- 1Biomedical Engineering Research Center, Tohoku Institute of Technology, Sendai, Japan
- 2Graduate Department of Environmental Information Engineering, Tohoku Institute of Technology, Sendai, Japan
- 3Graduate School of Environmental Studies, School of Engineering, Advanced Institute for Materials Research, Tohoku University, Sendai, Japan
- 4Center for General Education, Tohoku Institute of Technology, Sendai, Japan
- 5Graduate Department of Electronics, Tohoku Institute of Technology, Sendai, Japan
All living organisms bear its defense mechanism. Immune cells during invasion by foreign body undergoes phagocytosis during which monocyte and neutrophil produces reactive oxygen species (ROS). The ROS generated in animal cells are known to be involved in several diseases and ailments, when generated in excess. Therefore, if the ROS generated in cells can be measured and analyzed precisely, it can be employed in immune function evaluation and disease detection. The aim of the current study is to introduce our newly developed chip-type biosensor device with high specificity and sensitivity. It comprises of counter electrode and working electrodes I and II. The counter electrode is a platinum plate while the working electrodes I and II are platinum microelectrode and osmium-horseradish peroxidase modified gold electrode, respectively which acts as oxygen and hydrogen peroxide (H2O2) detection sensors. Simultaneous measurement of oxygen consumption and H2O2 generation were measured in animal cells under the effect of exogenous addition of differentiation inducer, phorbol 12-myristate 13-acetate. The results obtained showed considerable changes in reduction currents in the absence and presence of inducer. Our newly developed chip-type biosensor device is claimed to be a useful tool for real-time monitoring of the respiratory activity and precise detection of H2O2 in cells. It can thus be widely applied in biomedical research and in clinical trials being an advancement over other H2O2 detection techniques.
Introduction
Respiratory burst is the rapid release of reactive oxygen species (ROS) such as superoxide anion radical (O) and hydrogen peroxide (H2O2) involving a marked increase in oxygen consumption from different kinds of cells (Forman and Torres, 2002; Halliwell and Gutteridge, 2007). Bactericidal action and phagocytosis in immune cells such as neutrophils and monocytes is an important biological defense mechanism and are known to produce ROS (Halliwell and Gutteridge, 2007). Hydrogen peroxide is generated from O which is a primary ROS generated through the activation of NADPH oxidase (Halliwell and Gutteridge, 2007). Among the ROS generated, H2O2 is known to be relatively stable and thus function as a signal transducer in the cells (Groeger et al., 2009; Marques et al., 2009).
Reactive oxygen species besides its role in immune response has been known to be associated with various kind of human disease and its excessive production adversely affect the living organisms (Halliwell and Gutteridge, 2007; Auten and Davis, 2009; Brieger et al., 2012). The ROS disproportionately generated in the cell or organisms is known to oxidize nucleic acids, lipids and proteins including important biological components such as enzymes (Gutteridge and Halliwell, 2000; Halliwell and Gutteridge, 2007). Hydrogen peroxide is known to be poorly reactive with minimal capability to oxidize polyunsaturated fatty acids and nucleic acid however it can lead to oxidation of amino acids such as cysteine, tryptophan, tyrosine, histidine and methionine (Hoffmann and Meneghini, 1979; Halliwell et al., 2000). Being less reactive, it however acts as a substrate for highly oxidizing radicals such as hydroxyl (HOȂ) in the presence of metal ions (Barbouti et al., 2001).
Oxidative damage of these biological components are in recent years have been an extensive area of study with respect to its involvement in the onset of various diseases, including diabetes and high blood pressure as well as enhancement of the aging phenomenon and lifestyle-related diseases, such as arteriosclerosis (Bostwick et al., 2000; Oikawa, 2005). Reactive oxygen species detection at the cellular level has always been challenging due to non-availability of techniques for specific detection of different ROS and sensitive for low-level detection at the cellular level. Techniques such as low-level chemiluminescence/ultra-weak photon emission has been used as an indirect tool for deciphering the production of ROS and its role in oxidative radical reaction in various kind of living organism in the past few decades however, specific detection had not been possible (Cadenas et al., 1980; Kobayashi et al., 2009; Prasad and Pospišil, 2011). Direct methods involving electron paramagnetic resonance (EPR) spectroscopy have been used to detect species such as HO·, singlet oxygen, carbon centered radicals using spin trapping techniques (Buettner and Mason, 2003; Venkataraman et al., 2004; Rác et al., 2015). In addition, EPR spin trapping being an excellent technique for detection of various ROS, direct detection of H2O2 however is not possible. During the recent past, fluorescent probe such as amplex red has been used but is known to both lack specificity and limitation with respect to minimum detection limit of H2O2 (Yadav and Pospíšil, 2012). In addition to this, H2O2 detecting probes including 3,3 diaminobenzidine (DAB), amplex red (AR), amplex ultra red (AUR) and a europium-tetracycline complex (Eu3Tc) have been compared. The probes were tested for sensitivity to light, toxicity, subcellular localization and capacity to detect H2O2 in-vivo (Šnyrychová et al., 2009). The authors suggested that, the toxicity caused by the exogenous addition of fluorescent probes cannot be completely excluded and thus should be used with utmost caution to avoid artifacts.
