- 1Veterinary Faculty, University of Ljubljana, Ljubljana, Slovenia
- 2Institute for Biostatistics and Medical Informatics, University of Ljubljana, Ljubljana, Slovenia
- 3Department of Medicine, McMaster University Medical Centre Hamilton, Hamilton, ON, Canada
General anesthesia (GA) can cause abnormal lung fluid redistribution. Pulmonary circulation transvascular fluid fluxes (JVA) are attributed to changes in hydrostatic forces and erythrocyte volume (EV) regulation. Despite the very low hydraulic conductance of pulmonary microvasculature it is possible that GA may affect hydrostatic forces through changes in pulmonary vascular resistance (PVR), and EV through alteration of erythrocyte transmembrane ion fluxes (ionJVA). Furosemide (Fur) was also used because of its potential to affect pulmonary hydrostatic forces and ionJVA. A hypothesis was tested that JVA, with or without furosemide treatment, will not change with time during GA. Twenty dogs that underwent castration/ovariectomy were randomly assigned to Fur (n = 10) (4 mg/kg IV) or placebo treated group (Con, n = 10). Baseline arterial (BL) and mixed venous blood were sampled during GA just before treatment with Fur or placebo and then at 15, 30 and 45 min post-treatment. Cardiac output (Q) and pulmonary artery pressure (PAP) were measured. JVA and ionJVA were calculated from changes in plasma protein, hemoglobin, hematocrit, plasma and whole blood ions, and Q. Variables were analyzed using random intercept mixed model (P < 0.05). Data are expressed as means ± SE. Furosemide caused a significant volume depletion as evident from changes in plasma protein and hematocrit (P < 0.001). However; Q, PAP, and JVA were not affected by time or Fur, whereas erythrocyte fluid flux was affected by Fur (P = 0.03). Furosemide also affected erythrocyte transmembrane K+ and Cl−, and transvascular Cl− metabolism (P ≤ 0.05). No other erythrocyte transmembrane or transvascular ion fluxes were affected by time of GA or Fur. Our hypothesis was verified as JVA was not affected by GA or ion metabolism changes due to Fur treatment. Furosemide and 45 min of GA did not cause significant hydrostatic changes based on Q and PAP. Inhibition of Na+/K+/2Cl− cotransport caused by Fur treatment, which can alter EV regulation and JVA, was offset by the Jacobs Stewart cycle. The results of this study indicate that the Jacobs Stewart cycle/erythrocyte Cl− metabolism can also act as a safety factor for the stability of lung fluid redistribution preserving optimal diffusion distance across the blood gas barrier.
Introduction
Transvascular fluid fluxes in the pulmonary circulation (JVA) may influence the diffusion distance between the pulmonary capillary and the alveoli, and compromise or improve gas exchange when lungs function undergoes physiological (i.e., exercise) or pathological adaptations (inflammation) (Vengust et al., 2013; Apostolo et al., 2014). Transvascular fluid fluxes in the pulmonary circulation are traditionally attributed to changes in hydrostatic forces and perfused alveolar capillary surface area, which are determined by the rise in mean pulmonary artery pressure (PPA) (Coates et al., 1984; Sinha et al., 1996; Vengust et al., 2006a). Erythrocyte volume (EV) regulation has also been associated with JVA through transmembrane/transvascular ion redistribution (IonVA) and intracellular osmolality changes (Wickerts et al., 1992; Vengust et al., 2006a, 2013).
General anesthesia (GA) is a critical event, with a potential to change pulmonary vascular resistance (PVR) (hydrostatic forces) through alterations in cardiac output (Q) and pulmonary blood flow (Fischer et al., 2003). It affects ventilation-perfusion (V/Q) matching, which triggers a variable degree of the hypoxic pulmonary vasoconstriction (HPV) to match regional ventilation and perfusion and to maintain oxygenation. HPV increases PVR; higher pressures at the microvascular level then lead to greater transmural hydrostatic driving gradients and increased JVA (Starling, 1896; Fischer et al., 2003).
Transvascular fluid fluxes in healthy individuals at rest (steady state) are at or near zero (Coates et al., 1984; Newman et al., 1988; Schaffartzik et al., 1992; Wickerts et al., 1992; Vengust et al., 2006a, 2011, 2013). Several gravimetric studies identified an edemagenic PAP, which overcomes the hydraulic conductance of the lung microvasculature and the ability of pulmonary lymphatics to remove fluid from the lung parenchyma (Wickerts et al., 1992; Hoeper, 2009). Pulmonary microvasculature is very resilient to abnormal transvascular fluid redistribution (Schneeberger and Karnovsky, 1976; Bhattacharya, 1988; Maggiorini et al., 2001; Parker et al., 2006; Effros and Parker, 2009). However, JVA during GA may still progressively become abnormal at sub edemagenic levels of PAP (Chapman et al., 2005), and eventually compromise the diffusion distance across the blood gas barrier.
The purpose of this study was to investigate IonVA and JVA during GA, which has not been investigated to date. Vengust et al. (2006a,b, 2011, 2013) in a series of investigations in exercising horses employed the method, which can detect in vivo changes in JVA, and even follow JVA changes with time after the application of different therapeutic agents. Furosemide was used in this study to evaluate pulmonary circulation adaptations to changes in hydrostatic forces and/or ion fluxes (Mukherjee et al., 1981; Narins and Chusid, 1986; Boles Ponto and Schoenwald, 1990). Furosemide is used to treat abnormal lung fluid redistribution (lung edema) mostly due to its diuretic effect, but also the possibility to alter transmembrane ion fluxes through attenuation of Na+/K+/2Cl− cotransport (Dikshit et al., 1973; Kracke and Dunham, 1987; O'Donnell, 1993). However, hydrostatic force increase and transvascular ion metabolism changes would have to be substantial to overcome the ability of pulmonary vasculature to keep pulmonary transvascular fluid dynamics at a steady state level (Schneeberger and Karnovsky, 1976; Bhattacharya, 1988; Maggiorini et al., 2001; Parker et al., 2006). Therefore, we tested the hypothesis that JVA, with or without furosemide treatment, will not change with time during GA.
Methods
Ethical Approval
The study protocol was approved by the National Ethics Committee (document No.: U34401-23/2013/6), according to the relevant Slovene and European Union regulations.
Twenty dogs (10 males, 10 females), with a mean age of 26.3 months (range 11–65 months), mean weight of 29.35 kg (16.3–47.9 kg) were used. Gender was equally distributed between groups. The study was conducted while dogs underwent a routine castration or ovariectomy under GA. All dogs were classified as ASA I (healthy dogs without recognizable signs of disease) according to the American Society of Anesthesiologists. An informed client consent was obtained before the dogs entered the study.
Experimental Protocol
Dogs were randomly assigned to Fur (4 mg/kg IV) or placebo (Con) (0.9% saline solution at a volume corresponding to the Fur treatment IV) treatment. Dogs were premedicated with morphine (0.3 mg kg−1 SQ; Morfin Alkaloid, Alkaloid Skopje, FYROM). An intravenous catheter (BD Venflon, Becton Dickinson Infusion Therapy AB, Helsingborg, Sweden) was inserted into the left or right cephalic vein. Anesthesia was induced with midazolam (0.1 mg kg−1 IV; Midazolam Torrex, Chiesi -Pharmaceuticals GmbH, Austria), followed by propofol (3–4 mg kg−1 IV; Norofol, Norbrook Laboratories Limited, Northern Ireland). After endotracheal intubation, anesthesia was maintained with sevoflurane (Sevorane, AbbVie, Campoverdedi Aprilia, Italy) delivered in oxygen using a circle breathing system. Dogs were kept in dorsal position throughout the experiment to reduce variations in the distribution of blood flow and ventilation in the lung (Galvin et al., 2007). The electrocardiogram, end-tidal CO2 tension, and arterial oxygen saturation were monitored during anesthesia (BLT M9000 VET). Lactated Ringer's solution (5 mL kg−1 h−1 IV; B. Braun, Germany) was infused during the experiment.
