- 1Institute of Sport Science, University of Rostock, Rostock, Germany
- 2Department of Orthopaedics, Rostock University Medical Center, Rostock, Germany
During cycling before (PRE) and after exhaustion (POST) different modes of autonomic cardiac control might occur due to different interoceptive input and altered influences from higher brain centers. We hypothesized that heart rate variability (HRV) is significantly affected by an interaction of the experimental period (PRE vs. POST) and exercise intensity (HIGH vs. LOW; HIGH = HR > HR at the lactate threshold (HRLT), LOW = HR ≤ HRLT) despite identical average HR.
Methods: Fifty healthy volunteers completed an incremental cycling test until exhaustion. Workload started with 30 W at a constant pedaling rate (60 revolutions · min−1) and was gradually increased by 30 W · 5 min−1. Five adjacent 60 s inter-beat (R-R) interval segments from the immediate recovery period (POST 1–5 at 30 W and 60 rpm) were each matched with their HR-corresponding 60 s-segments during the cycle test (PRE 1–5). An analysis of covariance was carried out with one repeated-measures factor (PRE vs. POST exhaustion), one between-subject factor (HIGH vs. LOW intensity) and respiration rate as covariate to test for significant effects (p < 0.050) on the natural log-transformed root mean square of successive differences between adjacent R-R intervals (lnRMSSD60s).
Results: LnRMSSD60s was significantly affected by the interaction of experimental period × intensity [F(1, 242) = 30.233, p < 0.001, ηp2 = 0.111]. LnRMSSD60s was higher during PRE compared to POST at LOW intensity (1.6 ± 0.6 vs. 1.4 ± 0.6 ms; p < 0.001). In contrast, at HIGH intensity lnRMSSD60s was lower during PRE compared to POST (1.0 ± 0.4 vs. 1.2 ± 0.4 ms; p < 0.001).
Conclusion: Identical net HR during cycling can result from distinct autonomic modulation patterns. Results suggest a pronounced sympathetic-parasympathetic coactivation immediately after the cessation of peak workload compared to HR-matched cycling before exhaustion at HIGH intensity. On the opposite, at LOW intensity cycling, a stronger coactivational cardiac autonomic modulation pattern occurs during PRE-exhaustion if compared to POST-exhaustion cycling. The different autonomic modes during these phases might be the result of different afferent and/or central inputs to the cardiovascular control centers in the brainstem.
Introduction
Despite the interest in heart rate (HR) during exercise and recovery, the precise autonomic contributions to HR control across different intensities and exercise modes are still not fully understood, since direct measurements of autonomic outflow to the intact human heart is not feasible (Fisher, 2014). HR itself mainly reflects the chronotropic net effect of the autonomic nervous system. Thus, complementary information and tools are required to get a deeper understanding of the underlying mechanisms of HR control during exercise. Heart rate variability (HRV) is a non-invasive tool that has the potential to quantify autonomic influences on HR (Billman, 2011). However, it should be noted that HR per se profoundly influences HRV (Billman, 2013). Thus, in the current and previous studies (Weippert et al., 2013, 2015b), a HR-matched approach was applied to elucidate autonomic contributions to exercise HR.
Traditionally, the interplay between the sympathetic and parasympathetic nervous system during progressive exercise was thought to be organized in an antagonistic reciprocal fashion, with vagal withdrawal at the beginning of exercise leading to an HR increase of at most 30 beats · min−1 followed by an increase in sympathetic activity leading to a further HR acceleration (Robinson et al., 1966). Current data support the view of a continuum of balanced sympathetic-parasympathetic control throughout progressive exercise without clear on/off thresholds (Kannankeril et al., 2004; White and Raven, 2014). Thereby, the influence of both autonomic branches is supposed to be non-linear with a stronger increase of sympathetic and a stronger decrease of parasympathetic contributions at exercise intensities above 60% maximum oxygen consumption and HR > 150 beats · min−1 (White and Raven, 2014). Reciprocal antagonism appears to be the dominant mechanism of cardiac autonomic control during orthostatic regulation and many kinds of exercise. However, there are other modes of autonomic control that can generate instantaneous HR under pathological and physiological conditions. Different modulators (e.g., peripheral chemoreceptor, mechanoreceptor, and nociceptor input) can lead to a simultaneous activation (coactivation) of both autonomic branches (Berntson et al., 1991, 1993; Paton et al., 2005, 2006; Fadel and Raven, 2012; Fisher, 2014). Research evaluating the effect of sympathectomy/sympathicolysis in patients with hyperhidrosis speak for a strong cardiac sympathetic-parasympathetic coactivation even under resting conditions (Noppen et al., 1996; Fiorelli et al., 2017). The findings have been linked to a hyperactivity of the sympathetic nervous system during rest in hyperhidrosis, which—also in terms of HR and HRV—can be reversed by this clinical intervention. During physiological conditions, a sympatho-vagal coactivation may allow (i) a greater cardiac output under certain circumstances, such as during the peripheral chemoreceptor reflex and diving responses, as well as (ii) a finer tuning of cardiac function (Paton et al., 2006). Matsukawa (2012) reported that contrary to the traditional idea that vagal withdrawal causes an increase in HR at the onset of exercise, central command did not decrease cardiac vagal efferent activity but allowed sympathetic efferent nerve activity to produce cardiac acceleration in an animal model. Accordingly, recent studies in humans imply that various patterns of autonomic HR control, quantified by HRV, can occur during different exercise modalities (Gonzalez-Camarena et al., 2000; Weippert et al., 2013, 2015b). For example, a significantly different HRV pattern has been shown in response to dynamic and static exercise (Weippert et al., 2014, 2015b) or during dynamic upper and lower body exercise (Leicht et al., 2008) despite similar net effects on HR itself. Different feedback from baro-, metabo-, and mechanosensitive fibers as well as changes in central command and “effort sense” might contribute to these distinct autonomic cardiovascular adjustments during exercise (Boushel, 2010; Matsukawa, 2012; Fisher, 2014; Ichinose et al., 2014).
