- 1Department of Biological and Environmental Sciences, Qatar University, Doha, Qatar
- 2Department of Pharmacology and Toxicology, Faculty of Medicine, American University of Beirut, Beirut, Lebanon
The use of herbal therapies for treatment and management of cardiovascular diseases (CVDs) is increasing. Plants contain a bounty of phytochemicals that have proven to be protective by reducing the risk of various ailments and diseases. Indeed, accumulating literature provides the scientific evidence and hence reason d'etre for the application of herbal therapy in relation to CVDs. Slowly, but absolutely, herbal remedies are being entrenched into evidence-based medical practice. This is partly due to the supporting clinical trials and epidemiological studies. The rationale for this expanding interest and use of plant based treatments being that a significant proportion of hypertensive patients do not respond to Modern therapeutic medication. Other elements to this equation are the cost of medication, side-effects, accessibility, and availability of drugs. Therefore, we believe it is pertinent to review the literature on the beneficial effects of herbs and their isolated compounds as medication for treatment of hypertension, a prevalent risk factor for CVDs. Our search utilized the PubMed and ScienceDirect databases, and the criterion for inclusion was based on the following keywords and phrases: hypertension, high blood pressure, herbal medicine, complementary and alternative medicine (CAM), nitric oxide, vascular smooth muscle cell (VSMC) proliferation, hydrogen sulfide, nuclear factor kappa-B, oxidative stress, and epigenetics/epigenomics. Each of the aforementioned keywords was co-joined with herb in question, and where possible with its constituent molecule(s). In this first of a two-part review, we provide a brief introduction of hypertension, followed by a discussion of the molecular and cellular mechanisms. We then present and discuss the plants that are most commonly used in the treatment and management of hypertension.
Introduction
Cardiovascular disease (CVD) remains the leading cause of debility and premature death (WHO, 2013), and hence a major public health problem. Out of the major risk factors, which include diabetes, smoking, and dyslipidemia, hypertension is by far the most prevalent trigger for CVDs, and its comorbidity with other risk factors is even more puissant (Yang et al., 2011; WHO, 2013). Hypertension is responsible for around 16.5% of annual deaths worldwide (WHO, 2013), and is indeed the main cause of morbidity and mortality associated with CVDs (Kizhakekuttu and Widlansky, 2010). By 2030, the annual death toll is estimated to reach 23.5 million people (WHO, 2013). In addition to being a major player in the onset of diseases such as atherosclerosis, stroke, peripheral artery disease, heart failure, and coronary artery disease, hypertension can also lead to kidney damage, dementia, or blindness (August, 2004; Freedman and Cohen, 2016). It is important to note that May 17th of every year has been designated World Hypertension Day by the International Society of Hypertension (ISH), and the theme for 2013 World Health Day (7th April) was Hypertension, and hence a focus of considerable attention.
Hypertension is defined as having a systolic blood pressure (SBP) of ≥140 mmHg and a diastolic blood pressure (DBP) of ≥90 mmHg (≥140/≥90 mmHg; Tabassum and Ahmad, 2011). Every 20/10 (SBP/DBP) mmHg increase indicates a higher risk stage of hypertension; stage 1 (140–159/90–99 mmHg), stage 2 (≥160/≥100 mmHg; Archer, 2000; Weber et al., 2014) with the latter stage requiring immediate medical attention (Weber et al., 2014). Importantly, the American Society of Hypertension and ISH recommend that individuals with blood pressure of 120–139/80–89 mmHg be considered as pre-hypertensives (Weber et al., 2014). For targeted therapeutic interest, it is essential to realize that pre-hypertensive individuals are three times more likely to succumb to hypertension at a later stage of life than their normotensive counterparts (Archer, 2000). It is important to note that according to the Eighth Joint National Committee, it is recommended that for the general population, pharmacologic treatment be started at an SBP of 150 mmHg or DBP of 90 mmHg. However, for patients with Chronic Kidney Disease, treatment shall begin when the values of SBP and DBP reach 140 or 90 mmHg or higher, respectively (James et al., 2014).
Elevated blood pressure is categorized into types: primary (essential) and secondary hypertension. Secondary hypertension, which affects 5–10% of hypertensive individuals, is due to identifiable causes, such as diabetes and renal damage, and thus has a relatively higher chance of being treated. On the other hand, essential hypertension is acquired by multiple factors such as diet, age, lifestyle, neurohumoral activity, and interactions (Tabassum and Ahmad, 2011). Since its etiology may be more difficult to ascertain or establish, essential hypertension is more difficult to manage. Interestingly, the percentage of patients with essential hypertension (90–95%) far exceed those with secondary hypertension (Tabassum and Ahmad, 2011).
Many drugs, ranging from diuretics (Indapamide, Furosemide, Amiloride), sympathoplegic agents (clonidine, reserpine), renin inhibitor (Aliskiren), angiotensin converting enzymes (ACE) inhibitors (Enalapril, Captopril, Quinapril), angiotensin receptors blockers (ARBs—Losartan, Irbesartan, Olmesartan), calcium channel blockers (Nifedipine, Verapamil, Diltiazem), α-adrenergic blockers (Prazosin, Doxazosin), β-adrenergic blockers (Nebivolol, Atenolol) to vasodilators (Minoxidil, sodium nitroprusside), are used to manage blood pressure levels in hypertensive patients (Archer, 2000; Susalit et al., 2011). However, a point of interest to physicians and health-care practitioners is the alarming, and rather unfortunate, reality that high blood pressure is managed in only 34% of hypertensive patients (August, 2004; Wang and Xiong, 2012). The major concerns that often delay treatment allude to higher costs of antihypertensive drugs (Susalit et al., 2011), their availability and accessibility (Wang and Xiong, 2012), the undesired side effects of antihypertensive drugs (Susalit et al., 2011; Wang and Xiong, 2012) and the reduced patient compliance to consume more than a pill per day (August, 2004). Taking this into account, hypertensive patients, especially those dwelling in rural areas, seek alternative approaches such as herbal remedies for their treatment of hypertension and other diseases.
Herbal Remedies
The use of herbal medicine as a treatment modality has significantly increased over the last decade (Frishman et al., 2009). This is due to several factors, principal of which is that herbal medicine is a cheaper alternative with fewer undesired side effects (Frishman et al., 2009; Susalit et al., 2011; Tabassum and Ahmad, 2011). However, the increased desire to use herbal treatment is not a reflection of the economic status of an individual from a certain region or a country. Indeed, 70% of the population in developed nations have resorted to Complementary and Alternative Medicine (CAM) for treatment purposes, and herbal medicine forms a large proportion of its application (WHO, 2008). Further, the usage of CAM in developing countries is becoming even more pronounced (WHO, 2008). Evidently, the rationale for the use of herbal and plant remedies is definitely not surprising, considering the fact that they contain thousands of bioactive components that have known therapeutic applications (Pan et al., 2013). Indeed, plants and herbs have actually provided a starting point for synthesis of over 50% of currently used pharmaceutical drugs (Pan et al., 2013). The pharmacopeia includes ephedrine (from Ephedra sinica), aspirin (from Salix alba), lovastatin (from Monascus purpureus), reserpine (from Rauwolfia serpentina), and taxol (from Taxus brevifolia; Frishman et al., 2009). Remarkably, reserpine (which depletes adrenergic neurotransmitters) still remains an effective treatment for hypertension (Weber et al., 2014).
Importance of plants and herbs, per se, in the medical field must not be overlooked as they have been used throughout human history. Herbal plant-based formulations or drugs are pivotal to Traditional practices in Chinese, Ayurvedic, and Unani Tibb medicine, which is practiced worldwide. Overall, this may explain the increasing interest in panning out the beneficial health effects of various plants and herbs in different diseases including hypertension (Tabassum and Ahmad, 2011). In this review, we focus to provide a summary of different plants that have been reported to exhibit antihypertensive properties, and that can specifically mitigate anti-inflammatory causes in arterial hypertension. Moreover, where information is available, we have discussed botanical-induced improvements in renal biology.
Molecular Pathogenesis of Hypertension
Hypertension is characterized by arterial derangement in the vascular tree, affecting large conduit arteries (such as aorta), small resistance size arteries (150–400 μm), and the microcirculation (arterioles and capillaries). Increased arterial reactivity (sensitivity and potency) due to dysregulation in endothelial nitric oxide synthase (eNOS) and pro-oxidant enzymes, enhanced basal and activated calcium levels due to overactive transmembrane calcium permeability through calcium channels, and/or coexistence of vascular smooth muscle cell (VSMC) hyperplasia and hypertrophy (vascular remodeling) can all lead to increased vasoconstriction. These pathological events lead to an increased ratio of vessel wall thickness as compared to the dimensions of the arterial lumen (Folkow, 1990). It is this increased ratio that plays a major role in precipitating hypertension. Below we discuss some of the major mechanisms implicated in the pathogenesis of hypertension. Then we discuss the most commonly used herbs that ameliorate blood pressure by modulating these mechanisms.
Vascular Smooth Muscle Cell (VSMC) Proliferation
VSMCs participate in the pathogenesis of hypertension (Oparil et al., 2003; Lacolley et al., 2012), and their proliferation contributes to increased peripheral resistance by decreasing arterial diameters (Oparil et al., 2003; Figure 1). For an understanding of these intricate alterations, it is essential to examine the modulating factors that stimulate or inhibit VSMC growth for the treatment of hypertension. Growth factors impel the cell into entering cell cycle until the G1 phase, the first check point (Marx et al., 2011). These growth factors include: platelet-derived growth factor (PDGF; Itoh et al., 1993; Rudijanto, 2007; Marx et al., 2011), fibroblast growth factor (FGF; Itoh et al., 1993; Rudijanto, 2007; Marx et al., 2011) endothelin-1, thrombin, interleukin-1 (IL-1; Rudijanto, 2007), and visfatin (Miao and Li, 2012). Angiotensin II (Ang II) can also promote cell cycle progression especially that it can regulate the expression of both basic FGF (Itoh et al., 1993) and epidermal growth factor receptor (EGFR; Inagami and Eguchi, 2000).
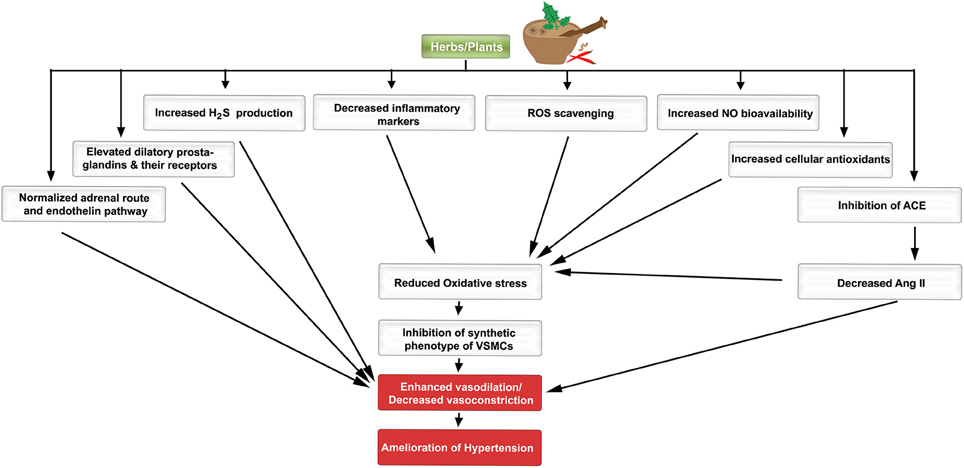
Figure 1. A schematic diagram indicating the favorable effects of plants/herbs on the molecular pathogenesis of hypertension. Different molecular, biochemical, and cellular pathways are favorably modulated by herbs/plants or their extracts.
Amongst the several VSMC growth inhibitors are nitric oxide (NO), cyclic guanosine monophosphate (cGMP; Pilz and Casteel, 2003), transforming growth factor-beta (TGFβ; Itoh et al., 1993; Rudijanto, 2007), and adenosine monophosphate-activated protein kinase (AMPK; Song and Zou, 2012). For example, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), a membrane-permeable activator of AMPK, inhibits cell cycle progression, and migration of VSMCs as well as vascular remodeling following injury (Stone et al., 2013). Moreover, rosiglitazone, a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, acts via protein kinase G (PKG) to exert an anti-proliferative effect on VSMCs in models of angioplasty-induced vascular injury (Yang et al., 2013). Contextually, complex regulatory interactions exist between growth factors and the VSMC phenotype (Itoh et al., 1993). The balance between the pro-proliferative and anti-proliferative signals determines, to a large extent, the phenotype of VSMCs. As will be discussed later in this review, many plants and herbs indeed ameliorate increased blood pressure by favorably modulating the VSMC phenotype.
Endothelial Cells
The endothelial cell layer is no longer considered as an inert entity. It rather plays significant roles in many aspects of homeostasis along the cardiovascular network. Endothelial function is regulated and maintained by a variety of cell surface receptors, some of which induce the release of vasoactive substances to regulate vascular tone and smooth muscle cell proliferation (Drexler and Hornig, 1999). Local and circulating cues stimulate the vascular endothelium to release vasodilators (NO, prostacyclin, and endothelial-derived hyperpolarizing factor) and vasoconstrictors (endothelin, thromboxane, and PDGF; Iglarz and Clozel, 2007). When there is an imbalance between these vasoactive agents, an increased production of reactive oxygen species (ROS) may result, which then leads to endothelial dysfunction and eventually hypertension (Virdis and Taddei, 2011; Montezano and Touyz, 2012; Silva et al., 2012; Figure 1).
Endothelial dysfunction anticipates the progression of anatomically overt vascular disease, which robustly correlates with hypertension. However, endothelial derangement could be reversed by modification therapy, such as with herbal remedies. This fact will be highlighted in different sections of this review.
Repertoire of Signaling Molecules
Assuming a homeostatic imbalance, the following signaling molecular entities become integral to the pathogenesis of hypertension (Ong and Whitworth, 2011; Montezano and Touyz, 2012). Fortunately, a diverse range of plant and herbal extracts and their individual metabolites can modulate signaling cascades implicated in the physiology of the cardiovascular system (see Section Herbs and Spices Most Commonly Used for Treatment of Hypertension, below). These herbs are not only vasculo-protective but they could also potentially reverse the changes in hypertension. This particularly applies if the alterations in hypertensive patients are addressed prior to reaching a decompensated state.
Reactive Oxygen Species
In good health, the activity of pro-oxidants is balanced by anti-oxidative agents. However, when this equilibrium is disturbed, the un-orchestrated milieu leads to pathologic states, such as hypertension, atherosclerosis, and other vascular complications (Figure 1; Montezano and Touyz, 2014). ROS such as superoxide anions () and hydroxyl ions (OH−) play a major role by promoting an environment of oxidative stress, which is a primary cause in the pathogenesis of hypertension (Zeng et al., 2009; Song and Zou, 2012; Montezano et al., 2015). ROS are produced from the reactivity of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase and many other enzymatic reactions particularly ones related to the electron transport chain within the mitochondria (Zeng et al., 2009; Dharmashankar and Widlansky, 2010; Drummond et al., 2011; Song and Zou, 2012). The oxidant molecules are inactivated by antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and non-enzymatic substances like reduced glutathione (GSH; Song and Zou, 2012). Recent evidence suggests that AMPK can also suppress the activity of NADPH oxidase (Song and Zou, 2012). An increase in the amount of ROS, and the corresponding fall in intracellular endothelial antioxidant levels (Zeng et al., 2009; Drummond et al., 2011; Song and Zou, 2012), signals an oxidative stress state. The oxidative stress leads to endothelial cell dysfunction and vascular smooth muscle remodeling by reducing NO bioavailability (Zeng et al., 2009; Drummond et al., 2011). ROS are also responsible for the oxidation of low-density lipoprotein (LDL), which results in inflammation and increased proliferation of VSMCs (Slevin et al., 2012). Both inflammation and enhanced proliferation significantly trigger plaque formation (Slevin et al., 2012) which in turn may contribute to increased blood pressure. Interestingly, blocking ROS production by exposure to antioxidants has been shown to reduce blood pressure in rodents (Zeng et al., 2009).
A key molecule that regulates oxidative stress in VSMCs is the transcription factor erythroid 2-related factor 2 (Nrf2, or nuclear factor erythroid 2-like 2). Under physiological conditions, Nrf2 activity is inhibited by its endogenous inhibitor Kelch-like ECH-associated inhibitor 1 (Keap1; Villeneuve et al., 2013). On the other hand, when oxidative stress ensues, Keap1 releases Nrf2, which then translocates to the nucleus and promotes expression of different antioxidant enzymes such as CAT, GPX, heme oxygenase-1 (HO-1), SOD (Kern et al., 2007). Pertinently, various phytochemicals suppress oxidative stress by upregulating antioxidant enzymes through the Keap1–Nrf2 pathway (Tao et al., 2013; Niture et al., 2014).
Nitric Oxide
Nitric oxide (NO) is often recognized as an important indicator of vascular health. It plays an important role in blood pressure regulation due to its vasodilating potency (Francis et al., 2010) as well as its ability to inhibit aggregation of platelets and proliferation of VSMCs (Zeng et al., 2009). NO is produced from L-arginine (Francis et al., 2010) by NO synthases (NOS) such as endothelial NOS (eNOS; Francis et al., 2010). After its release, NO translocates into VSMCs and activates soluble guanylate cyclase (sGC), which then catalyzes the conversion and cyclization of guanosine triphosphate (GTP) to cGMP (Francis et al., 2010). cGMP then binds to cGMP-dependent protein kinases (PKGs; Francis et al., 2010) which modulate calcium levels and the consequent contraction of VSMCs (Francis et al., 2010). Within seconds of its production, NO reacts with superoxide anions (Drummond et al., 2011) to produce peroxynitrite (ONOO−; Drummond et al., 2011). This in itself is a selective oxidant and can precipitate oxidative stress as well as diminish the vasodilatory effect of NO (Drummond et al., 2011). As such, the concentration of ROS has a direct impact on the bioavailability of NO. Interestingly, oxidative stress can also induce eNOS uncoupling, so that eNOS generates free radicals instead of NO. For example, stimulation of NADPH oxidase leads to the generation of superoxide anion (), which reacts with NO to form the potent oxidizing agent, peroxynitrite. This peroxynitrite then oxidatively degrades the eNOS cofactor tetrahydrobiopterin (BH4) to the inactive dihydrobiopterin (BH2). Effectively, this yields more superoxide anion that eventually depletes BH4. Therefore, a shift in balance from NO to superoxide formation ensues; this is referred to as uncoupling of NOS (Michel and Vanhoutte, 2010). As such, the interplay between NO and the regulatory mechanisms governing its bioavailability are thus intimately intertwined with vascular tone and endothelial dysfunction (Montezano and Touyz, 2012).
