Corrigendum: Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy
- 1Department of Medicine, Maimonides Medical Center, Brooklyn, NY, USA
- 2Department of Pediatrics, Maimonides Medical Center, Brooklyn, NY, USA
- 3Medical Director, Yuma Regional Cancer Center, Yuma, AZ, USA
The indications of immune checkpoint inhibitors (ICIs) are set to rise further with the approval of newer agent like atezolimumab for use in patients with advanced stage urothelial carcinoma. More frequent use of ICIs has improved our understanding of their unique side effects, which are known as immune-related adverse events (irAEs). The spectrum of irAEs has expanded beyond more common manifestations such as dermatological, gastrointestinal and endocrine effects to rarer presentations involving nervous, hematopoietic and urinary systems. There are new safety data accumulating on ICIs in patients with previously diagnosed autoimmune conditions. It is challenging for clinicians to continuously update their working knowledge to diagnose and manage these events successfully. If diagnosed timely, the majority of events are completely reversible, and temporary immunosuppression with glucocorticoids, infliximab or other agents is warranted only in the most severe grade illnesses. The same principles of management will possibly apply as newer anti- cytotoxic T lymphocytes-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1/PD-L1) antibodies are introduced. The current focus of research is for prophylaxis and for biomarkers to predict the onset of these toxicities. In this review we summarize the irAEs of ICIs and emphasize their growing spectrum and their management algorithms, to update oncology practitioners.
Introduction
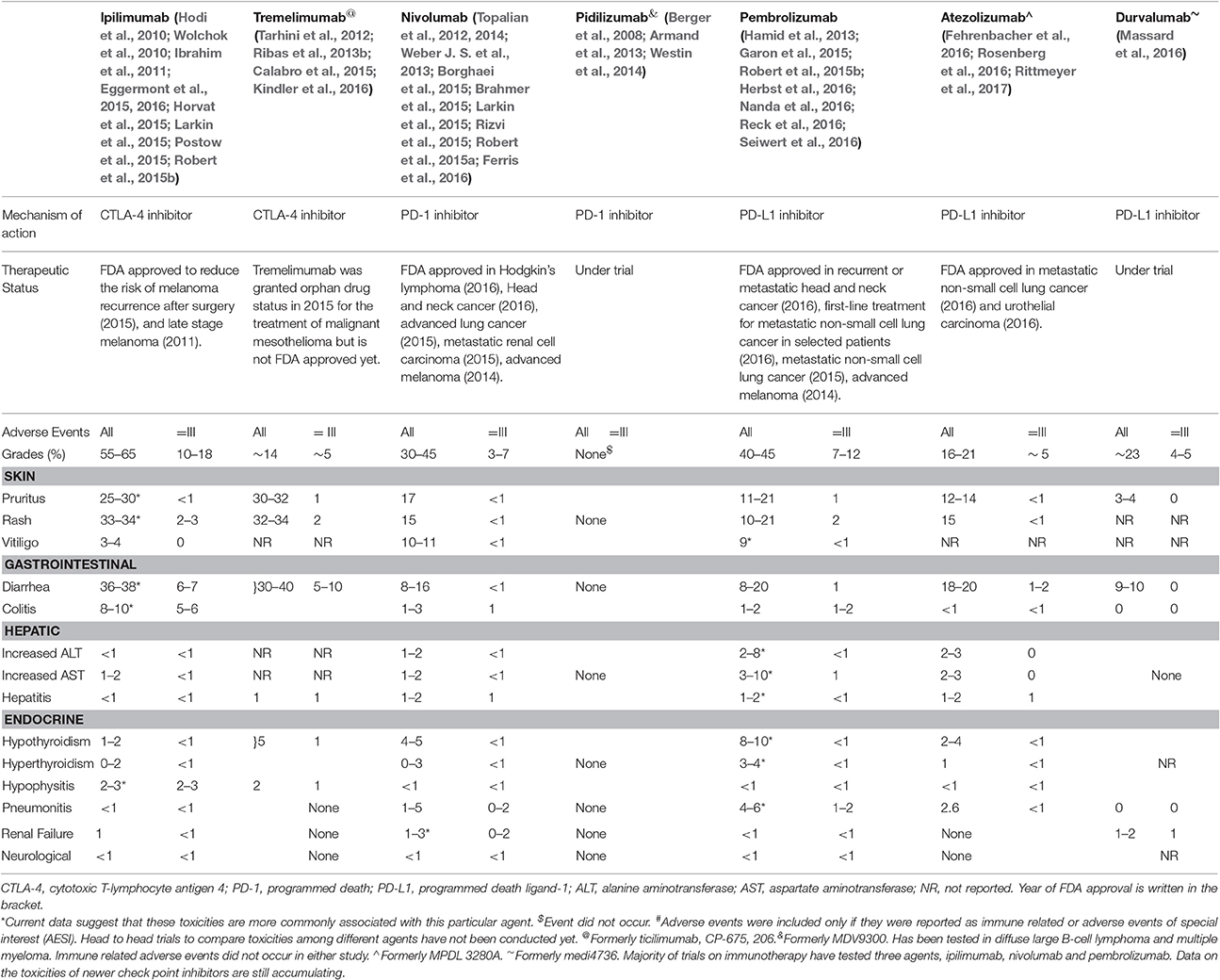
Recent advances in cancer immunotherapy are notable for the introduction of a novel class of drugs known as immune checkpoint inhibitors (ICIs). These agents inhibit negative regulatory components of the immune response, such as the cytotoxic T lymphocytes-associated antigen 4 (CTLA-4) and the programmed cell death protein-1 and its ligand (PD-1/PD-L1), which lead to enhanced T cell action against the cancer cells (Hodi et al., 2010). Ipilimumab is an anti- CTLA-4 antibody and was the first agent to receive food and drug administration (FDA) approval for use against advanced-stage melanoma1. Since then anti- CTLA-4 antibody tremelimumab, anti-PD-1 antibodies pembrolizumab and nivolumab and PD-L1 antibodies, atezolizumab and durvalumab, have shown beneficial effects in several cancers (Wolchok et al., 2010; Topalian et al., 2012, 2014; Ribas et al., 2013b; Larkin et al., 2015; Robert et al., 2015a; Postow et al., 2015; Fehrenbacher et al., 2016; Ferris et al., 2016; Massard et al., 2016; Reck et al., 2016; Rittmeyer et al., 2017) (Table 1). In contrast to conventional chemotherapy, boosting the immune system leads to a unique constellation of inflammatory toxicities known as immune-related adverse events (irAEs) that may warrant the discontinuation of therapy and/or the administration of immunosuppressive agents (Gangadhar and Vonderheide, 2014; Cousin and Italiano, 2016). The use of these agents is set to increase due to their dramatic impact on survival in a variety of advanced-stage cancers (Horvat et al., 2015). Thus, it is imperative for personnel involved in the care of oncology patients to be well versed with the heterogeneous presentations of irAEs in terms of recognition and management. Here, we review the current literature on appropriate steps in patient evaluation for the prompt diagnosis of irAEs and describe strategies for optimizing patient outcome with suitable treatment.
