Thirty Years of Sweat Chloride Testing at One Referral Center
- 1Department of Pediatrics, School of Medical Sciences, University of Campinas, Campinas, Brazil
- 2Laboratory of Pulmonary Physiology, Center for Pediatrics Investigation, School of Medical Sciences, University of Campinas, Campinas, Brazil
- 3Department of Medical Genetics, School of Medical Sciences, University of Campinas, Campinas, Brazil
Objective: To conduct a descriptive analysis of the sweat test (ST), associating ST results with epidemiological data, CFTR (cystic fibrosis transmembrane conductance regulator) mutations and reasons to indicate the ST, as well as correlating sweat sodium and sweat chloride concentrations in subjects.
Methods: Retrospective survey and descriptive analysis of 5,721 ST at a university referral center.
Results: The inclusion of the subjects was based on clinical data related with cystic fibrosis (CF) phenotype. The samples were grouped by (i) sweat chloride concentrations (mEq/L): <30: 3,249/5,277 (61.6%); ≥30 to <60: 1,326/5,277 (25.1%); ≥60: 702/5,277 (13.3%) and (ii) age: (Group A––GA) 0 to <6 months; (Group B––GB) ≥6 months to <18 years; (Group C––GC) ≥18 years. Digestive symptoms showed higher prevalence ratio for the CF diagnosis as well as association between younger age and higher values of sweat chloride, sweat sodium, and chloride/sodium ratio. The indication of ST due to respiratory symptoms was higher in GB and associated with greater age, lower values of sweat chloride, sweat sodium, and chloride/sodium ratio. There was higher prevalence of ST with sweat chloride levels <30 mEq/L in GB, ≥60 mEq/L in GC, and with borderline level in GB. There was positive correlation between sweat sodium and sweat chloride. Sweat chloride/sweat sodium and sweat sodium–sweat chloride indexes showed association with sex, reason for ST indication, and CFTR mutations. Sex alters some values presented in the ST. The number of ST/year performed before and after the newborn screening implementation was the same; however, we observed a higher number of borderlines values. A wide spectrum of CFTR mutation was found. Severe CFTR mutations and F508del/F508del genotype were associated with highest probability of ST chloride levels ≥60 mEq/L, and the absence of CFTR mutations identified was associated with borderline ST and respiratory symptoms.
Conclusions: ST data showed wide variability dependent on age, sex, reason for examination indication, CFTR mutations, and weight of the collected sweat sample. Sweat sodium concentration is directly correlated with sweat chloride levels and it could be used as a quality parameter.
Introduction
Cystic fibrosis (CF) (OMIM: #219700) is a chronic disease that leads to variability of genotypic and phenotypic expression. The CF diagnosis is based on neonatal screening findings and/or phenotypic manifestations, family history, and higher chloride ion (Cl−) concentration in sweat, in addition to two mutations in the CFTR gene (cystic fibrosis transmembrane conductance regulator) (OMIM: *602421) (1). If mutations in the CFTR gene and/or altered CFTR protein functions cannot be detected by any method, a definitive CF diagnosis cannot be made.
The function and/or presence of the CFTR protein has been demonstrated in the sweat glands by measurement of ion concentrations in sweat [sweat test (ST) and evaporimeter] (2–5), nasal epithelium (nasal potential difference) (6), salivary gland (ions in saliva) (7), and in the digestive tract (presence and function of the CFTR protein in rectal biopsy) (8, 9). Although CFTR gene mutations are the most important and appropriate markers for CF diagnosis, the analysis of ion chloride concentration in sweat is still considered the gold standard for the diagnosis, as well as the simplest method used to assess functional properties of the CFTR protein. In 1938, Dr. Dorothy Hansine Andersen pointed out that the high concentration of salt in sweat of patients with CF was almost accepted as pathognomonic for CF (10).
Over the past 65 years, there have been advances in the implementation and interpretation of the ST (3). Sweat chloride concentrations ≥60 mEq/L in at least two tests performed at different collection times are considered the gold standard for CF diagnosis. Individuals with borderline values ranging from 30 to 59 mEq/L require evaluation of the mutations in the CFTR gene (11).
The application of appropriate methods to perform ST is essential for accurate CF diagnosis. Therefore, referral centers should follow internal procedures which are in line with the guidelines provided by the cystic fibrosis foundation (CFF) (12). One of the ST collection methods approved by the CFF was developed by Gibson and Cooke (2). The quantitative pilocarpine iontophoresis ST determines the weight of sweat collected with analytical balance. Special care should be taken to avoid evaporation of the sample. This procedure is likely to fail unless it is carried out by experienced and trained personnel (13).
Currently, sweat chloride concentration dosage has also been useful to demonstrate the function of the CFTR protein after the administration of correctors, potentiators, or stabilizers drugs by personalized/precision medicine (14). Therefore, the future role of ST should include the successful monitoring of personalized medicine therapy.
The aim of this study was to conduct a descriptive analysis of the ST, associating ST results with epidemiological data, CFTR mutations screening, and reasons to indicate the test (respiratory, digestive, or nutritional symptoms), as well as correlating concentrations of sweat sodium and sweat chloride in subjects.
Materials and Methods
A retrospective study between 1986 and 2016, characterizing 30 years of monitoring, was conducted on 5,721 sweat samples at a CF reference center.
The sweat was collected following the traditional Gibson–Cooke method (2), and concentrations of sweat chloride and sweat sodium were determined by chloridometry and flame photometry, respectively.
This study was carried out in accordance with the recommendations of Ethics Committee of the University of Campinas (Protocol no. 474326), with written informed consent from the institution in accordance with the Declaration of Helsinki. The ST and subjects’ clinical data were obtained from medical records of the Pediatric Gastroenterology Laboratory, and from the Gastroenterology Center at the University Hospital, namely, name, subject’s age at the time of the examination, sex, indication for the ST (respiratory, digestive, or nutritional symptoms), CFTR mutation screening, immunoreactive trypsinogen (IRT) inclusion in our center, weight of the sweat sample collected, sweat chloride and sweat sodium concentrations, sweat chloride/sweat sodium ratio, and sweat sodium–sweat chloride in the ST samples. Samples were excluded according to the following criteria: sweat weight <75 mg and >400 mg, sweat chloride level ≤10 mEq/L or >160 mEq/L, sweat sodium level ≤10 mEq/L or >150 mEq/L, or absence of descriptive data (sweat weight and sweat sodium concentrations).
The age of patients was divided into three groups: (i) from birth to <6 months; (ii) ≥6 months to <18 years; (iii) ≥18 years (11, 15). The sweat chloride levels were used to group the samples according to the CF diagnosis: (i) chloride <30 mEq/L; (ii) chloride ≥30 to <60 mEq/L; (iii) chloride ≥60 mEq/L (group of patients with CF) (11).
All requested tests have been analyzed. For some subjects, the test involved more than one sweat sample.
Identification of Mutations in the CFTR Gene
CFTR mutations were analyzed by polymerase chain reaction techniques for F508del and enzymatic digestion for G542X, R1162X, R553X, G551D, and N1303K. Other mutations in CFTR were also identified by sequencing or using the SALSA MLPA technique (multiplex ligation-dependent probe amplification) Kit P091-C1 CFTR-MRC-Holland: S4X, 2183 A > G, 1717-G > A, I618T, with MegaBace1000® (GE Healthcare Biosciences, Pittsburgh, PA, USA) and ABI 3500 (Applied Biosystems-Thermo Fisher Scientific, São Paulo, Brazil) (16).
Considering the CFTR genotypes, patients were divided into three groups: (i) with two identified mutations belonging to class I, II, and/or III; (ii) with one identified mutation belonging to class I, II, and/or III; (iii) no identified mutation belonging to class I, II, and/or III. Other identified mutations in the CFTR gene, belonging to class IV, V, and/or VI, were not included in the statistical analysis. The CFTR mutation classification is according to the literature (17).
Statistical Analysis
A descriptive analysis was used with a number of observations, mean value, standard deviation, median, and minimum and maximum values for continuous variables. Confidence interval (95%) was calculated for proportions. For categorical variables, the data are presented by frequencies and percentages.
Statistical analyses were performed using the Statistical Package for the Social Sciences software version 23.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and OpenEpi version 3.03a. The comparison between the variables with categorical distribution was carried out by chi-square test and Fisher’s exact test, depending on the data distribution. Mann–Whitney and Kruskal–Wallis non-parametric tests were used for the analysis of variables with numerical distribution. Spearman’s rank correlation test and linear regression were used to compare the association between two variables with numerical distribution. Significance level was set at 0.05 for all analyses. The level of power in sample size calculations was >80%.
Results
This study assessed 5,721 sweat samples of STs that had been requested due to clinical presentations compatible with CF and/or neonatal screening with abnormal IRT values. One excluded 444 (7.78%) of these sweat samples, as follows: (i) 23 showed sweat weight <75 mg; (ii) four lacked sweat weight described in the examination; (iii) one lacked laboratory data; (iv) one showed no sweat sodium value described in the examination; (v) 395 showed sweat chloride level <10 mEq/L; (vi) nine showed sweat chloride level >160 mEq/L; (vii) four showed sweat sodium level <10 mEq/L; (viii) one showed sweat sodium level >150 mEq/L; (ix) six showed sweat weight >400 mg. Thus, the final study included 5,277 sweat samples. Sex of 15 (0.28%) subjects was not obtained because the examination was carried out on the newborn’s mother’s name after neonatal screening. Thus, 2,786/5,262 (52.9%) samples of male subjects were included and analyzed.
