- 1Department of Cardiovascular Medicine, Juntendo University School of Medicine, Tokyo, Japan
- 2Cardiovascular Respiratory Sleep Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan
Background: Acute effects of positive airway pressure (PAP) [including continuous PAP (CPAP) and adaptive servo-ventilation, an advanced form of bi-level PAP] on functional mitral regurgitation (fMR) in patients with heart failure (HF) with left ventricular (LV) systolic dysfunction remain unclear. Thus, whether PAP therapy reduces fMR in such patients with HF was investigated.
Methods and Results: Twenty patients with HF and LV systolic dysfunction defined as LV ejection fraction (LVEF) <50% (14 men; mean LVEF, 35.0 ± 11.5%) with fMR underwent echocardiography during 10-min CPAP (4 and 8 cm H2O) and adaptive servo-ventilation. For fMR assessment, MR jet area fraction, defined as the ratio of MR jet on color Doppler to the left atrial area, was measured. The forward stroke volume (SV) index (fSVI) was calculated from the time-velocity integral, cross-sectional area of the aortic annulus, and body surface area. fMR significantly reduced on CPAP at 8 cm H2O (0.30 ± 0.12) and adaptive servo-ventilation (0.29 ± 0.12), compared with the baseline phase (0.37 ± 0.12) and CPAP at 4 cm H2O (0.34 ± 0.12) (P < 0.001). The fSVI did not change in any of the PAP sessions (P = 0.888). However, significant differences in fSVI responses to PAP were found between sexes (P for interaction, 0.006), with a significant reduction in fSVI in women (P = 0.041) and between patients with baseline fSVI ≥ and < the median value (27.8 ml/m2, P for interaction, 0.018), with a significant fSVI reduction in patients with high baseline fSVI (P = 0.028). In addition, significant differences were found in fSVI responses to PAP between patients with LV end-systolic volume (LVESV) index ≥ and < the median value (62.0 ml/m2, P for interaction, 0.034), with a significant fSVI increase in patients with a high LVESV index (P = 0.023).
Conclusion: In patients with HF, LV systolic dysfunction, and fMR, PAP can alleviate fMR without any overall changes in forward SV. However, MR alleviation due to PAP might be associated with a decrease in forward SV in women with high baseline SV, whereas MR alleviation due to PAP might be accompanied by increased forward SV in patients with a dilated LV.
Introduction
Positive airway pressure (PAP) delivered by face masks has been used in the wide spectrum of heart failure (HF) care (Bradley et al., 2005; Arzt et al., 2007; Wang et al., 2007; Gray et al., 2008; Kasai et al., 2008; Kato et al., 2014; Khayat et al., 2015; Momomura et al., 2015; Nakano et al., 2015; Kinoshita et al., 2017). For instance, in patients with acute decompensated HF, continuous PAP (CPAP) resulted in early physiologic improvements and reduced the need for intubation and mechanical ventilation, indicating that PAP has beneficial hemodynamic and respiratory effects (Bersten et al., 1991). Several studies have suggested that bi-level PAP, which provides fixed pressure support (PS) during inspiration in addition to expiratory PAP (EPAP) and backup ventilation with a preset respiratory rate, is also an effective mode of PAP in patients with acute decompensated HF (Hoffmann and Welte, 1999; Rusterholtz et al., 1999; Masip et al., 2000). More recently, adaptive servo-ventilation (ASV), which is an advanced mode of bi-level PAP and automatically provides altering PS for each inspiration with an EPAP in addition to backup ventilation with variable respiratory rates, leading to stabilization of respiration (see Figure S1), was reported to be useful for patients with acute decompensated HF (Nakano et al., 2015; Kinoshita et al., 2017).
In contrast, patients with chronic stable HF had inconsistent acute hemodynamic responses to PAP, which were possibly associated with differences in the type of PAP and the patients' profiles and conditions (Bradley et al., 1992; De Hoyos et al., 1995; Liston et al., 1995; Kiely et al., 1998; Philip-Joet et al., 1999; Mehta et al., 2000; Steiner et al., 2008; Haruki et al., 2011; Yoshida et al., 2012; Yamada et al., 2013). CPAP increases stroke volume (SV) in patients with HF with a high LV filling pressure and/or LV chamber size (Bradley et al., 1992; De Hoyos et al., 1995; Steiner et al., 2008; Yoshida et al., 2012). Similar responses in those patient populations were observed when bi-level PAP or ASV was applied (Philip-Joet et al., 1999; Yamada et al., 2013). Recently, adding PS on CPAP was reported to more favorably affect SV than isolated CPAP, possibly through lung inflation and its induced reflex inhibition of sympathetic nerve activity (Yoshida et al., 2012). In addition, stabilization of respiration by altering PS and servo-control ventilation of ASV was suggested to play important roles in sympathoinhibitory effects (Ushijima et al., 2014).
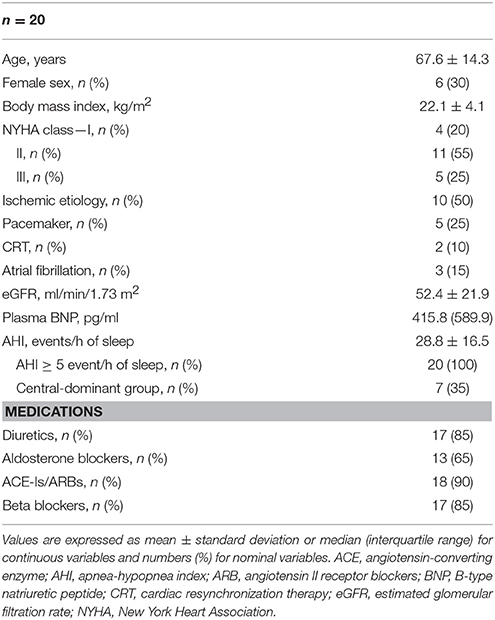
In patients with chronic HF, PAP has been used to alleviate sleep-disordered breathing (SDB), which is a frequent complication known to be associated with worse clinical outcomes (Kasai and Bradley, 2011; Kasai, 2012; Kasai et al., 2012). In patients with HF and SDB, PAP resulted in improvements in cardiac function in many small-scale and relatively short-term randomized controlled trials (Kasai and Bradley, 2011; Kasai, 2012; Kasai et al., 2012; Kato et al., 2014); however, two clinical trials that investigated the effects of SDB treatment with PAP on long-term clinical outcomes in patients with chronic HF failed to show any benefit of PAP against mortality and/or adverse clinical events (Bradley et al., 2005; Cowie et al., 2015). Even in those studies, PAP therapy might be beneficial in specific subgroups of patients, such as those with central sleep apnea, which can be alleviated by CPAP, those with less Cheyne–Stokes respiration, and those with less impaired left ventricular (LV) ejection fraction (LVEF) (Arzt et al., 2007; Cowie et al., 2015). Considering these facts, there might be subgroups of patients with HF with specific profiles or conditions who are responsive to PAP therapy. These profiles or conditions may include functional mitral regurgitation (fMR).
