- 1Department of Radiation Oncology, University of Rochester Medical Center, Rochester, NY, United States
- 2Department of Radiation Oncology, Duke University Health System, Raleigh, NC, United States
- 3Department of Radiation and Cellular Oncology, University of Chicago, Chicago, IL, United States
Non-small cell lung cancer (NSCLC) typically presents at an advanced stage, which is often felt to be incurable, and such patients are usually treated with a palliative approach. Accumulating retrospective and prospective clinical evidence, including a recently completed randomized trial, support the existence of an oligometastatic disease state wherein select individuals with advanced NSCLC may experience historically unprecedented prolonged survival with aggressive local treatments, consisting of radiotherapy and/or surgery, to limited sites of metastatic disease. This is reflected in the most recent AJCC staging subcategorizing metastatic disease into intra-thoracic (M1a), a single extra thoracic site (M1b), and more diffuse metastases (M1c). In the field of radiation oncology, recent technological advances have allowed for the delivery of very high, potentially ablative, doses of radiotherapy to both intra- and extra-cranial disease sites, referred to as stereotactic radiosurgery and stereotactic body radiotherapy (or SABR), in much shorter time periods compared to conventional radiation and with minimal associated toxicity. At the same time, significant improvements in systemic therapy, including platinum-based doublet chemotherapy, molecular agents targeting oncogene-addicted NSCLC, and immunotherapy in the form of checkpoint inhibitors, have led to improved control of micro-metastatic disease and extended survival sparking newfound interest in combining these agents with ablative local therapies to provide additive, and in the case of radiation and immunotherapy, potentially synergistic, effects in order to further improve progression-free and overall survival. Currently, despite the tantalizing potential associated with aggressive local therapy in the setting of oligometastatic NSCLC, well-designed prospective randomized controlled trials sufficiently powered to detect and measure the possible added benefit afforded by this approach are desperately needed.
Introduction
Although lung cancer incidence and mortality are declining, due in large part to public health smoking cessation efforts, it remains the leading cause of cancer-related mortality both in the United States and worldwide (1). The majority of lung cancer patients have non-small cell lung cancer (NSCLC) and commonly present with metastases involving distant organ sites. Historically, palliative treatment with chemotherapy has been the standard of care for metastatic NSCLC, and outcomes with this approach have been frustratingly dismal, resulting in a median overall survival (OS) of only 8–10 months (2). However, in the past 5 years, clinical trials evaluating the efficacy of targeted therapy with receptor tyrosine kinase inhibitors (TKIs) and immunotherapy with checkpoint inhibitors represent a breakthrough in the care of advanced NSCLC offering improvements in progression-free survival (PFS) and OS (3–5). Despite these advances, long-term PFS remains limited to a relatively small subset of metastatic NSCLC patients.
Radiation therapy, a critical component of curative treatment for non-metastatic NSCLC, has classically been reserved for tumor-related symptom palliation in the metastatic disease setting. The past decade has brought about dramatic improvements in the planning and delivery of radiation treatments due to technical advancements in computing power, diagnostic imaging, and motion management. This has led to the increased use of precisely targeted highly conformal radiation, often in large doses per treatment.
Hypofractionated image-guided radiotherapy (HIGRT), typically referred to as “stereotactic radiosurgery” (SRS) when delivered to an intracranial target in one or more fractions or “stereotactic body radiotherapy” or “stereotactic ablative radiotherapy” (SBRT or SABR) when given to extra-cranial body sites in one or more fractions, has been shown to result in high rates of treated tumor control (6–8) with a favorable toxicity profile and improved convenience (9) when compared to conventionally fractionated external beam radiation (EBRT). Although not yet elucidated entirely, it is postulated that hypofractionated radiotherapy may accomplish tumor killing via different biological mechanisms than conventional fractionation, one being the possible infliction of endothelial or vascular damage (10, 11).
The role of radiotherapy, delivered as SBRT or conventionally fractionated EBRT with or without systemic therapy, is rapidly evolving with dramatically increased utilization in the treatment of advanced NSCLC patients with limited sites of metastatic disease termed “oligometastases” (12). Despite these advances, the appropriate selection of oligometastatic patients for curative-intent local treatment, optimal integration of radiotherapy with systemic therapy, and the added long-term benefit such as aggressive treatment approach provides have not been sufficiently clarified.
The Oligometastatic State
One of the hallmarks of cancer is the development of pioneer cells that are able to release from the primary tumor site and metastasize to regional lymph nodes and/or distant organs via the lymphatics, blood stream, or direct extension (13). The risk of subclinical dissemination in solid tumors, even in the setting of apparently localized disease, is variable and dependent on tumor histology, size, grade, stage, genetics, and a host of other factors, many of which are not yet understood. Hellman and Weichselbaum postulated the existence of an oligometastatic state in which tumors develop sites of distant metastasis in a single or limited number of organs as a function of the underlying biology of tumor cells and the unique receptiveness of distant organ sites (“seed and soil”) (14). This concept of oligometastases is derived from the spectrum theory that bridges the gap between the Fisher and Halstedian viewpoints on cancer. Fisher argued many tumors are micro-metastatic from inception even when presenting without clinical/radiographic evidence of distant metastatic disease (15), whereas Halsted postulated orderly spread from the primary tumor into regional lymph nodes and ultimately distant organs (16). In the spectrum theory of cancer, the oligometastatic state may reflect patients with more indolent disease courses that may be cured or rendered disease free for long time intervals with aggressive local treatment of distant metastases. We will further discuss the rationale and review the ever-growing clinical evidence supporting an aggressive treatment approach in select NSCLC patients presenting with oligometastatic disease.
Historically, before the development of systemic therapies with increased efficacy, aggressive metastasis-directed treatments were relied upon to palliate, and occasionally cure, patients with limited metastases (17). In the setting of systemic therapy, which may be able to sterilize micrometastases, control of clinically detectable tumors is perhaps of even more importance. Although prospective clinical trials do not routinely report PFS of individual metastases, observational studies report that the predominant pattern of recurrence in patients with oligometastatic NSCLC treated with first-line systemic therapy appears to be local only (18, 19). This pattern of progression would support the potential PFS benefit of delivering aggressive local therapy to all appreciable metastatic sites, as well as the thoracic primary, if feasible. As unchecked growth of oligometastases may culminate in progressive organ dysfunction eventually leading to death, improved PFS with aggressive local therapy may ultimately result in longer OS. In recent decades, the bulk of published clinical series have included patients with oligometastatic sarcoma, colorectal, or breast cancer (20–22); however, there are an increasing number of single institution studies, the majority retrospective, which report long-term PFS and OS associated with aggressive treatment to all known sites of disease in oligometastatic NSCLC (23).
One of the difficulties in interpreting and applying the available data to predict which patients will benefit from an aggressive treatment approach including local therapies is establishing the appropriate cutoff to define the oligometastatic state. Nearly all published studies of oligometastatic NSCLC have limited inclusion to patients with ≤5 metastases; however, the majority enrolled patients with ≤3 metastases and over half of all patients included in a recent meta-analysis had only a single metastasis (24). The oligometastatic state is believed to be a relatively common presentation of advanced NSCLC; however, its exact incidence is dependent on the cutoff used for its definition. The relative prevalence of oligometastatic disease in advanced NSCLC has been reported to range from 26 to 50% using cutoffs of ≤3–5 metastases (18, 25). The reported rates of oligorecurrence after definitive surgical treatment of NSCLC are even higher, with 33–50% of patients recurring with ≤3 lesions (26, 27). It is important to note that the studies reporting rates of oligometastases are likely subjected to selection bias as patients with more metastatic burden may be less likely to be enrolled on protocols and/or treated at tertiary or quaternary referral centers that often report their large institutional experiences.