The IRT inclusion in our center was dated since 2010. From our subjects, 4,020/5,265 (76.4%) were included before 2010 and 1,245/5,265 (23.6%) after this date. In this context, there were performed 174.78 and 177.86 ST/year on average before and after IRT inclusion, respectively.
The STs were carried out at an outpatient clinic which is primarily intended for individuals under 25 years of age. Therefore, only a few examinations were performed in adult individuals. This explains the low inclusion rate of adults compared with children and teenagers.
The mean age of subjects was 12.84 ± 18.28 years; median of five (ranging from 0 to 85.58) years. However, the age in 80/5,277 (1.5%) samples was not entered at the time of the examination. Thus, three age groups were built with the following frequencies: (i) birth to six months: 567/5,197 (10.9%) samples; (ii) ≥6 months to <18 years: 3,558/5,197 (68.5%) samples; (iii) ≥18 years: 1,072/5,197 (20.6%) samples. Moreover, in 61/5277 samples, we had the age groups without the exact age by years and/or months.
The mean level of sweat chloride was 34.01 ± 27.16 mEq/L, median of 23.74 (ranging from 10 to 159.2) mEq/L. The mean level of sweat sodium was 37.76 ± 21.31 mEq/L, median of 30.9 (ranging from 10 to 149.7) mEq/L. Subjects were referred to the ST due to the following symptoms (each symptom was analyzed separately): (i) respiratory symptoms: 2,623/3,400 (77.1%); (ii) digestive symptoms: 419/3,400 (12.3%); (iii) nutritional symptoms: 391/3,400 (11.5%). In addition, we analyzed the presence of simultaneous digestive and respiratory symptoms, and we observed (i) no symptom––577/3,400 (17%); (ii) one symptom––2,604/3,400 (76.6%); (iii) two symptoms––219/3,400 (6.4%).
Initial ST request information could not be obtained for 1,877/5,277 (35.57%) sweat samples. The chloride–sodium ratio showed mean level of 0.84 ± 0.23 mEq/L; median of 0.82 mEq/L (ranging from 0.29 to 2.31 mEq/L). The sodium–chloride difference showed mean level of 3.75 ± 10.38 mEq/L; median of 5.3 mEq/L (ranging from −82.7 to 42.4 mEq/L). The weight of the sweat samples showed mean level of 170 ± 40 mg and median of 170 mg (ranging from 80 to 370 mg).
The sweat samples were divided into three groups of sweat chloride levels: (i) <30 mEq/L: 3,249/5,277 (61.6%); (ii) ≥30 to <60 mEq/L: 1,326/5,277 (25.1%); (iii) ≥60 mEq/L: 702/5,277 (13.3%).
Table 1 shows the association between sweat chloride value, sex of the subject, and reason for the indication of the examination. The indication due to nutritional symptoms showed no positive association (p = 0.678). The indication due to respiratory reasons showed no correlation with the CF diagnosis, unlike the indication due to digestive reasons. In the ST, for sweat chloride, males had lower prevalence ratio for borderline values and values ≥60 mEq/L, as well as higher prevalence ratio for values <30 mEq/L.
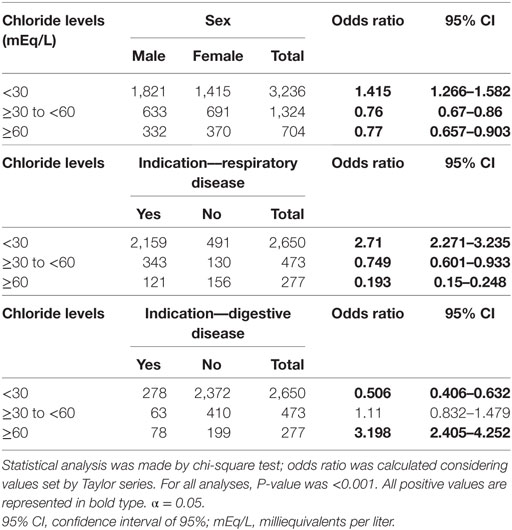
Table 1. Association between sweat chloride levels, sex of the subject, and reason for indication of sweat test.
Figure 1 shows the association of ST results with the weight of sweat, sweat sodium value and subject’s age.
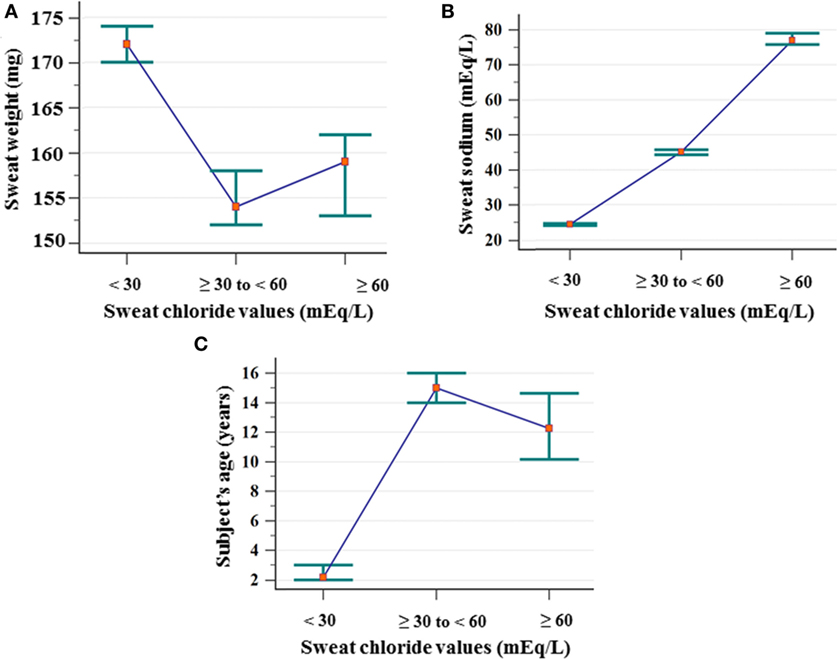
Figure 1. Association between the ST results considering chloride cutoff values and sweat weight (mg), sodium value (mEq/L), and subject’s age (years). (A) Sweat weight: (chloride <30 mEq/L) N = 3,249; mean of 175 ± 43; median of 172 (amplitude from 75 to 367); (chloride ≥30 to <60 mEq/L) N = 1,326; mean of 162 ± 41; median of 154 (amplitude from 78 to 299); (chloride ≥60 mEq/L) N = 702; mean of 164 ± 42; median of 159 (amplitude from 78 to 326). Chloride <30 mEq/L ≠ chloride ≥30 to <60 mEq/L, and chloride ≥60 mEq/L. (B) Sweat–sodium concentration: (chloride <30 mEq/L) N = 3,249; mean of 25.24 ± 7.03; median of 24.4 (amplitude from 10 to 68.5); (chloride ≥30 to <60 mEq/L) N = 1,326; mean of 45.82 ± 9.89; median of 45.11 (amplitude from 20.3 to 81.8); (chloride ≥60 mEq/L) N = 702; mean of 80.54 ± 17.98; median of 77 (amplitude from 42.9 to 149.7). All groups differ from each other. (C) Subject’s age: (<30 mEq/L) N = 3.233; mean of 6.66 ± 11.91; median of 2.17 (amplitude from 0 to 76.33); (chloride ≥30 to <60 mEq/L) N = 1,282; mean of 24.34 ± 21.74; median of 15 (amplitude from 0 to 85.58); (chloride ≥60 mEq/L) N = 621; mean of 21.27 ± 22.52; median of 12.25 (amplitude from 0 to 81.17). All groups differ from each other. Statistical analysis was performed by Kruskal–Wallis test. P-value for all analyses was <0.001. α = 0.05. The median is set in red marker and the 95% confidence interval is set in green line. mEq/L, milliequivalents per liter; ST, sweat test.
Table 2 presents the subjects’ age distribution at the time of the ST, as well as the comparison between ST results and reason for the examination indication. There was a higher prevalence of sweat samples with sweat chloride levels <30 mEq/L in subjects aged ≥6 months to <18 years. The CF diagnosis (chloride ≥60 mEq/L) showed higher prevalence in the group aged over 18 years. Higher prevalence ratio for the group older than 18 years of age with borderline sweat chloride values was also observed. The indication for the ST due to respiratory manifestations was more often in the group aged ≥6 months to <18 years. For digestive manifestation, indication was more often in the group under six months of age.