fMR, arising from mitral annular dilation and/or papillary muscle dysfunction in association with LV dysfunction, is a common finding in patients with HF (Asgar et al., 2015). It contributes to the elevation of LV end-diastolic pressure as well as left and right atrial pressure and causes progressive dilatation of the LV (Vanoverschelde et al., 1990), leading to a vicious cycle. However, the presence of significant fMR suggests that intensive treatment would be beneficial (Keren et al., 1989; Evangelista-Masip et al., 1992). Indeed, short-term application of CPAP or bi-level PAP was reported to alleviate fMR rapidly in patients with acute decompensated HF (Bellone et al., 2004). In contrast, short-term application of ASV did not rapidly alleviate fMR in a study on patients with stable HF (Haruki et al., 2011). In any case, inconsistent acute responses of fMR to PAP might possibly be associated with differences in the type of PAP and patients' profiles and conditions.
The aim of our study was to evaluate the acute effect of several settings of PAP therapy on fMR, taking into account changes in other hemodynamic parameters. Our specific hypothesis is that fMR will be alleviated because an increased CPAP level and ASV have similar effects and that alleviation of fMR will be accompanied by changes in SV, which will be more prominent in certain subgroups of patients.
Materials and Methods
Subjects
We enrolled patients with systolic HF at Juntendo University Hospital (Tokyo, Japan) if they met following criteria: (1) men and women aged ≥20 years, (2) HF due to ischemic or non-ischemic cardiomyopathy, (3) LVEF <50% on echocardiography, (4) mild to severe fMR, (5) stable clinical status evidenced by the absence of acute exacerbations of dyspnea, and (6) underwent overnight polysomnography to assess for SDB. The exclusion criteria were (1) patients with shock, (2) those requiring catecholamine and/or inotrope infusion, (3) those requiring mechanical ventilation or supplemental oxygen, (4) those with organic MR, (5) those with moderate to severe aortic regurgitation and/or stenosis, (6) those with a history of chronic lung disease, and (7) those with dialysis.
The subjects' characteristics and medications at the time of this study were recorded. The estimated glomerular filtration rate (Matsuo et al., 2009) and B-type natriuretic peptide levels were assessed in the early morning of the day of the experiment. All patients underwent overnight polysomnography on the night before the experiment. Apneas and hypopneas were quantified, and the severity of SDB was assessed using the frequency of apneas and hypopneas per hour of sleep (i.e., apnea–hypopnea index, AHI). Patients were divided into an obstructive-dominant group (≥50% had obstructive apnea) and a central-dominant group (>50% had central apnea).
This study was approved by the Juntendo University Hospital Institutional Review Board, and the study complied with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Echocardiography
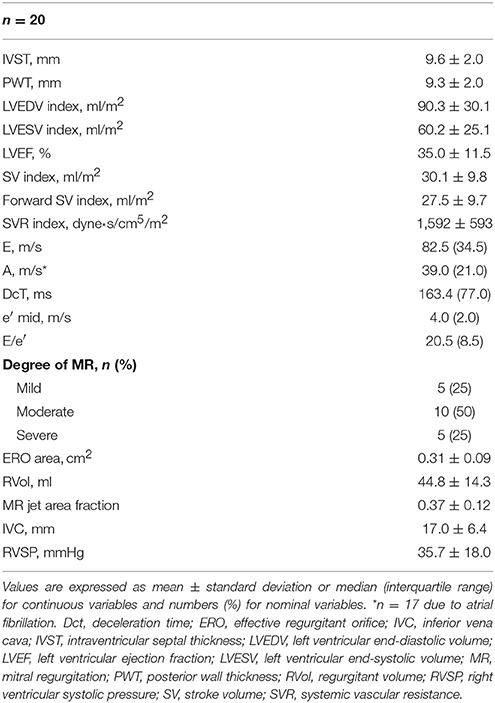
Standard two-dimensional echocardiography and Doppler ultrasound examinations were performed using a commercially available ultrasound imaging system (Vivid I, GE Healthcare, Milwaukee, WI, USA). Left ventricular end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), stroke volume (SV), and LVEF were calculated using Simpson's biplane method. LVEDV, LVESV, and SV were divided by the body surface area and expressed as the LVEDV, LVESV, and SV indexes. The degree of MR was assessed from 0 (none) to 4 (severe) and was based on the MR jet area fraction, which was defined as the ratio of MR jet area to the left atrial (LA) area. Because SV determined using Simpson's method may consist of both forward and MR flows (Giannuzzi et al., 1991), forward SV was also calculated as the LV outflow tract area × velocity time integral of the LV outflow velocity. Systemic vascular resistance (SVR) was calculated as (mean BP − central vein pressure [CVP]/[forward SV × HR]) × 80. CVP was estimated by the diameter of the inferior vena cava (IVC), and by the presence or absence and degree of the respiratory variation of the IVC (Lanzarini et al., 2002). Forward SV and SVR were also divided by the body surface area and expressed as indices. Diastolic function was evaluated using transmitral flow velocity and average e′ from the septal and lateral tissue Doppler samplings (Nagueh et al., 2009). All images were recorded by one investigator in at least three cardiac cycles and were assessed by another investigator who was blinded to the clinical data and settings of PAP. The final values represented the mean of at least three measurements for patients with regular rhythm and five measurements for those with atrial fibrillation (AF).
Intra-observer variability was determined by having one observer repeat the measurement of MR jet area fraction and forward SV in 10 randomly selected patients three times. Repeated measurements were performed 1 week apart. Inter-observer variability was determined by having a second observer measure these variables in the same datasets. Intra- and inter-observer variabilities were assessed by values calculated as the standard deviation of the corresponding three or two measurements as a percentage of the mean.
PAP and Study Protocol
All PAPs were applied with a full-face mask, using the BiPAP Auto-SV Advanced system (Philips Respironics, Murrysville, PA, USA), with the patients awake. After BP and HR were stabilized, the baseline step was started without PAP for 10 min, during which baseline echocardiography was performed. BP and HR were measured twice on the right upper arm in each step, using an automated vital signs monitoring device (DASH3000, GE Health Care, UK Ltd., Milwaukee, WI, USA). Subsequently, CPAP at 4 cm H2O, 8 cm H2O, and ASV with an EPAP of 4 cm H2O, an automated PS ranging from 0 to 4 cm H2O, and an automated backup ventilation mode (Figure S1) were applied for 10 min in a random order. In each 10-min period, repeated echocardiography was performed. After these three steps with PAP, all measurements were repeated without PAP (i.e., post step).