The main goal of this study was to assess whether autonomic HR control, mirrored by HRV, differs between cycling before volitional exhaustion (PRE) and cycling at a low workload immediately after exhaustion (POST). It was assumed that sympathetic-parasympathetic coactivation is more pronounced during early POST compared to HR-corresponding PRE periods, probably due to a strong vagal rebound following reduced central command and maintained sympathetic activity stimulated by metabo- and baroreceptors. Therefore, five adjacent 60 s-segments of the active recovery period immediately after peak exercise (POST 1–5) were matched with their HR-corresponding 60 s-segments during progressive exercise before exhaustion (PRE 5–1). It was hypothesized that the pattern of autonomic HR modulation, is significantly affected by (i) the experimental period (PRE vs. POST exhaustion) and (ii) the exercise intensity (HIGH vs. LOW intensity) despite identical net effects on average HR. Exercise intensity was dichotomized into LOW [ = HR ≤ HR at the individual lactate threshold (HRLT)] and HIGH (= HR > HRLT). HR-matched PRE and POST exhaustion periods at HIGH or LOW intensity served as a physiological model for states of different afferent and central inputs to the cardiovascular control center in the Medulla oblongata.
Autonomic HR control was evaluated by the analysis of HR as well as of the root mean square of the successive differences (RMSSD60s) of adjacent inter-beat intervals (R-R) over a period of 60 s. It is supposed, that RMSSD60s mirrors predominantly parasympathetic HR modulation (Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology, 1996; Goldberger et al., 2006).
Before we tested the above-mentioned hypothesis, we assessed the internal and external validity of ultra-short-term HRV (= 60 s-segments). Traditionally, short-term evaluation of HRV bases on the analysis of 180–300 s-segments; however, these time windows are not appropriate to give a representative picture of transient processes like recovery from exercise. Validity and reproducibility of HRV ultra-short measures have been tested under resting conditions and recovery from exercise (Thong et al., 2003; Nussinovitch et al., 2011; Esco and Flatt, 2014; Munoz et al., 2015; Nakamura et al., 2017); however, a paucity of research exists regarding the validity and agreement with standard short-term measures during low to high intensity exercise. Therefore, agreement of natural log-transformed 60 s-RMSSD (lnRMSSD60s), -high frequency power (lnHFP60s), and -low frequency power (lnLFP60s) of the R-R spectra with their respective traditional short-term HRV indices, calculated for a time interval of 180 s (lnRMSSD180s, lnHFP180s and lnLFP180s), were assessed across different exercise intensities. In addition, lnRMSSD60s was plotted against the corresponding HR during incremental exercise. The aim was to correlate the behavior of lnRMSSD60s during incremental cycling with the current model of parasympathetic-sympathetic HR control during progressive exercise (White and Raven, 2014).
In conclusion, this paper comprised two studies: (i) a validation study and (ii) a comparison of HRV during HR-matched cycling before and after volitional exhaustion. The validation study (i) was a prerequisite to the second experiment and aimed to fill the gap of knowledge regarding the agreement of ultra-short-term measures of HRV with their respective measures of traditional recording length during various exercise intensities. The comparison study (ii) aimed to elucidate the mechanism of autonomic HR-control during conditions of distinct afferent and central inputs to the cardiovascular control center in the medulla oblongata. Therefore, HRV was compared between exercise and active recovery segments of corresponding net HR.
Material and Methods
Ethics Statement
This study was performed in compliance with the Declaration of Helsinki and approval of the local ethics committee at Rostock University was obtained. All participants gave their written informed consent to take part.
Validation Study
Twenty participants (10 females, 22.8 ± 2.2 years, 62.0 ± 5.3 kg, 169.2 ± 5.8 cm; 10 males, 23.9 ± 1.3 years, 76.1 ± 6.6 kg, 182.5 ± 5.9 cm) volunteered in this study. All participants were non-habitual smokers, free of medication and abstained from any exhaustive exercise and alcohol for >24 h prior to the experiment. Furthermore, the consumption of caffeine or nicotine was not allowed during the night and on the morning of the experiment. After a medical clearance participants performed an incremental cycling test (ER 900, Ergoline, Germany) until volitional exhaustion (initial load 30 W + 30 W 5 · min−1) at a constant pedaling rate (60 revolutions · min−1). Beat-to-beat HR (S810i, Polar, Finland) was continuously measured throughout the test. Individual R-R recordings were screened for five stationary 180 s-segments that covered a wide physiological activity range from low to maximal HR. HRV analysis of the resulting 100 R-R interval segments was performed using the free software Kubios HRV 2.2 (University of Kuopio, Finland). All analyzed R-R recordings exhibited low noise (rate of erroneous R-R intervals below 5%). Before the computation, R-R time series were detrended and corrected for artifacts using adaptive filtering. Frequency analysis was performed using a Fast Fourier Transform (Welch's periodogram: 256 s-window with 50% overlap). Internal validity was assessed for the parasympathetic HRV indices lnHFP and lnRMSSD as well as for lnLFP—mirroring both parasympathetic and sympathetic effects on HR (Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology, 1996; Goldberger et al., 2006; Smith et al., 2013). Agreement of the 180 s- with the 60 s-HRV indices was assessed using estimation equation, mean and standardized mean bias, typical error of estimate, and validity correlation. Statistical indices were calculated and interpreted according to Hopkins (2015). In a second step, lnRMSSD60s was plotted against the corresponding HR to visualize the behavior of the vagally mediated HRV during incremental exercise.
Comparison of HRV During HR-Matched Cycling before and after Exhaustion
Fifty healthy participants (13 females, 25.7 ± 5.8 years, 65.1 ± 10.6 kg, 169.7 ± 5.3 cm; 37 males, 26.4 ± 7.1 years, 77.9 ± 9.6 kg, 182.6 ± 6.3 cm) volunteered in this study. Inclusion criteria as well as load protocol are identical with and described under (i) validation study. Immediately after exhaustion, workload was reduced to 30 W and participants kept pedaling at 60 revolutions · min−1 for additional 10 min (active recovery = POST). Beat-to-beat HR (S810i, Polar, Finland), oxygen uptake (VO2), and carbon dioxide output (VCO2) (EOS Sprint, Jaeger, Germany) were continuously recorded during the test. Blood lactate concentration was determined from capillary blood samples (LactateScout, SensLab, Germany), drawn from the left ear lobe at the end of each stage. Cardiorespiratory data and blood lactate concentration at exhaustion are described in Table 1. HRLT was determined at the lowest value of the lactate-equivalent (ratio of blood lactate concentration and VO2) during the incremental test (Dickhuth et al., 1999). For the statistical testing, exercise intensity was dichotomized into LOW [ = HR ≤ HR at the individual lactate threshold (HRLT)] and HIGH (= HR > HRLT) for both, PRE and POST, periods.