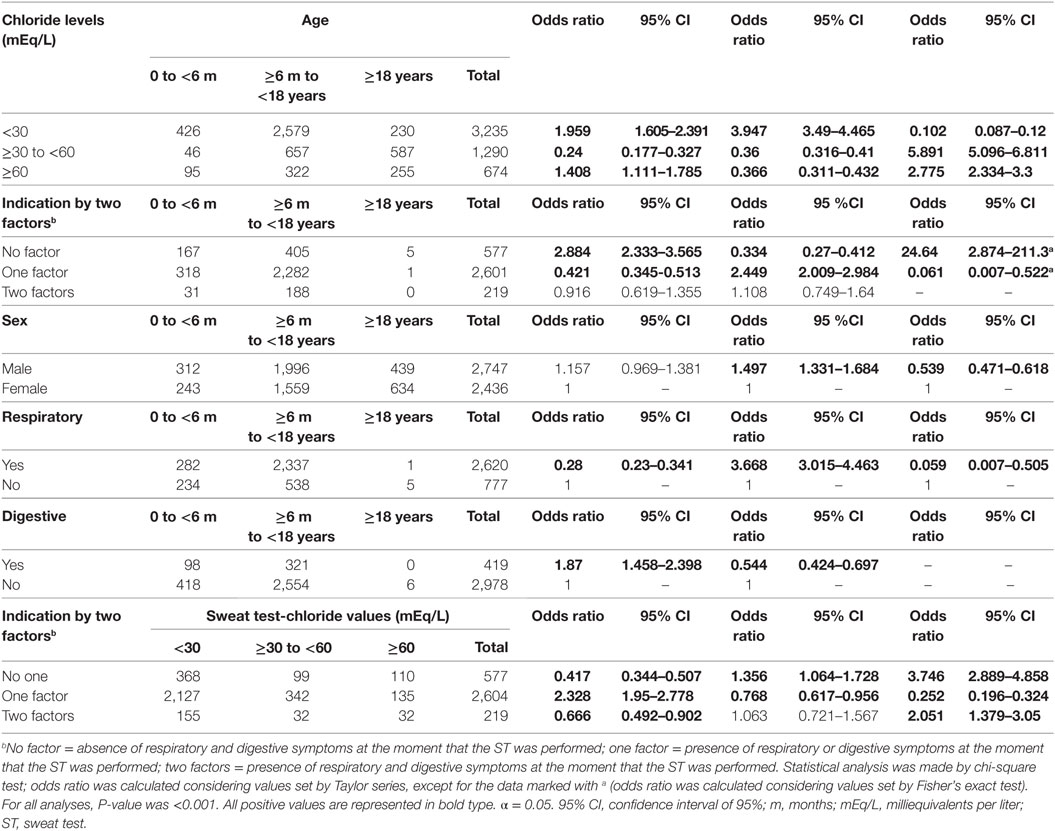
Table 2. Association between sweat chloride levels, sex of the subjects, and reason for indication of sweat test with age and sweat test result.
There was no association between the reason for ST indication and the sex of the subjects [respiratory symptoms (p = 0.362), digestive symptoms (p = 1), or nutritional symptoms (p = 0.384)]. However, male patients were less prevalent in the group aged over 18 years and showed higher volume of sweat and lower levels of sweat sodium, sweat chloride, and chloride/sodium ratio (Tables 2 and 3).
Subjects referred to the ST because of respiratory symptoms were older and presented higher sweat weight, lower levels of sweat chloride, sweat sodium and chloride/sodium ratio, as well as higher sodium–chloride level. For the indication due to digestive reasons, there was no association with sweat weight (p = 0.426) (Table 3). However, there was association with digestive symptom and higher levels of sweat chloride, sodium and chloride/sodium ratio, as well as subject’s younger age and lower sodium–chloride level (Table 3).
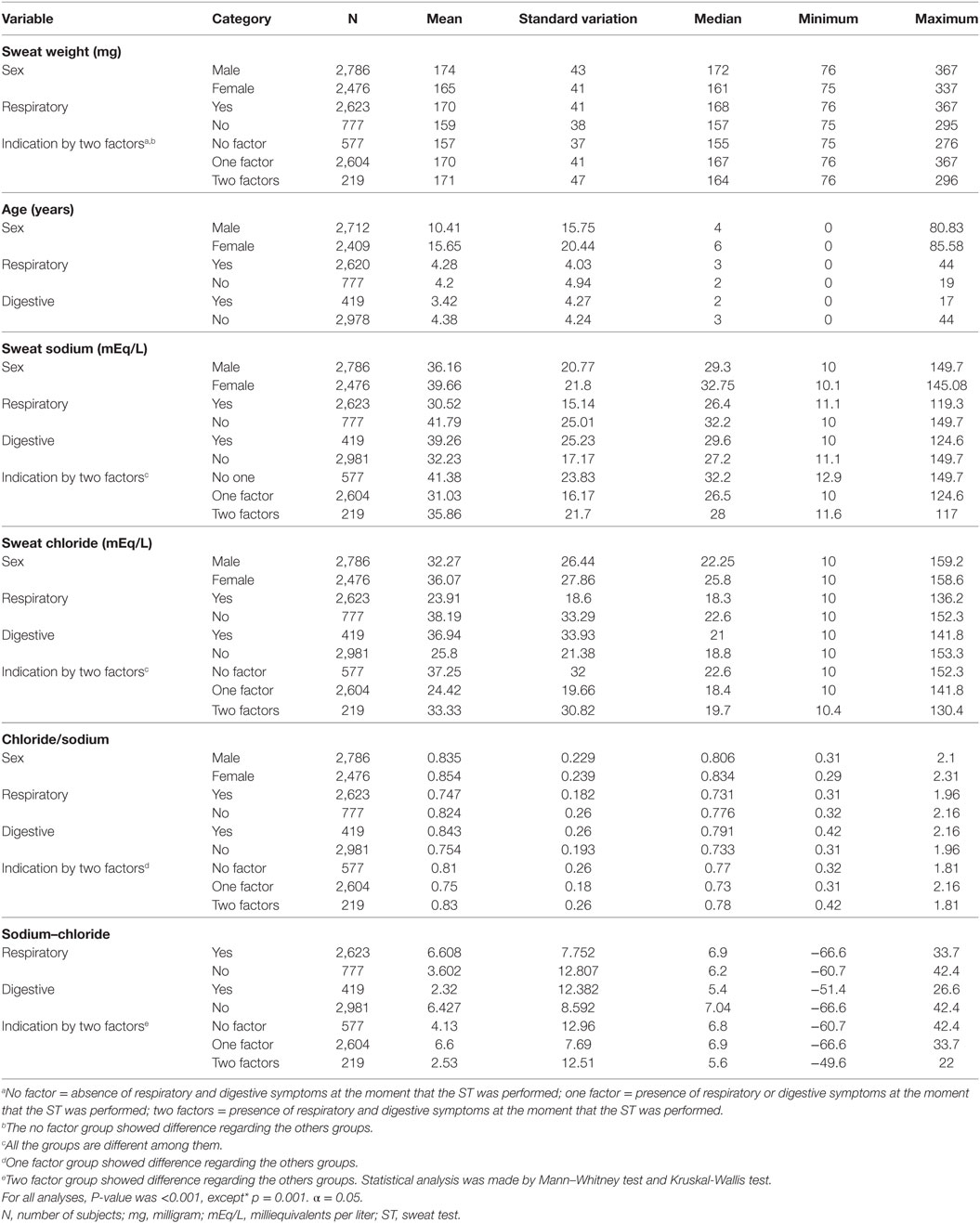
Table 3. Association between sweat weight, sex of the subjects, sweat chloride, sweat sodium, and reason for indication of sweat test.
Figure 2 brings the correlation between sweat weight and levels of sweat chloride and sweat sodium, subject’s age, difference between sodium and chloride concentrations and chloride/sodium ratio.