Statistical Analysis
In this study, values are expressed as mean ± standard deviation or median (interquartile range) for continuous variables and numbers (%) for nominal variables. Variations in echocardiography parameters across each step were assessed with one way repeated-measures analysis of variance (ANOVA) and the Tukey test for multiple comparisons. To assess the potential heterogeneity of the effect of PAP on fMR, forward SV index (fSVI), and SVR index, we performed subgroup analyses as exploratory analyses. The subgroups included sex, age (cutoff, median of 71 years), body mass index ([BMI], cutoff, median of 21.7 kg/m2), presence or absence of AF, type of predominant SDB, BNP level (cutoff, median of 415.8 pg/ml), right ventricular systolic pressure (RVSP) by echocardiography (cutoff, median of 34.2 mmHg), baseline LVEDV index (cutoff, median of 91.2 ml/m2), baseline LVESV index (cutoff, median of 62.0 ml/m2), baseline fSVI (cutoff, median of 27.8 ml/m2), and baseline MR degree. The first-order interactions in two-way repeated measures ANOVA models for fMR, forward SV and SVR were examined by entering interaction terms between PAP steps (i.e., baseline, CPAP at 4 cm H2O, 8 cm H2O, ASV, and post steps) and the above-mentioned subgroup (i.e., step-by-subgroup interaction). If significant step-by-subgroup interactions were found, one-way repeated-measures ANOVA was performed within each subgroup. A P < 0.05 indicated statistical significance. Analyses were performed using SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline Patient Characteristics
Overall, 23 eligible patients were enrolled in this study. However, three were excluded because of technical difficulty while measuring echocardiography parameters; thus, the data of 20 patients were assessed. The characteristics of these patients, including 14 men and 6 women, are summarized in Table 1. Most of them were elderly, non-obese, and had HF with New York Heart Association class II symptoms. Half of them had ischemic etiology, and three had AF. Of the five patients with pacemaker, none were pacemaker dependent during the study period. All of them had an AHI ≥ 5 events/h of sleep, and seven of them were in the central-dominant group.
Echocardiography data are shown in Table 2. The patients had severely impaired LVEF and dilated LV chamber sizes, which were associated with a high LV filling pressure. None of the subjects had significant adverse effects due to PAP application, and all of them completed the PAP trial. During ASV mode, the mean applied PS was 1.0 ± 0.4 cm H2O above EPAP of 4 cm H2O.
Effects of PAP on BP, HR, and Echocardiography Parameters
Intra-observer variabilities for measuring MR jet area fraction and fSVI were 4.8 and 5.0%, respectively. Corresponding inter-observer variabilities were 7.9 and 8.7%, respectively.
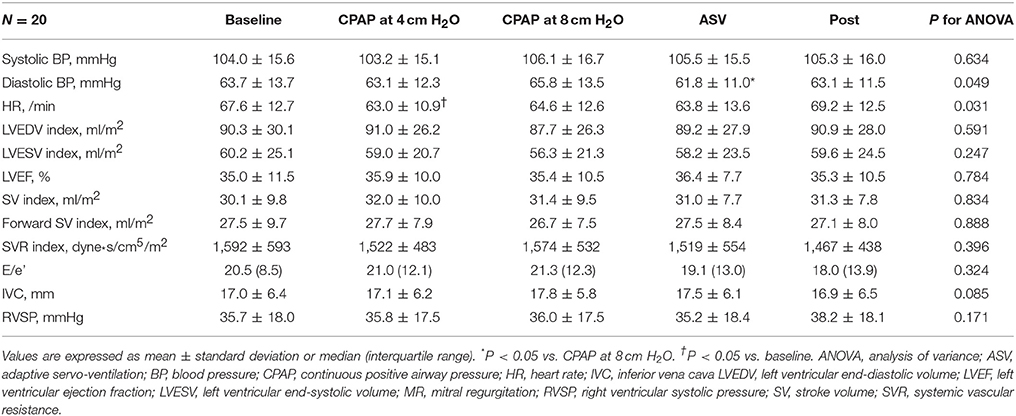
As shown in Figure 1, the MR jet area fraction was significantly reduced with the use of CPAP at 8 cm H2O and ASV compared with that at baseline and CPAP at 4 cm H2O. A dose-effect relationship was found across CPAP pressures from 0 (baseline) and 4–8 cm H2O, although no difference was observed in the reduction of MR jet area fraction between CPAP at 8 cm H2O and ASV. Representative color Doppler images for fMR are shown in Figure 2 (also see Supplementary Video). The fSVI did not change across the steps (Table 3). The changes in BP, HR, and other echocardiography data are summarized in Table 3. Diastolic BP significantly decreased in ASV compared to CPAP at 8 cm H2O. HR significantly decreased in CPAP at 4 cm H2O compared to baseline. However, no significant variations were found in the other parameters, although they tended to increase the diameter of the IVC.
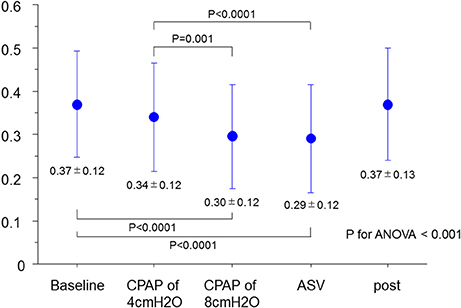
Figure 1. Changes in MR jet area fraction. ANOVA, analysis of variance; ASV, adaptive-servo ventilation; CPAP, continuous positive airway pressure; MR, mitral regurgitation.
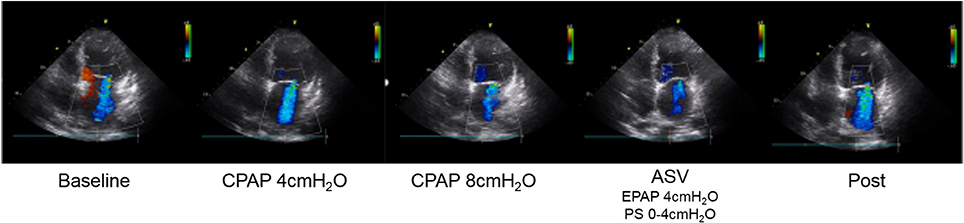
Figure 2. Representative color Doppler images of functional MR. MR jet area fraction reduced due to PAP therapy, especially CPAP at 8 cm H2O and ASV. ASV, adaptive-servo ventilation; CPAP, continuous positive airway pressure; EPAP, expiratory positive airway pressure; MR, mitral regurgitation; PAP, positive airway pressure; PS, pressure support.
