- 1Department of Oncology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Gastroenterology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
- 4Department of Pathology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 5Department of Dermatology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 6Department of Dermatology, Affiliated Wuxi People’s Hospital, Nanjing Medical University, Wuxi, China
Background: Topical calcineurin inhibitors including tacrolimus and pimecrolimus are used in the treatment of many inflammatory skin diseases mainly via blocking T-cell proliferation. Our previous studies found that pimecrolimus 1% cream could reverse high-dose ultraviolet B (UVB) irradiation-induced epidermal Langerhans cell (LC) reduction via inhibition of LC migration. We conducted this study to investigate the effects of topical tacrolimus 0.03% ointment on high-dose UVB-irradiated human epidermal LCs.
Methods: Twenty fresh human foreskin tissues were randomly divided into four groups as follows: Control, Tacrolimus (0.03%), UVB (180 mJ/cm2), and UVB (180 mJ/cm2) + Tacrolimus (0.03%). Four time points were set as follows: 0, 18, 24, and 48 h. We collected culture medium and tissues at each time point. The percentage of CD1a+ cells in the medium was detected by means of flow cytometry. Each tissue was prepared for immunohistochemistry, real-time quantitative PCR, and western blot. HaCaT cells were cultured and divided into four groups: Control, Tacrolimus (1 μg/ml), UVB (30 mJ/cm2), and UVB (30 mJ/cm2) + Tacrolimus (1 μg/ml). The cells were incubated for 24 h and prepared for real-time quantitative PCR and western blot.
Results: Topical tacrolimus significantly reversed high-dose UVB irradiation-induced epidermal LC reduction and CD1a+ cell increment in culture medium. Tacrolimus significantly inhibited UVB irradiation-induced tumor necrosis factor-α (TNF-α) and nuclear factor kappa B (NF-κB)/p65 mRNA and protein expression in HaCaT cells. Tacrolimus also significantly inhibited high-dose UVB irradiation-induced TNF-α expression in cultured tissues. Finally, TNF-α antagonist (recombinant human TNF-α receptor II: IgG Fc fusion protein) could significantly reverse UVB irradiation-induced epidermal LC reduction.
Conclusion: Topical tacrolimus 0.03% could reverse UVB irradiation-induced epidermal LC reduction by inhibiting TNF-α secretion in keratinocytes via regulation of NF-κB/p65.
Introduction
Topical calcineurin inhibitors (TCIs), including tacrolimus ointment and pimecrolimus cream, are widely used in the treatment of atopic dermatitis and many other inflammatory skin diseases, where the central therapeutic mechanism is to block T-cell proliferation and inhibit the activation of T-cells and thereby diminish inflammation (Yin et al., 2014; Nygaard et al., 2017). In contrast with topical corticosteroids, TCIs are not related to atrophy or increased percutaneous absorption after long-term use and have much lower possibility for systemic effects (Siegfried et al., 2016).
There is no evidence that TCIs have direct action on epidermal Langerhans cells (LCs). Epidermal LCs have the action of immunosurveillance and process antigen and migrate to local draining lymphnodes from epidermis, expressing CD1a (Rowden, 1980). Single high-dose ultraviolet B (UVB) irradiation can induce significant epidermal LC depletion in human skin, and the increased migration of LCs might be the main mechanism (Kölgen et al., 2002). UVB-induced apoptotic cells are phagocytosed by LCs ex vivo, which has an important anti-inflammatory effect in the resolution of UVB-induced cutaneous inflammation (Hatakeyama et al., 2017).
Our previous studies found that high-dose UVB irradiation significantly decreased the number of epidermal LCs, and pimecrolimus 1% cream could reverse these changes via inhibition of LCs migration by regulation of tumor necrosis factor-α (TNF-α) and E-cadherin (Yin et al., 2012b, 2014). Whether topical tacrolimus (another kind of widely used TCI) can also inhibit LC migration in UVB-irradiated skin is still unclear.
Lan et al. (2005) reported UVB irradiation-induced secretion of TNF-α from keratinocytes, which could be inhibited by tacrolimus by downregulation of nuclear factor kappa B (NF-κB) expression. Wu et al. (2012) found that tacrolimus didn’t affect the nuclear activation and translocation of NF-κB/p50; however, UVB irradiation-induced NF-κB/p65 nuclear expression was suppressed by tacrolimus. This study aimed to investigate the effect of topical tacrolimus 0.03% ointment on high-dose UVB-irradiated human epidermal LCs and the possible relation with TNF-α secretion and NF-kB/p65 regulation, which would contribute to further understanding of the mechanism of TCI action and treatment.
Materials and Methods
Ethics Statement
This study was carried out in accordance with the recommendations of institutional guidelines and Local Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (approval number 2013-SRFA-074). Written informed consent was obtained from all subjects.
Study Design
Twenty fresh human foreskin tissues were obtained from Department of Urology by circumcision, consented by the patients (age range 18–30 years). All subjects gave written informed consent in accordance with the Declaration of Helsinki. The tissues were randomly divided into four groups of five each, as follows: Control, Tacrolimus (tissues were applied once with topical tacrolimus 0.03% on the epidermis), UVB (tissues were irradiated once with 180 mJ/cm2 UVB on the epidermis), and UVB + Tacrolimus (tissues were applied on the epidermis with topical tacrolimus 0.03% after 180 mJ/cm2 UVB irradiation). The tissues were processed and cultured as previously described (Yin et al., 2012b). Recombinant human TNF-α receptor II: IgG Fc fusion protein (Yisaipu; CP Guojian Pharmaceutical Co., Ltd., Shanghai, China; 50 μg/ml) was added into culture medium to block the effect of TNF-α.
