- 1Department of Biology, National Museum of Natural Science, Taichung, Taiwan
- 2Graduate School of Engineering, Chiba University, Chiba, Japan
- 3Guangzhou Key Laboratory of Insect Development Regulation and Application Research, Institute of Insect Sciences and School of Life Sciences, South China Normal University, Guangzhou, China
In this study, phosphorylation of c-Jun N-terminal kinase (JNK) by the prothoracicotropic hormone (PTTH) was investigated in prothoracic glands (PGs) of the silkworm, Bombyx mori. Results showed that JNK phosphorylation was stimulated by the PTTH in time- and dose-dependent manners. In vitro activation of JNK phosphorylation in PGs by the PTTH was also confirmed in an in vivo experiment, in which a PTTH injection greatly increased JNK phosphorylation in PGs of day-6 last instar larvae. JNK phosphorylation caused by PTTH stimulation was greatly inhibited by U73122, a potent and specific inhibitor of phospholipase C (PLC) and an increase in JNK phosphorylation was also detected when PGs were treated with agents (either A23187 or thapsigargin) that directly elevated the intracellular Ca2+ concentration, thereby indicating involvement of PLC and Ca2+. Pretreatment with an inhibitor (U0126) of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK) and an inhibitor (LY294002) of phosphoinositide 3-kinase (PI3K) failed to significantly inhibit PTTH-stimulated JNK phosphorylation, indicating that ERK and PI3K were not related to JNK. We further investigated the effect of modulation of the redox state on JNK phosphorylation. In the presence of either an antioxidant (N-acetylcysteine, NAC) or diphenylene iodonium (DPI), PTTH-stimulated JNK phosphorylation was blocked. The JNK kinase inhibitor, SP600125, markedly inhibited PTTH-stimulated JNK phosphorylation and ecdysteroid synthesis. The kinase assay of JNK in PGs confirmed its stimulation by PTTH and inhibition by SP600125. Moreover, PTTH treatment did not affect JNK or Jun mRNA expressions. Based on these findings, we concluded that PTTH stimulates JNK phosphorylation in Ca2+- and PLC-dependent manners and that the redox-regulated JNK signaling pathway is involved in PTTH-stimulated ecdysteroid synthesis in B. mori PGs.
Introduction
Ecdysteroids regulate insect growth, molting, and metamorphosis; they are synthesized and secreted by the prothoracic glands (PGs) (Marchal et al., 2010; De Loof, 2011; Smith and Rybczynski, 2012; De Loof et al., 2013, 2015; Yamanaka et al., 2013). A neuropeptide, known as the prothoracicotropic hormone (PTTH) and produced by brain neurosecretory cells, activates ecdysteroidogenesis in PGs (Marchal et al., 2010; De Loof, 2011; Smith and Rybczynski, 2012; De Loof et al., 2013, 2015). Numerous studies were conducted to examine the complex PTTH signaling network. PTTH activates PGs by binding to its receptor, Torso, which is a receptor tyrosine kinase (Rewitz et al., 2009, 2013; Smith and Rybczynski, 2012). A complex signaling transduction network is activated downstream of PTTH receptor activation (Rewitz et al., 2009; Marchal et al., 2010; Smith and Rybczynski, 2012). This network includes a rapid increase in Ca2+ (Gu et al., 1998; Birkenbeil and Dedos, 2002; Fellner et al., 2005), cAMP generation (Smith et al., 1984, 1985; Gu et al., 1996), and activation of protein kinase A (PKA), phospholipase C (PLC), protein kinase C (PKC), p70S6 kinase (S6K), ribosomal protein S6, and tyrosine kinase (Song and Gilbert, 1995, 1997; Smith et al., 2003; Rybczynski and Gilbert, 2006; Lin and Gu, 2011). Our recent studies further indicated that reactive oxygen species (ROS) and phosphoinositide 3-kinase (PI3K)/adenosine 5′-monophosphate-activated protein kinase (AMPK)/target of rapamycin (TOR) signaling are involved in PTTH-stimulated ecdysteroidogenesis in Bombyx mori PGs (Gu et al., 2011, 2012, 2013; Hsieh et al., 2013, 2014).
Mitogen-activated protein kinase (MAPK) cascades transduce a variety of signals in eukaryotic cells in response to multiple extracellular stimuli (Roux and Blenis, 2004). Depending on the cell type, duration of the stimulus, and pathway, they mediate a range of cellular responses including proliferation, differentiation, development, inflammation, and apoptosis. The most thoroughly characterized subgroups of the MAPK family include extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases/stress-activated protein kinases (JNKs/SAPKs), and the p38 family of kinases (Widmann et al., 1999; Wetzker and Böhmer, 2003). Activated MAPKs are translocated to nuclei, where they phosphorylate a variety of target transcription factors (Roux and Blenis, 2004; Krishna and Narang, 2008). In insects, ERK phosphorylation appears to be involved in PTTH-stimulated ecdysteroidogenesis in both Manduca sexta and B. mori (Rybczynski et al., 2001; Lin and Gu, 2007; Gu et al., 2010; Gu and Hsieh, 2015). However, it is not clear whether other MAPK family members are involved in PTTH-stimulated ecdysteroidogenesis.
JNKs are a member of the MAPK family of protein kinases (Ip and Davis, 1998; Lewis et al., 1998; Weston and Davis, 2002). Mammalian JNKs were described as SAPKs, since they are activated by a variety of cellular stresses, such as UV light, heat, hyperosmotic shock, ROS, antioxidants, protein synthesis inhibitors, and inflammatory cytokines (Davis, 2000). In addition, JNKs are also activated by various growth factors, including prolactin, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), insulin, insulin-like growth factor, and ligands for some G protein-coupled receptors. Phosphorylated JNKs subsequently bind to the NH2-terminal activation domain of c-Jun on Ser-63 and Ser-73, resulting in mediation of gene expression regulation (Weston and Davis, 2002, 2007). Similar to mammalian cells, the JNK signaling pathway is also conserved in Drosophila. The Drosophila JNK pathway consists of Drosophila JNK or basket (DJNK) and JNK kinase Hep, which are respective homologs of JNK and upstream JNK kinases in mammals (Sluss et al., 1996). Drosophila JNK signaling appears to be involved in various developmental processes, such as dorsal and thorax closure, wing development, control of morphogenetic apoptosis, regulation of imaginal disc proliferation, wound healing, and regeneration (Stronach and Perrimon, 1999; Bogoyevitch and Kobe, 2006). Both the ERK- and JNK-dependent signaling pathways appear to contribute to Bombyx nucleopolyhedrovirus infection (Katsuma et al., 2007). More recently, we reported that JNK signaling together with other MAPK signaling pathways, which are rapidly induced by injury, are related to diapause termination in dechorionated Bombyx eggs (Gu and Chen, 2017).
In the present study, we investigated the involvement of JNK in PTTH-stimulated ecdysteroidogenesis by B. mori PGs. We demonstrated that JNK phosphorylation was stimulated by PTTH both in vitro and in vivo. The kinase assay of JNK in PGs confirmed its stimulation by PTTH. The JNK inhibitor, SP600125, markedly inhibited PTTH-stimulated JNK phosphorylation and ecdysteroid synthesis, indicating the involvement of JNK phosphorylation in PTTH-stimulated ecdysteroidogenesis.
Materials and Methods
Animals
Larvae of an F1 racial hybrid, Guofu × Nongfong, of B. mori were reared on fresh mulberry leaves at 25°C under a 12-h light: 12-h dark photoperiod. Newly-ecdysed last instar larvae were collected and used for each experiment.