Subgroup Analyses
No interactions were found between each subgroup and variation of MR jet area fraction. On the contrary, although no step-by-subgroup interactions were found between the variation of the fSVI and the subgroups of age, BMI, AF, type of predominant SDB, BNP, baseline RVSP, baseline LVEDV index, and baseline MR degree, significant differences were found in the variations of fSVI based on sex, baseline LVESV index, and baseline fSVI (P for interaction = 0.006, 0.034, and 0.017, respectively; Figures 3A–C). In men, the fSVI remained stable (P = 0.454), whereas in women, the fSVI decreased (P = 0.041) as the applied PAP levels increased. In addition, in patients with a low LVESV index, the fSVI tended to decrease (P = 0.071), whereas in those with a high LVESV index, fSVI significantly increased (P = 0.023). In patients with a low fSVI at baseline, fSVI remained stable (P = 0.438), whereas in those with a high fSVI at baseline, fSVI significantly decreased (P = 0.028). In terms of SVR index, although no step-by-subgroup interactions were found between variations of the SVR index and the subgroups of BMI, AF, central-dominant, BNP, baseline RVSP, baseline LVEDV index, baseline LVESV index, and baseline MR degree, a significant difference was found in the variations of the SVR index based on the baseline fSVI (P for interaction = 0.003), and sex and age subgroups tended to have different variations of the SVR index (P for interaction = 0.051, and 0.087, respectively; Figures S2A–C). Patients with a low fSVI at baseline showed a significant reduction in the SVR index as the applied PAP level increased (P < 0.001), whereas those with a high fSVI at baseline showed no change in the SVR index (P = 0.204).
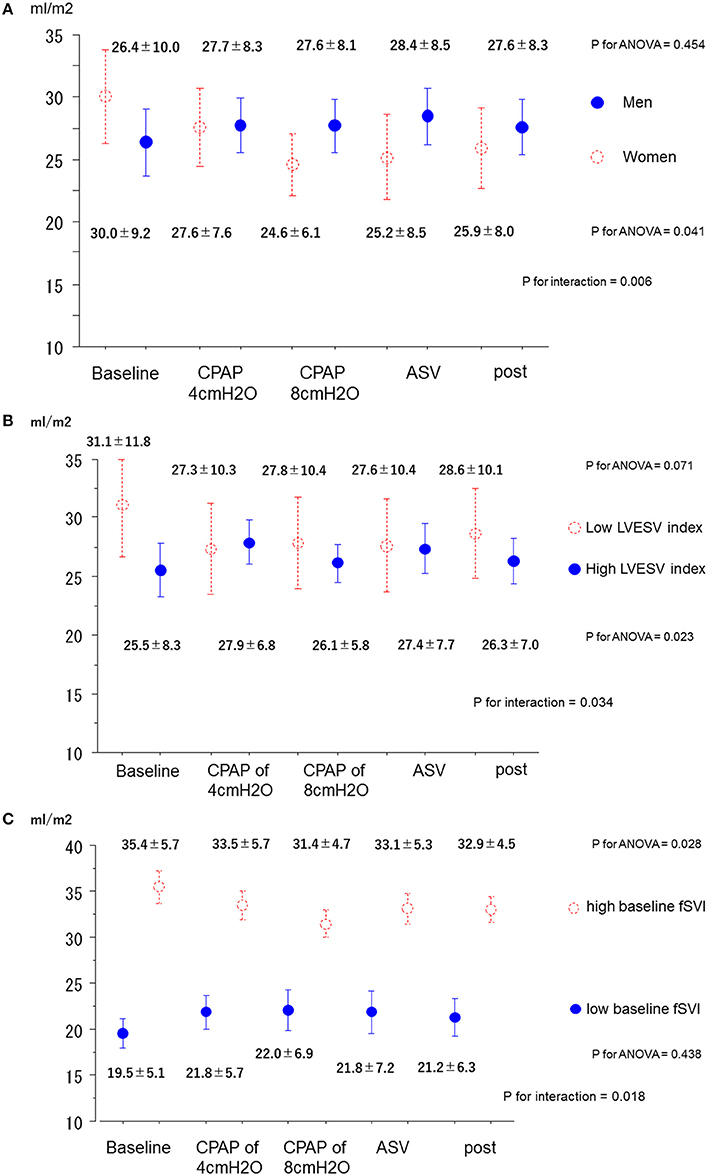
Figure 3. Changes in the forward SV index in the subgroups. (A) Men and women. (B) High and low LVESV index. (C) High and low baseline fSVI. ANOVA, analysis of variance; ASV, adaptive-servo ventilation; CPAP, continuous positive airway pressure; fSVI, forward stroke volume index; LVESV, left ventricular end-systolic volume.
Discussion
This study has several important findings that provide insight into the alleviation of fMR due to PAP in patients with HF and LV systolic dysfunction. First, the degree of fMR in patients with HF and LV systolic dysfunction was rapidly alleviated by PAP therapy. In addition, fMR revealed a stepwise reduction as the CPAP level increased, but no difference was found between CPAP at 8 cm H2O and ASV. Second, alleviation of fMR due to PAP was not accompanied by any overall changes in the other echocardiographic parameters, such as the SV index, fSVI, SVR index, LVEF, E/e′, and RVSP. Third, although no significant interactions were found between subgroups and alleviation of fMR, variations of the fSVI across each step were significantly affected by sex, baseline LVESV index, and baseline fSVI. fSVI increased in a subgroup of patients with a high LVESV index, whereas fSVI decreased in women and the subgroup of patients with a high baseline fSVI. Furthermore, variations of the SVR index across each step were significantly affected by the baseline fSVI. The SVR index decreased in a subgroup of patients with a low baseline fSVI. Altogether, in patients with HF, LV systolic dysfunction, and fMR, CPAP at 8 cm H2O and similarly ASV (EPAP of 4 cm H2O and automated PS ranging between 0 and 4 cm H2O with auto backup ventilation mode) can result in a significant alleviation of fMR, although no changes were found in the overall fSVI. Despite being results from exploratory analyses, sex, degree of LV dilatation, and degree of baseline forward SV may play some roles in hemodynamic changes that are accompanied by alleviation of fMR in response to PAP therapy.
