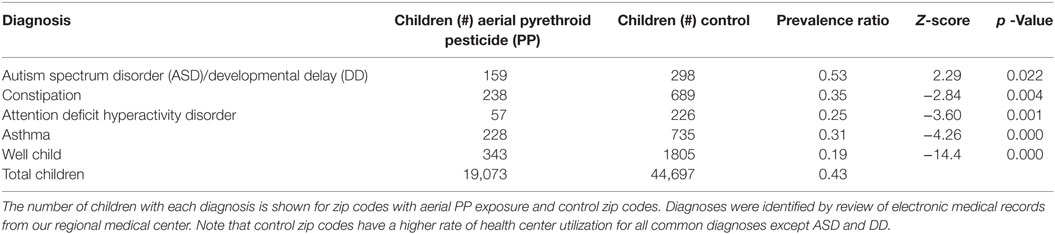
Neurodevelopmental Delay Diagnosis Rates Are Increased in a Region with Aerial Pesticide Application
- 1Department of Pediatrics, State University of New York, Upstate Medical University, Syracuse, NY, United States
- 2Department of Pediatrics, Milton S. Hershey Medical Center of Penn State University, Hershey, PA, United States
- 3Department of Public Health Sciences, Milton S. Hershey Medical Center of Penn State University, Hershey, PA, United States
- 4Department of Pediatrics, Palo Alto Medical Foundation, Palo Alto, CA, United States
- 5Department of Pediatrics, University of California at San Francisco, San Francisco, CA, United States
A number of studies have implicated pesticides in childhood developmental delay (DD) and autism spectrum disorder (ASD). The influence of the route of pesticide exposure on neurodevelopmental delay is not well defined. To study this factor, we examined ASD/DD diagnoses rates in an area near our regional medical center that employs yearly aerial pyrethroid pesticide applications to combat mosquito-borne encephalitis. The aim of this study was to determine if areas with aerial pesticide exposure had higher rates of ASD/DD diagnoses. This regional study identified higher rates of ASD/DD diagnoses in an area with aerial pesticides application. Zip codes with aerial pyrethroid exposure were 37% more likely to have higher rates of ASD/DD (adjusted RR = 1.37, 95% CI = 1.06–1.78, p = 0.02). A Poisson regression model controlling for regional characteristics (poverty, pesticide use, population density, and distance to medical center), subject characteristics (race and sex), and local birth characteristics (prematurity, low birthweight, and birth rates) identified a significant relationship between aerial pesticide use and ASD/DD rates. The relationship between pesticide application and human neurodevelopment deserves additional study to develop safe and effective methods of mosquito prevention, particularly as communities develop plans for Zika virus control.
Introduction
The recent emergence of the Zika virus and the discovery that perinatal Zika exposure increases fetal morbidity and mortality has led to a call for swift prevention measures (1). Among these measures is the expansion of aerial pesticide application to control the Aedes aegypti mosquito. Pesticides, which are designed to interfere with synaptic transmission in the central nervous system (2, 3), are indeed successful at reducing mosquito-borne illness. However, a growing number of studies suggest that the neurotoxic properties of pesticides may have unintended consequences in the brain of developing children (4–7). Although the instinct of communities is to protect children at all costs, it may be prudent to recall the Hippocratic Oath of physicians: “Primum non-nocere,” or “First, do no harm.”
The Childhood Autism Risks from Genetics and Environment study assessed the association of prenatal agricultural pesticide exposure and risk of autism spectrum disorder (ASD) or developmental delay (DD) in 970 children aged 2–5 years (6). The study found that children with ASD or DD were more likely than typically developing controls to have had organophosphate (OP), pyrethroid, or carbamate pesticides applied near their mother’s home, particularly during the third trimester of gestation. Although subsequent reviews have pointed out that such evidence does not establish causation, it does bolster the hypothesis that exposure to pesticides may contribute to neurodevelopmental disorders in genetically vulnerable children (8). Acetylcholine, a neurotransmitter that plays a critical role in learning, attention, and memory (9), is reduced in frontal and parietal cortices of children with ASD (10) and is a target of OP and pyrethroid pesticides (PPs) (11). This may represent one mechanism through which pesticides target the developing brain.
Pyrethroids are one specific class of pesticides that may increase the risk of ASD and DD (6, 12). In a study by Roberts and colleagues (4), maternal exposure to the pyrethroid bifenthrin during critical periods of gestation was associated with an increased risk of ASD diagnosis. In comparison, a recent study of 287 children showed that urinary pyrethroid levels at 6 years were negatively correlated with verbal comprehension and working memory, but maternal pyrethroid levels during gestation were not (7). These studies contribute to a growing body of evidence that pyrethroids can negatively affect neurodevelopment. The influence of exposure route and timing remain unclear. This question is an important one given that pyrethroid use has increased following a ban of OP pesticides in residential areas (13) and is a primary platform in Zika virus prevention.
Pyrethroid pesticides are employed in the central New York (NY, USA) county of Onondaga as a preventive tool against mosquitoes carrying eastern equine encephalitis (EEE) and West Nile virus (WNV). Each summer, the Onondaga County Department of Health supervises the aerial application (using aircraft) of 220 L (66 kg) of PP (Masterline® Kontrol 30-30 Concentrate; Austin, TX, USA) to a four zip-code area collectively known as the Cicero Swamp. The effects of this application on neurodevelopmental patterns in local children have not been investigated.