A Swan-Ganz catheter (Baxter Healthcare Corp., Irvine, CA, USA) was placed via the left or right jugular vein into the pulmonary artery for mixed venous blood sampling, pulmonary artery pressure (PAP) and core body temperature measurement. Correct catheter placement was ascertained by observing characteristic pressure waveforms (HP Model 66S, Hewlett-Packard Company, Palo Alto, Calif.). Cardiac output was measured by the thermodilution technique (10 mL of 0.9% NaCl; injectate temperature, 23 to 25°C). Injectate volume and temperature were used according to the manufacturer's instructions (HP Component monitoring system anesthesia/standard; Ganz et al., 1971; Nemec et al., 2003).
Blood Sampling and Analysis
Baseline (BL) arterial and mixed venous blood were sampled simultaneously just before treatment with Fur or Con and at 15, 30, and 45 min post-treatment. Surgery (castration/ovariectomy) then commenced within few min after the 45 min sample was taken. Blood samples were collected into lithium-heparinized syringes (Gaslyte, arterial blood sampler, Vital Signs, Inc., Englewood, CO, USA) and analyzed immediately in duplicates with the Rapid Point 500 analyzer (Siemens Healthcare, Erlangen, Germany). Rapid Point 500 uses ion selective electrode method (potentiometry) for the determination of electrolyte activity, including PCO2 (potentiometry based on Severinghaus). It uses amperometric oxygen electrode for PO2. Total hemoglobin (Hb) is determined by multiwavelength spectrophotometry. The analyzer automatically calibrates sensors several times a day. Intra-and inter- assay coefficients of variation for Rapid Point 500 have coefficients of determination (CV) higher than 0.91 (Nicolas et al., 2013), whereas for variables included in this study coefficients of determination was higher than 0.96. Hematocrit (Hct) was measured using microhematocrit method (CV = 0.96). Total plasma protein (PP) was measured using a clinical refractometer (Attago 331; Attago, Tokyo, Japan) (CV = 0.92). For whole blood [Na+], [K+], and [Cl−] determination, blood samples were repeatedly frozen (−80°C) and thawed (room temperature) to induce red cell lysis.
Calculations
Calculation methods have been reported previously (Vengust et al., 2006a, 2011, 2013). Plasma volume changes across the lung (ΔPV) were calculated from changes in PP at the same time point from central venous to arterial blood according to Dill and Costill (1974). Changes in erythrocyte volume (ΔEV) across the lungs were calculated from changes in Hb and Hct (Costill et al., 1974). Fluid fluxes across the lung were calculated from plasma and EV changes. Fluid flux was quantified based on Q (Costill et al., 1974; Dill and Costill, 1974; Vengust et al., 2006a, 2013):
for plasma fluid fluxes (JPL) where [PPv] is the plasma protein concentration in venous and [PPa] the plasma protein concentration in arterial blood, and
for erythrocyte fluid fluxes (JER) where [Hbv] is Hb concentration in venous, [Hba] Hb concentration in arterial blood, (Hctv) is Hct in venous and (Hcta) Hct concentration in arterial blood.
Fluid flux from or into the pulmonary vasculature was then calculated as the sum JPL and JER:
Erythrocyte ion concentrations (ER[Ion]) were calculated from whole blood (WB) and plasma (PL) ion concentration according to Buono and Yeager (1986) and McKelvie et al. (1991).
Veno-arterial differences across the lung were corrected for ΔPVVA, ΔEVVA and ΔBVVA according to McKenna et al. (1997):
Erythrocyte electrolyte fluxes across the lung (JERIon) were calculated from changes in ER[Ion]VA, Hcta and Q (Vengust et al., 2013):
Whole blood electrolyte fluxes across the lung (JWBIon) were calculated from changes in WB[Ion]VA and Q:
Statistical Analysis
This was randomized double blind placebo controlled study. Mean and standard error (±SE) are reported for each variable. The data were analyzed with the random intercept mixed model. The preplanned differences were carried out with the contrast analysis, where P-values of the non-orthogonal contrasts were corrected with the Benjamini–Hocberg method for multiple comparisons. A P-value smaller than 0.05 was considered statistically significant. The computations were performed with R language for statistical computing (R version 3.0.3) (R Core Team, 2014).
Results
All dogs were successfully recovered form anesthesia. No complications related to castration or ovariectomy were reported, nor were there any post-procedures adverse effect reported 6 months after the procedure.
Cardiac Output and Pulmonary Artery Pressure
Cardiac output did not change with the duration of GA (time), nor was there a significant effect of Fur (BL: 3.3 ± 0.4 L/min in Con, 3.8 ± 0.3 L/min in Fur; 45 min: 3.3 ± 0.4 L/min in Con and 3.6 ± 0.5 L/min in Fur) (P = 0.5) (Figure 1A). Baseline PAP was 15.5 ± 1.5 L/mmHg and 16.0 ± 0.8 mmHg in Con and Fur, respectively. There was only a slight increase in PAP from baseline to 45 min in Con and Fur (P = 0.07) (45 min: 17.5 ± 1.4 mmHg in Con and 16.5 ± 1.0 mmHg in Fur) (Figure 1B).
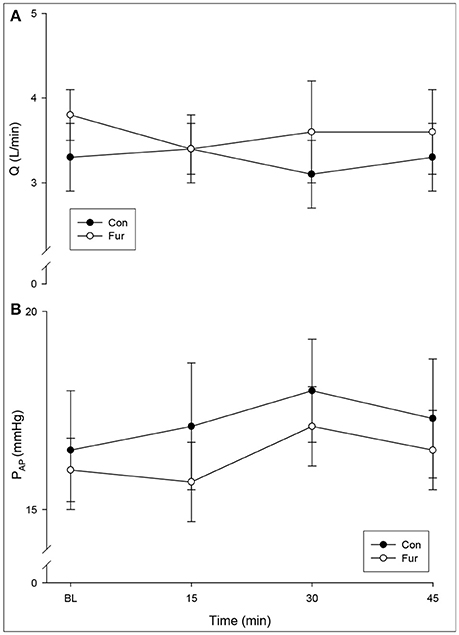
Figure 1. (A) Cardiac output (Q) and (B) mean pulmonary artery pressure (PAP) at Baseline (BL) and at 15, 30, and 45 min of general anesthesia. Values are means ± SE.
Haematocrit, Hemoglobin, Plasma Protein, and Blood Gas Difference across the Lung
No effect of time on Hctv, Hcta, Hbv, Hba, PPv, and PPa was observed in Con, whereas Hctv, Hcta, PPv, and PPa, but not Hbv and Hba, increased significantly in Fur (P < 0.001) (Table 1).
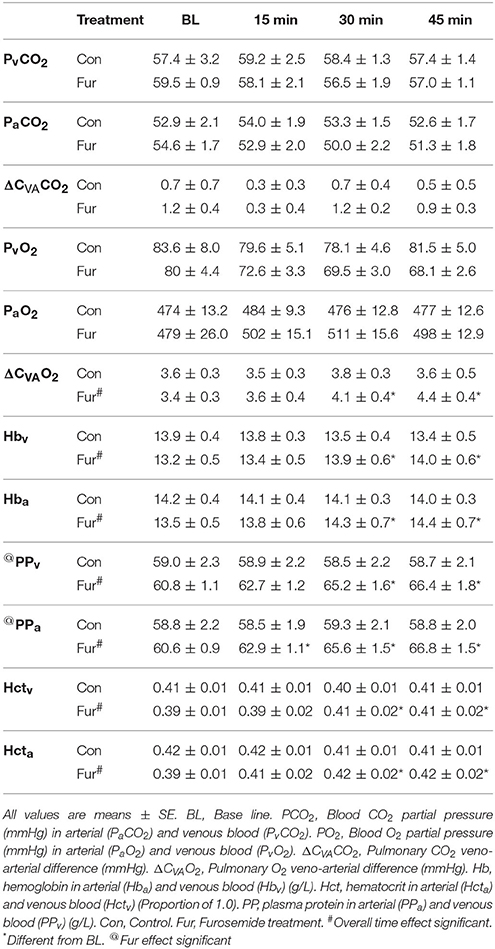
Table 1. Respiratory changes across the lung, hemoglobin (Hb), Hematocrit (Hct), and Plasma protein (PP).
