- 1Department of Physiology, University of Auckland, Auckland, New Zealand
- 2Department of Pharmacology, Oxford University, Oxford, United Kingdom
- 3Department of Physiology, McGill University, Montreal, QC, Canada
- 4Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand
Synaptic plasticity is defined as the ability of synapses to change their strength of transmission. Plasticity of synaptic connections in the brain is a major focus of neuroscience research, as it is the primary mechanism underpinning learning and memory. Beyond the brain however, plasticity in peripheral neurons is less well understood, particularly in the neurons innervating the heart. The atria receive rich innervation from the autonomic branch of the peripheral nervous system. Sympathetic neurons are clustered in stellate and cervical ganglia alongside the spinal cord and extend fibers to the heart directly innervating the myocardium. These neurons are major drivers of hyperactive sympathetic activity observed in heart disease, ventricular arrhythmias, and sudden cardiac death. Both pre- and postsynaptic changes have been observed to occur at synapses formed by sympathetic ganglion neurons, suggesting that plasticity at sympathetic neuro-cardiac synapses is a major contributor to arrhythmias. Less is known about the plasticity in parasympathetic neurons located in clusters on the heart surface. These neuronal clusters, termed ganglionated plexi, or “little brains,” can independently modulate neural control of the heart and stimulation that enhances their excitability can induce arrhythmia such as atrial fibrillation. The ability of these neurons to alter parasympathetic activity suggests that plasticity may indeed occur at the synapses formed on and by ganglionated plexi neurons. Such changes may not only fine-tune autonomic innervation of the heart, but could also be a source of maladaptive plasticity during atrial fibrillation.
Introduction
Cardiac arrhythmias are devastating disorders in which normal sinus rhythm is disrupted, resulting in the heart beating too rapidly, slowly, or erratically, thereby impairing cardiac function. The most common cardiac arrhythmia is atrial fibrillation (AF): AF affects 2.5–3.2% of people worldwide, with ~5 million new cases reported annually (Chugh et al., 2014). In AF, atrial electrical activation is rapid and disorganized leading to irregular and often rapid ventricular rhythm. AF disrupts the reservoir and contactile functions of the atria, which impairs ventricular filling and also results in stasis of blood in the left atrium in particular (Staerk et al., 2017). The prevalence of AF increases with aging (Benjamin et al., 1994; Chugh et al., 2014) and it has significant clinical consequences including a 5-fold increase in stroke, a 3-fold increase in heart failure and a doubling of risk for dementia (Benjamin et al., 1994; Chugh et al., 2014).
The hallmark of AF is rapid activation of the atria from one or more localized sources, which can be either focal discharges or self-sustaining circuits of re-entrant activity. Atrial myocardium distal to the arrhythmia source cannot follow the high frequency driver and consequently conduction becomes slow and irregular (Schotten et al., 2011). The progressive nature of this rhythm disturbance is acknowledged in the observation that “AF begets AF” (Wijffels et al., 1995). Repeated episodes of paroxysmal AF, which terminate spontaneously in hours, lead eventually to persistent AF. In persistent AF, atrial electrical and structural remodeling amplifies the electrophysiological instability that drives AF and the re-entrant substrates that sustain it (Iwasaki et al., 2011). It is well established that the autonomic nervous system contributes significantly to this process (Esler, 1992; Chen et al., 2014; Linz et al., 2014; Ardell and Armour, 2016). Sympathovagal discharge is a common trigger for paroxysmal AF (Tan et al., 2008; Chou and Chen, 2009). Specifically, it is thought to be proarrhythmic by enhancing delayed afterdepolarisation related ectopic activity through increasing β-adrenoceptor-dependent diastolic Ca2+ leak (Dobrev et al., 2011), and stabilizing re-entrant activity by reducing atrial action potential duration through increased acetylcholine-dependent K+ current (Kneller et al., 2002). Atrial sympathetic hyperinnervation and remodeling of the autonomic nervous system are both contributors to positive feedback loops that promote persistent and recurrent AF (Gould et al., 2006; Tan et al., 2008; Chou and Chen, 2009; Iwasaki et al., 2011). There is evidence of imbalance between sympathetic and parasympathetic components of the autonomic nervous system at both effector and end-organ levels (Chen and Tan, 2007; Czick et al., 2016; Kuyumcu et al., 2017). Furthermore, it is argued that progressive remodeling of the atrial neural plexus in persistent AF contributes to the maintenance of electrical instability (Chen et al., 2010, 2014; Shen et al., 2012). Despite this, we lack detailed knowledge of the structure and function of synapses formed on and by neurons within the atrial neural plexus and how these change with AF.
