Developmental Programming of Renal Function and Re-Programming Approaches
- Pediatric Nephrology, Department of Pediatrics, Medical Faculty, University of Cologne, Cologne, Germany
Chronic kidney disease affects more than 10% of the population. Programming studies have examined the interrelationship between environmental factors in early life and differences in morbidity and mortality between individuals. A number of important principles has been identified, namely permanent structural modifications of organs and cells, long-lasting adjustments of endocrine regulatory circuits, as well as altered gene transcription. Risk factors include intrauterine deficiencies by disturbed placental function or maternal malnutrition, prematurity, intrauterine and postnatal stress, intrauterine and postnatal overnutrition, as well as dietary dysbalances in postnatal life. This mini-review discusses critical developmental periods and long-term sequelae of renal programming in humans and presents studies examining the underlying mechanisms as well as interventional approaches to “re-program” renal susceptibility toward disease. Clinical manifestations of programmed kidney disease include arterial hypertension, proteinuria, aggravation of inflammatory glomerular disease, and loss of kidney function. Nephron number, regulation of the renin–angiotensin–aldosterone system, renal sodium transport, vasomotor and endothelial function, myogenic response, and tubuloglomerular feedback have been identified as being vulnerable to environmental factors. Oxidative stress levels, metabolic pathways, including insulin, leptin, steroids, and arachidonic acid, DNA methylation, and histone configuration may be significantly altered by adverse environmental conditions. Studies on re-programming interventions focused on dietary or anti-oxidative approaches so far. Further studies that broaden our understanding of renal programming mechanisms are needed to ultimately develop preventive strategies. Targeted re-programming interventions in animal models focusing on known mechanisms will contribute to new concepts which finally will have to be translated to human application. Early nutritional concepts with specific modifications in macro- or micronutrients are among the most promising approaches to improve future renal health.
Introduction
Prevention of chronic kidney disease is a major public health challenge (1). Although diabetes mellitus is the most common cause of chronic kidney disease worldwide (2), developmental programming processes that have been reviewed by us (3, 4) and others (5–7) before substantially contribute to differences in morbidity and mortality between individuals. The normal development of the kidney can be disturbed by multiple environmental factors, including intrauterine deficiencies by disturbed placental function or maternal malnutrition, prematurity, intrauterine and postnatal stress, intrauterine and postnatal overnutrition, as well as dietary dysbalances of macro- and micronutrients. Since developmental steps take place during unique developmental periods, timing, and duration of an adverse environment specifically impact on developmental programming. Adverse kidney programming increases the incidence of severe renal and cardiovascular sequels later in life. This includes arterial hypertension and associated end organ damage, the aggravation of inflammatory glomerular disease and the occurrence of end-stage renal disease. Specific “re-programming” interventions may mitigate or even prevent programmed disease. Consequently, our mini-review will address the following topics:
(1) Which developmental stages are especially vulnerable?
(2) What are the long-term sequelae of adverse renal programming?
(3) Which environmental factors may have impact on kidney development and what are potential mechanisms of developmental kidney programming?
(4) What are potential therapeutic “re-programming” interventions?
Critical Developmental Periods of Renal Programming in Humans
In humans the pronephros begins to form around day 22, urine production starts after 10 weeks (8), and maximum renal growth occurs between 26 and 34 weeks of gestation (9). Around week 36, nephrogenesis is completed and the number of nephrons is determined (8, 10). In preterm infants, adaptation to extra-uterine conditions impairs nephrogenesis, and the children end up with fewer nephrons and a higher percentage of morphologically abnormal glomeruli (5, 6, 11, 12). In small for gestational age (SGA) fetuses, intrauterine renal growth is reduced compared to appropriate for gestational age controls (9). In both term and preterm infants, glomerular and tubular functions undergo further maturational changes during the first months of life (8, 13). In these vulnerable periods, babies are often exposed to nephrotoxic medication, such as non-steroidal anti-inflammatory drugs (14), antibiotics, or diuretics, during neonatal intensive care unit treatment (15, 16).
Long-Term Sequelae of Renal Programming in Humans
Blood Pressure and Loss of Kidney Function
Hypertension is the most important risk factor for cardiovascular events and mortality worldwide (17). Elevated blood pressure contributes to progression of renal insufficiency (18) and is a strong independent risk factor for end-stage renal disease (19). Vice versa, decreased renal function is associated with increased blood pressure and cardiovascular morbidity (20). Early detection of blood pressure elevation plays a major role in the prevention of end organ damage (21). Many studies, including a systematic meta-analysis of studies tracking blood pressure during life course, demonstrated that childhood blood pressure predicts blood pressure (22–24) and vascular end organ damage in adulthood (25). Abnormal birth weight, either low or high, increases the risk for blood pressure elevation and loss of renal function in a U-shaped manner (26–31). In SGA individuals, some studies demonstrated elevated blood pressure in childhood (32) or adulthood (33, 34) especially when rapid postnatal catch-up growth and later adiposity were present (35). Further risk factors include high maternal BMI (36) or elevated protein/carbohydrate ratios in maternal diet during pregnancy (37, 38), rapid postnatal weight gain (39), or being born large for gestational age (36, 40, 41).
Proteinuria and Loss of Kidney Function
Several risk factors during early life predispose toward proteinuria and related decline of renal function. Accordingly, the prevalence of microalbuminuria among adults whose mothers had been exposed to the Dutch Hunger Winter 1944/45 was elevated (42). Chinese women born in the famine years 1959–1961 had a higher risk to develop more severe stages of proteinuria in their forties (43). Low birthweight itself is associated with elevated risks for albuminuria (OR, 1.81; 95% CI, 1.19–2.77), end-stage renal disease (OR, 1.58; 95% CI, 1.33–1.88), or low estimated glomerular filtration rate (GFR) (29, 44, 45). A birthweight-dependent decline in GFR may already be seen in childhood (29, 46, 47).
Glomerular Disease and Inflammation
Furthermore, a number of studies have evaluated the association between perinatal risk factors and later glomerular disease. Thus, SGA individuals have a higher risk to experience steroid resistance and a more severe course in nephrotic syndrome (48, 49). In IgA-nephropathy, they develop arterial hypertension and glomerulosclerosis more often (50).
