- 1Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy
- 2Department of Translational Medical Sciences, Center for Basic and Clinical Immunology Research, University of Naples Federico II, Naples, Italy
- 3Clinic of Cardiovascular Diseases, IRCCS San Martino IST, Genova, Italy
- 4Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 5Department of Molecular Biotechnology and Health Sciences, Molecular Biotechnology Center, University of Turin, Turin, Italy
- 6Institute of Cardiology, Center of Excellence on Aging, Università degli Studi “G. d'Annunzio” Chieti – Pescara, Chieti, Italy
- 7Department of Internal Medicine, Texas Heart Institute and Center for Cardiovascular Biology and Atherosclerosis Research, University of Texas Health Science Center, Houston, TX, United States
- 8Section of Hygiene, Department of Public Health, University of Naples Federico II, Naples, Italy
- 9Monaldi Hospital Pharmacy, Naples, Italy
- 10Department of General Surgery and Medical-Surgery Specialities, University of Catania, Catania, Italy
- 11U.O.C. Magnetic Resonance Imaging, Fondazione Toscana G. Monasterio C.N.R., Pisa, Italy
- 12Division of Clinical and Experimental Cardiology, Department of Medicine and Pharmacology, Policlinico “G. Martino” University of Messina, Messina, Italy
- 13Department of Clinical and Biological Sciences, University of Turin, Turin, Italy
Antineoplastic drugs can be associated with several side effects, including cardiovascular toxicity (CTX). Biochemical studies have identified multiple mechanisms of CTX. Chemoterapeutic agents can alter redox homeostasis by increasing the production of reactive oxygen species (ROS) and reactive nitrogen species RNS. Cellular sources of ROS/RNS are cardiomyocytes, endothelial cells, stromal and inflammatory cells in the heart. Mitochondria, peroxisomes and other subcellular components are central hubs that control redox homeostasis. Mitochondria are central targets for antineoplastic drug-induced CTX. Understanding the mechanisms of CTX is fundamental for effective cardioprotection, without compromising the efficacy of anticancer treatments. Type 1 CTX is associated with irreversible cardiac cell injury and is typically caused by anthracyclines and conventional chemotherapeutic agents. Type 2 CTX, associated with reversible myocardial dysfunction, is generally caused by biologicals and targeted drugs. Although oxidative/nitrosative reactions play a central role in CTX caused by different antineoplastic drugs, additional mechanisms involving directly and indirectly cardiomyocytes and inflammatory cells play a role in cardiovascular toxicities. Identification of cardiologic risk factors and an integrated approach using molecular, imaging, and clinical data may allow the selection of patients at risk of developing chemotherapy-related CTX. Although the last decade has witnessed intense research related to the molecular and biochemical mechanisms of CTX of antineoplastic drugs, experimental and clinical studies are urgently needed to balance safety and efficacy of novel cancer therapies.
Introduction
Antineoplastic treatments have improved overall survival and progression-free survival in the treatment of an increasing number of malignancies (Jemal et al., 2011). However, different antineoplastic drugs can cause a wide spectrum of cardiovascular (CV) toxicities (CTX), particularly in long-term cancer survivors (Oeffinger et al., 2006; Tocchetti et al., 2013; Moslehi and Deininger, 2015; Mercurio et al., 2016; Zamorano et al., 2016; Armenian et al., 2017). CTX include vasospastic and thromboembolic ischemia, hypertension, dysrhythmia, myocarditis and left ventricular (LV) dysfunction, leading to heart failure (Yeh and Bickford, 2009; Ky et al., 2013; Suter and Ewer, 2013; Zamorano et al., 2016). Figure 1 schematically illustrates the wide spectrum of cardiovascular toxicities associated with different antineoplastic drugs in patients with cancer. Anthracyclines (ANTs) can cause irreversible type 1 CTX through the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Ewer and Lenihan, 2008; Ewer and Ewer, 2010; Scott et al., 2011). Intracellular signaling inhibitors (e.g., tyrosine kinase inhibitors) block pathways that are main regulators of myocardial function, especially under conditions of cardiac stress, such as hypertension or hypertrophy (Suter and Ewer, 2013). The toxicity induced by biological drugs (e.g., trastuzumab) is often reversible, and has been classified as type 2 CTX (Ewer and Lippman, 2005; Ewer et al., 2005). However, these two forms of CTX may overlap. For example, trastuzumab, a monoclonal antibody anti-HER-2 (Shinkai et al., 1999), can cause irreversible LV dysfunction in patients previously treated with ANTs (Timolati et al., 2006; Suter and Ewer, 2013; Zamorano et al., 2016). More recently, immune myocarditis has entered as a novel challenge in the cardio-oncologic arena, due to a growing number of patients treated with immune checkpoint inhibitors (Swain and Vici) that unleash immune responses (Johnson et al., 2016; Varricchi et al., 2017d).
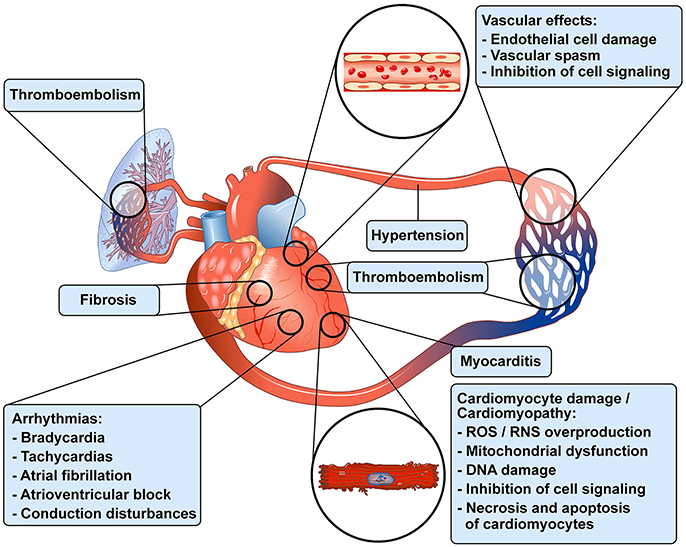
Figure 1. Schematic representation of some of the cardiovascular toxicities associated with antineoplastic drugs in patients with cancer. Modified with permission from Albini et al. (2010).
Here, we review the cellular and molecular mechanisms of CTX of antineoplastic drugs from a redox perspective, since plenty of evidence supports the importance of redox homeostasis for the maintainance of cardiovascular health, while anticancer drugs can disrupt such delicate balance in the myocardium and in the endothelium (Ferroni et al., 2011; Vincent et al., 2013; Zamorano et al., 2016).
Oxidative and Nitrosative Stress in Cardiovascular Toxicity
ROS is a collective term that includes oxygen radicals, like superoxide (•) and hydroxyl radicals (OH•), and other non-radicals such as hydrogen peroxide (H2O2), singlet oxygen (1O2), etc. (Del Río, 2015). The term RNS includes radicals like nitric oxide (NO•) and nitric dioxide (NO2•), as well as non-radicals such as nitrous acid (HNO2) and dinitrogen tetroxide (N2O4), among others. Redox stress, resulting from overproduction of ROS and RNS, may directly or indirectly induce cardiac injury (Nediani et al., 2011; Willis and Patterson, 2013).
Physiological levels of ROS and RNS are fundamental for the regulation of many cellular functions (Egea et al., 2017). For example, H2O2 is an endothelium-derived vasodilator of the coronary vessels (Saitoh et al., 2007). In pathological conditions (e.g., cancer growth) there is a deregulation of homeostatic control of ROS production leading to DNA damage, inhibition of cellular repair mechanisms and abnormal cell proliferation. ROS/RNS contribute to dysregulation of gene expression and genome stability, but also influence epigenetic pathways affecting the functions/expression of histone and DNA modifying enzymes (Mikhed et al., 2015; Niu et al., 2015). Several antineoplastic drugs induce CTX through an unbalanced generation of ROS/RNS, leading to the so-called oxidative/nitrosative stress. ROS/RNS imbalance derives from increased production or inactivation of endogenous antioxidant enzymes by antineoplastic drugs. Figure 2 schematically illustrates the transition from the homeostatic role of ROS/RNS in healthy subjects to the pathological role in cancer patients and during chemotherapy or radiotherapy.
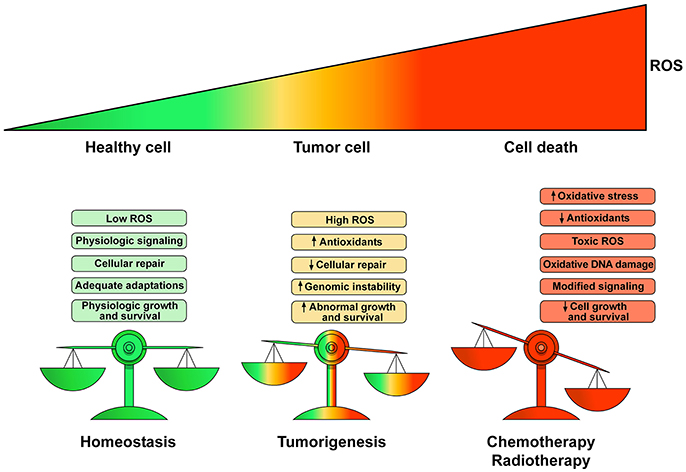
Figure 2. Schematic representation of the homeostatic role of ROS and their pathologic role in tumor growth and cell death. Low production of ROS and balanced antioxidant activity play a fundamental role in cellular signaling and repair resulting in controlled growth and survival. Proliferation of tumor cells yields elevated ROS concentrations enhancing cell survival and proliferation leading to DNA damage and genetic instability causing cell dysfunction. Chemotherapeutic agents and radiotherapy increase ROS production to toxic concentrations resulting in irreparable damage to the cell, inadequate adaptations and eventually cell death. The heart is particularly vulnerable to ROS/RNS injury because antioxidant resources are lower than other tissues. Modified with permission from Moloney and Cotter (2017).
The heart is particularly vulnerable to ROS/RNS injury because antioxidant resources are lower than other tissues (e.g., liver) (Minotti et al., 2004, 2010). High levels of ROS/RNS, by exhausting endogeneous antioxidant defenses, can hamper cellular signaling pathways in the CV system. Oxidative stress and low grade inflammation are interdependent processes implicated in cardiovascular diseases and cancer (Galdiero et al., 2016; Varricchi et al., 2017a). Tissue resident (e.g., macrophages, mast cells) and circulating inflammatory cells (e.g., neutrophils, monocytes) can also release ROS increasing oxidative stress (Varricchi et al., 2017a), interestingly ROS can initiate intracellular signaling increasing proinflammatory gene expression (Biswas, 2016).
High levels of ROS/RNS induce membrane lipid peroxidation and membrane damage, DNA damage and trigger death cell and apoptosis, leading to cardiomyocyte death and replacement by connective tissue, which results in irreversible cardiac damage (Li and Singal, 2000; Menna et al., 2008, 2012; Zang et al., 2012; Ky et al., 2013; Suter and Ewer, 2013; Hahn et al., 2014; Salvatorelli et al., 2015; Mercurio et al., 2016).
The major intracellular sources of ROS include the mitochondrial electron transport and the NADPH oxidase family (NOXs) (Lassègue and Griendling, 2010; Zhang et al., 2013). Mitochondria are key organelles for the regulation of redox signaling and redox homeostasis (Egea et al., 2017). Mitochondria function as a central hub that directly and indirectly controls redox homeostasis by hosting several redox-active complexes and enzymes that generate ROS and RNS. Mitochondria represent ≅35% of the myocyte volume and produce ≅90% of the cellular energy. Therefore, impairment of mitochondrial function is critical in cardiomyocytes (Pagliaro et al., 2011; Pagliaro and Penna, 2015; Tocchetti et al., 2015b). At present, the NOX family is composed of five isoforms (NOX1, NOX2, NOX3, NOX4, and NOX5). Cardiomyocytes (Varga et al., 2013) and macrophages (Moon et al., 2016) express NOX4. Mitochondrial and extramitochondrial NOX4 is a source of ROS and can be affected by anticancer drugs. Activated myocardial NOX2 produces , whereas NOX4 generates H2O2. Moreover, superoxide dismutases (SODs) convert to H2O2. In mitochondria, H2O2 may be converted to O2 and H2O by catalase and by glutathione peroxidase (GPx). In the presence of iron complexes, these ROS may be converted to the more toxic OH• within and outside mitochondria (Zhao et al., 2010; Pagliaro et al., 2011; Penna et al., 2014; Pagliaro and Penna, 2015; Tocchetti et al., 2015a). Interestingly, mitochondrial ROS are involved in the modulation of immune cells, including human neutrophils (Vorobjeva et al., 2017).
Peroxisomes, cytoplasmic organelles specialized for carrying out oxidative reactions, also play a role in ROS production/regulation in cardiomyocytes. Several substrates (i.e., amino acids, uric acid, and fatty acids) are broken down by oxidative reactions in peroxisomes. Fatty acid metabolism is very active in cardiomyocytes and peroxisomes are critical for processing long carbon chain fatty acids. The contribution of peroxisomes in the mechanism of CTX is largely unknown (Zanardelli et al., 2014).
Nitric oxide (NO) is a key regulator of cellular functions. It is a redox species with both oxidant and antioxidant properties (Takimoto and Kass, 2007; Pagliaro and Penna, 2015; Tocchetti et al., 2015a) produced produced from the metabolism of the amino acid, L-arginine by three isoforms of nitric oxide synthase (NOS): the endothelial (eNOS or NOS3) and neuronal (nNOS or NOS1) NOSs, constitutively expressed in cardiomyocytes, and the inducible NOS2 (iNOS), which is induced by pro-inflammatory mediators or by ischemia (Pagliaro and Penna, 2015; Tocchetti et al., 2015a). NO is also produced by other reactions termed “non-NOS” processes (Penna et al., 2014; Pagliaro and Penna, 2015). ROS can react with NO to form different RNS, thus amplifying the production of oxidant compounds, and NOS itself may produce ROS (Fogli et al., 2004; Penna et al., 2014; Pagliaro and Penna, 2015; Tocchetti et al., 2015b). NO together with RNS has an important role in mediating proteotoxic stress and modifications of mitochondrial activities, resulting in cytotoxicity and cell necrosis (Lala and Chakraborty, 2001). S-nitrosylation (SNO) is the covalent attachment of a NO moiety to a protein thiol group. SNO is a redox-dependent modification that exerts an antioxidant effect, shielding critical cysteine residues from oxidation and affecting protein functions (Penna et al., 2014; Pagliaro and Penna, 2015).
