- 1Muscle Cellular and Molecular Physiology Research Group, Department of Sport Science and Physical Activity, Institute of Sport and Physical Activity Research, University of Bedfordshire, Bedford, United Kingdom
- 2Sport Science Program, College of Arts and Sciences, Qatar University, Doha, Qatar
- 3Centre for Human Performance, Exercise and Rehabilitation, Division of Sport, Health and Exercise Sciences, Department of Life Sciences, Brunel University London, London, United Kingdom
- 4Department of Neurobiology, Physiology and Behavior, University of California, Davis, Davis, CA, United States
- 5School of Exercise and Health Sciences, Edith Cowan University, Perth, WA, Australia
- 6Department of Sport and Physical Activity, Edgehill University, Ormskirk, United Kingdom
- 7Milton Keynes University Hospital, Milton Keynes, United Kingdom
- 8National Centre for Sport and Exercise Medicine, School of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, United Kingdom
- 9School of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, United Kingdom
- 10ASPETAR, Qatar Orthopedic and Sports Medicine Hospital, Doha, Qatar
The leukocyte heat shock response (HSR) is used to determine individual's thermotolerance. The HSR and thermotolerance are enhanced following interventions such as preconditioning and/or acclimation/acclimatization. However, it is unclear whether the leukocyte HSR is an appropriate surrogate for the HSR in other tissues implicated within the pathophysiology of exertional heat illnesses (e.g., skeletal muscle), and whether an acute preconditioning strategy (e.g., downhill running) can improve subsequent thermotolerance. Physically active, non-heat acclimated participants were split into two groups to investigate the benefits of hot downhill running as preconditioning strategy. A hot preconditioning group (HPC; n = 6) completed two trials (HPC1HOTDOWN and HPC2HOTDOWN) of 30 min running at lactate threshold (LT) on −10% gradient in 30°C and 50% relative humidity (RH) separated by 7 d. A temperate preconditioning group (TPC; n = 5) completed 30 min running at LT on a −1% gradient in 20°C and 50% (TPC1TEMPFLAT) and 7 d later completed 30 min running at LT on −10% gradient in 30°C and 50% RH (TPC2HOTDOWN). Venous blood samples and muscle biopsies (vastus lateralis; VL) were obtained before, immediately after, 3, 24, and 48 h after each trial. Leukocyte and VL Hsp72, Hsp90α, and Grp78 mRNA relative expression was determined via RT-QPCR. Attenuated leukocyte and VL Hsp72 (2.8 to 1.8 fold and 5.9 to 2.4 fold; p < 0.05) and Hsp90α mRNA (2.9 to 2.4 fold and 5.2 to 2.4 fold; p < 0.05) responses accompanied reductions (p < 0.05) in physiological strain [exercising rectal temperature (−0.3°C) and perceived muscle soreness (~ −14%)] during HPC2HOTDOWN compared to HPC1HOTDOWN (i.e., a preconditioning effect). Both VL and leukocyte Hsp72 and Hsp90α mRNA increased (p < 0.05) simultaneously following downhill runs and demonstrated a strong relationship (p < 0.01) of similar magnitudes with one another. Hot downhill running is an effective preconditioning strategy which ameliorates physiological strain, soreness and Hsp72 and Hsp90α mRNA responses to a subsequent bout. Leukocyte and VL analyses are appropriate tissues to infer the extent to which the HSR has been augmented.
Introduction
Preconditioning of an individual using environmental stressors, with the intent of ameliorating physiological and cellular stress in extreme conditions has applications for athletic, military and occupational populations (Taylor et al., 2012; Lee et al., 2014). One pathway for preconditioning these populations is the initiation of the heat shock response (HSR) which is characterized by induction of heat shock proteins (Hung et al., 2005; Madden et al., 2008; Taylor et al., 2012). The leukocyte HSR, principally heat shock protein 72 (HSP72; protein and mRNA) is used to indicate the extent of cellular heat acclimation (Amorim et al., 2015), and identify individuals at risk of exertional heat illnesses within athletic, military and occupational settings (Moran et al., 2006; Marshall et al., 2007; Ruell et al., 2007). This is primarily due to the role of Hsp72 mRNA and HSP72 as markers of the cellular stress response and thermotolerance [attenuated cellular stress response suggests a greater likelihood of cellular survival (Kampinga et al., 1995; Theodorakis et al., 1999)] in response to isolated, combined, and cross-environmental stressors (Gibson et al., 2017). Ideally the assessment of thermotolerance would take place in skeletal muscle due to its important role in locomotion and exertional heat illness pathophysiology (Sawka et al., 2011). Unfortunately, obtaining multiple muscle biopsies prior to relocation to a hot environment is not always viable for ethical, performance, cost, comfort and medical reasons (MacInnis et al., 2017). Leukocytes are a desirable tissue site for determining thermotolerance given the relative ease by which they can be collected, and because leukocytes, as circulating cells, are exposed to both systemic signals and to signals of the perfused tissues (Sonna et al., 2007). As such Hsp72 mRNA from leukocytes has been utilized as a surrogate to skeletal muscle samples with inferences made from changes in circulating intracellular sites across many exercise, heat, and nutritional experiments whereby the cellular stress response and thermotolerance are augmented (Fehrenbach et al., 2000a,b, 2001; Niess et al., 2002; Connolly et al., 2004; Marshall et al., 2007; Selkirk et al., 2009; Gibson et al., 2015a,c; Tuttle et al., 2015; Mee et al., 2016). Consequently, determining whether the HSR occurs concurrently within both tissues (leukocytes and the vastus lateralis; VL) following an acute stressor (initial experimental trial), and whether this response is attenuated in both tissues following a second trial (i.e., following preconditioning), requires elucidation to assess the viability of the leukocyte HSR to represent the skeletal muscle HSR.
The Hsp72 mRNA response is particularly pertinent during this acute stress response because HSP72 protein concentrations (due to translational inhibition) may not necessary directly represent the magnitude of the cellular stress response, particularly during the early stages of adaptation to stress (Paulsen et al., 2007) and within heat intolerant individuals (Moran et al., 2006). The differential kinetics of the Hsp72 response in the VL [typically delayed, peak between 24 h and 7d; (Morton et al., 2006; Tupling et al., 2007)] compared to leukocyte subsets [0–24 h (Fehrenbach et al., 2000a; Oehler et al., 2001)] suggests the leukocyte Hsp72 mRNA specific response which peaks within 0–3 h (Fehrenbach and Northoff, 2001; Neubauer et al., 2014), is more practical (shorter sampling time course required) for assessing the cellular stress response in the VL for comparative purposes. In addition to Hsp72 mRNA, Hsp90α mRNA is of interest due to its important role within restoration of proteostasis (Kourtis and Tavernarakis, 2011; van Oosten-Hawle et al., 2013), regulation of the transmission of signaling cascades (Taipale et al., 2010), recovery of global protein synthesis (Duncan, 2005) and regulation of cellular repair (Erlejman et al., 2014). Additionally it is unknown if the physiological signals e.g., increases in systemic temperature (Gibson et al., 2016), which elicit increases in leukocyte Hsp72 and Hsp90α mRNA transcription to damaging (Tuttle et al., 2015), and non-damaging exercise-heat stress (Gibson et al., 2015c), are as relevant in skeletal muscle. The current study also sought to investigate the gene transcript response of another HSP, glucose regulated protein 78 mRNA (Grp78 mRNA) given its ability to indicate when the unfolded protein response ends (Ron and Walter, 2007). Importantly Grp78 mRNA may also act as a biomarker of thermotolerance within heat intolerant individuals where Heat Shock factor-1 (HSF-1) signaling and Hsp72 and Hsp90α mRNA transcription are attenuated (McMillan et al., 1998). However, it is currently unclear if previous in vitro observations demonstrating the role of Hsp72 and Hsp90α mRNA in the cellular stress response (Heldens et al., 2011) occur within human leukocytes and skeletal muscle (VL) in vivo (i.e., following exercise, and exercise and heat related stressors).
Experimental aims were to determine whether a prior bout of hot downhill running [eliciting large changes in exercising rectal temperature (Tre) and delayed onset muscle soreness (DOMS)], when compared to a temperate flat run, could provide a preconditioning effect relative to attenuation of the VL Hsp responses (Hsp72, Hsp90α, and Grp78 mRNA) during a subsequent trial of hot downhill running 7 d later. The second experimental aim was to determine whether this response occurred concurrently within leukocytes and the VL. It was hypothesized that a prior bout of hot downhill running would attenuate both the VL and leukocyte Hsp72 and Hsp90 mRNA responses during a second trial, and that a significant relationship between the VL and leukocyte Hsp72 and Hsp90 mRNA responses following the first trial would exist.
Methods
Ethical Approval
The protocol was approved by the University of Bedfordshire's Sport Science and Physical Activity Departmental Human Ethics Committee and all participants signed informed consent in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki.
Participants
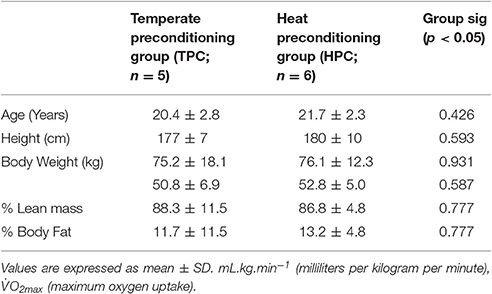
Demographic variables were recorded for 11 male Caucasian participants (see Table 1) who were non-smokers and were not heat acclimated (experimental trials completed between January and March, within the UK; average temperatures 1.5°C–8.1°C). Body mass (kg) and height (cm) were measured with a single set of mechanical scales (Weylux Marsden 424 London, UK) and a stadiometer (Harpenden HAR- 98.602, Crymych, UK) respectively. Body composition was measured using air displacement plethysmology (Bod Pod 2000A, Cranlea, UK). The lactate threshold (LT) and maximum oxygen uptake (O2max) were determined using a graded treadmill test (Winter et al., 2007). This test consisted of 6–8 incremental 3 min stages at a 1% gradient. Participants started running at 8–9 km.h−1 and running velocity was increased by 1 km.h−1 per stage until exhaustion. Fingertip capillary blood samples (40 μL) were taken at rest and the end of each 3 min stage to determine blood lactate concentrations (B[La]). Blood lactate concentrations were plotted against running velocity to determine LT which was defined as the first sustained B[La] increase above baseline. Pulmonary gas exchange was measured breath by breath using an online gas analysis system (Cortex Metalyser 3B, Biophysik, Leipzig, Germany) to determine changes in oxygen uptake (O2) with the highest O2 attained over a 30 s period accepted as O2max.