The UVB source was a BLE-1T158 UV lamp (Spectronics Corp., Westbury, NY, United States) by which 180 mJ/cm2 UVB was delivered once to the epidermis. After UVB irradiation or not, tacrolimus 0.03% ointment (Protopic; Astellas Toyama Co., Toyama, Japan) was applied on the epidermis. Ten minutes after application or irradiation, 1 ml culture medium was added to each well to immerse the whole tissue. All tissues were cultured at 37°C.
Four time points were set as follows: 0, 18, 24, and 48 h. For each group, each tissue was cut into four pieces corresponding to four time points. We collected culture medium and tissues at each time point, following which each tissue was cut into three parts. The percentage of CD1a+ cells in the medium was detected by means of flow cytometry. Each tissue was prepared for immunohistochemistry, real-time quantitative PCR, and western blot.
Keratinocyte line HaCaT cells were cultured as previously described (Zhou et al., 2013), and seeded in 12-well culture plates and divided into four groups, as follows: Control, Tacrolimus (Prograf; Astellas Ireland Co., Ltd., Killorglin, Co. Kerry, Ireland; 1 μg/ml), UVB (30 mJ/cm2), and UVB (30 mJ/cm2) + Tacrolimus (1 μg/ml). The cells were incubated for 24 h, and then prepared for real-time quantitative PCR and western blot. Experiments were repeated independently at least three times.
Flow Cytometry
Detection of CD1a expression on cells in the culture medium was performed using anti-human CD1a-PE antibody (BioLegend, Inc., San Diego, CA, United States) (Yin et al., 2014). A FACS CaliburTM Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, United States) was used to gather data and images.
Immunohistochemistry
Slides were prepared using a Ventana autoimmunostainer (Loche, United States) and available CD1a monoclonal antibody (Maixin-Bio, Fuzhou, Fujian, China) and active caspase-3 polyclonal antibody (Abcam, New Territories, Hong Kong, China). Detection utilized Polymer-HRP, with 3,3′-diaminobenzidine chromogen, and slides were visualized at 40× with a Nikon Eclipse microscope (Yin et al., 2012b). The number of typical CD1a positive epidermal LCs was counted for five successive fields in high magnification (HM, 400×). The number of LCs was calculated and expressed as CD1a+ LC/HM.
Real-Time Quantitative PCR
Total mRNA was extracted from part (50 mg) of the aforementioned collected tissue, using TRIzol® Reagent (Invitrogen; Life Technologies Corp., Carlsbad, CA, United States). The HaCaT cells attached to the culture plates were washed three times using PBS and then dissolved in TRIzol® Reagent. First-strand cDNA was synthesized from 2 μg of total RNA. PCR amplification was performed in a total volume of 20 μl containing 1 μl template cDNA and 10 μl Real-Time PCR Master Mix (SYBR Green) (TOYOBO, Japan), and transcripts quantified using StepOnePlusTM Real-Time PCR System (Applied Biosystems, United States) (Yin et al., 2017). All values were normalized to the expression of glyceraldehyde phosphate dehydrogenase (GAPDH). Primer sequences are shown in Table 1.
Western Blot
The collected tissues and HaCaT cells were homogenized in cold lysis buffer containing protease inhibitor. Centrifugal separation was conducted at 4°C, at 14,000 rpm for 15 min. The upper layer of the solution was tested for protein using the Bradford method. SDS–PAGE was performed (Yin et al., 2017). The primary antibody was added as below: TNF-α, β-actin (Biosynthesis Bio, Beijing, China), and NF-κB/p65 (KeyGEN Biotech, Nanjing, Jiangsu, China), following the manufacturer’s instructions. Differences in protein expression were examined using Gel-Pro 32 (Media Cybernetics, Rockville, MD, United States).
Statistical Analysis
Data analysis was conducted by using GraphPad Prism for Windows (GraphPad Software, San Diego, CA, United States). All data were presented as mean ±SD. Data were tested for normality and statistical significance calculated using a Student’s t-test, Mann–Whitney U-test, or Friedman’s test, as appropriate (Yin et al., 2017). P < 0.05 was considered statistically significant.
Results
Topical Tacrolimus Reverses High-Dose UVB Irradiation-Induced Epidermal Langerhans Cell Reduction
Immunohistochemistry showed that the number of epidermal CD1a+ LCs had no significant differences at different time points, for Control and Tacrolimus group. One hundred and eighty millijoules per centimeter squared UVB irradiation induced significant CD1a+ LC reduction at 18, 24, and 48 h (P < 0.01, respectively), which could be significantly reversed by topical tacrolimus 0.03% at 24 and 48 h (P < 0.05 and P < 0.01) (Figures 1A–D, upper panel, E).