Reagents
SP600125, N-acetylcysteine (NAC), and diphenylene iodonium (DPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Grace's insect cell culture medium was purchased from Invitrogen (Carlsbad, CA, USA). A MAPK/ERK kinase (MEK) inhibitor (U0126), a PI3K inhibitor (LY294002), A23187, and thapsigargin were purchased from Calbiochem (San Diego, CA, USA). All other reagents used were of analytical grade. [23, 24-3H] Ecdysone was obtained from New England Nuclear (Boston, MA, USA). Recombinant B. mori PTTH (PTTH) was kindly provided by Dr. David R. O'Reilly; it was produced by infection of Spodoptera frugiperda-SF21 cells with the vWTPTTHM baculovirus as described previously (O'Reilly et al., 1995). The same PTTH as that previously reported (O'Reilly et al., 1995; Gu et al., 2010) was used in the present study. In the present study, extracellular fluid from cells infected with vWTPTTHM was used as the PTTH source, and it was diluted 500 times with Grace's medium. Each incubation (50 μl) contained about 0.15 ng of PTTH.
In Vitro Incubation of PGs, Radioimmunoassay (RIA) of Ecdysteroids, and in Vivo Injection of the PTTH
In vitro incubation of PGs and the RIA of ecdysteroids followed protocols described in a previous study (Lin and Gu, 2007). To study the in vivo activation of JNK phosphorylation of PGs by the PTTH, day-6 last instar larvae were injected with 10 μl saline containing 0.3 μl of the original PTTH solution. Larvae injected with 10 μl saline only were used as controls.
Antibodies
Anti-phospho-SAPK/JNK (Thr183/Tyr185) (#9251), anti-phospho-ERK (#9101), anti-phospho-4E-BP (#9459), anti-total ERK (#9102), and anti-α-tubulin (#2144) antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The anti-JNK1/3 antibody (sc-474) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-labeled anti-rabbit immunoglobulin G (IgG) was purchased from PerkinElmer Life Sciences (Boston, MA, USA).
Western Blot Analysis
Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were performed as described previously (Lin and Gu, 2007; Gu et al., 2010). Briefly, PGs from day-6 last instar larvae were homogenized in lysis buffer (10 mM Tris and 0.1% Triton x100) at 4°C, and then boiled in an equal volume of SDS sample buffer for 4 min followed by centrifugation at 15,800 g for 3 min to remove any particulate matter. One percent protease inhibitor cocktail (Sigma-Aldrich, cat. no. P8340) and 1% phosphatase inhibitor cocktail (Calbiochem, cat. no. 524629) were added at the time of homogenization. Aliquots of the supernatants were loaded onto SDS gels. Following electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes using an Owl (Portsmouth, NH, USA) Bandit™ Tank Electroblotting System, and then washed with Tris-buffered saline (TBS) for 5 min at room temperature. Blots were blocked at room temperature for 1 h in TBS containing 0.1% Tween 20 (TBST) and 5% (w/v) nonfat powdered dry milk, followed by washing three times for 5 min each with TBST. Blots were incubated overnight at 4°C with primary antibodies against phospho-SAPK/JNK (1:1,000), phospho-ERK (1:10,000), phospho-4E-BP (1:3,000), JNK (1:1,000), ERK (1:1,000), or α-tubulin (1:10,000) in TBST with 5% bovine serum albumin (BSA). Blots were then washed three times in TBST for 10 min each and further incubated with an HRP-linked second antibody in TBST with 1% BSA. Following three additional washes, immunoreactivity was visualized by chemiluminescence using Western Lightning Chemiluminescence Reagent Plus from PerkinElmer Life Sciences. Films exposed to the chemiluminescent reaction were scanned and quantified using an AlphaImager Imaging System and AlphaEaseFC software (Alpha Innotech, San Leandro, CA, USA).
Kinase Assay for JNK Activity
To measure JNK activity, control or treated PGs (each of a pair of glands for control and treatment from 10 larvae) were lysed in JNK lysis buffer (20 mM Tris at pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin). Subsequently, 200 μl of gland lysate was incubated with 20 μl of c-Jun fusion protein beads overnight at 4°C, and then a kinase assay was performed following the manufacturer's instructions (Cell Signaling Technology). The reaction was carried out for 30 min at 30°C and was stopped by boiling the samples in 3x SDS sample buffer. Levels of phospho-c-Jun were measured by Western blotting as described in the section “Western blot analysis”.
Lambda Protein Phosphatase Treatment
PG lysates were treated with lambda protein phosphatase (New England Biolabs, Beverly, MA, USA), which removes phosphates from serine, threonine, and tyrosine residues (Zhuo et al., 1993). The lysates were incubated for 30 min at 30°C with 1000 units of lambda protein phosphatase in 50 μl of reaction buffer according to the manufacturer's specifications. At the end of the incubation period, the sample was boiled for 10 min and stored at −70°C until the PAGE analysis.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA from PGs was extracted using the TRI Reagent (New England Biolabs, Beverly, MA, USA) according to the manufacturer's protocol. Purification of total RNA, reverse transcription and real-time qRT-PCR were performed as described previously (Young et al., 2012). Transcript levels were normalized to the Bombyx ribosomal protein 49 (rp49, GenBank accession: NM_001098282.1). The real-time qRT-PCR was performed using the following primers: JNK forward, 5′-ACCAAGACTGATATTGTGATAC-3′ and JNK reverse, 5′-AAGTTGTCCGTGTCCATA-3′; Jun forward, 5′-ATGACGAGCAATATCCTC-3′ and Jun reverse, 5′-GATCCGAGCTTCAACATT-3′; and rp49 forward, 5′-CAGGCGGTTCAAGGGTCAATAC-3′ and rp49 reverse, 5′-TGCTGGGCTCTTTCCACGA-3′.
Data Analysis
Data are shown as the mean ± standard error of the mean (SEM). Statistical comparisons between different groups were done using either Student's t-test for comparison of two groups or a one-way analysis of variance (ANOVA) followed by Tukey's test for more than two groups. A p < 0.05 was considered significant.
Results
Bombyx mori PGs Expressed JNK, and PTTH Stimulated JNK Phosphorylation Both in Vitro and in Vivo
Although there are 10 different splicing isoforms of the three JNK genes (JNK 1, 2, and 3) in mammals, only one JNK (DJNK) exists in Drosophila. B. mori JNK (BmJNK) was cloned as previously reported by Katsuma et al. (2007). BmJNK was respectively reported to have 79.8 and 74.5% amino acid sequence identities with Drosophila DJNK (Basket) and human JNK1 (Katsuma et al., 2007). In the kinase domain, Bombyx JNK contains all conserved residues of protein kinases, and the TPY motif between kinase subdomains VII and VIII, which is phosphorylated on threonine and tyrosine residues to activate JNKs (Riesgo-Escovar et al., 1996; Sluss et al., 1996; Davis, 2000). High similarities of B. mori JNK with those of human and Drosophila suggest that commercial antibodies against mammalian JNK can likely be used in B. mori.
Previous studies showed that PTTH stimulates ERK phosphorylation in both Manduca and Bombyx PGs (Rybczynski et al., 2001; Lin and Gu, 2007). In the present study, we further examined whether PTTH stimulates JNK phosphorylation. Figure 1A shows whole blots of lysates of PGs. Using an anti-phospho-JNK (Thr183/Tyr185) antibody, one immunoreactive protein with a molecular weight (MW) of about 46 kDa was detected in the lysate of a control PG from day-6 last instar larvae. The PTTH greatly increased the phosphorylation level of this protein. In addition, the phosphorylation level of another immunoreactive protein with an MW of ~38 kDa was also strongly increased upon PTTH stimulation, although it remained low in the lysate of the control PG. Between these two bands, there was another small immunoreactive protein with an MW of ~40 kDa. Currently, it is not clear whether these two bands (of 38 and 40 kDa) correspond to minor splice variants of Bombyx JNK or were due to non-specificity of the antibody. Considering the difference in MW between Bombyx JNK and these two bands, we only focused on the 46 kDa Bombyx JNK in subsequent experiments.
