- 1Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
- 2Shanghai Sixth People’s Hospital East Campus, Shanghai University of Medicine and Health, Shanghai, China
- 3Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 4School of Pharmacy, Shanghai Jiao Tong University, Shanghai, China
- 5Department of Neurology, Jinshan Hospital, Fudan University, Shanghai, China
miR-132 is an endogenous small RNA and controls post-transcriptional regulation of gene expression via controlled degradation of mRNA or transcription inhibition. In the nervous system, miR-132 is significant for regulating neuronal differentiation, maturation and functioning, and widely participates in axon growth, neural migration, and plasticity. The miR-132 is affected by factors like mRNA expression, functional redundancy, and signaling cascades. It targets multiple downstream molecules to influence physiological and pathological neuronal activities. MiR-132 can influence the pathogenesis of many diseases, especially in the nervous system. The dysregulation of miR-132 results in the occurrence and exacerbation of neural developmental, degenerative diseases, like Alzheimer’s disease, Parkinson’s disease and epilepsy, neural infection and psychiatric disorders including disturbance of consciousness, cognition and memory, depression and schizophrenia. Regulation of miR-132 expression relieves symptoms, alleviates severity and finally effects a cure. This review aims to discuss the clinical potentials of miR-132 in the nervous system.
Introduction
MicroRNA (miRNA) is an endogenic RNA composed of 20 to 24 nucleotides, in combination with the 3′ untranslated region (3′UTR) to regulate the expression of gene transcription (Reschke and Henshall, 2015). Currently, around 1500 genes encoded by miRNAs have been confirmed in the human genome, regulating the expression of various mRNAs in different physiological and biological conditions. Among these miRNAs, miR-132 has been mentioned in many articles that illustrate its role in the neuronal development and functioning. Besides, its part in diverse diseases of the nervous system has not been elucidated clearly yet. miR-132 was initially found in nerve tissues of mice (Lagos-Quintana et al., 2001), later in human (Calin et al., 2004), zebrafish (Chen et al., 2005), and cow (Coutinho et al., 2007). Mature miR-132 sequences with a length of 22 base pairs (bp) are processed by precursor sequences with 66 bp in length (Coutinho et al., 2007). miR-132 in the human body is composed of two homologous miRNAs, hsa-miR-132-5p and hsa-miR-132-3p. The latter miRNA is a significant part of the miR-132/212 cluster, targets and decreases spinal AMPA receptor subunit GluA1 as a negative regulator of chronic neuropathic pain (Leinders et al., 2016). miR-132 has undergone conservative evolution in humans, rats, mice, apes, and other species, exhibiting the same sequence and structure (Sun et al., 2015).
Regulation of miR-132 in a biological process is critical to physiological balance among various systems in human bodies. It is influenced by several factors including expression levels of miR-132 and its mRNA target, overlapping functional redundancy, temporal patterns of miR-132 changes, signaling feedbacks and other control mechanisms, as well as miR-132 longevity with long-lasting effects on protein output (Ebert and Sharp, 2012). Once the balance is broken, dysregulation of miR-132 will lead to occurrence and exacerbation of multiple disorders from different systems, including the nervous system.
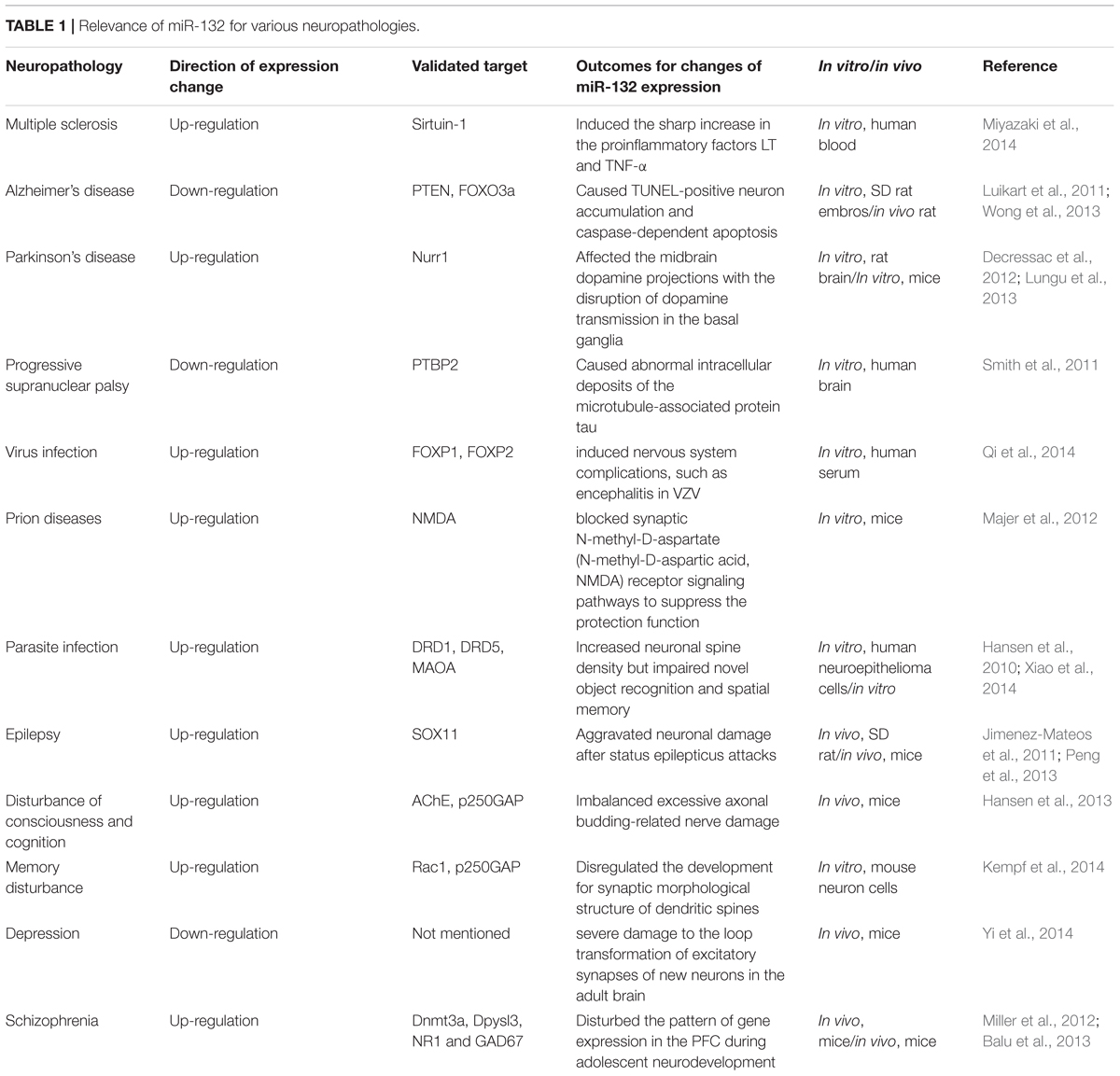
miR-132 represents a broad category of nerve regulatory networks in the dynamic regulation of neuronal differentiation, maturation and functioning, and participates in axon growth, neural migration, and plasticity. The dysfunction of miR-132 occurs widely in neural development, neurodegenerative diseases and psychological and mental disorders (Table 1). Regulation of miR-132 expression and downstream signaling cascades potentially relieves symptoms, alleviates severity and finally effects a cure.