Sample size calculations of Hsp72 mRNA were determined via G.Power 3.1, (Universität Dusseldorf, Germany; Faul et al., 2009) using data from a previous paper (Mestre-Alfaro et al., 2012). For a two tailed test with an alpha of 0.05 and power of 0.8, Six participants were required to find an Hsp72 mRNA increase of 3.8-fold significant. This sample size is ≥ others in the field (Puntschart et al., 1996; Febbraio and Koukoulas, 2000; Fehrenbach and Northoff, 2001; Fehrenbach et al., 2003; Liu et al., 2004; Mee et al., 2016).
Experimental Design
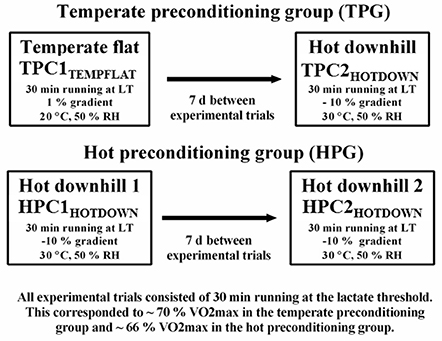
Participants were split into two experimental groups (see Figure 1). The temperate (TPC; five participants) and HOT (HPC; six participants) preconditioning groups (conditions) both featured two exercise trials separated by 7 d:
TPC Exercise trial (1): Temperate flat (TPC1TEMPFLAT) which involved 30 min running at the LT on a 1% gradient in 20°C, 50% RH. TPC Exercise trial (2): 7 d post TPC1TEMPFLAT, hot downhill (TPC2HOTDOWN) which involved 30 min downhill running at the LT on a −10% gradient in 30°C, 50% RH.
HPC Exercise trial (1): Hot downhill (HPC1HOTDOWN) which involved 30 min downhill running at the LT on a −10% gradient in 30°C, 50% RH. HPC Exercise trial (2): 7 d post HPC1HOTDOWN, hot downhill 2 (HPC2HOTDOWN) which involved 30 min downhill running at the LT on a −10% gradient in 30°C, 50% RH.
Previous work from our research group has demonstrated that the leukocyte Hsp72 and Hsp90α mRNA responses are larger following exercise in hot compared to temperate environments (Gibson et al., 2015c, 2016), and following downhill compared to flat running (Tuttle et al., 2015). It is known that downhill running is an effective whole body preconditioning strategy (Shima et al., 2008; Touchberry et al., 2012; Isanejad et al., 2015), consequently, an acute preconditioning trial featuring both stressors (hot environmental conditions and downhill running; hot downhill running) was selected in the current experimental design to maximize stimuli to initiate the HSR and subsequent cellular preconditioning. This was compared to a temperate flat trial (flat running in a temperate environment) where no change in leukocyte Hsp72 and Hsp90α mRNA has been previously observed (Tuttle et al., 2015) and thus no preconditioning effect was hypothesized to occur. A 7 d period between trials was selected to ensure any spontaneous preconditioning effect from exercise stress on core temperature (Barnett and Maughan, 1993) and leukocyte HSP72 (Fehrenbach et al., 2001; Lee et al., 2014), had returned to baseline following TPC1TEMPFLAT.
All experimental trials were completed at the running velocity which elicited the LT to minimize differences in metabolic strain between experimental trials (Baldwin et al., 2000). However, environmental temperature mediated differences still remained as relative exercise intensity is higher at the same velocity during exercise in hot environments (Lorenzo et al., 2011). All experimental trials were completed at the same time of day to minimize the influence of diurnal and circadian variations on exercise performance (Drust et al., 2005). Confounding variables were controlled for via abstinence prior to testing and throughout the testing period (see brackets for duration). These confounding variables were caffeine and alcohol (72 h), non-steroidal anti-inflammatory medications [48 h (Nielsen and Webster, 1987; Van Wijck et al., 2012)], dietary supplementation (vitamins, ergogenic aids; 30 d), exercise [7 d (Morton et al., 2006)], thermal stressors [3 months (Gibson et al., 2014)] and hypoxic and hyperbaric stressors [3 months (Taylor et al., 2010a, 2011, 2012)]. A questionnaire was administered prior to each experimental trial to determine adherence to the aforementioned experimental control measures with apparent adherence 100% in all participants.
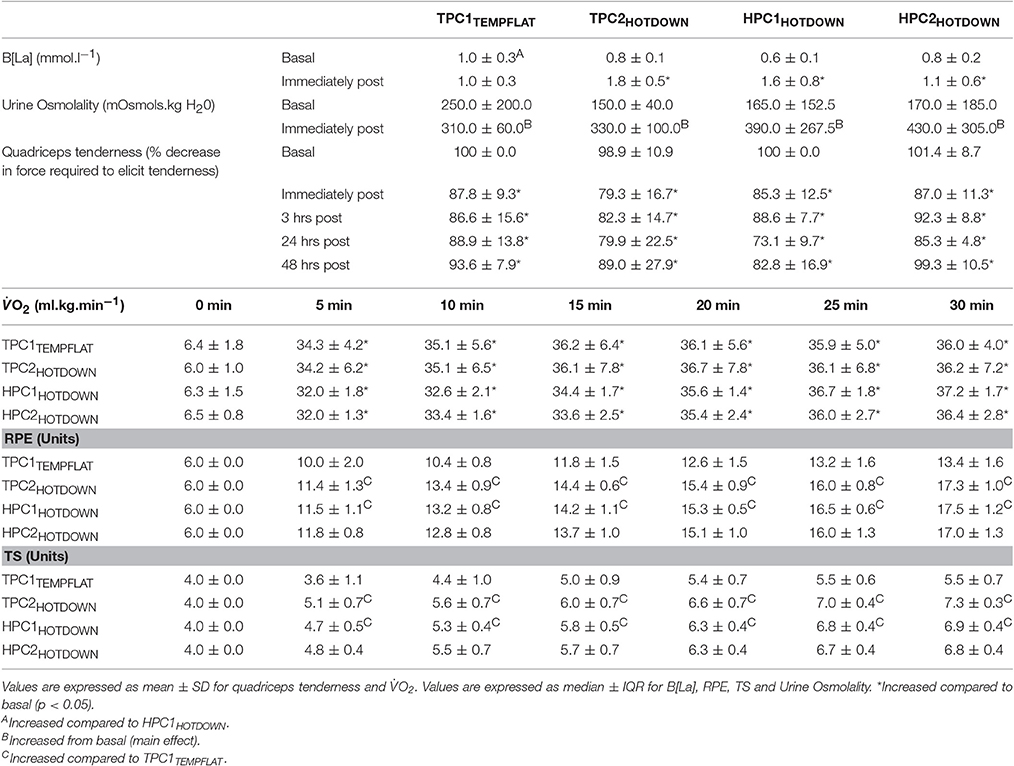
Participants were instructed to drink 500 mL of water 2 h before each experimental trial as per the ACSM position stand (Sawka et al., 2007). Hydration status was assessed via urine osmolality (UOsm) using a handheld digital refractometer (Osmocheck, Vitech Scientific Ltd, Horsham, UK) before any pre exercise measures were obtained and immediately after exercise. All participants were euhydrated [UOsm was <600 mOsmols.kg.H20 (Hillman et al., 2011, 2013)] prior to all experimental conditions and remained euhydrated during each experimental trial despite UOsm increasing (Time; F = 63.7, p < 0.001) immediately post exercise compared to basal.
Molecular Physiology Measures
Blood Sampling and Leukocyte Isolation
Venous blood was obtained from the antecubital vein into a 6 mL EDTA tube immediately before (basal), immediately post, 3 h post, 24 h post, and 48 h post exercise. Using an adaptation of a previously validated method (Taylor et al., 2010b), 500 μL of venous blood was pipetted into 10 mL of 1 in 10 red blood cell lysis solution (10X Red Blood Cell Lysis Solution, Miltenyi Biotech, UK). Samples were incubated for 15 min at room temperature and then isolated via centrifugation at 400G for 5 min and washed twice in 2 mL phosphate-buffered saline (PBS) at 400 G for 5 min. The pellet was suspended in 1 mL of PBS, pipetted into a 1.5 mL RNase free microtube and then centrifuged at 17 000 G for 5 min at 4°C. The remaining supernatant was aspirated prior to the pellet being completely re-suspended in 200 μL of TRIzol reagent (Sigma Aldrich, Dorset, UK) and stored at −80°C for subsequent RNA extraction.
Muscle Biopsies
All biopsies were taken by medically qualified Orthopedic Surgeons, with full UK General Medical Council registration. Muscle Biopsies were obtained using a previously validated and HSP specific in vivo technique (Morton et al., 2006, 2007, 2008, 2009) applied to the lateral portion of the vastus lateralis. Biopsies were taken 3 cm apart in a proximal to distal fashion, under local anesthetic (2% lidocaine hydrochloride). The fascia of the muscle was specifically avoided (Trappe et al., 2013). Disposable manually primed biopsy needle guns were utilized (12 × 16, Disposable Monopty Core Biopsy Instrument, Bard Biopsy Systems, USA). Samples collected (20–30 mg) were immediately frozen in liquid nitrogen (−196°C) and stored at −80°C for later analysis. Serial biopsies were separated by 3 cm to ensure muscle damage from previous incisions did not influence the Hsp72, Hsp90α, and Grp78 mRNA responses (Khassaf et al., 2001).
Biopsy samples were later ground under liquid nitrogen to remove surrounding tissue (i.e., adipose, and connective tissue) prior to homogenization with a sonicator (T10 Basic, IKA, Thermo Fisher Scientific, Loughborough, UK) on ice in 1 mL TRIzol reagent followed by a 10 min incubation period on ice, in preparation for RNA extraction.
RNA Extraction
The TRIzol method was used to extract RNA from the biopsy samples and the leukocytes in accordance with manufacturer instructions (Invitrogen, Life Technologies, Carlsbad, USA). Quantity was determined at an optical density of 260 nm while quality was determined via the 260/280 and 260/230 ratios using a nanodrop spectrophotometer (Nanodrop 2000c, Thermo Scientific). Only samples with a 260:280 ratio of between 1.9 and 2.15 were carried forward for reverse transcription and PCR amplification detailed below.
