- 1State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China
- 2University of Chinese Academy of Sciences, Beijing, China
Carbon dioxide (CO2) is involved in plant growth as well as plant responses to abiotic stresses; however, it remains unclear whether CO2 is involved in the response of rice (Oryza sativa) to aluminum (Al) toxicity. In the current study, we discovered that elevated CO2 (600 μL·L−1) significantly alleviated Al-induced inhibition of root elongation that occurred in ambient CO2 (400 μL·L−1). This protective effect was accompanied by a reduced Al accumulation in root apex. Al significantly induced citrate efflux and the expression of OsALS1, but elevated CO2 had no further effect. By contrast, elevated CO2 significantly decreased Al-induced accumulation of hemicellulose, as well as its Al retention. As a result, the amount of Al fixed in the cell wall was reduced, indicating an alleviation of Al-induced damage to cell wall function. Furthermore, elevated CO2 decreased the Al-induced root nitric oxide (NO) accumulation, and the addition of the NO scavenger c-PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) abolished this alleviation effect, indicating that NO maybe involved in the CO2-alleviated Al toxicity. Taken together, these results demonstrate that the alleviation of Al toxicity in rice by elevated CO2 is mediated by decreasing hemicellulose content and the Al fixation in the cell wall, possibly via the NO pathway.
Introduction
Atmospheric levels of carbon dioxide (CO2), which is the most important gas contributing to global climate change (IPCC, 2007), have risen from 280 μL·L−1 during the Industrial Revolution to about 391 μL·L−1 today (Conway and Tans, 2011), and are predicted to peak between 490 and 1,260 μL·L−1 in the coming decades (Houghton et al., 2001). As a critical substance converted by plants into sugars and other carbohydrates, the increasing concentration of atmospheric CO2 has major influences on plant growth, such as affecting the acquisition of nutrients. Kogawara et al. (2006) demonstrated that the phosphate (P) demand of Japanese red pine (Pinus densiflora) was altered under different CO2 concentrations, and Jin et al. (2009) proposed that elevated CO2 improves tomato (Solanum lycopersicum) iron nutrition; however, whether elevated CO2 influences plant responses to metal toxicity such as Al is still a key unresolved issue.
Aluminum (Al), the most abundant metal on earth, inhibits root elongation in plants at very low concentrations, which in turn hinders their acquisition of nutrients (Kochian, 1995). As a result, the production and the quality of crops growing on Al-polluted land are decreased (Matsumoto, 2000). Al mainly exists as Al3+ in soils with a pH below 5, which is the most toxic form to plants. Extensive studies have reported that Al disrupts a set of physiological and molecular processes, such as cell wall biosynthesis and calcium metabolism, as well as distorting the structure of the plasma membrane (Panda et al., 2009); however, the primary mechanisms underlying the Al-induced inhibition of root growth remain elusive.
Plants use both internal and external mechanism to cope with Al stress (Kochian et al., 2005). To achieve external detoxification, plants either exclude Al from the root tip through secretion of Al-binding organic acids such as citrate, oxalate, and malate (Kochian et al., 2004), or bind Al in the cell wall (Delhaize et al., 1993; Zhu et al., 2013), both of which directly prevent Al from entering the cell (Magalhaes et al., 2007). The internal detoxification mechanism requires the chelation of Al inside the vacuoles (Ma, 2007). However, it is unclear how plants sense and respond to Al stress under conditions of elevated CO2.
Rice (Oryza sativa) is one of the most important crops worldwide and also is the most Al-resistant crop (Fageria, 1989), which is mediated by the expression of Al-responsive gene (Tsutsui et al., 2012), such as ART1 (for Al resistance transcription factor 1), STAR1 (for sensitive to Al rhizotoxicity1), STAR2 and ASR5 (Abscisic acid, Stress, and Ripening 5). ART1 is a C2H2-type zinc finger transcription factor that localized in the nucleus of all root cells, regulates the expression of 31 genes related to Al tolerance in rice (Oryza sativa; Yamaji et al., 2009), including STAR1 and STAR2. STAR1 encodes a nucleotide binding domain, and interacts with OsSTAR2 to form a ABC transporter that responsible for the transporation of the UDP-glucose, a substrate used to modify the cell wall and mask the Al-binding sites, while ASR5 is a key transcription factor that mediates the expression of STAR1 through binding to the STAR1 promoter region (Arenhart et al., 2014). Using two typical rice cultivars, “Kasalath” (Kas) and “Nipponbare” (Nip), with different sensitivities to Al stress (Yokosho et al., 2016), the current study demonstrates that elevated CO2 can alleviate Al toxicity through the induction of physiological and molecular changes that reduce Al accumulation in the plants.
Materials and Methods
Plant Materials and Growth Conditions
Rice subspecies O. sativa ssp. indica cultivar “Kasalath” and O. sativa ssp. japonica cultivar “Nipponbare” were used in the present study. Seeds were surface-sterilized using a 1% sodium hypochlorite solution prior to germination on filter paper soaked in sterilized water. After germination the seedlings were transferred to a CaCl2 solution (0.5 mM, pH 4.5) for 3 days. The 3-day-old seedlings were then subjected to a 24 h treatment of the same CaCl2 solution in either ambient CO2 (400 μL·L−1), termed “CK” (control check), or elevated CO2 (600 μL·L−1), termed “CO2”. Two further treatments were generated by the inclusion of 50 μM Al to the CaCl2 solution in either the CK conditions (termed “Al”) or to the elevated CO2 condition (termed “Al + CO2”). The rice seedlings were grown in an environmentally controlled glasshouse, with a photoperiod of 14 h light (26°C) and 10 h dark (23°C). The relative humidity was around 60%.
