Preterm Hypoxic–Ischemic Encephalopathy
- 1Monash Children’s Hospital, Melbourne, VIC, Australia
- 2The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, VIC, Australia
- 3Department of Paediatrics, Monash University, Melbourne, VIC, Australia
- 4The Royal Children’s Hospital, Melbourne, VIC, Australia
- 5Murdoch Childrens Research Institute, Melbourne, VIC, Australia
- 6Department of Obstetrics and Gynaecology, Monash University, Melbourne, VIC, Australia
Hypoxic–ischemic encephalopathy (HIE) is a recognizable and defined clinical syndrome in term infants that results from a severe or prolonged hypoxic–ischemic episode before or during birth. However, in the preterm infant, defining hypoxic–ischemic injury (HII), its clinical course, monitoring, and outcomes remains complex. Few studies examine preterm HIE, and these are heterogeneous, with variable inclusion criteria and outcomes reported. We examine the available evidence that implies that the incidence of hypoxic–ischemic insult in preterm infants is probably higher than recognized and follows a more complex clinical course, with higher rates of adverse neurological outcomes, compared to term infants. This review aims to elucidate the causes and consequences of preterm hypoxia–ischemia, the subsequent clinical encephalopathy syndrome, diagnostic tools, and outcomes. Finally, we suggest a uniform definition for preterm HIE that may help in identifying infants most at risk of adverse outcomes and amenable to neuroprotective therapies.
Introduction
Worldwide, 11.1% of all live births every year are preterm (born before 37 weeks’ of completed gestation) (1), and the rate appears to be rising (2). In high-income settings, advances in neonatal care for preterm babies have greatly increased survival rates; however, premature babies remain at risk of serious health problems, including respiratory distress syndrome, bronchopulmonary dysplasia, retinopathy of prematurity, feeding difficulties, necrotizing enterocolitis, infections, longer hospital stays, and adverse long-term outcomes. In low-income countries, prematurity is a leading cause of neonatal and infant mortality (3).
Infants born prematurely have a high incidence of neonatal brain injury, with detrimental effects on motor, cognitive, behavioral, social, attentional, and sensory outcomes. Increased survival in lower gestational ages is accompanied by increased suboptimal neurodevelopmental outcomes (4–9). The incidence of any adverse neurodevelopmental outcomes varies with up to 17% of preterm infants described as having major impairments and, for babies weighing less than 1000 g at birth, up to 42% of survivors having minor impairment. The incidence of cerebral palsy (CP) and other adverse neurodevelopmental outcomes increases with decreasing gestational age at birth [from 5 to 10% in very low birth weight infants (<1500 g), 6 to 20% in extremely premature babies (<26 weeks’ gestation), and up to 25% in those born at a gestational age of less than 25 weeks] (10).
Historically, the best described neuropathological correlates of “encephalopathy of prematurity” (11) have been periventricular leukomalacia (PVL) with associated axonal/neuronal disruption and severe germinal matrix/intraventricular hemorrhage, with or without, posthemorrhagic ventricular dilatation (12). More recently, abnormalities of white matter (WM), with disrupted development of other cerebral structures (such as hippocampus, basal ganglia, corpus callosum) have been described in the context of premature brain abnormalities observed in the neonatal period (13, 14).
Preterm cerebral hypoxic–ischemic injury (HII) may occur rarely because of a recognized sentinel event. However, in the setting of coexistent factors, such as infection, inflammation, growth restriction, severe hypoglycemia, or hyperoxia, the contribution of individual pathologies may be difficult. For these reasons, perinatal HII to the premature brain, its clinical manifestations, recognition, and monitoring has not been well studied. In contrast, the diagnosis of HIE in full-term infants is aided by defined objective criteria involving perinatal factors, such as acidosis, Apgar scores, and the need for resuscitation, with standardized neurological examination and neurodevelopmental outcomes (15–17).
In this review, we highlight the contribution of preterm HIE to the injury complex in the developing brain. We propose an algorithm for a universal definition of preterm HIE, based on recognition of the specific manifestations of a perinatal HII. An accurate and uniform definition of preterm HIE may help to identify a homogeneous population of infants, who may be eligible for future clinical intervention trials.
Premature Brain Development and Pathophysiology of Hypoxic–Ischemic Insult
Between 24 and 40 weeks of gestation, the human brain undergoes rapid changes that make the developing brain vulnerable to injury from hypoxia–ischemia, inflammation, free radical, and excitotoxic damage (Figure 1). There is growing understanding of the etiology of preterm brain injury, involving interactions between an immature brain and vulnerable WM developmental processes (18). Key developmental processes during this time include the development of cerebral WM, proliferation zones, and neuronal structures. WM development involves pre-myelinating oligodendrocytes (OLs), axons, microglia, and neurons (subplate and late migrating GABAergic neurons). Pre-OLs are the predominant cell lineage present in human cerebral WM from 24 to 40 weeks of gestation that differentiate into myelin-producing OLs. These mature forms of OLs do not become abundant until after term, and tolerate hypoxic insult better than pre-OLs. Microglial cells, which become abundant during the third trimester, are capable of generating inflammatory cytokines, enhancing cytotoxicity and generating free radicals when exposed to hypoxia and infection. Subplate neurons are a collection of neurons located beneath the cortical plate, reaching their peak mass and developmental impact by 24–32 weeks of gestation. Development of the subplate is closely linked with development of cerebral cortex, deep nuclear structures (especially thalamus and formation of area-specific thalamocortical connections), and axons. The second significant developmental difference is the presence of two proliferative zones [dorsal cerebral subventricular zone (SVZ) and the ventral germinative epithelium of the ganglionic eminence]. The fetal SVZ is directly related to the evolutionary expansion of the human cerebral cortex. The third key process is the development of neuronal structures, such as thalamus, cerebral cortex, and basal ganglia (11, 12, 18, 19).
