- 1Department of Molecular Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
- 2Department of Pathology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Twenty-eight days subacute toxicity studies performed in rats using sclerotial powder of Lignosus cameronensis cultivar was conducted to assess its safety for consumption prior to other scientific investigations on its medicinal benefits, nutraceutical or pharmaceutical application of the mushroom. The study was conducted at 250, 500, and 1000 mg/kg sclerotial powder of L. cameronensis cultivar (n = 5 for each respective dose, on both male and female groups) while control groups received only distilled water. At the end of the study (29th day), the animals were sacrificed followed by blood and organs collection for analysis. Subacute toxicity studies done shows that sclerotial powder of L. cameronensis cultivar at 250, 500, and 1000 mg/kg did not induce treatment related changes on behavioral patterns, gross physical appearance, growth pattern, body weight gain, values of hematological and clinical biochemical panels as well as histopathological findings on kidney, spleen, heart, lung and liver of the experimental rats. The no-observed-adverse-effect level dose for sclerotial powder of L. cameronensis cultivar in 28-days sub-acute toxicity study is determined to be 1000 mg/kg.
Introduction
Lignosus, a genus of mushroom belonging to the Polyporaceae family, is mainly distributed in the tropical forest of South China, Thailand, Malaysia, Indonesia, Philippines, Papua New Guinea, Africa, and Australia (Ryvarden and Johansen, 1980; Cui et al., 2011; Tan et al., 2012). Six species of the genus (Lignosus) have been identified, namely L. dimiticus, L. ekombitii, L. goetsii, L. sacer, L. hainanensis, and L. rhinocerotis (Douanla-Meli and Langer, 2003; Cui et al., 2011; Tan et al., 2012). Two new species of Lignosus mushrooms have been collected from the tropical forest in the state of Pahang, Malaysia. They are identified and subsequently named as L. tigris and L. cameronensis (Tan et al., 2013). These two newly discovered Lignosus species and L. rhinocerotis were collectively known as Tiger Milk mushroom prior to their discovery and have now been successfully cultivated in the laboratory (Tan et al., 2013; Lau et al., 2015).
The sclerotium of Lignosus species are the part with medicinal value. In Peninsular Malaysia, the sclerotia of Tiger Milk mushroom are widely utilized by aborigines for the treatment of various ailments including cough, asthma, fever, food poisoning, cancer and as a general tonic (Lee et al., 2009). In China, the sclerotium is sold at a high price and is considered expensive among the other folk medicine in China. It has been used to treat gastric ulcers, chronic hepatitis and liver cancer (Wong and Cheung, 2009). Previous studies demonstrated that the sclerotia of L. rhinocerotis exhibit anti-proliferative, anti-oxidant, anti-microbial, anti-inflammatory, and immunomodulatory effect as well as stimulatory effect on neurite outgrowth (Lai et al., 2008; Wong et al., 2009, 2011; Eik et al., 2012; Lee et al., 2012; Mohanarji et al., 2012; Lau et al., 2013; Phan et al., 2013; Yap et al., 2013; Zaila et al., 2013; Lee et al., 2014). Non-digestible carbohydrates (NDCs) from the sclerotia may function as novel prebiotics (Gao et al., 2009). L. tigris was also reported to have anti-oxidant activity (Yap et al., 2014a; Kong et al., 2015).
Wide ethno-botanical usages of Tiger Milk mushrooms as well as several scientific reports on medicinal properties of L. rhinocerotis and L. tigris suggest that L. cameronensis likely has similar health benefit and has the potential to be developed into a nutraceutical. Prior to its successful cultivation, there were no studies done on the medicinal properties of L. cameronensis due to its limited supply from the wild. The advent of successful cultivation has enabled the exploration into its safety profile, medicinal and nutritional values.
In the present inquest, a 28-days sub-acute toxicity study on the sclerotial powder of L. cameronensis cultivar in rats was conducted to assess its safety for consumption prior to other scientific investigations on its medicinal benefits, nutraceutical or pharmaceutical application of the mushroom. In the experiments, male and female rats (n = 5 each) were randomly assigned into three treatment groups per gender and were orally administered with the sclerotial powder at three ascending doses while control groups received only distilled water. At the end of the study (29th day), the animals were sacrificed followed by blood and organs collection for analysis. The sub-acute toxicity study was conducted in accordance with the Organization of Economic Cooperation and Development guidelines (Organization for Economic Cooperation and Development [OECD], 2008).
Materials and Methods
Preparation of the Sclerotial Powder of L. cameronensis Cultivar
Lignosus cameronensis cultivar was obtained from Ligno Biotech Sdn. Bhd. (Selangor, Malaysia). The cultivation tissue of the cultivar originated from the wild type L. cameronensis which was collected from the tropical forest in Lata Iskandar (4°17.46′N 101°34.41′E), Pahang, Malaysia. The wild type mushroom was identified by internal transcribed spacer (ITS) regions of the ribosomal DNA via PCR technology (Yap et al., 2014b) [ITS-1 (5′-TT > GGCCCTT > CCCTT > CTGGCGG-3′) and ITS-2 (5′-GAGGCGCAGCCCTTCACTT-3′)]. Fifty milligram of clean sclerotial tissue was inoculated on potato dextrose agar (PDA) supplemented with 100 μg/L of streptomycin. Mycelial culture of 200 mg was sub-cultured into sterilized substrate containing 100 g of brown rice and 100 ml of distilled water in a polypropylene container. Spawn container were incubated in darkness at 24°C for 3 months. Sclerotia formed were then harvested, freeze dried and ground into powder by using 0.2 μm mesh filter. The sclerotial powder of the Lignosus cameronensis cultivar appears to be beige in color and light powdery in texture, similar to that of the wild type sclerotial powder.
Animals
Male and female Sprague Dawley (SD) rats aged 5 weeks old were purchased from Sterling Ascent Sdn Bhd (Penang, Malaysia). Rats were housed in Animal Experimental Unit in University of Malaya under standard housing conditions of 12 h light/12 h darkness and 22 ± 2°C. Rats were given ad libitum access to standard chow diets and water. All animals were quarantined for at least 14 days prior to use. Transportation and care of the animals were performed in compliance with the relevant laws and experimental protocols reported in this study were approved by Institutional Animal Care and Use Committee, University of Malaya (UM IACUC-Ethics reference no.2015-180505/MOL/R/FSY).
Oral Administration of L. cameronensis Cultivar Sclerotial Powder in Rats
Male (7 weeks old) and female (7–8 weeks old) Sprague Dawley (SD) rats were randomly divided into three treatment groups (250, 500, or 1000 mg/kg) and one control group per gender (one female and one male control group). Each experimental group consists of five animals. Sclerotial powder (at various dosages) was suspended in distilled water (10 ml/kg) and orally fed to the rats using oral gavage needles, once daily for 28 consecutive days. Control group received distilled water only during the study. Body weight of each rat was recorded daily and any changes in behavioral patterns and gross physical appearance were also observed every day. The dosages for treatment were selected based on OECD guidelines. Two lower doses, 250 mg/kg and 500 mg/kg were used to examine the presence or absence of dose related responses.
Blood Analysis
On the final day of experimental study (day 28), rats were fasted for 18 h followed by anesthesia with ketamine (45 mg/kg) and xylazine (4.5 mg/kg). Blood collection was carried out by cardiac puncture. Examination of hematological and clinical biochemical parameters from blood sample were performed by using Advia 2120 Hematology System (Siemen, Germany) and Advia 2400 Chemistry System (Siemen, Germany), respectively. The parameters for hematological examination encompass red blood cell (RBC) count, hemoglobin concentration, packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, white blood cell (WBC) count, neutrophil count, lymphocyte count, monocyte count, eosinophil count, basophil count and atypical lymphocyte count. Biochemical tests include glucose, urea, creatinine, calcium, inorganic phosphate, uric acid, sodium, potassium, chloride, total cholesterol, total protein, albumin, globulin, total bilirubin, alkaline phosphatase, serum glutamic oxaloacetic transaminase (SGOT or AST), serum glutamic pyruvic transaminase (SGPT or ALT) and gamma-glutamyl transpeptidase (GTT) (Lee et al., 2013).
Histopathological Evaluations
Vital organs (kidney, spleen, heart, lung, and liver) were harvested and immersed in fixative (10% buffered formalin). The tissues were dehydrated by ethanol solution in serial concentrations, cleared with xylene and paraffin embedded. Paraffin blocks were sectioned with microtome into 5 μm thickness and each tissue section was stained with hematoxylin and eosin followed by microscopic examination by light microscope.