For experiments involving the application of the NO scavenger c-PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), six further treatments were used. The CK treatment with additional c-PTIO (10 μM) were denoted as “c-PTIO”, while an elevated CO2 treatment using the CaCl2 solution containing 10 μM c-PTIO was also performed “CO2 + c-PTIO”. The Al treatments were also performed with the addition of 10 μM c-PTIO (“Al+c-PTIO”), and a final treatment combining Al + CO2 with 10 μM c-PTIO was termed “Al + CO2 + c-PTIO”. The final pH of all treatment solutions was 4.5.
Effect of Al and Lanthanum (La) on Root Elongation
The elongation of the root before and after various treatments (50 μM Al or 10 μM La under “CK” or “CO2” condition) was measured using a ruler. Relative root growth was defined as the increase in root length as a percentage of the root for a treatment compared to the increase in the CK-treated plants.
Root Apex and Root Cell Wall Extraction
The root apex of rice seedlings was cut to extract the cell wall as described by Yang et al. (2008). Briefly, the root apex (0–1 cm root tip) was ground in liquid nitrogen, after which 5 mL of 75% ethanol was added for a 20 min incubation. The homogenate was centrifuged for 8 min at 12,000 g, and the resulting pellet was washed with 5 mL acetone, then with 5 mL methanol:chloroform (1:1), and finally with 5 mL methanol. The remaining precipitate was defined as the cell wall, and was freeze-dried for further use.
Al Content Measurement
The Al in the root apex and the root cell walls was extracted in 1 mL 2 M HCl with occasional shaking for 24 h, followed by centrifugation at 12,000 g for 5 min. The Al content in the supernatant was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES).
Root Cell Wall Hemicellulose and Total Sugar Measurement
To extract the cell wall hemicellulose, the cell wall material was treated with sterilized water at 100°C three times, after which 24% KOH containing 0.1% NaBH4 was added to the remaining pellet twice, for 12 h each time, before centrifugation at 12,000 g for 15 min. After each KOH extraction, the supernatant was collected and combined to yield the hemicellulose.
The content of total sugars in hemicellulose was used to quantify the hemicellulose content of the cell walls. First, 200 μL of the extracted hemicellulose, 10 μL 80% phenol (v/v), and 1 mL 98% H2SO4 were incubated together at 30°C for 15 min. This solution was subsequently boiled in 100°C water bath for another 15 min, after which the solution was chilled and its absorbance of 490 nm light was determined.
Examination of Citrate Efflux
Exudates from the roots of seedlings subjected to various treatments were collected, and their roots were weighed. The citrate in the root exudates was isolated and measured as described by Zhu et al. (2015).
Measurement of Root NO Accumulation
The accumulation of NO in Kas roots was visualized using a NO probe, DAF-FM DA (4-amino-5-methylamino-2,7-difluorofluorescein diacetate). First, after washing with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-KOH (pH 7.4), the root apex was immersed in 200 μL DAF-FM DA (10 μM) for 30 min in dark. Excess fluorescence was removed by washing with HEPES-KOH (pH 7.4) three times, then visualized using an Eclipse 80i upright microscope with the following filters: EX 460-500, DM 505, and BA 510-560 (Nikon, Minato, Tokyo, Japan). The intensity of the fluorescence was calculated using Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA).
Gene Expression Analysis
RNA was extracted from the root apexes of the treated rice seedlings and reverse-transcribed according to Zhu et al. (2012). The total volume of the real-time PCR mixture was 10 μL, which comprised 0.2 μL each of the forward and reverse primers, 3.6 μL RNase-free water, 5 μL SYBR Premix ExTaq (Takara Bio, Inc., Kusatsu, Shiga, Japan) and 1 μL cDNA. The sequences for the gene-specific primers were as follows: OsNRAT1 (forward: 5′-GAGGCCGTCTGCAGGAGAGG-3′; reverse: 5′-GGAAGTATCTGCAAGCAGCTCTGATGC-3′); OsFRDL4 (forward: 5′-CGTCATCAGCACCATCCACAG-3′; reverse: 5′-TCATTTGCGAAGAAACTTCCACG-3′); OsSTAR1 (forward: 5′-TCGCATTGGCTCGCACCCT-3′; reverse: 5′-TCGTCTTCTTCAGCCGCACGAT-3′); and OsALS1 (forward: 5′-GGTCGTCAGTCTCTGCCTTCTC-3′; reverse: 5′-CCTCCCCATCATTTTCATTTGT-3′). Expression data were normalized with the expression level of OsHISTONE (forward: 5′-AGTTTGGTCGCTCTCGATTTCG-3′; reverse: 5′-TCAACAAGTTGACCACGTCACG-3′; Xia et al., 2010; Yokosho et al., 2011; Huang et al., 2012). Each cDNA sample was run in triplicate.
Statistical Analysis
Each experiment was repeated at least three times, with one set of data shown in Results. The data were analyzed using a one-way analysis of variance (ANOVA) and a Duncan's multiple range test. Different letters on the histograms indicate statistically difference at P < 0.05.