Hydrogen Sulfide
Hydrogen sulfide (H2S) is an important biological mediator produced in VSMCs and potentially endothelial cells via the catalysis of L-cysteine by the enzyme cystathionine γ-lyase (CSE; Calvert et al., 2010; Pan et al., 2012). It has been reported that deficiency in H2S production is positively correlated with pathophysiology of hypertension in several animal models (Benavides et al., 2007; Liu et al., 2012). Indeed, CSE activity and H2S levels were notably decreased in the aorta and plasma of SHR, respectively (Yan et al., 2004). In addition, there was a significant increase in BP levels of Wistar Kyoto (WKY) rats treated with DL-propargylglycine (PPG), a CSE inhibitor (Yan et al., 2004). Furthermore, administration of H2S is sufficient to abolish the Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME, an inhibitor of nitric oxide synthase)-induced hypertension in WKY rats (Zhong et al., 2003).
At the molecular and cellular levels, H2S exhibits a vasorelaxant effect on VSMCs by increasing intracellular levels of cGMP (Bucci et al., 2012) and opening ATP-dependent potassium channels (KATP; Banerjee et al., 2002; Calvert et al., 2010; Bucci et al., 2012; Pan et al., 2012). Interestingly, H2S has also been reported to prevent vascular inflammation (Calvert et al., 2010; Pan et al., 2012), reduce ROS production, depress Ang II and ACE levels, potentiate antioxidant mechanisms, diminish VSMC proliferation, and induce NO synthesis (Benavides et al., 2007).
Angiotensin II
The Renin–Angiotensin–Aldosterone (RAA) system plays a pivotal role in regulating blood pressure (Nguyen Dinh Cat and Touyz, 2011). Activation of the RAA system in response to a decrease in cardiac output leads to the secretion of renin, which in turn catalyzes the conversion of angiotensinogen to angiotensin I (Ang I). Ang I is then cleaved by Angiotensin Converting Enzyme (ACE)-1 (ACE-1) to form Angiotensin II (Ang II). Ang II-mediated hypertension occurs by promotion of sodium and water retention as well as enhancement of vasoconstriction by binding to the angiotensin type 1 (AT1) receptor (Morgan, 2003; Nguyen Dinh Cat and Touyz, 2011; Savoia et al., 2011). Activation of AT1 receptors is known to trigger the proliferation of VSMCs (Castro et al., 2010; discussed earlier). The fact that ACE is present in several tissues, including arteries, indicates that Ang II can be formed locally within the arteries themselves. Indeed, there is a higher concentration of Ang II in the vasculature of hypertensive compared to normotensive rats (Ma et al., 2010). Taken together, these observations may explain why inhibiting ACE is an attractive approach for antihypertensive therapies (Morgan, 2003; Bernstein et al., 2013). Ang II induces aldosterone production (Oparil et al., 2003; Manrique et al., 2009; Ma et al., 2010) and cardiac muscle cell growth (Manrique et al., 2009; Ma et al., 2010; Song and Zou, 2012). Blockade of AT1 receptors improves endothelial dysfunction (Virdis et al., 2011) and has been reported to decrease BP (Kang et al., 2009). Accumulating evidence demonstrates that Ang II also stimulates NADPH oxidase to generate ROS (Ma et al., 2010; Virdis et al., 2011). In addition, Ang II may increase the sympathetic nervous system (SNS) activity (Manrique et al., 2009), which participates in pathogenesis of hypertension by elevating cardiac output and increasing peripheral vascular resistance (Oparil et al., 2003).
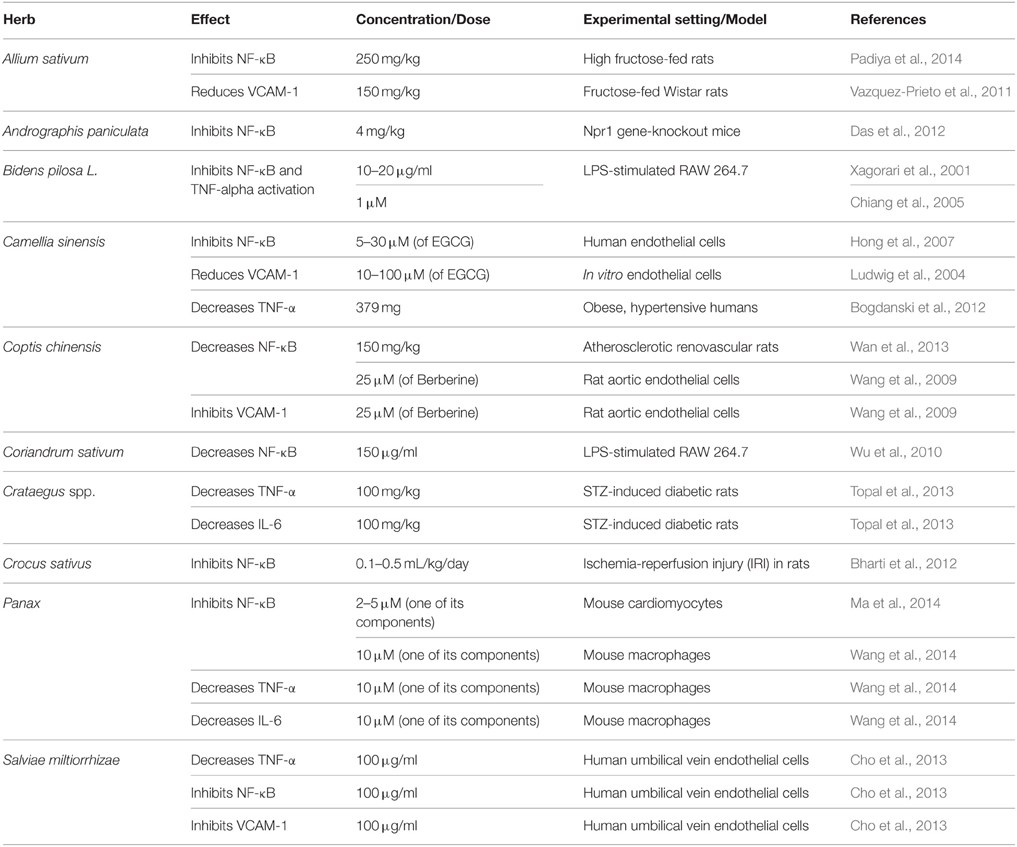
Nuclear Factor Kappa B
Inflammation is well-documented to contribute to vascular remodeling and consequent hypertension (Vazquez-Prieto et al., 2011). Nuclear Factor kappa B (NF-κB), a transcription factor, is known to partake in the pathology of hypertension. It induces endothelial cell dysfunction, oxidative stress, and inflammation (Li and Zhuo, 2008; Kang et al., 2009) through the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6; Kang et al., 2009).
It has been shown that increased expression and activation of NF-κB contributes to renal injury and hypertension (Elks et al., 2009). Indeed, suppressing NF-κB with pyrrolidine dithiocarbamate (PDTC) decreased SBP in spontaneous hypertensive rats (SHRs; Elks et al., 2009). Interestingly, PDTC also suppressed the higher concentration of cytosolic and mitochondrial ROS in kidneys of these SHRs (Elks et al., 2009).
Activation of NF-κB could be induced by several factors such as Ang II (Kang et al., 2009), ROS (Kang et al., 2009), and/or tumor necrosis factor-alpha (TNF-α; Mathew and Biju, 2008; Zhang et al., 2009; Vazquez-Prieto et al., 2011). Interestingly, TNF-α-induced ROS production, which drastically impacts endothelial dysfunction, is shown to be mediated by NF-κB (Zhang et al., 2009). Moreover, NF-κB plays an important role in attenuation of ANG II-induced pressor response (Kang et al., 2009), regulation of AT1 receptors (Bhatt et al., 2014; Luo et al., 2015), as well as induction of oxidative stress (Mathew and Biju, 2008; Zhang et al., 2009; Vazquez-Prieto et al., 2011). Further, NF-κB can increase proliferation, decrease apoptosis of endothelial cells, as well as stimulate expression of vascular cell adhesion molecule-1 (VCAM-1; Vazquez-Prieto et al., 2011; Jiang et al., 2013b). Taken together, these NF-κB-induced changes can lead to the derangement of endothelial function and vascular tone.
Herbs and Spices Most Commonly Used for Treatment of Hypertension
Secondary metabolites of herbs and spices exhibit anti-hypertensive effects. Here, we present a comprehensive alphabetical list of herbs for which sound evidence suggests they could be beneficial in hypertension therapy (Tables 1–6; Figure 1).
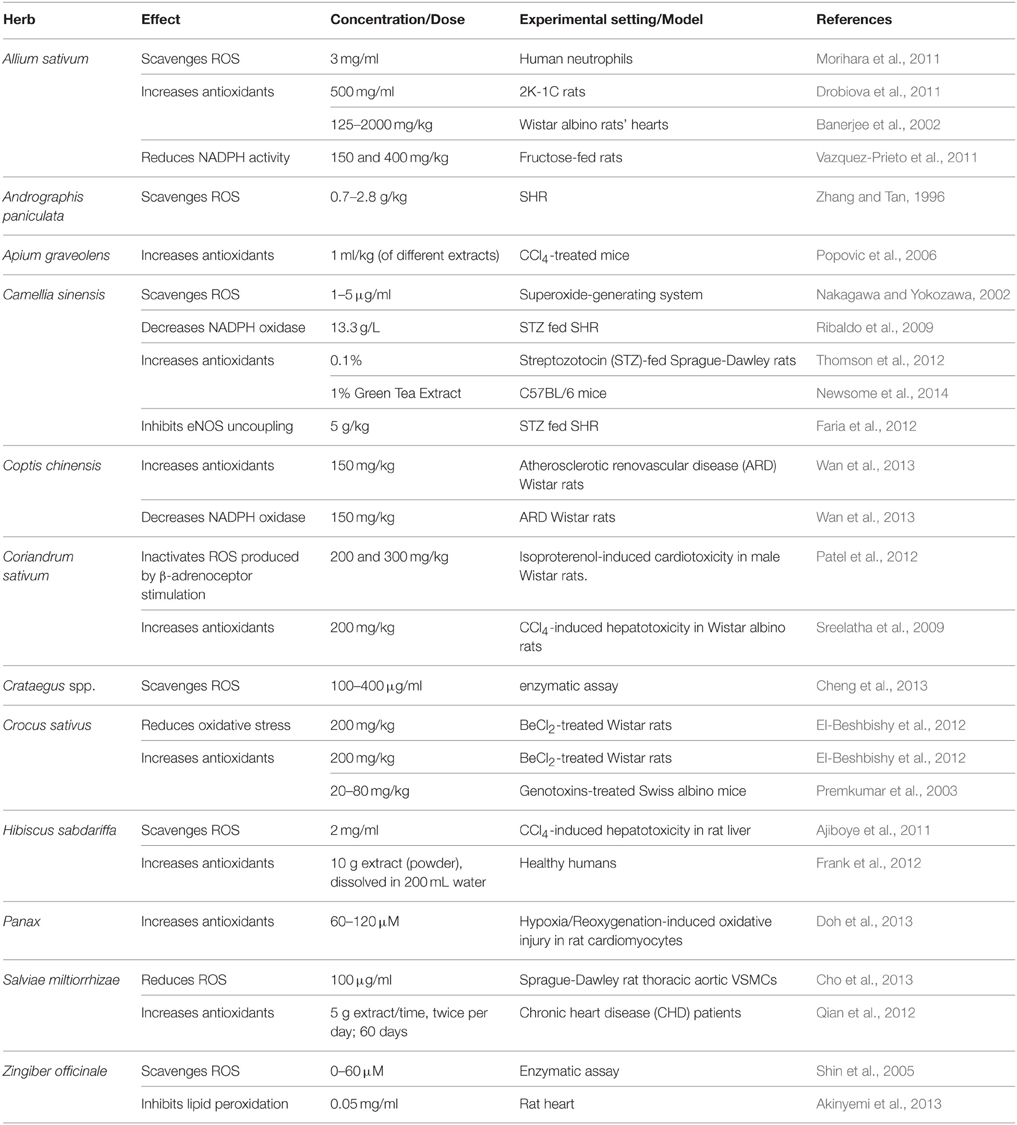
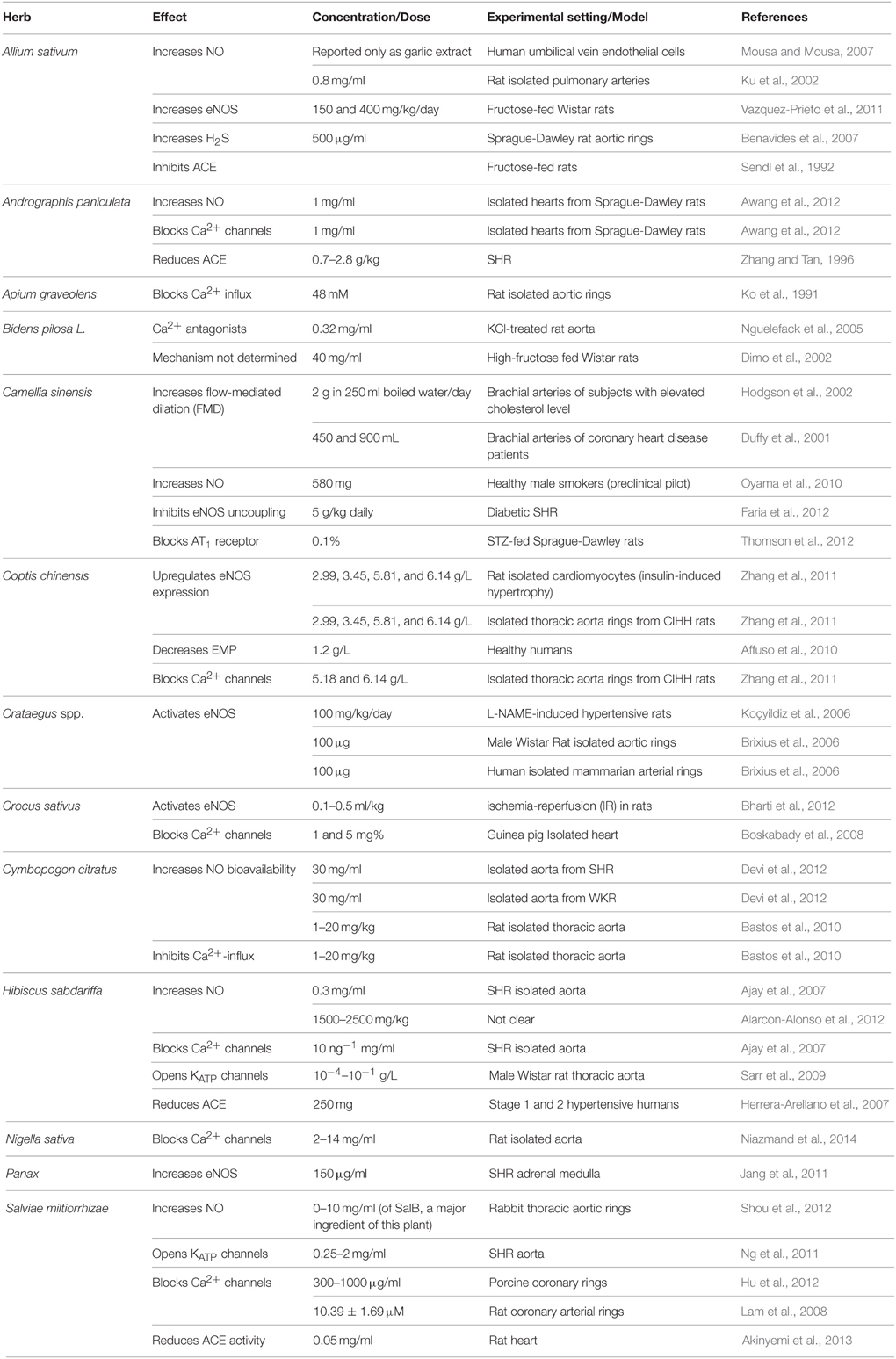
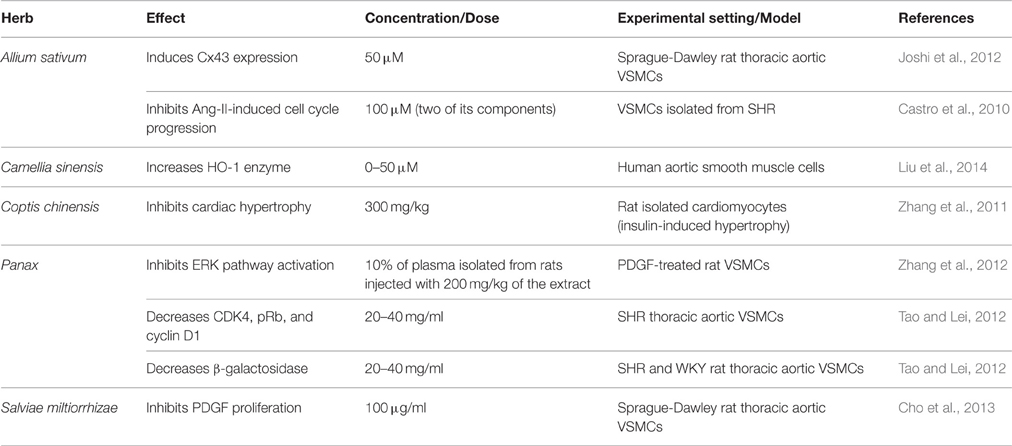
Allium sativum (Garlic)
Garlic's multi-fold therapeutic effects have been recognized for thousands of years amongst different cultures around the world, and continues to attract interest from pharmacologists and health practitioners (Frishman et al., 2009; Qidwai and Ashfaq, 2013; Table 2). This herb is not only known for its hypotensive capacity, but is also characterized by anti-inflammatory, antioxidant, antibacterial, hypocholesteremic, and anti-cancer properties (Banerjee et al., 2002; Mousa and Mousa, 2007; Frishman et al., 2009; Qidwai and Ashfaq, 2013). For health benefits, garlic can be consumed in different forms, such as raw, aged, an aqueous extract, oil, and in powder form (Banerjee et al., 2002; Frishman et al., 2009; Ried et al., 2013).
Several mechanisms have been alluded to in the explanation of garlic's hypotensive effects (Shouk et al., 2014). These are based on garlic's organo-sulfur constituents such as Allicin, S-allylcysteine (SAC), diallyl disulfides (DADS), diallyl trisulfides (DATS), and methyl thiosulfonate (Banerjee et al., 2002; Qidwai and Ashfaq, 2013).