Statistical Analysis
All data were presented as mean ± standard deviation. Statistical differences between the means of control and treatment groups were determined by One-way Analysis of Variance (ANOVA) followed by Dunnett’s t (two-sided) test. Dunnett T3 test was employed when homogeneity of variance was not fulfilled. Homogeneity of variances was calculated by using Levene statistics. Results were considered statistically significant when p < 0.05.
Results
General Observations
Oral administration of the L. cameronensis cultivar sclerotial powder at various doses (250, 500, or 1000 mg/kg) did not induce alteration of fur and eye color. There was no occurrence of piloerection and diarrhea or impairment of locomotor activity in all the sclerotial administered rats. All animals survived. Growth rate for all treated rats were found to be similar to that of control rats from the same gender (Figures 1, 2). The net body weight gain of rats from all treatment groups were found to be comparable to the control rats (p > 0.05; Table 1).
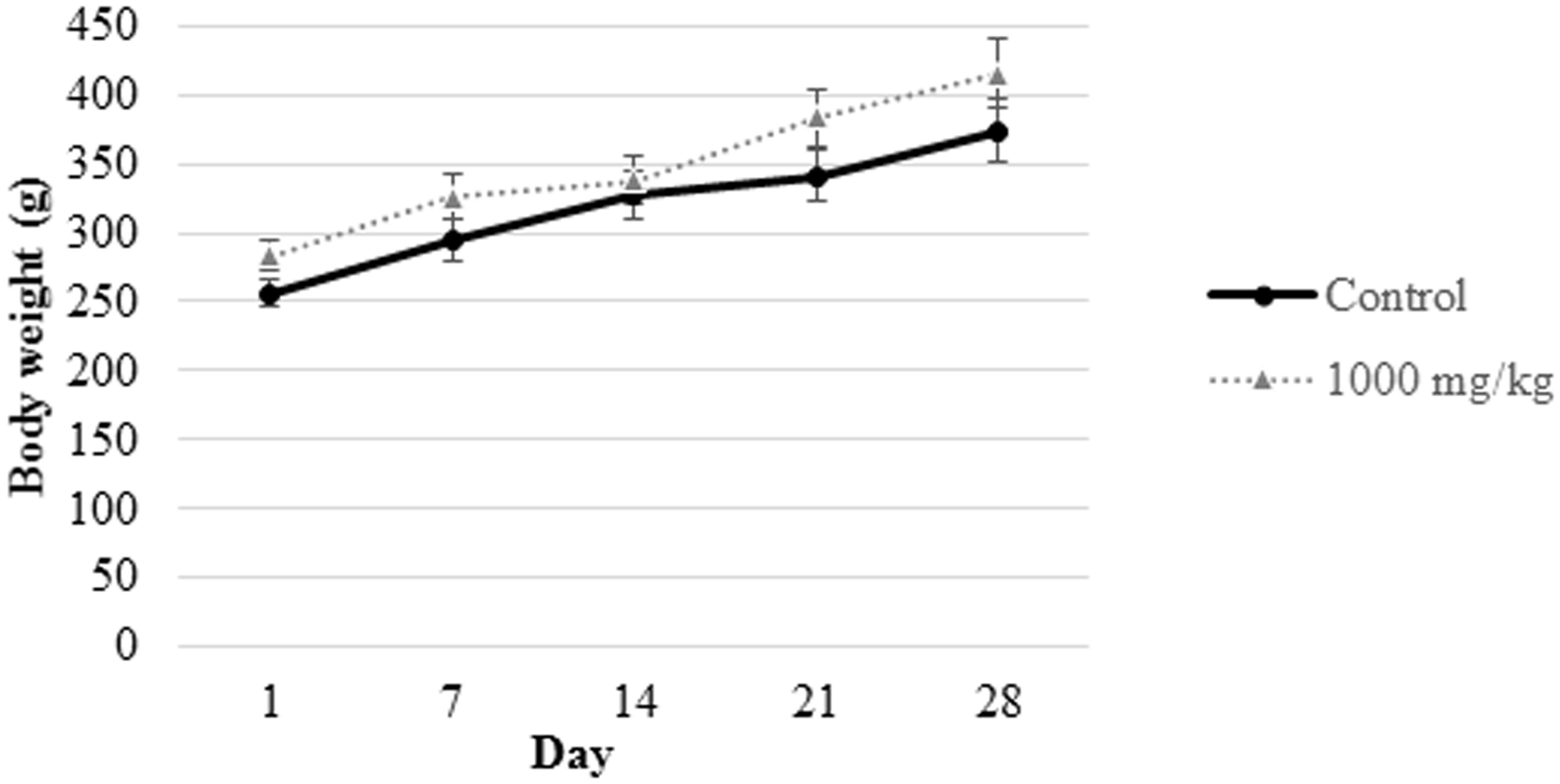
FIGURE 1. Body weight measurement of male rats from control group and those subjected to treatment with 1000 mg/kg L. cameronensis cultivar sclerotial powder. Body weight of rats are presented as mean ± SD (n = 5).
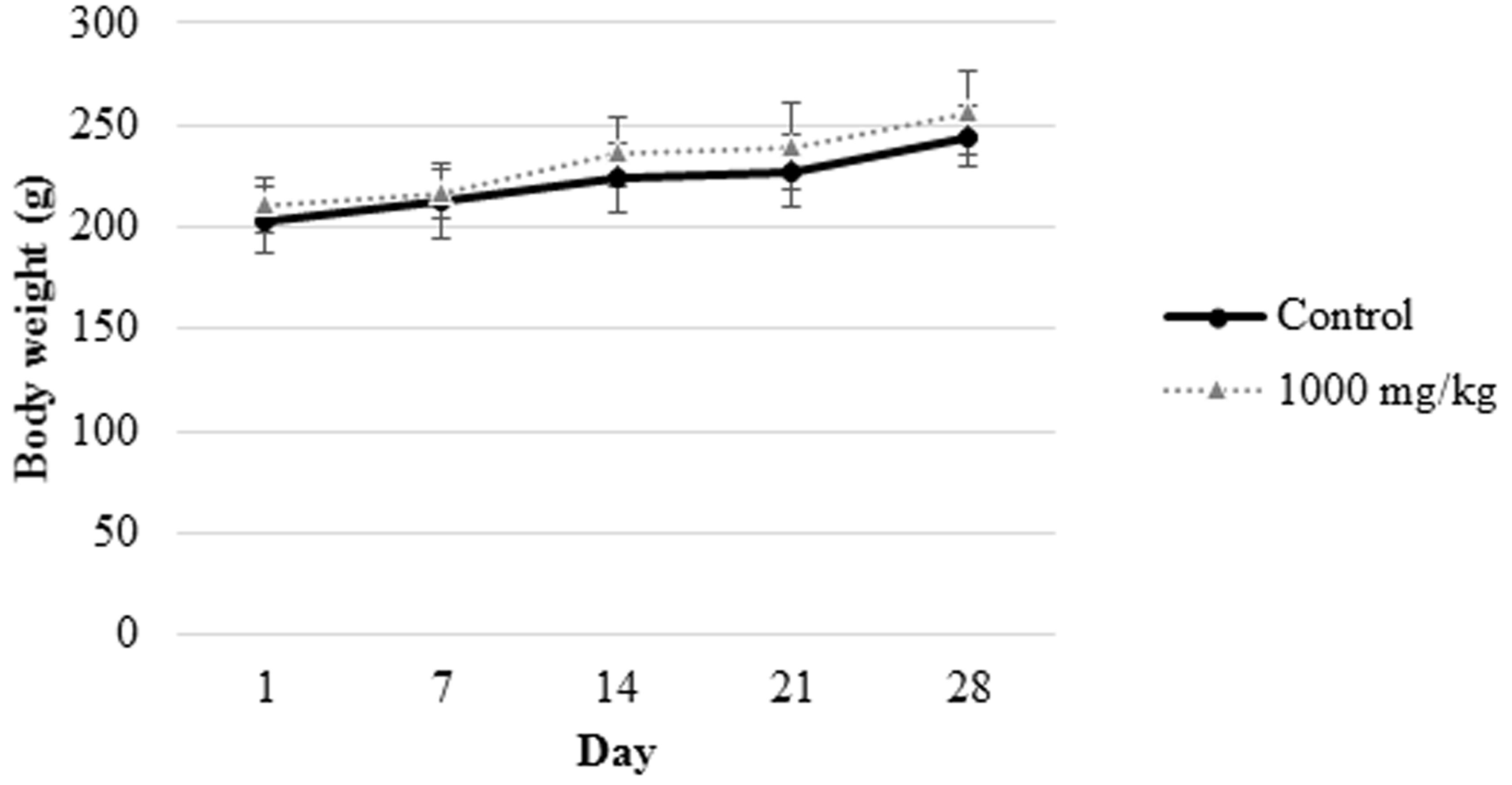
FIGURE 2. Body weight measurement of female rats from control group and those treated with 1000 mg/L. cameronensis cultivar sclerotial powder. Body weight of rats are presented as mean ± SD (n = 5).