Anthracyclines
The production of ROS/RNS is central in the CTX of several anti-cancer drugs. Some agents alter the activity of redox enzymes within and outside the mitochondria, including NOSs, respiratory complexes, the Krebs cycle, oxidative phosphorylation, and β-oxidation (Tocchetti et al., 2017). This impairment results in oxidative/nitrosative stress, a reduction in antioxidant capacity, and induction of cell death (Fogli et al., 2004; Albini et al., 2010; Mele et al., 2016a,b).
ANTs (doxorubicin, epirubicin and daunorubicin), widely used as anticancer agents, are recognized as prototype of type 1 CTX since the 1960s (Tan et al., 1967). ANTs can induce LV dysfunction, leading to HF in up to 9% of patients (Cardinale et al., 2015). ANT can cause CTX via a series of many cellular and molecular mechanisms (Zhang et al., 2012; Zamorano et al., 2016). Figure 3 schematically illustrates the complex interplay of the major mechanisms by which ANTs can induce injury to cardiac cells. The administration of ANTs can alter redox homeostasis in cardiomyocytes and tissue resident (e.g., fibroblasts, endothelial cells, mast cells, macrophages) and circulating inflammatory cells (e.g., neutrophils, eosinophils) in the heart by producing ROS and RNS (Pagliaro and Penna, 2015; Ghigo et al., 2016; Tocchetti et al., 2017).
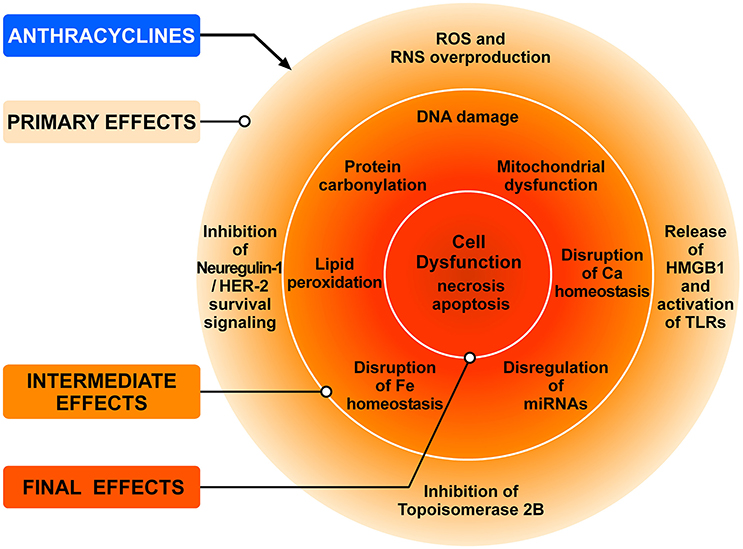
Figure 3. Schematic representation of the main mechanisms of anthracycline-induced injury to cardiac cells. The classic model of anthracycline (ANT) cardiotoxicity involves the generation of ROS by the quinone moiety common to all anthracyclines. ROS and RNS hyperproduction results in damage to DNA, protein carbonylation and lipid peroxidation leading to cellular dysfunction and cardiomyocyte death. ANTs can also bind and block the functions of both topoisomerases 2A (TOP2A) and 2B (TOP2B). Tumor cells express high levels of TOP2A, whereas TOP2B is ubiquitously expressed. Cardiomyocytes express TOP2B, but not TOP2A. ANTs form a complex with TOP2B inhibiting its enzymatic activity. Without functional TOP2B, DNA breaks accrue, leading to the activation of p53 tumor-suppressor protein, mitochondrial dysfunction, and the generation of ROS that result in cardiomyocyte death. Another mechanism underlying doxorubicin-dependent oxidative stress is linked to the ability of the drug to directly interfere with the activity of NADPH oxidase and nitric oxide synthase (NOS). Both NADPH oxidase and NOS can transfer electron from NADPH to doxorubicin, causing the formation of semiquinone doxorubicin (SQ-DOX). SQ-DOX in turn transfers electron to O2 and generates . In the NOS compartment, can react with NO to form peroxynitrite (ONOO−), a powerful oxidant that can generate free radicals. An alternative mechanism by which ANTs exert their cardiotoxic effects is the inhibition of neuregulin-1 (NRG-1)-HER-2 in cardiomyocytes. Doxorubicin also induces necrosis of immune (i.e., macrophages) and cancer cells releasing HMGB1 which activates TLR-2 and TLR-4 in cardiomyocytes and inflammatory cells inducing the release of proinflammatory cytokines. These primary effects induce a plethora of secondary effects in cardiomyocytes (e.g., DNA damage, lipid peroxidation, mitochondrial dysfunction, etc.,) which result in cell dysfunction and death. Modified with permission from Tocchetti et al. (2017).
A basic mechanism by which ANTs can cause CTX is the interaction with topoisomerase 2 (TOP2) A and -B highly expressed in cardiomyocytes (Lyu et al., 2007). The former is present in rapidly dividing cells, such as cancer cells, and forms the ternary TOP2-doxorubicin-DNA complex, inducing cell apoptosis. TOP2B, highly expressed in human cardiomyocytes, forms the TOP2B-doxorubicin-DNA complex, which causes DNA damage leading to cell apoptosis. The tumor suppressor protein p53, a pivotal enzyme for activating DNA repair proteins, can cause mitochondrial dysfunction and metabolic failure (Sawyer, 2013). The metabolic alterations caused by doxorubicin-activated p53 damage mitochondria in the cardiomyocytes, result in enhanced ROS/RNS generation and ultimately cell death. Collectively, these results indicate that oxidative reactions play a central role in ANT-induced LV dysfunction. Therefore, drugs that interfere with molecules involved in heart metabolism (e.g., p53) may represent a potential approach in limiting LV dysfunction (Sawyer, 2013; Mercurio et al., 2016).
Besides directly damaging cardiomyocytes, doxorubicin induces apoptosis of immune (e.g., macrophages) and cancer cells releasing high mobility group box 1 (HMGB1) which, in turn, triggers toll-like receptor (TLR)-2 and-4 (Ma et al., 2012; Yao et al., 2012). TLR-2 and TLR-4 are found in cardiomyocytes and inflammatory cells and their engagement induces the release of proinflammatory cytokines (i.e., IL-6, IL-1β, TNF-α). Overall, these findings emphasize the contribution of TLRs in mediating ANT-induces inflammation and CTX and envisage the possibility of targeting this pathway for therapeutic purposes.
A better characterization of the multiple molecular mechanisms of ANT-related toxicity of blood vessels and cardiomyocytes appears fundamental to select the best approach to prevent and treat CTX (Van Cutsem et al., 2002; Scott et al., 2011; Madonna et al., 2015a,b; Cadeddu et al., 2016).
As mentioned in a previous section, mitochondrial ROS (mtROS) represent a prominent source (≅80%) of ROS, expecially in the heart (Russell and Cotter, 2015). mtROS play a pivotal role in ANT-induced CTX (Minotti et al., 2004, 2010). Doxorubicin binds with high affinity to the mitochondrial phospholipid cardiolipin, inhibits its function, stimulates ROS/RNS production, inhibits oxidative phosphorylation, and causes mitochondrial DNA damage (Pereira et al., 2016). ANTs also cause mitochondrial calcium accumulation, leading to mitochondrial injury (Pereira, Pereira et al., 2016). ANTs can also affect cardiac progenitor cells following myocardial injury (Huang et al., 2010; Oliveira et al., 2013).
The production of ROS is a central event in ANT-induced CTX. ROS are effectors of membrane lipid peroxidation, irreversible damage, and myocyte replacement by connective tissue (Menna et al., 2008, 2012; Zhang et al., 2012; Ky et al., 2013; Suter and Ewer, 2013; Salvatorelli et al., 2015). ROS generated by ANTs affect mitochondrial enzymes, NOSs, NAD(P)H oxidases, and catalase, leading to oxidative stress and cell injury. ANTs are metabolized to unstable compounds (such as doxorubicin-semiquinone), which react with O2, producing H2O2 and .
ANTs chelate free intracellular iron, forming iron-doxorubicin complexes. ANTs also interfere with iron-transporting and -binding proteins (Gammella et al., 2014; Ghigo et al., 2016). Ardehali and collaborators found that doxorubicin impairs a mitochondrial iron exporter with consequent iron accumulation and subsequent ROS generation (Ichikawa et al., 2014). Cardiac dysfunction following ANT treatment is associated with high mitochondrial iron levels compared with normal hearts (Ichikawa et al., 2014). Collectively, these findings indicate that oxidative stress and mitochondrial iron accumulation play a key role in ANT-induced CTX.
ANTs interact with cardiolipin leading to concentration of the drug in mitochondrial membrane phospholipids (Goormaghtigh et al., 1990). In mitochondria, the drug exerts adverse effects (e.g., ROS generation, inhibition of oxidative phosphorylation, and mitochondrial DNA damage). ROS cause peroxidation of cardiolipin, which induces the release of mitochondrial factors, such as cytochrome c, which in turn triggers cardiolipin peroxidation. This cycle exacerbates ANT-induced injury. NO inhibits both the peroxidase activity of cytochrome c and cardiolipin oxidation. NO, which possesses antioxidant properties, may counteract the toxic effects of ANTs (Vlasova et al., 2006; Gonzalvez and Gottlieb, 2007; Pointon et al., 2010).
Enzymes located outside the mitochondria also able to produce ROS. A nonexhaustive list includes NADPH oxidases (NOXs), xanthine oxidase (XO), and monoamine oxidase. Xanthine oxidase and NADPH, may be targeted by ANTs. Doxorubicin deoxyaglycone can be obtained by a reduction process and accumulates in membranes, altering the function of NADH dehydrogenase in mitochondria or the NOXs in the plasma membrane (Thorn et al., 2011). Among other mechanisms involved in cardiotoxicity caused by ANTs, recent studies have highlighted the role of altered myocardial energetics, expressed by a lower phosphocreatine/adenosine triphosphate (ATP) ratio, which precedes LV dysfunction (Maslov et al., 2010). Indeed, ANTs can oxidize sulfhydryl groups of creatine kinase (CK), reducing its function, thus impairing myocardial energetics (Maslov et al., 2010), hence causing LV dysfunction. More studies on such an interesting mechanism could be helpful in order to identify new protective therapeutic strategies. Indeed, overexpression of myofibrillar CK in mice with HF induced by transverse aortic constriction increased heart function (Gupta et al., 2012) supporting a role for CK in HF prevention and treatment. Accordingly, the same group demonstrated that CK overexpression also ameliorated myocardial energetics, contractile function, and survival in murine anthracyclines cardiotoxicity (Gupta et al., 2013). These results provide novel strategies for limitation of anthracycline-related cardiotoxicity.
ANTs are also able to alter cardiac energy metabolism by lowering the level of 5′ AMP-activated protein kinase (AMPK, activated in the response to energy stress) and phosphorylation of anti-acetyl-CoA carboxylase, leading to impairment of fatty acid oxidation (Tokarska-Schlattner et al., 2005). The mechanisms underlying inhibition of AMPK need to be fur-ther elucidated (Mercurio et al., 2016).
Importantly, along with ANTs (Menna et al., 2012; Sawyer, 2013; Sterba et al., 2013; Ghigo et al., 2016), redox abnormalities are central in the pathophysiology of cardiotoxicity caused by other anticancer drugs, among which are new biologic antineoplastic agents, such as intracellular signaling inhibitors, that are increasingly being used (Tocchetti et al., 2017). Such agents may cause cardiotoxicity, since they block pathways important for the modulation of myocardial function, especially under conditions of cardiac stress, such as hypertension or hypertrophy (Suter and Ewer, 2013), with mechanisms of action that often involve redox dysregulation as well.
Antimetabolites
Fluoropyrimidines [i.e., 5-fluorouracil (5-FU), capecitabine, and gemcitabine] are used in the treatment of several tumors. 5-FU administered intravenously has a short half-life, but active metabolites concentrate in cardiac and cancer cells, resulting in a prolonged exposure to the drug (Kosmas et al., 2008; Miura et al., 2010; Lestuzzi et al., 2011). Capecitabine is converted into its active form preferentially within tumors (Ng et al., 2005; Aprile et al., 2009; Khan et al., 2014; Petrelli et al., 2016). 5-FU and its main metabolite can induce CTX after few days of treatment (Jensen and Sorensen, 2006; Jensen and Sørensen, 2012). The enzyme involved in the conversion of capecitabine to 5-FU is expressed in both atherosclerotic plaques and cancer cells, explaining the CTX in patients with coronary artery disease. The incidence of CTX caused by 5-FU ranges from 0 to 35%, with a mortality rate between 2 and 13%. Myocardial ischemia is the strongest risk factors for fluoropyrimidine-induced CTX (Koca et al., 2011; Polk et al., 2013, 2014). Silent ischemia due to cardiac stress test has been reported in 6–7% of 5-FU-treated patients (Lestuzzi et al., 2014). The mechanisms involved in the CTX of 5-FU and its metabolites involve inhibition of NO (Cianci et al., 2003; Shoemaker et al., 2004), enhanced generation of ROS/RNS (Lamberti et al., 2014), higher endothelial thrombogenicity (Kalam and Marwick, 2013) and senescence (Altieri et al., 2017), and DNA and RNA damage. 5-FU can induce oxidative stress in cardiomyocytes and endothelial cells. This drug causes eNOS dysregulation, endothelin 1 upregulation and the activation of protein kinase C. These effects lead to endothelium-dependent and -independent vasoconstriction, and eventually to coronary spasm (Alter et al., 2006; Sorrentino et al., 2012).
Her-2 Inhibitors
Epidermal growth factor receptor 2 (ErbB2) (also called HER-2), ErbB1, ErbB3, and ErbB4 are members of the human epidermal growth factor receptor family. When activated by their ligands, these transmembrane receptors homodimerize or heterodimerize and are phosphorylated, initiating several cellular responses (Force et al., 2007). HER-2, present on human heart and overexpressed in approximately 30% of breast cancers, can interact with HER-1 and HER-3, independently from ligand stimulation, thus triggering signaling pathways that stimulate tumor growth (Slamon et al., 1987). Trastuzumab, a humanized mAb that binds the extracellular domain IV of HER-2 (Force et al., 2007; Suter et al., 2007), can cause type 2 CTX (Ewer and Lippman, 2005; Ewer et al., 2005) in approximately 30% of patients when combined with ANTs (Slamon et al., 2001; Suter et al., 2007; De Keulenaer et al., 2010).