Two studies have shown the acute effects of PAP on fMR in patients with systolic HF. In one study, Bellone et al. (2004) found that either CPAP at 10 cm H2O or bi-level PAP with an EPAP of 5 cm H2O and PS at 10 cm H2O for 30 min each significantly reduced the area of MR, accompanied by an increase in LVEF and a reduction in LVEDV.
The results of the present study were in line with these previous studies, although we used lower applied PAP levels over a shorter period. In addition, all patients in their study had signs of exacerbation of congestive HF, whereas all our patients were in a stable clinical state as evidenced by the absence of acute exacerbations of dyspnea. On the contrary, in another study that enrolled patients with stable HF, Haruki et al. found that a 30-min application of ASV with an EPAP of 5 cm H2O, PS of 3–10 cm H2O, and the automated backup mode did not affect the severity of MR despite significant increases in LVEF and SV and significant reductions in LVESV and SVR (Haruki et al., 2011). This lack of changes in severity of MR in their study may be explained by the fact that their study was not specifically focused on the alleviation of MR and may have included some patients without MR. Considering the findings of Bellone's study and ours, short-term application of any form of PAP (i.e., CPAP, bi-level PAP, or ASV) can alleviate fMR in patients with HF, LV systolic dysfunction, and fMR. However, acute responses of hemodynamic parameters to the PAP were inconsistent.
One mechanism for the alleviation of fMR may be that limited venous return (cardiac preload) by PAP through an increase in intrathoracic pressure leads to decongestion and reductions in LV volume and filling pressure, enhancing LVEF, and SV will occur in patients with highly dilated LV based on the Frank-Starling principle. In most previous studies on the effects of the acute application of PAP on SV, patients with high LV filling pressure and/or enlarged LV had increased SV by application of PAP (Bradley et al., 1992; De Hoyos et al., 1995; Philip-Joet et al., 1999; Steiner et al., 2008; Yamada et al., 2013). In the present study, responses of PAP on fSVI differed between subgroups, particularly subgroups divided by baseline LVESV index; in patients with a high baseline LVESV index, fSVI was increased significantly by PAP, whereas no such changes were observed in their counterparts. In addition, an increase in intrathoracic pressure by PAP can reduce the LV afterload through reduction of the LV transmural pressure and through production of a pressure gradient between the thoracic and systemic vascular system (Buda et al., 1979; Rudikoff et al., 1980; Pinsky et al., 1983). Because a failing heart is afterload-dependent, forward SV appears to be responsive to changes in afterload in patients with HF (Pinsky et al., 1983, 1985), particularly in patients at the limit of the preload reserve (i.e., close to the maximal compensatory dilation of LV) based on the Frank-Starling principle (Ross, 1976). These also explain that patients with a high LVESV index at baseline showed an increase in fSVI and that patients with a low baseline fSVI showed a decrease in the SVR index as the applied PAP level increased. By reducing both preload and afterload in addition to the increase in forward SV, PAP can improve not only dilated LV but also LV geometry (Steiner et al., 2008). Consequently, in patients with a high LVESV index, fMR can be diminished by PAP through enhanced forward SV and possibly through improved annular dilatation and increased leaflet coaptation of the mitral valve in association with the improved LV geometry (Asgar et al., 2015).
In contrast, in previous studies wherein hemodynamic responses to various PAP therapies were investigated, healthy subjects with normal or high SV and patients with HF who had relatively low LV filling pressure had reduced SV by PAP (Bradley et al., 1992; Philip-Joet et al., 1999; Steiner et al., 2008; Yoshida et al., 2012; Yamada et al., 2013). In the present study, the subgroup of patients with a high baseline fSVI had significant reduction of forward SV by PAP despite similar alleviation of MR to their counterparts. In patients with high forward SV, who may not be in an afterload-dependent state, limited preload by PAP results in a reduction of blood flow throughout the heart. This may directly cause reductions in both MR and fSVI in patients with high forward SV. Consequently, such contrasting responses of forward SV across subgroups may explain why PAP did not change fSVI despite significant alleviation of fMR in the overall patient population.
Interestingly, in the subgroup analysis of the present study, we found a significant difference in the response of fSVI to PAP between the sexes; women had reduced forward SV by PAP, whereas men did not. This may have been due to the fact that women generally have smaller LV chamber sizes and better LV systolic function than men (Redfield et al., 2005). However, because women had a similar LV chamber size and LVEF to men in the present study (see Supplementary Data), this was not the case. Because these findings are based on subgroup analysis, an exploratory analysis to generate further hypotheses, these findings should be interpreted with caution. Furthermore, the underlying mechanisms for differing responses in forward SV between the sexes may be multifactorial, and we may have to take into account the differences between the sexes in lung mechanics (Bode et al., 1976). Furthermore, lung/airway resistance and lung compliance differed based on the cardiac function (Witte et al., 2002), and it was reported that PAP improved lung compliance and lung/airway resistance in patients with HF and the work of breathing decreased as the PAP level increased (Lenique et al., 1997). Thus, investigations regarding responses of fMR and its accompanying hemodynamic parameters to PAP in consideration of lung mechanics are of interest.
A recent study suggested that adding PS favorably affects SV compared with isolated CPAP, possibly through lung inflation and reflex inhibition of sympathetic nerve activity (Yoshida et al., 2012). However, in the present study, we did not find any differences in responses of fSVI and fMR between CPAP at 8 cm H2O and ASV. This may be explained because much less PS than ASV was applied in the present study compared with those in the previous study (mean PS of 1 cm H2O in addition to 4 cm H2O of EPAP in the present study vs. fixed PS at 5 cm H2O in addition to 4 cm H2O of EPAP in the previous study). However, it is of great interest that ASV with an even lower net applied PAP (i.e., 5 cm H2O) showed similar fMR alleviation and fSVI changes to CPAP at 8 cm H2O (net applied PAP, 8 cm H2O) in the present study. This may be associated with the stabilization of respiration by ASV, which has sympathoinhibitory effects (Ushijima et al., 2014). In a study by Ushijima et al. (2014), the sympathoinhibitory effects by ASV were predominantly observed in patients with periodic breathing while awake, which is generally observed in patients with advanced HF (Tomita et al., 2015). Despite the lack of assessment of such periodic breathing while awake, the findings of Ushijima's study and our findings that patients with a low baseline fSVI who are also likely to have periodic breathing while awake showed significant reductions in the SVR index in addition to the fact that all of our patients have SDB, which frequently coexists with periodic breathing while awake (Emdin et al., 2017), may help explain why ASV showed similar alleviation of MR and changes in fSVI to CPAP at 8 cm H2O. Further studies assessing the effects of ASV on fMR and fSVI in consideration of the presence or absence of periodic breathing while being awake are needed to clarify this.