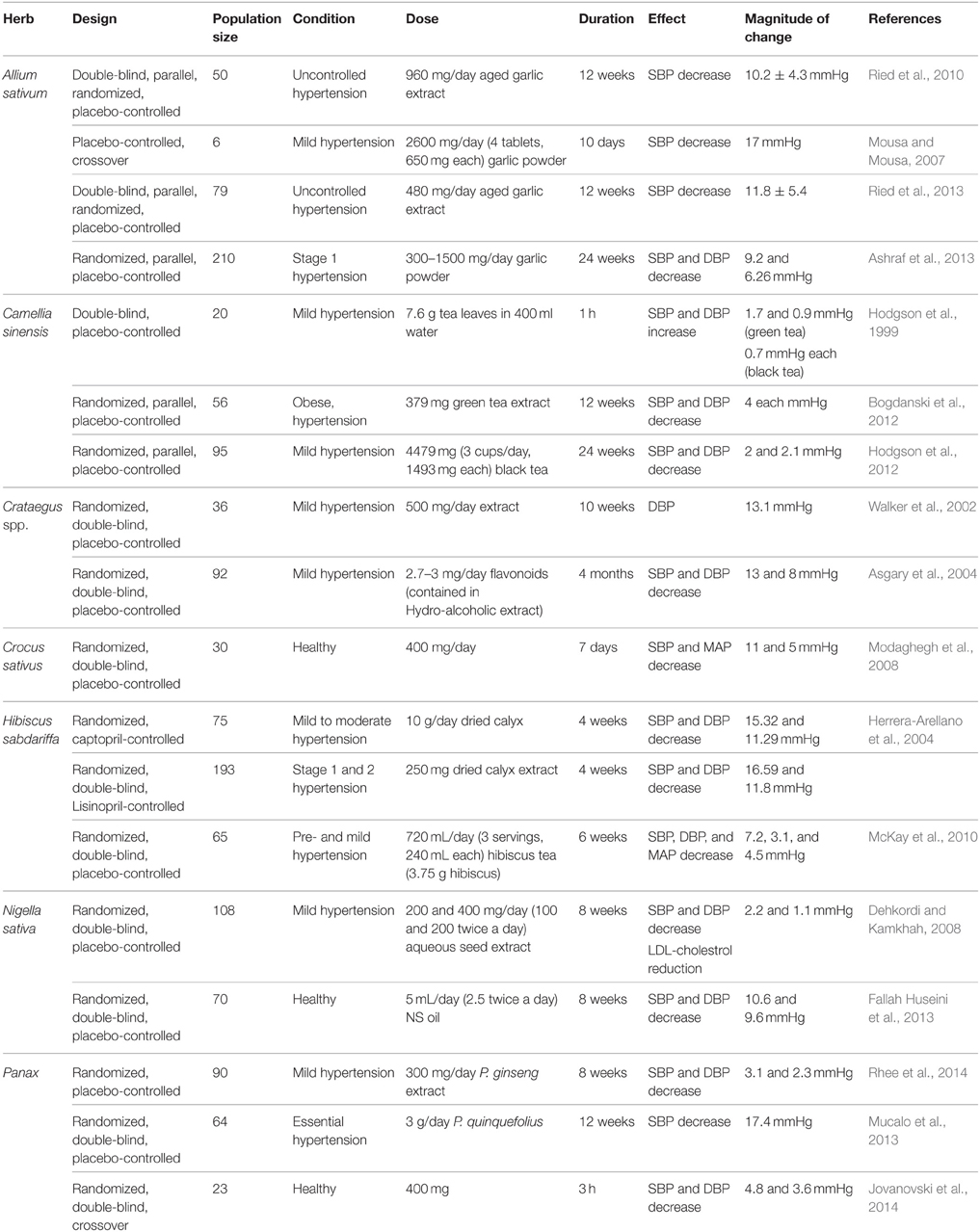
Many hypertensive patients use garlic to lower their blood pressure (Qidwai and Ashfaq, 2013). The reported effects vary from significant reduction in mean arterial pressure, drop in either SBP or DBP only, to no alteration in blood pressure at all (Banerjee et al., 2002; Mousa and Mousa, 2007; Frishman et al., 2009; Yang et al., 2011; Augusti et al., 2012). However, most studies confirm the induction of hypotensive effects by garlic and its constituents. Banerjee and Maulik reviewed pertinent literature and concluded that different forms of garlic can reduce SBP, DBP, or both (Banerjee et al., 2002). Also, an investigation by Qidwai and Ashfaq indicated that an almost 80% efficacy in anti-hypertensive effects of garlic was reported (Qidwai and Ashfaq, 2013). Interestingly, evidence from meta-analysis studies indicates that aged garlic extract (AGE) produces consistent lowering of blood pressure compared to other forms of garlic. A recent meta-analysis of randomized, controlled trials concluded that garlic supplements induce a significant reduction in both SBP and DBP by 3.75 and 3.39 mmHg, respectively (Wang et al., 2015). Similarly, another meta-analysis on randomized, controlled trials also reported a significant decrease in SBP by 4.6 ± 2.8 mmHg compared to placebo (Ried et al., 2008). Moreover, in a double-blind, parallel randomized placebo-controlled study, individuals with uncontrolled hypertension (≥140 mmHg) who were treated with 960 mg/day of AGEs for 12 weeks exhibited an average decrease of 10.2 ± 4.3 mmHg in SBP (Ried et al., 2010). This effect appears to be due to the principle constituent S-allylcysteine, which is relatively more stable in relation to allicin (Ried et al., 2010). Furthermore, in another randomized, parallel, placebo-controlled trial, patients with stage 1 hypertension ingested garlic tablets (300–1500 mg/day) for 24 weeks (Ashraf et al., 2013). This study reported a significant decrease in SBP and DBP by a maximum of 9.2 and 6.27 mmHg, respectively (Ashraf et al., 2013).
Analysis of assays on antioxidant ability of different forms of garlic demonstrate far greater potency in aged extracts than other types of garlic clove derivatives (Mathew and Biju, 2008). Indeed, AGE potently scavenges ROS (Drobiova et al., 2011; Morihara et al., 2011) leading to an increase in cellular antioxidants (Banerjee et al., 2002; Drobiova et al., 2011). In comparable studies, Drobiova et al. (2011) treated two-kidney, one-clip (2K-1C) hypertensive rats for 3 weeks with an aqueous extract of garlic (500 mg/ml), which raised the antioxidant levels by over 60% and resulted in reduction of SBP by 50%. The superoxide scavenging abilities of AGE have also been demonstrated in human neutrophils (Morihara et al., 2011). In addition, a daily 150 or 400 mg/kg dose of garlic extracts has also been reported to decrease NADPH oxidase in fructose-fed rats' aorta (Vazquez-Prieto et al., 2011).
The endogenous signaling gases, NO and H2S, are recognized as mediators of garlic's antihypertensive properties (Banerjee et al., 2002; Mousa and Mousa, 2007; Ried et al., 2013). In a clinical trial performed by Mousa and Mousa (2007) on stage 1 hypertensive subjects (≥140 mmHg), who consumed a daily dose of 2600 mg of garlic (one tablet comprising of 650 mg of garlic bulb—Allium sativum—powder) for a period of 10 days, the authors reported a significant reduction of 17 mmHg in SBP, but the DBP remained unchanged (Mousa and Mousa, 2007; Table 6). In mechanistic support of the blood pressure measurements, the group exposed human umbilical vein endothelial cells (HUVECs) to a garlic extract, which caused an increase in the bioavailability of NO, a potent vasodilator, by 200% (Mousa and Mousa, 2007). This is thought to occur through the reaction between NO and the sulfide components of garlic (Ku et al., 2002). An ethanolic extract of garlic (0.8 mg/ml) caused relaxation in rat pulmonary arteries pre-contracted with phenylephrine (Ku et al., 2002). Another study has demonstrated that extracts (150 and 400 mg/kg daily) of garlic not only upregulate eNOS, but also induce an increase in eNOS activity in fructose-fed rats (Vazquez-Prieto et al., 2011). In addition, garlic does not merely increase H2S production, but induces its synthesis for vasorelaxant activity (Benavides et al., 2007). In their study, Benavides et al. (2007) demonstrated that red blood cells synthesize H2S from polysulfides that were extracted from garlic. They also reported that garlic (500 μg/ml) and garlic compounds-mediated increase in H2S is correlated with an increase in vasorelaxant activities in rat aortic rings (Benavides et al., 2007). Moreover, treatment with 50 μM garlic-derived DADS induce an increase in expression of Connexin-43 (Cx43), a gap junction protein whose expression is correlated with a reduced VSMC proliferation and DNA synthesis (Joshi et al., 2012; Table 4).
Garlic's ability to inhibit ACE activity has also been recognized (Sendl et al., 1992), and in this regard gamma-glutamyl-cysteines have been identified as the antagonists (Sendl et al., 1992). In addition, a daily dose of 150 and 400 mg/kg of aqueous garlic extracts caused a reduction of VCAM-1 in fructose-fed rats (Vazquez-Prieto et al., 2011). Constituents of Garlic dampen Ang II-induced vasoconstrictor responses, antagonize endothelin-1 induced vasoconstriction, inhibit VSMCs proliferation in smooth muscles isolated from SHR and abrogate the activation of NF-κB (Banerjee et al., 2002; Castro et al., 2010; Pan et al., 2012). These effects are modulated by Allicin after the reaction of Alliin with the enzyme Alliinase (Frishman et al., 2009; Qidwai and Ashfaq, 2013).
Despite having these multifarious therapeutic effects, garlic also produces a few minor side effects. Several articles report garlic's ability to cause abdominal swelling, heartburn, flatulence, and acid reflux (Yang et al., 2011; Ried et al., 2013). Individuals under anticoagulant management are advised to avoid garlic consumption throughout the duration of treatment as the anti-hemostatic effect may be far more potent and detrimental (Qidwai and Ashfaq, 2013).
Andrographis paniculata (King of Bitter)
Andrographis paniculata is a plant that is commonly known as the “King of bitter” (Awang et al., 2012). A. paniculata has been part of eastern and southeastern Asian traditional medicine as a treatment for cold, CVDs (Awang et al., 2012) and fever (Kunwar et al., 2010). It has been shown to possess anti-bacterial, anti-inflammatory (Awang et al., 2012), and antioxidant effects. Several hypotensive labdane-type diterpenoid compounds have been identified in Andrographis paniculatia extracts. These include andrographolide, 14-deoxy-11,12-didehydroandrographolide and 14-deoxyandrographolide (Awang et al., 2012). However, no clinical trials have yet been conducted using A. paniculata.
Treatments with extracts of A. paniculata decrease ACE and ROS activities in spontaneously hypertensive rats (SHR) leading to a decrease in BP (Zhang and Tan, 1996; Table 1). Both 14-deoxy-11,12-didehydroandrographolide and 14-deoxyandrographolide (1 mg/ml in 40% ethanol, dose used: 0.1 mg for each substance) reduce vascular resistance reflected by decreased coronary perfusion pressure (an index of vascular tone) in rat isolated hearts (Langendorff model; Awang et al., 2012; Table 2). Moreover, crude extracts with high content of 14-deoxy-11,12-didehydroandrographolide induced dramatic hypotensive effects (Awang et al., 2012). This was apparently due to increased NO release which consequently induced vasodilation (Awang et al., 2012). In addition, 14-deoxy-11,12-didehydroandrographolide inhibited the rise in intracellular Ca2+ via receptor- and voltage-gated Ca2+ channels (Awang et al., 2012).
In addition to its antioxidant (Lobo et al., 2010) and anti-inflammatory (Kunwar et al., 2010) characteristics, this herb can potently inhibit the activation of NFκB (Das et al., 2012; Table 3). Other reports also indicate that A. paniculata exhibits anti-inflammatory activities in natriuretic peptide receptor-A (Npr1)-gene knockout mice (Das et al., 2012). Indeed, a daily 4 mg/kg dose of andrographolide caused a significant reduction in the production of NF-κB (Das et al., 2012).
Apium graveolens (Celery)
The hypotensive effect of celery has been reported in in vivo animal studies. Seed extracts [300 mg/kg body weight, aqueous-ethanolic (20/80, v/v), hexanic and methanolic] of Apium graveolens reduce blood pressure in deoxycorticosterone acetate-induced hypertensive rats (Moghadam et al., 2013). Hexanic extract was by far the more potent in lowering BP in comparison to other solvent extracts. This was explained by greater retention of n-butylphthalide, which has been identified as the source of celery's flavor and aromatic odor (Moghadam et al., 2013). Similarly, this phthalide was reported to decrease BP in another animal model of hypertension (SHRs; Tsi and Tan, 1997).
Apigenin, a flavone isolate of A. graveolens, blocked aortic ring contractions caused by cumulative calcium increases in high potassium (60 mM) Krebs' solution; this was suggested to result from blocking of Ca2+ influx via calcium channels (voltage and receptor gated; Ko et al., 1991). The inhibition of calcium entry from extracellular sources was also responsible for apigenin-dependent relaxation of noradrenaline-preconstricted rat isolated aorta. This action was not influenced by either methylene blue, endothelial denudation, indomethacin, nifedipine, or caffeine, and the concentration of intracellular signaling molecules (cAMP, cGMP, and inositol monophosphate) were unaltered (Ko et al., 1991). Importantly, extracts and constituents of celery have been reported to lower arterial pressure in humans, possibly by lowering levels of circulating catecholamines and decreasing vascular resistance (Houston, 2005). Interestingly, this herb can reduce oxidative stress by virtue of its flavonoid content that potentiates antioxidant mechanisms (Popovic et al., 2006).
Bidens pilosa L. (Beggar's Tick, Black-Jack, etc.)
This plant belongs to the family Asteraceae and has several common names: beggar's tick, black-jack, and broom stick. In addition to exhibiting antihypertensive effects, B. pilosa also possesses anti-cancer, anti-bacterial, anti-malarial, and anti-obesity properties (Bartolome et al., 2013). As of yet, no clinical trials have determined the potential effect of this plant on hypertension. However, extracts of its leaves were able to prevent and attenuate high blood pressure in different hypertensive rat models (SHRs and fructose-fed hypertensive rats) as well as normotensive Wistar rats (Dimo et al., 2002; Bartolome et al., 2013; Tables 2, 3). In fructose-fed rats, after 6 h of treatment with 75 and 150 mg/kg of methanolic leaf extract of B. pilosa, SBP was reduced by 17 and 21%, respectively (Dimo et al., 2002). Interestingly, the extract also showed preventive effect on SBP by 9 and 11% at 75 and 150 mg/kg, respectively. Using the same animal model, it was also shown that a 3-week treatment with aqueous and methylene chloride extracts of B. pilosa can prevent fructose-induced hypertension (Dimo et al., 2001).
There are conflicting reports regarding B. piloas's effect on insulin sensitivity, with some studies showing improvement in insulin sensitivity (Dimo et al., 2002; Bartolome et al., 2013), while others reporting no effect on plasma insulin concentration (Dimo et al., 2001). However, there is a definite agreement on its vasorelaxant responses (Dimo et al., 2002; Bartolome et al., 2013). Cumulative doses of a neutral extract of B. pilosa (with an optimum concentration of 0.32 mg/ml) relaxed potassium chloride and noradrenaline pre-constricted rat aortas (Nguelefack et al., 2005). The mechanism of vasodilation has not been completely deciphered. It appears to be independent of ATP-sensitive potassium channels, but possibly involves a calcium channel antagonism and cyclooxygenase metabolite (Nguelefack et al., 2005). Importantly, the crude extract, its fractions or the isolated phytochemicals of B. pilosa have been reported to display radical scavenging ability (Bartolome et al., 2013). Two of these compounds, luteolin (a flavonoid) and ethyl caffeate (ester of hydroxycinnamic acid), exhibit potent anti-inflammatory activities. For example, luteolin blocked the effects of inflammatory cytokines, TNF-α, and IL-6 (Xagorari et al., 2001). Moreover, both luteolin (Xagorari et al., 2001) and ethyl caffeate (Chiang et al., 2005) can inhibit the pro-inflammatory transcription factor, NF-κB.
Camellia sinensis (Tea)
Collectively, the teas prepared from Camellia sinensis are the most frequently consumed beverages and are second only to water, worldwide (Faria et al., 2012). Tea has pleiotropic effects including antibacterial, anti-inflammatory (Deka and Vita, 2011), anti-cancer, anti-diabetic, as well as antihypertensive actions (Deka and Vita, 2011; Table 1). The data on the hypotensive effect of tea are not concrete. Hodgson et al. reported increases in BP after 30 min of consuming tea, with the BP returning to its baseline value after 60 min (Hodgson et al., 1999). Another study suggested that green tea causes a decrease in SBP for participants with BP of 140 mmHg or higher (Nagao et al., 2007). A meta-analysis of five trials came to conclusion of no change in blood pressure subsequent to drinking tea (Taubert et al., 2007). However, a more recent meta-analysis study of randomized controlled trials demonstrated that green tea reduces both SBP and DBP by 1.98 and 1.92, respectively (Peng et al., 2014). Likewise, other meta-analysis of randomized controlled trials came to the same conclusion that green tea reduces SBP and DBP by 1.8 and 1.4 mmHg, respectively. Interestingly, it was reported that green tea evoked a more powerful hypotensive effect compared to black tea, and that long-term tea consumption produced a more significant SBP and DBP reduction. Moreover, in a double-blind, placebo-controlled trial, obese hypertensive patients who received 379 mg green tea extract for 12 weeks exhibited a significant decrease in SBP and DBP by 4 mmHg each (Bogdanski et al., 2012). Another randomized double-blind, placebo-controlled trial concluded that hypertensive subjects who consumed 4479 mg (3 cups/day, 1493 mg each) of black tea for 24 weeks exhibited a significant reduction in both SBP and DBP by 2 and 2.1 mmHg, respectively (Hodgson et al., 2012). It is important to note here that ingestion of green or black tea (7.6 g in 400 ml water) by mild hypertensive subjects did not decrease blood pressure. On the contrary, green or black tea ingestion caused a non-significant increase in SBP/DBP by 1.7/0.9 and 0.7/0.7 mmHg, respectively (Hodgson et al., 1999).