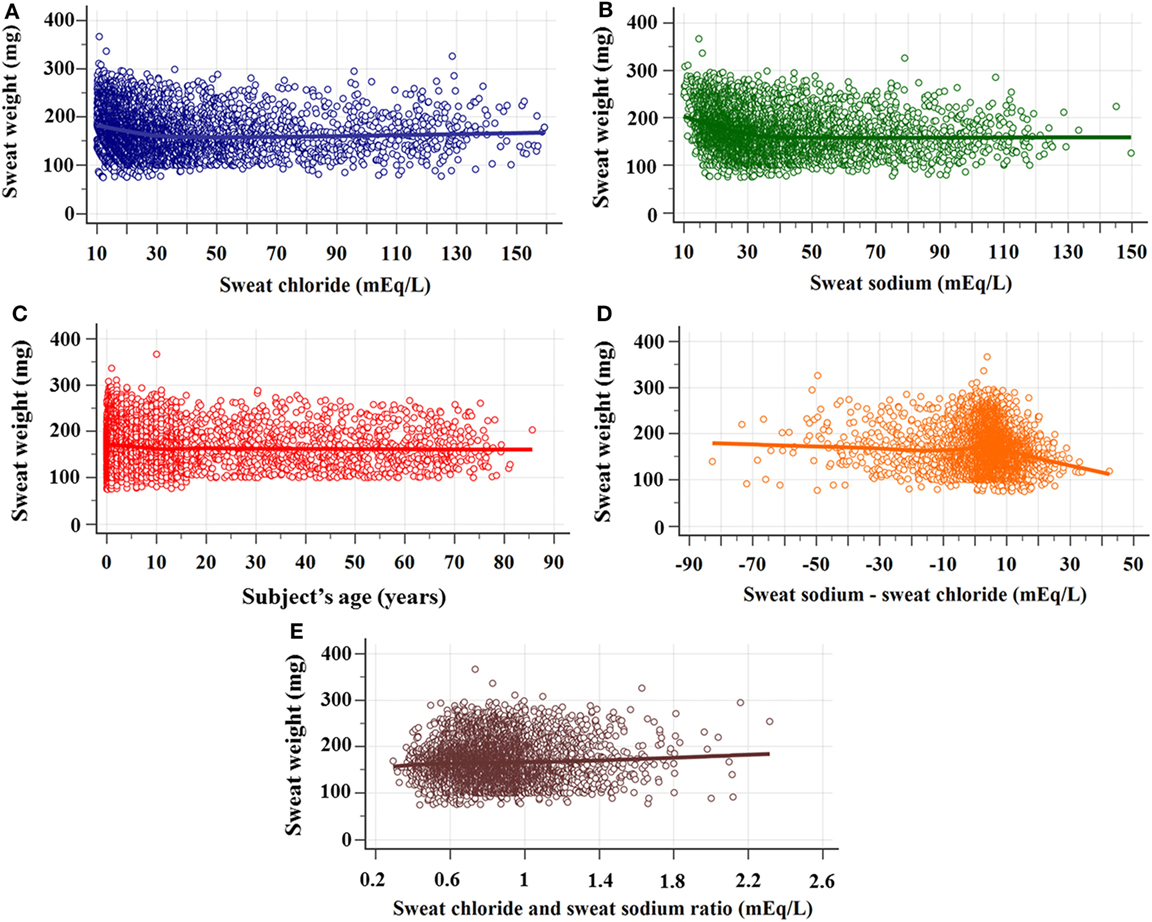
Figure 2. Correlation between sweat weight and sweat chloride and sodium concentrations, subject’s age, difference between sweat chloride and sodium concentrations, and sodium/chloride ratio. (A) Correlation between sweat weight and chloride concentrations. N = 5,277; rho = −0.201 (95% CI = −0.227 to −0.175), p < 0.0001. (B) Correlation between sweat weight and sweat sodium concentrations. N = 5,277; rho = −0.257 (95% CI = −0.282 to −0.232), p < 0.001. (C) Correlation between sweat weight and subject’s age. N = 5,136; rho = –0.075 (95% CI = –0.102 to –0.048), p < 0.0001. (D) Correlation between sweat weight and the difference between sweat sodium and chloride concentrations. N = 5,277; rho = –0.094 (95% CI = –0.121 to 0.067), p < 0.0001. (E) Correlation between sweat weight and chloride/sodium ratio. N = 5,277; rho = 0.007 (95% CI = –0.02 to 0.034), p = 0.621. The statistical analysis was performed by Spearman’s rank correlation test. α = 0.05. 95% CI, confidence interval of 95%.
Figure 3 shows the sex of the subjects distribution by the result of CF diagnosis regarding the sweat weight and the sweat chloride.
Figure 3. Distribution of sex of subject, considering sweat chloride (mEq/L) and sweat weight. (A) Sweat weight (mg). (chloride <30 mEq/L) Female: N = 1,415; mean of 171 ± 43; median of 167 (amplitude from 75 to 337); male: N = 1,821; mean of 178 ± 43; median of 175 (amplitude from 76 to 367) (p < 0.001); (chloride ≥30 to <60 mEq/L) female: N = 691; mean of 156 ± 38; median of 149 (amplitude from 87 to 279); male: N = 633; mean of 168 ± 44; median of 162 (amplitude from 78 a 299) (p < 0.001); (chloride ≥60 mEq/L) Female: N = 370; mean of 157 ± 39; median of 150 (amplitude from 78 to 295); male: N = 332; mean of 170 ± 44; median of 168 (amplitude from 79 to 326) (p < 0.001). (B) Sweat chloride concentrations obtained in the sweat test. (<30 mEq/L) Female: N = 1,821; mean of 18.15 ± 5.26; median of 17.4 (amplitude from 10 to 29.9); male: N = 1,415; mean of 18.54 ± 5.29; median of 17.7 (amplitude from 10 to 29.9) (p = 0.038); (chloride ≥30 to < 60 mEq/L) female: N = 691; mean of 42.08 ± 8.29; median of 41.1 (amplitude from 30 to 59.8); male: N = 633; mean of 40.89 ± 8.28; median of 39.3 (amplitude from 30 to 59.9) (p = 0.006); (chloride ≥60 mEq/L) female: N = 370; mean of 91.87 ± 24.53; median of 86.85 (amplitude from 60 to 158.6); male: N = 332; mean of 93.28 ± 25.06; median of 91.2 (amplitude from 60 to 159.2) (p = 0.618). The statistical analysis was performed by Mann–Whitney test. α = 0.05. The median is set in red marker and the 95% confidence interval is set in green line. The second graphic is a box plot. mEq/L, milliequivalents per liter.
Figure 4 shows the correlation between the levels of sweat sodium and sweat chloride by the result of CF diagnosis.
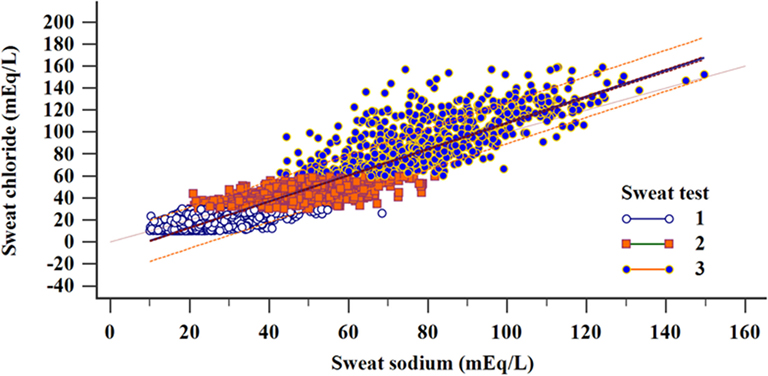
Figure 4. Regression and correlation of sweat sodium and chloride concentrations distributed according to the diagnostic results considering the sweat test values (total sample). Linear regression: N = 5,277; R2 = 0.877; y = −11.05 + 1.1932x (p < 0.001). Correlation: N = 5,277; correlation coefficient r = 0.9365; 95% CI = 0.9331–0.9397 (p < 0.001); (1) N = 3,249; R2 = 0.4347; y = 5.8091 + 0.4955x (p < 0.001); (2) N = 1,324; R2 = 0.4913; y = 14.5228 + 0.5888x (p < 0.001); (3) N = 702; R2 = 0.5336; y = 11.4797 + 1.0064x (p < 0.001). (i) chloride <30 mEq/L; (ii) chloride ≥30 to <60 mEq/L; and (iii) chloride ≥60 mEq/L. α = 0.05. mEq/L, milliequivalents per liter; 95% CI, confidence interval of 95%.
In addition, the association between the IRT inclusion in our sample and sex of the subjects, CF diagnosis, and age are presented in Table 4. The association with indication to the ST performance was not made regarding the bias by the IRT inclusion.
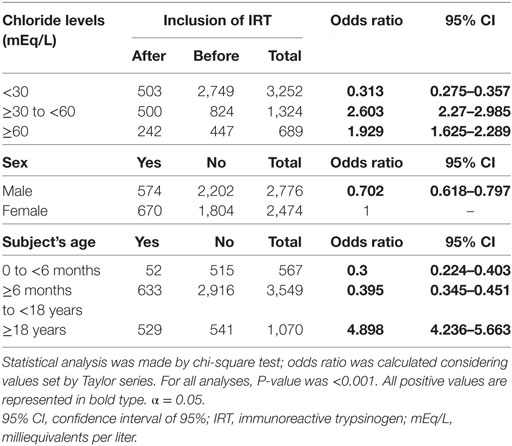
Table 4. Association between immunoreactive trypsinogen (IRT) inclusion in our center and subject’s sex and age and cystic fibrosis diagnosis.