One Step Reverse Transcription Quantitative Polymerase Chain Reaction (RT-QPCR)
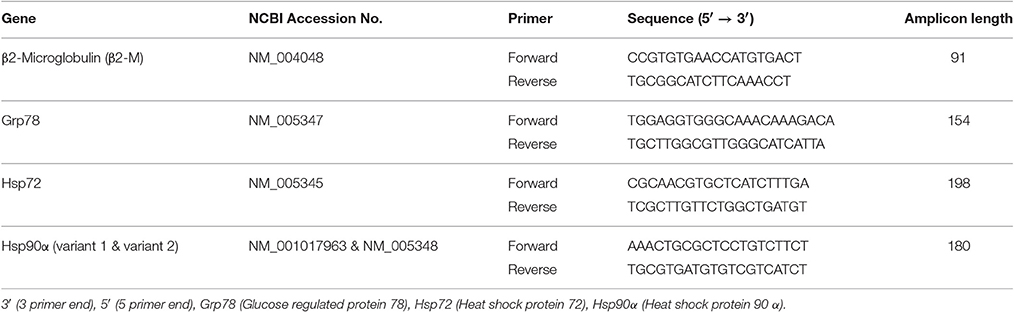
Primers (see Table 2) were designed using primer design software (Primer Quest and Oligoanalyzer—Integrated DNA technologies). During primer design sequence homology searches were performed against the Genbank database to ensure the primers matched the gene of interest. Primers were designed to span exon-intron boundaries and avoided three or more GC bases within the last 5 bases at the 3′ end of primer to avoid non-specific binding. Further searches were performed to ensure primers did not contain secondary structures and inter or intra molecular interactions (hairpins, self-dimer and cross dimers), which can inhibit product amplification. Hsp72, Hsp90α and Grp78 relative mRNA expression was then quantified using RT-QPCR. 20 μL reactions containing 10 μL SYBR-Green RT-PCR Mastermix (Quantifast SYBRgreen Kit, Qiagen, Manchester, UK), 0.15 μL forward primer, 0.15 μL reverse primer, 0.2 μL reverse transcription mix (Quantifast RT Mix, Qiagen) and 9.5 μL sample (70 ng RNA/ μL) were prepared using the Qiagility automated pipetting system (Qiagen). Each reaction was amplified in a thermal cycler (Rotorgene Q, Qiagen) and involved reverse transcription lasting 10 min at 50°C and a transcriptase inactivation and initial denaturation phase lasting 5 min at 95°C. The PCR reaction then followed with a denaturation step lasting 10 s at 95°C and a primer annealing and extension stage lasting 30 s at 60°C repeated for 40 cycles. Fluorescence was measured following each cycle as a result of the incorporation of SYBR green dye into the amplified PCR product. Melt curves (50 to 95°C; Ramp protocol 5 s stages) were analyzed for each reaction to ensure only the single gene of interest was amplified.
The relative quantification of mRNA expression for each sample (Hsp72, Hsp90α, and Grp78) was assessed by determining the ratio between the cycling threshold (CT) value of the target mRNA and the CT values for β2-Microglobulin (β2-M) mRNA. Fold change in relative mRNA expression was calculated using the 2-ΔΔCT method (Schmittgen and Livak, 2008). β2-Microglobulin was used as a housekeeping gene as it was stable between experimental trials and across time in both the VL and leukocytes, as previously observed following exercise (Mahoney et al., 2004, 2008; Tuttle et al., 2015). The coefficient of variation for β2-M mRNA, Hsp72 mRNA, Hsp90α mRNA and Grp78 mRNA were 0.55, 0.34, and 0.28% respectively.
Statistical Analysis
Central tendency and dispersion are reported as the mean and standard deviation for normally distributed data and as the median and interquartile range for non-normally distributed data. Inferential statistical analyses were completed using linear mixed models for repeated measures (IBM SPSS Statistics 19, Chicago, IL) with comparisons made for main effects, two way interactions (experimental trial × time) and three way interactions (group × experimental trial × time). The best fitting covariance structure was selected by minimizing the Hurvich and Tsai's criterion (Field, 2013). Changes in Hsp72, Hsp90α, and Grp78 mRNA are presented as fold change from basal in accordance with previous literature (Tuttle et al., 2015; Gibson et al., 2015c). Where significant F ratios for main and interaction effects occurred, post-hoc pairwise comparisons were made with Bonferroni adjusted p-values. Pearson's product correlation was performed between leukocyte and vastus lateralis Hsp72 mRNA and Hsp90α mRNA before, immediately after and 3 h after TPC1TEMPFLAT and HPC1HOTDOWN. Pearson's product correlations were also performed between physiological variables Tre and HR, and leukocyte and VL Hsp72 mRNA and Hsp90α mRNA immediately and 3 h after the corresponding TPC1TEMPFLAT and HPC1HOTDOWN. The mRNA responses to TPC2HOTDOWN and HPC2HOTDOWN were not included in the correlational analyses given the likelihood of the prior trials to be a confounding factor due to the hypothesized preconditioning effect i.e., increase gene transcription and therefore signal post translational events to increase basal HSP (Tuttle et al., 2015). Statistical significance was accepted at p < 0.05 (two tailed).
Results
Thermoregulatory Response
Exercising Tre (Figure 2) increased as main effect between 5 and 30 min (p < 0.001) compared to basal. Average exercising Tre was higher during the hot downhill running trials (HPC1HOTDOWN; 38.3°C; F = 14.3, p = 0.002, and TPC2HOTDOWN (37.9°C; F = 6.1, p = 0.017) compared to the temperate flat trial (TPC1TEMPFLAT; 37.7°C). Exercising Tre was greater during the hot downhill trials (TPC2HOTDOWN; 20–30 min, p < 0.05, HPC1HOTDOWN; 5–30 min, p < 0.05) compared to the temperate flat trial (TPC1TEMPFLAT). Exercising Tre was also 0.3°C higher (39.3 ± 0.3°C compared to 39.0 ± 0.4°C) at 30 min during HPC1HOTDOWN compared to HPC2HOTDOWN (F = 6.1, p = 0.017).
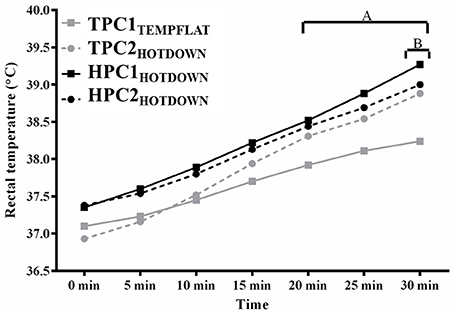
Figure 2. Rectal temperature (Tre) at 0–30 min of exercise. A, Tre increased (p < 0.005) during TPC2HOTDOWN compared to TPC1TEMPFLAT. B, Tre decreased (p = 0.017) during HPC2HOTDOWN compared to HPC1HOTDOWN. Mean data presented. Error bars omitted to maintain clarity.
Heart rate (Figure 3) was increased compared to basal between 5 and 30 min (p < 0.001). Average HR was higher during TPC2HOTDOWN (162 beats.min−1) compared to TPC1TEMPFLAT (147 beats.min−1; F = 22.3, p = 0.001). No difference in average HR was observed between HPC1HOTDOWN (161 beats.min−1) and TPC1TEMPFLAT (F = 3.3, P = 0.096) or HPC2HOTDOWN (157 beats.min−1; F = 2.8, p = 0.128). Heart rate was higher during the hot downhill trials (TPC2HOTDOWN; 5–30 min, p < 0.05 and HPC1HOTDOWN; 20–30 min, p < 0.05) compared to the temperate flat trial (TPC1TEMPFLAT). A trend for HR to be reduced during HPC2HOTDOWN compared to HPC1HOTDOWN between 20 and 30 min (8 beats.min−1; ~ F = 3.8, p = ~ 0.069) was observed.
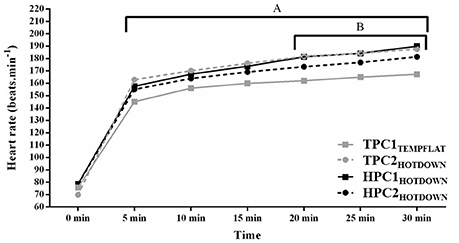
Figure 3. Heart rate (HR) at 0–30 min of exercise. A, increased (p < 0.010) during TPC2HOTDOWN compared to TPC1TEMPFLAT at 5–30 min. B, increased (p < 0.050) during HPC1HOTDOWN increased compared to TPC1TEMPFLAT at 20–30 min. Mean data presented. Error bars omitted to maintain clarity.
Perceived muscle soreness (indicated by the VAS; Figure 4) was increased over time as a main effect immediately post to 48 h post exercise compared to basal (p < 0.001). Perceived muscle soreness also increased from basal between immediately post to 48 h post exercise following TPC2HOTDOWN and HPC1HOTDOWN (p < 0.001) and between immediately post—3 h post HPC2HOTDOWN (p < 0.05). Perceived muscle soreness was greater following the hot downhill running trials (TPC2HOTDOWN and HPC1HOTDOWN) compared to the temperate flat running trial (TPC1TEMPFLAT) immediately post, (F = 7.2, p = 0.011 and F = 11.8, p = 0.002), 3 h post (F = 6.1, p = 0.019 and F = 9.1, p = 0.005), 24 h post (F = 12.2, p = 0.001 and F = 25.0, p < 0.001) and 48 h post exercise (F = 14.3, p = 0.001 and F = 30.4, p < 0.001) respectively. Perceived muscle soreness was attenuated 24 and 48 h after the second hot downhill running trial (HPC2HOTDOWN) compared to the first hot downhill trial (HPC1HOTDOWN; F = 12.6, p = 0.001 and F = 11.3, p = 0.002, respectively) in the HPC.
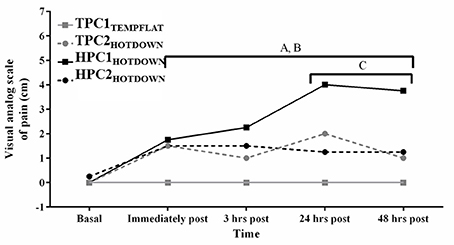
Figure 4. Perceived muscle soreness measured via the Visual analog scale of pain (VAS) immediately before, immediately post, 3 h post, 24 h post, and 48 h post exercise. A, increased (p < 0.005) during TPCHEATDOWN compared to TPC1TEMPFLAT. B, increased (p < 0.001) during HPC1HOTDOWN compared to TPC1TEMPFLAT. C, decreased (p < 0.001) during HPC2HOTDOWN compared to HPC1HOTDOWN. Median data presented. Error bars omitted to maintain clarity.
Quadriceps tenderness (QT; Table 3) was increased as a main effect immediately post to 48 h post exercise (p < 0.05) compared to basal. No difference in QT was observed between experimental trials (P > 0.05).
Metabolic and Perceptual Responses
Compared to basal, blood lactate concentrations (Table 3) increased following the hot downhill running trials (TPC2HOTDOWN; F = 11.0, p = 0.006, HPC1HOTDOWN; F = 13.3, p = 0.003 and HPC2HOTDOWN; F = 5.7, p = 0.035), but not the temperate flat trial (TPC1TEMPFLAT; F = 0.0, p = 0.874). Oxygen uptake (O2) increased (F = 236.0, p < 0.001) over time as a main effect but there was no difference between experimental trials (P < 0.05). Participants exercised at an average % O2max of 70.2 ± 6.0% during the TPC1TEMPFLAT trial, 70.8 ± 6.9% during the TPC2HOTDOWN trial, 66.2 ± 6.0% during the HPC1HOTDOWN trial and 65.8 ± 8.4% during the HPC2HOTDOWN trial.