Results
Effect of Elevated CO2 on Al-Induced Inhibition of Root Growth
Three-day-old Kas and Nip rice plants grown for a further 24 h in ambient CO2 (CK) in a medium containing 50 μM Al showed a significant reduction in root length relative to CK, producing roots 69 and 52% shorter than the control, respectively (Figure 1; Supplemental Table 1). This inhibition of root elongation was significantly less severe in plants grown in elevated CO2, with reductions of only 49% in Kas and 32% in Nip, indicating that elevated CO2 can alleviate Al toxicity in rice. Furthermore, the elongation of the Kas and Nip roots in control conditions (without Al) showed almost no difference between ambient and elevated CO2 treatments (Figure 1; Supplemental Table 1), showing that elevated CO2 did not affect root growth directly. However, elevated CO2 did not affect the root growth under other trivalent metals stress such as La (Supplemental Figure 1), indicating that elevated CO2 only can alleviate Al toxicity. Furthermore, as elevated CO2 had a similar effect on the root elongation of Nip and Kas in the presence of Al, and Kas is the more Al-sensitive cultivar (Figure 1; Yokosho et al., 2016), thus all of the following experiments were performed in Kas.
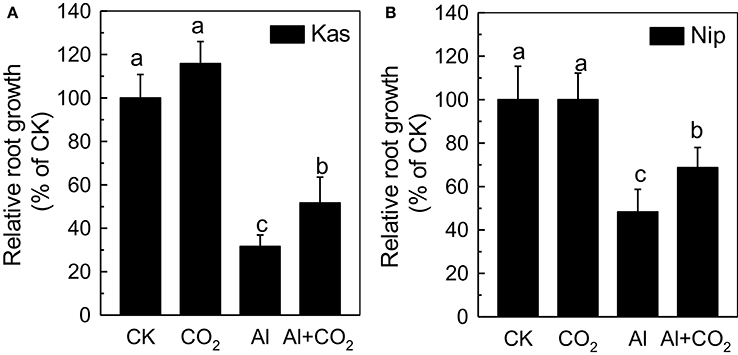
Figure 1. Effects of elevated CO2 on the relative root growth of “Kasalath” (Kas) (A) and “Nipponbare” (Nip) (B). Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). Root length was measured before and after treatment. Data are means ± SD (n = 10). Columns with different letters are significantly different at P < 0.05.
Effect of Elevated CO2 on Rice Root Apex Al Content
To uncover the potential mechanism behind the alleviation of Al toxicity by elevated CO2, Al accumulation in root apex was determined. There was ~0.01 μg Al in the root apex in the absence of Al, which may be due to the impurity of the chemicals used (Figure 2A). Under Al stress, elevated CO2 significantly decreased the root apex Al content when compared with the ambient CO2 treatment, indicating that the alleviation of Al-induced root growth inhibition by elevated CO2 might achieved by reducing Al accumulation. This conclusion was further confirmed by the reduced expression level of NRAMP ALUMINUM TRANSPORTER 1 (NRAT1) in the Al-stressed plants in elevated CO2 compared to those in ambient CO2 (Figure 2B), as NRAT1 is a plasma membrane-localized protein responsible for the uptake of the trivalent Al ion (Xia et al., 2010).
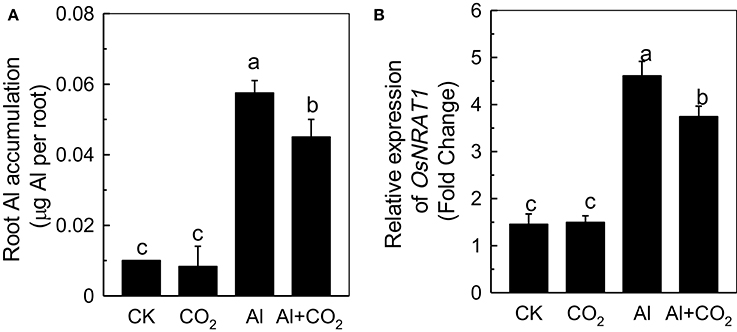
Figure 2. Effects of elevated CO2 on Al accumulation (A) and the expression of OsNRAT1 (B) in Kas roots. Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). The root apex was excised for Al content measurement (A) and for RNA extraction (B). Expression levels of plants grown in ambient CO2 without Al treatment were assigned as expression level of 1. Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Elevated CO2 on Citrate Secretion
The amount of citrate in the root exudate was quantified to determine whether the reduced Al accumulation in roots under elevated CO2 was due to an alteration of the Al-induced secretion of citrate (Yang et al., 2008). Although Al significantly induced the secretion of citrate (Figure 3A), there was no significant difference between the ambient CO2 and elevated CO2 treatments, indicating that the differences in root Al accumulation in the two conditions could not be attributed to the secretion of citrate. This conclusion was further confirmed by the similar levels of FERRIC REDUCTASE DEFECTIVE LIKE4 (FRDL4) expression between plants in the ambient and elevated CO2 conditions (Figure 3B), which is known to be correlated well with the secretion of citrate (Yokosho et al., 2011, 2016).