In general, patients with fewer metastases tend to have better outcomes than those with a more widespread presentation irrespective of the potential impact of aggressive metastasis-directed local therapy. This has been shown in multiple retrospective studies (25, 27, 28) including a comprehensive analysis of advanced NSCLC patients treated on consecutive Southwest Oncology Group prospective protocols that revealed a significantly longer OS in patients developing a single metastasis (8.7 months) vs multiple metastases in a single organ (6.2 months) or multiple organs (5.1 months) (28). Some argue that oligometastases do not reflect a more indolent biology but rather lead-time bias in which patients are found to have metastatic disease at an earlier point in the natural history of their disease. However, this explanation cannot fully account for the long-term survival of some individuals with oligometastatic disease with up to one-quarter of patients surviving long-term with aggressive treatment to all sites of disease (24, 29). The recent 8th edition of the AJCC staging manual now considers a single extrathoracic metastasis to be M1b vs more widespread extrathoracic M1c disease (30). The M1a substage interestingly includes patients with potentially more metastatic burden than M1b, such as numerous lung metastases and/or malignant pleural effusion(s), as long as it is contained to the thorax.
Another important consideration when defining the oligometastatic state is appropriate patient evaluation/staging. Advancements in modern diagnostic imaging, including more widespread use of brain MRI and FDG-PET/CT, have improved the detection of both intra- and extra-cranial metastatic disease. MRI is superior to CT in staging the brain and may detect the presence of metastases, particularly small lesions, unappreciated by CT (31). The use of PET/CT staging is associated with improved OS, likely due in part to stage migration where patients are bumped into a higher stage category by the detection of otherwise clinically unapparent metastases (32, 33). Based on published studies, approximately 15% of NSCLC patients initially thought to be stage I–III may be upstaged to stage IV with use of PET/CT in addition to contrast CT imaging alone (34, 35). Furthermore, modern imaging may detect widespread metastases in patients thought to have oligometastatic disease, thereby avoiding aggressive metastasis-directed local therapy in those who are unlikely to have a PFS benefit. It is important to consider that the bulk of published studies in oligometastatic NSCLC included patients treated before the routine use of PET/CT for staging (24).
Despite a strong focus on using a strict number of metastases to define oligometastatic disease, other factors including age and performance status, volume of disease, histology, tumor location(s), rate of progression, and genetics may be important in predicting benefit from aggressive local therapy (36). For appropriate clarification of distinct clinical scenarios, oligometastatic disease can be subdivided based on the development of metastases in relation to initial diagnosis and systemic therapy (37). Synchronous or de novo oligometastases refers to presentation with a limited number of lesions at initial diagnosis, while oligorecurrence is the metachronous development of new metastases after definitive treatment of initial locoregional thoracic disease. Patients with more widespread presentation experiencing relative disease stability on “mostly effective” systemic therapy aside from a limited number of persistent or recurrent/growing metastases may be referred to as having oligoresistance (or “induced oligometastases”) and oligoprogression disease, respectively. The latter two scenarios are fairly common in the setting of oncogene-addicted NSCLC (those patients with ALK rearrangements or EGFR mutations) and are due predominantly to acquired resistance to treatment with TKIs in progressing/resistant tumor clonogens (38).
Timing does appear to be important and an improved prognosis has been observed in patients presenting with oligorecurrence compared to those with de novo oligometastases as evidenced by an individual patient data meta-analysis by Ashworth and colleagues including 757 oligometastatic NSCLC patients treated with ablative treatments to all sites of disease which reported the latter to be associated with a HR of 1.96 (p < 0.001) on multivariate analysis (24). It is worth mentioning that a more recent publication did not show worse OS outcomes in synchronous patients if treated with aggressive thoracic therapy (ATT) (39). Additional adverse prognostic factors for OS reported in the meta-analysis by Ashworth (24) included higher thoracic stage and/or mediastinal node positivity, presence of brain metastases, non-adenocarcinoma histology, and non-surgical treatment. Nearly 90% of patients included had a single metastatic lesion and the presence of >1 oligometastatic lesion and/or multiple organ involvement was significantly associated with worse PFS. As alluded to previously, there is evidence to support the premise that larger volume, rather than number, of metastases is more predictive of worse outcome. A retrospective study conducted at MD Anderson Cancer Center including 1,284 patients with advanced NSCLC found that the number of extra-cranial metastases correlated with OS; however, among patients with brain- or lung-only metastases, there was an even stronger association with cumulative metastatic tumor volume (40).
Definitive Radiotherapy-Based Treatment of Oligometastases
Historically, surgical resection has been the preferred metastasis-directed treatment for patients with limited metastases from NSCLC (41). Surgery has the attributes of being both diagnostic, by providing pathologic confirmation of metastatic disease, and therapeutic, by eliminating tumor and/or alleviating tumor-related symptoms (42, 43). The benefit of aggressive metastasis-directed therapy was first shown in patients with limited brain metastases. Patchell and colleagues (44) performed a phase III randomized controlled trial evaluating the impact of surgical resection added to palliative whole brain radiotherapy (WBRT) in a predominantly NSCLC population of patients with a single brain metastasis reporting resected patients lived significantly longer (40 vs 15 weeks, p < 0.01). Following this, a number of studies examined the effect of extra-cranial metastasis-directed therapy, typically consisting of surgery and/or radiotherapy, with nearly two-thirds of patients included in a recent meta-analysis of oligometastatic NSCLC patients managed with surgery as the primary treatment. Although surgery was found to be significantly associated with improved OS, there was a strong potential selection bias favoring outcomes in the surgical group (for example, medical co-morbidities) (24).
Unfortunately, many patients with oligometastases present with one or more unresectable deposits and/or may be poor operative candidates due to advanced age and/or medical co-morbidities. There is interest in utilizing less invasive, potentially ablative techniques to treat oligometastases including thermal, cryo-, chemical, or irreversible electroporation ablation, but experience with these techniques is limited and restricted to select institutions (45). Furthermore, their role in the treatment of oligometastatic disease, NSCLC, or otherwise is not well defined.
The ability of modern radiotherapy techniques to deliver potentially ablative HIGRT doses, including SRS and SBRT, to numerous organ sites throughout the body has allowed for the aggressive treatment of unresectable metastases. Techniques intrinsic to SRS were initially developed to treat small targets in the brain that were not amenable to conventional surgery (46), however, have since been greatly refined due to improved brain imaging, treatment planning software allowing for MRI-CT image fusion and accurate dose calculation, more widely available LINAC-based delivery, and non-invasive immobilization. SRS alone has replaced WBRT as the recommended upfront treatment for NSCLC patients with oligometastases in the brain (47).
Extra-cranially, SBRT is associated with treated tumor control rates rivaling surgery, often in excess of 90%, among NSCLC patients when escalated to a biologically effective dose (BED) of at least 100 Gy (6, 48). As these treatments deliver very high, potentially ablative, doses of radiation to tumor, often near critical normal organs (i.e., spinal cord, kidney, bowel, and heart), the safe delivery of HIGRT is highly dependent on effective patient immobilization, accurate and reproducible image-guided setup, and respiratory motion analysis and management. As the technical expertise and availability of equipment required to deliver HIGRT rapidly expands, there are a growing number of institutions reporting their experiences, both retrospective and prospective, using these techniques in advanced stage NSCLC patients to target metastases located throughout the body (see Table 1).
