- 1Department of Pharmacology, University of the Basque Country, UPV/EHU, Leioa, Spain
- 2Centro de Investigación Biomédica en Red de Salud Mental, Madrid, Spain
Schizophrenia is a chronic psychiatric disorder which substantially impairs patients’ quality of life. Despite the extensive research in this field, the pathophysiology and etiology of schizophrenia remain unknown. Different neurotransmitter systems and functional networks have been found to be affected in the brain of patients with schizophrenia. In this context, postmortem brain studies as well as genetic assays have suggested alterations in Group II metabotropic glutamate receptors (mGluRs) in schizophrenia. Despite many years of drug research, several needs in the treatment of schizophrenia have not been addressed sufficiently. In fact, only 5–10% of patients with schizophrenia successfully achieve a full recovery after treatment. In recent years mGluRs have turned up as novel targets for the design of new antipsychotic medications for schizophrenia. Concretely, Group II mGluRs are of particular interest due to their regulatory role in neurotransmission modulating glutamatergic activity in brain synapses. Preclinical studies have demonstrated that orthosteric Group II mGluR agonists exhibit antipsychotic-like properties in animal models of schizophrenia. However, when these compounds have been tested in human clinical studies with schizophrenic patients results have been inconclusive. Nevertheless, it has been recently suggested that this apparent lack of efficacy in schizophrenic patients may be related to previous exposure to atypical antipsychotics. Moreover, the role of the functional heterocomplex formed by 5-HT2A and mGlu2 receptors in the clinical response to Group II mGluR agonists is currently under study.
Introduction
Schizophrenia is a severe, chronic, and disabling mental disorder affecting approximately 0.6% of the population worldwide (McGrath et al., 2008). Among psychiatric disorders, it is considered the most disabling one, requiring a disproportionate share of mental health services (Mueser and McGurk, 2004). Individuals diagnosed with schizophrenia have impaired social and occupational functioning. Thus, schizophrenia is placed among the world’s top leading causes of years lived with disability (World Health Organization, 2008; Vos et al., 2015), being also the seventh most costly medical illness in our society (Freedman, 2003).
The clinical features of schizophrenia are clustered in three categories: positive symptoms, negative symptoms and cognitive deficits. Positive or psychotic symptoms include delusions (false beliefs held with strong conviction in spite of contradictory evidence), hallucinations (perceptions in the absence of external stimulus, commonly experienced as hearing voices distinct from one’s own thoughts), thought disorder (e.g., loose associations), and abnormal psychomotor activity (e.g., grossly disorganized behavior, posturing, or catatonia). Negative symptoms comprise social withdrawal, impairments in initiative and motivation, a reduced capacity to recognize and express emotional states and poverty in the amount or content of speech. Cognitive impairments include disturbances in selective attention, working memory, executive control, episodic memory, language comprehension, and social-emotional processing. Symptomatic onset occurs in late adolescence and early adulthood in males and somewhat later in females, who tend to be less severely affected (Abel et al., 2010). The course of schizophrenia is typically characterized by psychotic exacerbations or relapses alternating with periods of partial remissions.
The principal pharmacological treatment for schizophrenia is antipsychotic medication. In general terms, antipsychotic drugs are effective in reducing the severity of positive symptoms such as hallucinations and delusions and have made it possible for many individuals with schizophrenia to live outside hospital settings. Nevertheless, antipsychotics have minimal impact on both negative symptoms and cognitive impairments (see Miyamoto et al., 2012 for review). Thus, the treatment of schizophrenia with antipsychotics rarely, if ever, produces a cure or entirely reverses symptoms of the illness. Only 5–10% of persons with schizophrenia successfully achieve a full recovery with or without these medications. There is a good response to antipsychotic medication in 30–40% of patients. However, about 20% are resistant to standard antipsychotics and an additional 30–40% show an improvement but are residually symptomatic despite antipsychotic treatment (Smith et al., 2009).
The Glutamate Hypothesis of Schizophrenia
Glutamate is the major excitatory neurotransmitter in the brain. It interacts with two types of receptors: (i) the ionotropic receptors, with NMDA, Kainate, and AMPA receptor subtypes connected to or representing ion channels, and (ii) the metabotropic glutamate receptors (mGluRs), which activate G protein-coupled signal transduction and comprising groups I to III with a total of eight identified subtypes (Nakanishi, 1992).
The observation that the administration of phencyclidine (PCP) and the dissociative anesthetic ketamine —two NMDA receptor antagonists— could mimic schizophrenia symptoms in healthy individuals led to the hypothesis of a functional impairment of NMDA receptors in this disease (Javitt and Zukin, 1991; Olney and Farber, 1995; Stone et al., 2008). Importantly, it was described that NMDA receptor antagonists, besides inducing positive-like symptoms, also found to be induced by other stimulant drugs such as amphetamine and other dopaminergic agonists, were also able to evoke cognitive- and negative-like symptoms (Krystal et al., 2005). Since dopamine release is under control of NMDA receptors in several brain circuits, some authors sustain that the glutamatergic dysfunction may be underlying the dopaminergic deficits found in schizophrenia (Javitt, 2010).
Additionally, genome-wide association studies have shown that genes involved in glutamatergic neurotransmission and synaptic plasticity, e.g., mGluR3 (GRM3), glutamate ionotropic receptor NMDA type subunit 2A (GRIN2A), serine racemase (SRR), glutamate ionotropic receptor AMPA type subunit 1 (GRIA1) or neurogranin (NRGN), are associated with schizophrenia (Stefansson et al., 2009; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Moreover, postmortem and neuroimaging studies have found that several components of glutamatergic signaling system are affected in schizophrenic patients (Gao et al., 2000; Stone, 2009; Coyle et al., 2012). Thus, drugs targeted to restore the glutamatergic imbalance could provide better outcomes than those obtained with current antipsychotics in the treatment of schizophrenia. In this sense, preclinical and clinical studies have provided evidence for mGluRs 2, 3, and 5, muscarinic receptors M1 and M4 or the Glycine transporter GlyT1 among others as potential targets to retrieve the glutamatergic functioning in schizophrenia (Field et al., 2011).
Group II Metabotropic Glutamate Receptors
Genes encoding eight mGluR subtypes —many of them with multiple splice variants— have been identified and are classified into three groups (I–III) according to their sequence homology, coupling mechanism and pharmacology (Conn and Pin, 1997; Niswender and Conn, 2010; Figure 1). Group II includes mGlu2 and mGlu3 receptors, that are coupled predominantly to Gi/o proteins, which mediate the downstream inhibition of adenylyl cyclase activity, modulation of voltage-dependent ion channels (inhibition of calcium and activation of potassium channels), and the regulation of other downstream signaling partners via released Gβγ subunits. Recent studies have shown that Group II mGluRs can also modulate additional signaling pathways, such as activation of PI3K and MAPK pathways (Iacovelli et al., 2002; Niswender and Conn, 2010; Nicoletti et al., 2011).
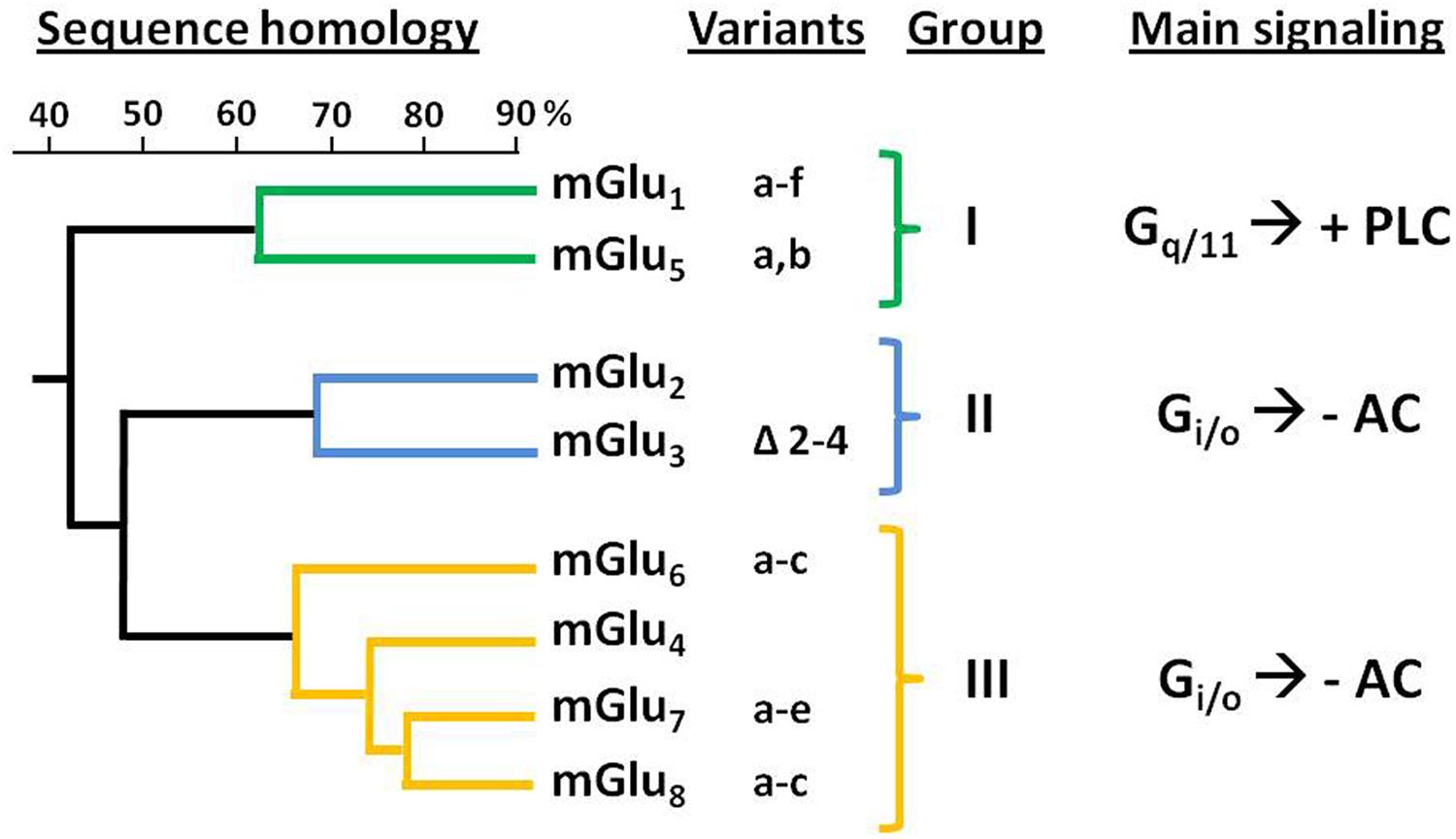
FIGURE 1. Classification and sequence homology dendrogram of mGluRs. The known splice variants and main signaling pathways are indicated. –AC, inhibition of adenylyl cyclase activity; +PLC, activation of phospholipase C.