Catechins, the major flavonoids in tea, include (−)-epicatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin-3-gallate (EGCG; Deka and Vita, 2011). EGCG constitutes the primary component of tea's total catechins (Babu and Liu, 2008; Faria et al., 2012; Slevin et al., 2012; Thomson et al., 2012). Constituents of tea lessen the risk of hypertension through several mechanisms, such as attenuation of oxidative stress (Table 1). Green tea has been reported to increase CAT antioxidant enzyme while simultaneously blocking AT1 receptors in streptozotocin-treated rats (Thomson et al., 2012). It has also been reported to upregulate the expression of antioxidants genes such as SOD1 and GST in C57BL/6 mice (Newsome et al., 2014). Another mechanism for oxidative stress reduction by tea is the inhibition of eNOS uncoupling (Faria et al., 2012). In addition, green tea has the capacity to scavenge superoxides (Nakagawa and Yokozawa, 2002) in vitro as well as attenuate NAPDH oxidase production (Ribaldo et al., 2009) in diabetic SHRs.
Flavonoids are noted for their vasorelaxant responses, including flow-mediated (Ras et al., 2011), and endothelial-dependent dilation (Oyama et al., 2010; Table 2). Black tea catechins are converted by an enzymatic (polyphenol oxidase and peroxidase) oxidative polymerization reaction to tannins: theaflavins (benztropolone ring) and thearubigins, both of which are orange-red colored polyphenolic pigments that are also potent vasodilators (Yang et al., 2011). A couple of clinical studies have reported black tea's positive effect on flow-mediated dilation (FMD), an index of endothelial function (Duffy et al., 2001; Hodgson et al., 2002). In subjects with moderately elevated cholesterol and/or triglyceride levels, consumption of black tea increased FMD in brachial arteries (Hodgson et al., 2002) as well as in patients with coronary arterial disease (450 mL of tea or water—short-term, after 2 h; and 900 mL of tea or water/day for 4 weeks long-term, n = 50 subjects; Duffy et al., 2001). In a clinical trial, Oyama et al. (2010) used venous occlusion strain-gauge plethysmography to demonstrate the beneficial effects of green tea catechins (580 mg/day for 4 weeks) on blood flow in forearms of smokers. The data revealed a significant increase in blood flow due to an augmented release of NO with a concomitant decrease in levels of both asymmetrical dimethylarginine (an endogenous inhibitor of eNOS) and oxidative stress (Oyama et al., 2010; Table 6).
The bioactive components of tea are reported to express anti-inflammatory properties (Table 3), reflected by mitigated expression and release of different cytokines. In a clinical double-blind, placebo-controlled study on 56 obese, hypertensive males and females, green tea extract (379 mg/day for 3 months) caused a decrease in BP as well as TNF-α levels (Bogdanski et al., 2012). In other studies, EGCG (Ludwig et al., 2004) and theaflavin (Lü et al., 2005) derived from tea have been reported to reduce VCAM-1 levels. In addition, EGCG was able to inhibit NF-κB activation in human endothelial cells (Hong et al., 2007). Interestingly, EGCG derived from green tea also elicited a concentration-dependent inhibition of proliferation in human aortic VSMCs by increasing HO-1 enzyme expression (Liu et al., 2014).
Coptis chinensis (Goldthread)
Coptis chinensis, commonly known as Chinese goldthread, is widely used in Chinese folk medicine (Affuso et al., 2010). Evidence indicates that goldthread, and its main component Berberine (BBR), have the ability to lower blood pressure (Affuso et al., 2010; Xiong et al., 2013). Indeed, a recent meta-analysis of twenty-seven randomized controlled trials involving 2569 patients reported that BBR can cause a significant hypotensive effect (Lan et al., 2015). In addition, this meta-analysis concluded that combined with an oral hypotensor, BBR can significantly reduce BP more than the hypotensor alone can do. The magnitude of the decrease was determined to be an average of 4.91 and 2 mmHg for SBP and DBP, respectively (Lan et al., 2015).
Several mechanisms have been proposed for Chinese goldthread's antihypertensive effect. One mechanism appears to be via amelioration of oxidative stress (Zhang et al., 2011; Wan et al., 2013; Table 1). Indeed, BBR (150 mg/kg) is reported to scavenge ROS, inhibit NADPH oxidase (Wan et al., 2013), and increase the antioxidant enzyme, SOD, in rats with atherosclerotic renovascular disease.
Constituents of goldthread also act by relaxing arterial tissues through endothelial-dependent and independent routes (Affuso et al., 2010). In chronic intermittent hypobaric hypoxic and normoxic animal models, goldthread has been shown to relax NE-induced contractions in rat isolated thoracic aortic rings (Zhang et al., 2011). The same authors also reported BBR's vasorelaxant activity on KCl-induced contractions using the same models (Zhang et al., 2011). Apparently, BBR elevates the expression of eNOS with a concomitant rise in NO release that leads to enhanced flow-mediated vasodilation (Affuso et al., 2010; Zhang et al., 2011). This dilation is likely mediated by the vasodilator PGI2 as well as the opening of KATP channels and blockage of Ca2+ influx (Zhang et al., 2011). In a clinical study, BBR (1.2 g/day for 1 month) decreased the formation of endothelial microparticles (EMPs) which are known to induce endothelial dysfunction and pro-coagulant activity in healthy humans (Wang et al., 2009; Affuso et al., 2010).
In addition, BBR isolate of Chinese goldthread inhibits endothelial injury (Wang et al., 2009) modulates inflammatory pathways through suppression of transcription factor NF-κB, VCAM-1 expression, VSMC proliferation (Affuso et al., 2010; Wan et al., 2013; Table 4). It also improves lipid profile by reducing total and LDL cholesterol, and cardiac muscle hypertrophy (Zhang et al., 2011).
Coriandrum sativum (Cilantro or Coriander)
In several countries, coriander (also known as cilantro or dhania) is not only used as a culinary ingredient (Anilakumar et al., 2010; Wu et al., 2010) but also as a traditional medicine for the treatment of cardiovascular and gastrointestinal diseases (Jabeen et al., 2009).
To the best of our knowledge, no clinical trials have been conducted to assess coriander's effects on BP. However, coriander has been reported to exhibit antioxidant properties (Sreelatha et al., 2009; Cioanca et al., 2013; Ramkissoon et al., 2013). In isoproterenol-induced myocardial infarction (MI) model of cardiotoxicity, coriander extract (200 and 300 mg/kg) inactivated β-adrenoceptor-induced ROS production and also prevented MI by inhibiting myofibrillar damage (Patel et al., 2012; Table 1). Coriander leaves' extracts also increased antioxidants enzymes (Sreelatha et al., 2009) and its seeds' powder (5 and 10%) showed similar effect on the antioxidant GPX (Anilakumar et al., 2010). Other studies have also reported similar antioxidant activities of coriander (Cioanca et al., 2013; Ramkissoon et al., 2013).
Vasodilatory effects of coriander are well-established. Indeed, intravenous application of aqueous methanolic extract of dried, ground coriander seeds (1–30 mg/ml) produced a dose-dependent fall in SBP, DBP, and mean arterial blood pressure (MABP) in normotensive Sprague-Dawley rats by 40.84 ± 6.34% (Jabeen et al., 2009). The same report also showed that coriander fruit extracts produced dose-dependent relaxation of pre-constricted (phenylephrine and potassium chloride) rabbit aortas, and this response was atropine and calcium-channel dependent (Jabeen et al., 2009). Further, the same extracts showed diuretic affects as well. The active component of which should act synergistically with the vasoactive constituent to complement the treatment and management of hypertension (Jabeen et al., 2009). Moreover, coriander acts as an inhibitory agent to reduce the activities of NF-κB and iNOS (Wu et al., 2010).
Crataegus spp. (Hawthorns)
Hawthorns (hawberry or thorn apple) plants are shrubs that belong to a genus comprising almost 300 species (Tassell et al., 2010) that have been used in traditional medicine for thousands of years (Tassell et al., 2010; Asher et al., 2012). Hawthorns have been used for treatment of CVDs since the seventeenth century (Asher et al., 2012).
Modest decreases in blood pressure have been observed in a few human-based studies with a demographic of hypertensive patients (Walker et al., 2002; Tassell et al., 2010). In a randomized, double-blind, placebo-controlled study where mildly hypertensive subjects were treated with 500 mg of hawthorn extract for 10 weeks, a promising tendency for a reduction in DBP (by 13.1 mmHg) was reported (Walker et al., 2002). It is argued that the dose and duration were not sufficient for a more effective result to be noted. Indeed, in phytotherapy practice, a significant decrease in BP is only noted after a longer duration and higher doses (Bone and Mills, 2013), In another randomized, double-blind, placebo-controlled clinical study, administration of hydro-alcoholic extracts of Crataegus curvisepala Lind flowers to hypertensive patients (age range 40–60 years) for 3 months induced a decrease in both SBP and DBP by around 13 and 8 mmHg, respectively (Asgary et al., 2004).
The aforemnetioned antihypertensive actions are credited to the plant's multiple components: flavonoids (hyperoside, quercetin, rutin, and vitexin) and oligomeric proanthocyanidins (OPCs, epicatechin, procyanidin, and procyanidin B-2; Valli and Giardina, 2002; Houston, 2005; Asher et al., 2012; Yang and Liu, 2012). Quercetin, a major polyphenolic flavonoid in hawthorn shrubs, expresses numerous bioactive functions including anti-oxidant, anti-inflammatory, and vasorelaxant effects. Quercetin supplement intervention studies have demonstrated a reduction in blood pressure of hypertensive subjects (Larson et al., 2012). Interestingly, hawthorn extracts have an effect on both VSMCs and endothelial cells (Tassell et al., 2010). The latter interaction is attributable to increased NOS activity and hence NO release (Brixius et al., 2006; Anselm et al., 2009), possibly due to an enhanced phosphorylation of eNOS at serine 1177 (Brixius et al., 2006; Anselm et al., 2009; Table 2). In porcine isolated coronary arterial rings, WS 1442 (an extract of Crataegus leaves with flowers) induced endothelium-dependent relaxation through the activation of multiple signaling pathways, including ROS, Src, PI3-kinase, Akt, and eNOS (Anselm et al., 2009). Moreover, WS 1442 caused endothelium-dependent and NO-mediated vasorelaxation of phenylephrine-preconstricted rings of rat aorta as well as human internal mammaria (Brixius et al., 2006). In the L-NAME-induced hypertension model, the blood pressure decreased after the administration of Crataegus tanacetifolia leaf extract or its isolate, hyperoside. Both C. tanacetifolia extract and hyperoside display protective effects at multiple levels. These include improving hyperlipidemia, decreasing body weight, resolving hyperplasia, reducing thickness of the vascular medial layer as well as improving kidney function. Such effects appear to be mediated by an increase in diuretic activity, efflux of water and sodium, as well as expression of NOS enzyme. Together, all of these mechanistic actions contribute to the amelioration of hypertensive outcome (Koçyildiz et al., 2006).
As part of the integrated cardiovascular beneficial bioprocesses, hawthorn has the capacity to scavenge ROS (Tassell et al., 2010; Cheng et al., 2013; Table 1), up-regulate antioxidant enzymes (SOD, CAT) and augment the concentration of the reducing glutathione (GSH; Tassell et al., 2010). Moreover, hawthorn extracts express anti-inflammatory activity, which is mirrored by the decline in concentrations of NF-κB, TNF-α (Topal et al., 2013), VCAM-1 (Shin et al., 2012), iNOS, and IL-6 (Topal et al., 2013).
Crocus sativus (Saffron)
Saffron (common name), a plant indigenous to Southwest Asia (Iran, Pakistan, and India), Spain, Greece, and Morocco, is a stemless herb whose medicinal values have been sought for over 4000 years (Srivastava et al., 2010). Saffron's main components include crocin, picrocrocin, safranal, and crocetin (Srivastava et al., 2010; Mehdizadeh et al., 2013) and these molecules exhibit different mechanisms of action (Mokhtari-Zaer et al., 2015).
Several reports support the use of saffron for anti-hypertensive benefits. A clinical study reported that 400 mg of saffron tablets administered for 7 days were able to significantly reduce the SBP and mean arterial pressure in healthy humans by 11 and 5 mmHg, respectively (Modaghegh et al., 2008). Saffron demonstrates vasorelaxant activities in different animal models. Extracts of C. sativus petals (rich in flavonoids and anthocyanins) dose-dependently reduced the BP of male Sprague-Dawley rats, possibly by modulating peripheral vascular resistance (Fatehi et al., 2003). Moreover, extract of C. sativus stigma (10 mg/kg), and two of its primary components [Crocin (200 mg/kg) and Safranal (1 mg/kg)], attenuated MABP in normotensive and desoxycorticosterone acetate (DOCA)-salt induced hypertensive male Wistar rats (Imenshahidi et al., 2010). More recently, it was shown that chronic administration of safranal (1, 2, and 4 mg/Kg/day) reduced SBP in DOCA-salt hypertensive but not normotensive rats (Imenshahidi et al., 2015).
Saffron relaxes non-vascular muscles as well. Indeed, extracts of saffron decreased contractility and heart rate of guinea-pig isolated perfused hearts (Langendorff procedure) by blocking Ca2+ channels, opening potassium channels, and antagonizing β-adrenoceptors (Boskabady et al., 2008; Table 2). In addition, safranal (0.1–0.5 mL/kg daily) offers protection in a rat model of myocardial ischemia-reperfusion injury via enhanced phosphorylation of protein kinase B (Akt)/glycogen synthase kinase-3β (GSK-3β)/eNOS pathway, attenuation of IKK-β/NF-κB activity, normalization of the antioxidant reserve and up-regulation of the anti-apoptotic route (Bharti et al., 2012; Table 2).
Saffron's antioxidant ability has also been widely reported. It was shown to reduce oxidative stress (El-Beshbishy et al., 2012; Mehdizadeh et al., 2013) and increase antioxidant enzymes, such as SOD (Premkumar et al., 2003; El-Beshbishy et al., 2012), CAT, GPX, and reduced GSH (Premkumar et al., 2003;Table 1). 20–80 mg/kg of saffron's aqueous extracts were able to increase antioxidants levels in genotoxin-treated mice (Premkumar et al., 2003). Crocin treatment (200 mg/kg for 7 days) of beryllium chloride-induced model of oxidative stress in male Wistar rats resulted in a significant decline in oxidative stress and the corresponding up-regulation of antioxidant enzymes (El-Beshbishy et al., 2012). Moreover, saffron and its constituents also possess an inherent ability to inhibit inflammatory pathways, including NF-κB (Nam et al., 2010; Bharti et al., 2012) and TNF-α expression (Nam et al., 2010).
Cymbopogon citratus (Lemongrass)
Lemongrass (common name) has been widely used in traditional medicine of Brazil, China, and Southern Asia (Devi et al., 2012). It has been reported to possess antihypertensive properties, which have been attributed to its active phytochemicals, the principle of which being Citral (Devi et al., 2011, 2012). However, no clinical trials have yet investigated the effect of Lemongrass on BP.
The relaxant effect of lemongrass has been demonstrated in several different tissues, including the rabbit ileum (Devi et al., 2011), rat aortic rings (Devi et al., 2012), and the rat mesentery (Bastos et al., 2010; Table 2). For instance, Citral or crude extracts of C. citratus (leaves, stems, or roots) generated a dose-dependent vasorelaxation in phenylephrine pre-constricted aortic rings from male WKRs or SHRs (Devi et al., 2012). Further, the underlying mechanism for this relaxation appeared to be mediated by activation of NO and/or the inhibition of calcium channels (Devi et al., 2012). Likewise, administration of an intravenous bolus of Citronellol, an acyclic monoterpenoid isolated from lemongrass, to male Wistar rats produced a hypotensive response. This hypotensive effect was not affected by L-NAME, indomethacin, atropine, or hexamethonium (Bastos et al., 2010). Citronellol also induced relaxation of rat superior mesenteric artery via an endothelium-independent mechanism. Moreover, arteries denuded of endothelium were not reliant on tetraethylammonium-dependent potassium channels. Rather, citronellol acted by inhibiting Ca2+-influx through voltage operated calcium channels (VOCCs) as well as regulating IP3- and caffeine-gated intracellular Ca2+ stores (Bastos et al., 2010).
Lemongrass is known to display moderate antioxidant activity. In male rats treated with H2O2, 100 mg/kg of lemongrass was able to reduce oxidative stress and increase GSH expression in testes (Rahim et al., 2013). Results from another study showed an increase in antioxidant enzymes and molecules such as SOD and GSH in murine lungs after administering 5 and 10 μg of lemongrass' extracts (Tiwari et al., 2010). Lemongrass was reported to suppress ROS molecular activity (Tiwari et al., 2010; Koh et al., 2012). In addition, lemongrass' Citral contributes to the anti-inflammatory pathways by inhibiting NF-κB (Lee et al., 2008; Francisco et al., 2013) and iNOS activity (Lee et al., 2008; Figueirinha et al., 2010).
Hibiscus sabdariffa (Roselle)
Hibiscus, widely known as roselle, is used for hypertension, fever, and other diseases in folk medicine. Different parts of this plant (buds, calyx, flowers, leaves, and petals—fresh or dried) are used for health purposes and as refreshing beverages, food items (jams, preserves), or lotions.
Roselle's blood pressure lowering effects have been extensively reported in both animal (Odigie et al., 2003; Ali et al., 2005; Ajay et al., 2007; Mojiminiyi et al., 2007; McKay et al., 2010; Ojeda et al., 2010; Inuwa et al., 2012; Hopkins et al., 2013) and human studies (Onyenekwe et al., 1999; Herrera-Arellano et al., 2004, 2007; Mojiminiyi et al., 2007; Mozaffari-Khosravi et al., 2009; Inuwa et al., 2012; Hopkins et al., 2013). In a randomized, double-blind, Lisinopril-controlled clinical trial, antihypertensive effects were notable subsequent to treatment with dried extract of calyx (250 mg) for 4 weeks in patients with stage 1 or 2 hypertension (Herrera-Arellano et al., 2007; Table 6). Indeed, a drop of BP from 146.48/97.77–129.89/85.96 mmHg was noticed (Herrera-Arellano et al., 2007). In yet another randomized controlled trial, hypertensive patients ingested 10 g/day of Roselle's calyx. After 4 weeks, a significant decrease in SBP and DBP by 15.32 and 11.29 mmHg, respectively was reported (Herrera-Arellano et al., 2004). Additional support for hibiscus' therapeutic role in ameliorating hypertension is provided by a report which shows that in mild and pre-hypertensive patients (65 subjects—30–70 years old), consuming roselle's tea (240 ml—three times a day for 6 weeks) significantly reduces SBP, DBP, and MAP by 7.2, 3.1, and 4.5 mmHg, respectively (McKay et al., 2010).