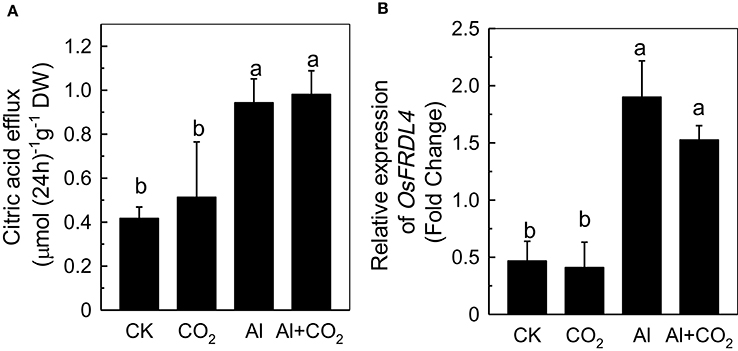
Figure 3. Effects of elevated CO2 on citric acid efflux (A) and the expression of OsFRDL4 (B) in Kas roots. Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). Root exudates were collected (A) and the root apex was detached for RNA extraction (B). Expression levels of plants grown in ambient CO2 without Al treatment were assigned as expression level of 1. Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Elevated CO2 on Root Cell Wall Al Content and Hemicellulose Content
Recently, more and more evidences indicate that cell wall plays pivotal roles when plant in response to Al toxicity (Horst et al., 2010; Zhu et al., 2013), thus we determined the influence of elevated CO2 on cell wall Al accumulation in Kas root. As shown in Figure 4, less Al was accumulated in the root cell walls of Al-treated plants grown in elevated CO2 than those in ambient conditions (Figure 4). One of the component cell wall polysaccharides, hemicellulose, was previously shown to significantly contribute to the Al-binding capacity of the cell wall (Yang et al., 2011); therefore, its involvement in root cell wall Al accumulation was investigated. As expected, the cell walls of Al-treated plants contained significantly more hemicellulose (indicated by the total sugar content in hemicellulose) than the controls; however, when Al-treated plants were grown in elevated CO2, their cell walls contained significantly less hemicellulose than those grown in ambient CO2 (Figure 5B). This correlated well with the reduced Al accumulation in the hemicellulose of the cell walls in plants grown in elevated CO2 (Figure 5A), as the content of hemicellulose correlated well with the Al retention in cell wall and hemicellulose (Zhu et al., 2012), and indicated that less Al entered into the cells under elevated CO2, making the plants more Al resistant. It is noteworthy that Al largely accumulates in the hemicellulose of the cell wall, although a 2-fold difference in hemicellulose content in the Al vs. Al + CO2 treatments was found, there's only a 25% change in Al accumulation in the wall. One possible explanation is that there may be a threshold of the hemicellulose to bind Al, thus a 2-fold increment of hemicelulose under Al treatment does not lead to a 2-fold Al accumulation here, which needs further study.
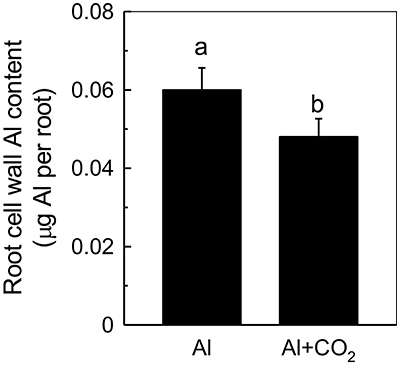
Figure 4. Effects of elevated CO2 treatment on root cell wall Al accumulation in Kas. Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). The root apex was excised for cell wall extraction. Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
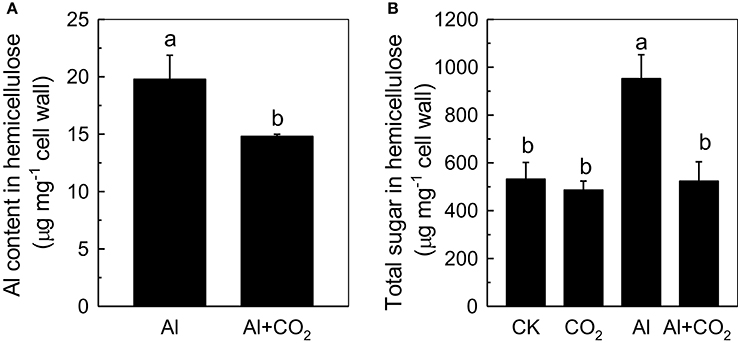
Figure 5. Effects of elevated CO2 treatment on the Al content (A) and the total sugar content (B) of Kas root cell wall hemicellulose. Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
Effect of Elevated CO2 on the Expression of OsALS1 in Roots
Although, most Al is fixed in the root cell wall (Ma et al., 2004), a fraction of the metal can rapidly enter into the cells, initiating an internal detoxification mechanism. OsALS1, a tonoplast transporter localized in rice root tips, can reallocate Al from the cytoplasm to the vacuole (Huang et al., 2012), and is thus essential for the internal detoxification of Al. While the expression of OsALS1 was significantly increased in plants treated with Al, there was almost no difference in its expression between plants grown in ambient or elevated CO2 (Figure 6), suggesting that the OsALS1-based internal detoxification mechanism is not involved in the elevated CO2-enhanced Al resistance.
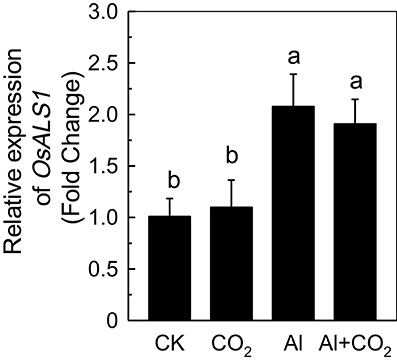
Figure 6. Effects of elevated CO2 treatment on the expression of OsALS1 in Kas. Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). The root apex was excised for RNA extraction. Expression levels of plants grown in ambient CO2 were assigned as expression level of 1. Data are means ± SD (n = 4). Columns with different letters are significantly different at P < 0.05.