The mGlu2 and mGlu3 receptors share about 70% of their amino acid sequence (Pin and Duvoisin, 1995). The gene encoding the human mGlu2R (GRM2) has been mapped to chromosome 3p21.1-p21.2 (Marti et al., 2002a). For its part, mGlu3R gene (GRM3) was mapped to human chromosome 7q21.1-q21.2 (Scherer et al., 1996). While no splicing variants have been reported for GRM2, alternative splicing has been described for GRM3 leading to four different variants: full length mGlu3R, GRM3Δ2 (lacking exon 2), GRM3Δ4 (lacking exon 4), and GRM3A2Δ3 (lacking exons 2 and 3); being the GRM3Δ4 the most abundant one (Sartorius et al., 2006). Despite this variant lacks the transmembrane domain—which is encoded by exon 4— it has been shown that it can be translated in cells, thus suggesting its potential function as a unique glutamate receptor (Niswender and Conn, 2010).
Group II mGluRs are widely expressed throughout the central nervous system. The expression levels are moderate to high in different brain regions such as the prefrontal cortex (PFC), the dorsal and ventral striatum, the thalamus, the hippocampus, and the amygdala (Petralia et al., 1996; Wright et al., 2001; Gu et al., 2008); regions that have been shown to be involved in cognition and emotional states. The mGlu3Rs expression across these regions is more disperse than that of mGlu2Rs (Ohishi et al., 1993; Gu et al., 2008). In the PFC mGlu2Rs present a high but restricted expression with a bilaminar distribution in layer I and layer Va while mGlu3Rs distribution is more homogenous throughout the cortex with a slight higher expression in layers I–III than in layers IV–VI (Marek, 2010). At the neuronal level, mGlu2Rs are localized at the perisynapse (Cartmell and Schoepp, 2000) mainly acting as autoreceptors where they function as a feedback negative mechanism to suppress the excessive glutamate release keeping the homeostasis of the synapse (Cartmell and Schoepp, 2000; Schoepp, 2001). However, both presynaptic and postsynaptic cortical immunoreactivity of mGlu2Rs has been described. For its part mGlu3Rs immunoreactivity has been shown mainly presynaptic (Neki et al., 1996; Ohishi et al., 1998; Tamaru et al., 2001) and whereas mGlu2Rs expression is restricted to neurons, mGlu3Rs are also found on glial cells (Ohishi et al., 1993; Tamaru et al., 2001) where they may interact with glutamate transporters (Aronica et al., 2003).
Besides modulation of glutamate physiology, Group II mGluRs, also control the neurotransmitter release of other systems acting as heteroreceptors in GABAergic, dopaminergic, noradrenergic, or serotonergic synapses (Cartmell and Schoepp, 2000).
A limited number of molecules possess agonist activity across all mGluRs. The endogenous agonist L-glutamate, L-CCG-I [(2S,10 S,20 S)-2-(carboxycyclopropyl)glycine] and ABHxD-I (2-aminobicyclo[2.1.1]hexane-2,5-dicarboxylic acid-I) are the most potent (Acher, 2011). More recently, systemically active and highly selective agonists of Group II mGluRs have been developed, providing valuable insights into the in vitro and in vivo functions of these receptors (Niswender and Conn, 2010). LY354740 ((1S,2S,5R,6S)-2-Aminobicyclo[3.1.0]hexane-2, 6-di carboxylic acid) was the first Group II mGluR selective agonist reported to exhibit a nanomolar affinity (Monn et al., 1997). It has been followed by more recent compounds, including LY379268 ((1S,2R,5R,6R)-2-amino-4-oxabicyclo[3.1.0]hexane-2,6-dicarboxylic acid), now a commonly used tool for studies of Group II mGluR function (Schoepp et al., 1999). These compounds are highly selective for Group II mGluRs relative to other mGluR subtypes but do not differentiate between mGlu2R and mGlu3R. Other Group II selective agonists have been described with submicromolar affinity, including (2R,4R)-APDC ((2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate) and DCG-IV ((2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine). Additiona lly, an analog of LY354740 with a methyl substituent at the C4α-position was reported to have mGlu2R agonist and mGlu3R antagonist activity (Dominguez et al., 2005). Thus far, no orthosteric antagonists have been discovered that are entirely specific for Group II mGluRs. However, LY341495 ((2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid) provides relatively high selectivity with nanomolar potency as a Group II mGluR antagonist with submicromolar to micromolar potencies at all other mGluR subtypes (Schoepp et al., 1999).
To date, besides orthosteric ligands, multiple selective positive allosteric modulators (PAMs) of mGlu2R have been identified. The majority are structurally related to either LY487379 (2,2,2-trifluoro-N-[4-(2-methoxyphenoxy)phenyl]-N-(3-pyridinylmethyl)ethanesulfonamide hydrochloride) or BINA (biphenyl-indanone A), two prototypical mGlu2R PAMs (Conn et al., 2009; Niswender and Conn, 2010). Many of these compounds are highly selective for mGlu2R and do not potentiate responses to activation of mGlu3R or any other mGluR subtype (Cid et al., 2015). In addition, group II mGluR negative allosteric modulators (NAMs) have also been developed, but in this case acting at both mGlu2R and mGlu3R (Hemstapat et al., 2007; Woltering et al., 2008a,b).
Alterations of Group II mGluRs in Schizophrenia
Postmortem Brain Studies
Different approaches have been used to determine the possible alterations of both, mRNA and protein expression, of Group II mGluRs in the postmortem brain of schizophrenic subjects. The majority of the findings suggest that the level of expression of GRM3 mRNA is unaffected in schizophrenia (Ohnuma et al., 1998; Richardson-Burns et al., 2000; Egan et al., 2004; Bullock et al., 2008; Ghose et al., 2008; Gonzalez-Maeso et al., 2008). Fewer studies have investigated GRM2 mRNA expression in postmortem human brain of schizophrenic subjects. Semi-quantitative approaches such as in situ hybridization have reported unaffected levels of GRM2 mRNA in thalamus (Richardson-Burns et al., 2000), and higher GRM2 mRNA expression in the PFC white matter (Ghose et al., 2008). However, quantitative real-time PCR assays showed lower level of expression of GRM2 mRNA in the PFC (Gonzalez-Maeso et al., 2008) and cerebellum (Bullock et al., 2008) of schizophrenic subjects.
Studies using immunolabeling techniques have reported different outcomes in regard to Group II mGluRs protein expression levels in schizophrenia. Differentiation of mGlu3R from mGlu2R has been problematic because of the lack of selective ligands and antibodies. An early study, using non-specific antibodies that detect both mGlu2 and mGlu3 receptor proteins, found no change in mGlu2/3R expression in the PFC (BA46) of schizophrenic subjects compared to controls (Crook et al., 2002). A second study, using also non-specific antibodies, found a significant increase in mGlu2/3R expression in the BA46 of schizophrenic subjects compared to controls, but not in other cortical regions including BA9 and BA11 (Gupta et al., 2005). The availability of specific antibodies allowed the evaluation of the protein expression of each subtype of Group II mGluRs. Corti et al. (2007) found a significant decrease in the dimeric form of mGlu3Rs in the PFC (BA10) of schizophrenic subjects compared to controls, with unaffected levels of the monomeric forms. Similarly, another study reported a decrease in mGlu3R protein in the PFC (BA46) of schizophrenic subjects, but not in other areas such as temporal or motor cortices (Ghose et al., 2009). This study reported unchanged mGlu2R protein expression in schizophrenia (Ghose et al., 2009), however, the antibody used to assess the mGlu2R immunoreactivity was not previously validated and was actually measuring the subtype 2 of the AMPA ionotropic glutamate receptor1.