With the employment of electrochemical techniques, quantitative analysis, real-time monitoring and imaging of ROS has been made possible (Ahammad, 2013). Also, it is conceivable to improve the selectivity by the use of modified electrode (Ahammad, 2013; Enomoto et al., 2013). Attempts have been made to detect ROS and reactive nitrogen species at the cellular level (Amatore et al., 2003, 2008a,b). During the respiratory burst, oxygen consumption was measured under the effect of phorbol 12-myristate 13-acetate (PMA) using Platinum (Pt) microelectrode in immune cells with a scanning electrochemical microscope (SECM; Kasai et al., 2009, 2014). In addition, Inoue et al. measured in real-time, the production of H2O2 in human progenitor myeloid leukemia cells differentiated into neutrophil-like (HL-60) using Os-HRP modified electrode (Inoue et al., 2010). Polymer used for the Os-HRP modified electrode was developed by Vreeke et al. (1992). The enzyme HRP is converted to its oxidized form, which is than reduced at the surface of the electrode at 0.0 V by the transfer of the electron via the mediator/electron donor (Os) to generate the electrochemical signal (Ohara, 1995; Prasad et al., 2015; Scheme I).
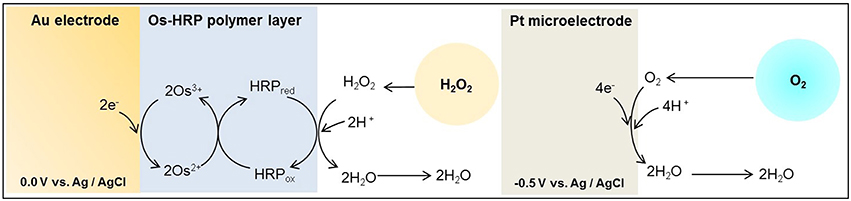
Scheme I. Schematic illustration of the reaction mechanism of catalytic amperometric method of detection of oxygen consumption using platinum (Pt) microelectrode and H2O2 generation using Os-HRP modified gold (Au) electrode. A Pt plate acts as reference electrode.
Knowing the mechanism and quantitative estimation of ROS formation is crucial and extremely important in biomedical research and clinical applications. In the current study, an electrochemical method for simultaneous detection and quantitative estimation of ROS formation have been demonstrated in THP-1 cells (cell lines of myeloid leukemia patients) during the respiratory burst (Tsuchiya et al., 1980, 1982). THP-1 is a monocyte and are known to differentiate into macrophages under the effect of PMA which acts as a differentiation inducer (Prasad et al., 2015). PMA is a powerful carcinogenic substance, with a similar physiological activity as diacylglycerol (DAG). By directly activating protein kinase C (PKC), it has been known to cause the activation of NADPH oxidase (Castagna et al., 1982; Kikkawa et al., 1983). In the current study, we aimed to design and develop electrochemical sensors that can simultaneously detects in real-time, the consumption of oxygen and production of H2O2 in animal cells. The real time measurements also reflects oxygen consumption volume and response time during the process of respiratory burst.
Materials and Methods
Cell Culture and Reagents
Human monocytic leukemia cell line, THP-1 was purchased from Japanese Collection of Research Bioresources (JCRB) cell bank (Cosmo Bio. Co. Ltd., Tokyo, Japan). The cells were maintained in RPMI 1640 supplemented with 2mM L-glutamine with incubation at 37°C in 5% CO2 in humidified atmosphere. The cells were passaged every 3 days.
Glucose, L-glutamine, H2O2 and PMA were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and RPMI 1640 medium and phosphate buffered saline (PBS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Fabrication of the Chip-Type Electrochemical Biosensors and Measurement Well
Chip-type electrochemical biosensor was fabricated as follows. Glass plate (S1127, Matsunami Glass Ind, Ltd., Japan) was cleaned using the oxygen plasma generator (LTA-101, Yanaco Co., Japan) with conditions as follows: power: 100 W; frequency, 13.6 MHz ; oxygen flow rate, 40 ml/min; time, 3 min. By photolithography, we prepared platinum and gold pattern on a glass substrate. The platinum plate (Pt plate) (1 × 3 mm) was prepared to act as the counter electrode. Platinum (diameter, 20 μm) and gold (diameter, 1 mm) circuit pattern was designed for the detection of oxygen and H2O2, respectively. For the preparation of Os-HRP modified Au electrode, volume of 0.5 μL Os-HRP polymer solution was immobilized over the electrode surface of the Au electrode and dried overnight at 4°C in the dark (Supplementary data 1). It has been known that, in HRP modified electrodes, electrons from the electrode are relayed to the enzyme through a redox epoxy network to which the enzyme HRP is bound covalently (Vreeke et al., 1992) (Scheme II).
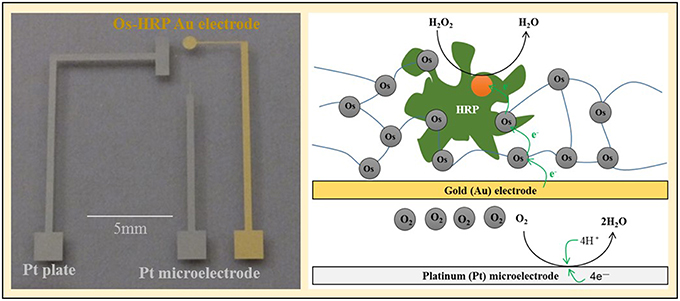
Scheme II. The chip device showing the Os-HRP modified Au electrode, the Pt microelectrode and Pt plate (left panel) and schematic representation of the mechanism of catalytic amperometry for measurement of oxygen reduction current and reduction current for H2O2 using the chip device (right panel).