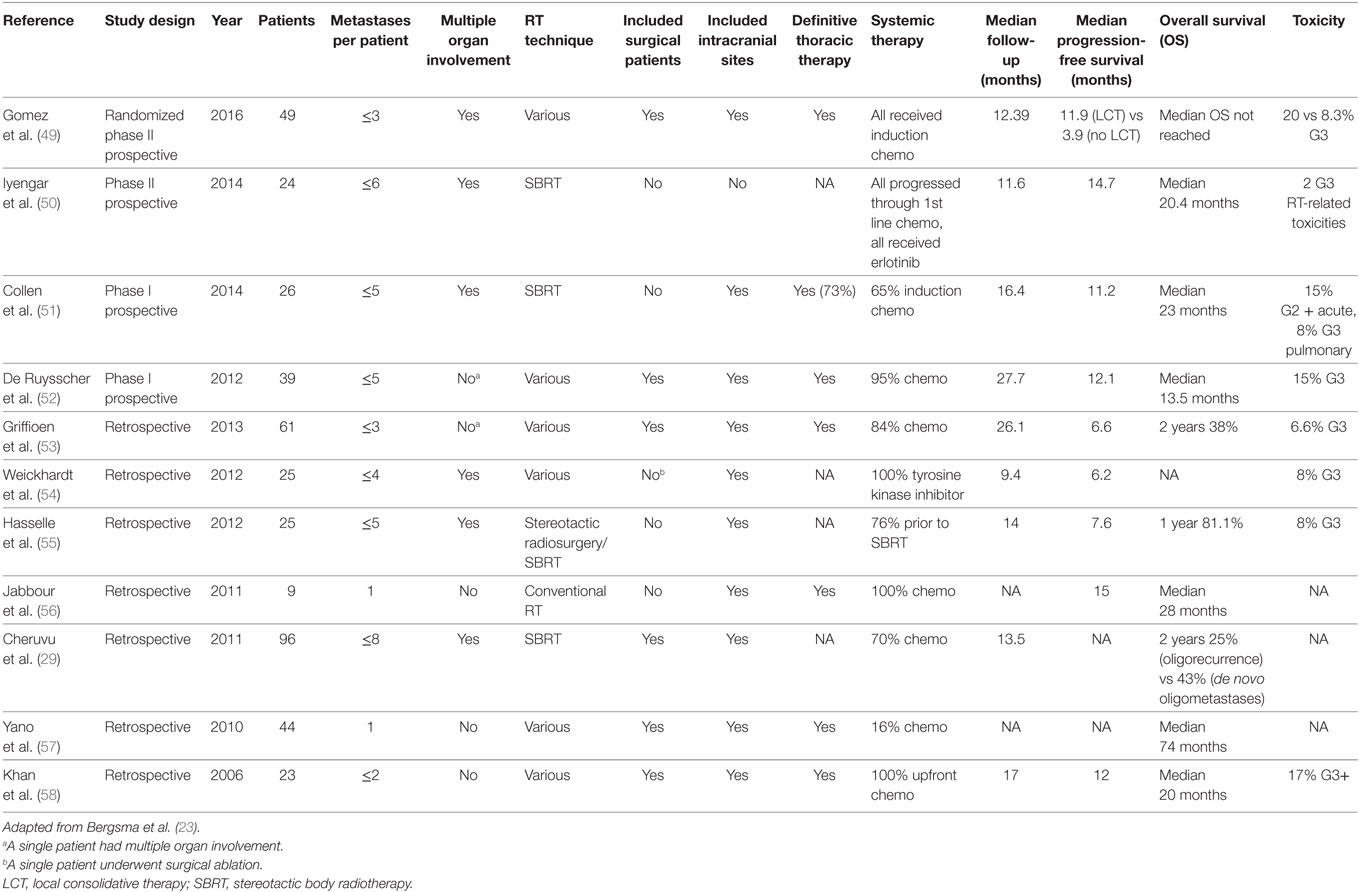
Table 1. Summary of select studies of high dose radiation therapy as part of an aggressive local treatment approach targeting oligometastases from non-small cell lung cancer.
Treatment of Individual Organ Sites
Brain
Brain metastases ultimately develop in 30–50% of NSCLC patients and are typically associated with a very poor prognosis. The increased availability of brain MRI as well as improved systemic therapies that improve survival but often poorly penetrate the blood–brain barrier have led to an increase in the number of NSCLC patients ultimately diagnosed with brain metastases (59). Historically, upfront WBRT constituted the standard of care treatment for brain metastases, despite a lack of proven OS benefit, due to its ability to provide improved central nervous system (CNS) control and decrease the risk of neurologic death, at the risk of potential late neurocognitive toxicity, compared to optimal supportive care (OSC). The utility of WBRT has come under significant scrutiny, particularly based on the results of a recently published phase III, non-inferiority, randomized trial from the United Kingdom (UK) (QUARTZ) which compared WBRT to OSC in NSCLC patients with brain metastases unsuitable for surgical resection or SRS. The study authors concluded that although the OSC alone arm did not meet the predetermined primary endpoint of non-inferiority in regard to quality-adjusted life-years, the absolute benefits of WBRT were clinically insignificant and it should not routinely be used to treat this patient population. Proponents of WBRT argue that the patients enrolled on the QUARTZ trial had extremely poor prognosis, evidenced by the reported median OS of 8–9 weeks, which precluded significant benefit from WBRT. In fact, the prognosis for NSCLC patients with brain metastases varies widely and the anticipated benefit of WBRT may be more substantial in patients with a greater ratio of intra- to extra-cranial disease burden who are at high risk of severe mortality and/or mortality with uncontrolled progression of CNS metastases (60). Furthermore, select patients with adequate performance status, limited brain metastases, and low burden extra-cranial disease may experience improved survival from the improved brain control associated with aggressive CNS-directed local therapies including surgery and/or SRS (61). An aforementioned trial demonstrated that in good performance status patients with a single intracranial metastasis, adding surgical resection to WBRT significantly improved OS (44). Similarly, RTOG 9508 studied the impact of SRS boost after WBRT for patients with 1–3 newly diagnosed brain metastases and revealed an OS benefit with the addition of SRS in patients with a single brain metastasis, mean survival time (MST) of 6.5 vs 4.9 months (p = 0.039), as well as in NSCLC patients, MST of 5.9 vs 3.9 months (p = 0.012) (62).
The benefit afforded by the addition of upfront WBRT to surgical resection and/or SRS has been intensely studied given the potential for prolonged survival in the most favorable subset of NSCLC patients with brain metastases as well as the appreciable risk of late neurocognitive toxicity with WBRT (61). The Alliance group recently published the results of a trial comparing upfront SRS with or without WBRT in patients (approximately two-third NSCLC) with one to three brain metastases and reported no detriment to OS and less cognitive deterioration in the SRS alone group (63, 64). Furthermore, two additional randomized trials each enrolling surgically resected patients with limited brain metastases, one (in which 20% had NSCLC) comparing SRS vs observation (65) and the other (in which 58% had NSCLC) SRS with or without WBRT (66), show adjuvant SRS may provide an optimal balance between maximizing brain metastasis control in the resection cavity and preservation of neurocognition without compromising survival provided patients are followed closely with salvage therapy (either additional SRS or WBRT) initiated at the time of intracranial progression.
An additional consideration in the treatment of brain oligometastases includes the relatively lower prescribed dose, limited by potential toxicity including the risk of radionecrosis, and resulting high local failure rates associated with single fraction SRS for large lesions (>2 cm). Recently published retrospective data show a multifraction SRS (three to five fractions) approach yields increased local control (LC) and decreased risk of radionecrosis in large brain metastases compared to single fraction SRS (67). There are also emerging data supporting the premise that the cumulative volume of intracranial metastatic burden may matter more than brain metastasis number and OS may not be inferior for patients with 4–10 vs 2–3 brain metastases (68). Overall, although the presence of brain metastases has historically felt to be an adverse prognostic factor (24), the development and widespread implementation of SRS has proven to be a powerful tool in the radiation oncologist’s armamentarium for the treatment of oligometastatic NSCLC within the brain.
Lung
For decades, pulmonary metastasectomy has been utilized for lung metastases from several cancer types, predominantly sarcoma and colorectal cancer. Published experiences confirm that resection can lead to prolonged PFS and OS in selected patients. Pulmonary oligometastases in NSCLC patients have been reported to carry a favorable prognosis that is now reflected in the recently published 8th edition of the TNM classification for lung cancer that separates intra-thoracic metastases, including metastatic lung or pleural nodules, as M1a rather than M1b or M1c (69). The safety and efficacy of SBRT delivered to primary NSCLC tumors and lung oligometastases has been well studied in multiple prospective studies (7, 70). Rusthoven and colleagues reported an actuarial 2-year LC of 96% in a prospective phase II study enrolling patients with one to three lung metastases from various solid tumor primaries (13% from primary lung cancer). Treatment was very well tolerated with only 8% grade 3 events and no grades 4 or 5 toxicity reported. A more recent retrospective study of SBRT for lung oligometastases limited to NSCLC reported 88.9% LC and 74.6% OS at 2 years with no grade 4 pulmonary toxicity, chest pain, or rib fractures (71). Of note, the use of PET/CT along with pathological confirmation (if acceptable risk) can be quite helpful as the presence of a contralateral lung nodule in newly diagnosed advanced NSCLC can be difficult to differentiate between a metastasis and a synchronous lung primary (72). Robust motion management is critical when delivering lung SBRT as metastases may be subjected to significant respiratory-induced motion and resulting target uncertainty which can be minimized by abdominal compression, breath hold, respiratory gating, real-time tumor tracking, and/or generation of an internal target volume based on four-dimensional CT at time of simulation (73).
