Venous Thromboembolism in Critical Illness and Trauma: Pediatric Perspectives
- 1Division of Critical Care Medicine, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, USA
- 2Medical College of Wisconsin, Children’s Hospital of Wisconsin, Milwaukee, WI, USA
Critically ill children and those sustaining severe traumatic injuries are at higher risk for developing venous thromboembolism (VTE) than other hospitalized children. Multiple factors including the need for central venous catheters, immobility, surgical procedures, malignancy, and dysregulated inflammatory state confer this increased risk. As well as being at higher risk of VTE, this population is frequently at an increased risk of bleeding, making the decision of prophylactic anticoagulation even more nuanced. The use of pharmacologic and mechanical prophylaxis remains variable in this high-risk cohort. VTE pharmacologic prophylaxis is an accepted practice in adult trauma and intensive care to prevent VTE development and associated morbidity, but it is not standardized in critically ill or injured children. Given the lack of pediatric specific guidelines, prevention strategies are variably extrapolated from the successful use of mechanical and pharmacologic prophylaxis in adults, despite the differences in developmental hemostasis and thrombosis risk between children and adults. Whether the burden of VTE can be reduced in the pediatric critically ill or injured population is not known given the lack of robust data. There are no trials in children showing efficacy of mechanical compression devices or prophylactic anticoagulation in reducing the rate of VTE. Risk stratification using clinical factors has been shown to identify those at highest risk for VTE and allows targeted prophylaxis. It remains unproven if such a strategy will mitigate the risk of VTE and its potential sequelae.
Introduction
Venous thromboembolism (VTE) diagnosis in hospitalized children appears to have increased markedly over the past decade (1). Critically ill and/or severely injured children are at a disproportionately higher risk of VTE events due to the presence of multiple VTE risk factors (2, 3). Clinical diagnosis of VTE can be especially challenging in a critically ill and severely injured child as extremity swelling and erythema may be non-specific signs and self-reporting of pain is limited by sedation, immobility, and physical state. Hence, a high degree of suspicion is needed on the part of a clinician to perform imaging and diagnose VTE.
In critically ill and injured adults, mechanical and pharmacologic prophylaxis is an accepted practice to mitigate VTE (4); however, this is not the case in children. In addition, VTE risk prediction and stratification remains a challenge and robust risk scoring systems remain elusive in children. Even if one were to develop the perfect risk screen, there are no data showing a benefit from prophylaxis in critically ill children. Despite the paucity of evidence surrounding screening and prophylaxis, health-care providers are motivated to develop strategies to reduce the incidence of VTE in children. While mechanical prophylaxis is relatively risk free, pharmacologic prophylaxis may increase the bleeding risk in this group of patients. This review will summarize the epidemiology/incidence and current controversies in regards to VTE in critically ill and severely injured children.