Possible Involvement of NO in the Alleviation of Al Toxicity by Elevated CO2
As elevated CO2 can influence endogenous NO content under different stress, such as Fe deficiency in tomato (Jin et al., 2009) and P deficiency in Arabidopsis (Niu et al., 2013), thus we then examined root NO level. Although Al significantly induced the accumulation of NO in the rice roots, the elevated CO2 treatment significantly reduced their NO content (Figure 7). To determine whether this reduction of NO content was involved in the enhancement of Al resistance by elevated CO2, a NO scavenger c-PTIO was added. The c-PTIO treatment had no influence on root growth in the absence of Al, however, Al-treated plants grown in elevated CO2 (Al + CO2 + c-PTIO) condition had a similar level of root growth as those treated with c-PTIO in ambient CO2 condition (Al + c-PTIO; Figure 8B; Supplemental Table 2), indicating that alleviation of Al toxicity by CO2 depends on NO accumulation as eliminating NO abolishes this beneficial effect (Figure 8).
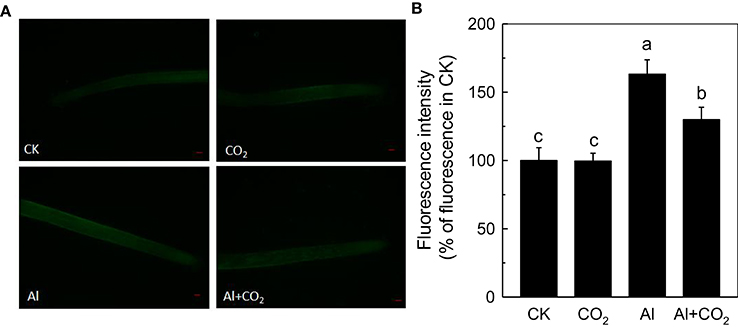
Figure 7. Effects of elevated CO2 treatment on the NO production in Kas. Three-day-old rice seedlings were treated with 0.5 mM CaCl2 solution with or without 50 μM Al under ambient (400 μL·L−1; CK) or elevated (600 μL·L−1; CO2) CO2 for 24 h (pH 4.5). (A) Photographs of NO production shown as green fluorescence in representative roots and (B) NO production expressed as relative fluorescence intensity (% of control). Data are means ± SD (n = 10). Scale bar = 1 mm. Columns with different letters are significantly different at P < 0.05.
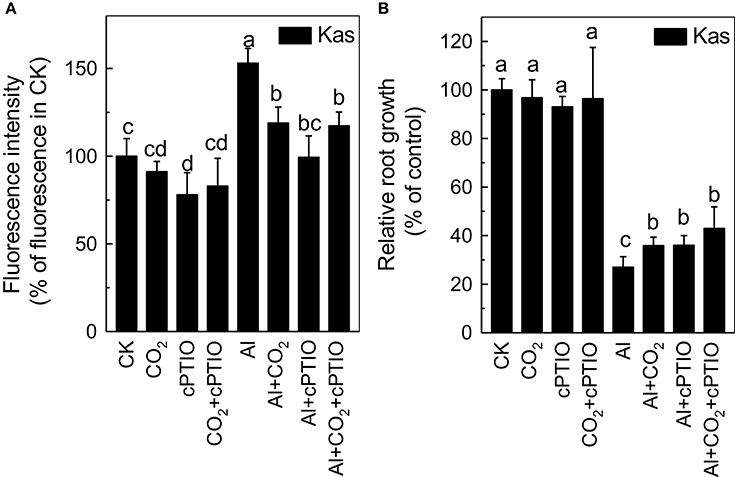
Figure 8. Role of NO in the elevated CO2alleviated Al-toxicity in Kas. Three-day-old rice seedlings were treated with a 0.5 mM CaCl2 solution with or without 50 μM Al under ambient or elevated CO2 for 24 h in the presence or absence of c-PTIO for the NO production analysis (A) and root length measurement (B). NO production was expressed as relative fluorescence intensity (% of control). Root length was measured before and after treatment. Data are means ± SD (n = 10). Columns with different letters are significantly different at P < 0.05.
Discussion
Increases in atmospheric CO2 have previously been shown to stimulate plant growth, increasing the demands for macronutrients such as phosphorus and nitrogen (Lagomarsino et al., 2008), and enhancing the reutilization efficiency of iron and phosphorus where these nutrients are limited (Jin et al., 2009; Niu et al., 2013). Until now, the interaction of elevated CO2 with Al toxicity remained elusive. In this study, we found that elevated CO2 alleviated the Al-induced inhibition of root growth in rice (Figure 1) and demonstrated that elevated CO2 decreased the hemicellulose content of the cell wall and the amount of Al bound to hemicellulose (Figure 5). This in turn decreased the cell wall Al content (Figure 4), resulting in a more Al-resistant rice (Figure 1). Neither the external exclusion mechanism involving the secretion of the organic acids (Figure 3) nor the internal detoxification mechanism regulated by OsALS1 (Figure 6) were found to be involved in this elevated CO2 alleviation of Al toxicity, but NO signaling does appear to participate in this process (Figures 7, 8). To our knowledge, this is the first study to show that elevated CO2 can affect Al sensitivity in rice through the modification of the Al-binding capacity of root cell wall hemicellulose.
The reduced inhibition of Al-treated plant root elongation under elevated CO2 is likely to be related to the reduced Al accumulation (Figures 1, 2A), as root Al levels have been shown to correlate well with Al toxicity in several studies (Frantzios et al., 2001; Wang et al., 2010; Zhu et al., 2012). OsNRAT1, part of the NRAMP (natural resistance-associated macrophage protein) family, is known to be involved in root Al accumulation in rice, as the nrat1 loss-of-function mutant had a reduced root Al content (Xia et al., 2010). In the present study, the expression of OsNRAT1 was up-regulated by Al (Figure 2B), as previously shown by Xia et al. (2010). We found that the OsNRAT1 expression decreased in the elevated CO2 treatment, which might be responsible for the lower root Al accumulation in Al-treated plants grown in elevated rather than ambient CO2 (Figure 1B).