Physiological Function of miR-132 in the Nervous System
Neural Growth and Migration
The physiological involvement of miR-132 in the process of neural growth and migration requires multiple interactions with other cells. Radial glial progenitors, regulated by the Notch signaling pathway, have significant implications in the neural growth and patterning of spinal cord. Salta and colleagues identified a bidirectional regulatory circuit in a temporal manner between miR-132 and its target Ctbp2 (C-terminal binding protein 2). This circuit dynamically contributes to the transduction of Notch signaling and its downstream effects on glial progenitors. During early stage of embryogenesis, increasing Ctbp2 represses transcriptional level of Sirtuin-1 to initiate Notch signaling cascade, resulting in the progressive accumulation of the glial progenitor cells. With significant increase of Ctbp2, miR-132 expression becomes much higher and suppresses Ctbp2 expression after reaching a certain threshold. These processes contribute to a bidirectional Notch signaling pathway in Gfap+ glial progenitors (Salta et al., 2014). Moreover, block of miR132-mediated repression contributed to the overexpression of MeCP2 and BDNF (brain-derived neurotrophic factor) levels in vivo (Klein et al., 2007).
Besides, miR-132 impacts on the neural migration in mammals. By profiling gene expression and knocking out miR-132 in the mice dorsal root ganglion, Clovis and colleagues identified the ectopic expression of a new form of transcription factor Foxp2 in cortex projecting neurons. Therefore, the group believes knocking out miR-132 leads to a reduction of Foxp2 expression, a delay of neurite outgrowth and blockage of radial migration for neural cells. Moreover, proliferation and branching is precisely regulated by a coordinated effort of miR-9 and miR-132 for Foxp2 derived 3′UTR (Clovis et al., 2012).
Cell Differentiation
The relationship between miRNAs and cell differentiation has long been studied. miR-132 directly targets the expression of nuclear receptor related protein 1 (Nurr1) to induce the differentiation of embryonic stem cells (ESCs) into dopamine neurons. In the mouse ESCs, Yang and colleagues noticed that endogenous miR-132 expression was directly down-regulated by the antisense oligonucleotide, thereby promoting the cell differentiation of tyrosine hydroxylase-positive nerve cells. Furthermore, the antisense oligonucleotide is even capable of reversing the neuronal differentiation of the miR-132-mediated inhibitory effect (Yang D. et al., 2012). Besides, Yoshimura and colleagues pointed out that silencing miR-132 or suppressing ERK1/2 signaling pathway activation led to the increased expression of a variety of synaptic proteins, including PSD-95, GluR1 and synapsin I (Yoshimura et al., 2016). Therefore, miR-132 can be regarded as a negative regulator of synapse maturation and cell differentiation.
Neural Plasticity
Apart from cell proliferation and differentiation, miR-132 is also implicated in the process of modulation of specific neuronal and immune function. Remenyi and colleagues found that in miR-132/212 knockout mouse models, miR-132 could affect cortex synaptic transmission and plasticity (Remenyi et al., 2013). Developing axons can localize protein synthesis. In the axon, miR-132 is present at high concentrations and regulates axonal growth through the target gene Rasa1. Hancock and colleagues proposed two possible mechanisms for the regulation of the activity of miR-132: potential axonal guidance and the temporal regulation associated with the developmental stage (Hancock et al., 2014). Another mechanism for regulation of miR-132 is raised by Cheng and colleagues. They believe that miR-132 attenuates the entraining effects of light, induced by photic stimulation via a MAPK/CREB signaling pathway (Cheng et al., 2007).
Apart from miR-132 knock out, silencing other molecules can also contribute to negative regulation of neural plasticity together with miR-132. Luteolin, a type of flavonoid, acts as a nerve nutrition factor. By knocking out luteolin, Lin and colleagues demonstrated that the inhibition of axon growth with the combined effect of miR-132 and luteolin, suggesting miR-132 might regulate nerve axon formation. They think that luteolin can activate the cAMP response element binding protein via phosphorylation to improve the miR-132 expression level and axon growth. According to the findings, luteolin therapy can enhance the level of ERK phosphorylation and PKA activity by inducing miR-132 expression and promoting axonal growth (Lin et al., 2012). Based on the researches above, we conclude the main roles of miR-132 in neurological conditions (Figure 1).