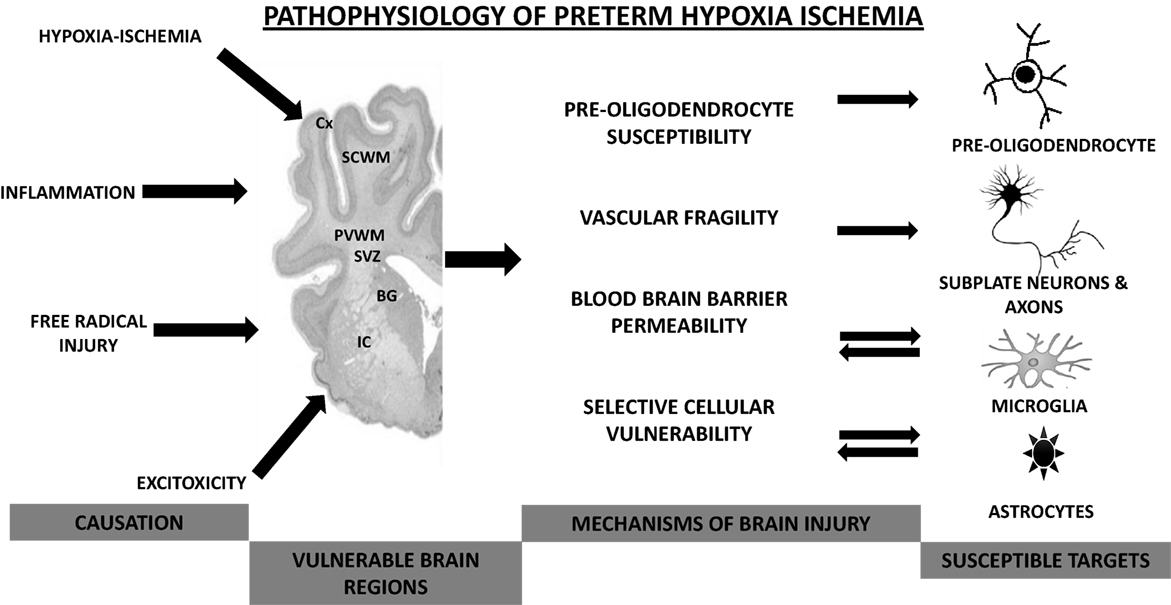
Figure 1. Pathophysiological mechanisms involved in preterm hypoxic–ischemic encephalopathy. Cx, cortex; SCWM, subcortical white matter; PVWM, periventricular white matter; BG, basal ganglia; IC, internal capsule.
Even without exacerbating factors, preterm birth is associated with subtle WM pathology (20). One theory is that the OL precursors and subplate neurons are exquisitely sensitive to pro-inflammatory cytokines, hypoxia, and oxidative stress (21). The principle pathogenic mechanism underlying neurological damage in HIE resulting from hypoxemia, ischemia, or both, is deprivation of glucose, and oxygen supply that causes a primary energy failure and initiates a cascade of biochemical events leading to cell dysfunction and ultimately to cell death (22–24).
The phases of cell death and biochemical interactions at cellular level in HII are well described and studied. Primary energy failure, where depletion of oxygen prevents oxidative phosphorylation, disrupting Na–K pump activity is followed by anaerobic metabolism with accumulation of lactic acid (25). The failure of the transmembrane Na–K pump results in the intracellular accumulation of sodium, calcium, and water (cytotoxic edema) leading to membrane depolarization, excessive release of excitatory neurotransmitters (particularly glutamate), increase in the intracellular concentration of calcium, activation of phospholipase, and generation of free radicals. The release of glutamate results in further calcium accumulation and sodium retention contributing to cell damage (26–28). Excitotoxicity refers to cell death mediated by excessive stimulation of excitatory amino acid receptors in response to dicarboxylic acid glutamate and, at the cellular level, is believed to be fundamental in hypoxic–ischemic damage to neurons (28). With the restoration of blood flow, there is a brief period of normalization of cerebral metabolism called a latent period. The latent period is believed to vary depending on the severity of the hypoxic–ischemic insult. The more severe the insult, the shorter is the recovery period (29–31). The secondary energy failure phase begins 6–48 h after the initial insult. The exact mechanisms of secondary energy failure are unclear but are attributed to oxidative stress, excitotoxicity, and inflammation (32, 33) and ultimately results in cell death through either apoptosis or necrosis, depending on the region of the brain affected and the severity of the insult. Necrosis dominates in severe injury, whereas apoptosis is observed in milder insults (10, 34). Following HII, a cell may undergo nitric oxide-mediated necrosis, when endogenous inhibitors of apoptosis are abundant, or apoptosis, when the inhibitors are deficient (35, 36). Mitochondrial dysfunction appears to play a crucial role in determining whether the cells affected by hypoxia–ischemia undergo necrosis or apoptosis (37, 38). Apoptosis may be predominant in the premature brain through upregulation of the key elements, such as caspase-3, caspase-12, and BAX (39, 40).
Hypoxic Ischemia in Preterm Population
The pathophysiology of HII in the premature brain is particularly complex. How HI damage affects the developing brain is determined by the severity, intensity, timing of asphyxia, in addition to selective cellular vulnerability, and immaturity of the brain (36, 41). The following summarizes the distinguishing effect of HII on key components of the developing brain and the susceptibility of the immature brain to the effects of HII.
Vascular Fragility
The thin, delicate vessels of the developing brain may not sustain effective blood flow to compensate for HII because of the underdeveloped distal arterial network and an immature cerebral auto regulatory capacity (12). The peripheral arteries in the growing brain lack collateral vessels and have limited vasodilatory function in response to HII, making them more susceptible to hypoxic injury (42).