The secretion of a variety of organic acids from the root apex has been demonstrated to play an essential role in preventing Al from entering the root (Kochian et al., 2015), with citrate in soybean (Shen et al., 2005), malate in wheat (Sasaki et al., 2004), and oxalate in buckwheat (Zheng et al., 1998), all being shown to reduce Al toxicity to plants. In the present study, Al significantly induced the secretion of citrate (Figure 3A) and up-regulated the expression of OsFRDL4 (Figure 3B), which is able to transport citrate and correlates well with the secretion of citrate in rice (Yokosho et al., 2011, 2016). Elevated CO2 had no further effect on the secretion of citrate in Al-treated plants (Figure 3A), indicating that citrate efflux does not contribute to the CO2-mediated reduction of Al accumulation in rice roots.
A large proportion of the accumulated Al in the roots is bound to the cell wall, thus, restricting Al to the cell wall is another class of exclusion-based Al-resistance mechanisms in rice. Bound Al is detrimental to the function of the cell wall, making it more rigid and decreasing the wall viscosity and elasticity, which in turn inhibits root elongation (Ma et al., 2004). Studies in rice (Yang et al., 2008) and maize (Eticha et al., 2005) have shown that the more Al that binds to the cell wall, the more sensitive the plant is to Al. Moreover, we recently identified two Arabidopsis mutants, xth31 and xth15, that accumulated less Al in their root cell walls and thus were highly Al resistant (Zhu et al., 2012, 2013). Here, we found that elevated CO2 significantly reduced Al retention in the cell wall (Figure 4), which in turn increased Al resistance in rice (Figure 1A), indicating the operation of the cell-wall-based Al exclusion mechanism. However, the expression of OsSTAR1 is not further up-regulated by elevated CO2 under Al stress (Supplemental Figure 2), indicating that the OsSTAR1 is not involved in CO2-alleviated Al toxicity.
Moreover, as hemicellulose is regarded as the major site for Al binding in the cell wall (Yang et al., 2011), we investigated whether hemicellulose contributed to the reduced cell wall Al accumulation. As expected, elevated CO2 significantly reduced hemicellulose content and Al retention within this polysaccharide (Figure 5), indicating that hemicellulose contributes to the decreased Al accumulation in rice root cell walls under elevated CO2. However, the reason why elevated CO2 significantly reduced the production of the hemicellulose is not clear, we speculated that this may be attributed to the reduced NO accumulation, which has yet to be clarified.
Once Al enters the root cell, an internal detoxification mechanism is activated to compartmentalize Al into the vacuole; for example, Al was sequestered into the vacuole in its Al-organic acids form in hydrangea cell saps (Ma et al., 1997), and in its Al-oxalate form in the protoplasts of buckwheat leaves (Shen et al., 2002). Later, Larsen et al. (2005, 2007) identified ALS3 and ALS1, both of which can sequester Al from more sensitive organs to less sensitive tissues in Arabidopsis, with each knockout mutant exhibiting hypersensitivity to Al. Zhu et al. (2013) demonstrated that auxin negatively regulates Al tolerance by altering AtALS1 expression, which made the growing root suffer Al toxicity. Recently, OsALS1 was characterized, and found to be localized in the tonoplast and responsible for compartmentalizing Al into the vacuoles (Huang et al., 2012). OsALS1 is expressed in all root cells, and the knockout of OsALS1 results in an extremely Al-sensitive plant. In the present study, we tested the expression of OsALS1 in Al-treated plants growing in either ambient or elevated CO2, however, they showed similar levels of expression (Figure 6), suggesting this internal detoxifying mechanism is not involved in CO2-alleviated Al toxicity.
Then, how does the elevated CO2 decrease cell wall Al accumulation and alleviate Al toxicity? NO has previously been shown to be a crucial signaling molecule involved in Al toxicity; for instance, exacerbating Al toxicity in the rice bean (Zhou et al., 2012). In the current study, elevated CO2 significantly decreased the Al-induced production of NO in the roots (Figure 7), and the addition of the NO scavenger c-PTIO made no difference to the Al tolerance of plants in ambient or elevated CO2 conditions (Figure 8). This suggests that the protective effect of elevated CO2 to plants under Al stress may be related to the decreased accumulation NO. Interestingly, elevated CO2 had no effect on the root NO level when in the absence of Al (Figure 7), which may explain why elevated CO2 only specifically improved root elongation in plants faced with Al toxicity. Furthermore, a question is raised as to why CO2 elevation decreases NO levels in roots. Jin et al. (2009) reported that elevated CO2 can induce NO accumulation under Fe-deficient condition in tomato (Solanum lycopersicum), and they speculated that the elevated CO2 first increased the auxin levels (Teng et al., 2006), which subsequently induced the NO accumulation (Du et al., 2008). However, in the present study, CO2 elevation decreased NO accumulation under Al stress, and this inconsistency may be attributed to different plant cultivars, different culture condition, and etc, which needs further investigation.
In conclusion, we have demonstrated for the first time that elevated CO2 can alleviate Al toxicity in rice. This protection is mediated by a cell wall-based Al-exclusion mechanism involving a decrease in hemicellulose content and a reduction in the Al fixation of the hemicellulose. NO may act downstream of CO2 to control these processes; however, this putative mechanism requires further investigation.
