- 1Li Ka Shing Faculty of Medicine, School of Biomedical Sciences, University of Hong Kong, Hong Kong, China
- 2School of Medicine, Imperial College London, London, UK
- 3Department of Physiology, McGill University, Montreal, QC, Canada
- 4Department of Medicine and Therapeutics, Institute of Digestive Disease, LKS Institute of Health Sciences, Chinese University of Hong Kong, Hong Kong, China
The gastrointestinal (GI) tract is an electrically excitable organ system containing multiple cell types, which coordinate electrical activity propagating through this tract. Disruption in its normal electrophysiology is observed in a number of GI motility disorders. However, this is not well characterized and the field of GI electrophysiology is much less developed compared to the cardiac field. The aim of this article is to use the established knowledge of cardiac electrophysiology to shed light on the mechanisms of electrical activation and propagation along the GI tract, and how abnormalities in these processes lead to motility disorders and suggest better treatment options based on this improved understanding. In the first part of the article, the ionic contributions to the generation of GI slow wave and the cardiac action potential (AP) are reviewed. Propagation of these electrical signals can be described by the core conductor theory in both systems. However, specifically for the GI tract, the following unique properties are observed: changes in slow wave frequency along its length, periods of quiescence, synchronization in short distances and desynchronization over long distances. These are best described by a coupled oscillator theory. Other differences include the diminished role of gap junctions in mediating this conduction in the GI tract compared to the heart. The electrophysiology of conditions such as gastroesophageal reflux disease and gastroparesis, and functional problems such as irritable bowel syndrome are discussed in detail, with reference to ion channel abnormalities and potential therapeutic targets. A deeper understanding of the molecular basis and physiological mechanisms underlying GI motility disorders will enable the development of better diagnostic and therapeutic tools and the advancement of this field.
Introduction
The heart is responsible for delivering oxygenated blood to, and removing deoxygenated blood from, active respiring tissues in the periphery. Its electrical and mechanical activity is tightly regulated and further modulated by neuroendocrine signals. By contrast, in the gastrointestinal (GI) tract, the stomach initiates digestion and delivers gastric contents via the pylorus to the small intestine in a regulated manner. The intestines then further digest and absorb the contents. Both the heart and the gastrointestinal (GI) tract are electrically excitable. Normal mechanical functions of these organ systems depend on the highly coordinated activity of this electrical excitation, whose disruptions can lead to arrhythmias (Tse, 2015, 2016a,b,c; Chen Z. et al., 2016; Choy et al., 2016; Tse et al., 2016a,b,c,e,f,g; Tse and Yan, 2016). Whilst the electrophysiological properties of the heart have been extensively studied, those of the GI tract are relatively less well characterized. This is perhaps because arrhythmias in this system is usually not life-threatening (O'Grady et al., 2014), whereas those in the ventricles can cause sudden cardiac death (Murakoshi and Aonuma, 2013). However, there is increasing evidence that electrophysiological abnormalities play important roles in GI motility disorders such as gastroesophageal reflux disease (Shafik et al., 2005), achalasia (Faussone-Pellegrini and Cortesini, 1985a; Goldblum et al., 1994), Allgrove syndrome (Khelif et al., 2003), gastroparesis (O'Grady et al., 2012), pyloric stenosis (Langer et al., 1995; Vanderwinden et al., 1996), functional dyspepsia (Jung et al., 2012), idiopathic rapid gastric emptying (Bharucha et al., 2011), unexplained nausea and vomiting (Abell et al., 2009), mesenteric ischaemia (Irimia and Wikswo, 2008), functional diarrhea (Dellon and Ringel, 2006), or constipation (Camilleri, 2011), irritable bowel syndrome (Saito et al., 2009), Hirschsprung disease (Yamataka et al., 1995), chronic pseudo-obstruction (Feldstein et al., 2003), slow transit constipation (Lyford et al., 2002), and colonic hypomotility associated with anorectal malformations (Kenny et al., 1998). These conditions cause significant morbidity in the population and it is therefore important to understand their underlying mechanisms for devising effective treatment. A comparison between these systems may provide some insight for the GI electrophysiologists and physicians. Thus, the aim of this article is to examine the conduction pathways, the ionic currents responsible for electrical activation of different cell types, and mechanisms of their propagation in both the heart and the GI tract. This is followed by a discussion on the clinical relevance and molecular targets for future therapy.
Specialized Conduction Pathways
There are specialized conduction pathways responsible for electrical conduction through the heart and the GI tract (Figure 1; Veeraraghavan et al., 2014a). Pacemaker cells are responsible for the spontaneous initiation of electrical activity in both systems. In cardiac tissue, the dominant pacemaker is the sinoatrial node, which is responsible for initiating action potentials (APs) that spread along the cardiac conduction system, reaching all parts of the myocardium. They first spread radially through the right and left atria, converging on the atrioventricular (AV) node, where conduction velocity (CV) is reduced. This brief delay ensures atrial and ventricular systole occurs sequentially. The APs then propagate along the Bundle of His, and then the left and right bundle branches, activating the Purkinje fibers. From there they propagate to the apex and then into the ventricular myocardium. The SA node receives innervation from both the sympathetic and parasympathetic nervous systems, which exert positive and negative chronotropic effects, respectively.
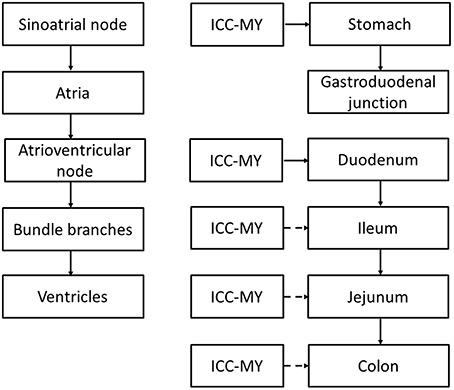
Figure 1. Comparison between the cardiac and gastrointestinal conduction pathways. ICC-MY: interstitial cells of Cajal in the myenteric plexus, pacemaker cells of the GI tract. Broken arrows indicate that the ICC is normally reset by the dominant pacemaker upstream that has the highest rate of discharge.
By contrast, the pacemaker region of the gastrointestinal tract is the interstitial cells of Cajal (ICC) of the myenteric plexus (ICC-MY) found in stomach, small intestine and colon, generating slow wave activity that spread into the circular and longitudinal muscle layers (Suzuki et al., 1986; Langton et al., 1989; Ward et al., 1994; Huizinga et al., 1995; Dickens et al., 1999). Interestingly, although ICC have been found in the esophagus, very few of these are associated with the myenteric plexus and therefore they have little role in pacemaker activity (Faussone-Pellegrini and Cortesini, 1985b). Instead, they are of the intramuscular subtype (ICC-IM) in close contact with nerves and smooth muscles. Nevertheless, there is some evidence to suggest that ICC-IM, in conjunction with PDGFRα-positive fibroblast-like cells, together are responsible for electrical and mechanical activation of the esophagus (Chen et al., 2013). In the stomach, ICC-MY is located high up at the greater curvature. The slow waves propagate radially in both the longitudinal and circumferential directions and the main direction of propagation is along the longitudinal direction toward the distal antrum (Lammers et al., 2009). Interestingly, the slow wave does not propagate from the stomach to the small intestine, suggesting either a loss of ICC (Wang et al., 2005) or electrical coupling between these regions (Lammers et al., 1998). The latter is unlikely because conduction of slow waves from the stomach into the pylorus has been observed (Sanders and Vogalis, 1989; Lammers et al., 2000; Wang et al., 2005). Thus, slow wave activity of the stomach and small intestine occurs independently of each other. This is consistent with the predominant role of the pylorus in controlling the flow of contents out of the stomach rather than conducting slow waves to the small intestine. Nevertheless, upon relaxation of the pylorus, there is coordinated activity between the stomach and small intestine, where synchronized propagation of peristaltic activity in the duodenum occur after stomach contractions. This coordination may involve stretch-activated or neural activation mechanisms rather than direct electrical coordination per se, as occurs in the heart for atrioventricular delays. Peristalsis in the small intestine propagates from the gastroduodenal junction toward the terminal ileum at a rate of 8–12 bpm, finally arriving at the jejunum (Christensen et al., 1966). In the colon, slow waves are generated and conducted throughout its length (Smith et al., 1987). ICC located within the muscle layers (ICC-IM) have additional physiological functions. These cells serve as a target for innervation, influencing GI motility in response to neural inputs (Beckett et al., 2003, 2005; Powley et al., 2008), set the resting membrane potential of smooth muscle cells by releasing the hyperpolarizing gasotransmitter, carbon monoxide (Farrugia et al., 2003; Sha et al., 2007) and mechanoreception (Won et al., 2005).
Pacemaker Activity
Pacemaker activity in the heart produces rhythmic atrial and ventricular contractions. By contrast, GI motility in the baseline is regulated by slow wave activity generated by the ICC network, but significant contractions are regulated by a complex interplay between neurogenic and myogenic factors locally, and endocrine signals systemically (Cheung and Wu, 2013). The GI tract produces two types of motion: peristalsis, which are rhythmic contractions that propel intraluminal contents and encourage mixing, and segmentation, which are ring contractions that divide the intraluminal contents but do not produce net movement along the GI tract (Weisbrodt, 1981; Table 1). In the human heart, the normal rate of the discharge of the SA node is between 60 and 100 beats per min (bpm). Subsidiary pacemakers discharge at slower rates and are normally reset by the SA node. These include the AV node, which discharges at 40–60 bpm, and the Purkinje system that discharges at 20–40 bpm. In the GI tract, ICC-MY at the greater curvature of the stomach discharges with a rate of 5–8 bpm with a rate of 5–8 bpm (Kelly and Code, 1971; Rhee et al., 2011; Cheng, 2015). Thus, ICC are organized into a continuous network, such that most regions of the smooth muscle in the small intestine are capable of generating pacemaker activity. This network runs circumferentially and longitudinally within the tunica muscularis of the GI tract. For the intestines, the dominant pacemaker with the highest rate of discharge is the duodenum, discharging at 12 bpm, with a decreasing frequency along the length to 8 bpm in the ileum (Christensen et al., 1966; Diamant and Bortoff, 1969; Szurszewski et al., 1970). There is a conduction delay between proximal and distal sites, ensuring coordinated contraction in this sequence for moving the luminal contents along the GI tract. Loss of dominant pacemaker will result in the takeover of subsidiary pacemakers in both the heart and GI tract (Homma et al., 2004; Tse, 2015). In the GI tract, three distinct population of cells are found: smooth muscle, ICC, PDGFR-positive cells, which together constitute an integrated united called the SIP syncytium (Sanders et al., 2006). It remains to be elucidated whether smooth muscle or PDGFR-positive cells are capable of taking over pacemaker activity in many GI motility disorders where ICC are lost or absent.