Incidence and Risk Factors for VTE in Critically Ill or Injured Children
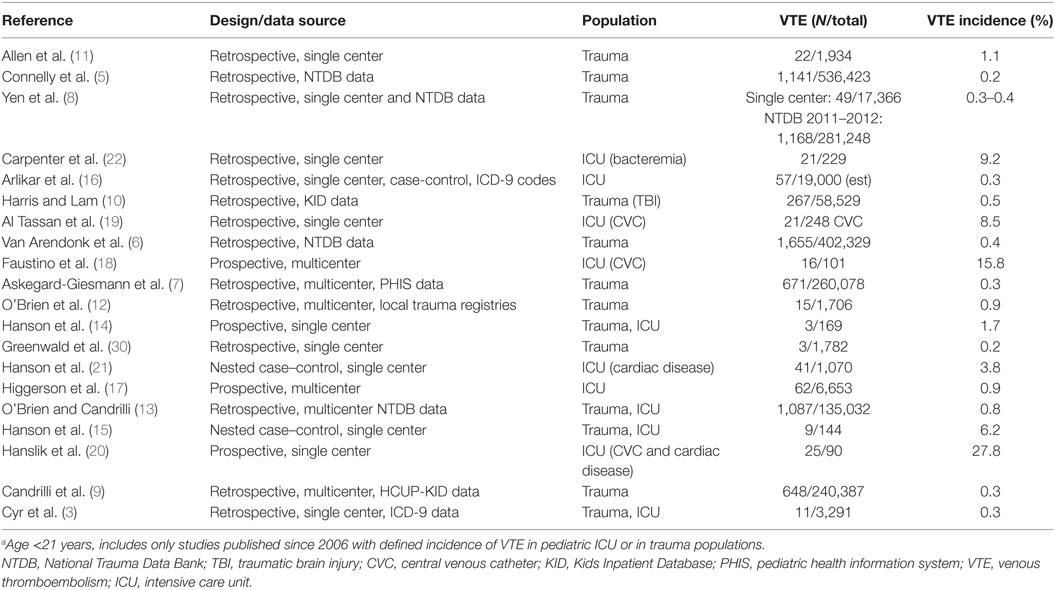
An increase in the diagnosis for VTE has been reported for hospitalized children from 2001 to 2008 (1); however, it is not known if this increase is equivalent among the subpopulations of critically ill or injured children. The interpretation of reported rates of VTE is confounded by the lack of standard VTE screening or for diagnosis of VTE. The reported incidence of VTE in critically ill and/or severely injured children is summarized in Table 1. There is a wide variation in incidence based on the study design and the specific population included. For instance, the incidence of VTE in pediatric trauma ranges from 0.2 to 0.5% in large, retrospective studies of hospitalized children using databases (5–10), while higher rates of 0.9–1.1% were found in smaller studies using data from the patient records (11, 12). For injured children admitted to the intensive care unit (ICU), the incidence rate (0.3–6.2%) is higher especially in prospective studies (3, 13–15).
For the general pediatric ICU population, the incidence of VTE ranges 0.3–0.9% (16–22), with the higher incidence being reported from prospective studies. Specific subpopulations of critically ill children report higher incidence of VTE including those with central venous catheters (CVCs) (17–19), cardiac disease (20, 21), and bacteremia (22).
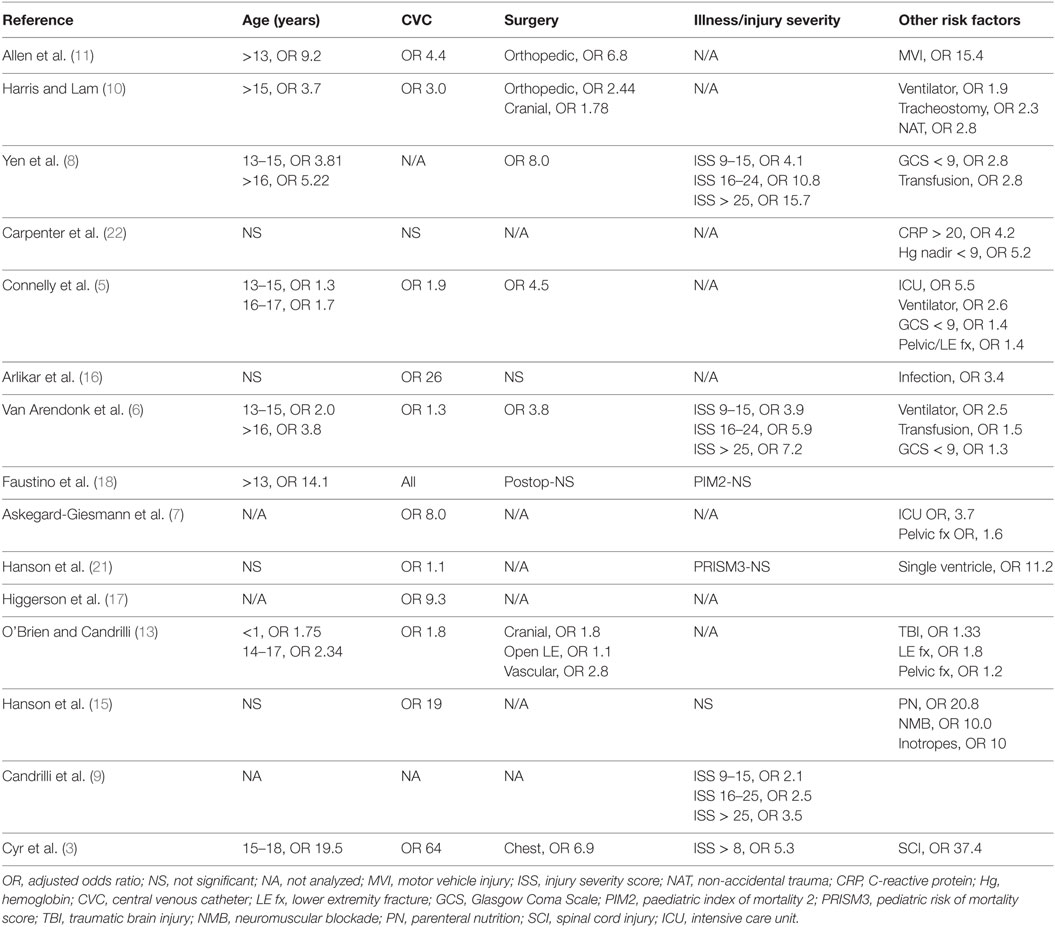
The risk factors for VTE in critically ill and/or severely injured children are summarized in Table 2. Critically ill and/or severely injured children are at a disproportionately higher risk of VTE events than any other cohort of children due to the presence of multiple risk factors: including endothelial injury from trauma, CVC placement, and/or operative procedures; alterations in blood flow from immobility and poor perfusion requiring inotropic support; and hypercoagulability from sepsis, trauma, blood transfusion, or other dysregulated inflammatory states (see Table 2). The exact contribution of each of these factors is unclear; however, the presence of a CVC is probably the most important risk factor for VTE in this cohort of children. Most, but not all, studies found increasing age to be a risk factor for VTE in injured and critically ill children (see Table 2). In studies that performed a separate analysis for infants, <1 year of age was also associated with increased risk. For trauma patients, the risk of VTE appears to increase with higher injury severity scores (ISS) (see Table 2).