Author Contributions
XFZ and RFS designed the research, XFZ, XSZ, BW, and QW performed research, XSZ and BW analyzed data. XFZ and RFS wrote the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the National Key Basic Research Program of China (grant number 2014CB441000), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (grant numbers XDB15030302 and XDB15030202), the Field Frontier Program of the Institute of Soil Science (ISSASIP1601) and Natural Science Foundation of China (grant number 31501825).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2017.00512/full#supplementary-material
References
Arenhart, R. A., Bai, Y., de Oliveira, L. F. V., Neto, L. B., Schunemann, M., dos Santos Maraschin, F., et al. (2014). New insights into aluminum tolerance in rice: the ASR5 protein binds the STAR1 promoter and other aluminum-responsive genes. Mol. Plant 7, 709–721. doi: 10.1093/mp/sst160
Conway, T., and Tans, P. (2011). Trends in Atmospheric Carbon Dioxide. NOAA/ESRL. Available online at: http://www.esrl.noaa.gov/gmd/ccgg/trends
Delhaize, E., Ryan, P. R., and Randall, P. J. (1993). Aluminum tolerance in wheat (Triticum aestivum L.)(II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol. 103, 695–702. doi: 10.1104/pp.103.3.695
Du, S. T., Zhang, Y. S., Lin, X. Y., Wang, Y., and Tang, C. X. (2008). Regulation of nitrate reductase by its partial product nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L. cv. Baoda). Plant Cell Environ. 31, 195–204. doi: 10.1111/j.1365-3040.2007.01750.x
Eticha, D., Stass, A., and Horst, W. J. (2005). Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ. 28, 1410–1420. doi: 10.1111/j.1365-3040.2005.01375.x
Frantzios, G., Galatis, B., and Apostolakos, P. (2001). Aluminium effects on microtubule organization in dividing root-tip cells of Triticum turgidum. II. Cytokinetic cells. J. Plant Res. 114, 157–170. doi: 10.1007/PL00013979
Horst, W. J., Wang, Y., and Eticha, D. (2010). The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann. Bot. 106, 185–197. doi: 10.1093/aob/mcq053
Houghton, J. E. T., Ding, Y. H., Griggs, J., Noguer, M., Pj, V. D. L., Dai, X., et al. (2001). IPCC, 2001: Climate Change 2001: The Scientific Basis. Contribution of Working Group 1 to the Third Assessment Report of the Intergovernmental Panel on Climate Change, Edited by J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, K. Maskell and C. A. Johnson. Cambridge, UK; New York, NY: Cambridge University Press.
Huang, C. F., Yamaji, N., Chen, Z., and Ma, J. F. (2012). A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 69, 857–867. doi: 10.1111/j.1365-313X.2011.04837.x
IPCC (2007). Climate Change 2007: The Fourth IPCC Assessment Report. Valencia: Intergovernmental Panel on Climate Change.
Jin, C. W., Du, S. T., Chen, W. W., Li, G. X., Zhang, Y. S., and Zheng, S. J. (2009). Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol. 150, 272–280. doi: 10.1104/pp.109.136721
Kochian, L. V. (1995). Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Biol. 46, 237–260. doi: 10.1146/annurev.pp.46.060195.001321
Kochian, L. V., Hoekenga, O. A., and Pineros, M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Plant Biol. 55, 459–493. doi: 10.1146/annurev.arplant.55.031903.141655
Kochian, L. V., Pineros, M. A., and Hoekenga, O. A. (2005). The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274, 175–195. doi: 10.1007/s11104-004-1158-7
Kochian, L. V., Pineros, M. A., Liu, J., and Magalhaes, J. V. (2015). Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 66, 571–598. doi: 10.1146/annurev-arplant-043014-114822
Kogawara, S., Norisada, M., Tange, T., Yagi, H., and Kojima, K. (2006). Elevated atmospheric CO2 concentration alters the effect of phosphate supply on growth of Japanese red pine (Pinus densiflora) seedlings. Tree Physiol. 26, 25–33. doi: 10.1093/treephys/26.1.25
Lagomarsino, A., Moscatelli, M. C., Hoosbeek, M. R., De Angelis, P., and Grego, S. (2008). Assessment of soil nitrogen and phosphorous availability under elevated CO2 and N-fertilization in a short rotation poplar plantation. Plant Soil 308, 131–147. doi: 10.1007/s11104-008-9614-4
Larsen, P. B., Cancel, J., Rounds, M., and Ochoa, V. (2007). Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 225, 1447–1458. doi: 10.1007/s00425-006-0452-4
Larsen, P. B., Geisler, M. J. B., Jones, C. A., Williams, K. M., and Cancel, J. D. (2005). ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 41, 353–363. doi: 10.1111/j.1365-313X.2004.02306.x
Ma, J. F. (2007). Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 264, 225–252. doi: 10.1016/S0074-7696(07)64005-4
Ma, J. F., Hiradate, S., Nomoto, K., Iwashita, T., and Matsumoto, H. (1997). Internal detoxification mechanism of Al in hydrangea - Identification of Al form in the leaves. Plant Physiol. 113, 1033–1039. doi: 10.1104/pp.113.4.1033