Incidence
The incidence of any grade irAEs is reported to range from 15 to 90% (Hodi et al., 2010; Eggermont et al., 2016; Ferris et al., 2016) in single agent trials. The rate of severe irAEs requiring immunosuppression and withdrawal of immunotherapy is estimated to be 0.5–13% (Hodi et al., 2010; Wolchok et al., 2010; Topalian et al., 2012, 2014; Ribas et al., 2013b; Larkin et al., 2015; Postow et al., 2015; Robert et al., 2015a; Ferris et al., 2016; Table S1)1. The high incidence reported in a recent study was attributed to subjectivity in toxicity evaluations among investigators and the accumulation of experience leading to early diagnosis (Horvat et al., 2015). The risk of severe grade adverse events increased from 7 to 25% with an increase in the dose of ipilimumab from 3 mg/kg to 10 mg/kg9. This was mostly due to increase in the episodes of diarrhea. However, this pattern was not observed when nivolumab dosing was increased from 0.3 mg/kg to 10 mg/kg10. Severe grade toxicities with pembrolizumab were also similar at doses of 10 mg/kg every 2 or 3 weeks and its FDA-approved dosage of 2 mg/kg every 3 weeks (Herbst et al., 2016). Thus, it may be argued that toxicities due to anti-CTLA-4 antibodies are dose dependent whereas toxicities with anti-PD-1/anti-PDL-1 antibodies are independent of doses. Ipilimumab, a CTLA-4 inhibitor is associated with higher rates of gastrointestinal (GI) toxicities, pruritus, rash, and hypophysitis whereas use of PD-1/PD-L1 antagonists is associated with higher risk of vitiligo, dysthyroidism, hepatotoxicity, and pneumonitis (Hamid et al., 2013; Weber J. S. et al., 2013; Brahmer et al., 2015; Eggermont et al., 2015, 2016; Herbst et al., 2016; Table 1 and Table S1). The data on toxicities from newer agents continue to accumulate. The irAEs in select clinical trials have been summarized in Table S1.
Timing
The majority of toxicities appear temporally, with skin manifestations the earliest to appear at 2–3 weeks after the 1st dose of ipilimumab. Immune-mediated colitis and hepatitis appear approximately 5–10 and 12–16 weeks after the 2nd and 3rd dose, respectively. Endocrine dysfunctions present from the 9th week onwards following the 4th dose (Hodi et al., 2010; Ryder et al., 2014). Immune-mediated pneumonitis is seen 8–14 weeks after treatment initiation (Hodi et al., 2010). Immune-mediated nephritis appears much later, after 14–42 weeks on immunotherapy (Izzedine et al., 2014). However, similar temporal association of the appearance of irAEs has not been described for PD-1/PDL-1 antagonists (Naidoo et al., 2015).
General Principles of Management
Current guidelines are formulated by manufacturers in collaboration with the FDA and are based on the experience from clinical trials examining the efficacy of ICIs and expert consensus. The guidelines are incorporated in the packaging inserts of these agents2,3,4,5. Although anti-PD-1/ anti-PDL-1 antibodies may be less toxic than anti-CTLA-4 antibodies, the approach to managing irAEs due to these agents is similar, with slight variations (Postow and Wolchok, 2016). The severity of adverse events is graded using Common Terminology Criteria for Adverse Events (CTCAE) on a scale from 1 to 5 (1 = mild, 2 = moderate, 3 = severe, 4 = life threatening, and 5 = death related to toxicity) (National Cancer Institute, 2009). However, the grading of irAEs may be challenging, due to arbitrary distinctions between grade 2 and 3 toxicities, such as the number of stools in a day, may be affected by recall bias. Thus, this system of grading may not be entirely suitable to grade ICIs toxicities (Horvat et al., 2015). Therefore, it is prudent to use clinical judgment rather than strictly adhering to the guidelines. We have outlined several general principles that should be followed irrespective of affected organs2,3,4,5.
➢ The ICIs are interrupted in moderate (grade 2) irAEs and are resumed when symptoms and/or lab values decrease below grade 1. Glucocorticoids (prednisone 0.5–1 mg/kg/day or equivalent) should be started if symptoms persist beyond 1 week.
➢ For grade 3/4 toxicities, high doses of glucocorticoids (prednisone 1–2 mg/kg/day or equivalent) should be given. The glucocorticoids should be tapered gradually when symptoms subside to grade 1 or less. The eligible patients (those on prednisone 20 mg or equivalent doses for at least 4 weeks) should also receive appropriate prophylaxis against Pneumocystis jirovecii as per the established guidelines6.
➢ Alternative immunosuppressive agents should be considered (infliximab 5 mg/kg; mycophenolate mofetil in hepatitis) if symptoms continue beyond 3 days on intravenous glucocorticoids. Infliximab 5 mg/kg should be repeated after 2 weeks for persistent symptoms.
➢ For grade 4 toxicities, ICIs should be stopped permanently except in endocrinopathies controlled on hormone replacement. Therapy can be resumed in selected patients with grade 3 toxicities, as discussed in the organ-specific toxicities section.
ICIs should also be stopped permanently in the following circumstances 2,3,4,5:
➢ Grade 2 reactions lasting for 6 weeks or longer. However, anti- PD-1/anti-PD-L1 antibodies can be continued in endocrinopathies controlled with hormone replacement.
➢ Inability to reduce glucocorticoids dose to 7.5 mg prednisone or equivalent per day for patients treated with anti-CTLA-4 antibodies and less than 10 mg /day within 12 weeks for anti-PD-1 antibodies.