Four independent studies have investigated the radioligand binding density of mGlu2/3Rs in the postmortem brain of schizophrenic subjects. Gonzalez-Maeso et al. (2008), reported a decrease in the binding density of mGlu2/3Rs in the PFC (BA9) of schizophrenic subjects respect to matched controls using the mGlu2/3R antagonist [3H]LY341495. Other studies, however, reported no differences in mGlu2/3R binding density in the PFC (BA46) between schizophrenic subjects and controls when using either the mGlu2/3R agonist [3H]LY354740 (Frank et al., 2011) or the antagonist [3H]LY341495 (McOmish et al., 2016). Two studies have evaluated the mGlu2/3Rs density in other areas besides PFC, founding no differences between schizophrenia and control groups neither in the anterior cingulated cortex (BA24; Matosin et al., 2014; McOmish et al., 2016) nor in the visual cortex (BA17; McOmish et al., 2016).
Taking into account the different findings from postmortem studies the status of Group II mGluRs in schizophrenia remains unclear. Thus, further investigation of the level of expression and function of mGlu2/3Rs in postmortem human brain of schizophrenic subjects and controls is needed.
Genetic Studies
While other factors besides genetics are definitely involved, investigation of the genetic alterations responsible for schizophrenia represents a useful approach to better understand the cause of the disease (Harrison and Weinberger, 2005). As mentioned above, mGlu2R gen (GRM2) has been mapped to chromosome 3p21.1–p21.2 (Marti et al., 2002a), and linkage studies of schizophrenia show no positive results regarding this region (Moreno et al., 2009). Moreover, in a population-based genetic study for candidate polymorphisms in alleles of the mGlu2R gene, no association was found between such polymorphisms and schizophrenia (Joo et al., 2001).
Genetic association analyses have consistently suggested an association between SNPs in the mGlu3R gene (GRM3) and schizophrenia (Fujii et al., 2003; Egan et al., 2004; Chen et al., 2005; Sartorius et al., 2008; Cherlyn et al., 2010) including a recent multi-stage schizophrenia genome-wide association study (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). However, this association was not replicated in other population-based genetic studies (Marti et al., 2002b; Tochigi et al., 2006). GRM3 polymorphisms associated with schizophrenia are often located in a non-coding region. Therefore, the mechanism underlying the association between GRM3 and schizophrenia is not clear. GRM3 polymorphisms have been associated with negative symptom improvement during olanzapine treatment (Bishop et al., 2005). Egan et al. (2004) proposed a specific pathway by which GRM3 genotype could alter the glutamatergic transmission leading to an increase in the risk for schizophrenia. These authors found an association between an intronic variation in GRM3 and a reduced performance on cognitive tests of prefrontal and hippocampal function, which are schizophrenia related phenotypes. Moreover, in postmortem human PFC, GRM3 variant carriers showed lower mRNA levels of the glial glutamate transporter EAAT2. Therefore, authors suggest that the pathophysiological mechanism underlying schizophrenia may involve altered mGlu3 transcription/expression and altered glutamate neurotransmission related to a reduced expression of the glial glutamate transporter EAAT2 (Egan et al., 2004).
Group II mGluRs As Targets for Novel Antipsychotic Drugs
Metabotropic glutamate receptors have received significant interest as potential drug targets. Such interest is due to the belief that metabotropic receptor targeting provides a way for modulating glutamate tone and phasic release in a more subtle manner than that which can be achieved through glutamate ionotropic receptors. Specifically, emerging preclinical and clinical data suggest that activation of Group II mGluRs is a mechanistically novel and promising approach for the treatment of schizophrenia (Marek, 2004; Harrison, 2008; Krivoy et al., 2008; Sodhi et al., 2008; Conn et al., 2009; Chaki, 2010; Fell et al., 2012; Vinson and Conn, 2012; Wierońska et al., 2016).
Preclinical Evidences of Antipsychotic Activity
Extensive preclinical data proved that orthosteric Group II mGluR agonists, including LY354740, LY379268, and LY404039 exhibit antipsychotic-like properties in animal models of schizophrenia. The dissociative drugs PCP and ketamine have been shown to increase the activity of glutamatergic synapses in the PFC (Adams and Moghaddam, 1998; Lorrain et al., 2003) and different studies have confirmed that Group II mGluR agonists are able to reverse this effect (Moghaddam and Adams, 1998; Marek et al., 2000; Lorrain et al., 2003). It has been also shown that the systemic administration of the mGlu2/3R agonists LY379268 and LY404039 is able to increase dopamine extracellular levels in rodent’s frontal cortex (Cartmell et al., 2001; Rorick-Kehn et al., 2007b). Increases in cortical dopamine levels have been linked to the improvement of negative symptoms in schizophrenia. Additionally, atypical antipsychotic drugs such as clozapine and risperidone also produce a cortical increase of this neurotransmitter (Cartmell et al., 2001). Besides dopamine, cortical serotonin is also enhanced by the systemic administration of mGlu2/3R agonists as well as by the atypical antipsychotic risperidone (Cartmell et al., 2001; Rorick-Kehn et al., 2007b). These neurochemical similarities between mGlu2/3R agonists and already known antipsychotic drugs provide support for the potential antipsychotic properties of the firsts.
In addition to this neurochemical evidence, Group II mGluR agonists have also shown the ability to reverse the behavioral effects induced by psychotomimetic drugs in several animal models predicting their potential as antipsychotic agents (Wierońska et al., 2016). In this sense, locomotor response to psychostimulants in rodents represents an animal correlate of schizophrenia positive symptoms (Arguello and Gogos, 2006). Regarding this aspect, the mGlu2/3R agonist LY354740 administered at a dose that did not affect spontaneous locomotor activity itself has proved to attenuate PCP-induced locomotor hyperactivity and stereotypies (Moghaddam and Adams, 1998). Similar results have been reported with other mGlu2/3R agonists, like LY404039 (Rorick-Kehn et al., 2007a,b), LY379268 (Cartmell et al., 2000), MGS0008 and MGS0028 (Nakazato et al., 2000). Moreover, the hyperactivity induced by amphetamine has also been shown to be inhibited by both LY379268 and LY404039 (Galici et al., 2005; Rorick-Kehn et al., 2007a,b). Furthermore, the agonists MGS0008, MGS0028, and LY404039 have been reported to inhibit conditioned avoidance responses (Takamori et al., 2003; Rorick-Kehn et al., 2007b) and LY354740 has also shown ability to prevent the PCP-induced deficits on a working memory task (Moghaddam and Adams, 1998), a paradigm that correlates with the cognitive dimension of schizophrenia symptoms (Arguello and Gogos, 2006). Interestingly, Group II mGluR agonists have also been shown to reverse the effects induced by 5-HT2AR hallucinogenic agonists. Thus, mGlu2/3R orthosteric agonists, such as LY379268 and LY354740, reduce the cellular (Zhai et al., 2003; Gonzalez-Maeso et al., 2008), electrophysiological (Marek et al., 2000) and behavioral (Gewirtz and Marek, 2000; Gonzalez-Maeso et al., 2008) effects induced by the hallucinogen 2,5-Dimethoxy-4-iodoamphetamine (DOI). Similar findings have been reported for the selective mGlu2R positive allosteric modulator (PAM) BINA (Benneyworth et al., 2007). In fact, in recent years, more attention has been paid to mGluRs PAMs, especially to those selective for mGlu2R subtype (Ellaithy et al., 2015). In this sense, several preclinical studies have shown efficacy for selective mGlu2R PAMs, i.e., CBiPES, JNJ-40411813, JNJ-42153605, and TASP0433864, in reversing psychotic-like symptoms (Johnson et al., 2005; Hiyoshi et al., 2014; Hikichi et al., 2015; Lavreysen et al., 2015). All of the above-mentioned findings support the potential use of mGlu2/3R agonists for the treatment of schizophrenia symptoms.
In regard to Group II mGluR agonists’ selectivity, Seeman et al. (2008) have suggested that the antipsychotic effects of these compounds may be due to their affinity for D2 receptors (Seeman et al., 2008). However, other laboratories have convincingly demonstrated that this direct effect of Group II mGluR agonists over dopamine receptors is not replicable (Fell et al., 2009; Zysk et al., 2011). In terms of subtype selectivity, it has been suggested that the antipsychotic effects exerted by Group II mGluR agonists are mediated by mGlu2R rather than by mGlu3R (Woolley et al., 2008; Fell et al., 2012; Vinson and Conn, 2012). This hypothesis is based on the results obtained in studies performed with mGlu2R-KO and mGlu3R-KO mice. Thus, antipsychotic actions of Group II agonists LY404039 and LY314582 (racemic mixture of LY354740) were absent in mGlu2R-KO mice but present in mGlu3R-KO mice, strongly implicating mGlu2R as the predominant player in this effect (Spooren et al., 2000; Fell et al., 2008). Similar results were observed for the Group II agonist LY379268 that reversed PCP- and amphetamine-evoked hyperactivity in wild type and mGlu3R-KO mice but not in mGlu2R-KO mice (Woolley et al., 2008). This finding is further supported by the results observed with selective mGlu2R PAMs mentioned above, which have shown efficacy in animal behavioral paradigms used to assess the antipsychotic activity regarding both positive-like symptoms and cognitive impairments (Johnson et al., 2003; Galici et al., 2005, 2006, Govek et al., 2005; Johnson et al., 2005; Pinkerton et al., 2005; Benneyworth et al., 2007; Duplantier et al., 2009; Hiyoshi et al., 2014; Hikichi et al., 2015; Lavreysen et al., 2015). The action of PAMs depends on the presence of a threshold level of agonist, since they do not activate the receptor directly. Hence, it has been postulated that PAMs may provide a safer and better tolerated therapeutic profile than orthosteric compounds, with a more regulated action and a lower potential receptor desensitization (Johnson et al., 2005; Urwyler, 2011). On the other hand, mGlu3Rs have been recently postulated as potential targets to treat the cognitive dysfunction in schizophrenia. Walker et al. (2015) showed that mGlu3Rs can influence synaptic plasticity within mice PFC and that the specific blockade of this receptor impairs learning in a mPFC-dependent fear extinction task. Thus, these authors propose selective PAMs of mGlu3Rs as a novel therapeutic strategy for enhancing prefrontal function in schizophrenic patients (Walker et al., 2015).