The present study has several limitations. First, the number of enrolled subjects was small. However, the patients' hemodynamic responses to PAP therapy in the present study are basically compatible with the results of previous studies, which indicated that PAP therapy resulted in favorable hemodynamic responses in patients who are thought to have high filling pressure (Bradley et al., 1992; Philip-Joet et al., 1999; Steiner et al., 2008; Yoshida et al., 2012; Yamada et al., 2013). In contrast, because of the small number of subjects, and because of the complex study design to evaluate the acute effect of several settings of PAP therapy on fMR and accompanying changes in hemodynamic parameters, conducting more advanced multivariate analyses is difficult, and only exploratory subgroup analyses were performed. Thus, a future study recruiting a larger number of patients in a simple comparison, for instance, between on and off PAP, is certainly required to confirm the findings. Second, the 10-min duration of each step may be too short to detect changes in echocardiography parameters. Although the duration of the application of PAP varied between 5 and 30 min in previous studies that investigated similar acute hemodynamic responses (Bradley et al., 1992; Philip-Joet et al., 1999; Haruki et al., 2011; Yoshida et al., 2012; Yamada et al., 2013), our results were similar in this perspective. In addition, it is not feasible to have a longer duration of PAP application because our protocol has five steps. Third, because the study subjects were not asleep during PAP application, extrapolation of the hemodynamic responses to PAP during sleep is difficult. Therefore, further studies are needed to determine whether the present findings can be extrapolated to patients with HF who are asleep. Fourth, although the assessment of the severity of fMR was made by previously validated methods (Helmcke et al., 1987; Spain et al., 1989; Kang et al., 2008), the color Doppler appearance of the MR jets is influenced by several parameters unrelated to MR severity, such as gain settings, packet size, aliasing velocities, and frame rate. Finally, this study was designed to assess the acute effects of PAP on the alleviation of fMR, and whether chronic application of PAP can also alleviate fMR and improve LV function remains unclear. Therefore, whether a responder in the acute phase has a similar benefit in the chronic phase needs to be determined.
In conclusion, PAP therapy, including CPAP at 8 cm H2O and ASV, can alleviate fMR without any overall changes in fSVI in patients with HF, LV systolic dysfunction, and fMR. However, significant differences might be found in the responses of fSVI between sexes, patients with and without dilated LV, and those with and without low baseline SV despite no such differences in the alleviation of MR. These suggest that alleviation of MR due to PAP is not always a good sign and should be interpreted with caution, particularly in patients with HF and a less dilated LV. Although the effectiveness of the long-term application of PAP for the alleviation of fMR and improvement of LV function in these patients remains unclear, patients with dilated LV who have fMR may benefit from CPAP at 8 cm H2O or ASV.
Author Contributions
TakaoK: Experimental design, data acquisition, and analysis, drafting of the manuscript, and final approval of the manuscript submitted. TakatoshiK and SY: Experimental design, execution, data acquisition, and analysis, drafting of the manuscript, and final approval of the manuscript submitted. AM, MH, and SM: Experimental design and analysis, drafting of the manuscript, and final approval of the manuscript submitted. HM and NS: Data analysis and drafting of the manuscript, and final approval of the manuscript submitted. MK, FK, and SS: Data analysis, revision of the manuscript, and final approval of the manuscript submitted. HD: Research financing, experimental design, data analysis, revision and final approval of the manuscript submitted.
Funding
This study is partly supported by a Grant-in-Aid for Scientific Research (C) [Grant Number 26507010]; by a grant to the Respiratory Failure Research Group from the Ministry of Health, Labour and Welfare of Japan; by a Health, Labour, and Welfare Sciences Research Grants, Research on Region Medical from the Ministry of Health, Labour and Welfare of Japan; and by MEXT*-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (*Ministry of Education, Culture, Sports, Science and Technology); Japanese Center for Research on Women in Sport, Juntendo University. These funding sources do not have any other roles in this study.
Conflict of Interest Statement
TakatoshiK, SS, HM, NS, MK, and FK are affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi. HD received manuscript fees, research funds, and scholarship funds from Kirin Co., Ltd., Kaken Pharmaceutical Co., Ltd., Abbott Japan Co., Ltd., Astellas Pharma Inc., Astrazeneca K.K., Bayer Yakuhin, Ltd., Boston Scientific Japan K.K., Bristol-Myers Squibb, Daiichi Sankyo Company, MSD K.K., Pfizer Inc., Philips Respironics, Sanofi K.K., and Takeda Pharmaceutical Co. Ltd.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2017.00921/full#supplementary-material
References
Arzt, M., Floras, J. S., Logan, A. G., Kimoff, R. J., Series, F., Morrison, D., et al. (2007). Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 115, 3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482
Asgar, A. W., Mack, M. J., and Stone, G. W. (2015). Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J. Am. Coll. Cardiol. 65, 1231–1248. doi: 10.1016/j.jacc.2015.02.009
Bellone, A., Monari, A., Cortellaro, F., Vettorello, M., Arlati, S., and Coen, D. (2004). Myocardial infarction rate in acute pulmonary edema: noninvasive pressure support ventilation versus continuous positive airway pressure. Crit. Care Med. 32, 1860–1865. doi: 10.1097/01.CCM.0000139694.47326.B6
Bersten, A. D., Holt, A. W., Vedig, A. E., Skowronski, G. A., and Baggoley, C. J. (1991). Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N. Engl. J. Med. 325, 1825–1830. doi: 10.1056/NEJM199112263252601
Bode, F. R., Dosman, J., Martin, R. R., Ghezzo, H., and Macklem, P. T. (1976). Age and sex differences in lung elasticity, and in closing capacity in nonsmokers. J. Appl. Physiol. 41, 129–135.
