standard in modern clinical trials and daily clinical usage (5). Modern HT technology has been developed and optimized for minimal hot spot occurrence as a main focus (51, 52). As a consequence, therapy-limitation due to focal hot spots has not been an issue in many recent HT trials (62–64).
Temperature Control
As important as heat generation, measurement of the actual tissue temperature distribution is crucial for effective heating of tumors. A homogenous temperature distribution is necessary for optimal treatment effects. Local dose-limiting hotspots have to be avoided. Originally, temperature assessment was restricted to single-point measurements. It can be performed either invasively by insertion of intratumoral catheters or, as applicable in tumors with close proximity to natural cavities such as rectal, cervical, vaginal, urethral or vesical tumors, equally efficient by endoluminal catheters. Insertion of catheters inherits the risk of complications such as pain, inflammation, or abscess formation (65). Thus, the latter option should be used if possible. In superficial tumors, surface skin measurements by contact electrodes constitute a further alternative. In addition, by using infrared thermography cameras, two-dimensional data can be obtained for superficial tumors even though calibration with contact electrodes is necessary for absolute temperature assessment (56). A promising method for deep temperature monitoring is MRI-guided thermometry capable of measuring three-dimensional temperature distributions non-invasively. Temperature can be measured by exploiting either T1w-imaging, diffusion weighted imaging or proton resonance frequency shift-imaging (66). Proton resonance frequency shift-imaging appears to be the most accurate method in the clinical setting. By combining HT with online MRI thermometry, direct changes of temperature delivery can be performed to optimize temperature distribution and suppress hot spots (67). To this end, an adaptive iterative algorithm has been developed (68). First studies have confirmed its applicability in the clinical setting (69).
Interval of Administration
In general, a dose–effect relationship of HT has been shown (70). The interval of administration of HT relative to RT has great influence on its effectiveness. The actual effect is quantified with the thermal enhancement ratio (TER) defined as the ratio of the respective radiation doses of RT alone divided by the RT + HT dose necessary to receive equal survival curves (4). However, biological aspects of HT react differently to the extent of heating sequence of HT. In the clinical setting, the maximal achievable TER should be combined with the most limited TER for healthy tissue to retain a tumor-specific effect reducing toxicity.
The inhibition of DNA repair has its highest effect when HT is given simultaneously to RT. The effect declines with the end of DNA repair mechanisms approximately 4 h after RT. However, inhibition of DNA repair mechanisms is not tumor specific since it is also present in normal tissues (71, 72). In contrast, direct cell killing is specific to malignant tissue at target temperatures. The respective TER is estimated at 1.5 deriving most likely from direct radiation-independent cell damage to radioresistant hypoxic cells (73). To summarize, optimal effects can be achieved by simultaneous RTHT treatment with no tumor-directed specificity. Treatment selectiveness would completely depend on accuracy of radiation delivery. In the time frame of 1–4 h before or after radiation, a maximal selective TER can be achieved by adding up DNA repair inhibition and direct cell damage. For optimization of the oxygenation-effect, RT should be applied shortly after HT (72). So far, the shortest interval between HT and RT has been described for wIRA followed by RT (56).
When considering time schedules and fractionation schemes, one should also take into account two phenomena termed cellular and vascular thermotolerance. The underlying mechanisms remain incompletely understood (74–77). For this reason, detailed description of these phenomena is not performed in the context of this review. Most probably, thermotolerance in currently used clinical HT schedules (i.e., once or twice/week) seems to play no limiting role.
Clinical Evidence of Combined Thermoradiotherapy
In the following, key studies providing evidence for a clinical benefit of a combined treatment with HT and RT is presented, sorted by tumor entity and the extent of existing evidence (see Tables 1–3 for a detailed overview). In the following paragraph, existing clinical evidence of combined HT with CT is summarized (see Table 4).
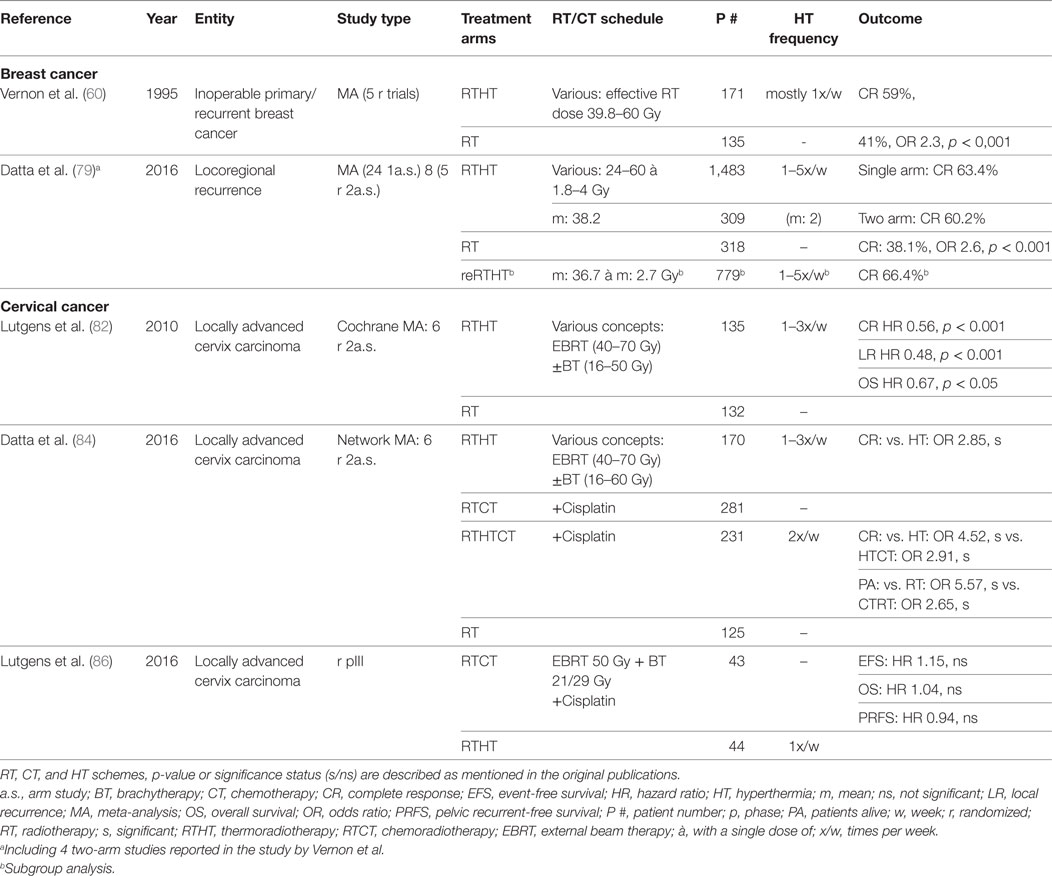
Table 1. Summary of cited meta-analyses and randomized trials for breast cancer and cervical cancer.
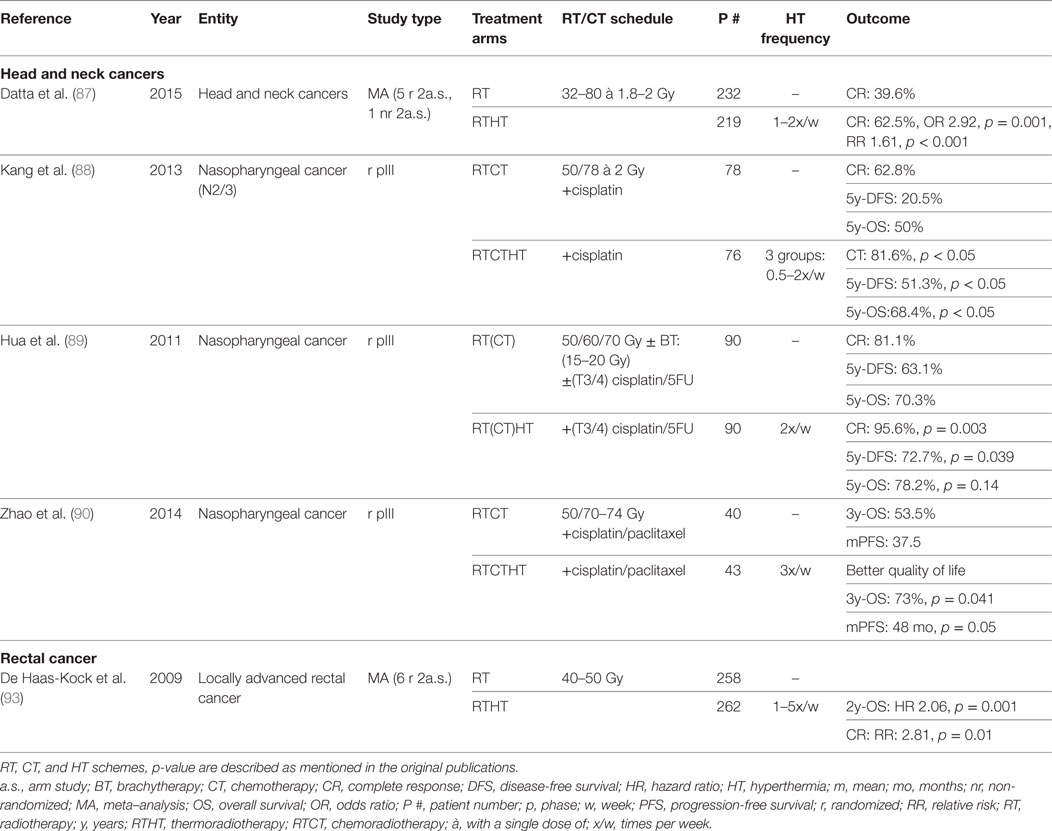
Table 2. Summary of cited meta-analyses and randomized trials for head and neck cancer and rectal cancer.