Different mechanisms for roselle's antihypertensive effect have been reported. Roselle primes the vasorelaxant pathways of both endothelial cells (Ajay et al., 2007; Herrera-Arellano et al., 2007) and VSMCs (Ali et al., 2005; Ajay et al., 2007). Its relaxant effect is mediated through an increased production of NO (Ajay et al., 2007; Alarcon-Alonso et al., 2012), inhibition of Ca2+ channels (Ajay et al., 2007) and opening of KATP channels (Sarr et al., 2009). Additionally, it has been shown to inhibit cardiac hypertrophy (Odigie et al., 2003; Inuwa et al., 2012), and decrease heart rates in rats (Odigie et al., 2003).
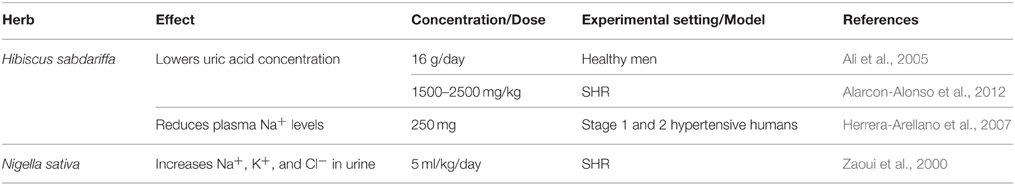
A strong body of evidence is reported to support roselle's ability as a diuretic agent (Onyenekwe et al., 1999; Ali et al., 2005; Herrera-Arellano et al., 2007; Alarcon-Alonso et al., 2012). One clinical study detected a lower concentration of uric acid in urine of healthy humans consuming roselle (Ali et al., 2005). Another clinical study reflected the decrease in blood sodium content of stage 1 and 2 hypertensive humans after 4 weeks administration of 250 mg of roselle's extracts (Herrera-Arellano et al., 2007), thus verifying its multiple diuretic properties. This diuretic activity is related to the vasorelaxant effect, as NO elevation is positively correlated with increases in renal filtration rates (Alarcon-Alonso et al., 2012).
Roselle exhibits potent antioxidant function (Ali et al., 2005; McKay et al., 2010). Its anthocyanin extracts (2 mg/ml) were reported to reduce oxidative stress, potentially by scavenging free radicals in livers of CCl4-treated rats (Ajiboye et al., 2011). In a clinical study, roselle's aqueous extracts enhanced the concentration of cellular antioxidants in healthy humans (Frank et al., 2012; Table 1). Moreover, it blocks the oxidation of LDL, indicating its role as an anti-atherogenic (Lin et al., 2011). Roselle also shows anti-inflammatory capacity by inhibiting not only ACE activity (Herrera-Arellano et al., 2007; Ojeda et al., 2010) but also the proliferation of VSMCs (Lin et al., 2011). The effect of roselle on ACE activity was confirmed in a Lisinopril-controlled clinical trial, where 250 mg of its extract was administered to patients with stage 1 or 2 hypertension (Herrera-Arellano et al., 2007).
Nigella sativa (Black Cumin; Seed of Blessing)
Black cumin, also known as Habbatul barakah (seed of blessing), has been used in the kitchens of Europe, the Middle East, Africa, and South and Southwest Asia for centuries (Ahmad et al., 2013; Leong et al., 2013; Tables 2, 5). In addition to its antihypertensive role, black cumin is also effective against diabetes and gastrointestinal diseases (Leong et al., 2013). Thymoquinone (TQ), one of the most abundant and bioactive components in Nigella sativa's seeds, has been identified as the major element in its healing effects (Ahmad et al., 2013).
N. sativa, and its constituents, have been reported to lower blood pressure in humans and in different animal models of hypertension (Zaoui et al., 2000; Khattab and Nagi, 2007; Dehkordi and Kamkhah, 2008; Fallah Huseini et al., 2013) and also decrease heart rate (Leong et al., 2013). For example, L-NAME-induced hypertension in rats was ameliorated by concomitant treatment with thymoquinone (0.5 and 1 mg/kg/day for 4 weeks; Khattab and Nagi, 2007). Improvement in renal function and antioxidant activity were also noted, evident by the reduced serum creatinine or increased glutathione in control vs. L-NAME treated rats, respectively (Khattab and Nagi, 2007). Likewise, in SHRs, an oral dose of dichloromethane extract of black cumin seeds (0.6 ml/kg/day for 15 days) reduced mean arterial pressure by 22% and increased diuretic activity (Khattab and Nagi, 2007), mirrored by urinary excretion of urea, Cl−, Na+, and K+ (Zaoui et al., 2000; Table 5).
A randomized, double-blind placebo-controlled clinical trial of the effects of N. sativa's seed extract administrated orally (either 100 or 200 mg, two times per 24 h for 8 weeks) to mild hypertensive male patients recorded a dose-dependent fall in both SBP and DBP, in the two treated groups compared to placebo (Dehkordi and Kamkhah, 2008; Table 6). For the 200 mg dose, there was a decrease of around 2.2 and 2 mmHg in SBP and DBP, respectively. Moreover, the NS's extract reduced total cholesterol as well as low density lipoprotein (LDL)-cholesterol relative to pretreatment concentrations (Dehkordi and Kamkhah, 2008). Again, in a randomized, placebo-controlled, double-blind study with 70 healthy subjects, N. sativa oil caused a significant decrease of 10.6 and 9.6 mmHg, respectively in both SBP and DBP (Fallah Huseini et al., 2013).
Another route by which black cumin attenuates hypertension is by vasorelaxant means. This is illustrated by its ability to inhibit Ca2+ channels (voltage-gated and ligand-gated) leading to concomitant relaxation of rat aorta (Leong et al., 2013). Moreover, TQ is reported to inhibit the release of vasoconstrictor metabolites of COX-1 (Ahmad et al., 2013) and COX-2 (Ahmad et al., 2013; Kundu et al., 2013).
TQ's antihypertensive effects are partly due to its antioxidant activities as it lowers oxidative stress (Khattab and Nagi, 2007; Ahmad et al., 2013). Another mechanism that may explain the hypotensive effect of black cumin pertains to its diuretic action (Zaoui et al., 2000; Ahmad et al., 2013; Leong et al., 2013). This has been highlighted by the ability of black cumin seeds to increase urea, calcium, sodium, and potassium in urine of rats (Zaoui et al., 2000; Leong et al., 2013), where it also increases renal filtration and urinary output (Leong et al., 2013). Yet another mechanism that underlies black cumin's actions is its anti-inflammatory property since it inhibits the generation of TNF-α and NF-κB (Kundu et al., 2013).
Panax (Ginseng)
For centuries, the species Panax, especially the Asian variety, has been used in folk medicine (Jang et al., 2011; Kim, 2012). Ginseng is prepared and administered in various forms, either as a solid: tablets, capsules, dried roots; or as a liquid: oil, extracts or tea (Valli and Giardina, 2002). P. ginseng (Asian or Korean ginseng), P. quinquefolius (American ginseng), P. japonicas (Japanese ginseng) and P. notoginseng (Chinese ginseng) are the four most common species of ginseng (Valli and Giardina, 2002; Kim, 2012). Interestingly, these—as a group—have the most reported hypotensive effects. Heterogeneous triterpenoid saponins and steroid glycosides or ginsenosides (or panaxosides) are the active principle components of ginseng (Valli and Giardina, 2002; Kim, 2012). In addition to anti-hypertensive effects, ginseng also plays anti-carcinogenic and antidiabetic roles (Choi et al., 2013).
Although ginseng's blood pressure lowering effect is widely reported (Jeon et al., 2000; Valli and Giardina, 2002; Jang et al., 2011; Kim, 2012; Mucalo et al., 2013; Tables 1, 2), conflicting reports of elevated blood pressure also exist (Valli and Giardina, 2002; Jang et al., 2011; Kim, 2012). Contextually, low doses of ginseng raise BP, while higher concentrations are hypotensive (Jang et al., 2011). A probable explanation for this phenomenon is the varied action of different ginsenosides (Valli and Giardina, 2002). Interestingly, Kim (2012) has used the term “normalize” to describe hypotensive and hypertensive actions of ginseng. Therefore, these studies suggest that ginseng rheostatically adjusts the BP level in hypotensive patients (Kim, 2012), possibly by tuning vascular function, modulating the autonomic nervous system, or regulating the arterial baroreflex.
Several clinical trials have been conducted to assess the efficacy of ginseng in modulating BP (Table 6). In one trial, P. ginseng extract rich in ginsenoside protopanaxatriol (300 mg/day) was administered to mild hypertensive patients and caused a significant decrease of 3.1 and 2.3 mmHg in SBP and DBP, respectively (Rhee et al., 2014). Furthermore, a randomized, double-blind, placebo-controlled trial where hypertensive patients ingested American ginseng (3 g/day) for 12 weeks showed that SBP was significantly lowered by 11.7% (17.4 mmHg; Mucalo et al., 2013). Along the same line, another randomized, double-blind, crossover trial reported that central SBP and DBP of healthy subjects was significantly reduced by 4.8 ± 6.8 and 3.6 ± 6.4 mmHg, respectively, after hours of ingesting Ginsenoside Rg3-enriched P. ginseng (400 mg; Jovanovski et al., 2014).
The primary mechanism associated with ginseng-induced hypotensive effect is attributed to an improvement in arterial function. Indeed, ginseng causes a dramatic increase in eNOS expression and NO production (Valli and Giardina, 2002; Jang et al., 2011, 2012; Hong et al., 2012; Pan et al., 2012; Table 2). Ginsenoside Rg3 (red ginseng) is known to activate eNOS (Valli and Giardina, 2002; Jang et al., 2011), increase NO and cGMP levels, as well as activate Ca2+-gated potassium channels (Kim et al., 1999b). Ginsenosides mediate vasorelaxation of different vessels in different animals: rat aortas (Kim et al., 1999a), murine coronary arteries (Pan et al., 2012), and monkey cerebral arteries (Toda et al., 2001). In the same context, this relaxation may be aided by ginseng's ability to diminish secretion of adrenal catecholamines in hypertensive rats (Jang et al., 2011).
Ginseng also elicits an anti-proliferative effect on VSMCs, and hence, it can be expected to possess antihypertensive and anti-atherosclerotic capacities (Table 4). Importantly, results of a clinical trial indicate that administering 3 g/day of P. quinquefolius for 12 weeks improves arterial stiffness in hypertensive patients (Mucalo et al., 2013; Table 6). Further support for this beneficial effect of ginseng comes from another clinical trial, where taking 400 mg of P. ginseng caused a significant reduction in aortic Alx, a marker of arterial stiffness (Jovanovski et al., 2014; Table 6). It has been reported that Chinese ginseng blocks the activation of extracellular signal-regulated protein kinases (ERK) pathway, and therefore inhibits PDGF-induced VSMCs proliferation (Zhang et al., 2012). Moreover, Chinese and other ginsengs reduced vascular aging of SHRs compared to WKY rats; this was reflected by inhibition of proliferation of VSMCs isolated from both WKY and SHRs. The mechanistic pathway for the effect of ginseng on SHR VSMCs entailed the decrease in the number of senescence-associated β-galactosidase (SA-β-gal) positive cells. In the SHR, expression of p16, and retinoblastoma protein (Rb) was elevated whereas that of cyclin D1 and cyclin-dependent kinase 4 (CDK4) was decreased (Tao and Lei, 2012). Further, red ginseng also attenuated Ang II-induced VSMC growth (Kim, 2012).
Amongst ginseng's other hypotensive mechanisms is its antioxidant ability (Table 1). In this regard, it has been reported that ginsenoside Rg1 (60–120 μM) inhibits oxidative stress (Doh et al., 2013), possibly by increasing antioxidant enzymes (Zhu et al., 2009) and scavenging free radicals (Zhu et al., 2009). In addition to its antioxidant effect, ginseng exhibits anti-inflammatory properties. Indeed, Asian ginseng (red) inhibits the release of TNF-α (Kim, 2012) as well as attenuates NF-κB and p38 mitogen activated protein kinase (MAPK) pathways (Bak et al., 2012). It has been suggested that the consequence of this effect is a reduction in the vasoconstrictor activity of COX-2 enzyme (Bak et al., 2012; Table 3).
Salviae miltiorrhizae (Chinese Sage)
Salviae miltiorrhizae, known as danshen or red/Chinese sage, is one of the oldest and most frequently consumed Chinese traditional herbs (Cho et al., 2013; Jiang et al., 2013a) and is commonly used for the treatment of CVDs (Ng et al., 2011; Cho et al., 2013; Jiang et al., 2013a). Danshen's most effective components include: salvianolic acid A (SalA), salvianolic acid B (SalB), danshensu, and tanshinones (Ng et al., 2011; Jiang et al., 2013a).
Danshen relaxes the vasculature via endothelium-dependent and endothelium-independent mechanisms. A combination treatment of danshen and gegen (Pueraria lobata) was shown to lower blood pressure in SHRs (Ng et al., 2011) and to induce relaxation of porcine coronary arteries (Hu et al., 2012), rat aorta (Ng et al., 2011), and basilar arteries (Lam et al., 2010; Table 2). Similarly, dihydrotanshinone (lipophilic constituent of danshen) is a vasorelaxant of rat coronary arteries (Lam et al., 2008). Danshen's endothelium-dependent relaxations occur via an NO-dependent mechanism (Chan et al., 2011; Ng et al., 2011; Shou et al., 2012). SalB-derived vasodilation in rabbit aorta is mediated by the NO-sGC-cGMP pathway (Shou et al., 2012). Other studies have also demonstrated that L-NAME inhibits eNOS and blocks the activity of danshen (Chan et al., 2011; Shou et al., 2012), further verifying the NO-dependent mechanism of vasodilation. In VSMCs, danshen exhibits its vasodilating effect by opening of KATP, Kir, and Kv channels (Ng et al., 2011; Jiang et al., 2013a) as well as blocking the Ca2+ influx (Lam et al., 2008; Hu et al., 2012).
Apart from its vasodilatory capacity, danshen expresses additional anti-hypertensive parameters such as antioxidative, anti-proliferative, and anti-inflammatory activities. Its extracts have been shown to decrease ROS production in rat thoracic aorta (Cho et al., 2013). In a randomized, placebo-controlled clinical study involving chronic heart disease patients, danshen's hydrophilic extract (5 g/twice a day/60 days) increased antioxidative enzymes like CAT, SOD, and the tripeptide glutathione (Qian et al., 2012; Table 1). Danshen was also reported to inhibit PDGF-induced proliferation of VSMCs (Cho et al., 2013; Table 4). The anti-inflammatory capacity of danshen was demonstrated by virtue of its ability to inhibit TNF-α, NF-κB production, and VCAM-1 expression (Cho et al., 2013) in HUVECs (Table 3). Taken together, these results illustrate the underlying molecular mechanism for danshen's antihypertensive effect.
Zingiber officinale (Ginger)
Ginger, a very common culinary ingredient, is reported to possess hypotensive properties. In a clinical study, oral (70–140 mg/kg) or intravenous (1.75–3.5 mg/kg) administration of two bioactive constituents of ginger, namely (6)-gingerol and (6)-shogoal, produced triphasic blood pressure profiles: initial rapid fall, intermediate rise, and finally a delayed decrease in BP (Suekawa et al., 1984). Indeed, [6]-gingerol is now considered a novel angiotensin II type 1 receptor antagonist with an IC50 of 8.17 × 10−6 M (Liu et al., 2013).
The aqueous extract of ginger (0.05 mg/ml) has also been reported to inhibit lipid peroxidation as well as ACE in rat hearts (Akinyemi et al., 2013). In addition, zingerone, another active compound in ginger, can potently scavenge oxidant molecules like peroxynitrite (Shin et al., 2005). Recently, it was found that ginger not only reduces levels of total cholesterol, triglyceride, LDL, and vLDL, but it can also inhibit ACE-1 activity (Akinyemi et al., 2014).
Conclusion
With CVD remaining as a leading cause of worldwide mortality, the search for more effective treatments ought to be of prime importance. An approach that appears promising is CAM (Frishman et al., 2009; Su and Li, 2011; Orekhov et al., 2013). Not surprisingly, of all small-molecule new chemical entities introduced as drugs during the last three decades, a significant fraction were either obtained from or inspired by nature (Newman and Cragg, 2012). Perhaps this could explain, at least partly, the “phenomenon” that more American patients visit CAM providers than primary care physicians (Eisenberg et al., 1998; Tachjian et al., 2010). Of relevant interest, it is important to note that herbal consumption appear to be the most common type of CAM among CVD patients (Yeh et al., 2006).
In this first part of our review, we discussed the mechanisms of action of several plants that are most commonly used in the treatment or management of hypertension.
The evidence presented is strongly indicative of the notion that herbs and plants are becoming part of evidence-based medicine in the prevention and/or treatment of CVD. The pharmacological actions of herbs or herbal isolates appear to favorably modulate several parameters implicated in the pathogenesis of blood pressure, including but not limited to ROS production, VSMC phenotype, endothelial function, platelet activation, pro-inflammatory signaling, and gene expression. With such a broad spectrum of actions, one may predict that herbal remedies will receive even more attention in the coming years, perhaps accentuating the need for further experimentations and clinical trials. Indeed, the lack of sufficient clinical trials constitutes a significant limitation on their use at the present time. Of equal importance, it may be advisable that patients be appropriately educated, particularly in relation to herbs whose consumption has been considered safe for thousands of years (black cumin, Chinese sage, coriander, garlic, ginger, ginseng, and tea), and has been supported by sound scientific evidence such as one based on clinical trials with large population groups. It is important to note that there are herbs and plants that could actually raise blood pressure and thus should be avoided by hypertensive patients. There are also other limitations for herbal therapy of hypertension. These limitations would be discussed in the second part of this review.
Author Contributions
All authors contributed to the writing. AE conceived, designed and revised the manuscript.
Funding
This publication was made possible by grant # NPRP 4-571-3-171 from the Qatar National Research Fund (a member of Qatar Foundation). The Statements made herein are solely the responsibility of the authors. This grant was awarded to AE.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Miss Tuqa Saleh Al Shehabi for her assistance in drawing the figures.