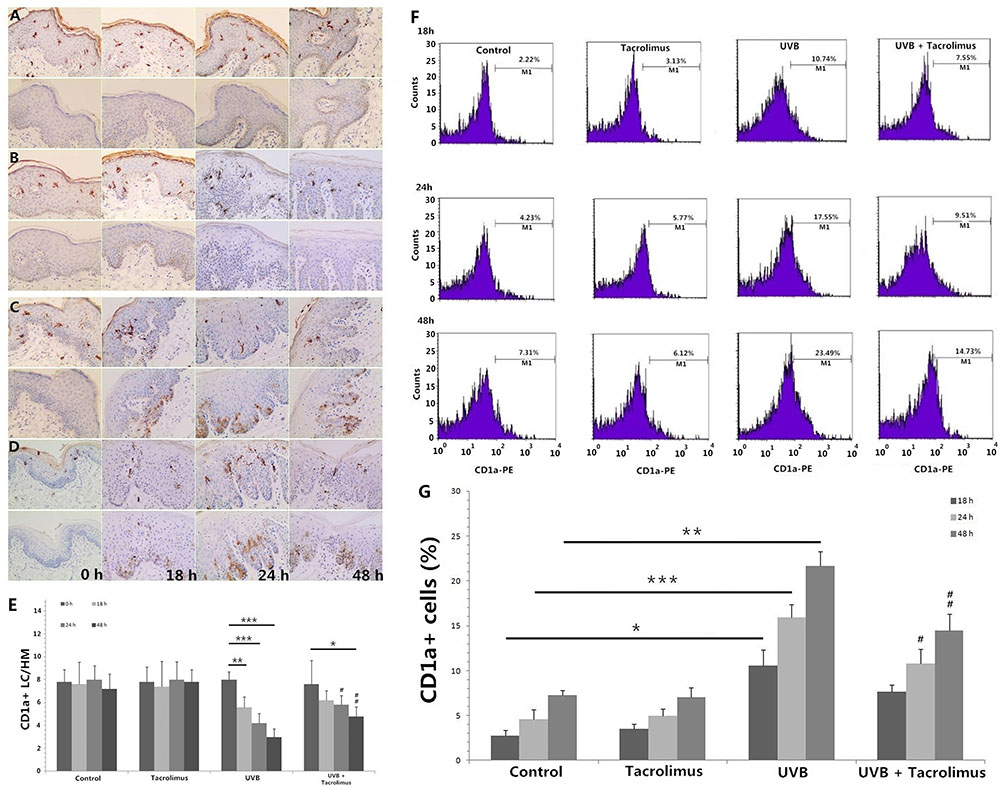
FIGURE 1. Immunohistochemical staining of CD1a and active caspase 3 in foreskin tissues (A–E, HM × 400) and flow cytometry of CD1a expression on cells in the culture medium (F,G) at different time points. (A) Control group, (B) Tacrolimus group, (C) UVB group, (D) UVB + Tacrolimus group, (E) Topical tacrolimus significantly reversed 180 mJ/cm2 UVB irradiation-induced marked epidermal CD1a+ LC reduction in tissues at 24 and 48 h, (F) Flow cytometry of CD1a, (G) Topical tacrolimus significantly inhibited UVB irradiation-induced marked CD1a+ cell increment in the culture medium at 24 and 48 h. Statistical significance indicated: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05, ##P < 0.01, ###P < 0.001. Abbreviation: UVB, ultraviolet B; LCs, Langerhans cells; HM, high magnification.
The lower panel of Figures 1A,B show there were no obvious active caspase 3-positive cells in the epidermis for Control and Tacrolimus group. High-dose UVB irradiation induced obvious cell apoptosis in stratum spinosum and basal layer of epidermis, and UVB + Tacrolimus group seemed to have no visible difference in comparison with UVB group (Figures 1C,D, lower panel).
Topical Tacrolimus Reverses High-Dose UVB Irradiation-Induced CD1a+ Cell Increment in Culture Medium
Flow cytometry (Figures 1F,G) showed 180 mJ/cm2 UVB irradiation induced significant increment of the percentage of CD1a+ cells in culture medium at 18, 24, and 48 h (18 h, P < 0.05; 24 h, P < 0.001; 48 h, P < 0.01), compared with Control group. Topical tacrolimus had no direct effect on the percentage of CD1a+ cells in culture medium; however, it could reverse UVB irradiation-induced CD1a+ cell increment (24 h, P < 0.05; 48 h, P < 0.01).
Tacrolimus Inhibits UVB Irradiation-Induced TNF-α and NF-κB/p65 Expression in HaCaT Cells
We irradiated HaCaT cells with 30 mJ/cm2 UVB and observed statistically significant increases in TNF-α and NF-κB/p65 but not interleukin-18 (IL-18) or E-cadherin mRNA after 24 h of incubation (P < 0.01, respectively) (Figure 2A). One microgram per milliliter tacrolimus had no direct effect on these cytokines and adhesion molecule mRNA expression in HaCaT cells; however, it could significantly inhibit UVB irradiation-induced TNF-α and NF-κB/p65 expression (P < 0.001, respectively) (Figure 2A), which was also confirmed at the protein level (Figures 2B,C).