Clinical Evidences of Antipsychotic Activity
The selective Group II mGluR agonists have been well-characterized and optimized and have entered into clinical trials for treatment of schizophrenia. The oral prodrug of LY404039, (1R,4S,5S,6S)-2-thiabicyclo[3.1.0]- hexane-4,6-dicarboxylic acid,4-[(2S)-2-amino-4-(methylthio)-1-oxobutyl]amino-, 2,2-dioxide monohydrate (LY2140023) developed by Eli Lilly and Co, showed significant antipsychotic efficacy for both positive and negative symptoms with no major side effects in a trial involving patients suffering from schizophrenia and also showed a better metabolic profile than the comparator olanzapine (Patil et al., 2007). Unfortunately, in the follow-up study, neither LY2140023 nor the comparator olanzapine were more efficacious than placebo as measured by the Positive and Negative Syndrome Scale (PANSS) total score due to higher-than expected placebo response (Kinon et al., 2011), thus, the results of this study were considered to be inconclusive (Kinon et al., 2011). In a more recent multicenter, randomized, double-blind, phase II study, LY2140023 monohydrate was again tested against placebo and the active control risperidone in schizophrenic patients with an acute exacerbation of symptoms. The primary outcome assessed change from baseline in the PANSS total score in an overall schizophrenia population and a predefined subpopulation which excluded non-Hispanic white patients with the A/A genotype at the serotonin 2A receptor (5-HT2AR) single nucleotide polymorphism rs7330461 (Downing et al., 2014). Neither LY2140023 dose showed significant improvement compared to placebo in either population. Conversely, risperidone showed a better efficacy than placebo in both populations (Downing et al., 2014). Finally, another study found no benefit of adjunctive treatment with LY2140023 versus placebo for negative symptoms in patients with schizophrenia receiving treatment with second-generation antipsychotics (Stauffer et al., 2013). The mGlu2R PAM JNJ-40411813/ADX71149 from Janssen Pharmaceuticals, Inc. and Addex Therapeutics has also been evaluated in clinical trials for schizophrenia treatment. Data reported in 2012 showed that JNJ-40411813/ADX71149 met the primary objectives of safety and tolerability and demonstrated an effect in patients with residual negative symptoms (Hopkins, 2013). The latest data in two phase-1 studies showed efficacy of the drug reducing the continuity of attention score, improving the quality of episodic memory and reducing the ketamine-induced negative symptoms in healthy volunteers (Salih et al., 2015). Another mGlu2R PAM that has advanced into clinical trials is the AZD8529 from AstraZeneca. Despite this compound failed to be effective in a phase 2 study when administered as monotherapy at a single dose in schizophrenic patients, it remains to be determined whether different treatment regimens or adjunct treatment would provide benefit (Litman et al., 2014).
The Role of the 5-Ht2AR/mGlu2R Heterocomplex
5-HT2AR and mGlu2R have been both implicated in the pathophysiology of schizophrenia and also have been considered as targets for antipsychotic drug development. Previous electrophysiological (Marek et al., 2000), cellular (Benneyworth et al., 2007), neurochemical (Martin-Ruiz et al., 2001), and behavioral (Gewirtz and Marek, 2000) data have suggested an interaction between 5-HT2A and mGlu2 receptors. At present, it has been convincingly proved the existence of a specific functional heteromeric complex formed by 5-HT2A and mGlu2 receptors through which serotonin and glutamate ligands modulate the pattern of G protein-coupling in living cells (Gonzalez-Maeso et al., 2008; Moreno et al., 2012; Baki et al., 2016).
This serotonin-glutamate heterocomplex has been involved in the mechanism of action of both hallucinogenic (Gonzalez-Maeso et al., 2003, 2007; Moreno et al., 2011a) and antipsychotic drugs (Fribourg et al., 2011). Thus, it has been reported that mGlu2R is necessary for at least some of the cellular and behavioral responses induced by hallucinogenic 5-HT2AR agonists such as lysergic acid diethylamide (LSD). It has been shown in [35S]GTPγS binding assays followed by immunoprecipitation with anti-Gq/11 or anti-Gi1,2,3 antibodies that the hallucinogenic 5-HT2AR agonist DOI activates both Gq/11 and Gi proteins only when the 5-HT2AR is expressed as a receptor heterocomplex with the mGlu2R (Gonzalez-Maeso et al., 2008). Moreover, the head-twitch response was not produced by the hallucinogens DOI and LSD in mGlu2R-KO mice (Moreno et al., 2011a). Furthermore, it has been recently proved that the disruption of heteromeric expression with mGlu2R attenuates the psychosis-like effects induced in mice by hallucinogenic 5-HT2AR agonists (Moreno et al., 2012; Figure 2). These authors, not only validate the 5-HT2A/mGlu2 receptor heterocomplex as necessary for the behavioral effects induced by LSD-like drugs in rodents, but also provide the first evidence for the specific residues responsible for a G protein-coupled receptor (GPCR) heteromeric complex formation (Moreno et al., 2012).
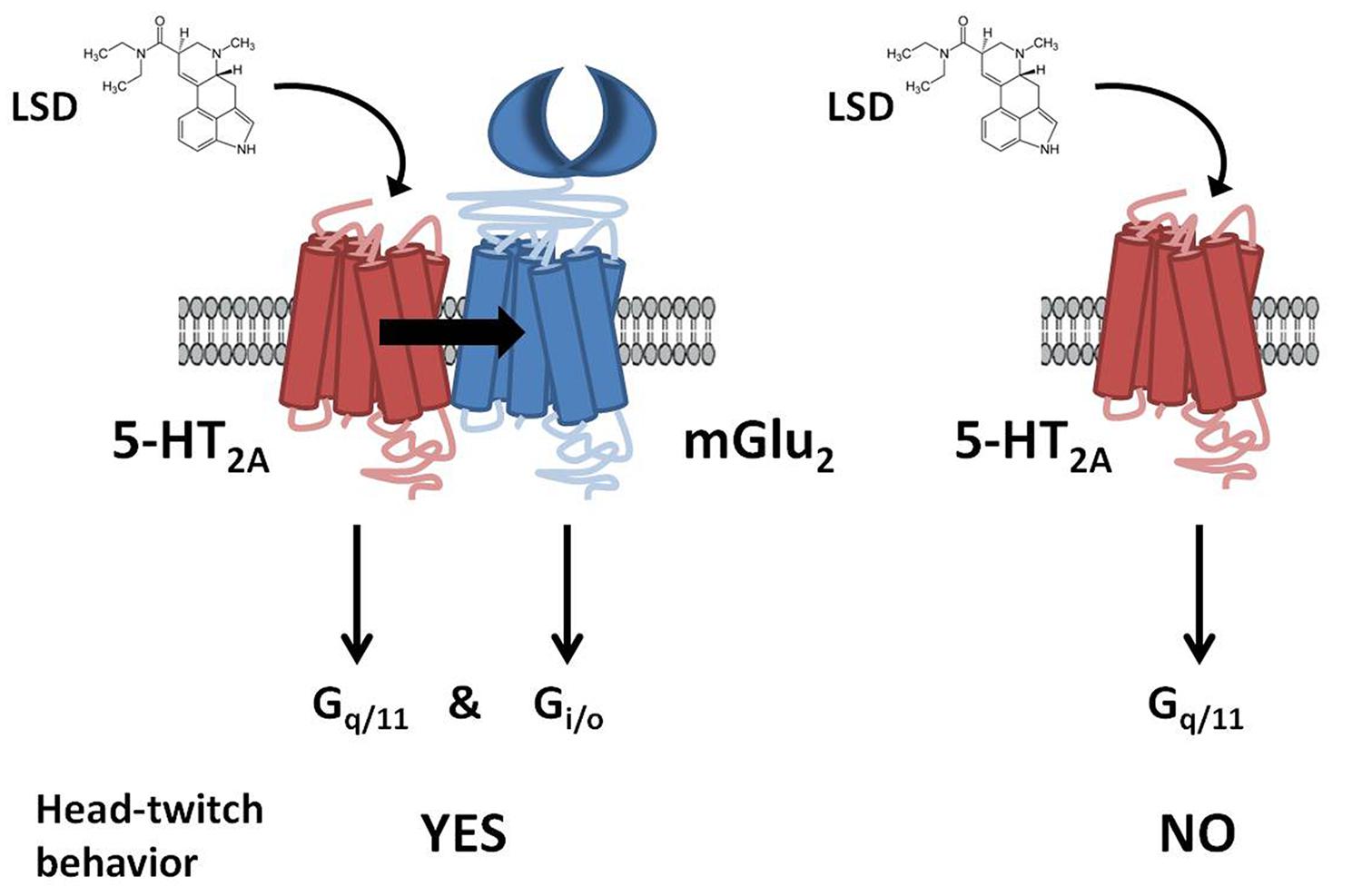
FIGURE 2. G protein-dependent signaling and behavioral responses that require the 5-HT2AR/mGlu2R heterocomplex. LSD acting at the 5-HT2AR/mGlu2R heterocomplex activates both Gq/11- and Gi/o-dependent signaling. In contrast, when 5-HT2AR and mGlu2R are prevented from forming a receptor heterocomplex, activation of 5-HT2AR by LSD elicits characteristic signaling of Gq/11-protein subtypes. Head-twitch behavior is reliably and robustly elicited by hallucinogenic 5-HT2AR agonists, and is absent in mGlu2R-KO mice. Adapted from Gonzalez-Maeso (2011).