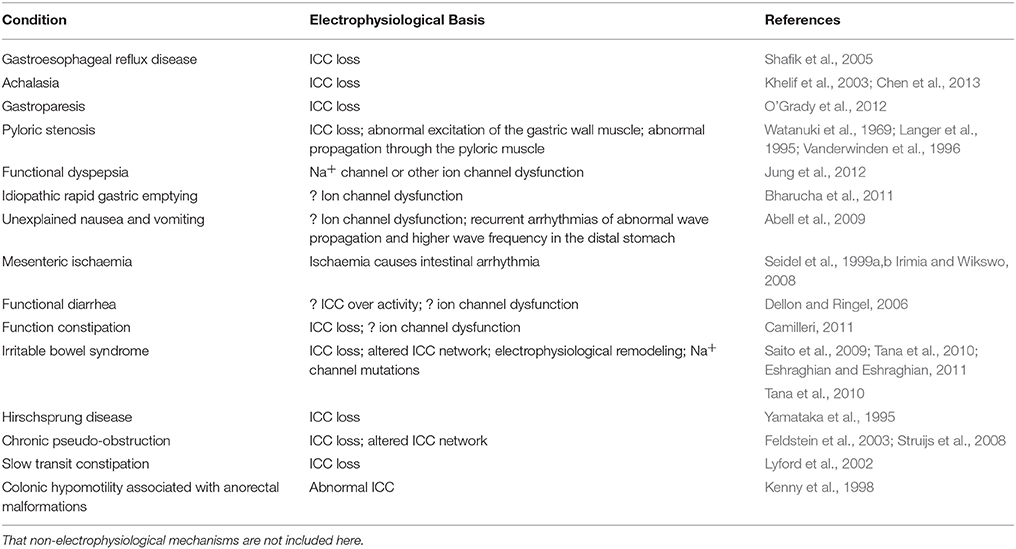
Table 1. Conditions caused by abnormal gastrointestinal electrophysiology and their proposed mechanisms.
Molecular mechanisms underlying cardiac and GI pacemaker activity involve both voltage- and Ca2+-dependent processes (Lakatta et al., 2006; Takaki et al., 2010). In the heart, the voltage-dependent mechanism involves the funny current (If) (Baruscotti et al., 2005) that has several unusual characteristics (DiFrancesco, 1993), including activation by hyperpolarization, permeability to both Na+ and K+ ions with a small single channel conductance, and modulation by cAMP. The Ca2+ mechanism involves spontaneous Ca2+ release from the endoplasmic reticulum (ER) (Vinogradova et al., 2005), activating the Na+−Ca2+ exchanger (INCX). Some have contended that the one mechanism is more important, entraining the other (Lakatta and DiFrancesco, 2009). However, numerical studies suggest that both voltage- and Ca2+ mechanisms are synergistically coupled to each other, called the coupled clock theory (Maltsev and Lakatta, 2013), a notion that is supported by experimental studies (Yaniv et al., 2014). Interestingly, transient receptor potential channels and store-operated Ca2+ entry have been demonstrated in SA node cells, suggesting that these channels may play a role in modulating pacemaker activity in the heart (Ju et al., 2007).
By contrast, ICC-MY are the pacemaker cells of the GI tract (Lees-Green et al., 2011). The maximum diastolic potential is set mainly by the K+ channel called ether-a-go-go-related (ERG) channel (Huizinga et al., 2004). Ca2+ release from inositol 1,4,5-trisphosphate (IP3) receptor-operated stores is followed by Ca2+ stimulated uptake by the mitochondria and back to the ER (Suzuki et al., 2000; Ward et al., 2000, 2003). Depletion of intracellular Ca2+ activates the Ca2+-inhibited, non-selective cationic conductance (Koh et al., 2002). The identity of this channel may be the transient receptor potential canonical 4 or related channels (Walker et al., 2002; Jin et al., 2009). Other currents, such as the voltage-independent, dihydropyridine-insensitive Ca2+ conductances and Ca2+-activated Cl− conductance (called Ano1) (Gomez-Pinilla et al., 2009; Zhu et al., 2009), may also contribute to pacemaker activity (Lee et al., 2007). This pacemaker current depolarization then activates the T-type Ca2+ channels (Lee et al., 2007). Furthermore, experimental evidence suggests that tetrodotoxin-resistant Na+ channels play a role in slow wave generation, at least in humans (Strege et al., 2003). The Na+ channels found in ICC are encoded by SCN5A, bearing similar electrophysiological properties to the cardiac isoform. Interestingly, these channels are likely to have a role in setting the resting membrane potential and modulate the rate of upstroke and frequency of the slow waves, rather than directing contributing to the pacemaker current per se (Strege et al., 2003). The repolarization phase of the slow wave involves several currents, mediated by Ano1 described above (Zhu et al., 2009) and K+ currents mediated by the ERG (McKay et al., 2006), Big K+ (Zhu and Huizinga, 2008), Ca2+-activated K+ (Fujita et al., 2001), and rectifier channels (Hatton et al., 2001; Huizinga et al., 2004).
Ionic Contributions of the Cardiac Action Potential and GI Slow Wave
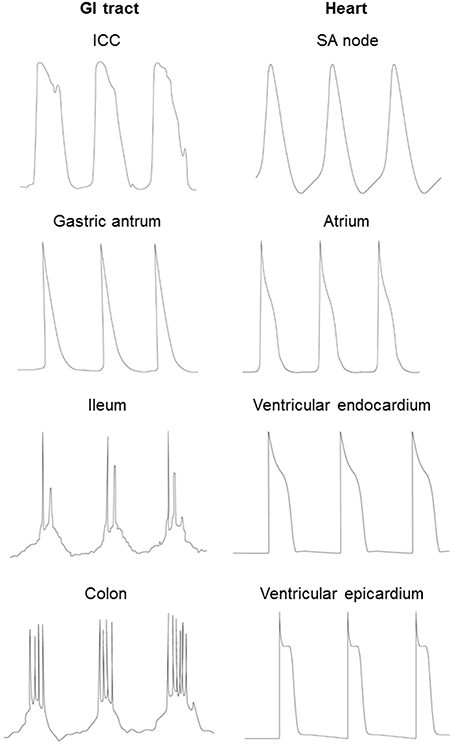
Both the cardiac AP and the GI slow wave result from the sequential opening and closing of ion channels that are located in the plasma membrane (Roden et al., 2002). In both systems, differences in the expression and properties of ion channels are responsible for the heterogeneities in signal waveforms found in different cell types and ensures its normal unidirectional spread through the respective conduction pathways (Figure 2; Nerbonne and Guo, 2002; van Helden et al., 2010; Tse and Yeo, 2015). In the heart, the different cell types are pacemaker cells (sinoatrial and atrioventricular nodes) and cardiomyocytes (epicardium, myocardium and endocardium). In the GI tract, three distinct population of cells are found: smooth muscle, ICC, PDGFR-positive cells, as discussed above (Sanders et al., 2006).
Generation of the APs or slow waves is dependent upon voltage-gated conductances, and their durations are determined by the balance between inward and outward currents (Figure 2). APs of cardiomyocytes have a fast upstroke followed by a spike and plateau morphology, and then delayed repolarization back to the resting membrane potential. Nodal cells have a pacemaker current leading to spontaneous depolarization before the upstroke of the AP.By contrast, in the GI tract, electric activity produced by a gastric antral cell has a triangular morphology, with a fast upstroke followed by rapid repolarization. Smooth muscle in small intestine and colon generate slow waves, which have two phases: an initial depolarizing phase, the pacemaker potential, generated by the ICC-MY (Dickens et al., 1999). The second phase is produced by ICC-IM within the smooth muscle (Dickens et al., 2001). Some cells are capable of producing regenerative Ca2+-mediated spikes superimposed upon the slow wave (Suzuki and Hirst, 1999; Lammers and Slack, 2001). The durations of both cardiac APs and GI slow waves can decrease with increasing pacing rates. In cardiac tissue, this was originally described by the restitution hypothesis, which related AP duration to the previous diastolic interval (DI) (Nolasco and Dahlen, 1968). Increased restitution gradient can lead to the formation of APD alternans, wave break and ventricular arrhythmias (Hsieh et al., 2009, 2015; Tse et al., 2016c,e). However, in the GI smooth muscle cells, the restitution curve is flat (Aliev et al., 2000) and therefore breaks in the slow wave conduction are unlikely to be attributed to steep APD restitution.
Conduction of the electrical excitation through the cardiac myocardium and along the GI tract involve mechanisms common to both systems, but additional differences are observed (Saez et al., 2003; van Helden et al., 2010; Veeraraghavan et al., 2014d, 2015; Tse and Yeo, 2015). In the heart, action potential conduction was originally described by the cable theory, positing that the myocardium functions as a syncytium coupled by resistive pathways with capacitances due to the phospholipid bilayer of the cell membranes (Weidmann, 1952). The related core conductor equation makes no assumptions on the structure of the cell membrane. Subsequent experiments revealed that the myocardium consisted of individual cardiomyocytes, which are coupled to each other via nexuses termed gap junctions (Dewey and Barr, 1962; Barr et al., 1965). The latter are hexagonal proteins originally described in the Mauthner cell synapses in goldfish brains (Robertson, 1963). They are non-specific pores that allow the spread of ions and molecules up to 1 kDa in molecular mass (Harris, 2001; Weber et al., 2004). Each gap junction consists of two hemi-channels termed connexons, with each connexon made of six connexin (Cx) subunits. Over 20 Cx isoforms have been identified thus far. In cardiac tissue, Cx43 is expressed in the atria and ventricles (Koval et al., 2014). Cx40 are expressed in the atria and His-Purkinje system and responsible for increasing electrical coupling and CV of the APs in this area (Schrickel et al., 2009). Cx45 alone is sufficient for maintaining electrical conduction through the atrioventricular node, and is also found in the ventricles (Schrickel et al., 2009). Cx30.2 has the lowest unitary conductance out of the cardiac connexins, and its expression in atrioventricular node is responsible for decreasing the CV of AP propagation through this node (Kreuzberg et al., 2006). Other isoforms of Cx, such as 30, 37, and 46 are also found throughout the heart, the reader is referred to this article here for additional information on their respective functions (Verheule and Kaese, 2013). Recent studies have proposed the role of ephaptic coupling in mediating cardiac conduction (Rhett and Gourdie, 2012; Lin and Keener, 2013; Rhett et al., 2013; Veeraraghavan et al., 2014d, 2015, 2014b,c; George et al., 2015).