Blood–Brain Barrier Function
The effect of hypoxia on the blood–brain barrier function in the developing brain can be profound. HII results in altered function and increased permeability of the BBB. The hypoxic insult affects the important cellular and functional components of the BBB, the astrocytes, the tight junctions of endothelial cells, and the pericytes (43, 44).
Developing Neuroglial Cells
Astrocytes are the predominant cell population in the CNS. They provide structural and metabolic support; they play a crucial role in scavenging high levels of excitatory neurotransmitters and are an important constituent of the BBB (6). In response to HII, astrocytes influence neuronal survival. Astrocytes play a neuroprotective role following insult, by promoting erythropoiesis (45); however, sustained HII can lead to a decreased functioning astrocyte population and, thereby, greatly decrease neuronal regeneration (46).
Pre-Oligodendrocytes and the Immature Oligodendrocytes
Oligodendroglial maturation involves four sequential stages, the oligodendroglial progenitor, the pre-OL (or late oligodendroglial progenitor), the immature OL, and the mature myelin-producing OL (12). The late oligodendroglial progenitors predominate in cerebral WM and SVZ and, at 28 weeks of gestation, account for 90% of the total OL population (47). Pre-OLs and OL progenitors are highly susceptible to hypoxia, and this vulnerability is central to the pathogenesis of preterm WM injury and PVL (48, 49). The mechanism of damage to OLs results from release of excitatory amino acids (glutamate, GABA, and aspartate) cytokine and free radical-mediated injuries, and the inflammatory response induced by HII (12, 49–52).
Selective Vulnerability
In the developing brain, certain regions and cells appear vulnerable depending on timing and the severity of the insult. In the preterm brain, the subplate neurons and OL precursors are most vulnerable, and in the term brain, projection neurons especially in the deep gray nuclei are at greatest risk during ischemic insults. Subplate neurons are the earliest and the most transient cell population of the neocortex – and are affected by HI (53). Loss of these cells results in abnormal thalamocortical connectivity and may explain the visual and somatosensory impairment seen in prematurely born infants suffering perinatal HII (15, 54). Several studies have shown that the late OL progenitors appear to be the most vulnerable in this lineage, and they neither mature nor develop following injury. The selective vulnerability could result from expression of the receptor subtypes that favor calcium entry and excitability and inefficient endogenous antioxidant mechanisms (48, 55, 56).
Preterm Hypoxic–Ischemic Encephalopathy – Definitions, Incidence, Clinical Spectrum, and Recognition
Definitions used to identify preterm HIE vary across available studies. Chalak et al. (57) screened preterm babies (33–35 weeks) using NICHD criteria for hypothermia, whereas Logitharajah et al. (58) included Apgar scores of less than 5 and 7 at 1 and 5 min, cord pH <7, sentinel event, and need for resuscitation. Schmidt and Walsh (59) included babies with 5-min Apgar score less than 6, cord or initial pH <7, base deficit >15 mmol/L, sentinel event, and clinical evidence of encephalopathy, and the severity of HIE was based on modified Sarnat HIE staging.
Incidence/Epidemiology
There is a paucity of literature on the true incidence and epidemiology of HIE in infants born preterm. Depending on the definition used, the reported incidence across studies varies from 1.3/1000 live births to 5–9/1000 live births (57, 59, 60). The gestational age for inclusion also varies slightly across studies. In a study by Salhab and Perlman (60), most babies were close to 34 weeks (range 31–36), whereas Chalak et al. (57) and Schmidt and Walsh (59) reported outcomes on infants between 32 and 35 weeks. The major limitations in these studies are small sample sizes, retrospective examination, and variable inclusion criteria used to identify the babies with hypoxic ischemia. The Salhab study included babies with umbilical cord pH <7, whereas the Schmidt study screened infants of 32–36 weeks of gestation for HIE on the basis of a 5-min Apgar score less than 6. In a study by Logitharajah et al. (58), up to 30% of babies with preterm HIE had a cord pH >7, so only including infants with a pH <7 might underestimate preterm HIE. Most of the studies quoted above had an incidence for moderate to severe HIE in a preterm population, not the milder forms of HIE. It is likely that the true incidence of preterm HIE is higher than in the term population that is around 1–2/1000 live births (61).
Clinical Spectrum
The clinical manifestations of HIE in the preterm population are vague, variable, and not well studied (Table 1). The difficulties in diagnosing HIE in preterm infants exist because the infant’s developmental immaturity adds ambiguity to differentiating clinical features (62).
The neurological signs in the study by Chalak et al. used the NICHD guidelines for hypothermia and concluded that standard neurological assessment, including tone, posture, level of consciousness, spontaneous activity, primitive reflexes, and autonomic nervous system, can be applied reliably for gestational age of 33–35 weeks (64). There are no reported studies, which describe neurological signs of HIE in lower gestational (<32 weeks) age groups. The cord or initial pH was lower than 7.2 in babies with HIE in most of the studies, and persistent or delayed resolution of metabolic acidosis was associated with development of HIE (57).
Term vs. Preterm Hypoxic–Ischemic Encephalopathy
The clinical features among term and preterm babies in HIE do overlap at many junctures, but what characteristically sets apart the preterm from term HIE are as follows:
• The higher rates of neurodevelopmental impairment as the effect of hypoxia–ischemia exaggerates vulnerability of the preterm brain.
• Preterm infants with moderate acidosis often appear well initially and often receive less intervention than term infants with the same degree of acidosis.
• Distinctive selective vulnerability of developing brain.
• Seizures in preterm infants with HIE have been reported as a marker of more severe outcome (24).