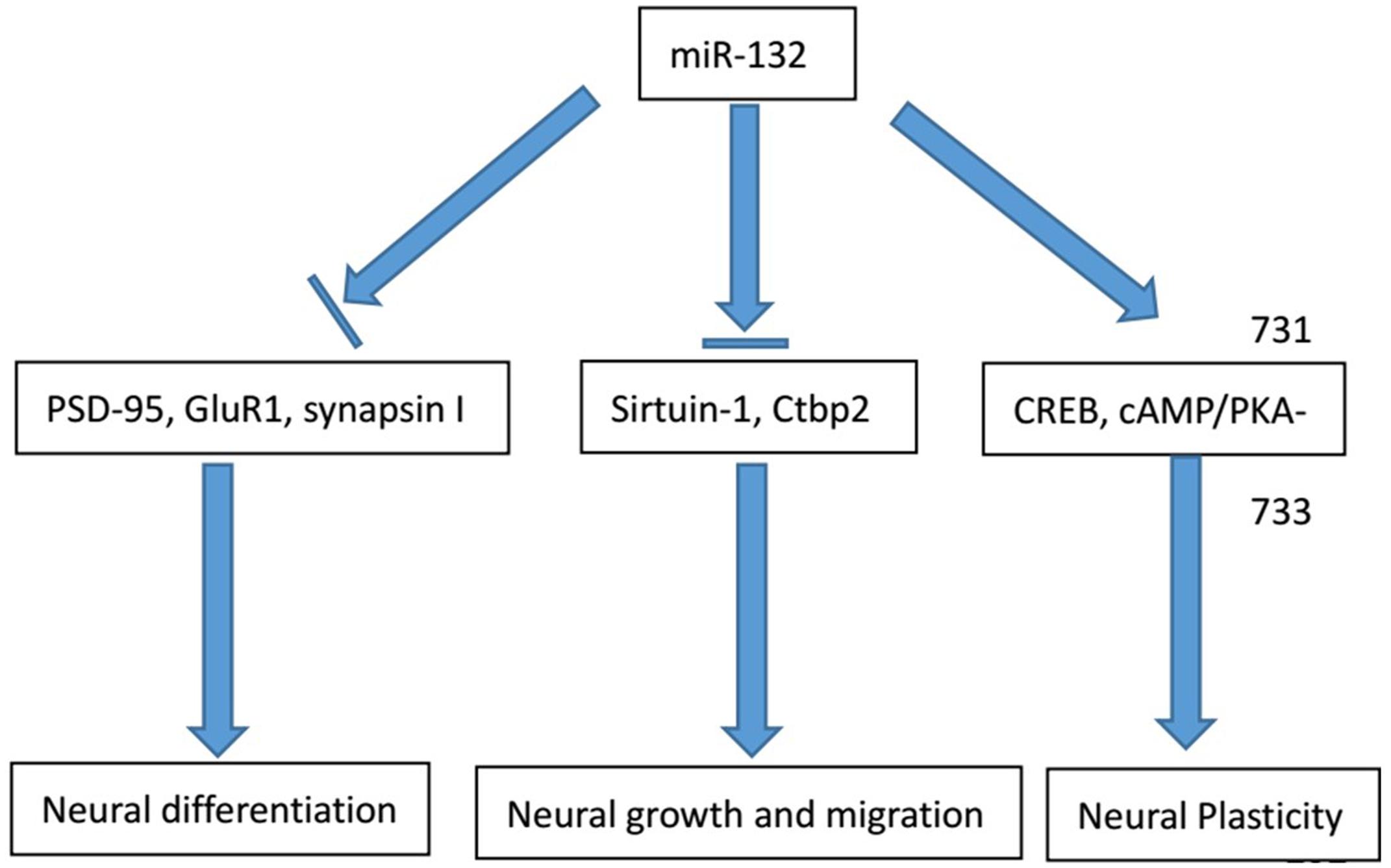
FIGURE 1. Major physiological functions of miR-132 in the nervous system. The physiological involvement of miR-132 in the process of neural growth and migration, cell differentiation and neural plasticity and major regulator genes in the process are listed above.
The Relationship Between miR-132 and Neurological Disorders
Neurodegenerative Diseases
Multiple Sclerosis
Multiple sclerosis (MS) is a demyelinating disorder with damaged insulating covers of neurons in the brain and spinal cord. This damage destroyed partial ability of communication in the nervous system, resulting in plenty of signs and symptoms, involving mental, physical, and sometimes psychiatric problems (Compston and Coles, 2002). Miyazaki and colleagues found a significant rise in miR-132 expression in B cells accompanied with the disease onset. Compared with the healthy group, dual B-cell antigen receptor (BCR)+CD40 stimulation considerably increased the expression of lymphotoxin (LT) and TNF-α, along with a significant increase in miR-132 (p = 0.0143). MiR-132 does not directly target LT and TNF-α, indicating an indirect pathway. (Miyazaki et al., 2014; Table 1).
Nakahama and colleagues used Sirtuin-1, a negative regulator of miR-132 to study the underlying mechanism. Sirtuin-1, member of the histone acetylation enzyme III family, plays an important role in aging, metabolism, tumor formation, inflammation, and a series of immune reactions. Sirtuin-1 possibly inhibits the transcription factors NF-kB and AP-1 in BCR signaling pathways, thus inhibiting the downstream LT and TNF-α expression. Excitation of Sirtuin-1 is regulated by resveratrol, a small molecule that can pass the blood brain barrier and suppress pro-inflammatory cellular responses in the peripheral circulation and target organs (Nakahama et al., 2013). Yeung and colleagues also proposed that Sirtuin-1 could reduce nerve damage and regulate nerve protection in muscle atrophy sclerosis and Alzheimer’s disease (Yeung et al., 2004). Sirtuin-1 down-regulates miR-132 expression and regulates downstream targets, offering a meaningful alternative therapy in MS treatment.
Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disease with a progressive development style and one of the most common types of dementia. In 30 years, it is predicted that there will be over 100 million AD patients. The synapses and neurons are lost at a significant rate in the basal forebrain, cortex and hippocampus in these patients, resulting in cognitive decline and other symptoms. The abnormal expression of miRNA regulates major AD-related genes, such as APP and BACE1, affects Aβ cytotoxicity and neural death, and even marks characteristic changes in disease initiation and progression (Fang et al., 2012). Wong and colleagues found that the decrease of miR-132/212 could be detected at the onset of AD, and it became especially distinct at the late stage. In primary cultured neurons, the inhibition of miR-132/212 expression will lead to TUNEL-positive neuron accumulation and caspase-dependent apoptosis. They prove that PTEN and FOXO3a are two direct targets and suppress the AKT signaling pathway (Wong et al., 2013; Table 1). The inhibition of either miR-132 or miR-212 will eliminate the inhibition of PTEN and FOXO3a and trigger programmed death.
Similarly, the miR-132/212 cluster in late-onset Alzheimer’s disease (LOAD) appears to be in significant decline. miR-132-3p exhibits different levels of decreased expression in three different parts – the hypothalamus, the frontal cortex and medial temporal area (Luikart et al., 2011). Through the analysis of the LOAD samples from the hypothalamus, expression levels are found to be promoted in glial-rich miR-142-3p, miR-150-5p and miR-223-3p. However, these miRNAs can only be detected in the middle-late stage, so they only explain the loss of neurons of neurodegenerative diseases in late stages. miR-132-3p is dysregulated at early courses of the disease, especially before neuronal loss occurs. Therefore, miR-132 is closely related to LOAD in different stages (Magill et al., 2010). miR-132 with a high synaptic activity and plasticity, regulates neural outgrowth under the effect of nerve growth factors. Moreover, it controls the combination of newborn neurons in adult hypothalamus and dentate gyrus, and shapes synapse formation and plasticity in the visual cortex. miR-132 has many molecular targets, such as Tau, EP300, Sirtuin-1 and FOXO1a (Min et al., 2010). In the AD samples of the hypothalamus, the protein content of FOXO1a obviously increased (Lau et al., 2013). By examining the expression of different downstream targets of miR-132, we are provided with a new way to predict disease progression.