The present study examines the prevalence of neurodevelopmental delay (rates of ASD and DD measured as the proportion of children diagnosed) in a central New York locale exposed to yearly aerial pyrethroid applications. The study tests the hypothesis that rates of regional ASD/DD diagnoses are related to route (aerial application) of PP exposure.
Materials and Methods
Subject Identification
This study employed an ecological cross-sectional design. Permission for a retrospective review of pediatric charts from outpatient electronic medical records at the State University of New York (SUNY) Upstate Medical University was obtained from the Institutional Review Board. On April 16, 2015, an electronic chart review was used to identify all children younger than 20 years evaluated during a 5-year period (March 15, 2010 to March 15, 2015) with ICD-9 diagnostic codes corresponding to ASD (F84.0) or DD (R62.50) at one of six pediatric outpatient clinics (psychiatry and behavioral sciences private practice; pediatric general provider clinic; psychiatry; pediatrics autism, behavior, and feeding program; pediatric child development center; or pediatric genetics). Neurodevelopmental characteristics, including developmental test scores [Vineland Adaptive Behavioral Scales; Autism Diagnostic Observation Schedule (ADOS); Baylee Scales of Infant and Toddler Development; and Developmental Assessment of Young Children (DAY-C)], genetic test results, psychiatric comorbidities [attention deficit hyperactivity disorder (ADHD) and anxiety], parental drug/cigarette use, age at diagnosis, and birth month were recorded for all subjects when available in the electronic medical record.
Subjects were divided by zip code of residence into aerial-exposed and control zip codes. Aerial-exposed zip codes included four zip codes directly exposed to aerial application of PPs by the Onondaga County Health Department EEE prevention program and four adjacent zip codes (all within 2.5 km of the aerial application area). The distance of 2.5 km was chosen based upon the study by Shelton et al. (6), which determined that children within this exposure distance had increased risk of ASD or DD. Sixteen surrounding zip codes greater than 2.5 km from the application area served as control regions. The control zip codes were not exempt from standard methods of pesticide application (e.g., hydraulic spraying, manual dispensing of granules, or controlled droplet application) by state-certified commercial applicators, but had no state-approved aerial applications.
Population Demographics
Publicly available databases were employed to determine demographic data, perinatal data, and total pesticide exposure for each of the control and aerial-exposed zip codes. The total number of children younger than 20 years in each zip code was obtained from the 2013 American Community Survey (ACS).1 This database also provided the percentage of male children, the percentage of children below the poverty level, and the ethnic breakdown of children younger than 20 years in each zip code. The 2010–2012 New York State Vital Statistics Database2 was used to identify the number of births, the percentage of premature births (less than 37 weeks gestation), and the percentage low birthweight deliveries (less than 2,500 g) for each zip code over the specified 3-year period.
Regional Pesticide Exposure
Pesticide exposure within each zip code was estimated using data from the New York State Department of Environmental Conservation (DEC) Database.3 This database is maintained in cooperation with Cornell University and uses standard quality assurance processes to correct and validate mandated submissions of pesticide use by commercial applicators. Average pesticide exposure (in kilograms) over a 3-year period (2007–2009) was determined for each zip code using the most recently available data on the DEC website. Pesticide exposure in kilograms per square kilometer was calculated for each zip code. Details regarding the timing and volume of aerial pyrethroid application over the exposed zip codes (application of 220L between late July and early September of each calendar year from 2003 to present) were provided by the Onondaga County Department of Health and are reflected in the totals on the DEC website.
Prevalence of Neurodevelopmental Delay
The prevalence of neurodevelopmental delay (ASD and DD) for each zip code was calculated by dividing the number of observed cases at SUNY Upstate outpatient clinics by the total number of children residing in that zip code (as reported by the 2013 ACS). Comparisons were made between the 8 aerial-exposed zip codes and the 16 control zip codes. To validate the rates of ASD/DD diagnoses seen in control and aerial-exposed regions, the rates of ASD/DD in 30 additional zip codes in Central New York were recorded using electronic medical record review as described above. These zip codes had standard pesticide application procedures, but no aerial pesticide application practices. Total number of children and total pesticide application (kilograms) were recorded for these regions as well. Eight zip-code combinations were generated 360 times from this group, and mean rates of ASD/DD diagnoses were compared against the control and aerial-exposed zip-code rates.
Regional Rates of Health Center Utilization
To prevent bias introduced by regional rates of health center utilization, we examined whether children from aerial-exposed zip codes were seen at SUNY Upstate outpatient clinics more often than children residing in control zip codes. The number of children from each region who were seen at our regional health center for four common pediatric visits were recorded: constipation, ADHD, asthma, and well-child checks. A retrospective chart review was used to identify subjects aged 20 years or older seen at the institution’s outpatient clinics during the same 5-year period (March 15, 2010 to March 15, 2015) with an ICD-9 diagnostic code corresponding to constipation (K59.00), ADHD (F90.9), asthma (J45.909), or well-child check (Z00.129). A health center utilization ratio was calculated using the total number of children with a specified diagnosis from the 8 aerial-exposed zip codes divided by the total number of children with that same diagnosis from the 16 control zip codes. These utilization ratios were then compared to the ratio seen for ASD/DD, as well as the ratio of the total number of children living in aerial-exposed and control zip codes.