Several oral small molecules inhibiting tyrosine kinase (TK) associated with HER are clinically used or under development (De Keulenaer et al., 2010; Ades et al., 2014). Lapatinib and neratinib are novel HER-2/HER-4 TK inhibitors undergoing clinical development in HER-2+ breast cancer. Their cardiac safety data show a favorable profile (Ades et al., 2014). Several clinical trials have demonstrated that lapatinib is less toxic than trastuzumab (Ades et al., 2014). Pertuzumab is a humanized mAb blocking domain II of the extracellular part of HER-2, thus stopping HER-2/HER-3 homo-heterodimeration. Several clinical trials have assessed the cardiac toxicity of pertuzumab (Bowles et al., 2012; Molinaro et al., 2015). Pertuzumab causes a modest (≅10%) reduction of LVSD in patients with HER-2+ breast cancer (Baselga et al., 2012; Gianni et al., 2012; Swain et al., 2013).
Importantly, in breast cancer treatment, the co-administration of trastuzumab with ANTs enhances the latter's toxicity and is now avoided. In fact, anti-HER-2 mAbs block the protective mechanisms of HER-2, exhacerbating the oxidative damage caused by doxorubicin (Ewer and Ewer, 2010). Indeed, redox mechanisms have also been advocated for the neuregulin/ErbB2 pathway. This pathway can modulate the increase in ROS caused by doxorubicin in animal models (Timolati et al., 2006), suggesting that cardiotoxicity from ErbB2 blockade can also involve a dysregulation of redox homeostasis (Gordon et al., 2009; Mercurio et al., 2016).
In the heart, endothelial cells release neoregulin 1 (NRG-1), especially the NRG-1β isoform (Lim et al., 2015), which triggers HER-4/HER-4 homodimerization and HER-4/HER-2 heterodimerization on cardiomyocytes to activate protective pathways in response to stress (De Keulenaer et al., 2010; Odiete et al., 2012; Lim et al., 2015; Figure 4). The HER-2 pathway mediates cell survival and possibly regeneration (D'Uva et al., 2015) and is stimulated when the heart experiences stress, including hypertension (de Korte et al., 2007; Ewer and Ewer, 2010) and ANT therapy (Gabrielson et al., 2007). Anti-HER-2 agents interfere with the NRG-1/HER-4/HER-2 axis and can cause cardiomyocyte damage. This hypothesis is corroborated by ErbB2 KO-mice that present with LV dilation and increased susceptibility to ANT-induced cardiac damage (Crone et al., 2002; Ozcelik et al., 2002), supporting a fundamental role of HER-2 in the heart. Conversely, cardiac ErbB2 overexpressed mice exhibited reduced levels of ROS in mitochondria, with lower ROS levels and less cell death after treatment of neonatal cardiomyocytes isolated from ErbB2 (Bosch et al., 2013) hearts with anthracyclines. This was due to higher levels of glutathione peroxidase 1 (GPx1) protein and GPx activity, with increased levels of two known GPx activators, c-Abl and Arg (Belmonte et al., 2015; Tocchetti et al., 2017).
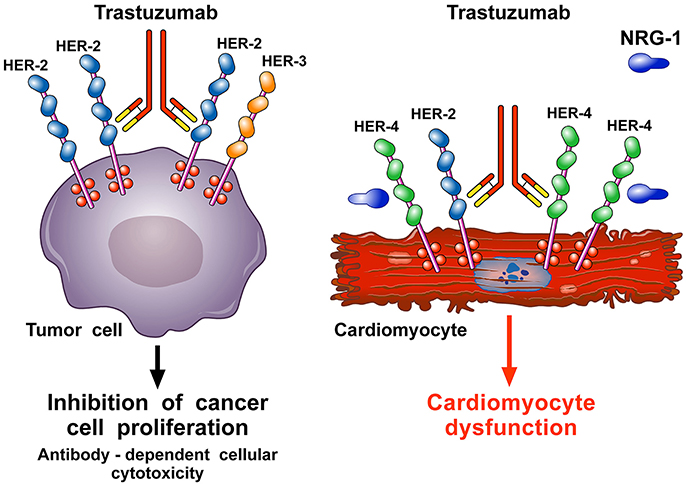
Figure 4. Schematic representation of the mechanism of action of trastuzumab and pathogenesis of its cardiotoxicity. Trastuzumab is a mAb that binds the extracellular domain IV of HER-2. It is used to treat breast cancer patients (≅30%) in which HER-2 is overexpressed and spontaneously homodimerizes or forms heterodimers with other HER receptors, especially HER-3. This ligand-independent activation of HER-2 promotes proliferation and survival of tumor cells. Trastuzumab blocks the interaction HER-2/HER-3 and downstream signaling halting the growth of tumor cells. Moreover, trastuzumab induces the antibody-dependent immune cell-mediated cytotoxicity of cancer cells (left side). In the heart, neuregulin-1 (NRG-1) triggers HER-4/HER-4 homodimerization and HER-4/HER-2 heterodimerization on cardiomyocytes to induce protective pathways in response to stress. Blockade of cardiac HER-2 by trastuzumab results in the disruption of NRG-1-dependent signaling and consequently in alterations of structure and functions that cause cardiomyocyte death (right side).
HER-2 and HER-4 receptor expression and activation/phosphorylation are lower in failing human myocardium, a condition characterized by increased oxidative stress (Rohrbach et al., 1999). Dogs with HF showed increased phosphorylation of ErbB4 and ErbB2 (Doggen et al., 2009). NRG-1 expression is enhanced in HF (Rohrbach et al., 1999; Doggen et al., 2009). Collectively, these results indicate that NRG-1/HER-4/HER-2 activity is involved in the pathophysiology of HF (Mercurio et al., 2016). (Mercurio et al., 2016). Importantly, NRG-1 exerts a lusitropic effect on isolated cardiac muscle preparations via a NO-dependent mechanism (Lemmens et al., 2004): this requires a functional NO synthase, with preserved NO bioavailability, a condition which can be hampered by the increased oxidative stress in HF (Nediani et al., 2011; Arcaro et al., 2016).
Based on cardioprotective properties of NRG-1 via HER-4/HER-2, the neuregulin-HER pathway is currently being assessed in clinical studies for HF treatment (Galindo et al., 2014a,b). NRG-1β increases LV function and reduces cardiac dimensions in experimental failing hearts (Liu et al., 2006; Li et al., 2011; Galindo et al., 2014a,b; Mercurio et al., 2016). NRG-1 also inhibits cardiac fibroblasts and prevents fibrosis (Galindo et al., 2014a,b). NRG-1 administration after myocardial infarction is able to blunt remodeling of the damaged heart (Liu et al., 2006; Galindo et al., 2014a,b). Clinical trials have shown that NRG-1 is well-tolerated and ameliorates heart dimensions and LVEF up to 3 months after treatment (Gao et al., 2010; Jabbour et al., 2011). However, NRG-1 may be a growth factor for cancer cells, and further studies are necessary to assess the safety and efficacy of NRG-1 in HF (Lim et al., 2015; Mercurio et al., 2016).
Inhibitors of Vascular Endothelial growth Factor (VEGF) Signaling
Vascular endothelial growth factors (VEGF-A, VEGF-B, VEGF-C, VEGF-D. and PlGF) activate specific tyrosine kinase (TK) receptors (VEGFR-1, VEGFR-2, and VEGFR-3) on blood endothelial cells (Loffredo et al., 2016; Staiano et al., 2016) and on endothelial colony forming cells (Dragoni et al., 2011) and have a major role in myocardial angiogenesis at rest and in pressure-overload hearts (Oka et al., 2014). Inhibitors of VEGF signaling (i.e., mAbs anti-VEGF-A and “specific” TK inhibitors) are used for the treatment of several malignancies (Hurwitz et al., 2004; Sandler et al., 2006). VEGFs also regulate several myocardial functions and the integrity of coronary and systemic blood vessels (Folkman, 2007; Eschenhagen et al., 2011; Curigliano et al., 2012; Tocchetti et al., 2013; Marone and Granata, 2014), hence, not surprisingly, beside fighting cancer proliferation by inhibiting angiogenesis, VEGF antagonists may produce different forms of CTX, mainly hypertension, thromboembolism, LV dysfunction, and HF (Gressett and Shah, 2009; Nazer et al., 2011; Welti et al., 2013).
Bevacizumab (anti-VEGF mAb), sunitinib and sorafenib (TK inhibitors: TKIs) are used for the treatment of different types of cancer (Hurwitz et al., 2004; Sandler et al., 2006). Bevacizumab can induce hypertension and cardiac dysfunction in 1–3% patients undergoing chemotherapy (Miller et al., 2005). Regorafenib is a multi-target TKI that inhibits VEGFR1, endothelial-specific receptor tyrosine kinase (trk2), PDGFR, fibroblast growth factor receptor (FGFR), KIT, and RET. Regorafenib, used in therapeutic protocolos for gastrointestinal tumors, can induce hypertension (Brinda et al., 2016) and less frequently cardiac ischemia and myocardial infarction (Bronte et al., 2015). Treatment with pazopanib and axitinib (inhibitors of VEGFRs, PDGFRA and B, and KIT) can lead to hypertension (Motzer et al., 2013). 40% of patients treated with axitinib can experience hypertension (Hutson et al., 2013). Novel anti-angiogenic drugs such as cediranib, vatalanib and nintedanib also exhibit a potential risk of hypertension and HF (Goss et al., 2010; Van Cutsem et al., 2011; Reck et al., 2014).
Sunitinib and sorafenib are not selective TKIs and inhibit several kinases other than VEGFR (Cheng and Force, 2010). Sunitinib inhibits more than 30 TKs, including platelet-derived growth factor receptor (PDGFR), KIT, and colony-stimulating factor 1 receptor (CSF1R) (Force et al., 2007; Cheng and Force, 2010; Hasinoff and Patel, 2010). All these kinases are regulators of CV functions (Lévy, 2006; Anisimov et al., 2009). Up to 28% of patients can develop cardiac dysfunction from sunitinib (Chu et al., 2007; Motzer et al., 2007; Khakoo et al., 2008; Telli et al., 2008). The CTX induced by sunitinib is also due to interference with ribosomal S6 kinase (Tokarska-Schlattner et al., 2005) that then triggers apoptosis (Force et al., 2007; Kerkela et al., 2009). Sunitinib prolongs opening of the mitochondrial permeability transition pore (mPTP) and mitochondrial swelling in myocytes from heart subjected to pressure overload (Chu et al., 2007). Also, treatment of different myocardial preparations with sunitinib produces a dose-dependent negative inotropic effect, paralleled by a decline in intracellular Ca2+ and increase of ROS production (Rainer et al., 2012; Tocchetti et al., 2013). Interestingly, our preliminary data show that CK might play a role in the regulation of sunitinib cardiac effects (Tocchetti et al., 2015b). In addition, sutinitib can harm pericytes in cardiac vessels (Chintalgattu et al., 2013). Sorafenib inhibits at least 15 kinases, including the VEGFR, PDGFR, and KIT (Force et al., 2007; Cheng and Force, 2010; Tocchetti et al., 2013).
In conclusion, cardiac dysfunction can be induced by many mechanisms in patients treated with mAbs anti-VEGF and TKIs including alterations of mitochontrial function and energy production with increase in ROS generation, as well as induction of arterial hypertension (Mourad and Levy, 2011). Bevacizumab and sunitinib can cause hypertension because of functional (inactivation of endothelial NO synthase and production of vasoconstrictors such as endothelin-1) and anatomic modifications, bringing to vasoconstriction and to an increase in peripheral vascular resistance (Ku et al., 1993; Mourad and Levy, 2011; Nazer et al., 2011; Hahn et al., 2014). Arterial and venous thrombosis is due to reduction of NO synthesis, endothelial dysfunction, and plaque instability.
Antioxidant Properties of Cardiovascular Drugs: A Useful Tool for the Protection from Cardiotoxicity of Antineoplastic Drugs
It has been suggested that drugs with antioxidant properties can prevent CTX induced by an increase in ROS (Swain et al., 1997; Li and Singal, 2000; Spallarossa et al., 2004; Cadeddu et al., 2010; Lipshultz et al., 2012; Dessí et al., 2013; Broeyer et al., 2014). Dexrazoxane, an iron-chelating drug, is a cardioprotective agent approved by the FDA for ANT-induced CTX. It is a pro-drug that enters the cardiomyocyte, is rapidly metabolized into its active form, and inhibits the formation of ANT-iron complexes and the production of ROS (Simunek et al., 2009). Its efficacy in several types of tumors has been demonstrated in clinical trials and two pooled analyses (Swain et al., 1997; Seymour et al., 1999; Swain and Vici, 2004; Lipshultz et al., 2012). Other iron chelators have not shown any cardioprotective effect suggesting that dexrazoxane exerts its effects by means of additional protective mechanisms (Simunek et al., 2009). Dexrazoxane changes the Top2β configuration preventing its interface with ANTs, thereby impeding the formation of the Top2-DNA complexes (Lyu et al., 2007; Lencova-Popelova et al., 2016). Stěrba and coworkers have shown that the cardioprotective effects of dexrazoxane are due to its interaction with Top2-β, rather than to its iron chelating activity (Sterba et al., 2013). Derivatives of dexrazoxane lacking effects on Top2β were found not to be protective in models of ANT-induced CTX (Martin et al., 2009; Tocchetti et al., 2017) suggesting the relevance of Top2β in the cardioprotective mechanism.
Antioxidant Properties of Beta Blockers: Beyond the Antiadrenergic Effects
β-blockers are cornerstone treatments for patients with low LVEF (Ponikowski et al., 2016), and there is evidence to encourage their use in asymptomatic ANT-related LV dysfunction (Curigliano et al., 2012; Cadeddu et al., 2016). The rationale for β blocker utilization in ANT-induced CTX is based on clinical and experimental results. Alterations of β-adrenergic receptor (β-AR) signaling are present in LV dysfunction caused by ANTs and in other types of dilated cardiomyopathies (Fu et al., 1994). Furthermore, a positive effect of β-AR blockage in reducing oxidative stress and myocardial calcium overload (Nakamura et al., 2002; Asanuma et al., 2004) has been shown in experimental models. New-generation β blockers (i.e., carvedilol and nebivolol) have been taken into consideration for their cardioprotective properties. Carvedilol, a non-selective β- and α1-AR antagonist with strong antioxidant properties, was compared to atenolol, a β blocker devoid of antioxidant properties. Only carvedidol conferred protection from ANT-induced LV-dysfunction and such effect has been attributed to its antioxidant properties rather than to the β-AR blocking action (Matsui et al., 1999). Carvedilol inhibits ANT-induced ROS release, cardiomyocyte apoptosis (Spallarossa et al., 2004), and mitochondrial alterations (Santos et al., 2002). In a small clinical trial evaluating the cardioprotective effect of carvedilol in patients treated with ANTs a reduced incidence of LV dysfunction was reported (Kalay et al., 2006). More studies are needed in order to confirm this cardioprotective effect.