Both the rate of perceived exertion (RPE; Table 3) and thermal sensation (TS; Table 3) were greater during the hot downhill running trials (TPC2HOTDOWN and HPC1HOTDOWN; p < 0.05) compared to the temperate flat trial (TPC1TEMPFLAT). No difference in RPE or TS was observed between the HPC1HOTDOWN and HPC2HOTDOWN trials (p > 0.05).
Cellular Stress (Hsp mRNA) Response
The responses of Hsp72, Hsp90α, and Grp78 mRNA were assessed to determine their suitability as markers of the cellular stress response. Vastus lateralis Hsp72 mRNA (Figure 5A) increased as a main effect immediately post (p < 0.001) and 3 h post exercise (p = 0.002) compared to basal. Vastus lateralis Hsp72 mRNA increased immediately post exercise compared to basal in the hot downhill running trials (TPC2HOTDOWN and HPC1HOTDOWN; p < 0.001). Vastus lateralis Hsp72 mRNA expression was greater immediately post TPC2HOTDOWN and HPC1HOTDOWN compared to the temperate flat trial (TPC1TEMPFLAT; F = 24.2, p < 0.001 and F = 9.2, p = 0.004, respectively) and the second hot downhill trial in the hot preconditioning group (HPC2HOTDOWN; F = 9.7, p = 0.003 and F = 5.0, p = 0.028, respectively). Vastus lateralis Hsp72 mRNA was also greater 3 h after HPC1HOTDOWN compared to TPC1TEMPFLAT (F = 6.6, p = 0.013).
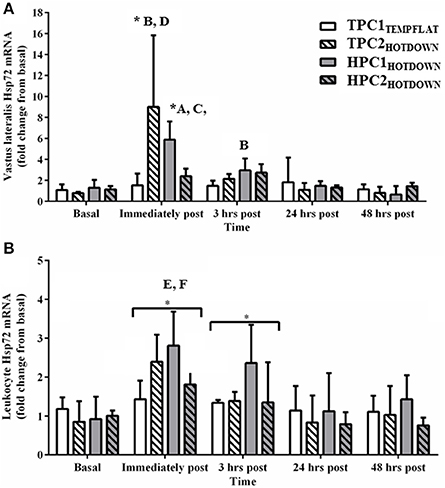
Figure 5. Hsp72 mRNA response immediately before, immediately post, 3 h post, 24 h post and 48 h post exercise in the Vastus lateralis (A) and Leukocytes (B). * Increased (p < 0.001) compared to basal. A, increased (p = 0.020) in HPC1HOTDOWN compared to TPC1TEMPFLAT. B, increased (p < 0.001) in TPC2HOTDOWN compared to TPC1TEMPFLAT. C, increased (p = 0.028) in HPC1HOTDOWN compared to HPC2HOTDOWN. D, increased in TPC2HOTDOWN compared to HPC2HOTDOWN. E, HPC1HOTDOWN increased (p = 0.049) compared to HPC2HOTDOWN. F, HPC1HOTDOWN increased (p = 0.003) compared to TPC1TEMPFLAT. Data presented as median ± interquartile range.
Leukocyte Hsp72 mRNA expression (Figure 5B) increased as a main effect immediately post (p < 0.001) and 3 h post exercise (p = 0.004) compared to basal. Leukocyte Hsp72 mRNA expression was greater following HPC1HOTDOWN compared to TPC1TEMPFLAT (F = 4.2, p = 0.049) and HPC2HOTDOWN (F = 10.2, p = 0.003).
Vastus lateralis Hsp90α mRNA (Figure 6A) increased compared to basal following the hot downhill running trials TPC2HOTDOWN (immediately post exercise; p < 0.001) and HPC1HOTDOWN (immediately post; p < 0.001 and 3 h post; p = 0.020). Vastus lateralis Hsp90α mRNA expression was greater immediately post TPC2HOTDOWN compared to TPC1TEMPFLAT (F = 8.4, p = 0.006), and HPC2HOTDOWN (F = 7.4, p = 0.010). Vastus lateralis Hsp90α mRNA expression was also greater following HPC1HOTDOWN compared to TPC1TEMPFLAT (immediately post; F = 4.3, p = 0.044 and 3 h post; F = 4.4, p = 0.043) and HPC2HOTDOWN (immediately post; F = 19.4, p < 0.001).
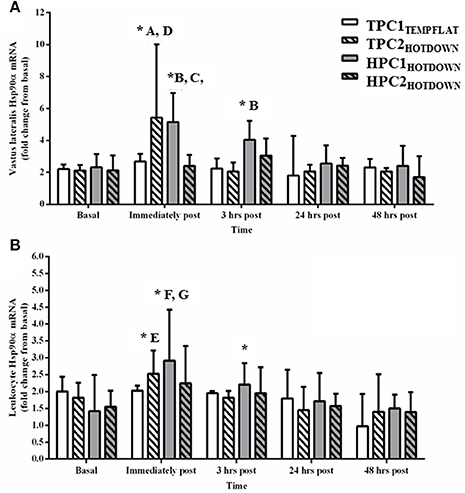
Figure 6. Hsp90α mRNA response immediately before, immediately post, 3 h post, 24 h post, and 48 h post exercise in the Vastus lateralis (A) and Leukocytes (B). * Increased (p < 0.02) compared to basal. A, increased (p = 0.006) during TPC2HOTDOWN compared to TPC1TEMPFLAT. B, increased (p < 0.001) during HPC1HOTDOWN compared to HPC2HOTDOWN. C, increased (p < 0.05) during HPC1HOTDOWN compared to TPC1TEMPFLAT. D, increased (p = 0.01) during TPC2HOTDOWN compared to HPC2HOTDOWN. E, (p = 0.024) during TPC2HOTDOWN compared to TPC1TEMPFLAT. F, increased (p = 0.030) during HPC1HOTDOWN compared to HPC2HOTDOWN. G, (p = 0.002) during HPC1HOTDOWN compared to TPC1TEMPFLAT. Data presented as median ± interquartile range.
Leukocyte Hsp90α mRNA expression increased as a main effect immediately post exercise compared to basal (p < 0.001). Leukocyte Hsp90α mRNA expression also increased following TPC2HOTDOWN (immediately post; p = 0.024) and HPC1HOTDOWN (immediately post; p < 0.001 and 3 h post; p = 0.041) compared to basal. Leukocyte Hsp90α mRNA expression was greater immediately after TPC2HOTDOWN compared to TPC1TEMPFLAT (F = 5.3, p = 0.024). Leukocyte Hsp90α mRNA expression was also greater immediately after HPC1HOTDOWN compared to TPC1TEMPFLAT (F = 10.1, p = 0.002) and HPC2HOTDOWN (F = 4.9, p = 0.030).
Vastus lateralis Grp78 mRNA (Figure 7A) increased as a main effect immediately post to 48 h post exercise compared to basal (p < 0.002). Vastus lateralis Grp78 mRNA also increased within the TPC immediately post (p = 0.003) and within the HPC at 3 h (p < 0.001) and 24 h post (p < 0.001). Vastus lateralis Grp78 mRNA increased compared to basal following the hot downhill running trials, TPC2HOTDOWN (immediately post; p < 0.001), HPC1HOTDOWN (3 and 24 h post; p < 0.010) and HPC2HOTDOWN (24 h post; p = 0.003), but did not change following the temperate flat trial (TPC1TEMPFLAT; p > 0.05).
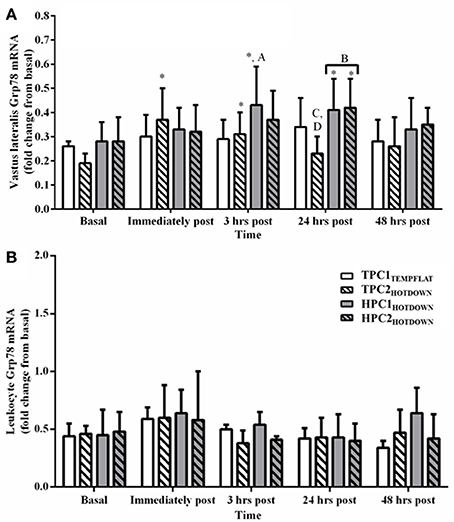
Figure 7. Grp78 mRNA response immediately before, immediately post, 3 h post, 24 h post, and 48 h post exercise in the Vastus lateralis (A) and Leukocytes (B). * Increased (p < 0.01) compared to basal. * Increased (p < 0.01) compared to basal. A, increased (p = 0.031) during HPC1HOTDOWN compared to TPC1TEMPFLAT. B, increased (p < 0.001) during HPC compared to TPC. C, decreased (p = 0.006) during TPC2HOTDOWN compared to HPC2HOTDOWN. D, decreased (p = 0.01) during TPC2HOTDOWN compared to TPC1TEMPFLAT. Data presented as median ± interquartile range.
All main effects and interactions had no effect (p > 0.05) on leukocyte Grp78 mRNA (Figure 7B).
Relationship between mRNA Responses
A strong correlation was observed between vastus lateralis Hsp72 and Hsp90α mRNA expression (r = 0.863, p < 0.001; Figure 8A), and between leukocyte Hsp72 and Hsp90α mRNA expression (r = 0.844, p < 0.001; Figure 8B). Modest correlations were also observed between leukocyte Hsp72 mRNA and vastus lateralis Hsp72 mRNA (r = 0.651, p < 0.001; Figure 8C), and between leukocyte Hsp90α mRNA and vastus lateralis Hsp90α mRNA. (r = 0.640, p < 0.001; Figure 8D). Relationships between Hsp72 and Hsp90α mRNA, and Grp78 mRNA were not analyzed given the absence of a change in leukocyte Grp78 mRNA (Figure 7B).
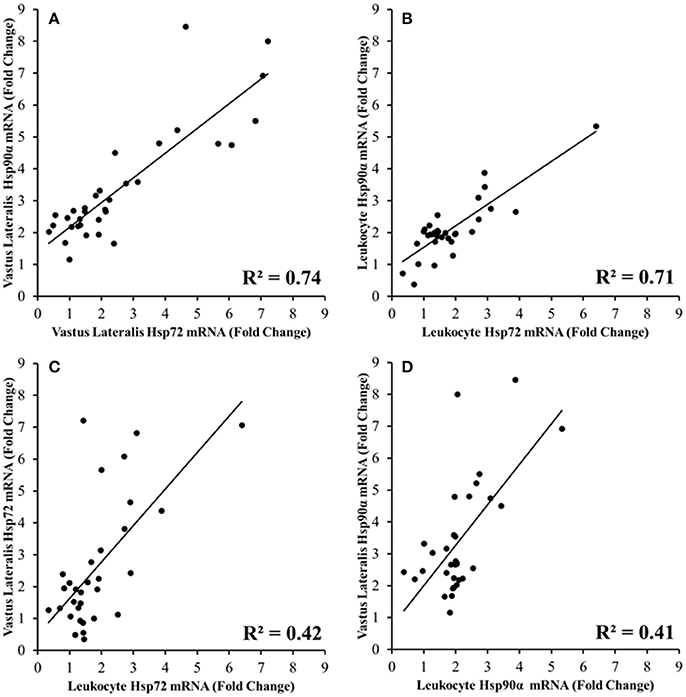
Figure 8. Relationships between Hsp72 (A,C) and Hsp90α mRNA (B,D) responses in the Vastus Lateralis and Leukocytes immediately before, immediately post, 3 h post TPC1TEMPFLAT and HPC1HOTDOWN (all p < 0.001).