Monitoring/Recognition of Preterm HIE
The recognition of HIE in preterm babies is difficult. Although a standard neurological examination may be applicable in the late premature group, between 33 and 35 weeks of gestation, the clinical features in the younger preterm group can be masked by physiological immaturity and, thus, can be even more challenging to diagnose. The low initial arterial pH may be a subtle marker of hypoxic ischemia and is associated with abnormal cognitive outcome in apparently low-risk preterm babies (65–68).
Preterm babies with acidotic cord or initial pH, delayed resolution of acidosis, renal impairment, raised creatinine, elevated liver enzymes, prolonged assisted ventilation, and abnormal neurological examination can be presumed to have suffered some degree of hypoxic insult. But these features may also result from etiology other than HIE (e.g., chorioamnionitis, fetal growth restriction), and patterns of enzyme changes following an asphyxial event are only described for term-born infants and older children (69, 70).
Diagnostic Evaluation
Magnetic resonance imaging (MRI) is considered the most sensitive imaging modality for many pathological conditions of the newborn brain. Techniques, such as MR spectroscopic imaging and diffusion tensor imaging (DTI), aid in assessment of neonatal brain development. MR spectroscopic imaging measures regional brain biochemistry and is useful in assessing metabolic changes associated with brain development and injury.
Imaging Pattern in Preterm vs. Term Brain with HIE
The classical imaging abnormalities in HIE involve three major types of lesions – PVL, basal ganglia thalami lesions, and multicystic encephalopathy. Focal non-cystic WMI is the most commonly recognized pattern of brain injury in the preterm population whereas, in term babies with HIE, two major types of injury are involved (i) a watershed predominant pattern involving the WM, particularly in the vascular watershed, extending to cortical gray matter following severe insult (ii) a basal ganglia predominant pattern involving the deep gray nuclei and perirolandic cortex, involving the cortex in severe injury (70).
In the study by Logitharajah et al. (58), WM injury was noted in around 82% of the study population and was usually diffuse and mild. Most of the infants with WM injury also had basal ganglia/thalamus injury. Infants with brain stem lesions were most likely to have incurred a severe insult. Isolated WM injury in which the cortex was spared and there was no cystic PVL was an uncommon finding, consistent with an earlier study by Barkovich and Sargent (63). The reduced susceptibility of the preterm cortex to HII may result from the lower density of a subtype of glutamate receptor allowing calcium influx into the cell leading to calcium induced excitotoxicity in the early third trimester (71, 72).
In contrast to the advantage of lesion identification, the use of MRI has a major limitation in early diagnosis since brain abnormalities might only be apparent several days after insult (73). Diffusion-weighted (DWI) MRI detects abnormalities from day 1 after birth asphyxia, but it remains unclear if the detected abnormalities relate to outcome (74, 75). However, early MRI, especially DWI, may help in detecting diffuse WM injury, which is the most dominant form of neuropathology in the premature brain (76). Given that all potential neuroprotective treatments have a limited therapeutic window of opportunity (77, 78), alternative diagnostic and predictive tools that can promptly and accurately detect abnormalities are required.
Amplitude-Integrated Electroencephalogram/EEG
Amplitude-integrated electroencephalogram (aEEG)/electroencephalogram (EEG) are powerful tools for the diagnosis and prediction of neurological outcomes in asphyxiated infants (79, 80). Because of their practicality, and real-time interpretation, continuous monitoring with aEEG has become standard in the care of term infants with HIE (81–83). It has been suggested that the predictive accuracy of aEEG is limited compared to conventional EEG due to its data reduction and artifacts (84). However, the provision of prolonged conventional multichannel EEG with expert interpretation is not readily available in most NICUs. Thus, one or two channel aEEG is currently used more commonly in NICU (83). With respect to the assessment in preterm infants, EEGs change with uneventful maturity of the developing brain, making interpretation more complex (85–87). Specifically, the abnormal background patterns that are predictive of poor outcome at term are normal at early gestational ages. Various classification systems using conventional EEG were reported for abnormal EEGs of the preterm infants, and the findings characterized by increased discontinuity, decreased faster frequency activities, and lowered amplitudes at day 1–2 have demonstrated high predictive value of neurological outcome in infants born 27–32 weeks of gestation (88). The absence or mild depression of background activity is associated with a favorable outcome in 89%, and severe depression is associated with death in 38% and moderate or severe CP in 52% of surviving infants (85). The background pattern of aEEG 6 h after birth in asphyxiated late preterm infants born between 34 and 36 weeks of gestation has prognostic value for neurodevelopmental outcome (88). Although the data are not limited to asphyxiated infants, a recent study showed that single-channel aEEG/EEG can predict long-term outcome with 75–80% accuracy at 24 h postnatal age, even in very preterm infants born 22–30 weeks (89). In preclinical animal studies, decrease of EEG spectral edge and power are indicators of preterm brain injury following acute HI (90). Where EEG spectral edge and power were markedly and rapidly suppressed after 25–30 min HI in fetal sheep at 0.65–0.7 gestation (corresponding to 24–32 weeks gestation of brain development in human), these parameters neither recovered to baseline values within 3 days (91, 92) nor were they suppressed after 15-min HI, but did not lead to significant histopathological brain damage. These findings indicate that the changes of EEG post HI reflect the degree of injury sensitively and instantly in the preterm brain. The development of aEEG/EEG measurements and analysis may provide an effective early assessment in preterm HI injury.
Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) can be useful in analyzing parameters, such as tissue oxygenation index (TOI) and regional tissue oxygen saturation (rSO2), of brain in a variety of pathological conditions (93). Cerebral blood flow can be indirectly measured by TOI and rSO2 under stable arterial oxygen saturation, and NIRS measurement of hemoglobin total (HbT) can be used to measure the cerebral blood volume (94). The abovementioned NIRS parameters may provide important information on metabolic dynamics in different brain regions and predict long-term prognosis in asphyxiated neonates (95), although this remains to be demonstrated. The utility of NIRS in evaluating oxygenation and parameters, such as blood volume, has been employed in asphyxiated term neonates (96). Tax et al. have used NIRS in assessing peripheral oxygenation in asphyxiated neonates beyond 34 weeks of gestation (97). The utility of NIRS, along with other modalities such as MRI/aEEG, has been studied in term asphyxiated neonates (98, 99). The utility of NRIS in monitoring HII in preterm neonates has not been well established.