Due to measurement artifacts, data of one male subject had to be excluded from further analysis. For all other participants (N = 49), the first five adjacent 60 s-segments during the immediate active recovery (POST 1–5) were matched with corresponding 60 s-segments yielding the same average HR during the incremental cycling test. Thus, POST 1 was matched with PRE 5, POST 2 with PRE 4 and so on (Figure 1). All analyzed R-R interval recordings exhibited low noise (rate of erroneous R-R intervals below 5%). LnRMSSD60s was used to quantify vagal HR modulation. Before the computation, R-R time series were detrended and corrected for artifacts using adaptive filtering (Kubios HRV 2.2, University of Kuopio, Finland). Bland-Altman Plots (Bland and Altman, 2007) and Student's t-test statistics were applied to statistically verify the agreement of the matched R-R segments. A repeated-measures analysis of variance (IBM SPSS Statistics 22.0, USA) was carried out to test the effect of measurement time, experimental period (PRE vs. POST) and their interaction on lnRMSSD. To test the hypothesis of exercise intensity being a significant contributor to distinct HRV patterns at similar HR levels, experimental period was used as repeated measures factor (PRE vs. POST) and intensity as between subject factor (HIGH vs. LOW). Additionally, high frequency peak values (HFpeak), a measure of respiration rate (Thayer et al., 2002), was entered as covariate to control for potential bias. If data violated the assumption of sphericity, Greenhouse-Geisser corrected p-values and respective degrees of freedom were reported. For post-hoc pair-wise comparisons significance levels were adjusted using Bonferroni's correction.
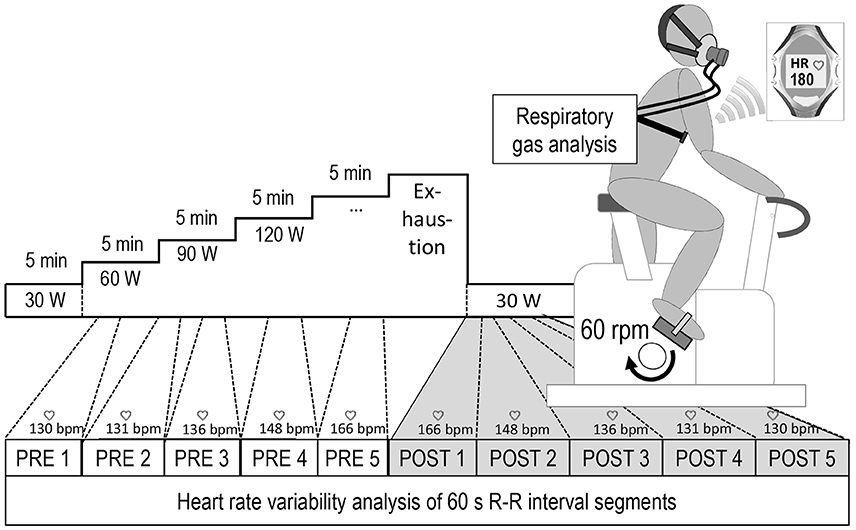
Figure 1. Overview of the experimental setup and the heart rate based data matching procedure, POST 1–5 = five consecutive 60 s-segments during active recovery from cycling until exhaustion, PRE 5–1 = corresponding HR-matched 60 s-periods during exercise.
Results
Validation Study
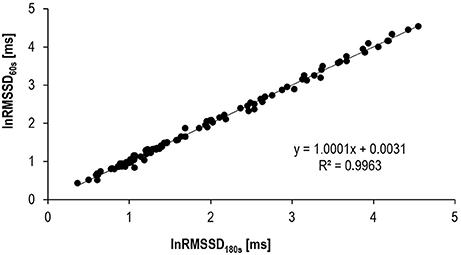
Results of the internal validation study are provided in Table 2 and Figure 2. Mean biases between HRV180s and HRV60s as well as typical errors were very low and the correlations between the very short and the traditional short-term HRV very high.
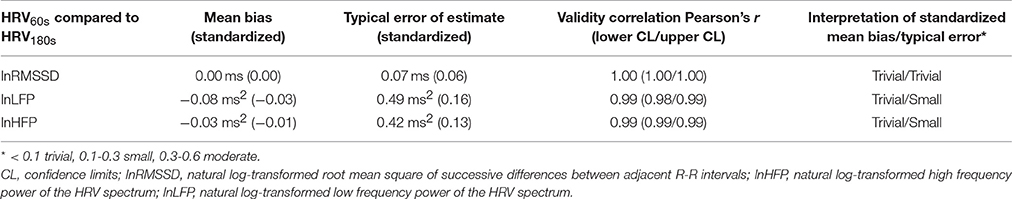
Table 2. Validation indices and interpretation of standardized mean bias and standardized typical error for ultra-short-term HRV indices (60 s) in comparison to traditional short-term HRV (180 s).
LnHFP60s, lnLFP60s, and lnRMSSD60s showed high agreement with their respective traditional short-term 180 s-HRV indices. Confidence limits of the validity correlation coefficients r were between 0.98 and 1.00.
Results of the external validity approach showed a three-phase behavior for lnRMSSD60s. It almost linearly decreased to a minimum, plateauing at HR-values around 170 beats · min−1, followed by a small rebound close to peak exercise (Figure 3).
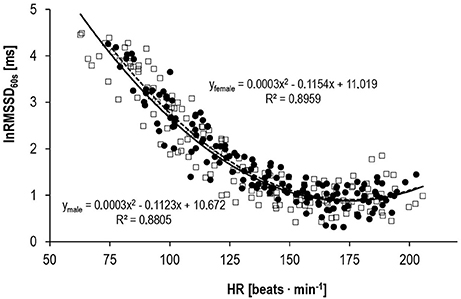
Figure 3. Correlation between lnRMSSD60s and HR for male (□, solid line, n = 10) and female subjects (•, dashed line, n = 10) during progressive exercise.