Extrinsic and Intrinsic Innervation of the Atria
The atria receive rich innervation from the autonomic branch of the peripheral nervous system (Hillarp, 1960; Skok, 1973; Pardini et al., 1989; Tan et al., 2006; Choi et al., 2010; Chen et al., 2014; Linz et al., 2014). Specifically, the autonomic sympathetic and parasympathetic nervous systems control normal heart rhythm and the heart's susceptibility to atrial and ventricular arrhythmias (Armour, 2008; Choi et al., 2010; Gibbons et al., 2012; Chen et al., 2014; Linz et al., 2014). Sympathetic nerves mediating control of cardiac function originate within the intermediolateral column of the spinal cord and extend to paravertebral ganglia situated from levels C1 to T5, which include the superior cervical ganglia as well as the cervico-thoracic (stellate) ganglia and thoracic ganglia (Kawashima, 2005). Cardiac nerves originating from these ganglia track to the base of the heart along the brachiocephalic trunk, common carotid and subclavian arteries as well as the superior vena cava (Kawashima, 2005). Parasympathetic cardiomotor neurons are situated in medial regions of the medulla oblongata (nucleus ambiguus and dorsal motor nucleus) and issue fibers to the atria via the bilateral vagus nerves (Spyer, 2011).
Most of the sympathetic efferent fibers directly innervate the myocardium or form synapses with neurons in cardiac ganglia located throughout the heart (Armour et al., 1997; Tan et al., 2006; Linz et al., 2014). These synapses consist of presynaptic axonal varicosities invaginated by the postsynaptic cardiomyocyte membrane which contains high densities of adrenergic receptors, adhesion and scaffold proteins (Landis, 1976; Shcherbakova et al., 2007). Hyperactive sympathetic activity is a major feature of heart disease, significantly contributing to the high arrhythmia burden and sudden cardiac death (Chen et al., 2001; Shanks et al., 2013; Ajijola et al., 2015), and recent research has revealed that this is predominantly driven by the postganglionic sympathetic neurons (Larsen et al., 2016a,b). Specifically, hypertension induces increases in membrane calcium currents, intracellular calcium, and cyclic nucleotide signaling in sympathetic stellate neurons, resulting in an increase in noradrenaline release (Shanks et al., 2013; Larsen et al., 2016a,b). Stimulation of sympathetic neurons can redistribute postsynaptic adrenergic receptors on the surface of cardiomyocytes (Shcherbakova et al., 2007). Together these data show that both pre- and post-synaptic changes can readily occur in transmission at sympathetic neuro-cardiac synapses.
Parasympathetic fibers form synapses with clusters of cardiac ganglia neurons located on the surface of the heart (Figure 1; Armour, 2008; Linz et al., 2014; Wake and Brack, 2016). These clusters are termed ganglionated plexi (GP), or “little brains” (Armour, 2008), and they are proposed to act as local coordinators of cardiac electrical and mechanical properties (Horackova and Armour, 1995; Choi et al., 2010; Linz et al., 2014; Ardell and Armour, 2016). In humans, approximately 14,000 GP neurons are located on the heart surface, with many clustered around the pulmonary veins (Armour et al., 1997). Increasing evidence supports the hypothesis that GP neurons can independently modulate neural control of the heart (Horackova and Armour, 1995; Arora et al., 2003; Heaton et al., 2007; Choi et al., 2010; Gibbons et al., 2012; Chen et al., 2014; Linz et al., 2014). For example, GP neurons are proposed to play a critical role in the development and propagation of arrhythmias such as AF (Choi et al., 2010; Gibbons et al., 2012; Chen et al., 2014; Linz et al., 2014), and AF can be induced by direct stimulation of GP sites (Lim et al., 2011; Gibbons et al., 2012). In addition, changes in parasympathetic tone (which increase the risk of arrhythmias, heart failure and mortality), have been proposed to occur in GP (Bibevski and Dunlap, 1999; Arora et al., 2003; Heaton et al., 2007).
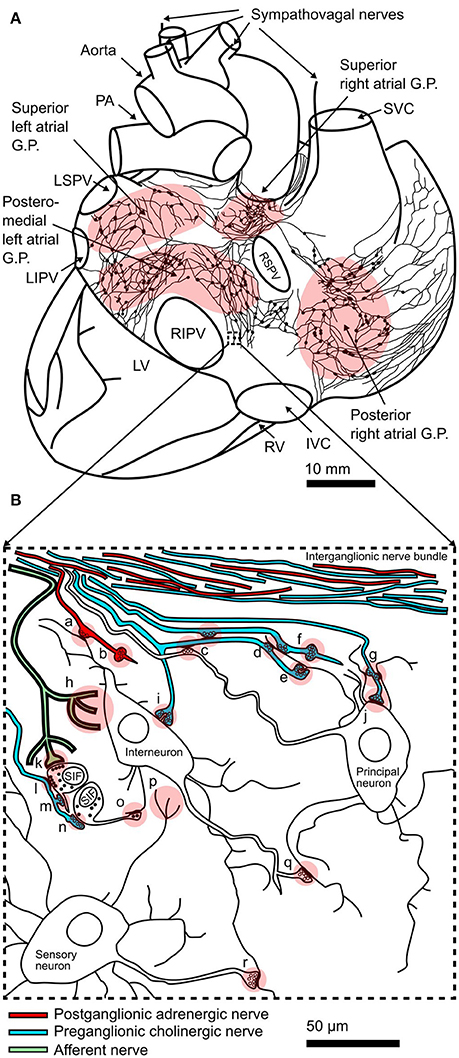
Figure 1. Structure of the intracardiac plexus and synaptic connections within: (A) Drawing of a posterior view of the human heart and major vessels showing the locations of posterior atrial ganglionated plexi (GP) and interconnecting nerves. This schematic representation of the plexus is derived from example reconstructions of acetylcholinesterase positive nerves and ganglia on the surface of juvenile atria (Pauza et al., 2000). Sympathovagal nerves enter the heart by coursing down the aorta and superior vena cava (SVC) to innervate the superior atrial GP. The locations of the pulmonary arteries (PA), left superior pulmonary vein (LSPV), left inferior pulmonary vein (LIPV), right superior pulmonary vein (RSPV), right inferior pulmonary vein (RIPV), left ventricle (LV), right ventricle (RV), and inferior vena cava (IVC) are shown. (B) Schematic representation of interconnectivity in cardiac ganglia showing