Atypical antipsychotic drugs, such as clozapine and risperidone, have a high affinity for the serotonin 5-HT2AR, which preferred signaling pathway is via Gq/11 proteins. Closely related non-antipsychotic drugs, such as ritanserin and methysergide, also block 5-HT2AR function, but they lack comparable neuropsychological effects. In this regard, it has been reported that these ligands inputs are actually integrated by the 5-HT2AR/mGlu2R heterocomplex that modulates signaling outputs and behavioral changes (Fribourg et al., 2011). Thus, serotonergic and glutamatergic drugs would bind to the 5-HT2AR/mGlu2R heterocomplex, which then balances Gi/o- and Gq/11-dependent signaling. The authors also state that 5-HT2AR/mGlu2R -mediated changes in Gi/o and Gq/11 activity could predict the psychoactive behavioral effects of different pharmacological compounds.
Importantly, it has been demonstrated a dysregulation in the binding density of the receptors comprising this heterocomplex in postmortem PFC of schizophrenic subjects (Gonzalez-Maeso et al., 2008). Thus, increased 5-HT2AR and decreased mGlu2/3R binding was found in schizophrenic subjects compared to matched controls (Gonzalez-Maeso et al., 2008). Interestingly, 5-HT2AR density was comparable to control values in those subjects that were under antipsychotic treatment at time of death, whereas mGlu2/3R density remains decreased. Furthermore, the ligand binding interaction between the components of the 5-HT2A/mGlu2 receptor heterocomplex was found up-regulated in the postmortem PFC of schizophrenic subjects as compared with controls (Moreno et al., 2012). Additionally, at the level of signaling, a recent study by same authors showed that mGlu2R-dependent activation of Gq/11, but not Gi/o proteins, is reduced in the postmortem PFC from schizophrenic patients (Moreno et al., 2016). Moreover, recent studies have reported altered densities and behavioral functions of 5-HT2A and mGlu2 receptors in different animal models that resemble some aspects of schizophrenia. Thus, in frontal cortex of mice born to influenza virus-infected mothers, stressed mothers or lipopolysaccharide (LPS)-treated mothers the 5-HT2AR is upregulated (Moreno et al., 2011b; Holloway et al., 2013; Wischhof et al., 2015) and the mGlu2R receptor is downregulated (Moreno et al., 2011b; Holloway et al., 2013). Furthermore, these changes are translated into behavioral alterations, since increased head-twitch response to the hallucinogenic 5-HT2AR agonist DOI and decreased mGlu2-dependent antipsychotic-like effect of the mGlu2/3 agonist LY379268 were observed in these three studies (Moreno et al., 2011b; Holloway et al., 2013; Wischhof et al., 2015).
A pharmacogenetic analysis of the efficacy of LY2140023 monohydrate in the treatment of schizophrenia has demonstrated a genetic association between several single nucleotide polymorphisms located in the gene encoding the 5-HT2AR and the response to LY2140023 treatment (Liu et al., 2012). Thus, a 30-point PANSS total reduction was seen in schizophrenic patients in the most responsive genotype group that presented the single nucleotide polymorphism rs7330461 for the 5-HT2AR gene (Liu et al., 2012). Additionally, a recent study has confirmed that the T/T genotype at rs7330461 is consistently associated with an increased treatment response to pomaglumetad methionil (LY2140023) compared to the A/A genotype (Nisenbaum et al., 2016).
All these facts point to a putative role of the 5-HT2AR/mGlu2R heterocomplex in the antipsychotic-like properties of the Group II mGluR agonists that could also explain the controversial results reported in clinical trials. In this way, it has been demonstrated that chronic atypical antipsychotics downregulate the transcription of mGlu2R through epigenetic modifications (Kurita et al., 2012). This change occurs in concert with a 5-HT2AR-dependent up-regulation and increased binding of histone deacetylase 2 to the mGlu2 promoter (Kurita et al., 2012). This decrease in the mGlu2R expression could induce a lower response to Group II mGluR agonists as LY2140023 in patients previously treated with atypical antipsychotics. Accordingly, a recent study has reanalyzed previous clinical data on LY2140023 treatment defining two patients subpopulations based upon medication exposure during the 2 years before study entry (Kinon et al., 2015). This analysis has demonstrated that patients previously treated with antipsychotics with prominent dopamine 2 receptor antagonist activity who were subsequently treated with LY2140023 monohydrate showed a significantly greater improvement on the PANSS total score from baseline than placebo treated patients. Conversely, patients previously treated with antipsychotics with prominent 5-HT2AR antagonist activity demonstrated no greater response than placebo (Kinon et al., 2015). Thus, as LY2140023 monohydrate treatment is targeted to mGlu2R receptor activation it will induce lower efficacy if the mGlu2R receptor levels are reduced as a consequence of previous treatment with atypical antipsychotics.
Conclusion and Future Directions
Several studies have showed that Group II mGluR agonists exhibit antipsychotic-like properties in preclinical assays. However, when these compounds have been used in human clinical trials the results have been controversial. Recent data suggest that this apparent lack of efficacy in schizophrenic patients may be related to previous exposure to atypical antipsychotics. Moreover, pharmacogenetic assays have demonstrated the influence of genetic variants on response to Group II mGluR agonists in patients with schizophrenia. The fact that Group II mGluRs represent a new target for the treatment of schizophrenia supports the need for additional investigation to establish the real efficacy of these new compounds. Moreover, it is mandatory to clarify if specific subgroups of patients could obtain a greater benefit from using these new drugs.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Spanish MINECO (SAF2013-48586-R), the Basque Government (IT616/13). CM was recipient of a postdoctoral fellowship from the Basque Government, Spain.
Footnotes
References
Abel, K. M., Drake, R., and Goldstein, J. M. (2010). Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428. doi: 10.3109/09540261.2010.515205
Adams, B., and Moghaddam, B. (1998). Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 18, 5545–5554.
Arguello, P. A., and Gogos, J. A. (2006). Modeling madness in mice: one piece at a time. Neuron 52, 179–196. doi: 10.1016/j.neuron.2006.09.023
Aronica, E., Gorter, J. A., Ijlst-Keizers, H., Rozemuller, A. J., Yankaya, B., Leenstra, S., et al. (2003). Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur. J. Neurosci. 17, 2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x
Baki, L., Fribourg, M., Younkin, J., Eltit, J. M., Moreno, J. L., Park, G., et al. (2016). Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells. Pflugers Arch. 468, 775–793. doi: 10.1007/s00424-015-1780-7
Benneyworth, M. A., Xiang, Z., Smith, R. L., Garcia, E. E., Conn, P. J., and Sanders-Bush, E. (2007). A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 72, 477–484. doi: 10.1124/mol.107.035170
Bishop, J. R., Ellingrod, V. L., Moline, J., and Miller, D. (2005). Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr. Res. 77, 253–260. doi: 10.1016/j.schres.2005.04.001
Bullock, W. M., Cardon, K., Bustillo, J., Roberts, R. C., and Perrone-Bizzozero, N. I. (2008). Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am. J. Psychiatry 165, 1594–1603. doi: 10.1176/appi.ajp.2008.07121845
Cartmell, J., Monn, J. A., and Schoepp, D. D. (2000). The mGlu(2/3) receptor agonist LY379268 selectively blocks amphetamine ambulations and rearing. Eur. J. Pharmacol. 400, 221–224. doi: 10.1016/S0014-2999(00)00423-4
Cartmell, J., Perry, K. W., Salhoff, C. R., Monn, J. A., and Schoepp, D. D. (2001). Acute increases in monoamine release in the rat prefrontal cortex by the mGlu2/3 agonist LY379268 are similar in profile to risperidone, not locally mediated, and can be elicited in the presence of uptake blockade. Neuropharmacology 40, 847–855. doi: 10.1016/S0028-3908(01)00034-X
Cartmell, J., and Schoepp, D. D. (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 75, 889–907. doi: 10.1046/j.1471-4159.2000.0750889.x
Chaki, S. (2010). Group II metabotropic glutamate receptor agonists as a potential drug for schizophrenia. Eur. J. Pharmacol. 639, 59–66. doi: 10.1016/j.ejphar.2009.12.041
Chen, Q., He, G., Chen, Q., Wu, S., Xu, Y., Feng, G., et al. (2005). A case-control study of the relationship between the metabotropic glutamate receptor 3 gene and schizophrenia in the Chinese population. Schizophr. Res. 73, 21–26. doi: 10.1016/j.schres.2004.07.002