For cellular assays, a polydimethylsiloxane (PDMS) prepolymer (SILPOTW/C, DOW Corning Toray, Japan) with a glass tube with an inner and outer diameter of 5 and 7 mm, respectively and height 30 mm (Figure 1A) was poured on an acrylic master plate which was then allowed to solidify with incubation at 90°C for 30 min followed by peeling off from the master plate. Photographs of the chip type device with microelectrodes have been depicted in Figure 1B. A photograph (Figure 2A) and detailed representation of the setup for the electrochemical measurements using chip type device have also been shown (Figure 2B).
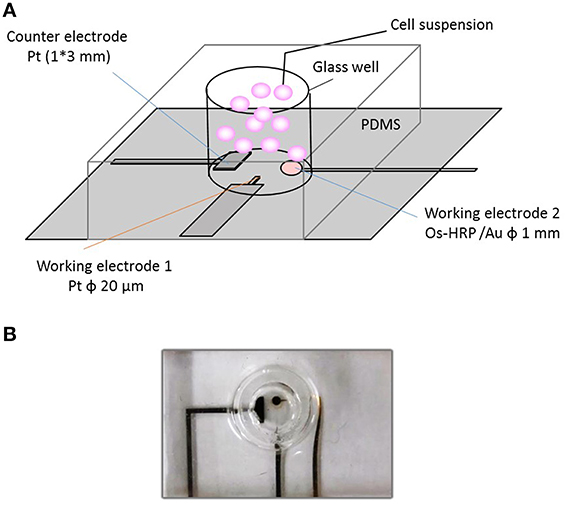
Figure 1. Experimental setup of catalytic amperometry using newly developed chip-type electrochemical biosensor device. The setup illustrated shows (A); the schematic representation of the chip-type device showing the counter electrode, CE; working electrode 1, WE1 (Pt microelectrode) and working electrode 2, WE2 (Os-HRP modified Au electrode) (B); photograph of the chip-type device showing the glass well consisting of electrodes.
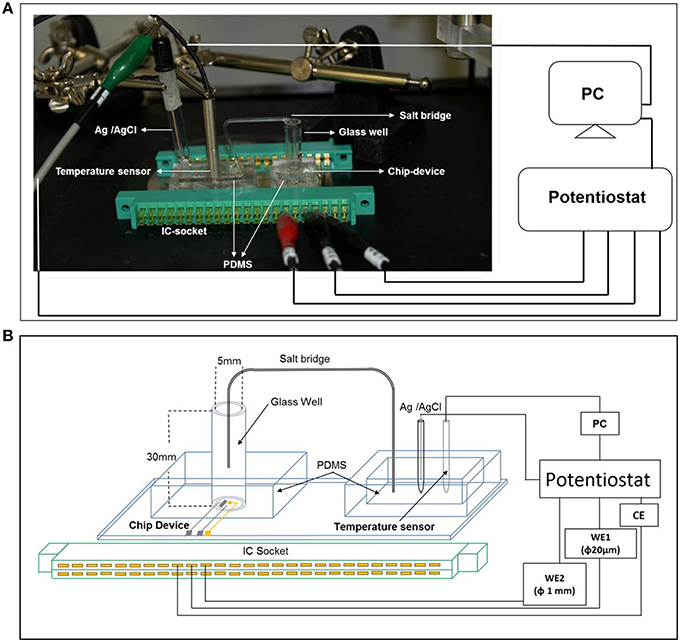
Figure 2. The photograph showing the arrangement of electrodes (A) and the schematic representation of the experimental setup (B) of catalytic amperometry for measurement of oxygen reduction current and reduction current for H2O2. The setup shows the PDMS prepolymer with a glass tube with an inner and outer diameter of 5 and 7 mm, respectively and height 30 mm containing the chip device; PDMS prepolymer with reference electrode (Ag/AgCl) and the temperature sensor. To avoid any concentration gradient between the 2 wells, a salt bridge was created using a glass tube. Changes in reduction current in WE1 and WE2 were measured using a potentiostat.
Equipment and Methods for Electrochemical Measurement
All electrochemical measurements were performed using potentiostat (HA1010mM4S; Hokuto denko Co., Ltd., Japan). An Ag/AgCl electrode was used as a reference electrode. For the basic characterization of the Pt microelectrode, Au electrode and Os-HRP modified Au electrode, cyclic voltammetry was conducted at room temperature (Figure 3). Calibration curve of Os-HRP modified Au electrode against standard H2O2 solution was performed in the concentration range of 1–4 nM at room temperature in PBS solution (Figure 4). Additionally, amperometric response of standard H2O2 solution in PBS at the final concentration of 0.25 nM H2O2 (A) and 0.5 nM H2O2 were also tested (B) (Supplementary data 2). To experimentally demonstrate the stability and interaction of Os-HRP on Au electrode, we tested the effect of 10 μM H2O2 solution by dipping Os-HRP modified Au electrode into it for a span of 3 h. This was followed by measuring the cyclic voltammogram before (red trace) and after 3 h (blue trace). No considerable change in cyclic voltamogram was observed indicating the long term stability of coated Os-HRP on Au electrode (Figure 5).