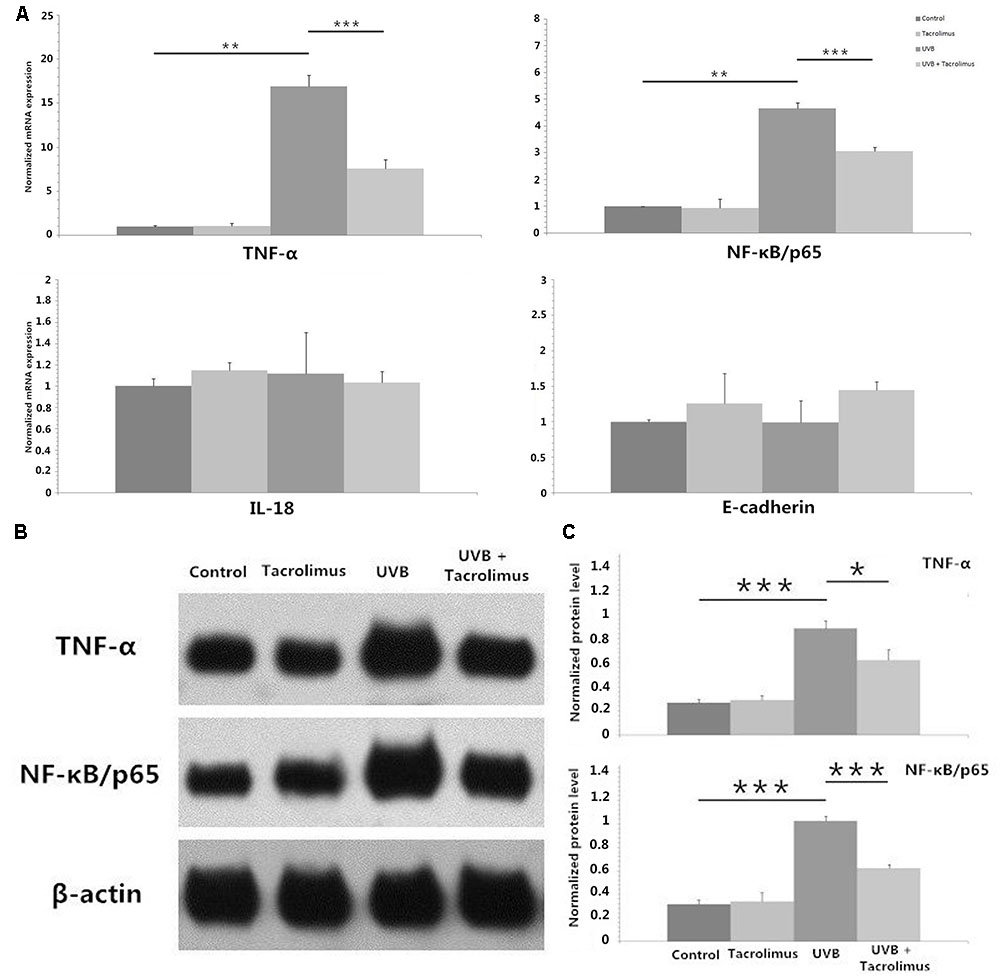
FIGURE 2. Real-time quantitative PCR analyses of TNF-α, NF-κB/p65, IL-18, and E-cadherin mRNA expression in HaCaT cells (A) and western blot of TNF-α and NF-κB/p65 protein expression in HaCaT cells (B,C) after 24 h of incubation. (A) One microgram per milliliter tacrolimus significantly inhibited 30 mJ/cm2 UVB irradiation-induced marked TNF-α and NF-κB/p65 mRNA expression. (B) Western blot of TNF-α and NF-κB/p65. (C) The inhibition of tacrolimus on UVB irradiation-induced TNF-α and NF-κB/p65 mRNA expression was confirmed at the protein level. Statistical significance indicated: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviation: UVB, ultraviolet B; IL-18, interleukin-18; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor kappa B.
Topical Tacrolimus Inhibits High-Dose UVB Irradiation-Induced TNF-α Expression in Cultured Tissues
One hundred and eighty millijoules per centimeter squared UVB irradiation induced significant increases in TNF-α mRNA expression in cultured tissues at 18, 24, and 48 h (18 h, P < 0.001; 24 h, P < 0.01; 48 h, P < 0.01) (Figure 3A), which was confirmed at the protein level (24 h, P < 0.01; 48 h, P < 0.01) (Figures 3B,C). Topical tacrolimus could significantly inhibit high-dose UVB irradiation-induced TNF-α mRNA expression (18 h, P < 0.001; 24 h, P < 0.01; 48 h, P < 0.05) (Figure 3A), which was also confirmed at the protein level (24 h, P < 0.05; 48 h, P < 0.05) (Figures 3B,C). High-dose UVB irradiation also induced significant increases in matrix metallopeptidase 9 (MMP-9) mRNA expression in cultured tissues at 24 and 48 h (24 h, P < 0.001; 48 h, P < 0.001); however, it could not be regulated by topical tacrolimus (Figure 3A). Neither high-dose UVB single irradiation nor topical tacrolimus had direct effect on IL-1β, C–C chemokine receptor type 7 (CCR7), C–C motif ligand 19 (CCL19), and integrin alpha-6 (ITGA6) mRNA expression in tissues.
