- 1Department of Biochemistry, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
- 2Biosciences Eastern and Central Africa, International Livestock Research Institute, Nairobi, Kenya
- 3Department of Chemistry, University of Nairobi, Nairobi, Kenya
- 4Department of Pharmacology and Pharmacognosy, School of Pharmacy, University of Nairobi, Nairobi, Kenya
- 5National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, Oxford, MS, United States
Infections caused by Mycoplasma species belonging to the ‘mycoides cluster’ negatively affect the agricultural sector through losses in livestock productivity. These Mycoplasma strains are resistant to many conventional antibiotics due to the total lack of cell wall. Therefore, there is an urgent need to develop new antimicrobial agents from alternative sources such as medicinal plants to curb the resistance threat. Recent studies on extracts from Solanum aculeastrum and Piliostigma thonningii revealed interesting antimycoplasmal activities hence the motivation to investigate the antimycoplasmal activities of constituent compounds. The CH2Cl2/MeOH extracts from the berries of S. aculeastrum yielded a new β-sitosterol derivative (1) along with six known ones including; lupeol (2), two long-chain fatty alcohols namely undecyl alcohol (3) and lauryl alcohol (4); two long-chain fatty acids namely; myristic acid (5) and nervonic acid (6) as well as a glycosidic steroidal alkaloid; (25R)-3β-O-α-L-rhamnopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranosyloxy-22α-N-spirosol-5-ene (7) from the MeOH extracts. A new furan diglycoside, (2,5-D-diglucopyranosyloxy-furan) (8) was also characterized from the CH2Cl2/MeOH extract of stem bark of P. thonningii. The structures of the compounds were determined on the basis of spectroscopic evidence and comparison with literature data. Compounds 1, 3, 4, 7, and 8 isolated in sufficient yields were tested against the growth of two Mycoplasma mycoides subsp. mycoides (Mmm), two M. mycoides. capri (Mmc), and one M. capricolum capricolum (Mcc) using broth dilution methods, while the minimum inhibitory concentration (MIC) was determined by serial dilution. The inhibition of Mycoplasma in vitro growth was determined by the use of both flow cytometry (FCM) and color change units (CCU) methods. Compounds 4 and 7 showed moderate activity against the growth of Mmm and Mmc but were inactive against the growth of Mcc. The lowest MIC value was 50 μg/ml for compound 7 against Mmm. The rest of the compounds showed minimal or no activity against the strains of Mycoplasma mycoides tested. This is the first report on the use of combined FCM and CCU to determine inhibition of in vitro growth of Mycoplasma mycoides. The activity of these compounds against other bacterial strains should be tested and their safety profiles determined.
Introduction
Mycoplasmas are distinguished phenotypically from other bacteria by their small size and total lack of a cell wall (Weisburg et al., 1989). Infections caused by these Mycoplasma constitute a threat to both humans and animals (Arjoon et al., 2012). Many species belonging to these classes are pathogenic and of great economic concern in livestock production (Tambi et al., 2006). In particular, the so-called “Mycoplasma mycoides cluster” consists of ruminant pathogens (Fischer et al., 2012) and they negatively affect the agricultural sector through losses in livestock productivity, mortality and international trade restrictions (Tambi et al., 2006; Jores et al., 2013). The members of this cluster include: M. mycoides subsp. mycoides (Mmm) the causative agent of contagious bovine pleuropneumonia (CBPP), M. capricolum subsp. capripneumoniae (Mccp) the agent of contagious caprine pleuropneumonia (CCPP), M. capricolum subsp. capricolum (Mcc), M. leachii, and M. mycoides subsp. capri (Mmc). CBPP and CCPP are major livestock diseases, also called transboundary animal diseases (TADs) which negatively impact the agricultural sector specifically in developing countries through livestock product trade, food-supply reduction and decrease productivity (FAO, 2016). Furthermore, these diseases remain a threat to disease-free countries (Tambi et al., 2006); therefore, better control measures are needed (Jores et al., 2013).
The Mycoplasma are resistant to many antimicrobials as they lack a cell wall. The current antimicrobials widely used to treat infections with Mycoplasma are limited to tetracyclines, macrolide-lincosamide-streptogramin-ketolide antibiotic group and fluoroquinolones (Arjoon et al., 2012; Kama-Kama et al., 2016). Therefore, there is need to develop new antimicrobials from plant origin because of their availability and affordability and because they might provide new pathways for interfering with mycoplasma survival.
Solanum aculeastrum belongs to the genus of Solanum in the family of Solanaceae. It is used as a medicinal plant in many African countries (Beaman-Mbaya and Muhammed, 1976; Odhiambo et al., 2011). In Kenya, S. aculeastrum is used traditionally to treat sexually transmitted diseases by the Luo and Kuria communities (Wanyonyi et al., 2002; Owuor et al., 2012). Piliostigma thonningii, on the other hand, belongs to the genus Piliostigma in the family of Fabaceae. Its roots and twigs are used as remedy for dysentery, fever, respiratory ailments and snakebites, leaves are edible, and chewed by Maasai to relieve thirst (Jimoh and Oladiji, 2005; Bako et al., 2005; Kama-Kama et al., 2016).
Following our latest work on the preliminary screening of 152 extracts from 20 selected medicinal plants from the Kenyan flora (Kama-Kama et al., 2016), the current work focuses on investigating the phytochemistry and the antimycoplasmal activities of compounds from the berries of S. aculeastrum and the stem bark of P. thonningii. The crude extracts from these two plants showed interesting activities against selected Mycoplasma mycoides strains in previous studies, hence the motivation to carry out this research (Kama-Kama et al., 2016).
Growth of Mycoplasma has been determined by color changing units (CCU), measuring a decrease in pH after sufficient growth by a change in color (red to yellow) of the pH indicator (Stemke and Robertson, 1982). In this paper, we have used both CCU and a flow cytometric (FCM) method (Assunção et al., 2005, 2006) to follow growth of Mycoplasma and its inhibition by plant compounds.
Experimental Section
General Experimental
Column chromatography was carried out using Merck silica gel 40 (70–230 mesh) as the stationary phase. Analytical thin layer chromatography (TLC) was carried out using Merck pre- coated 60 F254. Compounds were visualized under UV light at 254 or 365 nm, followed by placing the plates in iodine tanks to view spots that were inactive under the two UV wavelengths used. 1D and 2D Nuclear Magnetic Resonance (NMR) spectra were recorded in deuterated chloroform (CDCl3) on a 400 MHz Bruker AVANCE NMR instrument at room temperature. Chemical shifts were expressed in ppm and referenced against the solvent resonances at 7.26 and 77.23 ppm for 1H and 13C NMR, respectively. Electron ionization mass spectroscopy (EIMS) spectra were recorded on 70 eV, SSQ 710 MAT mass spectrometer.