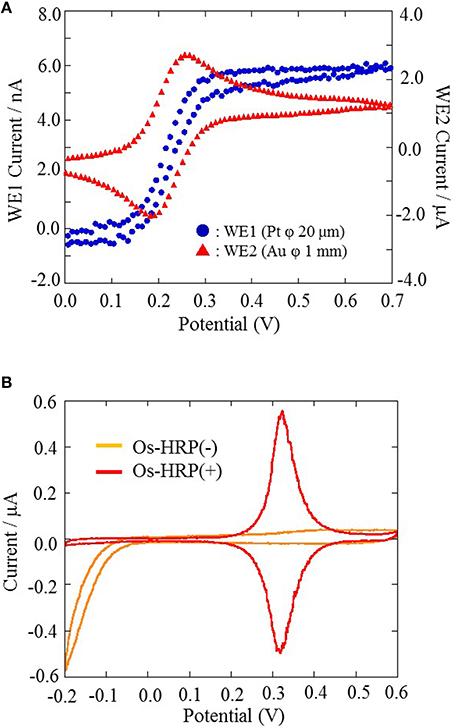
Figure 3. The characterization of Pt microelectrode and Au electrode performed using cyclic voltammetry at a scan rate of 20 mV/s from 0.0 to +0.7 V at room temperature (A). The characterization of Os-HRP modified Au electrode conducted at a scan rate of 20 mV/s from −0.2 to +0.6 V at room temperature (B).
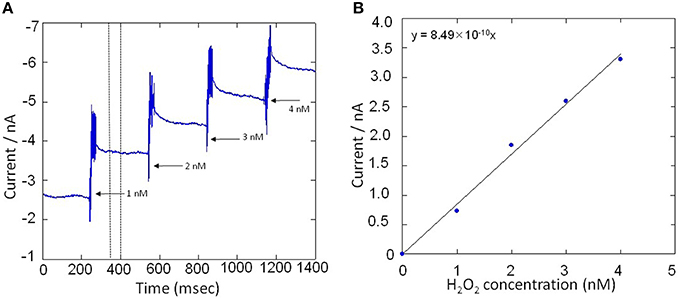
Figure 4. (A) Amperometric response of standard H2O2 solution in PBS in the concentration range of 1–4 nM. Addition of H2O2 solution was done in real-time under continuous stirring condition and reduction current for H2O2 was monitored using Os-HPR modified Au electrode. (B), Calibration curve was plotted by taking an average of 50 s of the constant phase (as indicated in A, dotted lines) obtained after the addition of standard known concentration of H2O2.
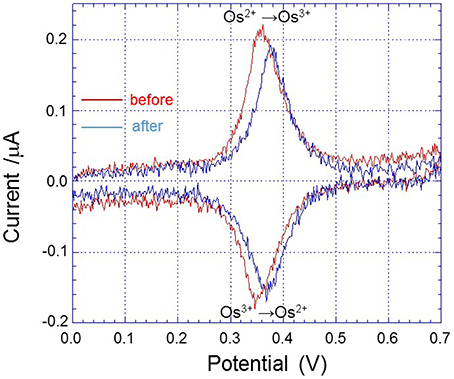
Figure 5. Cyclic voltammogram of Os-HRP modified Au electrode conducted at a scan rate of 20 mV/s from 0.0 to +0.7 V at room temperature before (red trace) and after 3 h of incubation in 10 μM H2O2 (blue trace).
Experimental Conditions
THP-1 cells were measured in the sensor well at a constant temperature of 30 ± 0.5°C and density of 3.0 × 105 cells/well. Cells were suspended in PBS in presence of glucose at the concentration of 11.4 mM. PMA was added dropwise after 5 min of starting of measurement with concentration of 200 nM and simultaneous reduction currents were measured. Oxygen reduction current was measured at −0.5 V vs. Ag/AgCl and reduction current for H2O2was measured at 0.0 V vs. Ag/AgCl at room temperature.
Result and Discussion
Characterization and Calibration of Electrodes
For the basic characterization of Pt microelectrode and Au electrode, a portion of the electrode were soaked in 10 ml PBS containing 4mM Potassium ferrocyanide [K4Fe(CN)6] and 0.1 M potassium chloride (KCl) and cyclic voltammetry were performed. Cyclic voltammetry were conducted at a scan rate of 20 mV/s from 0.0 to +0.7 V at room temperature (Figure 3A). For the characterization of the Os-HRP modified Au electrode, cyclic voltammetry was conducted at a scan rate of 20 mV/s from −0.2 to +0.6 V at room temperature. The oxidation and reduction current were obtained at 0.3 V vs. Ag/AgCl (Figure 3B). Based on the data obtained, the surface concentration of Os-HRP on the Au electrode was calculated to be 3.6 × 10−9 mol/cm2.
Calibration curve of the Os-HRP modified Au electrode was performed to establish the sensitivity and the concentration of H2O2 formed in THP-1 cells under the effect of PMA. The calibration was done using standard H2O2 solution in the concentration range of 1–4 nM (Figure 4A). Figure 4B shows a gradual increase in reduction current for H2O2 upon addition of exogenous standard H2O2 solution. The change in reduction current for H2O2 was observed to be approximately 1 nA with each step of 1 nM increase in concentration of H2O2(Figure 4B). In addition, the response on reduction current for H2O2 were also measured at concentration below 1 nM (Supplementary data 2). It is thus claimed that the Os-HRP modified Au electrode with the diameter of 1 mm is sufficiently sensitive for H2O2detection with high precision in concentration range of fractions of nM concentration.