Overall interpretation and generalizability of data in regards to incidence and risk factors is limited, given the significant differences in the population included and study design. Several, recent, large studies in children have used diagnostic codes for the identification of VTE (5, 6). Using diagnostic codes for identification of pediatric VTE has a low specificity and sensitivity (23). Hence, misidentification of children with and without VTE could result in differences in incidence rates and risk factors. Likewise, studies with smaller numbers of patients may fail to identify significant risk factors. Despite these limitations in study populations and methodologies, the incidence of VTE appears to increase in patients with multiple risk factors, with the presence of a CVC being the most important risk factor in critically ill and/or injured patients. Certain subpopulations of critically ill children have a greater risk for VTE, with an incidence of VTE >1%.
Prevention of VTE in Critically Ill or Injured Children
Efforts for prevention of VTE in critically ill or injured patients hinge on early mobilization and the use of mechanical and/or pharmacologic prophylaxis. Mechanical prophylaxis includes the use of sequential compression devices (SCD) or graduated compression stockings, both of which are limited by size and cannot be used in smaller children and on injured extremities. There are no pediatric studies showing efficacy of mechanical prophylaxis in preventing VTE.
There is little evidence to guide the use of pharmacologic thromboprophylaxis in critically ill and injured children. Published pediatric guidelines are based on weak evidence and recommend against the use of pharmacologic prophylaxis except in children with cyanotic congenital heart disease, dilated cardiomyopathy, cavopulmonary anastomosis, end-stage renal disease, and primary pulmonary hypertension (24). A recently published consensus of experts in regards to pediatric trauma recommended against prophylaxis in children <12 years of age and gave a strong recommendation for pharmacologic prophylaxis in patients with a history of VTE, while a weak recommendation for patients with CVCs (25). Given the lack of data, it is not surprising that there is a wide variation in thromboprophylactic practices in critically ill children as shown in the PROTRACT study (26). This global point-prevalence study clearly demonstrated that the use of both mechanical and pharmacologic prophylaxes was center dependent with a wide variation in the use of prophylaxis. Data were collected on the type of pharmacologic thromboprophylaxis used in the ICU including aspirin, low-molecular-weight heparin, IV unfractionated heparin (UFH), subcutaneous UFH, warfarin, and clopidogrel. Aspirin was the most commonly used agent (143 of 308 patients, 46.4%), primarily because of patients with congenital heart disease. LMWH, almost exclusively enoxaparin, was the next most commonly used agent (113 of 308 patients, 36.7%). Warfarin was rarely used in the ICU setting (26).