Arterial (PaO2) and venous (PvO2) blood O2 tensions were not affected by time or Fur. Similarly, arterial (PaCO2) and venous (PvCO2) blood CO2 tensions were not affected by time or Fur. Veno-arterial O2 difference (ΔCVAO2) increased with time in Fur (P = 0.001) from 3.4 ± 0.3 mmHg at BL to 4.1 ± 0.3 mmHg at 45 min. Veno-arterial CO2 difference (ΔCVACO2) was not affected by time or Fur (P = 0.9) (Table 1).
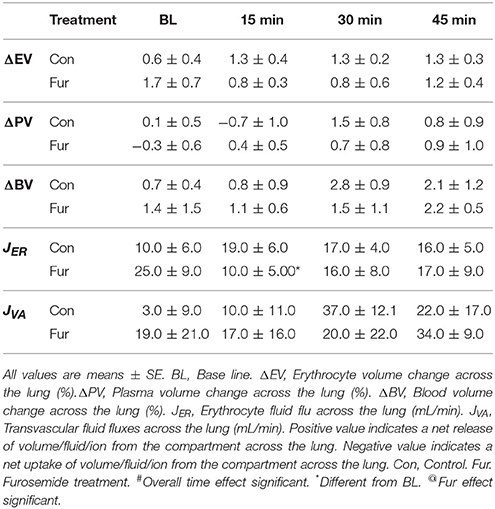
Volume and Fluid Changes across the Lung
Baseline ΔEV was 0.6 ± 0.4% and 1.7 ± 0.7% in Con and Fur, respectively (P = 0.2), indicating a decrease in EV across the lung. Erythrocyte volume across the lung did not change with time (P = 0.9), and was not affected by Fur (P = 0.8). Baseline JER was 10.0 ± 6.0 and 25.0 ± 9.0 mL/min in Con and Fur, respectively (P = 0.1). In Con JER remained unchanged throughout the experiment, whereas in Fur at 15 min JER declined to 10.0 ± 3.0 mL/min (P = 0.03), and then returned to BL value (Table 2).
Baseline ΔBV were 0.7 ± 0.5% and 1.4 ± 1.5% in Con and Fur, respectively (P = 0.6), indicating a decrease in BV across the lung. Blood volume changes across the lung were not affected by time (P = 0.5) or Fur (P = 0.8). Baseline JVA was 3.0 ± 9.0 and 19.0 ± 21.0 mL/min in Con and Fur, respectively (P = 0.5). Transvascular fluid fluxes remained unchanged over time (P = 0.4) and were not affected by Fur (P = 0.8) (Table 2).
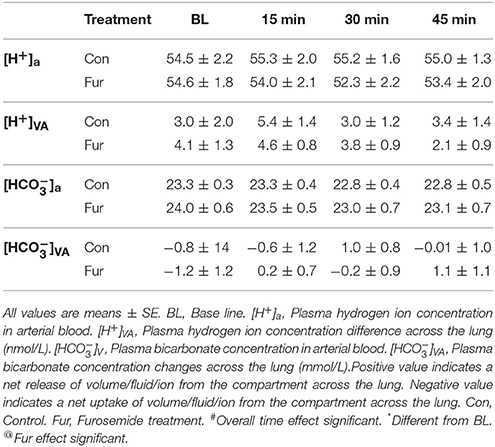
Ion Changes across the Lung
Plasma [H+] and [] changes across the lung were not affected by time or Fur (P ≤ 0.1) (Table 3).
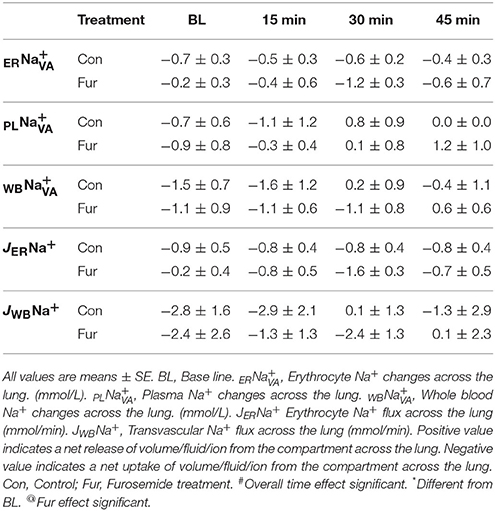
Baseline ER was −0.7 ± 0.3 mmol/L and −0.2 ± 0.3 mmol/L in Con and Fur, respectively (P = 0.4), indicating no or minimal increase in ERNa+. Throughout the experiment ERNa+ remained at BL levels in Con (45 min: −0.4 ± 0.7 mmol/L) and Fur (45 min:−0.6 ± 0.3 mmol/L), and was not affected by time (P = 0.8) or Fur (P = 0.9). Some increase in ERNa+ was evident in Fur at 30min (−1.2 ± 0.3 mmol/L); however, this value remained non-significant (P = 0.08). Baseline PL was −0.7 ± 0.6 mmol/L and −0.9 ± 0.7 mmol/L in Con and Fur (P = 0.9), respectively, indicating a modest increase in PLNa+ across the lung. Throughout the experiment PLNa+ remained at BL level. Baseline WB was −1.5 ± 1.4 and −1.1 ± 0.9 mmol/L in Con and Fur, respectively (P = 0.9). Throughout the experiment WBNa+ continue to show a weak tendency to move into the vascular compartment in Con and Fur. Effects of time (P = 0.3) or Fur (P = 0.9) were not evident (Table 4).
Baseline JERNa+ was −0.9 ± 0.5 mmol/min and −0.2 ± 0.4 mmol/min in Con and Fur. Sodium erythrocyte fluxes remained unchanged over time in Con (45 min: 0.8 ± 0.4 mmol/min). In Fur with time ERNa+ influx increased to 1.6 ± 0.3 mmol/min at 30 min (P = 0.08), but then returned to BL vales at −0.7 ± 0.6 mmol/min at 45 min. Baseline JWBNa+ was −2.8 ± 1.6 mmol/min and −2.4 ± 1.8 mmol/min in Con and Fur (P = 0.9), respectively. Time (P = 0.4) and Fur (0.09) had no effect on JWBNa+ (Table 4).
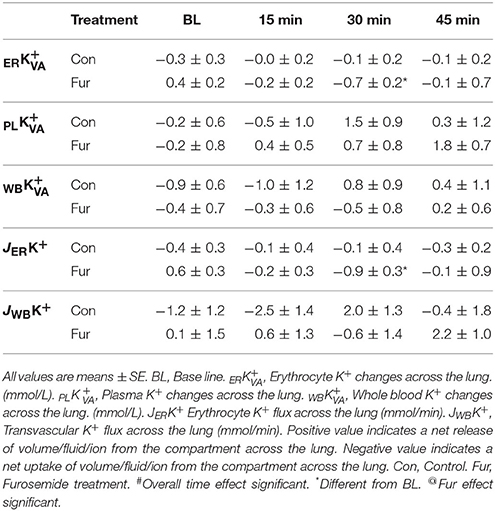
Baseline ER was −0.3 ± 0.2 and 0.4 ± 0.2 mmol/L in Con and Fur, respectively (P = 0.3). Time did not affect ER in Con (P = 0.9), whereas in Fur ERK+ started to increase at 30 min (P = 0.03) and the returned to BL value at 45 min. Baseline PL was −0.2 ±0.6 and −0.2 ±0.8 mmol/L in Con and Fur, respectively (P = 1.0). Although PL started to decrease within plasma compartment with time in Con and Fur, this effect was not found to be significant (P = 0.3). Baseline WB was −0.9 ±0.6 and −0.4 ±0.7 mmol/L in Con and Fur, respectively (P = 0.9). Time (P = 0.5) and Fur (P = 0.9) did not affect WB (Table 5).