A strong relationship was also observed between the peak Tre during TPC1TEMPFLAT and HPC1HOTDOWN and the immediately post measured leukocyte Hsp72 (r = 0.665, p = 0.026) and Hsp90α mRNA (r = 0.708, p = 0.015), and the 3 h measured leukocyte (r = 0.786, p = 0.004) and vastus lateralis (r = 0.720, p = 0.013) Hsp72 mRNA, and vastus lateralis Hsp90α mRNA (r = 0.682, p = 0.021). A strong relationship was also observed between peak heart rate during TPC1TEMPFLAT and HPC1HOTDOWN, and leukocyte (r = 0.739, p = 0.009) and vastus lateralis (r = 0.766, p = 0.006) Hsp72 mRNA, and leukocyte (r = 0.677, p = 0.022) and vastus lateralis Hsp90α mRNA (r = 0.746, p = 0.008) at 3 h post exercise. No significant relationship was observed immediately post TPC1TEMPFLAT or HPC1HOTDOWN.
Discussion
The current study demonstrated that both VL and leukocyte Hsp72 and Hsp90α mRNA increases following the first trial of downhill running in a hot environment (HPC1HOTDOWN) were attenuated concurrently with reductions in exercising Tre and DOMS during the second trial of downhill running in a hot environment (HPC2HOTDOWN; see Figures 5, 6). This suggests that the cellular stress response (Hsp72 and Hsp90α mRNA) occurred simultaneously within both tissues (Figure 8) and likely contributed to the preconditioning effect. This was not demonstrated in GRP78 mRNA (Figure 7). The absence of change in GRP78 mRNA in leukocytes suggests this is not an appropriate tissue to determine changes in its expression levels. Therefore, the leukocyte Hsp72 and Hsp90α mRNA responses could potentially be a useful surrogate for the VL response. At a physiological level the attenuated Tre (Figure 2), HR (Figure 3) and VAS (Figure 4) responses to an equivalent downhill run following HPC demonstrates an acute preconditioning response was attained. This was not discernible in the TPC group whom demonstrated the known responses to downhill running under heat stress in comparison to level gradient running in temperate conditions i.e., increased Tre (Figure 2), HR (Figure 3) and VAS (Figure 4).
Cellular Stress Response and Surrogate Hsp mRNA Response
Increased Hsp72 mRNA transcription has frequently been demonstrated following exercise [leukocytes and VL (Walsh et al., 2001; Mestre-Alfaro et al., 2012)], muscle damaging exercise [VL (Vissing et al., 2009)] and exercise heat stress [leukocytes (Mestre-Alfaro et al., 2012)] within humans. However, there is less data available regarding the Hsp72 mRNA response being attenuated during repeated trials of muscle damaging exercise, or exercise heat stress as observed frequently during repeated trials of in vitro heat shock (Kiang et al., 1996; Theodorakis et al., 1999). Studies have previously only observed a blunted response following muscle damaging exercise in the VL (Paulsen et al., 2007) and exercise heat stress within leukocytes (Fehrenbach et al., 2001; Marshall et al., 2007). Within these studies reductions in thermal strain [exercising Tre (Fehrenbach et al., 2001; Marshall et al., 2007)] and muscle damage (Paulsen et al., 2007) during subsequent experimental trials were suggested to be responsible for the attenuated Hsp72 mRNA response observed. The current study also observed a reduction in thermal strain (Tre −0.3°C) equivalent to that of various heat acclimation regimes (Gibson et al., 2015b; Tyler et al., 2016), and an attenuated perceived muscle soreness (24 h post = +12.2%, 48 h post = −16.5%) response that is indicative of muscle damage (Fridén et al., 1981) from near identical exercise trials [HPC2HOTDOWN compared to HPC1HOTDOWN (see Figure 2 and Table 3)]. Together these responses indicate that downhill running models may be able to elicit a beneficial preconditioning effect (Dolci et al., 2015; Tuttle et al., 2015). Given that acute non-damaging exercise heat stress does not improve thermal responses to a greater extent than equivalent temperate condition exercise [Figure 2, (Lee et al., 2014)], the cellular responses to the eccentric muscle action of the damaging downhill running is important. The attenuated exercising Tre response could be suggestive of a reduction in relative exercise intensity and therefore potentially reduced requirement for ATP production (Febbraio et al., 1996), though no statistical difference in absolute intensity as indicated by O2 was observed (Table 3). Therefore, metabolic strain was likely reduced. Protein denaturation, the key cellular change associated with heat shock factor-1 (HSF-1) activation and Hsp72 and Hsp90α mRNA transcription, is temperature (Mestre-Alfaro et al., 2012), metabolic strain (Beckmann et al., 1992) and muscle damage (Michailidis et al., 2013) dependent. This suggests the observed attenuated thermal strain and muscle damage responses could be an important mechanism explaining the attenuated Hsp72 mRNA response in leukocytes (HPC1HOTDOWN = +207%; HPC2HOTDOWN = +79%) and VL (HPC1HOTDOWN = +353%; HPC2HOTDOWN = +109%) observed following the HPC2HOTDOWN trial, compared to HPC2HOTDOWN trial. Although, the expression of VL (Neubauer et al., 2014) and leukocyte (Moran et al., 2006) Hsp90α mRNA have previously been observed to increase following exercise and exercise heat stress, respectively, with equality of physiological stimuli i.e., Tre maintaining Hsp90α mRNA transcription (Gibson et al., 2015c), no studies have determined whether Hsp90α mRNA is attenuated during repeated trials of muscle damaging exercise. Consequently, the attenuated Hsp90α mRNA response in both leukocytes (HPC1HOTDOWN = +106%, HPC2HOTDOWN = +45%) and skeletal muscle (HPC1HOTDOWN = +122%, HPC2HOTDOWN = +113%) following reductions in physiological strain is a novel observation (see Figure 6). It is a novel finding that the relationship between Hsp72 and Hsp72 mRNA transcription is equivalent in the VL (Figure 8A, R2 = 0.74), as has been previously shown in leukocytes [R2 = 0.77 (Gibson et al., 2016)]. It has also been observed that the Hsp72 and Hsp72 mRNA transcription response is comparable following damaging exercise (Figure 8B, R2 = 0.71), as it has previously in non-damaging exercise models (Gibson et al., 2016). Within leukocytes, it has been observed that Hsp72 mRNA transcription (Gibson et al., 2015a,c; Mee et al., 2016), and Hsp90α mRNA transcription (Gibson et al., 2015c) returns to baseline 24 h following non-damaging exercise heat stress (Moran et al., 2006). The heat shock factor-1 (HSF-1) transcription pathway likely highlights the mechanism between equality of increases in Hsp72 and Hsp90α mRNA as demonstrated in this experiment (Figure 8), and others utilizing a non-damaging model (Gibson et al., 2016) with the attenuated mRNA response in the HPC2HOTDOWN trial reflecting a reduction in the physiological stimuli as a result of the prior HPC for all participants (Figure 9).
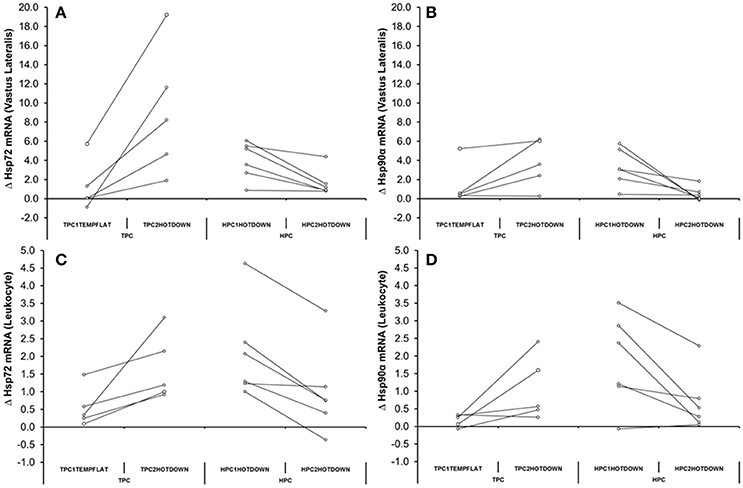
Figure 9. Individual responses reflecting the change in mRNA (A = Hsp72 mRNA in Vastus Lateralis, B = Hsp90α mRNA in Vastus Lateralis, C = Hsp72 mRNA in Leukocytes, DS Hsp90α mRNA in Leukocytes) from baseline to immediately post TPC1TEMPFLAT and TPC2HOTDOWN (A,C), and HPC1HOTDOWN and HPC2HOTDOWN (B,D).
HSP72 protein concentrations (due to translational inhibition) may not necessary directly represent the magnitude of the cellular stress response therefore the mRNA response has been proposed as more appropriate (Amorim et al., 2015; Gibson et al., 2015a; Lee et al., 2015). A reduction in the mRNA response is therefore representative of a gain in protein concentration (Marshall et al., 2007). The VL cellular adaptations associated with the repeated bout effect include a strengthened cytoskeleton [increased desmin concentrations (Feasson et al., 2002)] and elevated small HSP concentrations [αβ-crystallin and HSP27 (Paulsen et al., 2009)] and therefore, could be responsible for the attenuated Hsp72 and Hsp90α mRNA responses observed following HPC2HOTDOWN. Optimization of transcriptional and translational processes (Touchberry et al., 2012) and elevated concentrations of anti-apoptotic (Horowitz, 2014) and antioxidant (Horowitz and Kodesh, 2010) proteins, which are implicated in enhanced thermotolerance, could also be responsible for the attenuated Hsp72 and Hsp90α mRNA responses observed following HPC2HOTDOWN within both the VL and leukocytes.