Parkinson’s Disease
Parkinson’s disease (PD) is closely associated with age and the second most common neurodegenerative disorder after Alzheimer’s disease. It attacks 1–2% people aged 60 and above (Yoshimura et al., 2016). The neurodegenerative process in PD affects the midbrain dopamine projections from the substantia nigra with the disruption of dopamine transmission in the motor loop of the basal ganglia. Lungu and colleagues developed a spontaneous autosomal recessive rat model for neurodegeneration and found a decreased expression of BDNF in the midbrain and plasma. Increased synaptic nucleoprotein α and decreased Nurr1 transcription factor lead to a significant increase in the miR-132 level (Lungu et al., 2013; Table 1). Decressac and colleagues showed the downstream target of miR-132, Nurr1 nucleoprotein, has an anti- toxic effect on nucleoprotein α (Decressac et al., 2012; Table 1). Therefore, Nurr1 is the most important transcription factor for midbrain dopamine neurons to survive and to maintain their differentiation ability, serving as a potential new target for nerve protection.
Tauopathies: Progressive Supranuclear Palsy
Tauopathies account for a major group of neurological and movement diseases with a significant characteristics that its main cause, protein tau exhibits abnormal deposits in the cells. The miRNA expression information from patients who had progressive supranuclear palsy (PSP), a primary 4R-tau disorder was comprehensively analyzed, which displayed a downregulation of miR-132. Moreover, miR-132 can target polypyrimidine tract binding protein 2 (PTBP2) directly, which is a major neuronal splicing factor and increased in protein level from PSP patients. Overexpressed miR-132 or silenced PTBP2 can influence endogenous 4R:3R-tau proportion in neurons (Smith et al., 2011; Table 1).
Epilepsy
Epilepsy is a chronically progressive neurological disease originating from unusual neuronal discharges in the brain. There are nearly 50 million epileptic patients in the world, which puts heavy burden on societies. The present anti-epileptic therapy aims at inhibiting convulsant or epileptic effects. They can control the frequency of seizure attacks, but attempts on revealing the underlying pathophysiology of epilepsy show little progress. Nudelman and colleagues found that pilocarpine induced epilepsy led to a significant upregulation of primiR-132 and mature miR-132. miR-132 in patients with epilepsy tolerance presents a significant ability to react to different stimuli (Nudelman et al., 2010). Studies on the functional sequence of miR-132 showed that before status epilepticus occurs, miR-132 antagomirs, which can inhibit expression of miR-132, does not reduce epilepsy risks but can significantly reduce the hippocampal nerve damage, which confirms that the excessive expression of miR-132 aggravates neuronal damage after status epilepticus attacks (Peng et al., 2013; Table 1). Researchers also found that levels of miR-132 in the acute and chronic phases were significantly increased. In the latency stage, the expression of miR-132 was still at a high level (Jimenez-Mateos et al., 2011; Table 1). Moreover, micro-injection of the antagomirs can significantly decrease miR-132 and reduce mortality caused by the abnormal epileptic discharge of neurons. The increase of miR-132 in the latency stage of epilepsy provides new insight into the prediction and control of the disease.
The role of miR-132 in the pathogenesis of epilepsy also involves the participation of other genes and factors. In a focal medial temporal lobe epilepsy model, the 3′UTR binding sites of the transcription factor SOX11 were found to be connected with miR-132-3p to regulate the branching and bifurcation of newborn neurons. Using a status epilepticus hippocampus neuron model, Lei and colleagues found that miR-132 and BDNF mRNA increased significantly. Meanwhile changes in epileptic discharge were noticed as well. BDNF preprocessing catabatic TrkB receptor activation can partially reduce high frequency discharge, but the excessive expression of miR-132 deteriorates the symptoms of epileptic discharge, proving the control of miR-132 on the BDNF/TrkB signaling pathway as an important mechanism of epilepsy (Xiang et al., 2015).
Neural Infection
Virus and Prion Infection
Varicella, also called chickenpox, is a highly contagious disease caused by varicella zoster virus (VZV). As novel and non-invasive indicators for the diagnosis of contagious disease, circulating miRNAs have been intensively researched. Qi and colleagues found that miR-132 could distinguish varicella patients from healthy controls and other microbial infections with moderate sensitivity and specificity (Qi et al., 2014; Table 1).
Prion disease, a kind of deadly disease can cause neurodegeneration and affect mammals. The underlying pathogenesis is probably that normal cellular prion protein, (PrPC) is turned into the infectious type, PrPSc (Scrapie prion protein). Although this happens over a long preclinical latency stage, compromised nerve function is noticed at asymptomatic stages. Particularly, in animal models of prion disease, loss of synaptic function occurs before the onset of clinical symptoms. Majer and colleagues separated hippocampal CA1 neurons, determined the preclinical transcriptional response, and found the abnormal transcription of miR-132-3p blocked synaptic N-methyl-D-aspartate (N-methyl-D-aspartic acid, NMDA) receptor signaling pathways to suppress the protection function (Majer et al., 2012; Table 1). miR-132 plays an indirect role in the pathogenesis of prion disease and target management of miR-132 seems to be a promising method for prevention.
Parasite Infection
Toxoplasma gondii (T. gondii) is a highly infectious parasite to living creatures. It is discovered that T. gondii impacts intermediate hosts on their behavioral changes as long as it progresses to a late stage, distinguished by the parasite cysts existing in the brain. Li and colleagues found that miR-132, was the only miRNA that was apparently increased in all three prototype Toxoplasma strains. They predicted the potential target and identified 20 genes and the strongest associated pathway dopamine receptor signaling cascade (Li et al., 2015; Table 1).
In chronically infected mice models, it was discovered that miR-132 overexpression upregulated nerve spine density in transgenic mice at the cost of recognition and memory functions (Xiao et al., 2014; Table 1). Wang and colleagues reported that when the expression of miR-132 was decreased in hippocampus, their ability for tracing fear memory was compromised at the same time. Therefore, in Toxoplasma gondii infection, the memory of infected patients will be impaired whether miR-132 is increased or decreased. The detection of five brain regions showed that women were infected in the hypothalamus, hippocampus, striatum and brainstem. However, men were infected only in the hippocampus. This confirms that sex is an important factor in the severity of disease (Hansen et al., 2010; Table 1). In spite of the regulatory ability of BDNF for miR-132 in neurons, it is not correlated in the brains of individuals with Toxoplasma infection. Wanet and colleagues found that other transcription factors, such as CREB and REST, may affect the expression level of miR-132 in patients with chronic infection (Wanet et al., 2012).