Baseline JERK+ was −0.4 ±0.3 mmol/min and 0.6 ±0.3 mmol/min in Con and Fur (P = 0.3), respectively. Potassium erythrocyte flux remained unchanged over time (P = 0.6) and was not affected by Fur (45 min: −0.3 ± 0.2 mmol/min in Con and −0.1 ± 0.9 mmol/min in Fur) (P = 0.9). Baseline JWBK+ was −1.2 ± 1.2 and 0.1 ±1.5 mmol/min in Con and Fur (P = 0.6), respectively. Potassium transvascular flux remained unchanged over time (P = 0.3) and was not affected by Fur (P = 0.3) (Table 5).
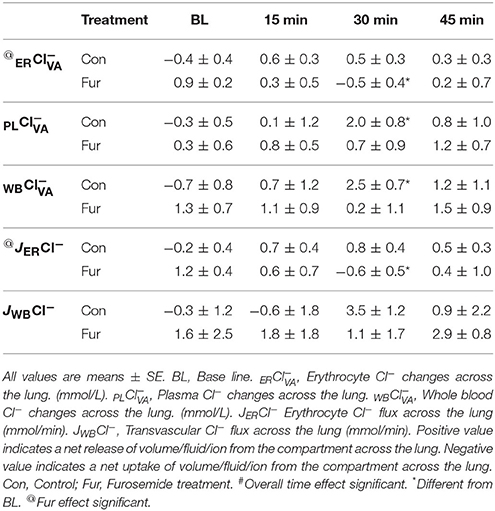
Baseline ER was −0.4 ± 0.4 and 0.9 ± 0.3 mmol/L in Con and Fur, respectively (P = 0.7). Throughout the experiment ERCl− remained within BL values in Con (P = 0.8). In Fur at 30 min ERCl− efflux reversed to influx at −0.5 ± 0.4 mmol/L (P = 0.02). Overall effect of Fur was significant at P = 0.04. Baseline PL was −0.3 ± 0.5 and 0.3 ± 0.6 mmol/L in Con and Fur, respectively (P = 0.9). In Con PLCl− efflux increased to 2.0 ± 0.8 mmol/L (P = 0.04) and returned o BL value by 45 min. Similar was not evident in Fur (P = 0.7). Baseline WB was −0.7 ±0.8 and 1.3 ±0.7 mmol/L in Con and Fur, respectively (P = 0.2). With time WBCl− showed a weak tendency to efflux from the vascular compartment, which was most prominent at 30 min in Con (2.6 ± 0.7 mmol/L; P = 0.02). Similar was not evident in Fur (P = 0.7) (Table 6).
Baseline JERCl− was −0.02 ± 0.4 mmol/min and 1.2 ± 0.4 mmol/min in Con and Fur (P = 0.1), respectively. Chloride erythrocyte flux did not change with time in Con, whereas in Fur at 30 min it changed to influx at 0.6 ± 0.4 mmol/min (P = 0.02). The overall effect of Fur on JERCl− was significant at P = 0.05. Baseline JWBCl− was −0.3 ± 1.2 and 1.6 ± 2.5 mmol/min in Con and Fur (P = 0.4), respectively. In general, Cl− showed the tendency to flux out of the vascular compartment throughout the experiment. In Con at 30 min JWBCl− was significantly different from BL (P = 0.04). No overall effect of Fur on JERCl− was observed (P = 0.6) (Table 6).
Discussion
This is the first report of fluid and ion fluxes across the pulmonary circulation during GA. In the present study we observed erythrocyte and blood volume changes across the lung (~1.0–1.5%), which are in line with those reported from horses at rest (Vengust et al., 2006a,b, 2011, 2013). Volume changes created JVAof ~20 mL/min. Dogs were treated with Fur to reduce hydrostatic forces and/or influence erythrocyte and transvascular ion metabolism (Mukherjee et al., 1981; Narins and Chusid, 1986; Boles Ponto and Schoenwald, 1990). Treatment with Fur caused dehydration and partially affected Cl− metabolism across pulmonary vascular compartments; however, it did not change Q, PAP, and/or JVA. Our hypothesis was, therefore, verified.
Effects of Furosemide on JVA
Furosemide is used in patients with pulmonary edema. The reduction in lung water is due to a decrease in preload through venodilatation and diuresis (Dikshit et al., 1973), which decreases transmural hydrostatic pressures (Bake et al., 1968; Hlastala et al., 1996). Furosemide treatment in this study dehydrated dogs and caused volume depletion due to diuresis (Dikshit et al., 1973), which was not translated into reduced Q, PPA, and JVA. Transvascular fluid fluxes, however, were also not affected in previous studies where a significant decrease in Q due to Fur was reported (Wickerts et al., 1992; Vengust et al., 2011).
Effects of General Anesthesia on JVA
General anesthesia can cause some degree of ventilation perfusion (V/Q) mismatch (Gunnarsson et al., 1991), which is mitigated by a variable degree of HPV (Dueck et al., 1984). The most consistent triggering factor is the decrease in lung compliance and a fall in functional residual capacity (Mead and Collier, 1959; Bendixen et al., 1963). HPV optimizes systemic O2 delivery by constricting and increasing pressure in pulmonary microcirculation away from hypoxic lung regions (Madden et al., 1992). Increased pulmonary microvascular pressures change the balance between intra- and extravascular Starling forces and may influence JVA (Starling, 1896; Vengust et al., 2013). In normal lungs, however, it is unlikely that the level of alveolar hypoxia during GA using normal concentration of volatile anesthetics would create edemagenic PAP and clinical edema (Domino et al., 1986; Marshall et al., 1991). In the present study a steady but non-significant increase in JVAwas observed within 45 min of GA on Con and Fur. This was equal in Con and Fur and, therefore, cannot be attributed to fluid therapy because of the diuretic effect of Fur (Mitchell et al., 1992). It is most likely that the supine (dorsal) position during GA was the reason for modest increase in JVA (Wiener et al., 1990).
Opioid drugs, benzodiazepines and propofol used for premedication and induction of GA in this study do not affect pulmonary vascular reactivity, and are not considered a significant initiator for V/Q mismatch (Gibbs and Johnson, 1978; Benumof et al., 1987; Reves et al., 2010). Sevoflurane and other modern inhaled anesthetic on the other hand have a moderate inhibitory effect on HPV (Marshall et al., 1984; Wang et al., 1998; Kerbaul et al., 2006) and may even cause a reduction in JVA. Because sevoflurane in this study was delivered in O2, some degree of atelectasis would theoretically be expected (Sylvester et al., 2012). However, studies in animals and humans failed to generate significant O2 related shunt during GA (Wagner et al., 1974; Dantzker et al., 1975; Lundquist et al., 1988; Sylvester et al., 2012).