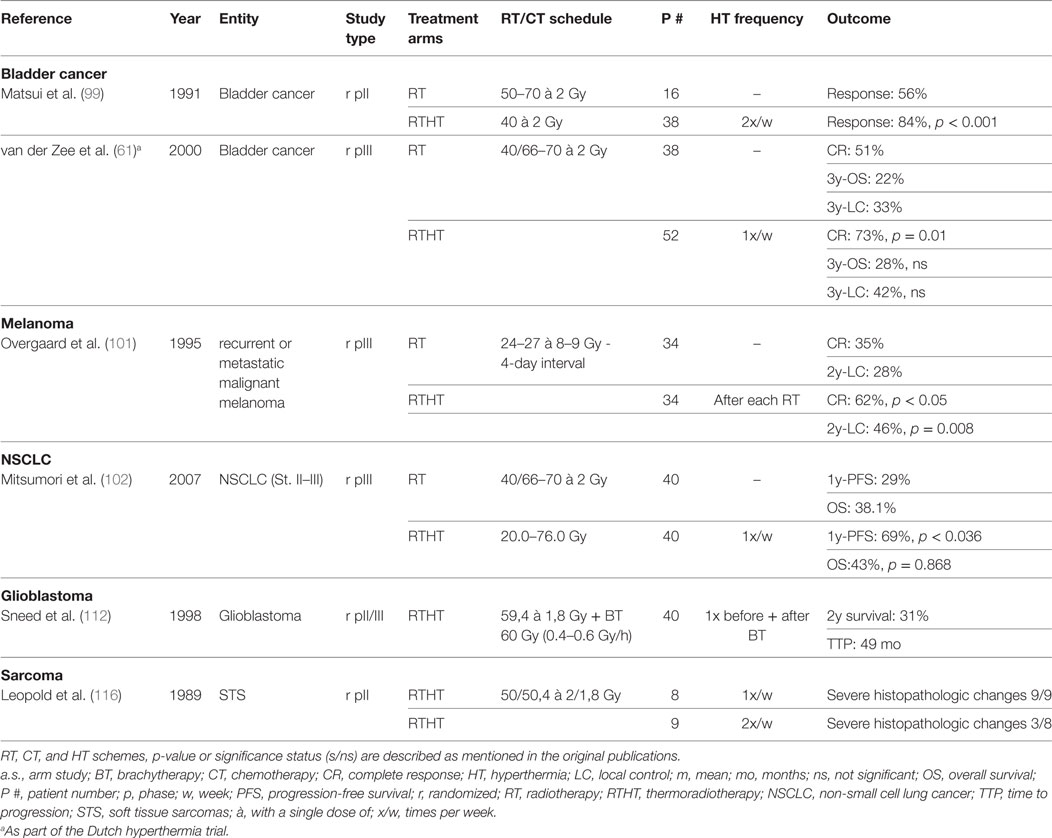
Table 3. Summary of cited randomized trials for bladder cancer, melanoma, NSCLC, glioblastoma, and sarcoma.
Breast Cancer
Breast cancer constitutes the most widely investigated malignant entity. Vernon et al. published the results of five randomized trials conducted between 1981 and 1991 that were combined due to insufficient patient accrual. The pooled analysis of 306 patients with inoperable primary or recurrent disease yielded a significantly better complete response (CR) (RTHT: 59%, RT alone: 41%, odds ratio (OR): 2.3, p < 0.001) but no survival benefit. However, 50% of patients had metastases at the time of randomization. The effect was most prominent in preirradiated recurrent lesions. Skin toxicities such as blisters, ulceration, and necrosis were higher in the HT group, however with low impact on the patients well-being and generally treatable with conservative measures (60).
In cases of locoregional recurrence, breast surgery is recommended if possible. However, radical resection only appears to be feasible in 65% of patients and may cause significant treatment-related morbidity (78). Thus, RT constitutes a significant alternative, and depending on the time interval between first and second RT and other pretreatment characteristics offers substantial clinical benefit. Since nowadays most patients receive RT in the primary situation, HT may help to enhance the effects of reirradiation in the recurrence scenario, especially since in some clinical situations only reduced radiation doses may be prescribed. A recent meta-analysis by Datta et al. included 8 two-arm studies and 24 single-arm studies involving 2,110 patients with locoregional recurrent disease (79). CR was similar between one-arm studies (CR: 63.4%) and two-arm studies as well as significantly higher compared to RT alone (RTHT: 60.2%, RT: 38.1%, OR 2.6, p < 0.001). In preirradiated patients (779 patients), a CR of 66.4% was achieved (mean re-RT dose: 36.7 Gy). Treatment-related toxicity was overall not increased with mean acute grade 3/4 toxicities of 14.4%. Among the analyzed studies, there was great heterogeneity in RT dose, HT fraction schedules, total number of HT fractions, HT duration, or average achieved temperatures. However, no prognostic treatment variables could be identified in a subgroup analysis and meta-regression. Similar results were recently published by Linthorst et al. In a retrospective study encompassing 248 patients with unresectable recurrences, reirradiation + HT yielded a CR of 70% (80). In resectable cases, a regimen including surgery, RT, HT, and partly CT or hormone therapy, a local control (LC) rate of 78% after 5 years was achieved (81). Notter et al. have treated patients with locally recurrent breast cancer with RTHT using a hypofractionation scheme of 5 × 4 Gy with one fraction per week. A wIRA system was used for superficial HT. A CR rate of 61% was achieved without any treatment-related toxicities (56). In summary, there is sustained evidence demonstrating a value of adjunct HT in locoregional, recurrent breast cancer as definitive or adjuvant treatment.
Cervical Cancer
Several randomized trials have been conducted to test HT in combination with RT. A Cochrane database meta-analysis was performed analyzing six trials involving a total of 487 patients (82). The studies included mostly patients with locally advanced disease (74% FIGO stage IIIB). Except in one study, RT was delivered as a combination of external beam therapy (EBRT) and brachytherapy (BT). The pooled data analysis showed a significantly higher CR [CR relative risk (RR): 0.56, p < 0.001] and OS [hazard ratio (HR): 0.67, p = 0.05] in favor of RTHT as well as a reduced local recurrence rate (HR: 0.48, p < 0.001). No difference was found regarding acute and late toxicity rates. The authors criticized a lack of uniformity among the trials concerning HT delivery, HT schedules, as well as RT treatment protocols, RT dose, and RT techniques. Therefore, the authors conclude that the results do not suffice for a definitive recommendation to apply HT along standard treatment regimen. The included Dutch deep HT trial was updated for long-term results after 12 years of follow-up showing sustained improved LC (RT: 37%, RTHT: 57%, p = 0.01) and survival (RT: 20%, RTHT: 37%, p = 0.03) (83). All studies have in common that concurrent CT was not included in the treatment regimen.
In recent years, many trials have discussed the value of the combined treatment regimes such as RTHT, RTCT, or trimodal RTHTCT. A recently published meta-analysis has revised all relevant publications including 23 articles with a total patient number of 1,160 (84). In a network meta-analysis (NMA), all four possible treatment modalities (RT, RTHT, RTCT, RTHTCT) were compared. All studies, but one, included patients with locally advanced disease. RT always comprised EBRT + BT. The comparison of RTHT and RT yielded similar results as the previously described Cochrane analysis. The same six trials with minor updates were included. For the direct comparison of RTHTCT and RTCT, only one study including 68 patients was available (85). It showed a significant better CR for the trimodal treatment arm (RTCT: 46.7%, RTHTCT: 83.3%, risk difference 36.7%, p = 0.0001). No significantly increased grade 3/4 toxicities were found. The NMA showed a significant advantage of RTHTCT over all other treatment combinations in direct and indirect comparisons as well as in the SUCRA value-based ranking for CR and patients alive. RTCT and RTHT had similar performance even though RTHT had a small advantage over RTCT regarding CR. In coherence, a recent randomized phase III trial, which was closed early due to poor accrual with only 87 of 376 planned patients, did not show any significant difference in event-free survival and pelvic recurrence-free survival between RTHT and RTCT (86). In summary, by adding radiosensitizing treatments such as CT or HT to RT, better treatment outcomes are achievable. In locally advanced cervical cancers, RTHT appears to be a valuable substitute for RTCT if CT is not applicable. By combining all three modalities, the best treatment effect may be possible. Additional phase III trials are necessary directly comparing RTHT, RTCT, and RTHTCT for optimal treatment stratification.
Head and Neck Cancers
To evaluate the effect of HT in head and neck carcinomas, a meta-analysis was recently performed including six 2-armed studies encompassing 451 patients. Five of the six studies were randomized trials. The CR rate appeared to be significantly better in patients treated with combined RTHT compared to RT alone (RT alone: 39.6%, RTHT: 62.5%, OR 2.92, p < 0.0001). Acute and late grade 3/4 toxicities were not significantly different (87). One study included exclusively nasopharyngeal carcinomas, whereas all other studies considered all cancer sites of the head and neck. However, all studies involving surgery or concurrent CT were excluded.
Three other randomized trials with a total of 417 patients recently analyzed the effects of trimodal treatment combing RT, CT, and HT in patients with nasopharyngeal carcinomas (88–90). Two studies reporting CR showed a significant advantgae for RTHTCT treatment. The same patients had an increased OS in two of the studies. Progression-free survival (PFS) or disease-free survival (DFS) was significantly better in the RTHTCT group in all three studies. Patients with higher tumor temperatures and higher HT fraction numbers showed a better outcome (88). In all three studies, no difference in treatment-related toxicity has been described. Patients receiving HT showed even better quality of life scores after completion of therapy (90). These studies demonstrate that trimodal therapy including RT, CT with different agents, and HT constitute an effective and safe treatment alternative. To our knowledge, no other randomized studies have been published in other head and neck sites investigating trimodal therapy. As shown in other malignancies, re-treatment may constitute a further clinical situation in which HT may be a valuable treatment option. In a small cohort receiving reirradiations combined with HT, a CR of 46% was achieved showing feasibility of such an approach (91). To conclude, HT constitutes a valuable treatment option in cancers of the head and neck. However, due to relatively high perfusion rates and fast adaptation to local temperature changes, HT delivery appears to be especially challenging. By using a site-tailored radiative heating device, treatment outcomes may be better in the future (92). HT could be used in multimodal treatment schemes further improving treatment outcome. Alternatively, it may constitute a toxicity-sparing alternative for concurrent CT in elderly or multimorbid patients. Further clinical studies are necessary to evaluate the true clinical value.