Bradley, T. D., Holloway, R. M., McLaughlin, P. R., Ross, B. L., Walters, J., and Liu, P. P. (1992). Cardiac output response to continuous positive airway pressure in congestive heart failure. Am. Rev. Respir. Dis. 145, 377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377
Bradley, T. D., Logan, A. G., Kimoff, R. J., Series, F., Morrison, D., Ferguson, K., et al. (2005). Continuous positive airway pressure for central sleep apnea and heart failure. N. Engl. J. Med. 353, 2025–2033. doi: 10.1056/NEJMoa051001
Buda, A. J., Pinsky, M. R., Ingels, N. B. Jr., Daughters, G. T. 2nd, Stinson, E. B., and Alderman, E. L. (1979). Effect of intrathoracic pressure on left ventricular performance. N. Engl. J. Med. 301, 453–459. doi: 10.1056/NEJM197908303010901
Cowie, M. R., Woehrle, H., Wegscheider, K., Angermann, C., d'Ortho, M. P., Erdmann, E., et al. (2015). Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N. Engl. J. Med. 373, 1095–1105. doi: 10.1056/NEJMoa1506459
De Hoyos, A., Liu, P. P., Benard, D. C., and Bradley, T. D. (1995). Haemodynamic effects of continuous positive airway pressure in humans with normal and impaired left ventricular function. Clin. Sci. 88, 173–178. doi: 10.1042/cs0880173
Emdin, M., Mirizzi, G., Giannoni, A., Poletti, R., Iudice, G., Bramanti, F., et al. (2017). Prognostic significance of central apneas throughout a 24-hour period in patients with heart failure. J. Am. Coll. Cardiol. 70, 1351–1364. doi: 10.1016/j.jacc.2017.07.740
Evangelista-Masip, A., Bruguera-Cortada, J., Serrat-Serradell, R., Robles-Castro, A., Galve-Basilio, E., Alijarde-Guimera, M., et al. (1992). Influence of mitral regurgitation on the response to captopril therapy for congestive heart failure caused by idiopathic dilated cardiomyopathy. Am. J. Cardiol. 69, 373–376. doi: 10.1016/0002-9149(92)90236-R
Giannuzzi, P., Shabetai, R., Imparato, A., Temporelli, P. L., Bhargava, V., Cremo, R., et al. (1991). Effects of mental exercise in patients with dilated cardiomyopathy and congestive heart failure. An echocardiographic Doppler study. Circulation 83(4 Suppl.), II155–II165.
Gray, A., Goodacre, S., Newby, D. E., Masson, M., Sampson, F., Nicholl, J., et al. (2008). Noninvasive ventilation in acute cardiogenic pulmonary edema. N. Engl. J. Med. 359, 142–151. doi: 10.1056/NEJMoa0707992
Haruki, N., Takeuchi, M., Kaku, K., Yoshitani, H., Kuwaki, H., Tamura, M., et al. (2011). Comparison of acute and chronic impact of adaptive servo-ventilation on left chamber geometry and function in patients with chronic heart failure. Eur. J. Heart Fail. 13, 1140–1146. doi: 10.1093/eurjhf/hfr103
Helmcke, F., Nanda, N. C., Hsiung, M. C., Soto, B., Adey, C. K., Goyal, R. G., et al. (1987). Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 75, 175–183. doi: 10.1161/01.CIR.75.1.175
Hoffmann, B., and Welte, T. (1999). The use of noninvasive pressure support ventilation for severe respiratory insufficiency due to pulmonary oedema. Intens. Care Med. 25, 15–20. doi: 10.1007/s001340050781
Kang, S. J., Lim, H. S., Hwang, J., Choi, J. H., Seo, K. W., Choi, B. J., et al. (2008). Impact of changes in myocardial velocity assessed by tissue Doppler imaging during exercise on dynamic mitral regurgitation in patients with nonischemic cardiomyopathy. Echocardiography 25, 394–400. doi: 10.1111/j.1540-8175.2007.00621.x
Kasai, T. (2012). Sleep apnea and heart failure. J. Cardiol. 60, 78–85. doi: 10.1016/j.jjcc.2012.05.013
Kasai, T., and Bradley, T. D. (2011). Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J. Am. Coll. Cardiol. 57, 119–127. doi: 10.1016/j.jacc.2010.08.627
Kasai, T., Floras, J. S., and Bradley, T. D. (2012). Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 126, 1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813
Kasai, T., Narui, K., Dohi, T., Yanagisawa, N., Ishiwata, S., Ohno, M., et al. (2008). Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 133, 690–696. doi: 10.1378/chest.07-1901
Kato, T., Suda, S., and Kasai, T. (2014). Positive airway pressure therapy for heart failure. World J. Cardiol. 6, 1175–1191. doi: 10.4330/wjc.v6.i11.1175
Keren, G., Katz, S., Strom, J., Sonnenblick, E. H., and LeJemtel, T. H. (1989). Dynamic mitral regurgitation. an important determinant of the hemodynamic response to load alterations and inotropic therapy in severe heart failure. Circulation 80, 306–313. doi: 10.1161/01.CIR.80.2.306
Khayat, R., Jarjoura, D., Porter, K., Sow, A., Wannemacher, J., Dohar, R., et al. (2015). Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur. Heart J. 36, 1463–1469. doi: 10.1093/eurheartj/ehu522
Kiely, J. L., Deegan, P., Buckley, A., Shiels, P., Maurer, B., and McNicholas, W. T. (1998). Efficacy of nasal continuous positive airway pressure therapy in chronic heart failure: importance of underlying cardiac rhythm. Thorax 53, 957–962. doi: 10.1136/thx.53.11.957
Kinoshita, M., Okayama, H., Kawamura, G., Shigematsu, T., Takahashi, T., Kawata, Y., et al. (2017). Beneficial effects of rapid introduction of adaptive servo-ventilation in the emergency room in patients with acute cardiogenic pulmonary edema. J. Cardiol. 69, 308–313. doi: 10.1016/j.jjcc.2016.05.015
Lanzarini, L., Fontana, A., Lucca, E., Campana, C., and Klersy, C. (2002). Noninvasive estimation of both systolic and diastolic pulmonary artery pressure from Doppler analysis of tricuspid regurgitant velocity spectrum in patients with chronic heart failure. Am. Heart J. 144, 1087–1094. doi: 10.1067/mhj.2002.126350
Lenique, F., Habis, M., Lofaso, F., Dubois-Rande, J. L., Harf, A., and Brochard, L. (1997). Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am. J. Respir. Crit. Care Med. 155, 500–505. doi: 10.1164/ajrccm.155.2.9032185
Liston, R., Deegan, P. C., McCreery, C., Costello, R., Maurer, B., and McNicholas, W. T. (1995). Haemodynamic effects of nasal continuous positive airway pressure in severe congestive heart failure. Eur. Respir. J. 8, 430–435. doi: 10.1183/09031936.95.08030430
Masip, J., Betbese, A. J., Paez, J., Vecilla, F., Canizares, R., Padro, J., et al. (2000). Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet 356, 2126–2132. doi: 10.1016/S0140-6736(00)03492-9
Matsuo, S., Imai, E., Horio, M., Yasuda, Y., Tomita, K., Nitta, K., et al. (2009). Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992. doi: 10.1053/j.ajkd.2008.12.034
Mehta, S., Liu, P. P., Fitzgerald, F. S., Allidina, Y. K., and Douglas Bradley, T. (2000). Effects of continuous positive airway pressure on cardiac volumes in patients with ischemic and dilated cardiomyopathy. Am. J. Respir. Crit. Care Med. 161, 128–134. doi: 10.1164/ajrccm.161.1.9903055
Momomura, S., Seino, Y., Kihara, Y., Adachi, H., Yasumura, Y., Yokoyama, H., et al. (2015). Adaptive servo-ventilation therapy for patients with chronic heart failure in a confirmatory, multicenter, randomized, controlled study. Circ. J. 79, 981–990. doi: 10.1253/circj.CJ-15-0221
Nagueh, S. F., Appleton, C. P., Gillebert, T. C., Marino, P. N., Oh, J. K., Smiseth, O. A., et al. (2009). Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 22, 107–133. doi: 10.1016/j.echo.2008.11.023
Nakano, S., Kasai, T., Tanno, J., Sugi, K., Sekine, Y., Muramatsu, T., et al. (2015). The effect of adaptive servo-ventilation on dyspnoea, haemodynamic parameters and plasma catecholamine concentrations in acute cardiogenic pulmonary oedema. Eur. Heart. J. Acute. Cardiovasc. Care. 4, 305–315. doi: 10.1177/2048872614549103
Philip-Joet, F. F., Paganelli, F. F., Dutau, H. L., and Saadjian, A. Y. (1999). Hemodynamic effects of bilevel nasal positive airway pressure ventilation in patients with heart failure. Respiration 66, 136–143. doi: 10.1159/000029355
Pinsky, M. R., Matuschak, G. M., and Klain, M. (1985). Determinants of cardiac augmentation by elevations in intrathoracic pressure. J. Appl. Physiol. 58, 1189–1198.