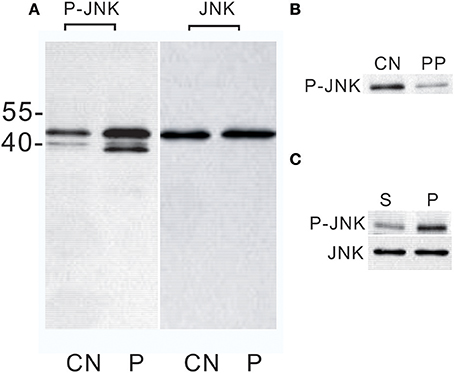
Figure 1. Western blot analysis of JNK phosphorylation in Bombyx PGs. (A) Effect of the PTTH in vitro. PGs were pre-incubated in control medium for 30 min and then transferred to control medium (CN) or medium containing the PTTH (P). PGs were incubated for 1 h. MW markers are shown on the left side of the gel. (B) Effect of lambda protein phosphatase treatment. CN, treated with buffer only; PP, treated with lambda protein phosphatase. (C) Effects of an in vivo PTTH injection on JNK phosphorylation. Larvae from day-6 last instar were injected with saline containing the PTTH (P) or saline only (S). At 30 min after the injection, PGs were quickly dissected out, and then immediately flash-frozen. Gland lysates were prepared and subjected to an immunoblot analysis with anti-phospho-JNK (P-JNK) and anti-JNK (JNK) antibodies. Results shown are representative of three independent experiments.
Studies were conducted with an antibody that recognizes total JNK. Results (Figure 1A) showed that the JNK level did not change after PTTH treatment, thus confirming that PTTH activates phosphorylation levels of this kinase. Lysates of PTTH-stimulated PGs were incubated with lambda protein phosphatase prior to electrophoresis, and the immunoreactivity detected by the antibody directed against human anti-phospho-JNK decreased (Figure 1B); this indicates that the above antibody, raised against mammalian sequences, indeed recognized phosphorylated epitopes in B. mori PGs.
The above results clearly showed that the PTTH activated JNK phosphorylation of B. mori PGs in vitro. In subsequent experiments, we examined in vivo activation of JNK phosphorylation of PGs by PTTH. PTTH was injected into day-6 last instar larvae. After 30 min, the PGs were quickly dissected out, and JNK phosphorylation was examined and compared to that of control larvae. We chose 30 min after the in vivo PTTH injection because in vitro time-dependent effects of the PTTH revealed that the highest stimulation was detected 30 min after the PTTH injection (see below). Results showed that the PTTH injection greatly increased the JNK phosphorylation level, compared to that of the controls, verifying the in vitro stimulation of JNK phosphorylation (Figure 1C).
PTTH Stimulated JNK Phosphorylation in Time- and Dose-Dependent Manners, and PTTH Stimulation Was Tissue-Specific
In a subsequent experiment, we studied the in vitro JNK phosphorylation by the PTTH in greater detail. Figure 2A shows that a time-dependent increase in JNK phosphorylation in vitro was detected when PGs from day-6 last instar larvae were treated with the PTTH. Activation of JNK by the PTTH was detected after 10 min and reached a peak at 30 min after treatment, and activation persisted for 120 min (Figure 2A). Figure 2B shows the dose-dependent effects of the PTTH on JNK phosphorylation. To examine whether or not PTTH specifically exerts its action on B. mori PGs, PGs, subesophageal ganglions, wing disks, salivary glands, and a small piece of fat body were incubated with or without the PTTH. Results showed that only PGs, but not other tissues, exhibited activation of JNK phosphorylation after stimulation by the PTTH (Figure 2C), indicating the specificity of PTTH's action on PGs.
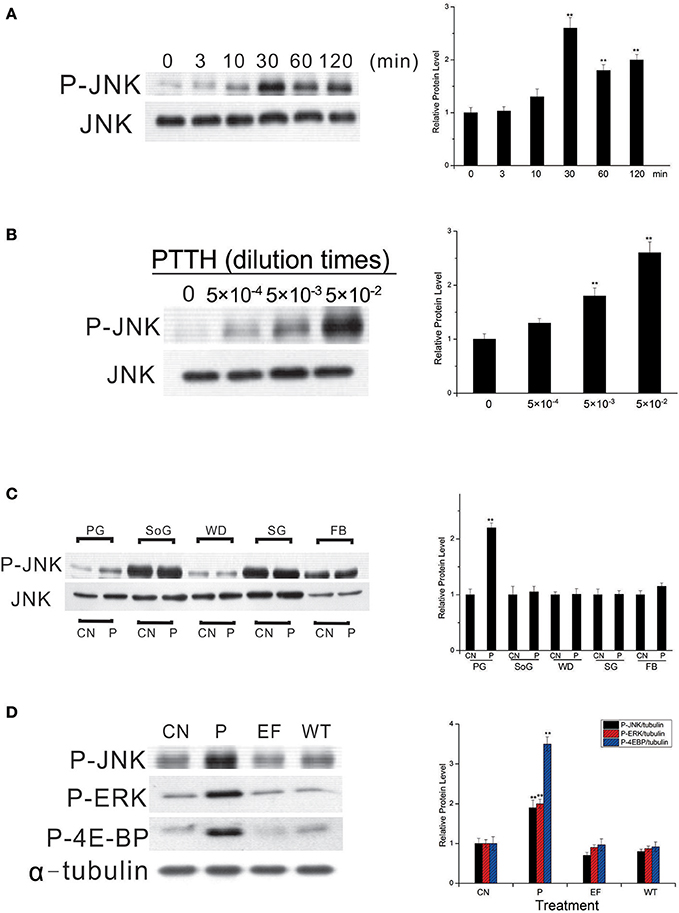
Figure 2. Time- (A) and dose- (B) dependent effects of JNK phosphorylation by the PTTH, tissue specificity of PTTH-stimulated JNK phosphorylation (C), and the effect of treatment with extracellular fluid from cells only or cells infected with the wild-type (WT) AcMNPV on protein phosphorylation (D). (A,B) Time- and dose-dependent effects. PGs were either treated with PTTH for the indicated time points (A), treated with the indicated concentrations of PTTH, or incubated with control medium (0) for 60 min (B). (C) Tissue specificity. PGs, subesophageal ganglia (SoG), wing disks (WD), salivary glands (SG), and fat body (FB) from day 6-last instar larvae were incubated with control medium (CN) or medium containing the PTTH (P) for 60 min. (D) Effect of treatment with either control medium (CN), the PTTH (P), extracellular fluid from cells only (EF), or cells infected with WT AcMNPV (WT) on the phosphorylation of JNK, ERK, and 4E-BP. Each lysate was prepared and subjected to an immunoblot analysis with anti-phospho-JNK (P-JNK), anti-phospho-ERK (P-ERK), anti-phospho-4E-BP (P-4E-BP), anti-JNK (JNK), and anti-α-tubulin (α-tubulin) antibodies. Results shown in the left panels are representative of three independent experiments. Data are expressed as multiples of change over the respective control after being normalized to the level of JNK (for A–C) or α-tubulin (for D). Asterisks indicate a significant difference compared to the respective control (by Student's t-test, **p < 0.01).
In addition, when PGs were treated with extracellular fluid from cells only or cells infected with WT AcMNPV, no activation of JNK phosphorylation was observed (O'Reilly et al., 1995); this indicates the specificity of the recombinant PTTH (Figure 2D). Treatment with the PTTH, but not with extracellular fluid from cells only or cells infected with the WT AcMNPV, stimulated ERK and 4E-BP phosphorylation, two well-documented signaling pathways downstream of PTTH stimulation (Gu et al., 2010, 2017), further confirming the specificity of the recombinant PTTH in the present study.