In an experimental model of ANT-induced CTX, nebivolol, a cardio-selective β blocker with limited vasodilating properties, improved LV function, while enhancing NO levels and lowering oxidative stress (de Nigris et al., 2008; Tocchetti et al., 2015a). In a small clinical trial the prophylactic use of nebivolol in patients undergoing ANT-based treatments was associated with lower incidence of LV dilatation and systolic dysfunction in the nebivolol group compared to the placebo group (Kaya et al., 2013).
Interestingly, β blockers have been associated with reduced risk of cardiac dysfunction in patients on trastuzumab, ANTs, or both (Seicean et al., 2013). More recently, β blockers such as bisoprolol (Pituskin et al., 2017) and metoprolol have not shown promising results in the prevention of trastuzumab-induced LV dysfunction, suggesting that blockade of β1 alone is not cardioprotective (Gulati et al., 2016). This supports the use of non-selective β1 and β2 blockers (Sysa-Shah et al., 2016).
The Redox Role of Renin-Angiotensin-Aldosterone System Antagonists
The renin-angiotensin-aldosterone system (RAAS) is a key player in ANT-induced CTX (Arnolda et al., 1985). Angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin II receptor blockers (ARBs) can reduce the progression of heart dysfunction and prevent HF in high-risk patients (Ponikowski et al., 2016). Experimental studies have shown the efficacy of ACE-Is in fighting ANT-induced CTX (Abd El-Aziz et al., 2001; Boucek et al., 2003). ACE-Is can confer protection from ANT-related CTX by reducing ROS damage, intracellular calcium overload and fibrosis, and by enhancing mitochondrial respiration and cardiomyocyte metabolism (Abd El-Aziz et al., 2001; Boucek et al., 2003). Enalapril, captopril, and lisinopril can improve acute and chronic ANT-induced cardiotoxicity in experimental models (Abd El-Aziz et al., 2001). In ANTs-treated patients, enalapril reduced the incidence of LV dysfunction compared to placebo (Cardinale et al., 2015). Candesartan modulates experimental cardiotoxicity induced by ANTs (Soga et al., 2006). Pre- and post-treatment with telmisartan protects against acute doxorubicin-induced LV dysfunction in rats (Iqbal et al., 2008). Telmisartan affects the bioavailability of NO and inhibits the production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (Yamagishi and Takeuchi, 2005). In a small prospective study, telmisartan blunted subclinical cardiotoxic effects of epirubicin (EPI) (Cadeddu et al., 2010). Telmisartan reversed early EPI-induced myocardial dysfunction and maintained a normal systolic function up to the 18-month follow-up (Dessí et al., 2011, 2013). Valsartan exerted a cardioprotective effect in patients treated with ANTs (Nakamae et al., 2005).
The combination of ACE-Is (enalapril) and β blockers (carvedidol) seems to be beneficial in treating ANT-induced CTX (Bosch et al., 2013). Several clinical trials have evaluated the role of ACE-Is and ARBs as cardiopreventive agents in patients undergoing chemotherapy (Lim et al., 2015; Molinaro et al., 2015). A recent meta-analysis showed that the prophylactic administration of ACE-Is and ARBs in patients treated with ANTs reduced the risk of developing CTX compared with placebo (Kalam and Marwick, 2013). Unfortunately, recent studies have failed to show promising results about prevention of cardiotoxicity with beta blockers or ACE-Is or ARBs (Boekhout et al., 2016; Gulati et al., 2016; Pituskin et al., 2017).
Non-dihydropyridine calcium channel blockers are not indicated in patients with anti-angiogenic drug-induced hypertension, due to the pharmacokinetic interaction of sorafenib and sunitinib with CYP3A4 (Maitland et al., 2010; Cadeddu et al., 2016). Experimental and clinical studies should evaluate the safety and efficacy of the combination of ACE-Is and β blockers in preventing sunitinib-induced CTX.
Experimental Antioxidant Drugs in Cardioprotection against Cardiotoxic Effects of Anthracyclines
Several drugs (e.g., ranolazine, statins, and phosphodiesterase-5 inhibitors) have been assessed in counteracting ANT-induced CTX. The efficacy of different statins in preventing ANT-induced CTX is so far unproven, due to controversial data. Statins (i.e., lovastatin and fluvastatin) were cardioprotective in cellular studies performed on proliferating H9c2 cell line, but not on cardiomyocytes (Riad et al., 2009; Huelsenbeck et al., 2011). Lovastatin did not modify LV dysfunction induced by doxorubicin (Henninger et al., 2015). Small clinical studies have reported protective/marginal effects of statins in patients treated with ANTs (Seicean et al., 2012; Chotenimitkhun et al., 2015). Hence, several experimental models of cardiac dysfunction have suggested a cardioprotective effect with ranolazine (Sabbah et al., 2002; Rastogi et al., 2008; Coppini et al., 2013, 2017). Ranolazine can preserve cardiac function in mice treated with ANTs by reducing oxidative stress (Tocchetti et al., 2014; Cappetta et al., 2017). Ranolazine can prevent calcium overload and the occurrence of oxidative damage by suppressing ROS production (Kohlhaas et al., 2010). Although the INTERACT study indicated that ranolazine was a promising agent for the prevention of DOX-induced cardiotoxicity, more studies are needed to confirm such evidence (Minotti, 2013).
Sildenafil, a phosphodiesterase-5 inhibitor, seems to protect from ANT-induced cardiac dysfunction by opening mitochondrial KATP channels, preserving mitochondrial membrane potential and myofibrillar integrity, and preventing cardiomyocyte apoptosis (Fisher et al., 2005). Tadalafil blunted ANT-induced LV dysfunction through NO-mediated rises in cGMP levels (Koka et al., 2010; Jin et al., 2013).
Hydrogen sulfide (H2S), a redox compound, also attracted the interest of cardio-oncologists. Cystathionine gamma-lyase, a key enzyme in the synthesis of H2S, is involved in ANT-induced CTX in cardiomyocytes and exogenous H2S has been shown to protect against CTX (Papapetropoulos et al., 2015; Cadeddu et al., 2016; Mele et al., 2016b). Further experimental research and randomized trials will be needed to assess the safety and efficacy of H2S.
Experimental data show that VEGF-B favors coronary artheriogenesis, physiological cardiac hyperthrophy, and resistance to ischemia (Bry et al., 2010; Kivelä et al., 2014). Furthermore, VEGF-B has been proposed as a candidate for the therapy of dilated cardiomyophaty (Kivelä et al., 2014; Woitek et al., 2015). There is preliminary evidence that VEGF-B gene therapy can inhibit doxorubicin-induced CTX (Räsänen et al., 2016).
Beyond Pharmacologic Approaches
Nutritional supplementation and exercise training may also exert antioxidant properties (Andreadou et al., 2009; Haykowsky et al., 2009; Scott et al., 2011, 2013; Kirkham and Davis, 2015; Stefani et al., 2015; Singh et al., 2016). While in experimental models, dietary supplementation of antioxidants can mitigate LV dysfunction induced by ANTs (Rephaeli et al., 2007; Andreadou et al., 2009; Xi et al., 2012), evidence suggesting that antioxidant supplementation may modulate ANT-induced CTX in cancer patients is still scant (Fuchs-Tarlovsky, 2013).
Exercise has a positive impact on CV risk factors (e.g., hypertension, high cholesterol and lipids, overweight and diabetes; Kirkham and Davis, 2015) and it has been hypothesized that aerobic exercise can reduce ROS production and restore calcium cycling, thus improving myocardial energetics (Scott et al., 2011). There is some evidence that physical exercise can be beneficial to cancer patients (Stefani et al., 2015). Preliminary studies showed a role for aerobic exercise in combating ANT- (Schermuly et al., 2005) and trastuzumab-induced CTX (Haykowsky et al., 2009). Further studies will be necessary to assess the effects of exercise on CTX caused by anticancer agents (Scott et al., 2013).
Redox-Related Biomarkers of Cardiotoxicity
One of the main obstacles that renders difficult the prevention of several types of CTX is their complex pathogenesis and lack of reliable biomarkers. Biomarkers ideally should be simple to measure, widely available, low-cost, and used in other pathological conditions. Rather than using single biomarkers, the complexity of CTX is likely to be captured by the association of two or more biomarkers or by modern high-throughout “omics” platform (Chen et al., 2012). At the moment, troponins (Oztop et al., 2004; Suter and Ewer, 2013; Zamorano et al., 2016), brain natriuretic peptide (BNP) and its N-terminal fragment (NT-proBNP), mainly released from cardiomyocytes may be used as biomarkers of CTX in clinical practice (Cardinale et al., 2015; Novo et al., 2016b).
In the setting of cardiac toxicity induced by redox alterations from anticancer drugs, most ROS/RNS are very unstable, with half-lives of 10−6–10−9s. Also more long-lasting ROS, such as H2O2, have a half-life of less than a millisecond (Garcia-Garcia et al., 2012). Hence, it is still difficult to assess ROS/RNS generation due to limitations that affect their detection. Therefore, there is a need to identify alternative biomarkers of oxidative/nitrosative CTX. The metabolomic identification of acetate and succinate can be used as a redox-biomarker (Andreadou et al., 2009). Decrease in NAD(P)H:quinone oxidoreductase 1 activity and increased ROS production by NAD(P)H oxidases have been proposed as early biomarkers of LV dysfunction due to ANTs (Novo et al., 2016b). An increase of IL-6 and its soluble receptor (sIL-6R), has been correlated with an early alteration in systolic function in patients treated with EPI (Dessí et al., 2011, 2013). Other potential redox-related biomarkers are high-sensitivity C-reactive protein (CRP), heart-type fatty acid-binding protein (H-FABP), and glycogen phosphorylase BB (GPBB), while some miRNAs that could be used in the assessment of acute coronary syndromes (Novo et al., 2016b) may also be helpful in early detection of CTX (Horacek et al., 2010; Horie et al., 2010; Wang et al., 2013).
Conclusions and Perspectives
Novel anticancer drugs (e.g., targeted therapies and immune checkpoint inhibitors) have revolutioned the management of a wide spectrum of malignancies (Johnson et al., 2016; Menzies et al., 2017; Varricchi et al., 2017b). However, CTX caused by both conventional and novel antineoplastic drugs remains a critical issue (Tocchetti et al., 2013; Ghigo et al., 2016). Chemotherapeutics such as doxorubicin are the prototype of drugs causing CTX (Ghigo et al., 2016). Targeted therapies, initially thought to be safer, can also be responsible of some degree of CTX. Moreover, there is increasing evidence that immune checkpoint inhibitors (i.e., mAbs blocking CTLA-4, PD-1, and PD-L1 on immune cells) can also produce a spectrum of immune-related adverse events, including CTX (Varricchi et al., 2017c,d). Importantly, certain drugs used to prevent cardiovascular complications can even contribute to cancer induction (De Caterina, 2015). Several strategies have been proposed to prevent CTX from antineoplastic agents. None of these is completely safe and satisfactory. This is, at least in part, due to the complexity of different types of CTX. Moreover, it is important to note that heart dysfunction can also manifest years after cancer therapy, making it difficult to evaluate preventive and treatment strategies. It is important to understand the biochemical and molecular mechanisms by which anticancer agents affect cardiomyocytes and immune cells for implementing optimal drug design.
Although oxidative and nitrosative stress elicited by chemotherapeutic agents can harm the heart, indiscriminate elimination of ROS and RNS by antioxidant drugs may not provide beneficial effect, and may even impair physiological cellular functions (Aon et al., 2010; Cortassa et al., 2014; Nickel et al., 2014; Münzel et al., 2015; Arcaro et al., 2016). Indeed, anti-oxidants have been shown to fight LV remodeling and ameliorate contractility in many HF experimental models. Nevertheless, when translated to the clinical arena, these therapeutic approaches did not lead to much benefit or even worsened mortality (Kirk and Paolocci, 2014; Arcaro et al., 2016), when the antioxidant effect was not coupled to other pharmaceutical and biological properties (Fonarow, 2009). Importantly, the site of generation of ROS can determine their biological effects on cardiomyocytes. Hence, more specific, targeted, and “compartmentalized” antioxidant strategies that blunt local ROS/RNS production might be more successful than broad indiscriminate approaches.
In conclusion, although in the last decade research implicating ROS/RNS in antineoplastic drug-induced CTX has greatly advanced, experimental studies and clinical trials are needed to close several gaps in our knowledge of molecular and clinical aspects of CTX in order to balance safety and efficacy of cancer therapy.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The authors are supported by Regione Campania CISI-Lab, CRÈME Project and TIMING Project (GV and CT), the University of Cagliari (CC and GM), and MeccaSaric of the University of Turin (PasP).
Conflict of Interest Statement
CT received speaking fees from Alere.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Prof. Gianni Marone for his invaluable intellectual inputs, and the Italian Society of Cardiovascular Research and the Working Group on Drug Cardiotoxicity and Cardioprotection of the Italian Society of Cardiology for having made possible the meeting of scientists who participated in the drafting of this manuscript. The authors thank Dr. Gjada Criscuolo for critical reading of the manuscript and Fabrizio Fiorbianco for the elaboration of the figures.