Comparison of HRV between HR-Matched Cycling before and after Exhaustion
Bland-Altman plot and t-test statistics confirmed the high correlation (r = 1.0, p < 0.001) and agreement (mean difference: 0.02 ± 1.29 ms, p = 0.792) between matched R-R segments, thus verifying the validity of the experimental approach (Figure 4). Despite identical HR for the matched R-R segments, lnRMSSD60s was significantly affected by an interaction of measurement time × experimental period [F(4, 192) = 30.733, p < 0.001, ηp2 = 0.390]. Figure 5 illustrates the behavior of HR and lnRMSSD60s during cycling at baseline, peak exercise, and during PRE and POST exhaustion cycling. HR and lnRMSSD60s at peak exercise were significantly higher (p < 0.001) and lower (p = 0.017), respectively, if compared to the first minute of POST. While HR further decreased from POST 1 to POST 5, no additional rebound from POST 1 to POST 5 was evident for lnRMSSD60s. In contrast, lnRMSSD60s progressively decreased with increasing HR during PRE. Both HR and lnRMSSD60s did not reach baseline values after 5 min of recovery (POST 5).
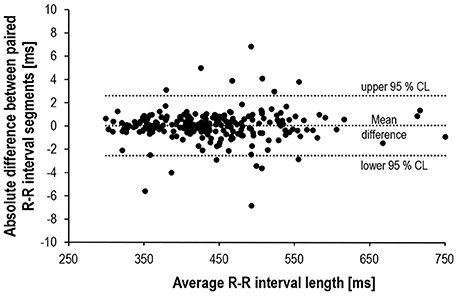
Figure 4. Bland-Altman plot of the absolute R-R differences (N = 245) between matched pairs (PRE vs. POST).
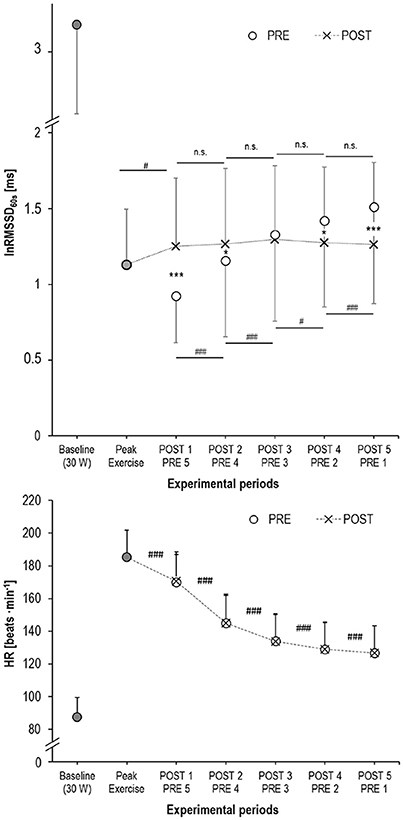
Figure 5. Mean and standard deviation of lnRMSSD60s (upper panel) and HR (lower panel) at baseline (cycling with 30 W, filled circles), at peak exercise (filled circles), during active recovery from exhaustion (POST, crosses) and the HR-matched segments during cycling before exhaustion (PRE, open circles); significance of the lnRMSSD60s-differences: ***p < 0.001, *p < 0.050 between PRE vs. POST; ###p < 0.001, #p < 0.050 between adjacent measurement time points.
Individual workload responses during the different experimental periods may not be perfectly comparable between subjects due to differences in aerobic capacity, training state and individual recovery behavior. Therefore, metabolic state—estimated by individual lactate threshold—was used as a factor potentially influencing the behavior of lnRMSSD60s. ANCOVA revealed that—despite no interaction effect on average R-R interval [F(1, 242) = 1.888; p = 0.665; ņp2 = 0.001]—lnRMSSD60s was affected by the interaction of experimental period × intensity [F(1, 242) = 30.233, p < 0.001, ņp2 = 0.111; Figure 6].
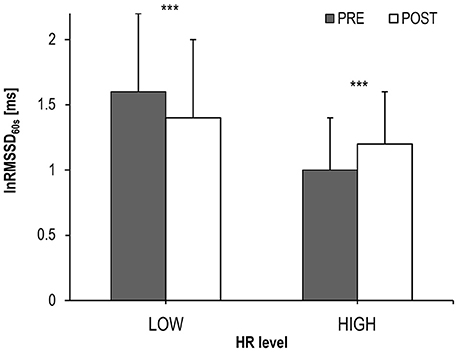
Figure 6. LnRMSSD60s (Mean and SD) during cycling before (PRE) and after exhaustion (POST) at HIGH (HR > individual lactate threshold HR) and LOW (HR ≤ individual lactate threshold HR) intensity; significance of the lnRMSSD60s-differences between PRE vs. POST: ***p < 0.001.
Discussion
Aim of this study was twofold: (i) validity of ultra-short-term HRV measures against traditional HRV was tested across a broad range of exercise intensities and (ii) autonomic HR modulation, assessed by HRV, was compared during different exercise conditions.
Validation Study
Our data confirmed the internal validity of the 60 s HRV indices lnRMSSD60s, lnLFP60s, and lnHFP60s compared to their respective 180 s indices. LnRMSSD60s performed even better than the frequency domain indices. Based on the standardized mean bias as well as the standardized mean error, the difference between lnRMSSD60s and lnRMSSD180s was rated trivial. Thus, very short-term lnRMSSD60s can be used interchangeably with traditional lnRMSSD180s data segments. Furthermore, the behavior of lnRMSSD60s during progressive exercise in our study was coherent with the current model of sympathetic-parasympathetic interaction during progressive exercise. According to this model and empirical data, vagal modulations of HR should be detectable until submaximal workloads around 150 · beats min−1 (Ng et al., 2009; White and Raven, 2014). Thus, our results indirectly speak for the external validity of lnRMSSD60s and its potential to reflect these vagal effects on HR control during submaximal exercise. Previous autonomic blocking experiments have also provided direct evidence for lnRMSSD60s being a valid tool to describe parasympathetic reactivation following exercise (Goldberger et al., 2006).