In the GI tract, two schools of thought were proposed to describe the conduction of slow waves through the GI tract, the core conductor and the coupled oscillator theories (Publicover and Sanders, 1989; Daniel et al., 1994). Like in the heart, the core conductor theory was used to describe electrical propagation along the GI tract, but this theory alone does not fully explain the differing waveforms and frequencies as well as periods of quiescence observed for slow wave propagation. These can be explained by the oscillator model, a clock governs the frequency and a transformer determines the morphology of the waveform (Bardakjian and Diamant, 1994). Two popular models are the Hodgkin-Huxley and Fitzhugh-Nagumo oscillators, incorporating features of both oscillator and conductor theories (Aliev et al., 2000). They accurately describing the physiology of slow wave propagation: changes in frequency along the GI tract, synchronization in short distances and desynchronization over long distances (Aliev et al., 2000; Lin et al., 2006). Recent mapping experiments demonstrated that the pyloric junction is the dominant pacemaker, initiating slow waves that propagate through the small intestine, with decreasing CV and spontaneous conduction blocks along its length (Lammers and Stephen, 2008). Gap junctions may be responsible for facilitating the spread of electrical activity within the ICC network and between different cell types (Daniel et al., 1998; Daniel and Wang, 1999; Seki and Komuro, 2001; Cousins et al., 2003; Daniel, 2004; Hanani et al., 2005). They regulate many aspects of GI physiology, including motility, stomach acid secretion, mucosal barrier function and mediate oral tolerance by antigen transfer (Iino et al., 2001; Ey et al., 2009; Fukushi et al., 2014; Mazzini et al., 2014). Thus, Cx26, 32, and 43 have been found in the stomach, predominantly in the antrum and greater curvature, but rarely in the fundus and pylorus (Maes et al., 2015). Experiments in mouse intestine showed that they may not be necessary for conduction of pacing activity from the ICC to circular smooth muscle (Cho and Daniel, 2005; Daniel et al., 2007). However, gap junction blocker carbonaloxone did decrease the amplitude, frequency and velocity of circular smooth muscle contraction and within the circular smooth muscle network (Schultz et al., 2003), suggesting a modulatory role of gap junctions. However, another blocker, heptanol, did not affect these parameters (Parsons and Huizinga, 2015). Other experiments have shown that gap junctions are not needed for electrical coupling, but instead an electric field mechanism, i.e., ephaptic coupling, is sufficient (Vigmond and Bardakjian, 1995; Sperelakis and McConnell, 2002). It is thus likely that gap junctions play a diminished role in electrical conduction through the GI tract and play more important roles in metabolic regulation, as opposed to electrical coupling in cardiac tissue. Moreover, anisotropic conduction is more important in the heart compared to the GI tract (Spach, 1999; Lammers et al., 2002). In cardiac tissue, CV is much more rapid in the longitudinal compared to the transverse direction. In the gut, the presence of an ICC network means that propagation does not differ significantly in the longitudinal and circumferential directions (Huizinga et al., 1995). In one study, CV was more rapid in the circular compared to longitudinal direction (Lammers et al., 2002), although no significant anisotropy was observed in a different study (Gao et al., 2013). Exacerbations in anisotropic conduction can lead to arrhythmias in both the heart and the GI tract (Allessie et al., 1989; Angeli et al., 2013).
Electrical excitation of cardiac muscle and GI smooth muscle cells induce mechanical contractions via excitation-contraction coupling. In the heart, activation of cardiac muscle elicits an AP, during which Ca2+ entry through L-type Ca2+ channels provides the necessary current for transverse tubular depolarization and subsequent Ca2+-induced Ca2+ release from the sarcoplasmic reticulum via the ryanodine receptors (Ozaki et al., 1991). By contrast, phasic contractions in the GI tract involve coordination within the SIP syncytium (Sanders et al., 2014). GI smooth muscle cells receive direct depolarizing current from the ICC for L-type Ca2+ channel activation. Its distension also causes depolarization by stretch-sensitive ion channels (Bülbring, 1955; Thorneloe and Nelson, 2005; Kraichely and Farrugia, 2007). Moreover, the migrating myoelectric complex is a band of excitation that travels slowly across the stomach and intestine, and within this band, slow wave-driven peristalsis occurs (Hall et al., 1982; Sarna et al., 1983; Siegle and Ehrlein, 1987).
Studying Cardiac and GI Electrophysiology in Humans and Animal Models
In clinical practice, it is possible to record electrical activity of the heart and the stomach from the skin surface non-invasively using electrocardiography and electrogastrography. Magnetic resonance imaging is an excellent non-invasive method for characterization of structural and metabolic abnormalities in both the cardiovascular and gastrointestinal systems (Leung et al., 2004; Vassiliou et al., 2014; Chan et al., 2015; Tse et al., 2015a,b). Recent developments have focused on the measurement of magnetic signals for studying electrical characteristics in these organs. Thus, magnetocardiography can be used to diagnose and predict the risk of cardiac arrhythmias (Steinhoff et al., 2004; Sato et al., 2012; Kwong et al., 2013; Ito et al., 2014; Yoshida et al., 2015). Similarly, magnetogastrography can be used to study the electrophysiological basis of GI motility disorders (Bradshaw et al., 2016).
Many physiological findings described above have been derived from experiments conducted in animal models, but their limitations must be recognized. Significant differences in physiology are observed between different species, largely due to isometric scaling. For example, heart rate in humans are between 60 and 100 bpm but occurs at 600 bpm in mice (Tse et al., 2016c). Similarly, for the gastrointestinal tract, peristalsis occurs at a frequency of 8–12 bpm in humans but 30–40 bpm in mice (Christensen et al., 1966; Huizinga et al., 1995). There are also differences in the cellular electrophysiology. Thus, atrial and ventricular APs in mouse hearts have a triangular morphology without the characteristic plateau phase seen in humans and other species such as guinea pigs (Nerbonne and Kass, 2005; Tse et al., 2012, 2016c,d,i; Osadchii, 2014a,b, 2016). In the GI tract, the morphology of gastric slow waves between mouse and humans is largely similar, and in the small intestine and colon, slow waves with superimposed spikes are observed in both species (Sanders et al., 2014), meaning that results from mouse studies are highly translatable to human GI electrophysiology. Animal models are useful as a variety of experimental recording techniques can be used. Thus, cardiac electrical recordings can be obtained from single cells using microelectrode techniques intracellularly (Sano et al., 1959; Allessie et al., 1976), or from the intact organ by extracellular recording techniques such as the monophasic action potential or bipolar electrogram methods (Vigmond and Leon, 1999; Vigmond, 2005; Vigmond et al., 2009; Tse et al., 2016h), which are techniques used routinely in cardiac electrophysiological studies (Yoshida et al., 2012). Optical mapping can provide high resolution of electrical activation patterns and used to determine depolarization or repolarization abnormalities locally in both organ systems (Hsieh et al., 2009; O'Grady et al., 2012; Angeli et al., 2013).
Clinical Relevance: Motility Disorders and Targets for Future Therapy
A number of diseases can arise from disturbances in GI electrophysiology, which is summarized in Table 1. Starting from the top of the GI tract, gastroesophageal reflux disease occurs when the reflux of gastric contents causes troublesome symptoms and has been associated with a loss of ICC in the esophagogastric junction (Shafik et al., 2005). Achalasia, defined as absence of esophageal peristalsis and impaired relaxation of the lower esophageal sphincter, has been attributed to a loss of ICC-IM in the lower esophagus (Chen et al., 2013). Allgrove syndrome is a rare autosomal recessive disorder characterized by a triad of achalasia, alacrima and Addsonian features. The underlying pathology involves lymphocytic infiltration of myenteric plexus and may involve loss of ICC-MY or ICC-IM (Khelif et al., 2003). Gastroparesis, i.e., paralysis of the stomach muscle, is characterized by delayed emptying and caused by abnormal slow wave initiation secondary to ICC-MY loss (O'Grady et al., 2012). Pyloric stenosis, which affects infants, is characterized by hyperplasia and hypertrophy of the pyloric muscle. As far back as 1969, it was suggested that the “hypertrophy” of the pylorus may be caused by spasm of the pyloric muscle, which was associated with abnormal excitation of the gastric wall muscle or disturbance of propagation in the pyloric portion (Watanuki et al., 1969). Reduced number of ICC has also been implicated in this disorder (Langer et al., 1995; Vanderwinden et al., 1996). Functional dyspepsia is persistent epigastric pain and fullness and early satiety without an organic cause. Some proposed mechanisms are abnormal gastric motor function, altered visceral sensitivity and Helicobacter pylori infection. Loss-of-function in the SCN5A gene encoding for the α-subunit of the Na+ channel is known to cause Brugada syndrome, a primarily right ventricular arrhythmogenic disorder (Brugada and Brugada, 1992). A recent study of patients with either Brugada syndrome or functional dyspepsia revealed that some Brugada patients with SCN5A mutations have functional dyspepsia (Jung et al., 2012). Thus, the role of abnormalities in Na+ and other channels in functional dyspepsia awaits further clarification.
By contrast, idiopathic rapid gastric emptying could involve increased gastric contractility or reduced resistance to gastric outflow at the pylorus (Bharucha et al., 2011). Its mechanism is uncertain but could conceivably involve ion channel dysfunction, such as stretch-activated channels involved in mechanosensation or any of the ion channels mediating gastric slow wave initiation and propagation. In unexplained nausea and vomiting, both neuropathic changes and abnormal gastric electrophysiology have been detected (Abell et al., 2009). Gastric serosal electrophysiological study using serosal electrogastrographic recordings demonstrated recurrent arrhythmias of abnormal wave propagation and higher frequency in the distal stomach. The precise electrophysiological mechanism of arrhythmia, such as focal activity or reentry, will need to be elucidated. Like idiopathic rapid gastric emptying and unexplained nausea and vomiting, arrhythmogenesis is also observed in mesenteric ischaemia, but this is a consequence of rather than the cause (Seidel et al., 1999b). The problem of this condition is that it has a high mortality, due to the disease process itself and the fact that diagnosis is often delayed because of non-specific symptoms. Thus, there is a need of developing means to detect ischaemia early. A highly sensitive magnetometer, such as superconducting quantum interference device (SQUID), could detect a decrease in basic electrical rhythm frequency, which is suggestive of ischaemia (Seidel et al., 1999a). Indeed, a recent study used magnetoenterographic imaging with a SQUID biomagnetometer to measure spatiotemporal return map in pigs. Before induction of ischemia, no intestinal arrhythmias were observed, and the spatiotemporal return map perimeter was relatively constant in time. However, after mesenteric artery ligation, arrhythmias were detected, and associated with spatiotemporal return map perimeter showing statistically significant variations in information dimensionality (Irimia and Wikswo, 2008). However, the need to use a magnetically shielded room and high costs have prohibited the use of SQUID in clinical practice.