Plant Material
The plant materials (about 1kg of S. aculeastrum berries and stem bark of P. thonningii, each) were collected from Migori and Kisumu counties (Kenya) in June and July 2014, respectively. The plant materials were immediately transported in open polythene bags from the collection site to the Department of Chemistry, University of Nairobi. The identification of these plants was done by Mr. Patrick Chyalo Mutiso, a plant taxonomist at the University of Nairobi herbarium, School of Biological Sciences (SBS), College of Biological and Physical Sciences (CBPS) where voucher specimens (Fmk2012/14 and Fmk2012/9) were deposited.
The berries of S. aculeastrum and the stem bark of P. thonningii were dried at room temperature under the shade away from direct sunlight for 3 weeks. The plant materials were then ground into fine powder using a MM 20 grinder (Wiley laboratory mill, standard model No. 2, Arthur H. Thomas Company).
Extraction with 50% MeOH in CH2Cl2 and Pure MeOH
Powdered ground berries of S. aculeastrum and stem bark of P. thonningii (1 Kg, each) were extracted by cold percolation using a mixture of 50% of MeOH in CH2Cl2 and the resultant extracts filtered under pressure. The solvent was removed in vacuo using a rotary evaporator resulting in 94 and 150 g of the crude extracts from S. aculeastrum and P. thonningii respectively. The insoluble residues from S. aculeastrum (see the earlier extractions) was soaked into MeOH and left overnight. The extracts obtained were filtered and concentrated as earlier described yielding 20.6 g of MeOH extract.
Isolation of the Compounds from the Berries of S. aculeastrum and the Stem Bark of P. thonningii
About 50 g of crude 50% MeOH–CH2Cl2 extracts from the berries of S. aculeastrum was adsorbed onto 50 g of silica gel, left to dry and loaded on a column packed as follows: 300 g of silica gel was packed into a column using 30% of dichloromethane (CH2Cl2) in n-hexane (n-C6H14) and left standing overnight. Elution was done with n-C6H14 containing an increasing percentage of CH2Cl2. One hundred and sixty fractions (500 ml each) were collected and combined on the basis of the similarities of their TLC profiles. This resulted in a total of 11 fractions that were labeled as FA to FK. Fraction D from the main column yielded 48 mg of the first compound, (17-(5-ethyl-3-hydroxy-6-methylheptan-2-yl)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-10,13,14-tri- methyl-3-oxo-1H-cyclopenta[a]phenanthren-6-yl benzoate) (1), while fraction B yielded 8.7 mg of triterpene lupeol (2) and 12 mg of compound 3. Fraction C yielded 32.5 mg of compound 4 while compound 5 and 6 were isolated from fractions D and F to yielded 7 mg and 11 mg respectively. The methanol extract (20 g) from the berries of this plant was chromatographed on a silica gel column (200 g) starting with 100% EtOAc and increasing the polarity with MeOH to yield 45 mg of the last compound (7), (25R)-3β-O-α-L-rhamnopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranosyloxy-22α-N-spirosol-5-ene (7).
As for the isolation of compounds from the CH2Cl2/MeOH extract from the stem bark of P. thonningii, 100 g of crude extracts from this plant was adsorbed onto 100 g of silica gel, left to dry and loaded on a column packed as follows: One kilogram of silica gel was used to pack a column under 80% CH2Cl2 in n-C6H14 and left standing overnight. The extract was loaded onto the pre-packed column and eluted initially with 2000 ml of 80% CH2Cl2 in n-C6H14 increasing the polarity with CH2Cl2 up to 100% CH2Cl2, followed by 1% MeOH in CH2Cl2 with increased polarity up to 20% MeOH in CH2Cl2. This column yielded 350 mg of compound 8.
Preparation of Compounds for Antimycoplasmal Activity Tests
The pure compounds (5 mg) were reconstituted into 1 ml of dimethyl sulfoxide (DMSO) to make a stock solution of 5 mg/ml. The mixture was prepared by vortexing to ensure homogenization of the solution and 20 μl from the stock solution (containing 100 μg of the compound) were used as a starting concentration for the antimycoplasmal activity tests.
Bacterial Strains and Culture Conditions
All laboratory manipulations with Mycoplasma were carried out under BSL2 conditions (Mwirigi et al., 2016). Two Mycoplasma mycoides subsp. capri (211/94 and 95010), two Mycoplasma mycoides subsp. Mycoides (Afadé and Gladysdale), and one Mycoplasma capricolum subsp capricolum (6443-90) (Tables 2, 3) were cultured in Pleuropneumonia Like-Organism (PPLO) broth (DifcoTM PPLO Broth) media prepared as follows: 21 g of PPLO was dissolved in 700 ml of distilled water and autoclaved for 15 min at 121°C. The mixture was cooled in a water bath to 55°C and supplemented with phenol red (Carl Roth GmbH) to a final concentration of 3%, 200 ml horse serum (Sigma), 0.25% of glucose (Carl Roth GmbH), 0.15% of penicillin G (Carl Roth GmbH) and 0.25% of thallium acetate (Carl Roth GmbH). A 48 well-plate was used to grow Mycoplasma strains where they were incubated at 37°C for a minimum period of seven days. Growth of Mycoplasma cells was determined by color change from red to yellow (Stemke and Robertson, 1982), as a result of pH change due to the growth. Stock cultures of Mmc, Mmm, and Mcc were grown to a density of approximately 1–3 106 cells per ml as measured by both the colony forming units (CFU) method (Stemke and Robertson, 1982) and the use of FCM (Assunção et al., 2006) after cryopreservation in a freezer at -80°C for further antimycoplasmal activity tests.
Analysis of Mycoplasma Using Flow Cytometer
Growth of Mycoplasma (Mmm, Mmc, and Mcc) cells in culture were followed using FCM (Assunção et al., 2006). The modern FACs Canto II (BD Biosciences) with a 488 nm solid-state laser was used. To minimize the extent of contamination with small particles that could show up as Mycoplasma on the flow cytometer, a solution of 0.9% saline was filtered and used as sheath fluid and for sample dilution. Only forward scatter (FSc) and side scatter (SSc) were recorded and the number of events was recorded after every 30 seconds when its rate was constant. The concentration of Mycoplasma (cells per microliter) was calculated as the number of events acquired within the FSc/SSc window correlating with Mycoplasma parameters (see Figure 1) in one minute, and divided by the flow rate (12 μl/min). BD Diva software was used to analyze the data.
Antimicrobial Susceptibility Testing
The broth microdilution method as described by Arjoon et al. (2012) was used to characterize the in vitro antimycoplasmal activity of compounds 1, 3, 4, 7, and 8 against the growth of the Mycoplasma strains used in this study. Briefly, a stock solution of 5 mg was reconstituted into 1 ml of DMSO (5 mg/ml). From the stock solution, 20 μl was used and made up to 1 ml with cell culture (giving a concentration of 100 μg/ml) for the antimycoplasmal test. Two control samples were tested in parallel, consisting of the following: media plus Mycoplasma (negative control) and media plus Mycoplasma plus tetracycline (positive control). The kinetics of the bioassays were determined by monitoring the concentrations of the Mycoplasma cells used for the inhibition assays from the first day – to the seventh day (day 0– day 6). The cultures were also monitored for color change (from red–yellow) due to the change of pH, an indication of the growth of Mycoplasma cells. No color change meant that the compounds in the well prevented growth of Mycoplasma. Data were analyzed using Graphprism software version 6.0.