Mechanisms of Renal Programming
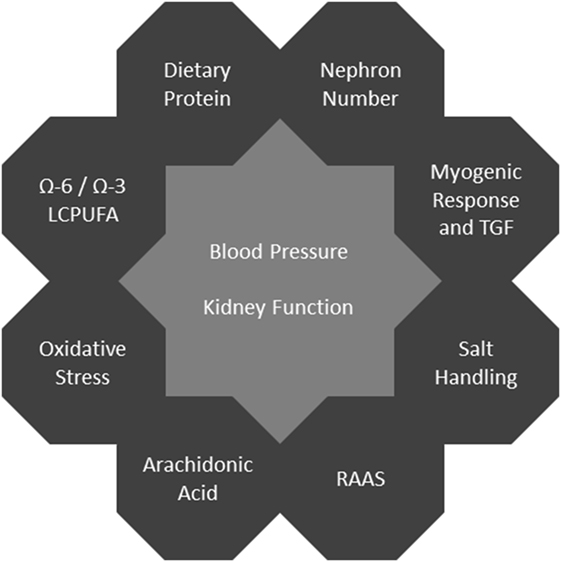
In human studies, it is difficult to establish mechanistic links in the field of developmental programming since there usually is a large delay between an adverse event and the related clinical phenotype. This makes it very challenging to distinguish the underlying causes from multiple modifying factors. Thus, animal models providing the possibility of equalized postnatal conditions and specific interventions are especially valuable. In rodents, kidney development during the early postnatal period corresponds to the third trimester in humans (10). For an overview of mechanisms see Figure 1.
Nephron Number
Nephron number in humans ranges from ~200,000 to >2.5 million nephrons per kidney (51). The well-known hypothesis of Brenner et al. linked decreased glomerular number with increased glomerular size, hyperfiltration, hypertension, and progressive glomerular injury (7, 52). Nephron number positively correlates with birth weight (53, 54) and is reduced after low-protein (LP) diet throughout pregnancy (55–59), utero-placental insufficiency (60–62), intrauterine glucocorticoid exposure (63), preterm birth (11, 64), and oxidative stress (65). In addition, a diet deficient in vitamin A (58, 66), zinc (67), or iron (68) is associated with low nephron count. Finally, nephrons get lost with age (69). Interestingly, low nephron number in young individuals is not necessarily associated with hypertension (70, 71). Thus, modulating factors such as early hyperalimentation and aging processes certainly have an impact on renal outcome in individuals with low nephron count (71, 72).
Renin–Angiotensin–Aldosterone System (RAAS)
Dysregulation of all or single components of the RAAS system may severely impair renal development (73, 74). Both activating and deactivating effects on the RAAS can induce a vicious circle of persisting hormonal dysbalances which may finally contribute to the development of arterial hypertension and renal failure.
In the fetal and perinatal period, downregulation of the RAAS has been identified as a relevant mechanism. In neonatal rats after LP diet during gestation, renal renin and angiotensin II levels (75) as well as angiotensin II receptors type 1 (AT1R) and 2 (AT2R) protein expressions (76) were reduced. Similarly, renal AT2R gene and protein expressions were reduced in fetal rats after prenatal caffeine exposure (77). Fetal angiotensin II levels in plasma were decreased after maternal high-salt diet in sheep (78).
Later in life, most environmental influences during early childhood end up with a RAAS activation. Adult rat offspring from the LP diet model showed elevated blood pressure (59), increased AT1R expression (79, 80) and elevated plasma angiotensin-converting enzyme (ACE) activity going along with slightly elevated angiotensin II levels (81). When challenged with angiotensin II infusion, adult LP offspring reacted with a greater decline in GFR than controls (80). In another LP study, there were more angiotensin II-positive cells in the cortical tubulointerstitium of adult offspring (82). Offspring from diabetic mothers had marked upregulation of angiotensinogen (AGT) and AT1R gene expression as well as increased ACE:ACE2 mRNA ratio (83). Some environmental influences induce RAAS activation already in fetal life. Thus, ovine offspring exposed to high salt during gestation presented with increased gene expression of AGT, ACE, AT1R, and increased ACE:ACE2 and AT1R:AT2R mRNA ratio (78). In the human situation, plasma renin concentrations were elevated in umbilical veins of SGA infants, and birth weight was inversely associated with circulating aldosterone concentrations (84). Treatment of human proximal tubule epithelial cells with palmitic acid demonstrated susceptibility to nutritional factors, as it induced intracellular endoplasmic reticulum (ER) stress and increased angiotensin II concentrations in cell medium. Co-treatment with AT1R-blocker or renin-inhibitor prevented ER stress (85).
Renal Sodium Transport
In rats, LP nutrition or dexamethasone treatment during gestation both resulted in an upregulation of the bumetanide-sensitive Na-K-2Cl cotransporter and of the thiazide-sensitive Na-Cl cotransporter in the offspring (86, 87). Adult offspring exposed to LP nutrition during gestation and lactation presented with a reduced diuretic response after a single dose of furosemide (88). After prenatal dexamethasone treatment, proximal tubule Na/H exchanger protein expression was increased, going along with an increase in proximal tubule sodium and volume reabsorption (86, 89). Sodium uptake in renal proximal tubule cells from adult male sheep was enhanced after prenatal betamethasone exposure (90). In rat offspring exposed to experimental utero-placental insufficiency (91) or maternal diabetes (92), sodium-dependent hypertension was observed.
Vasomotor and Endothelial Function
Another interesting aspect is the maturation of vascular smooth muscle function and small artery resistance regulation. Sympathectomy suppresses maturation of the gene program involved in small artery resistance regulation (93). Intrauterine and perinatal stress could, therefore, have a major impact on vascular tone regulation. In addition, vasomotor function can be impaired by perinatal hyperoxia (65) and LP diet (94). Endothelial dysfunction has also been described after intrauterine deficiency and may add to hypertension and glomerular damage (95).
Myogenic Response and Tubuloglomerular Feedback (TGF)
An impaired myogenic response as well as a disturbed TGF are important contributors to glomerular damage in diabetic and hypertensive nephropathy (96). An altered myogenic response has been described in intrauterine growth-restricted (IUGR) neonates, which may be beneficial postnatally, but harmful in the long run (97). The TGF mechanism matures during fetal life and could, therefore, be susceptible to programming in utero (98). However, no study has examined the specific consequences of disturbed intrauterine environment for TGF function.