Rectal Cancer
In 2009, a Cochrane analysis of six phase II and III randomized-controlled trials including 520 patients with locally advanced rectal carcinomas was performed. Patients received neoadjuvant RT with or without HT. Increased CR (RR 2.81, p = 0.01) as well as increased OS at 2y follow-up (HR 2.06, p = 0.001) could be observed. The survival benefit, however, could not be measured for any later time point. No difference in acute toxicity was found in the two studies reporting on this side effect (93). A positive impact on pathologic complete response (pCR) could as well be shown in a retrospective study of 106 patients. Sphincter-sparing surgery was higher for tumors in close proximity to the anal verge (94). In a further retrospective study encompassing 235 patients, HT appeared to confer better downstaging of the primary tumor and involved lymph nodes (95). Two small studies evaluated hypofractionated RTHTCT schemes showing principal efficacy and safety (96, 97). Additional HT appears to be well tolerated without increased impairment of quality of life (98). To conclude, the Cochrane analysis demonstrated a fundamental possibility of increased response by applying adjunct HT. However, further randomized prospective trials are necessary to evaluate the true value of neoadjuvant RTHTCT as well as of treatment of recurrent disease.
Bladder Cancer
For the treatment of bladder carcinomas, HT has been predominantly applied in combination with intravesical CT. However, a few trials have evaluated RTHT. In an early study, 56 patients with bladder carcinomas were treated with intravesical CT (bleomycin) simultaneously to RTHT with reduced total dose (40 Gy) or RT alone with higher dose prescription (50–70 Gy). HT was delivered by intravesical infusion of warmed saline solution containing bleomycin. The RTHT group had a higher response rates (RTHT: 84%, RT: 56%, p < 0.001) with decreased toxicity rates (less bladder capacity reduction) (99). In a different approach, 49 patients with nodal-negative disease of all T stages were treated with a hypofractionated RT scheme (24 Gy, 4 Gy/fraction) with or without HT. The HT group was split into a high (Tmean > 41.5°C) and a low temperature cohort (Tmean < 41.5°C). The high temperature cohort showed significantly better downstaging compared to both other groups indicating the importance of adequate temperature delivery (100). In a more recent German trial, high-risk T1 and T2 cancers were treated with transurethral resection followed by RTHTCT (50.4 Gy + 5.4–9 Gy; cisplatin and 5FU). At six weeks follow-up, a pCR of 96% was achieved. After a median follow-up of 34 months, OS was 89% with 80% of the patients being satisfied with their bladder function (63). The Dutch deep HT trial also included bladder carcinomas besides cervical and rectal carcinomas. In a randomized protocol, 101 patients with T2–T4 N0 M0 bladder carcinoma were treated with either RT or RTHT. RTHT yielded a significantly better CR (RTHT: 73%, RT: 51%, p = 0.01). However at 3y, LC and OS were not significantly different. There was no difference in toxicity (61). Taken together, some studies exist demonstrating a clinical benefit for adjunct HT with no additional toxicity. However, the patient cohorts in the different studies appeared to be quite heterogeneous by mixing locally restricted and advanced tumors. The treatment regimens used differed among studies impairing adequate comparisons. Randomized studies are necessary with clearly defined risk profiles and adequate direct comparisons with guideline-based treatment regiments.
Melanoma
One multicentric randomized trial analyzed the benefit of adjunct HT in melanomas treated with RT. Patients either received RT (24 Gy or 27 Gy in three fractions) alone or with HT (43°C for 60 min). There was no significant difference in toxicity. CR (RT alone: 35%, RTHT: 62%, p < 0.05) and LC after 2y were significantly increased (RT alone: 28%, RTHT: 46%, p = 0.008) (101). As these results are very promising, more randomized trials would help to establish a distinct role for RTHT in the treatment of melanomas, for example in combination with less hypofractionated treatment schemes.
Non-Small Cell Lung Cancer (NSCLC)
Only few studies have investigated the role of HT for the treatment of NSCLC. A multi-institutional prospective randomized trial investigated the role of HT in addition to primary RT for locally advanced NSCLCs. No significant difference of OS, local response, or treatment-related toxicity could be observed. However, with a significantly higher 1y-PFS, a certain benefit was apparent (67.5%, 29%, p = 0.036) (102). In a small case–control study encompassing 13 patients with direct bone invasion treated with RTHT (60–70 Gy) showed a possibly high efficacy in LC and survival under this unfavorable condition (103). The benefit of HT in addition to reirradiation for recurrent NSCLC after primary RT was investigated in a small retrospective study involving 33 patients. Median doses used initially and for reirradiation were 70 and 50 Gy, respectively. Toxicity was moderate and limited to a maximum toxicity of grade 3 in 9% of patients. In patients with smaller tumors (<4 cm) and no distant metastases, long time survival was partly achieved (104). All three studies employed radiofrequency capacitive heating systems. So far, only limited evidence exists showing a true benefit of HT in NSCLC treatment. More studies are necessary to explore potential areas of application.
Prostate Cancer
Feasibility of adjuvant HT treatment for prostate carcinomas has first been shown in two phase I/II studies involving locally advanced disease or recurrences after radical prostatectomy. Toxicity was limited to grades 2 and 3, respectively. Quality of life was not significantly changed by addition of HT to RT treatment (105). A HT-dependent burn occurred in one patient indicating critical temperature delivery (106, 107). Similar results were obtained in a larger phase II study involving 144 patients with high-risk disease (T2 + serum-PSA > 10 ng/ml or Gleason score ≥ 7) or locally advanced disease (T3/4) treated with RTHT and antihormonal therapy (108). A 5y-OS of 87% and 5y-biochemical PFS of 49% was observed with limited toxicity (maximum grade 2). Hurwitz et al. combined radiation with or without hormone deprivation therapy and transrectal ultrasound HT in locally advanced disease showing promising results with a 2y-DFS of 84% compared to historical 2y-DFS of 64% observed in patients in the 4-month androgen suppression cohort of the RTOG 92-02 trial (62). Currently, a phase II study examines the safety of combining HT and dose-escalated external salvage RT for recurrent prostate cancer (109). Another study is examining salvage BT combined with interstitial HT (110). In a retrospective analysis of 146 patients, no significant difference was found between patients receiving HT or not. The authors discussed that this might be due to insufficient heat delivery, since a significant difference was apparent for patients receiving a high thermal dose (111). To summarize, a set of phase II studies show promising results. However, randomized phase III trials are necessary to evaluate the actual value of adjuvant HT in the treatment of prostate carcinomas.
Glioblastoma Multiforme (GBM)
In 1998, Sneed et al. investigated the impact of adjuvant interstitial HT after a BT boost for patients with newly diagnosed, supratentorial GBM smaller than 5 cm treated with postoperative RT and concomitant hydroxyurea. After patient exclusions, 68 patients were randomized to BT with or without HT (112). HT was administered 30 min before and after a BT boost via placement of helical-coil microwave antennas. The HT group showed significantly increased survival (2y-survival 31% vs. 15%, p = 0.02) and time to progression (TTP) (median TTP 49 vs. 33 months, p = 0.045); however, this treatment was accompanied by increased grade 3 toxicity rates. In recent years, efforts were made to optimize HT delivery by improving interstitial catheters or applying focused ultrasound (113, 114). No data exist so far validating these new techniques or the combination with temozolomide.
Sarcomas
Randomized trials have shown a significant benefit of HT in addition to CT (115). However, evidence of combined RTHT in sarcoma treatment remains scarce. An early phase II study involving 17 soft tissue sarcomas (STS) patients neoadjuvant RTHT with twice weekly HT showed significantly more extensive changes in histopathological examinations than the once weekly HT group (116). In another study, 16 patients received irradiation with concurrent HT for the treatment of radiation-associated sarcomas (predominantly angiosarcoma) showing a total response rate of 75%. Toxicity was mild except one grade 4 adverse event (117). First clinical results show general feasibility of applying RTHT to sarcoma treatment. However, randomized trials are necessary to assess whether a similar benefit exists as it has been shown for neoadjuvant HTCT.
Esophageal Cancer
Several phase II studies have investigated the feasibility of trimodal neoadjuvant RTHTCT treatment. Nakajima et al. treated 24 patients with neoadjuvant RTHTCT using docetaxel. A general response rate of 41.7% with a pCR rate of 17.6% was observed (118). Described toxicities were limited to grade 2 and grade 3. In a further study, 28 patients received RTHTCT with carboplatin and paclitaxel. R0 resection was possible in all patients with mild toxicity. Pathologic evaluation yielded a pCR rate of 19%. Treatment was tolerated well with mild toxicity rates (maximum grade 2) (119). A third study treated 35 patients with advanced disease with RTHTCT (bleomycin/cisplatin and 5FU) yielding a good CR rate of 33.3% (120). In addition, multiple retrospective analyses have shown a significantly increased survival benefit in favor of RTHTCT over RTCT (121–123). In summary, there is existing evidence promising a substantial clinical value of RTHTCT. However, randomized trials are necessary directly comparing RTHTCT to standardized treatment protocols involving RTCT.
RTHT in Indications with Limited Data
Apart from the described entities, in multiple trials, RTHT has been applied to rather rare RT indications with only low levels of evidence (see Table 4 for a detailed summary of the cited trials).
A Dutch retrospective trial analyzed the efficacy of hypofractionated (28/32 Gy with a single dose of 4 Gy) reirradiation with concurrent HT for the palliation of painful unresectable recurrent rectal cancer with good to complete response in 72% of 47 patients (124). Similar results (70% pain relief) were reproduced in a prospective phase II trial by Milani et al. with normofractionated RTCTHT (125). Klaver et al. proposed a novel treatment strategy in a case series for locally advanced rectal cancer with concurrent peritoneal carcinomatosis by combing hyperthermic intraperitoneal chemotherapy with intraoperative RT showing general feasibility (126).
Thermoradiotherapy was evaluated for the palliation of symptomatic locally advanced or recurrent hormone-refractory prostate cancer in a small phase I/II study. All patients demonstrated partial response or complete response as well as complete symptom relief (127). However, two of eight preirradiated patients developed grade IV toxicities.