Comparison of HRV between HR-Matched Cycling before and after Exhaustion
The behavior of HR and lnRMMSD60s during the final minute of cycling at maximum workload and during POST 1 indicated a strong parasympathetic reactivation immediately after peak exercise. In our experiment, RMSSD60s plateaued at this increased level throughout the following POST periods. This is in contrast to the findings of Goldberger et al. (2006) who found RMSSD to progressively increase across the first 3 min of recovery after a shorter submaximal exercise protocol, but in line with other studies that have shown a slower recovery of vagally mediated HRV after maximal intensity exercise (see Michael et al. (2017) for review). Assuming that cardiac vagal activity plateaus on a high level as suggested by lnRMSSD60s, the progressive HR decrease during POST can be mainly attributed to sympathetic withdrawal.
The main finding of an interaction effect of experimental period (PRE vs. POST exhaustion cycling) × intensity (LOW vs. HIGH) confirmed the hypothesis of a different HRV pattern during these kinds of exercise, despite an identical net effect on average R-R interval length. We cautiously conclude that lnRMSSD reflects a different chronotropic pattern generated by the autonomic network in the medulla oblongata, which arises from differences in central command and afferent feedback during the different experimental periods. When HR is still above HRLT during early POST, vagal HR modulation is stronger compared to cycling at corresponding HRs during PRE. Thus, during the very first minutes after exhaustive exercise, net HR might result from a stronger sympathetic-parasympathetic coactivation if compared to high intensity cycling at similar HR (Berntson et al., 1991, 1993). An alternative explanation for a decreased vagally modulated HRV during late PRE might be a high or even saturated sympathetic tone (White and Raven, 2014). However, because direct nerve recordings in the intact human heart are impracticable and HRV reflects only the end-organ response that integrates different (autonomic) facilitatory and inhibitory effects on HR, the underlying mechanisms remain speculative.
Several neural feedback and feedforward mechanisms and their interaction may play a role in generating the distinct HRV pattern during HR-matched PRE and POST periods. The reactivation of vagal activity during early POST might counteract or even overpower the potential sympathetically mediated HR elevation by the metaboreflex (Fisher, 2014). The vagal rebound may occur due to the removal of inhibitory inputs from central command and the muscle mechanoreceptors and/or baroreflexes (Fisher, 2014 and references cited herein). This model is consistent with our results and the assumption of a pronounced sympathetic-parasympathetic coactivation during early POST.
Principally, afferent signaling from several types of receptors, including peripheral and central chemoreceptors, arterial baroreceptors, metabolite-, mechano-, and nocisensitive afferents within and around the working muscle (Fisher, 2014; Amann et al., 2015; Michelini et al., 2015) should profoundly differ during PRE and POST. For example, baroreceptor inputs change by the rapid decline of arterial blood pressure upon cessation of heavy exercise, inter alia by a decreased venous return due to a reduced activity of the skeletal muscle pump during POST (Takahashi et al., 2005). Along with decreases in central command at the beginning of POST, the cardiac baroreflex sensitivity increases via an augmented vagal activity (Ogoh et al., 2002, 2005; Gallagher et al., 2006). An alteration of the baroreflex sensitivity might in turn affect HRV (Di Rienzo et al., 1991). Additionally, altered feedback from muscle mechano- and metabolite-sensitive afferents during POST compared to PRE might contribute to different ANS outflow to the heart (Gladwell et al., 2005; Amann et al., 2010; Fisher, 2014). Mechanoreceptor inputs to the cardiovascular control centers in the brainstem decrease due to lesser muscular engagement during active recovery and would thereby alter cardiac autonomic drive (Crisafulli et al., 2003; Trinity et al., 2010). A stronger phasic compromised muscle blood flow in the exercising muscles (Holtz, 1996) during PRE might have also contributed to a distinct activity pattern of metabolite-sensitive afferents, resulting in a different autonomic HR modulation during PRE and POST (Alam and Smirk, 1938; O'Leary, 1993; Spranger et al., 2013; Fisher, 2014; Amann et al., 2015). Altered feedback from central and peripheral chemoreceptors during PRE and POST periods might have also modulated cardiac control. During mild to moderate dynamic exercise, arterial CO2 pressure slightly increases toward hypercapnic tension, whereas it returns to resting values or even hypocapnic tension during heavy to exhaustive exercise due to the respiratory compensation of acidosis (Dempsey, 1988; Forster and Pan, 1988; Raven et al., 2013). The compensatory hyperventilation during heavy exercise can result in a respiratory alkalosis in the cerebral fluid that is expected to reduce central chemoreceptor discharge and to stimulate neural projections that inhibit the carotid afferent chemosensitive activity (Whipp and Ward, 1998). Taken together, it can be assumed that a different chemical milieu of the arterial blood and/or cerebral fluid during the matched PRE and POST leads to an altered signaling from peripheral and/or central chemosensitive afferents to the cardiovascular control centers in the medulla oblongata. Despite chemoreflexes being powerful modulators of the neural ventilatory and circulatory control, they are itself subject to important negative feedback interactions with baro- and pulmonary stretch receptors as well as to modulatory effects of circulating catecholamines (Kara et al., 2003; Mansukhani et al., 2015). The inhibitory effect of the baro- and pulmonary stretch reflexes in turn depends on arterial CO2-pressure (Somers et al., 1989, 1991), which—in turn—depends on exercise intensity and the ventilatory response.
Limitations
Despite indirectly controlling for different respiratory rates by using the HFpeak from the HRV spectrum as a covariate, it cannot be fully neglected that ventilation has differently affected HRV during the two experimental periods. However, previous studies that systematically and profoundly manipulated breathing pattern, have shown that RMSSD is robust against different breathing patterns at physiological respiration rates >0.1 Hz (Schipke et al., 1999; Weippert et al., 2015a). Thus, a significant impact of breathing on RMSSD is not very likely, especially since breathing rates did not profoundly differ between the matched experimental periods. It has generally to be considered that RMSSD is not a direct measure of vagal tone. Finally, alterations in circulating plasma catecholamines during PRE and POST (Hagberg et al., 1979) may have also slightly affected HRV via direct effects on the sinus node (Kienzle et al., 1992; Breuer et al., 1993; Grossman and Taylor, 2007). One strength of this study is also its weakness: because we measured effects on HR control during physiological conditions without artificially manipulating afferent feedback and/or pharmacological blocking of parasympathetic or sympathetic receptors, conclusions regarding the precise contributions of afferent feedback and central command modulation on autonomic HR control remain elusive.