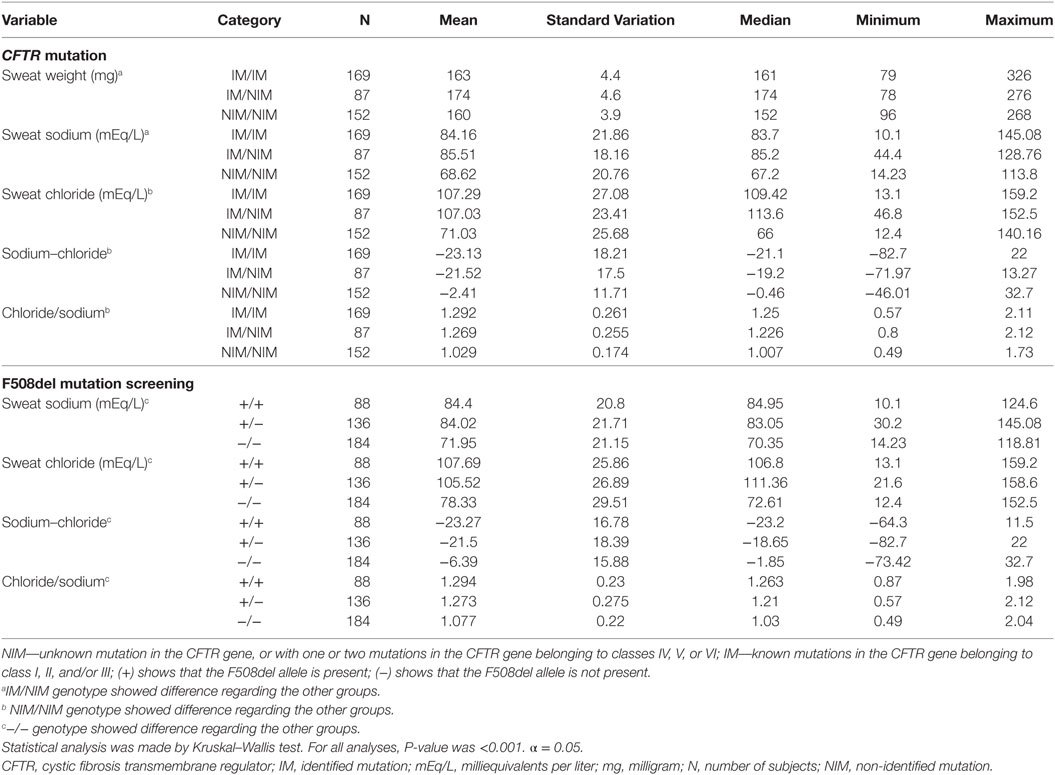
In our sample, 408 subjects were screened for at least F508del mutation in the CFTR gene. From these subjects, we found (i) 169/408 (41.4%) patients with unknown mutation in the CFTR gene, or with one or two mutations in the CFTR gene belonging to classes IV, V, or VI; (ii) 87/408 (21.3%) patients with one mutation in the CFTR gene belonging to class I, II, or III, and unknown mutation or one mutation in the CFTR gene belonging to class IV, V, or VI; (iii) 152/408 (37.3%) patients with two identified mutations in the CFTR gene belonging to class I, II, and/or III (see Table 5).
The CFTR genotype was associated with sex, reason for indication of ST, result achieved from ST, age of subjects, and ST markers regarding CFTR mutation screening genotype and F508del genotype (Tables 6 and 7).
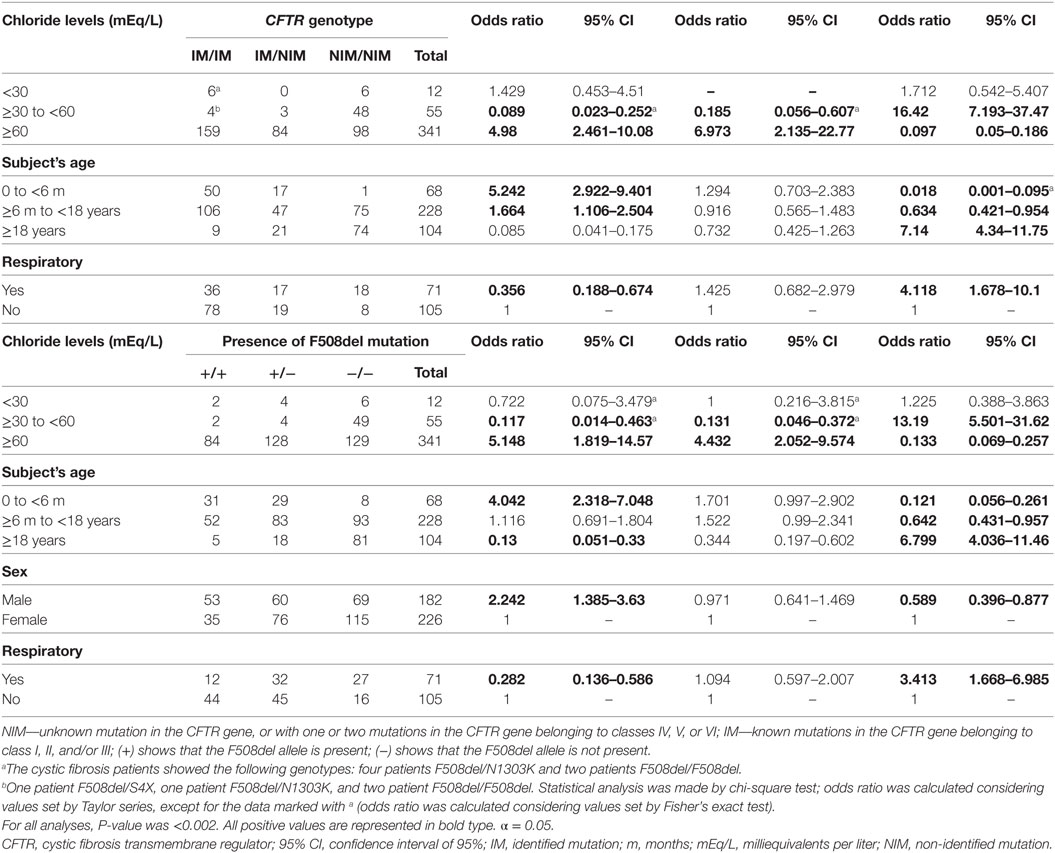
Table 6. Association between sex of the subject, reason for indication of sweat test, result achieved from sweat test, and subjects’ ages with CFTR mutation screening.
The normal variability enrolled with the ST could direct the diagnosis to CF, mainly in the limits associated with the ST cutoff points. In addition, the sex, CFTR mutation, sweat weight, digestive symptoms, pulmonary symptoms, and neonatal screening influenced the values achieved in the ST, and this influence is present in all possible ST classes: (i) <30 mEq/L; (ii) ≥30 to <60 mEq/L; and (iii) ≥60 mEq/L (Figure 5).
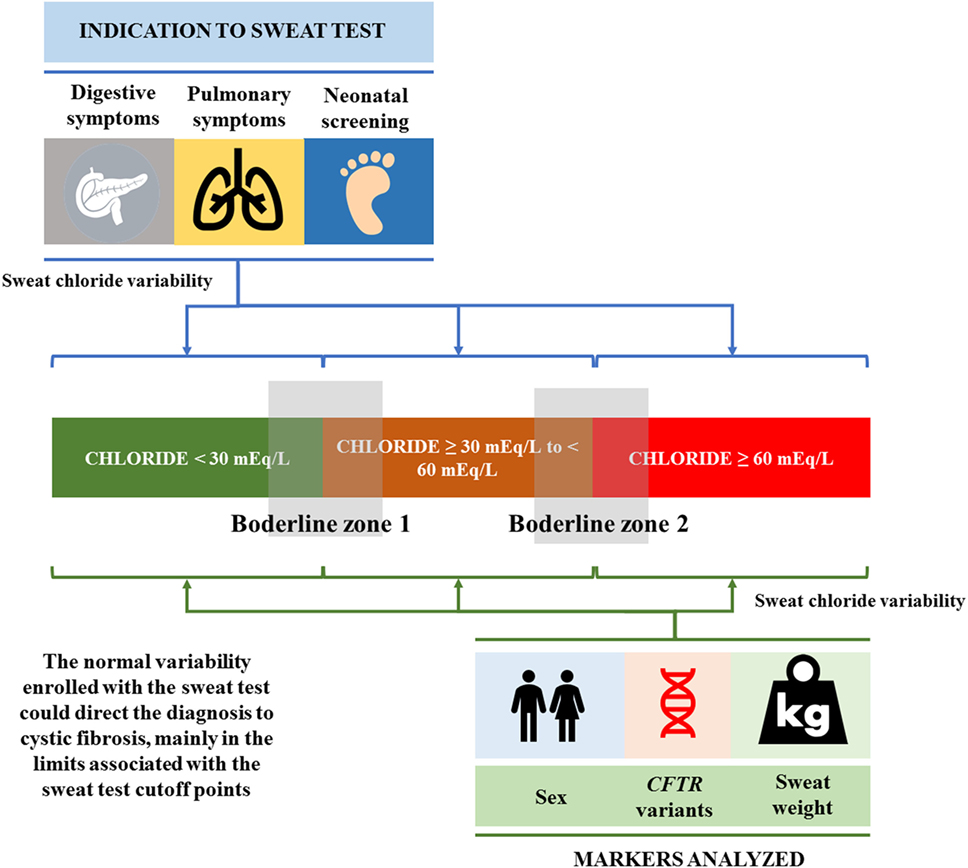
Figure 5. The normal variability enrolled with the sweat test, which could direct the diagnosis to cystic fibrosis, mainly in the limits (borderline zones 1 and 2) associated with the sweat test cutoff points. In addition, sex, CFTR mutation, sweat weight, digestive symptoms, pulmonary symptoms, and neonatal screening influenced the values achieved in the sweat test, and this influence is present in all possible sweat test classes: (i) <30 mEq/L; (ii) ≥30 to <60 mEq/L; and (iii) ≥60 mEq/L. CFTR, cystic fibrosis transmembrane regulator.