Auditory Brain Stem Responses
Universal neonatal hearing screening is commonly done in most NICUs. Otoacoustic emissions and automated auditory brain stem response (AABR) are standard in a number of screening programs, and infants with a history of perinatal asphyxia are overrepresented in the abnormal outcomes group (100). Generally, AABR is recommended for screening of high-risk infants. A Dutch nationwide cohort of preterm infants (born before 30 weeks gestation and less than 1000 g birth weight) who underwent AABR hearing screening, recognized severe birth asphyxia as an independent risk factor for hearing loss (101). AABRs may have a diagnostic and prognostic role to play in preterm HIE survivors.
Neurological Outcome of Preterm HIE
There are few well-designed studies available to answer the question of neurological outcomes following preterm HIE. In the study by Chalak et al. (57), all babies with Stage 1 and Stage 2 HIE had normal neurological examinations and typical developmental milestones at 12 months of age. All surviving babies with HIE Stage 3 had poor neurological outcomes, two of nine babies died. In Logitharajah’s study (58), 15 (33%) of babies with HIE died and 15 had normal outcome, though this was not matched to severity of HIE stage. The most common type of CP observed was quadriplegic 13 (25%) followed by diplegic CP 2 (4%). A small percentage of babies had minor motor delay.
Standard Definition
Having a standard definition of HIE in preterm infants would enable the treating physician to recognize, investigate, and prognosticate brain injury earlier during the preterm neonate’s journey. It also provides a platform base on which future studies and therapeutic interventions can be planned and implemented.
Proposed Definition of Preterm Hypoxic–Ischemic Encephalopathy
Definite pHIE (Both Criteria Needed)
• pH≤7 and base deficit ≥12 mmol/L in fetal/cord/first neonatal blood sample (within 1 h of birth).
• Neonatal encephalopathy – Sarnat staging [staged according to all criteria (except EEG) for infants between 33 and 35 weeks of gestation], significant changes in neurological examination and/or seizures (for infants less than 33 weeks of gestation).
Probable pHIE (Any Two Criteria)
• pH 7.01–7.2 in fetal/cord/first neonatal blood sample.
• Early (less than 48 h) multisystem involvement, e.g., renal, liver, cardiac dysfunction.
• Preceding identifiable sentinel event (e.g., placental abruption, uterine rupture, cord prolapse) with cardiotocograph abnormalities with previously normal pattern.
• Prolonged (more than 72 h) need for assisted ventilation in absence of respiratory illness/neuromuscular disorder.
• Delayed (more than 24 h) resolution of metabolic acidosis.
• Specific region injury (predominant WM and basal ganglia injury, relative sparing of cortex) on MRI brain performed within the first week of life.
Conclusion
Preterm HIE produces a complex, heterogeneous, and characteristic pattern of injury to the developing brain with a wide spectrum of clinical manifestations. It poses a great challenge for the treating physician to recognize, evaluate, and prognosticate. An acknowledgment and accurate definition of preterm HIE, as a distinct entity, will help in designing future studies and implementing neuroprotective strategies for treatment of this high-risk premature population.
Author Contributions
KG drafted the initial manuscript, did the literature search, edited, and approved the final manuscript for submission. JL helped with writing section on EEG in preterm HIE, revised, and approved the manuscript for final submission. MF edited the manuscript, provided insights into the variable aspects of preterm HIE, and revised the manuscript for final submission. RH, GJ, and SM provided insights into the various aspects of preterm HIE, edited the manuscript, and approved the final submission. AM conceptualized and structured the review article, edited, and finalized the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
SM is a supported by an ARC (Australian Research Council) Future Fellowship, AM is supported by a RACP (Royal Australasian College of Physicians) Foundation Research Scholarship. The authors also wish to acknowledge the Victorian Government’s Operational Infrastructure Support program.
Abbreviations
AABR, automated auditory brain stem response; aEEG, amplitude-integrated EEG; BBB, blood–brain barrier; BPD, bronchopulmonary dysplasia; CP, cerebral palsy; CTG, cardiotocograph; EEG, electroencephalography; HbT, hemoglobin total; HIE, hypoxic–ischemic encephalopathy; HII, hypoxic–ischemic injury; MRI, magnetic resonance imaging; NIRS, near-infrared spectroscopy; OLs, oligodendrocytes; PVL, periventricular leukomalacia; rSO2, regional tissue oxygen saturation; SVZ, subventricular zone; TOI, tissue oxygenation index; WHO, World Health Organization; WM, white matter; WMI, white mater injury.