types of synapses seen in electron microscopy studies (Shvalev and Sosunov, 1985; Armour et al., 1997; Pauziene and Pauza, 2003): (a) axo-dendritic synapse formed by adrenergic nerve terminal; (b) adrenergic varicosity without glial sheath; (c) axo-axonal synapses; (d) two axons forming axo-dendritic synapses on a single dendrite; (e) axo-dendritic synapse on dendritic spine; (f) cholinergic varicosity without glial sheath; (g) a single axon forming axo-dendritic synapses on two dendrites; (h) afferent nerve ending; (i) axo-dendritic synapse on small spine like protrusion from soma; (j) axo-somatic synapse; (k) contact of afferent nerve terminal with SIF cell; (l) efferent (soma-axonal) synapse made by SIF cell with cholinergic nerve terminal; (m) synapse formed between cholinergic nerve terminal and process of SIF cell; (n) afferent (axo-somatic) synapse made between SIF cell and cholinergic nerve terminal; (o) synapse formed between process of SIF cell and neuronal dendrite; (p) sensory neuron afferent nerve ending; (q) axo-dendritic synapse between neurons within the GP; (r) axo-dendritic synapse between sensory neuron and other neuronal types. Modified from Shvalev and Sosunov (1985).
Ganglionated Plexi
GP Structure and Neuron Function
Although initially defined as clusters of cholinergic neurons, GP neurons show significant heterogeneity in their morphology, chemical composition, and physiology (Edwards et al., 1995; Horackova et al., 1999; Richardson et al., 2003; Rimmer and Harper, 2006; Tan et al., 2006; McAllen et al., 2011; Wake and Brack, 2016). Immunocytochemical analysis has revealed multiple neurochemical subtypes of GP neurons: while choline acetyltransferase (ChAT) is expressed in all principal neurons, subpopulations express other transmitters and neuropeptides including nitric oxide, serotonin, and neuropeptide Y (Mawe et al., 1996; Horackova et al., 1999; Singh et al., 1999; Richardson et al., 2003; Adams and Cuevas, 2004; Wake and Brack, 2016). Small clusters of catecholaminergic neurons, termed SIF (small intensely fluorescent) cells, constitute 5% of GP neurons (Horackova et al., 1999; Slavíková et al., 2003) where they modulate synaptic transmission of the cholinergic neurons (McGrattan et al., 1987; Gagliardi et al., 1988; Adams and Cuevas, 2004). The presence of multiple neurochemical variants suggests differential roles for peptides and neurotransmitters in modulating GP neuron function. Distinct subtypes of neurons within GP have also been defined electrophysiologically based on action potential kinetics, ability to fire bursts of action potentials, rectification properties and synaptic input (Edwards et al., 1995; McAllen et al., 2011). This heterogeneity within GP suggests the neurons play different roles in controlling electrical signals to the heart (Ardell and Armour, 2016). Moreover, the ability of GP neurons to modulate the level of parasympathetic activity to the heart also suggests that synaptic communication from GP neurons can be altered. These synaptic changes may not only fine-tune autonomic activation of the heart, but could also likely be a source of maladaptive changes including arrhythmogenesis (Choi et al., 2010; Gibbons et al., 2012; Chen et al., 2014; Ajijola et al., 2015; Ardell et al., 2016).
Clinical Importance of GP Neurons
Clinically, AF is treated pharmacologically with rate and rhythm controllers (Lafuente-Lafuente et al., 2015; Hanley et al., 2016; which have variable efficacy and trigger ventricular arrhythmias), and by the pulmonary vein isolation procedure. In this technique, an interior ring or “firebreak” is created within each pulmonary vein to stop aberrant electrical impulses that can trigger AF from reaching the heart (Haissaguerre et al., 1998; Lancaster et al., 2016). This procedure has been most effective in reversing paroxysmal AF compared with persistent AF, however initial success rates at 12 months drop significantly beyond 2 years (Kron et al., 2010; Weerasooriya et al., 2011; Calkins et al., 2012; Zheng et al., 2013). Ablation of the GP sites has been combined with pulmonary vein isolation, and these two procedures appear to increase the numbers of patients free of AF, however results are variable, and long-term efficacy is unknown (Scherlag et al., 2006; Pokushalov et al., 2009; Kron et al., 2010; Katritsis et al., 2011; Calkins et al., 2012; Zheng et al., 2013). Moreover, GP neurons also innervate the ventricles and modulate ventricular function, raising concern of increased susceptibility to ventricular arrhythmias after ablation procedures (Pappone et al., 2004; Osman et al., 2010; Buckley et al., 2016; Jungen et al., 2017). It is critical that in order to advance GP ablation techniques and increase their reproducibility and success rates that we gain a detailed understanding of the physiological properties of the GP neurons in the aged or arrhythmic states, and how changes in their function may trigger and drive AF.