Ma, J. F., Shen, R. F., Nagao, S., and Tanimoto, E. (2004). Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 45, 583–589. doi: 10.1093/pcp/pch060
Magalhaes, J. V., Liu, J., Guimaraes, C. T., Lana, U. G. P., Alves, V. M. C., Wang, Y. H., et al. (2007). A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 39, 1156–1161. doi: 10.1038/ng2074
Matsumoto, H. (2000). Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev. Cytol. 200, 1–46. doi: 10.1016/S0074-7696(00)00001-2
Niu, Y., Chai, R., Dong, H., Wang, H., Tang, C., and Zhang, Y. (2013). Effect of elevated CO2 on phosphorus nutrition of phosphate-deficient Arabidopsis thaliana (L.) Heynh under different nitrogen forms. J. Exp. Bot. 64, 355–367. doi: 10.1093/jxb/ers341
Panda, S. K., Baluska, F., and Matsumoto, H. (2009). Al stress signaling in plants. Plant Signal. Behav. 4, 592–597. doi: 10.4161/psb.4.7.8903
Sasaki, T., Yamamoto, Y., Ezaki, B., Katsuhara, M., Ahn, S. J., Ryan, P. R., et al. (2004). A wheat gene encoding an aluminum-activated malate transporter. Plant J. 37, 645–653. doi: 10.1111/j.1365-313X.2003.01991.x
Shen, H., He, L. F., Sasaki, T., Yamamoto, Y., Zheng, S. J., Ligaba, A., et al. (2005). Citrate secretion coupled with the modulation of soybean root tip under aluminum stress. Up-regulation of transcription, translation, and threonine-oriented phosphorylation of plasma membrane H+-ATPase. Plant Physiol. 138, 287–296. doi: 10.1104/pp.104.058065
Shen, R. F., Ma, J. F., Kyo, M., and Iwashita, T. (2002). Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta 215, 394–398. doi: 10.1007/s00425-002-0763-z
Teng, N. J., Wang, J., Chen, T., Xu, W., Wang, Y., and Li, J. (2006). Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 172, 92–103. doi: 10.1111/j.1469-8137.2006.01818.x
Tsutsui, T., Yamaji, N., Huang, C. F., Motoyama, R., Nagamura, Y., and Ma, J. F. (2012). Comparative genome-wide transcriptional analysis of Al-responsive genes reveals novel Al tolerance mechanisms in rice. PLoS ONE 7:e48197. doi: 10.1371/journal.pone.0048197
Wang, H. H., Huang, J. J., and Bi, Y. R. (2010). Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 179, 281–288. doi: 10.1016/j.plantsci.2010.05.014
Xia, J., Yamaji, N., Kasai, T., and Ma, J. F. (2010). Plasma membrane-localized transporter for aluminum in rice. Proc. Natl Acad. Sci. U.S.A. 107, 18381–18385. doi: 10.1073/pnas.1004949107
Yamaji, N., Huang, C. F., Nagao, S., Yano, M., Sato, Y., Nagamura, Y., et al. (2009). A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 21, 3339–3349. doi: 10.1105/tpc.109.070771
Yang, J. L., Li, Y. Y., Zhang, Y. J., Zhang, S. S., Wu, Y. R., Wu, P., et al. (2008). Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 146, 602–611. doi: 10.1104/pp.107.111989
Yang, J. L., Zhu, X. F., Peng, Y. X., Zheng, C., Li, G. X., Liu, Y., et al. (2011). Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 155, 1885–1892. doi: 10.1104/pp.111.172221
Yokosho, K., Yamaji, N., and Ma, J. F. (2011). An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 68, 1061–1069. doi: 10.1111/j.1365-313X.2011.04757.x
Yokosho, K., Yamaji, N., Kashino-Fujii, M., and Ma, J. F. (2016). Retrotransposon-mediated aluminum tolerance through enhanced expression of the citrate transporter OsFRDL4. Plant Physiol. 172, 2327–2336. doi: 10.1104/pp.16.01214
Zheng, S. J., Ma, J. F., and Matsumoto, H. (1998). High aluminum resistance in buckwheat - I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 117, 745–751. doi: 10.1104/pp.117.3.745
Zhou, Y., Xu, X. Y., Chen, L. Q., Yang, J. L., and Zheng, S. J. (2012). Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 76, 46–51. doi: 10.1016/j.phytochem.2011.12.004
Zhu, X. F., Lei, G. J., Wang, Z. W., Shi, Y. Z., Braam, J., Li, G. X., et al. (2013). Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol. 162, 1947–1955. doi: 10.1104/pp.113.219147
Zhu, X. F., Shi, Y. Z., Lei, G. J., Fry, S. C., Zhang, B. C., Zhou, Y. H., et al. (2012). XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 24, 4731–4747. doi: 10.1105/tpc.112.106039
Keywords: aluminum (Al) stress, cell wall, CO2, hemicellulose, nitric oxide (NO), root elongation
Citation: Zhu XF, Zhao XS, Wang B, Wu Q and Shen RF (2017) Elevated Carbon Dioxide Alleviates Aluminum Toxicity by Decreasing Cell Wall Hemicellulose in Rice (Oryza sativa). Front. Physiol. 8:512. doi: 10.3389/fphys.2017.00512
Received: 24 January 2017; Accepted: 04 July 2017;
Published: 18 July 2017.
Edited by:
Adriano Nunes-Nesi, Universidade Federal de Viçosa, BrazilReviewed by:
Xiao Feng Li, Guangxi University, ChinaLauro Bücker Neto, UNICENTRO, Brazil
Peter Ryan, Commonwealth Scientific and Industrial Research Organisation, Australia
Copyright © 2017 Zhu, Zhao, Wang, Wu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren Fang Shen, rfshen@issas.ac.cn
 Xiao Fang Zhu1
Xiao Fang Zhu1 Xu Sheng Zhao
Xu Sheng Zhao Qi Wu
Qi Wu Ren Fang Shen
Ren Fang Shen