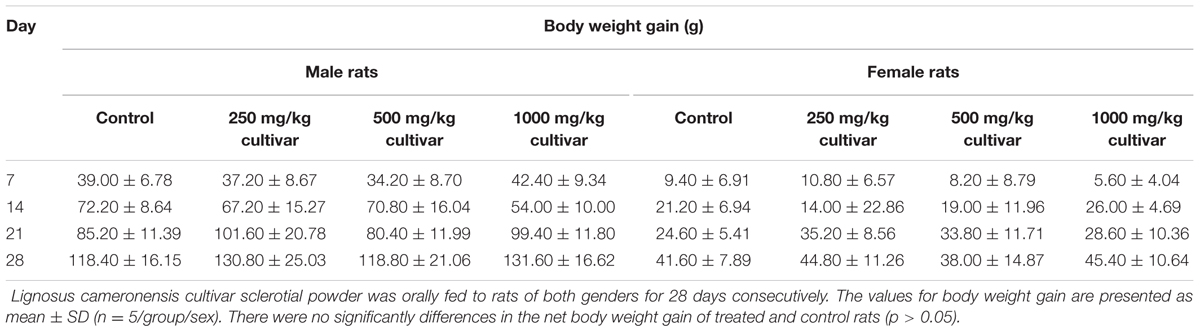
TABLE 1. Body weight gain of male and female rats treated with varying doses of L. cameronensis cultivar sclerotial powder for 28 days.
Hematology
Hematological examinations of all male rats from the sclerotial administered groups (250, 500, and 1000 mg/kg) showed that the treatment did not induce significant changes in the values of RBC, hemoglobin, PCV, MCV, MCH, MCHC, platelet count, WBC, neutrophil, lymphocyte, monocyte, eosinophil, basophil and atypical lymphocyte (p > 0.05; Table 2). Other than MCV, MCHC and platelet counts, all of the hematological panels tested in all treatment groups of female rats were also found to be not significantly different from the control group (p > 0.05; Table 3). MCV levels in female groups treated with 500 mg/kg (53.40 ± 1.14 fL) and 1000 mg/kg (53.20 ± 0.84 fL) sclerotial powder were slightly lower than the control group (56.60 ± 1.34 fL). MCHC level in rats treated with 250 mg/kg (33.60 ± 0.55 g/dL) sclerotial powder was slightly lower than the control group (34.40 ± 0.55 g/dL). Platelet count in groups treated with 250 mg/kg (935.40 ± 45.37 ×109/L), 500 mg/kg (923.20 ± 57.35 ×109/L) and 1000 mg/kg (923.80 ± 45.23 ×109/L) sclerotial powder were significantly different from the control group (795.80 ± 48.00 ×109/L).
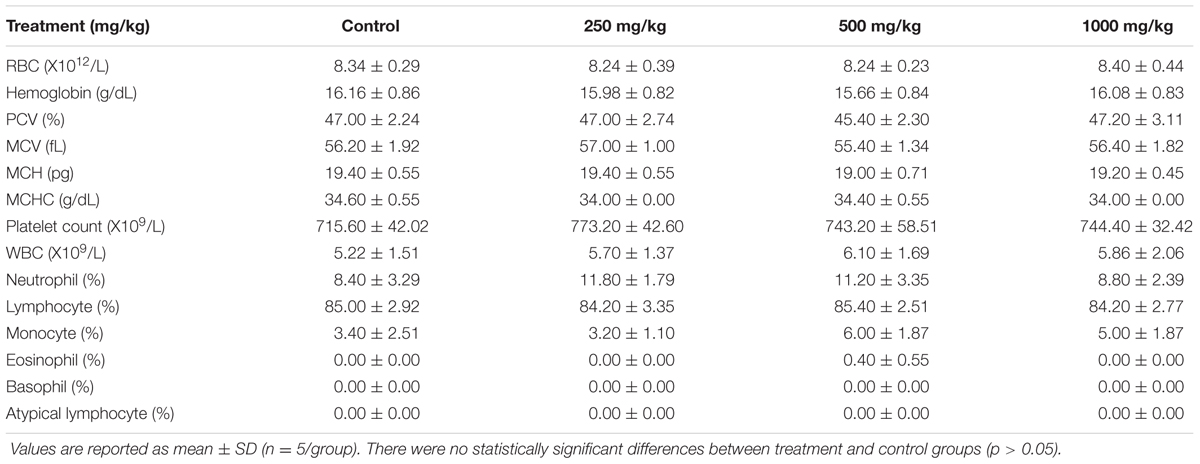
TABLE 2. Hematological values of male rats treated with three doses (250, 500, 1000 mg/kg) of L. cameronensis cultivar sclerotial powder for 28 days.
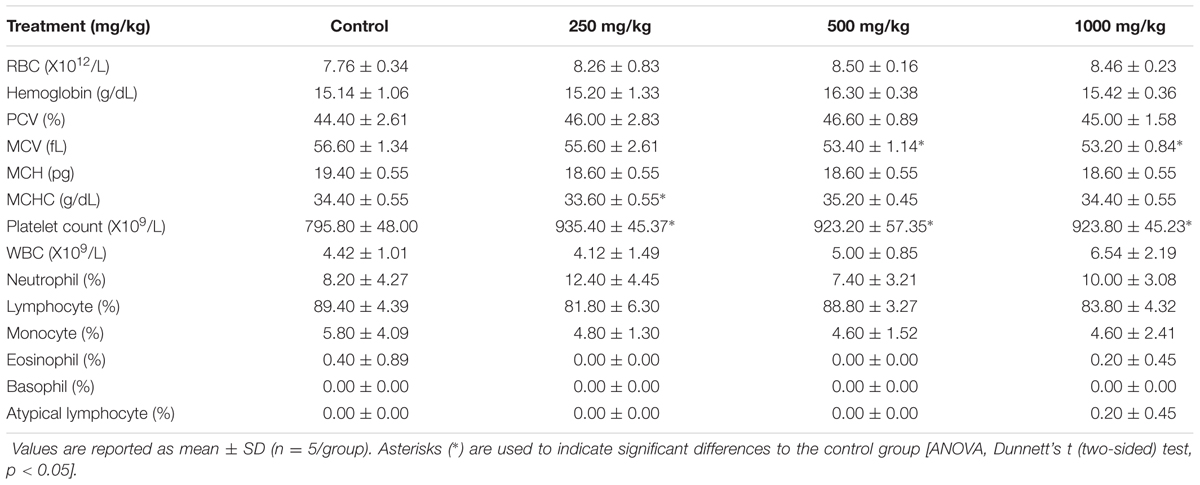
TABLE 3. Hematological values of female rats treated with three doses (250, 500, 1000 mg/kg) of L. cameronensis cultivar sclerotial powder for 28 days.
Clinical Biochemistry
Tables 4, 5 shows the results of clinical biochemistry for male and female rats, respectively. After 28 days of feeding with the L. cameronensis cultivar sclerotial powder by oral gavage, serum biochemical parameters (serum glucose, urea, creatinine, calcium, inorganic phosphate, uric acid, sodium, potassium, chloride, total cholesterol, total protein, albumin, globulin, total bilirubin, alkaline phosphatase, SGOT, SGPT and GGT) of female rats from treatment groups were not markedly different from the control group (p > 0.05). In the male rats, most of the tested serum biochemical parameters showed insignificant differences between the treated and control rats, except for sodium and chloride levels. Sodium level in the male rats (146.60 ± 0.55 mmol/L) treated with 500 mg/kg sclerotial powder was slightly higher than in the control rats (144.00 ± 0.71 mmol/L, p < 0.05). Chloride level in male rats (102.40 ± 0.89 mmol/L) administered with 250 mg/kg sclerotial powder was slightly lower than the level in the control group (104.40 ± 0.55 mmol/L, p < 0.05).