Summary
Our experiment evidences a different HRV during HR-matched cycling before and after exhaustion that is also dependent on HR level in healthy subjects free of cardiovascular diseases. The HRV results speak for distinct patterns of autonomic neural drive to the heart during these conditions, exerting a similar net effect on HR (Berntson et al., 1991, 1993). The different autonomic control pattern might be the result of complex and distinct interactions of afferent feedback and/or central command during PRE and POST periods (Table 3). Whether this differential HRV response during and after exercise is also evident in subjects with cardiovascular diseases remains to be investigated. However, since ANS function may play a role in exercise related cardiovascular events like sudden cardiac death (Thompson et al., 2007; Franciosi et al., 2017), a different response pattern or amplitude in persons at risk is not unlikely.
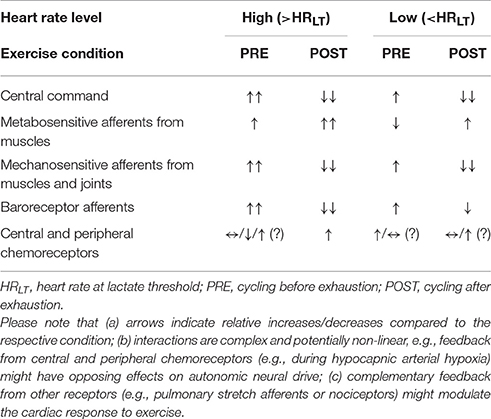
Table 3. Potential feedback and feedforward contributions to autonomic cardiac control during PRE and POST at high and low HR level, respectively.
Author Contributions
MW, MB, and KB designed this study; MW, collected, analyzed, and interpreted the data; MW drafted the manuscript, all authors revised the manuscript and approved the final version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Part of this work was technically supported by the Rostock University Medical Center. We further acknowledge financial support by German Research Foundation (DFG) and University of Rostock/University Medicine Rostock within the funding programme Open Access Publishing.
References
Alam, M., and Smirk, F. H. (1938). Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. J. Physiol. 92, 167–177. doi: 10.1113/jphysiol.1938.sp003592
Amann, M., Blain, G. M., Proctor, L. T., Sebranek, J. J., Pegelow, D. F., and Dempsey, J. A. (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 109, 966–976. doi: 10.1152/japplphysiol.00462.2010
Amann, M., Sidhu, S. K., Weavil, J. C., Mangum, T. S., and Venturelli, M. (2015). Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton. Neurosci. Basic Clin. 188, 19–23. doi: 10.1016/j.autneu.2014.10.018
Berntson, G. G., Cacioppo, J. T., and Quigley, K. S. (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol. Rev. 98, 459–487. doi: 10.1037/0033-295X.98.4.459
Berntson, G. G., Cacioppo, J. T., and Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychol. Bull. 114, 296–322. doi: 10.1037/0033-2909.114.2.296
Billman, G. E. (2011). Heart rate variability - a historical perspective. Front. Physiol. 2:86. doi: 10.3389/fphys.2011.00086
Billman, G. E. (2013). The effect of heart rate on the heart rate variability response to autonomic interventions. Front. Physiol. 4:222. doi: 10.3389/fphys.2013.00222
Bland, J. M., and Altman, D. G. (2007). Agreement between methods of measurement with multiple observations per individual. J. Biopharmaceut. Stat. 17, 571–582. doi: 10.1080/10543400701329422
Boushel, R. (2010). Muscle metaboreflex control of the circulation during exercise. Acta Physiol. 199, 367–383. doi: 10.1111/j.1748-1716.2010.02133.x
Breuer, H. W., Skyschally, A., Schulz, R., Martin, C., Wehr, M., and Heusch, G. (1993). Heart rate variability and circulating catecholamine concentrations during steady state exercise in healthy volunteers. Br. Heart J. 70, 144–149. doi: 10.1136/hrt.70.2.144
Crisafulli, A., Orru, V., Melis, F., Tocco, F., and Concu, A. (2003). Hemodynamics during active and passive recovery from a single bout of supramaximal exercise. Eur. J. Appl. Physiol. 89, 209–216. doi: 10.1007/s00421-003-0796-4
Dempsey, J. A. (1988). Problems with the hyperventilatory response to exercise and hypoxia. Adv. Exp. Med. Biol. 227, 277–276. doi: 10.1007/978-1-4684-5481-9_24
Di Rienzo, M., Parati, G., Castiglioni, P., Omboni, S., Ferrari, A. U., Ramirez, A. J., et al. (1991). Role of sinoaortic afferents in modulating BP and pulse-interval spectral characteristics in unanesthetized cats. Am. J. Physiol. Heart Circ. Physiol. 261, 30–36.
Dickhuth, H. H., Yin, L., Niess, A., Rocker, K., Mayer, F., Heitkamp, H. C., et al. (1999). Ventilatory, lactate-derived and catecholamine thresholds during incremental treadmill running: relationship and reproducibility. Int. J. Sports Med. 20, 122–127. doi: 10.1055/s-2007-971105
Esco, M. R., and Flatt, A. A. (2014). Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J. Sports Sci. Med. 13, 535–541.