Involvement of PLC and Ca2+ in PTTH-Stimulated JNK Phosphorylation
U73122, a selective pharmacological inhibitor of phosphoinositide-specific PLC (Smith et al., 1990), was used to demonstrate the involvement of PLC in PTTH-stimulated JNK phosphorylation. As shown in Figure 3A, pretreatment of PGs with U73122 (10 μM) blocked PTTH-stimulated JNK phosphorylation, thus indicating PLC's involvement.
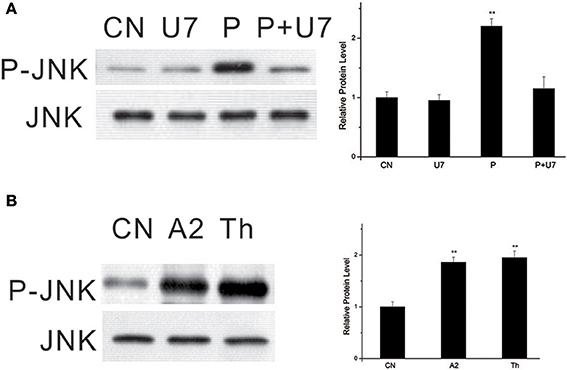
Figure 3. Effect of U73122 on PTTH-stimulated JNK phosphorylation (A) and stimulation of JNK phosphorylation by A23187 and thapsigargin (B). (A) Effect of U73122. PGs were pretreated with either 10 μM U73122, or the vehicle alone for 30 min and transferred to medium containing the same dose of U73122, with or without the PTTH. CN, glands incubated in control medium; P, glands incubated in medium containing the PTTH only; U7: glands incubated in medium containing U73122 only; P+U7, glands incubated in medium containing both the PTTH and U73122. (B) Effects of A23187 and thapsigargin. Gland lysates were prepared and subjected to an immunoblot analysis with anti-phospho-JNK (P-JNK) and anti-JNK (JNK) antibodies. Results shown in the left panels are representative of three independent experiments. Data are expressed as multiples of change over the respective control after being normalized to the JNK level. Asterisks indicate a significant difference compared to the respective control (by Student's t-test, **p < 0.01).
To further examine whether PTTH-stimulated JNK phosphorylation is dependent on Ca2+, we used A23187, a Ca2+ ionophore. Figure 3B shows that treatment with A23187 greatly increased JNK phosphorylation. Similar to A23187, thapsigargin, an inhibitor of endoplasmic reticulum Ca2+-ATPase, also increased JNK phosphorylation. Gu et al. (2010) previously reported that A23187 and thapsigargin increase ERK phosphorylation and ecdysteroid secretion in Bombyx PGs.
Effects of Inhibitors of MEK and PI3K on PTTH-Stimulated JNK Phosphorylation
We used U0126, a specific MEK inhibitor, to determine whether PTTH-stimulated JNK phosphorylation is linked to ERK phosphorylation. As shown in Figure 4A, U0126 did not block PTTH-stimulated JNK phosphorylation. We further examined the effect of LY294002, a PI3K inhibitor, on PTTH-stimulated JNK phosphorylation. PGs pretreated with LY294002 were subsequently challenged with the PTTH. Figure 4B shows that LY294002 did not affect PTTH-stimulated JNK phosphorylation, which indicates that PI3K signaling is not related in PTTH-stimulated JNK phosphorylation.
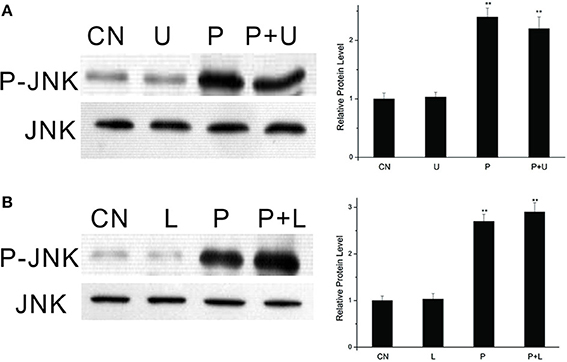
Figure 4. Effects of U0126 (A) and LY294002 (B) on PTTH-stimulated JNK phosphorylation. PGs were pretreated with either 10 μM U0126, 50 μM LY294002, or control medium for 30 min, and then transferred to medium containing the same dose of each inhibitor, with or without the PTTH. CN, PGs incubated in control medium; P, PGs incubated in medium containing the PTTH only; U, PGs incubated in medium containing U0126 only; P+U, PGs incubated in medium containing both the PTTH and U0126; L, PGs incubated in medium containing LY294002 only; P+L, PGs incubated in medium containing both the PTTH and LY294002. The incubation time was 60 min. Gland lysates were prepared and subjected to an immunoblot analysis with anti-phospho-JNK (P-JNK) and anti-JNK (JNK) antibodies. Results shown in the left panels are representative of three independent experiments. Data are expressed as multiples of change over the respective control after being normalized to the level of JNK. Asterisks indicate a significant difference compared to the respective control (by Student's t-test, **p < 0.01).
Activation of JNK Signaling by the PTTH Is Dependent on ROS
In our previous study, we found that PTTH-stimulated phosphorylation of ERK is dependent on the redox status (Hsieh et al., 2014). To determine whether the JNK signaling pathway is redox-sensitive, PGs were pretreated with NAC, a superoxide scavenger, to block PTTH-stimulated ROS production (Hsieh et al., 2013), and then were stimulated with the PTTH. As shown in Figure 5A, blocking the PTTH-stimulated production of ROS by NAC completely blocked PTTH-stimulated JNK phosphorylation compared to PTTH treatment only. Treatment with a mitochondrial oxidative phosphorylation inhibitor (DPI) also greatly decreased PTTH-stimulated JNK phosphorylation (Figure 5B). In addition, treatment with NAC also reduced the basal JNK phosphorylation level compared to the controls, possibly due to inhibition of ROS production. Figure 5C shows that treatment with 1 mM of H2O2 greatly stimulated JNK phosphorylation. This result demonstrated that ROS alone can regulate JNK phosphorylation, thus confirming that ROS play a critical role in PTTH-stimulated JNK signaling. These findings suggest that JNK phosphorylation might be a critical component of the redox-sensitive signaling pathway activated by the PTTH in B. mori PGs.
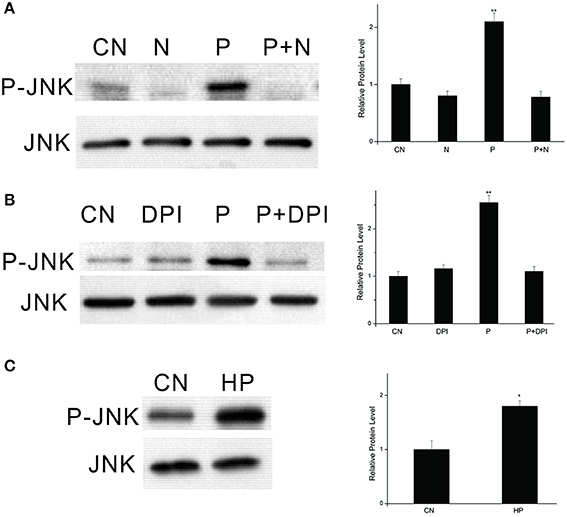
Figure 5. Effects of NAC (A) and DPI (B) on PTTH-stimulated JNK phosphorylation and effect of exogenous H2O2 on JNK phosphorylation (C). PGs were pretreated with either NAC (20 mM), DPI (10 μM), or vehicle alone for 30 min, and then transferred to medium containing the same dose of the inhibitors, with or without the PTTH. Incubation was maintained for 60 min. CN, PGs incubated in control medium; N, PGs incubated in medium containing NAC only; P, PGs incubated in medium containing the PTTH only; P+N, PGs incubated in medium containing both the PTTH and NAC; DPI, PGs incubated in medium containing DPI only; P+DPI, PGs incubated in medium containing both the PTTH and DPI; HP, PGs treated with H2O2 (1 mM). Gland lysates were prepared and subjected to an immunoblot analysis with anti-phospho-JNK (P-JNK) and anti-JNK (JNK) antibodies. Results shown in the left panels are representative of three independent experiments. Data are expressed as multiples of change over the respective control after being normalized to the JNK level. Asterisks indicate a significant difference compared to the respective control (by Student's t-test, *p < 0.05; **p < 0.01).