Simultaneous Measurement of Oxygen Consumption and Hydrogen Peroxide Production
Using the chip-type biosensor device comprising of Pt microelectrode and Os-HPR modified Au electrode, the oxygen reduction current and reduction current for H2O2, respectively were measured at −0.5 V vs. Ag/AgCl and 0.0 V vs. Ag/AgCl. Dimethyl sulfoxide (DMSO) (0.001 %) was used as solvent to solubilize PMA. The amperometric response of PMA (200 nM) in system without THP-1 cells were measured as control to monitor for any changes due to its interference. No significant changes in reduction current for either oxygen or H2O2 were observed (Figure 6). A variation however, in oxygen reduction current in cell (blue trace) and cell-free system (green trace) was observed because of the fact that the dissolved oxygen concentration is lowered by cell respiration (Figure 7).
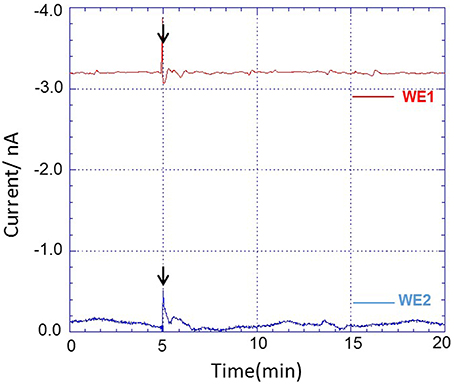
Figure 6. Amperometric response of addition of PMA dissolved in DMSO (0.001%) in system without THP-1 cells on oxygen reduction current (red trace) measured using WE1 and reduction current for H2O2 (blue trace) measured using WE2. PMA at final concentration of 200 nM was added after 5 min of start of measurement (indicated by arrow).
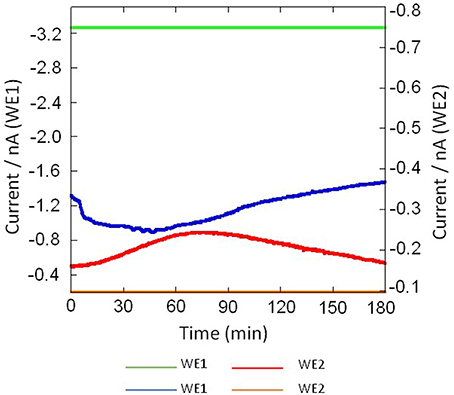
Figure 7. Real-time monitoring of oxygen reduction current in system containing no THP-1 cell (green trace) and in THP-cell under the effect of 200 nM PMA (blue trace) were measured using Pt microelectrode (WE1) and real-time monitoring of reduction current for H2O2 in system containing no THP-1 cell (yellow trace) and during respiratory burst in THP-1 (red trace) using Os-HRP modified Au electrode (WE2) under the effect of 200 nM PMA cells using a chip-type biosensor device.
Figure 7 shows the result of the simultaneous measurement of the oxygen reduction current and reduction current for H2O2 during the respiratory burst in THP-1 cells with a chip type biosensor device. Oxygen reduction current value was found to decrease immediately after drop wise addition of 200 nM PMA. However, after ~120 min, it showed the same reduction current value as measured before the addition of PMA. This sudden drop is most likely because of the rapid oxygen consumption as a result of respiratory burst in THP-1 cells (Figures 7, 8A). On contrary to immediate change in oxygen reduction current after PMA addition, gradual increase in reduction current for H2O2 was observed after about 15–20 min of PMA addition. As evident from the kinetics of the reduction current for H2O2, it can be seen that there occurs continuous production of H2O2 until ~70 min after PMA addition followed by a gradual decrease (Figures 7, 8B). This indicates that H2O2 is produced for a longer period of time. From the above observations, it can be concluded that oxygen consumption occurs rapidly as a result of respiratory burst while production of H2O2 is delayed. In addition, it can be concluded that oxygen consumption and H2O2 production during respiratory burst are phenomenon which are linked together. The continuous production of H2O2 during respiratory burst and its longer half-life due to low reactivity can be correlated to involvement of H2O2 in signaling.
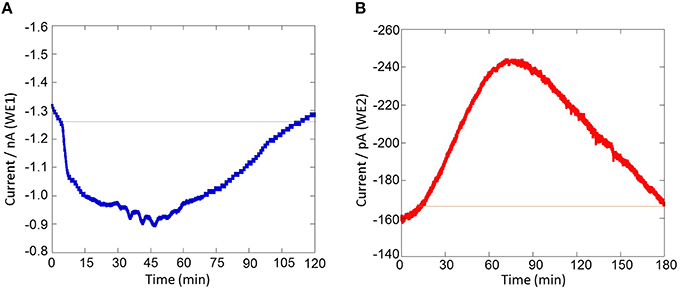
Figure 8. Real-time monitoring of oxygen reduction current and reduction current for H2O2 during respiratory burst in THP-1 under the effect of 200 nM PMA cells using a chip-type biosensor device. Changes in oxygen reduction current was measured using WE1 (A) while changes in reduction current for H2O2 was measured using WE2 (B).
Sensitivity of the Chip-Type Biosensor Device
The results presented shows the real-time monitoring of oxygen consumption and H2O2 production during respiratory burst in THP-1 cells under the effect of PMA (Figures 7, 8). Before the addition of PMA, an oxygen reduction current value of −1.3 nA was monitored (Figure 8A). It shows the dissolved oxygen concentration in the normal respiratory activity of cells. After the addition of PMA, the oxygen reduction current value was found to rapidly drop by about ~0.3 nA (300 pA) at a concentration of 200 nM PMA (Figure 8A).