Determination of the Minimum Inhibitory Concentration (MIC)
Active compounds were serially diluted in order to determine the minimum inhibitory concentration (MIC), which was defined as the lowest concentration that inhibited the growth of different Mycoplasma strains for an incubation time of seven days. The MICs of all the active compounds were determined by broth microdilution as previously described by Arjoon et al. (2012) starting from 100 to 5 μg/ml. The experiment was repeated thrice for each assay and mean was recorded. The standard error of mean was obtained at P < 0.05.
Results
Chromatographic purification of compounds from the 50% MeOH in CH2Cl2 extract of the berries of S. aculeastrum led to the isolation of the following compounds; a β-sitosterol derivative;(17-(5-ethyl-3-hydroxy-6-methylheptan-2-yl)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-10,13,14-trimethyl-3-oxo-1H-cyclopenta[a]phenanthren-6-yl benzoate) (1) and the commonly known triterpene, lupeol (2), two long-chain fatty alcohols namely lauryl alcohol (3) and undecyl alcohol (4); two long-chain fatty acids namely myristic acid (5) and nervonic acid (6) while the MeOH extract of the same plant material led to the isolation of a known triglycosidic steroidal alkaloid; (25R)-3β-O-α-L-rhamnopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranosyloxy-22α-N-spirosol-5-ene (7). Using the same method, the CH2Cl2/MeOH extracts from the stem bark of Piliostigma thonningii led to the isolation of a new diglycoside furan (8). The structures of these compounds (1,3–8) were determined on the basis of spectroscopic techniques while the triterpene, lupeol (2) was identified by comparison with an authentic sample. Both NMR and MS data of compounds 1 and 8 are presented in the Supplementary Material. The antimycoplasmal activity test of the isolated compounds showed that compounds 4 and 7 exhibited moderate activities as compared to the control (tetracycline). The lowest MIC value was 50 μg/ml for compound 7 against Mmm.
Compounds from the Berries of S. aculestrum
Compound 1
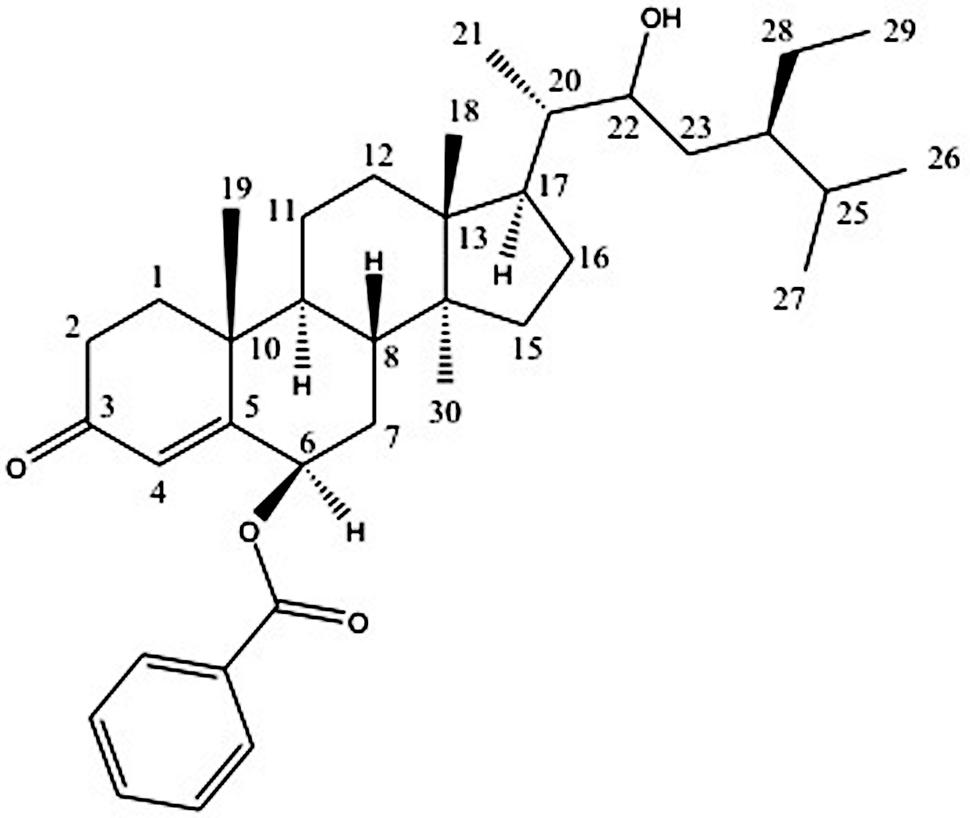
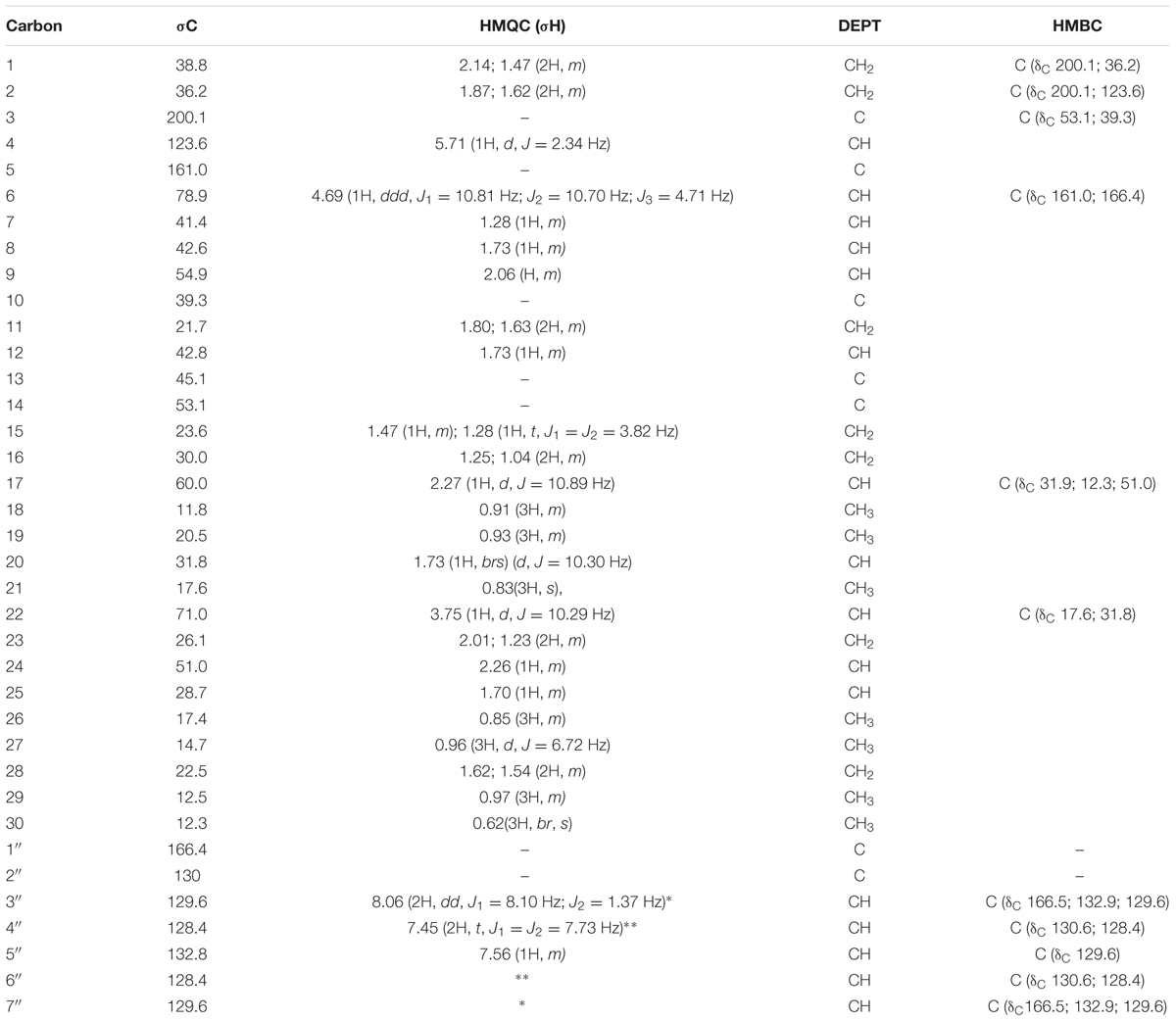
Compound 1 was isolated as white crystals, with melting point (mp) above 210°C. The HRMS (Supplementary 1) of this compound showed an ion peak at 563.4011 (calculated mass was of 562.40) corresponding to a molecular formula of C37H54O4. Most of the peaks in the 1H and 13C NMR spectra of this compound match those of β-sitosterol (Quillez et al., 2003) with an additional benzoate substituent at position C-6, as shown in Figure 1.
The 1H NMR (Supplementary 1) indicated seven characteristic methyls signals at δH 0.62 (3H, br, s), δH 0.83 (3H, m), δH 0.91 (3H, m), δH 0.93 (3H, m), δH 0.96 (3H, d, J = 6.72), δH1.10 (3H, d, J = 5.91 Hz), and δH1.31 (3H, m). Six doublets were observed at δH 0.96 (3H, d, J = 6.72 Hz), δH 1.10 (3H, d, J = 5.91 Hz), δH 1.20 (1H, d, J = 4.85 Hz), δH 2.27 (1H, d, J = 10.89 Hz), δH 3.73(1H, d, J = 0.69 Hz), and δH 5.70 (1H, d, J = 2.34 Hz). The doublet at δH 5.70 (1H, d, J = 2.34 Hz) at carbon C-4 is a characteristic of olefinic protons. The Characteristic aromatic protons were observed at δH 8.06 (2H, dd, J1 = 8.10 Hz, and J2 = 1.37 Hz), δH 7.56 (1H, m), and δH 7.45 (2H, t, J1 = J2 = 7.73 Hz). The protons H-6 and H-22 were downfield resonating at δH 4.69 and at δH 3.75 because of the oxygenation.