Effects of SP600125 on PTTH-Stimulated JNK Phosphorylation and Ecdysteroid Synthesis
The above data clearly show that the PTTH stimulates JNK phosphorylation. We further confirmed the role of JNK signaling in regulating PTTH-stimulated ecdysteroid synthesis with SP600125, a specific JNK kinase inhibitor. After PGs were pretreated with SP600125, they were challenged with the PTTH. Phosphorylated JNK and ERK levels in PGs were examined, and ecdysteroid secretion was determined. Results (Figure 6) showed that SP600125 treatment decreased PTTH-stimulated JNK phosphorylation levels, but did not affect PTTH-stimulated ERK phosphorylation levels. The basal ERK phosphorylation level was inhibited by treatment with SP600125, indicating that basal ERK phosphorylation is sensitive to this inhibitor. Moreover, although SP600125 treatment did not completely block PTTH-stimulated ecdysteroid secretion, it greatly inhibited it. These results confirm the involvement of JNK signaling in PTTH-stimulated ecdysteroid secretion.
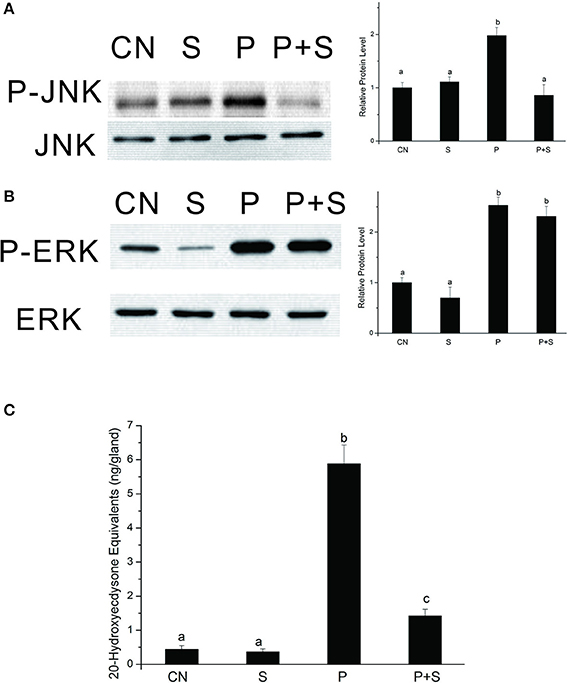
Figure 6. Effects of SP600125 on PTTH-stimulated phosphorylation of JNK (A) and ERK (B) and ecdysteroid secretion (C). PGs were pretreated with 20 μM SP600125 or vehicle alone for 30 min, and then transferred to medium containing the same dose of SP600125, with or without the PTTH. Incubation was maintained for 60 min. Gland lysates were prepared and subjected to an immunoblot analysis with anti-phospho-JNK (P-JNK), anti-JNK (JNK), anti-phospho-ERK (P-ERK), and anti-ERK (ERK) antibodies. CN, glands incubated in control medium; S, glands incubated in medium containing 20 μM SP600125 only; P, glands incubated in medium containing the PTTH only; P+S, glands incubated in medium containing both the PTTH and 20 μM SP600125. Results shown in the left panels (for A,B) are representative of three independent experiments. Data are expressed as multiples of change over the respective control after being normalized to the level of JNK (for A) or ERK (for B). Ecdysteroid released into the medium was determined by an RIA. Different letters above the bars indicate a significant difference (ANOVA followed by Tukey's multiple-comparisons test, p < 0.05).
Effect of the PTTH on the Kinase Activity of JNK
We further examined the effect of the PTTH on the kinase activity of JNK. Lysates from PGs were incubated with 20 μl of c-Jun fusion protein beads overnight, and then a kinase assay was performed per the manufacturer's instructions. Results (Figure 7) show that the kinase activity of JNK from PTTH-stimulated PG lysates greatly increased compared to that from control gland lysates, and that SP600125 inhibited the PTTH-stimulated kinase activity of JNK. In addition, SP600125 also inhibited the basal kinase activity of JNK. These results confirmed that the PTTH-activated phosphorylation of JNK is indeed related to its kinase activity.
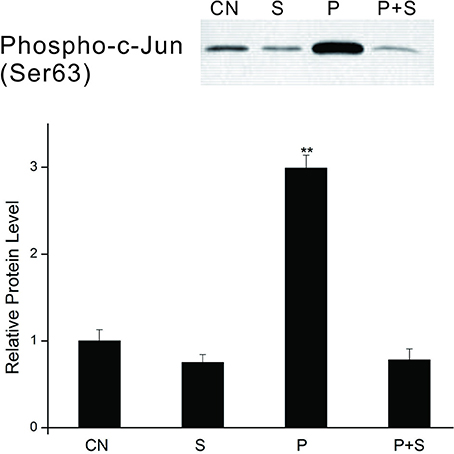
Figure 7. Effects of SP600125 on the PTTH-stimulated kinase activity of JNK. PGs were pretreated with 20 μM SP 600125 or vehicle alone for 30 min, and then transferred to medium containing the same dose of SP600125, with or without the PTTH. Incubation was maintained for 60 min. CN, glands incubated in control medium; S, glands incubated in medium containing 20 μM SP600125 only; P, glands incubated in medium containing the PTTH only; P+S, glands incubated in medium containing both the PTTH and 20 μM SP600125. In vitro kinase assays using c-Jun fusion protein beads were performed on gland lysates and subsequently analyzed by immunoblotting with an antibody to phospho-c-Jun. Results shown in the top panels are representative of three independent experiments. Data are expressed as multiples of change over the control. Asterisks indicate a significant difference compared to the control (by Student's t-test, **p < 0.01).
Effect of the PTTH on JNK and Jun Gene Expressions
We further conducted an experiment to determine changes in JNK (GenBank accession: NP_001103396.1) and Jun (http://silkworm.genomics.org.cn/: BGIBMGA004164) mRNA expression levels upon PTTH treatment. As shown in Figure 8, no significant differences were detected in JNK or Jun transcription levels between control glands and those treated with the PTTH during the 1- or 2-h incubation periods after in vitro treatment with PTTH.