IBS is a chronic relapsing and remitting functional disorder of the GI tract. It consists of a triad of altered bowel habits, bloating, and abdominal pain without an organic cause (Sinagra et al., 2016). It can take on a diarrhea- or constipation- predominant phenotype, which must be distinguished from functional diarrhea (Dellon and Ringel, 2006) or constipation, respectively (Dellon and Ringel, 2006; Camilleri, 2011; Cheung et al., 2013). Loss of ICC has been suggested as a pathophysiological mechanism underlying this condition (Eshraghian and Eshraghian, 2011). Mutations in Na+ channels have been implicated in IBS (Saito et al., 2009). There is increasing evidence that altered microbiota profile in the intestines may be responsible for the symptoms of irritable bowel syndrome (Tana et al., 2010; Ng et al., 2013), and may increase the activity of intestinal Cl− channels (Chang and Talley, 2010). Inflammatory changes in IBS (Der et al., 2000) can alter ICC network and result in electrophysiological remodeling (Akbarali et al., 2010). Hyperexcitability of nociceptive dorsal root ganglia could explain the abdominal pain (Beyak and Vanner, 2005). Finally, the following disorders involve reduced motion of the colon: Hirschsprung disease (Yamataka et al., 1995), chronic pseudo-obstruction (Feldstein et al., 2003; Struijs et al., 2008), slow transit constipation (Lyford et al., 2002) and colonic hypomotility associated with anorectal malformations (Kenny et al., 1998). In each of these condition, loss or abnormal ICCs has been demonstrated.
How can elucidation of the electrophysiological mechanisms underlying GI motility disorders enable the development of better treatment? Ion channels have been targets for anti-arrhythmic therapy in the heart, and have enormous potential to be targeted for the management of GI motility disorders. For example, lubiprostone is an agonist of the chloride channel protein 2, which promotes the Cl− efflux out of intestinal cells into the lumen, for the management of functional constipation or constipation-predominant IBS (Camilleri et al., 2006; Andresen et al., 2007). Crofelemer, by contrast, inhibits both chloride channel protein 2 and also the cystic fibrosis transmembrane regulator channel to reduce Cl− efflux. It has been licensed for HIV-associated diarrhea (Yeo et al., 2013), and has therapeutic potential in conditions such as functional diarrhea or diarrhea-predominant IBS (Manabe et al., 2010). TRPCs, which mediate nociception, may be useful targets for managing abdominal pain symptoms in IBS (Hicks, 2006). Analogous to bradyarrhythmias such as sick sinus syndrome or atrioventricular blocks in the heart, pharmacotherapy or pacemakers can be used to manage GI hypomotility disorders, depending on the disease severity. In gastroparesis, dopamine receptor antagonists with prokinetic effects such as metoclopramide and domperidone, or macrolide antibiotics, can be used. In more severe cases, pacemaker implantation for gastric electrical stimulation is an effective treatment with improvements in gastric emptying rates (Abrahamsson, 2007). Colonic electrical stimulation has demonstrated efficacy of increasing colon transit time in animal studies, and is a potential treatment option for chronic functional constipation or constipation-predominant IBS refractory to medical therapy in humans (Chen S. et al., 2016). For fast rhythms in the heart, anti-tachycardia pacing is a treatment option (Aonuma et al., 1985); whether this could also be useful in gastrointestinal tachyarrhythmias remains to be elucidated.
In conclusion, most GI motility disorders have an electrophysiological basis, and ion channel dysfunction is increasingly recognized as potential causes. GI electrophysiology is a fascinating subject with much to be learnt. A detailed understanding of the complex spatiotemporal dynamics of GI excitation will enable the development of novel therapy for managing GI motility disorders effectively.
Author Contributions
GT design of manuscript; drafted and critically revised the manuscript for important intellectual content; preparation of figures. EL acquired and interpreted primary research papers; critically revised the manuscript for important intellectual content; preparation of figures. VT acquired and interpreted primary research papers; critically revised the manuscript for important intellectual content. JY analyzed and interpreted primary research papers; critically revised the manuscript for important intellectual content. SW drafted and critically revised the manuscript for important intellectual content; preparation of figures. All authors approved the final version, ensured that the text is accurate and agreed to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
GT was awarded a doctoral training award (DTA) from the Biotechnology and Biological Sciences Research Council (BBSRC) at the University of Cambridge. SW is grateful to the Croucher Foundation for their generous support.
References
Abell, T. L., Familoni, B., Voeller, G., Werkman, R., Dean, P., Waters, B., et al. (2009). Electrophysiologic, morphologic, and serologic features of chronic unexplained nausea and vomiting: lessons learned from 121 consecutive patients. Surgery 145, 476–485. doi: 10.1016/j.surg.2008.12.006
Abrahamsson, H. (2007). Treatment options for patients with severe gastroparesis. Gut 56, 877–883. doi: 10.1136/gut.2005.078121
Akbarali, H. I. G., Hawkins, E., Ross, G. R., and Kang, M. (2010). Ion channel remodeling in gastrointestinal inflammation. Neurogastroenterol. Motil. 22, 1045–1055. doi: 10.1111/j.1365-2982.2010.01560.x
Aliev, R. R., Richards, W., and Wikswo, J. P. (2000). A simple nonlinear model of electrical activity in the intestine. J. Theor. Biol. 204, 21–28. doi: 10.1006/jtbi.2000.1069
Allessie, M. A., Bonke, F. I., and Schopman, F. J. (1976). Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block, as studied with multiple microelectrodes. Circ. Res. 39, 168–177. doi: 10.1161/01.RES.39.2.168
Allessie, M. A., Schalij, M. J., Kirchhof, C. J., Boersma, L., Huybers, M., and Hollen, J. (1989). Experimental electrophysiology and arrhythmogenicity. Anisotropy and ventricular tachycardia. Eur. Heart J. 10, 2–8. doi: 10.1093/eurheartj/10.suppl_E.2
Andresen, V., Camilleri, M., Busciglio, I. A., Grudell, A., Burton, D., McKinzie, S., et al. (2007). Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 133, 761–768. doi: 10.1053/j.gastro.2007.06.067
Angeli, T. R., O'Grady, G., Du, P., Paskaranandavadivel, N., Pullan, A. J., Bissett, I. P., et al. (2013). Circumferential and functional re-entry of in vivo slow-wave activity in the porcine small intestine. Neurogastroenterol. Motil. 25, e304–e314. doi: 10.1111/nmo.12085
Aonuma, K., Rozanski, J. J., Barold, S. S., DeWitt, P. L., Gosselin, A. J., and Lister, J. W. (1985). Externally activated antitachycardia pacemaker with noninvasive electrophysiologic re-testing capability. Pacing Clin. Electrophysiol. 8, 215–224. doi: 10.1111/j.1540-8159.1985.tb05752.x
Bardakjian, B. L., and Diamant, N. E. (1994). A mapped clock oscillator model for transmembrane electrical rhythmic activity in excitable cells. J. Theor. Biol. 166, 225–235. doi: 10.1006/jtbi.1994.1020
Barr, L., Dewey, M. M., and Berger, W. (1965). Propagation of action potentials and the structure of the nexus in cardiac muscle. J. Gen. Physiol. 48, 797–823. doi: 10.1085/jgp.48.5.797
Baruscotti, M., Bucchi, A., and Difrancesco, D. (2005). Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol. Ther. 107, 59–79. doi: 10.1016/j.pharmthera.2005.01.005
Beckett, E. A., McGeough, C. A., Sanders, K. M., and Ward, S. M. (2003). Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation. J. Physiol. 553, 545–559. doi: 10.1113/jphysiol.2003.050419
Beckett, E. A., Takeda, Y., Yanase, H., Sanders, K. M., and Ward, S. M. (2005). Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J. Comp. Neurol. 493, 193–206. doi: 10.1002/cne.20746
Beyak, M. J., and Vanner, S. (2005). Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol. Motil. 17, 175–186. doi: 10.1111/j.1365-2982.2004.00596.x
Bharucha, A. E., Manduca, A., Lake, D. S., Fidler, J., Edwards, P., Grimm, R. C., et al. (2011). Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol. Motil. 23, 617–e252. doi: 10.1111/j.1365-2982.2011.01710.x
Bradshaw, L. A., Cheng, L. K., Chung, E., Obioha, C. B., Erickson, J. C., Gorman, B. L., et al. (2016). Diabetic gastroparesis alters the biomagnetic signature of the gastric slow wave. Neurogastroenterol. Motil. 28, 837–848. doi: 10.1111/nmo.12780
Brugada, P., and Brugada, J. (1992). Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 20, 1391–1396. doi: 10.1016/0735-1097(92)90253-J
Bülbring, E. (1955). Correlation between membrane potential, spike discharge and tension in smooth muscle. J. Physiol. 128, 200–221. doi: 10.1113/jphysiol.1955.sp005299
Camilleri, M. (2011). New treatment options for chronic constipation: mechanisms, efficacy and safety. Can. J. Gastroenterol. 25, 29B–35B. doi: 10.1155/2011/527583
Camilleri, M., Bharucha, A. E., Ueno, R., Burton, D., Thomforde, G. M., Baxter, K., et al. (2006). Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G942–G947. doi: 10.1152/ajpgi.00264.2005
Chan, R., Wong, V. W., Chu, W. C., Wong, G. L., Li, L. S., Leung, J., et al. (2015). Diet-quality scores and prevalence of nonalcoholic fatty Liver Disease: a population study using proton-magnetic resonance spectroscopy. PLoS ONE 10:e0139310. doi: 10.1371/journal.pone.0139310
Chang, J. Y., and Talley, N. J. (2010). Current and emerging therapies in irritable bowel syndrome: from pathophysiology to treatment. Trends Pharmacol. Sci. 31, 326–334. doi: 10.1016/j.tips.2010.04.008
Chen, J. H., Wang, X. Y., Liu, L. W., Yu, W., Yu, Y., Zhao, L., et al. (2013). On the origin of rhythmic contractile activity of the esophagus in early achalasia, a clinical case study. Front. Neurosci. 7:77. doi: 10.3389/fnins.2013.00077
Chen, S., Liu, L., Guo, X., Yao, S., Li, Y., Chen, S., et al. (2016). Effects of colonic electrical stimulation using different individual parameter patterns and stimulation sites on gastrointestinal transit time, defecation, and food intake. Int. J. Colorectal Dis. 31, 429–437. doi: 10.1007/s00384-015-2457-6
Chen, Z., Sun, B., Tse, G., Jiang, J., and Xu, W. (2016). Reversibility of both sinus node dysfunction and reduced HCN4 mRNA expression level in an atrial tachycardia pacing model of tachycardia-bradycardia syndrome in rabbit hearts. Int. J. Clin. Exp. Pathol. 9.