Psychological Diseases
Disturbance of Consciousness and Cognition
Apart from the involvement in the neurologic diseases, miR-132 also deeply affects the psychological changes and formation of mental problems. In three different stress rat models, Shaltiel and colleagues found long-term stress can significantly improve the individual differences and expression levels of hippocampus miR-132. At the same time, the expression for two miR-132 targets AChE and GTP enzyme agonist p250GAP is restrained (Shaltiel et al., 2013). In a foot shock model, knocking out AChE significantly restricted the increase of miR-132. The results confirmed that the hippocampus miR-132 mediated the sustainability of the cholinergic signaling pathway, which was interrupted by AChE overexpression (Shaltiel et al., 2013). The inhibition of AChE will hinder cognitive defects stimulated by the plantar electric shocks, which indicates that miR-132 regulates the process of cognitive impairment after stress (Wayman et al., 2008). Under the AChE glucocorticoid reactive component activation, the ACh level drops and suppresses the CREB-induced transcription for miR-132, later achieving balance again (Vo et al., 2005). The inhibition of cortical neuron p250GAP will improve axial budding and neuronal activity, the GABAα inhibitor dicentrine can induce miR-132 transcription and reduce p250GAP expression levels to further promote axonal growth (Impey et al., 2010). These findings confirm the long-term double inhibition of AChE and p250GAP will balance excessive axonal budding-related nerve damages and become an important compensatory mechanism.
Hansen and colleagues showed miR-132 expression was upregulated prominently via a spatial memory mission by 1.5 folds, in excitatory hippocamal layers confirming the positive role of miR-132 in cognitive capacity (Hansen et al., 2013; Table 1).
Memory Disturbance
Hippocampal miR-132 is vital to the formation of trace fear memory. Mature miR-132 was up-regulated and reached a peak expression in 30 min with trace fear conditioning (TFC) and returned to a normal value after 2 h. The above information demonstrated the transient, expression of miR-132 was induced by activity (Lambert et al., 2010). The increased level of hippocampus miR-132 assists in memory regulation in dendritic spines and synaptic transmission. However, the excessive expression of miR-132 in transgenic mice, can increase the dendritic spine density but significantly damage the cognition of spatial memory (Wang et al., 2013). This indicates that proper miR-132 expression promotes good memory formation, and the lack or overexpression of miR-132 will damage cognitive function.
Besides, stimuli like high-dose ionizing radiation lead to severe learning and memory impairments. Kempf and colleagues observed radiation-induced reduction of Rac1 and miR-132 inhibits the GTPase-activating protein p250GAP to regulate Rac1 activity. In the irradiated hippocampus and cortex, the researchers observed similar changes in the signaling cascades compared with in vitro results. The reduced expression of miR-132 and Rac1 led to up-regulation in hippocampal cofilin which contributes to correct learning and memory for its necessity of correct spine and synapse morphology (Kempf et al., 2014; Table 1). This process is essential for the correct development of the synaptic morphological structure of dendritic spines to ensure proper memory and cognitive function.
Depression
Depression has become an increasingly prevalent health problem throughout the world. Yi and colleagues found that single anti-depression therapy has a poor effect in 30% of depression patients. miR-132 can improve nerve growth, synaptic function, and cognition and offers several antidepressant effects (Yi et al., 2014; Table 1). Natural Extracts compose of a major part in depression treatment. Oleanolic acid is a pentacyclic terpene that can secrete serotonin and inhibit ischemic brain damage (Yang Y.C. et al., 2012). BDNF and TrkB are closely related to neurotransmitter release and postsynaptic signals and participate in the antidepressant response of oleanolic acid. Researchers silenced miR-132 expression to inhibit oleanolic acid-mediated behavior improvement and nerve nutritional signal transduction pathways. Oleanolic acid regulates and inhibits the signal transduction pathway and neuron proliferation of chronic stress injury through hypothalamus TrkB receptor-mediated BDNF-ERK after CREB miR-132 (Luikart et al., 2011; Table 1). Previous studies have reported that BDNF can increase cortical cultivation and miR-132 expression levels of dopamine neurons (Numakawa et al., 2011a,b), which proves a great potential in antidepressant treatment.
Schizophrenia
Schizophrenia is comprised of affective, cognitive, neuromorphological, and molecular abnormalities that originate from a problematic neurodevelopment. Accurate diagnosis of this disease seems to be a priority for early control and management. Yu and colleagues used dizocilpine to create a schizophrenia-like rat model and found that decrease of miR-132 in peripheral blood of 105 schizophrenia controls compared to 130 healthy controls. Besides, they also proved an apparent increase of miR-132 after risperidone treatment compared to that before treatment, further indicating the diagnostic role for miR-132 in schizophrenia (Yu et al., 2015).
As for the primary mechanism for schizophrenia onset, the dysregulation through NMDA/AMPA receptor-related signaling pathway may potentially disrupt cognitive development. Miller and colleagues found that miR-132 contributed to activity-oriented synaptic plasticity via NMDARs’ involvement in the positive feedback loop. A four-fold upregulation was noticed in miR-132 expression in the prefrontal cortex(PFC) in 3 weeks, after birth, a period with large quantity of synaptic modulation, via NMDAR signaling. Decreased miR-132 expression and the abnormal expression of Dpysl3 could affect synaptic activity and outgrowth negatively (Miller et al., 2012; Table 1). The above conclusions indicated that miR-132 was significant for gene expression mode in the PFC neurodevelopment.
The maturation of prefrontal GABAergic interneurons, which aim at excitatory nerve cells, is the second major process during late neuron development. With the possible functions of miR-132 in the initiation of NMDAR cascade taken into account, dysregulated miR-132 expression may interrupt the maturation of GABA interneurons at a molecular level where decreased GAD67 was encoded by GAD1 gene and synthesized by GABA (Balu et al., 2013; Table 1).
Interrelationship Between miR-132 and miR-212 in the Nervous System
miR-212, categorized to the same cluster as miR-132, also serves as a significant gene in the process of neuronal development in both physiological and pathological conditions. Close sequences and identical seeding region are noticed in miR-132 and miR-212, indicating their potential targeting the same mRNAs. When it comes to the role in neurologic diseases, a down-regulation of miR-212 is detected in anencephaly (Zhang et al., 2010). Besides, in the prefrontal cortex of individuals, the dysfunction of miR-132 and miR-212 is greatly influenced by psychological diseases (Kim et al., 2010). Moreover, down-regulation is detected in miR-132 and miR-212expression in the α-synuclein overexpressed tissue, which is a classic stereotype of PD (Gillardon et al., 2008). The interrelationship between miR-132 and miR-212 deserves further investigation and attention as to their promising roles in the physiological and pathological regulation and functioning in the nervous system.