Adrenal Glands
Adrenal gland metastases may be present in 5–10% of NSCLC patients (74) at initial presentation and solitary metastases occur in approximately 2–3% of cases. As solitary adrenal masses found on CT may in fact be benign adenomas, further diagnostic workup with PET/CT or dedicated MRI and possible histological confirmation should be pursued (75). Surgery with adrenalectomy of solitary metastases has been reported to provide favorable outcomes in NSCLC patients per several single and multi-institutional series (76) and conventionally fractionated EBRT can be used for palliation of pain with good response rates as measured by analgesic requirements (77). The use of SBRT in the treatment of adrenal oligometastases is gaining traction as an alternative to adrenalectomy resulting in high rates of palliation and LC rates (≥74%) which appear to correlate well with greater BED (78). Definitive radiation for adrenal metastases is an attractive non-invasive alternative particularly given the not infrequent adverse pathological features of positive margins and incomplete resections seen after adrenalectomy. Treatment appears to be well tolerated with only rare severe toxicity (79, 80); however, care must be taken during treatment planning as adrenal metastases may also exhibit significant motion with respiration and are often near the kidney, spinal cord, and sensitive gastrointestinal organs including the liver, colon, stomach, and small bowel.
Liver
Liver involvement at diagnosis is a relatively uncommon presentation of advanced NSCLC with hepatic metastases reportedly occurring in less than 5% of new diagnoses (81). Histology plays a role in the comparative number of metastases with solitary presentation in 50% of patients with squamous cell carcinoma vs 5% of those with adenocarcinoma. Ultimately, the development of hepatic metastases is not uncommon in the natural history of advanced stage NSCLC. Although there is a wealth of data establishing the benefit of partial hepatectomy for isolated or limited liver metastases from colorectal cancer, the published experience of surgical resection for liver metastases from NSCLC, oligometastatic, or otherwise is lacking. The safety and efficacy of SBRT delivered to hepatic oligometastases from solid tumors has been well established in both retrospective and prospective (82) series with Goodman and colleagues reporting 91% LC at 4 years with only 4.9% grade 3 or greater liver toxicity (83). A major limitation of using these studies in the context of oligometastatic NSCLC is the wide variety of tumor histologies included, with NSCLC comprising a minority of treated cases (21% of patients enrolled on the prospective phase I/II trial by Rusthoven and colleagues); however, a large retrospective study from Moffitt Cancer Center showed that liver metastases of NSCLC origin may exhibit relative radiosensitivity compared to other histologies (84). Contrast (ideally tri-phasic) should be given at simulation to help delineate the target given the similar CT density of metastasis and normal liver. Fiducials may be helpful in aligning to the target for image guidance radiotherapy and motion assessment and management is mandatory due to potential for respiratory-induced tumor motion during treatment (85).
Other Sites Including Bone, Kidney, Spleen, Skin, and Lymph Nodes
There is relatively little data on management of oligometastatic NSCLC involving these sites with the majority being surgical series for solitary bone (86) or skin (87) lesions. Although not limited to patients with oligometastases, Gerszten and colleagues report a single institutional experience detailing outcomes in 500 cases of spine radiosurgery documenting remarkable 100% long-term radiographic control and 93% long-term pain improvement in a subset of 80 lung cases (88). A recent retrospective study from Mayo Clinic analyzed outcomes after SBRT for non-spine bone oligometastases reporting a 91.8% LC at 1 year and acceptable acute and late toxicities; however, a minority of patients included had NSCLC (89). RTOG 0631 is a randomized phase II/III study of image-guided SRS/SBRT for localized spine metastasis, not limited to NSCLC patients, which has recently closed after adequate accrual with the primary endpoints of feasibility and palliation of pain (NCT00922974). Although the use of SRS or SBRT for bone metastases is promising, more studies are needed evaluating its impact in the context of oligometastatic NSCLC.
Role of ATT
The potential benefit of aggressive therapy directed to the primary tumor (and involved nodes) has been proven in the setting of multiple histologies of advanced stage cancers. For example, in metastatic renal cell carcinoma, randomized controlled trials have shown the significant OS advantage of cytoreductive nephrectomy added to immunotherapy (90). For advanced NSCLC patients with de novo oligometastases or oligorecurrence including initial thoracic disease, unchecked growth of locoregional chest disease may lead to significant tumor-related morbidity including cough, pain, shortness of breath, endobronchial obstruction causing airway collapse or post-obstructive pneumonia, superior vena cava syndrome, and/or severe hemoptysis, which may ultimately result in death. Radiotherapy can alleviate symptoms associated with bulky thoracic disease and is often utilized in the palliative treatment of advanced NSCLC patients.
Historical trials conducted by the RTOG in the 1970s showed that dose escalation up to 60 Gy utilizing conventional fractionation, relative to lower doses, led to improved thoracic tumor control in inoperable NSCLC patients treated with definitive radiotherapy (91). The optimal dose of chest radiotherapy in the setting of oligometastatic NSCLC has been debated given the need to balance palliation, including prevention of morbidity related to thoracic disease progression, while also minimizing treatment toxicity and duration as prolonged breaks in systemic therapy could heighten competing risks of systemic disease progression. A large retrospective study using the National Cancer Database evaluated the comparative effectiveness of chest radiotherapy dose escalation and found a positive association between improved survival and higher-dose radiotherapy (BED above 50 Gy) (92). However, a recent meta-analysis of 14 randomized controlled trials with 3,576 patients concluded there was no strong evidence to support extended fractionation schedules of radiotherapy to palliate thoracic symptoms in incurable NSCLC patients as all patients (including those receiving shorter treatment schedules) appeared to benefit in regard to palliation with no apparent difference in OS (93). Of note, patients with good performance status had longer 1-year OS with more fractions (33.3 vs 25.6%); however, the relative effect was not reported due to a high level of heterogeneity. Acute toxicity was an issue with higher radiotherapy doses though most patients were treated before the era of 3D conformal radiotherapy that has the potential to decrease exposure of normal organs to the full prescribed dose.
Although robust prospective randomized evidence is lacking, Li and colleagues recently published a meta-analysis that included 7 retrospective observational cohort studies and 668 synchronous oligometastatic NSCLC patients, of whom 227 (34.0%) received ATT consisting of surgery and/or radiotherapy to a total dose more than 40 Gy (94). Receipt of ATT was associated with significantly improved OS (HR 0.48, p < 0.00001) in the entire cohort, as well as in subgroup analyses of patients with single organ metastases (HR 0.42, p < 0.00001), solitary (HR 0.49, 95% CI 0.31–0.75) or two to four brain metastases (HR 0.44, 95% CI 0.26–0.73), and patients with thoracic stage I–II (HR 0.38, p = 0.004) or stage III (HR 0.32, p = 0.01) disease. Pooled cumulative OS at 3 years was significantly higher in the ATT group (23.0 vs 3.7%). A recent prospective phase II study by Li and colleagues evaluating the efficacy and toxicity of definitive thoracic concurrent chemoradiation (BED ≥ 60 Gy) followed by consolidation chemotherapy for oligometastatic NSCLC (≤5 metastases) enrolled 64 patients yielding encouraging 14-month median PFS and 26-month median OS at a median follow-up of 28 months (95). These prospectively accrued data are consistent with PFS and OS outcomes reported in other retrospective studies of ATT in oligometastatic NSCLC (25, 96, 97). While most published studies employed conventionally fractionated radiotherapy schedules with sequential or concurrent chemotherapy, HIGRT has also been used as definitive local treatment of smaller primary lung tumors in the oligometastatic setting (51, 53). Whether treatment is surgical or radiotherapy based, the use of ATT for controlling presenting or potential symptoms of thoracic disease is an integral component of an aggressive treatment approach for oligometastatic NSCLC patients with synchronous presentation given the potential for prolonged survival and significant morbidity and/or mortality resulting from uncontrolled locoregional progression.