References
Affuso, F., Mercurio, V., Fazio, V., and Fazio, S. (2010). Cardiovascular and metabolic effects of Berberine. World J. Cardiol. 2, 71–77. doi: 10.4330/wjc.v2.i4.71
Ahmad, A., Husain, A., Mujeeb, M., Khan, S. A., Najmi, A. K., Siddique, N. A., et al. (2013). A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 3, 337–352. doi: 10.1016/S2221-1691(13)60075-1
Ajay, M., Chai, H. J., Mustafa, A. M., Gilani, A. H., and Mustafa, M. R. (2007). Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J. Ethnopharmacol. 109, 388–393. doi: 10.1016/j.jep.2006.08.005
Ajiboye, T. O., Salawu, N. A., Yakubu, M. T., Oladiji, A. T., Akanji, M. A., and Okogun, J. I. (2011). Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem. Toxicol. 34, 109–115. doi: 10.3109/01480545.2010.536767
Akinyemi, A. J., Ademiluyi, A. O., and Oboh, G. (2013). Aqueous extracts of two varieties of ginger (Zingiber officinale) inhibit angiotensin I-converting enzyme, iron(II), and sodium nitroprusside-induced lipid peroxidation in the rat heart in vitro. J. Med. Food 16, 641–646. doi: 10.1089/jmf.2012.0022
Akinyemi, A. J., Ademiluyi, A. O., and Oboh, G. (2014). Inhibition of angiotensin-1-converting enzyme activity by two varieties of ginger (Zingiber officinale) in rats fed a high cholesterol diet. J. Med. Food 17, 317–323. doi: 10.1089/jmf.2012.0264
Alarcón-Alonso, J., Zamilpa, A., Aguilar, F. A., Herrera-Ruiz, M., Tortoriello, J., and Jimenez-Ferrer, E. (2012). Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J. Ethnopharmacol. 139, 751–756. doi: 10.1016/j.jep.2011.12.005
Ali, B. H., Al Wabel, N., and Blunden, G. (2005). Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: a review. Phytother. Res. 19, 369–375. doi: 10.1002/ptr.1628
Anilakumar, K. R., Khanum, F., and Bawa, A. S. (2010). Effect of coriander seed powder (CSP) on 1, 2-dimethyl hydrazine-induced changes in antioxidant enzyme system and lipid peroxide formation in rats. J. Diet. Suppl. 7, 9–20. doi: 10.3109/19390210903534970
Anselm, E., Socorro, V. F., Dal-Ros, S., Schott, C., Bronner, C., and Schini-Kerth, V. B. (2009). Crataegus special extract WS 1442 causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of endothelial NO synthase but not via activation of estrogen receptors. J. Cardiovasc. Pharmacol. 53, 253–260. doi: 10.1097/FJC.0b013e31819ccfc9
Archer, J. S. (2000). Evaluation and treatment of hypertension. Prim. Care Update Ob Gyns 7, 1–6. doi: 10.1016/S1068-607X(99)00032-3
Asgary, S., Naderi, G. H., Sadeghi, M., Kelishadi, R., and Amiri, M. (2004). Antihypertensive effect of Iranian Crataegus curvisepala Lind.: a randomized, double-blind study. Drugs Exp. Clin. Res. 30, 221–225.
Asher, G. N., Viera, A. J., Weaver, M. A., Dominik, R., Caughey, M., and Hinderliter, A. L. (2012). Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: a randomized, controlled cross-over trial. BMC Complement. Altern. Med. 12:26. doi: 10.1186/1472-6882-12-26
Ashraf, R., Khan, R. A., Ashraf, I., and Qureshi, A. A. (2013). Effects of Allium sativum (garlic) on systolic and diastolic blood pressure in patients with essential hypertension. Pak. J. Pharm. Sci. 26, 859–863.
August, P. (2004). Overview: mechanisms of hypertension: cells, hormones, and the kidney. J. Am. Soc. Nephrol. 15, 1971–1973. doi: 10.1097/01.ASN.0000133197.23478.76
Augusti, K. T., Jose, R., Sajitha, G. R., and Augustine, P. (2012). A rethinking on the benefits and drawbacks of common antioxidants and a proposal to look for the antioxidants in allium products as ideal agents: a review. Indian J. Clin. Biochem. 27, 6–20. doi: 10.1007/s12291-011-0146-y
Awang, K., Abdullah, N. H., Hadi, A. H. A., and Fong, Y. S. (2012). Cardiovascular activity of Labdane Diterpenes from andrographis paniculata in isolated rat hearts. J. Biomed. Biotechnol. 2012:876458. doi: 10.1155/2012/876458
Babu, P. V., and Liu, D. (2008). Green tea catechins and cardiovascular health: an update. Curr. Med. Chem. 15, 1840–1850. doi: 10.2174/092986708785132979
Bak, M. J., Hong, S. G., Lee, J. W., and Jeong, W. S. (2012). Red ginseng marc oil inhibits iNOS and COX-2 via NFkappaB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules 17, 13769–13786. doi: 10.3390/molecules171213769
Banerjee, S. K., Maulik, M., Mancahanda, S. C., Dinda, A. K., Gupta, S. K., and Maulik, S. K. (2002). Dose-dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci. 70, 1509–1518. doi: 10.1016/S0024-3205(01)01514-4
Bartolome, A. P., Villaseñor, I. M., and Yang, W. C. (2013). Bidens pilosa L. (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid Based Complement. Alternat. Med. 2013:340215. doi: 10.1155/2013/340215
Bastos, J. F., Moreira, I. J., Ribeiro, T. P., Medeiros, I. A., Antoniolli, A. R., De Sousa, D. P., et al. (2010). Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 106, 331–337. doi: 10.1111/j.1742-7843.2009.00492.x
Benavides, G. A., Squadrito, G. L., Mills, R. W., Patel, H. D., Isbell, T. S., Patel, R. P., et al. (2007). Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. U.S.A. 104, 17977–17982. doi: 10.1073/pnas.0705710104
Bernstein, K. E., Ong, F. S., Blackwell, W. L., Shah, K. H., Giani, J. F., Gonzalez-Villalobos, R. A., et al. (2013). A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 65, 1–46. doi: 10.1124/pr.112.006809
Bharti, S., Golechha, M., Kumari, S., Siddiqui, K. M., and Arya, D. S. (2012). Akt/GSK-3beta/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur. J. Nutr. 51, 719–727. doi: 10.1007/s00394-011-0251-y
Bhatt, S. R., Lokhandwala, M. F., and Banday, A. A. (2014). Vascular oxidative stress upregulates angiotensin II type I receptors via mechanisms involving nuclear factor kappa B. Clin. Exp. Hypertens. 36, 367–373. doi: 10.3109/10641963.2014.943402
Bogdanski, P., Suliburska, J., Szulinska, M., Stepien, M., Pupek-Musialik, D., and Jablecka, A. (2012). Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 32, 421–427. doi: 10.1016/j.nutres.2012.05.007
Bone, K., and Mills, S. (2013). Principles and Practice of Phytotherapy. Edinburgh: Churchill Livingstone.
Boskabady, M. H., Shafei, M. N., Shakiba, A., and Sefidi, H. S. (2008). Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother. Res. 22, 330–334. doi: 10.1002/ptr.2317
Brixius, K., Willms, S., Napp, A., Tossios, P., Ladage, D., Bloch, W., et al. (2006). Crataegus special extract WS 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 20, 177–184. doi: 10.1007/s10557-006-8723-7
Bucci, M., Papapetropoulos, A., Vellecco, V., Zhou, Z., Zaid, A., Giannogonas, P., et al. (2012). cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS ONE 7:e53319. doi: 10.1371/journal.pone.0053319
Calvert, J. W., Coetzee, W. A., and Lefer, D. J. (2010). Novel insights into hydrogen sulfide–mediated cytoprotection. Antioxid. Redox Signal. 12, 1203–1217. doi: 10.1089/ars.2009.2882
Castro, C., Lorenzo, A. G., González, A., and Cruzado, M. (2010). Garlic components inhibit angiotensin II-induced cell-cycle progression and migration: involvement of cell-cycle inhibitor p27(Kip1) and mitogen-activated protein kinase. Mol. Nutr. Food Res. 54, 781–787. doi: 10.1002/mnfr.200900108
Chan, P., Liu, J. C., Lin, L. J., Chen, P. Y., Cheng, T. H., Lin, J. G., et al. (2011). Tanshinone IIA inhibits angiotensin II-induced cell proliferation in rat cardiac fibroblasts. Am. J. Chin. Med. 39, 381–394. doi: 10.1142/S0192415X11008890
Cheng, N., Wang, Y., Gao, H., Yuan, J., Feng, F., Cao, W., et al. (2013). Protective effect of extract of Crataegus pinnatifida pollen on DNA damage response to oxidative stress. Food Chem. Toxicol. 59, 709–714. doi: 10.1016/j.fct.2013.07.015
Chiang, Y. M., Lo, C. P., Chen, Y. P., Wang, S. Y., Yang, N. S., Kuo, Y. H., et al. (2005). Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br. J. Pharmacol. 146, 352–363. doi: 10.1038/sj.bjp.0706343
Cho, Y. H., Ku, C. R., Hong, Z. Y., Heo, J. H., Kim, E. H., Choi, D. H., et al. (2013). Therapeutic effects of water soluble danshen extracts on atherosclerosis. Evidence Based Complement. Alternat. Med. 2013:623639. doi: 10.1155/2013/623639
Choi, J., Kim, T. H., Choi, T. Y., and Lee, M. S. (2013). Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS ONE 8:e59978. doi: 10.1371/journal.pone.0059978
Cioanca, O., Hritcu, L., Mihasan, M., and Hancianu, M. (2013). Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid beta(1-42) rat model of Alzheimer's disease. Physiol. Behav. 120, 193–202. doi: 10.1016/j.physbeh.2013.08.006
Das, S., Periyasamy, R., and Pandey, K. N. (2012). Activation of IKK/NF-kappaB provokes renal inflammatory responses in guanylyl cyclase/natriuretic peptide receptor-A gene-knockout mice. Physiol. Genomics 44, 430–442. doi: 10.1152/physiolgenomics.00147.2011
Dehkordi, F. R., and Kamkhah, A. F. (2008). Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam. Clin. Pharmacol. 22, 447–452. doi: 10.1111/j.1472-8206.2008.00607.x
Deka, A., and Vita, J. A. (2011). Tea and cardiovascular disease. Pharmacol. Res. 64, 136–145. doi: 10.1016/j.phrs.2011.03.009
Devi, R. C., Sim, S. M., and Ismail, R. (2011). Spasmolytic effect of citral and extracts of Cymbopogon citratus on isolated rabbit ileum. J. Smooth Muscle Res. 47, 143–156. doi: 10.1540/jsmr.47.143
Devi, R. C., Sim, S. M., and Ismail, R. (2012). Effect of cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid. Based Complement. Alternat. Med. 2012:539475. doi: 10.1155/2012/539475
Dharmashankar, K., and Widlansky, M. E. (2010). Vascular endothelial function and hypertension: insights and directions. Curr. Hypertens. Rep. 12, 448–455. doi: 10.1007/s11906-010-0150-2
Dimo, T., Azay, J., Tan, P. V., Pellecuer, J., Cros, G., Bopelet, M., et al. (2001). Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J. Ethnopharmacol. 76, 215–221. doi: 10.1016/S0378-8741(01)00229-X
Dimo, T., Rakotonirina, S. V., Tan, P. V., Azay, J., Dongo, E., and Cros, G. (2002). Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J. Ethnopharmacol. 83, 183–191. doi: 10.1016/S0378-8741(02)00162-9
Doh, K. C., Lim, S. W., Piao, S. G., Jin, L., Heo, S. B., Zheng, Y. F., et al. (2013). Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am. J. Nephrol. 37, 421–433. doi: 10.1159/000349921
Drexler, H., and Hornig, B. (1999). Endothelial dysfunction in human disease. J. Mol. Cell Cardiol. 31, 51–60. doi: 10.1006/jmcc.1998.0843
Drobiova, H., Thomson, M., Al-Qattan, K., Peltonen-Shalaby, R., Al-Amin, Z., and Ali, M. (2011). Garlic increases antioxidant levels in diabetic and hypertensive rats determined by a modified peroxidase method. Evid. Based Complement. Alternat. Med. 2011, 703049. doi: 10.1093/ecam/nep011
Drummond, G. R., Selemidis, S., Griendling, K. K., and Sobey, C. G. (2011). Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 10, 453–471. doi: 10.1038/nrd3403
Duffy, S. J., Keaney, J. F. Jr., Holbrook, M., Gokce, N., Swerdloff, P. L., Frei, B., et al. (2001). Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 104, 151–156. doi: 10.1161/01.CIR.104.2.151
Eisenberg, D. M., Davis, R. B., Ettner, S. L., Appel, S., Wilkey, S., van Rompay, M., et al. (1998). Trends in alternative medicine use in the United States, 1990-1997 - Results of a follow-up national survey. JAMA 280, 1569–1575. doi: 10.1001/jama.280.18.1569
El-Beshbishy, H. A., Hassan, M. H., Aly, H. A., Doghish, A. S., and Alghaithy, A. A. (2012). Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol. Environ. Saf. 83, 47–54. doi: 10.1016/j.ecoenv.2012.06.003
Elks, C. M., Mariappan, N., Haque, M., Guggilam, A., Majid, D. S., and Francis, J. (2009). Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am. J. Physiol. Renal Physiol. 296, F298–F305. doi: 10.1152/ajprenal.90628.2008
Fallah Huseini, H., Amini, M., Mohtashami, R., Ghamarchehre, M. E., Sadeqhi, Z., Kianbakht, S., et al. (2013). Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: a randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 27, 1849–1853. doi: 10.1002/ptr.4944
Faria, A. M., Papadimitriou, A., Silva, K. C., Lopes de Faria, J. M., and Lopes de Faria, J. B. (2012). Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 61, 1838–1847. doi: 10.2337/db11-1241
Fatehi, M., Rashidabady, T., and Fatehi-Hassanabad, Z. (2003). Effects of Crocus sativus petals' extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. J. Ethnopharmacol. 84, 199–203. doi: 10.1016/S0378-8741(02)00299-4
Figueirinha, A., Cruz, M. T., Francisco, V., Lopes, M. C., and Batista, M. T. (2010). Anti-inflammatory activity of Cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: contribution of the polyphenols. J. Med. Food 13, 681–690. doi: 10.1089/jmf.2009.0115
Folkow, B. (1990). “Structural factor” in primary and secondary hypertension. Hypertension 16, 89–101. doi: 10.1161/01.HYP.16.1.89
Francis, S. H., Busch, J. L., Corbin, J. D., and Sibley, D. (2010). cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563. doi: 10.1124/pr.110.002907
Francisco, V., Costa, G., Figueirinha, A., Marques, C., Pereira, P., Miguel Neves, B., et al. (2013). Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-kappaB pathway inhibition: contribution of chlorogenic acid. J. Ethnopharmacol. 148, 126–134. doi: 10.1016/j.jep.2013.03.077
Frank, T., Netzel, G., Kammerer, D. R., Carle, R., Kler, A., Kriesl, E., et al. (2012). Consumption of Hibiscus sabdariffa L. aqueous extract and its impact on systemic antioxidant potential in healthy subjects. J. Sci. Food Agric. 92, 2207–2218. doi: 10.1002/jsfa.5615
Freedman, B. I., and Cohen, A. H. (2016). Hypertension-attributed nephropathy: what's in a name? Nat. Rev. Nephrol. 12, 27–36. doi: 10.1038/nrneph.2015.172
Frishman, W. H., Beravol, P., and Carosella, C. (2009). Alternative and complementary medicine for preventing and treating cardiovascular disease. Dis. Mon. 55, 121–192. doi: 10.1016/j.disamonth.2008.12.002
Herrera-Arellano, A., Flores-Romero, S., Chávez-Soto, M. A., and Tortoriello, J. (2004). Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine 11, 375–382. doi: 10.1016/j.phymed.2004.04.001
Herrera-Arellano, A., Miranda-Sanchez, J., Avila-Castro, P., Herrera-Alvarez, S., Jimenez-Ferrer, J. E., Zamilpa, A., et al. (2007). Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med. 73, 6–12. doi: 10.1055/s-2006-957065
Hodgson, J. M., Puddey, I. B., Burke, V., Beilin, L. J., and Jordan, N. (1999). Effects on blood pressure of drinking green and black tea. J. Hypertens. 17, 457–463. doi: 10.1097/00004872-199917040-00002
Hodgson, J. M., Puddey, I. B., Burke, V., Watts, G. F., and Beilin, L. J. (2002). Regular ingestion of black tea improves brachial artery vasodilator function. Clin. Sci. 102, 195–201. doi: 10.1042/cs1020195
Hodgson, J. M., Puddey, I. B., Woodman, R. J., Mulder, T. P., Fuchs, D., Scott, K., et al. (2012). Effects of black tea on blood pressure: a randomized controlled trial. Arch. Intern. Med. 172, 186–188. doi: 10.1001/archinte.172.2.186
Hong, M. H., Kim, M. H., Chang, H. J., Kim, N. H., Shin, B. A., Ahn, B. W., et al. (2007). (-)-Epigallocatechin-3-gallate inhibits monocyte chemotactic protein-1 expression in endothelial cells via blocking NF-kappaB signaling. Life Sci. 80, 1957–1965. doi: 10.1016/j.lfs.2007.02.024
Hong, S. Y., Kim, J. Y., Ahn, H. Y., Shin, J. H., and Kwon, O. (2012). Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J. Agric. Food Chem. 60, 3086–3091. doi: 10.1021/jf204447y
Hopkins, A. L., Lamm, M. G., Funk, J. L., and Ritenbaugh, C. (2013). Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia 85, 84–94. doi: 10.1016/j.fitote.2013.01.003
Houston, M. C. (2005). Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog. Cardiovasc. Dis. 47, 396–449. doi: 10.1016/j.pcad.2005.01.004
Hu, F., Koon, C. M., Chan, J. Y., Lau, K. M., Kwan, Y. W., and Fung, K. P. (2012). Involvements of calcium channel and potassium channel in Danshen and Gegen decoction induced vasodilation in porcine coronary LAD artery. Phytomedicine 19, 1051–1058. doi: 10.1016/j.phymed.2012.07.007
Iglarz, M., and Clozel, M. (2007). Mechanisms of ET-1-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 50, 621–628. doi: 10.1097/FJC.0b013e31813c6cc3
Imenshahidi, M., Hosseinzadeh, H., and Javadpour, Y. (2010). Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 24, 990–994. doi: 10.1002/ptr.3044
Imenshahidi, M., Razavi, B. M., Faal, A., Gholampoor, A., Mousavi, S. M., and Hosseinzadeh, H. (2015). The effect of chronic administration of safranal on systolic blood pressure in rats. Iran. J. Pharm. Res. 14, 585–590.