Cheng, L. K. (2015). Slow wave conduction patterns in the stomach: from waller's foundations to current challenges. Acta Physiol. 213, 384–393. doi: 10.1111/apha.12406
Cheung, C. K., Lee, Y. Y., Chan, Y., Cheong, P. K., Law, W. T., Lee, S. F., et al. (2013). Decreased Basal and postprandial plasma serotonin levels in patients with functional dyspepsia. Clin. Gastroenterol. Hepatol. 11, 1125–1129. doi: 10.1016/j.cgh.2013.03.026
Cheung, C. K., and Wu, J. C. (2013). Role of ghrelin in the pathophysiology of gastrointestinal disease. Gut Liver 7, 505–512. doi: 10.5009/gnl.2013.7.5.505
Cho, W. J., and Daniel, E. E. (2005). Proteins of interstitial cells of Cajal and intestinal smooth muscle, colocalized with caveolin-1. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G571–G585. doi: 10.1152/ajpgi.00222.2004
Choy, L., Yeo, J. M., Tse, V., Chan, S. P., and Tse, G. (2016). Cardiac disease and arrhythmogenesis: mechanistic insights from mouse models. Int. J. Cardiol. Heart Vasc. 12, 1–10. doi: 10.1016/j.ijcha.2016.05.005
Christensen, J., Schedl, H. P., and Clifton, J. A. (1966). The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with variety of diseases. Gastroenterology 50, 309–315.
Cousins, H. M., Edwards, F. R., Hickey, H., Hill, C. E., and Hirst, G. D. (2003). Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J. Physiol. 550, 829–844. doi: 10.1113/jphysiol.2003.042176
Daniel, E. E. (2004). Communication between interstitial cells of Cajal and gastrointestinal muscle. Neurogastroenterol. Motil. 16, 118–122. doi: 10.1111/j.1743-3150.2004.00486.x
Daniel, E. E., Bardakjian, B. L., Huizinga, J. D., and Diamant, N. E. (1994). Relaxation oscillator and core conductor models are needed for understanding of GI electrical activities. Am. J. Physiol. 266, G339–G349.
Daniel, E. E., and Wang, Y. F. (1999). Gap junctions in intestinal smooth muscle and interstitial cells of Cajal. Microsc. Res. Tech. 47, 309–320.
Daniel, E. E., Wang, Y. F., and Cayabyab, F. S. (1998). Role of gap junctions in structural arrangements of interstitial cells of Cajal and canine ileal smooth muscle. Am. J. Physiol. 274, G1125–G1141.
Daniel, E. E., Yazbi, A. E., Mannarino, M., Galante, G., Boddy, G., Livergant, J., et al. (2007). Do gap junctions play a role in nerve transmissions as well as pacing in mouse intestine? Am. J. Physiol. Gastrointest. Liver Physiol. 292, G734–G745. doi: 10.1152/ajpgi.00428.2006
Dellon, E. S., and Ringel, Y. (2006). Treatment of functional diarrhea. Curr. Treat. Options Gastroenterol. 9, 331–342. doi: 10.1007/s11938-006-0015-6
Der, T., Bercik, P., Donnelly, G., Jackson, T., Berezin, I., Collins, S. M., et al. (2000). Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology 119, 1590–1599. doi: 10.1053/gast.2000.20221
Dewey, M. M., and Barr, L. (1962). Intercellular connection between smooth muscle cells: the nexus. Science 137, 670–672. doi: 10.1126/science.137.3531.670-a
Diamant, N. E., and Bortoff, A. (1969). Nature of the intestinal slow-wave frequency gradient. Am. J. Physiol. 216, 301–307.
Dickens, E. J., Edwards, F. R., and Hirst, G. D. (2001). Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J. Physiol. 531, 827–833. doi: 10.1111/j.1469-7793.2001.0827h.x
Dickens, E. J., Hirst, G. D., and Tomita, T. (1999). Identification of rhythmically active cells in guinea-pig stomach. J. Physiol. 514, 515–531. doi: 10.1111/j.1469-7793.1999.515ae.x
DiFrancesco, D. (1993). Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 55, 455–472. doi: 10.1146/annurev.ph.55.030193.002323
Eshraghian, A., and Eshraghian, H. (2011). Interstitial cells of Cajal: a novel hypothesis for the pathophysiology of irritable bowel syndrome. Can. J. Gastroenterol. 25, 277–279. doi: 10.1155/2011/478370
Ey, B., Eyking, A., Gerken, G., Podolsky, D. K., and Cario, E. (2009). TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J. Biol. Chem. 284, 22332–22343. doi: 10.1074/jbc.M901619200
Farrugia, G., Lei, S., Lin, X., Miller, S. M., Nath, K. A., Ferris, C. D., et al. (2003). A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc. Natl. Acad. Sci. U.S.A. 100, 8567–8570. doi: 10.1073/pnas.1431233100
Faussone-Pellegrini, M. S., and Cortesini, C. (1985a). The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J. Submicrosc. Cytol. 17, 673–685.
Faussone-Pellegrini, M. S., and Cortesini, C. (1985b). Ultrastructural features and localization of the interstitial cells of Cajal in the smooth muscle coat of human esophagus. J. Submicrosc. Cytol. 17, 187–197.
Feldstein, A. E., Miller, S. M., El-Youssef, M., Rodeberg, D., Lindor, N. M., Burgart, L. J., et al. (2003). Chronic intestinal pseudoobstruction associated with altered interstitial cells of cajal networks. J. Pediatr. Gastroenterol. Nutr. 36, 492–497. doi: 10.1097/00005176-200304000-00016
Fujita, A., Takeuchi, T., Saitoh, N., Hanai, J., and Hata, F. (2001). Expression of Ca(2+)-activated K(+) channels, SK3, in the interstitial cells of Cajal in the gastrointestinal tract. Am. J. Physiol. Cell Physiol. 281, C1727–C1733.
Fukushi, Y., Sakurai, T., and Terakawa, S. (2014). Cell-to-cell propagation of intracellular signals fluorescently visualized with acridine orange in the gastric glands of guinea pigs. Biochem. Biophys. Res. Commun. 447, 38–43. doi: 10.1016/j.bbrc.2014.03.095
Gao, J., Du, P., O'Grady, G., Archer, R., Farrugia, G., Gibbons, S. J., et al. (2013). Numerical metrics for automated quantification of interstitial cell of Cajal network structural properties. J. R. Soc. Interface 10:20130421. doi: 10.1098/rsif.2013.0421
George, S. A., Sciuto, K. J., Lin, J., Salama, M. E., Keener, J. P., Gourdie, R. G., et al. (2015). Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Arch. 467, 2287–2297. doi: 10.1007/s00424-015-1698-0
Goldblum, J. R., Whyte, R. I., Orringer, M. B., and Appelman, H. D. (1994). Achalasia. A morphologic study of 42 resected specimens. Am. J. Surg. Pathol. 18, 327–337. doi: 10.1097/00000478-199404000-00001
Gomez-Pinilla, P. J., Gibbons, S. J., Bardsley, M. R., Lorincz, A., Pozo, M. J., Pasricha, P. J., et al. (2009). Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370–G1381. doi: 10.1152/ajpgi.00074.2009
Hall, K. E., El-Sharkawy, T. Y., and Diamant, N. E. (1982). Vagal control of migrating motor complex in the dog. Am. J. Physiol. Gastrointest. Liver Physiol. 243, G276–G284.
Hanani, M., Farrugia, G., and Komuro, T. (2005). Intercellular coupling of interstitial cells of cajal in the digestive tract. Int. Rev. Cytol. 242, 249–282. doi: 10.1016/S0074-7696(04)42006-3
Harris, A. L. (2001). Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34, 325–472. doi: 10.1017/S0033583501003705
Hatton, W. J., Mason, H. S., Carl, A., Doherty, P., Latten, M. J., Kenyon, J. L., et al. (2001). Functional and molecular expression of a voltage-dependent K(+) channel (Kv1.1) in interstitial cells of Cajal. J. Physiol. 533, 315–327. doi: 10.1111/j.1469-7793.2001.0315a.x
Hicks, G. A. (2006). TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterol. Motil. 18, 590–594. doi: 10.1111/j.1365-2982.2006.00823.x
Homma, S., Satoh, K., Matsuo, H., Yagi, M., Hasegawa, J., Maruta, T., et al. (2004). Electrogastrographic activity in patients who received proximal gastrectomy plus jejunal interposition or total gastrectomy plus jejunal interposition. J. Smooth Muscle Res. 40, 271–280. doi: 10.1540/jsmr.40.271
Hsieh, Y. C., Lin, J. C., Hung, C. Y., Li, C. H., Lin, S. F., Yeh, H. I., et al. (2015). Gap junction modifier rotigaptide decreases the susceptibility to ventricular arrhythmia by enhancing conduction velocity and suppressing discordant alternans during therapeutic hypothermia in isolated rabbit hearts. Heart Rhythm 13, 251–261. doi: 10.1016/j.hrthm.2015.07.023
Hsieh, Y. C., Lin, S. F., Lin, T. C., Ting, C. T., and Wu, T. J. (2009). Therapeutic hypothermia (30 degrees C) enhances arrhythmogenic substrates, including spatially discordant alternans, and facilitates pacing-induced ventricular fibrillation in isolated rabbit hearts. Circ. J. 73, 2214–2222. doi: 10.1253/circj.CJ-09-0432
Huizinga, J. D., Golden, C. M., Zhu, Y., and White, E. J. (2004). Ion channels in interstitial cells of Cajal as targets for neurotransmitter action. Neurogastroenterol. Motil. 16 (Suppl. 1), 106–111. doi: 10.1111/j.1743-3150.2004.00484.x
Huizinga, J. D., Thuneberg, L., Klüppel, M., Malysz, J., Mikkelsen, H. B., and Bernstein, A. (1995). W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349. doi: 10.1038/373347a0
Iino, S., Asamoto, K., and Nojyo, Y. (2001). Heterogeneous distribution of a gap junction protein, connexin43, in the gastroduodenal junction of the guinea pig. Auton. Neurosci. 93, 8–13. doi: 10.1016/S1566-0702(01)00320-4
Irimia, A., and Wikswo, J. P. Jr. (2008). Gastrointestinal arrhythmias are associated with statistically significant fluctuations in systemic information dimension. Physiol. Meas. 29, N33–N40. doi: 10.1088/0967-3334/29/5/N01
Ito, Y., Shiga, K., Yoshida, K., Ogata, K., Kandori, A., Inaba, T., et al. (2014). Development of a magnetocardiography-based algorithm for discrimination between ventricular arrhythmias originating from the right ventricular outflow tract and those originating from the aortic sinus cusp: a pilot study. Heart Rhythm 11, 1605–1612. doi: 10.1016/j.hrthm.2014.05.032
Jin, N. G., Koh, S. D., and Sanders, K. M. (2009). Caffeine inhibits nonselective cationic currents in interstitial cells of Cajal from the murine jejunum. Am. J. Physiol. Cell Physiol. 297, C971–C978. doi: 10.1152/ajpcell.00155.2009
Ju, Y.-K., Chu, Y., Chaulet, H., Lai, D., Gervasio, O. L., Graham, R. M., et al. (2007). Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ. Res. 100, 1605–1614. doi: 10.1161/CIRCRESAHA.107.152181
Jung, K. T., Park, H., Kim, J. H., Shin, D. J., Joung, B. Y., Lee, M. H., et al. (2012). The relationship between gastric myoelectric activity and SCN5A mutation suggesting sodium channelopathy in patients with brugada syndrome and functional dyspepsia - a pilot study. J. Neurogastroenterol. Motil. 18, 58–63. doi: 10.5056/jnm.2012.18.1.58
Kelly, K. A., and Code, C. F. (1971). Canine gastric pacemaker. Am. J. Physiol. Legacy Content 220, 112–118.