Cherlyn, S. Y., Woon, P. S., Liu, J. J., Ong, W. Y., Tsai, G. C., and Sim, K. (2010). Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci. Biobehav. Rev. 34, 958–977. doi: 10.1016/j.neubiorev.2010.01.002
Cid, J. M., Trabanco, A. A., and Lavreysen, H. (2015). Metabotropic glutamate receptor 2 activators. Top. Med. Chem. 13, 101–142. doi: 10.1007/7355_2014_48
Conn, P. J., Lindsley, C. W., and Jones, C. K. (2009). Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 30, 25–31. doi: 10.1016/j.tips.2008.10.006
Conn, P. J., and Pin, J. P. (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237. doi: 10.1146/annurev.pharmtox.37.1.205
Corti, C., Crepaldi, L., Mion, S., Roth, A. L., Xuereb, J. H., and Ferraguti, F. (2007). Altered dimerization of metabotropic glutamate receptor 3 in schizophrenia. Biol. Psychiatry 62, 747–755. doi: 10.1016/j.biopsych.2006.12.005
Coyle, J. T., Basu, A., Benneyworth, M., Balu, D., and Konopaske, G. (2012). Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb. Exp. Pharmacol. 213, 267–295. doi: 10.1007/978-3-642-25758-2_10
Crook, J. M., Akil, M., Law, B. C., Hyde, T. M., and Kleinman, J. E. (2002). Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann’s area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol. Psychiatry 7, 157–164. doi: 10.1038/sj.mp.4000966
Dominguez, C., Prieto, L., Valli, M. J., Massey, S. M., Bures, M., Wright, R. A., et al. (2005). Methyl substitution of 2-aminobicyclo[3.1.0]hexane 2,6-dicarboxylate (LY354740) determines functional activity at metabotropic glutamate receptors: identification of a subtype selective mGlu2 receptor agonist. J. Med. Chem. 48, 3605–3612. doi: 10.1021/jm040222y
Downing, A. M., Kinon, B. J., Millen, B. A., Zhang, L., Liu, L., Morozova, M. A., et al. (2014). A double-blind, placebo-controlled comparator study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry 14:351. doi: 10.1186/s12888-014-0351-3
Duplantier, A. J., Efremov, I., Candler, J., Doran, A. C., Ganong, A. H., Haas, J. A., et al. (2009). 3-Benzyl-1,3-oxazolidin-2-ones as mGluR2 positive allosteric modulators: hit-to lead and lead optimization. Bioorg. Med. Chem. Lett. 19, 2524–2529. doi: 10.1016/j.bmcl.2009.03.032
Egan, M. F., Straub, R. E., Goldberg, T. E., Yakub, I., Callicott, J. H., Hariri, A. R., et al. (2004). Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 101, 12604–12609. doi: 10.1073/pnas.0405077101
Ellaithy, A., Younkin, J., Gonzalez-Maeso, J., and Logothetis, D. E. (2015). Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 38, 506–516. doi: 10.1016/j.tins.2015.06.002
Fell, M. J., McKinzie, D. L., Monn, J. A., and Svensson, K. A. (2012). Group II metabotropic glutamate receptor agonists and positive allosteric modulators as novel treatments for schizophrenia. Neuropharmacology 62, 1473–1483. doi: 10.1016/j.neuropharm.2011.06.007
Fell, M. J., Perry, K. W., Falcone, J. F., Johnson, B. G., Barth, V. N., Rash, K. S., et al. (2009). In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-bicaroxylate monohydrate (LY354740) and (-)-2-oxa-4-aminobicyclo[3.1.0] Hexane-4,6-dicarboxylic acid (LY379268). J. Pharmacol. Exp. Ther. 331, 1126–1136. doi: 10.1124/jpet.109.160598
Fell, M. J., Svensson, K. A., Johnson, B. G., and Schoepp, D. D. (2008). Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J. Pharmacol. Exp. Ther. 326, 209–217. doi: 10.1124/jpet.108.136861
Field, J. R., Walker, A. G., and Conn, P. J. (2011). Targeting glutamate synapses in schizophrenia. Trends Mol. Med. 17, 689–698. doi: 10.1016/j.molmed.2011.08.004
Frank, E., Newell, K. A., and Huang, X. F. (2011). Density of metabotropic glutamate receptors 2 and 3 (mGluR2/3) in the dorsolateral prefrontal cortex does not differ with schizophrenia diagnosis but decreases with age. Schizophr. Res. 128, 56–60. doi: 10.1016/j.schres.2011.01.008
Fribourg, M., Moreno, J. L., Holloway, T., Provasi, D., Baki, L., Mahajan, R., et al. (2011). Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147, 1011–1023. doi: 10.1016/j.cell.2011.09.055
Fujii, Y., Shibata, H., Kikuta, R., Makino, C., Tani, A., Hirata, N., et al. (2003). Positive associations of polymorphisms in the metabotropic glutamate receptor type 3 gene (GRM3) with schizophrenia. Psychiatr. Genet. 13, 71–76. doi: 10.1097/01.ypg.0000056682.82896.b0
Galici, R., Echemendia, N. G., Rodriguez, A. L., and Conn, P. J. (2005). A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J. Pharmacol. Exp. Ther. 315, 1181–1187. doi: 10.1124/jpet.105.091074
Galici, R., Jones, C. K., Hemstapat, K., Nong, Y., Echemendia, N. G., Williams, L. C., et al. (2006). Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J. Pharmacol. Exp. Ther. 318, 173–185. doi: 10.1124/jpet.106.102046
Gao, X. M., Sakai, K., Roberts, R. C., Conley, R. R., Dean, B., and Tamminga, C. A. (2000). Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am. J. Psychiatry 157, 1141–1149. doi: 10.1176/appi.ajp.157.7.1141
Gewirtz, J. C., and Marek, G. J. (2000). Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23, 569–576. doi: 10.1016/S0893-133X(00)00136-6
Ghose, S., Crook, J. M., Bartus, C. L., Sherman, T. G., Herman, M. M., Hyde, T. M., et al. (2008). Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int. J. Neurosci. 118, 1609–1627. doi: 10.1080/00207450802330702
Ghose, S., Gleason, K. A., Potts, B. W., Lewis-Amezcua, K., and Tamminga, C. A. (2009). Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am. J. Psychiatry 166, 812–820. doi: 10.1176/appi.ajp.2009.08091445
Gonzalez-Maeso, J. (2011). GPCR oligomers in pharmacology and signaling. Mol. Brain 4:20. doi: 10.1186/1756-6606-4-20
Gonzalez-Maeso, J., Ang, R. L., Yuen, T., Chan, P., Weisstaub, N. V., Lopez-Gimenez, J. F., et al. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97. doi: 10.1038/nature06612
Gonzalez-Maeso, J., Weisstaub, N. V., Zhou, M., Chan, P., Ivic, L., Ang, R., et al. (2007). Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452. doi: 10.1016/j.neuron.2007.01.008
Gonzalez-Maeso, J., Yuen, T., Ebersole, B. J., Wurmbach, E., Lira, A., Zhou, M., et al. (2003). Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 23, 8836–8843.
Govek, S. P., Bonnefous, C., Hutchinson, J. H., Kamenecka, T., McQuiston, J., Pracitto, R., et al. (2005). Benzazoles as allosteric potentiators of metabotropic glutamate receptor 2 (mGluR2): efficacy in an animal model for schizophrenia. Bioorg. Med. Chem. Lett. 15, 4068–4072. doi: 10.1016/j.bmcl.2005.06.017
Gu, G., Lorrain, D. S., Wei, H., Cole, R. L., Zhang, X., Daggett, L. P., et al. (2008). Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: implication in emotional responses and central disinhibition. Brain Res. 1197, 47–62. doi: 10.1016/j.brainres.2007.12.057
Gupta, D. S., McCullumsmith, R. E., Beneyto, M., Haroutunian, V., Davis, K. L., and Meador-Woodruff, J. H. (2005). Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57, 123–131. doi: 10.1002/syn.20164
Harrison, P. J. (2008). Metabotropic glutamate receptor agonists for schizophrenia. Br. J. Psychiatry 192, 86–87. doi: 10.1192/bjp.bp.107.045088
Harrison, P. J., and Weinberger, D. R. (2005). Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry 10, 40–68. doi: 10.1038/sj.mp.4001558
Hemstapat, K., Da Costa, H., Nong, Y., Brady, A. E., Luo, Q., Niswender, C. M., et al. (2007). A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 322, 254–264. doi: 10.1124/jpet.106.117093
Hikichi, H., Hiyoshi, T., Marumo, T., Tomishima, Y., Kaku, A., Iida, I., et al. (2015). Antipsychotic profiles of TASP0443294, a novel and orally active positive allosteric modulator of metabotropic glutamate 2 receptor. J. Pharmacol. Sci. 127, 352–361. doi: 10.1016/j.jphs.2015.02.004
Hiyoshi, T., Marumo, T., Hikichi, H., Tomishima, Y., Urabe, H., Tamita, T., et al. (2014). Neurophysiologic and antipsychotic profiles of TASP0433864, a novel positive allosteric modulator of metabotropic glutamate 2 receptor. J. Pharmacol. Exp. Ther. 351, 642–653. doi: 10.1124/jpet.114.218651
Holloway, T., Moreno, J. L., Umali, A., Rayannavar, V., Hodes, G. E., Russo, S. J., et al. (2013). Prenatal stress induces schizophrenia-like alterations of serotonin 2A and metabotropic glutamate 2 receptors in the adult offspring: role of maternal immune system. J. Neurosci. 33, 1088–1098. doi: 10.1523/JNEUROSCI.2331-12.2013
Hopkins, C. R. (2013). Is there a path forward for mGlu(2) positive allosteric modulators for the treatment of schizophrenia? ACS Chem. Neurosci. 4, 211–213. doi: 10.1021/cn400023y
Iacovelli, L., Bruno, V., Salvatore, L., Melchiorri, D., Gradini, R., Caricasole, A., et al. (2002). Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol-3-kinase pathways. J. Neurochem. 82, 216–223. doi: 10.1046/j.1471-4159.2002.00929.x
Javitt, D. C. (2010). Glutamatergic theories of schizophrenia. Isr. J. Psychiatry Relat. Sci. 47, 4–16.