The current study observed for the first time that the leukocyte and VL Hsp72 and Hsp90α mRNA response occurs concurrently (Figures 8C,D). This novel data supports the notion that leukocytes are a desirable tissue site for determining the cellular stress response due to accessibility for analysis following exposure to both systemic signals and to signals of the perfused tissues (Sonna et al., 2007). Some caution should be raised as this experiment did not quantify the leukocyte infiltration to skeletal muscle, a known component of the intramuscular response which follows damaging exercise (Malm et al., 2004), though the time course and magnitude of this response are controversial (St. Pierre Schneider and Tiidus, 2007). A resolution to this issue within future experiments would be quantification of total mRNA (Sanders et al., 2014). As previously discussed the reduction in thermal and metabolic strain mediated within both leukocytes and the VL likely attenuated the increases in protein denaturation during HPC2HOTDOWN and thus could explain the attenuated Hsp72 and Hsp90α mRNA response observed in both tissues. Muscle damage mediated release of ligands [damage associated molecular patterns (DAMPs), circulating cell free DNA and extracellular HSPs (Neubauer et al., 2014)] from skeletal muscle could also explain the concurrent Hsp72 and Hsp90α mRNA responses via a toll like receptor mediated stress response within leukocytes, as previously observed following muscle damaging exercise (Fernandez-Gonzalo et al., 2012). Although elevations in these ligands may be exercise related (Neubauer et al., 2013), evidence for these ligands actually being released from skeletal muscle following exercise is limited. Consequently, the concurrent Hsp72 and Hsp90α mRNA responses are probably dependent on increases in thermal strain and metabolic strain within both leukocytes and the VL, and are unlikely to be muscle damage dependent.
Increases in VL Grp78 mRNA were observed following both HPC1HOTDOWN and HPC2HOTDOWN despite the observed reductions in exercising Tre and DOMS, which are associated with reduced protein denaturation, the key cellular change regulating Grp78 mRNA transcription. Activation of the unfolded protein response also occurs when the endoplasmic reticulum protein load increases during cellular remodeling (Ron and Walter, 2007). Therefore, the Grp78 mRNA response may reflect the need to increase ER protein folding capacity to aid cellular adaptation (Ron and Walter, 2007). These observations combined with the absence of Grp78 mRNA increases within leukocytes suggest that Grp78 mRNA cannot be used as a marker of the cellular stress response, or thermotolerance, at least within the current experimental model.
Practical Applications and Future Directions
The results of this experiment highlight that an acute bout of downhill running in a hot environment is an effective preconditioning strategy to attenuate the increase in thermal strain experienced during a subsequent, equivalent exercise in hot conditions. Typically it is proposed that athletes, workers and the military should perform acclimation/acclimatization prior to traveling to unfamiliar, hot conditions (Racinais et al., 2015). An acute bout of downhill running in hot conditions i.e., whole body preconditioning may therefore be there an appropriate method to expediently elicit thermal protection i.e., a reduction in thermal strain prior to exercise in hot conditions. Given recent evidence of cross acclimation between stressors (Gibson et al., 2015c; Lee et al., 2016; White et al., 2016), it is also possible that this whole body preconditioning strategy will induce physiological and cellular adaptations which are beneficial in unfamiliar stressors e.g., hypoxia. These adaptations may become greater with repeated stress, i.e., repeated HPC, thus providing either a greater magnitude of cytoprotection, or a more prolonged post-HPC level of protection, or a combination of both. It is currently unknown how long the preconditioning effect elicited by HPC1HOTDOWN is retained beyond the 7 d duration we have observed. Without evidencing the decay in HSP72 and HSP90α content this is difficult to estimate, as such this remains an area for future investigation. Measurement of RNA/protein ratios may also aid understanding of the cytoprotective dynamics. The current study suggests that the leukocyte Hsp72 and Hsp90α mRNA responses could potentially be used as a surrogate measure of the HSR within skeletal muscle, at least within the current experimental model (preconditioning via downhill running in a hot environment). Consequently, the leukocyte Hsp72 and Hsp90α mRNA responses are potentially a relevant marker of individuals thermotolerance and thus could be useful for allocating appropriate athletic or occupational workloads without the potential reductions in performance and increased infection risk (within the biopsy incision) associated with skeletal muscle biopsies. The current experimental model utilized a combination of exercise heat stress and downhill running. Consequently, leukocyte Hsp72 mRNA and Hsp90α mRNA responses could be useful for suggesting thermotolerance within situations where exercise heat stress occur, such as military exercises or during athletic competition. Although the concurrent Hsp72 and Hsp90α mRNA responses are unlikely to be mechanistically linked exclusively to a muscle damage response, future work should set out to confirm whether this concurrent leukocyte and skeletal muscle response also occurs within a non-damaging exercise heat stress trial.
Summary and Conclusions
Hot downhill running is an effective preconditioning strategy which ameliorates physiological strain, muscle soreness and the cellular stress response (Hsp72 and Hsp90α mRNA transcription) to a subsequent bout of exercise-heat stress. This preconditioning strategy has applications for athletic, occupational and military populations. The current study suggests that Hsp72 and Hsp90α mRNA act as markers of the cellular stress response within both the VL and leukocytes. Consequently, the leukocyte Hsp72 mRNA and Hsp90α mRNA responses appear to be a surrogate measure of the cellular stress response in the VL. Accordingly, venepuncture to obtain circulating leukocytes provides a viable alternative to muscle sampling via biopsies to determine the cellular stress response to exercise-heat stress.
Author Contributions
JT, PC, LT, and ML conception and design of research; JT, JB, DH, AJM, OP, CK, FR, and SA performed experiments; JT, BC, OG, PC, AWM, LT, and ML analyzed data; JT, BC, OG, PC, LT, and ML interpreted results of experiments; JT and OG prepared figures; JT drafted manuscript; JT, BC, OG, JB. DH, AJM, OP, CK, FR, SA, PC, AJM, AWM, LT, and ML edited and revised manuscript; JT, BC, OG, JB, DH, AJM, OP, CK, FR, SA, PC, AJM, AWM, LT, and ML approved the final version of manuscript.
Funding
No external funding was received in the preparation of this article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the participants who took part in this study. For ML this activity was conducted under the auspices of the National Center for Sport and Exercise Medicine (NCSEM), collaboration between several universities, NHS trusts and sporting and public bodies. The views expressed are those of the author and not necessarily those of NCSEM or the partners involved.
Abbreviations
CT, cycling threshold; DOMS, Delayed onset muscle soreness; Grp78, Glucose regulated protein 78; HOT, Hot testing conditions; HPC, Hot preconditioning group; Hsp, Heat shock protein (number indicates molecular weight); HSF-1, Heat Shock factor-1; HSR, Heat shock response; LT, Lactate threshold; mRNA, Messenger RNA; PBS, Phosphate-buffered saline; QT, Quadriceps tenderness; RH, Relative humidity; RNA, Ribonucleic acid; RPE, Rating of perceived exertion; RT-QPCR, Reverse transcription quantitative polymerase chain reaction; TEMP, Temperate testing conditions; TPC, Temperate preconditioning group; TS, Thermal sensation; UOsm, Urine Osmolality; VL, Vastus lateralis; O2, Oxygen uptake; O2max, Maximal oxygen uptake.
References
Amorim, F. T., Fonseca, I. T., Machado-Moreira, C. A., and de Castro Magalhães, F. (2015). Insights into the role of heat shock proteins 72 to whole-body heat acclimation in humans. Temperature 2, 499–505. doi: 10.1080/23328940.2015.1110655
Baldwin, J., Snow, R. J., and Febbraio, M. A. (2000). Effect of training status and relative exercise intensity on physiological responses in men. Med. Sci. Sports Exerc. 32, 1648–1654. doi: 10.1097/00005768-200009000-00020
Barnett, A., and Maughan, R. J. (1993). Response of unacclimatized males to repeated weekly bouts of exercise in the heat. Br. J. Sports Med. 27, 39–44. doi: 10.1136/bjsm.27.1.39
Beckmann, R. P., Lovett, M., and Welch, W. J. (1992). Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J. Cell Biol. 117, 1137–1150. doi: 10.1083/jcb.117.6.1137
Connolly, P. H., Caiozzo, V. J., Zaldivar, F., Nemet, D., Larson, J., Hung, S.-P., et al. (2004). Effects of exercise on gene expression in human peripheral blood mononuclear cells. J. Appl. Physiol. 97, 1461–1469. doi: 10.1152/japplphysiol.00316.2004
Dolci, A., Fortes, M. B., Walker, F. S., Haq, A., Riddle, T., and Walsh, N. P. (2015). Repeated muscle damage blunts the increase in heat strain during subsequent exercise heat stress. Eur. J. Appl. Physiol. 115, 1577–1588. doi: 10.1007/s00421-015-3143-7
Drust, B., Waterhouse, J., Atkinson, G., Edwards, B., and Reilly, T. (2005). Circadian rhythms in sports performance–an update. Chronobiol. Int. 22, 21–44. doi: 10.1081/CBI-200041039
Duncan, R. F. (2005). Inhibition of Hsp90 function delays and impairs recovery from heat shock. FEBS J. 272, 5244–5256. doi: 10.1111/j.1742-4658.2005.04921.x
Erlejman, A. G., Lagadari, M., Toneatto, J., Piwien-Pilipuk, G., and Galigniana, M. D. (2014). Regulatory role of the 90-kDa-heat-shock protein (Hsp90) and associated factors on gene expression. Biochim. Biophys. Acta 1839, 71–87. doi: 10.1016/j.bbagrm.2013.12.006
Faul, F., Erdfelder, E., Buchner, A., and Lang, A.-G. G. (2009). Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. doi: 10.3758/BRM.41.4.1149
Feasson, L., Stockholm, D., Freyssenet, D., Richard, I., Duguez, S., Beckmann, J. S., et al. (2002). Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J. Physiol. 543, 297–306. doi: 10.1113/jphysiol.2002.018689
Febbraio, M. A., Carey, M. F., Snow, R. J., Stathis, C. G., and Hargreaves, M. (1996). Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. Am. J. Physiol. 271, R1251–R1255.
Febbraio, M. A., and Koukoulas, I. (2000). HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J. Appl. Physiol. 89, 1055–1060. Available online at: http://jap.physiology.org/content/89/3/1055.full.pdf+html
Fehrenbach, E., Niess, A. M., Schlotz, E., Passek, F., Dickhuth, H.-H., and Northoff, H. (2000a). Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J. Appl. Physiol. 89, 704–710. Available online at: http://jap.physiology.org/content/89/2/704.long
Fehrenbach, E., Niess, A. M., Veith, R., Dickhuth, H. H., and Northoff, H. (2001). Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J. Leukoc. Biol. 69, 747–754. Available online at: http://www.jleukbio.org/content/69/5/747.abstract
Fehrenbach, E., and Northoff, H. (2001). Free radicals, exercise, apoptosis, and heat shock proteins. (Radicaux libres, exercice, apoptose et proteines du choc thermique). Exerc. Immunol. Rev. 7, 66–89.