➢ Grade 2–4 ocular reactions not improving to grade 1 within 2 weeks after treatment with topical immunosuppression or requiring systemic treatment.
Impact of irAEs and Immunosuppression on Efficacy
The immunosuppressive agents used to treat irAEs do not appear to affect the response to further immunotherapy (Attia et al., 2005). In contrast to previous studies, a recent retrospective analysis reported similar overall survival in patients who received immunosuppression (Horvat et al., 2015). The association between irAEs and the efficacy of ICIs is also controversial (Attia et al., 2005).
Biomarkers
Biomarkers which could predict the development of toxicities have been described in the patients on ipilimumab. An increase from baseline in eosinophils and interleukin 17 (IL-17) after treatment has been shown to be associated with irAEs (Callahan et al., 2011; Schindler et al., 2014). On gene profiling, two markers of neutrophil activation, CD177 and CEACAM1 also show promise as biomarkers of ICIs toxicity. These genes are expressed increasingly in the blood of patients, who developed GI toxicity after treatment with anti-CTLA-4 antibodies (Shahabi et al., 2013). Higher risk of GI toxicity was also seen in patients who exhibited evidence of inflammation on colon biopsies like infiltration of lamina propria by neutrophils and presence of cryptic abscesses, erosions and gland destruction prior to the initiation of treatment (Berman et al., 2010). However, routine testing of these biomarkers is not recommended yet.
Organ-Specific Immune Related Adverse Events
Systemic Adverse Events
Fatigue is the most common symptom reported by up to 40% of patients after treatment with anti-CTLA-4 antibodies (Weber, 2009; Hodi et al., 2010; Ibrahim et al., 2011; Tarhini et al., 2012; Calabro et al., 2015; Larkin et al., 2015; Kindler et al., 2016) and 16–24% of patients treated with anti-PD-1/anti-PD-L1 antibodies in single-agent trials (Borghaei et al., 2015; Garon et al., 2015; Rizvi et al., 2015; Robert et al., 2015a,b; Nanda et al., 2016; Reck et al., 2016; Rosenberg et al., 2016; Seiwert et al., 2016). This fatigue is usually mild, and the presence of severe fatigue should trigger an assessment for underlying disorders such as endocrinopathies2,3,4,5. Infusion reactions, including fever and chills, are more common with CTLA-4 inhibitors accounting for AEs in phase III studies (Momtaz et al., 2015). They are rarely high grade and may be managed supportively with antipyretics and antihistamines (Villadolid and Amin, 2015).
Dermatological
Skin manifestations, such as rash/pruritus and mucositis, are the most common irAEs associated with ICIs. Approximately 47–68% of patients treated with anti-CTLA-4 antibodies and 30–40% patients treated with anti-PD-1/anti-PD-L1 antibodies suffer skin toxicities of any grade (Weber, 2009; Hodi et al., 2010; Wolchok et al., 2010; Ibrahim et al., 2011; Tarhini et al., 2012; Topalian et al., 2012; Ribas et al., 2013b; Topalian et al., 2014; Postow et al., 2015; Borghaei et al., 2015; Calabro et al., 2015; Garon et al., 2015; Larkin et al., 2015; Rizvi et al., 2015; Robert et al., 2015a,b; Ferris et al., 2016; Kindler et al., 2016; Nanda et al., 2016; Rosenberg et al., 2016; Seiwert et al., 2016)1. The characteristic rash is faintly erythematous and maculopapular, involves the trunk and extremities and may be pruritic (Jaber et al., 2006). Vitiligo is also common and has delayed appearance after several months of treatment with ICIs (Weber, 2012). Histological analysis shows perivascular lymphocytic infiltration deep in the dermis with CD4+ and CD8+ T cells in close proximity with melanocytes (Jaber et al., 2006). The treatment of symptomatic cases with topical glucocorticoids (betamethasone 0.1% cream) or urea-containing creams and oral antipruritic agents (diphenhydramine, hydroxyzine, GABA agonists, or NK-1 receptor antagonists) for troublesome pruritus is usually sufficient (Weber, 2012; Horvat et al., 2015). The majority of skin eruptions are mild, and immunotherapy can be continued in most patients (Weber, 2012). Severe (Grade 3 defined as papules and/or rash covering >30% BSA) cases should be treated with oral glucocorticoids for 3–4 weeks, with temporary discontinuation of ICIs (Table 2). Permanent discontinuation should be considered in more severe cases, such as Stevens–Johnson syndrome, but fortunately severe irAEs are rare. Patients who fail to respond to steroids or have bullae formation merit dermatologic evaluation and skin biopsy. Oral mucositis and dryness are seen more frequently with anti-PD-1/anti-PD-L1 antibodies than with anti-CTLA-4 antibodies (Weber, 2009; Ibrahim et al., 2011; Tarhini et al., 2012; Borghaei et al., 2015; Calabro et al., 2015; Garon et al., 2015; Rizvi et al., 2015; Robert et al., 2015b; Kindler et al., 2016; Nanda et al., 2016; Rosenberg et al., 2016; Seiwert et al., 2016). Topical glucocorticoids and lidocaine are used for treatment. These lesions may mimic oral candidiasis, which should be ruled out2,3,4,5.
Gastrointestinal
Diarrhea and colitis are more common with anti-CTLA-4 antibodies and are reported in 30–40% of patients treated with ipilimumab (Hodi et al., 2010). Grade 3/4 diarrhea is seen in up to 10% of patients on ipilimumab therapy and 1–2% of cases treated with anti-PD-1/anti-PD-L1 antibodies alone (Weber, 2009; Ibrahim et al., 2011; Tarhini et al., 2012; Borghaei et al., 2015; Calabro et al., 2015; Garon et al., 2015; Rizvi et al., 2015; Robert et al., 2015b; Kindler et al., 2016; Nanda et al., 2016; Rosenberg et al., 2016; Seiwert et al., 2016). Enteritis without colonic involvement leading to small bowel obstruction can also be seen. Colitis predominantly affects the descending colon (Oble et al., 2008). Patients with significant diarrhea/colitis during ipilimumab treatment have subsequently been treated with anti-PD-1/anti-PD-L1 antibodies without developing diarrhea/colitis (Naidoo et al., 2015).