Kenny, S. E., Connell, M. G., Rintala, R. J., Vaillant, C., Edgar, D. H., and Lloyd, D. A. (1998). Abnormal colonic interstitial cells of Cajal in children with anorectal malformations. J. Pediatr. Surg. 33, 130–132. doi: 10.1016/S0022-3468(98)90379-7
Khelif, K., De Laet, M. H., Chaouachi, B., Segers, V., and Vanderwinden, J. M. (2003). Achalasia of the cardia in Allgrove's (triple A) syndrome: histopathologic study of 10 cases. Am. J. Surg. Pathol. 27, 667–672. doi: 10.1097/00000478-200305000-00010
Koh, S. D., Jun, J. Y., Kim, T. W., and Sanders, K. M. (2002). A Ca(2+)-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J. Physiol. 540, 803–814. doi: 10.1113/jphysiol.2001.014639
Koval, M., Isakson, B. E., and Gourdie, R. G. (2014). Connexins, pannexins and innexins: protein cousins with overlapping functions. FEBS Lett. 588, 1185. doi: 10.1016/j.febslet.2014.03.001
Kraichely, R. E., and Farrugia, G. (2007). Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol. Motil. 19, 245–252. doi: 10.1111/j.1365-2982.2006.00880.x
Kreuzberg, M. M., Schrickel, J. W., Ghanem, A., Kim, J. S., Degen, J., Janssen-Bienhold, U., et al. (2006). Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc. Natl. Acad. Sci. U.S.A. 103, 5959–5964. doi: 10.1073/pnas.0508512103
Kwong, J. S., Leithäuser, B., Park, J. W., and Yu, C. M. (2013). Diagnostic value of magnetocardiography in coronary artery disease and cardiac arrhythmias: a review of clinical data. Int. J. Cardiol. 167, 1835–1842. doi: 10.1016/j.ijcard.2012.12.056
Lakatta, E. G., and DiFrancesco, D. (2009). JMCC Point-Counterpoint: what keeps us ticking, a funny current, a Calcium clock, or both? J. Mol. Cell. Cardiol. 47, 157–170. doi: 10.1016/j.yjmcc.2009.03.022
Lakatta, E. G., Vinogradova, T., Lyashkov, A., Sirenko, S., Zhu, W., Ruknudin, A., et al. (2006). The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart's pacemaker. Ann. N.Y. Acad. Sci. 1080, 178–206. doi: 10.1196/annals.1380.016
Lammers, W. J., and Slack, J. R. (2001). Of slow waves and spike patches. News Physiol. Sci. 16, 138–144. doi: 10.1053/gast.2001.27713
Lammers, W. J., Slack, J. R., Stephen, B., and Pozzan, O. (2000). The spatial behaviour of spike patches in the feline gastroduodenal junction in vitro. Neurogastroenterol. Motil. 12, 467–473. doi: 10.1046/j.1365-2982.2000.00223.x
Lammers, W. J., and Stephen, B. (2008). Origin and propagation of individual slow waves along the intact feline small intestine. Exp. Physiol. 93, 334–346. doi: 10.1113/expphysiol.2007.039180
Lammers, W. J., Stephen, B., Adeghate, E., Ponery, S., and Pozzan, O. (1998). The slow wave does not propagate across the gastroduodenal junction in the isolated feline preparation. Neurogastroenterol. Motil. 10, 339–349. doi: 10.1046/j.1365-2982.1998.00113.x
Lammers, W. J., Stephen, B., Slack, J. R., and Dhanasekaran, S. (2002). Anisotropic propagation in the small intestine. Neurogastroenterol. Motil. 14, 357–364. doi: 10.1046/j.1365-2982.2002.00340.x
Lammers, W. J., Ver Donck, L., Stephen, B., Smets, D., and Schuurkes, J. A. (2009). Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1200–G1210. doi: 10.1152/ajpgi.90581.2008
Langer, J. C., Berezin, I., and Daniel, E. E. (1995). Hypertrophic pyloric stenosis: ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal. J. Pediatr. Surg. 30, 1535–1543. doi: 10.1016/0022-3468(95)90151-5
Langton, P., Ward, S. M., Carl, A., Norell, M. A., and Sanders, K. M. (1989). Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc. Natl. Acad. Sci. U.S.A. 86, 7280–7284. doi: 10.1073/pnas.86.18.7280
Lee, H. T., Hennig, G. W., Fleming, N. W., Keef, K. D., Spencer, N. J., Ward, S. M., et al. (2007). The mechanism and spread of pacemaker activity through myenteric interstitial cells of cajal in human small intestine. Gastroenterology 132, 1852–1865. doi: 10.1053/j.gastro.2007.02.049
Lees-Green, R., Du, P., O'Grady, G., Beyder, A., Farrugia, G., and Pullan, A. J. (2011). Biophysically based modeling of the interstitial cells of cajal: current status and future perspectives. Front. Physiol. 2:29. doi: 10.3389/fphys.2011.00029
Leung, W. K., Lam, W. W., Wu, J. C., So, N. M., Fung, S. S., Chan, F. K., et al. (2004). Magnetic resonance colonography in the detection of colonic neoplasm in high-risk and average-risk individuals. Am. J. Gastroenterol. 99, 102–108. doi: 10.1046/j.1572-0241.2003.04008.x
Lin, A. S.-H., Buist, M. L., Smith, N. P., and Pullan, A. J. (2006). Modelling slow wave activity in the small intestine. J. Theor. Biol. 242, 356–362. doi: 10.1016/j.jtbi.2006.03.004
Lin, J., and Keener, J. P. (2013). Ephaptic coupling in cardiac myocytes. IEEE Trans. Biomed. Eng. 60, 576–582. doi: 10.1109/TBME.2012.2226720
Lyford, G. L., He, C. L., Soffer, E., Hull, T. L., Strong, S. A., Senagore, A. J., et al. (2002). Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 51, 496–501. doi: 10.1136/gut.51.4.496
Maes, M., Cogliati, B., Crespo Yanguas, S., Willebrords, J., and Vinken, M. (2015). Roles of connexins and pannexins in digestive homeostasis. Cell. Mol. Life Sci. 72, 2809–2821. doi: 10.1007/s00018-015-1961-8
Maltsev, V. A., and Lakatta, E. G. (2013). Numerical models based on a minimal set of sarcolemmal electrogenic proteins and an intracellular Ca(2+) clock generate robust, flexible, and energy-efficient cardiac pacemaking. J. Mol. Cell. Cardiol. 59, 181–195. doi: 10.1016/j.yjmcc.2013.03.004
Manabe, N., Rao, A. S., Wong, B. S., and Camilleri, M. (2010). Emerging pharmacologic therapies for irritable bowel syndrome. Curr. Gastroenterol. Rep. 12, 408–416. doi: 10.1007/s11894-010-0124-1
Mazzini, E., Massimiliano, L., Penna, G., and Rescigno, M. (2014). Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 40, 248–261. doi: 10.1016/j.immuni.2013.12.012
McKay, C. M., Ye, J., and Huizinga, J. D. (2006). Characterization of depolarization-evoked ERG K currents in interstitial cells of Cajal. Neurogastroenterol. Motil. 18, 324–333. doi: 10.1111/j.1365-2982.2006.00764.x
Murakoshi, N., and Aonuma, K. (2013). Epidemiology of arrhythmias and sudden cardiac death in Asia. Circ. J. 77, 2419–2431. doi: 10.1253/circj.CJ-13-1129
Nerbonne, J. M., and Guo, W. (2002). Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias. J. Cardiovasc. Electrophysiol. 13, 406–409. doi: 10.1046/j.1540-8167.2002.00406.x
Nerbonne, J. M., and Kass, R. S. (2005). Molecular physiology of cardiac repolarization. Physiol. Rev. 85, 1205–1253. doi: 10.1152/physrev.00002.2005
Ng, S. C., Lam, E. F., Lam, T. T., Chan, Y., Law, W., Tse, P. C., et al. (2013). Effect of probiotic bacteria on the intestinal microbiota in irritable bowel syndrome. J. Gastroenterol. Hepatol. 28, 1624–1631. doi: 10.1111/jgh.12306
Nolasco, J. B., and Dahlen, R. W. (1968). A graphic method for the study of alternation in cardiac action potentials. J. Appl. Physiol. 25, 191–196.
O'Grady, G., Angeli, T. R., Du, P., Lahr, C., Lammers, W. J., Windsor, J. A., et al. (2012). Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 143, 589–598 e581–e583. doi: 10.1053/j.gastro.2012.05.036
O'Grady, G., Wang, T. H., Du, P., Angeli, T., Lammers, W. J., and Cheng, L. K. (2014). Recent progress in gastric arrhythmia: pathophysiology, clinical significance and future horizons. Clin. Exp. Pharmacol. Physiol. 41, 854–862. doi: 10.1111/1440-1681.12288
Osadchii, O. E. (2014a). Impact of hypokalemia on electromechanical window, excitation wavelength and repolarization gradients in guinea-pig and rabbit hearts. PLoS ONE 9:e105599. doi: 10.1371/journal.pone.0105599
Osadchii, O. E. (2014b). Impaired epicardial activation-repolarization coupling contributes to the proarrhythmic effects of hypokalaemia and dofetilide in guinea pig ventricles. Acta Physiol. 211, 48–60. doi: 10.1111/apha.12259
Osadchii, O. E. (2016). Flecainide attenuates rate adaptation of ventricular repolarization in guinea-pig heart. Scand. Cardiovasc. J. 50, 28–35. doi: 10.3109/14017431.2015.1099721
Ozaki, H., Stevens, R. J., Blondfield, D. P., Publicover, N. G., and Sanders, K. M. (1991). Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am. J. Physiol. 260, C917–925.