Electrolyte and Volume Changes across the Lung
In the present study Cl− metabolism was the most affected by Fur, which coincided with the reduction of JER but did not influence JVA. Transvascular fluid fluxes in healthy individuals seem to be dependent on ΔEV (Vengust et al., 2006a, 2011, 2013). Erythrocytes have a complex and specific regulation of their volume through changes in their osmolality (van't Hoff, 1887; Hamburger, 1891, 1918). In peripheral tissues in deoxygenated blood, Cl− (and water) is exchanged for across the erythrocyte plasma membrane (Hamburger, 1891, 1918; Bretcher, 1971). Na+/K+/2Cl− cotransport across the erythrocyte plasma membrane is activated by similar stimuli and contributes to solute concentration in the erythrocyte. Erythrocyte osmolality persists at rather higher levels also due to lower PO2 in peripheral tissues, which inhibits K+/Cl− cotransport/egress from erythrocytes. On contrary, the Na+/K+ ATPase activity across the erythrocyte plasma membrane decreases erythrocyte [Na+] and consequently erythrocyte osmolality. Na+/K+ ATPase effect, however, is inferior to combined activity of other ion channels, which work toward the increase of intracellular osmolality and EV. In the lung capillary bed increased PO2, efflux of Cl−, decreased [H+], and active K+/Cl− cotransport across the erythrocyte plasma membrane reverse the process to erythrocyte regulatory volume decrease and fluid egress from erythrocytes (Fievet et al., 1990; Gibson et al., 1993, 1994, 2000; Honess et al., 1996; Speake et al., 1997; Juel et al., 1999). Previous studies in horses demonstrated, that JVA is mostly dependent upon the Jacobs-Stewart cycle (Vengust et al., 2013), which is a cycle of intracellular-extracellular exchanges involving CO2, , Cl−, and H+ across the erythrocyte membrane during capillary transit that speeds and enhances CO2 elimination (Jacobs and Stewart, 1942). As erythrocytes traverse the pulmonary microvasculature their membranes come into close contact with the capillary endothelium to form a functional single semi-permeable barrier. This semi-permeable “membrane” has the osmotic characteristics of the erythrocyte membrane itself (Hansen, 1961), and so may permit ionJVA and JVA (Vengust et al., 2013).
Increase in ERK+, and near significant increase of ERNa+ at 30 min indicated that Na+/K+/2Cl− cotransport was affected by Fur. These changes were only detected across the erythrocyte membrane at 30 min of GA, which is consistent with furosemide pharmacokinetics in dogs (Hirai et al., 1992). However, changes in Cl− metabolism across the lung were evident throughout the vascular compartment and not only across the erythrocyte membrane. These changes are not exclusive to Na+/K+/2Cl− cotransport inhibition and should also be attributed to the Jacobs-Stewart cycle. Because Na+/K+/2Cl− cotransport is also important at the vascular endothelial level where it contributes to the integrity of the permeability barrier (O'Donnell, 1993), the Jacobs-Stewart cycle assumed a transvascular role in maintaining the volume and ion equilibrium after Fur treatment.
Other Effects of Furosemide Relevant to Pulmonary Transvascular Fluid Fluxes
It would also be possible that Fur influences JVA through other effects not directly related to diuresis. Furosemide causes direct pulmonary vasodilatation and improved pulmonary compliance, which should reduce the risk for JVA (Lundergan et al., 1988; Silke, 1993; Greenberg et al., 1994) Hemodynamic properties of Fur are beneficial in patients with mild physical impairments due to ventricular dysfunction, whereas it seems that in healthy subjects are unlikely to show any quantifiable effect (Silke, 1993). Furosemide also induces a weak bronchodilator effect when inhaled in asthmatic humans (Bianco et al., 1988) or given intravenously to horses with (Rubie et al., 1993) or without the pulmonary obstructive disease (Olsen et al., 1992). Bronchodilation reduces the effect of exercise induced alveolar hypoxia and consequent pulmonary vasoconstriction of small pulmonary arteries, which increases pulmonary microvascular pressure and affects pulmonary capillary water permeability (Mairbäurl et al., 2002). The combination of Fur effect related to volume depletion, pulmonary vasodilatation and bronchodilation most probably contributed to better ΔCVAO2 in Fur in this study.
Effects of [H+] on JVA
Changes in [H+] can influence vascular tone by regulating endothelium and vascular smooth muscle function (Aalkjaer, 1990). No acid base imbalance was noted in dogs in this study, which could potentially affect JVA. Alkalosis is consistently associated with the reduction in pulmonary microvascular pressures (Loeppky et al., 1985). Effects of acidosis, however, on pulmonary circulation vascular resistance is inconsistent. Pulmonary vasculature in general, unlike systemic circulation, shows resistance to vasodilator effect of extracellular acidosis (Aalkjaer, 1990; Barnes and Liu, 1995). Extracellular acidosis has also been shown to increase PVR in isolated dogs' pulmonary lobes, and calves and children with congenital heart disease and associated pulmonary hypertension (Lloyd, 1966; Rudolph and Yuan, 1966; Morray et al., 1988). Increase in pulmonary smooth muscle intracellular [H+], however, decreases PVR in isolated animal lungs (Raffestin and McMurtry, 1987; Ketabchi et al., 2009).
Methodological Considerations and Limitations
The experimental methodology used in the present study has previously been validated (Costill et al., 1974; Dill and Costill, 1974; Vengust et al., 2006a, 2011, 2013). Variables measured are reproducible, have excellent CV and are able to detect small changes across different compartments. It is important to realize that methodology used herein enables an “in-vivo” investigation of lung fluid physiology. In contrast, lung lymph flow or pulmonary gravimetric techniques, two other methods to study lung fluid physiology, are more invasive, require post mortem examination, and/or require static investigation employing nuclear medicine. Lung lymph flow studies would also require better defined attention to the uncertainty concerning the tissues drained by the lymphatics and the effect of the lymph nodes themselves on lymph constituents (Coates et al., 1984; Newman et al., 1988). Gravimetric lung fluid dynamic studies only detect variations in the presence of lung water and are unable to account for alterations when changes are to be contributed to the vascular, interstitial, and/or cellular compartments in lungs (Lin et al., 1998; Hanel et al., 2003).
Most dog breeds have a very low Na+/K+ ATPase activity with consequent high erythrocyte Na+ and low erythrocyte K+ concentrations (Maede and Inaba, 1985). The importance of Na+/K+ ATPase activity with regards to JVA is minimal, as discussed above. Reduced Na+/K+ ATPase causes erythrocyte Na+ and K+ concentrations to be similar to those in plasma, making intra-erythrocyte ion analyses in dogs less prone to an analytical error arising from high or low intracellular ion concentrations present in other species.
This study does not provide evidence and comparison between awake and anesthetized dogs. However, it is relevant to assume that JVA in an awake dog is similar to BL values in this study (Wickerts et al., 1992; Vengust et al., 2006a). It is ethically unacceptable to instrument awake dogs in a manner such as used in this study, and physical restraint would cause a variety of stress related physiological changes.
Conclusion
The dynamics of water movement in the pulmonary circulation are complex events encompassing Starling forces, gas exchange mechanisms, and EV regulation. Adaptations in ion metabolism in this study complimented the very low hydraulic conductance of the lung microvasculature, and prevented changes in JVA. The Jacobs Stewart cycle also seems to be an important safety factor for the stability of lung fluid dynamics. Differences in JVA should be expected when alveolar epithelial and endothelial permeability are compromised due to e.g., mechanical ventilation and/or inflammation. Lung microvascular and alveolar permeability to proteins would then alter Starling forces and EV regulation and cause more prominent and abnormal lung fluid redistribution.
Author Contributions
MV, GH, and RB: participated in research design; OF, AD, AS, and MV: conducted experiments; OF, RB, GH, MV: performed data analysis. All authors wrote/contributed to the writing of the manuscript.
Funding
This study was supported by the Slovenian Research Agency grant P4-0053 and the Canadian Institutes of Health Research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mitja Miklavcic, DVM, and Jerneja Sredensek, DVM for their assistance during general anesthesia.