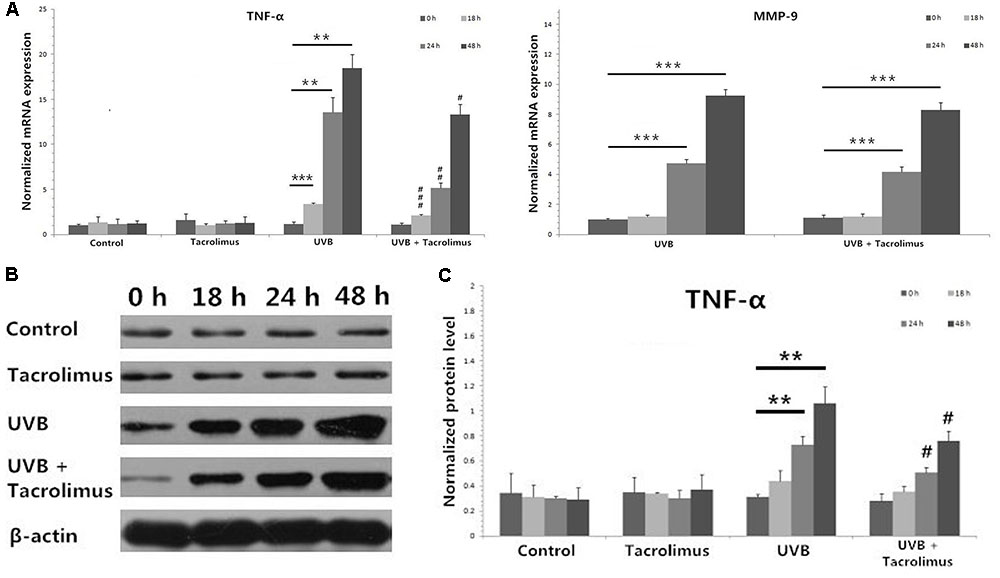
FIGURE 3. Real-time quantitative PCR analyses of cultured tissues (A) and protein detection by using western blot (B,C) at different time points. (A) Topical tacrolimus significantly inhibited 180 mJ/cm2 UVB irradiation-induced TNF-α mRNA expression in cultured tissues at 18, 24, and 48 h; however, it could not regulate UVB irradiation-induced significant increases in MMP-9 mRNA expression. (B) Western blot of TNF-α. (C) The inhibition of tacrolimus on UVB irradiation-induced TNF-α mRNA expression was confirmed at the protein level at 24 and 48 h. Statistical significance indicated: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05, ##P < 0.01, ###P < 0.001. Abbreviation: UVB, ultraviolet B; TNF-α, tumor necrosis factor-α; MMP-9, matrix metallopeptidase 9.
TNF-α Antagonist Reverses High-Dose UVB Irradiation-Induced Epidermal Langerhans Cell Reduction and CD1a+ Cell Increment in Culture Medium
Figures 4A,B show that TNF-α antagonist (recombinant human TNF-α receptor II: IgG Fc fusion protein, 50 μg/ml) significantly reversed high-dose UVB irradiation-induced epidermal LC reduction at 24 h (P < 0.05), and inhibited UVB irradiation-induced significant increment of the percentage of CD1a+ cells in culture medium (P < 0.01) (Figures 4C,D).
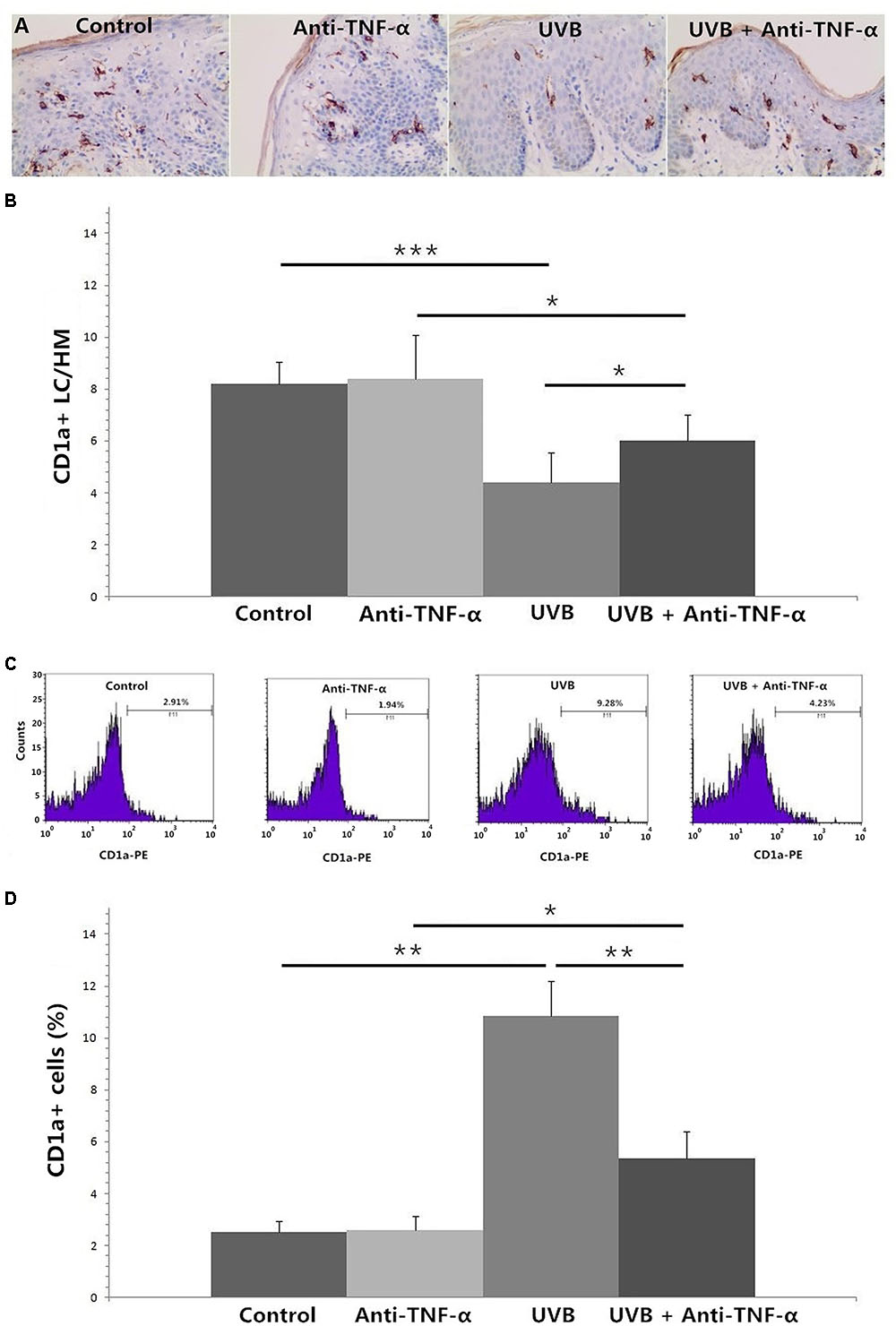
FIGURE 4. Immunohistochemical staining of CD1a in foreskin tissues (A,B, HM × 400) and flow cytometry of CD1a expression on cells in the culture medium (C,D) after 24 h of incubation. (A) Less CD1a+ LCs were seen in epidermis after 180 mJ/cm2 UVB irradiation. (B) TNF-α antagonist significantly reversed high-dose UVB irradiation-induced epidermal LC reduction at 24 h. (C) A higher percentage of CD1a+ cells in culture medium were observed in UVB group by using flow cytometry. (D) TNF-α antagonist significantly inhibited UVB irradiation-induced increment of the percentage of CD1a+ cells in culture medium. Statistical significance indicated: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviation: UVB, ultraviolet; LCs, Langerhans cells; HM, high magnification; TNF-α, tumor necrosis factor-α.
Discussion