Beyond the Brain – Does Synaptic Plasticity Occur in Neurons Innervating the Heart?
“Plasticity” is defined as the ability of neurons to alter their strength of communication at synapses (Bliss and Lomo, 1973; Dudek and Bear, 1992; Genoux and Montgomery, 2007; Nabavi et al., 2014). Synapse plasticity is a critical process in the brain, and a major area of neuroscience research as it has been shown to underlie learning and memory, as well as changes in sensory and motor functions (Genoux and Montgomery, 2007; Huang et al., 2007; Lee et al., 2014; Nabavi et al., 2014; Leighton and Lohmann, 2016). High-frequency stimulation paradigms induce increases in synaptic efficacy that last for seconds (i.e., short-term plasticity; Dobrunz et al., 1997; Jackman and Regehr, 2017), or from minutes to hours or days, referred to as long-term potentiation (LTP; Bliss and Lomo, 1973). Alternatively, low frequency stimulation paradigms induce long term depression (LTD) of synaptic efficacy (Dudek and Bear, 1992). The induction of LTP and LTD is dependent on activation of NMDA-type glutamate receptors (Harris et al., 1984; Morris et al., 1986; Dudek and Bear, 1992). The mechanisms underpinning the expression of these changes in synaptic strength vary between brain regions. Specifically, LTP/LTD paradigms can induce changes in postsynaptic receptor surface number, conductance, and distribution, the probability of presynaptic transmitter release, and/or ultrastructural changes in synaptic protein localisation (Hayashi et al., 2000; Montgomery et al., 2001; Castillo et al., 2002; Mellor et al., 2002; Ehlers et al., 2007; Volk et al., 2015; Tang et al., 2016). However, in contrast to the brain, less is known about the mechanisms of short and long-term synaptic plasticity in the neurons that innervate the heart.
Synaptic Plasticity in Cardiac Sympathetic Ganglia
Both short and long-term plasticity mechanisms have been described in the peripheral synapses within sympathetic ganglia. In the stellate and superior cervical sympathetic ganglia that innervate the heart, short-term increases in the strength of synaptic transmission occur in response to a single action potential or a short train of impulses (Bennett et al., 1976; Lin et al., 1998), and longer bursts of high frequency stimulation of the preganglionic nerve result in enhancement of postsynaptic responses and heart rate (Alonso-deFlorida et al., 1991; Bachoo and Polosa, 1991; Aileru et al., 2004). Conversely, low frequency stimulation can induce LTD (Alkadhi et al., 2008). Induction of ganglionic LTP and LTD (gLTP/gLTD) is not dependent on transmission via nicotinic, adrenergic, muscarinic or adenosine receptors, but requires activation of 5-HT3 receptors by serotonin, potentially released from SIF cells (Alkadhi et al., 1996, 2008). Both pre- and postsynaptic expression mechanisms have been implicated in gLTP (Alkadhi et al., 2005), with increases in evoked acetylcholine (ACh) release (Briggs et al., 1985) and postsynaptic sensitivity to ACh observed (Bachoo and Polosa, 1991). More recently, both pre- and postsynaptic intracellular calcium changes have been shown to contribute equally to gLTP (Vargas et al., 2011), and the potential involvement of nitric oxide signaling (Altememi and Alkadhi, 1999) supports a trans-synaptic form of gLTP (Vargas et al., 2011) that can be enhanced by neurotrophins to regulate sympathetic tone (Arias et al., 2014).
The mechanisms underpinning gLTP likely contribute to the enhanced sympathetic drive seen in conditions associated with heart disease and AF (Alkadhi and Alzoubi, 2007). In spontaneously hypertensive rats (SHRs), synaptic transmission is augmented as shown by increased ACh release (Magee and Schofield, 1992, 1994), greater recruitment of postganglionic neurons (Magee and Schofield, 1992), and faster spike frequency adaptation in SHR ganglia (Yarowsky and Weinreich, 1985). Increased sympathetic stimulation may increase presynaptic activity to induce gLTP observed in vivo in sympathetic ganglia in SHRs (Alzoubi et al., 2010). Additional evidence of gLTP in vivo is the inhibition of baseline ganglionic transmission by 5-HT3 receptor antagonists in sympathetic ganglia from SHRs but not age-matched controls (Alkadhi et al., 2001). Further gLTP cannot be induced, indicating occlusion of the plasticity mechanism (Alkadhi and Alzoubi, 2007). Chronic treatment with 5-HT3 receptor antagonists also reduces blood pressure in SHRs (Alkadhi et al., 2001) but its effect on atrial arrhythmia burden in this model is unknown.