References
Abd El-Aziz, M. A., Othman, A. I., Amer, M., and El-Missiry, M. A. (2001). Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J. Appl. Toxicol. 21, 469–473. doi: 10.1002/jat.782
Ades, F., Zardavas, D., Pinto, A. C., Criscitiello, C., Aftimos, P., and de Azambuja, E. (2014). Cardiotoxicity of systemic agents used in breast cancer. Breast 23, 317–328. doi: 10.1016/j.breast.2014.04.002
Albini, A., Pennesi, G., Donatelli, F., Cammarota, R., De Flora, S., and Noonan, D. M. (2010). Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J. Natl. Cancer Inst. 102, 14–25. doi: 10.1093/jnci/djp440
Alter, P., Herzum, M., Soufi, M., Schaefer, J. R., and Maisch, B. (2006). Cardiotoxicity of 5-fluorouracil. Cardiovasc. Hematol. Agents Med. Chem. 4, 1–5. doi: 10.2174/187152506775268785
Altieri, P., Murialdo, R., Barisione, C., Lazzarini, E., Garibaldi, S., Fabbi, P., et al. (2017). 5-fluorouracil causes endothelial cell senescence: potential protective role of glucagon-like peptide 1. Br. J. Pharmacol. 174, 3713–3726. doi: 10.1111/bph.13725
Andreadou, I., Papaefthimiou, M., Zira, A., Constantinou, M., Sigala, F., Skaltsounis, A. L., et al. (2009). Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 22, 585–592. doi: 10.1002/nbm.1370
Anisimov, A., Alitalo, A., Korpisalo, P., Soronen, J., Kaijalainen, S., Leppänen, V. M., et al. (2009). Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ. Res. 104, 1302–1312. doi: 10.1161/CIRCRESAHA.109.197830
Aon, M. A., Cortassa, S., and O'Rourke, B. (2010). Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta 1797, 865–877. doi: 10.1016/j.bbabio.2010.02.016
Aprile, G., Mazzer, M., Moroso, S., and Puglisi, F. (2009). Pharmacology and therapeutic efficacy of capecitabine: focus on breast and colorectal cancer. Anticancer. Drugs 20, 217–229. doi: 10.1097/CAD.0b013e3283293fd4
Arcaro, A., Pirozzi, F., Angelini, A., Chimenti, C., Crotti, L., Giordano, C., et al. (2016). Novel perspectives in redox biology and pathophysiology of failing myocytes: modulation of the intramyocardial redox milieu for therapeutic interventions-a review article from the working group of cardiac cell biology, italian society of cardiology. Oxid. Med. Cell. Longev. 2016:6353469. doi: 10.1155/2016/6353469
Armenian, S. H., Lacchetti, C., Barac, A., Carver, J., Constine, L. S., Denduluri, N., et al. (2017). Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J. Clin. Oncol. 35, 893–911. doi: 10.1200/JCO.2016.70.5400
Arnolda, L., McGrath, B., Cocks, M., Sumithran, E., and Johnston, C. (1985). Adriamycin cardiomyopathy in the rabbit: an animal model of low output cardiac failure with activation of vasoconstrictor mechanisms. Cardiovasc. Res. 19, 378–382. doi: 10.1093/cvr/19.6.378
Asanuma, H., Minamino, T., Sanada, S., Takashima, S., Ogita, H., Ogai, A., et al. (2004). Beta-adrenoceptor blocker carvedilol provides cardioprotection via an adenosine-dependent mechanism in ischemic canine hearts. Circulation 109, 2773–2779. doi: 10.1161/01.CIR.0000130917.12959.04
Baselga, J., Cortés, J., Kim, S. B., Im, S. A., Hegg, R., Im, Y. H., et al. (2012). Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366, 109–119. doi: 10.1056/NEJMoa1113216
Belmonte, F., Das, S., Sysa-Shah, P., Sivakumaran, V., Stanley, B., Guo, X., et al. (2015). ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 309, H1271–H1280. doi: 10.1152/ajpheart.00517.2014
Biswas, S. K. (2016). Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016:5698931. doi: 10.1155/2016/5698931
Boekhout, A. H., Gietema, J. A., Milojkovic Kerklaan, B., van Werkhoven, E. D., Altena, R., Honkoop, A., et al. (2016). Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2, 1030–1037. doi: 10.1001/jamaoncol.2016.1726
Bosch, X., Rovira, M., Sitges, M., Doménech, A., Ortiz-Pérez, J. T., de Caralt, T. M., et al. (2013). Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 61, 2355–2362. doi: 10.1016/j.jacc.2013.02.072
Boucek, R. J. Jr., Steele, A., Miracle, A., and Atkinson, J. (2003). Effects of angiotensin-converting enzyme inhibitor on delayed-onset doxorubicin-induced cardiotoxicity. Cardiovasc. Toxicol. 3, 319–329. doi: 10.1385/CT:3:4:319
Bowles, E. J., Wellman, R., Feigelson, H. S., Onitilo, A. A., Freedman, A. N., Delate, T., et al. (2012). Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J. Natl. Cancer Inst. 104, 1293–1305. doi: 10.1093/jnci/djs317
Brinda, B. J., Viganego, F., Vo, T., Dolan, D., and Fradley, M. G. (2016). Anti-VEGF-induced hypertension: a review of pathophysiology and treatment options. Curr. Treat. Options Cardiovasc. Med. 18, 33. doi: 10.1007/s11936-016-0452-z
Broeyer, F. J., Osanto, S., Suzuki, J., de Jongh, F., van Slooten, H., Tanis, B. C., et al. (2014). Evaluation of lecithinized human recombinant super oxide dismutase as cardioprotectant in anthracycline-treated breast cancer patients. Br. J. Clin. Pharmacol. 78, 950–960. doi: 10.1111/bcp.12429
Bronte, G., Bronte, E., Novo, G., Pernice, G., Lo Vullo, F., Musso, E., et al. (2015). Conquests and perspectives of cardio-oncology in the field of tumor angiogenesis-targeting tyrosine kinase inhibitor-based therapy. Expert Opin. Drug Saf. 14, 253–267. doi: 10.1517/14740338.2015.986092
Bry, M., Kivela, R., Holopainen, T., Anisimov, A., Tammela, T., Soronen, J., et al. (2010). Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation 122, 1725–1733. doi: 10.1161/CIRCULATIONAHA.110.957332
Cadeddu, C., Mercurio, V., Spallarossa, P., Nodari, S., Triggiani, M., Monte, I., et al. (2016). Preventing antiblastic drug-related cardiomyopathy: old and new therapeutic strategies. J. Cardiovasc. Med. (Hagerstown) 17(Suppl. 1), e64–e75. doi: 10.2459/JCM.0000000000000382
Cadeddu, C., Piras, A., Mantovani, G., Deidda, M., Dessì, M., Madeddu, C., et al. (2010). Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am. Heart J. 160, e481–e487. doi: 10.1016/j.ahj.2010.05.037
Cappetta, D., Esposito, G., Coppini, R., Piegari, E., Russo, R., Ciuffreda, L. P., et al. (2017). Effects of ranolazine in a model of doxorubicin-induced left ventricle diastolic dysfunction. Br. J. Pharmacol. 174, 3696–3712. doi: 10.1111/bph.13791
Cardinale, D., Colombo, A., Bacchiani, G., Tedeschi, I., Meroni, C. A., Veglia, F., et al. (2015). Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131, 1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777
Chen, R., Mias, G. I., Li-Pook-Than, J., Jiang, L., Lam, H. Y., Miriami, E., et al. (2012). Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 148, 1293–1307. doi: 10.1016/j.cell.2012.02.009
Cheng, H., and Force, T. (2010). Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ. Res. 106, 21–34. doi: 10.1161/CIRCRESAHA.109.206920
Chintalgattu, V., Rees, M. L., Culver, J. C., Goel, A., Jiffar, T., Zhang, J., et al. (2013). Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci. Transl. Med. 5:187ra169. doi: 10.1126/scitranslmed.3005066
Chotenimitkhun, R., D'Agostino, R. Jr., Lawrence, J. A., Hamilton, C. A., Jordan, J. H., Vasu, S., et al. (2015). Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can. J. Cardiol. 31, 302–307. doi: 10.1016/j.cjca.2014.11.020
Chu, T. F., Rupnick, M. A., Kerkela, R., Dallabrida, S. M., Zurakowski, D., Nguyen, L., et al. (2007). Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370, 2011–2019. doi: 10.1016/S0140-6736(07)61865-0
Cianci, G., Morelli, M. F., Cannita, K., Morese, R., Ricevuto, E., Di Rocco, Z. C., et al. (2003). Prophylactic options in patients with 5-fluorouracil-associated cardiotoxicity. Br. J. Cancer 88, 1507–1509. doi: 10.1038/sj.bjc.6600967
Coppini, R., Ferrantini, C., Yao, L., Fan, P., Del Lungo, M., Stillitano, F., et al. (2013). Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127, 575–584. doi: 10.1161/CIRCULATIONAHA.112.134932
Coppini, R., Mazzoni, L., Ferrantini, C., Gentile, F., Pioner, J. M., Laurino, A., et al. (2017). Ranolazine prevents phenotype development in a mouse model of hypertrophic cardiomyopathy. Circ. Heart Fail. 10. doi: 10.1161/CIRCHEARTFAILURE.116.003565
Cortassa, S., O'Rourke, B., and Aon, M. A. (2014). Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochim. Biophys. Acta 1837, 287–295. doi: 10.1016/j.bbabio.2013.11.007
Crone, S. A., Zhao, Y. Y., Fan, L., Gu, Y., Minamisawa, S., Liu, Y., et al. (2002). ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 8, 459–465. doi: 10.1038/nm0502-459
Curigliano, G., Cardinale, D., Suter, T., Plataniotis, G., de Azambuja, E., Sandri, M. T., et al. (2012). Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 23(Suppl. 7), vii155–vii166. doi: 10.1093/annonc/mds293
De Caterina, R. (2015). Cancer after intense and prolonged antiplatelet therapies–fact or fiction? Thromb. Haemost. 114, 1100–1103. doi: 10.1160/TH15-11-0842
De Keulenaer, G. W., Doggen, K., and Lemmens, K. (2010). The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ. Res. 106, 35–46. doi: 10.1161/CIRCRESAHA.109.205906
de Korte, M. A., de Vries, E. G., Lub-de Hooge, M. N., Jager, P. L., Gietema, J. A., van der Graaf, W. T., et al. (2007). 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: a clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur. J. Cancer 43, 2046–2051. doi: 10.1016/j.ejca.2007.06.024
Del Río, L. A. (2015). ROS and RNS in plant physiology: an overview. J. Exp. Bot. 66, 2827–2837. doi: 10.1093/jxb/erv099
de Nigris, F., Rienzo, M., Schiano, C., Fiorito, C., Casamassimi, A., and Napoli, C. (2008). Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur. J. Cancer 44, 334–340. doi: 10.1016/j.ejca.2007.12.010
Dessí, M., Madeddu, C., Piras, A., Cadeddu, C., Antoni, G., Mercuro, G., et al. (2013). Long-term, up to 18 months, protective effects of the angiotensin II receptor blocker telmisartan on Epirubin-induced inflammation and oxidative stress assessed by serial strain rate. Springerplus 2:198. doi: 10.1186/2193-1801-2-198
Dessí, M., Piras, A., Madeddu, C., Cadeddu, C., Deidda, M., Massa, E., et al. (2011). Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction. Exp. Ther. Med. 2, 1003–1009. doi: 10.3892/etm.2011.305
Doggen, K., Ray, L., Mathieu, M., Mc Entee, K., Lemmens, K., and De Keulenaer, G. W. (2009). Ventricular ErbB2/ErbB4 activation and downstream signaling in pacing-induced heart failure. J. Mol. Cell. Cardiol. 46, 33–38. doi: 10.1016/j.yjmcc.2008.10.010
Dragoni, S., Laforenza, U., Bonetti, E., Lodola, F., Bottino, C., Berra-Romani, R., et al. (2011). Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells. 29, 1898–1907. doi: 10.1002/stem.734
D'Uva, G., Aharonov, A., Lauriola, M., Kain, D., Yahalom-Ronen, Y., Carvalho, S., et al. (2015). ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638. doi: 10.1038/ncb3149
Egea, J., Fabregat, I., Frapart, Y. M., Ghezzi, P., Görlach, A., Kietzmann, T., et al. (2017). European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 13, 94–162. doi: 10.1016/j.redox.2017.05.007
Eschenhagen, T., Force, T., Ewer, M. S., de Keulenaer, G. W., Suter, T. M., Anker, S. D., et al. (2011). Cardiovascular side effects of cancer therapies: a position statement from the heart failure association of the european society of cardiology. Eur. J. Heart Fail. 13, 1–10. doi: 10.1093/eurjhf/hfq213
Ewer, M. S., and Ewer, S. M. (2010). Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction. J. Clin. Oncol. 28, 3901–3904. doi: 10.1200/JCO.2010.30.6274
Ewer, M. S., and Lenihan, D. J. (2008). Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J. Clin. Oncol. 26, 1201–1203. doi: 10.1200/JCO.2007.14.8742
Ewer, M. S., and Lippman, S. M. (2005). Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J. Clin. Oncol. 23, 2900–2902. doi: 10.1200/JCO.2005.05.827
Ewer, M. S., Vooletich, M. T., Durand, J. B., Woods, M. L., Davis, J. R., Valero, V., et al. (2005). Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J. Clin. Oncol. 23, 7820–7826. doi: 10.1200/JCO.2005.13.300
Ferroni, P., Della-Morte, D., Palmirotta, R., McClendon, M., Testa, G., Abete, P., et al. (2011). Platinum-based compounds and risk for cardiovascular toxicity in the elderly: role of the antioxidants in chemoprevention. Rejuvenat. Res. 14, 293–308. doi: 10.1089/rej.2010.1141
Fisher, P. W., Salloum, F., Das, A., Hyder, H., and Kukreja, R. C. (2005). Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111, 1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2
Fogli, S., Nieri, P., and Breschi, M. C. (2004). The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. FASEB J. 18, 664–675. doi: 10.1096/fj.03-0724rev
Folkman, J. (2007). Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 6, 273–286. doi: 10.1038/nrd2115
Fonarow, G. C. (2009). Role of carvedilol controlled-release in cardiovascular disease. Expert Rev. Cardiovasc. Ther. 7, 483–498. doi: 10.1586/erc.09.15
Force, T., Krause, D. S., and Van Etten, R. A. (2007). Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer 7, 332–344. doi: 10.1038/nrc2106
Fu, M., Matoba, M., Liang, Q. M., Sjögren, K. G., and Hjalmarson, A. (1994). Properties of G-protein modulated receptor-adenylyl cyclase system in myocardium of spontaneously hypertensive rats treated with adriamycin. Int. J. Cardiol. 44, 9–18. doi: 10.1016/0167-5273(94)90061-2
Fuchs-Tarlovsky, V. (2013). Role of antioxidants in cancer therapy. Nutrition 29, 15–21. doi: 10.1016/j.nut.2012.02.014
Gabrielson, K., Bedja, D., Pin, S., Tsao, A., Gama, L., Yuan, B., et al. (2007). Heat shock protein 90 and ErbB2 in the cardiac response to doxorubicin injury. Cancer Res. 67, 1436–1441. doi: 10.1158/0008-5472.CAN-06-3721
Galdiero, M. R., Varricchi, G., and Marone, G. (2016). The immune network in thyroid cancer. Oncoimmunology 5:e1168556. doi: 10.1080/2162402X.2016.1168556
Galindo, C. L., Kasasbeh, E., Murphy, A., Ryzhov, S., Lenihan, S., Ahmad, F. A., et al. (2014a). Anti-remodeling and anti-fibrotic effects of the neuregulin-1β glial growth factor 2 in a large animal model of heart failure. J. Am. Heart Assoc. 3:e000773. doi: 10.1161/JAHA.113.000773
Galindo, C. L., Ryzhov, S., and Sawyer, D. B. (2014b). Neuregulin as a heart failure therapy and mediator of reverse remodeling. Curr. Heart Fail. Rep. 11, 40–49. doi: 10.1007/s11897-013-0176-2
Gammella, E., Maccarinelli, F., Buratti, P., Recalcati, S., and Cairo, G. (2014). The role of iron in anthracycline cardiotoxicity. Front. Pharmacol. 5:25. doi: 10.3389/fphar.2014.00025
Gao, R., Zhang, J., Cheng, L., Wu, X., Dong, W., Yang, X., et al. (2010). A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 55, 1907–1914. doi: 10.1016/j.jacc.2009.12.044
Garcia-Garcia, A., Rodriguez-Rocha, H., Madayiputhiya, N., Pappa, A., Panayiotidis, M. I., and Franco, R. (2012). Biomarkers of protein oxidation in human disease. Curr. Mol. Med. 12, 681–697. doi: 10.2174/156652412800792543
Ghigo, A., Li, M., and Hirsch, E. (2016). New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim. Biophys. Acta 1863, 1916–1925. doi: 10.1016/j.bbamcr.2016.01.021
Gianni, L., Pienkowski, T., Im, Y. H., Roman, L., Tseng, L. M., Liu, M. C., et al. (2012). Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32. doi: 10.1016/S1470-2045(11)70336-9
Gonzalvez, F., and Gottlieb, E. (2007). Cardiolipin: setting the beat of apoptosis. Apoptosis 12, 877–885. doi: 10.1007/s10495-007-0718-8
Goormaghtigh, E., Huart, P., Praet, M., Brasseur, R., and Ruysschaert, J. M. (1990). Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys. Chem. 35, 247–257. doi: 10.1016/0301-4622(90)80012-V
Gordon, L. I., Burke, M. A., Singh, A. T., Prachand, S., Lieberman, E. D., Sun, L., et al. (2009). Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 284, 2080–2087. doi: 10.1074/jbc.M804570200
Goss, G. D., Arnold, A., Shepherd, F. A., Dediu, M., Ciuleanu, T. E., Fenton, D., et al. (2010). Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J. Clin. Oncol. 28, 49–55. doi: 10.1200/JCO.2009.22.9427
Gressett, S. M., and Shah, S. R. (2009). Intricacies of bevacizumab-induced toxicities and their management. Ann. Pharmacother. 43, 490–501. doi: 10.1345/aph.1L426
Gulati, G., Heck, S. L., Ree, A. H., Hoffmann, P., Schulz-Menger, J., Fagerland, M. W., et al. (2016). Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 37, 1671–1680. doi: 10.1093/eurheartj/ehw022
Gupta, A., Akki, A., Wang, Y., Leppo, M. K., Chacko, V. P., Foster, D. B., et al. (2012). Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J. Clin. Invest. 122, 291–302. doi: 10.1172/JCI57426
Gupta, A., Rohlfsen, C., Leppo, M. K., Chacko, V. P., Wang, Y., Steenbergen, C., et al. (2013). Creatine kinase-overexpression improves myocardial energetics, contractile dysfunction and survival in murine doxorubicin cardiotoxicity. PLoS ONE 8:e74675. doi: 10.1371/journal.pone.0074675
Hahn, V. S., Lenihan, D. J., and Ky, B. (2014). Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J. Am. Heart Assoc. 3:e000665. doi: 10.1161/JAHA.113.000665
Hasinoff, B. B., and Patel, D. (2010). The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol. Appl. Pharmacol. 249, 132–139. doi: 10.1016/j.taap.2010.08.026
Haykowsky, M. J., Mackey, J. R., Thompson, R. B., Jones, L. W., and Paterson, D. I. (2009). Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin. Cancer Res. 15, 4963–4967. doi: 10.1158/1078-0432.CCR-09-0628
Henninger, C., Huelsenbeck, S., Wenzel, P., Brand, M., Huelsenbeck, J., Schad, A., et al. (2015). Chronic heart damage following doxorubicin treatment is alleviated by lovastatin. Pharmacol. Res. 91, 47–56. doi: 10.1016/j.phrs.2014.11.003
Horacek, J. M., Vasatova, M., Tichy, M., Pudil, R., Jebavy, L., and Maly, J. (2010). The use of cardiac biomarkers in detection of cardiotoxicity associated with conventional and high-dose chemotherapy for acute leukemia. Exp. Oncol. 32, 97–99.