Use of Radiotherapy with Systemic Therapy
Systemic therapy is the standard palliative treatment option for reasonably fit NSCLC patients presenting with either synchronous or metachronous disseminated disease with the agent (chemotherapy, TKIs, or immunotherapy with checkpoint inhibitors) selected based on histological and genotypic information about the primary and/or metastatic tumor. Randomized trials supporting the use of these systemic therapies typically included patients with widespread, rather than limited, metastases. Regardless, the promise of improved systemic control only heightens the importance of effective local treatment modalities to address isolated persistent or progressive metastases. For example, oligoprogression is well documented during treatment of onco-addicted NSCLC (ALK gene rearrangements or EGFR mutations) with TKIs such as crizotinib for ALK+ and erlotinib for EGFR mutated NSCLC. In these patients, local ablative treatment with HIGRT has allowed continuation of targeted therapy with greater than 6 months of additional disease control (50, 54).
The optimal integration of definitive local therapy with systemic therapy in oligometastatic NSCLC is not yet certain. It is common clinical practice to address limited brain metastases with upfront SRS or surgery (if symptomatic or warranted for diagnosis) followed by SRS or WBRT with initiation of systemic therapy or definitive thoracic therapy (if brain only metastases and synchronous presentation); however, medical oncologists typically treat patients with extra-cranial oligometastases with upfront systemic therapy. As alluded to earlier, the use of induction systemic therapy may allow for selection of patients who are less likely to develop new metastases and may experience improved PFS after consolidation with aggressive local therapy to the chest and limited residual metastases (typically ≤3) (49). The selective use of aggressive metastasis-directed and thoracic therapy in non-progressing patients is a potential source of selection bias in published retrospective studies, namely immortal time bias, which is best controlled for with a randomized controlled study. The recently reported multicenter, phase II randomized controlled trial by Gomez and colleagues utilized local consolidative therapy (LCT) of all active disease and reported a dramatic median PFS benefit (11.9 vs 3.9 months, p = 0.0054) compared to maintenance treatment in oligometastatic NSCLC patients (46 of 49 de novo) with three or fewer metastatic disease lesions without progression after first-line systemic therapy (49). These randomized prospective data reinforce the sum of available retrospective evidence signaling the significant PFS benefit of adding aggressive local therapy to standard systemic treatment for patients with NSCLC oligometastases.
It should be noted that the administration of systemic therapy may be associated with significant acute toxicities, including chemotherapy-related nausea and myelosuppression and autoimmune phenomena related to immunotherapy, which adversely affect patient quality of life. In the setting of oligometastatic NSCLC, Collen and colleagues reported the results of a small prospective study that showed receipt of induction chemotherapy prior to undergoing SBRT was not prognostic for LC or PFS (51). It is possible that select patients may experience prolonged PFS with aggressive local therapy directed at all metastatic sites in lieu of systemic therapy; however, it is reasonable to consider chemotherapy, or other appropriate systemic agents, as upfront treatment in oligometastatic patients given the OS benefit afforded in both early (adjuvant after resection in node positive and/or larger primary tumors) and advanced stage NSCLC (98). This treatment approach is reflected in the NCCN guidelines version 1.2017. As mentioned earlier, the use of aggressive local therapy in the setting of oncogene-addicted NSCLC is an area of significant interest as studies have reported excellent PFS and OS with the addition of SBRT to erlonitib in patients progressing after first-line platinum-based chemotherapy (50). Salvage of oligoprogression in the setting of advanced NSCLC with a driver mutation may allow continuation of otherwise efficacious and well-tolerated systemic therapy in patient who may not have other effective treatment options (99). Reported toxicities of the above approaches have been quite low with rare reports of severe (grades 4–5) adverse events.
Although the benefit of aggressive primary and metastasis-directed local therapy in the metastatic setting has commonly felt to be due to improved LC of targeted gross tumor(s), Gomez and colleagues reported a significantly prolonged time interval to the appearance of a new lesion among patients randomized to LCT (11.9 vs 5.7 months) (49). This is a provocative finding that suggests an aggressive local treatment approach may alter the natural history of metastatic disease either by limiting the potential for later spread or stimulating systemic immune-surveillance. Furthermore, there are fascinating reports of radiation inducing an “abscopal effect” whereby treating a single lesion results in regression of metastases far away from the treated site, however, these remain mostly anecdotal at present. Preclinical studies show synergistic antitumor effects with radiotherapy via pro-immunogenic properties resulting from increased tumor antigen presentation and activation of cytotoxic T cells (100) and emerging clinical data also support this concept with a recent study reporting previous treatment with extra-cranial radiotherapy was associated with significantly improved median OS (11.6 vs 5.3 months, p = 0.034) among patients treated with pembrolizumab on the KEYNOTE-001 phase I trial (101). Importantly, predominantly retrospective data to date suggest that the contemporaneous administration of immunotherapy and intracranial SRS or palliative dose extra-cranial radiotherapy is relatively safe without dramatically increased risk of synergistic toxicity; however, efficacy nor the safety of more aggressive extra-cranial dose schedules has not been studied (102, 103). There may even be a detrimental effect of more protracted palliative radiotherapy schedules given the extreme radiosensitivity of circulating lymphocytes and our growing understand of the importance of the immune system in combatting metastatic disease (104).
Future Directions and Ongoing Prospective Trials
The emerging evidence supports the existence of a subset of advanced NSCLC patients who will benefit from definitive local treatment to limited sites of disease with unparalleled PFS and OS. The challenge has been defining the appropriate patient population and proving the added benefit of aggressive local therapy in a randomized fashion. The phase II study by Gomez and colleagues represents the first randomized controlled trial addressing the question at hand and supports the premise that select advanced NSCLC patients may progress predominantly in known disease sites and aggressive thoracic and metastasis-directed local therapy can result in improved PFS. As follow-up remains short (median of 12.4 months), it is unclear whether the PFS benefit observed will translate into improved OS. It is possible that crossover of patients in the maintenance arm to LCT after progression could minimize the potential OS benefit similar to that seen in randomized trials of targeted agents in NSCLC (105). In addition, as the study was powered to assess the primary outcome, PFS, and was closed early at the recommendation of the data safety monitoring committee due to an overwhelming probability of concluding in favor of the LCT group, it may be insufficiency powered to measure a true difference in OS between arms. Regardless of whether an improvement in OS is ultimately shown, an improvement in PFS is certainly meaningful as a prolonged disease-free interval off of systemic therapy may represent a significant quality of life benefit to the patient.
Further randomized studies are necessary. NRG Oncology has recently opened NRG-LU002 (NCT03137771), a randomized phase II/III trial enrolling NSCLC patients with ≤3 oligometastatic sites that will build upon the experience of Gomez and colleagues and seeks to evaluate the PFS and OS benefit, if it exists, of consolidative SBRT and definitive thoracic therapy after first-line/induction systemic therapy in a national cooperative group setting. SABR-COMET (NCT01446744) is another multi-institutional randomized phase II trial that has completed accrual of 99 patients (not limited to NSCLC histology) with ≤5 metastases and a controlled primary tumor. Patients were randomized to standard of care with or without SBRT consolidation to all sites of known disease with OS as the primary outcome measure. SARON (NCT02417662) is a UK-based multicenter randomized phase III study enrolling (target of 340) patients with oligometastatic NSCLC and examining the feasibility, safety, and efficacy of consolidation with SBRT or conventional RT to primary and sites of metastases after standard platinum-based doublet chemotherapy. CORE (NCT02759783) is another randomized phase II trial (anticipated accrual of 206 patients) that has opened in the UK enrolling breast, prostate, and NSCLC patients with oligorecurrence and is evaluating the impact of adding SBRT to standard of care with PFS as the primary outcome. These larger randomized studies should increase the power to uncover an OS benefit with the addition of SBRT as comprehensive local therapy in the setting of oligometastatic NSCLC.