Epigenetic Mechanisms
The molecular details of kidney development have been extensively studied (99, 100). Altered DNA methylation, histone modification, and other mechanisms modifying the renal transcriptome may significantly impair renal organogenesis and predispose toward renal disease which has lately been reviewed in detail (101). In this context, it is important to separate epigenetic changes during kidney disease (102) from epigenetic changes during early life leading to “programmed” disease. So far, there is little evidence that single, kidney-specific epigenetic alterations during early life might actually cause renal disease later on. Candidate genes would be all genes which are activated during specific developmental windows. Pax-2, for example, is essential for kidney development, ontogenetically regulated and can be reactivated in repair processes after acute kidney injury (103, 104). Global alterations of methylation associated with hypertension were observed after significant periconceptional deficiency of B vitamins and methionine (105). Thus, nutritional modifications may induce temporary or permanent epigenetic alterations that certainly have the potential to modulate kidney disease.
Oxidative Stress
Oxidative stress and inflammation are major contributors to vascular remodeling and hypertension (106). In LP (94, 107) and maternal smoking models (108), it was shown that oxidative stress during critical developmental steps may significantly contribute to renal susceptibility toward disease. In addition, both IUGR offspring after global undernutrition of the dam (109) and after high-fat died during gestation and lactation (110) showed increased oxidative stress and elevated blood pressure later in life. Reduction of oxidative stress during early life can prevent programmed hypertension and renal damage (94, 107, 108).
Metabolism
Rapid postnatal weight gain and early life obesity have been associated with adverse renal outcome (111, 112). Interplay between adiposity, leptin, and insulin resistance with RAAS regulation and sympathetic activity has been described (113, 114). Early postnatal overfeeding in rats by litter size reduction induced increased early postnatal weight gain and was associated with increased blood pressure, glomerulosclerosis, and proteinuria in adulthood (71). In a similar study, postnatal overfeeding resulted in decreased GFR, increased proteinuria and increased deposition of collagens. On the molecular level, intrinsic renal leptin resistance could be demonstrated (115). Dysregulation of renal leptin and Akt/AMPKα signaling associated with increased renal matrix deposition could also be shown in overweight offspring from mothers fed a high-fat diet during gestation and lactation (116). Maternal LP nutrition during rat gestation persistently decreased the expression of renal 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) (117, 118) and increased the expression of the renal glucocorticoid receptor in the offspring (118). The same was shown for sheep offspring exposed to temporary maternal calorie restriction (119).
Arachidonic Acid Metabolism Pathway
Finally, there is evidence that the arachidonic acid metabolism pathway could be involved in the development of programmed hypertension (120, 121). 20-hydroxyeicosatetraenoic acid (20-HETE), a metabolite of arachidonic acid, contributes to the normal myogenic pressure response. Physiologically, arachidonic acid is released from cell membranes by phospholipase A2, converted to 20-HETE, which then adds to vasoconstriction of the afferent arteriole (96, 122). However, 20-HETE has also been linked to systemic hypertension and endothelial dysfunction in rats (123). Further arachidonic acid metabolites like Cox-2 derived prostaglandins contribute to counter regulatory vasodilation of the afferent arteriole after TGF-mediated vasoconstriction (124) and oxidative stress in the kidney (125), and therefore modulate intraglomerular pressure and GFR as well as renal inflammation (82). Thus, nutritional intake of arachidonic acid may significantly affect blood pressure, kidney function, and kidney survival.
Potential Therapeutic “Re-Programming” Interventions
The ultimate goal of all research on programmed disease is to develop preventive strategies. So far, the number of studies on re-programming interventions is still limited and mainly restricted to dietary or anti-oxidative approaches.
Early Dietary Interventions
Data on nutritional interventions are available from both animal and human studies. A meta-analysis showed slightly, but significantly lower blood pressure in infants, children, and adolescents who were breast fed during infancy compared to those being formula fed (126). Micronutrient (127), calcium (128), vitamin A (129, 130), and iron (131) supplementation during pregnancy as well as long-chain polyunsaturated fatty acid (LCPUFA) supplementation in infant formula (132) may be beneficial to renal outcome.
In detail, children of women receiving a multiple micronutrient supplementation during the second and third trimesters of pregnancy were heavier and had lower systolic blood pressure during infancy (127). Calcium supplementation from the 20th gestational week until delivery lowered systolic blood pressure in children aged 7 years, with a stronger effect when children were overweight (128). Supplementation of iron and folate until the end of pregnancy in rural Bangladesh caused a slightly decreased diastolic blood pressure and a slightly increased GFR in infants at the age of 4.5 years when started at the ninth, but not when started at the 20th gestational week (131). Another dietary intervention with micronutrient supplementation in malnourished pregnant Nepalese women until 3 months postpartum showed that folic acid or the combination of folic acid, iron, and zinc reduced the risk of microalbuminuria, but not blood pressure in the children aged 6–8 years (133). The effects of retinoic acid have mainly been studied in animals. Decreased availability of retinoic acid induced by down-regulated vitamin A metabolism after previous overexposure to vitamin A strongly impairs metanephric kidney development, which can be restored by adequate retinoic acid supplementation (129). In rat offspring exposed to LP diet of the dam during pregnancy, a single injection of retinoic acid to the dam at midgestation increased postnatal nephron number at 4 weeks of age (130). Postnatal administration of retinoic acid in preterm baboons, however, did not alter kidney growth or nephron number, presumably because the timing of the intervention was chosen too late (134).
Long-chain polyunsaturated fatty acid supplementation with arachidonic acid and docosahexaenoic acid (ratio 2:1) in infant milk formula (IF) during the first 4 months of life lowered blood pressure at 6 years of age compared to IF without LCPUFAs. Blood pressure of children fed LCPUFA-IF was similar compared to breast fed children (132). A diet sufficient in ω-3 PUFAs reduced blood pressure levels compared to a diet almost free of ω-3 PUFAs in TGR(mRen-2)27 rats which have high angiotensin II levels (135). Finally, the importance of the amino acid composition was demonstrated. Addition of glycine to maternal LP diet throughout gestation normalized body weight and blood pressure at 4 weeks of age in rat offspring, whereas alanine or urea had no effect (136).