Horie, T., Ono, K., Nishi, H., Nagao, K., Kinoshita, M., Watanabe, S., et al. (2010). Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 87, 656–664. doi: 10.1093/cvr/cvq148
Huang, C., Zhang, X., Ramil, J. M., Rikka, S., Kim, L., Lee, Y., et al. (2010). Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 121, 675–683. doi: 10.1161/CIRCULATIONAHA.109.902221
Huelsenbeck, J., Henninger, C., Schad, A., Lackner, K. J., Kaina, B., and Fritz, G. (2011). Inhibition of Rac1 signaling by lovastatin protects against anthracycline-induced cardiac toxicity. Cell Death Dis. 2:e190. doi: 10.1038/cddis.2011.65
Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342. doi: 10.1056/NEJMoa032691
Hutson, T. E., Lesovoy, V., Al-Shukri, S., Stus, V. P., Lipatov, O. N., Bair, A. H., et al. (2013). Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 14, 1287–1294. doi: 10.1016/S1470-2045(13)70465-0
Ichikawa, Y., Ghanefar, M., Bayeva, M., Wu, R., Khechaduri, A., Naga Prasad, S. V., et al. (2014). Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Invest. 124, 617–630. doi: 10.1172/JCI72931
Iqbal, M., Dubey, K., Anwer, T., Ashish, A., and Pillai, K. K. (2008). Protective effects of telmisartan against acute doxorubicin-induced cardiotoxicity in rats. Pharmacol. Rep. 60, 382–390.
Jabbour, A., Hayward, C. S., Keogh, A. M., Kotlyar, E., McCrohon, J. A., England, J. F., et al. (2011). Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur. J. Heart Fail. 13, 83–92. doi: 10.1093/eurjhf/hfq152
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., and Forman, D. (2011). Global cancer statistics. CA Cancer J. Clin. 61, 69–90. doi: 10.3322/caac.20107
Jensen, S. A., and Sorensen, J. B. (2006). Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother. Pharmacol. 58, 487–493. doi: 10.1007/s00280-005-0178-1
Jensen, S. A., and Sørensen, J. B. (2012). 5-fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer Chemother. Pharmacol. 69, 57–64. doi: 10.1007/s00280-011-1669-x
Jin, Z., Zhang, J., Zhi, H., Hong, B., Zhang, S., Guo, H., et al. (2013). Beneficial effects of tadalafil on left ventricular dysfunction in doxorubicin-induced cardiomyopathy. J. Cardiol. 62, 110–116. doi: 10.1016/j.jjcc.2013.03.018
Johnson, D. B., Balko, J. M., Compton, M. L., Chalkias, S., Gorham, J., Xu, Y., et al. (2016). Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 375, 1749–1755. doi: 10.1056/NEJMoa1609214
Kalam, K., and Marwick, T. H. (2013). Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur. J. Cancer 49, 2900–2909. doi: 10.1016/j.ejca.2013.04.030
Kalay, N., Basar, E., Ozdogru, I., Er, O., Cetinkaya, Y., Dogan, A., et al. (2006). Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 48, 2258–2262. doi: 10.1016/j.jacc.2006.07.052
Kaya, M. G., Ozkan, M., Gunebakmaz, O., Akkaya, H., Kaya, E. G., Akpek, M., et al. (2013). Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int. J. Cardiol. 167, 2306–2310. doi: 10.1016/j.ijcard.2012.06.023
Kerkela, R., Woulfe, K. C., Durand, J. B., Vagnozzi, R., Kramer, D., Chu, T. F., et al. (2009). Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2, 15–25. doi: 10.1111/j.1752-8062.2008.00090.x
Khakoo, A. Y., Kassiotis, C. M., Tannir, N., Plana, J. C., Halushka, M., Bickford, C., et al. (2008). Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 112, 2500–2508. doi: 10.1002/cncr.23460
Khan, M. F., Gottesman, S., Boyella, R., and Juneman, E. (2014). Gemcitabine-induced cardiomyopathy: a case report and review of the literature. J. Med. Case Rep. 8:220. doi: 10.1186/1752-1947-8-220
Kirk, J. A., and Paolocci, N. (2014). New redox-related arrows in the arsenal of cardiac disease treatment. Antioxid. Redox Signal. 21, 1945–1948. doi: 10.1089/ars.2014.6124
Kirkham, A. A., and Davis, M. K. (2015). Exercise prevention of Cardiovascular disease in breast cancer survivors. J. Oncol. 2015:917606. doi: 10.1155/2015/917606
Kivelä, R., Bry, M., Robciuc, M. R., Räsänen, M., Taavitsainen, M., Silvola, J. M., et al. (2014). VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Mol. Med. 6, 307–321. doi: 10.1002/emmm.201303147
Koca, D., Salman, T., Unek, I. T., Oztop, I., Ellidokuz, H., Eren, M., et al. (2011). Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy 57, 381–387. doi: 10.1159/000331645
Kohlhaas, M., Liu, T., Knopp, A., Zeller, T., Ong, M. F., Böhm, M., et al. (2010). Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 121, 1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911
Koka, S., Das, A., Zhu, S. G., Durrant, D., Xi, L., and Kukreja, R. C. (2010). Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J. Pharmacol. Exp. Ther. 334, 1023–1030. doi: 10.1124/jpet.110.170191
Kosmas, C., Kallistratos, M. S., Kopterides, P., Syrios, J., Skopelitis, H., Mylonakis, N., et al. (2008). Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J. Cancer Res. Clin. Oncol. 134, 75–82. doi: 10.1007/s00432-007-0250-9
Ku, D. D., Zaleski, J. K., Liu, S., and Brock, T. A. (1993). Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am. J. Physiol. 265, H586–H592. doi: 10.1152/ajpheart.1993.265.2.H586
Ky, B., Vejpongsa, P., Yeh, E. T., Force, T., and Moslehi, J. J. (2013). Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ. Res. 113, 754–764. doi: 10.1161/CIRCRESAHA.113.300218
Lala, P. K., and Chakraborty, C. (2001). Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2, 149–156. doi: 10.1016/S1470-2045(00)00256-4
Lamberti, M., Porto, S., Zappavigna, S., Addeo, E., Marra, M., Miraglia, N., et al. (2014). A mechanistic study on the cardiotoxicity of 5-fluorouracil in vitro and clinical and occupational perspectives. Toxicol. Lett. 227, 151–156. doi: 10.1016/j.toxlet.2014.03.018
Lassègue, B., and Griendling, K. K. (2010). NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 30, 653–661. doi: 10.1161/ATVBAHA.108.181610
Lemmens, K., Fransen, P., Sys, S. U., Brutsaert, D. L., and De Keulenaer, G. W. (2004). Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation 109, 324–326. doi: 10.1161/01.CIR.0000114521.88547.5E
Lencová-Popelová, O., Jirkovský, E., Jansová, H., Jirkovská-Vavrová, A., Vostatková-Tichotová, L., Mazurová, Y., et al. (2016). Cardioprotective effects of inorganic nitrate/nitrite in chronic anthracycline cardiotoxicity: comparison with dexrazoxane. J. Mol. Cell. Cardiol. 91, 92–103. doi: 10.1016/j.yjmcc.2015.12.021
Lestuzzi, C., Tartuferi, L., and Corona, G. (2011). Capecitabine (and 5 fluorouracil) cardiotoxicity. Metabolic considerations. Breast J. 17, 564–565. doi: 10.1111/j.1524-4741.2011.01120.x
Lestuzzi, C., Vaccher, E., Talamini, R., Lleshi, A., Meneguzzo, N., Viel, E., et al. (2014). Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann. Oncol. 25, 1059–1064. doi: 10.1093/annonc/mdu055
Lévy, B. I. (2006). Microvascular plasticity and experimental heart failure. Hypertension 47, 827–829. doi: 10.1161/01.HYP.0000215283.53943.39
Li, B., Zheng, Z., Wei, Y., Wang, M., Peng, J., Kang, T., et al. (2011). Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc. Diabetol. 10:69. doi: 10.1186/1475-2840-10-69
Li, T., and Singal, P. K. (2000). Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation 102, 2105–2110. doi: 10.1161/01.CIR.102.17.2105
Lim, S. L., Lam, C. S., Segers, V. F., Brutsaert, D. L., and De Keulenaer, G. W. (2015). Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur. Heart J. 36, 2050–2060. doi: 10.1093/eurheartj/ehv132
Lipshultz, S. E., Miller, T. L., Lipsitz, S. R., Neuberg, D. S., Dahlberg, S. E., Colan, S. D., et al. (2012). Continuous versus bolus infusion of doxorubicin in children with all: long-term cardiac outcomes. Pediatrics 130, 1003–1011. doi: 10.1542/peds.2012-0727
Liu, X., Gu, X., Li, Z., Li, X., Li, H., Chang, J., et al. (2006). Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J. Am. Coll. Cardiol. 48, 1438–1447. doi: 10.1016/j.jacc.2006.05.057
Loffredo, S., Bova, M., Suffritti, C., Borriello, F., Zanichelli, A., Petraroli, A., et al. (2016). Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy 71, 989–996. doi: 10.1111/all.12862
Lyu, Y. L., Kerrigan, J. E., Lin, C. P., Azarova, A. M., Tsai, Y. C., Ban, Y., et al. (2007). Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 67, 8839–8846. doi: 10.1158/0008-5472.CAN-07-1649
Ma, Y., Zhang, X., Bao, H., Mi, S., Cai, W., Yan, H., et al. (2012). Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin induced cardiomyopathy in mice. PLoS ONE 7:e40763. doi: 10.1371/journal.pone.0040763
Madonna, R., Cadeddu, C., Deidda, M., Giricz, Z., Madeddu, C., Mele, D., et al. (2015a). Cardioprotection by gene therapy: a review paper on behalf of the working group on drug cardiotoxicity and cardioprotection of the Italian Society of Cardiology. Int. J. Cardiol. 191, 203–210. doi: 10.1016/j.ijcard.2015.04.232
Madonna, R., Cadeddu, C., Deidda, M., Mele, D., Monte, I., Novo, G., et al. (2015b). Improving the preclinical models for the study of chemotherapy-induced cardiotoxicity: a Position Paper of the Italian Working Group on Drug Cardiotoxicity and Cardioprotection. Heart Fail. Rev. 20, 621–631. doi: 10.1007/s10741-015-9497-4
Maitland, M. L., Bakris, G. L., Black, H. R., Chen, H. X., Durand, J. B., Elliott, W. J., et al. (2010). Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J. Natl. Cancer Inst. 102, 596–604. doi: 10.1093/jnci/djq091
Marone, G., and Granata, F. (2014). Angiogenesis, lymphangiogenesis and clinical implications. Preface. Chem. Immunol. Allergy 99, XI–XII. doi: 10.1159/000352074
Martin, E., Thougaard, A. V., Grauslund, M., Jensen, P. B., Bjorkling, F., Hasinoff, B. B., et al. (2009). Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicology 255, 72–79. doi: 10.1016/j.tox.2008.10.011
Maslov, M. Y., Chacko, V. P., Hirsch, G. A., Akki, A., Leppo, M. K., Steenbergen, C., et al. (2010). Reduced in vivo high-energy phosphates precede adriamycin-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 299, H332–337. doi: 10.1152/ajpheart.00727.2009
Matsui, H., Morishima, I., Numaguchi, Y., Toki, Y., Okumura, K., and Hayakawa, T. (1999). Protective effects of carvedilol against doxorubicin-induced cardiomyopathy in rats. Life Sci. 65, 1265–1274. doi: 10.1016/S0024-3205(99)00362-8
Mele, D., Nardozza, M., Spallarossa, P., Frassoldati, A., Tocchetti, C. G., Cadeddu, C., et al. (2016a). Current views on anthracycline cardiotoxicity. Heart Fail. Rev. 21, 621–634. doi: 10.1007/s10741-016-9564-5
Mele, D., Tocchetti, C. G., Pagliaro, P., Madonna, R., Novo, G., Pepe, A., et al. (2016b). Pathophysiology of anthracycline cardiotoxicity. J Cardiovasc Med (Hagerstown). 17(Suppl. 1), e3–e11. doi: 10.2459/JCM.0000000000000378
Menna, P., Paz, O. G., Chello, M., Covino, E., Salvatorelli, E., and Minotti, G. (2012). Anthracycline cardiotoxicity. Expert Opin. Drug Saf. 11(Suppl. 1), S21–S36. doi: 10.1517/14740338.2011.589834
Menna, P., Salvatorelli, E., and Minotti, G. (2008). Cardiotoxicity of antitumor drugs. Chem. Res. Toxicol. 21, 978–989. doi: 10.1021/tx800002r
Menzies, A. M., Johnson, D. B., Ramanujam, S., Atkinson, V. G., Wong, A. N. M., Park, J. J., et al. (2017). Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28, 368–376. doi: 10.1093/annonc/mdw443
Mercurio, V., Pirozzi, F., Lazzarini, E., Marone, G., Rizzo, P., Agnetti, G., et al. (2016). Models of heart failure based on the cardiotoxicity of anticancer drugs. J. Card. Fail. 22, 449–458. doi: 10.1016/j.cardfail.2016.04.008
Mikhed, Y., Görlach, A., Knaus, U. G., and Daiber, A. (2015). Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. 5, 275–289. doi: 10.1016/j.redox.2015.05.008
Miller, K. D., Chap, L. I., Holmes, F. A., Cobleigh, M. A., Marcom, P. K., Fehrenbacher, L., et al. (2005). Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 23, 792–799. doi: 10.1200/JCO.2005.05.098
Minotti, G. (2013). Pharmacology at work for cardio-oncology: ranolazine to treat early cardiotoxicity induced by antitumor drugs. J. Pharmacol. Exp. Ther. 346, 343–349. doi: 10.1124/jpet.113.204057
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., and Gianni, L. (2004). Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56, 185–229. doi: 10.1124/pr.56.2.6
Minotti, G., Salvatorelli, E., and Menna, P. (2010). Pharmacological foundations of cardio-oncology. J. Pharmacol. Exp. Ther. 334, 2–8. doi: 10.1124/jpet.110.165860
Miura, K., Kinouchi, M., Ishida, K., Fujibuchi, W., Naitoh, T., Ogawa, H., et al. (2010). 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers 2, 1717–1730. doi: 10.3390/cancers2031717
Molinaro, M., Ameri, P., Marone, G., Petretta, M., Abete, P., Di Lisa, F., et al. (2015). Recent advances on pathophysiology, diagnostic and therapeutic insights in cardiac dysfunction induced by antineoplastic drugs. Biomed Res. Int. 2015:138148. doi: 10.1155/2015/138148
Moloney, J. N., and Cotter, T. G. (2017). ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. doi: 10.1016/j.semcdb.2017.05.023. [Epub ahead of print].