Fadel, P. J., and Raven, P. B. (2012). Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp. Physiol. 97, 39–50. doi: 10.1113/expphysiol.2011.057554
Fiorelli, A., Messina, G., Chiodini, P., Costanzo, S., Viggiano, A., Monda, M., et al. (2017). Cardiac autonomic changes after thoracic sympathectomy: a prospective, randomized study. Ann. Thorac. Surg. 103, 216–224. doi: 10.1016/j.athoracsur.2016.10.055
Fisher, J. P. (2014). Autonomic control of the heart during exercise in humans: role of skeletal muscle afferents. Exp. Physiol. 99, 300–305. doi: 10.1113/expphysiol.2013.074377
Forster, H. V., and Pan, L. G. (1988). Breathing during exercise: demands, regulation, limitations. Adv. Exp. Med. Biol. 227, 257–276. doi: 10.1007/978-1-4684-5481-9_23
Franciosi, S., Perry, F. K. G., Roston, T. M., Armstrong, K. R., Claydon, V. E., and Sanatani, S. (2017). The role of the autonomic nervous system in arrhythmias and sudden cardiac death. Auton. Neurosci. 205, 1–11. doi: 10.1016/j.autneu.2017.03.005
Gallagher, K. M., Fadel, P. J., Smith, S. A., Strømstad, M., Ide, K., Secher, N. H., et al. (2006). The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp. Physiol. 91, 79–87. doi: 10.1113/expphysiol.2005.032110
Gladwell, V. F., Fletcher, J., Patel, N., Elvidge, L. J., Lloyd, D., Chowdhary, S., et al. (2005). The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J. Physiol. 567(Pt 2), 713–721. doi: 10.1113/jphysiol.2005.089243
Goldberger, J. J., Le, F. K., Lahiri, M., Kannankeril, P. J., Ng, J., and Kadish, A. H. (2006). Assessment of parasympathetic reactivation after exercise. Am. J. Physiol. Heart Circ. Physiol. 290, H2446–H2452. doi: 10.1152/ajpheart.01118.2005
Gonzalez-Camarena, R., Carrasco-Sosa, S., Roman-Ramos, R., Gaitan-Gonzalez, M. J., Medina-Banuelos, V., and Azpiroz-Leehan, J. (2000). Effect of static and dynamic exercise on heart rate and blood pressure variabilities. Med. Sci. Sports Exerc. 32, 1719–1728. doi: 10.1097/00005768-200010000-00010
Grossman, P., and Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 74, 263–285. doi: 10.1016/j.biopsycho.2005.11.014
Hagberg, J. M., Hickson, R. C., McLane, J. A., Ehsani, A. A., and Winder, W. W. (1979). Disappearance of norepinephrine from the circulation following strenuous exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 1311–1314.
Holtz, J. (1996). “Hemodynamics in regional circulatory beds and local vascular reactivity,” in Comprehensive Human Physiology, eds R. Greger and U. Windhorst (Heidelberg: Springer), 1917–1939.
Ichinose, M., Maeda, S., Kondo, N., and Nishiyasu, T. (2014). Blood pressure regulation II: what happens when one system must serve two masters-oxygen delivery and pressure regulation? Eur. J. Appl. Physiol. 114, 451–465. doi: 10.1007/s00421-013-2691-y
Kannankeril, P. J., Le, F. K., Kadish, A. H., and Goldberger, J. J. (2004). Parasympathetic effects on heart rate recovery after exercise. J. Investig. Med. 52, 394–401. doi: 10.1136/jim-52-06-34
Kara, T., Narkiewicz, K., and Somers, V. K. (2003). Chemoreflexes–physiology and clinical implications. Acta Physiol. Scand. 177, 377–384. doi: 10.1046/j.1365-201X.2003.01083.x
Kienzle, M. G., Ferguson, D. W., Birkett, C. L., Myers, G. A., Berg, W. J., and Mariano, D. J. (1992). Clinical, hemodynamic and sympathetic neural correlates of heart rate variability in congestive heart failure. Am. J. Cardiol. 69, 761–767. doi: 10.1016/0002-9149(92)90502-P
Leicht, A. S., Sinclair, W. H., and Spinks, W. L. (2008). Effect of exercise mode on heart rate variability during steady state exercise. Eur. J. Appl. Physiol. 102, 195–204. doi: 10.1007/s00421-007-0574-9
Mansukhani, M. P., Wang, S. H., and Somers, V. K. (2015). Chemoreflex physiology and implications for sleep apnoea: insights from studies in humans. Exp. Physiol. 100, 130–135. doi: 10.1113/expphysiol.2014.082826
Matsukawa, K. (2012). Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp. Physiol. 97, 20–28. doi: 10.1113/expphysiol.2011.057661
Michael, S., Graham, K. S., and Davis, G. M. (2017). Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Front. Physiol. 8:301. doi: 10.3389/fphys.2017.00301
Michelini, L. C., O'Leary, D. S., Raven, P. B., and Nobrega, A. C. (2015). Neural control of circulation and exercise: a translational approach disclosing interactions between central command, arterial baroreflex, and muscle metaboreflex. Am. J. Physiol. Heart Circ. Physiol. 309, H381–H392. doi: 10.1152/ajpheart.00077.2015
Munoz, M. L., Van Roon, A., Riese, H., Thio, C., Oostenbroek, E., Westrik, I., et al. (2015). Validity of (ultra-)short recordings for heart rate variability measurements. PLoS ONE 10:e0138921. doi: 10.1371/journal.pone.0138921
Nakamura, F. Y., Pereira, L. A., Cal Abad, C. C., Cruz, I. F., Flatt, A. A., Esco, M. R., et al. (2017). Adequacy of the ultra-short-term HRV to assess adaptive processes in youth female basketball players. J. Hum. Kinet. 56, 73–80. doi: 10.1515/hukin-2017-0024
Ng, J., Sundaram, S., Kadish, A. H., and Goldberger, J. J. (2009). Autonomic effects on the spectral analysis of heart rate variability after exercise. Am. J. Physiol. Heart Circ. Physiol. 297, H1421–H1428. doi: 10.1152/ajpheart.00217.2009
Noppen, M., Dendale, P., Hagers, Y., Herregodts, P., Vincken, W., and D'Haens, J. (1996). Changes in cardiocirculatory autonomic function after thoracoscopic upper dorsal sympathicolysis for essential hyperhidrosis. J. Auton. Nerv. Syst. 60, 115–120. doi: 10.1016/0165-1838(96)00034-3
Nussinovitch, U., Elishkevitz, K. P., Katz, K., Nussinovitch, M., Segev, S., Volovitz, B., et al. (2011). Reliability of ultra-short ECG indices for heart rate variability. Ann. Noninvasive Electrocardiol. 16, 117–122. doi: 10.1111/j.1542-474X.2011.00417.x
Ogoh, S., Fisher, J. P., Dawson, E. A., White, M. J., Secher, N. H., and Raven, P. B. (2005). Autonomic nervous system influence on arterial baroreflex control of heart rate during exercise in humans. J. Physiol. 566, 599–611. doi: 10.1113/jphysiol.2005.084541
Ogoh, S., Wasmund, W. L., Keller, D. M., Gallagher, K. M., Mitchell, J. H., et al. (2002). Role of central command in carotid baroreflex resetting in humans during static exercise. J. Physiol. 543, 349–364. doi: 10.1113/jphysiol.2002.019943
O'Leary, D. S. (1993). Autonomic mechanisms of muscle metaboreflex control of heart rate. J. Appl. Physiol. 74, 1748–1754.