Primary or recurrent locally advanced pancreatic cancer was subject to an open-label study comparing RTCT with RTHTCT (gemcitabine ± 5FU/cisplatin/oxaliplatin) showing a significantly increased survival benefit without increased toxicity (mean OS: RTHTCT: 15 months, RTCT: 11 months, p = 0.025) (128). Moreover, the influence of adjunct HT for the treatment of liver lesions has been assessed. In a randomized Chinese trial of hepatocellular carcinoma patients, 1y-recurrence (RTHT: 10%, RT: 15%, p < 0.001) and mortality rates (RTHT: 12.5, RT: 20%, p < 0.001) were significantly lower for combined RTHT compared to RT alone (129). Chemorefractory colorectal cancer metastases were treated with whole-liver RT and concimittant HT showing partial response and pain relief in 30% of treated patients, respectively (130). Vaginal cancers have been chosen as target for HT in small prospective Dutch trial. Patients with vaginal carcinomas with a tumor size larger than 4 cm were treated with RTHT, whereas smaller tumors were primarily treated with RT showing no significant difference in 5y-survival (131).
In a more general approach, Jones et al. performed a randomized prospective trial pooling superficial tumors of various entities (breast carcinoma, melanoma, head and neck cancer, and others). Only tumors that appeared to be heatable on a pretest were randomized. Addition of HT to RT lead to significantly increased CR (RTHT: 66.1%, RT 42.3%, OR 1.7, p = 0.02). In coherence with many other studies, the highest difference was achieved in preirradiated patients (CR: RTHT: 68.2%, CR 23.5%) (64).
Despite the limited amount of evidence, substantial benefits of RTHT, especially for preirradiated, locally advanced and recurrent tumors, became apparent. Since there is a lack of randomized trials, definite recommendations for treatment cannot be made. In situations of recurrent or metastatic disease, RTHT may be justifiable as “individual treatment approach” on the basis of the existing evidence. In order to reach necessary patient number in potential future randomized trials, multiple entities with a similar condition (such as “recurrent,” “preirradiated,” or “locally advanced”) could be combined, following the approach of Jones et al. (64). In addition, HT centers should work more closely together for the establishment of multicenter trials capable of gathering critical patient numbers.
Combined Thermochemotherapy (CTHT)
Thermochemotherapy has been evaluated in multiple clinical trials. In contrast to RTHT, CTHT is also being combined with WBHT. A limited number of phase II studies have shown feasibility of applying WBHT to CTHT treatment of various entities such as recurrent ovarian cancer, malignant pleural mesothelioma, metastatic STS, melanoma, and pretreated metastatic colorectal cancer (132–136). Up to date, no phase III trials exist. In contrast, regional HT has been evaluated in a large phase III trial (341 patients) of STS performed as joint effort by the European Organization for the Research and Treatment of Cancer and European Society for Hyperthermic Oncology. It showed a substantially and significantly improved local PFS, DFS, and OS after adding HT to EIA (etoposide, ifosfamide, doxorubicin) CT (HR for local progression/death: 0.58, p = 0.003, local 2y-PFS: HTCT: 76%, CT: 61%; 2y-DFS: CTHT: 58%, CT: 44%, p = 0.011; per-protocol OS: HR 0.66, p = 0.038) changing daily clinical practice in HT treatment centers (115). Further randomized trials have evaluated neoadjuvant CTHT in esophageal carcinoma (40 patients; histologic effectiveness: CTHT 58.3, CT: 14.3, p < 0.05) and adjuvant CTHT after transurethral resection of bladder carcinomas (83 patients; 10y-DFS: CTHT: 53%, CT: 15%, p < 0.001) demonstrating increased treatment efficacy (137, 138). A randomized trial with NSCLC showed a small benefit regarding “clinical benefit response” (80 patients, CTHT: 82.5%, CT: 47.5%, p < 0.05) (139). General feasibility of local CTHT has also been shown in other entities such as refractory or recurrent non-testicular germ cell carcinomas, recurrent or persistent ovarian cancer, breast carcinoma, or peritoneal carcinomatosis in several phase II studies (140–143). As alternative to regional HT, hyperthermic isolated limb perfusion has been established for the treatment of STS and unresectable melanomas showing favorable results (144, 145). In summary, the few existing randomized trials suggest substantial benefit by adding HT to CT. More randomized trials are necessary to broaden the spectrum of CTHT.
Outlook
Review of the current literature has shown various retrospective and prospective trials exploring the value of adding HT to RT or RTCT regiments in multiple tumor entities. As in some entities, the real benefit of HT remains elusive, in other malignancies sustained evidence has been acquired. When considering the currently existing studies, a substantial part of evidence was gathered between the 1980s and early 2000s. Apart from technical aspects of HT, RT techniques have dramatically evolved since then. Nowadays, even better results in regard to treatment outcome as well as toxicity could be expected. Still, no widespread use of HT has been established in the last decades. Several reasons, such as reimbursement issues in certain countries, technical complexities, and challenges of homogenous heating and temperature monitoring, may have contributed to this fact. As more and more clinical trials are being published, the willingness/memorandum to make the effort of establishing HT in a rising number of institutions has increased.
The development of novel techniques with more exact heat delivery and temperature monitoring capacities may help to gain higher acceptance among physicians. HT may as well be further improved by better planning techniques. Mathematical modeling of the earlier mentioned HT effects on cell biology has paved the way to actual thermoradiotherapy planning (4). By integrating the biological HT effect into the LQ-model, a more exact RTHT treatment planning becomes possible. By adding online temperature control, as it can be achieved by MRI thermometry, temperature distribution could be optimized even further by real-time adjustments.
In the trials performed so far, HT delivery specifications were highly heterogeneous. HT frequency varied between once weekly to daily applications. Mean achieved temperature profiles differed vastly between 39 and 43°C. In trials using multiple HT specification, higher temperatures or HT frequencies were associated with better outcome (88, 100, 116). This underlines the necessity of optimizing HT schedules to optimize the treatment effect. To this end, larger randomized studies are necessary directly comparing distinct HT specifications.
Even though there are still a lot of open questions, basic research has revealed many ways of action of HT. Regarding the biological mechanisms of HT, combining HT with drugs exploiting underlying mechanisms may further increase radiosensitization. As an example, HT has been combined with antiangiogenesis agents. HT itself appears to directly impair angiogenesis at least in part by plasminogen activator inhibitor 1 induction (146). By combining HT with VEGFR2-inhibitor treatment, a synergistic antiangiogenesis effect in vivo has been shown to inhibit tumor growth (147). Regarding the immunogenic effects of HT, adjunct immunotherapy, such as checkpoint inhibition, constitutes a further interesting field of research. Before clinical trials can be designed, more basic research is necessary to evaluate the effects of thermo-immunotherapy in preclinical models.
All studies mentioned above have used HT in combination with photon-based irradiation. However, all over the world, an increasing number of particle beam facilities are being installed. Due to technical improvements, smaller and low-cost proton facilities may become available in the future possibly enabling a further widespread use. In contrast, facilities capable of delivering heavy ion-based irradiation with 12C remain scarce. In a brief summary, proton and 12C-ions share similar favorable dose distributions with low entry dose, a high dose in the “Bragg peak” followed by a more or less steep dose decline (148). In contrast to protons, 12C-ions inherit a significantly higher linear energy transfer and relative biological effectiveness (149). Several biological factors have been identified such as a low oxygen enhancement ratio (OER), less cell-cycle-dependent cell killing, inhibition of non-homologous DNA repair, cluster damages to the DNA, and more efficient cell killing of tumor stem cells (150). HT triggers killing of hypoxic, acidic as well as energy-deprived tumor cells, decreases OER, and confers direct killing of S-phased cells. Therefore, it has been proposed that combined therapy of proton irradiation with HT may have similar effectiveness as 12C beam therapy alone (151, 152). To the best of our knowledge, no data of the clinical use of simultaneous thermo-particle therapy has been published. The HYPROSAR phase I/II study is currently recruiting patients with unresectable STS at the Paul Scherrer Institut in Switzerland. Weekly HT is combined with proton beam therapy to achieve tumor downstaging with subsequent resection. There has only been one randomized trial treating 151 patients with uveal melanoma with or without adjuvant transpupillary thermotherapy months after the end of proton irradiation (153). Indeed, the rate of secondary enucleation was significant lower. Since the therapy was not conducted in direct temporal proximity, the abovementioned factors would not have taken effect. Hence, clinical trials are necessary to explore the benefit of combinational therapy. Direct comparison of thermo-particle therapy with 12C beam therapy should be performed in randomized trials.
Different technical solutions of external HT delivery have been discussed. Advances in the field of nanomedicine have introduced a novel approach for targeted HT by development of magnetic or superparamagnetic nanoparticles as recently reviewd by Datta et al. (154). Particles with cores of iron oxide or gold shells have already made their way into clinical phase I trials. Bergs et al. recently reviewed the existing particle constructs (155). Tumor-specific targeting might be achieved by passive accumulation in the tumor due to the aberrant vasculature with increased leakage and simultaneous impairment of lymphatic drainage (156). Alternatively, active targeting could be achieved by coating with tumor-specific antibodies or ligands (157). Heat can then be generated by applying external magnetic fields with rapid field alternations (158). In clinical phase I trials, dispersed nanoparticles were directly deposited at the tumor side either by percutaneous injection or intraoperatively. Only mild toxicities and quality of life impairments were observed. A maximum temperature of up to 55°C was achieved but target coverage remained insufficient (159–161). The advantage of nanoparticle-based HT may lead to a more selective heat delivery to the tumor with possibly higher temperatures and lower toxicity to adjacent normal tissues. By carrying chemotherapeutic agents, antibodies, or gene silencing RNA residues, nanoparticles may open completely new therapeutic opportunities (154). On the other side, tumor volume coverage is still far from optimal. Poorly perfused regions of tumors, in which HT has its greatest potential for radiosensitization, tend to “collect” lower particle numbers. Accumulation of particles in non-malignant tissues such as the reticuloendothelial system or by the glomerular filter of the kidney carries the risk of side effects (162). Microscopic disease, e.g., in lymphatic tissue may not be reached by sufficient high temperatures. Until safe appliance in the clinic becomes possible, more research is necessary to assess the biological risks and to optimize particle distribution and targeting. If these problems can be addressed, nanoparticles may be a valuable alternative to external HT.
Besides enhancement of RT effects, HT may also be used for radiation dose reduction. As described above, Notter et al. used a hypofractionation treatment scheme (5 × 4 Gy, one fraction per week) to treat patients with locally recurrent breast cancer. A similar CR rate of 61% was achieved compared to other studies using mean doses of 38.2 Gy (79). The relatively low dose enabled the authors to perform re-reirradiations. 17 patients with re-recurrences of lymphangiosis carcinomatosa received re-reirradiation following the same therapy scheme. A CR rate of 31% could be achieved (56).