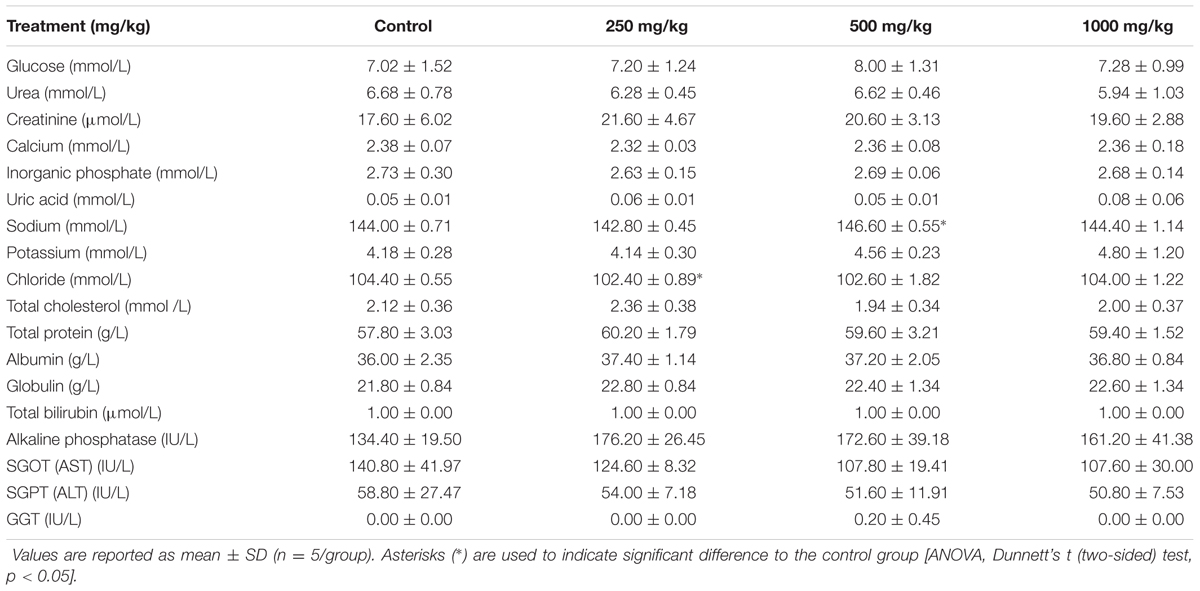
TABLE 4. Clinical biochemistry of male rats treated with three doses (250, 500, 1000 mg/kg) of L. cameronensis cultivar sclerotial powder for 28 days.
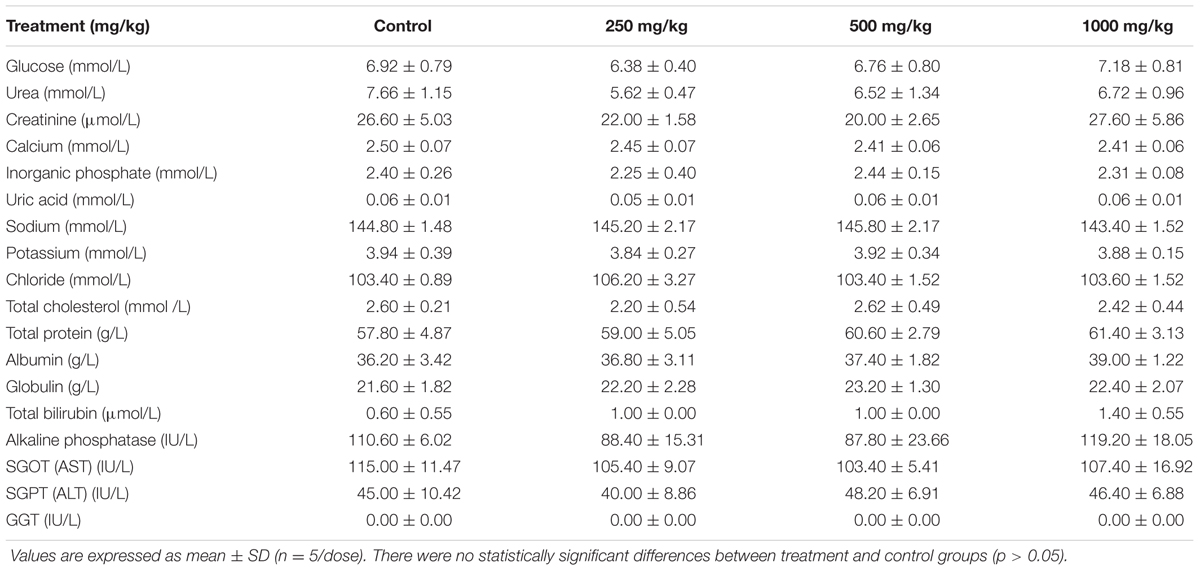
TABLE 5. Clinical biochemistry of female rats treated with three doses (250, 500, 1000 mg/kg) of L. cameronensis cultivar sclerotial powder for 28 days.
Histopathological Examinations
There were no treatment-related pathological changes in microscopic examination of kidney, spleen, heart, lung, and liver of female rats from both control and treatment groups, following oral feeding of L. cameronensis cultivar sclerotial powder for 28 consecutive days. Glomeruli and tubules were found to be normal in kidney tissues of control and treated groups (Figure 3). Histology of spleen (Figure 4), heart (Figure 5) and lung (Figure 6) of treated rats were also devoid of abnormality in which the spleen shows normal red and white pulps; the heart shows normal myocardial fibers; and the lung shows normal bronchi and alveoli without evidence of acute or chronic inflammation in the tissues. Figure 7 shows normal portal tracts, central vein and hepatocytes in liver tissues of control and treatment groups. Neither necrosis of hepatocytes nor portal inflammation was observed. Similar histological observations of organs were noted in male rats, except for mild fatty change in liver tissue of control male rats (Figure 8). Similar findings were not observed in male rats that were administered L. cameronensis cultivar sclerotial powder at all the dosages in this sub-acute toxicity study.
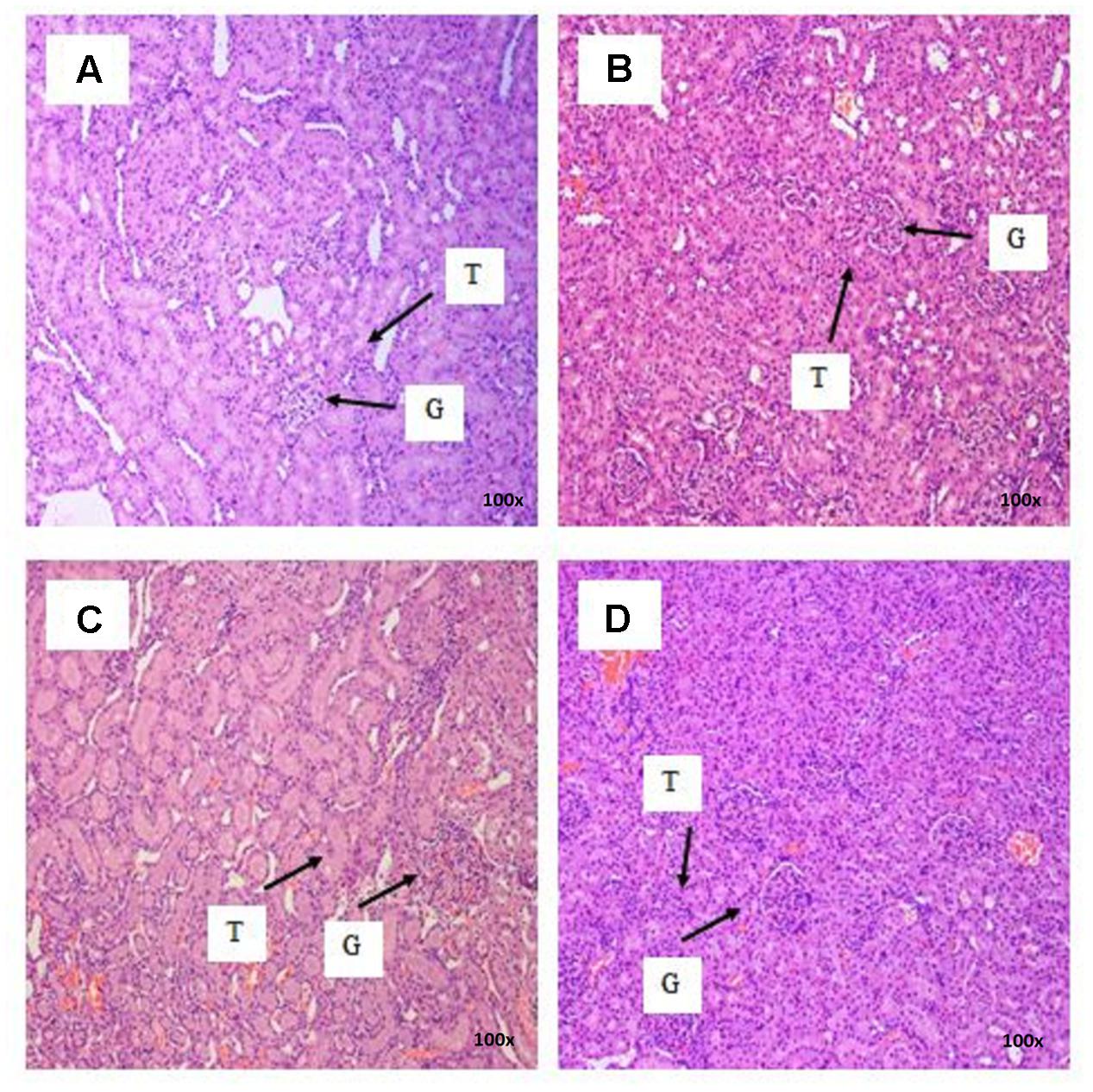
FIGURE 3. Photomicrograph of kidney sections from female rats treated with L. cameronensis cultivar sclerotial powder at different doses exhibited normal histological structure of glomerulus and tubules (x100 magnification; H&E stain). (A) control; (B) cultivar, 250 mg/kg; (C) cultivar, 500 mg/kg; (D) cultivar, 1000mg/kg. ‘G’ and ‘T’ indicate the position of glomerulus and tubule, respectively.