The 13C NMR (Supplementary 1) spectrum of the compound 1 showed 37 carbon signals, 30 of which are from the sitosterol base skeleton, and 7 from the benzoate group. The carbon C-3 at δC 200.1 is characteristic of a keto carbon while carbon C-1′ at δC 166.4 corresponds to an ester carbonyl carbon. The HMBC (Supplementary 1) experiment showed cross peaks between H-6 resonating at δH 4.69 and C-5 (δC 161.0) and C-1′ (δC 166.4) confirming the placement of the benzoate moiety at C-6. Furthermore, the correlation observed between H-22 at δH 3.75 with C-21 resonating at dC 17.6 also confirms the placement of the hydroxyl group at C-22. The stereochemistry of the base skeleton, the β-sitosterol is biogenetically known where the methyls CH3-19 and CH3-18 are on the same side while H-9 is on the opposite side. The 1H NMR data showed that H-6 was coupling with H-8 (each 1H, J = 3.41 Hz) suggesting that they are on the opposite direction of the β-sitosterol skeleton. Similarly, H-8 was also coupling with H-9 (each 1H, J = 4.71 Hz) suggesting that H-8 and H-9 are on the opposite side (Garbisch and Griffith, 1968). This was confirmed by the NOESY (Supplementary 1) experiment, which showed a correlation between H-9 and H-6 suggesting they are on the same side. The NOESY experiment further showed that there was a correlation between H-21 and H-22 (1H each, J = 10.29 Hz) suggesting they are on the same side. However, the NOESY experiment did not show any correlation between the methyls CH3-18 and CH3-30 suggesting that they are in trans configuration.
Based on these data coupled with comparison of these spectra with those in the literature, compound 1 was identified as (17-(5-ethyl-3-hydroxy-6-methylheptan-2-yl)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-10,13,14-trimethyl-3-oxo-1H-cyclopenta[a]phenanthren-6β-yl benzoate). This is the first report of a derivative of β-sitosterol with a benzoate substituent at C-6 position. Therefore, compound 1 was identified as a novel β-sitosterol derivative namely β-sitosterol-6β-benzoate. The 1H (400 MHz) and 13C NMR data for compound 1 are presented in Table 1 while its structure is shown in Figure 1.
Compound 7
Compound 7 was isolated as light brown crystals, with a mp of 200–205°C. The 1H-NMR and 13C-NMR spectra of this compound was comparable with that of a steroidal alkaloid glycoside, solasodine triglycoside (Radeglia et al., 1977; Ripperger and Schreiber, 1981; Murakami et al., 1985; Ripperger and Himmelreich, 1994; Ripperger, 1995; Ripperger and Porzel, 1997; Vázquez et al., 1997). The HRMS showed an intense ion peak at m/z 870 (calculated 869.07) corresponding to a molecular formula C45H74015N.
Additional peaks were evident at m/z 724 and 578 attributed to the loss the first and the second molecules of deoxyhexose units respectively. This fragmentation pattern was characteristic of a triglycoside steroidal alkaloids previously characterized from a number of species belonging to the genus Solanum including, the seeds of S. robustum (Ripperger and Schreiber, 1981) and the berries of S. aculeastrum (Wanyonyi et al., 2003).