Javitt, D. C., and Zukin, S. R. (1991). Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 148, 1301–1308. doi: 10.1176/ajp.148.10.1301
Johnson, M. P., Baez, M., Jagdmann, G. E. Jr., Britton, T. C., Large, T. H., Callagaro, D. O., et al. (2003). Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2- trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J. Med. Chem. 46, 3189–3192. doi: 10.1021/jm034015u
Johnson, M. P., Barda, D., Britton, T. C., Emkey, R., Hornback, W. J., Jagdmann, G. E., et al. (2005). Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology (Berl.) 179, 271–283. doi: 10.1007/s00213-004-2099-9
Joo, A., Shibata, H., Ninomiya, H., Kawasaki, H., Tashiro, N., and Fukumaki, Y. (2001). Structure and polymorphisms of the human metabotropic glutamate receptor type 2 gene (GRM2): analysis of association with schizophrenia. Mol. Psychiatry 6, 186–192. doi: 10.1038/sj.mp.4000841
Kinon, B. J., Millen, B. A., Zhang, L., and McKinzie, D. L. (2015). Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol. Psychiatry 78, 754–762. doi: 10.1016/j.biopsych.2015.03.016
Kinon, B. J., Zhang, L., Millen, B. A., Osuntokun, O. O., Williams, J. E., Kollack-Walker, S., et al. (2011). A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 31, 349–355. doi: 10.1097/JCP.0b013e318218dcd5
Krivoy, A., Fischel, T., and Weizman, A. (2008). The possible involvement of metabotropic glutamate receptors in schizophrenia. Eur. Neuropsychopharmacol. 18, 395–405. doi: 10.1016/j.euroneuro.2007.11.001
Krystal, J. H., Perry, E. B. Jr., Gueorguieva, R., Belger, A., Madonick, S. H., Abi-Dargham, A., et al. (2005). Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch. Gen. Psychiatry 62, 985–994. doi: 10.1001/archpsyc.62.9.985
Kurita, M., Holloway, T., Garcia-Bea, A., Kozlenkov, A., Friedman, A. K., Moreno, J. L., et al. (2012). HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat. Neurosci. 15, 1245–1254. doi: 10.1038/nn.3181
Lavreysen, H., Langlois, X., Donck, L. V., Nunez, J. M., Pype, S., Lutjens, R., et al. (2015). Preclinical evaluation of the antipsychotic potential of the mGlu2-positive allosteric modulator JNJ-40411813. Pharmacol. Res. Perspect. 3:e00097. doi: 10.1002/prp2.97
Litman, R. E., Smith, M. A., Doherty, J., Cross, A., Raines, S., and Zukin, S. (2014). AZD8529, A positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: a proof of principle study. Schizophr. Res. 153, S176. doi: 10.1016/j.schres.2016.02.001
Liu, W., Downing, A. C., Munsie, L. M., Chen, P., Reed, M. R., Ruble, C. L., et al. (2012). Pharmacogenetic analysis of the mGlu2/3 agonist LY2140023 monohydrate in the treatment of schizophrenia. Pharmacogenomics J. 12, 246–254. doi: 10.1038/tpj.2010.90
Lorrain, D. S., Baccei, C. S., Bristow, L. J., Anderson, J. J., and Varney, M. A. (2003). Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117, 697–706. doi: 10.1016/S0306-4522(02)00652-8
Marek, G. J. (2004). Metabotropic glutamate 2/3 receptors as drug targets. Curr. Opin. Pharmacol. 4, 18–22. doi: 10.1016/j.coph.2003.10.003
Marek, G. J. (2010). Metabotropic glutamate2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur. J. Pharmacol. 639, 81–90. doi: 10.1016/j.ejphar.2010.02.058
Marek, G. J., Wright, R. A., Schoepp, D. D., Monn, J. A., and Aghajanian, G. K. (2000). Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 292, 76–87.
Marti, S. B., Cichon, S., Propping, P., and Nothen, M. (2002a). Human metabotropic glutamate receptor 2 gene (GRM2): chromosomal sublocalization (3p21.1-p21.2) and genomic organization. Am. J. Med. Genet. 114, 12–14. doi: 10.1002/ajmg.1622
Marti, S. B., Cichon, S., Propping, P., and Nothen, M. (2002b). Metabotropic glutamate receptor 3 (GRM3) gene variation is not associated with schizophrenia or bipolar affective disorder in the German population. Am. J. Med. Genet. 114, 46–50. doi: 10.1002/ajmg.1624
Martin-Ruiz, R., Puig, M. V., Celada, P., Shapiro, D. A., Roth, B. L., Mengod, G., et al. (2001). Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J. Neurosci. 21, 9856–9866.
Matosin, N., Fernandez-Enright, F., Frank, E., Deng, C., Wong, J., Huang, X. F., et al. (2014). Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: implications for novel mGluR-based therapeutics. J. Psychiatry Neurosci. 39, 407–416. doi: 10.1503/jpn.130242
McGrath, J., Saha, S., Chant, D., and Welham, J. (2008). Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 30, 67–76. doi: 10.1093/epirev/mxn001
McOmish, C. E., Pavey, G., Gibbons, A., Hopper, S., Udawela, M., Scarr, E., et al. (2016). Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J. Affect. Disord. 190, 241–248. doi: 10.1016/j.jad.2015.10.004
Miyamoto, S., Miyake, N., Jarskog, L. F., Fleischhacker, W. W., and Lieberman, J. A. (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry 17, 1206–1227. doi: 10.1038/mp.2012.47
Moghaddam, B., and Adams, B. W. (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349–1352. doi: 10.1126/science.281.5381.1349
Monn, J. A., Valli, M. J., Massey, S. M., Wright, R. A., Salhoff, C. R., Johnson, B. G., et al. (1997). Design, synthesis, and pharmacological characterization of (++)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J. Med. Chem. 40, 528–537. doi: 10.1021/jm9606756
Moreno, J. L., Holloway, T., Albizu, L., Sealfon, S. C., and Gonzalez-Maeso, J. (2011a). Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 493, 76–79. doi: 10.1016/j.neulet.2011.01.046
Moreno, J. L., Kurita, M., Holloway, T., Lopez, J., Cadagan, R., Martinez-Sobrido, L., et al. (2011b). Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT(2)A and mGlu(2) receptors in the adult offspring. J. Neurosci. 31, 1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011
Moreno, J. L., Miranda-Azpiazu, P., Garcia-Bea, A., Younkin, J., Cui, M., Kozlenkov, A., et al. (2016). Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci. Signal. 9:ra5. doi: 10.1126/scisignal.aab0467
Moreno, J. L., Muguruza, C., Umali, A., Mortillo, S., Holloway, T., Pilar-Cuellar, F., et al. (2012). Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J. Biol. Chem. 287, 44301–44319. doi: 10.1074/jbc.M112.413161
Moreno, J. L., Sealfon, S. C., and Gonzalez-Maeso, J. (2009). Group II metabotropic glutamate receptors and schizophrenia. Cell Mol. Life Sci. 66, 3777–3785. doi: 10.1007/s00018-009-0130-3
Mueser, K. T., and McGurk, S. R. (2004). Schizophrenia. Lancet 363, 2063–2072. doi: 10.1016/S0140-6736(04)16458-1
Nakanishi, S. (1992). Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597–603. doi: 10.1126/science.1329206
Nakazato, A., Kumagai, T., Sakagami, K., Yoshikawa, R., Suzuki, Y., Chaki, S., et al. (2000). Synthesis, SARs, and pharmacological characterization of 2-amino-3 or 6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent, selective, and orally active group II metabotropic glutamate receptor agonists. J. Med. Chem. 43, 4893–4909. doi: 10.1021/jm000346k
Neki, A., Ohishi, H., Kaneko, T., Shigemoto, R., Nakanishi, S., and Mizuno, N. (1996). Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci. Lett. 202, 197–200. doi: 10.1016/0304-3940(95)12248-6
Nicoletti, F., Bockaert, J., Collingridge, G. L., Conn, P. J., Ferraguti, F., Schoepp, D. D., et al. (2011). Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041. doi: 10.1016/j.neuropharm.2010.10.022
Nisenbaum, L. K., Downing, A. M., Zhao, F., Millen, B. A., Munsie, L., Kinon, B. J., et al. (2016). Serotonin 2A Receptor SNP rs7330461 association with treatment response to pomaglumetad methionil in patients with schizophrenia. J. Pers. Med. 6:E910.3390/jm6010009. doi: 10.3390/jpm6010009
Niswender, C. M., and Conn, P. J. (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322. doi: 10.1146/annurev.pharmtox.011008.145533
Ohishi, H., Neki, A., and Mizuno, N. (1998). Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci. Res. 30, 65–82. doi: 10.1016/S0168-0102(97)00120-X
Ohishi, H., Shigemoto, R., Nakanishi, S., and Mizuno, N. (1993). Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J. Comp. Neurol. 335, 252–266. doi: 10.1002/cne.903350209
Ohnuma, T., Augood, S. J., Arai, H., McKenna, P. J., and Emson, P. C. (1998). Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res. Mol. Brain Res. 56, 207–217. doi: 10.1016/S0169-328X(98)00063-1
Olney, J. W., and Farber, N. B. (1995). Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry 52, 998–1007. doi: 10.1001/archpsyc.1995.03950240016004
Patil, S. T., Zhang, L., Martenyi, F., Lowe, S. L., Jackson, K. A., Andreev, B. V., et al. (2007). Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 13, 1102–1107. doi: 10.1038/nm1007-1264
Petralia, R. S., Wang, Y. X., Niedzielski, A. S., and Wenthold, R. J. (1996). The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience 71, 949–976. doi: 10.1016/0306-4522(95)00533-1
Pin, J. P., and Duvoisin, R. (1995). The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26. doi: 10.1016/0028-3908(94)00129-G
Pinkerton, A. B., Cube, R. V., Hutchinson, J. H., James, J. K., Gardner, M. F., Rowe, B. A., et al. (2005). Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorg. Med. Chem. Lett. 15, 1565–1571. doi: 10.1016/j.bmcl.2005.01.077
Richardson-Burns, S. M., Haroutunian, V., Davis, K. L., Watson, S. J., and Meador-Woodruff, J. H. (2000). Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol. Psychiatry 47, 22–28. doi: 10.1016/S0006-3223(99)00207-3
Rorick-Kehn, L. M., Johnson, B. G., Burkey, J. L., Wright, R. A., Calligaro, D. O., Marek, G. J., et al. (2007a). Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039). J. Pharmacol. Exp. Ther. 321, 308–317.