Anti-Oxidative Substances
Re-programming interventions with anti-oxidative substances have only been performed in animals. Supplementation of maternal LP diet with anti-oxidative (ACH09)-derived polyphenols extracted from grape skins reduced signs of renal oxidative stress in the offspring on the first postnatal day and attenuated the adverse effects of maternal LP diet on glomerular number and maturity (107). Administration of a lipid peroxidation inhibitor along with LP diet in gestation reduced prenatal oxidative stress and prevented programming of elevated blood pressure, enhanced vasoconstriction after angiotensin II administration and reduced vasodilation after sodium nitroprusside administration in adult animals (94). Similarly, treatment of previously malnourished dams with α-tocopherol during lactation prevented the development of hypertension in the offspring. In addition, upregulated angiotensin II levels and down-regulated Cox-2 expression in the tubulointersititum were brought back to control levels and oxidative stress as well as macrophage infiltration was prevented. However, treatment of control dams with α-tocopherol resulted in arterial hypertension of the offspring (82).
Conclusion and Future Directions
The concept of “developmental origins of health and disease” highlights the interrelationship between environmental factors throughout life and differences in morbidity and mortality between individuals. Chronic kidney disease affects more than 10% of the population (1). High blood pressure, childhood underweight, and suboptimal breastfeeding are among the top risk factors contributing to global burden of disease (137). Prematurity, IUGR, overweight in early life, and other conditions have been associated with the development of arterial hypertension, proteinuria, and decline of renal function. Around 11% of all live-born infants worldwide are born preterm (138). IUGR is seen in 3–7% of all pregnancies (139). During childhood, 5–6% of girls and 7–8% of boys become overweight (140). Thus, renal programming is not a rare phenomenon but affects large parts of the population. Further studies that broaden our understanding of renal programming mechanisms are needed to ultimately develop preventive strategies. Targeted re-programming interventions in animal models focusing on known mechanisms will contribute to new concepts which finally will have to be translated to human application. Early nutritional concepts with specific modifications in macro- or micronutrients are among the most promising approaches to improve future renal health.
Author Contributions
EN performed the majority of literature research, and designed and wrote the review. K-DN contributed to literature research, and designed and wrote the review. JD and LW contributed to literature research and writing. All authors revised and approved the review.
Conflict of Interest Statement
The authors declare that the article was written in absence of any commercial or financial relationships that could be a potential conflict of interest to the topic.
References
1. Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet (2013) 382(9887):158–69. doi:10.1016/S0140-6736(13)60439-0
2. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet (2013) 382(9888):260–72. doi:10.1016/S0140-6736(13)60687-X
3. Nüsken E, Nüsken K-D, Dötsch J. Diet and epigenetic alteration of renal function. In: Patel V, Preedy V, editors. Handbook of Nutrition, Diet, and Epigenetics. Cham: Springer International Publishing (2017). p. 1–20.
4. Dötsch J, Alejandre-Alcazar M, Janoschek R, Nüsken E, Weber LT, Nüsken KD. Perinatal programming of renal function. Curr Opin Pediatr (2016) 28(2):188–94. doi:10.1097/MOP.0000000000000312
5. Stritzke A, Thomas S, Amin H, Fusch C, Lodha A. Renal consequences of preterm birth. Mol Cell Pediatr (2017) 4(1):2. doi:10.1186/s40348-016-0068-0
6. Luyckx VA. Preterm birth and its impact on renal health. Semin Nephrol (2017) 37(4):311–9. doi:10.1016/j.semnephrol.2017.05.002
7. Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl (2005) 97:S68–77. doi:10.1111/j.1523-1755.2005.09712.x
8. Quigley R. Developmental changes in renal function. Curr Opin Pediatr (2012) 24(2):184–90. doi:10.1097/MOP.0b013e32834fe863
9. Konje JC, Bell SC, Morton JJ, de Chazal R, Taylor DJ. Human fetal kidney morphometry during gestation and the relationship between weight, kidney morphometry and plasma active renin concentration at birth. Clin Sci (Lond) (1996) 91(2):169–75. doi:10.1042/cs0910169
10. Seely JC. A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: juvenile animal relevancy. J Toxicol Pathol (2017) 30(2):125–33. doi:10.1293/tox.2017-0006
11. Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol (2004) 7(1):17–25. doi:10.1007/s10024-003-3029-2
12. Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol (2011) 22(7):1365–74. doi:10.1681/ASN.2010121266
13. Aperia A, Broberger O, Herin P, Thodenius K, Zetterström R. Postnatal control of water and electrolyte homeostasis in pre-term and full-term infants. Acta Paediatr Scand Suppl (1983) 305:61–5. doi:10.1111/j.1651-2227.1983.tb09861.x
14. Giniger RP, Buffat C, Millet V, Simeoni U. Renal effects of ibuprofen for the treatment of patent ductus arteriosus in premature infants. J Matern Fetal Neonatal Med (2007) 20(4):275–83. doi:10.1080/14767050701227950
15. Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med (2014) 27(14):1485–90. doi:10.3109/14767058.2013.860522
16. Girardi A, Raschi E, Galletti S, Poluzzi E, Faldella G, Allegaert K, et al. Drug-induced renal damage in preterm neonates: state of the art and methods for early detection. Drug Saf (2015) 38(6):535–51. doi:10.1007/s40264-015-0288-6
17. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet (2006) 367(9524):1747–57. doi:10.1016/S0140-6736(06)68770-9