Besides these two compounds, two long-chain fatty alcohols namely; lauryl alcohol (3) and undecyl alcohol (4); two long-chain fatty acids namely; myristic acid (5) and nervonic acid (6) were also isolated. This is the first report of these compounds from S. aculeastrum. The identification of these four compounds was done by comparing the generated data (1H NMR and 13C NMR) with those in the literature (Davidson and Contrill, 1985; Boven et al., 1997; Bond, 2003).
Compounds from the Stem Bark of P. thonningii
Compound 8
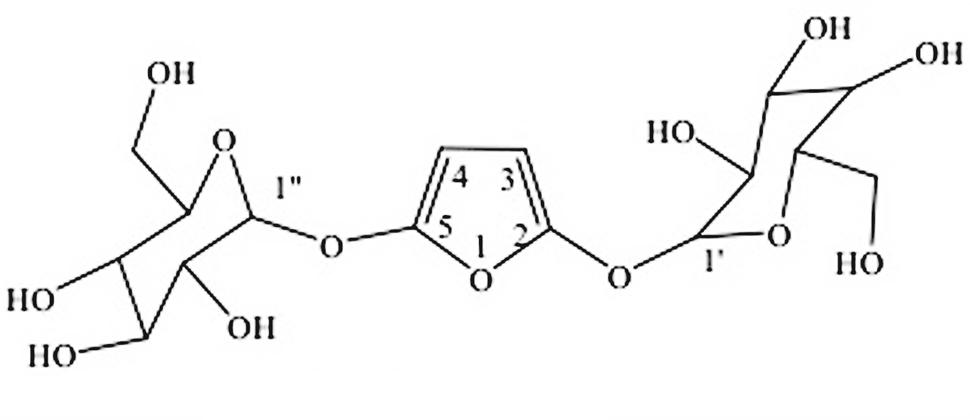
Compound 8 was isolated as amorphous crystals. HRMS (Supplementary 2) showed an ion peak at m/z 423 (calculated mass was 424) corresponding to a molecular formula C16H24O13. The compound was identified as a furan diglycoside. The 1H NMR (Supplementary 2) spectra showed four doublets, two of which were attributed to the olefinic protons, and two to anomeric protons. The two doublets resulting from the olefinic protons appeared at δ 6.02 (1H, dd, J1 = 9.58 Hz, J2 = 3.25 Hz) and δ 6.22 (1H, dd, J1 = 10.11 Hz, J2 = 1.71 Hz) assigned to C-3 and C-4, of the furan ring, respectively. However, the doublets resulting from the two-downfield shifted anomeric protons appeared at δ 5.17 (1H, dd, J1 = 7.21 Hz, J2 = 3.16 Hz) and at δ 5.68 (1H, br, s) and were placed at C1′ and C1″, respectively. The 13C NMR (Supplementary 2) showed 16 carbons assigned to a substituted furan ring, with two glucose molecules at C-2 and C-5. The HMBC (Supplementary 2) experiments showed a correlation between H-3 and C-2; and between H-1′ and C-3. Similarly, the HMBC showed a correlation between H-1″ with C-5 confirming the placement of the two glycosides on C-2 and C-5 positions of the furan ring. Based on the above spectroscopic data and comparison with literature values, it was evident that this is the first time this compound has been characterized from nature. The 1H (400 MHz) and 13C NMR data for compound 8 are presented in Table 2 while its structure is shown in Figure 2.
Determination of the Antimycoplasmal Activity Tests of Pure Compounds Using Flow Cytometer
Compounds 1, 3, 4, 7, and 8 were tested against the growth of five strains of Mycoplasma (two stains of Mmm, two strains of Mmc and one strain of Mcc) together with two controls which included: media plus Mycoplasma (negative control), and media plus Mycoplasma plus tetracycline (positive control). The FCM profile analysis of Mycoplasma is shown in Figure 3.
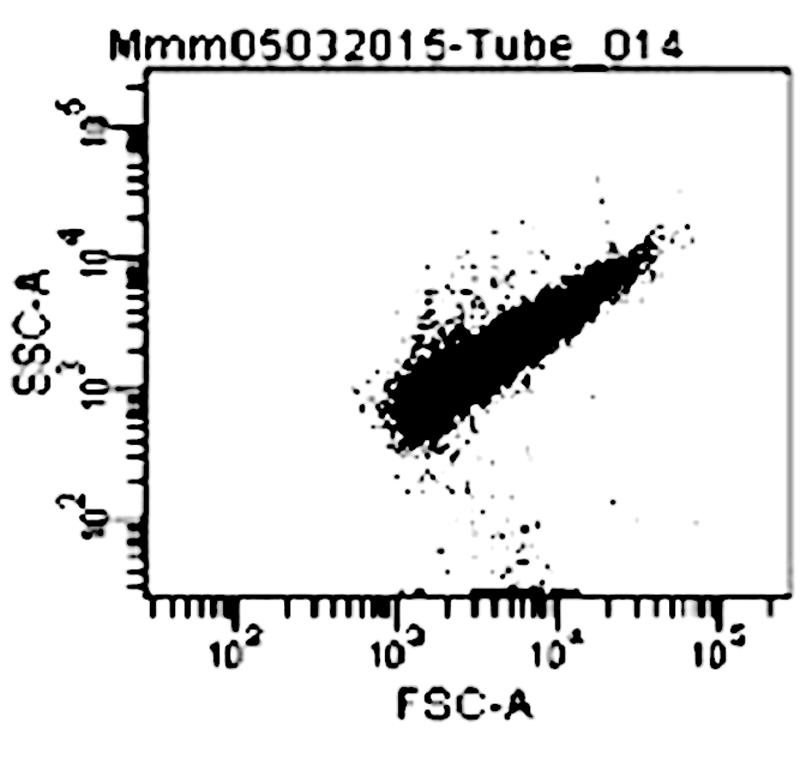
FIGURE 3. Dot plot (forward versus 90° scatter signal) of M. mycoides mycoides on Flow cytometer Keys: FSc (Forward scatter); SSc (Side scatter); Mmm (Mycoplasma mycoides subsp. mycoides).
The results of the antimycoplasmal activity tests of the pure compounds showed that only compounds 4 and 7 inhibited the growth of Mmm (Afadé and Gladysdale) and Mmc (211/94 and 95010) for 3 days only. The inhibition was observed as a delay of the growth and a partial reduction of maximum concentration. The growth of the selected Mycoplasma strains was clearly observed when the color of the media (PPLO) changed from red to yellow due to the change of pH as well as the increase in the concentration of the Mycoplasma strains used. However, it is important to note that even in the case of the inhibition control with tetracycline, where no color change was observed, the concentration of the Mycoplasma as measured by flow cytometry did also increase from day 3, although the concentration remained lower as compared to that of the Mycoplasma treated with the plant compounds.
The results of the antimycoplasmal activity using CCU only are presented in Table 3. We have in this case presented only two strains (one Mmm and one Mmc)
The ability of the pure compounds to inhibit the growth of Mycoplasma was monitored for 6 days and the results of the kinetics of antimycoplasmal activity tests determined by the use of the FCM are presented below in Figures 4, 5. The summary of the antimycoplasmal activity tests of the isolated compounds is presented in Table 4.