Discussion
Recently, microRNA (miR) modulators in neuronal processes convey diagnostic and therapeutic implications for regulating miR levels in many neurological diseases (Glass et al., 2010). As one of the most significant neural-related miRNAs, miR-132 mainly targets transcription factors and directly or indirectly affects neural signaling to the central and peripheral nervous systems. From the bidirectional regulatory circuit between miR-132 and Ctbp2 in neural migration, to cAMP/PKA- and ERK dependent CREB signaling cascade in neural modification, changing miR-132 expression manipulates neural signaling transduction and results in physiological activities. Moreover, miR-132 is considered to play a significant role in neural diseases, including caspase-dependent apoptosis, synaptic signaling blockage and excessive axonal damages, and to activate or inhibit downstream cascades, resulting in irreversible devastation in humans. Its upregulation or downregulation in some nerve tissues significantly affects normal neuron functions and activity. Besides, at different stages of neural diseases, the dysregulation of miR-132 could be regulated to different directions under many transcriptional factors. Therefore, miRNA target therapy and diagnosis pose a significant implication in various situations. The major advantages of miR-132 in therapeutic and diagnostic applications lie in its accuracy to track down signaling transduction in a series of neurological disorders and in its ability to multi-directionally regulate message feedback circuits. Moreover, its significance also enlightens us to improve drug development of target therapy, with the help of miRNA mimics and miRNA antagonists that function as regulators of important communication cascades. For now, the mechanism of miR-132’s regulation of downstream targets is not completely understood because the present research is limited by brain blood barrier and thus it is difficult to study miR-132 in nanomedicine transport in brain diseases. To overcome this problem, we need to further our investigation in the future.
Limited research has been carried out on the miR-132 physiological functions related to peripheral nervous system disorders. Yao and colleagues found miR-132 expression increased after hypoxia dependent sciatic nerve injury. The overexpression of miR-132 in Schwann cells significantly promoted cell migration, and the injection of a miR-132 agonist into a sciatic nerve lesion in mouse models accelerated the migration of the distal Schwann cells proximally (Yao et al., 2016). However, the molecular mechanism and the main signaling pathways have not been fully elucidated. miR-132 is an important neural-related miRNA, which promotes nerve growth, migration and differentiation in the central nervous system and peripheral nervous system. Without the intervention of the blood brain barrier, miR-132 is directly involved in the various complex physiological phenomena that regulate the progress of peripheral neuropathy through similar feedback as is in the hippocampus and thalamus. As an outrageously important neural-related indicator, miR-132 significantly contributes to the control of the neural network. It provides new opportunities for clinical diagnosis and treatment of central and peripheral nervous system diseases. Ongoing research is destined to transform our understanding of disease mechanism and future treatment clinically. Identifying new factors associated with the inhibition of nerve regeneration and providing more molecular targets for intervention will have a profound effect on miRNA research in the future.
Author Contributions
YQ, JS, YO, QH, WC, XZ, YX, YC, CF, and WY participated in its design, searched databases, extracted and assessed studies and helped to draft the manuscript. WY conceived the initial idea and the conceptualization, participated in the data extraction and analysis. YC, CF, and WY revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was supported by the Projects of National Science Foundation of China (Nos. 81373366, 81570992 and 81571261), Projects of the Shanghai Committee of Science and Technology, China (No. 12XD1403800), Programs Foundation of Shanghai Municipal Health Bureau (No. 201540075) and Funds for Interdisciplinary Projects of Medicine and Engineering of Shanghai Jiao Tong University (Nos. YG2014QN06 and YG2015MS06), and Science and Technology Development Foundation of Pudong New District, Shanghai, China (PKJ2016-Y55).
References
Balu, D. T., Li, Y., Puhl, M. D., Benneyworth, M. A., Basu, A. C., Takagi, S., et al. (2013). Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc. Natl. Acad. Sci. U.S.A. 110, E2400–E2409. doi: 10.1073/pnas.1304308110
Calin, G. A., Sevignani, C., Dumitru, C. D., Hyslop, T., Noch, E., Yendamuri, S., et al. (2004). Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. U.S.A. 101, 2999–3004. doi: 10.1073/pnas.0307323101
Chen, P. Y., Manninga, H., Slanchev, K., Chien, M., Russo, J. J., Ju, J., et al. (2005). The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 19, 1288–1293. doi: 10.1101/gad.1310605
Cheng, H. Y., Papp, J. W., Varlamova, O., Dziema, H., Russell, B., Curfman, J. P., et al. (2007). microRNA modulation of circadian-clock period and entrainment. Neuron 54, 813–829. doi: 10.1016/j.neuron.2007.05.017
Clovis, Y. M., Enard, W., Marinaro, F., Huttner, W. B., and De-Pietri, T. D. (2012). Convergent repression of Foxp2 3’UTR by miR-9 and miR-132 in embryonic mouse neocortex: implications for radial migration of neurons. Development 139, 3332–3342. doi: 10.1242/dev.078063
Compston, A., and Coles, A. (2002). Multiple sclerosis. Lancet 359, 1221–1231. doi: 10.1016/S0140-6736(02)08220-X
Coutinho, L. L., Matukumalli, L. K., Sonstegard, T. S., Van-Tassell, C. P., Gasbarre, L. C., Capuco, A. V., et al. (2007). Discovery and profiling of bovine microRNAs from immune-related and embryonic tissues. Physiol. Genomics 29, 35–43. doi: 10.1152/physiolgenomics.00081.2006