Grade 1 gastrointestinal events are classified as increase in stool frequency of less than 4 per day or mildly increased ostomy output from baseline (Table 2). Mild cases should be managed symptomatically with loperamide, oral hydration and electrolyte replacement (Weber et al., 2015). Other etiologies such as infection with Clostridium difficile or other viral/bacterial pathogens should be excluded (Du-Thanh et al., 2015). The American Dietary Association colitis diet may also be beneficial2,3,4,5. Treatment with glucocorticoids is indicated for worsening of symptoms or persistence beyond 3 days. Grade 2 severity events are 4–6 stools per day over baseline or a moderate increase in ostomy output. These events are managed by oral diphenoxylate, atropine or budesonide, in addition to symptomatic treatment. It is important to rule out colitis by performing a colonoscopy in persistent grade 2 or grade 1 diarrhea with hematochezia. Grade 2 diarrhea with bleeding/ulceration should be treated with oral glucocorticoids and interruption of immunotherapy. Grade 3 events include diarrhea with 7 or more stools per day above the baseline, incontinence or severe increase in ostomy limiting self-care activities. Grade 4 is any life-threatening complication, such as bowel perforation, requiring acute intervention. Grade 3 and 4 events should be treated with intravenous glucocorticoids in addition to symptomatic treatment2,3,4,5.
The symptomatic improvement should be noted in 48–72 h. Hospitalization is warranted for parenteral glucocorticoids with fluid and electrolyte management in cases refractory to oral glucocorticoids (Weber, 2012; Weber et al., 2015). If the symptoms fail to resolve after 3 days on intravenous glucocorticoids (methylprednisolone 2 mg/kg or equivalent), then infliximab should be given at a dose of 5 mg/kg every 2 weeks2,3,4,5. The use of infliximab for this indication is based on its efficacy in Crohn's disease (Minor et al., 2009). However, the recommendations are not clear for non-resolving symptoms on infliximab therapy, and prolonged courses of glucocorticoids are preferred. These patients should be cautiously observed for GI complications, including perforation and obstruction. The prompt intervention is mandatory because colitis related mortality has been attributed to delayed reporting and lack of ICI withholding (Naidoo et al., 2015). The efficacy of matrix budesonide for prophylaxis against colitis has not been proven and is not recommended (Weber et al., 2009).
Hepatic
Hepatotoxicity can be caused by both anti-CTLA-4 and anti-PD-1/anti-PD-L1 antibodies. Hepatotoxicity occurs in 2–9% of patients (Weber, 2009; Ibrahim et al., 2011; Tarhini et al., 2012; Borghaei et al., 2015; Calabro et al., 2015; Garon et al., 2015; Rizvi et al., 2015; Robert et al., 2015b; Kindler et al., 2016; Nanda et al., 2016; Rosenberg et al., 2016; Seiwert et al., 2016). Liver function should be tested at baseline and prior to each cycle of immunotherapy. The patients should also be monitored regularly during the post-treatment period. An asymptomatic elevation of hepatic transaminases and hyperbilirubinemia is common, and concomitant fever can also occur (Weber, 2012). Liver biopsy is reserved for unclear cases and reveals prominent sinusoidal histiocytic infiltrates and central vein damage with endotheliitis suggestive of ipilimumab-associated hepatitis (Johncilla et al., 2015). Grade 2 reactions require interruption of cancer treatment, with daily or alternate-day monitoring of liver enzymes until they decrease, and then subsequent weekly assessments (Table 2). Grade 3 or greater irAEs involve AST/ALT levels >5 times upper limit of normal (ULN) or bilirubin >3 times ULN. Severe hepatotoxicity requires high-dose intravenous glucocorticoids for 24–48 h followed by a slow taper for the next 30 days. The glucocorticoids should be switched to mycophenolate 500 mg every 12 h if the liver enzymes are still elevated after 48 h of treatment2,3,4,5. The use of infliximab is contraindicated due its potential hepatotoxicity. The differential diagnoses of immunotherapy-induced liver damage include metastasis to the liver, viral hepatitis and other drug toxicity meriting extensive analysis. Hepatitis persisting for longer periods requires prolonged or repeated glucocorticoids tapering (≥4 weeks) and/or additional immunosuppression2,3,4,5.
Endocrine
Endocrinopathies can occur secondary to inflammation of the pituitary, thyroid and adrenal glands or may be related to development of type-1 diabetes mellitus. Clinical presentation is confounded by nonspecific symptoms such as behavioral changes, nausea, headache, fatigue and visual complaints (Corsello et al., 2013). Hypophysitis and hypothyroidism are the most common endocrinopathies seen in up to 10% of patients treated with anti-CTLA-4 and anti-PD-1/anti-PD-L1 antibodies (Weber, 2009; Hodi et al., 2010; Wolchok et al., 2010; Ibrahim et al., 2011; Topalian et al., 2012, 2014; Tarhini et al., 2012; Ribas et al., 2013b; Borghaei et al., 2015; Calabro et al., 2015; Garon et al., 2015; Larkin et al., 2015; Postow et al., 2015; Rizvi et al., 2015; Robert et al., 2015a,b; Ferris et al., 2016; Kindler et al., 2016; Nanda et al., 2016; Seiwert et al., 2016; Rosenberg et al., 2016)1.
Hypophysitis with pituitary dysfunction requires testing for thyroid stimulating hormone (TSH), serum cortisol, adrenocorticotropic hormone (ACTH), growth hormone (GH), prolactin, luteinizing hormone (LH), and follicular stimulating hormone (FSH) in women or testosterone levels in men. Diagnosis is based on clinical symptoms with radiographic abnormalities (pituitary enlargement with enhancement) and biochemical test results (low tropic hormones)2,3,4,5. The role of high dose glucocorticoids (1 mg/kg prednisone daily) is controversial in cases with suspected hypophysitis. The use of physiological replacement doses has been suggested, and high doses should be reserved for patients with symptoms related to mass effects such as severe headaches or visual disturbances (Albarel et al., 2015). A recent study reported that TSH and FSH normalized after a follow-up period of 33 months. However, ACTH remained low, with persistent pituitary abnormalities on MRI irrespective of glucocorticoid dose (Albarel et al., 2015; Min et al., 2015).
The routine monitoring of thyroid function is indicated before each dose of ipilimumab. Hypothyroidism is more common than hyperthyroidism. It is important to distinguish primary hypothyroidism (low free T4 with high TSH) from secondary disease (low free T4 with low TSH) caused by hypophysitis. The treatment of hypo and hyperthyroidism should be consistent with standard guidelines (Table 2)2,3,4,5. Immunotherapy can be continued with hormone replacement.