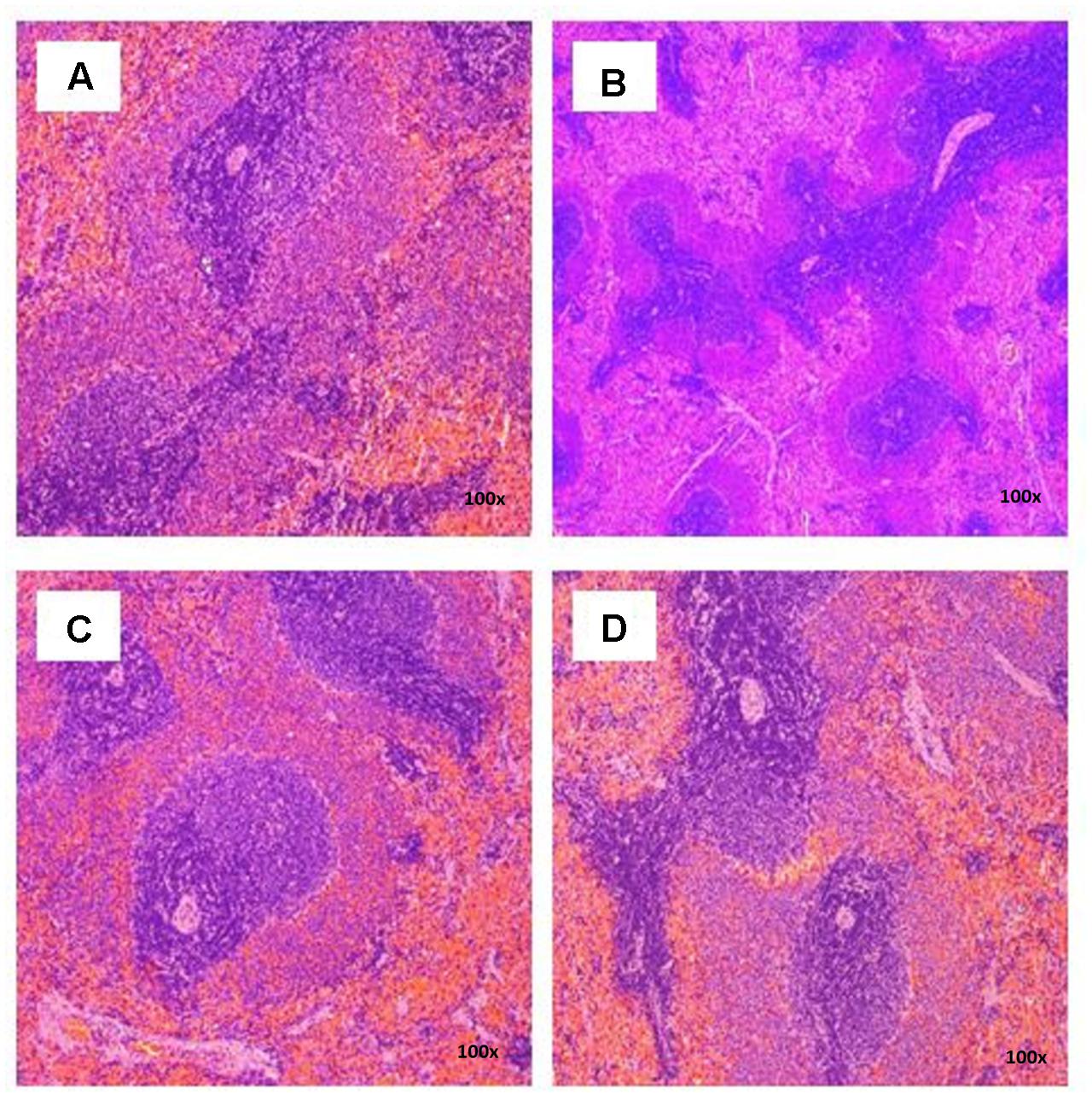
FIGURE 4. Photomicrograph of spleen sections from female rats treated with L. cameronensis cultivar sclerotial powder exhibited normal architecture on red and white pulp (x100 magnification; H&E stain). (A) control; (B) cultivar, 250 mg/kg; (C) cultivar, 500 mg/kg; (D) cultivar, 1000mg/kg.
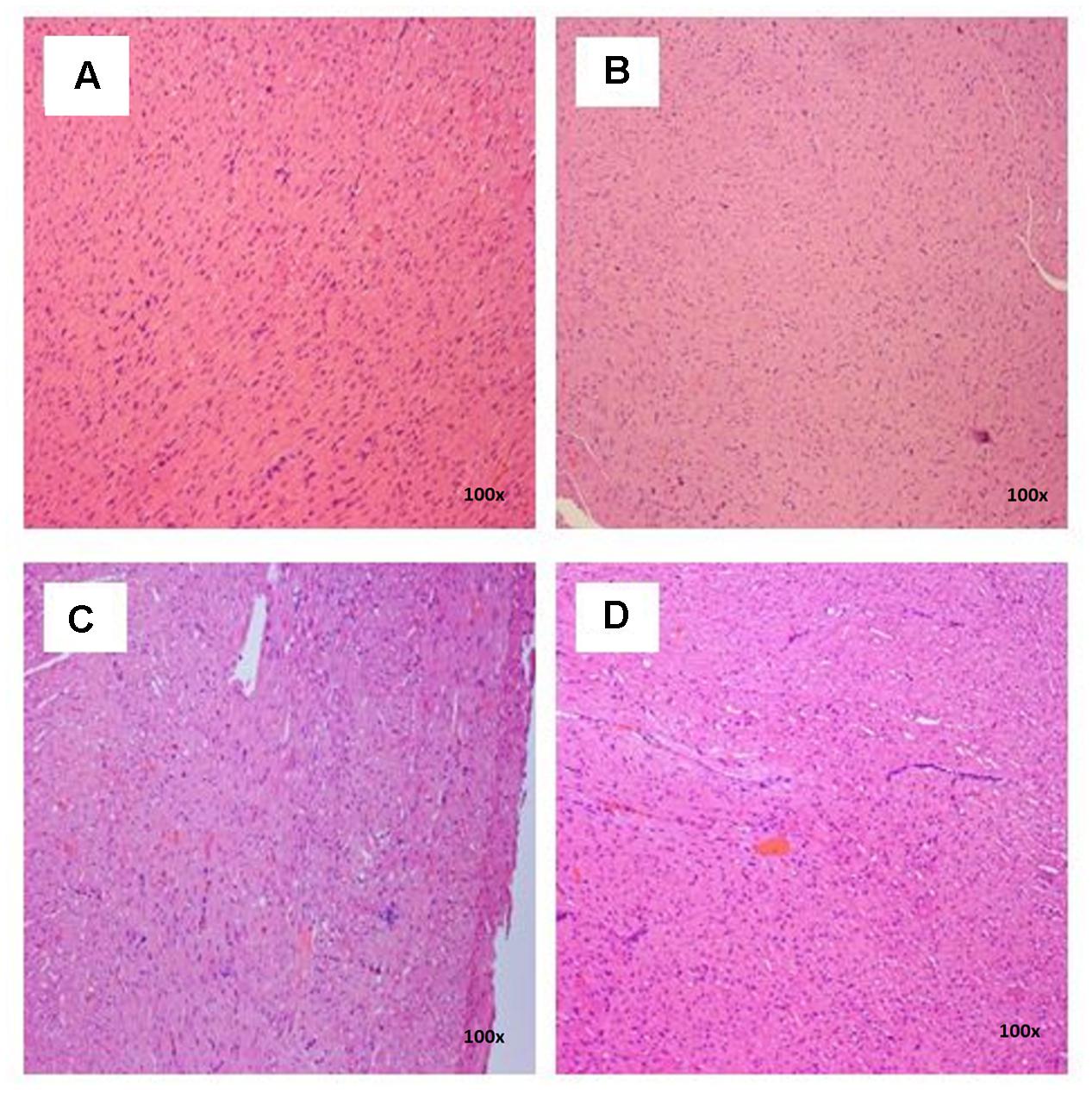
FIGURE 5. Photomicrograph of heart sections of female rats treated with L. cameronensis cultivar sclerotial powder exhibited normal appearance of myocardial fibers (x100 magnification; H&E stain). (A) control; (B) cultivar, 250 mg/kg; (C) cultivar, 500 mg/kg; (D) cultivar, 1000 mg/kg.