Statistics
Two-sample t-test or Wilcoxon rank-sum test was used for continuous variables, and Pearson’s chi-square test or Fisher’s exact test was used for categorical variables to identify between-group differences in demographic, perinatal, pesticide, and ASD prevalence data, as appropriate. Two-proportion Z-tests were used to examine health center utilization ratios for common pediatric diagnoses. A prevalence ratio for ASD/DD diagnosis was calculated for children living in the aerial-exposed zip codes compared with control zip codes. Pearson’s statistic was used to determine correlations between ASD/DD prevalence, aerial pesticide exposure, birth month, pesticide exposure per square kilometer, percent of premature births, percent of low birthweight births, race (% Caucasian), percent of children below the poverty level, and percent of male children for each zip code. A Poisson regression model was fitted to estimate the association between ASD/DD prevalence and aerial pyrethroid exposure (as a binary exposure variable) across zip codes adjusted to potential confounders including socioeconomic status (percent of children below the poverty level), incidence of premature births, number of births, percent of low birthweight births, distance to the tertiary referral center, race (percent of Caucasian children within the zip code), pesticide exposure per square kilometer, population density, and sex (percent of male children within the zip code). The overdispersion of the Poisson model was tested based on z-statistic, and the goodness-of-fit test was performed based on the residual deviance. The confounders that might influence pesticide exposure or ASD/DD prevalence were initially identified with Bayesian approach with a drected acyclic graph (Figure S2 in Supplementary Materials) and formally evaluated with stepwise regression procedures based on deviance chi-square tests. Factors without statistically significant impact remained in the final model due to projected clinical relevance. Statistical analyses were conducted using SAS version 9.4, and MetaboAnalyst 3.0 (14).
Results
Demographics
Demographic data from 8 aerial-exposed zip codes were compared with 16 control zip codes (Table 1). There were no significant differences between aerial-exposed and control zip codes in land area (p = 0.92), number of children younger than 20 years (p = 0.59), premature births (p = 0.47), low birthweight births (0.24), children with white race (p = 0.82), children below the poverty level (p = 0.14), or child sex (p = 0.63). There was an increased rate of neonatal death per 1,000 births in the control zip codes (p = 0.02).
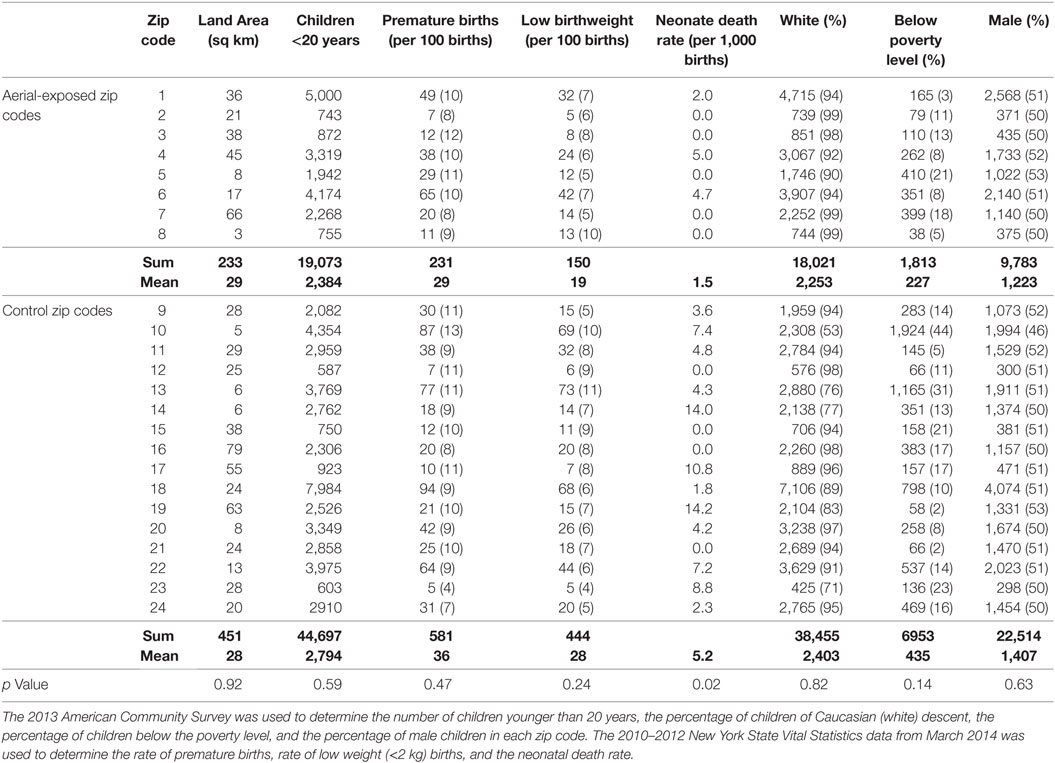
Table 1. Demographic data from 8 zip codes exposed to aerial pyrethroid pesticides and 16 control zip codes.
Health Center Utilization Rates
There were less children living in the 8 aerial-exposed zip codes (n = 19,073) compared with the 16 control zip codes (n = 44,697). The ratio of children younger than 20 years in aerial-exposed zip codes compared with control zip codes was 0.43 (Table 2). In comparison, lower ratios of children were seen at our health center from aerial-exposed zip codes for common pediatric diagnoses: constipation (Z = −2.84), ADHD (Z = −3.60), asthma (Z = −4.26), and well-child checks (Z = −14.36). Despite reduced utilization of our health center by aerial-exposed zip regions, the utilization ratio for ASD/DD diagnoses by these regions exceeded the population ratio (Z = 2.29; p = 0.02).