Decressac, M., Kadkhodaei, B., Mattsson, B., Laguna, A., Perlmann, T., and Björklund, A. (2012). α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 4:163ra156. doi: 10.1126/scitranslmed.3004676
Ebert, M. S., and Sharp, P. A. (2012). Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515–524. doi: 10.1016/j.cell.2012.04.005
Fang, M., Wang, J., Zhang, X., Geng, Y., Hu, Z., Rudd, J. A., et al. (2012). The miR-124 regulates the expression of BACE1/beta secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 209, 94–105. doi: 10.1016/j.toxlet.2011.11.032
Gillardon, F., Mack, M., Rist, W., Schnack, C., Lenter, M., Hildebrandt, T., et al. (2008). MicroRNA and proteome expression profiling in early-symptomatic alpha-synuclein(A30P)- transgenic mice. Proteomics Clin. Appl. 2, 697–705. doi: 10.1002/prca.200780025
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., and Gage, F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140, k918–934. doi: 10.1016/j.cell.2010.02.016
Hancock, M. L., Preitner, N., Quan, J., and Flanagan, J. G. (2014). MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J. Neurosci. 34, 66–78. doi: 10.1523/JNEUROSCI.3371-13.2014
Hansen, K. F., Karelina, K., Sakamoto, K., Wayman, G. A., Impey, S., and Obrietan, K. (2013). MiRNA-132: a dynamic regulator of cognitive capacity. Brain Struct. Funct. 218, 817–831. doi: 10.1007/s00429-012-0431-4
Hansen, K. F., Sakamoto, K., Wayman, G. A., Impey, S., and Obrietan, K. (2010). Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLOS ONE 5:e15497. doi: 10.1371/journal.pone.0015497
Impey, S., Davare, M., Lesiak, A., Fortin, D., Ando, H., Varlamova, O., et al. (2010). An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol. Cell Neurosci. 43, 146–156. doi: 10.1016/j.mcn.2009.10.005
Jimenez-Mateos, E. M., Bray, I., Sanz-Rodriguez, A., Engel, T., McKiernan, R. C., Mouri, G., et al. (2011). MiRNA expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am. J. Pathol. 179, 2519–2532. doi: 10.1016/j.ajpath.2011.07.036
Kempf, S. J., Buratovic, S., von-Toerne, C., Moertl, S., Stenerlöw, B., Hauck, S. M., et al. (2014). Ionising radiation immediately impairs synaptic plasticity-associated cytoskeletal signalling pathways in HT22 cells and in mouse brain: an In Vitro / In Vivo comparison study. PLOS ONE 9:e110464. doi: 10.1371/journal.pone.0110464
Kim, A. H., Reimers, M., Maher, B., Williamson, V., McMichael, O., McClay, J. L., et al. (2010). MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 124, 183–191. doi: 10.1016/j.schres.2010.07.002
Klein, M. E., Lioy, D. T., Ma, L., Impey, S., Mandel, G., and Goodman, R. H. (2007). Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 12, 1513–1514. doi: 10.1038/nn2010
Lagos-Quintana, M., Rauhut, R., Lendeckel, W., and Tuschl, T. (2001). Identification of novel genes coding for small expressed RNAs. Science 294, 853–858. doi: 10.1126/science.1064921
Lambert, T. J., Storm, D. R., and Sullivan, J. M. (2010). MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLOS ONE 5:e15182. doi: 10.1371/journal.pone.0015182
Lau, P., Bossers, K., Janky, R., Salta, E., Frigerio, C. S., Barbash, S., et al. (2013). Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 5, 1613–1634. doi: 10.1002/emmm.201201974
Leinders, M., Üçeyler, N., Pritchard, R. A., Sommer, C., and Sorkin, L. S. (2016). Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 283, 276–286. doi: 10.1016/j.expneurol.2016.06.025
Li, Y. E., Kannan, G., Pletnikov, M. V., Yolken, R. H., and Xiao, J. (2015). Chronic infection of Toxoplasma gondii downregulates miR-132 expression in multiple brain regions in a sex-dependent manner. Parasitology. 142, 623–632. doi: 10.1017/S003118201400167X
Lin, L. F., Chiu, S. P., Wu, M. J., Chen, P. Y., and Yen, J. H. (2012). Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLOS ONE 7:e43304. doi: 10.1371/journal.pone.0043304
Luikart, B. W., Bensen, A. L., Washburn, E. K., Perederiy, J. V., Su, K. G., Li, Y., et al. (2011). MiR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLOS ONE 6:e19077. doi: 10.1371/journal.pone.0019077
Lungu, G., Stoica, G., and Ambrus, A. (2013). MicroRNA profiling and the role of microRNA-132 in neurodegeneration using a rat model. Neurosci. Lett. 553, 153–158. doi: 10.1016/j.neulet.2013.08.001
Magill, S. T., Cambronne, X. A., Luikart, B. W., Lioy, D. T., Leighton, B. H., Westbrook, G. L., et al. (2010). MicroRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. U.S.A. 107, 20382–20387. doi: 10.1073/pnas.1015691107
Majer, A., Medina, S. J., Niu, Y., Abrenica, B., Manguiat, K. J., Frost, K. L., et al. (2012). Early mechanisms of pathobiology are revealed by transcriptional temporal dynamics in hippocampal CA1 neurons of prion infected mice. PLOS Pathog. 8:e1003002. doi: 10.1371/journal.ppat.1003002
Miller, B. H., Zeier, Z., Xi, L., Lanz, T. A., Deng, S., Strathmann, J., et al. (2012). MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. U.S.A. 109, 3125–3130. doi: 10.1073/pnas.1113793109
Min, S. W., Cho, S. H., Zhou, Y., Schroeder, S., Haroutunian, V., Seeley, W. W., et al. (2010). Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966. doi: 10.1016/j.neuron.2010.08.044
Miyazaki, Y., Li, R., Rezk, A., Misirliyan, H., Moore, C., Farooqi, N., et al. (2014). A novel MicroRNA-132-Surtuin-1 axis underlies aberrant B-cell cytokine regulation in patients with relapsing-remitting multiple sclerosis. PLOS ONE 9:e105421. doi: 10.1371/journal.pone.0105421
Nakahama, T., Hanieh, H., Nguyen, N. T., Chinen, I., Ripley, B., Millrine, D., et al. (2013). Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 10, 11964–11969. doi: 10.1073/pnas.1311087110
Nudelman, A. S., DiRocco, D. P., Lambert, T. J., Garelick, M. G., Le, J., Nathanson, N. M., et al. (2010). Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20, 492–498. doi: 10.1002/hipo.20646
Numakawa, T., Richards, M., Adachi, N., Kishi, S., Kunugi, H., and Hashido, K. (2011a). MicroRNA function and neurotrophin BDNF. Neurochem. Int. 59, 551–558. doi: 10.1016/j.neuint.2011.06.009
Numakawa, T., Yamamoto, N., Chiba, S., Richards, M., Ooshima, Y., Kishi, S., et al. (2011b). Growth factors stimulate expression of neuronal and glial miR-132. Neurosci. Lett. 505, 242–247. doi: 10.1016/j.neulet.2011.10.025