Pinsky, M. R., Summer, W. R., Wise, R. A., Permutt, S., and Bromberger-Barnea, B. (1983). Augmentation of cardiac function by elevation of intrathoracic pressure. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 54, 950–955.
Redfield, M. M., Jacobsen, S. J., Borlaug, B. A., Rodeheffer, R. J., and Kass, D. A. (2005). Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112, 2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078
Ross, J. Jr. (1976). The concept of afterload mismatch and its implications in the clinical assessment of cardiac contractility. Jpn. Circ. J. 40, 865–875. doi: 10.1253/jcj.40.865
Rudikoff, M. T., Maughan, W. L., Effron, M., Freund, P., and Weisfeldt, M. L. (1980). Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation 61, 345–352. doi: 10.1161/01.CIR.61.2.345
Rusterholtz, T., Kempf, J., Berton, C., Gayol, S., Tournoud, C., Zaehringer, M., et al. (1999). Noninvasive pressure support ventilation (NIPSV) with face mask in patients with acute cardiogenic pulmonary edema (ACPE). Intens. Care Med. 25, 21–28. doi: 10.1007/s001340050782
Spain, M. G., Smith, M. D., Grayburn, P. A., Harlamert, E. A., and DeMaria, A. N. (1989). Quantitative assessment of mitral regurgitation by Doppler color flow imaging: angiographic and hemodynamic correlations. J. Am. Coll. Cardiol. 13, 585–590. doi: 10.1016/0735-1097(89)90597-4
Steiner, S., Schannwell, C. M., and Strauer, B. E. (2008). Left ventricular response to continuous positive airway pressure: role of left ventricular geometry. Respiration 76, 393–397. doi: 10.1159/000150442
Tomita, Y., Kasai, T., Kisaka, T., Rossiter, H. B., Kihara, Y., Wasserman, K., et al. (2015). Altered breathing syndrome in heart failure: newer insights and treatment options. Curr. Heart Fail. Rep. 12, 158–165. doi: 10.1007/s11897-014-0250-4
Ushijima, R., Joho, S., Akabane, T., Oda, Y., and Inoue, H. (2014). Differing effects of adaptive servoventilation and continuous positive airway pressure on muscle sympathetic nerve activity in patients with heart failure. Circ. J. 78, 1387–1395. doi: 10.1253/circj.CJ-13-1468
Vanoverschelde, J. L., Raphael, D. A., Robert, A. R., and Cosyns, J. R. (1990). Left ventricular filling in dilated cardiomyopathy: relation to functional class and hemodynamics. J. Am. Coll. Cardiol. 15, 1288–1295. doi: 10.1016/S0735-1097(10)80016-6
Wang, H., Parker, J. D., Newton, G. E., Floras, J. S., Mak, S., Chiu, K. L., et al. (2007). Influence of obstructive sleep apnea on mortality in patients with heart failure. J. Am. Coll. Cardiol. 49, 1625–1631. doi: 10.1016/j.jacc.2006.12.046
Witte, K. K., Morice, A., Clark, A. L., and Cleland, J. G. (2002). Airway resistance in chronic heart failure measured by impulse oscillometry. J. Card. Fail. 8, 225–231. doi: 10.1054/jcaf.2002.126916
Yamada, S., Sakakibara, M., Yokota, T., Kamiya, K., Asakawa, N., Iwano, H., et al. (2013). Acute hemodynamic effects of adaptive servo-ventilation in patients with heart failure. Circ. J. 77, 1214–1220. doi: 10.1253/circj.CJ-12-1088
Keywords: adaptive servo-ventilation, continuous positive airway pressure, cardiac output, congestion, filling pressure
Citation: Kato T, Kasai T, Yatsu S, Murata A, Matsumoto H, Suda S, Hiki M, Shiroshita N, Kato M, Kawana F, Miyazaki S and Daida H (2017) Acute Effects of Positive Airway Pressure on Functional Mitral Regurgitation in Patients with Systolic Heart Failure. Front. Physiol. 8:921. doi: 10.3389/fphys.2017.00921
Received: 18 July 2017; Accepted: 31 October 2017;
Published: 23 November 2017.
Edited by:
Jean-Pierre Montani, University of Fribourg, SwitzerlandReviewed by:
Jie Liu, Fourth Military Medical University, ChinaDiego Arroyo, University of Fribourg, Switzerland
Shin-ichi Ando, Kyushu University Hospital, Japan
Copyright © 2017 Kato, Kasai, Yatsu, Murata, Matsumoto, Suda, Hiki, Shiroshita, Kato, Kawana, Miyazaki and Daida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takatoshi Kasai, kasai-t@mx6.nisiq.net
 Takao Kato
Takao Kato Takatoshi Kasai
Takatoshi Kasai Shoichiro Yatsu
Shoichiro Yatsu Azusa Murata
Azusa Murata Hiroki Matsumoto
Hiroki Matsumoto Shoko Suda
Shoko Suda Masaru Hiki
Masaru Hiki Nanako Shiroshita2
Nanako Shiroshita2 Sakiko Miyazaki
Sakiko Miyazaki