Abbreviations
Fur, Furosemide treatment; VA, Veno-arterial difference across the lung; JVA, Transvascular fluid fluxes in the pulmonary circulation; PPA, Pulmonary artery pressure; ionJVA, Transmembrane/transvascular ion fluxes; BL, Baseline; EV, Erythrocyte volume; PV, Plasma volume; JPL, Plasma fluid fluxes; JER, Erythrocyte fluid fluxes; ER[Ion], Erythrocyte ion concentrations; WB[Ion], Whole blood concentrations; JERIon, Erythrocyte ion fluxes across the lung; JWBIon, Whole blood fluxes across the lung; SID, Strong ion difference.
References
Aalkjaer, C. (1990). Regulation of intracellular pH and its role in vascular smooth muscle function. J. Hypertens. 8, 197–206. doi: 10.1097/00004872-199003000-00001
Apostolo, A., Agostoni, P., Contini, M., Antonioli, L., and Swenson, E. R. (2014). Acetazolamide and inhaled carbon dioxide reduce periodic breathing during exercise in patients with chronic heart failure. J. Card. Fail. 4, 278–288. doi: 10.1016/j.cardfail.2014.01.007
Bake, B., Bjure, J., and Widimsky, J. (1968). The effect of sitting and graded exercise on the distribution of pulmonary blood flow in healthy subjects studied with the 133Xenon technique. Scand. J. Clin. Lab. Invest. 22, 99–106. doi: 10.3109/00365516809160952
Barnes, P. J., and Liu, S. F. (1995). Regulation of pulmonary vascular tone. Pharmacol. Rev. 47, 87–131.
Bendixen, H. H., Hedley-Whyte, J., and Laver, M. B. (1963). Impaired oxygen in surgical patients during general anesthesia with controlled ventilation: a concept of atelectasis. N. Engl. J. Med. 269, 991–996. doi: 10.1056/NEJM196311072691901
Benumof, J. L., Augustine, S. D., and Gibbons, J. A. (1987). Halothane and isoflurane only slightly impair arterial oxygenation during one-lung ventilation in patients undergoing thoracotomy. Anesthesiology 67, 910–915. doi: 10.1097/00000542-198712000-00006
Bhattacharya, J. (1988). Hydraulic conductivity of lung venules determined by split droplet technique. J. Appl. Physiol. 64, 2562–2567. doi: 10.1152/jappl.1988.64.6.2562
Bianco, S., Vaghi, A., Robuschi, M., and Pasargiklian, M. (1988). Prevention of exercise-induced bronchoconstriction by inhaled frusemide. Lancet 2, 252–255. doi: 10.1016/S0140-6736(88)92540-8
Boles Ponto, L. L., and Schoenwald, R. D. (1990). Furosemide (frusemide): a pharmacokinetic/pharmacodynamic review (Part I). Clin. Pharmacokinet. 18, 381–408. doi: 10.2165/00003088-199018050-00004
Bretcher, M. S. (1971). A major protein, which spans the human erythrocyte membrane. J. Mol. Biol. 59, 351–357. doi: 10.1016/0022-2836(71)90055-6
Buono, M. J., and Yeager, J. E. (1986). Intraerythrocyte and plasma lactate concentrations during exercise in humans. Eur. J. Appl. Physiol. 55, 326–329. doi: 10.1007/BF02343807
Chapman, M. J., Myburgh, J. A., Kluger, M. T., and Runciman, W. B. (2005). Crisis management during anaesthesia: pulmonary oedema. Qual. Saf. Health Care 14:e8. doi: 10.1136/qshc.2002.004267
Coates, G., O'Brodovich, H., Jefferies, A. L., and Gray, G. W. (1984). Effects of exercise on lung lymph flow in sheep and goats during normoxia and hypoxia. J. Clin. Invest. 74, 133–141. doi: 10.1172/JCI111393
Costill, D. L., Branam, L., Eddy, D., and Fink, W. (1974). Alterations in red cell volume following exercise and dehydration. J. Appl. Physiol. 37, 912–916. doi: 10.1152/jappl.1974.37.6.912
Dantzker, D. R., Wagner, P. D., and West, J. B. (1975). Instability of lung units with low calcium sensitivity by acute hypoxia in rat distal pulmonary arteries VA/Q ratios during O2 breathing. J. Appl. Physiol. 38, 886–895. doi: 10.1152/jappl.1975.38.5.886
Dikshit, K., Vyden, J. K., Forrester, J. S., Chatterjee, K., Prakash, R., and Swan, H. J. (1973). Renal and extrarenal haemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N. Engl. J. Med. 288, 1087–1090. doi: 10.1056/NEJM197305242882102
Dill, D. B., and Costill, D. L. (1974). Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 37, 247–248. doi: 10.1152/jappl.1974.37.2.247
Domino, K. B., Borowec, L., Alexander, C. M., Williams, J. J., Chen, L., Marshall, C., et al. (1986). Influence of isoflurane on hypoxic pulmonary vasoconstriction in dogs. Anesthesiol 64, 423–429. doi: 10.1097/00000542-198604000-00002
Dueck, R., Rathbun, M., and Greenburg, A. G. (1984). Lung volume and VA/Q distribution response to intravenous versus inhalation anesthesia in sheep. Anesthesiol 61, 55–65. doi: 10.1097/00000542-198407000-00010
Effros, R. M., and Parker, J. C. (2009). Pulmonary vascular heterogeneity and the Starling hypothesis. Microvasc. Res. 78, 71–77. doi: 10.1016/j.mvr.2009.03.004
Fievet, B., Caroff, J., and Motais, R. (1990). Catecholamine release controlled by blood oxygen tension during deep hypoxia in trout. Effect on red blood cell Na/H exchanger. Respir. Physiol. 79, 81–90. doi: 10.1016/0034-5687(90)90062-4
Fischer, L. G., Van Aken, H., and Bürkle, H. (2003). Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists. Anesth. Analg. 96, 1603–1616. doi: 10.1213/01.ANE.0000062523.67426.0B
Galvin, I., Drummond, G. B., and Nirmalan, M. (2007). Distribution of blood flow and ventilation in the lung: gravity is not the only factor. Br. J. Anaesth. 98, 420–428. doi: 10.1093/bja/aem036
Ganz, W., Donoso, R., Marcus, H. S., Forrester, J. S., and Swan, H. J. (1971). A new technique for measurement of cardiac output by thermodilution in man. Am. J. Cardiol. 27, 392–396. doi: 10.1016/0002-9149(71)90436-X
Gibbs, J. M., and Johnson, H. (1978). Lack of effect of morphine and buprenorphine on hypoxic pulmonary vasoconstriction in the isolated perfused cat lung and the perfused lobe of the dog lung. Br. J. Anaesth. 50, 1197–1201. doi: 10.1093/bja/50.12.1197
Gibson, J. S., Cossins, A. R., and Ellory, J. C. (2000). Oxygen-sensitive transporters in vertebrate red cells. J. Exp. Biol. 203, 1395–1407. Available online at: http://jeb.biologists.org/content/203/9/1395
Gibson, J. S., Ellory, J. C., Culliford, S. J., and Fincham, D. A. (1993). Volume-sensitive KCl co-transport and taurine fluxes in horse red blood cells. Exp. Physiol. 78, 685–695. doi: 10.1113/expphysiol.1993.sp003716
Gibson, J. S., Godart, H., Ellory, J. C., Staines, H., Honess, N. A., and Cossins, A. R. (1994). Modulation of K1-Cl2 cotransport in equine red blood cells. Exp. Physiol. 79, 997–1009. doi: 10.1113/expphysiol.1994.sp003824
Greenberg, S., McGowan, C., Xie, J., and Summer, W. R. (1994). Selective pulmonary and venous smooth muscle relaxation by furosemide: a comparison with morphine. J. Pharmacol. Exp. Ther. 270, 1077–1085.