Similarly, the reduction currents for H2O2 was measured under the effect of exogenous addition of PMA. With the addition of 200 nM PMA, a net change of 80 pA from −160 pA to −240 pA in reduction current for H2O2 was observed (Figure 8B). To determine the concentration of H2O2generated in THP-1 cells, the calibration curve was established for various concentrations obtained using H2O2 standard solution (Figure 4). Figure 9 depicts the concentration of oxygen consumption and H2O2 generation in THP-1 cells during respiratory burst. The total oxygen consumption during respiratory burst was found to be equivalent to 297 mM (Table 1A) in the time span of about 110 min recalculated using the standard bulk oxygen concentration as described in Hitoshi and co-workers (Hitoshi et al., 2005) while total concentration of H2O2 generation in THP-1 cells was recalculated using calibration curve of H2O2 (Figure 4) and was found equivalent to 494 nM in the time span of about 175 min (Table 1B).
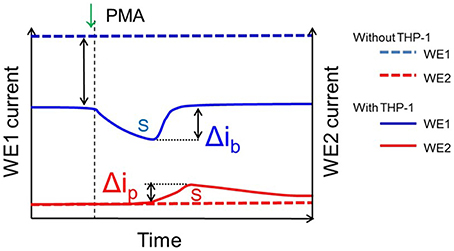
Figure 9. Details showing parameters utilized for calculation of oxygen consumption and H2O2 generation. The blue line (dotted) shows the bulk oxygen reduction current in system containing no THP-1 cells while the blue line (non-dotted) shows the oxygen reduction current in system containing THP-1 cells; the difference (blue dotted and blue un-dotted represents the change in dissolved oxygen concentration due to cell respiration and Δib represent the change in oxygen reduction current with the exogenous addition of PMA. Similarly, the red line (dotted) shows the reduction current for H2O2 in system containing no THP-1 cells while the red line (non-dotted) shows reduction current for H2O2 in system containing THP-1 cells and Δip represent the change in reduction current for H2O2 with the exogenous addition of PMA. The parameters were considered for calculations of oxygen consumption and generation of H2O2 as in Table 1.
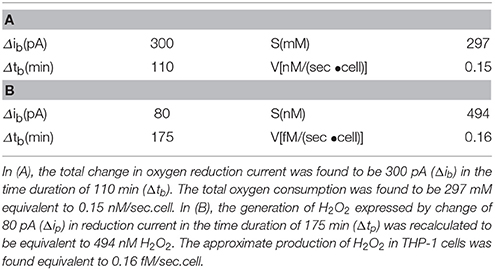
Table 1. Oxygen consumption calculated using standard bulk oxygen of 209 μM and H2O2 generation during the respiratory burst calculated using calibration curve (equation y = 8.49 × 10−10×).
Application of the Chip-Type Biosensor Device
To validate the potential of chip-type biosensor device for its wide application, we have performed experiments on a different cell line, HL-60 which is a human promyelocytic leukemia cell under the same experimental conditions as in Figures 7, 8. Real-time monitoring of oxygen consumption and H2O2 generation during respiratory burst in HL-60 cells under the effect of 200 nM PMA at a constant temperature of 30 ± 0.5°C and density of 3.0 × 105 cells/well were measured. Changes in oxygen reduction current was measured using Pt microelectrode, WE1 (red trace) while changes in reduction current for H2O2 was measured using Os-HRP modified Au electrode, WE2 (blue trace) (Supplementary data 3). Also, real-time monitoring of oxygen reduction current during respiratory burst in HL-60 cells under the effect of 200 nM and 2 μM PMA (n = 2) were tested. The time span of oxygen reduction current at different concentration of PMA was observed to vary considerably (Supplementary data 4).
In addition to this, real-time monitoring of oxygen reduction current and reduction current for H2O2 during respiratory burst in THP-1 cells (A) and HL-60 cells (B) under the effect of 200 nM PMA at two different cell densities (6.0 × 104 cells/well- red trace and 3.0 × 105 cells/well- blue trace) using a chip-type biosensor device were also tested (Figure 10). Based on these results, it is thus claimed that the chip-type biosensor device can be widely applied in different cell culture with high reproducibility, sensitivity and precision.
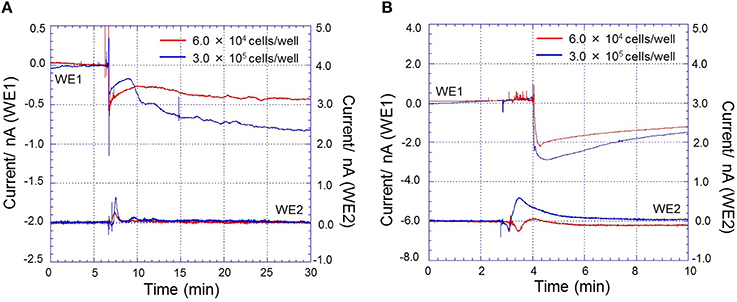
Figure 10. Real-time monitoring of oxygen reduction current and reduction current for H2O2 during respiratory burst in THP-1 cells (A) and HL-60 cells (B) under the effect of 200 nM PMA at a constant temperature of 30 ± 0.5°C and density of 6.0 × 104 cells/well (red trace) and 3.0 × 105 cells/well (blue trace) using a chip-type biosensor device. Changes in oxygen reduction current was measured using Pt microelectrode, WE1 while changes in reduction current for H2O2 was measured using Os-HRP modified Au electrode, WE2.