Hypophysitis2,3,4,5 with clinically significant adrenal insufficiency (hypotension, dehydration, and dyselectrolytemia) is equivalent to adrenal crisis and is a medical emergency that mandates hospitalization, evaluation by an endocrinologist and treatment with methylprednisolone. It is important to distinguish this condition from sepsis, and prompt testing with cultures is mandatory (Table 2).
Pulmonary
Grade 3 or higher pneumonitis has been reported in 5–7% of NSCLC patients treated with nivolumab and pembrolizumab (Langer, 2015; Abdel-Rahman and Fouad, 2016). The incidence of symptomatic pneumonitis is only 1% with ipilimumab (Barjaktarevic et al., 2013). The risk increased in patients with prior thoracic radiation. There have been reported granulomatous reactions similar to sarcoidosis (Berthod et al., 2012). The presence of infiltrates on chest radiographs or CT imaging is more common and resolves rapidly after withholding the drug. Pneumonitis should be excluded by CT imaging in any patient with cough, shortness of breath, and fever2,3,4,5. A bronchoscopy may reveal diffuse lymphocytic infiltration and should be performed in moderate to severe cases to exclude infections. Severe cases should be treated with glucocorticoids using 2 mg/kg intravenous methyl prednisolone. Immunotherapy should be permanently discontinued in cases with recurrent grade 2–4 irAEs (Table 2)2,3,4,5.
Rare Events
Ocular
Common ocular manifestations include episcleritis, conjunctivitis and uveitis. The incidence of these events is higher with ipilimumab but remains less than 1% (Huillard et al., 2014; Abu Samra et al., 2016). The patient should be referred to an ophthalmologist and treatment with topical glucocorticoids is required in most cases. The use of oral glucocorticoid therapy is reserved for severe events.
Renal
ICIs can cause acute kidney injury that presents similar to other drug-induced tubulointerstitial nephritis. The median duration for the appearance of the kidney injury is 13 weeks (Cortazar et al., 2016). In addition to nephritis, granulomatous lesions and thrombotic microangiopathy can also be seen on renal biopsy. A previous study demonstrated that renal function partially improved after glucocorticoid treatment, and that one-third of patients required dialysis (Cortazar et al., 2016). Grade 2 or higher toxicity is treated with glucocorticoids (Table 2). The immunotherapy should be withheld for Grade 2–3 events and permanently discontinued for Grade 4 events or resistant Grade 2–3 irAEs2,3,4,5.
Pancreatic
Routine monitoring of amylase/lipase in otherwise asymptomatic individuals is not recommended. Asymptomatic elevation does not require treatment. The significance of elevated amylase and lipase in a large number of patients remains unclear (Ribas et al., 2013b; Postow et al., 2015; Herbst et al., 2016).
Neurological
The reported neurologic complications of immunotherapies include posterior reversible encephalopathy syndrome (Maur et al., 2012), Guillain-Barre Syndrome (Wilgenhof and Neyns, 2011), myasthenia gravis (Liao et al., 2014), transverse myelitis (Liao et al., 2014), and neuropathy (de Maleissye et al., 2016). Serious cases should be treated with glucocorticoids and a neurologist should be consulted for additional therapies such as intravenous immunoglobulin and plasmapheresis. (for detailed management see Table 2)
Hematological
Autoimmune anemia (Kong et al., 2016), neutropenia (Akhtari et al., 2009), thrombocytopenia (Ahmad et al., 2012), and acquired hemophilia A have been reported (Delyon et al., 2011). Symptom management is similar to other irAEs and involves the use of glucocorticoids and alternative immunosuppression in refractory cases.
Combination Therapy
The distinct mechanisms of action of anti-CTLA-4 and anti-PD-1/anti-PD-L1 antibodies have led to trials examining combination therapies in a variety of malignancies. The incidence of severe adverse events due to the combination of ipilimumab and nivolumab is reported to be 55%, which is significantly higher than either agent individually and leads to discontinuation of treatment in one-third of patients (Larkin et al., 2015). The toxicity profile of ipilimumab varies with the chemotherapy agent used in combination (Weber J. et al., 2013). The combination with dacarbazine is more hepatotoxic (Robert et al., 2011), and carboplatin and taxanes treatment leads to more cutaneous manifestations (Arriola et al., 2016). There are more nephrotoxic and hepatotoxic events observed when combined with vemurafenib (Ribas et al., 2013a). The combination of ipilimumab (dose 10 mg/kg) with granulocyte-macrophage colony-stimulating factor showed fewer gastrointestinal and pulmonary irAEs than ipilimumab alone (45 vs. 58%)(Hodi et al., 2014). However, the efficacy of this combination is not clear at the FDA-approved dose of 3 mg/kg and requires further confirmation.
Autoimmune Conditions
The safety of ICIs in patients with preexisting autoimmune diseases is not clear, and there is a theoretical concern regarding the exacerbation of preexisting conditions. There have been anecdotal reports of patients with anti-muscle antibodies developing rhabdomyolysis with polymyositis (Bilen et al., 2016), and neurotoxicity has been described in patients with anti-neuronal antibodies (Williams et al., 2016). Ipilimumab treatment led to the exacerbation of previously diagnosed autoimmune conditions in 27% of patients within 6 weeks of treatment, and these conditions were easily managed with steroids (Johnson et al., 2015). Clinicians should engage patients in discussions for trial of these agents due to the significant benefit of these antibodies in life-threatening malignancies. The development of new autoimmune syndromes (30% sicca syndromes, 70% inflammatory arthritis and 40% ANA positivity) have also been reported in patients without any prior history of rheumatic conditions (Cappelli et al., 2017).
Conclusion
ICIs targeting CTLA-4 and PD-1/PDL-1 have dramatically changed the outcomes of patients with many advanced-stage malignancies. However, their introduction is associated with unique irAEs that are mostly transient and mild but can occasionally be fatal. Rapid identification and appropriate treatment can improve outcomes without compromising the efficacy of these agents. In the absence of prospective data, these patients should be managed as per established guidelines based upon pooled clinical experience. Additional data on toxicities will enable us to utilize the full therapeutic potential of these novel drugs.