Langerhans cells represent the first line of immunological defense, which has been proved to belong to the macrophage lineage recently (Doebel et al., 2017). The proliferation, maturation, and migration of epidermal LCs can be affected by skin inflammation, and increased migration during inflammation can lead to a partial depletion of epidermal LCs. Adhesion between keratinocytes and LCs, mediated by E-cadherin, is important in the retention of LCs in the skin (Matthews et al., 2003). Epidermal LCs disengage from their adhesion with surrounding keratinocytes during the migration in part by downregulation of E-cadherin (Tang et al., 1993). Intradermal injection of antagonists against TNF-α, IL-1β, and IL-18 impairs epidermal LC migration (Cumberbatch et al., 1997, 2001). The increment of the expression of CCR7, CCL19, MMP-9, and ITGA6 also promotes LC migration from epidermis to dermis (Price et al., 1997; Randolph, 2001; Koch et al., 2006).
Previous studies observed that high-dose UVB irradiation could decrease the number of epidermal LCs, and found the possible mechanism was the increment of LC migration rather than LC apoptosis (Kölgen et al., 2002; Yin et al., 2012b). The cited study has also proved that high-dose UVB irradiation-induced epidermal LC reduction was related to LCs migration (Yin et al., 2014). Our study showed topical tacrolimus could reverse high-dose UVB irradiation-induced epidermal LC reduction by inhibiting TNF-α secretion in keratinocytes via regulation of NF-κB/p65. Anti-TNF-α treatment significantly reversed UVB irradiation-induced epidermal LC reduction and CD1a+ cell increment in culture medium. We thought the CD1a staining positive cells in the culture medium were mainly made up of migrated LCs and dermal dendritic cells, and the increased CD1a+ cells in the medium induced by UVB irradiation should most probable be LCs.
Epidermal LCs play important role in immunosurveillance, which is an important pathway against tumorigenesis. Epidermal immature LCs can discern and ingest antigen, then gradually mature during migration. Interestingly, Modi et al. (2012) reported that LCs facilitated epithelial DNA damage and squamous cell carcinoma, suggesting the complex relation between immune cells and carcinogenesis. Lewis et al. (2015) also reported that LCs could facilitate UVB-induced epidermal carcinogenesis, which was counter to the concept of LCs as key players in tumor immunosurveillance that indicated the complexity of LC functions. Transforming growth factor beta-1 (TGF-β1) signaling promoted UVB-induced skin carcinogenesis that was mediated partly through its role in UVB-induced migration of dermal dendritic cell and cutaneous inflammation (Ravindran et al., 2014).