Gunnarsson, L., Tokics, L., Gustavsson, H., and Hedenstierna, G. (1991). Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br. J. Anaesth. 66, 423–432. doi: 10.1093/bja/66.4.423
Hamburger, H. (1891). Uber der einfluss der athmung auf die permeabilitit der blutkörperchen. Biochem. Z. 28:405.
Hamburger, H. (1918). Anionenwanderungen in Serum und Blut unter dem Einfluss von CO2, Saure und Akali. Biochem. Z. 86, 309–324.
Hanel, B., Law, I., and Mortensen, J. (2003). Maximal rowing has an acute effect on the blood-gas barrier in elite athletes. J. Appl. Physiol. 95, 1076–1082. doi: 10.1152/japplphysiol.00082.2002
Hansen, A. T. (1961). Osmotic pressure effect of the red blood cells – possible physiological significance. Nature 190, 504–508. doi: 10.1038/190504a0
Hirai, J., Miyazaki, H., and Taneike, T. (1992). The pharmacokinetics and pharmacodynamics of furosemide in the anaesthetized dog. J. Vet. Pharmacol. Ther. 15, 231–239. doi: 10.1111/j.1365-2885.1992.tb01011.x
Hlastala, M. P., Bernard, S. L., Erickson, H. H., Fedde, M. R., Gaughan, E. M., McMurphy, R., et al. (1996). Pulmonary blood flow distribution in standing horses is not dominated by gravity. J. Appl. Physiol. 81, 1051–1061. doi: 10.1152/jappl.1996.81.3.1051
Hoeper, M. M. (2009). Definition, classification, and epidemiology of pulmonary arterial hypertension. Semin. Respir. Crit. Care Med. 4, 369–375. doi: 10.1055/s-0029-1233306
Honess, N. A., Gibson, J. S., and Cossins, A. R. (1996). The effects of oxygenation upon the Cl dependent K flux pathway in equine red cells. Pflugers Arch. 432, 270–277. doi: 10.1007/s004240050133
Jacobs, M. H., and Stewart, D. R. (1942). The role of carbonic anhydrase in certain ionic exchanges involving the erythrocyte. J. Gen. Physiol. 25, 539–552. doi: 10.1085/jgp.25.4.539
Juel, C., Hellsten, Y., Saltin, B., and Bangsbo, J. (1999). Potassium fluxes in contracting human skeletal muscle and red blood cells. Am. J. Physiol. 276, R184–R188. doi: 10.1152/ajpregu.1999.276.1.R184
Kerbaul, F., Bellezza, M., Mekkaoui, C., Feier, H., Guidon, C., Gouvernet, J., et al. (2006). Sevoflurane alters right ventricular performance but not pulmonary vascular resistance in acutely instrumented anesthetized pigs. J. Cardiothorac. Vasc. Anesth. 20, 209–216. doi: 10.1053/j.jvca.2005.05.017
Ketabchi, F., Egemnazarov, B., Schermuly, R. T., Ghofrani, H. A., Seeger, W., Grimminger, F., et al. (2009). Effects of hypercapnia with and without acidosis on hypoxic pulmonary vasoconstriction. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, 977–983. doi: 10.1152/ajplung.00074.2009
Kracke, G. R., and Dunham, P. B. (1987). Effect of membrane potential on furosemide-inhibitable sodium influxes in human red blood cells. J. Membr. Biol. 98, 117–124. doi: 10.1007/BF01872124
Lin, W., Jacobs, E., Schapira, R. M., Presberg, K., and Effros, R. M. (1998). Stop-flow studies of distribution of filtration in rat lungs. J. Appl. Physiol. 84, 47–52. doi: 10.1152/jappl.1998.84.1.47
Lloyd, T. C. Jr. (1966). Influence of blood pH on hypoxic pulmonary vasoconstriction. J. Appl. Physiol. 21, 358–364. doi: 10.1152/jappl.1966.21.2.358
Loeppky, J. A., Scotto, P., Riedel, C. E., Roach, R. C., and Chick, T. W. (1985). Effects of acid-base status on acute hypoxic pulmonary vasoconstriction and gas exchange. J. Appl. Physiol. 72, 1787–1797.
Lundergan, C. F., Fitzpatrick, T. M., Rose, J. C., Ramwell, P. W., and Kot, P. A. (1988). Effect of cyclooxygenase inhibition on the pulmonary vasodilator response to furosemide. J. Pharmacol. Exp. Ther. 246, 102–106.
Lundquist, H., Hedenstierna, G., and Ringertz, H. (1988). Barbiturate anaesthesia does not cause pulmonary densities in dogs: a study using computerized axial tomography. Acta Anaesthesiol. Scand. 32, 162–165. doi: 10.1111/j.1399-6576.1988.tb02708.x
Madden, J. A., Vadula, M. S., and Kurup, V. P. (1992). Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am. J. Physiol. 263, L384–L393. doi: 10.1152/ajplung.1992.263.3.L384
Maede, Y., and Inaba, M. (1985). (Na,K)-ATPase and ouabain binding in reticulocytes from dogs with high K and low K erythrocytes and their changes during maturation. J. Biol. Chem. 260, 3337–3343.
Maggiorini, M., Mélot, C., Pierre, C., Pfeiffer, F., Greve, I., Sartori, C., et al. (2001). High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 103, 2078–2083. doi: 10.1161/01.CIR.103.16.2078
Mairbäurl, H., Mayer, K., Kim, K. J., Borok, Z., Bärtsch, P., and Crandall, E. D. (2002). Hypoxia decreases active Na transport across primary rat alveolar epithelial cell monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L659–L665. doi: 10.1152/ajplung.00355.2001
Marshall, B. E., Marshall, C., Magno, M., Lilagan, P., and Pietra, G. G. (1991). Influence of bronchial arterial PO2 on pulmonary vascular resistance. J. Appl. Physiol. 70, 405–415. doi: 10.1152/jappl.1991.70.1.405
Marshall, C., Lindgren, L., and Marshall, B. E. (1984). Effects of halothane, enflurane, and isoflurane on hypoxic pulmonary vasoconstriction in rat lungs in vitro. Anesthesiology 60, 304–308. doi: 10.1097/00000542-198404000-00006
McKelvie, R. S., Lindinger, M. L., Heigenhauser, G. J. F., and Jones, N. L. (1991). Contribution of erythrocytes to the control of the electrolyte changes of exercise. Can. J. Physiol. Pharmacol. 69, 984–993. doi: 10.1139/y91-148
McKenna, M. J., Heigenhauser, G. J., McKelvie, R. S., MacDougall, J. D., and Jones, N. L. (1997). Sprint training enhances ionic regulation during intense exercise in men. J. Physiol. 501, 687–702. doi: 10.1111/j.1469-7793.1997.687bm.x
Mead, J., and Collier, C. (1959). Relation of volume history of lungs to respiratory mechanics in anesthetized dogs. J. Appl. Physiol. 14, 669–678. doi: 10.1152/jappl.1959.14.5.669
Mitchell, J. P., Schuller, D., Calandrino, F. S., and Schuster, D. P. (1992). Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am. Rev. Respir. Dis. 145, 990–998. doi: 10.1097/00132586-199306000-00019
Morray, J. P., Lynn, A. M., and Mansfield, P. B. (1988). Effect of pH and PCO2 on pulmonary and systemic hemodynamics after surgery in children with congenital heart disease and pulmonary hypertension. J. Pediatr. 113, 474–479. doi: 10.1016/S0022-3476(88)80631-0
Mukherjee, S. K., Katz, M. A., Michael, U. R., and Ogden, D. A. (1981). Mechanisms of hemodynamic actions of furosemide: differentiation of vascular and renal effects on blood pressure in functionally anephric hypertensive patients. Am. Heart J. 101, 313–318. doi: 10.1016/0002-8703(81)90196-4
Narins, R. G., and Chusid, P. (1986). Diuretic use in critical care. Am. J. Cardiol. 57, 26A−32A. doi: 10.1016/0002-9149(86)91003-9
Nemec, A., Pecar, J., Seliskar, A., Kompan, L., and Butinar, J. (2003). Assessment of acid-base status and plasma lactate concentrations in arterial, mixed venous, and portal blood from dogs during experimental hepatic blood inflow occlusion. Am. J. Vet. Res. 64, 599–608. doi: 10.2460/ajvr.2003.64.599
Newman, J. H., Butka, B. J., Parker, R. E., and Roselli, R. J. (1988). Effect of progressive exercise on lung fluid balance in sheep. J. Appl. Physiol. 64, 2125–2131. doi: 10.1152/jappl.1988.64.5.2125
Nicolas, T., Cabrolier, N., Bardonnet, K., and Davani, S. (2013). Evaluation of a new blood gas analysis system: RapidPoint 500®. Ann. Biol. Clin. 71, 305–311. doi: 10.1684/abc.2013.0821
O'Donnell, M. E. (1993). Role of Na-K-Cl cotransport in vascular endothelial cell volume regulation. Am. J. Physiol. 264, C1316–C1326. doi: 10.1152/ajpcell.1993.264.5.C1316
Olsen, S. C., Coyne, C. P., Lowe, B. S., Pelletier, N., Raub, E. M., and Erickson, H. H. (1992). Influence of furosemide on hemodynamic responses during exercise in horses. Am. J. Vet. Res. 53, 742–747.