Critically ill and/or injured children represent a high-risk cohort for VTE, especially in the setting of CVCs, and may merit from thromboprophylaxis. This is especially true as patients approach adulthood wherein heparin-based prophylaxis regimens have been shown to be effective in preventing VTE in critically ill adults (4). Whether such strategies are of benefit in critically ill and/or injured children remain unproven. However, a standardized systematic approach to VTE prevention may result in a reduction in VTE. This was demonstrated in a single-center study in the setting of pediatric trauma where a reduction in incidence of VTE was noted after implementation of standardized thromboprophylaxis guidelines (14). Notably in this study, the reduced incidence of VTE was not associated with an increase in pharmacologic prophylaxis. The authors speculate that the decrease in VTE was a result of standardized, focused pharmacologic prophylaxis to those patients at high risk for VTE.
Pharmacologic prophylaxis should be instituted thoughtfully especially in patients at high risk for bleeding. There are minimal data on bleeding in the setting of pharmacologic prophylaxis for VTE in the critical care or trauma setting in pediatrics. In a multicenter review of trauma registry data to assess pharmacologic prophylaxis, the rate of major bleeding was 0.3% (12). However, single-center data demonstrated a higher rate of 4% in hospitalized pediatric patients receiving pharmacologic prophylaxis (27). A recent prospective observational study of hospitalized children receiving prophylactic anticoagulation showed a similar incidence of major bleeding especially in patients following orthopedic surgery (28). Taken together, the data demonstrate a low but definite risk of bleeding children receiving pharmacologic prophylaxis. Hence, it is imperative that any preventive strategy utilizing pharmacologic prophylaxis account for the bleeding risk, especially in a high bleeding-risk cohort, such as children who are critically ill or severely injured.
In summary, VTE prevention in critically ill and/or injured children needs a standardized approach with VTE risk stratification. Interventions should include early mobilization and removal of CVCs alongside mechanical and pharmacologic prophylaxes, especially in children >12 years of age.
Predicting VTE Risk in Children after Trauma
Recently, two scoring systems to predict the risk of VTE in children hospitalized after trauma have been developed (5, 8). Both studies used the National Trauma Data Bank to derive and validate the VTE risk score over similar time periods. The model from Connelly et al. had good performance with an area under the curve of 93–94% (5). This model incorporated 10 VTE risk factors: age (increased risk for <1 year and adolescence), sex, Glasgow Coma Scale (GCS), CVC, intubation, blood transfusion, ICU admission, major surgery, pelvic fracture, and lower extremity fracture. Varying points for each risk factor are summed for a total score. Categorical risk was assigned based on this score: low risk (VTE incidence <1%), medium risk (VTE incidence 1–5%), and high risk (VTE incidence >5%). The authors suggest a potential management strategy to implement screening ultrasounds and SCD for the medium-risk group, with the addition of pharmacologic prophylaxis for the high-risk group. By contrast, Yen et al. used a combination of local trauma registry data and the national trauma data bank for development and validation of a VTE risk score model with good performance as shown by the area under curve of 91% (8). The preferred model incorporates six risk factors, for which varying points are accumulated: older age, GCS, ISS, blood transfusion, intubation, and major surgery. CVC was not analyzed as a risk factor for the model. A score >17 is associated with VTE risk >2%, referenced as a threshold for prophylaxis.
These studies provide the framework to convert epidemiologic risk factors into tools clinicians can use to predict the overall VTE risk for their injured patient. Both studies recognize the limitations of the national trauma database: surveillance bias, no temporal association of risks (intubation, surgery, and transfusion) with the development of VTE, and the confounding effect of variable use of thromboprophylaxis. The rare occurrence of VTE in the overall hospitalized pediatric trauma population makes large database studies necessary to provide adequate power of associated risks, with the risks studied limited to those captured in the database. As injured children in the ICU have a higher VTE rate compared to the overall hospitalized children after trauma, this high-risk population may be appropriate to prospectively validate and refine an optimal VTE prediction tool.
Future Directions
The ever-increasing medical complexity of critically ill and injured children implies that the risk of VTE will continue to be present especially in the setting of CVCs. Hence, standardized risk prediction and stratification will be the key to implementing any thromboprophylactic strategy. Validation of risk prediction tools will be challenging given the low overall incidence for VTE in children. Currently, most risk assessment algorithms use clinical variables, and whether the addition of biomarkers bolsters their performance remains unclear. A recent prospective study in critically ill children with CVCs showed an association between factor VIII activity and catheter-related thrombosis (29).