Conclusion
In this study, we have developed a catalytic amperometric chip-type electrochemical biosensor device for simultaneous and real-time monitoring of the respiratory activity and H2O2 production in animal cells. This device comprises of an Os-HRP modified Au electrode and Pt microelectrode for detection of H2O2 production and oxygen consumption, respectively. The device is composed of PDMS wells designed to accommodate living cells under controlled conditions. This electrochemical chip-type biosensor device monitored by changes in reduction currents reflecting oxygen and hydrogen peroxide generation is a useful tool for real-time monitoring of the respiratory activity and precise detection of level of oxygen and H2O2 in cellular systems. It is thus claimed to bear the potential for its application in biomedical research and for paving its way to clinical application being an advancement over other H2O2 detection techniques.
Author Contributions
HK, TS, MS, AT, and YS carried out the fabrication of the device and the measurements. AP analyzed, interpreted the data and drafted the manuscript. KI, AP, TM, and SK contributed to the conception and design of the work. KI and SK revised it critically for important content. All authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, Japan.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2016.00109
References
Ahammad, A. J. S. (2013). Hydrogen peroxide biosensors based on horseradish peroxidase and hemoglobin. J. Biosens. Bioelectron. S9:001. doi: 10.4172/2155-6210.S9-001
Amatore, C., Arbault, S., Bouton, C., Drapier, J. C., Ghandour, H., and Koh, A. C. (2008a). Real-time amperometric analysis of reactive oxygen and nitrogen species released by single immunostimulated macrophages. ChemBioChem 9, 1472–1480. doi: 10.1002/cbic.200700746
Amatore, C., Arbault, S., Chen, Y., Crozatier, C., and Tapsoba, I. (2003). Electrochemical detection in a microfluidic device of oxidative stress generated by macrophage cells. Lab Chip 7, 233–238. doi: 10.1039/B611569A
Amatore, C., Arbault, S., Ferreira, D. C. M., Tapsoba, I., and Verchier, Y. (2008b). Vitamin C stimulates or attenuates reactive oxygen and nitrogen species (ROS, RNS) production depending on cell state: quantitative amperometric measurements of oxidative bursts at PLB-985 and RAW 264.7 cells at the single cell level. J. Electroanal. Chem. 615, 34–44. doi: 10.1016/j.jelechem.2007.11.037
Auten, R. L., and Davis, J. M. (2009). Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr. Res. 66, 121–127. doi: 10.1203/PDR.0b013e3181a9eafb
Barbouti, A., Doulias, P. T., Zhu, B. Z., Frei, B., and Galaris, D. (2001). Intracellular iron, but not copper, plays a critical role in hydrogen peroxide-induced DNA damage. Free Radic. Biol. Med. 31, 490–498. doi: 10.1016/S0891-5849(01)00608-6
Bostwick, D. G., Alexander, E. E., Singh, R., Shan, A., Qian, J., Santella, R. M., et al. (2000). Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 89, 123–134. doi: 10.1002/1097-0142(20000701)89:1<123::AID-CNCR17>3.0.CO;2-9
Brieger, K., Schiavone, S., Miller, F. J. Jr., and Krause, K. H. (2012). Reactive oxygen species: from health to disease. Swiss Med. Wkly. 17, 142. doi: 10.4414/smw.2012.13659
Buettner, G. R., and Mason, R. P. (2003). “Spin-trapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo,” in Critical Reviews of Oxidative Stress and Aging: Advances in Basic Science, Diagnostics and Intervention, eds R. G. Cutler and H. Rodriguez (Singapore: World Scientific Publishing Co. Pte. Ltd.), 27–38.
Cadenas, E., Arad, I. D., Boveris, A., Fisher, A. B., and Chance, B. (1980). Partial spectral-analysis of the hydroperoxide-induced chemi-luminescence of the perfused lung. FEBS Lett. 111, 413–418. doi: 10.1016/0014-5793(80)80839-8
Castagna, M., Takai, Y., Kaibuchi, K., Sano, K., Kikkawa, U., and Nishizuka, Y. (1982). Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 257, 7847–7851.
Enomoto, J., Matharu, Z., and Revzin, A. (2013). Electrochemical biosensors for on-chip detection of oxidative stress from cells. Methods Enzymol. 526, 107–121. doi: 10.1016/B978-0-12-405883-5.00006-5
Forman, H. J., and Torres, M. (2002). Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care 166, S4–S8. doi: 10.1164/rccm.2206007
Groeger, G., Quiney, C., and Cotter, T. G. (2009). Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Sign. 11, 2655–2671. doi: 10.1089/ars.2009.2728
Gutteridge, J. M. C., and Halliwell, B. (2000). Free radicals and antioxidants in the year 2000 - A historical look to the future. Ann. N.Y. Acad. Sci. 899, 136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x
Halliwell, B., Clement, M. V., and Long, L. H. (2000). Hydrogen peroxide in the human body. FEBS Lett. 486, 10–13. doi: 10.1016/S0014-5793(00)02197-9
Halliwell, B., and Gutteridge, J. M. C. (2007). Free Radicals in Biology and Medicine, 4th Edn. New York, NY: Oxford University Press.