Synaptic Plasticity in the Intracardiac Plexus
Within GP, the cholinergic and catecholaminergic neurons possess large numbers of asymmetrical axodendritic synapses and project axons to neurons within the same or different ganglion (Figure 1; Armour et al., 1997; Klemm et al., 1997; Horackova et al., 1999; Richardson et al., 2003; Tan et al., 2006; Armour, 2008), suggesting that significant synaptic communication occurs between networks of GP neurons. Electrophysiological recordings from synapses within the intracardiac plexus are hampered by difficulty accessing GP neurons given their proximity to the heart and great vessels, and the extensive connective tissue surrounding the ganglia. Intracellular recordings from GP neurons have been performed in the working heart-brainstem preparation (McAllen et al., 2011). These recordings revealed the presence of subthreshold synaptic potentials and silent synapses, indicating that significant capacity exists for increasing synaptic strength within GP, which could alter and/or restore vagal tone (McAllen et al., 2011). Multiple studies have measured changes in postsynaptic neuronal excitability as an indication of synaptic efficacy in the intracardiac plexus following chronic spinal cord stimulation or myocardial infarction, suggesting altered neurotransmission in the intracardiac plexus contributes to altered parasympathetic control of the heart (Bibevski and Dunlap, 1999; Ardell et al., 2014; Hardwick et al., 2014; Rajendran et al., 2016; Smith et al., 2016). After myocardial infarction, the observed overall reduction in network connectivity (Rajendran et al., 2016) suggests depression of synaptic transmission (i.e., LTD) within the intracardiac nervous system, however this may differ at afferent versus efferent synaptic inputs. With regards to AF, enhanced interaction at the level of the GP network through changes in local circuit neuron function have been proposed to be a factor contributing to AF substrate (Beaumont et al., 2013; Ardell et al., 2016). Possible factors altering ganglionic neurotransmission include changes in postsynaptic nicotinic receptor expression (Bibevski and Dunlap, 2011) and dysfunctional NO-cGMP signaling in postganglionic neurons (Heaton et al., 2007). Intriguingly, NMDA receptors are abundantly expressed in the atrium, including in the GP (Gill et al., 2007), and their activation is associated with increased arrhythmogenesis, AF inducibility, and atrial fibrosis (Shi et al., 2014, 2017). NMDA receptors are critical for the induction of synaptic plasticity in the brain, suggesting that NMDA receptors in the heart also play a role in inducing plasticity within GP, and contribute to autonomic dysfunction in arrhythmias such as AF.
Significant evidence indicates the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) is involved in plasticity at GP synapses. PACAP is localized to parasympathetic preganglionic fibers (Calupca et al., 2000; Richardson et al., 2003) and GP neurons express PAC1 receptors (Braas et al., 1998). PACAP modulates nicotinic neurotransmission in the ciliary ganglion by enhancing presynaptic quantal ACh release via trans-synaptic action of NO (Pugh et al., 2010; Jayakar et al., 2014). Postsynaptically, PACAP increases the agonist affinity of GP nicotinic receptors through G-protein signaling (Liu et al., 2000). High frequency stimulation of nerve bundles within the intracardiac plexus results in a slow postsynaptic depolarisation and a sustained increase in excitability of GP neurons that is thought to be at least partially mediated by PACAP (Tompkins et al., 2007). This increase in excitability is driven by enhanced current through hyperpolarization-induced nonselective cationic (Ih; Tompkins et al., 2009) and T/R-type calcium channels (Tompkins et al., 2015). The enhanced excitability of GP neurons likely contributes to the PACAP induced AF seen in dogs (Hirose et al., 1997) and guinea pigs (Chang et al., 2005).
Future Directions
The detailed knowledge of plasticity mechanisms in the brain has resulted from precise imaging and electrophysiological analysis of synaptic properties (e.g., Hayashi et al., 2000; Montgomery et al., 2001; Ehlers et al., 2007; Fourie et al., 2014; Tang et al., 2016). To gain comparable knowledge of short and long-term plasticity mechanisms in the innervation of the heart, similar high-resolution techniques need to be applied to synapses formed on and by cardiac parasympathetic and sympathetic neurons, especially in human tissue where some aspects of GP circuitry and cell composition appear to differ (Armour et al., 1997; Pauziene and Pauza, 2003; Hoover et al., 2009). Animal models of AF are also important, as recording changes in GP neuron and synapse function during and after the onset of arrhythmia will provide evidence of whether plasticity does occur at GP synapses with changes in heart rhythm. In more intact systems, such as the working heart-brainstem and the innervated heart preparations (Brack et al., 2004; Ng et al., 2007; McAllen et al., 2011; Ashton et al., 2013), this would enable researchers to determine whether changes in synaptic strength can increase or decrease autonomic tone to the heart, and play a major role in generating the aberrant electrical impulses in the GP around the pulmonary veins that can trigger and drive AF.
Author Contributions
JM and JA initiated the review topic and designed the review. All authors contributed to the writing, editing, and approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the University of Auckland Faculty Research Development Fund awarded to JM and BS to support JA. We also acknowledge funding from the Heart Foundation New Zealand and the Maurice and Phyllis Paykel Trust awarded to JM to establish neuro-cardiac research. RB holds a Sir Henry Dale Royal Society and Wellcome Trust Fellowship (109371/Z/15/Z) and acknowledges support from the Nuffield Benefaction for Medicine and the Wellcome Institutional Strategic Support Fund (ISSF) Oxford and Medical Research Council; RB and JM hold a Colin Pillinger International Exchange Award (Royal Society).