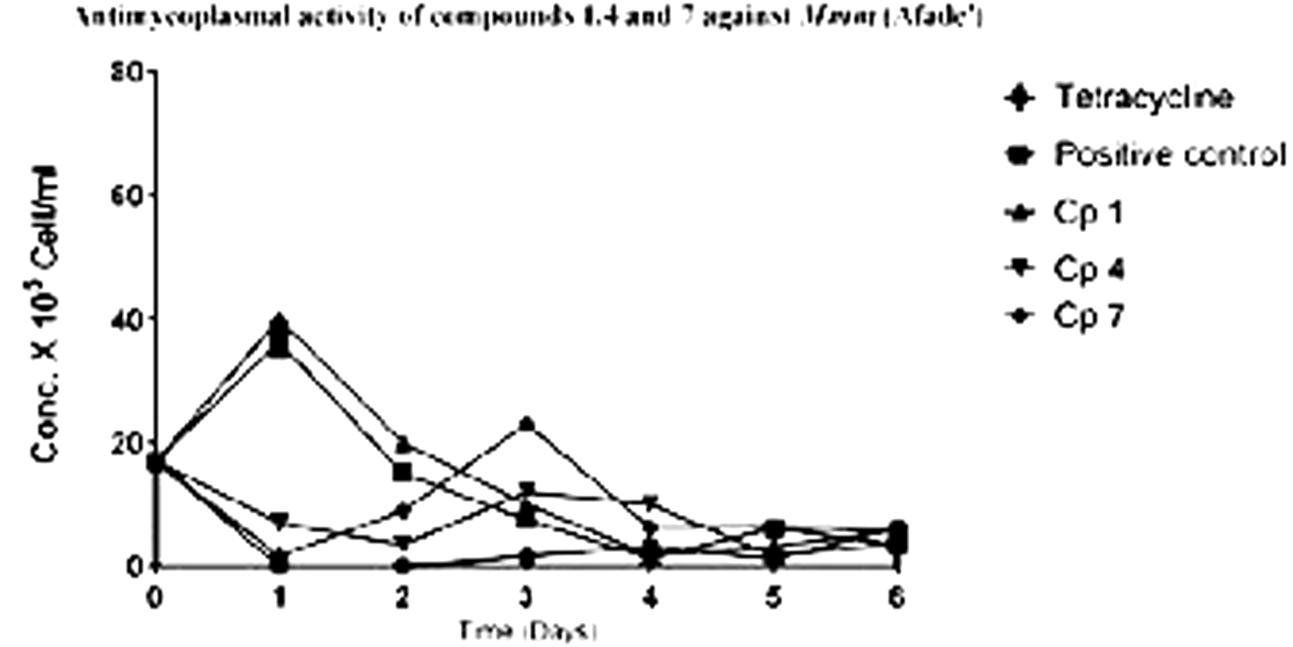
FIGURE 4. Antimycoplasmal activity tests of compounds 1, 4, and 7 against the growth of Mmm (Afade) Keys; Cp 1 (compound l); Cp4 (compound 4); Cp 7 (compound 7).
FIGURE 5. Anlimycoptasmal activity tests of compounds 1, 4, and 7 against the growth of Mmc (211/94) Keys: Cp I (compound 1); Cp 4 (compound 4); Cp 7 (compound 7).
Determination of the MICs of the Pure Compounds
Minimum inhibitory concentrations were regarded as the lowest concentration of the pure compound able to inhibit the growth of Mycoplasma for a period of 7 days. However, in this case, there was growth on the third day meaning that there was only a delay in growth of Mycoplasma but not complete inhibition of the Mycoplasma. The results of the MICs are presented in Table 5.
Discussion
The results from this study showed that compounds 4 and 7 exhibited moderate antimycoplasmal activities as compared to the control, tetracycline. The lowest minimum inhibitory concentration of active compound, 7, was 50 μg/ml against Mmm.
Although the crude extracts from these two plants showed very good activities against the growth of Mycoplasma strains (Kama-Kama et al., 2016), these activities could not be observed with pure compounds. This could be most probably attributed to synergistic effects of all or some of the compounds occurring in these plants. The activities observed in the crude extracts and not in the isolated compounds could also be due to minor compounds which were not isolated in the current study.
The antimycoplasmal activity of compound 4 observed in this study could be attributed to the fact that it is a long chain fatty alcohol which in previous studies is known to have antibacterial activities (Kubo et al., 1995; Mukherjee et al., 2013). According to Kubo et al. (1995) the activity of long chain fatty alcohols against selected Gram-positive bacteria suddenly dropped off from C13 but the MICs obtained from their investigation showed that long fatty alcohol with a C12 exhibited a better MIC value than C11. However, the results from our study showed that lauryl alcohol, a C12 long chain fatty alcohol exhibited moderate activity while undecyl, a C11 long fatty alcohol was inactive against Mycoplasma mycoides strains tested. The MIC value of compound 4 was also higher (100 μg/ml) as compared to the control, tetracycline (5 μg/ml). This was also observed in previous studies on other plant compounds (Kubo et al., 1995) where the MIC value ranged from 12.5 to 25 μg /ml. This could be because Mycoplasmas are different from other bacteria strains as they have a much smaller genome (Westberg et al., 2004).
Compound 7 is a steroidal alkaloid glycoside and previous reports on its biological activity showed that this compound exhibited antimicrobial as well as anticancer activity (Wanyonyi et al., 2002; Shabana et al., 2013). The minimal activity exhibited by 7 could be explained by the fact that it is a steroidal alkaloid glycoside and contains in its structure so many OH groups that could have been hydrolyzed before reaching the target and consequently losing its activity. Nevertheless, it is clear that the pure compounds exhibited weak activity as compared to the crude extracts (Kama-Kama et al., 2016).
The use of both FCM and CCU methods for the antimycoplasmal activity tests has proven to be more efficient that than the CCU alone. The color change method only tells us when the mycoplasma have grown enough to make the medium acidic, while the FCM method allows us to see growth before that time or to see weak growth that is not able to change the pH. Using FCM, the antimycoplasmal activity test of compounds 4 and 7 showed that the two compounds delayed the growth of the Mycoplasma and to lower maxima than the control samples without compound or samples with compounds 1, 3, or 8 that showed no activity. While tetracycline was a much better inhibitor than the plant compounds, we did find using FCM that there still was weak growth with the antibiotic after a number of days. But this could not be observed with the CCU method. The latter method did confirm that the two compounds, 4 and 7, had activity against Mmm and Mmc as they delayed the change in pH-induced color change.
The weak increase in Mycoplasma concentration 3 days after tetracycline treatment could have several causes; one is that tetracycline at this concentration may not kill all Mycoplasma. In vivo experiments have shown that the antibiotic does reduce mycoplasmemia and fever in Mmm-infected cattle, but a resurgence of fever occurred after several days (Schieck et al., 2014). Alternatively, these Mycoplasmas might have developed resistance to tetracycline. Previous works also confirmed that Mycoplasma have developed resistance to tetracycline (Dégrange et al., 2008).