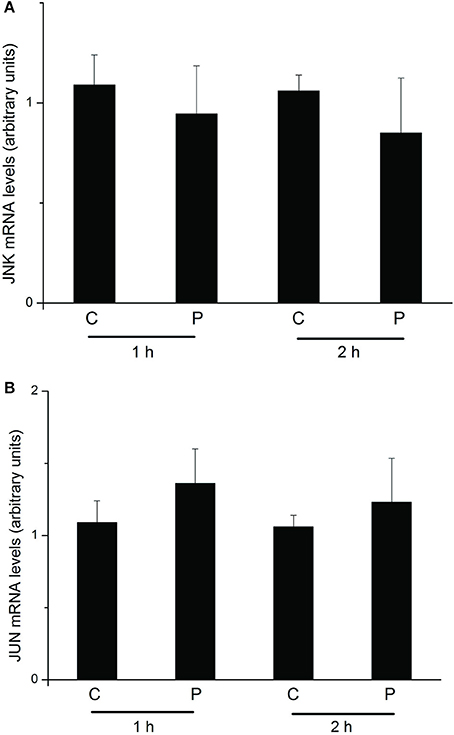
Figure 8. Changes in mRNA expression levels of JNK (A) and Jun (B) upon treatment with the PTTH. PGs were preincubated in medium for 30 min and then transferred to medium containing the PTTH (P) or control medium (C). Incubation was maintained for 1 and 2 h. After each incubation, total RNA was extracted from PGs, and mRNA expression levels of JNK and Jun were determined by an RT-qPCR. Each bar represents the mean ± SEM of four separate assays.
Discussion
The pathways that mediate ecdysteroidogenesis in response to PTTH stimulation are complex and not fully understood at the molecular level. Several studies demonstrated the involvement of the PKA, PKC, ERK, and AMPK/TOR signaling pathways (Smith and Rybczynski, 2012; Gu et al., 2017). In the present study, we demonstrated that PTTH stimulates phosphorylation of JNK in B. mori PGs. In vitro activation by the PTTH of JNK phosphorylation in B. mori PGs was also confirmed by in vivo experiments: a PTTH injection into day-6 last instar larvae greatly augmented JNK phosphorylation of PGs. The kinase assay of JNK in PGs confirmed its stimulation by the PTTH. However, the PTTH did not affect JNK or Jun mRNA expressions. Although ERK/MAPK signaling was previously demonstrated to be involved in PTTH-stimulated ecdysteroidogenesis in PGs of both M. sexta (Rybczynski et al., 2001) and B. mori (Lin and Gu, 2007; Gu et al., 2010), this is the first study, to our knowledge, to demonstrate JNK phosphorylation by the PTTH both in vitro and in vivo.
We further showed that PTTH-stimulated ecdysteroidogenesis in B. mori PGs was suppressed by SP600125, a specific inhibitor of JNK (Bennett et al., 2001). SP600125 was found to truly attenuate JNK phosphorylation induced by the PTTH in PGs. A kinase assay of JNK confirmed its inhibitory effect. ERK was previously reported to be involved in phosphorylation induced by the PTTH (Rybczynski et al., 2001; Lin and Gu, 2007). However, SP600125 did not affect PTTH-stimulated ERK phosphorylation. Therefore, SP600125-induced inhibition of PTTH-stimulated ecdysteroid synthesis is due to inhibition of JNK phosphorylation. Based on these findings, it is most likely that ecdysteroidogenesis stimulated by the PTTH is mediated through phosphorylation of both ERK and JNK in B. mori PGs (Figure 9). However, it should be stated that PTTH-mediated signaling transduction pathways are far more complex than illustrated in Figure 9, and that many downstream effectors are still being explored. It is suggested that PTTH binding alters the protein conformation of Torso such that it stimulates nearby effector proteins which catalyze the production of second messengers, and finally propagate the signal through various intricate signaling pathways to induce new gene expressions. In addition, our previous study demonstrated that TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis in Bombyx PGs (Gu et al., 2012). PGs may integrate signaling from the PTTH, insulin, nutrients, or other factors to regulate the AMPK/TOR signaling pathway in order to modulate cell growth and ecdysteroidogenesis (Gu et al., 2012, 2015; Smith et al., 2014).
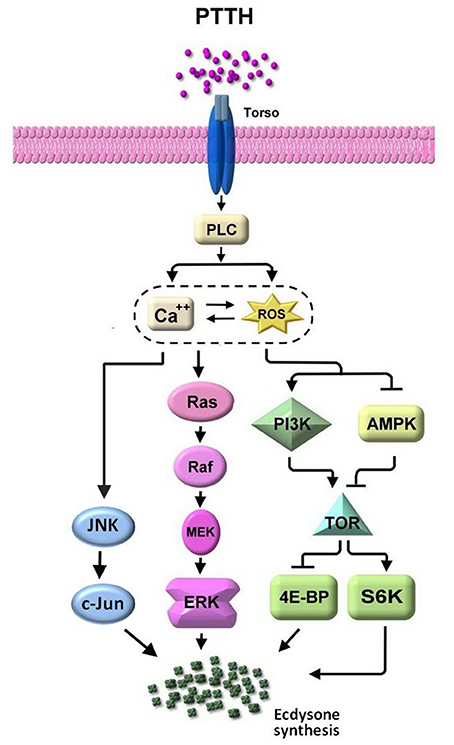
Figure 9. The signaling network involved in PTTH-stimulated ecdysteroidogenesis in Bombyx PGs. See text for details.
The MAPK superfamily was found to mediate intracellular signaling of extracellular agonists and play important roles in various cellular functions including proliferation, differentiation, and apoptosis in a variety of cells (Roux and Blenis, 2004). Conventional MAPK proteins consist of three family members (Johnson and Lapadat, 2002): ERK, JNK, and p38. ERK is mainly activated by mitogens and differentiation signals, while JNK and p38 are activated by stress stimuli and many different factors, including EGF, PDGF, transforming growth factor (TGF)-β, and tumor necrosis factor (Roux and Blenis, 2004). In the present study, we found that pretreatment with neither U0126, a MEK inhibitor, nor LY294002, a PI3K inhibitor, greatly inhibited JNK phosphorylation stimulated by the PTTH. This result suggests that PTTH-stimulated JNK signaling is independent of both ERK and PI3K. The independence of PTTH-stimulated ERK signaling from PI3K was previously demonstrated (Gu et al., 2010). In addition, we detected increased JNK phosphorylation after PGs were treated with agents that directly elevated the intracellular Ca2+ concentration (either A23187 or thapsigargin). U73122 was also found to block PTTH-stimulated JNK phosphorylation. These results clearly indicate that PTTH-stimulated JNK signaling is both PLC- and /Ca2+-dependent.
Our previous study demonstrated that mitochondrion-derived ROS signaling and Ca2+ signaling are together involved in PTTH-stimulated ecdysteroidogenesis (Hsieh et al., 2013). We further found that ROS signaling lies upstream of ERK, 4E-BP, and AMPK phosphorylation (Hsieh et al., 2014). In the present study, we investigated the regulatory function of the redox state on JNK phosphorylation. Our results indicate that pretreatment with either an antioxidant (NAC) or DPI blocked PTTH-regulated JNK phosphorylation. In addition, we found that ROS alone could activate JNK phosphorylation. These results indicate that similar to ERK signaling, PTTH-stimulated JNK phosphorylation is a redox-regulated signaling pathway. Although redox regulation of MAPK signaling is well-documented in mammalian systems (Son et al., 2013), this, to our knowledge, is the first study to demonstrate redox regulation of JNK signaling in the endocrine system of an insect. However, it is necessary to view this conclusion with caution, because the specificities of the experimental inhibitors we used have not been proven in insects.