References
Adams, D. J., and Cuevas, J. (2004). “Electrophysiological properties of intrinsic cardiac neurons,” in Basic and Clinical Neurocardiology, eds J. A. Armour and J. L. Ardell (New York, NY: Oxford University Press), 1–60.
Aileru, A. A., Logan, E., Callahan, M., Ferrario, C. M., Ganten, D., and Diz, D. I. (2004). Alterations in sympathetic ganglionic transmission in response to angiotensin II in (mRen)27 transgenic rats. Hypertension 43, 270–275. doi: 10.1161/01.HYP.0000112422.81661.f3
Ajijola, O. A., Howard-Quijano, K., Scovotti, J., Vaseghi, M., Lee, C., Mahajan, A., et al. (2015). Augmentation of cardiac sympathetic tone by percutaneous low-level stellate ganglion stimulation in humans: a feasibility study. Physiol. Rep. 3:e12328. doi: 10.14814/phy2.12328
Alkadhi, K. A., Al-Hijailan, R. S., and Alzoubi, K. H. (2008). Long-term depression in the superior cervical ganglion of the rat. Brain Res. 1234, 25–31. doi: 10.1016/j.brainres.2008.07.112
Alkadhi, K. A., Alzoubi, K. H., and Aleisa, A. M. (2005). Plasticity of synaptic transmission in autonomic ganglia. Prog. Neurobiol. 75, 83–108. doi: 10.1016/j.pneurobio.2005.02.002
Alkadhi, K. A., Otoom, S. A., Tanner, F. L., Sockwell, D., and Hogan, Y. H. (2001). Inhibition of ganglionic long-term potentiation decreases blood pressure in spontaneously hypertensive rats. Exp. Biol. Med. 226, 1024–1030. doi: 10.1177/153537020122601109
Alkadhi, K. A., Salgado-Commissariat, D., Hogan, Y. H., and Akpaudo, S. B. (1996). Induction and maintenance of ganglionic long-term potentiation require activation of 5-hydroxytryptamine (5-HT3) receptors. J. Physiol. 496, 479–489. doi: 10.1113/jphysiol.1996.sp021700
Alkadhi, K., and Alzoubi, K. (2007). Role of long-term potentiation of sympathetic ganglia (gLTP) in hypertension. Clin. Exp. Hypertens. 29, 267–286. doi: 10.1080/10641960701500356
Alonso-deFlorida, F., Morales, M. A., and Minzoni, A. A. (1991). Modulated long-term potentiation in the cat superior cervical ganglion in vivo. Brain Res. 544, 203–210. doi: 10.1016/0006-8993(91)90055-Z
Altememi, G. F., and Alkadhi, K. A. (1999). Nitric oxide is required for the maintenance but not initiation of ganglionic long-term potentiation. Neuroscience 94, 897–902. doi: 10.1016/S0306-4522(99)00362-0
Alzoubi, K. H., Aleisa, A. M., and Alkadhi, K. A. (2010). In vivo expression of ganglionic long-term potentiation in superior cervical ganglia from hypertensive aged rats. Neurobiol. Aging 31, 805–812. doi: 10.1016/j.neurobiolaging.2008.06.007
Ardell, J. L., and Armour, J. A. (2016). Neurocardiology: structure-based function. Comp. Physiol. 6, 1635–1653. doi: 10.1002/cphy.c150046
Ardell, J. L., Andresen, M. C., Armour, J. A., Billman, G. E., Chen, P. -S., Foreman, R. D., et al. (2016). Translational neurocardiology: preclinical models and cardioneural integrative aspects. J. Physiol. 594, 3877–3909. doi: 10.1113/JP271869
Ardell, J. L., Cardinal, R. R., Beaumont, E., Vermeulen, M., Smith, F. M., and Armour, A. J. (2014). Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton. Neurosci. Basic. Clin. 186, 38–44. doi: 10.1016/j.autneu.2014.09.017
Arias, E. R., Valle-Leija, P., Morales, M. A., and Cifuentes, F. (2014). Differential contribution of BDNF and NGF to long-term potentiation in the superior cervical ganglion of the rat. Neuropharm 81, 206–214. doi: 10.1016/j.neuropharm.2014.02.001
Armour, J. A. (2008). Potential clinical relevance of the little brain on the mammalian heart. Exp. Physiol. 93, 165–176. doi: 10.1113/expphysiol.2007.041178
Armour, J. A., Murphy, D. A., Yuan, B. X., MacDonald, S., and Hopkins, D. A. (1997). Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 47, 289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L
Arora, R. C., Cardinal, R., Smith, F. M., Ardell, J. L., Dell'Italia, L. J., and Armour, J. A. (2003). Intrinsic cardiac nervous system in tachycardia induced heart failure. Am. J. Physiol. 285, R1212–1223. doi: 10.1152/ajpregu.00131.2003
Ashton, J. L., Paton, J. F., Trew, M. L., LeGrice, I. J., and Smaill, B. H. (2013). A working heart-brainstem preparation of the rat for the study of reflex mediated autonomic influences on atrial arrhythmia development. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 3785–3788. doi: 10.1109/EMBC.2013.6610368
Bachoo, M., and Polosa, C. (1991). Long-term potentiation of nicotinic transmission by a heterosynaptic mechanism in the stellate ganglion of the cat. J. Neurophysiol. 65, 639–647. doi: 10.1152/jn.1991.65.3.639
Beaumont, E., Salavatian, S., Southerland, E. M., Vinet, A., Jacquemet, V., Armour, J. A., et al. (2013). Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J. Physiol. 591, 4515–4533. doi: 10.1113/jphysiol.2013.259382
Benjamin, E. J., Levy, D., Vaziri, S. M., D'Agostino, R. B., and Belanger, A. J. (1994). Independent risk factors for atrial fibrillation in a population-based cohort the framingham heart study. JAMA 271, 840–844.