Additional questions remain unanswered beyond the measurable added benefit, if any, of an aggressive treatment approach including definitive local therapy. Both SABR-COMET and the randomized phase II study by Gomez and colleagues were evaluating the use of definitive local therapy to sites of limited disease as consolidation after upfront systemic therapy. However, the optimal timing of aggressive local treatment remains undefined. The ongoing Chinese OITROLC trial (NCT02076477) may help answer this question as it randomizes oligometastatic patients (≤5 distant organ metastases) between upfront definitive local therapy to the primary and all sites of metastases vs a consolidative approach after two cycles of induction chemotherapy with 3-month response rate as the primary outcome and 3-year PFS and toxicity as secondary outcomes. As use of SBRT combined with erlotinib showed remarkable outcomes in oligoprogressive metastatic NSCLC (50), a pilot study from Memorial Sloan Kettering Cancer Center (NCT02450591) is now evaluating outcomes when SBRT or surgery is added to erlotinib for newly diagnosed oligometastatic lung adenocarcinoma harboring a sensitizing EGFR mutation with the goal to evaluate feasibility and PFS.
Although crucial before more broadly adopting an aggressive local therapy approach to oligometastatic disease, the safety and optimal dose fractionation when treating multiple oligometastases in various organ sites remains unknown as the bulk of published literature studied the use of SBRT for single metastases within individual organs (8, 106). NRG BR001 (NCT02206334), a phase I study enrolling patients with oligometastatic NSCLC, prostate, or breast cancer, attempts to clarify the tolerability of SBRT when treating patients with multiple metastases at pre-defined doses in seven organ sites including bone and lymph nodes, where little safety data currently exist.
The era of personalized medicine has arrived in the field of oncology, ushered in by advances in imaging and molecular biology. Broad molecular profiling is expected to be a key component of future advancements in the care of patients with NSCLC. Prospective clinical trials are underway to generate clinical and molecular predictors, including comprehensive molecular profiling and/or primary tumor microRNA expression, to guide selection of patients for oligometastases-directed ablative therapy (107, 108). Further investigation, including independent validation, is needed before clinical implementation. Given the transition toward earlier incorporation of immunotherapy, such as the upfront administration of pembrolizumab in some newly diagnosed advanced NSCLC patients, there is considerable interest in combining immunotherapy and radiotherapy to improve outcomes and perhaps even induce the abscopal effect (109). A web search of http://clinicaltrials.gov revealed that there are now at least 14 actively recruiting studies evaluating immunotherapy in NSCLC as of March 31, 2017. These studies will hopefully add knowledge as to the added benefit and optimal incorporation of radiation with immunotherapy, including the most appropriate timing, sequencing, and dosing of each.
Despite the emerging evidence supporting the use of aggressive local treatments in addition to standard of care systemic therapy for oligometastatic NSCLC, as well as the increasing availability of non-invasive potentially ablative radiotherapy techniques, there are practical limitations that must be considered as our society increasingly recognizes the rising costs of health care in the modern era and begins to transition toward a value-based reimbursement model for providers and hospital systems (110). “Payers,” including governmental and private health insurers, are increasingly emphasizing an evidence-based approach to justify potentially costly treatments in patients with relatively poor prognosis. This can make obtaining insurance approval for novel and/or investigational uses for expensive treatment modalities, including SRS or SBRT, an onerous challenge for the treating radiation oncology team, as well as increase the financial burden and stress patients and their families experience during cancer treatment (111). This new reality reinforces the need for high level evidence to justify and guide the recommendation for aggressive local treatments in the setting of oligometastatic NSCLC.
Conclusion
Tremendous developments in the field of oncology within the past decade, including improvements in imaging and radiotherapy technique allowing for the safe delivery of potentially ablative doses of radiation with minimal toxicity or interruption in quality of life or systemic therapy, have ushered in the next frontier of NSCLC treatment. A steadily increasing number of published retrospective and prospective clinical experiences, including the first successfully completed randomized trial, support the concept that NSCLC patients with limited metastatic disease, termed as oligometastases, will experience improved outcomes with aggressive local treatment with surgery and/or radiation therapy targeting all sites of appreciable disease. The challenge for the oncology community moving forward is to design and accrue to prospective randomized controlled trials that will allow for an accurate assessment of the added benefit of aggressive local therapy as well as the optimal integration with existing and emerging systemic therapies.
Author Contributions
DB wrote the initial draft of this review, with edits and revisions from each of the other authors: JS, DS, SC, and MM.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin (2016) 66:7–30. doi:10.3322/caac.21332
2. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev (2010) 5:CD007309. doi:10.1002/14651858.CD007309.pub2
3. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med (2016) 375:1823–33. doi:10.1056/NEJMoa1606774
4. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373:1627–39. doi:10.1056/NEJMoa1507643
5. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12:735–42. doi:10.1016/S1470-2045(11)70184-X
6. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol (2015) 16:630–7. doi:10.1016/S1470-2045(15)70168-3
7. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA (2010) 303:1070–6. doi:10.1001/jama.2010.261
8. Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys (2012) 83:878–86. doi:10.1016/j.ijrobp.2011.08.036
9. Nyman J, Hallqvist A, Lund JA, Brustugun OT, Bergman B, Bergstrom P, et al. SPACE – a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol (2016) 121:1–8. doi:10.1016/j.radonc.2016.08.015
10. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell (2005) 8:89–91. doi:10.1016/j.ccr.2005.07.014
11. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res (2012) 177:311–27. doi:10.1667/RR2773.1
12. Lewis SL, Porceddu S, Nakamura N, Palma DA, Lo SS, Hoskin P, et al. Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: an international survey of >1000 radiation oncologists. Am J Clin Oncol (2015) 40:418–22. doi:10.1097/COC.0000000000000169
13. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell (2000) 100:57–70. doi:10.1016/S0092-8674(00)81683-9
14. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol (1995) 13:8–10. doi:10.1200/JCO.1995.13.1.8
15. Fisher B. From Halsted to prevention and beyond: advances in the management of breast cancer during the twentieth century. Eur J Cancer (1999) 35:1963–73. doi:10.1016/S0959-8049(99)00217-8
16. Halsted WS. I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg (1907) 46:1–19. doi:10.1097/00000658-190707000-00001
17. Rubin P. Comment: are metastases curable? JAMA (1968) 204:612–3. doi:10.1001/jama.1968.03140200052016
18. Mehta N, Mauer AM, Hellman S, Haraf DJ, Cohen EE, Vokes EE, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment. Int J Oncol (2004) 25:1677–83. doi:10.3892/ijo.25.6.1677
19. Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol (2009) 48:578–83. doi:10.1080/02841860802662722
20. Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol (2012) 4:283–301. doi:10.2147/CLEP.S34285
21. Predina JD, Puc MM, Bergey MR, Sonnad SS, Kucharczuk JC, Staddon A, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol (2011) 6:913–9. doi:10.1097/JTO.0b013e3182106f5c
22. Salama JK, Chmura SJ. The role of surgery and ablative radiotherapy in oligometastatic breast cancer. Semin Oncol (2014) 41:790–7. doi:10.1053/j.seminoncol.2014.09.016
23. Bergsma DP, Salama JK, Singh DP, Chmura SJ, Milano MT. The evolving role of radiotherapy in treatment of oligometastatic NSCLC. Expert Rev Anticancer Ther (2015) 15:1459–71. doi:10.1586/14737140.2015.1105745
24. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer (2014) 15:346–55. doi:10.1016/j.cllc.2014.04.003
25. Parikh RB, Cronin AM, Kozono DE, Oxnard GR, Mak RH, Jackman DM, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2014) 89:880–7. doi:10.1016/j.ijrobp.2014.04.007
26. Yano T, Okamoto T, Haro A, Fukuyama S, Yoshida T, Kohno M, et al. Local treatment of oligometastatic recurrence in patients with resected non-small cell lung cancer. Lung Cancer (2013) 82:431–5. doi:10.1016/j.lungcan.2013.08.006
27. Torok J, Kelsey C, Salama JK. Patterns of distant metastases in surgically managed early stage non-small cell lung cancer. Paper Presentation, 55th Annual ASTRO Meeting. Atlanta, GA (2013). 2013 p.
28. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol (1991) 9:1618–26. doi:10.1200/JCO.1991.9.9.1618
29. Cheruvu P, Metcalfe SK, Metcalfe J, Chen Y, Okunieff P, Milano MT. Comparison of outcomes in patients with stage III versus limited stage IV non-small cell lung cancer. Radiat Oncol (2011) 6:80. doi:10.1186/1748-717X-6-80
30. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:39–51. doi:10.1016/j.jtho.2015.09.009
31. Zakaria R, Das K, Bhojak M, Radon M, Walker C, Jenkinson MD. The role of magnetic resonance imaging in the management of brain metastases: diagnosis to prognosis. Cancer Imaging (2014) 14:8. doi:10.1186/1470-7330-14-8
32. Li J, Xu W, Kong F, Sun X, Zuo X. Meta-analysis: accuracy of 18FDG PET-CT for distant metastasis staging in lung cancer patients. Surg Oncol (2013) 22:151–5. doi:10.1016/j.suronc.2013.04.001
33. Tonnies S, Tonnies M, Kollmeier J, Bauer TT, Forster GJ, Kaiser D, et al. Impact of preoperative 18F-FDG PET/CT on survival of resected mono-metastatic non-small cell lung cancer. Lung Cancer (2016) 93:28–34. doi:10.1016/j.lungcan.2015.12.008
34. Dinan MA, Curtis LH, Carpenter WR, Biddle AK, Abernethy AP, Patz EF Jr, et al. Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998–2003. J Clin Oncol (2012) 30:2725–30. doi:10.1200/JCO.2011.40.4392
35. Weber WA, Dietlein M, Hellwig D, Kirsch CM, Schicha H, Schwaiger M. [PET with (18)F-fluorodeoxyglucose for staging of non-small cell lung cancer]. Nuklearmedizin (2003) 42:135–44.
36. Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, et al. The oligometastatic state – separating truth from wishful thinking. Nat Rev Clin Oncol (2014) 11:549–57. doi:10.1038/nrclinonc.2014.96
37. Patel PR, Yoo DS, Niibe Y, Urbanic JJ, Salama JK. A call for the aggressive treatment of oligometastatic and oligo-recurrent non-small cell lung cancer. Pulm Med (2012) 2012:480961. doi:10.1155/2012/480961
38. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol (2014) 11:473–81. doi:10.1038/nrclinonc.2014.104
39. Fleckenstein J, Petroff A, Schafers HJ, Wehler T, Schope J, Rube C. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer (2016) 16:348. doi:10.1186/s12885-016-2379-x
40. Oh Y, Taylor S, Bekele BN, Debnam JM, Allen PK, Suki D, et al. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer (2009) 115:2930–8. doi:10.1002/cncr.24333
41. Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer (2010) 69:251–8. doi:10.1016/j.lungcan.2010.05.003
42. Collaud S, Stahel R, Inci I, Hillinger S, Schneiter D, Kestenholz P, et al. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer (2012) 78:234–8. doi:10.1016/j.lungcan.2012.09.011
43. Congedo MT, Cesario A, Lococo F, De Waure C, Apolone G, Meacci E, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg (2012) 144:444–52. doi:10.1016/j.jtcvs.2012.05.051
44. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med (1990) 322:494–500. doi:10.1056/NEJM199002223220802
45. Di Lascio S, Pagani O. Oligometastatic breast cancer: a shift from palliative to potentially curative treatment? Breast Care (Basel) (2014) 9:7–14. doi:10.1159/000358750
46. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand (1951) 102:316–9.
47. Sahgal A, Larson D, Knisely J. Stereotactic radiosurgery alone for brain metastases. Lancet Oncol (2015) 16:249–50. doi:10.1016/S1470-2045(14)71106-4
48. Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol (2007) 2:S94–100. doi:10.1097/JTO.0b013e318074de34
49. Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol (2016) 17:1672–82. doi:10.1016/S1470-2045(16)30532-0
50. Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol (2014) 32:3824–30. doi:10.1200/JCO.2014.56.7412
51. Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol (2014) 25:1954–9. doi:10.1093/annonc/mdu370
52. De Ruysscher D, Wanders R, van Baardwijk A, Dingemans AM, Reymen B, Houben R, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol (2012) 7:1547–55. doi:10.1097/JTO.0b013e318262caf6
53. Griffioen GH, Toguri D, Dahele M, Warner A, de Haan PF, Rodrigues GB, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer (2013) 82:95–102. doi:10.1016/j.lungcan.2013.07.023
54. Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol (2012) 7:1807–14. doi:10.1097/JTO.0b013e3182745948
55. Hasselle MD, Haraf DJ, Rusthoven KE, Golden DW, Salgia R, Villaflor VM, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol (2012) 7:376–81. doi:10.1097/JTO.0b013e31824166a5
56. Jabbour SK, Daroui P, Moore D, Licitra E, Gabel M, Aisner J. A novel paradigm in the treatment of oligometastatic non-small cell lung cancer. J Thorac Dis (2011) 3:4–9. doi:10.3978/j.issn.2072-1439.2010.12.09
57. Yano T, Haro A, Yoshida T, Morodomi Y, Ito K, Shikada Y, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol (2010) 102:852–5. doi:10.1002/jso.21750
58. Khan AJ, Mehta PS, Zusag TW, Bonomi PD, Penfield Faber L, Shott S, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol (2006) 81:163–7. doi:10.1016/j.radonc.2006.09.006
59. Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol (1999) 44:275–81. doi:10.1023/A:1006308808769
60. Mehta MP, Aoyama H, Gondi V. The changing role of whole-brain radiotherapy: demise or time for selective usage? JAMA Oncol (2017) 3:1021–2. doi:10.1001/jamaoncol.2016.5414
61. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol (2012) 30:419–25. doi:10.1200/JCO.2011.38.0527
62. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (2004) 363:1665–72. doi:10.1016/S0140-6736(04)16250-8
63. Brown PD, Asher AL, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. NCCTG N0574 (Alliance): a phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J Clin Oncol (2015) 33(15_Suppl):lba4. doi:10.1200/jco.2015.33.15_suppl.lba4
64. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA (2016) 316:401–9. doi:10.1001/jama.2016.9839
65. Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol (2017) 18:1040–8. doi:10.1016/S1470-2045(17)30414-X
66. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol (2017) 18:1049–60. doi:10.1016/S1470-2045(17)30441-2
67. Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-fraction versus multifraction (3 x 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys (2016) 95:1142–8. doi:10.1016/j.ijrobp.2016.03.013
68. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol (2014) 15:387–95. doi:10.1016/S1470-2045(14)70061-0
69. Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A III, et al. The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol (2015) 10:1515–22. doi:10.1097/JTO.0000000000000673
70. Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol (2009) 27:1579–84. doi:10.1200/JCO.2008.19.6386
71. De Rose F, Cozzi L, Navarria P, Ascolese AM, Clerici E, Infante M, et al. Clinical outcome of stereotactic ablative body radiotherapy for lung metastatic lesions in non-small cell lung cancer oligometastatic patients. Clin Oncol (R Coll Radiol) (2016) 28:13–20. doi:10.1016/j.clon.2015.08.011
72. Patel PR, Milano MT, Onaitis MW, Salama JK. Treatment considerations for synchronous primary NSCLC and oligometastatic disease. In: Bogart J, Detterbeck FC, editors. Treatment of High-Risk Early Stage Lung Cancer. New York, NY: Springer-Verlag (2015).