In 2006, the US Food and Drug Administration issued “black box” warnings for TCIs due to potential safety risks including skin cancers and lymphomas, which is still lack of evidence presently (Yin et al., 2012a). Voskamp et al. (2013) reported oral tacrolimus did not enhance UV irradiation-induced skin carcinogenesis. Mitamura et al. (2011) found both topical tacrolimus 0.03% and tacrolimus 0.1% could inhibit tumor induction in a mouse model of an initiation–promotion skin tumor. Topical tacrolimus did not enhance photocarcinogenesis or induce any dermal carcinogenicity in hairless mice (Lerche et al., 2008).
Pimecrolimus cream, another kind of TCI, did not affect LC number in healthy and atopic skin (Hoetzenecker et al., 2005; Yin et al., 2012b). In this study, tacrolimus 0.03% ointment used alone has also no obvious effect on human epidermal LC number; however, topical tacrolimus reverses high-dose UVB irradiation-induced epidermal LC reduction by inhibiting LC migration via downregulation TNF-α secretion in epidermal keratinocytes. In light of the fact that LCs could facilitate UVB-induced epidermal carcinogenesis (Lewis et al., 2015), the regulation effect of tacrolimus ointment on UVB irradiation-induced LC migration would promote photocarcinogenesis or inhibit skin carcinogenesis, which needs further investigation.
Ethics Statement
This study was carried out in accordance with the recommendations of institutional guidelines and Local Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (approval number 2013-SRFA-074). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
JX and YF were responsible for cell and tissue culture and qRT-PCR and western blot. GS and QG were responsible for immunohistochemistry. LY and YH were responsible for flow cytometry and data analysis. DL provided guidance to experiment design. ZY was responsible for the quality of the overall manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81301387) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231802).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Cumberbatch, M., Dearman, R.-J., Antonopoulos, C., Groves, R.-W., and Kimber, I. (2001). Interleukin (IL)-18 induces Langerhans cell migration by a tumour necrosis factor-alpha- and IL-1beta-dependent mechanism. Immunology 102, 323–330. doi: 10.1046/j.1365-2567.2001.01187.x
Cumberbatch, M., Dearman, R.-J., and Kimber, I. (1997). Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology 92, 388–395. doi: 10.1046/j.1365-2567.1997.00360.x
Doebel, T., Voisin, B., and Nagao, K. (2017). Langerhans cells - the macrophage in dendritic cell clothing. Trends Immunol. 38, 817–828. doi: 10.1016/j.it.2017.06.008
Hatakeyama, M., Fukunaga, A., Washio, K., Taguchi, K., Oda, Y., Ogura, K., et al. (2017). Anti-inflammatory role of Langerhans Cells and apoptotic keratinocytes in ultraviolet-B-induced cutaneous inflammation. J. Immunol. 199, 2937–2947. doi: 10.4049/jimmunol.1601681
Hoetzenecker, W., Ecker, R., Kopp, T., Stuetz, A., Stingl, G., and Elbe-Bürger, A. (2005). Pimecrolimus leads to an apoptosis-induced depletion of T cells but not Langerhans cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 115, 1276–1283. doi: 10.1016/j.jaci.2005.02.011
Koch, S., Kohl, K., Klein, E., von Bubnoff, D., and Bieber, T. (2006). Skin homing of Langerhans cell precursors: adhesion, chemotaxis, and migration. J. Allergy Clin. Immunol. 117, 163–168. doi: 10.1016/j.jaci.2005.10.003
Kölgen, W., Both, H., van Weelden, H., Guikers, K.-L., Bruijnzeel-Koomen, C.-A., Knol, E.-F., et al. (2002). Epidermal Langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration. J. Invest. Dermatol. 118, 812–817. doi: 10.1046/j.1523-1747.2002.01742.x
Lan, C.-C., Yu, H.-S., Wu, C.-S., Kuo, H.-Y., Chai, C.-Y., and Chen, G.-S. (2005). FK506 inhibits tumour necrosis factor-alpha secretion in human keratinocytes via regulation of nuclear factor-kappaB. Br. J. Dermatol. 153, 725–732. doi: 10.1111/j.1365-2133.2005.06779.x
Lerche, C.-M., Philipsen, P.-A., Poulsen, T., and Wulf, H.-C. (2008). Topical tacrolimus in combination with simulated solar radiation does not enhance photocarcinogenesis in hairless mice. Exp. Dermatol. 17, 57–62.
Lewis, J.-M., Bürgler, C.-D., Freudzon, M., Golubets, K., Gibson, J.-F., Filler, R.-B., et al. (2015). Langerhans cells facilitate UVB-induced epidermal carcinogenesis. J. Invest. Dermatol. 135, 2824–2833. doi: 10.1038/jid.2015.207
Matthews, K., Leong, C.-M., Baxter, L., Inglis, E., Yun, K., Bäckström, B.-T., et al. (2003). Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J. Virol. 77, 8378–8385. doi: 10.1128/JVI.77.15.8378-8385.2003
Mitamura, T., Doi, Y., Kawabe, M., Motomura, M., Oishi, Y., Yoshizawa, K., et al. (2011). Inhibitory potency of tacrolimus ointment on skin tumor induction in a mouse model of an initiation-promotion skin tumor. J. Dermatol. 38, 562–570. doi: 10.1111/j.1346-8138.2010.01046.x
Modi, B.-G., Neustadter, J., Binda, E., Lewis, J., Filler, R.-B., Roberts, S.-J., et al. (2012). Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science 335, 104–108. doi: 10.1126/science.1211600
Nygaard, U., Deleuran, M., and Vestergaard, C. (2017). Emerging treatment options in atopic dermatitis: topical therapies. Dermatology doi: 10.1159/000484407 [Epub ahead of print].