Combining FCM and CCU methods in the antimycoplasmal activity test gave more detailed information and could be used for slow growing bacteria such as Mycoplasma in order to quickly establish the resistance of these organisms to the antimicrobial agents.
Conclusion and Recommendation
From the berries of S. aculeastrum, a new β-sitosterol benzoate namely; (17-(5-ethyl-3-hydroxy-6-methylheptan-2-yl)-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-10,13,14-trimethyl-3-oxo-1H-cyclopenta[a]phenanthren-6β-yl benzoate) (1) and six known compounds; lupeol (2) two long-chain fatty alcohols namely; lauryl alcohol (3) and undecyl alcohol (4); two long-chain fatty acids namely; myristic acid (5) and nervonic acid (6) and a steroidal alkaloid triglycoside; (25R)-3β-O-α-L-rhamnopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranosyloxy-22αN-spirosol-5-ene ion (7), were isolated. Furthermore, the stem bark of Piliostigma thonnignii yielded a new diglycoside furan (8). The antimycoplasmal activity tests of these compounds against the growth of selected Mycoplasma mycoides strains showed that compounds 4 and 7 had moderate activities with the lowest mean MIC value of 50 μg/ml for 7 against Mmm. The activities of these compounds were lower as compared to those of the crude extracts. Structural modification of these compounds could increase their antimycoplasmal activities. Alternatively, isolation and purification of compounds from these plants could yield additional compounds with interesting activity. These compounds should also be tested against other strains of bacteria, as well as their safety profiles ascertained.
Flow cytometry measurement of Mycoplasma concentrations allows one to obtain a more precise growth kinetics and measure growth before a color change occurs. However, the technique needs the right equipment and is more labor intensive.
Author Contributions
FK-K, JM, LO, and JN conceived and designed the experiments. FK-K performed the experiments. FK-K, LO, MI, and SY analyzed the data. FK-K, LO, SY, JNG, NM, JM, GO, and JN wrote the paper.
Funding
FK-K has been supported by DAAD (Grant No: A/12/94884). The laboratory aspects of this project was partly supported by the BecA-ILRI Hub through the Africa Biosciences Challenge Fund (ABCF) program. The ABCF Program is funded by the Australian Department for Foreign Affairs and Trade (DFAT) (Grant No: BS01 NBO SID004 SID004D1 ABC105) through the BecA-CSIRO partnership; the Syngenta Foundation for Sustainable Agriculture (SFSA); the Bill and Melinda Gates Foundation (BMGF); the United Kingdom Department for International Development (DFID) and; the Swedish International Development Cooperation Agency (Sida, Grant No: 51000131). We gratefully acknowledge support from German Federal Ministry for Economic Cooperation and Development (project no: 09.7860.1-001.00, contract no: 81121408) and the International Science Program, Uppsala University, Sweden (ISP)-KEN-02 Project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mr. Boni Gisacho for helping in the extraction of crude from these two plants. We thank our herbarium technician Mr. Patrick C. Mutiso for his help in identification of plants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2017.00920/full#supplementary-material
References
Arjoon, A. V., Saylor, C. V., and May, M. (2012). In vitro efficacy of antimicrobial extracts against the atypical ruminant pathogen Mycoplasma mycoides subsp capri. BMC Complement. Altern. Med. 12:169. doi: 10.1186/1472-6882-12-169
Assunção, P., Antunes, N. T., Rosales, R. S., Poveda, C., Poveda, J. B., and Davey, H. M. (2005). Flow cytometric determination of the effects of antibacterial agents on Mycoplasma agalactiae, Mycoplasma putrefaciens, Mycoplasma capricolum subsp capricolum, and Mycoplasma mycoides subsp mycoides large colony type. Antimicrob. Agents Chemother. 50, 2845–2849. doi: 10.1128/AAC.01582-05
Assunção, P., de la Fe, C., Antunes, N. T., Rosales, R. S., De Galarreta, C. M. R., and Poveda, J. B. (2006). Use of flow cytometry for enumeration of Mycoplasma mycoides subsp mycoides large-colony type in broth medium. J. Appl. Microbiol. 100, 878–884. doi: 10.1111/j.1365-2672.2005.02858.x
Bako, S. P., Bakur, M. J., John, I., and Bala, E. I. (2005). Ethnomedicinal and phytochemical profile of some savanna species in Nigeria. Int. J. Bot. 1, 147–150. doi: 10.3923/ijb.2005.147.150
Beaman-Mbaya, V., and Muhammed, S. I. (1976). Antibiotic action of Solanum incanum Linnaeus. Antimicrob. Agents Chemother. 9, 920–924. doi: 10.1128/AAC.9.6.920
Bond, A. D. (2003). On the crystal structures and melting point alternation of the n-alkyl carboxylic acids. New J. Chem. 28, 104–114. doi: 10.1039/B307208H
Boven, M. V., Da, P., Ma, K., and Cokela, M. (1997). Content and composition of free sterols and free fatty alcohols from Jojoba oil. J. Agric. Food Chem. 45, 1180–1184. doi: 10.1021/jf960488g
Dégrange, S., Renaudin, H., Charron, A., and Be, C. (2008). Tetracycline resistance in Ureaplasma spp. and Mycoplasma hominis: prevalence in Bordeaux, France, from 1999 to 2002 and description of two tet(M)-positive isolates of M. hominis susceptible to tetracyclines. Antimicrob. Agents Chemother. 52, 742–744. doi: 10.1128/AAC.00960-07
FAO (2016). Economic Analysis of Animal Diseases, Vol. 18. Rome: FFAO Animal Production and Health Guidelines.