Pesticide Exposure
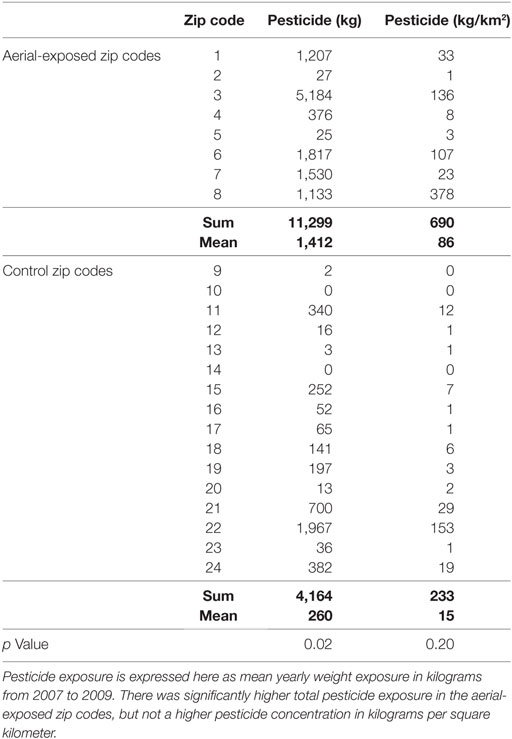
Between 2007 and 2009, the average yearly pesticide burden for the eight aerial-exposed zip codes was 11,299 kg (Table 3). This was a higher burden than the 4,164 kg of pesticide exposure seen across the 16 control zip codes (p = 0.02). Concentration of pesticide exposure for the 8 aerial-exposed zip codes (86 kg/km2) was not significantly higher (p = 0.20) than that in the 16 control zip codes (15 kg/km2). These data included the aerial application of 220 L of PPs over the aerial-exposed zip codes each year. A geographical representation of the aerial application zone along with mean yearly pesticide exposures for each zip code is displayed in Figure 1.
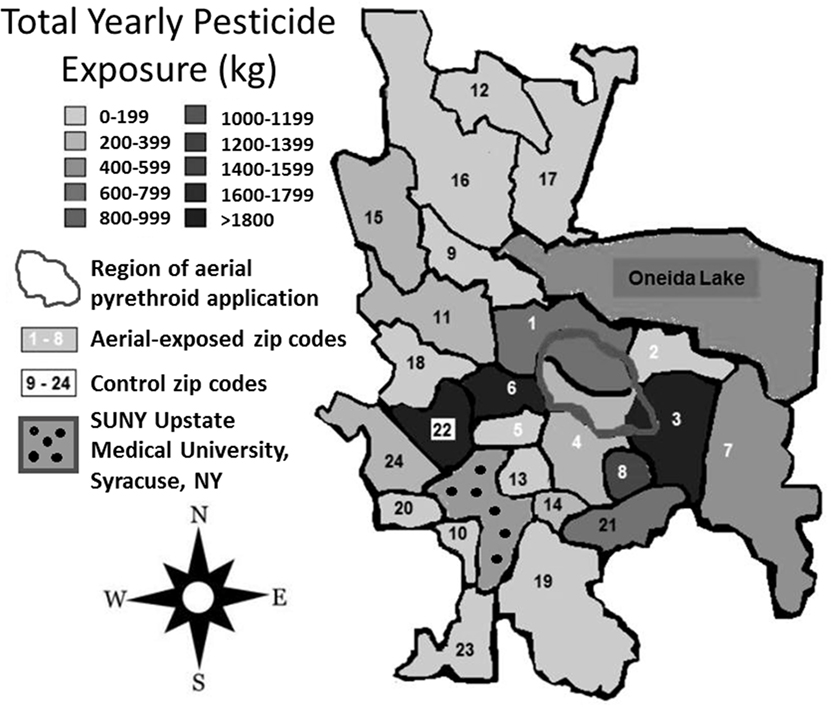
Figure 1. Regional pesticide exposure. Mean yearly pesticide exposure for each zip code is shown in kilograms. These levels were determined using the most recently available data from the New York State Department of Environmental Conservation Mandated Reporting Database. Total pesticide exposure includes pyrethroids, organophosphates, and other pesticide families regardless of class or application method employed. Aerial-exposed zip codes are numbered 1–8 in white, and control zip codes are numbered 9–24 in black. Note that there was no statistical difference between mean concentration of pesticide exposure between aerial-exposed zip codes (49 kg/km2) and control zip codes (9 kg/km2).
A review of SUNY Upstate electronic medical records revealed 159 children from aerial-exposed zip codes with a visit diagnosis of ASD or DD between March 15, 2010, and March 15, 2015 (Table 4). Of those 159 children, 121 (76%) were on the autistic spectrum. There were 251 children seen at SUNY Upstate with a diagnosis of ASD or DD from the 16 control zip codes during this same period. Of those children, 197 (78%) were on the autistic spectrum. These figures yield mean observed ASD/DD diagnosis rates of 1 of 115 (0.0087) in the aerial-exposed zip codes compared with 1 of 196 (0.0051) in the control zip codes. A geographical representation of the observed ASD/DD diagnosis rate for aerial-exposed and control zip codes is displayed in Figure 2. The adjusted prevalence ratio for ASD/DD diagnosis in the children exposed to aerial pyrethroids was 1.37 (95% CI: 1.06–1.78; p = 0.02) compared with the children living in the control zip codes.