Fehrenbach, E., Passek, F., Niess, A. M., Pohla, H., Weinstock, C., Dickhuth, H. H., et al. (2000b). HSP expression in human leukocytes is modulated by endurance exercise. (L'expression des proteines HSP dans les leucocytes humains est modulee par un effort d'endurance). Med. Sci. Sport. Exerc. 32, 592–600.
Fehrenbach, E., Veith, R., Schmid, M., Dickhuth, H.-H., Northoff, H., and Niess, A. M. (2003). Inverse response of leukocyte heat shock proteins and DNA damage to exercise and heat. Free Radic. Res. 37, 975–982. doi: 10.1080/10715760310001595748
Fernandez-Gonzalo, R., De Paz, J. A., Rodriguez-Miguelez, P., Cuevas, M. J., and Gonzalez-Gallego, J. (2012). Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J. Appl. Physiol. 112, 2011–2018. doi: 10.1152/japplphysiol.01499.2011
Field, A. (2013). Discovering Statistics Using IBM SPSS Statistics. 4th Edn. London: SAGE Publications.
Fridén, J., Sjöström, M., and Ekblom, B. (1981). A morphological study of delayed muscle soreness. Experientia 37, 506–507. doi: 10.1007/BF01986165
Gibson, O. R., Dennis, A., Parfitt, T., Taylor, L., Watt, P. W., and Maxwell, N. S. (2014). Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones 19, 389–400. doi: 10.1007/s12192-013-0468-1
Gibson, O. R., Mee, J. A., Taylor, L., Tuttle, J. A., Watt, P. W., Maxwell, N. S., et al. (2015a). Isothermic and fixed-intensity heat acclimation methods elicit equal increases in Hsp72 mRNA. Scand. J. Med. Sci. Sports 25, 259–268. doi: 10.1111/sms.12430
Gibson, O. R., Mee, J. A., Tuttle, J. A., Taylor, L., Watt, P. W., and Maxwell, N. S. (2015b). Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J. Therm. Biol. 49–50, 55–65. doi: 10.1016/j.jtherbio.2015.02.005
Gibson, O. R., Taylor, L., Watt, P. W., and Maxwell, N. S. (2017). Cross-adaptation: heat and cold adaptation to improve physiological and cellular responses to hypoxia. Sports Med. doi: 10.1007/s40279-017-0717-z
Gibson, O. R., Turner, G., Tuttle, J. A., Taylor, L., Watt, P. W., and Maxwell, N. S. (2015c). Heat acclimation attenuates physiological strain and the HSP72, but not HSP90α, mRNA response to acute normobaric hypoxia. J. Appl. Physiol. 119, 889–899. doi: 10.1152/japplphysiol.00332.2015
Gibson, O. R., Tuttle, J. A., Watt, P. W., Maxwell, N. S., and Taylor, L. (2016). Hsp72 and Hsp90α mRNA transcription is characterised by large, sustained changes in core temperature during heat acclimation. Cell Stress Chaperones 21, 1021–1035. doi: 10.1007/s12192-016-0726-0
Heldens, L., Hensen, S. M. M., Onnekink, C., van Genesen, S. T., Dirks, R. P., and Lubsen, N. H. (2011). An atypical unfolded protein response in heat shocked cells. PLoS ONE 6:e23512. doi: 10.1371/journal.pone.0023512
Hillman, A. R., Turner, M. C., Peart, D. J., Bray, J. W., Taylor, L., McNaughton, L. R., et al. (2013). A comparison of hyperhydration versus ad libitum fluid intake strategies on measures of oxidative stress, thermoregulation, and performance. Res. Sports Med. 21, 305–317. doi: 10.1080/15438627.2013.825796
Hillman, A. R., Vince, R. V., Taylor, L., McNaughton, L., Mitchell, N., and Siegler, J. (2011). Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl. Physiol. Nutr. Metab. 36, 698–706. doi: 10.1139/h11-080
Horowitz, M. (2014). Heat acclimation, epigenetics, and cytoprotection memory. Compr. Physiol. 4, 199–230. doi: 10.1002/cphy.c130025
Horowitz, M., and Kodesh, E. (2010). Molecular signals that shape the integrative responses of the heat-acclimated phenotype. Med. Sci. Sports Exerc. 42, 2164–2172. doi: 10.1249/MSS.0b013e3181e303b0
Hung, C.-H., Chang, N.-C., Cheng, B.-C., and Lin, M.-T. (2005). Progressive exercise preconditioning protects against circulatory shock during experimental heatstroke. Shock 23, 426–433. doi: 10.1097/01.shk.0000159557.95285.96
Isanejad, A., Saraf, Z. H., Mahdavi, M., Gharakhanlou, R., Shamsi, M. M., and Paulsen, G. (2015). The effect of endurance training and downhill running on the expression of IL-1β, IL-6, and TNF-α and HSP72 in rat skeletal muscle. Cytokine 73, 302–308. doi: 10.1016/j.cyto.2015.03.013
Kampinga, H., Brunsting, J. F., Stege, G. J., Burgman, P. W., and Konings, A. W. (1995). Thermal protein denaturation and protein aggregation in cells made thermotolerant by various chemicals: role of heat shock proteins. Exp. Cell Res. 219, 536–546. doi: 10.1006/excr.1995.1262
Khassaf, M., Child, R. B., McArdle, A., Brodie, D. A., Esanu, C., and Jackson, M. J. (2001). Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J. Appl. Physiol. 90, 1031–1035. Available online at: http://jap.physiology.org/content/90/3/1031.long
Kiang, J. G., Wang, X. D., Ding, X. Z., Gist, I. D., and Smallridge, R. C. (1996). Heat shock inhibits the hypoxia-induced effects on iodide uptake and signal transduction and enhances cell survival in rat thyroid FRTL-5 cells. Thyroid 6, 475–483. doi: 10.1089/thy.1996.6.475
Kourtis, N., and Tavernarakis, N. (2011). Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 30, 2520–2531. doi: 10.1038/emboj.2011.162
Lee, B. J., Emery-Sinclair, E. L., Mackenzie, R. W., Hussain, A., Taylor, L., James, R. S., et al. (2014). The impact of submaximal exercise during heat and/or hypoxia on the cardiovascular and monocyte HSP72 responses to subsequent (post 24 h) exercise in hypoxia. Extrem. Physiol. Med. 3:15. doi: 10.1186/2046-7648-3-15
Lee, B. J., Miller, A., James, R. S., and Thake, C. D. (2016). Cross acclimation between heat and hypoxia: Heat acclimation improves cellular tolerance and exercise performance in acute normobaric hypoxia. Front. Physiol. 7:78. doi: 10.3389/fphys.2016.00078
Lee, E. C., Muñoz, C. X., McDermott, B. P., Beasley, K. N., Yamamoto, L. M., Hom, L. L., et al. (2015). Extracellular and cellular Hsp72 differ as biomarkers in acute exercise/environmental stress and recovery. Scand. J. Med. Sci. Sports 27, 66–74. doi: 10.1111/sms.12621
Liu, Y., Lormes, W., Wang, L., Reissnecker, S., and Steinacker, J. M. (2004). Different skeletal muscle HSP70 responses to high-intensity strength training and low-intensity endurance training. Eur. J. Appl. Physiol. 91, 330–335. doi: 10.1007/s00421-003-0976-2
Lorenzo, S., Minson, C. T., Babb, T. G., and Halliwill, J. R. (2011). Lactate threshold predicting time-trial performance: impact of heat and acclimation. J. Appl. Physiol. 111, 221–227. doi: 10.1152/japplphysiol.00334.2011
MacInnis, M. J., McGlory, ‘C., Gibala, M. J., and Phillips, S. M. (2017). Investigating human skeletal muscle physiology with unilateral exercise models: when one limb is more powerful than two. Appl. Physiol. Nutr. Metab. 42, 563–570. doi: 10.1139/apnm-2016-0645
Madden, L. A., Sandström, M. E., Lovell, R. J., and McNaughton, L. (2008). Inducible heat shock protein 70 and its role in preconditioning and exercise. Amino Acids 34, 511–516. doi: 10.1007/s00726-007-0004-7
Mahoney, D. J., Carey, K., Fu, M.-H., Snow, R., Cameron-Smith, D., Parise, G., et al. (2004). Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol. Genomics 18, 226–231. doi: 10.1152/physiolgenomics.00067.2004
Mahoney, D. J., Safdar, A., Parise, G., Melov, S., Fu, M., MacNeil, L., et al. (2008). Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1901–R1910. doi: 10.1152/ajpregu.00847.2007
Malm, C., Sjödin, T. L. B., Sjöberg, B., Lenkei, R., Renström, P., Lundberg, I. E., et al. (2004). Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 556, 983–1000. doi: 10.1113/jphysiol.2003.056598
Marshall, H. C., Campbell, S. A., Roberts, C. W., and Nimmo, M. A. (2007). Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J. Therm. Biol. 32, 341–348. doi: 10.1016/j.jtherbio.2007.04.003
McMillan, D. R., Xiao, X., Shao, L., Graves, K., and Benjamin, I. J. (1998). Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 273, 7523–7528. doi: 10.1074/jbc.273.13.7523
Mee, J. A., Gibson, O. R., Tuttle, J. A., Taylor, L., Watt, P. W., Doust, J., et al. (2016). Leukocyte Hsp72 mRNA transcription does not differ between males and females during heat acclimation. Temperature 3, 549–556. doi: 10.1080/23328940.2016.1214336
Mestre-Alfaro, A., Ferrer, M. D., Banquells, M., Riera, J., Drobnic, F., Sureda, A., et al. (2012). Body temperature modulates the antioxidant and acute immune responses to exercise. Free Radic. Res. 46, 799–808. doi: 10.3109/10715762.2012.680193
Michailidis, Y., Karagounis, L. G., Terzis, G., Jamurtas, A. Z., Spengos, K., Tsoukas, D., et al. (2013). Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 98, 233–245. doi: 10.3945/ajcn.112.049163
Moran, D. S., Eli-Berchoer, L., Heled, Y., Mendel, L., Schocina, M., and Horowitz, M. (2006). Heat intolerance: does gene transcription contribute? J. Appl. Physiol. 100, 1370–1376. doi: 10.1152/japplphysiol.01261.2005
Morton, J. P., Holloway, K., Woods, P., Cable, N. T., Burniston, J., Evans, L., et al. (2009). Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve 39, 230–233. doi: 10.1002/mus.21182
Morton, J. P., MacLaren, D. P. M., Cable, N. T., Bongers, T., Griffiths, R. D., Campbell, I. T., et al. (2006). Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J. Appl. Physiol. 101, 176–182. doi: 10.1152/japplphysiol.00046.2006