Even with the ideal risk prediction tool, the appropriate interventions to prevent VTE are unknown. Hence, there is a pressing need to evaluate the efficacy of interventions in preventing VTE in children, including pharmacologic or mechanical prophylaxis, early ICU rehabilitation, and increased mobility. Given the low incidence of VTE in children, focusing on the subpopulations of critically ill or injured children at highest risk for VTE, including those with CVCs, will optimize the results of any clinical trial.
Author Contributions
RC and SH conceptualized and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics (2009) 124(4):1001–8. doi:10.1542/peds.2009-0768
2. Mahajerin A, Branchford BR, Amankwah EK, Raffini L, Chalmers E, van Ommen CH, et al. Hospital-associated venous thromboembolism in pediatrics: a systematic review and meta-analysis of risk factors and risk-assessment models. Haematologica (2015) 100(8):1045–50. doi:10.3324/haematol.2015.123455
3. Cyr C, Michon B, Pettersen G, David M, Brossard J. Venous thromboembolism after severe injury in children. Acta Haematol (2006) 115(3–4):198–200. doi:10.1159/000090935
4. Alhazzani W, Lim W, Jaeschke RZ, Murad MH, Cade J, Cook DJ. Heparin thromboprophylaxis in medical-surgical critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care Med (2013) 41(9):2088–98. doi:10.1097/CCM.0b013e31828cf104
5. Connelly CR, Laird A, Barton JS, Fischer PE, Krishnaswami S, Schreiber MA, et al. A clinical tool for the prediction of venous thromboembolism in pediatric trauma patients. JAMA Surg (2016) 151(1):50–7. doi:10.1001/jamasurg.2015.2670
6. Van Arendonk KJ, Schneider EB, Haider AH, Colombani PM, Stewart FD, Haut ER. Venous thromboembolism after trauma: when do children become adults? JAMA Surg (2013) 148(12):1123–30. doi:10.1001/jamasurg.2013.3558
7. Askegard-Giesmann JR, O’Brien SH, Wang W, Kenney BD. Increased use of enoxaparin in pediatric trauma patients. J Pediatr Surg (2012) 47(5):980–3. doi:10.1016/j.jpedsurg.2012.01.060
8. Yen J, Van Arendonk KJ, Streiff MB, McNamara L, Stewart FD, Conner KG, et al. Risk factors for venous thromboembolism in pediatric trauma patients and validation of a novel scoring system: the risk of clots in kids with trauma score. Pediatr Crit Care Med (2016) 17(5):391–9. doi:10.1097/PCC.0000000000000699
9. Candrilli SD, Balkrishnan R, O’Brien SH. Effect of injury severity on the incidence and utilization-related outcomes of venous thromboembolism in pediatric trauma inpatients. Pediatr Crit Care Med (2009) 10(5):554–7. doi:10.1097/PCC.0b013e3181a705d3
10. Harris DA, Lam S. Venous thromboembolism in the setting of pediatric traumatic brain injury. J Neurosurg Pediatr (2014) 13(4):448–55. doi:10.3171/2014.1.PEDS13479
11. Allen CJ, Murray CR, Meizoso JP, Ray JJ, Neville HL, Schulman CI, et al. Risk factors for venous thromboembolism after pediatric trauma. J Pediatr Surg (2016) 51(1):168–71. doi:10.1016/j.jpedsurg.2015.10.033
12. O’Brien SH, Klima J, Gaines BA, Betz S, Zenati MS. Utilization of low-molecular-weight heparin prophylaxis in pediatric and adolescent trauma patients. J Trauma Nurs (2012) 19(2):117–21. doi:10.1097/JTN.0b013e31825629c4
13. O’Brien SH, Candrilli SD. In the absence of a central venous catheter, risk of venous thromboembolism is low in critically injured children, adolescents, and young adults: evidence from the National Trauma Data Bank. Pediatr Crit Care Med (2011) 12(3):251–6. doi:10.1097/PCC.0b013e3181f36bd9
14. Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg (2012) 72(5):1292–7. doi:10.1097/TA.0b013e31824964d1
15. Hanson SJ, Punzalan RC, Greenup RA, Liu H, Sato TT, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma (2010) 68(1):52–6. doi:10.1097/TA.0b013e3181a74652
16. Arlikar SJ, Atchison CM, Amankwah EK, Ayala IA, Barrett LA, Branchford BR, et al. Development of a new risk score for hospital-associated venous thromboembolism in critically-ill children not undergoing cardiothoracic surgery. Thromb Res (2015) 136(4):717–22. doi:10.1016/j.thromres.2015.04.036
17. Higgerson RA, Lawson KA, Christie LM, Brown AM, McArthur JA, Totapally BR, et al. Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatr Crit Care Med (2011) 12(6):628–34. doi:10.1097/PCC.0b013e318207124a
18. Faustino EV, Spinella PC, Li S, Pinto MG, Stoltz P, Tala J, et al. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J Pediatr (2013) 162(2):387–91. doi:10.1016/j.jpeds.2012.06.059
19. Al Tassan R, Al Alem H, Al Harbi T. Temporary central line related thrombosis in a pediatric intensive care unit in central Saudi Arabia. Two-year incidence and risk factors. Saudi Med J (2014) 35(4):371–6.