Moon, J. S., Nakahira, K., Chung, K. P., DeNicola, G. M., Koo, M. J., Pabón, M. A., et al. (2016). NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 22, 1002–1012. doi: 10.1038/nm.4153
Moslehi, J. J., and Deininger, M. (2015). Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J. Clin. Oncol. 33, 4210–4218. doi: 10.1200/JCO.2015.62.4718
Motzer, R. J., Hutson, T. E., Cella, D., Reeves, J., Hawkins, R., Guo, J., et al. (2013). Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 369, 722–731. doi: 10.1056/NEJMoa1303989
Motzer, R. J., Hutson, T. E., Tomczak, P., Michaelson, M. D., Bukowski, R. M., Rixe, O., et al. (2007). Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124. doi: 10.1056/NEJMoa065044
Mourad, J. J., and Levy, B. I. (2011). Mechanisms of antiangiogenic-induced arterial hypertension. Curr. Hypertens. Rep. 13, 289–293. doi: 10.1007/s11906-011-0206-y
Münzel, T., Gori, T., Keaney, J. F. Jr., Maack, C., and Daiber, A. (2015). Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 36, 2555–2564. doi: 10.1093/eurheartj/ehv305
Nakamae, H., Tsumura, K., Terada, Y., Nakane, T., Nakamae, M., Ohta, K., et al. (2005). Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 104, 2492–2498. doi: 10.1002/cncr.21478
Nakamura, K., Kusano, K., Nakamura, Y., Kakishita, M., Ohta, K., Nagase, S., et al. (2002). Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation 105, 2867–2871. doi: 10.1161/01.CIR.0000018605.14470.DD
Nazer, B., Humphreys, B. D., and Moslehi, J. (2011). Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation 124, 1687–1691. doi: 10.1161/CIRCULATIONAHA.110.992230
Nediani, C., Raimondi, L., Borchi, E., and Cerbai, E. (2011). Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antioxid. Redox Signal. 14, 289–331. doi: 10.1089/ars.2010.3198
Ng, M., Cunningham, D., and Norman, A. R. (2005). The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur. J. Cance. 41, 1542–1546. doi: 10.1016/j.ejca.2005.03.027
Nickel, A., Kohlhaas, M., and Maack, C. (2014). Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 73, 26–33. doi: 10.1016/j.yjmcc.2014.03.011
Niu, Y., DesMarais, T. L., Tong, Z., Yao, Y., and Costa, M. (2015). Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 82, 22–28. doi: 10.1016/j.freeradbiomed.2015.01.028
Novo, G., Cadeddu, C., Sucato, V., Pagliaro, P., Romano, S., Tocchetti, C. G., et al. (2016b). Role of biomarkers in monitoring antiblastic cardiotoxicity. J. Cardiovasc. Med. (Hagerstown). 17(Suppl. 1), S27–S34. doi: 10.2459/JCM.0000000000000379
Odiete, O., Hill, M. F., and Sawyer, D. B. (2012). Neuregulin in cardiovascular development and disease. Circ. Res. 111, 1376–1385. doi: 10.1161/CIRCRESAHA.112.267286
Oeffinger, K. C., Mertens, A. C., Sklar, C. A., Kawashima, T., Hudson, M. M., Meadows, A. T., et al. (2006). Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582. doi: 10.1056/NEJMsa060185
Oka, T., Akazawa, H., Naito, A. T., and Komuro, I. (2014). Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ. Res. 114, 565–571. doi: 10.1161/CIRCRESAHA.114.300507
Oliveira, M. S., Melo, M. B., Carvalho, J. L., Melo, I. M., Lavor, M. S., Gomes, D. A., et al. (2013). Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. J. Cancer Sci. Ther. 5, 52–57. doi: 10.4172/1948-5956.1000184
Ozcelik, C., Erdmann, B., Pilz, B., Wettschureck, N., Britsch, S., Hübner, N., et al. (2002). Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 99, 8880–8885. doi: 10.1073/pnas.122249299
Oztop, I., Gencer, M., Okan, T., Yaren, A., Altekin, E., Turker, S., et al. (2004). Evaluation of cardiotoxicity of a combined bolus plus infusional 5-fluorouracil/folinic acid treatment by echocardiography, plasma troponin I level, QT interval and dispersion in patients with gastrointestinal system cancers. Jpn. J. Clin. Oncol. 34, 262–268. doi: 10.1093/jjco/hyh047
Pagliaro, P., Moro, F., Tullio, F., Perrelli, M. G., and Penna, C. (2011). Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal. 14, 833–850. doi: 10.1089/ars.2010.3245
Pagliaro, P., and Penna, C. (2015). Redox signalling and cardioprotection: translatability and mechanism. Br. J. Pharmacol. 172, 1974–1995. doi: 10.1111/bph.12975
Papapetropoulos, A., Foresti, R., and Ferdinandy, P. (2015). Pharmacology of the 'gasotransmitters' NO, CO and H2S: translational opportunities. Br. J. Pharmacol. 172, 1395–1396. doi: 10.1111/bph.13005
Penna, C., Angotti, C., and Pagliaro, P. (2014). Protein S-nitrosylation in preconditioning and postconditioning. Exp. Biol. Med. (Maywood) 239, 647–662. doi: 10.1177/1535370214522935
Pereira, G. C., Pereira, S. P., Tavares, L. C., Carvalho, F. S., Magalhães-Novais, S., Barbosa, I. A., et al. (2016). Cardiac cytochrome c and cardiolipin depletion during anthracycline-induced chronic depression of mitochondrial function. Mitochondrion 30, 95–104. doi: 10.1016/j.mito.2016.07.005
Petrelli, F., Barni, S., Bertocchi, P., and Zaniboni, A. (2016). TAS-102, the first “cardio-gentle” fluoropyrimidine in the colorectal cancer landscape? BMC Cancer 16:386. doi: 10.1186/s12885-016-2409-8
Pituskin, E., Mackey, J. R., Koshman, S., Jassal, D., Pitz, M., Haykowsky, M. J., et al. (2017). Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J. Clin. Oncol. 35, 870–877. doi: 10.1200/JCO.2016.68.7830
Pointon, A. V., Walker, T. M., Phillips, K. M., Luo, J., Riley, J., Zhang, S. D., et al. (2010). Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE 5:e12733. doi: 10.1371/journal.pone.0012733
Polk, A., Vaage-Nilsen, M., Vistisen, K., and Nielsen, D. L. (2013). Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 39, 974–984. doi: 10.1016/j.ctrv.2013.03.005
Polk, A., Vistisen, K., Vaage-Nilsen, M., and Nielsen, D. L. (2014). A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 15:47. doi: 10.1186/2050-6511-15-47
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G., Coats, A. J., et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975. doi: 10.1002/ejhf.592
Rainer, P. P., Doleschal, B., Kirk, J. A., Sivakumaran, V., Saad, Z., Groschner, K., et al. (2012). Sunitinib causes dose-dependent negative functional effects on myocardium and cardiomyocytes. BJU Int. 110, 1455–1462. doi: 10.1111/j.1464-410X.2012.11134.x
Räsänen, M., Degerman, J., Nissinen, T. A., Miinalainen, I., Kerkelä, R., Siltanen, A., et al. (2016). VEGF-B gene therapy inhibits doxorubicin-induced cardiotoxicity by endothelial protection. Proc. Natl. Acad. Sci. U.S.A. 113, 13144–13149. doi: 10.1073/pnas.1616168113
Rastogi, S., Sharov, V. G., Mishra, S., Gupta, R. C., Blackburn, B., Belardinelli, L., et al. (2008). Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am. J. Physiol. Heart Circ. Physiol. 295, H2149–2155. doi: 10.1152/ajpheart.00728.2008
Reck, M., Kaiser, R., Mellemgaard, A., Douillard, J. Y., Orlov, S., Krzakowski, M., et al. (2014). Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 15, 143–155. doi: 10.1016/S1470-2045(13)70586-2
Rephaeli, A., Waks-Yona, S., Nudelman, A., Tarasenko, I., Tarasenko, N., Phillips, D. R., et al. (2007). Anticancer prodrugs of butyric acid and formaldehyde protect against doxorubicin-induced cardiotoxicity. Br. J. Cancer. 96, 1667–1674. doi: 10.1038/sj.bjc.6603781
Riad, A., Bien, S., Westermann, D., Becher, P. M., Loya, K., Landmesser, U., et al. (2009). Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 69, 695–699. doi: 10.1158/0008-5472.CAN-08-3076
Rohrbach, S., Yan, X., Weinberg, E. O., Hasan, F., Bartunek, J., Marchionni, M. A., et al. (1999). Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation 100, 407–412.
Russell, E. G., and Cotter, T. G. (2015). New insight into the role of Reactive Oxygen Species (ROS) in cellular signal-transduction processes. Int. Rev. Cell Mol. Biol. 319, 221–254. doi: 10.1016/bs.ircmb.2015.07.004
Sabbah, H. N., Chandler, M. P., Mishima, T., Suzuki, G., Chaudhry, P., Nass, O., et al. (2002). Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J. Card. Fail. 8, 416–422. doi: 10.1054/jcaf.2002.129232
Saitoh, S., Kiyooka, T., Rocic, P., Rogers, P. A., Zhang, C., Swafford, A., et al. (2007). Redox-dependent coronary metabolic dilation. Am. J. Physiol. Heart Circ. Physiol. 293, H3720–H3725. doi: 10.1152/ajpheart.00436.2007
Salvatorelli, E., Menna, P., and Minotti, G. (2015). Managing anthracycline-induced cardiotoxicity: beginning with the end in mind. Future Cardiol. 11, 363–366. doi: 10.2217/FCA.15.35
Sandler, A., Gray, R., Perry, M. C., Brahmer, J., Schiller, J. H., Dowlati, A., et al. (2006). Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–2550. doi: 10.1056/NEJMoa061884
Santos, D. L., Moreno, A. J., Leino, R. L., Froberg, M. K., and Wallace, K. B. (2002). Carvedilol protects against doxorubicin-induced mitochondrial cardiomyopathy. Toxicol. Appl. Pharmacol. 185, 218–227. doi: 10.1006/taap.2002.9532
Sawyer, D. B. (2013). Anthracyclines and heart failure. N. Engl. J. Med. 368, 1154–1156. doi: 10.1056/NEJMcibr1214975
Schermuly, R. T., Dony, E., Ghofrani, H. A., Pullamsetti, S., Savai, R., Roth, M., et al. (2005). Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 115, 2811–2821. doi: 10.1172/JCI24838
Scott, J. M., Khakoo, A., Mackey, J. R., Haykowsky, M. J., Douglas, P. S., and Jones, L. W. (2011). Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation 124, 642–650. doi: 10.1161/CIRCULATIONAHA.111.021774
Scott, J. M., Koelwyn, G. J., Hornsby, W. E., Khouri, M., Peppercorn, J., Douglas, P. S., et al. (2013). Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Semin. Oncol. 40, 218–228. doi: 10.1053/j.seminoncol.2013.01.001
Seicean, S., Seicean, A., Alan, N., Plana, J. C., Budd, G. T., and Marwick, T. H. (2013). Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ. Heart Fail. 6, 420–426. doi: 10.1161/CIRCHEARTFAILURE.112.000055
Seicean, S., Seicean, A., Plana, J. C., Budd, G. T., and Marwick, T. H. (2012). Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J. Am. Coll. Cardiol. 60, 2384–2390. doi: 10.1016/j.jacc.2012.07.067
Seymour, L., Bramwell, V., and Moran, L. A. (1999). Use of dexrazoxane as a cardioprotectant in patients receiving doxorubicin or epirubicin chemotherapy for the treatment of cancer. The Provincial Systemic Treatment Disease Site Group. Cancer Prev. Control. 3, 145–159.