Fischer, A., Shapiro, B., Muriuki, C., Heller, M., Schneee, C., Bongcam-Rudoff, E., et al. (2012). The origin of the ‘Mycoplasma mycoides cluster’ coincides with domestication of ruminants. PLOS ONE 7:e36150. doi: 10.1371/journal.pone.0036150
Garbisch, E. W., and Griffith, M. (1968). Proton couplings in cyclohexane. J. Am. Chem. Soc. 480, 6543–6544. doi: 10.1021/ja01025a069
Jimoh, F. O., and Oladiji, A. T. (2005). Preliminary studies on Piliostigma thonningii seeds: proximate analysis, mineral composition and phytochemical screening. Afr. J. Biotechnol. 4, 1439–1442. doi: 10.4314/ajb.v4i12.71459
Jores, J., Mariner, J. C., and Naessens, J. (2013). Development of an improved vaccine for contagious bovine pleuropneumonia: an African perspective on challenges and proposed actions. Vet. Res. 44, 1–5. doi: 10.1186/1297-9716-44-122
Kama-Kama, F., Midiwo, J., Nganga, J., Maina, N., Schiek, E., Kerubo, L., et al. (2016). Selected ethno-medicinal plants from Kenya with in vitro activity against major African livestock pathogens belonging to the Mycoplasma mycoides cluster. J. Ethnopharmacol. 192, 524–534. doi: 10.1016/j.jep.2016.09.034
Kubo, I., Muroi, H., and Kubo, A. (1995). Structural functions of antimicrobial long-chain alcohols and phenols. Bioorg. Med. Chem. 3, 873–880. doi: 10.1016/0968-0896(95)00081-Q
Mukherjee, K., Tribedi, P., Mukhopadhyay, B., and Sil, A. K. (2013). Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol. Lett. 338, 177–183. doi: 10.1111/1574-6968.12043
Murakami, K., Ezima, H., Takaishi, Y., Takeda, Y., Fujita, T., Sato, A., et al. (1985). Studies on constituents of Solanum plants: the constituents of S. lyratum THUNB.II. Chem. Pharm. Bull. 33, 67–73. doi: 10.1248/cpb.33.67
Mwirigi, M., Nkando, I., Aye, R., Soi, R., Ochanda, H., Berberov, E., et al. (2016). Veterinary immunology and immunopathology experimental evaluation of inactivated and live attenuated vaccines against Mycoplasma mycoides subsp. mycoides. Vet. Immunol. Immunopathol. 169, 63–67. doi: 10.1016/j.vetimm.2015.12.006
Odhiambo, J. A., Lukhoba, C. W., and Dossaji, S. F. (2011). Evaluation of herbs as potential drugs/medicines. Afr. J. Tradit. Complement. Altern. Med. 8(Suppl. 5), 144–151. doi: 10.4314/ajtcam.v8i5S.20
Owuor, B. O., Ochanda, J. O., Kokwaro, J. O., Cheruiyot, A. C., Yeda, R. A, Okudo, C. A., et al. (2012). In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J. Ethnopharmacol. 144, 779–781. doi: 10.1016/j.jep.2012.09.045
Quillez, J., Garcila-Lorda, P., and Salas-Salvadó, J. (2003). Potential uses and benefits of phytosterols in diet: present situation and future directions. Clin. Nutr. 22, 343–351. doi: 10.1016/S0261-5614(03)00060-8
Radeglia, R., Ripperger, H., and Adams, G. (1977). 13C NMR spectroscopy of steroidal alkaloid. Tetrahedron Lett. 11, 903–906. doi: 10.1016/S0040-4039(01)92787-X
Ripperger, H. (1995). Steroid alkaloid glycosides from Solanum robustum. Phytochemistry 29, 1475–1477. doi: 10.1016/0031-9422(95)00150-6
Ripperger, H., and Himmelreich, U. (1994). Anguivine and isoanguivine steroid alkaloid glycosides from Solanum anguivi. Phytochemistry 17, 1725–1727. doi: 10.1016/S0031-9422(00)89600-4
Ripperger, H., and Porzel, A. (1997). Steroidal alkaloid glycosides from Solanum suaveolens. Phytochemistry 46, 1279–1282. doi: 10.1016/S0031-9422(97)80027-1
Schieck, E., Liljander, A., Hamsten, C., Gicheru, N., Scacchia, M., Sacchini, F., et al. (2014). High antibody titres against predicted Mycoplasma surface proteins do not prevent sequestration in infected lung tissue in the course of experimental contagious bovine pleuropneumonia. Vet. Microbiol. 172, 285–293. doi: 10.1016/j.vetmic.2014.04.018
Shabana, M. M., Salama, M. M., Ezzat, S. M., and Ismail, L. R. (2013). In vitro and in vivo anticancer activity of the fruit peels of Solanum. J. Carcinog. Mutagen. 4, 1–6. doi: 10.4172/2157-2518.1000149
Stemke, G. W., and Robertson, J. A. (1982). Comparison of two methods for enumeration of mycoplasmas. J. Clin. Microbiol. 16, 959–961.
Tambi, N. E., Maina, W. O., and Ndi, C. (2006). An estimation of the economic impact of contagious bovine pleuropneumonia in Africa. Rev. Sci. Tech. 25, 999–1011. doi: 10.20506/rst.25.3.1710
Vázquez, A., Gonzalaz, G., Ferreira, F., Moyna, P., and Kenne, L. (1997). Glycoalkaloids of Solanum commersonii Dun. ex Poir. Euphytica 95, 195–201. doi: 10.1023/A:1002997616784
Wanyonyi, A. W., Chhabra, S. C., Mkoji, G., Eilert, U., and Njue, W. M. (2002). Bioactive steroidal alkaloid glycosides from Solanum aculeastrum. Phytother. Res. 59, 79–84. doi: 10.1016/S0031-9422(01)00424-1
Wanyonyi, A. W., Tarus, P. K., and Chhabra, S. C. (2003). A novel glycosidic steroidal alkaloid from Solanum aculeastrum. Bull. Chem. Soc. Ethiop. 17, 61–66. doi: 10.4314/bcse.v17i1.61733
Weisburg, W. G., Tully, J. G., Rose, D. L., Petzel, J. P., Oyaizu, H., Yang, D., et al. (1989). A phylogenetic analysis of the mycoplasmas: basis for their classification. J. Bactriol. 171, 6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989
Westberg, J., Persson, A., Holmberg, A., Goesmann, A., Lundeberg J,Uhlén, M., et al. (2004). The genome sequence of Mycoplasma mycoides subsp. mycoides SC type strain PG1 T, the causative agent of contagious bovine the genome sequence of Mycoplasma mycoides subsp. mycoides SC type strain PG1 T, the causative agent of contagious bovine pleurop. Genome Res. 14, 221–227. doi: 10.1101/gr.1673304
Keywords: antimycoplasmal activities, Solanum aculeastrum, Piliostigma thonningii, Mycoplasma mycoides, glycosidic steroidal alkaloid
Citation: Kama-Kama F, Omosa LK, Nganga J, Maina N, Osanjo G, Yaouba S, Ilias M, Midiwo J and Naessens J (2017) Antimycoplasmal Activities of Compounds from Solanum aculeastrum and Piliostigma thonningii against Strains from the Mycoplasma mycoides Cluster. Front. Pharmacol. 8:920. doi: 10.3389/fphar.2017.00920
Received: 21 July 2017; Accepted: 04 December 2017;
Published: 21 December 2017.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Kannan R. R. Rengasamy, Alagappa University, IndiaJude Chinedu Chukwujekwu, University of KwaZulu-Natal, South Africa
Copyright © 2017 Kama-Kama, Omosa, Nganga, Maina, Osanjo, Yaouba, Ilias, Midiwo and Naessens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisca Kama-Kama, franciscayeye@yahoo.fr Jan Naessens, jnaessens@ymail.com
 Francisca Kama-Kama
Francisca Kama-Kama Leonidah K. Omosa3
Leonidah K. Omosa3 Souaibou Yaouba
Souaibou Yaouba Jan Naessens
Jan Naessens