Bennett, M. R., Florin, T., and Pettigrew, A. G. (1976). The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J. Physiol. 257, 597–620. doi: 10.1113/jphysiol.1976.sp011387
Bibevski, S., and Dunlap, M. E. (1999). Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation 99, 2958–2963. doi: 10.1161/01.CIR.99.22.2958
Bibevski, S., and Dunlap, M. E. (2011). Evidence for impaired vagus nerve activity in heart failure. Heart Fail. Rev. 16, 129–135. doi: 10.1007/s10741-010-9190-6
Bliss, T. V. P., and Lomo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. doi: 10.1113/jphysiol.1973.sp010273
Braas, K. M., May, V., Harakall, S. A., Hardwick, J. C., and Parsons, R. L. (1998). Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J. Neurosci. 18, 9766–9779.
Brack, K. E., Coote, J. H., and Ng, G. A. (2004). Interaction between direct sympathetic and vagus nerve stimulation on heart rate in the isolated rabbit heart. Exp. Physiol. 89, 128–39. doi: 10.1113/expphysiol.2003.002654
Briggs, C. A., Brown, T. H., and Mcafee, D. A. (1985). Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J. Pharmacol. Sci. 359, 503–521. doi: 10.1113/jphysiol.1985.sp015599
Buckley, U., Rajendran, P. S., and Shivkumar, K. (2016). Ganglionated plexus ablation for atrial fibrillation: just because we can, does that mean we should? Heart Rhythm 14, 133–134. doi: 10.1016/j.hrthm.2016.09.001
Calkins, H., Kuck, K. H., Cappato, R., Brugada, J., Camm, A. J., Chen, S. A., et al. (2012). 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14, 528–606. doi: 10.1093/europace/eus027
Calupca, M. A., Vizzard, M. A., and Parsons, R. L. (2000). Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J. Comp. Neurol. 423, 26–39. doi: 10.1002/1096-9861(20000717)423:1<26::AID-CNE3>3.0.CO;2-C
Castillo, P. E., Schoch, S., Schmitz, F., Südhof, T. C., and Malenka, R. C. (2002). RIM1alpha is required for presynaptic long-term potentiation. Nature 415, 327–330. doi: 10.1038/415327a
Chang, Y., Lawson, L. J., Hancock, J. C., and Hoover, D. B. (2005). Pituitary adenylate cyclase-activating polypeptide: localization and differential influence on isolated hearts from rats and guinea pigs. Regul. Pept. 129, 139–146. doi: 10.1016/j.regpep.2005.02.012
Chen, P. S., and Tan, A. Y. (2007). Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 4, S61–S64. doi: 10.1016/j.hrthm.2006.12.006
Chen, P. S., Chen, L. S., Fishbein, M. C., Lin, S. F., and Nattel, S. (2014). Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 114, 1500–1515. doi: 10.1161/CIRCRESAHA.114.303772
Chen, P.-S., Chen, L. S., Cao, J. M., Sharifi, B., Karagueuzian, H. S., and Fishbein, M. C. (2001). Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc. Res. 50, 409–416, doi: 10.1016/S0008-6363(00)00308-4
Chen, P.-S., Choi, E.-K., Zhou, S., Shien-Fong, L., and Chen, L. S. (2010). Cardiac neural remodelling and its role in arrhythmogenesis. Heart Rhythm. 7, 1512–1513. doi: 10.1016/j.hrthm.2010.05.020
Choi, E. K., Shen, M. J., Han, S., Kim, D., Hwang, S., Sayfo, S., et al. (2010). Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 121, 2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829
Chou, C. C., and Chen, P. S. (2009). New concepts in atrial fibrillation: neural mechanisms and calcium dynamics. Cardiol. Clin. 27, 35–43. doi: 10.1016/j.ccl.2008.09.003
Chugh, S. S., Havmoeller, R., Narayanan, K., Singh, D., Rienstra, M., Benjamin, E. J., et al. (2014). Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 129, 837–847. doi: 10.1161/CIRCULATIONAHA.113.005119
Czick, M. E., Shapter, C. L., and Silverman, D. I. (2016). Atrial Fibrillation: the science behind its defiance. Aging Dis. 7, 635–656. doi: 10.14336/AD.2016.0211
Dobrev, D., Voigt, N., and Wehrens, X. H. (2011). The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc Res. 89, 734–743. doi: 10.1093/cvr/cvq324
Dobrunz, L. E., Huang, E. P., and Stevens, C. F. (1997). Very short-term plasticity in hippocampal synapses. Proc. Natl. Acad. Sci. U.S.A. 94, 14843–14847. doi: 10.1073/pnas.94.26.14843
Dudek, S. M., and Bear, M. F. (1992). Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. U.S.A. 89, 4363–4367. doi: 10.1073/pnas.89.10.4363