Infections with human papilloma virus (HPV) are commonly found in carcinomas of the head and neck or cervix. HPV-positive tumors appear to be more radiosensitive than HPV-negative tumors. HT has been shown to trigger the degradation of the oncogenic HPV-derived E6 protein. As functional E6 binds and inactivates p53, HT may indirectly renew p53 activity favoring p53-dependent apoptosis. Thus, a combination of RT and HT may be particularly effective. To evaluate this effect, future trials should include HPV status for risk stratification (87). As a next step, clinical trials should test further RT dose de-escalation with concurrent HT in HPV-positive patients as it is already performed for RT alone (163, 164).
Conclusion
There is abundant evidence demonstrating that HT constitutes a valuable supplement to currently performed RT or RTCT schemes improving tumor response in tumor entities such as head and neck or cervix. It also inherits great potential to enhance RT-based therapy of recurrent disease as it has been shown for breast cancer. Through continuous improvement of HT delivery, planning, and monitoring techniques, treatment effects may further improve. Novel combinations with targeted therapy agents, immunotherapy, nanomedicine, or particle therapy constitute promising fields of further research and interesting areas for future clinical application.
Author Contributions
JP: reviewed literature and wrote the manuscript. PV: reviewed literature and wrote the manuscript. SC: reviewed literature and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BT, brachytherapy; CT, chemotherapy; DFS, disease-free survival; EBRT, external beam radiotherapy; HPV, human papilloma virus; HR, hazard ratio; HSP, heat shock protein; HT, hyperthermia; HTP, hyperthermia treatment planning; LC, local control; NK, natural killer; NMA, network meta-analysis; NSCLC, non-small cell lung cancer; OR, odds ratio; pCR, pathologic complete response; PFS, progression-free survival; RR, risk ratio; RT, radiation therapy; RTHT, thermoradiotherapy; RTCT, chemoradiotherapy; RTHTCT, thermochemoradiotherapy; SAR, specific absorption rate; TER, thermal enhancement ratio; TRTP, thermoradiotherapy planning; TTP, time to progression; WBHT, whole-body hyperthermia; wIRA, water-filtered infrared-A; y, year.
References
1. Coley W. The therapeutic value of the mixed toxins of the Streptococcus of erysipelas and Bacillus prodigiosus in the treatment of inoperable malignant tumors, with a report of one hundred and sixty cases. Am J Med Sci (1896) 112:251–81. doi:10.1097/00000441-189609000-00001
2. Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther (1994) 64(3):529–64. doi:10.1016/0163-7258(94)90023-X
3. Quinn SD, Gedroyc WM. Thermal ablative treatment of uterine fibroids. Int J Hyperthermia (2015) 31(3):272–9. doi:10.3109/02656736.2015.1010608
4. Crezee H, van Leeuwen CM, Oei AL, Stalpers LJ, Bel A, Franken NA, et al. Thermoradiotherapy planning: integration in routine clinical practice. Int J Hyperthermia (2016) 32(1):41–9. doi:10.3109/02656736.2015.1110757
5. Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia (2005) 21(8):779–90. doi:10.1080/02656730500271668
6. Vaupel PW, Kelleher DK. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: heterogeneity is the key issue. Int J Hyperthermia (2010) 26(3):211–23. doi:10.3109/02656731003596259
7. Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys (2000) 46(1):179–85. doi:10.1016/S0360-3016(99)00362-4
8. Song CW, Rhee JG, Haumschild DJ. Continuous and non-invasive quantification of heat-induced changes in blood flow in the skin and RIF-1 tumour of mice by laser Doppler flowmetry. Int J Hyperthermia (1987) 3(1):71–7. doi:10.3109/02656738709140374
9. Vaupel PW, Otte J, Manz R. Oxygenation of malignant tumors after localized microwave hyperthermia. Radiat Environ Biophys (1982) 20(4):289–300. doi:10.1007/BF01323754
10. Song CW, Shakil A, Griffin RJ, Okajima K. Improvement of tumor oxygenation status by mild temperature hyperthermia alone or in combination with carbogen. Semin Oncol (1997) 24(6):626–32.
11. Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci U S A (2010) 107(47):20477–82. doi:10.1073/pnas.1006646107
12. Thews O, Li YP, Kelleher DK, Chance B, Vaupel P. Microcirculatory function, tissue oxygenation, microregional redox status and ATP distribution in tumors upon localized infrared-A-hyperthermia at 42°C. Adv Exp Med Biol (2003) 530:237–47. doi:10.1007/978-1-4615-0075-9_23
13. Dewey WC, Thrall DE, Gillette EL. Hyperthermia and radiation – a selective thermal effect on chronically hypoxic tumor cells in vivo. Int J Radiat Oncol Biol Phys (1977) 2(1–2):99–103. doi:10.1016/0360-3016(77)90013-X
14. Overgaard J, Grau C, Lindegaard JC, Horsman MR. The potential of using hyperthermia to eliminate radioresistant hypoxic cells. Radiother Oncol (1991) 20(Suppl 1):113–6. doi:10.1016/0167-8140(91)90197-O
15. Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res (2001) 155(4):515–28. doi:10.1667/0033-7587(2001)155[0515:IOTOBM]2.0.CO;2
16. Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia (2006) 22(5):365–73. doi:10.1080/02656730600836386
17. Kelleher DK, Vaupel P. No sustained improvement in tumor oxygenation after localized mild hyperthermia. Adv Exp Med Biol (2010) 662:393–8. doi:10.1007/978-1-4419-1241-1_57
18. Mackey MA, Ianzini F. Enhancement of radiation-induced mitotic catastrophe by moderate hyperthermia. Int J Radiat Biol (2000) 76(2):273–80. doi:10.1080/095530000138925
19. Harmon BV, Corder AM, Collins RJ, Gobé GC, Allen J, Allan DJ, et al. Cell death induced in a murine mastocytoma by 42-47°C heating in vitro: evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int J Radiat Biol (1990) 58(5):845–58. doi:10.1080/09553009014552221
20. Roti Roti JL, Kampinga HH, Malyapa RS, Wright WD, vander Waal RP, Xu M. Nuclear matrix as a target for hyperthermic killing of cancer cells. Cell Stress Chaperones (1998) 3(4):245–55. doi:10.1379/1466-1268(1998)003<0245:NMAATF>2.3.CO;2
21. Lepock JR. Role of nuclear protein denaturation and aggregation in thermal radiosensitization. Int J Hyperthermia (2004) 20(2):115–30. doi:10.1080/02656730310001637334
22. Wong RS, Kapp LN, Dewey WC. DNA fork displacement rate measurements in heated Chinese hamster ovary cells. Biochim Biophys Acta (1989) 1007(2):224–7. doi:10.1016/0167-4781(89)90043-2
23. Vertrees RA, Das GC, Coscio AM, Xie J, Zwischenberger JB, Boor PJ. A mechanism of hyperthermia-induced apoptosis in ras-transformed lung cells. Mol Carcinog (2005) 44(2):111–21. doi:10.1002/mc.20124
24. Sakaguchi Y, Stephens LC, Makino M, Kaneko T, Strebel FR, Danhauser LL, et al. Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res (1995) 55(22):5459–64.
25. Calderwood SK, Stevenson MA, Hahn GM. Effects of heat on cell calcium and inositol lipid metabolism. Radiat Res (1988) 113(3):414–25. doi:10.2307/3577239
26. Dressler C, Beuthan J, Mueller G, Zabarylo U, Minet O. Fluorescence imaging of heat-stress induced mitochondrial long-term depolarization in breast cancer cells. J Fluoresc (2006) 16(5):689–95. doi:10.1007/s10895-006-0110-z
27. Yu D-Y, Matsuya Y, Zhao Q-L, Ahmed K, Wei Z-L, Nemoto H, et al. Enhancement of hyperthermia-induced apoptosis by a new synthesized class of furan-fused tetracyclic compounds. Apoptosis (2007) 12(8):1523–32. doi:10.1007/s10495-007-0080-x
28. Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A (2011) 108(24):9851–6. doi:10.1073/pnas.1101053108
29. Burgman P, Ouyang H, Peterson S, Chen DJ, Li GC. Heat inactivation of Ku autoantigen: possible role in hyperthermic radiosensitization. Cancer Res (1997) 57(14):2847–50.
30. Warters RL, Roti Roti JL. Excision of X-ray-induced thymine damage in chromatin from heated cells. Radiat Res (1979) 79(1):113–21. doi:10.2307/3575026
31. Ihara M, Suwa A, Komatsu K, Shimasaki T, Okaichi K, Hendrickson EA, et al. Heat sensitivity of double-stranded DNA-dependent protein kinase (DNA-PK) activity. Int J Radiat Biol (1999) 75(2):253–8. doi:10.1080/095530099140717
32. Oei AL, Vriend LEM, Crezee J, Franken NAP, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol (2015) 10:165. doi:10.1186/s13014-015-0462-0
33. Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia (2014) 30(8):531–9. doi:10.3109/02656736.2014.968640
34. Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol (2007) 82(5):1322–31. doi:10.1189/jlb.1106699
35. Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, et al. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci (2003) 94(3):308–13. doi:10.1111/j.1349-7006.2003.tb01438.x
36. Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, et al. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol (1999) 27(11):1627–36. doi:10.1016/S0301-472X(99)00104-6
37. Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJS, Kuppner MC, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol (2002) 169(10):5424–32. doi:10.4049/jimmunol.169.10.5424
38. Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat-shock protein-chaperoned peptides. Science (1995) 269(5230):1585–8. doi:10.1126/science.7545313
39. Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, et al. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res (2005) 11(20):7554–63. doi:10.1158/1078-0432.CCR-05-0810
40. Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia (2012) 28(1):9–18. doi:10.3109/02656736.2011.616182
41. Yoshioka H, Koga S, Maeta M, Shimizu N, Hamazoe R, Murakami A. The influence of hyperthermia in vitro on the functions of peritoneal macrophages in mice. Jpn J Surg (1990) 20(1):119–22. doi:10.1007/BF02470725
42. Basu S, Srivastava PK. Fever-like temperature induces maturation of dendritic cells through induction of hsp90. Int Immunol (2003) 15(9):1053–61. doi:10.1093/intimm/dxg104
43. Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res (2001) 61(7):3027–32.
44. Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest (2011) 121(10):3846–59. doi:10.1172/JCI44952
45. Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol (2002) 3(8):487–97. doi:10.1016/S1470-2045(02)00818-5
46. Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, et al. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer (1986) 58(8):1589–95. doi:10.1002/1097-0142(19861015)58:8<1589::AID-CNCR2820580802>3.0.CO;2-B
47. Kok HP, Crezee J. A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int J Hyperthermia (2017) 33(4):378–86. doi:10.1080/02656736.2016.1268726
48. Rhee JG, Lee CK, Osborn J, Levitt SH, Song CW. Precooling prevents overheating of subcutaneous fat in the use of RF capacitive heating. Int J Radiat Oncol Biol Phys (1991) 20(5):1009–15. doi:10.1016/0360-3016(91)90198-D
49. Seebass M, Beck R, Gellermann J, Nadobny J, Wust P. Electromagnetic phased arrays for regional hyperthermia: optimal frequency and antenna arrangement. Int J Hyperthermia (2001) 17(4):321–36. doi:10.1080/02656730110049529
50. Wust P, Fähling H, Wlodarczyk W, Seebass M, Gellermann J, Deuflhard P, et al. Antenna arrays in the SIGMA-eye applicator: interactions and transforming networks. Med Phys (2001) 28(8):1793–805. doi:10.1118/1.1388220
51. Crezee J, Van Haaren PMA, Westendorp H, De Greef M, Kok HP, Wiersma J, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: a preclinical study. Int J Hyperthermia (2009) 25(7):581–92. doi:10.3109/02656730903213374
52. Togni P, Rijnen Z, Numan WCM, Verhaart RF, Bakker JF, van Rhoon GC, et al. Electromagnetic redesign of the HYPERcollar applicator: toward improved deep local head-and-neck hyperthermia. Phys Med Biol (2013) 58(17):5997–6009. doi:10.1088/0031-9155/58/17/5997
53. Mittal BB, Sathiaseelan V, Kies MS. Simultaneous localized 915 MHz external and interstitial microwave hyperthermia to heat tumors greater than 3 cm in depth. Int J Radiat Oncol Biol Phys (1990) 19(3):669–75. doi:10.1016/0360-3016(90)90495-6
54. Seegenschmiedt MH, Klautke G, Walther E, Feldmann HJ, Katalinic A, Stuschke M, et al. Water-filtered infrared-A-hyperthermia combined with radiotherapy in advanced and recurrent tumors. Initial results of a multicenter phase I-II study. Strahlenther Onkol (1996) 172(9):475–84.
55. Vaupel P, Kelleher DK, Krüger W. Water-filtered infrared-A radiation: a novel technique to heat superficial tumors. Strahlenther Onkol (1992) 168(11):633–9.
56. Notter M, Piazena H, Vaupel P. Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: a retrospective study of 73 patients. Int J Hyperthermia (2017) 33(2):227–36. doi:10.1080/02656736.2016.1235731
57. Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, et al. Clinical evaluation and verification of the hyperthermia treatment planning system hyperplan. Int J Radiat Oncol Biol Phys (2000) 47(4):1145–56. doi:10.1016/S0360-3016(00)00425-9
58. Sreenivasa G, Gellermann J, Rau B, Nadobny J, Schlag P, Deuflhard P, et al. Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys (2003) 55(2):407–19. doi:10.1016/S0360-3016(02)04144-5
59. van Haaren PMA, Kok HP, van den Berg CAT, Zum Vörde Sive Vörding PJ, Oldenborg S, Stalpers LJA, et al. On verification of hyperthermia treatment planning for cervical carcinoma patients. Int J Hyperthermia (2007) 23(3):303–14. doi:10.1080/02656730701297538
60. Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys (1996) 35(4):731–44. doi:10.1016/0360-3016(96)00154-X
61. van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet (2000) 355(9210):1119–25. doi:10.1016/S0140-6736(00)02059-6
62. Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer. Cancer (2011) 117(3):510–6. doi:10.1002/cncr.25619
63. Wittlinger M, Rödel CM, Weiss C, Krause SF, Kühn R, Fietkau R, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol (2009) 93(2):358–63. doi:10.1016/j.radonc.2009.09.018
64. Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol (2005) 23(13):3079–85. doi:10.1200/JCO.2005.05.520
65. Wust P, Gellermann J, Harder C, Tilly W, Rau B, Dinges S, et al. Rationale for using invasive thermometry for regional hyperthermia of pelvic tumors. Int J Radiat Oncol Biol Phys (1998) 41(5):1129–37. doi:10.1016/S0360-3016(98)00165-5
66. Gellermann J, Wlodarczyk W, Feussner A, Fähling H, Nadobny J, Hildebrandt B, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia (2005) 21(6):497–513. doi:10.1080/02656730500070102
67. Weihrauch M, Wust P, Weiser M, Nadobny J, Eisenhardt S, Budach V, et al. Adaptation of antenna profiles for control of MR guided hyperthermia (HT) in a hybrid MR-HT system. Med Phys (2007) 34(12):4717–25. doi:10.1118/1.2804617
68. Stakhursky VL, Arabe O, Cheng K-S, Macfall J, Maccarini P, Craciunescu O, et al. Real-time MRI-guided hyperthermia treatment using a fast adaptive algorithm. Phys Med Biol (2009) 54(7):2131–45. doi:10.1088/0031-9155/54/7/019
69. Kok HP, Ciampa S, de Kroon-Oldenhof R, Steggerda-Carvalho EJ, van Stam G, Zum Vörde Sive Vörding PJ, et al. Toward online adaptive hyperthermia treatment planning: correlation between measured and simulated specific absorption rate changes caused by phase steering in patients. Int J Radiat Oncol Biol Phys (2014) 90(2):438–45. doi:10.1016/j.ijrobp.2014.05.1307
70. Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys (1984) 10(6):787–800. doi:10.1016/0360-3016(84)90379-1
71. Sapareto SA, Hopwood LE, Dewey WC. Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res (1978) 73(2):221–33. doi:10.2307/3574816
72. Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys (1980) 6:1507–17. doi:10.1016/0360-3016(80)90008-5
73. Marino C, Cividalli A. Combined radiation and hyperthermia: effects of the number of heat fractions and their interval on normal and tumour tissues. Int J Hyperthermia (1992) 8(6):771–81. doi:10.3109/02656739209005025
74. Rhee JG, Schuman VL, Song CW, Levitt SH. Difference in the thermotolerance of mouse mammary carcinoma cells in vivo and in vitro. Cancer Res (1987) 47(10):2571–5.
75. Overgaard J, Nielsen OS. The importance of thermotolerance for the clinical treatment with hyperthermia. Radiother Oncol (1983) 1(2):167–78. doi:10.1016/S0167-8140(83)80019-X
76. Nah BS, Choi IB, Oh WY, Osborn JL, Song CW. Vascular thermal adaptation in tumors and normal tissue in rats. Int J Radiat Oncol Biol Phys (1996) 35(1):95–101. doi:10.1016/S0360-3016(96)85016-4
77. Griffin RJ, Dings RPM, Jamshidi-Parsian A, Song CW. Mild temperature hyperthermia and radiation therapy: role of tumour vascular thermotolerance and relevant physiological factors. Int J Hyperthermia (2010) 26(3):256–63. doi:10.3109/02656730903453546
78. Petrella F, Radice D, Borri A, Galetta D, Gasparri R, Casiraghi M, et al. Chest wall resection and reconstruction for locally recurrent breast cancer: from technical aspects to biological assessment. Surgeon (2016) 14(1):26–32. doi:10.1016/j.surge.2014.03.001
79. Datta NR, Puric E, Klingbiel D, Gomez S, Bodis S. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys (2016) 94(5):1073–87. doi:10.1016/j.ijrobp.2015.12.361
80. Linthorst M, Baaijens M, Wiggenraad R, Creutzberg C, Ghidey W, van Rhoon GC, et al. Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: results in 248 patients. Radiother Oncol (2015) 117(2):217–22. doi:10.1016/j.radonc.2015.04.019
81. Linthorst M, van Geel AN, Baaijens M, Ameziane A, Ghidey W, van Rhoon GC, et al. Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol (2013) 109(2):188–93. doi:10.1016/j.radonc.2013.05.010
82. Lutgens L, van der Zee J, Pijls-Johannesma M, De Haas-Kock DFM, Buijsen J, Mastrigt GAV, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev (2010) 3:CD006377. doi:10.1002/14651858.CD006377
83. Franckena M, Stalpers LJA, Koper PCM, Wiggenraad RGJ, Hoogenraad WJ, van Dijk JDP, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch Deep Hyperthermia Trial. Int J Radiat Oncol Biol Phys (2008) 70(4):1176–82. doi:10.1016/j.ijrobp.2007.07.2348
84. Datta NR, Rogers S, Klingbiel D, Gomez S, Puric E, Bodis S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: a systematic review with conventional and network meta-analyses. Int J Hyperthermia (2016) 6736:1–34. doi:10.1080/02656736.2016.1195924
85. Chen HW, Jei JWL. A randomized trial of hyperthermoradiochemotherapy for uterine cervix. Chin J Oncol (1997) 24(249):51.