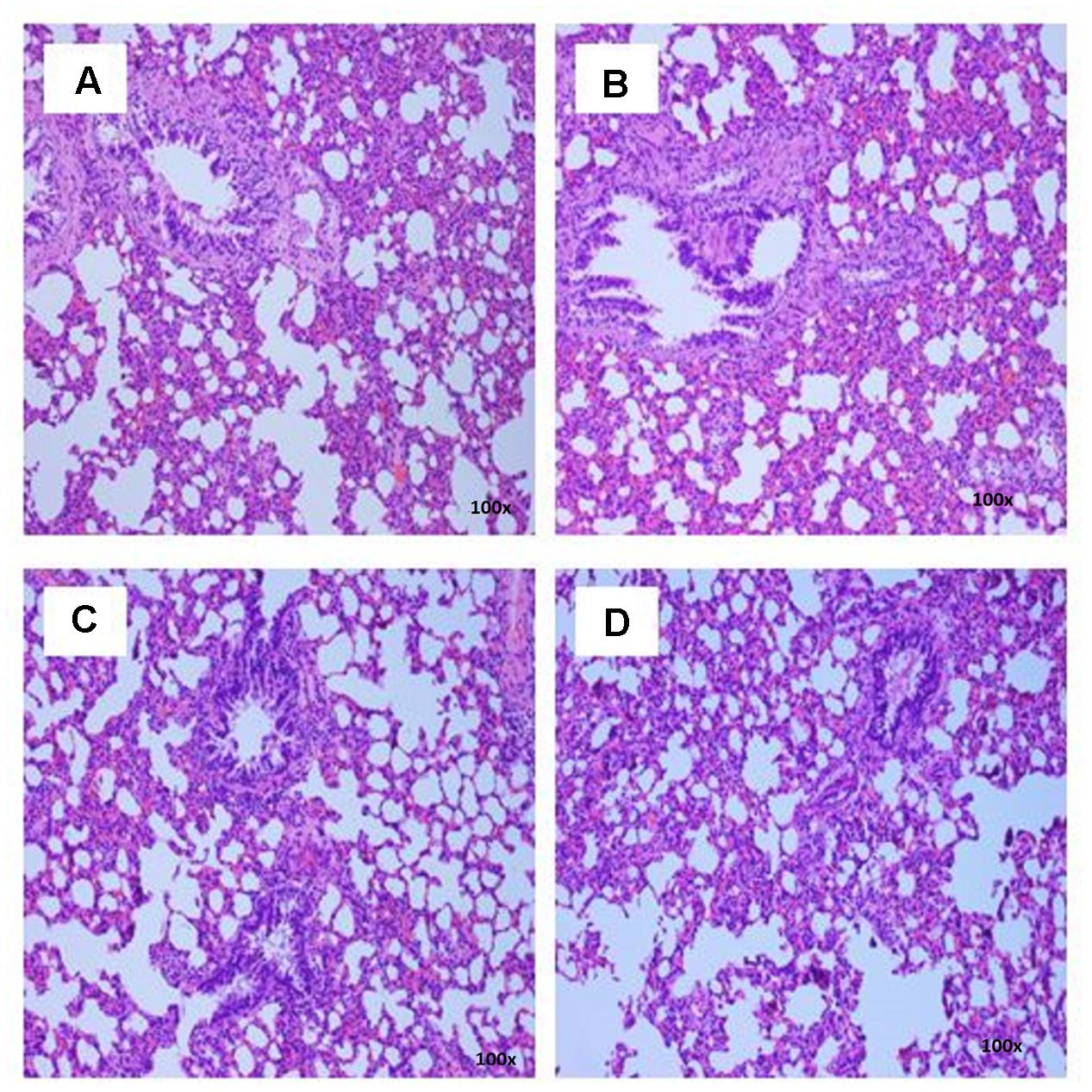
FIGURE 6. Photomicrograph of lung sections from female rats treated with sclerotial powder of L. cameronensis cultivar exhibited normal histological structure of bronchi and alveoli (x100 magnification; H&E stain). (A) control; (B) cultivar, 250 mg/kg; (C) cultivar, 500 mg/kg; (D) cultivar, 1000 mg/kg.
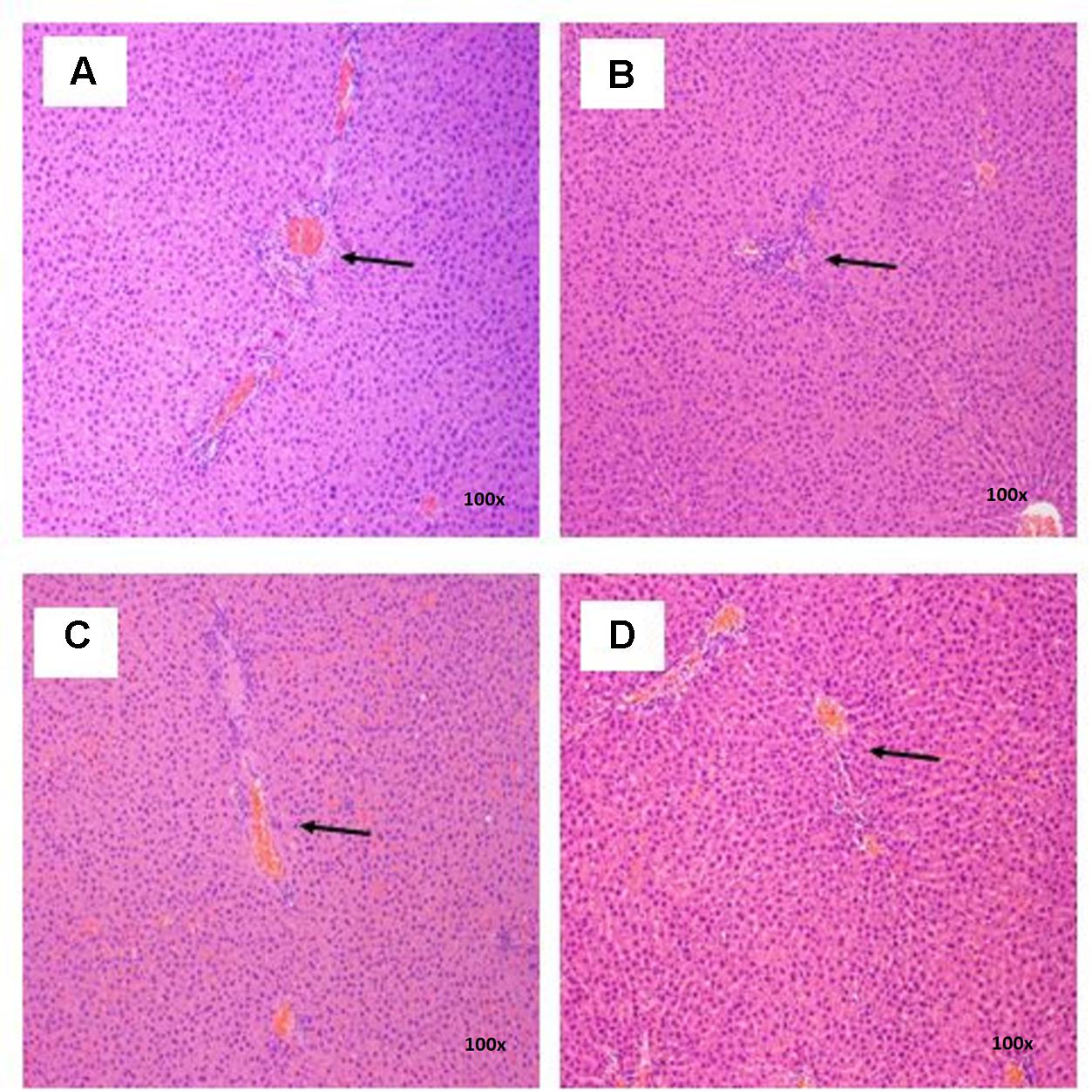
FIGURE 7. Photomicrograph of liver sections from female rats treated with sclerotial powder of L. cameronensis cultivar exhibited normal portal tracts and hepatocytes (x100 magnification; H&E stain). (A) control; (B) cultivar, 250 mg/kg; (C) cultivar, 500 mg/kg; (D) cultivar, 1000 mg/kg. Arrows show the position of portal tract.