Rorick-Kehn, L. M., Johnson, B. G., Knitowski, K. M., Salhoff, C. R., Witkin, J. M., Perry, K. W., et al. (2007b). In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl.) 193, 121–136. doi: 10.1007/s00213-007-0758-3
Salih, H., Anghelescu, I., Kezic, I., Sinha, V., Hoeben, E., Van Nueten, L., et al. (2015). Pharmacokinetic and pharmacodynamic characterisation of JNJ-40411813, a positive allosteric modulator of mGluR2, in two randomised, double-blind phase-I studies. J. Psychopharmacol. 29, 414–425. doi: 10.1177/0269881115573403
Sartorius, L. J., Nagappan, G., Lipska, B. K., Lu, B., Sei, Y., Ren-Patterson, R., et al. (2006). Alternative splicing of human metabotropic glutamate receptor 3. J. Neurochem. 96, 1139–1148. doi: 10.1111/j.1471-4159.2005.03609.x
Sartorius, L. J., Weinberger, D. R., Hyde, T. M., Harrison, P. J., Kleinman, J. E., and Lipska, B. K. (2008). Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology 33, 2626–2634. doi: 10.1038/sj.npp.1301669
Scherer, S. W., Duvoisin, R. M., Kuhn, R., Heng, H. H., Belloni, E., and Tsui, L. C. (1996). Localization of two metabotropic glutamate receptor genes, GRM3 and GRM8, to human chromosome 7q. Genomics 31, 230–233. doi: 10.1006/geno.1996.0036
Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. doi: 10.1038/nature13595
Schoepp, D. D. (2001). Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 299, 12–20.
Schoepp, D. D., Jane, D. E., and Monn, J. A. (1999). Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 38, 1431–1476. doi: 10.1016/S0028-3908(99)00092-1
Seeman, P., Caruso, C., and Lasaga, M. (2008). Dopamine partial agonist actions of the glutamate receptor agonists LY 354,740 and LY 379,268. Synapse 62, 154–158. doi: 10.1002/syn.20482
Smith, T. E., Weston, C. A., and Lieberman, J. A. (2009). Schizophrenia (maintenance treatment). BMJ Clin. Evid. 2009:1007.
Sodhi, M., Wood, K. H., and Meador-Woodruff, J. (2008). Role of glutamate in schizophrenia: integrating excitatory avenues of research. Expert Rev. Neurother 8, 1389–1406. doi: 10.1586/14737175.8.9.1389
Spooren, W. P., Gasparini, F., van der Putten, H., Koller, M., Nakanishi, S., and Kuhn, R. (2000). Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur. J. Pharmacol. 397, R1–R2. doi: 10.1016/S0014-2999(00)00269-7
Stauffer, V. L., Millen, B. A., Andersen, S., Kinon, B. J., Lagrandeur, L., Lindenmayer, J. P., et al. (2013). Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr. Res. 150, 434–441. doi: 10.1016/j.schres.2013.08.020
Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D., et al. (2009). Common variants conferring risk of schizophrenia. Nature 460, 744–747. doi: 10.1038/nature08186
Stone, J. M. (2009). Imaging the glutamate system in humans: relevance to drug discovery for schizophrenia. Curr. Pharm. Des. 15, 2594–2602. doi: 10.2174/138161209788957438
Stone, J. M., Erlandsson, K., Arstad, E., Squassante, L., Teneggi, V., Bressan, R. A., et al. (2008). Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology (Berl.) 197, 401–408. doi: 10.1007/s00213-007-1047-x
Takamori, K., Hirota, S., Chaki, S., and Tanaka, M. (2003). Antipsychotic action of selective group II metabotropic glutamate receptor agonist MGS0008 and MGS0028 on conditioned avoidance responses in the rat. Life Sci. 73, 1721–1728. doi: 10.1016/S0024-3205(03)00509-5
Tamaru, Y., Nomura, S., Mizuno, N., and Shigemoto, R. (2001). Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 106, 481–503. doi: 10.1016/S0306-4522(01)00305-0
Tochigi, M., Suga, M., Ohashi, J., Otowa, T., Yamasue, H., Kasai, K., et al. (2006). No association between the metabotropic glutamate receptor type 3 gene (GRM3) and schizophrenia in a Japanese population. Schizophr. Res. 88, 260–264. doi: 10.1016/j.schres.2006.07.008
Urwyler, S. (2011). Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol. Rev. 63, 59–126. doi: 10.1124/pr.109.002501
Vinson, P. N., and Conn, P. J. (2012). Metabotropic glutamate receptors as therapeutic targets for schizophrenia. Neuropharmacology 62, 1461–1472. doi: 10.1016/j.neuropharm.2011.05.005
Vos, T., Barber, R. M., Bell, B., Bertozzi-Villa, A., Biryukov, S., Bolliger, I., et al. (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800. doi: 10.1016/S0140-6736(15)60692-4
Walker, A. G., Wenthur, C. J., Xiang, Z., Rook, J. M., Emmitte, K. A., Niswender, C. M., et al. (2015). Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc. Natl. Acad. Sci. U.S.A. 112, 1196–1201. doi: 10.1073/pnas.1416196112
Wierońska, J. M., Zorn, S. H., Doller, D., and Pilc, A. (2016). Metabotropic glutamate receptors as targets for new antipsychotic drugs: historical perspective and critical comparative assessment. Pharmacol. Ther. 157, 10–27. doi: 10.1016/j.pharmthera.2015.10.007
Wischhof, L., Irrsack, E., Dietz, F., and Koch, M. (2015). Maternal lipopolysaccharide treatment differentially affects 5-HT(2A) and mGlu2/3 receptor function in the adult male and female rat offspring. Neuropharmacology 97, 275–288. doi: 10.1016/j.neuropharm.2015.05.029
Woltering, T. J., Adam, G., Wichmann, J., Goetschi, E., Kew, J. N., Knoflach, F., et al. (2008a). Synthesis and characterization of 8-ethynyl-1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 2. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg. Med. Chem. Lett. 18, 1091–1095. doi: 10.1016/j.bmcl.2007.12.005
Woltering, T. J., Wichmann, J., Goetschi, E., Adam, G., Kew, J. N., Knoflach, F., et al. (2008b). Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg. Med. Chem. Lett. 18, 2725–2729. doi: 10.1016/j.bmcl.2008.02.076
Woolley, M. L., Pemberton, D. J., Bate, S., Corti, C., and Jones, D. N. (2008). The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl.) 196, 431–440. doi: 10.1007/s00213-007-0974-x
World Health Organization (2008). The Global Burden of Disease: 2004 Update. Rome: World Health Organization.
Wright, R. A., Arnold, M. B., Wheeler, W. J., Ornstein, P. L., and Schoepp, D. D. (2001). [H-3]LY341495 binding to group II metabotropic glutamate receptors in rat brain. J. Pharmacol. Exp. Ther. 298, 453–460.
Zhai, Y., George, C. A., Zhai, J., Nisenbaum, E. S., Johnson, M. P., and Nisenbaum, L. K. (2003). Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 28, 45–52. doi: 10.1038/sj.npp.1300013
Keywords: antipsychotic, glutamate, human brain, mGlu2R receptors, schizophrenia
Citation: Muguruza C, Meana JJ and Callado LF (2016) Group II Metabotropic Glutamate Receptors as Targets for Novel Antipsychotic Drugs. Front. Pharmacol. 7:130. doi: 10.3389/fphar.2016.00130
Received: 01 March 2016; Accepted: 05 May 2016;
Published: 20 May 2016.
Edited by:
Francisco Ciruela, Universitat de Barcelona, SpainReviewed by:
Sâmia R. L. Joca, University of Sao Paulo, BrazilMarián Castro, University of Santiago de Compostela, Spain
Copyright © 2016 Muguruza, Meana and Callado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis F. Callado, lf.callado@ehu.eus
 Carolina Muguruza
Carolina Muguruza J. Javier Meana1,2
J. Javier Meana1,2 Luis F. Callado
Luis F. Callado