In larval insects, the PTTH is the major stimulator of ecdysteroid secretion in PGs. Early studies showed that the three-dimensional structure of the PTTH resembles those of mammalian growth factors including PDGF, NGF, and TGF-β (Noguti et al., 1995). Although the present study clearly showed that JNK activation is involved in PTTH-stimulated ecdysteroid secretion by B. mori PGs during a short-term incubation period, it is not clear whether or not PTTH-stimulated JNK signaling has additional roles. In mammalian cells, it was revealed that growth factors and oncogenes induced sustained activation of JNK to mediate their effects on survival, proliferation, and transformation (Davis, 2000). Activation of JNK in Rat-1 fibroblasts was shown to contribute to insulin-stimulated transcription of genes regulated by activating protein-1 (Miller et al., 1996). Furthermore, JNK is important for AP-1-mediated transcriptional activation and cell growth in response to insulin-like growth factor-1 in IEC-6 intestinal cells (Simmons et al., 1995). Like ERK, JNK may be re-localized from the cytoplasm to nuclei following stimulation (Roux and Blenis, 2004; Krishna and Narang, 2008). A wide range of nuclear proteins, predominantly transcription factors and nuclear hormone receptors, were found to be JNK substrates (Weston and Davis, 2002). These directly affect its gene expression. A well-known substrate for JNK is the transcription factor, c-Jun. Phosphorylation of c-Jun by JNK leads to increased c-Jun-dependent transcription (Weston and Davis, 2002). In the present study, phosphorylation of c-Jun by the PTTH was confirmed. Thus, it is possible that PTTH-stimulated JNK phosphorylation may play a role in regulating activation of transcription factors related to c-Jun, leading to a sustained increase in ecdysteroidogenesis. Further study is needed to clarify downstream signaling pathways of JNK phosphorylation stimulated by the PTTH.
In summary, our study demonstrates that JNK represents another class of signaling molecules which reside downstream of the PTTH receptor and which may be necessary for ecdysteroid biosynthesis. Understanding the roles of JNK in signaling and identifying mechanisms that regulate JNK activation in response to PTTH and its downstream signaling pathways are fundamental to a clearer understanding of signaling events that regulate ecdysteroidogenesis in insect PGs.
Author Contributions
S-HG: designed and conducted the experiments, and wrote the manuscript; GL and SL: provided some ideas and contributed important resources, techniques, and reagents; H-YH and P-LL performed the experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Taiwan's Ministry of Science and Technology for a grant (MOST104-2311-B-178-002-MY3) and the National Museum of Natural Science, Taiwan for its financial support. GL is funded by the Japan Society for the Promotion of Science (JP17K17641).
References
Bennett, B. L., Sasaki, D. T., Murray, B. W., O'Leary, E. C., Sakata, S. T., Xu, W., et al. (2001). SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 98, 13681–13686. doi: 10.1073/pnas.251194298
Birkenbeil, H., and Dedos, S. G. (2002). Ca2+ as second messenger in PTTH-stimulated prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 32, 1625–1634. doi: 10.1016/S0965-1748(02)00101-7
Bogoyevitch, M. A., and Kobe, B. (2006). Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 70, 1061–1095. doi: 10.1128/MMBR.00025-06
Davis, R. J. (2000). Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252. doi: 10.1016/S0092-8674(00)00116-1
De Loof, A. (2011). Longevity and aging in insects: Is reproduction costly; cheap; beneficial or irrelevant? a critical evaluation of the “trade-off” concept. J. Insect Physiol. 57, 1–11. doi: 10.1016/j.jinsphys.2010.08.018
De Loof, A., Boerjan, B., Ernst, U. R., and Schoofs, L. (2013). The mode of action of juvenile hormone and ecdysone: towards an epi-endocrinological paradigm? Gen. Comp. Endocrinol. 188, 35–45. doi: 10.1016/j.ygcen.2013.02.004
De Loof, A., Vandersmissen, T., Marchal, E., and Schoofs, L. (2015). Initiation of metamorphosis and control of ecdysteroid biosynthesis in insects: the interplay of absence of juvenile hormone, PTTH, and Ca2+-homeostasis. Peptides 68, 120–129. doi: 10.1016/j.peptides.2014.07.025
Fellner, S. K., Rybczynski, R., and Gilbert, L. I. (2005). Ca2+ signaling in prothoracicotropic hormone-stimulated prothoracic gland cells of Manduca sexta: evidence for mobilization and entry mechanisms. Insect Biochem. Mol. Biol. 35, 263–275. doi: 10.1016/j.ibmb.2004.11.006
Gu, S. H., and Chen, C. H. (2017). Injury-induced rapid activation of MAPK signaling in dechorionated eggs and larvae of the silkworm, Bombyx mori. Insect Sci. 24, 248–258. doi: 10.1111/1744-7917.12301
Gu, S. H., Chen, C. H., Hsieh, Y. C., Young, S. C., and Lin, P. L. (2015). Modulatory effects of bombyxin on ecdysteroidogenesis in Bombyx mori prothoracic glands. J. Insect Physiol. 72, 62–69. doi: 10.1016/j.jinsphys.2014.11.007
Gu, S. H., Chow, Y. S., Lin, F. J., Wu, J. L., and Ho, R. J. (1996). A deficiency in prothoracicotropic hormone transduction pathway during the early last larval instar of Bombyx mori. Mol. Cell. Endocrinol. 120, 99–105.
Gu, S. H., Chow, Y. S., and O'Reilly, D. R. (1998). Role of calcium in the stimulation of ecdysteroidogenesis by recombinant prothoracicotropic hormone in the prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 28, 861–867
Gu, S. H., and Hsieh, Y. C. (2015). Regulation of histone H3 phosphorylation at serine 10 in PTTH-stimulated prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 57, 27–33. doi: 10.1016/j.ibmb.2014.12.004
Gu, S. H., Hsieh, Y. C., and Lin, P. L. (2017). Signaling involved in PTTH-stimulated 4E-BP phosphorylation in prothoracic gland cells of Bombyx mori. J. Insect Physiol. 96, 1–8. doi: 10.1016/j.jinsphys.2016.10.007
Gu, S. H., Hsieh, Y. C., Young, S. C., and Lin, P. L. (2013). Involvement of phosphorylation of adenosine 5'-monophosphate-activated protein kinase in PTTH-stimulated ecdysteroidogenesis in prothoracic glands of the silkworm, Bombyx mori. PLoS ONE 8:e63102. doi: 10.1371/journal.pone.0063102
Gu, S. H., Lin, J. L., and Lin, P. L. (2010). PTTH-stimulated ERK phosphorylation in prothoracic glands of the silkworm, Bombyx mori: role of Ca2+/calmodulin and receptor tyrosine kinase. J. Insect Physiol. 56, 93–101. doi: 10.1016/j.jinsphys.2009.09.008
Gu, S. H., Yeh, W. L., Young, S. C., Lin, P. L., and Li, S. (2012). TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 42, 296–303. doi: 10.1016/j.ibmb.2011.12.010
Gu, S. H., Young, S. J., Lin, J. L., and Lin, P. L. (2011). Involvement of PI3K/Akt signaling in PTTH-stimulated ecdysteroidogenesis by prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 41, 197–202. doi: 10.1016/j.ibmb.2010.12.004
Hsieh, Y. C., Hsu, S. L., and Gu, S. H. (2013). Involvement of reactive oxygen species in PTTH-stimulated ecdysteroidogenesis in prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 43, 859–866. doi: 10.1016/j.ibmb.2013.06.008
Hsieh, Y. C., Lin, P. L., and Gu, S. H. (2014). Signaling of reactive oxygen species in PTTH-stimulated ecdysteroidogenesis in prothoracic glands of the silkworm, Bombyx mori. J. Insect Physiol. 63, 32–39. doi: 10.1016/j.jinsphys.2014.02.004
Ip, Y. T., and Davis, R. J. (1998). Signal transduction by the c-Jun N-terminal kinase (JNK) -from inflammation to development. Curr. Opin. Cell Biol. 10, 205–219.
Johnson, G. L., and Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912. doi: 10.1126/science.1072682
Katsuma, S., Mita, K., and Shimada, T. (2007). ERK- and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J. Virol. 81, 13700–13709. doi: 10.1128/JVI.01683-07
Krishna, M., and Narang, H. (2008). The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci. 65, 3525–3244. doi: 10.1007/s00018-008-8170-7
Lewis, T. S., Shapiro, P. S., and Ahn, N. G. (1998). Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74, 49–139.