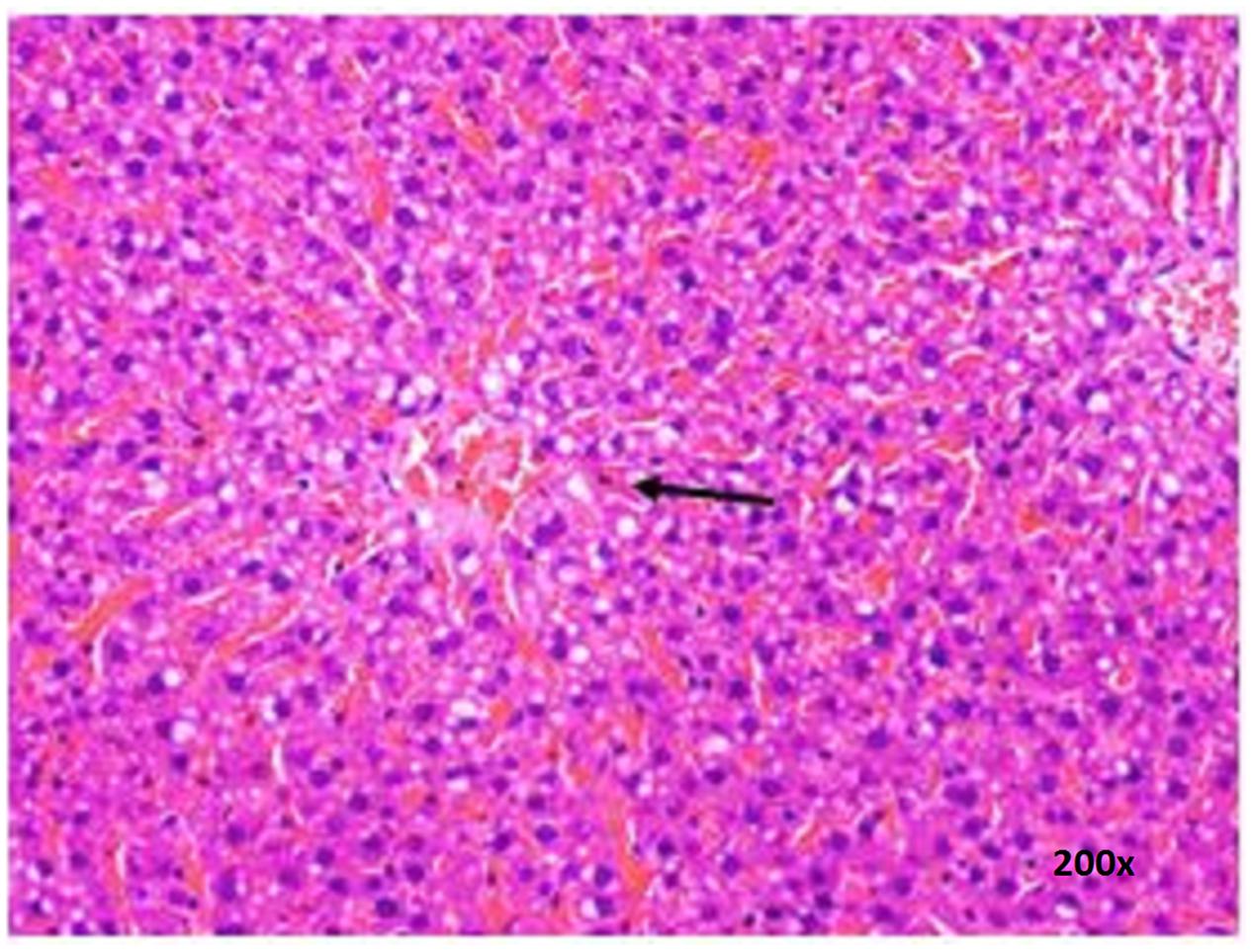
FIGURE 8. Photomicrograph of liver section of male rat displaying mild fatty change (x200 magnification; H&E stain) in control group. Arrow shows the area of fat contained in vacuolated cytoplasm of hepatocytes.
Discussion
In the present study, growth pattern (Figures 1, 2) and body weight gain (Table 1) of treated rats in both genders were found to be similar to that of the control group. This suggested that L. cameronensis cultivar sclerotial powder which was orally fed to the rats (up to 1000 mg/kg) for 28 consecutive days did not cause any harmful effect on the growth of the animals.
Hematopoietic system reflects physiological and pathological conditions in human and animal as it is one of the most susceptible targets for toxicant (Gautam and Goel, 2014). In general, our results show that the RBCs, white blood cells and platelet indices of rats administered with L. cameronensis cultivar sclerotial powder in three different doses (250, 500, and 1000 mg/kg) were not significantly different from the control group. Exceptions were noted in MCV, MCHC and platelet count of female rats. MCV levels of female rats administered with 500 and 1000 mg/kg of L. cameronensis cultivar sclerotial powder was slightly lower (p < 0.05) than that in the control group. The MCHC levels of female rats following administration of 250 mg/kg L. cameronensis cultivar sclerotial powder also showed slightly lower levels compared to the control group (p < 0.05). The minor variation in MCV and MCHC values were not of toxicological concern as they fell within normal reference range obtained from SD rats (Petterino and Argentino-Storino, 2006). The platelet counts in female rats administered with 250 mg/kg, 500 mg/kg, or 1000 mg/kg of the sclerotial powder showed significant difference from the control group. However, according to reference range reported by Patterino and Argentino-Storino, the values were considered normal for SD rats. Therefore, administration with L. cameronensis cultivar sclerotial powder for 28 days up to 1000 mg/kg did not induce any toxic effects on the blood cells and platelet of the rats.
Clinical biochemical examinations including liver function tests (total protein, albumin, globulin, total bilirubilin, alkaline phosphatase, SGOT, SGPT and GGT), and kidney function tests (urea, creatinine and uric acid levels) as well as serum electrolytes (calcium, inorganic phosphate, sodium, potassium, chloride), cholesterol and glucose levels of rats were carried out to further examine the safety of consumption of L. cameronensis cultivar sclerotial powder. In both groups of male and female rats administered with the sclerotial powder, the clinical biochemical parameters were not significantly different from the control group, except for sodium and chloride level of male rats. Sodium level of the male rats administered with 500 mg/kg L. cameronensis cultivar sclerotial powder was slightly higher (p < 0.05) than control. The chloride levels of male rats administered with 250 mg/kg L. cameronensis cultivar sclerotial powder was found to be slightly lower (p < 0.05) than the control group. Nevertheless, the values are within the normal ranges established by Patterino and Argentino-Storino for rats. Thus, the slight alterations in sodium and chloride levels are unlikely to be of toxicological concern.
Histological examinations of vital organs confirmed the findings obtained from clinical biochemistry. There were no observable histopathological changes in kidney, spleen, heart and lung of rats following oral administration of the L. cameronensis cultivar sclerotial powder up to 1000 mg/kg, for 28 consecutive days (Figures 3–6). Normal histological structures of liver were visualized in both male and female rats (Figure 7), except for mild fatty changes in the hepatocytes of the male control group (Figure 8). However, none of the treated groups exhibited fatty changes in hepatocytes.
Conclusion
The results obtained from 28-days sub-acute toxicity study of the L. cameronensis cultivar sclerotial powder demonstrated that the consumption of the sclerotial powder is safe at dosage level up to 1000 mg/kg. The experimental rats did not show any changes in general observations, growth pattern and body weight gain. There were also no significant changes in the hematological, clinical biochemical parameters and histopathology of vital organs of rats. Therefore, the no-observed-adverse-effect level (NOAEL) dose of the L. cameronensis cultivar sclerotial powder in 28-day sub-acute toxicity study is up to 1000 mg/kg. This dosing level can serve as reference for long term repeated dose toxicity testing as well as assessment of reproductive toxicity, mutagenicity and genotoxicity of the L. cameronensis cultivar sclerotial powder.
Author Contributions
S-YF and N-HT conceived and designed the study. S-SL performed the experiments, analyzed the data and drafted the manuscript. JP helped to perform and analyzed histopathological data. N-HT and S-YF helped to draft the manuscript.