Paton, J. F., Boscan, P., Pickering, A. E., and Nalivaiko, E. (2005). The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res. Brain Res. Rev. 49, 555–565. doi: 10.1016/j.brainresrev.2005.02.005
Paton, J. F., Nalivaiko, E., Boscan, P., and Pickering, A. E. (2006). Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: observations and functional implications. Clin. Exp. Pharmacol. Physiol. 33, 1245–1250. doi: 10.1111/j.1440-1681.2006.04518.x
Raven, P. B., Wasserman, D. H., Squires, W. G., and Murray, T. D. (2013). Exercise Physiology - An Integrated Approach. Belmont, CA: Wadsworth.
Robinson, B. F., Epstein, S. E., Beiser, G. D., and Braunwald, E. (1966). Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ. Res. 19, 400–411. doi: 10.1161/01.RES.19.2.400
Schipke, J. D., Arnold, G., and Pelzer, M. (1999). Effect of respiration on short-term heart-rate-variability. J. Clin. Basic Cardiol. 2, 92–95.
Smith, A. L., Owen, H., and Reynolds, K. J. (2013). Heart rate variability indices for very short-term (30 beat) analysis. Part 2: validation. J. Clin. Monit. Comput. 27, 577–585. doi: 10.1007/s10877-013-9473-2
Somers, V. K., Mark, A. L., and Abboud, F. M. (1991). Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J. Clin. Invest. 87, 1953–1957. doi: 10.1172/JCI115221
Somers, V. K., Mark, A. L., Zavala, D. C., and Abboud, F. M. (1989). Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J. Appl. Physiol. 67, 2095–2100.
Spranger, M. D., Sala-Mercado, J. A., Coutsos, M., Kaur, J., Stayer, D., Augustyniak, R. A., et al. (2013). Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R657–R663. doi: 10.1152/ajpregu.00601.2012
Takahashi, T., Hayano, J., Okada, A., Saitoh, T., and Kamiya, A. (2005). Effects of the muscle pump and body posture on cardiovascular responses during recovery from cycle exercise. Eur. J. Appl. Physiol. 94, 576–583. doi: 10.1007/s00421-005-1369-5
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065. doi: 10.1161/01.CIR.93.5.1043
Thayer, J. F., Sollers, J. J., Ruiz-Padial, E., and Vila, J. (2002). Estimating respiratory frequency from autoregressive spectral analysis of heart period. IEEE Eng. Med. Biol. Mag. 21, 41–45. doi: 10.1109/MEMB.2002.1032638
Thompson, P. D., Franklin, B. A., Balady, G. J., Blair, S. N., Corrado, D., Estes, N. A. III, et al. (2007). Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115, 2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485
Thong, T., Li, K., McNames, J., Aboy, M., and Goldstein, B. (2003). “Accuracy of ultra-short heart rate variability measures,” in A New Beginning for Human Health: Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology, eds R. S. Leder (Cancun), 2424–2427.
Trinity, J. D., Amann, M., McDaniel, J., Fjeldstad, A. S., Barrett-O'Keefe, Z., Runnels, S., et al. (2010). Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am. J. Physiol. Heart Circ. Physiol. 299, H1693–H1700. doi: 10.1152/ajpheart.00482.2010
Weippert, M., Behrens, K., Rieger, A., Kumar, M., and Behrens, M. (2015a). Effects of breathing patterns and light exercise on linear and nonlinear heart rate variability. Appl. Physiol. Nutr. Metab. 40, 762–768. doi: 10.1139/apnm-2014-0493
Weippert, M., Behrens, K., Rieger, A., Stoll, R., and Kreuzfeld, S. (2013). Heart rate variability and blood pressure during dynamic and static exercise at similar heart rate levels. PLoS ONE 8:833690. doi: 10.1371/journal.pone.0083690
Weippert, M., Behrens, M., Gonschorek, R., Bruhn, S., and Behrens, K. (2015b). Muscular contraction mode differently affects autonomic control during heart rate matched exercise. Front. Physiol. 6:156. doi: 10.3389/fphys.2015.00156
Weippert, M., Behrens, M., Rieger, A., and Behrens, K. (2014). Sample entropy and traditional measures of heart rate dynamics reveal different modes of cardiovascular control during low intensity exercise. Entropy 16, 5698–5711. doi: 10.3390/e16115698
Whipp, B. J., and Ward, S. A. (1998). Determinants and control of breathing during muscular exercise. Br. J. Sports Med. 32, 199–211.
Keywords: heart rate recovery, fatigue, sudden cardiac death, afferent feedback, central command, ultra short-term heart rate variability, validity, agreement
Citation: Weippert M, Behrens M, Mau-Moeller A, Bruhn S and Behrens K (2017) Cycling before and after Exhaustion Differently Affects Cardiac Autonomic Control during Heart Rate Matched Exercise. Front. Physiol. 8:844. doi: 10.3389/fphys.2017.00844
Received: 20 July 2017; Accepted: 10 October 2017;
Published: 01 November 2017.
Edited by:
Jun Sugawara, National Institute of Advanced Industrial Science and Technology, JapanReviewed by:
Naoto Fujii, University of Tsukuba, JapanGiovanni Messina, University of Foggia, Italy
Copyright © 2017 Weippert, Behrens, Mau-Moeller, Bruhn and Behrens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Weippert, matthias.weippert@uni-rostock.de
 Matthias Weippert
Matthias Weippert Martin Behrens
Martin Behrens Anett Mau-Moeller
Anett Mau-Moeller Sven Bruhn
Sven Bruhn Kristin Behrens1
Kristin Behrens1