Author Contributions
VK: original idea, reviewed literature and manuscript writing and editing. AC: mentored, writing and editing of manuscript. NC: reviewed literature and manuscript writing and editing. MG: writing and editing of manuscript. CF: editing and proofreading. PS: editing and proofreading.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphar.2017.00049/full#supplementary-material
Footnotes
1. ^Drugs@FDA: FDA Approved Drug Products. Available online at www.Accessdata.fda.gov/scripts/cder/drugsatfda/index.cf.
2. ^http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761034s000lbl.pdf
3. ^Yervoy® [package insert]. Princeton, NJ: Bristol-Myers Squibb Company, 2016. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2265ef30-253e-11df-8a39-0800200c9a66
4. ^Keytruda® [package insert]. Whitehouse Station, NJ: Merck, and Co., Inc., 2016. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9333c79b-d487-4538-a9f0-71b91a02b287
5. ^Opdivo® [package insert]. Princeton, NJ: Bristol-Myers Squibb Company, 2016. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394
6. ^http://www.nccn.org/professionals/physician_gls/pdf/infections.pdf.
References
Abdel-Rahman, O., and Fouad, M. (2016). Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther. Adv. Respir. Dis. 10, 183–193. doi: 10.1177/1753465816636557
Abu Samra, K., Valdes-Navarro, M., Lee, S., Swan, R., Foster, C. S., Anesi, S. D., et al. (2016). A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur. J. Ophthalmol. 26, e46–e48. doi: 10.5301/ejo.5000724
Ahmad, S., Lewis, M., Corrie, P., and Iddawela, M. (2012). Ipilimumab-induced thrombocytopenia in a patient with metastatic melanoma. J. Oncol. Pharm. Pract. 18, 287–292. doi: 10.1177/1078155211411001
Akhtari, M., Waller, E. K., Jaye, D. L., Lawson, D. H., Ibrahim, R., Papadopoulos, N. E., et al. (2009). Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 Antibody). J. Immunother. 32, 322–324. doi: 10.1097/CJI.0b013e31819aa40b
Albarel, F., Gaudy, C., Castinetti, F., Morange, I., Conte-Devolx, B., Grob, J. J., et al. (2015). Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur. J. Endocrinol. 172, 195–204. doi: 10.1530/EJE-14-0845
Armand, P., Nagler, A., Weller, E. A., Devine, S. M., Avigan, D. E., Chen, Y. B., et al. (2013). Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J. Clin. Oncol. 31, 4199–4206. doi: 10.1200/JCO.2012.48.3685
Arriola, E., Wheater, M., Galea, I., Cross, N., Maishman, T., Hamid, D., et al. (2016). Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J. Thor. Oncol. 11, 1511–1521. doi: 10.1016/j.jtho.2016.05.028
Attia, P., Phan, G. Q., Maker, A. V., Robinson, M. R., Quezado, M. M., Yang, J. C., et al. (2005). Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. 23, 6043–6053. doi: 10.1200/JCO.2005.06.205
Barjaktarevic, I. Z., Qadir, N., Suri, A., Santamauro, J. T., and Stover, D. (2013). Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 143, 858–861. doi: 10.1378/chest.12-1467
Berger, R., Rotem-Yehudar, R., Slama, G., Landes, S., Kneller, A., Leiba, M., et al. (2008). Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 14, 3044–3051. doi: 10.1158/1078-0432.CCR-07-4079
Berman, D., Parker, S. M., Siegel, J., Chasalow, S. D., Weber, J., Galbraith, S., et al. (2010). Blockade of cytotoxic T-lymphocyte antigen- 4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 10, 11.
Berthod, G., Lazor, R., Letovanec, I., Romano, E., Noirez, L., Mazza Stalder, J., et al. (2012). Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J. Clin. Oncol. 30, e156–e159. doi: 10.1200/JCO.2011.39.3298
Bilen, M. A., Subudhi, S. K., Gao, J., Tannir, N. M., Tu, S.-M., and Sharma, P. (2016). Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J. Immunother Cancer 4, 36. doi: 10.1186/s40425-016-0139-8
Borghaei, H., Paz-Ares, K. L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639. doi: 10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crin,ò, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135. doi: 10.1056/NEJMoa1504627
Calabro, L., Morra, A., Fonsatti, E., Cutaia, O., Fazio, C., Annesi, D., et al. (2015). Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 3, 301–309. doi: 10.1016/S2213-2600(15)00092-2
Callahan, M. K., Yang, A., Tandon, S., Xu, Y., Subudhi, S. K., Roman, R. A., et al. (2011). Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J. Clin. Oncol. 29s:2505.