18. Wingen AM, Fabian-Bach C, Schaefer F, Mehls O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet (1997) 349(9059):1117–23. doi:10.1016/S0140-6736(96)09260-4
19. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med (1996) 334(1):13–8. doi:10.1056/NEJM199601043340103
20. Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant (2005) 20(6):1048–56. doi:10.1093/ndt/gfh813
21. Schmieder RE. End organ damage in hypertension. Dtsch Arztebl Int (2010) 107(49):866–73. doi:10.3238/arztebl.2010.0866
22. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation (2008) 117(25):3171–80. doi:10.1161/CIRCULATIONAHA.107.730366
23. Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens (1995) 8(7):657–65. doi:10.1016/0895-7061(95)97901-3
24. Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: a 10-year longitudinal study. J Pediatr (2002) 141(6):770–9. doi:10.1067/mpd.2002.128113
25. Erlingsdottir A, Indridason OS, Thorvaldsson O, Edvardsson VO. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol (2010) 25(2):323–8. doi:10.1007/s00467-009-1350-3
26. Edvardsson VO, Steinthorsdottir SD, Eliasdottir SB, Indridason OS, Palsson R.Birth weight and childhood blood pressure. Curr Hypertens Rep (2012) 14(6):596–602. doi:10.1007/s11906-012-0311-6
27. Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med (2000) 160(10):1472–6. doi:10.1001/archinte.160.10.1472
28. Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes – a global concern. Nat Rev Nephrol (2015) 11(3):135–49. doi:10.1038/nrneph.2014.251
29. Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol (2008) 19(1):151–7. doi:10.1681/ASN.2007020252
30. Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) study. Am J Kidney Dis (2008) 51(1):10–20. doi:10.1053/j.ajkd.2007.09.013
31. Low Birth Weight and Nephron Number Working Group. The impact of kidney development on the life course: a consensus document for action. Nephron (2017) 136(1):3–49. doi:10.1159/000457967
32. Primatesta P, Falaschetti E, Poulter NR. Birth weight and blood pressure in childhood: results from the Health Survey for England. Hypertension (2005) 45(1):75–9. doi:10.1161/01.HYP.0000150037.98835.10
33. Barker DJ, Osmond C. Low birth weight and hypertension. BMJ (1988) 297(6641):134–5. doi:10.1136/bmj.297.6641.134-b
34. Spence D, Stewart MC, Alderdice FA, Patterson CC, Halliday HL. Intra-uterine growth restriction and increased risk of hypertension in adult life: a follow-up study of 50-year-olds. Public Health (2012) 126(7):561–5. doi:10.1016/j.puhe.2012.03.010
35. Eriksson JG, Forsén TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension (2007) 49(6):1415–21. doi:10.1161/HYPERTENSIONAHA.106.085597
36. Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol (2014) 24(11):793–800.e1. doi:10.1016/j.annepidem.2014.08.002
37. Hrolfsdottir L, Halldorsson TI, Rytter D, Bech BH, Birgisdottir BE, Gunnarsdottir I, et al. Maternal macronutrient intake and offspring blood pressure 20 years later. J Am Heart Assoc (2017) 6(4):e005808. doi:10.1161/JAHA.117.005808
38. Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring’s blood pressure 40 years later. Br J Obstet Gynaecol (1996) 103(3):273–80. doi:10.1111/j.1471-0528.1996.tb09718.x
39. Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Smith GD. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth study. Hypertension (2008) 52(4):638–44. doi:10.1161/HYPERTENSIONAHA.108.114256
40. Filler G, Yasin A, Kesarwani P, Garg AX, Lindsay R, Sharma AP. Big mother or small baby: which predicts hypertension? J Clin Hypertens (Greenwich) (2011) 13(1):35–41. doi:10.1111/j.1751-7176.2010.00366.x
41. Bowers K, Liu G, Wang P, Ye T, Tian Z, Liu E, et al. Birth weight, postnatal weight change, and risk for high blood pressure among Chinese children. Pediatrics (2011) 127(5):e1272–9. doi:10.1542/peds.2010-2213
42. Painter RC, Roseboom TJ, van Montfrans GA, Bossuyt PM, Krediet RT, Osmond C, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol (2005) 16(1):189–94. doi:10.1681/ASN.2004060474
43. Huang C, Guo C, Nichols C, Chen S, Martorell R. Elevated levels of protein in urine in adulthood after exposure to the Chinese famine of 1959–61 during gestation and the early postnatal period. Int J Epidemiol (2014) 43(6):1806–14. doi:10.1093/ije/dyu193
44. White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis (2009) 54(2):248–61. doi:10.1053/j.ajkd.2008.12.042
45. Ruggajo P, Skrunes R, Svarstad E, Skjærven R, Reisæther AV, Vikse BE. Familial factors, low birth weight, and development of ESRD: a nationwide registry study. Am J Kidney Dis (2016) 67(4):601–8. doi:10.1053/j.ajkd.2015.11.015
46. López-Bermejo A, Sitjar C, Cabacas A, Vázquez-Ruíz M, García-González MM, Mora C, et al. Prenatal programming of renal function: the estimated glomerular filtration rate is influenced by size at birth in apparently healthy children. Pediatr Res (2008) 64(1):97–9. doi:10.1203/PDR.0b013e31817282db
47. Franco MC, Nishida SK, Sesso R. GFR estimated from cystatin C versus creatinine in children born small for gestational age. Am J Kidney Dis (2008) 51(6):925–32. doi:10.1053/j.ajkd.2008.02.305
48. Sheu JN, Chen JH. Minimal change nephrotic syndrome in children with intrauterine growth retardation. Am J Kidney Dis (2001) 37(5):909–14. doi:10.1016/S0272-6386(05)80005-8
49. Zidar N, Avgustin Cavić M, Kenda RB, Ferluga D. Unfavorable course of minimal change nephrotic syndrome in children with intrauterine growth retardation. Kidney Int (1998) 54(4):1320–3. doi:10.1046/j.1523-1755.1998.00121.x
50. Zidar N, Cavić MA, Kenda RB, Koselj M, Ferluga D. Effect of intrauterine growth retardation on the clinical course and prognosis of IgA glomerulonephritis in children. Nephron (1998) 79(1):28–32. doi:10.1159/000044987
51. Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol (2011) 26(9):1529–33. doi:10.1007/s00467-011-1843-8
52. Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int (1996) 49(6):1774–7. doi:10.1038/ki.1996.265
53. Hughson M, Farris AB III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int (2003) 63(6):2113–22. doi:10.1046/j.1523-1755.2003.00018.x
54. Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol (1992) 99(4):296–301. doi:10.1111/j.1471-0528.1992.tb13726.x
55. Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci (1999) 64(11):965–74. doi:10.1016/S0024-3205(99)00022-3
56. Jones SE, Bilous RW, Flyvbjerg A, Marshall SM. Intra-uterine environment influences glomerular number and the acute renal adaptation to experimental diabetes. Diabetologia (2001) 44(6):721–8. doi:10.1007/s001250051681
57. Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int (2004) 65(4):1339–48. doi:10.1111/j.1523-1755.2004.00511.x
58. Sampogna RV, Schneider L, Al-Awqati Q. Developmental programming of branching morphogenesis in the kidney. J Am Soc Nephrol (2015) 26(10):2414–22. doi:10.1681/ASN.2014090886
59. Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int (2001) 59(1):238–45. doi:10.1046/j.1523-1755.2001.00484.x
60. Merlet-Bénichou C, Gilbert T, Muffat-Joly M, Lelièvre-Pégorier M, Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol (1994) 8(2):175–80. doi:10.1007/BF00865473
61. Schreuder MF, Nyengaard JR, Fodor M, van Wijk JA, Delemarre-van de Waal HA. Glomerular number and function are influenced by spontaneous and induced low birth weight in rats. J Am Soc Nephrol (2005) 16(10):2913–9. doi:10.1681/ASN.2004100875
62. Sanders MW, Fazzi GE, Janssen GM, de Leeuw PW, Blanco CE, De Mey JG. Reduced uteroplacental blood flow alters renal arterial reactivity and glomerular properties in the rat offspring. Hypertension (2004) 43(6):1283–9. doi:10.1161/01.HYP.0000127787.85259.1f
63. Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol (2007) 292(1):R453–61. doi:10.1152/ajpregu.00481.2006
64. Stelloh C, Allen KP, Mattson DL, Lerch-Gaggl A, Reddy S, El-Meanawy A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl Res (2012) 159(2):80–9. doi:10.1016/j.trsl.2011.10.004
65. Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, et al. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension (2008) 52(5):889–95. doi:10.1161/HYPERTENSIONAHA.108.116251
66. Lelièvre-Pégorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int (1998) 54(5):1455–62. doi:10.1046/j.1523-1755.1998.00151.x
67. Tomat AL, Inserra F, Veiras L, Vallone MC, Balaszczuk AM, Costa MA, et al. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol (2008) 295(2):R543–9. doi:10.1152/ajpregu.00050.2008
68. Lisle SJ, Lewis RM, Petry CJ, Ozanne SE, Hales CN, Forhead AJ. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr (2003) 90(1):33–9. doi:10.1079/BJN2003881
69. Tan JC, Workeneh B, Busque S, Blouch K, Derby G, Myers BD. Glomerular function, structure, and number in renal allografts from older deceased donors. J Am Soc Nephrol (2009) 20(1):181–8. doi:10.1681/ASN.2008030306
70. Mühle A, Mühle C, Amann K, Dötsch J, Nüsken KD, Boltze J, et al. No juvenile arterial hypertension in sheep multiples despite reduced nephron numbers. Pediatr Nephrol (2010) 25(9):1653–61. doi:10.1007/s00467-010-1512-3
71. Boubred F, Daniel L, Buffat C, Feuerstein JM, Tsimaratos M, Oliver C, et al. Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. Am J Physiol Renal Physiol (2009) 297(4):F943–51. doi:10.1152/ajprenal.90704.2008
72. Boubred F, Daniel L, Buffat C, Tsimaratos M, Oliver C, Lelièvre-Pégorier M, et al. The magnitude of nephron number reduction mediates intrauterine growth-restriction-induced long term chronic renal disease in the rat. A comparative study in two experimental models. J Transl Med (2016) 14(1):331. doi:10.1186/s12967-016-1086-3
73. Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens (2000) 18(2):123–37. doi:10.1097/00004872-200018020-00001
74. Terada T, Urushihara M, Saijo T, Nakagawa R, Kagami S. (Pro)renin and (pro)renin receptor expression during kidney development in neonates. Eur J Pediatr (2017) 176(2):183–9. doi:10.1007/s00431-016-2820-9
75. Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res (2001) 49(4):460–7. doi:10.1203/00006450-200104000-00005
76. Alwasel SH, Kaleem I, Sahajpal V, Ashton N. Maternal protein restriction reduces angiotensin II AT(1) and AT(2) receptor expression in the fetal rat kidney. Kidney Blood Press Res (2010) 33(4):251–9. doi:10.1159/000317739
77. Ao Y, Sun Z, Hu S, Zuo N, Li B, Yang S, et al. Low functional programming of renal AT2R mediates the developmental origin of glomerulosclerosis in adult offspring induced by prenatal caffeine exposure. Toxicol Appl Pharmacol (2015) 287(2):128–38. doi:10.1016/j.taap.2015.05.007
78. Mao C, Liu R, Bo L, Chen N, Li S, Xia S, et al. High-salt diets during pregnancy affected fetal and offspring renal renin-angiotensin system. J Endocrinol (2013) 218(1):61–73. doi:10.1530/JOE-13-0139
79. Luzardo R, Silva PA, Einicker-Lamas M, Ortiz-Costa S, do Carmo Mda G, Vieira-Filho LD, et al. Metabolic programming during lactation stimulates renal Na+ transport in the adult offspring due to an early impact on local angiotensin II pathways. PLoS One (2011) 6(7):e21232. doi:10.1371/journal.pone.0021232
80. Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) (2003) 104(6):607–14. doi:10.1042/CS20020355
81. Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol (1995) 110(3):223–8. doi:10.1016/0300-9629(94)00177-U
82. Vieira-Filho LD, Cabral EV, Farias JS, Silva PA, Muzi-Filho H, Vieyra A, et al. Renal molecular mechanisms underlying altered Na+ handling and genesis of hypertension during adulthood in prenatally undernourished rats. Br J Nutr (2014) 111(11):1932–44. doi:10.1017/S0007114513004236