References
1. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health (2013) 10:1–14. doi:10.1186/1742-4755-10-S1-S2
2. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (2012) 379(9832):2162–72. doi:10.1016/S0140-6736(12)60820-4
3. Beck S, Wojdyla D, Say L. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ (2010) 88(1):31–8. doi:10.2471/BLT.08.062554
4. Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ (2012) 345:e7961. doi:10.1136/bmj.e7961
5. Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network. Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993-1999. Pediatrics (2005) 115(6):1645–51. doi:10.1542/peds.2004-2215
6. Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen L, Tammela O, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996-1997. Pediatrics (2005) 116(6):1391–400. doi:10.1542/peds.2005-0171
7. Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol (2008) 21(2):123–8. doi:10.1097/WCO.0b013e3282f88bb4
8. Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain (2005) 128(11):2578–87. doi:10.1093/brain/awh618
9. Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev (2007) 83(4):247–54. doi:10.1016/j.earlhumdev.2006.05.021
10. Tronnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev Med Child Neurol (2014) 56(8):779–85. doi:10.1111/dmcn.12430
11. Volpe JJ. The encephalopathy of prematurity – brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol (2009) 16(4):167–78. doi:10.1016/j.spen.2009.09.005
12. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol (2009) 8(1):110–24. doi:10.1016/S1474-4422(08)70294-1
13. Murray AL, Thompson DK, Pascoe L, Leemans A, Inder TE, Doyle LW, et al. White matter abnormalities and impaired attention abilities in children born very preterm. Neuroimage (2016) 124(Pt A):75–84. doi:10.1016/j.neuroimage.2015.08.044
14. Ullman H, Spencer-Smith M, Thompson DK, Doyle LW, Inder TE, Anderson PJ, et al. Neonatal MRI is associated with future cognition and academic achievement in preterm children. Brain (2015) 138(Pt 11):3251–62. doi:10.1093/brain/awv244
15. MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ (1999) 319(7216):1054–9. doi:10.1136/bmj.319.7216.1054
16. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol (1976) 33(10):696–705. doi:10.1001/archneur.1976.00500100030012
17. Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol (2014) 123(4):896–901. doi:10.1097/01.AOG.0000445580.65983.d2
18. Kinney HC, Volpe JJ. Modelling the encephalopathy of prematurity in animals: the important role of translational research. Neurol Res Int (2012) 2012:295389. doi:10.1155/2012/295389
19. Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol (2005) 491(2):109–22. doi:10.1002/cne.20714
20. Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev (2005) 81(9):753–61. doi:10.1016/j.earlhumdev.2005.07.004
21. Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev (2006) 12:129–40. doi:10.1002/mrdd.20107
22. Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics (2006) 117(Pt 2):28–33. doi:10.1542/peds.2005-0620E
23. Rivkin MJ, Volpe JJ. Hypoxic-ischemic brain injury in the newborn. Semin Neurol (1993) 13(1):30–9. doi:10.1055/s-2008-1041104
25. Hanrahan JD, Sargentoni J, Azzopardi D, Manji K, Cowan FM, Rutherford MA, et al. Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res (1996) 39(4 Pt 1):584–90. doi:10.1203/00006450-199604000-00004
26. Du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol (2002) 15(2):151–7. doi:10.1097/00019052-200204000-00005
27. Johnston MV, Trescher W, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res (2001) 49:735–41. doi:10.1203/00006450-200106000-00003
28. Berger R, Garnier Y. Perinatal brain injury. J Perinat Med (2000) 28:261–85. doi:10.1515/JPM.2000.034
29. Shalak L, Perlman JM. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev (2004) 80(2):125–41. doi:10.1016/j.earlhumdev.2004.06.003
30. Iwata O, Iwata S, Thornton JS, De Vita E, Bainbridge A, Herbert L, et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res (2007) 1154:173–80. doi:10.1016/j.brainres.2007.03.083
31. Allen KA, Brandon DH. Hypoxic ischemic encephalopathy: pathophysiology and experimental treatments. Newborn Infant Nurs Rev (2011) 11(3):125–33. doi:10.1053/j.nainr.2011.07.004
32. Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res (1994) 36(6):699–706. doi:10.1203/00006450-199412000-00003
33. Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia-ischemia in the immature rat. J Cereb Blood Flow Metab (2004) 24(10):1090–7. doi:10.1097/01.WCB.0000133250.03953.63
34. Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull (1998) 46(4):281–309. doi:10.1016/S0361-9230(98)00024-0
35. Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, et al. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron (2002) 35(6):1067–83. doi:10.1016/S0896-6273(02)00905-4
36. Ferriero DM. Neonatal brain injury. N Engl J Med (2004) 351(19):1985–95. doi:10.1056/NEJMra041996
37. Ankarcrona M, Dypbukt J, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron (1995) 15:961–73. doi:10.1016/0896-6273(95)90186-8
38. Gilland E, Puka-Sundvall M, Hillered L, Hagberg H. Mitochondrial function and energy metabolism after hypoxia-ischemia in the immature rat brain: involvement of NMDA-receptors. J Cereb Blood Flow Metab (1998) 18(3):297–304. doi:10.1097/00004647-199803000-00008
39. Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ (2005) 12(2):162–76. doi:10.1038/sj.cdd.4401545
40. Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G, et al. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem (2003) 86(2):306–17. doi:10.1046/j.1471-4159.2003.01832.x
41. Walton M, Connor B, Lawlor P, Young D, Sirimanne E, Gluckman P, et al. Neuronal death and survival in two models of hypoxic-ischemic brain damage. Brain Res Brain Res Rev (1999) 29(2–3):137–68. doi:10.1016/S0165-0173(98)00053-8
42. Bauer R, Zwiener U, Buchenau W, Bergmann R, Beyer R, Beyer GJ, et al. Interaction between systemic circulation and brain injuries in newborns. Exp Pathol (1991) 42(4):197–203. doi:10.1016/S0232-1513(11)80065-4
43. Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol (2002) 282(4):485–94. doi:10.1152/ajpheart.00645.2001
44. Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs AR, Davis TP. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NF kappa B. J Cell Sci (2003) 116(4):693–700. doi:10.1242/jcs.00264
45. Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem (2006) 96(4):1101–10. doi:10.1111/j.1471-4159.2005.03597.x
46. Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab (2003) 23(2):137–49. doi:10.1097/01.WCB.0000044631.80210.3C
47. Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci (2001) 21(4):1302–12.
48. Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci (2002) 22(2):455–63.