Funding
This research is supported by Fundamental Research Grant Scheme (FRGS) FP029-2014A from Ministry of Science, Technology and Innovation, Malaysia, Special Assistance Funding (BKS045-2017) and Post Graduate Research Grant PG144-2014B from University of Malaya, Malaysia.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Cui, B. K., Tang, L. P., and Dai, Y. C. (2011). Morphological and molecular evidences for a new species of Lignosus (Polyporales, Basidiomycota) from tropical China. Mycol. Prog. 10, 267–271. doi: 10.1007/s11557-010-0697-y
Douanla-Meli, C., and Langer, E. (2003). A new species of Lignosus (Polyporaceae) from Cameroon. Mycotaxon 86, 389–394.
Eik, L. F., Naidu, M., David, P., Wong, K. H., Tan, Y. S., and Sabaratnam, V. (2012). Lignosus rhinocerus (Cooke) Ryvarden: a medicinal mushroom that stimulates neurite outgrowth in PC-12 cells. Evid. Based Complement. Alternat. Med. 2012:320308. doi: 10.1155/2012/320308
Gao, S., Lai, C. K. M., and Cheung, P. C. K. (2009). Nondigestible carbohydrates isolated from medicinal mushroom sclerotia as novel prebiotics. Int. J. Med. Mushrooms 11, 1–8. doi: 10.1615/IntJMedMushr.v11.i1.10
Gautam, M. K., and Goel, R. K. (2014). Toxicological study of Ocimum sanctum Linn leaves: hematological, biochemical, and histopathological studies. J. Toxicol. 2014, 135654. doi: 10.1155/2014/135654
Kong, B. H., Tan, N. H., Fung, S. Y., Pailoor, J., Tan, C. S., and Ng, S. T. (2015). Nutritional composition, antioxidant properties, and toxicology evaluation of the sclerotium of Tiger Milk Mushroom Lignosus tigris cultivar E. Nutr. Res. 36, 174–183. doi: 10.1016/j.nutres.2015.10.004
Lai, C. K. M., Wong, K. H., and Cheung, P. C. K. (2008). Antiproliferative effects of sclerotial polysaccharides from Polyporus rhinocerus Cooke (Aphyllophoromycetideae) on different kinds of leukemic cells. Int. J. Med. Mushrooms 10, 255–264. doi: 10.1615/IntJMedMushr.v10.i3.60
Lau, B. F., Abdullah, N., Aminudin, N., and Lee, H. B. (2013). Chemical composition and cellular toxicity of ethnobotanical-based hot and cold aqueous preparations of the tiger’s milk mushroom (Lignosus rhinocerotis). J. Ethnopharmacol. 150, 252–262. doi: 10.1016/j.jep.2013.08.034
Lau, B. F., Abdullah, N., Aminudin, N., Lee, H. B., and Tan, P. J. (2015). Ethnomedicinal uses, pharmacological activities, and cultivation of Lignosus spp. (Tiger′s Milk mushrooms) in Malaysia-a review. J. Ethnopharmacol. 169, 441–458. doi: 10.1016/j.jep.2015.04.042
Lee, M. L., Tan, N. H., Fung, S. Y., Tan, C. S., and Ng, S. T. (2012). The antiproliferative activity of sclerotia of Lignosus rhinocerus (Tiger Milk Mushroom). Evid. Based Complement. Alternat. Med. 2012:697603. doi: 10.1155/2012/697603
Lee, S. S., Chang, Y. S., and Noraswati, M. N. R. (2009). Utilization of macrofungi by some indigenous communities for food and medicine in Peninsular Malaysia. Forest Ecol. Manag. 257, 2062–2065. doi: 10.1016/j.foreco.2008.09.044
Lee, S. S., Enchang, F. K., Tan, N. H., Fung, S. Y., and Pailoor, J. (2013). Preclinical toxicological evaluations of the sclerotium of Lignosus rhinocerus (Cooke), the Tiger Milk mushroom. J. Ethnopharmacol. 147, 157–163. doi: 10.1016/j.jep.2013.02.027
Lee, S. S., Tan, N. H., Fung, S. Y., Sim, S. M., Tan, C. S., and Ng, S. T. (2014). Anti-inflammatory effect of the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden, the Tiger Milk mushroom. BMC Complement. Altern. Med. 14:359. doi: 10.1186/1472-6882-14-359
Mohanarji, S., Dharmalingam, S., and Kalusalingam, A. (2012). Screening of Lignosus rhinocerus extracts as antimicrobial agents against selected human pathogens. J. Pharm. Biomed. Sci. 18, 1–4.
Organization for Economic Cooperation and Development [OECD] (2008). “Repeated dose 28-day oral toxicity study in rodents,” in OECD Guideline for the Testing of Chemicals. Section 4: Health Effects, Paris: OECD.
Petterino, C., and Argentino-Storino, A. (2006). Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 57, 213–219. doi: 10.1016/j.etp.2005.10.002
Phan, C. W., David, P., Naidu, M., Wong, K. H., and Sabaratnam, V. (2013). Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement. Altern. Med. 13:261. doi: 10.1186/1472-6882-13-261
Ryvarden, L., and Johansen, I. (1980). A Preliminary Polypore Flora of East Africa. Oslo: Fungiflora.
Tan, C. S., Ng, S. T., and Tan, J. (2013). Two new species of Lignosus (Polyporaceae) from Malaysia- L. tigris and L. cameronensis. Mycotaxon 123, 193–204. doi: 10.5248/123.193
Tan, C. S., Ng, S. T., Yap, Y. H. Y., Lee, S. S., Lee, M. L., Fung, S. Y., et al. (2012). “Breathing new life to a Malaysia lost national treasure-the Tiger-Milk mushroom (Lignosus rhinocerotis),” in Proceedings of the 18th Congress of the International Society for Mushroom Science, eds J. Zhang, H. Wang, and M. Chen (Beijing: China Agriculture Press), 66–71.
Wong, K. H., and Cheung, P. C. K. (2009). “Sclerotia: emerging functional food derived from mushrooms,” in Mushrooms as Functional Foods, ed. P. C. K. Cheung (Hoboken, NJ: John Wiley), 111–146.
Wong, K. H., Lai, C. K., and Cheung, P. C. K. (2011). Immunomodulatory activities of mushroom sclerotial polysaccharides. Food Hydrocoll. 25, 150–158. doi: 10.1016/j.foodhyd.2010.04.008
Wong, K. H., Lai, C. K. M., and Cheung, P. C. K. (2009). Stimulation of human innate immune cells by medicinal mushroom sclerotial polysaccharides. Int. J. Med. Mushrooms 11, 215–223. doi: 10.1615/IntJMedMushr.v11.i3.10
Yap, H. Y. Y., Aziz, A. A., Fung, S. Y., Ng, S. T., Tan, C. S., and Tan, N. H. (2014a). Energy and nutritional composition of Tiger Milk Mushroom (Lignosus tigris Chon S. Tan) sclerotia and the antioxidant activity of its extracts. Int. J. Med. Sci. 11, 602–607. doi: 10.7150/ijms.8341
Yap, H. Y. Y., Fung, S. Y., Tan, N. H., Ng, S. T., and Tan, C. S. (2014b). DNA barcode markers for two new species of Tiger Milk Mushroom: Lignosus tigris and L. cameronensis. Int. J. Agric. Biol. 16, 841–844.
Yap, H. Y. Y., Tan, N. H., Fung, S. Y., Aziz, A. A., Tan, C. S., and Ng, S. T. (2013). Nutrient composition, antioxidant properties, and anti-proliferative activity of Lignosus rhinocerus Cooke sclerotium. J. Sci. Food Agric. 93, 2945–2952. doi: 10.1002/jsfa.6121
Keywords: Lignosus cameronensis, sclerotium, toxicity, hematological, histopathological
Citation: Lee S-S, Tan N-H, Pailoor J and Fung S-Y (2017) Safety Evaluation of Sclerotium from a Medicinal Mushroom, Lignosus cameronensis (Cultivar): Preclinical Toxicology Studies. Front. Pharmacol. 8:594. doi: 10.3389/fphar.2017.00594
Received: 07 April 2017; Accepted: 16 August 2017;
Published: 01 September 2017.
Edited by:
Banasri Hazra, Jadavpur University, IndiaReviewed by:
Pinarosa Avato, Università degli Studi di Bari Aldo Moro, ItalyAnita Mukherjee, University of Calcutta, India
Copyright © 2017 Lee, Tan, Pailoor and Fung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin-Yee Fung, fungshinyee@gmail.com
 Sook-Shien Lee
Sook-Shien Lee Nget-Hong Tan1
Nget-Hong Tan1 Shin-Yee Fung
Shin-Yee Fung