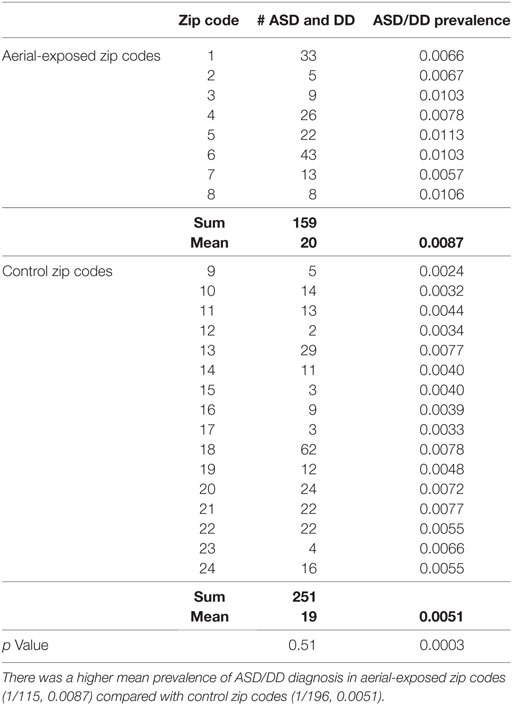
Table 4. Prevalence of autism spectrum disorder (ASD) or developmental delay (DD) diagnosis at the regional medical center for children from aerial-exposed and control zip codes.
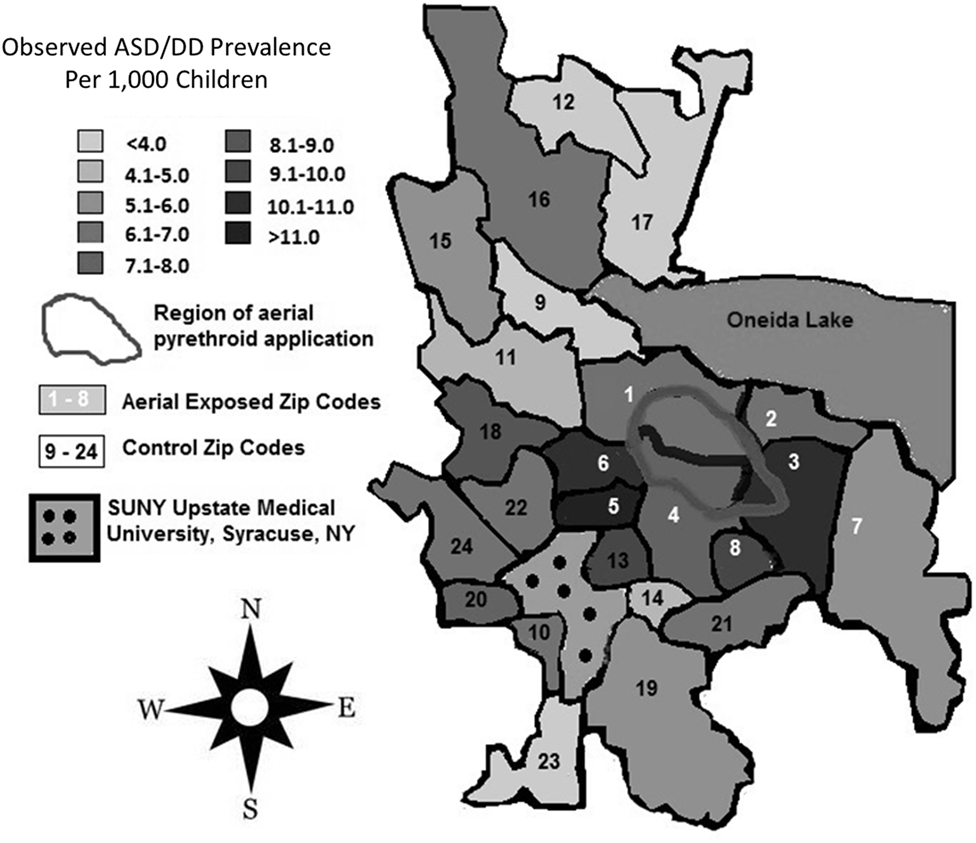
Figure 2. Regional rates of autism spectrum disorder (ASD) and developmental delay (DD) diagnoses. Total numbers of children diagnosed with ASD/DD in each zip code were identified with a retrospective review of records at the State University of New York (SUNY) Upstate Regional Medical Center, and prevalence rates were calculated using publicly available American Community Survey data for each zip code. Zip codes exposed to aerial pyrethroid pesticides had a higher prevalence of ASD/DD than control zip codes with standard pesticide application methods.
A cross-validation procedure was used to compare the rate of ASD/DD diagnoses in the aerial-exposed zip codes (0.0087) against ASD/DD diagnosis rates at our medical center from 30 additional zip codes in Central New York without aerial pesticide application. Random eight zip code combinations were generated 360 times from this group (Table S1 in Supplementary Material) and resulted in zero examples of prevalence exceeding that observed in the aerial-exposed zip codes (p < 0.001). Of the 360 combinations, 115 had ASD/DD diagnoses rates that exceeded that seen in the original control group (p = 0.32). Note that the mean total pesticide exposure in these 30 zip codes (1,689 kg) did not differ from that seen aerial-exposed regions (1,412 kg; p = 0.76).