Hitoshi, S., Torisawa, Y. S., Takagi, A., Aoyagi, S., Abe, H., Hoshi, H., et al. (2005). Metabolic and enzymatic activities of individual cells, spheroids and embryos as a function of the sample size. Sens. Actuat. B Chem. 108, 597–602. doi: 10.1016/j.snb.2004.12.030
Hoffmann, M. E., and Meneghini, R. (1979). Action of hydrogen peroxide on human fibroblast in culture. Photochem. Photobiol. 30, 151–155. doi: 10.1111/j.1751-1097.1979.tb07128.x
Inoue, K. Y., Ino, K., Shiku, H., Kasai, S., Yasukawa, T., Mizutani, F., et al. (2010). Electrochemical monitoring of hydrogen peroxide released from leucocytes on horseradish peroxidase redox polymer coated electrode chip. Biosens. Bioelectron. 25, 1723–1728. doi: 10.1016/j.bios.2009.12.014
Kasai, S., Kikuchi, H., Suzuki, M., Nakano, M., Inoue, K. Y., Tada, M., et al. (2014). “Respiratory burst evaluation of thp-1 cell chip using a scanning electrochemical microscopy,” in 225th ECS Meeting (Orlando). Available online at: https://ecs.confex.com/ecs/225/webprogram/Paper31427.html
Kasai, S., Numata, T., Shiku, H., Abe, H., Matsue, T., and Niizeki, Y. (2009). “Real-time monitoring of oxygen consumption during differentiation of human monocytic cell lines (THP-1) by scanning electrochemical microscopy,” in 215th ECS Meeting (San Francisco, CA).
Kikkawa, U., Takai, Y., Miyake, R., and Nishizuka, Y. (1983). Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J. Biol. Chem. 258, 11442–11445.
Kobayashi, M., Kikuchi, D., and Okamura, H. (2009). Imaging of ultraweak spontaneous photon emission from human body displaying diurnal rhythm. PLoS ONE 4:e6256. doi: 10.1371/journal.pone.0006256
Marques, V. O., Marinho, H. S., Cyrne, L., and Antunes, F. (2009). Role of hydrogen peroxide in NF-κB activation: from inducer to modulator. Antioxid. Redox Sign. 11, 2223–2243. doi: 10.1089/ars.2009.2601
Ohara, T. J. (1995). Osmium Bipyridyl redox polymers used in enzyme electrodes. Platin. Metals Rev. 39, 54–62.
Oikawa, S. (2005). Sequence-specific DNA damage by reactive oxygen species: implications for carcinogenesis and aging. Environ. Health Prev. Med. 10, 65–71. doi: 10.1007/BF02897995
Prasad, A., Kumar, A., Suzuki, M., Kikuchi, H., Sugai, T., Kobayashi, M., et al. (2015). Detection of hydrogen peroxide in Photosystem II (PSII) using catalytic amperometric biosensor. Front. Plant Sci. 6:862. doi: 10.3389/fpls.2015.00862
Prasad, A., and Pospišil, P. (2011). Two-dimensional imaging of spontaneous ultra-weak photon emission from the human skin: role of reactive oxygen species. J. Biophotonics 4, 840–849. doi: 10.1002/jbio.201100073
Rác, M., Křupka, M., Binder, S., Sedlářová, M., Matušková, Z., Raška, M., et al. (2015). Oxidative damage of U937 human leukemic cells caused by hydroxyl radical results in singlet oxygen formation. PLoS ONE 10:e0116958. doi: 10.1371/journal.pone.0116958
Šnyrychová, I., Ayaydin, F., and Hideg, É. (2009). Detecting hydrogen peroxide in leaves in vivo – a comparison of methods. Physiol. Plant. 135, 1–18. doi: 10.1111/j.1399-3054.2008.01176.x
Tsuchiya, S., Kobayashi, Y., Goto, Y., Okumura, H., Nakae, S., Konno, T., et al. (1982). Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 42, 1530–1536.
Tsuchiya, S., Yamabe, M., Yamaguchi, Y., Kobayashi, Y., Konno, Y., and Tada, K. (1980). Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26, 171–176. doi: 10.1002/ijc.2910260208
Venkataraman, S., Schafer, F. Q., and Buettner, G. R. (2004). Detection of lipid radicals using EPR. Antioxid. Redox Sign. 6, 631–638. doi: 10.1089/152308604773934396
Vreeke, M., Maidan, R., and Heller, A. (1992). Hydrogen peroxide and beta-nicotinamide adenine dinucleotide sensing amperometric electrodes based on electrical connection of horseradish peroxidase redox centers to electrodes through a three-dimensional electron relaying polymer network. Anal. Chem. 64, 3084–3090. doi: 10.1021/ac00048a004
Keywords: biosensors, hydrogen peroxide, reactive oxygen species, catalytic amperometric biosensor, THP-1 cells, chip-type device, oxidative stress
Citation: Prasad A, Kikuchi H, Inoue KY, Suzuki M, Sugiura Y, Sugai T, Tomonori A, Tada M, Kobayashi M, Matsue T and Kasai S (2016) Simultaneous Real-Time Monitoring of Oxygen Consumption and Hydrogen Peroxide Production in Cells Using Our Newly Developed Chip-Type Biosensor Device. Front. Physiol. 7:109. doi: 10.3389/fphys.2016.00109
Received: 21 December 2015; Accepted: 07 March 2016;
Published: 29 March 2016.
Edited by:
Francisco Monroy, Complutense University, SpainReviewed by:
Ashok K. Sundramoorthy, University of Wisconsin-Madison, USALuis J. Fernandez, University of Zaragoza, Spain
Copyright © 2016 Prasad, Kikuchi, Inoue, Suzuki, Sugiura, Sugai, Tomonori, Tada, Kobayashi, Matsue and Kasai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigenobu Kasai, kasai@tohtech.ac.jp
 Ankush Prasad
Ankush Prasad Hiroyuki Kikuchi2
Hiroyuki Kikuchi2 Tomoya Sugai
Tomoya Sugai Shigenobu Kasai
Shigenobu Kasai