20. Hanslik A, Thom K, Haumer M, Kitzmuller E, Albinni S, Wolfsberger M, et al. Incidence and diagnosis of thrombosis in children with short-term central venous lines of the upper venous system. Pediatrics (2008) 122(6):1284–91. doi:10.1542/peds.2007-3852
21. Hanson SJ, Punzalan RC, Christensen MA, Ghanayem NS, Kuhn EM, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children with cardiac disease. Pediatr Cardiol (2012) 33(1):103–8. doi:10.1007/s00246-011-0098-2
22. Carpenter SL, Goldman J, Sherman AK, Jeremiah Bell J, Selveraju S, Newland JG, et al. Clinical variables and Staphylococcus aureus virulence factors associated with venous thromboembolism in children. Thromb Res (2016) 138:69–73. doi:10.1016/j.thromres.2015.11.029
23. Branchford BR, Gibson E, Manco-Johnson MJ, Goldenberg NA. Sensitivity of discharge diagnosis ICD-9 codes for pediatric venous thromboembolism is greater than specificity, but still suboptimal for surveillance and clinical research. Thromb Res (2012) 129(5):662–3. doi:10.1016/j.thromres.2011.10.032
24. Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2012) 141(2 Suppl):737S–801S. doi:10.1378/chest.11-2308
25. Hanson SJ, Faustino EV, Mahajerin A, O’Brien SH, Streck CJ, Thompson AJ, et al. Recommendations for venous thromboembolism prophylaxis in pediatric trauma patients: a national, multidisciplinary consensus study. J Trauma Acute Care Surg (2016) 80(5):695–701. doi:10.1097/TA.0000000000000962
26. Faustino EV, Hanson S, Spinella PC, Tucci M, O’Brien SH, Nunez AR, et al. A multinational study of thromboprophylaxis practice in critically ill children. Crit Care Med (2014) 42(5):1232–40. doi:10.1097/CCM.0000000000000147
27. Cavo M, Wang W, O’Brien SH. Use of low molecular weight heparin for thromboprophylaxis in a pediatric inpatient population: reasons for use and incidence of bleeding complications. Thromb Res (2010) 125(4):370–2. doi:10.1016/j.thromres.2009.03.004
28. Thompson AJ, McSwain SD, Webb SA, Stroud MA, Streck CJ. Venous thromboembolism prophylaxis in the pediatric trauma population. J Pediatr Surg (2013) 48(6):1413–21. doi:10.1016/j.jpedsurg.2013.02.059
29. Faustino EV, Li S, Silva CT, Pinto MG, Qin L, Tala JA, et al. Factor VIII may predict catheter-related thrombosis in critically ill children: a preliminary study. Pediatr Crit Care Med (2015) 16(6):497–504. doi:10.1097/PCC.0000000000000409
Keywords: venous thromboembolism, deep vein thrombosis, pediatric critical illness, pediatric trauma, child, prophylaxis
Citation: Chima RS and Hanson SJ (2017) Venous Thromboembolism in Critical Illness and Trauma: Pediatric Perspectives. Front. Pediatr. 5:47. doi: 10.3389/fped.2017.00047
Received: 30 November 2016; Accepted: 24 February 2017;
Published: 13 March 2017
Edited by:
Brian R. Branchford, University of Colorado Denver School of Medicine, USAReviewed by:
Daniele Zama, University of Bologna, ItalyYee Hui Mok, KK Women’s and Children’s Hospital, Singapore
Copyright: © 2017 Chima and Hanson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheila J. Hanson, shanson@mcw.edu
 Ranjit S. Chima
Ranjit S. Chima Sheila J. Hanson
Sheila J. Hanson