86. Lutgens LCHW, Koper PCM, Jobsen JJ, van der Steen-Banasik EM, Creutzberg CL, van den Berg HA, et al. Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: results of the randomized RADCHOC trial. Radiother Oncol (2016) 120(3):378–82. doi:10.1016/j.radonc.2016.02.010
87. Datta NR, Rogers S, Ordonez SG, Puric E, Bodis S. Hyperthermia and radiotherapy in the management of head and neck cancers: a systematic review and meta-analysis. Int J Hyperthermia (2016) 32(1):31–40. doi:10.3109/02656736.2015.1099746
88. Kang M, Liu WQ, Qin YT, Wei ZX, Wang RS. Long-term efficacy of microwave hyperthermia combined with chemoradiotherapy in treatment of nasopharyngeal carcinoma with cervical lymph node metastases. Asian Pac J Cancer Prev (2013) 14(12):7395–400. doi:10.7314/APJCP.2013.14.12.7395
89. Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: a phase III clinical study. Int J Hyperthermia (2011) 27(2):180–6. doi:10.3109/02656736.2010.503982
90. Zhao C, Chen J, Yu B, Chen X. Improvement in quality of life in patients with nasopharyngeal carcinoma treated with non-invasive extracorporeal radiofrequency in combination with chemoradiotherapy. Int J Radiat Biol (2014) 90(10):853–8. doi:10.3109/09553002.2014.916579
91. De Wee E, Verduijn GM, Rijnen Z, Togni P, Hardillo JAU, Ten Hove I, et al. OC-0335: feasibility of deep head and neck hyperthermia. Radiother Oncol (2015) 115:S165–6. doi:10.1016/S0167-8140(15)40333-0
92. Paulides MM, Verduijn GM, van Holthe N. Status quo and directions in deep head and neck hyperthermia. Radiat Oncol (2016) 11:21. doi:10.1186/s13014-016-0588-8
93. De Haas-Kock DFM, Buijsen J, Pijls-Johannesma M, Lutgens L, Lammering G, Mastrigt GAV, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev (2009) 3:CD006269. doi:10.1002/14651858.CD006269.pub2
94. Schroeder C, Gani C, Lamprecht U, von Weyhern CH, Weinmann M, Bamberg M, et al. Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int J Hyperthermia (2012) 28(8):707–14. doi:10.3109/02656736.2012.722263
95. Kang MK, Kim MS, Kim JH. Clinical outcomes of mild hyperthermia for locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Hyperthermia (2011) 27(5):482–90. doi:10.3109/02656736.2011.563769
96. Barsukov YA, Gordeyev SS, Tkachev SI, Fedyanin MY, Perevoshikov AG. Phase II study of concomitant chemoradiotherapy with local hyperthermia and metronidazole for locally advanced fixed rectal cancer. Colorectal Dis (2013) 15(9):1107–14. doi:10.1111/codi.12281
97. Rasulov AO, Gordeyev SS, Barsukov YA, Tkachev SI, Malikhov AG, Balyasnikova SS, et al. Short-course preoperative radiotherapy combined with chemotherapy, delayed surgery and local hyperthermia for rectal cancer: a phase II study. Int J Hyperthermia (2016) 33:1–9.
98. Schulze T, Wust P, Gellermann J, Hildebrandt B, Riess H, Felix R, et al. Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia (2006) 22(4):301–18. doi:10.1080/02656730600665504
99. Matsui K, Takebayashi S, Watai K, Kakehi M, Kubota Y, Yao M, et al. Combination radiotherapy of urinary bladder carcinoma with chemohyperthermia. Int J Hyperthermia (1991) 7(1):19–26. doi:10.3109/02656739109004973
100. Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, et al. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyperthermia (1993) 10(1):31–40. doi:10.3109/02656739409009329
101. Overgaard J, Bentzen SM, Overgaard J, Gonzalez DG, Hulshof MC, Arcangeli G, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet (1995) 345(8949):540–3. doi:10.1016/S0140-6736(95)90463-8
102. Mitsumori M, Zhi-Fan Z, Oliynychenko P, Park JH, Choi IB, Tatsuzaki H, et al. Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Clin Oncol (2007) 12(3):192–8. doi:10.1007/s10147-006-0647-5
103. Sakurai H, Hayakawa K, Mitsuhashi N, Tamaki Y, Nakayama Y, Kurosaki H, et al. Effect of hyperthermia combined with external radiation therapy in primary non-small cell lung cancer with direct bony invasion. Int J Hyperthermia (2002) 18(5):472–83. doi:10.1080/02656730210146917
104. Ohguri T, Imada H, Yahara K, Moon SD, Yamaguchi S, Yatera K, et al. Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: a potential modality for inducing long-term survival in selected patients. Lung Cancer (2012) 77(1):140–5. doi:10.1016/j.lungcan.2012.02.018
105. Van Vulpen M, de Leeuw JRJ, van Gellekom MPR, van der Hoeven J, de Graeff A, van Moorselaar RJA, et al. A prospective quality of life study in patients with locally advanced prostate cancer, treated with radiotherapy with or without regional or interstitial hyperthermia. Int J Hyperthermia (2003) 19(4):402–13. doi:10.1080/0265673031000063855
106. Tilly W, Gellermann J, Graf R, Hildebrandt B, Weißbach L, Budach V, et al. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol (2005) 181(1):35–41. doi:10.1007/s00066-005-1296-8
107. Van Vulpen M, de Leeuw AAC, Raaymakers BW, van Moorselaar RJA, Hofman P, Lagendijk JJW, et al. Radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate cancer: preliminary results. BJU Int (2004) 93(1):36–41. doi:10.1111/j.1464-410X.2004.04551.x
108. Maluta S, Dall’Oglio S, Romano M, Marciai N, Pioli F, Giri MG, et al. Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: preliminary results of a prospective phase II study. Int J Hyperthermia (2007) 23(5):451–6. doi:10.1080/02656730701553260
109. Müller A-C, Zips D, Heinrich V, Lamprecht U, Voigt O, Burock S, et al. Regional hyperthermia and moderately dose-escalated salvage radiotherapy for recurrent prostate cancer. Protocol of a phase II trial. Radiat Oncol (2015) 10:138. doi:10.1186/s13014-015-0442-4
110. Kukiełka AM, Strnad V, Stauffer P, Dabrowski T, Hetnał M, Nahajowski D, et al. Salvage brachytherapy in combination with interstitial hyperthermia for locally recurrent prostate carcinoma following external beam radiation therapy: a prospective phase II study. J Contemp Brachytherapy (2015) 7(3):254–8. doi:10.5114/jcb.2015.51871
111. Yahara K, Ohguri T, Yamaguchi S, Imada H, Narisada H, Ota S, et al. Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: thermal parameters correlated with biochemical relapse-free survival. Int J Hyperthermia (2015) 31(6):600–8. doi:10.3109/02656736.2015.1062214
112. Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys (1998) 40(2):287–95. doi:10.1016/S0360-3016(97)00731-1
113. Hulshof MC, Raaymakers BW, Lagendijk JJW, Koot RW, Crezee H, Stalpers LJA, et al. A feasibility study of interstitial hyperthermia plus external beam radiotherapy in glioblastoma multiforme using the multi electrode current source (MECS) system. Int J Hyperthermia (2004) 20(5):451–63. doi:10.1080/02656730410001668357
114. Borasi G, Nahum A, Paulides MM, Powathil G, Russo G, Fariselli L, et al. Fast and high temperature hyperthermia coupled with radiotherapy as a possible new treatment for glioblastoma. J Ther Ultrasound (2016) 4(1):32. doi:10.1186/s40349-016-0078-3
115. Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem B-C, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol (2010) 11(6):561–70. doi:10.1016/S1470-2045(10)70071-1
116. Leopold KA, Harrelson J, Prosnitz L, Samulski TV, Dewhirst MW, Oleson JR, et al. Preoperative hyperthermia and radiation for soft tissue sarcomas: advantage of two vs one hyperthermia treatments per week. Int J Radiat Oncol Biol Phys (1989) 16(1):107–15. doi:10.1016/0360-3016(89)90017-5
117. de Jong MAA, Oldenborg S, Bing Oei S, Griesdoorn V, Kolff MW, Koning CCE, et al. Reirradiation and hyperthermia for radiation-associated sarcoma. Cancer (2012) 118(1):180–7. doi:10.1002/cncr.26252
118. Nakajima M, Kato H, Sakai M, Sano A, Miyazaki T, Sohda M, et al. Planned esophagectomy after neoadjuvant hyperthermo-chemoradiotherapy using weekly low-dose docetaxel and hyperthermia for advanced esophageal carcinomas. Hepatogastroenterology (2015) 62(140):887–91.
119. Hulshof MC, van Haaren PMA, van Lanschot JJB, Richel DJ, Fockens P, Oldenborg S, et al. Preoperative chemoradiation combined with regional hyperthermia for patients with resectable esophageal cancer. Int J Hyperthermia (2009) 25(1):79–85. doi:10.1080/02656730802464078
120. Sakamoto T, Katoh H, Shimizu T, Yamashita I, Takemori S, Tazawa K, et al. Clinical results of treatment of advanced esophageal carcinoma with hyperthermia in combination with chemoradiotherapy. Chest (1997) 112(6):1487–93. doi:10.1378/chest.112.6.1487
121. Kuwano H, Sumiyoshi K, Watanabe M, Sadanaga N, Nozoe T, Yasuda M, et al. Preoperative hyperthermia combined with chemotherapy and irradiation for the treatment of patients with esophageal carcinoma. Tumori (1995) 81(1):18–22.
122. Liming S, Yongling J, Qiner W, Xianghui D. Regional hyperthermia combined with radiotherapy for esophageal squamous cell carcinoma with supraclavicular lymph node metastasis. Oncotarget (2016) 8(3):5339–48. doi:10.18632/oncotarget.14148
123. Nozoe T, Kuwano H, Sadanaga N, Watanabe M, Yasuda M, Sugimachi K. Hyperthermia combined with chemotherapy and irradiation for the prolongation of the postoperative prognosis in the patients with esophageal carcinoma invading neighbouring structures. Int Surg (1996) 81(1):21–6.
124. Juffermans JHM, Hanssens PEJ, van Putten WLJ, van Rhoon GC, van der Zee J. Reirradiation and hyperthermia in rectal carcinoma: a retrospective study on palliative effect. Cancer (2003) 98(8):1759–66. doi:10.1002/cncr.11719
125. Milani V, Pazos M, Issels RD, Buecklein V, Rahman S, Tschoep K, et al. Radiochemotherapy in combination with regional hyperthermia in preirradiated patients with recurrent rectal cancer. Strahlenther Onkol (2008) 184(3):163–8. doi:10.1007/s00066-008-1731-8
126. Klaver YLB, Lemmens VEPP, Nienhuijs SW, Nieuwenhuijzen GAP, Rutten HJT, de Hingh IHJT. Intraoperative radiotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Strahlenther Onkol (2013) 189(3):256–60. doi:10.1007/s00066-012-0282-1
127. Kalapurakal JA, Pierce M, Chen A, Sathiaseelan V. Efficacy of irradiation and external hyperthermia in locally advanced, hormone-refractory or radiation recurrent prostate cancer: a preliminary report. Int J Radiat Oncol Biol Phys (2003) 57(3):654–64. doi:10.1016/S0360-3016(03)00625-4
128. Maluta S, Schaffer M, Pioli F, Dall’Oglio S, Pasetto S, Schaffer PM, et al. Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer. Strahlenther Onkol (2011) 187(10):619–25. doi:10.1007/s00066-011-2226-6
129. Dong Y, Wu G. Analysis of short and long term therapeutic effects of radiofrequency hyperthermia combined with conformal radiotherapy in hepatocellular carcinoma. J BUON (2016) 21(2):407–11.