Morton, J. P., MacLaren, D. P. M., Cable, N. T., Campbell, I. T., Evans, L., Bongers, T., et al. (2007). Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiol. 190, 319–327. doi: 10.1111/j.1748-1716.2007.01711.x
Morton, J. P., Maclaren, D. P. M., Cable, N. T., Campbell, I. T., Evans, L., Kayani, A. C., et al. (2008). Trained men display increased basal heat shock protein content of skeletal muscle. Med. Sci. Sports Exerc. 40, 1255–1262. doi: 10.1249/MSS.0b013e31816a7171
Neubauer, O., Sabapathy, S., Ashton, K. J., Desbrow, B., Peake, J. M., Lazarus, R., et al. (2014). Time course-dependent changes in the transcriptome of human skeletal muscle during recovery from endurance exercise: from inflammation to adaptive remodeling. J. Appl. Physiol. 116, 274–287. doi: 10.1152/japplphysiol.00909.2013
Neubauer, O., Sabapathy, S., Lazarus, R., Jowett, J. B. M., Desbrow, B., Peake, J. M., et al. (2013). Transcriptome analysis of neutrophils after endurance exercise reveals novel signaling mechanisms in the immune response to physiological stress. J. Appl. Physiol. 114, 1677–1688. doi: 10.1152/japplphysiol.00143.2013
Nielsen, V. G., and Webster, R. O. (1987). Inhibition of human polymorphonuclear leukocyte functions by ibuprofen. Immunopharmacology 13, 61–71. doi: 10.1016/0162-3109(87)90027-0
Niess, A. M., Fehrenbach, E., Schlotz, E., Sommer, M., Angres, C., Tschositsch, K., et al. (2002). Effects of RRR-alpha-tocopherol on leukocyte expression of HSP72 in response to exhaustive treadmill exercise. Int. J. Sports Med. 23, 445–452. doi: 10.1055/s-2002-33741
Oehler, R., Pusch, E., Zellner, M., Dungel, P., Hergovics, N., Homoncik, M., et al. (2001). Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones 6, 306–15. doi: 10.1379/1466-1268(2001)006<0306:CTSVIT>2.0.CO;2
Paulsen, G., Lauritzen, F., Bayer, M. L., Kalhovde, J. M., Ugelstad, I., Owe, S. G., et al. (2009). Subcellular movement and expression of HSP27, alphaB-crystallin, and HSP70 after two bouts of eccentric exercise in humans. J. Appl. Physiol. 107, 570–582. doi: 10.1152/japplphysiol.00209.2009
Paulsen, G., Vissing, K., Kalhovde, J. M., Ugelstad, I., Bayer, M. L., Kadi, F., et al. (2007). Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R844–R853. doi: 10.1152/ajpregu.00677.2006
St. Pierre Schneider, B., and Tiidus, P. M. (2007). Neutrophil Infiltration in Exercise-Injured Skeletal Muscle. Sports Med. 37, 837–856. doi: 10.2165/00007256-200737100-00002
Puntschart, A., Vogt, M., Widmer, H. R., Hoppeler, H., and Billeter, R. (1996). Hsp70 expression in human skeletal muscle after exercise. Acta Physiol. Scand. 157, 411–417. doi: 10.1046/j.1365-201X.1996.512270000.x
Racinais, S., Alonso, J. M., Coutts, A. J., Flouris, A. D., Girard, O., González-Alonso, J., et al. (2015). Consensus recommendations on training and competing in the heat. Scand. J. Med. Sci. Sports 25, 6–19. doi: 10.1111/sms.12467
Ron, D., and Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529. doi: 10.1038/nrm2199
Ruell, P. A., Thompson, M. W., Hoffman, K. M., Brotherhood, J. R., and Richards, D. A. B. (2007). Lymphocyte HSP72 following exercise in hyperthermic runners: the effect of temperature. J. Therm. Biol. 32, 406–412. doi: 10.1016/j.jtherbio.2007.07.001
Sanders, R., Mason, D. J., Foy, C. A., and Huggett, J. F. (2014). Considerations for accurate gene expression measurement by reverse transcription quantitative PCR when analysing clinical samples. Anal. Bioanal. Chem. 406, 6471–6483. doi: 10.1007/s00216-014-7857-x
Sawka, M. N., Burke, L. M., Eichner, E. R., Maughan, R. J., Montain, S. J., and Stachenfeld, N. S. (2007). American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 39, 377–390. doi: 10.1249/mss.0b013e31802ca597
Sawka, M. N., Leon, L. R., Montain, S. J., and Sonna, L. A. (2011). Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr. Physiol. 1, 1883–1928. doi: 10.1002/cphy.c100082
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Selkirk, G. A., McLellan, T. M., Wright, H. E., and Rhind, S. G. (2009). Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R575–R586. doi: 10.1152/ajpregu.90683.2008
Shima, Y., Kitaoka, K., Yoshiki, Y., Maruhashi, Y., Tsuyama, T., and Tomita, K. (2008). Effect of heat shock preconditioning on ROS scavenging activity in rat skeletal muscle after downhill running. J. Physiol. Sci. 58, 341–348. doi: 10.2170/physiolsci.RP004808
Sonna, L. A., Sawka, M. N., and Lilly, C. M. (2007). Exertional heat illness and human gene expression. Prog. Brain Res. 162, 321–346. doi: 10.1016/S0079-6123(06)62016-5
Taipale, M., Jarosz, D. F., and Lindquist, S. (2010). HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528. doi: 10.1038/nrm2918
Taylor, L., Hillman, A., and Midgley, A. (2012). Hypoxia mediated prior induction of monocyte-expressed HSP72 and HSP32 provides protection to the disturbances to redox balance associated with human sub-maximal aerobic exercise. Amino Acids 43, 1933–1944. doi: 10.1007/s00726-012-1265-3
Taylor, L., Midgley, A., and Chrismas, B. (2010a). The effect of acute hypoxia on heat shock protein 72 expression and oxidative stress in vivo. Eur. J. Appl. Physiol. 109, 849–855. doi: 10.1007/s00421-010-1430-x
Taylor, L., Midgley, A. W., Chrismas, B., Hilman, A. R., Madden, L. A., Vince, R. V, et al. (2011). Daily hypoxia increases basal monocyte HSP72 expression in healthy human subjects. Amino Acids 40, 393–401. doi: 10.1007/s00726-010-0644-x
Taylor, L., Midgley, A. W., Chrismas, B., Madden, L. A., Vince, R. V., and McNaughton, L. R. (2010b). Daily quadratic trend in basal monocyte expressed HSP72 in healthy human subjects. Amino Acids 38, 1483–1488. doi: 10.1007/s00726-009-0360-6
Theodorakis, N. G., Drujan, D., and De Maio, A. (1999). Thermotolerant cells show an attenuated expression of Hsp70 after heat shock. J. Biol. Chem. 274, 12081–12086. doi: 10.1074/jbc.274.17.12081
Touchberry, C. D., Gupte, A. A., Bomhoff, G. L., Graham, Z. A., Geiger, P. C., and Gallagher, P. M. (2012). Acute heat stress prior to downhill running may enhance skeletal muscle remodeling. Cell Stress Chaperones 17, 693–705. doi: 10.1007/s12192-012-0343-5
Trappe, T. A., Standley, R. A., Liu, S. Z., Jemiolo, B., Trappe, S. W., and Harber, M. P. (2013). Local anesthetic effects on gene transcription in human skeletal muscle biopsies. Muscle Nerve 48, 591–593. doi: 10.1002/mus.23860
Tupling, A. R., Bombardier, E., Stewart, R. D., Vigna, C., and Aqui, A. E. (2007). Muscle fiber type-specific response of Hsp70 expression in human quadriceps following acute isometric exercise. J. Appl. Physiol. 103, 2105–2111. doi: 10.1152/japplphysiol.00771.2007
Tuttle, J. A., Castle, P. C., Metcalfe, A. J., Midgley, A. W., Taylor, L., and Lewis, M. P. (2015). Downhill running and exercise in hot environments increase leukocyte Hsp 72 (HSPA1A) and Hsp90α (HSPC1) gene transcripts. J. Appl. Physiol. 118, 996–1005. doi: 10.1152/japplphysiol.00387.2014
Tyler, C. J., Reeve, T., Hodges, G. J., and Cheung, S. S. (2016). The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med. 46, 1699–1724. doi: 10.1007/s40279-016-0538-5
van Oosten-Hawle, P., Porter, R. S., and Morimoto, R. I. (2013). Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 153, 1366–1378. doi: 10.1016/j.cell.2013.05.015
Van Wijck, K., Lenaerts, K., Van Bijnen, A. A., Boonen, B., Van Loon, L. J. C., Dejong, C. H. C., et al. (2012). Aggravation of exercise-induced intestinal injury by Ibuprofen in athletes. Med. Sci. Sports Exerc. 44, 2257–2262. doi: 10.1249/MSS.0b013e318265dd3d
Vissing, K., Bayer, M. L., Overgaard, K., Schjerling, P., and Raastad, T. (2009). Heat shock protein translocation and expression response is attenuated in response to repeated eccentric exercise. Acta Physiol. 196, 283–293. doi: 10.1111/j.1748-1716.2008.01940.x
Walsh, R. C., Koukoulas, I., Garnham, A., Moseley, P. L., Hargreaves, M., and Febbraio, M. A. (2001). Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6, 386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2
Keywords: downhill running, heat shock response, heat stress, heat tolerance, preconditioning, cross tolerance, thermotolerance
Citation: Tuttle JA, Chrismas BCR, Gibson OR, Barrington JH, Hughes DC, Castle PC, Metcalfe AJ, Midgley AW, Pearce O, Kabir C, Rayanmarakar F, Al-Ali S, Lewis MP and Taylor L (2017) The Hsp72 and Hsp90α mRNA Responses to Hot Downhill Running Are Reduced Following a Prior Bout of Hot Downhill Running, and Occur Concurrently within Leukocytes and the Vastus Lateralis. Front. Physiol. 8:473. doi: 10.3389/fphys.2017.00473
Received: 30 March 2017; Accepted: 21 June 2017;
Published: 12 July 2017.
Edited by:
Igor B. Mekjavic, Jožef Stefan Institute, SloveniaReviewed by:
Michal Horowitz, Hebrew University of Jerusalem, IsraelEric Rullman, Karolinska Institutet, Sweden
Copyright © 2017 Tuttle, Chrismas, Gibson, Barrington, Hughes, Castle, Metcalfe, Midgley, Pearce, Kabir, Rayanmarakar, Al-Ali, Lewis and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lee Taylor, lee.taylor@aspetar.com
†Joint senior authors.
 James A. Tuttle
James A. Tuttle Bryna C. R. Chrismas
Bryna C. R. Chrismas Oliver R. Gibson
Oliver R. Gibson James H. Barrington1
James H. Barrington1 David C. Hughes
David C. Hughes Alan J. Metcalfe
Alan J. Metcalfe Adrian W. Midgley
Adrian W. Midgley Lee Taylor
Lee Taylor