Price, A.-A., Cumberbatch, M., Kimber, I., and Ager, A. (1997). Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J. Exp. Med. 186, 1725–1735. doi: 10.1084/jem.186.10.1725
Randolph, G.-J. (2001). Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin. Immunol. 13, 267–274. doi: 10.1006/smim.2001.0322
Ravindran, A., Mohammed, J., Gunderson, A.-J., Cui, X., and Glick, A.-B. (2014). Tumor-promoting role of TGFβ1 signaling in ultraviolet B-induced skin carcinogenesis is associated with cutaneous inflammation and lymph node migration of dermal dendritic cells. Carcinogenesis 35, 959–966. doi: 10.1093/carcin/bgt486
Rowden, G. (1980). Expression of Ia antigens on Langerhans cells in mice, guinea pigs and man. J. Invest. Dermatol. 75, 22–31. doi: 10.1111/1523-1747.ep12521071
Siegfried, E.-C., Jaworski, J.-C., Kaiser, J.-D., and Hebert, A.-A. (2016). Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC. Pediatr. 16:75. doi: 10.1186/s12887-016-0607-9
Tang, A., Amagai, M., Granger, L.-G., Stanley, J.-R., and Udey, M.-C. (1993). Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature 361, 82–85. doi: 10.1038/361082a0
Voskamp, P., Bodmann, C.-A., Koehl, G.-E., Rebel, H.-G., Van Olderen, M.-G., Gaumann, A., et al. (2013). Dietary immunosuppressants do not enhance UV-induced skin carcinogenesis, and reveal discordance between p53-mutant early clones and carcinomas. Cancer Prev. Res. 6, 129–138. doi: 10.1158/1940-6207.CAPR-12-0361
Wu, C.-S., Lan, C.-C., Kuo, H.-Y., Chai, C.-Y., Chen, W.-T., and Chen, G.-S. (2012). Differential regulation of nuclear factor-kappa B subunits on epidermal keratinocytes by ultraviolet B and tacrolimus. Kaohsiung J. Med. Sci. 28, 577–585. doi: 10.1016/j.kjms.2012.04.023
Yin, L., Hu, Y.-Y., Xu, J.-L., Guo, J., Tu, J., and Yin, Z. (2017). Ultraviolet B inhibits IL-17A/TNF-α-stimulated activation of human dermal fibroblasts by decreasing the expression of IL-17RA and IL-17RC on fibroblasts. Front. Immunol. 8:91. doi: 10.3389/fimmu.2017.00091
Yin, Z.-Q., Xu, J.-L., Lu, Y., and Luo, D. (2012a). Topical pimecrolimus 1% reverses long-term suberythemal ultraviolet B-induced epidermal Langerhans cell reduction and morphologic changes in mice. J. Drugs Dermatol. 11, e25–e27.
Yin, Z.-Q., Xu, J.-L., Zhang, Z.-H., and Luo, D. (2012b). Effects of topical pimecrolimus 1% on high-dose ultraviolet B-irradiated epidermal Langerhans cells. Int. Immunopharmacol. 14, 635–640. doi: 10.1016/j.intimp.2012.10.002
Yin, Z.-Q., Xu, J.-L., Zhou, B.-R., Wu, D., Xu, Y., Zhang, J.-A., et al. (2014). Topical pimecrolimus inhibits high-dose UVB irradiation-induced epidermal Langerhans cell migration, via regulation of TNF-α and E-cadherin. Drug Des. Devel. Ther. 8, 1817–1825. doi: 10.2147/DDDT.S70790
Zhou, B.-R., Zhang, J.-A., Zhang, Q., Permatasari, F., Xu, Y., Wu, D., et al. (2013). Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1β, and tumor necrosis factor-α via a NF-κB-dependent mechanism in HaCaT keratinocytes. Mediators Inflamm. 2013:530429. doi: 10.1155/2013/530429
Keywords: tacrolimus, UVB, Langerhans cells, TNF-α, NF-κB
Citation: Xu J, Feng Y, Song G, Gong Q, Yin L, Hu Y, Luo D and Yin Z (2018) Tacrolimus Reverses UVB Irradiation-Induced Epidermal Langerhans Cell Reduction by Inhibiting TNF-α Secretion in Keratinocytes via Regulation of NF-κB/p65. Front. Pharmacol. 9:67. doi: 10.3389/fphar.2018.00067
Received: 30 November 2017; Accepted: 18 January 2018;
Published: 22 February 2018.
Edited by:
Timothy Joseph Keane, Imperial College London, United KingdomReviewed by:
Claudio Ferrante, Università degli Studi “G. d’Annunzio” Chieti – Pescara, ItalyLucia Recinella, Università degli Studi “G. d’Annunzio” Chieti – Pescara, Italy
Copyright © 2018 Xu, Feng, Song, Gong, Yin, Hu, Luo and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ZhiQiang Yin, yzq2802@sina.com
 JiaLi Xu
JiaLi Xu YaDong Feng2,3
YaDong Feng2,3 ZhiQiang Yin
ZhiQiang Yin