Pearson correlation testing (Figure S1 in Supplementary Material) revealed positive correlations between aerial-exposure and ASD/DD prevalence (r = 0.58), as well as total pesticide exposure (in kilograms per kilometer) and ASD/DD prevalence (r = 0.41). There was an inverse correlation between ASD/DD prevalence and distance to the medical center (r = −0.45), as well as neonatal death rate per 1,000 births (r = −0.43). There was no correlation between birth month and ASD/DD prevalence (r = −0.034).
A Poisson regression model controlling for regional poverty (% children below poverty line), race (% Caucasian), sex (% male), birth characteristics (e.g., prematurity, low birthweight, and birth rate), population density (children per square kilometer), distance from the medical center (kilometer), and total pesticide exposure (kilograms per square kilometer) identified a significant relationship between aerial pesticide exposure and ASD/DD rates (Table 5). The analysis demonstrated a significant association between aerial pyrethroid exposure and ASD/DD prevalence (adjusted RR = 1.37; p = 0.02).
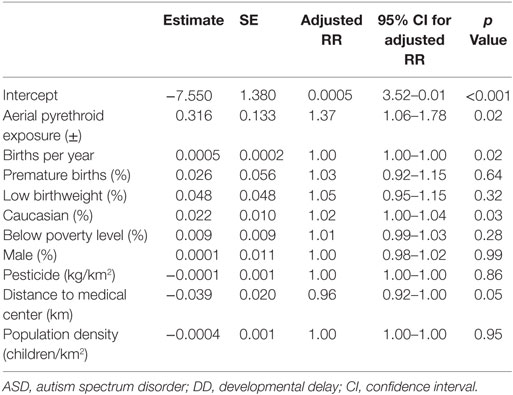
Table 5. Poisson regression analysis controlling for clinically relevant, confounding variables to estimate the direct effect of aerial pyrethroid exposure on ASD/DD prevalence.
Clinical characteristics for the ASD/DD subjects in both aerial-exposed and control zip codes are displayed in Table 6. There was no difference observed in child age, sex, age at ASD/DD diagnosis, or birth month between the two groups. No differences were noted in parental nicotine/alcohol use, and the groups had no difference in comorbid anxiety or ADHD. There were no difference between the two groups in percentage of children with ASD and no difference in those with known genetic disorders. In those children with developmental testing, no differences were detected in mean ADOS, DAY-C, or BSID scores. Composite adaptive behavior as measured by Vineland Scales was nominally higher in children from the aerial-exposed group, but this difference did not reach statistical significance (p = 0.06).
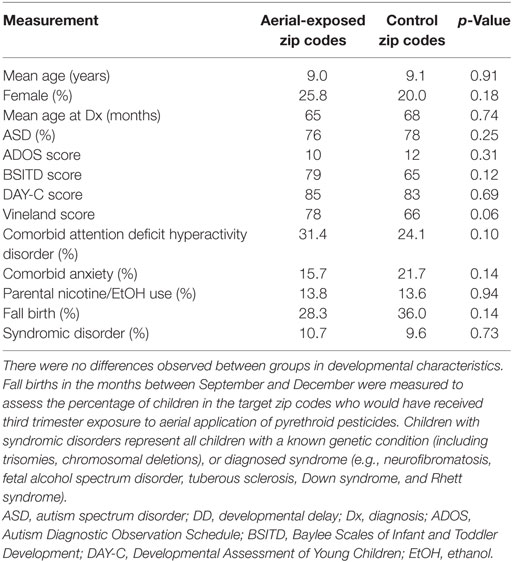
Table 6. Clinical characteristics of subjects with ASD and DD from aerial-exposed and control zip codes.
Discussion
This study examined the prevalence of ASD and DD in a central New York region with unique aerial exposure to the PP Masterline® Kontrol. When compared with surrounding areas, the zip codes exposed to yearly aerial pyrethroid spraying had a higher prevalence of ASD/DD. Total pesticide exposure (kilograms per square kilometer) was correlated with ASD/DD prevalence, but did not contribute significantly to a Poisson regression model of the data. There was also no correlation between birth month (i.e., timing of gestational pyrethroid exposure) and ASD/DD prevalence.
Although this study is observational and does not establish a causal relationship between pyrethroid exposure and ASD/DD, it adds to a growing body of literature that shows a relationship between pesticides and ASD/DD (4, 6, 7). To our knowledge, this is the first study to show a relationship between the route of pesticide exposure and rates of neurodevelopmental delay. It raises intriguing questions about the safety of pesticide use in our society and how the manner in which those pesticides are applied might affect brain development in children.
Why might the route of pesticide exposure be so important? Each summer, the Onondaga County Health Department in Upstate New York combats EEE and WNV through aerial application of Kontrol between July and September. Prior to this application, residents are advised to (1) stay indoors for up to 1 h following spraying and keep windows closed; (2) close outdoor vents of window-unit air conditioners; (3) remove outdoor children’s toys, outdoor furniture, and clotheslines; and (4) cover gardens or rinse homegrown produce with water prior to consumption (15). It is unknown what percent of residents receive and fully comply with these recommendations. There are obvious technical difficulties involved with removing outdoor playground equipment or covering large gardens. These areas represent potential exposure sites for pregnant mothers or young children in early stages of neurodevelopment. Pesticide levels in the urine or blood of children from these areas have not been specifically studied in areas with aerial pesticide application.