Discussion
High levels of sweat chloride with the presence of CF indicative signs and symptoms have been the gold standard for the CF diagnosis for more than 70 years (3). The amounts of ions in sweat have high specificity and sensitivity to demonstrate dysfunction or absence of chloride channels (CFTR protein) (18). However, despite the efficacy and importance of the ST for CF diagnosis, many methodological aspects of the stages related to its realization and interpretation continue and should be discussed as follows: (i) How do sweat chloride and sweat sodium concentrations vary with age? (15, 19); (ii) Is it necessary to measure sweat chloride alone or together with the analysis of sweat sodium? (15, 20); (iii) Which are the most appropriate types of waves and current intensity to stimulate sweating in the ST? (4); (iv) How are the ST stages performed at different reference centers? (21); How has the evolution of the ST been since its implementation for nearly 70 years? (3); Are the values of electrolytes obtained in the ST dependent on the variants of the CFTR gene? (16).
The importance of the ST is increasing day by day, and recently the sweat chloride level has been described as a predictive marker of CF severity lung disease (22).
In this context, in the present study we intended to demonstrate the characterization of the data presented in the ST regarding the influence of subject’s sex and age, CFTR mutation screening, and, mainly, ST clinical indication (nutritional, respiratory, and digestive symptoms). All aspects analyzed were discussed in part as follows.
ST Clinical Indication
Our laboratory, at a university referral center, receives requests to measure sweat sodium and sweat chloride ions in sweat from several specialties, mainly pediatric care. STs were more frequently requested for patients with chronic respiratory and digestive diseases.
Most patients with non-classic phenotype show symptoms later in life, often during adolescence or later, namely, less severe form of the disease, pancreatic sufficiency, mild chronic respiratory symptoms, and obstruction of the vas deferens with azoospermia. Levels of sweat chloride may be normal, abnormal, or borderline in patients with non-classical phenotypes. However, these levels are lower in patients with classic phenotypes (16, 23). In this context, the clinical manifestation could drive to the early or late CF diagnosis by the ST. In addition, early CF diagnosis provides numerous benefits, such as prevention of early malnutrition, reduced pulmonary complications, and long-term minor deterioration in lung function, allowing higher life quality and expectancy.
Our study found that the main reason for the ST requests was related to respiratory signs and symptoms (77.1%), followed by digestive symptoms (12.3%) and nutritional symptoms (11.5%). These data are in line with a recent study performed in France with 523 STs (24). Moreover, the ST request due to digestive manifestations prevailed in the group aged under six months, showing higher correlation with chloride values ≥60 mEq/L.
According to Ribeiro et al. (25), exocrine pancreatic insufficiency is often the earliest and most prevalent symptom in about 75% of patients with CF at birth, in 80–85% at the end of the first year and in 90% at adult age (25). As digestive symptoms are early identified, it allows differential diagnosis (26, 27). This fact was observed in our data, where the indication of ST due to digestive symptoms was also associated with higher values of sweat chloride, sweat sodium, and chloride/sodium ratio, as well as lower sodium–chloride level.
The low concordance between the ST indication by respiratory symptoms and the CF diagnosis might be related with the wide variability in the pulmonary symptoms, in addition to the higher number of diseases associated with pulmonary tract as compared with digestive tract. In this context, many solicitations of ST were performed to exclude the CF diagnosis hypothesis among many other pulmonary tract diseases.
Finally, the mutual association between respiratory and digestive symptoms enabled a better odds ratio for CF diagnosis and a lower odds ratio to achieve normal values in the ST. In literature, the clinical decision rule might be cited as a reliable index of clinical suspicion and timely referral for sweat testing in settings without newborn screening (NBS) programs, and may also be applied to false-negative individuals where such programs already exist (28).
Demographic Data Related with the Variability Presented in the ST
In this study, the analysis of a larger ST sample size indicated that the levels of sweat chloride and sweat sodium vary with age and sex.
Sex
In this study, males showed higher sweat weight. In addition, males showed higher sweat chloride levels only among those with sweat chloride values <30 mEq/mL cutoff in the ST as compared with females. This is likely to be explained by the fact that the sweat volume was higher in men due to the production of their sweat glands. Males have fewer active glands with higher sweat rate per gland and increased response to cholinergic and beta-adrenergic stimulation, as compared with females (5). In this context, these observations indicate prospects for the diagnosis of functional changes of the CFTR protein that include the difference between sexes (29).
Subject’s Age
Notably, the onset age of CF symptoms is widely variable: they may occur in the first years of life, childhood, adolescence, or even adulthood. This complex onset of symptoms can be explained by environmental factor, modifier genes, and different mutations in the CFTR (1). Therefore, the CF diagnosis should be made as soon as possible, with a high degree of certainty due to medical, financial, and psychosocial implications. Moreover, in our study group, we showed the correlation between subject’s age and sweat chloride levels regarding the ST results (19).
Subjects under six months and over 18 years of age showed greater probability of positive ST (≥60 mEq/L) and higher prevalence of sweat samples with chloride levels <30 mEq/L. This can be explained by the fact that STs had been indicated for children younger than six months due to clear clinical signs and probability of a CF diagnosis.
The highest prevalence ratio for borderline sweat chloride levels could be observed in the group over 18 years of age. The CFTR dysfunction causes a series of disorders in the organism, and the ST does not always distinguish CF from other pathological conditions also mediated by CFTR (30). However, we observed high probability to perform the ST with values ≥60 mEq/L in patients over 18 years of age, and this fact can be associated with the exclusion of other diseases, mainly for lung pathologies, during the first years of life. After the exclusion of common diseases, as asthma, the indication for the ST with a positive result could show higher probability.
The indication of the ST due to respiratory symptoms prevailed among subjects aged six months to 18 years. Lung diseases are more frequent after six months of age and their presence and deterioration have been assessed by lung function tests, bacteriological markers, and high-resolution computed tomography (31). The early diagnosis should be done once the lungs of patients with CF are often colonized and infected with microorganisms that cause damage to the epithelial surface at early childhood. Chronic infections are the main reason for reduced lung function and are associated with increased morbidity and mortality in CF (32).
Finally, clinical indication should consider the subject’s age in order to induce the ST performance. Even if the NBS was performed, the presence of false-negative tests may occur. In the presence of IRT false-negative test and symptoms related with CF, the ST performance should be recommended.
Correlation between Sweat Chloride and Sweat Sodium
The amounts of sweat sodium did not add discriminatory value to CF diagnosis. Current guidelines do not recommend using amounts of sweat sodium to CF diagnosis. However, some laboratories use this value for quality control purposes. The use of sweat sodium as quality control is given by the positive correlation between both ions (3), and this fact was showed in our study.
The association between sweat chloride and sweat sodium parameter to the ST quality control was analyzed by us previously, and we considered that the sweat sodium may be an indicative for quality discrimination, mainly in cases of doubts regarding the sweat chloride values (20). Even so, other studies should be done to clarify the biological variability, considering both ions dosage, observed in the ST among subjects that performed the test. Moreover, the studies should consider an ST performance overview, as a wide number of limitations and problems related in this examination at public and private centers is known (21). In this case, sweat sodium dosage should be stimulated in daily routine.
Finally, in all ages, levels of sweat sodium and sweat chloride were positively correlated, which had been documented in previous studies (3, 15).
CFTR Mutation Influencing the Data Presented in the ST
In 1989, mutations in the CFTR gene were described as a causative factor of CF. The identification of the CFTR mutations directed the diagnosis toward molecular biology, allowing greater understanding of CF, revision of the diagnostic criteria, and new therapeutic possibilities (1, 33–35). Moreover, the CFTR mutations classification showed changes along the years (17). In this context, with the advances in the molecular field, more than 2,000 mutations have been identified in the CFTR gene (33); however, its association with the results presented in the ST is not well known. Advances in this field are important to define the reference and levels of sweat chloride in the ST for each class of mutations in the CFTR gene (16, 36).
In addition, variants in the CFTR gene may cause several clinical phenotypes, such as chronic sinusitis, gastrointestinal disorders, and pulmonary diseases (1, 37). Thus, the ST should be an important tool for the CF diagnosis, particularly in the absence of the identification of mutations in the CFTR gene. It has also contributed to deeper understanding of the physiopathology aspects of CF and of the effects of some drugs to restore the function of the CFTR protein. The ST may show controversial results (although rare); therefore, further diagnostic methods are needed in some cases, as we showed in our data, when we identified 10 patients with CF and two CFTR mutations belonging to classes I, II, or III, and absence of positive ST.
Newborn Screening by Immunoreactive Trypsinogen
The implementation of NBS tends to increase the number of CF diagnosis in newborn infants. In CF, the release of trypsinogen in blood is increased as a result of the obstruction caused by secretion accumulation in the pancreatic ducts. High IRT levels are a marker of neonatal screening test for CF (38) and the diagnosis is confirmed by duplicate STs and/or the detection of two mutations in the CFTR gene (1).