Cappelli, L. C., Gutierrez, A. K., Baer, A. N., Albayda, J., Manno, R. L., Haque, U., et al. (2017). Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann. Rheum. Dis. 76, 43–50. doi: 10.1136/annrheumdis-2016-209595
Corsello, S. M., Barnabei, A., Marchetti, P., De Vecchis, L., Salvatori, R., and Torino, F. (2013). Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 98, 1361–1375. doi: 10.1210/jc.2012-4075
Cortazar, F. B., Marrone, K. A., Troxell, M. L., Ralto, K. M., Hoenig, M. P., Brahmer, J. R., et al. (2016). Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 90, 638–647. doi: 10.1016/j.kint.2016.04.008
Cousin, S., and Italiano, A. (2016). Molecular Pathways: Immune Checkpoint Antibodies and their Toxicities. Clin. Cancer Res. 22, 4550–4555. doi: 10.1158/1078-0432.CCR-15-2569
Delyon, J., Mateus, C., and Lambert, T. (2011). Hemophilia A induced by ipilimumab. N. Engl. J. Med. 365, 1747–1748. doi: 10.1056/NEJMc1110923
de Maleissye, M.-F., Nicolas, G., and Saiag, P. (2016). Pembrolizumab-Induced Demyelinating Polyradiculoneuropathy. N. Engl. J. Med. 375, 296–297. doi: 10.1056/NEJMc1515584
Du-Thanh, A., Pallure, V., Girard, C., Dereure, O., and Guillot, B. (2015). Clostridium difficile infection may loom behind ipilimumab-induced auto-immune colitis. Eur. J. Dermatol. 25, 344. doi: 10.1684/ejd.2015.2561
Eggermont, A. M., Chiarion-Sileni, V., Grob, J. J., Dummer, R., Wolchok, J. D., Schmidt, H., et al. (2016). Prolonged survival in stage III Melanoma with Ipilimumab adjuvant therapy. N. Engl. J. Med. 375, 1845–1855. doi: 10.1056/NEJMoa1611299
Eggermont, A. M., Chiarion-Sileni, V., Grob, J.-J., Dummer, R., Wolchok, J. D., Schmidt, H., et al. (2015). Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16, 522–530. doi: 10.1016/S1470-2045(15)70122-1
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. doi: 10.1016/S0140-6736(16)00587
Ferris, R. L., Blumenschein, G. Jr., Fayette, J., Guigay, J., Colevas, A. D., Licitra, L., et al. (2016). Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867. doi: 10.1056/NEJMoa1602252
Gangadhar, T. C., and Vonderheide, R. H. (2014). Mitigating the toxic effects of anticancer immunotherapy. Nat. Rev. Clin. Oncol. 11, 91–99. doi: 10.1038/nrclinonc.2013.245
Garon, E. B., Rizvi, N. A., Hui, R., Leighl, N., Balmanoukian, A. S., Eder, J. P., et al. (2015). Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028. doi: 10.1056/NEJMoa1501824
Hamid, O., Robert, C., Daud, A., Hodi, F. S., Hwu, W. J., Kefford, R., et al. (2013). Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144. doi: 10.1056/NEJMoa1305133
Herbst, R. S., Baas, P., Kim, D., Felip, E., Pérez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550. doi: 10.1016/S0140-6736(15)01281-7
Hodi, F., Lee, S., McDermott, D. F., Rao, U. N., Butterfield, L. H., Tarhini, A. A., et al. (2014). Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 312, 1744–1753. doi: 10.1001/jama.2014.13943
Hodi, F. S., O'Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. doi: 10.1056/NEJMoa1003466
Horvat, T. Z., Adel, N. G., Dang, T. O., Momtaz, P., Postow, M. A., Callahan, M. K., et al. (2015). Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J. Clin. Oncol. 33, 3193. doi: 10.1200/jco.2015.60.8448
Huillard, O., Bakalian, S., Levy, C., Desjardins, L., Lumbroso-Le Rouic, L., Pop, S., et al. (2014). Ocular adverse events of molecularly targeted agents approved in solid tumours: a systematic review. Eur. Cancer J. 50, 638–648. doi: 10.1016/j.ejca.2013.10.016
Ibrahim, R., Berman, D., de Pril, V. V., Humphrey, R. W., Chen, T., Messina, M., et al. (2011). Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J. Clin. Oncol. 29 (abstract).
Izzedine, H., Gueutin, V., Gharbi, C., Mateus, C., Robert, C., Routier, E., et al. (2014). Kidney injuries related to ipilimumab. Invest. New Drugs 32, 769. doi: 10.1007/s10637-014-0092-7
Jaber, S. H., Cowen, E. W., Haworth, L. R., Booher, S. L., Berman, D. M., Rosenberg, S. A., et al. (2006). Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch. Dermatol. 142, 166–172. doi: 10.1001/archderm.142.2.166
Johncilla, M., Misdraji, J., Pratt, D. S., Agoston, A. T., Lauwers, G. Y., Srivastava, A., et al. (2015). Ipilimumab-associated Hepatitis: Clinico pathologic Characterization in a Series of 11 Cases. Am. J. Surg. Pathol. 39, 1075–1084. doi: 10.1097/PAS.0000000000000453
Johnson, D. B., Sullivan, R. J., Ott, P. A., Carlino, M. S., Khushalani, N. I., Ye, F., et al. (2015). Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune. JAMA Oncol. 2, 234–240. doi: 10.1001/jamaoncol.2015.4368
Kindler, H. L., Scherpereel, A., Calabrò, L., Aerts, J., Perez, S. C., Bearz, A., et al. (2016). Tremelimumab as second- or third-line treatment of unresectable malignant mesothelioma (MM): Results from the global, double-blind, placebo-controlled DETERMINE study. J. Clin. Oncol. 34 (abstract).
Kong, B. Y., Micklethwaite, K. P., Swaminathan, S., Kefford, R. F., and Carlino, M. S. (2016). Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res. 26, 202–204. doi: 10.1097/CMR.0000000000000232
Langer, C. J. (2015). Emerging immunotherapies in the treatment of non-small cell lung cancer (NSCLC): the role of immune checkpoint inhibitors. Am. J. Clin. Oncol. 38, 422–430. doi: 10.1097/coc.0000000000000059
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., et al. (2015). Combined nivolumab and ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373, 23–34. doi: 10.1056/NEJMoa1504030
Liao, B., Shroff, S., Kamiya-Matsuoka, C., and Tummala, S. (2014). Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro-oncology 16, 589–593. doi: 10.1093/neuonc/nou001
Massard, C., Gordon, M. S., Sharma, S., Rafii, S., Wainberg, Z. A., Luke, J., et al. (2016). Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 34, 3119–3125. doi: 10.1200/JCO.2016.67.9761
Maur, M., Tomasello, C., Frassoldati, A., Dieci, M. V., Barbieri, E., Conte, P., et al. (2012). Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J. Clin. Oncol. 30, e76–e78. doi: 10.1200/JCO.2011.38.7886
Min, L., Hodi, F. S., Giobbie-Hurder, A., Ott, P. A., Luke, J. J., Donahue, H., et al. (2015). Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin. Cancer Res. 21, 749–755. doi: 10.1158/1078-0432.CCR-14-2353
Minor, D., Chin, K., and Kashani-Sabet, M. (2009). Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother. Radiopharm. 24, 321–325. doi: 10.1089/cbr.2008.0607
Momtaz, P., Park, V., Panageas, K. S., Postow, M. A., Callahan, M., Wolchok, J. D., et al. (2015). Safety of Infusing ipilimumab Over 30 Minutes. J. Clin. Oncol. 33, 3454–3458. doi: 10.1200/JCO.2015.61.0030
Naidoo, J., Page, D. B., Li, B. T., Connell, L. C., Schindler, K., Lacouture, M. E., et al. (2015). Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375. doi: 10.1093/annonc/mdv383
Nanda, R., Chow, L. Q., Dees, E. C., Berger, R., Gupta, S., Geva, R., et al. (2016). Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol. 34, 2460–2467. doi: 10.1200/JCO.2015.64.8931
National Cancer Institute (2009). Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available online at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
Oble, D. A., Mino-Kenudson, M., Goldsmith, J., Hodi, F. S., Seliem, R. M., Dranoff, G., et al. (2008). Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am. J. Surg. Pathol. 32, 1130–1137. doi: 10.1097/PAS.0b013e31817150e3
Postow, M. A., Chesney, J., Pavlick, A. C., Robert, C., Grossmann, K., McDermott, D., et al. (2015). Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017. doi: 10.1056/NEJMoa1414428
Postow, M., and Wolchok, J. (2016). “Toxicities associated with checkpoint inhibitor immunotherapy,” in UpToDate, ed T. W. Post (Waltham, MA). (Accessed: August 1, 2016).