A recent study of cortical neurons in vitro found that pesticide exposure caused specific transcriptional changes that mimic expression changes in the autistic human brain (16). Although transcriptome patterns across the groups of this study are unknown, there was no difference between the percentage of children with known genetic syndromes in aerial-exposed and control zip codes. There was also no difference between measures of autistic (ADOS), adaptive (Vineland), or social (DAY-C) behaviors. Future studies employing molecular sequencing analyses could be beneficial in identifying unique transcriptional profiles in children with ASD/DD exposed to PPs.
The recent emergence of Zika virus and associated cases of microcephaly may be relevant when considering the findings presented in this study. Zip codes employing aerial pesticides did so to combat WNV, another member of the flavivirus family. Although WNV is not known to cause overt dysmorphic features (17), its influence on long-term developmental outcomes is not well studied (18). Like Zika virus, WNV causes symptoms in less than 25% of infected individuals and less than 1% present with meningitis (19). Therefore, it is possible that children in zip codes with aerial pesticide exposure also experience increased rates of in utero exposure to WNV. Neither head circumferences nor WNV antibody titers have been studied from children in this region.
There are a number of difficulties in assessing the association between environmental exposures and neurodevelopmental outcomes. One of the primary difficulties involves accurate identification of children with ASD/DD within a defined geographic area (20). In the current study, that task has been accomplished through a review of records from a major tertiary referral center. The benefits of this approach are that SUNY Upstate is the only regional children’s hospital and the only academic medical center in an 80 mile radius, which includes a catchment area of approximately 1.8 million people. Children are diagnosed with ASD and DD at Upstate by regional experts in pediatrics, psychiatry, and childhood development using Diagnostic and Statistical Manual of Mental Disorders criteria. The limitation to this approach is that a number of children with ASD and DD may be managed by outlying primary pediatricians without specialist referral and are likely missed. This may explain why the average prevalence of ASD/DD in this study (0.0087 or 1/115) is roughly half that reported nationally (0.022 or 1/45) at the time of the records review. Rates of childhood constipation and asthma reported in this study (~1/67) are also one-third of the rates reported nationally (1/20). Thus, our data medical center likely provides care for one-third of children in the study region, and this is represented by consistent subsampling across diagnoses in the study data.
To increase the identification of ASD and DD in the community, records from schools and outlying evaluation centers could be employed. However, this approach carries its own set of limitations, such as reliance on diagnostic reporting by non-medical professionals (i.e., teachers and parents). To demonstrate that our sampling of subjects is an accurate snapshot of the region, we have provided a measure of regional health center utilization. The number of children in the exposed zip codes is 43% of the population of children in the control zip codes. Yet the number of children from aerial-exposed zip codes seen at SUNY Upstate Medical University for well-child checks and common pediatric diagnoses (asthma, constipation, ADHD) is consistently 20–30% of that seen from control zip codes. This suggests that diagnosis rates for ASD/DD may even underestimate the number of children in aerial-exposed zip codes with neurodevelopmental delay. The Poisson regression model employed in this study shows that the distance of each zip code to SUNY Upstate Medical Center does influence observed prevalence rates of ASD/DD. Even when accounting for this influence, the impact of aerial pyrethroid exposure remains a significant variable in relation to ASD/DD prevalence.
It should also be noted that this study was not designed to detect the influence of exposure timing on ASD/DD risk. The study was limited by availability of data resources, which restricted chronological overlap of birth statistics, population demographics, pesticide application, and ASD/DD diagnosis rates. The most up-to-date information for each respective resource was used in this study. In addition, we do not have information on where mothers resided during pregnancy, and this is a limitation of the current study. We observed no difference in ASD/DD diagnosis rates for children with birthdays between September and December (i.e., presumed third trimester exposure to July to August aerial pesticide spraying) relative to children born in other months of the year. The question of pesticide exposure timing is important and has been previously investigated by Shelton et al. (6).
A final consideration in studies of this nature is the principle of ecological fallacy or the use of group data to draw conclusions about individual health characteristics. While these results show an association between aerial pesticide exposure and rates of ASD/DD diagnosis at a regional health center, they do not support the conclusion that individual risk of autism increases with pesticide exposure. Furthermore, the application of these findings on a broader scale (statewide or nationwide) cannot be assessed without a broader multicenter study.
Aerial application of PPs has been an important public health tool in combating WNV and EEE virus. The results of this study suggest that this practice may have influences on pediatric neurodevelopment. Further studies are necessary to clarify the relationship between pesticide exposure and neurodevelopmental outcomes. It will be important to directly assess the biochemical burden of pesticide metabolites in the urine of children with ASD/DD and compare the epigenetic profiles of these children to those without pesticide exposure. In addition, knowledge about maternal and fetal exposure to flaviviruses such as WNV (assessed through antibody detection) could be critical in evaluating factors associated with ASD/DD risk in these groups. This information would help communities make decisions about the safety and utility of aerial pesticide application.