In our data, the annual number of the ST performed was the same as before and after the implementation of IRT dosage. In addition, we showed a higher number of ST performed in patients aged over 18 years after the implementation of IRT dosage. This fact can be associated with a higher number of solicitations by the adult pneumology, correlated with the improvement of knowledge of (i) CF and residual CFTR function; (ii) presence of CFTR mutations included in the classes IV, V, and VI; and (iii) better registry from the adult clinic in the last years.
Limitations of the Study
Our study shows the following limitations: (i) it did not include a control group, i.e., there may be patients with negative ST with CF; (ii) it was not possible to confirm a CF diagnosis for all patients through genetic studies; (iii) the reason for the ST referral for 1,877/5,277 (35.57%) sweat samples could not be obtained; (vi) there was inclusion of a higher number of subjects under 18 years of age than of older subjects.
Highlight
(i) High levels of sweat chloride with the presence of CF indicative signs and symptoms are the gold standard for the CF diagnosis.
(ii) Amount of chloride is greater in men than women in all ST reference ranges.
(iii) Indication to perform the ST regarding respiratory symptoms was associated with minor probability of presenting CF disease and age between six months and 18 years.
(iv) Indication to perform the ST regarding digestive symptoms was associated with higher probability of presenting CF disease and age under six months.
(v) CF diagnosis regarding the age cutoffs showed association between them.
(vi) Subjects with digestive symptoms showed higher chloride levels than subjects without this type of symptom. In addition, subjects with respiratory symptoms showed lower chloride levels in their tests than subjects without this type of symptom.
(vii) Indexes [(sweat chloride/sweat sodium) and (sweat sodium–sweat chloride)] used in the article showed association with sex, reason for ST indication, and CFTR mutations.
(viii) After the NBS implementation by the IRT, there was a higher chance of borderlines values in the ST and CF diagnosis.
(ix) A wide spectrum of CFTR mutation was found with the highest prevalence of F508del identification.
(x) CFTR mutation identification or presence of F508del/F508del genotype was associated with higher probability of ST chloride levels ≥60 mEq. Moreover, the absence of CFTR mutations identified was associated with borderlines values in the ST, as well as with presence of respiratory symptoms.
Perspectives
Some issues are not well known in CF disease. For example, a large number of subjects with intermediate amounts of ions in sweat will develop classic forms of CF in the future (39). This fact was observed by Groves et al. (39), who carried out a 15-year monitoring on patients with sweat chloride concentrations between 30 and 59 mEq/L. From all patients with positive NBS by IRT, a positive allele for F508del, and intermediate ST, 14/29 (48%) developed classic CF. These data should be analyzed prospectively in many centers worldwide regarding the values of chloride longitudinally and the inclusion of other ST markers, as we analyzed in the present study (sweat chloride and sweat sodium ratio; sweat sodium–sweat sodium; correlation with sweat chloride and sweat sodium). In addition, the intervals of chloride concentration to perform the ST should be analyzed and revised. The variability in the ST might be associated with the populational structure.
The outlier data from the ST should be considered, taking into account that 2% of US Americans with CF have normal sweat chloride levels in the ST (3, 5). Patients with CF and normal ST should be recognized among the health subjects, or regarding other disease. This fact is more important in the genetic era, when the personalized/precision medicine is available for some CFTR mutations and probability will be available to all classes of CFTR mutations. The innate variability of ST is associated with variants of the CFTR gene, presence of modifier genes, and individual biological variation in the production of chlorides, possibly through other ion channels (3, 30), as long as laboratory errors have not occurred. Furthermore, sweat chloride as a biomarker of CFTR activity in the personalized/precision medicine was already used and should be better understood (40).
The function of CFTR should be better studied. The sweat glands in patients with CF, unlike the glands of healthy individuals, do not excrete sweat in response to beta-adrenergic stimulation, but to cholinergic stimulation. Healthy individuals with one allele with mutation in the CFTR gene (e.g., parents of patients with CF) excrete 50% of sweat, due to beta-adrenergic stimulation. Function evaluation tests of the CFTR protein may provide, indirectly, identification of carriers of CFTR gene mutations, which enables proper genetic counseling (5, 9). However, this fact is not well studied to be used as daily practice. Other tools could be studied, including the sweat induction by evaporimeter, chloride dosage from saliva, nasal potential difference, rectal biopsies, and organoids so that to evaluate the CFTR presence and function (2–9).
Finally, it is necessary but not sufficient to organize and publish CF diagnosis consensus processes. In addition, monitoring implementation efforts and practices seem essential as recommended in the literature (41–43).
Conclusion
The ST data showed wide variability, which was dependent on age, sex, reason for examination indication (respiratory and digestive symptoms), CFTR mutations, and weight of the sweat sample collected. Sweat sodium concentration is directly correlated with sweat chloride levels and it should be used as a quality parameter.
Ethics Statement
This study was carried out in accordance with the recommendations of Ethics Committee of the University of Campinas (Protocol no. 474326), with written informed consent from the institution in accordance with the Declaration of Helsinki.
Author Contributions
AGF, FALM, and JDR made substantial contributions to conception and design, acquisition, analysis, and interpretation of data; they were involved in drafting the manuscript and revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. CCSG, MFS, and AFR made substantial contributions to conception and design of the study, acquisition, analysis, and interpretation of data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Carmen Sílvia Bertuzzo, Stéphanie Villa-Nova Pereira, Luciana Montes Rezende, Luciana Cardoso Bonadia, Maria Ângela Gonçalves de Oliveira Ribeiro, Carlos Emílio Levy, Adressa Oliveira Peixoto, Adyléia Aparecida Contrera Dalbo Toro, Renan Mauch, Roberto José Negrão Nogueira, Eulália Sakano, Natasha Matsunaga, Alfonso Eduardo Alvarez, Elizete Aparecida Lomazi, Paloma Lopes Francisco Parazzi, Larissa Lazzarini Furlan, Emília Gonçalves, Aline Gonçalves, Milena Baptistella Grotta Silva, who contribute to the studies carried out on cystic fibrosis at our reference center. The authors also thank Espaço da Escrita/Unicamp General Coordination for the language services provided.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) (grant number 2011/12939-4 and grant number 2015/12858-5 to FALM); Fund for the Support of Education, Research and Extension of University of Campinas (FAEPEX) (grant number 0648/2015 to FALM); FAPESP (grant number 2011/18845-1 and 2015/12183-8 to JDR); and FAEPEX (grant number 17616/2015 to JDR).
Abbreviations
CF, cystic fibrosis; CFF, cystic fibrosis foundation; CFTR, cystic fibrosis transmembrane conductance regulator; Cl−, chloride ion; IRT, immunoreactive trypsinogen; mEq/L, milliequivalents per liter; MLPA, multiplex ligation-dependent probe amplification; OMIM, online Mendelian inheritance in man; ST, sweat test.
References
1. Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guideline for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr (2008) 153(2):S4–14. doi:10.1016/j.jpeds.2008.05.005
2. Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics (1959) 23:23–5.