Parker, J. C., Stevens, T., Randall, J., Weber, D. S., and King, J. A. (2006). Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L30–L37. doi: 10.1152/ajplung.00317.2005
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Raffestin, B., and McMurtry, I. F. (1987). Effects of intracellular pH on hypoxic vasoconstriction in rat lungs. J. Appl. Physiol. 2, 2524–2531. doi: 10.1152/jappl.1987.63.6.2524
Reves, J. G., Glass, P. S., Lubarsky, D. A., McEvoy, M. D., and Martinez-Ruiz, R. (2010). “Intravenous anaesthesia,” in Miller's Anaesthesia, 7th Edn, ed R. D. Miller (Philedelphia,PA: Churchill Livingstone), 728–729.
Rubie, S., Robinson, N. E., Stoll, M., Broadstone, R. V., and Derksen, F. J. (1993). Flunixin meglumine blocks frusemide-induced bronchodilation in horses with chronic obstructive pulmonary disease. Equine Vet. J. 25, 138–142. doi: 10.1111/j.2042-3306.1993.tb02924.x
Rudolph, A. M., and Yuan, S. (1966). Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J. Clin. Invest. 45, 399–411. doi: 10.1172/JCI105355
Schaffartzik, W., Poole, D. C., Derion, T., Tsukimoto, K., Hogan, M. C., Arcos, J. P., et al. (1992). VA/Q distribution during heavy exercise and recovery in humans: implications for pulmonary edema. J. Appl. Physiol. 72, 1657–1667. doi: 10.1152/jappl.1992.72.5.1657
Schneeberger, E. E., and Karnovsky, M. J. (1976). Substructure of intercellular junctions in freeze-fractured alveolar-capillary membranes of mouse lung. Circ. Res. 38, 401–411. doi: 10.1161/01.RES.38.5.404
Silke, B. (1993). Central hemodynamic effects of diuretic therapy in chronic heart failure. Cardiovasc. Drugs Ther. Suppl. 1, 45–53. doi: 10.1007/BF00877957
Sinha, A. K., Gleed, R. D., Hakim, T. S., Dobson, A., and Shannon, K. J. (1996). Pulmonary capillary pressure during exercise in horses. J. Appl. Physiol. 80, 1792–1798. doi: 10.1152/jappl.1996.80.5.1792
Speake, P. F., Roberts, C. A., and Gibson, J. S. (1997). Effect of changes in respiratory blood parameters on equine red blood cell K–Cl cotransporter. Am. J. Physiol. 273, C1811–C1818. doi: 10.1152/ajpcell.1997.273.6.C1811
Starling, E. (1896). On the absorbtion of fluid from connective tissue spaces. J. Physiol. 19, 312–326. doi: 10.1113/jphysiol.1896.sp000596
Sylvester, J. T., Shimoda, L. A., Aaronson, P. I., and Ward, J. P. (2012). Hypoxic pulmonary vasoconstriction. Physiol. Rev. 92, 367–520. doi: 10.1152/physrev.00041.2010
van't Hoff, J. H. (1887). Die rolle osmotischen drucks in der analogie zwischen losungen und gasen. Z. Phys. Chem. 1, 481–508.
Vengust, M., Kerr, C., Staempfli, H. R., Pringle, J., Heigenhauser, G. J., and Viel, L. (2011). Effect of frusemide on transvascular fluid fluxes across the lung in exercising horses. Equine Vet. J. 43, 451–459. doi: 10.1111/j.2042-3306.2010.00301.x
Vengust, M., Staempfli, H. R., Viel, L., and Heigenhauser, G. J. (2006a). Transvascular fluid flux from the pulmonary vasculature at rest and during exercise in horses. J. Physiol. 570, 397–405. doi: 10.1113/jphysiol.2005.098723
Vengust, M., Staempfli, H. R., Viel, L., and Heigenhauser, G. J. (2006b). Effects of chronic acetazolamide administration on fluid flux from the pulmonary vasculature at rest and during exercise in horses. Equine Vet. J. Suppl. 36, 508–515. doi: 10.1111/j.2042-3306.2006.tb05596.x
Vengust, M., Staempfli, H., Viel, L., Swenson, E. R., and Heigenhauser, G. (2013). Acetazolamide attenuates transvascular fluid flux in equine lungs during intense exercise. J. Physiol. 591, 4499–4513. doi: 10.1113/jphysiol.2013.257956
Wagner, P. D., Laravuso, R. B., Uhl, R. R., and West, J. B. (1974). Continuous distributions of ventilation perfusion ratios in normal subjects breathing air and 100 per cent O2. J. Clin. Invest. 54, 54–68. doi: 10.1172/JCI107750
Wang, J. Y., Russell, G. N., Page, R. D., Jackson, M., and Pennefather, S. H. (1998). Comparison of the effects of sevoflurane and isoflurane on arterial oxygenation during one lung ventilation. Br. J. Anaesth. 81, 850–853. doi: 10.1093/bja/81.6.850
Wickerts, C. J., Berg, B., Frostell, C., Schmidt, J., Blomqvist, H., Rösblad, P. G., et al. (1992). Influence of hypertonic-hyperoncotic solution and furosemide on canine hydrostatic pulmonary oedema resorption. J. Physiol. 458, 425–438. doi: 10.1113/jphysiol.1992.sp019425
Keywords: general anesthesia, pulmonary circulation, transvascular fluid flux, pulmonary edema, starling forces, Jacobs Stewart cycle, furosemide
Citation: Frlic O, Seliškar A, Domanjko Petrič A, Blagus R, Heigenhauser G and Vengust M (2018) Pulmonary Circulation Transvascular Fluid Fluxes Do Not Change during General Anesthesia in Dogs. Front. Physiol. 9:124. doi: 10.3389/fphys.2018.00124
Received: 08 September 2017; Accepted: 07 February 2018;
Published: 21 February 2018.
Edited by:
Keith Russell Brunt, Dalhousie University, CanadaReviewed by:
Howard H. Erickson, Kansas State University, United StatesSotirios G. Zarogiannis, University of Thessaly, Greece
Copyright © 2018 Frlic, Seliškar, Domanjko Petrič, Blagus, Heigenhauser and Vengust. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Modest Vengust, modest.vengust@vf.uni-lj.si
 Olga Frlic1
Olga Frlic1 Alenka Seliškar
Alenka Seliškar Aleksandra Domanjko Petrič
Aleksandra Domanjko Petrič Modest Vengust
Modest Vengust