Ethics Statement
Permission for a retrospective review of the electronic medical records at the State University of New York (SUNY) Upstate Medical University was obtained from that institution’s Institutional Review Board. Not applicable. The study examined neurodevelopmental delay diagnoses in a pediatric population, but consent was not required for review of records. All collected health information was deidentified.
Author Contributions
SH conceived the study, collected and analyzed the data, and drafted the manuscript. EW contributed to study design and data collection. KF was involved in study design. VD contributed to manuscript preparation. MW participated in statistical analysis and manuscript preparation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, DS, and the handling editor declared their shared affiliation, and the handling editor states that the process nevertheless met the standards of a fair and objective review.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors’ time was supported by SUNY Upstate Medical University and Penn State College of Medicine.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/article/10.3389/fped.2017.00116/full#supplementary-material.
Footnotes
References
1. Heymann DL, Hodgson A, Freedman DO, Staples JE, Althabe F, Baruah K, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet (2016) 387(10020):719–21. doi: 10.1016/S0140-6736(16)00320-2
2. Brannen KC, Devaud LL, Liu J, Lauder JM. Prenatal exposure to neurotoxicants dieldrin or lindane alters tert-butylbicyclophosphorothionatebinding to GABA a receptors in fetal rat brainstem. Dev Neurosci (1998) 20(1):34–41. doi:10.1159/000017296
3. Briz V, Molina-Molina JM, Sánchez-Redondo S, Fernández MF, Grimalt JO, Olea N, et al. Differential estrogenic effects of the persistent organochlorine pesticides dieldrin, endosulfan, and lindane in primary neuronal cultures. Toxicol Sci (2011) 120(2):413–27. doi:10.1093/toxsci/kfr019
4. Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect (2007) 115:1482–9. doi:10.1289/ehp.10168
5. Eskenazi B, Marks AR, Bradman A, Harley K, Bart DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican–American children. Environ Health Perspect (2007) 115:792–8. doi:10.1289/ehp.9828
6. Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect (2014) 122(10):1103. doi:10.1289/ehp.1307044
7. Viel JF, Warembourg C, Le Maner-Idrissi G, Lacroix A, Limon G, Rouget F, et al. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: the PELAGIE mother–child cohort. Environ Int (2015) 82:69–75. doi:10.1016/j.envint.2015.05.009
8. Krishnan S. Prenatal pesticide exposure and neurodevelopmental disorders. AAP Grand Rounds (2015) 33(2):16–16. doi:10.1542/gr.33-2-16
9. Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci (2010) 41(3):340–6. doi:10.1007/s12031-010-9377-2
10. Deutsch SI, Urbano MR, Neumann SA, Burket JA, Katz E. Cholinergic abnormalities in autism: is there a rationale for selective nicotinic agonist interventions? Clin Neuropharmacol (2010) 33(3):114–20. doi:10.1097/WNF.0b013e3181d6f7ad
11. Hossain MM, Suzuki T, Sato I, Takewaki T, Suzuki K, Kobayashi H. Neuromechanical effects of pyrethroids, allethrin, cyhalothrin and deltamethrin on the cholinergic processes in rat brain. Life Sci (2005) 77(7):795–807. doi:10.1016/j.lfs.2005.01.014
12. Shelton JF, Hertz-Picciotto I, Pessah IN. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect (2012) 120(7):944. doi:10.1289/ehp.1104553
13. Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, et al. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 US Environmental Protection Agency restriction of organophosphates. Environ Health Perspect (2008) 116(12):1681. doi:10.1289/ehp.11367
14. Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0 – making metabolomics more meaningful. Nucleic Acids Res (2015) 43(W1):W251–7. doi:10.1093/nar/gkv380
15. Mahoney JA, Gupta I. Frequently Asked Questions about Aerial Mosquito Spraying. (2016). Available from: http://www.ongov.net/health/documents/FAQSpraying.pdf
16. Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun (2016) 7:11173. doi:10.1038/ncomms11173
17. O’Leary DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ, et al. Birth outcomes following West Nile virus infection of pregnant women in the United States: 2003–2004. Pediatrics (2006) 117(3):e537–45. doi:10.1542/peds.2005-2024
18. Smith JC, Mailman T, MacDonald NE. West Nile virus: should pediatricians care? J Infect (2014) 69:S70–6. doi:10.1016/j.jinf.2014.07.019
19. Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med (2002) 137(3):173–9. doi:10.7326/0003-4819-137-3-200208060-00009
Keywords: autism spectrum disorder, pyrethroid, neurodevelopmental disorder, pesticide, environment
Citation: Hicks SD, Wang M, Fry K, Doraiswamy V and Wohlford EM (2017) Neurodevelopmental Delay Diagnosis Rates Are Increased in a Region with Aerial Pesticide Application. Front. Pediatr. 5:116. doi: 10.3389/fped.2017.00116
Received: 13 September 2016; Accepted: 02 May 2017;
Published: 24 May 2017
Edited by:
Eugene R. Schnitzler, Loyola University Chicago, United StatesReviewed by:
M. Elizabeth Hodgson, Social and Scientific Systems Inc., United StatesDavid A. Shoham, Loyola University Chicago, United States
Copyright: © 2017 Hicks, Wang, Fry, Doraiswamy and Wohlford. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven D. Hicks, shicks1@hmc.psu.edu
 Steven D. Hicks
Steven D. Hicks Ming Wang3
Ming Wang3
 Eric M. Wohlford
Eric M. Wohlford