Peng, J., Omran, A., Ashhab, M. U., Kong, H., Gan, N., He, F., et al. (2013). Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J. Mol. Neurosci. 50, 291–297. doi: 10.1007/s12031-013-9953-3
Qi, Y., Zhu, Z., Shi, Z., Ge, Y., Zhao, K., Zhou, M., et al. (2014). Dysregulated microRNA expression in serum of non-vaccinated children with varicella. Viruses 6, 1823–1836. doi: 10.3390/v6041823
Remenyi, J., van-den Bosch, M. W., Palygin, O., Mistry, R. B., McKenzie, C., Macdonald, A., et al. (2013). MiR-132/212 knockout mice reveal roles for these miRNAs in regulating cortical synaptic transmission and plasticity. PLOS ONE 8:e62509. doi: 10.1371/journal.pone.0062509
Reschke, C. R., and Henshall, D. C. (2015). MicroRNA and epilepsy. Adv. Exp. Med. Biol. 888, 41–70. doi: 10.1007/978-3-319-22671-2_4
Salta, E., Lau, P., Sala, F. C., Coolen, M., Bally-Cuif, L., and De-Strooper, B. (2014). A self-organizing miR-132/Ctbp2 circuit regulates bimodal notch signals and glial progenitor fate choice during spinal cord maturation. Dev. Cell 30, k423–436. doi: 10.1016/j.devcel.2014.07.006
Shaltiel, G., Hanan, M., Wolf, Y., Barbash, S., Kovalev, E., Shoham, S., et al. (2013). Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct. Funct. 218, 59–72. doi: 10.1007/s00429-011-0376-z
Smith, P. Y., Delay, C., Girard, J., Papon, M. A., Planel, E., Sergeant, N., et al. (2011). MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum. Mol. Genet. 20, 4016–4024. doi: 10.1093/hmg/ddr330
Sun, M., Yamashita, T., Shang, J., Liu, N., Deguchi, K., Feng, J., et al. (2015). Time-dependent profiles of microRNA expression induced by ischemic preconditioning in the gerbil hippocampus. Cell Transplant. 24, 367–376. doi: 10.3727/096368915X686869
Vo, N., Klein, M. E., Varlamova, O., Keller, D. M., Yamamoto, T., Goodman, R. H., et al. (2005). A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, k16426–16431. doi: 10.1073/pnas.0508448102
Wanet, A., Tacheny, A., Arnould, T., and Renard, P. (2012). MiR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 40, 4742–4753. doi: 10.1093/nar/gks151
Wang, R. Y., Phang, R. Z., Hsu, P. H., Wang, W. H., Huang, H. T., and Liu, I. Y. (2013). In Vivo knockdown of hippocampal miR-132 expression impairs memory acquisition of trace fear conditioning. Hippocampus 23, 625–633. doi: 10.1002/hipo.22123
Wayman, G. A., Davare, M., Ando, H., Fortin, D., Varlamova, O., Cheng, H. Y., et al. (2008). An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc. Natl. Acad. Sci. U.S.A. 105, 9093–9098. doi: 10.1073/pnas.0803072105
Wong, H. K., Veremeyko, T., Patel, N., Lemere, C. A., Walsh, D. M., Esau, C., et al. (2013). De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet. 22, 3077–3092. doi: 10.1093/hmg/ddt164
Xiang, L., Ren, Y., Cai, H., Zhao, W., and Song, Y. (2015). MicroRNA-132 aggravates epileptiform discharges via suppression of BDNF/TrkB signaling in cultured hippocampal neurons. Brain Res. 1622, 484–495. doi: 10.1016/j.brainres.2015.06.046
Xiao, J., Li, Y., Prandovszky, E., Karuppagounder, S. S., Talbot, C. C. Jr., Dawson, V. L., et al. (2014). MicroRNA-132 dysregulation in Toxoplasma gondii infection has implications for dopamine signaling pathway. Neuroscience 268, 128–138. doi: 10.1016/j.neuroscience.2014.03.015
Yang, D., Li, T., Wang, Y., Tang, Y., Cui, H., Tang, Y., et al. (2012). MiR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J. Cell Sci. 125, 1673–1682. doi: 10.1242/jcs.086421
Yang, Y. C., Wei, M. C., and Huang, T. C. (2012). Optimisation of an ultrasound-assisted extraction followed by RP-HPLC separation for the simultaneous determination of oleanolic acid, ursolic acid and oridonin content in Rabdosia rubescens. Phytochem. Anal. 23, 627–636. doi: 10.1002/pca.2365
Yao, C., Shi, X., Zhang, Z., Zhou, S., Qian, T., Wang, Y., et al. (2016). Hypoxia-induced upregulation of miR-132 promotes schwann cell migration after sciatic nerve injury by targeting PRKAG3. Mol. Neurobiol. 53, 5129–5139. doi: 10.1007/s12035-015-9449-y
Yeung, F., Hoberg, J. E., Ramsey, C. S., Keller, M. D., Jones, D. R., Frye, R. A., et al. (2004). Modulation of NF-kB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380. doi: 10.1038/sj.emboj.7600244
Yi, L. T., Li, J., Liu, B. B., Luo, L., Liu, Q., and Geng, D. (2014). BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J. Psychiatry Neurosci. 39, 348–359. doi: 10.1503/jpn.130169
Yoshimura, A., Numakawa, T., Odaka, H., Adachi, N., Tamai, Y., and Kunugi, H. (2016). Negative regulation of microRNA-132 in expression of synaptic proteins in neuronal differentiation of embryonic neural stem cells. Neurochem. Int. 97, 26–33. doi: 10.1016/j.neuint.2016.04.013
Yu, H. C., Wu, J., Zhang, H. X., Zhang, G. L., Sui, J., Tong, W. W., et al. (2015). Alterations of miR-132 are novel diagnostic biomarkers in peripheral blood of schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 63, 23–29. doi: 10.1016/j.pnpbp.2015.05.007
Keywords: miR-132, nervous system diseases, signaling pathways, axon growth, neural migration
Citation: Qian Y, Song J, Ouyang Y, Han Q, Chen W, Zhao X, Xie Y, Chen Y, Yuan W and Fan C (2017) Advances in Roles of miR-132 in the Nervous System. Front. Pharmacol. 8:770. doi: 10.3389/fphar.2017.00770
Received: 01 July 2017; Accepted: 12 October 2017;
Published: 25 October 2017.
Edited by:
Chiranjib Chakraborty, Galgotias University, IndiaReviewed by:
Kevin J. O’Donovan, United States Military Academy, United StatesFei Geng, McMaster University, Canada
Copyright © 2017 Qian, Song, Ouyang, Han, Chen, Zhao, Xie, Chen, Yuan and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghui Chen, cyh1973131@163.com Weien Yuan, yuanweien@126.com Cunyi Fan, cyfan@sjtu.edu.cn
†These authors have contributed equally to this work.
 Yun Qian
Yun Qian Jialin Song
Jialin Song Yuanming Ouyang
Yuanming Ouyang Qixin Han
Qixin Han Wei Chen1,2
Wei Chen1,2 Xiaotian Zhao
Xiaotian Zhao Yangmei Xie
Yangmei Xie Yinghui Chen
Yinghui Chen Weien Yuan
Weien Yuan