73. Ehrbar S, Perrin R, Peroni M, Bernatowicz K, Parkel T, Pytko I, et al. Respiratory motion-management in stereotactic body radiation therapy for lung cancer – a dosimetric comparison in an anthropomorphic lung phantom (LuCa). Radiother Oncol (2016) 121:328–34. doi:10.1016/j.radonc.2016.10.011
74. Almaghrabi MY, Supiot S, Paris F, Mahe MA, Rio E. Stereotactic body radiation therapy for abdominal oligometastases: a biological and clinical review. Radiat Oncol (2012) 7:126. doi:10.1186/1748-717X-7-126
75. Chong S, Lee KS, Kim HY, Kim YK, Kim BT, Chung MJ, et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics (2006) 26:1811–24; discussion 1824–6. doi:10.1148/rg.266065057
76. Porte H, Siat J, Guibert B, Lepimpec-Barthes F, Jancovici R, Bernard A, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg (2001) 71:981–5. doi:10.1016/S0003-4975(00)02509-1
77. Soffen EM, Solin LJ, Rubenstein JH, Hanks GE. Palliative radiotherapy for symptomatic adrenal metastases. Cancer (1990) 65:1318–20. doi:10.1002/1097-0142(19900315)65:6<1318::AID-CNCR2820650611>3.0.CO;2-H
78. Chance WW, Nguyen QN, Mehran R, Welsh JW, Gomez DR, Balter P, et al. Stereotactic ablative radiotherapy for adrenal gland metastases: factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol (2016) 7:e195–203. doi:10.1016/j.prro.2016.09.005
79. Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys (2012) 82:919–23. doi:10.1016/j.ijrobp.2010.11.060
80. Ippolito E, D’Angelillo RM, Fiore M, Molfese E, Trodella L, Ramella S. SBRT: a viable option for treating adrenal gland metastases. Rep Pract Oncol Radiother (2015) 20(6):484–90. doi:10.1016/j.rpor.2015.05.009
81. Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M, Sekizawa K. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol (2003) 20:25–8. doi:10.1385/MO:20:1:25
82. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol (2009) 27:1572–8. doi:10.1200/JCO.2008.19.6329
83. Goodman BD, Mannina EM, Althouse SK, Maluccio MA, Cardenes HR. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract Radiat Oncol (2016) 6:86–95. doi:10.1016/j.prro.2015.10.011
84. Ahmed KA, Caudell JJ, El-Haddad G, Berglund AE, Welsh EA, Yue B, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys (2016) 95:1399–404. doi:10.1016/j.ijrobp.2016.03.050
85. Wu QJ, Thongphiew D, Wang Z, Chankong V, Yin FF. The impact of respiratory motion and treatment technique on stereotactic body radiation therapy for liver cancer. Med Phys (2008) 35:1440–51. doi:10.1118/1.2839095
86. Agarwala AK, Hanna NH. Long-term survival in a patient with stage IV non-small-cell lung carcinoma after bone metastasectomy. Clin Lung Cancer (2005) 6:367–8. doi:10.3816/CLC.2005.n.017
87. Ambrogi V, Nofroni I, Tonini G, Mineo TC. Skin metastases in lung cancer: analysis of a 10-year experience. Oncol Rep (2001) 8:57–61. doi:10.3892/or.8.1.57
88. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 32(2007):193–9. doi:10.1097/01.brs.0000251863.76595.a2
89. Owen D, Laack NN, Mayo CS, Garces YI, Park SS, Bauer HJ, et al. Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases. Pract Radiat Oncol (2014) 4:e143–9. doi:10.1016/j.prro.2013.05.006
90. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol (2004) 171:1071–6. doi:10.1097/01.ju.0000110610.61545.ae
91. Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer (1987) 59:1874–81. doi:10.1002/1097-0142(19870601)59:11<1874::AID-CNCR2820591106>3.0.CO;2-Z
92. Koshy M, Malik R, Mahmood U, Rusthoven CG, Sher DJ. Comparative effectiveness of aggressive thoracic radiation therapy and concurrent chemoradiation therapy in metastatic lung cancer. Pract Radiat Oncol (2015) 5:374–82. doi:10.1016/j.prro.2015.07.009
93. Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev (2015) 1:CD002143. doi:10.1002/14651858.CD002143.pub4
94. Li D, Zhu X, Wang H, Qiu M, Li N. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. J Thorac Dis (2017) 9:310–7. doi:10.21037/jtd.2017.02.21
95. Li T, Lv J, Liu L, Song Y, Li C, Wang J. A phase II prospective study of definitive thoracic concurrent chemoradiation followed by consolidation chemotherapy for oligometastatic non-small cell lung cancer. J Clin Oncol (2015) 33(15_suppl):e19008. doi:10.1200/jco.2015.33.15_suppl.e19008
96. Gray PJ, Mak RH, Yeap BY, Cryer SK, Pinnell NE, Christianson LW, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer (2014) 85:239–44. doi:10.1016/j.lungcan.2014.06.001
97. Xanthopoulos EP, Handorf E, Simone CB II, Grover S, Fernandes AT, Sharma S, et al. Definitive dose thoracic radiation therapy in oligometastatic non-small cell lung cancer: a hypothesis-generating study. Pract Radiat Oncol (2015) 5:e355–63. doi:10.1016/j.prro.2014.11.006
98. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol (2004) 22:3860–7. doi:10.1200/JCO.2004.02.109
99. Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. Br J Radiol (2016) 89:20160251. doi:10.1259/bjr.20160251
100. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulatingfactor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol (2015) 16:795–803. doi:10.1016/S1470-2045(15)00054-6
101. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi:10.1016/S1470-2045(17)30380-7
102. Kroeze SG, Fritz C, Hoyer M, Lo SS, Ricardi U, Sahgal A, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev (2017) 53:25–37. doi:10.1016/j.ctrv.2016.11.013
103. Bang A, Wilhite TJ, Pike LRG, Cagney DN, Aizer AA, Taylor A, et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys (2017) 98:344–51. doi:10.1016/j.ijrobp.2017.02.003
104. Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys (2014) 89:1084–91. doi:10.1016/j.ijrobp.2014.04.025
105. Clarke JM, Wang X, Ready NE. Surrogate clinical endpoints to predict overall survival in non-small cell lung cancer trials – are we in a new era? Transl Lung Cancer Res (2015) 4:804–8. doi:10.3978/j.issn.2218-6751.2015.05.03
106. Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer (2012) 118:2962–70. doi:10.1002/cncr.26611
107. Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer (2016) 122:2242–50. doi:10.1002/cncr.30058
108. Eke I, Makinde AY, Aryankalayil MJ, Ahmed MM, Coleman CN. Comprehensive molecular tumor profiling in radiation oncology: how it could be used for precision medicine. Cancer Lett (2016) 382:118–26. doi:10.1016/j.canlet.2016.01.041
109. Daly ME, Monjazeb AM, Kelly K. Clinical trials integrating immunotherapy and radiation for non-small-cell lung cancer. J Thorac Oncol (2015) 10:1685–93. doi:10.1097/JTO.0000000000000686
110. Aggarwal A, Hughes S. Palliative radiotherapy: evolving role and policy challenges. J Cancer Policy (2016) 10:21–9. doi:10.1016/j.jcpo.2016.05.003
Keywords: lung cancer, non-small cell lung cancer, oligometastases, stereotactic body radiotherapy, oligometastatic disease
Citation: Bergsma DP, Salama JK, Singh DP, Chmura SJ and Milano MT (2017) Radiotherapy for Oligometastatic Lung Cancer. Front. Oncol. 7:210. doi: 10.3389/fonc.2017.00210
Received: 28 July 2017; Accepted: 28 August 2017;
Published: 19 September 2017
Edited by:
John Varlotto, University of Massachusetts Medical Center, United StatesReviewed by:
Vivek Verma, University of Nebraska Medical Center, United StatesJohn Austin Vargo, West Virginia University Hospitals, United States
Copyright: © 2017 Bergsma, Salama, Singh, Chmura and Milano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael T. Milano, michael_milano@urmc.rochester.edu
 Derek P. Bergsma
Derek P. Bergsma Joseph K. Salama
Joseph K. Salama Deepinder P. Singh
Deepinder P. Singh Steven J. Chmura
Steven J. Chmura Michael T. Milano
Michael T. Milano