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Cőszi, T., Fülöp, A., et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1–Positive non–small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833. doi: 10.1056/NEJMoa1606774
Ribas, A., Hodi, F. S., Callahan, M., Konto, C., and Wolchok, J. (2013a). Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 368, 1365–1366. doi: 10.1056/nejmc1302338
Ribas, A., Kefford, R., Marshall, M. A., Punt, C. J., Haanen, J. B., Marmol, M., et al. (2013b). Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31, 616–622. doi: 10.1200/JCO.2012.44.6112
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265. doi: 10.1016/S0140-6736(16)32517-X
Rizvi, N. A., Mazières, J., Planchard, D., Stinchcombe, T. E., Dy, G. K., Antonia, S. J., et al. (2015). Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 16, 257–265. doi: 10.1016/S1470-2045(15)70054-9
Robert, C., Long, G. V., Brady, B., Dutriaux, C., Maio, M., Mortier, L., et al. (2015a). Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330. doi: 10.1056/NEJMoa1412082
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015b). Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532. doi: 10.1056/NEJMoa1503093
Robert, C., Thomas, L., Bondarenko, I., O'Day, S., Weber, J., Garbe, C., et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526. doi: 10.1056/NEJMoa1104621
Rosenberg, J. E., Jean, H., Tom, P., van der Heijden, M. S., Balar, A. V., Necchi, A., et al. (2016). Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920. doi: 10.1016/S0140-6736(16)00561-4
Ryder, M., Callahan, M., Postow, M., Wolchok, J., and Fagin, J. A. (2014). Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 21, 371–381. doi: 10.1530/ERC-13-0499
Schindler, K., Harmankaya, K., and Kuk, D. (2014). Correlation of absolute and relative eosinophil counts with immune-related adverse events in melanoma patients treated with ipilimumab. J. Clin. Oncol. 32:5s (abstract).
Seiwert, T. Y., Burtness, B., Mehra, R., Weiss, J., Berger, R., Eder, J. P., et al. (2016). Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965. doi: 10.1016/S1470-2045(16)30066-3
Shahabi, V., Berman, D., Chasalow, S. D., Wang, L., Tsuchihashi, Z., Hu, B., et al. (2013). Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune- related gastrointestinal adverse events. J. Transl. Med. 11:75. doi: 10.1186/1479-5876-11-75
Tarhini, A. A., Cherian, J., Moschos, S. J., Tawbi, H. A., Shuai, Y., Gooding, W. E., et al. (2012). Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J. Clin. Oncol. 30, 322–328. doi: 10.1200/JCO.2011.37.5394
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443. doi: 10.1056/NEJMoa1200690
Topalian, S. L., Sznol, M., McDermott, D., Kluger, F. H. M., Carvajal, R. D., Sharfman, W. H., et al. (2014). Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030. doi: 10.1200/JCO.2013.53.0105
Villadolid, J., and Amin, A. (2015). Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl. Lung Cancer Res. 4, 560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06
Weber, J. (2009). Ipilimumab: Controversies in its development, utility, and autoimmune adverse events. Cancer Immunol. Immunother. 58, 823–830. doi: 10.1007/s00262-008-0653-8
Weber, J., Hamid, O., Amin, A., Masson, E., Goldberg, S. M., Williams, D., et al. (2013). Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma. Cancer Immun. 13, 7
Weber, J. S. (2012). Practical Management of immune related adverse events from immune checkpoint protein antibodies for the oncologist. Am. Soc. Clin. Oncol. Educ. Book 2012, 174–177. doi: 10.14694/EdBook_AM.2012.32.174
Weber, J. S., Kudchadkar, R. R., and Yu, B. (2013). Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 31, 4311. doi: 10.1200/jco.2013.51.4802
Weber, J. S., Yang, J. C., Atkins, M. B., and Disis, M. L. (2015). Toxicities of Immunotherapy for the Practitioner. J. Clin. Oncol. doi: 10.1200/jco.2014.60.0379
Weber, J., Thompson, J. A., Hamid, O., Minor, D., Amin, A., Ron, I., et al. (2009). A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 15, 5591–5598. doi: 10.1158/1078-0432.CCR-09-1024
Westin, J. R., Chu, F., Zhang, M., Fayad, L. E., Kwak, L. W., Fowler, N., et al. (2014). Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 15, 69–77. doi: 10.1016/S1470-2045(13)70551-5
Wilgenhof, S., and Neyns, B. (2011). Anti-CTLA-4 antibody-induced Guillain-Barré syndrome in a melanoma patient. Ann. Oncol. 22, 991–993. doi: 10.1093/annonc/mdr028
Williams, T. J., Benavides, D. R., Patrice, K. A., Dalmau, J. O., de Ávila, A. L., Le, D. T., et al. (2016). Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 73, 928–933. doi: 10.1001/jamaneurol.2016.1399
Keywords: immune related adverse events, checkpoint blockade, irAEs, nivolumab, pembrolizumab, ipilimumab
Citation: Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P and Chandra AB (2017) Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 8:49. doi: 10.3389/fphar.2017.00049
Received: 16 August 2016; Accepted: 23 January 2017;
Published: 08 February 2017.
Edited by:
Raquel Abalo, King Juan Carlos University, SpainReviewed by:
James M. Rae, University of Michigan Health System, USARobert A. Rollins, Pfizer Inc., USA
Copyright © 2017 Kumar, Chaudhary, Garg, Floudas, Soni and Chandra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhinav B. Chandra, abhinavbck@hotmail.com
 Vivek Kumar
Vivek Kumar Neha Chaudhary2
Neha Chaudhary2 Charalampos S. Floudas
Charalampos S. Floudas