Inagami, T., and Eguchi, S. (2000). Angiotensin II-mediated vascular smooth muscle cell growth signaling. Braz. J. Med. Biol. Res. 33, 619–624. doi: 10.1590/S0100-879X2000000600002
Inuwa, I., Ali, B. H., Al-Lawati, I., Beegam, S., Ziada, A., and Blunden, G. (2012). Long-term ingestion of Hibiscus sabdariffa calyx extract enhances myocardial capillarization in the spontaneously hypertensive rat. Exp. Biol. Med. 237, 563–569. doi: 10.1258/ebm.2012.011357
Itoh, H., Mukoyama, M., Pratt, R. E., Gibbons, G. H., and Dzau, V. J. (1993). Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J. Clin. Invest. 91, 2268–2274. doi: 10.1172/JCI116454
Jabeen, Q., Bashir, S., Lyoussi, B., and Gilani, A. H. (2009). Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J. Ethnopharmacol. 122, 123–130. doi: 10.1016/j.jep.2008.12.016
James, P. A., Oparil, S., Carter, B. L., Cushman, W. C., Dennison-Himmelfarb, C., Handler, J., et al. (2014). 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520. doi: 10.1001/jama.2013.284427
Jang, H. A., Cho, S., Kang, S. G., Ko, Y. H., Kang, S. H., Bae, J. H., et al. (2012). The relaxant effect of ginseng saponin on the bladder and prostatic urethra: an in vitro and in vivo study. Urol. Int. 88, 463–469. doi: 10.1159/000337206
Jang, S. J., Lim, H. J., and Lim, D. Y. (2011). Inhibitory effects of total Ginseng Saponin on catecholamine secretion from the perfused adrenal medulla of SHRs. J. Ginseng Res. 35, 176–190. doi: 10.5142/jgr.2011.35.2.176
Jeon, B. H., Kim, C. S., Park, K. S., Lee, J. W., Park, J. B., Kim, K. J., et al. (2000). Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen. Pharmacol. 35, 135–141. doi: 10.1016/S0306-3623(01)00096-9
Jiang, B., Li, D., Deng, Y., Teng, F., Chen, J., Xue, S., et al. (2013a). Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS ONE 8:e59621. doi: 10.1371/journal.pone.0059621
Jiang, J., Qi, Y. X., Zhang, P., Gu, W. T., Yan, Z. Q., Shen, B. R., et al. (2013b). Involvement of Rab28 in NF-kappaB nuclear transport in endothelial cells. PLoS ONE 8:e56076. doi: 10.1371/journal.pone.0056076
Joshi, C. N., Martin, D. N., Shaver, P., Madamanchi, C., Muller-Borer, B. J., and Tulis, D. A. (2012). Control of vascular smooth muscle cell growth by connexin 43. Front. Physiol. 3:220. doi: 10.3389/fphys.2012.00220
Jovanovski, E., Bateman, E. A., Bhardwaj, J., Fairgrieve, C., Mucalo, I., Jenkins, A. L., et al. (2014). Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: a randomized controlled trial. J. Am. Soc. Hypertens. 8, 537–541. doi: 10.1016/j.jash.2014.04.004
Kang, Y. M., Ma, Y., Zheng, J. P., Elks, C., Sriramula, S., Yang, Z. M., et al. (2009). Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc. Res. 82, 503–512. doi: 10.1093/cvr/cvp073
Kern, J. T., Hannink, M., and Hess, J. F. (2007). Disruption of the Keap1-containing ubiquitination complex as an antioxidant therapy. Curr. Top. Med. Chem. 7, 972–978. doi: 10.2174/156802607780906825
Khattab, M. M., and Nagi, M. N. (2007). Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytother. Res. 21, 410–414. doi: 10.1002/ptr.2083
Kim, J. H. (2012). Cardiovascular Diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J. Ginseng Res. 36, 16–26. doi: 10.5142/jgr.2012.36.1.16
Kim, N. D., Kang, S. Y., Kim, M. J., Park, J. H., and Schini-Kerth, V. B. (1999a). The ginsenoside Rg3 evokes endothelium-independent relaxation in rat aortic rings: role of K+ channels. Eur. J. Pharmacol. 367, 51–57. doi: 10.1016/S0014-2999(98)00899-1
Kim, N. D., Kang, S. Y., Park, J. H., and Schini-Kerth, V. B. (1999b). Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur. J. Pharmacol. 367, 41–49. doi: 10.1016/S0014-2999(98)00898-X
Kizhakekuttu, T. J., and Widlansky, M. E. (2010). Natural antioxidants and hypertension: promise and challenges. Cardiovasc. Ther. 28, e20–e32. doi: 10.1111/j.1755-5922.2010.00137.x
Ko, F. N., Huang, T. F., and Teng, C. M. (1991). Vasodilatory action mechanisms of apigenin isolated from Apium graveolens in rat thoracic aorta. Biochim. Biophys. Acta 1115, 69–74. doi: 10.1016/0304-4165(91)90013-7
Koçyildiz, Z. C., Birman, H., Olgaç, V., Akgün-Dar, K., Melikoǧlu, G., and Meriçli, A. H. (2006). Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: a morphological study. Phytother. Res. 20, 66–70. doi: 10.1002/ptr.1808
Koh, P. H., Mokhtar, R. A., and Iqbal, M. (2012). Antioxidant potential of Cymbopogon citratus extract: alleviation of carbon tetrachloride-induced hepatic oxidative stress and toxicity. Hum. Exp. Toxicol. 31, 81–91. doi: 10.1177/0960327111407226
Ku, D. D., Abdel-Razek, T. T., Dai, J., Kim-Park, S., Fallon, M. B., and Abrams, G. A. (2002). Garlic and its active metabolite allicin produce endothelium- and nitric oxide-dependent relaxation in rat pulmonary arteries. Clin. Exp. Pharmacol. Physiol. 29, 84–91. doi: 10.1046/j.1440-1681.2002.03596.x
Kundu, J. K., Liu, L., Shin, J. W., and Surh, Y. J. (2013). Thymoquinone inhibits phorbol ester-induced activation of NF-kappaB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem. Biophys. Res. Commun. 438, 721–727. doi: 10.1016/j.bbrc.2013.07.110
Kunwar, R. M., Shrestha, K. P., and Bussmann, R. W. (2010). Traditional herbal medicine in far-west Nepal: a pharmacological appraisal. J. Ethnobiol. Ethnomed. 6:35. doi: 10.1186/1746-4269-6-35
Lacolley, P., Regnault, V., Nicoletti, A., Li, Z., and Michel, J. B. (2012). The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc. Res. 95, 194–204. doi: 10.1093/cvr/cvs135
Lam, F. F., Deng, S. Y., Ng, E. S., Yeung, J. H., Kwan, Y. W., Lau, C. B., et al. (2010). Mechanisms of the relaxant effect of a danshen and gegen formulation on rat isolated cerebral basilar artery. J. Ethnopharmacol. 132, 186–192. doi: 10.1016/j.jep.2010.08.015
Lam, F. F., Yeung, J. H., Chan, K. M., and Or, P. M. (2008). Dihydrotanshinone, a lipophilic component of Salvia miltiorrhiza (danshen), relaxes rat coronary artery by inhibition of calcium channels. J. Ethnopharmacol. 119, 318–321. doi: 10.1016/j.jep.2008.07.011
Lan, J., Zhao, Y., Dong, F., Yan, Z., Zheng, W., Fan, J., et al. (2015). Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 161, 69–81. doi: 10.1016/j.jep.2014.09.049
Larson, A. J., Symons, J. D., and Jalili, T. (2012). Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv. Nutr. 3, 39–46. doi: 10.3945/an.111.001271
Lee, H. J., Jeong, H. S., Kim, D. J., Noh, Y. H., Yuk, D. Y., and Hong, J. T. (2008). Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-kappa B activation in RAW264.7 cells. Arch. Pharm. Res. 31, 342–349. doi: 10.1007/s12272-001-1162-0
Leong, X. F., Rais Mustafa, M., and Jaarin, K. (2013). Nigella sativa and its protective role in oxidative stress and hypertension. Evid. Based Complement. Alternat. Med. 2013:120732. doi: 10.1155/2013/120732
Li, X. C., and Zhuo, J. L. (2008). Nuclear factor-kappaB as a hormonal intracellular signaling molecule: focus on angiotensin II-induced cardiovascular and renal injury. Curr. Opin. Nephrol. Hypertens. 17, 37–43. doi: 10.1097/MNH.0b013e3282f2903c
Lin, H. H., Chen, J. H., and Wang, C. J. (2011). Chemopreventive properties and molecular mechanisms of the bioactive compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 18, 1245–1254. doi: 10.2174/092986711795029663
Liu, P. L., Liu, J. T., Kuo, H. F., Chong, I. W., and Hsieh, C. C. (2014). Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1beta via heme oxygenase-1. Mediators Inflamm. 2014:523684. doi: 10.1155/2014/523684
Liu, Q., Liu, J. J., Guo, H. L., Sun, S. N., Wang, S. F., Zhang, Y. L., et al. (2013). [6]-Gingerol: a Novel AT(1) antagonist for the treatment of cardiovascular disease. Planta Med. 79, 322–326. doi: 10.1055/s-0032-1328262
Liu, Y. H., Lu, M., Hu, L. F., Wong, P. T., Webb, G. D., and Bian, J. S. (2012). Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal. 17, 141–185. doi: 10.1089/ars.2011.4005
Lobo, V., Patil, A., Phatak, A., and Chandra, N. (2010). Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4, 118–126. doi: 10.4103/0973-7847.70902
Lü, H., Hua, P., Yu, J., Jiang, X., and Leng, H. (2005). [Study on the effect of theaflavins on the adhesion between monocyte and endothelial cells]. Zhong Yao Cai 28, 304–306.
Ludwig, A., Lorenz, M., Grimbo, N., Steinle, F., Meiners, S., Bartsch, C., et al. (2004). The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 316, 659–665. doi: 10.1016/j.bbrc.2004.02.099
Luo, H., Wang, X., Wang, J., Chen, C., Wang, N., Xu, Z., et al. (2015). Chronic NF-kappaB blockade improves renal angiotensin II type 1 receptor functions and reduces blood pressure in Zucker diabetic rats. Cardiovasc. Diabetol. 14, 76. doi: 10.1186/s12933-015-0239-7
Ma, L., Liu, H., Xie, Z., Yang, S., Xu, W., Hou, J., et al. (2014). Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-kappaB pathway: a mouse cardiomyocyte model. PLoS ONE 9:e103628. doi: 10.1371/journal.pone.0103628
Ma, T. K., Kam, K. K., Yan, B. P., and Lam, Y. Y. (2010). Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 160, 1273–1292. doi: 10.1111/j.1476-5381.2010.00750.x
Manrique, C., Lastra, G., Gardner, M., and Sowers, J. R. (2009). The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med. Clin. North Am. 93, 569–582. doi: 10.1016/j.mcna.2009.02.014
Marx, S. O., Totary-Jain, H., and Marks, A. R. (2011). Vascular smooth muscle cell proliferation in restenosis. Circ. Cardiovasc. Interv. 4, 104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332
Mathew, B., and Biju, R. (2008). Neuroprotective effects of garlic a review. Libyan J. Med. 3, 23–33. doi: 10.4176/071110
McKay, D. L., Chen, C. Y., Saltzman, E., and Blumberg, J. B. (2010). Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J. Nutr. 140, 298–303. doi: 10.3945/jn.109.115097
Mehdizadeh, R., Parizadeh, M. R., Khooei, A. R., Mehri, S., and Hosseinzadeh, H. (2013). Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran. J. Basic Med. Sci. 16, 56–63.
Miao, C. Y., and Li, Z. Y. (2012). The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br. J. Pharmacol. 165, 643–658. doi: 10.1111/j.1476-5381.2011.01404.x
Michel, T., and Vanhoutte, P. M. (2010). Cellular signaling and NO production. Pflugers Archiv. 459, 807–816. doi: 10.1007/s00424-009-0765-9
Modaghegh, M. H., Shahabian, M., Esmaeili, H. A., Rajbai, O., and Hosseinzadeh, H. (2008). Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 15, 1032–1037. doi: 10.1016/j.phymed.2008.06.003
Moghadam, M. H., Imenshahidi, M., and Mohajeri, S. A. (2013). Antihypertensive effect of celery seed on rat blood pressure in chronic administration. J. Med. Food 16, 558–563. doi: 10.1089/jmf.2012.2664
Mojiminiyi, F. B., Dikko, M., Muhammad, B. Y., Ojobor, P. D., Ajagbonna, O. P., Okolo, R. U., et al. (2007). Antihypertensive effect of an aqueous extract of the calyx of Hibiscus sabdariffa. Fitoterapia 78, 292–297. doi: 10.1016/j.fitote.2007.02.011
Mokhtari-Zaer, A., Khazdair, M. R., and Boskabady, M. H. (2015). Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: possible mechanisms. Avicenna J. Phytomed. 5, 365–375.
Montezano, A. C., Dulak-Lis, M., Tsiropoulou, S., Harvey, A., Briones, A. M., and Touyz, R. M. (2015). Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can. J. Cardiol. 31, 631–641. doi: 10.1016/j.cjca.2015.02.008
Montezano, A. C., and Touyz, R. M. (2012). Reactive oxygen species and endothelial function–role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin. Pharmacol. Toxicol. 110, 87–94. doi: 10.1111/j.1742-7843.2011.00785.x
Montezano, A. C., and Touyz, R. M. (2014). Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid. Redox Signal. 20, 164–182. doi: 10.1089/ars.2013.5302
Morgan, T. (2003). Renin, angiotensin, sodium and organ damage. Hypertens. Res. 26, 349–354. doi: 10.1291/hypres.26.349
Morihara, N., Hayama, M., and Fujii, H. (2011). Aged garlic extract scavenges superoxide radicals. Plant Foods Hum. Nutr. 66, 17–21. doi: 10.1007/s11130-011-0216-6
Mousa, A. S., and Mousa, S. A. (2007). Cellular effects of garlic supplements and antioxidant vitamins in lowering marginally high blood pressure in humans: pilot study. Nutr. Res. 27, 119–123. doi: 10.1016/j.nutres.2007.01.001
Mozaffari-Khosravi, H., Jalali-Khanabadi, B. A., Afkhami-Ardekani, M., Fatehi, F., and Noori-Shadkam, M. (2009). The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. J. Hum. Hypertens. 23, 48–54. doi: 10.1038/jhh.2008.100
Mucalo, I., Jovanovski, E., Rahelic, D., Božikov, V., Romic, Z., and Vuksan, V. (2013). Effect of American ginseng (Panax quinquefolius L.) on arterial stiffness in subjects with type-2 diabetes and concomitant hypertension. J. Ethnopharmacol. 150, 148–153. doi: 10.1016/j.jep.2013.08.015
Nagao, T., Hase, T., and Tokimitsu, I. (2007). A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 15, 1473–1483. doi: 10.1038/oby.2007.176
Nakagawa, T., and Yokozawa, T. (2002). Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 40, 1745–1750. doi: 10.1016/S0278-6915(02)00169-2
Nam, K. N., Park, Y. M., Jung, H. J., Lee, J. Y., Min, B. D., Park, S. U., et al. (2010). Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 648, 110–116. doi: 10.1016/j.ejphar.2010.09.003
Newman, D. J., and Cragg, G. M. (2012). Natural Products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335. doi: 10.1021/np200906s
Newsome, B. J., Petriello, M. C., Han, S. G., Murphy, M. O., Eske, K. E., Sunkara, M., et al. (2014). Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. J. Nutr. Biochem. 25, 126–135. doi: 10.1016/j.jnutbio.2013.10.003
Ng, C. F., Koon, C. M., Cheung, D. W., Lam, M. Y., Leung, P. C., Lau, C. B., et al. (2011). The anti-hypertensive effect of Danshen (Salvia miltiorrhiza) and Gegen (Pueraria lobata) formula in rats and its underlying mechanisms of vasorelaxation. J. Ethnopharmacol. 137, 1366–1372. doi: 10.1016/j.jep.2011.08.006
Nguelefack, T. B., Dimo, T., Mbuyo, E. P., Tan, P. V., Rakotonirina, S. V., and Kamanyi, A. (2005). Relaxant effects of the neutral extract of the leaves of Bidens pilosa Linn on isolated rat vascular smooth muscle. Phytother. Res. 19, 207–210. doi: 10.1002/ptr.1646
Nguyen Dinh Cat, A., and Touyz, R. M. (2011). A new look at the renin-angiotensin system–focusing on the vascular system. Peptides 32, 2141–2150. doi: 10.1016/j.peptides.2011.09.010
Niazmand, S., Fereidouni, E., Mahmoudabady, M., and Mousavi, S. M. (2014). Endothelium-independent vasorelaxant effects of hydroalcoholic extract from Nigella sativa seed in rat aorta: the roles of Ca2+ and K+ channels. Biomed Res. Int. 2014:247054. doi: 10.1155/2014/247054
Niture, S. K., Khatri, R., and Jaiswal, A. K. (2014). Regulation of Nrf2-an update. Free Radic. Biol. Med. 66, 36–44. doi: 10.1016/j.freeradbiomed.2013.02.008
Odigie, I. P., Ettarh, R. R., and Adigun, S. A. (2003). Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J. Ethnopharmacol. 86, 181–185. doi: 10.1016/S0378-8741(03)00078-3
Ojeda, D., Jiménez-Ferrer, E., Zamilpa, A., Herrera-Arellano, A., Tortoriello, J., and Alvarez, L. (2010). Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 127, 7–10. doi: 10.1016/j.jep.2009.09.059
Ong, S. L., and Whitworth, J. A. (2011). How do glucocorticoids cause hypertension: role of nitric oxide deficiency, oxidative stress, and eicosanoids. Endocrinol. Metab. Clin. North Am. 40, 393–407, ix. doi: 10.1016/j.ecl.2011.01.010
Onyenekwe, P. C., Ajani, E. O., Ameh, D. A., and Gamaniel, K. S. (1999). Antihypertensive effect of roselle (Hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem. Funct. 17, 199–206.