Lin, J. L., and Gu, S. H. (2007). In vitro and in vivo stimulation of extracellular signal-regulated kinase (ERK) by prothoracicotropic hormone in prothoracic gland cells and its developmental regulation in the silkworm, Bombyx mori. J. Insect Physiol. 53, 622–631. doi: 10.1016/j.jinsphys.2007.03.004
Lin, J. L., and Gu, S. H. (2011). Prothoracicotropic hormone induces tyrosine phosphorylation in prothoracic glands of the silkworm, Bombyx mori. Arch. Insect Biochem. Physiol. 76, 144–155. doi: 10.1002/arch.20373
Marchal, E., Vandersmissen, H. P., Badisco, L., Van de Velde, S., Verlinden, H., Iga, M., et al. (2010). Control of ecdysteroidogenesis in prothoracic glands of insects: a review. Peptides 31, 506–519. doi: 10.1016/j.peptides.2009.08.020
Miller, B. S., Shankavaram, U. T., Horney, M. J., Gore, A. C. S., Kurtz, D. T., and Rosenzweig, S. A. (1996). Activation of cJun NH2-terminal kinase/stress-activated protein kinase by insulin. Biochemistry 35, 8769–8775.
Noguti, T., Adachi-Yamada, T., Katagiri, T., Kawakami, A., Iwami, M., Ishibashi, J., et al. (1995). Insect prothoracicotropic hormone: a new member of the vertebrate growth factor superfamily. FEBS Lett. 376, 251–256.
O'Reilly, D. R., Kelly, T. J., Masler, E. P., Thyagaraja, B. S., Robson, R. M., Shaw, T. C., et al. (1995). Overexpression of Bombyx mori prothoracicotropic hormone using baculovirus vectors. Insect Biochem. Mol. Biol. 25, 475–485.
Rewitz, K. F., Yamanaka, N., Gilbert, L. I., and O'Connor, M. B. (2009). The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science 326, 1403–1405. doi: 10.1126/science.1176450
Rewitz, K. F., Yamanaka, N., and O'Connor, M. B. (2013). Developmental checkpoints and feedback circuits time insect maturation. Curr. Top. Dev. Biol. 103, 1–33. doi: 10.1016/B978-0-12-385979-2.00001-0
Riesgo-Escovar, J. R., Jenni, M., Fritz, A., and Hafen, E. (1996). The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 10, 2759–2768.
Roux, P., and Blenis, J. (2004). The ERK- and p38 MAPK-activated protein kinases (MKs): A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344. doi: 10.1128/MMBR.68.2.320-344.2004
Rybczynski, R., Bell, S. C., and Gilbert, L. I. (2001). Activation of an extracellular signal-regulated kinase (ERK) by the insect prothoracicotropic hormone. Mol. Cell. Endocrinol. 184, 1–11. doi: 10.1016/S0303-7207(01)00664-5
Rybczynski, R., and Gilbert, L. I. (2006). Protein kinase C modulates ecdysteroidogenesis in the prothoracic gland of the tobacco hornworm, Manduca sexta. Mol. Cell. Endocrinol. 251, 78–87. doi: 10.1016/j.mce.2006.02.015
Simmons, J. G., Hoyt, E. C., Westwick, J. K., Brenner, D. A., Pucilowska, J. B., and Lund, P. K. (1995). Insulin-like growth factor-I and epidermal growth factor interact to regulate growth and gene expression in IEC-6 intestinal epithelial cells. Mol. Endocrinol. 9, 1157–1165.
Sluss, H. K., Han, Z., Barrett, T., Goberdhan, D. C., Wilson, C., Davis, R. J., et al. (1996). A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 10, 2745–2758.
Smith, R. J., Sam, L. M., Justen, J. M., Bundy, G. L., Bala, G. A., and Bleasdale, J. E. (1990). Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 253, 688–697.
Smith, W. A., Gilbert, L. I., and Bollenbacher, W. E. (1984). The role of cyclic AMP in the regulation of ecdysone synthesis. Mol. Cell. Endocrinol. 37, 285–294.
Smith, W. A., Gilbert, L. I., and Bollenbacher, W. E. (1985). Calcium-cyclic AMP interactions in prothoracicotropic hormone stimulation of ecdysone synthesis. Mol. Cell. Endocrinol. 39, 71–78.
Smith, W. A., Lamattina, A., and Collins, M. (2014). Insulin signaling pathways in lepidopteran ecdysone secretion. Front. Physiol. 5:19. doi: 10.3389/fphys.2014.00019
Smith, W., Priester, J., and Morais, J. (2003). PTTH-stimulated ecdysone secretion is dependent upon tyrosine phosphorylation in the prothoracic glands of Manduca sexta. Insect Biochem. Mol. Biol. 33, 1317–1325. doi: 10.1016/j.ibmb.2003.06.003
Smith, W. A., and Rybczynski, R. (2012). “Prothoracicotropic Hormone,” in Insect Endocrinology, ed L. I. Gilbert (New York, NY: Elsevier Academic Press), 1–62.
Son, Y., Kim, S., Chung, H. T., and Pae, H. O. (2013). Reactive oxygen species in the activation of MAP kinases. Meth. Enzymol. 528, 27–48. doi: 10.1016/B978-0-12-405881-1.00002-1
Song, Q., and Gilbert, L. I. (1995). Multiple phosphorylation of ribosomal protein S6 and specific protein synthesis are required for prothoracicotropic hormone-stimulated ecdysteroid biosynthesis in the prothoracic glands of Manduca sexta. Insect Biochem. Mol. Biol. 25, 591–602.
Song, Q., and Gilbert, L. I. (1997). Molecular cloning, developmental expression, and phosphorylation of ribosomal protein S6 in the endocrine gland responsible for insect molting. J. Biol. Chem. 272, 4429–4435.
Weston, C. R., and Davis, R. J. (2002). The JNK signal transduction pathway. Curr. Opin. Cell Biol. 12, 14–21. doi: 10.1016/S0959-437X(01)00258-1
Weston, C. R., and Davis, R. J. (2007). The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19, 142–149. doi: 10.1016/j.ceb.2007.02.001
Wetzker, R., and Böhmer, F. D. (2003). Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat. Rev. Mol. Cell Biol. 4, 651–657. doi: 10.1038/nrm1173
Widmann, C., Gibson, S., Jarpe, M. B., and Johnson, G. L. (1999). Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79,143–180.
Yamanaka, N., Rewitz, K. F., and O'Connor, M. B. (2013). Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58, 497–516. doi: 10.1146/annurev-ento-120811-153608
Young, S. C., Yeh, W. L., and Gu, S. H. (2012). Transcriptional regulation of the PTTH receptor in prothoracic glands of the silkworm, Bombyx mori. J. Insect Physiol. 58, 102–109. doi: 10.1016/j.jinsphys.2011.10.005
Keywords: PTTH, JNK, ERK, ecdysone, signaling, redox regulation
Citation: Gu S-H, Li G, Hsieh H-Y, Lin P-L and Li S (2018) Stimulation of JNK Phosphorylation by the PTTH in Prothoracic Glands of the Silkworm, Bombyx mori. Front. Physiol. 9:43. doi: 10.3389/fphys.2018.00043
Received: 26 September 2017; Accepted: 12 January 2018;
Published: 05 February 2018.
Edited by:
Elzbieta M. Pyza, Jagiellonian University, PolandReviewed by:
Takashi Koyama, Instituto Gulbenkian de Ciência (IGC), PortugalMakio None Takeda, Kobe University, Japan
Copyright © 2018 Gu, Li, Hsieh, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Hong Gu, gu330@mail.nmns.edu.tw
 Shi-Hong Gu
Shi-Hong Gu Gen Li2
Gen Li2