130. Yu JI, Park HC, Choi DH, Noh JM, Oh D, Park JS, et al. Prospective phase II trial of regional hyperthermia and whole liver irradiation for numerous chemorefractory liver metastases from colorectal cancer. Radiat Oncol J (2016) 34(1):34–44. doi:10.3857/roj.2016.34.1.34
131. Aktas M, de Jong D, Nuyttens JJ, van der Zee J, Wielheesen DHM, Batman E, et al. Concomitant radiotherapy and hyperthermia for primary carcinoma of the vagina: a cohort study. Eur J Obstet Gynecol Reprod Biol (2007) 133(1):100–4. doi:10.1016/j.ejogrb.2006.05.005
132. Atmaca A, Al-Batran S-E, Neumann A, Kolassa Y, Jäger D, Knuth A, et al. Whole-body hyperthermia (WBH) in combination with carboplatin in patients with recurrent ovarian cancer – a phase II study. Gynecol Oncol (2009) 112(2):384–8. doi:10.1016/j.ygyno.2008.11.001
133. Bruns I, Kohlmann T, Wiedemann GJ, Bakhshandeh A. Bewertung des therapeutischen Gewinns von Patienten mit Pleuramesotheliom unter Therapie mit 41,8°C Ganzkörperhyperthermie plus Ifosfamid, Carboplatin und Etoposid (ICE) anhand des Modifizierten Brunner-Score (MBS). Pneumologie (2004) 58(4):210–6. doi:10.1055/s-2003-812525
134. Richel O, zum Vörde Sive Vörding PJ, Rietbroek R, vander Velden J, van Dijk JDP, Schilthuis MS, et al. Phase II study of carboplatin and whole body hyperthermia (WBH) in recurrent and metastatic cervical cancer. Gynecol Oncol (2004) 95(3):680–5. doi:10.1016/j.ygyno.2004.08.023
135. Hegewisch-Becker S, Gruber Y, Corovic A, Pichlmeier U, Atanackovic D, Nierhaus A, et al. Whole-body hyperthermia (41.8°C) combined with bimonthly oxaliplatin, high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer: a phase II study. Ann Oncol (2002) 13(8):1197–204. doi:10.1093/annonc/mdf216
136. Engelhardt R, Müller U, Weth-Simon R, Neumann HA, Löhr GW. Treatment of disseminated malignant melanoma with cisplatin in combination with whole-body hyperthermia and doxorubicin. Int J Hyperthermia (1980) 6(3):511–5. doi:10.3109/02656739009140947
137. Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int (2011) 107(6):912–8. doi:10.1111/j.1464-410X.2010.09654.x
138. Sugimach K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: a prospective randomized trial. Int J Hyperthermia (1994) 10(4):485–93. doi:10.3109/02656739409009352
139. Shen H, Li X-D, Wu C-P, Yin Y-M, Wang R, Shu Y-Q. The regimen of gemcitabine and cisplatin combined with radio frequency hyperthermia for advanced non-small cell lung cancer: a phase II study. Int J Hyperthermia (2011) 27(1):27–32. doi:10.3109/02656736.2010.500645
140. Wessalowski R, Schneider DT, Mils O, Friemann V, Kyrillopoulou O, Schaper J, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol (2013) 14(9):843–52. doi:10.1016/S1470-2045(13)70271-7
141. Cho CH, Wust P, Hildebrandt B, Issels RD, Sehouli J, Kerner T, et al. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: evaluation of two annular-phased-array applicators. Int J Hyperthermia (2008) 24(5):399–408. doi:10.1080/02656730801929915
142. Fotopoulou C, Cho C-H, Kraetschell R, Gellermann J, Wust P, Lichtenegger W, et al. Regional abdominal hyperthermia combined with systemic chemotherapy for the treatment of patients with ovarian cancer relapse: results of a pilot study. Int J Hyperthermia (2010) 26(2):118–26. doi:10.3109/02656730903369200
143. Vujaskovic Z, Kim DW, Jones E, Lan L, Mccall L, Dewhirst MW, et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia (2010) 26(5):514–21. doi:10.3109/02656731003639364
144. Trabulsi NH, Patakfalvi L, Nassif MO, Turcotte RE, Nichols A, Meguerditchian AN. Hyperthermic isolated limb perfusion for extremity soft tissue sarcomas: systematic review of clinical efficacy and quality assessment of reported trials. J Surg Oncol (2012) 106(8):921–8. doi:10.1002/jso.23200
145. Koops HS, Vaglini M, Suciu S, Kroon BB, Thompson JF, Göhl J, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol (1998) 16(9):2906–12.
146. Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P, et al. Hyperthermia inhibits angiogenesis by plasminogen activator inhibitor. Cancer Res (2003) 63(7):1500–7.
147. Nie W, Ma XL, Sang YX, Li YL, Gao X, Xu GC, et al. Synergic antitumor effect of SKLB1002 and local hyperthermia in 4T1 and CT26. Clin Exp Med (2014) 14(2):203–13. doi:10.1007/s10238-012-0225-2
148. Wilkens J, Elfke U. Direct comparison of biologically optimized spread-out Bragg peaks for protons and carbon ions. Int J Radiat Oncol Biol Phys (2008) 70(1):262–6. doi:10.1016/j.ijrobp.2007.08.029
149. Dokic I, Mairani A, Niklas M, Zimmermann F, Jäkel O, Debus J, et al. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams. Oncotarget (2016) 7(35):56676–89. doi:10.18632/oncotarget.10996
150. Schlaff CD, Krauze A, Belard A, Connell JJO, Camphausen KA. Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol (2014) 9(1):1–19. doi:10.1186/1748-717X-9-88
151. Datta NR, Puric E, Schneider R, Weber DC, Rogers S, Bodis S. Could hyperthermia with proton therapy mimic carbon ion therapy? Exploring a thermo-radiobiological rationale. Int J Hyperthermia (2014) 30(7):524–30. doi:10.3109/02656736.2014.963703
152. Robinson JE, Wizenberg MJ, McCready WA. Combined hyperthermia and radiation suggest and alternative to heavy particle therapy for reduced oxygen enhancement ratios. Nature (1974) 251(5475):521–2. doi:10.1038/251521a0
153. Desjardins L, Lumbroso-Le Rouic L, Levy-Gabriel C, Dendale R, Delacroix S, Nauraye C, et al. Combined proton beam radiotherapy and transpupillary thermotherapy for large uveal melanomas: a randomized study of 151 patients. Ophthalmic Res (2006) 38(5):255–60. doi:10.1159/000094834
154. Datta NR, Krishnan S, Speiser DE, Neufeld E, Kuster N, Bodis S, et al. Magnetic nanoparticle-induced hyperthermia with appropriate payloads: Paul Ehrlich’s “magic (nano)bullet” for cancer theranostics? Cancer Treat Rev (2016) 50:217–27. doi:10.1016/j.ctrv.2016.09.016
155. Bergs JWJ, Wacker MG, Hehlgans S, Piiper A, Multhoff G, Rödel C, et al. The role of recent nanotechnology in enhancing the efficacy of radiation therapy. Biochim Biophys Acta (2015) 1856(1):130–43. doi:10.1016/j.bbcan.2015.06.008
156. Huynh E, Zheng G. Cancer nanomedicine: addressing the dark side of the enhanced permeability and retention effect. Nanomedicine (2015) 10:15–7. doi:10.2217/nnm.15.86
157. Zhang J, Dewilde AH, Chinn P, Foreman A, Barry S, Kanne D, et al. Herceptin-directed nanoparticles activated by an alternating magnetic field selectively kill HER-2 positive human breast cells in vitro via hyperthermia. Int J Hyperthermia (2011) 27(7):682–97. doi:10.3109/02656736.2011.609863
158. Carrey J, Mehdaoui B, Respaud M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: application to magnetic hyperthermia optimization. J Appl Phys (2011) 109(8):83921. doi:10.1063/1.3551582
159. Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol (2011) 103(2):317–24. doi:10.1007/s11060-010-0389-0
160. Stern JM, Kibanov Solomonov VV, Sazykina E, Schwartz JA, Gad SC, Goodrich GP. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol (2016) 35(1):38–46. doi:10.1177/1091581815600170
161. Wust P, Gneveckow U, Johannsen M, Böhmer D, Henkel T, Kahmann F, et al. Magnetic nanoparticles for interstitial thermotherapy – feasibility, tolerance and achieved temperatures. Int J Hyperthermia (2006) 22(8):673–85. doi:10.1080/02656730601106037
162. Durymanov MO, Rosenkranz AA, Sobolev AS. Current approaches for improving intratumoral accumulation and distribution of nanomedicines. Theranostics (2015) 5(9):1007–20. doi:10.7150/thno.11742
163. Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer (2015) 15(1):602. doi:10.1186/s12885-015-1598-x
164. Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol (2017) 35(5):490–7. doi:10.1200/JCO.2016.68.3300
Keywords: hyperthermia, radiation oncology, reirradiation, infrared-A, thermoradiotherapy
Citation: Peeken JC, Vaupel P and Combs SE (2017) Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 7:132. doi: 10.3389/fonc.2017.00132
Received: 28 February 2017; Accepted: 06 June 2017;
Published: 30 June 2017
Edited by:
William Small, Jr., Stritch School of Medicine, United StatesReviewed by:
Mark Hurwitz, Thomas Jefferson University, United StatesAaron Howard Wolfson, University of Miami, United States
Eric D. Donnelly, Northwestern Memorial Hospital, United States
Copyright: © 2017 Peeken, Vaupel and Combs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie E. Combs, stephanie.combs@tum.de
 Jan C. Peeken
Jan C. Peeken Peter Vaupel
Peter Vaupel Stephanie E. Combs
Stephanie E. Combs