Oparil, S., Zaman, M. A., and Calhoun, D. A. (2003). Pathogenesis of hypertension. Ann. Intern. Med. 139, 761–776. doi: 10.7326/0003-4819-139-9-200311040-00011
Orekhov, A. N., Sobenin, I. A., Korneev, N. V., Kirichenko, T. V., Myasoedova, V. A., Melnichenko, A. A., et al. (2013). Anti-atherosclerotic therapy based on botanicals. Recent Pat. Cardiovasc. Drug Discov. 8, 56–66. doi: 10.2174/18722083113079990008
Oyama, J., Maeda, T., Kouzuma, K., Ochiai, R., Tokimitsu, I., Higuchi, Y., et al. (2010). Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circ. J. 74, 578–588. doi: 10.1253/circj.CJ-09-0692
Padiya, R., Chowdhury, D., Borkar, R., Srinivas, R., Pal Bhadra, M., and Banerjee, S. K. (2014). Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE 9:e94228. doi: 10.1371/journal.pone.0094228
Pan, L. L., Liu, X. H., Gong, Q. H., Yang, H. B., and Zhu, Y. Z. (2012). Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxid. Redox Signal. 17, 106–118. doi: 10.1089/ars.2011.4349
Pan, S. Y., Zhou, S. F., Gao, S. H., Yu, Z. L., Zhang, S. F., Tang, M. K., et al. (2013). New perspectives on how to discover drugs from herbal medicines: CAM's outstanding contribution to modern therapeutics. Evid. Based Complement. Alternat. Med. 2013:627375. doi: 10.1155/2013/627375
Patel, D. K., Desai, S. N., Gandhi, H. P., Devkar, R. V., and Ramachandran, A. V. (2012). Cardio protective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem. Toxicol. 50, 3120–3125. doi: 10.1016/j.fct.2012.06.033
Peng, X., Zhou, R., Wang, B., Yu, X., Yang, X., Liu, K., et al. (2014). Effect of green tea consumption on blood pressure: a meta-analysis of 13 randomized controlled trials. Sci. Rep. 4:6251. doi: 10.1038/srep06251
Pilz, R. B., and Casteel, D. E. (2003). Regulation of gene expression by cyclic GMP. Circ. Res. 93, 1034–1046. doi: 10.1161/01.RES.0000103311.52853.48
Popovic, M., Kaurinovic, B., Trivic, S., Mimica-Dukic, N., and Bursac, M. (2006). Effect of celery (Apium graveolens) extracts on some biochemical parameters of oxidative stress in mice treated with carbon tetrachloride. Phytother. Res. 20, 531–537. doi: 10.1002/ptr.1871
Premkumar, K., Abraham, S. K., Santhiya, S. T., and Ramesh, A. (2003). Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother. Res. 17, 614–617. doi: 10.1002/ptr.1209
Qian, Q., Qian, S., Fan, P., Huo, D., and Wang, S. (2012). Effect of Salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: a randomized controlled trial. Phytother. Res. 26, 60–66. doi: 10.1002/ptr.3513
Qidwai, W., and Ashfaq, T. (2013). Role of garlic usage in cardiovascular disease prevention: an evidence-based approach. Evid. Based Complem. Alternat. Med. 2013:125649. doi: 10.1155/2013/125649
Rahim, S. M., Taha, E. M., Mubark, Z. M., Aziz, S. S., Simon, K. D., and Mazlan, A. G. (2013). Protective effect of Cymbopogon citratus on hydrogen peroxide-induced oxidative stress in the reproductive system of male rats. Syst. Biol. Reprod. Med. 59, 329–336. doi: 10.3109/19396368.2013.827268
Ramkissoon, J. S., Mahomoodally, M. F., Ahmed, N., and Subratty, A. H. (2013). Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 6, 561–569. doi: 10.1016/S1995-7645(13)60097-8
Ras, R. T., Zock, P. L., and Draijer, R. (2011). Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS ONE 6:e16974. doi: 10.1371/journal.pone.0016974
Rhee, M. Y., Cho, B., Kim, K. I., Kim, J., Kim, M. K., Lee, E. K., et al. (2014). Blood pressure lowering effect of Korea ginseng derived ginseol K-g1. Am. J. Chin. Med. 42, 605–618. doi: 10.1142/S0192415X14500396
Ribaldo, P. D., Souza, D. S., Biswas, S. K., Block, K., Lopes de Faria, J. M., and Lopes de Faria, J. B. (2009). Green tea (Camellia sinensis) attenuates nephropathy by downregulating Nox4 NADPH oxidase in diabetic spontaneously hypertensive rats. J. Nutr. 139, 96–100. doi: 10.3945/jn.108.095018
Ried, K., Frank, O. R., and Stocks, N. P. (2010). Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: a randomised controlled trial. Maturitas 67, 144–150. doi: 10.1016/j.maturitas.2010.06.001
Ried, K., Frank, O. R., and Stocks, N. P. (2013). Aged garlic extract reduces blood pressure in hypertensives: a dose-response trial. Eur. J. Clin. Nutr. 67, 64–70. doi: 10.1038/ejcn.2012.178
Ried, K., Frank, O. R., Stocks, N. P., Fakler, P., and Sullivan, T. (2008). Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc. Disord. 8:13. doi: 10.1186/1471-2261-8-13
Rudijanto, A. (2007). The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med. Indones. 39, 86–93.
Sarr, M., Ngom, S., Kane, M. O., Wele, A., Diop, D., Sarr, B., et al. (2009). In vitro vasorelaxation mechanisms of bioactive compounds extracted from Hibiscus sabdariffa on rat thoracic aorta. Nutr. Metab. 6:45. doi: 10.1186/1743-7075-6-45
Savoia, C., Burger, D., Nishigaki, N., Montezano, A., and Touyz, R. M. (2011). Angiotensin II and the vascular phenotype in hypertension. Expert Rev. Mol. Med. 13, e11. doi: 10.1017/S1462399411001815
Sendl, A., Elbl, G., Steinke, B., Redl, K., Breu, W., and Wagner, H. (1992). Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 58, 1–7. doi: 10.1055/s-2006-961378
Shin, I. S., Lee, M. Y., Lim, H. S., Ha, H., Seo, C. S., Kim, J. C., et al. (2012). An extract of Crataegus pinnatifida fruit attenuates airway inflammation by modulation of matrix metalloproteinase-9 in ovalbumin induced asthma. PLoS ONE 7:e45734. doi: 10.1371/journal.pone.0045734
Shin, S. G., Kim, J. Y., Chung, H. Y., and Jeong, J. C. (2005). Zingerone as an antioxidant against peroxynitrite. J. Agric. Food Chem. 53, 7617–7622. doi: 10.1021/jf051014x
Shou, Q., Pan, Y., Xu, X., Xu, J., Wang, D., Ling, Y., et al. (2012). Salvianolic acid B possesses vasodilation potential through NO and its related signals in rabbit thoracic aortic rings. Eur. J. Pharmacol. 697, 81–87. doi: 10.1016/j.ejphar.2012.09.044
Shouk, R., Abdou, A., Shetty, K., Sarkar, D., and Eid, A. H. (2014). Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr. Res. 34, 106–115. doi: 10.1016/j.nutres.2013.12.005
Silva, B. R., Pernomian, L., and Bendhack, L. M. (2012). Contribution of oxidative stress to endothelial dysfunction in hypertension. Front. Physiol. 3:441. doi: 10.3389/fphys.2012.00441
Slevin, M., Ahmed, N., Wang, Q., McDowell, G., and Badimon, L. (2012). Unique vascular protective properties of natural products: supplements or future main-line drugs with significant anti-atherosclerotic potential? Vasc. Cell 4:9. doi: 10.1186/2045-824X-4-9
Song, P., and Zou, M. H. (2012). Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic. Biol. Med. 52, 1607–1619. doi: 10.1016/j.freeradbiomed.2012.01.025
Sreelatha, S., Padma, P. R., and Umadevi, M. (2009). Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. Toxicol. 47, 702–708. doi: 10.1016/j.fct.2008.12.022
Srivastava, R., Ahmed, H., Dixit, R. K., and Dharamveer, Saraf, S. A. (2010). Crocus sativus L.: a comprehensive review. Pharmacogn. Rev. 4, 200–208. doi: 10.4103/0973-7847.70919
Stone, J. D., Narine, A., Shaver, P. R., Fox, J. C., Vuncannon, J. R., and Tulis, D. A. (2013). AMP-activated protein kinase inhibits vascular smooth muscle cell proliferation and migration and vascular remodeling following injury. Am. J. Physiol. Heart Circ. Physiol. 304, H369–H381. doi: 10.1152/ajpheart.00446.2012
Su, D., and Li, L. (2011). Trends in the use of complementary and alternative medicine in the United States: 2002-2007. J. Health Care Poor Underserved 22, 296–310. doi: 10.1353/hpu.2011.0002
Suekawa, M., Ishige, A., Yuasa, K., Sudo, K., Aburada, M., and Hosoya, E. (1984). Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J. Pharmacobiodyn. 7, 836–848. doi: 10.1248/bpb1978.7.836
Susalit, E., Agus, N., Effendi, I., Tjandrawinata, R. R., Nofiarny, D., Perrinjaquet-Moccetti, T., et al. (2011). Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine 18, 251–258. doi: 10.1016/j.phymed.2010.08.016
Tabassum, N., and Ahmad, F. (2011). Role of natural herbs in the treatment of hypertension. Pharmacogn. Rev. 5, 30–40. doi: 10.4103/0973-7847.79097
Tachjian, A., Maria, V., and Jahangir, A. (2010). Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 55, 515–525. doi: 10.1016/j.jacc.2009.07.074
Tao, L. L., and Lei, Y. (2012). [Effects of extracts from Panax ginseng, Panax notoginseng and Ligusticum chuanxiong on expression of beta-galactosidase and signal pathway p16-cyclin D/CDK-Rb in vascular smooth muscle cells]. Zhong Xi Yi Jie He Xue Bao 10, 76–84. doi: 10.3736/jcim20120112
Tao, S., Zheng, Y., Lau, A., Jaramillo, M. C., Chau, B. T., Lantz, R. C., et al. (2013). Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivo. Antioxid. Redox Signal. 19, 1647–1661. doi: 10.1089/ars.2012.5117
Tassell, M. C., Kingston, R., Gilroy, D., Lehane, M., and Furey, A. (2010). Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 4, 32–41. doi: 10.4103/0973-7847.65324
Taubert, D., Roesen, R., and Schömig, E. (2007). Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch. Intern. Med. 167, 626–634. doi: 10.1001/archinte.167.7.626
Thomson, M., Al-Qattan, K., Mansour, M. H., and Ali, M. (2012). Green tea attenuates oxidative stress and downregulates the expression of angiotensin II AT(1) receptor in renal and hepatic tissues of streptozotocin-induced diabetic rats. Evid. Based Complement. Alternat. Med. 2012:409047. doi: 10.1155/2012/409047
Tiwari, M., Dwivedi, U. N., and Kakkar, P. (2010). Suppression of oxidative stress and pro-inflammatory mediators by Cymbopogon citratus D. Stapf extract in lipopolysaccharide stimulated murine alveolar macrophages. Food Chem. Toxicol. 48, 2913–2919. doi: 10.1016/j.fct.2010.07.027
Toda, N., Ayajiki, K., Fujioka, H., and Okamura, T. (2001). Ginsenoside potentiates NO-mediated neurogenic vasodilatation of monkey cerebral arteries. J. Ethnopharmacol. 76, 109–113. doi: 10.1016/S0378-8741(01)00217-3
Topal, G., Koç, E., Karaca, C., Altuǧ, T., Ergin, B., Demirci, C., et al. (2013). Effects of Crataegus microphylla on vascular dysfunction in streptozotocin-induced diabetic rats. Phytother. Res. 27, 330–337. doi: 10.1002/ptr.4726
Tsi, D., and Tan, B. K. (1997). Cardiovascular pharmacology of 3-n-butylphthalide in spontaneously hypertensive rats. Phytother. Res. 11, 576–582.
Valli, G., and Giardina, E. G. (2002). Benefits, adverse effects and drug interactions of herbal therapies with cardiovascular effects. J. Am. Coll. Cardiol. 39, 1083–1095. doi: 10.1016/S0735-1097(02)01749-7
Vazquez-Prieto, M. A., Rodriguez Lanzi, C., Lembo, C., Galmarini, C. R., and Miatello, R. M. (2011). Garlic and onion attenuates vascular inflammation and oxidative stress in fructose-fed rats. J. Nutr. Metab. 2011:475216. doi: 10.1155/2011/475216
Villeneuve, N. F., Tian, W., Wu, T., Sun, Z., Lau, A., Chapman, E., et al. (2013). USP15 negatively regulates Nrf2 through deubiquitination of Keap1. Mol. Cell 51, 68–79. doi: 10.1016/j.molcel.2013.04.022
Virdis, A., Duranti, E., and Taddei, S. (2011). Oxidative stress and vascular damage in hypertension: role of angiotensin II. Int. J. Hypertens. 2011:916310. doi: 10.4061/2011/916310
Virdis, A., and Taddei, S. (2011). How to evaluate microvascular organ damage in hypertension: assessment of endothelial function. High Blood Pressure Cardiovasc. Prev. 18, 163–167. doi: 10.2165/11593630-000000000-00000
Walker, A. F., Marakis, G., Morris, A. P., and Robinson, P. A. (2002). Promising hypotensive effect of hawthorn extract: a randomized double-blind pilot study of mild, essential hypertension. Phytother. Res. 16, 48–54. doi: 10.1002/ptr.947
Wan, X., Chen, X., Liu, L., Zhao, Y., Huang, W. J., Zhang, Q., et al. (2013). Berberine ameliorates chronic kidney injury caused by atherosclerotic renovascular disease through the suppression of NFkappaB signaling pathway in rats. PLoS ONE 8:e59794. doi: 10.1371/journal.pone.0059794
Wang, H. P., Yang, J., Qin, L. Q., and Yang, X. J. (2015). Effect of garlic on blood pressure: a meta-analysis. J. Clin. Hypertens. 17, 223–231. doi: 10.1111/jch.12473
Wang, J., and Xiong, X. (2012). Outcome measures of chinese herbal medicine for hypertension: an overview of systematic reviews. Evid. Based Complem. Alternat. Med. 2012:697237. doi: 10.1155/2012/697237
Wang, Y., Huang, Y., Lam, K. S., Li, Y., Wong, W. T., Ye, H., et al. (2009). Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 82, 484–492. doi: 10.1093/cvr/cvp078
Wang, Y., Liu, Y., Zhang, X. Y., Xu, L. H., Ouyang, D. Y., Liu, K. P., et al. (2014). Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-kappaB and PI3K/Akt/mTOR pathways. Int. Immunopharmacol. 23, 77–84. doi: 10.1016/j.intimp.2014.07.028
Weber, M. A., Schiffrin, E. L., White, W. B., Mann, S., Lindholm, L. H., Kenerson, J. G., et al. (2014). Clinical practice guidelines for the management of hypertension in the community a statement by the american society of hypertension and the international society of hypertension. J. Hypertens. 32, 3–15. doi: 10.1097/HJH.0000000000000065
Wu, T. T., Tsai, C. W., Yao, H. T., Lii, C. K., Chen, H. W., Wu, Y. L., et al. (2010). Suppressive effects of extracts from the aerial part of Coriandrum sativum L. on LPS-induced inflammatory responses in murine RAW 264.7 macrophages. J. Sci. Food Agric. 90, 1846–1854. doi: 10.1002/jsfa.4023
Xagorari, A., Papapetropoulos, A., Mauromatis, A., Economou, M., Fotsis, T., and Roussos, C. (2001). Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 296, 181–187.
Xiong, X., Yang, X., Liu, W., Chu, F., Wang, P., and Wang, J. (2013). Trends in the treatment of hypertension from the perspective of traditional chinese medicine. Evid. Based Complement. Alternat. Med. 2013:275279. doi: 10.1155/2013/275279
Yan, H., Du, J., and Tang, C. (2004). The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem. Biophys. Res. Commun. 313, 22–27. doi: 10.1016/j.bbrc.2003.11.081
Yang, B., and Liu, P. (2012). Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 92, 1578–1590. doi: 10.1002/jsfa.5671
Yang, H. M., Kim, B. K., Kim, J. Y., Kwon, Y. W., Jin, S., Lee, J. E., et al. (2013). PPARgamma modulates vascular smooth muscle cell phenotype via a protein kinase G-dependent pathway and reduces neointimal hyperplasia after vascular injury. Exp. Mol. Med. 45, e65. doi: 10.1038/emm.2013.112
Yang, Y., Chan, S. W., Hu, M., Walden, R., and Tomlinson, B. (2011). Effects of some common food constituents on cardiovascular disease. ISRN Cardiol. 2011:397136. doi: 10.5402/2011/397136
Yeh, G. Y., Davis, R. B., and Phillips, R. S. (2006). Use of complementary therapies in patients with cardiovascular disease. Am. J. Cardiol. 98, 673–680. doi: 10.1016/j.amjcard.2006.03.051
Zaoui, A., Cherrah, Y., Lacaille-Dubois, M. A., Settaf, A., Amarouch, H., and Hassar, M. (2000). [Diuretic and hypotensive effects of Nigella sativa in the spontaneously hypertensive rat]. Therapie 55, 379–382.
Zeng, C., Villar, V. A., Yu, P., Zhou, L., and Jose, P. A. (2009). Reactive oxygen species and dopamine receptor function in essential hypertension. Clin. Exp. Hypertens. 31, 156–178. doi: 10.1080/10641960802621283
Zhang, C. Y., and Tan, B. K. (1996). Hypotensive activity of aqueous extract of Andrographis paniculata in rats. Clin. Exp. Pharmacol. Physiol. 23, 675–678. doi: 10.1111/j.1440-1681.1996.tb01756.x
Zhang, H., Park, Y., Wu, J., Chen, X. P., Lee, S., Yang, J., et al. (2009). Role of TNF-alpha in vascular dysfunction. Clin. Sci. 116, 219–230. doi: 10.1042/CS20080196
Zhang, P., Song, S. J., Liu, W. L., Li, L. L., Zhao, W. L., and Zhang, Y. (2011). [Eeffects of Coptis Chinensis on vasoconstrictive activity of isolated thoracic aorta of normoxic and chronic intermittent hypobaric hypoxic rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 27, 420–425.
Zhang, W., Chen, G., and Deng, C. Q. (2012). Effects and mechanisms of total Panax notoginseng saponins on proliferation of vascular smooth muscle cells with plasma pharmacology method. J. Pharm. Pharmacol. 64, 139–145. doi: 10.1111/j.2042-7158.2011.01379.x
Zhong, G., Chen, F., Cheng, Y., Tang, C., and Du, J. (2003). The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 21, 1879–1885. doi: 10.1097/00004872-200310000-00015
Keywords: herbal medicine, hypertension, epigenetics, oxidative stress, inflammation, nitric oxide
Citation: Al Disi SS, Anwar MA and Eid AH (2016) Anti-hypertensive Herbs and their Mechanisms of Action: Part I. Front. Pharmacol. 6:323. doi: 10.3389/fphar.2015.00323
Received: 13 October 2015; Accepted: 30 December 2015;
Published: 19 January 2016.
Edited by:
Hibah Omar Awwad, University of Oklahoma Health Sciences Center, USAReviewed by:
Javier Angulo, Hospital Ramón y Cajal, SpainBrett M. Mitchell, Texas A&M Health Science Center, USA
Copyright © 2016 Al Disi, Anwar and Eid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali H. Eid, ae81@aub.edu.lb;
ali.eid@qu.edu.qa
†These authors have contributed equally to this work.
 Sara S. Al Disi
Sara S. Al Disi M. Akhtar Anwar
M. Akhtar Anwar Ali H. Eid
Ali H. Eid