83. Chen YW, Chenier I, Tran S, Scotcher M, Chang SY, Zhang SL. Maternal diabetes programs hypertension and kidney injury in offspring. Pediatr Nephrol (2010) 25(7):1319–29. doi:10.1007/s00467-010-1506-1
84. Martinez-Aguayo A, Aglony M, Bancalari R, Avalos C, Bolte L, Garcia H, et al. Birth weight is inversely associated with blood pressure and serum aldosterone and cortisol levels in children. Clin Endocrinol (Oxf) (2012) 76(5):713–8. doi:10.1111/j.1365-2265.2011.04308.x
85. Li C, Lin Y, Luo R, Chen S, Wang F, Zheng P, et al. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am J Physiol Renal Physiol (2016) 310(5):F351–63. doi:10.1152/ajprenal.00223.2015
86. Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol (2008) 295(1):F29–34. doi:10.1152/ajprenal.00123.2008
87. Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol (2002) 283(1):F202–6. doi:10.1152/ajprenal.00358.2001
88. DuBois BN, Pearson J, Mahmood T, Nguyen D, Thornburg K, Cherala G. Perinatal growth restriction decreases diuretic action of furosemide in adult rats. Eur J Pharmacol (2014) 728:39–47. doi:10.1016/j.ejphar.2014.01.056
89. Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol (2007) 292(3):R1230–5. doi:10.1152/ajpregu.00669.2006
90. Su Y, Bi J, Pulgar VM, Figueroa J, Chappell M, Rose JC. Antenatal glucocorticoid treatment alters Na+ uptake in renal proximal tubule cells from adult offspring in a sex-specific manner. Am J Physiol Renal Physiol (2015) 308(11):F1268–75. doi:10.1152/ajprenal.00047.2015
91. Sanders MW, Fazzi GE, Janssen GM, Blanco CE, De Mey JG. High sodium intake increases blood pressure and alters renal function in intrauterine growth-retarded rats. Hypertension (2005) 46(1):71–5. doi:10.1161/01.HYP.0000171475.40259.d1
92. Nehiri T, Duong Van Huyen JP, Viltard M, Fassot C, Heudes D, Freund N, et al. Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes (2008) 57(8):2167–75. doi:10.2337/db07-0780
93. Reho JJ, Zheng X, Benjamin JE, Fisher SA. Neural programming of mesenteric and renal arteries. Am J Physiol Heart Circ Physiol (2014) 307(4):H563–73. doi:10.1152/ajpheart.00250.2014
94. Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Lê NL, et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol (2007) 292(3):R1236–45. doi:10.1152/ajpregu.00227.2006
95. Yzydorczyk C, Armengaud JB, Peyter AC, Chehade H, Cachat F, Juvet C, et al. Endothelial dysfunction in individuals born after fetal growth restriction: cardiovascular and renal consequences and preventive approaches. J Dev Orig Health Dis (2017) 8(4):448–64. doi:10.1017/S2040174417000265
96. Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol (2014) 12(6):845–58. doi:10.2174/15701611113116660149
97. Bauer R, Walter B, Brust P, Füchtner F, Zwiener U. Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur J Obstet Gynecol Reprod Biol (2003) 110(Suppl 1):S40–9. doi:10.1016/S0301-2115(03)00171-4
98. Brown RD, Turner AJ, Carlström M, Persson AE, Gibson KJ. Tubuloglomerular feedback response in the prenatal and postnatal ovine kidney. Am J Physiol Renal Physiol (2011) 300(6):F1368–74. doi:10.1152/ajprenal.00019.2011
99. Rumballe B, Georgas K, Wilkinson L, Little M. Molecular anatomy of the kidney: what have we learned from gene expression and functional genomics? Pediatr Nephrol (2010) 25(6):1005–16. doi:10.1007/s00467-009-1392-6
100. Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech Dev (2000) 92(1):31–45. doi:10.1016/S0925-4773(99)00323-8
101. Dressler GR, Patel SR. Epigenetics in kidney development and renal disease. Transl Res (2015) 165(1):166–76. doi:10.1016/j.trsl.2014.04.007
102. Reddy MA, Natarajan R. Recent developments in epigenetics of acute and chronic kidney diseases. Kidney Int (2015) 88(2):250–61. doi:10.1038/ki.2015.148
103. Soofi A, Levitan I, Dressler GR. Two novel EGFP insertion alleles reveal unique aspects of Pax2 function in embryonic and adult kidneys. Dev Biol (2012) 365(1):241–50. doi:10.1016/j.ydbio.2012.02.032
104. Imgrund M, Gröne E, Gröne HJ, Kretzler M, Holzman L, Schlöndorff D, et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int (1999) 56(4):1423–31. doi:10.1046/j.1523-1755.1999.00663.x
105. Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A (2007) 104(49):19351–6. doi:10.1073/pnas.0707258104
106. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension (2017) 70(4):660–7. doi:10.1161/HYPERTENSIONAHA.117.07802
107. Costa MR, Pires KM, Nalbones-Barbosa MN, Dos Santos Valença S, Resende ÂC, de Moura RS. Grape skin extract-derived polyphenols modify programming-induced renal endowment in prenatal protein-restricted male mouse offspring. Eur J Nutr (2016) 55(4):1455–64. doi:10.1007/s00394-015-0963-5
108. Stangenberg S, Nguyen LT, Chen H, Al-Odat I, Killingsworth MC, Gosnell ME, et al. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int J Biochem Cell Biol (2015) 64:81–90. doi:10.1016/j.biocel.2015.03.017
109. Rodríguez-Rodríguez P, de Pablo AL, Condezo-Hoyos L, Martín-Cabrejas MA, Aguilera Y, Ruiz-Hurtado G, et al. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J Nutr Biochem (2015) 26(12):1650–9. doi:10.1016/j.jnutbio.2015.08.004
110. Tain YL, Lin YJ, Sheen JM, Yu HR, Tiao MM, Chen CC, et al. High fat diets sex-specifically affect the renal transcriptome and program obesity, kidney injury, and hypertension in the offspring. Nutrients (2017) 9(4):E357. doi:10.3390/nu9040357
111. Yim HE, Yoo KH. Early life obesity and chronic kidney disease in later life. Pediatr Nephrol (2015) 30(8):1255–63. doi:10.1007/s00467-014-2922-4
112. Wang Y, Chen X, Klag MJ, Caballero B. Epidemic of childhood obesity: implications for kidney disease. Adv Chronic Kidney Dis (2006) 13(4):336–51. doi:10.1053/j.ackd.2006.07.016
113. Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am (2009) 93(3):569–82. doi:10.1016/j.mcna.2009.02.014
114. Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, et al. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta (2017) 1863(5):1106–14. doi:10.1016/j.bbadis.2016.07.019
115. Alcazar MA, Boehler E, Rother E, Amann K, Vohlen C, von Hörsten S, et al. Early postnatal hyperalimentation impairs renal function via SOCS-3 mediated renal postreceptor leptin resistance. Endocrinology (2012) 153(3):1397–410. doi:10.1210/en.2011-1670
116. Kasper P, Vohlen C, Dinger K, Mohr J, Hucklenbruch-Rother E, Janoschek R, et al. Renal metabolic programming is linked to the dynamic regulation of a Leptin-Klf15 axis and Akt/AMPKalpha signaling in male offspring of obese dams. Endocrinology (2017) 158(10):3399–415. doi:10.1210/en.2017-00489
117. Ostreicher I, Almeida JR, Campean V, Rauh M, Plank C, Amann K, et al. Changes in 11beta-hydroxysteroid dehydrogenase type 2 expression in a low-protein rat model of intrauterine growth restriction. Nephrol Dial Transplant (2010) 25(10):3195–203. doi:10.1093/ndt/gfq354
118. Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology (2001) 142(7):2841–53. doi:10.1210/endo.142.7.8238
119. Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin ii receptor in neonatal sheep. Endocrinology (2001) 142(7):2854–64. doi:10.1210/endo.142.7.8264
120. Sheen JM, Yu HR, Tiao MM, Chen CC, Huang LT, Chang HY, et al. Prenatal dexamethasone-induced programmed hypertension and renal programming. Life Sci (2015) 132:41–8. doi:10.1016/j.lfs.2015.04.005
121. Tain YL, Wu KL, Lee WC, Leu S, Chan JY. Maternal fructose-intake-induced renal programming in adult male offspring. J Nutr Biochem (2015) 26(6):642–50. doi:10.1016/j.jnutbio.2014.12.017
122. Imig JD, Zou AP, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol (1996) 270(1 Pt 2):R217–27.
123. Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA Jr, et al. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol (2008) 294(2):H1018–26. doi:10.1152/ajpheart.01172.2007
124. Ichihara A, Imig JD, Inscho EW, Navar LG. Cyclooxygenase-2 participates in tubular flow-dependent afferent arteriolar tone: interaction with neuronal NOS. Am J Physiol (1998) 275(4 Pt 2):F605–12.
125. Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther (2007) 323(1):85–93. doi:10.1124/jpet.107.123638
126. Owen CG, Whincup PH, Gilg JA, Cook DG. Effect of breast feeding in infancy on blood pressure in later life: systematic review and meta-analysis. BMJ (2003) 327(7425):1189–95. doi:10.1136/bmj.327.7425.1189
127. Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children’s weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet (2008) 371(9611):492–9. doi:10.1016/S0140-6736(08)60172-5
128. Belizán JM, Villar J, Bergel E, del Pino A, Di Fulvio S, Galliano SV, et al. Long-term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ (1997) 315(7103):281–5. doi:10.1136/bmj.315.7103.281
129. Lee LM, Leung CY, Tang WW, Choi HL, Leung YC, McCaffery PJ, et al. A paradoxical teratogenic mechanism for retinoic acid. Proc Natl Acad Sci U S A (2012) 109(34):13668–73. doi:10.1073/pnas.1200872109
130. Makrakis J, Zimanyi MA, Black MJ. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr Nephrol (2007) 22(11):1861–7. doi:10.1007/s00467-007-0572-5
131. Hawkesworth S, Wagatsuma Y, Kahn AI, Hawlader MD, Fulford AJ, Arifeen SE, et al. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. J Nutr (2013) 143(5):728–34. doi:10.3945/jn.112.168518
132. Forsyth JS, Willatts P, Agostoni C, Bissenden J, Casaer P, Boehm G. Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ (2003) 326(7396):953. doi:10.1136/bmj.326.7396.953
133. Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr (2009) 139(8):1575–81. doi:10.3945/jn.109.106666
134. Sutherland MR, Gubhaju L, Yoder BA, Stahlman MT, Black MJ. The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr Res (2009) 65(4):397–402. doi:10.1203/PDR.0b013e3181975f52
135. Jayasooriya AP, Begg DP, Chen N, Mathai ML, Sinclair AJ, Wilkinson-Berka J, et al. Omega-3 polyunsaturated fatty acid supplementation reduces hypertension in TGR(mRen-2)27 rats. Prostaglandins Leukot Essent Fatty Acids (2008) 78(1):67–72. doi:10.1016/j.plefa.2007.11.001
136. Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) (2002) 103(6):633–9. doi:10.1042/cs1030633
137. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease study 2010. Lancet (2012) 380(9859):2224–60. doi:10.1016/S0140-6736(12)61766-8
138. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (2012) 379(9832):2162–72. doi:10.1016/S0140-6736(12)60820-4
139. Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev (2009) 6(Suppl 3):332–6.
140. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet (2017) 390(10113):2627–42. doi:10.1016/S0140-6736(17)32129-3
Keywords: kidney development, nephron number, renin–angiotensin–aldosterone system, renal sodium transport, blood pressure, early nutrition, re-programming intervention
Citation: Nüsken E, Dötsch J, Weber LT and Nüsken KD (2018) Developmental Programming of Renal Function and Re-Programming Approaches. Front. Pediatr. 6:36. doi: 10.3389/fped.2018.00036
Received: 20 October 2017; Accepted: 08 February 2018;
Published: 27 February 2018
Edited by:
Miriam Schmidts, Radboud University Nijmegen, NetherlandsReviewed by:
Jan Michael Williams, University of Mississippi Medical Center School of Dentistry, United StatesLarry T. Patterson, Children’s National Health System, United States
Copyright: © 2018 Nüsken, Dötsch, Weber and Nüsken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai-Dietrich Nüsken, kai-dietrich.nuesken@uk-koeln.de
 Eva Nüsken
Eva Nüsken
 Lutz T. Weber
Lutz T. Weber Kai-Dietrich Nüsken
Kai-Dietrich Nüsken