Shinkai, A., Yoshisue, H., Koike, M., Shoji, E., Nakagawa, S., Saito, A., et al. (1999). A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J. Immunol. 163, 1602–1610.
Shoemaker, L. K., Arora, U., and Rocha Lima, C. M. (2004). 5-fluorouracil-induced coronary vasospasm. Cancer Control. 11, 46–49. doi: 10.1177/107327480401100107
Simunek, T., Stérba, M., Popelová, O., Adamcová, M., Hrdina, R., and Gersl, V. (2009). Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 61, 154–171. doi: 10.1016/S1734-1140(09)70018-0
Singh, C. K., Siddiqui, I. A., El-Abd, S., Mukhtar, H., and Ahmad, N. (2016). Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 60, 1406–1415. doi: 10.1002/mnfr.201500945
Slamon, D. J., Clark, G. M., Wong, S. G., Levin, W. J., Ullrich, A., and McGuire, W. L. (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 235, 177–182. doi: 10.1126/science.3798106
Slamon, D. J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792. doi: 10.1056/NEJM200103153441101
Soga, M., Kamal, F. A., Watanabe, K., Ma, M., Palaniyandi, S., Prakash, P., et al. (2006). Effects of angiotensin II receptor blocker (candesartan) in daunorubicin-induced cardiomyopathic rats. Int. J. Cardiol. 110, 378–385. doi: 10.1016/j.ijcard.2005.08.061
Sorrentino, M. F., Kim, J., Foderaro, A. E., and Truesdell, A. G. (2012). 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol. J. 19, 453–458. doi: 10.5603/CJ.2012.0084
Spallarossa, P., Garibaldi, S., Altieri, P., Fabbi, P., Manca, V., Nasti, S., et al. (2004). Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J. Mol. Cell. Cardiol. 37, 837–846. doi: 10.1016/j.yjmcc.2004.05.024
Staiano, R. I., Loffredo, S., Borriello, F., Iannotti, F. A., Piscitelli, F., Orlando, P., et al. (2016). Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 99, 531–540. doi: 10.1189/jlb.3HI1214-584R
Stefani, L., Maffulli, N., Mascherini, G., Francini, L., Petri, C., and Galanti, G. (2015). Exercise as prescription therapy: benefits in cancer and hypertensive patients. Transl Med UniSa. 11, 39–43.
Sterba, M., Popelová, O., Vávrová, A., Jirkovský, E., Kovaríková, P., Geršl, V., et al. (2013). Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signal. 18, 899–929. doi: 10.1089/ars.2012.4795
Suter, T. M., and Ewer, M. S. (2013). Cancer drugs and the heart: importance and management. Eur. Heart J. 34, 1102–1111. doi: 10.1093/eurheartj/ehs181
Suter, T. M., Procter, M., van Veldhuisen, D. J., Muscholl, M., Bergh, J., Carlomagno, C., et al. (2007). Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J. Clin. Oncol. 25, 3859–3865. doi: 10.1200/JCO.2006.09.1611
Swain, S. M., Ewer, M. S., Cortés, J., Amadori, D., Miles, D., Knott, A., et al. (2013). Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist 18, 257–264. doi: 10.1634/theoncologist.2012-0448
Swain, S. M., and Vici, P. (2004). The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J. Cancer Res. Clin. Oncol. 130, 1–7. doi: 10.1007/s00432-003-0498-7
Swain, S. M., Whaley, F. S., Gerber, M. C., Weisberg, S., York, M., Spicer, D., et al. (1997). Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 15, 1318–1332. doi: 10.1200/JCO.1997.15.4.1318
Sysa-Shah, P., Tocchetti, C. G., Gupta, M., Rainer, P. P., Shen, X., Kang, B. H., et al. (2016). Bidirectional cross-regulation between ErbB2 and beta-adrenergic signalling pathways. Cardiovasc. Res. 109, 358–373. doi: 10.1093/cvr/cvv274
Takimoto, E., and Kass, D. A. (2007). Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248. doi: 10.1161/01.HYP.0000254415.31362.a7
Tan, C., Tasaka, H., Yu, K. P., Murphy, M. L., and Karnofsky, D. A. (1967). Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 20, 333–353.
Telli, M. L., Witteles, R. M., Fisher, G. A., and Srinivas, S. (2008). Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann. Oncol. 19, 1613–1618. doi: 10.1093/annonc/mdn168
Thorn, C. F., Oshiro, C., Marsh, S., Hernandez-Boussard, T., McLeod, H., Klein, T. E., et al. (2011). Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet. Genomics. 21, 440–446. doi: 10.1097/FPC.0b013e32833ffb56
Timolati, F., Ott, D., Pentassuglia, L., Giraud, M. N., Perriard, J. C., Suter, T. M., et al. (2006). Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J. Mol. Cell. Cardiol. 41, 845–854. doi: 10.1016/j.yjmcc.2006.08.002
Tocchetti, C. G., Cadeddu, C., Di Lisi, D., Femminò, S., Madonna, R., Mele, D., et al. (2017). From molecular mechanisms to clinical management of antineoplastic drug-induced cardiovascular toxicity: A translational overview. Antioxid. Redox Signal. doi: 10.1089/ars.2016.6930
Tocchetti, C. G., Carpi, A., Coppola, C., Quintavalle, C., Rea, D., Campesan, M., et al. (2014). Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur. J. Heart Fail. 16, 358–366. doi: 10.1002/ejhf.50
Tocchetti, C. G., Gallucci, G., Coppola, C., Piscopo, G., Cipresso, C., Maurea, C., et al. (2013). The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. Eur. J. Heart Fail. 15, 482–489. doi: 10.1093/eurjhf/hft008
Tocchetti, C. G., Molinaro, M., Angelone, T., Lionetti, V., Madonna, R., Mangiacapra, F., et al. (2015a). nitroso-redox balance and modulation of basal myocardial function: an update from the Italian Society of Cardiovascular Research (SIRC). Curr. Drug Targets. 16, 895–903. doi: 10.2174/1389450116666150304103517
Tocchetti, C., Leppo, M. K., Bedja, D., Wang, Y., Weiss, R., and Paolocci, N. (2015b). Cardiac overexpression of creatine kinase improves cardiomyocytes function in heart failure and during increased redox stress. Circ. Res. 117:A338.
Tokarska-Schlattner, M., Zaugg, M., da Silva, R., Lucchinetti, E., Schaub, M. C., Wallimann, T., et al. (2005). Acute toxicity of doxorubicin on isolated perfused heart: response of kinases regulating energy supply. Am. J. Physiol. Heart Circ. Physiol. 289, H37–H47. doi: 10.1152/ajpheart.01057.2004
Van Cutsem, E., Bajetta, E., Valle, J., Köhne, C. H., Hecht, J. R., Moore, M., et al. (2011). Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J. Clin. Oncol. 29, 2004–2010. doi: 10.1200/JCO.2010.29.5436
Van Cutsem, E., Hoff, P. M., Blum, J. L., Abt, M., and Osterwalder, B. (2002). Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann. Oncol. 13, 484–485. doi: 10.1093/annonc/mdf108
Varga, Z. V., Kupai, K., Szucs, G., Gáspár, R., Pálóczi, J., Faragó, N., et al. (2013). MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell. Cardiol. 62, 111–121. doi: 10.1016/j.yjmcc.2013.05.009
Varricchi, G., Galdiero, M. R., Loffredo, S., Marone, G., Iannone, R., and Granata, F. (2017a). Are Mast Cells MASTers in Cancer? Front. Immunol. 8:424. doi: 10.3389/fimmu.2017.00424
Varricchi, G., Galdiero, M. R., Marone, G., Criscuolo, G., Triassi, M., Bonaduce, D., et al. (2017b). Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2:e000247. doi: 10.1136/esmoopen-2017-000247
Varricchi, G., Galdiero, M. R., Marone, G., Granata, F., and Borriello, F. (2017c). Controversial role of mast cells in skin cancers. Exp. Dermatol. 26, 11–17. doi: 10.1111/exd.13107
Varricchi, G., Galdiero, M. R., and Tocchetti, C. G. (2017d). Cardiac toxicity of immune checkpoint inhibitors: cardio-oncology meets immunology. Circulation 136, 1989–1992. doi: 10.1161/CIRCULATIONAHA.117.029626
Vincent, D. T., Ibrahim, Y. F., Espey, M. G., and Suzuki, Y. J. (2013). The role of antioxidants in the era of cardiooncology. Cancer Chemother. Pharmacol. 72, 1157–1168. doi: 10.1007/s00280-013-2260-4
Vlasova, I. I., Tyurin, V. A., Kapralov, A. A., Kurnikov, I. V., Osipov, A. N., Potapovich, M. V., et al. (2006). Nitric oxide inhibits peroxidase activity of cytochrome c.cardiolipin complex and blocks cardiolipin oxidation. J. Biol. Chem. 281, 14554–14562. doi: 10.1074/jbc.M509507200
Vorobjeva, N., Prikhodko, A., Galkin, I., Pletjushkina, O., Zinovkin, R., Sud'ina, G., et al. (2017). Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 96, 254–265. doi: 10.1016/j.ejcb.2017.03.003
Wang, X., Ha, T., Liu, L., Zou, J., Zhang, X., Kalbfleisch, J., et al. (2013). Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 97, 432–442. doi: 10.1093/cvr/cvs356
Welti, J., Loges, S., Dimmeler, S., and Carmeliet, P. (2013). Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Invest. 123, 3190–3200. doi: 10.1172/JCI70212
Willis, M. S., and Patterson, C. (2013). Proteotoxicity and cardiac dysfunction–Alzheimer's disease of the heart? N. Engl. J. Med. 368, 455–464. doi: 10.1056/NEJMra1106180
Woitek, F., Zentilin, L., Hoffman, N. E., Powers, J. C., Ottiger, I., Parikh, S., et al. (2015). Intracoronary cytoprotective gene therapy: a study of VEGF-B167 in a pre-clinical animal model of dilated cardiomyopathy. J. Am. Coll. Cardiol. 66, 139–153. doi: 10.1016/j.jacc.2015.04.071
Xi, L., Zhu, S. G., Das, A., Chen, Q., Durrant, D., Hobbs, D. C., et al. (2012). Dietary inorganic nitrate alleviates doxorubicin cardiotoxicity: mechanisms and implications. Nitric Oxide. 26, 274–284. doi: 10.1016/j.niox.2012.03.006
Yamagishi, S., and Takeuchi, M. (2005). Telmisartan is a promising cardiometabolic sartan due to its unique PPAR-gamma-inducing property. Med. Hypotheses. 64, 476–478. doi: 10.1016/j.mehy.2004.09.015
Yao, Y., Xu, X., Zhang, G., Zhang, Y., Qian, W., and Rui, T. (2012). Role of HMGB1 in doxorubicin-induced myocardial apoptosis and its regulation pathway. Basic Res. Cardiol. 107, 267. doi: 10.1007/s00395-012-0267-3
Yeh, E. T., and Bickford, C. L. (2009). Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 53, 2231–2247. doi: 10.1016/j.jacc.2009.02.050
Zamorano, J. L., Lancellotti, P., Rodriguez Muñoz, D., Aboyans, V., Asteggiano, R., Galderisi, M., et al. (2016). 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 37, 2768–2801. doi: 10.1093/eurheartj/ehw211
Zanardelli, M., Micheli, L., Cinci, L., Failli, P., Ghelardini, C., and Di Cesare Mannelli, L. (2014). Oxaliplatin neurotoxicity involves peroxisome alterations. PPARgamma agonism as preventive pharmacological approach. PLoS ONE 9:e102758. doi: 10.1371/journal.pone.0102758
Zang, J., Wu, S., Tang, L., Xu, X., Bai, J., Ding, C., et al. (2012). Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS ONE 7:e30353. doi: 10.1371/journal.pone.0030353
Zhang, M., Perino, A., Ghigo, A., Hirsch, E., and Shah, A. M. (2013). NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid. Redox Signal. 18, 1024–1041. doi: 10.1089/ars.2012.4550
Zhang, S., Liu, X., Bawa-Khalfe, T., Lu, L. S., Lyu, Y. L., Liu, L. F., et al. (2012). Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642. doi: 10.1038/nm.2919
Keywords: chemotherapy, HER-2 inhibitors, oxidative/nitrosative stress, vascular endothelial growth factor, tyrosine kinase inhibitors
Citation: Varricchi G, Ameri P, Cadeddu C, Ghigo A, Madonna R, Marone G, Mercurio V, Monte I, Novo G, Parrella P, Pirozzi F, Pecoraro A, Spallarossa P, Zito C, Mercuro G, Pagliaro P and Tocchetti CG (2018) Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol. 9:167. doi: 10.3389/fphys.2018.00167
Received: 05 January 2018; Accepted: 20 February 2018;
Published: 07 March 2018.
Edited by:
Gerald A. Meininger, University of Missouri, United StatesReviewed by:
Francesco Moccia, University of Pavia, ItalyMichele Samaja, Università degli Studi di Milano, Italy
Copyright © 2018 Varricchi, Ameri, Cadeddu, Ghigo, Madonna, Marone, Mercurio, Monte, Novo, Parrella, Pirozzi, Pecoraro, Spallarossa, Zito, Mercuro, Pagliaro and Tocchetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilda Varricchi, gilda.varricchi@unina.it
Carlo G. Tocchetti carlogabriele.tocchetti@unina.it
 Gilda Varricchi
Gilda Varricchi Pietro Ameri
Pietro Ameri Christian Cadeddu
Christian Cadeddu Alessandra Ghigo
Alessandra Ghigo Rosalinda Madonna
Rosalinda Madonna Giancarlo Marone
Giancarlo Marone Valentina Mercurio
Valentina Mercurio Ines Monte10
Ines Monte10 Flora Pirozzi
Flora Pirozzi Antonio Pecoraro
Antonio Pecoraro Paolo Spallarossa
Paolo Spallarossa Giuseppe Mercuro
Giuseppe Mercuro Carlo G. Tocchetti
Carlo G. Tocchetti