Parsons, S. P., and Huizinga, J. D. (2015). Effects of gap junction inhibition on contraction waves in the murine small intestine in relation to coupled oscillator theory. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G287–G297. doi: 10.1152/ajpgi.00338.2014
Powley, T. L., Wang, X. Y., Fox, E. A., Phillips, R. J., Liu, L. W., and Huizinga, J. D. (2008). Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 20, 69–79. doi: 10.1111/j.1365-2982.2007.00990.x
Publicover, N. G., and Sanders, K. M. (1989). Are relaxation oscillators an appropriate model of gastrointestinal electrical activity? Am. J. Physiol. 256, G265–274.
Rhee, P. L., Lee, J. Y., Son, H. J., Kim, J. J., Rhee, J. C., Kim, S., et al. (2011). Analysis of pacemaker activity in the human stomach. J. Physiol. 589, 6105–6118. doi: 10.1113/jphysiol.2011.217497
Rhett, J. M., and Gourdie, R. G. (2012). The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9, 619–623. doi: 10.1016/j.hrthm.2011.10.003
Rhett, J. M., Veeraraghavan, R., Poelzing, S., and Gourdie, R. G. (2013). The perinexus: sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc. Med. 23, 222–228. doi: 10.1016/j.tcm.2012.12.005
Robertson, J. D. (1963). The occurrence of a subunit pattern in the unit membranes of club endings in mauthner cell synapses in goldfish brains. J. Cell Biol. 19, 201–221. doi: 10.1083/jcb.19.1.201
Roden, D. M., Balser, J. R., George, A. L. J., and Anderson, M. E. (2002). Cardiac ion channels. Annu. Rev. Physiol. 64, 431–475. doi: 10.1146/annurev.physiol.64.083101.145105
Saez, J. C., Berthoud, V. M., Branes, M. C., Martinez, A. D., and Beyer, E. C. (2003). Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 83, 1359–1400. doi: 10.1152/physrev.00007.2003
Saito, Y. A., Strege, P. R., Tester, D. J., Locke, G. R. III., Talley, N. J., Bernard, C. E., et al. (2009). Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G211–G218. doi: 10.1152/ajpgi.90571.2008
Sanders, K. M., Koh, S. D., and Ward, S. M. (2006). Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 68, 307–343. doi: 10.1146/annurev.physiol.68.040504.094718
Sanders, K. M., and Vogalis, F. (1989). Organization of electrical activity in the canine pyloric canal. J. Physiol. 416, 49–66. doi: 10.1113/jphysiol.1989.sp017748
Sanders, K. M., Ward, S. M., and Koh, S. D. (2014). Interstitial cells: regulators of smooth muscle function. Physiol. Rev. 94, 859–907. doi: 10.1152/physrev.00037.2013
Sano, T., Takayama, N., and Shimamoto, T. (1959). Directional difference of conduction velocity in the cardiac ventricular syncytium studied by microelectrodes. Circ. Res. 7, 262–267. doi: 10.1161/01.RES.7.2.262
Sarna, S., Condon, R. E., and Cowles, V. (1983). Enteric mechanisms of initiation of migrating myoelectric complexes in dogs. Gastroenterology 84, 814–822.
Sato, Y., Yoshida, K., Ogata, K., Inaba, T., Tada, H., Sekiguchi, Y., et al. (2012). An increase in right atrial magnetic strength is a novel predictor of recurrence of atrial fibrillation after radiofrequency catheter ablation. Circ. J. 76, 1601–1608. doi: 10.1253/circj.CJ-11-1419
Schrickel, J. W., Kreuzberg, M. M., Ghanem, A., Kim, J. S., Linhart, M., Andrie, R., et al. (2009). Normal impulse propagation in the atrioventricular conduction system of Cx30.2/Cx40 double deficient mice. J. Mol. Cell. Cardiol. 46, 644–652. doi: 10.1016/j.yjmcc.2009.02.012
Schultz, T., Daniel, V., and Daniel, E. E. (2003). Does ICC pacing require functional gap junctions between ICC and smooth muscle in mouse intestine? Neurogastroenterol. Motil. 15, 129–138. doi: 10.1046/j.1365-2982.2003.00401.x
Seidel, S. A., Bradshaw, L. A., Ladipo, J. K., Wikswo, J. P. Jr., and Richards, W. O. (1999a). Noninvasive detection of ischemic bowel. J. Vasc. Surg. 30, 309–319. doi: 10.1016/S0741-5214(99)70142-4
Seidel, S. A., Hegde, S. S., Bradshaw, L. A., Ladipo, J. K., and Richards, W. O. (1999b). Intestinal tachyarrhythmias during small bowel ischemia. Am. J. Physiol. 277, G993–G999.
Seki, K., and Komuro, T. (2001). Immunocytochemical demonstration of the gap junction proteins connexin 43 and connexin 45 in the musculature of the rat small intestine. Cell Tissue Res. 306, 417–422. doi: 10.1007/s00441-001-0470-2
Sha, L., Farrugia, G., Harmsen, W. S., and Szurszewski, J. H. (2007). Membrane potential gradient is carbon monoxide-dependent in mouse and human small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G438–G445. doi: 10.1152/ajpgi.00037.2007
Shafik, A., Ahmed, I., El Sibai, O., and Shafik, A. A. (2005). Interstitial cells of cajal in reflux esophagitis: role in the pathogenesis of the disease. Med. Sci. Monit. 11, BR452–BR456.
Siegle, M. L., and Ehrlein, H. J. (1987). Interdigestive contractile patterns of the ileum in dogs. Am. J. Physiol. Gastrointest. Liver Physiol. 253, G452–G460.
Sinagra, E., Pompei, G., Tomasello, G., Cappello, F., Morreale, G. C., Amvrosiadis, G., et al. (2016). Inflammation in irritable bowel syndrome: Myth or new treatment target? World J. Gastroenterol. 22, 2242–2255. doi: 10.3748/wjg.v22.i7.2242
Smith, T. K., Reed, J. B., and Sanders, K. M. (1987). Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am. J. Physiol. Cell Physiol. 252, C215–C224.
Spach, M. S. (1999). Anisotropy of Cardiac Tissue. J. Cardiovasc. Electrophysiol. 10, 887–890. doi: 10.1111/j.1540-8167.1999.tb00271.x
Sperelakis, N., and McConnell, K. (2002). Electric field interactions between closely abutting excitable cells. IEEE Eng. Med. Biol. Mag. 21, 77–89. doi: 10.1109/51.993199
Steinhoff, U., Knappe-Grueneberg, S., Schnabel, A., Trahms, L., Smith, F., Langley, P., et al. (2004). Magnetocardiography for pharmacology safety studies requiring high patient throughput and reliability. J. Electrocardiol. 37(Suppl.), 187–192. doi: 10.1016/j.jelectrocard.2004.08.055
Strege, P. R., Ou, Y., Sha, L., Rich, A., Gibbons, S. J., Szurszewski, J. H., et al. (2003). Sodium current in human intestinal interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G1111–G1121. doi: 10.1152/ajpgi.00152.2003
Struijs, M.-C., Diamond, I. R., Pencharz, P. B., Chang, K. T. E., Viero, S., Langer, J. C., et al. (2008). Absence of the interstitial cells of Cajal in a child with chronic pseudoobstruction. J. Pediatr. Surg. 43, e25–e29. doi: 10.1016/j.jpedsurg.2008.09.017
Suzuki, H., and Hirst, G. D. S. (1999). Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J. Physiol. 517, 563–573. doi: 10.1111/j.1469-7793.1999.0563t.x
Suzuki, H., Takano, H., Yamamoto, Y., Komuro, T., Saito, M., Kato, K., et al. (2000). Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J. Physiol. 525(Pt 1), 105–111. doi: 10.1111/j.1469-7793.2000.00105.x
Suzuki, N., Prosser, C. L., and Dahms, V. (1986). Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am. J. Physiol. 250, G287–G294.
Szurszewski, J. H., Elveback, L. R., and Code, C. F. (1970). Configuration and frequency gradient of electric slow wave over canine small bowel. Am. J. Physiol. 218, 1468–1473.
Takaki, M., Suzuki, H., and Nakayama, S. (2010). Recent advances in studies of spontaneous activity in smooth muscle: ubiquitous pacemaker cells. Prog. Biophys. Mol. Biol. 102, 129–135. doi: 10.1016/j.pbiomolbio.2010.05.007
Tana, C., Umesaki, Y., Imaoka, A., Handa, T., Kanazawa, M., and Fukudo, S. (2010). Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 22, e512–e115. doi: 10.1111/j.1365-2982.2009.0142
Thorneloe, K. S., and Nelson, M. T. (2005). Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can. J. Physiol. Pharmacol. 83, 215–242. doi: 10.1139/y05-016
Tse, G. (2015). Mechanisms of Cardiac Arrhythmias. J. Arrhythmia 32, 75–81. doi: 10.1016/j.joa.2015.11.003
Tse, G. (2016a). Both transmural dispersion of repolarization and transmural dispersion of refractoriness are poor predictors of arrhythmogenicity: a role for the index of cardiac electrophysiological balance (QT/QRS)? J. Geriatr. Cardiol.
Tse, G. (2016b). Novel indices for stratifying arrhythmic risk: (Tpeak-Tend)/QRS and (Tpeak-Tend)/(QT x QRS). J. Geriatr. Cardiol.
Tse, G. (2016c). (Tpeak-Tend)/QRS and (Tpeak-Tend)/(QT x QRS): novel markers for predicting arrhythmic risk in Brugada syndrome. Europace.
Tse, G., Ali, A., Alpendurada, F., Prasad, S., Raphael, C. E., and Vassiliou, V. (2015a). Tuberculous constrictive pericarditis. Res. Cardiovasc. Med. 4:e29614. doi: 10.5812/cardiovascmed.29614
Tse, G., Ali, A., Prasad, S. K., Vassiliou, V., and Raphael, C. E. (2015b). Atypical case of post-partum cardiomyopathy: an overlap syndrome with arrhythmogenic right ventricular cardiomyopathy? BJR Case Rep. 1:20150182. doi: 10.1259/bjrcr.20150182
Tse, G., Hothi, S. S., Grace, A. A., and Huang, C. L. (2012). Ventricular arrhythmogenesis following slowed conduction in heptanol-treated, Langendorff-perfused mouse hearts. J. Physiol. Sci. 62, 79–92. doi: 10.1007/s12576-011-0187-2
Tse, G., Lai, E. T., Tse, V., and Yeo, J. M. (2016a). Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus. J. Diab. Res.