3. Collie JT, Massie RJ, Jones OH, LeGrys VA, Greaves RF. Sixty five years since the New York heat wave: advances in sweat testing for cystic fibrosis. Pediatr Pulmonol (2014) 49:106–17. doi:10.1002/ppul.22945
4. Gomez CC, Servidoni MF, Marson FAL, Canavezi PJ, Vinagre AM, Costa ET, et al. Pulsed direct and constant direct currents in the pilocarpine iontophoresis sweat chloride test. BMC Pulm Med (2014) 14:198. doi:10.1186/1471-2466-14-198
5. Quinton P, Molyneux L, Ip W, Dupuis A, Avolio J, Tullis E, et al. Beta adrenergic sweat secretion as a diagnostic test for cystic fibrosis. Am J Respir Crit Care Med (2012) 186(8):732–9. doi:10.1164/rccm.201205-0922OC
6. Ng RT, Marson FA, Ribeiro JD, Ribeiro AF, Bertuzzo CS, Ribeiro MA, et al. Nasal potential difference in cystic fibrosis considering severe CFTR mutations. Dis Markers (2015) 2015:306825. doi:10.1155/2015/306825
7. Gonçalves AC, Marson FA, Mendonça RM, Ribeiro JD, Ribeiro AF, Paschoal IA, et al. Saliva as a potential tool for cystic fibrosis diagnosis. Diagn Pathol (2013) 8:46. doi:10.1186/1746-1596-8-46
8. Sousa M, Servidoni MF, Vinagre AM, Ramalho AS, Bonadia LC, Felício V, et al. Measurements of CFTR-mediated Cl2 secretion in human rectal biopsies constitute a robust biomarker for cystic fibrosis diagnosis and prognosis. PLoS One (2012) 7(10):e47708. doi:10.1371/journal.pone.0047708
9. Servidoni MF, Sousa M, Vinagre AM, Cardoso SR, Ribeiro MA, Meirelles LR, et al. Rectal forceps biopsy procedure in cystic fibrosis: technical aspects and patients perspective for clinical trials feasibility. BMC Gastroenterol (2013) 13:91. doi:10.1186/1471-230X-13-91
10. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child (1938) 56:344–99. doi:10.1001/archpedi.1938.01980140114013
11. Farrell PM, White TB, Howenstine MS, Munck A, Parad RB, Rosenfeld M, et al. Diagnosis of cystic fibrosis in screened populations. J Pediatr (2017) 181S:S33–44. doi:10.1016/j.jpeds.2016.09.065
12. LeGrys V, Yankansas J, Quitelli L, Marshall B, Mogayzel P. Diagnostic sweat testing: the cystic fibrosis foundation guidelines. J Pediatr (2007) 151:85–9. doi:10.1016/j.jpeds.2007.03.002
13. Mattar A, Gomes E, Adde F, Leone C, Rodrigues J. Comparison between classic Gibson and Cooke technique and sweat conductivity test in patients without cystic fibrosis. J Pediatr (2010) 86:109–14. doi:10.2223/JPED.1979
14. Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in CF clinical trials: the Ivacaftor experience. Chest (2013) 143(1):14–8. doi:10.1378/chest.12-1430
15. Traeger N, Shi Q, Dozor AJ. Relationship between sweat chloride, sodium, and age in clinically obtained samples. J Cyst Fibros (2014) 13(1):10–4. doi:10.1016/j.jcf.2013.07.003
16. Bonadia LC, Marson FAL, Ribeiro JD, Paschoal IA, Pereira MC, Ribeiro AF, et al. CFTR genotype and clinical outcomes of adult patients carried as cystic fibrosis disease. Gene (2014) 540(2):183–90. doi:10.1016/j.gene.2014.02.040
17. Marson FAL, Bertuzzo CS, Ribeiro JD. Classification of CFTR mutation classes. Lancet Respir Med (2016) 4(8):e37–8. doi:10.1016/S2213-2600(16)30188-6
18. Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, et al. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J Pediatr (2017) 181S:S4–15. doi:10.1016/j.jpeds.2016.09.064
19. Faria AG, Marson FAL, Ribeiro AF, Ribeiro JD. The correlation between age and sweat chloride levels in sweat tests. Rev Port Pneumol (2017) 23(4):227–30. doi:10.1016/j.rppnen.2016.11.001
20. Faria AG, Marson FAL, Gomez CC, Ribeiro MÂ, Morais LB, Servidoni MF, et al. Quality of sweat test (ST) based on the proportion of sweat sodium (Na) and sweat chloride (Cl) as diagnostic parameter of cystic fibrosis: are we on the right way? Diagn Pathol (2016) 11(1):103. doi:10.1186/s13000-016-0555-6
21. Servidoni MF, Gomez CCS, Marson FAL, Toro AADC, Ribeiro MÂGO, Ribeiro JD, et al. Sweat test and cystic fibrosis: overview of test performance at public and private centers in the state of São Paulo, Brazil. J Bras Pneumol (2017) 43(2):121–8. doi:10.1590/S1806-37562016000000076
22. Caudri D, Zitter D, Bronsveld I, Tiddens H. Is sweat chloride predictive of severity of cystic fibrosis lung disease assessed by chest computed tomography? Pediatr Pulmonol (2017) 52(9):1135–41. doi:10.1002/ppul.23739
23. Bombieri C, Claustres M, De Boecck K, Derichs N, Dodge J, Girondon E, et al. Recommendations for the classification of disease as CFTR related disorders. J Cyst Fibros (2011) 2:86–102. doi:10.1016/S1569-1993(11)60014-3
24. Grimaldi C, Brémont F, Berlioz-Baudoin M, Brouard J, Corvol H, Couderc L, et al. Sweat test practice in pediatric pulmonology after introduction of cystic fibrosis newborn screening. Eur J Pediatr (2015) 174(12):1613–20. doi:10.1007/s00431-015-2579-4
25. Ribeiro JD, Ribeiro MA, Ribeiro AF. Controversies in cystic fibrosis: from pediatrician to specialist. J Pediatr (2002) 78:171–86. doi:10.2223/JPED.896
26. Feranchak A. Hepatobiliary complications in cystic fibrosis. Curr Gastroenterol Rep (2004) 6:231–9. doi:10.1007/s11894-004-0013-6
27. Milla CE. Nutrition and lung disease in cystic fibrosis. Clin Chest Med (2007) 28:319–30. doi:10.1016/j.ccm.2007.02.006
28. Camargos P, Gomes DL, Alvim CG, Gomes FS, Cajazeiro JM. From lip to lab: salty tasting skin is the main clue that raises clinical suspicion of cystic fibrosis in young infants. Acta Paediatr (2015) 104(5):e210–5. doi:10.1111/apa.12958
29. Behm JK, Hagiwara G, Lewiston NJ, Quinton PM, Wine JJ. Hyposecretion of beta-adrenergically induced sweating in cystic fibrosis heterozygotes. Pediatr Res (1987) 22(3):271–6. doi:10.1203/00006450-198709000-00007
30. Koerbin G, Greaves R, Robins H, Farquhar J, Hickman P. Total intra individual variation in sweat sodium and chloride concentration for diagnosis of cystic fibrosis. Clin Chem Acta (2008) 393:128–9. doi:10.1016/j.cca.2008.02.022
31. Castellani C, Cuppens H, Macek M, Cassiman J, Kerem E, Durie P, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros (2008) 7:179–176. doi:10.1016/j.jcf.2008.03.009
32. Lyczak J, Cannon C, Pier G. Lung infections associated with cystic fibrosis. Clin Microbol Rev (2002) 15:194–222. doi:10.1128/CMR.15.2.194-222.2002
33. Marson FAL, Bertuzzo CS, Ribeiro JD. Personalized drug therapy in cystic fibrosis: from fiction to reality. Curr Drug Targets (2015) 16(9):1007–17. doi:10.2174/1389450115666141128121118
34. Smyth A, Bell S, Bojcin S, Bryon M, Duff A, Flume P, et al. European respiratory standards of care: best practice guideline. J Cyst Fibros (2014) 13:S23–42. doi:10.1016/j.jcf.2014.03.010
35. Marson FAL, Bertuzzo CS, Ribeiro JD. Personalized or precision medicine? The example of cystic fibrosis. Front Pharmacol (2017) 8:390. doi:10.3389/fphar.2017.00390
36. Cutting G. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci (2010) 1214:57–69. doi:10.1111/j.1749-6632.2010.05879.x
37. Tiddens H, Puderbach M, Venegas J, Ratjen F, Donaldson S, Davis S, et al. Novel outcome measures for clinical trials in cystic fibrosis. Pediatr Pulmonol (2015) 50:302–15. doi:10.1002/ppul.23146
38. Comeau A, Accurso F, White T, Campbell P, Hoffman G, Parad R, et al. Cystic fibrosis foundation. Guidelines for implementation of cystic fibrosis foundation. Guideline for implementation of cystic fibrosis newborn screening programs: cystic fibrosis foundation workshop report. Pediatrics (2007) 119:495–518. doi:10.1542/peds.2006-1993
39. Groves T, Robinson P, Wiley V, Fitzgerald DA. Long-term outcomes of children with intermediate sweat chloride values in infancy. J Pediatr (2015) 166(6):.e1–3. doi:10.1016/j.jpeds.2015.01.052
40. Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, et al. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros (2014) 13(2):139–47. doi:10.1016/j.jcf.2013.09.007
41. Farrell PM, White TB, Derichs N, Castellani C, Rosenstein BJ. Cystic fibrosis diagnostic challenges over 4 decades: historical perspectives and lessons learned. J Pediatr (2017) 181S:S16–26. doi:10.1016/j.jpeds.2016.09.067
42. Aralica M, Krleza JL. Evaluating performance in sweat testing in medical biochemistry laboratories in Croatia. Biochem Med (Zagreb) (2017) 27(1):122–30. doi:10.11613/BM.2017.016
Keywords: cystic fibrosis, diagnosis, sweat chloride, sweat sodium, sweat test
Citation: Faria AG, Marson FAL, Gomez CCS, Servidoni MF, Ribeiro AF and Ribeiro JD (2017) Thirty Years of Sweat Chloride Testing at One Referral Center. Front. Pediatr. 5:222. doi: 10.3389/fped.2017.00222
Received: 19 July 2017; Accepted: 03 October 2017;
Published: 26 October 2017
Edited by:
Ron Rubenstein, Children’s Hospital of Philadelphia, United StatesReviewed by:
James Francis Chmiel, Rainbow Babies & Children’s Hospital, United StatesJayesh Mahendra Bhatt, Nottingham Children’s Hospital, United Kingdom
Copyright: © 2017 Faria, Marson, Gomez, Servidoni, Ribeiro and Ribeiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando Augusto Lima Marson, fernandolimamarson@hotmail.com;
José Dirceu Ribeiro, jdirceuribeiro@gmail.com
†These authors have contributed equally to this work.
 Alethéa Guimarães Faria1,2†
Alethéa Guimarães Faria1,2†
 Fernando Augusto Lima Marson
Fernando Augusto Lima Marson José Dirceu Ribeiro
José Dirceu Ribeiro