49. Kinney HC, Back SA. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol (1998) 5(3):180–9. doi:10.1016/S1071-9091(98)80033-8
50. Noetzel MJ, Brunstrom JE. The vulnerable oligodendrocyte: inflammatory observations on a cause of cerebral palsy. Neurology (2001) 56(10):1254–5. doi:10.1212/WNL.56.10.1254
51. Vannucci RC, Brucklacher RM, Vannucci SJ. CSF glutamate during hypoxia-ischemia in the immature rat. Brain Res Dev Brain Res (1999) 118(1–2):147–51. doi:10.1016/S0165-3806(99)00142-X
52. Rosin C, Bates TE, Skaper SD. Excitatory amino acid induced oligodendrocyte cell death in vitro: receptor-dependent and -independent mechanisms. J Neurochem (2004) 90(5):1173–85. doi:10.1111/j.1471-4159.2004.02584.x
53. McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci (2003) 23(8):3308–15.
54. Hoon AH Jr, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol (2009) 51(9):697–704. doi:10.1111/j.1469-8749.2009.03306.x
55. Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci (1998) 18(16):6241–53.
56. Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci (1993) 13(4):1441–53.
57. Chalak LF, Rollins N, Morriss MC, Brion LP, Heyne R, Sánchez PJ. Perinatal acidosis and hypoxic-ischemic encephalopathy in preterm infants of 33 to 35 weeks’ gestation. J Pediatr (2012) 160:388–94. doi:10.1016/j.jpeds.2011.09.001
58. Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res (2009) 66(2):222–9. doi:10.1203/PDR.0b013e3181a9ef34
59. Schmidt JW, Walsh WF. Hypoxic-ischemic encephalopathy in preterm infants. J Neonatal Perinatal Med (2010) 3:277–84.
60. Salhab WA, Perlman JM. Severe fetal acidemia and subsequent neonatal encephalopathy in the larger premature infant. Pediatr Neurol (2005) 32(1):25–9. doi:10.1016/j.pediatrneurol.2004.06.016
61. Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev (2013) 1:CD003311. doi:10.1002/14651858.CD003311.pub3
62. Volpe JJ. Neurologic outcome of prematurity. Arch Neurol (1998) 55(3):297–300. doi:10.1001/archneur.55.3.297
63. Barkovich AJ, Sargent SK. Profound asphyxia in the premature infant: imaging findings. AJNR Am J Neuroradiol (1995) 16(9):1837–46.
64. Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med (2005) 353(15):1574–84. doi:10.1056/NEJMcps050929
65. Stevens CP, Raz S, Sander CJ. Peripartum hypoxic risk and cognitive outcome: a study of term and preterm birth children at early school age. Neuropsychology (1999) 13(4):598–608. doi:10.1037/0894-4105.13.4.598
66. Hopkins-Golightly T, Raz S, Sander CJ. Influence of slight to moderate risk for birth hypoxia on acquisition of cognitive and language function in the preterm infant: a cross-sectional comparison with preterm-birth controls. Neuropsychology (2003) 17(1):3–13. doi:10.1037/0894-4105.17.1.3
67. Espy KA, Senn TE, Charak DA, Tyler J, Wiebe SA. Perinatal pH and neuropsychological outcomes at age 3 years in children born preterm: an exploratory study. Dev Neuropsychol (2007) 32(2):669–82. doi:10.1080/87565640701376003
68. Seth B, Datta V, Bhakhri BK. Umbilical artery pH at birth and neurobehavioral outcome in early preterm infants: a cohort study. J Pediatr Neurosci (2014) 9(1):7–10. doi:10.4103/1817-1745.131470
69. Lackmann GM, Tollner U, Mader R. Serum enzyme activities in full-term asphyxiated and healthy newborns: enzyme kinetics during the first 144 hours of life. Enzyme Protein (1993) 47(3):160–72.
70. Karlsson M, Blennow M, Nemeth A, Winbladh B. Dynamics of hepatic enzyme activity following birth asphyxia. Acta Paediatr (2006) 95(11):1405–11.
71. McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol (2004) 30(4):227–35. doi:10.1016/j.pediatrneurol.2003.10.001
72. Talos DM, Follett PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol (2006) 497(1):61–77. doi:10.1002/cne.20972
73. Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med (2006) 355(7):685–94. doi:10.1056/NEJMoa053792
74. Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, et al. Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics (2004) 114(4):1004–14. doi:10.1542/peds.2004-0222
75. Liauw L, van Wezel-Meijler G, Veen S, van Buchem MA, van der Grond J. Do apparent diffusion coefficient measurements predict outcome in children with neonatal hypoxic-ischemic encephalopathy? AJNR Am J Neuroradiol (2009) 30(2):264–70. doi:10.3174/ajnr.A1318
76. Inder T, Huppi PS, Zientara GP, Maier SE, Jolesz FA, Di salvo D, et al. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr (1999) 134(5):631–4. doi:10.1016/S0022-3476(99)70251-9
77. Gunn AJ, Bennet L. Brain cooling for preterm infants. Clin Perinatol (2008) 35(4):735–48. doi:10.1016/j.clp.2008.07.012
78. Robertson NJ, Tan S, Groenendaal F, Van Bel F, Juul SE, Bennet L, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr (2012) 160(4):544–52. doi:10.1016/j.jpeds.2011.12.052
79. Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics (2009) 124(3):459–67. doi:10.1542/peds.2008-2190
80. Al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics (1999) 103(6 I):1263–71. doi:10.1542/peds.103.6.1263
81. Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, et al. The TOBY study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr (2008) 8:17. doi:10.1186/1471-2431-8-17
82. Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet (2005) 365(9460):663–70. doi:10.1016/S0140-6736(05)17946-X
83. Walsh BH, Murray DM, Boylan GB. The use of conventional EEG for the assessment of hypoxic ischaemic encephalopathy in the newborn: a review. Clini Neurophysiol (2011) 122(7):1284–94. doi:10.1016/j.clinph.2011.03.032
84. Toet MC, van der Meij W, de Vries LS, Uiterwaal CS, van Huffelen KC. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics (2002) 109(5):772–9. doi:10.1542/peds.109.5.772
85. Watanabe K, Hayakawa F, Okumura A. Neonatal EEG: a powerful tool in the assessment of brain damage in preterm infants. Brain Dev (1999) 21(6):361–72. doi:10.1016/S0387-7604(99)00034-0
86. Olischar M, Klebermass K, Kuhle S, Hulek M, Kohlhauser C, Rucklinger E, et al. Reference values for amplitude-integrated electroencephalographic activity in preterm infants younger than 30 weeks’ gestational age. Pediatrics (2004) 113(1 Pt 1):61–6. doi:10.1542/peds.113.1.e61
87. Klebermass K, Olischar M, Waldhoer T, Fuiko R, Pollak A, Weninger M. Amplitude-integrated EEG pattern predicts further outcome in preterm infants. Pediatr Res (2011) 70(1):102–8. doi:10.1038/pr.2011.327
88. Jiang CM, Yang YH, Chen LQ, Shuai XH, Lu H, Xiang JH, et al. Early amplitude-integrated EEG monitoring 6 h after birth predicts long-term neurodevelopment of asphyxiated late preterm infants. Eur J Pediatr (2015) 174(8):1043–52. doi:10.1007/s00431-015-2490-z
89. Wikstrom S, Pupp IH, Rosen I, Norman E, Fellman V, Ley D, et al. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr (2012) 101(7):719–26. doi:10.1111/j.1651-2227.2012.02677.x
90. Inder TE, Buckland L, Williams CE, Spencer C, Gunning MI, Darlow BA, et al. Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants. Pediatrics (2003) 111(1):27–33. doi:10.1542/peds.111.1.27
91. Fraser M, Bennet L, Gunning M, Williams C, Gluckman PD, George S, et al. Cortical electroencephalogram suppression is associated with post-ischemic cortical injury in 0.65 gestation fetal sheep. Brain Res Dev Brain Res (2005) 154(1):45–55. doi:10.1016/j.devbrainres.2004.10.002
92. Keogh MJ, Drury PP, Bennet L, Davidson JO, Mathai S, Gunn ER, et al. Limited predictive value of early changes in EEG spectral power for neural injury after asphyxia in preterm fetal sheep. Pediatr Res (2012) 71(4):345–53. doi:10.1038/pr.2011.80
93. Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TA, Parkinson C, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res (2013) 74(5):525–35. doi:10.1038/pr.2013.132
94. Nakamura S, Koyano K, Jinnai W, Hamano S, Yasuda S, Konishi Y, et al. Simultaneous measurement of cerebral hemoglobin oxygen saturation and blood volume in asphyxiated neonates by near-infrared time-resolved spectroscopy. Brain Dev (2015) 37(10):925–32. doi:10.1016/j.braindev.2015.04.002
95. Liem KD, Greisen G. Monitoring of cerebral haemodynamics in newborn infants. Early Hum Dev (2010) 86(3):155–8. doi:10.1016/j.earlhumdev.2010.01.029
96. Greisen G. Is near-infrared spectroscopy living up to its promises? Semin Fetal Neonatal Med (2006) 11(6):498–502. doi:10.1016/j.siny.2006.07.010
97. Tax N, Urlesberger B, Binder C, Pocivalnik M, Morris N, Pichler G. The influence of perinatal asphyxia on peripheral oxygenation and perfusion in neonates. Early Hum Dev (2013) 89(7):483–6. doi:10.1016/j.earlhumdev.2013.03.011
98. Wintermark P, Hansen A, Warfield SK, Dukhovny D, Soul JS. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage (2014) 85(1):287–93. doi:10.1016/j.neuroimage.2013.04.072
99. Huang L, Ding H, Hou X, Zhou C, Wang G, Tian F. Assessment of the hypoxic-ischemic encephalopathy in neonates using non-invasive near-infrared spectroscopy. Physiol Meas (2004) 25(3):749–61. doi:10.1088/0967-3334/25/3/014
100. Xu ZM, Cheng WX, Yang XL. Performance of two hearing screening protocols in NICU in Shanghai. Int J Pediatr Otorhinolaryngol (2011) 75(10):1225–9. doi:10.1016/j.ijporl.2011.07.004
Keywords: cerebral palsy, asphyxia, preterm brain injury, excitotoxicity, encephalopathy
Citation: Gopagondanahalli KR, Li J, Fahey MC, Hunt RW, Jenkin G, Miller SL and Malhotra A (2016) Preterm Hypoxic–Ischemic Encephalopathy. Front. Pediatr. 4:114. doi: 10.3389/fped.2016.00114
Received: 14 July 2016; Accepted: 05 October 2016;
Published: 20 October 2016
Edited by:
Charles Christoph Roehr, Oxford Health NHS Foundation Trust, UKReviewed by:
Helmut Dietmar Hummler, University of Ulm, GermanyDaniele Trevisanuto, Azienda Ospedaliera di Padova, Italy
Roland H. Hentschel, University Medical Center Freiburg, Germany
Copyright: © 2016 Gopagondanahalli, Li, Fahey, Hunt, Jenkin, Miller and Malhotra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atul Malhotra, atul.malhotra@monash.edu
 Krishna Revanna Gopagondanahalli
Krishna Revanna Gopagondanahalli Jingang Li
Jingang Li Michael C. Fahey
Michael C. Fahey Rod W. Hunt4,5
Rod W. Hunt4,5
 Graham Jenkin
Graham Jenkin Suzanne L. Miller
Suzanne L. Miller Atul Malhotra
Atul Malhotra