Tse, G., Lai, E. T., Yeo, J. M., and Yan, B. P. (2016b). Electrophysiological mechanisms of Bayés syndrome: insights from clinical and mouse studies. Front. Physiol. 7:188. doi: 10.3389/fphys.2016.00188
Tse, G., Tse, V., and Yeo, J. M. (2016c). Ventricular anti-arrhythmic effects of heptanol in hypokalaemic, Langendorff-perfused mouse hearts. Biomed. Rep. 4, 313–324. doi: 10.3892/br.2016.577
Tse, G., Tse, V., Yeo, J. M., and Sun, B. (2016d). Atrial anti-arrhythmic effects of heptanol in Langendorff-perfused mouse hearts. PLoS ONE 11:e0148858. doi: 10.1371/journal.pone.0148858
Tse, G., Wong, S. T., Tse, V., Lee, Y. T., Lin, H. Y., and Yeo, J. M. (2016e). Cardiac dynamics: alternans and arrhythmogenesis. J. Arrhythmia. doi: 10.1016/j.joa.2016.02.009. [Epub ahead of print].
Tse, G., Wong, S. T., Tse, V., and Yeo, J. M. (2016f). Depolarization vs. repolarization: what is the mechanism of ventricular arrhythmogenesis underlying sodium channel haploinsufficiency in mouse hearts? Acta Physiol. doi: 10.1111/apha.12694
Tse, G., Wong, S. T., Tse, V., and Yeo, J. M. (2016g). Determination of action potential wavelength restitution in Scn5a+/- mouse hearts modelling human Brugada syndrome. J. Physiol.
Tse, G., Wong, S. T., Tse, V., and Yeo, J. M. (2016h). Monophasic action potential recordings: which is the recording electrode? J. Basic Clin. Physiol. Pharmacol. doi: 10.1515/jbcpp-2016-0007. [Epub ahead of print].
Tse, G., Wong, S. T., Tse, V., and Yeo, J. M. (2016i). Restitution analysis of alternans using dynamic pacing and its comparison with S1S2 restitution in heptanol-treated, hypokalaemic Langendorff-perfused mouse hearts. Biomed. Rep. 4, 673–680. doi: 10.3892/br.2016.659
Tse, G., and Yan, B. P. (2016). Novel arrhythmic risk markers incorporating QRS dispersion: QRSd x (Tpeak-Tend) / QRS and QRSd x (Tpeak-Tend) / (QT x QRS). Ann. Noninvasive. Electrocardiol.
Tse, G., and Yeo, J. M. (2015). Conduction abnormalities and ventricular arrhythmogenesis: the roles of sodium channels and gap junctions. IJC Heart Vascul. 9, 75–82. doi: 10.1016/j.ijcha.2015.10.003
Vanderwinden, J. M., Liu, H., De Laet, M. H., and Vanderhaeghen, J. J. (1996). Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology 111, 279–288. doi: 10.1053/gast.1996.v111.pm8690192
van Helden, D. F., Laver, D. R., Holdsworth, J., and Imtiaz, M. S. (2010). Generation and propagation of gastric slow waves. Clin. Exp. Pharmacol. Physiol. 37, 516–524. doi: 10.1111/j.1440-1681.2009.05331.x
Vassiliou, V., Chin, C., Perperoglou, A., Tse, G., Ali, A., Raphael, C., et al. (2014). 93 Ejection fraction by cardiovascular magnetic resonance predicts adverse outcomes post aortic valve replacement. Heart 100, A53–A54. doi: 10.1136/heartjnl-2014-306118.93
Veeraraghavan, R., Gourdie, R. G., and Poelzing, S. (2014a). Mechanisms of cardiac conduction: a history of revisions. Am. J. Physiol. Heart Circ. Physiol. 306, H619–H627. doi: 10.1152/ajpheart.00760.2013
Veeraraghavan, R., Lin, J., Hoeker, G. S., Keener, J. P., Gourdie, R. G., and Poelzing, S. (2015). Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 467, 2093–2105. doi: 10.1007/s00424-014-1675-z
Veeraraghavan, R., Poelzing, S., and Gourdie, R. G. (2014b). Intercellular electrical communication in the heart: a new, active role for the intercalated disk. Cell Commun. Adhes. 21, 161–167. doi: 10.3109/15419061.2014.905932
Veeraraghavan, R., Poelzing, S., and Gourdie, R. G. (2014c). Novel ligands for zipping and unzipping the intercalated disk: today's experimental tools, tomorrow's therapies? Cardiovasc. Res. 104, 229–230. doi: 10.1093/cvr/cvu216
Veeraraghavan, R., Poelzing, S., and Gourdie, R. G. (2014d). Old cogs, new tricks: a scaffolding role for connexin43 and a junctional role for sodium channels? FEBS Lett. 588, 1244–1248. doi: 10.1016/j.febslet.2014.01.026
Verheule, S., and Kaese, S. (2013). Connexin diversity in the heart: insights from transgenic mouse models. Front. Pharmacol. 4:81. doi: 10.3389/fphar.2013.00081
Vigmond, E. J. (2005). The electrophysiological basis of MAP recordings. Cardiovasc. Res. 68, 502–503. doi: 10.1016/j.cardiores.2005.07.020
Vigmond, E. J., and Bardakjian, B. L. (1995). The effect of morphological interdigitation on field coupling between smooth muscle cells. IEEE Trans. Biomed. Eng. 42, 162–171. doi: 10.1109/10.341829
Vigmond, E. J., and Leon, L. J. (1999). Electrophysiological basis of mono-phasic action potential recordings. Med. Biolog. Eng. Comput. 37, 359–365. doi: 10.1007/BF02513313
Vigmond, E. J., Tsoi, V., Yin, Y., Pagé, P., and Vinet, A. (2009). Estimating atrial action potential duration from electrograms. IEEE Trans. Biomed. Eng. 56, 1546–1555. doi: 10.1109/TBME.2009.2014740
Vinogradova, T. M., Maltsev, V. A., Bogdanov, K. Y., Lyashkov, A. E., and Lakatta, E. G. (2005). Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann. N.Y. Acad. Sci. 1047, 138–156. doi: 10.1196/annals.1341.013
Walker, R. L., Koh, S. D., Sergeant, G. P., Sanders, K. M., and Horowitz, B. (2002). TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am. J. Physiol Cell Physiol. 283, C1637–C1645. doi: 10.1152/ajpcell.00266.2002
Wang, X. Y., Lammers, W. J., Bercik, P., and Huizinga, J. D. (2005). Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G539–G549. doi: 10.1152/ajpgi.00046.2005
Ward, S. M., Baker, S. A., de Faoite, A., and Sanders, K. M. (2003). Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J. Physiol. 549, 207–218. doi: 10.1113/jphysiol.2003.040097
Ward, S. M., Burns, A. J., Torihashi, S., and Sanders, K. M. (1994). Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 480(Pt 1), 91–97. doi: 10.1113/jphysiol.1994.sp020343
Ward, S. M., Ordog, T., Koh, S. D., Baker, S. A., Jun, J. Y., Amberg, G., et al. (2000). Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J. Physiol. 525(Pt 2), 355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x
Watanuki, S., Ogata, H., Yokoyama, H., Kaiho, M., Honda, M., Ono, K., et al. (1969). Electrophysiological studies on congenital pyloric stenosis. Jpn. J. Smooth Muscle Res. 5, 117–125. doi: 10.1540/jsmr1965.5.117
Weber, P. A., Chang, H.-C., Spaeth, K. E., Nitsche, J. M., and Nicholson, B. J. (2004). The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 87, 958–973. doi: 10.1529/biophysj.103.036350
Weidmann, S. (1952). The electrical constants of Purkinje fibres. J. Physiol. 118, 348–360. doi: 10.1113/jphysiol.1952.sp004799
Weisbrodt, N. W. (1981). Patterns of intestinal motility. Annu. Rev. Physiol. 43, 21–31. doi: 10.1146/annurev.ph.43.030181.000321
Won, K. J., Sanders, K. M., and Ward, S. M. (2005). Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc. Natl. Acad. Sci. U.S.A. 102, 14913–14918. doi: 10.1073/pnas.0503628102
Yamataka, A., Kato, Y., Tibboel, D., Murata, Y., Sueyoshi, N., Fujimoto, T., et al. (1995). A lack of intestinal pacemaker (c-kit) in aganglionic bowel of patients with Hirschsprung's disease. J. Pediatr. Surg. 30, 441–444. doi: 10.1016/0022-3468(95)90051-9
Yaniv, Y., Lyashkov, A. E., Sirenko, S., Okamoto, Y., Guiriba, T. R., Ziman, B. D., et al. (2014). Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to-beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells. J. Mol. Cell. Cardiol. 77, 1–10. doi: 10.1016/j.yjmcc.2014.09.008
Yeo, Q. M., Crutchley, R., Cottreau, J., Tucker, A., and Garey, K. W. (2013). Crofelemer, a novel antisecretory agent approved for the treatment of HIV-associated diarrhea. Drugs Today 49, 239–252. doi: 10.1358/dot.2013.49.4.1947253
Yoshida, K., Ogata, K., Inaba, T., Nakazawa, Y., Ito, Y., Yamaguchi, I., et al. (2015). Ability of magnetocardiography to detect regional dominant frequencies of atrial fibrillation. J. Arrhythm 31, 345–351. doi: 10.1016/j.joa.2015.05.003
Yoshida, K., Tada, H., Ogata, K., Sekiguchi, Y., Inaba, T., Ito, Y., et al. (2012). Electrogram organization predicts left atrial reverse remodeling after the restoration of sinus rhythm by catheter ablation in patients with persistent atrial fibrillation. Heart Rhythm 9, 1769–1778. doi: 10.1016/j.hrthm.2012.06.033
Zhu, M. H., Kim, T. W., Ro, S., Yan, W., Ward, S. M., Koh, S. D., et al. (2009). A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 587, 4905–4918. doi: 10.1113/jphysiol.2009.176206
Keywords: cardiac electrophysiology, gastrointestinal electrophysiology, electrical excitation, conduction, propagation
Citation: Tse G, Lai ETH, Yeo JM, Tse V and Wong SH (2016) Mechanisms of Electrical Activation and Conduction in the Gastrointestinal System: Lessons from Cardiac Electrophysiology. Front. Physiol. 7:182. doi: 10.3389/fphys.2016.00182
Received: 23 March 2016; Accepted: 06 May 2016;
Published: 31 May 2016.
Edited by:
Ghanshyam Upadhyay, City University of New York, USAReviewed by:
Suni Tang, Texas Tech University Health Sciences Center, USANagaraja Nagre, Eastern Virginia Medical School, USA
Manish Mishra, Trinity School of Medicine, Saint Vincent and the Grenadines
Tanvirul Hye, Texas Tech University Health Sciences Center, USA
Copyright © 2016 Tse, Lai, Yeo, Tse and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gary Tse, gary.tse@doctors.org.uk
 Gary Tse
Gary Tse Eric Tsz Him Lai1
Eric Tsz Him Lai1