Edwards, F. R., Hirst, G. D., Klemm, M. F., and Steele, P. A. (1995). Different types of ganglion cell in the cardiac plexus of guinea-pigs. J. Physiol. 486, 453–471. doi: 10.1113/jphysiol.1995.sp020825
Ehlers, M. D., Heine, M., Groc, L., Lee, M. C., and Choquet, D. (2007). Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron 54, 447–460. doi: 10.1016/j.neuron.2007.04.010
Esler, M. (1992). The autonomic nervous system and cardiac arrhythmias. Clin. Auton. Res. 2, 133–135. doi: 10.1007/BF01819669
Fourie, C., Madison, D. V., and Montgomery, J. M. (2014). Paired whole cell recordings in hippocampal organotypic slices. J. Vis. Exp. 91:e51958. doi: 10.3791/51958
Gagliardi, M., Randall, W. C., Bieger, D., Wurster, R. D., and hopkins, D. A. (1988). Activity of in vivo canine cardiac plexus neurons. Am. J. Physiol. 255, H789–H800. doi: 10.1152/ajpheart.1988.255.4.H789
Genoux, D., and Montgomery, J. M. (2007). Glutamate receptor plasticity at excitatory synapses in the brain. Clin. Exp. Pharmacol. Physiol. 34, 1058–1063. doi: 10.1111/j.1440-1681.2007.04722.x
Gibbons, D. D., Southerland, E. M., Hoover, D. B., Beaumont, E., Armour, J. A., and Ardell, J. L. (2012). Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. J. Physiol. 302, R357–R364. doi: 10.1152/ajpregu.00535.2011
Gill, S., Veinot, J., Kavanagh, M., and Pulido, O. (2007). Human heart glutamate receptors—implications for toxicology, food safety, and drug discovery. Toxicol. Pathol. 35, 411–417. doi: 10.1080/01926230701230361
Gould, P. A., Yii, M., McLean, C., Finch, S., Marshall, T., Lambert, G. W., et al. (2006). Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin. Electrophysiol. 29, 821–829. doi: 10.1111/j.1540-8159.2006.00447.x
Haïssaguerre, M., Jaïs, P., Shah, D. C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New Engl. J. Med. 339, 659–666. doi: 10.1056/NEJM199809033391003
Hanley, C. M., Robinson, V. M., and Kowey, P. R. (2016). Status of antiarrhythmic drug development for atrial fibrillation: new drugs and new molecular mechanisms. Circ. Arrhythm. Electrophysiol. 9:e002479. doi: 10.1161/CIRCEP.115.002479
Hardwick, J. C., Ryan, S. E., Beaumont, E., Ardell, J. L., and Southerland, E. M. (2014). Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton. Neurosci. 181, 4–12. doi: 10.1016/j.autneu.2013.10.008
Harris, E. W., Ganong, A. H., and Cotman, C. W. (1984). Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 323, 132–137. doi: 10.1016/0006-8993(84)90275-0
Hayashi, Y., Shi, S. H., Esteban, J. A., Piccini, A., Poncer, J. C., and Malinow, R. (2000). Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267. doi: 10.1126/science.287.5461.2262
Heaton, D. A., Li, D., Almond, S. C., Dawson, T. A., Wang, L., Channon, K. M., et al. (2007). Gene transfer of neuronal nitric oxide synthase into intracardiac Ganglia reverses vagal impairment in hypertensive rats. Hypertension 49, 380–388. doi: 10.1161/01.HYP.0000255792.97033.f7
Hillarp, N.-A. (1960). “Peripheral autonomic mechanisms,” in Handbook of Physiology, Section I, Neurophysiology, ed J. Field (Washington, DC: American Physiological Society), 979–1006.
Hirose, M., Furukawa, Y., Nagashima, Y., Lakhe, M., and Chiba, S. (1997). Pituitary adenylate cyclase-activating polypeptide-27 causes a biphasic chronotropic effect and atrial fibrillation in autonomically decentralized, anesthetized dogs. J. Pharmacol. Exp. Ther. 283, 478–487.
Hoover, D. B., Isaacs, E. R., Jacques, F., Hoard, J. L., Pagé, P., and Armour, J. A. (2009). Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164, 1170–1179. doi: 10.1016/j.neuroscience.2009.09.001
Horackova, M., and Armour, J. A. (1995). Role of peripheral autonomic neurones in maintaining adequate cardiac function. Cardiovasc. Res. 30, 326–335. doi: 10.1016/0008-6363(95)00105-0
Horackova, M., Armour, J. A., and Byczko, Z. (1999). Distribution of intrinsic cardiac neurons in whole-mount guinea pig atria identified by multiple neurochemical coding a confocal microscope study. Cell. Tissue Res. 297, 409–421. doi: 10.1007/s004410051368
Huang, L. C., Thorne, P. R., Housley, G. D., and Montgomery, J. M. (2007). Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development 134, 2925–2933. doi: 10.1242/dev.001925
Iwasaki, Y.-K., Nishida, K., Kato, T., and Nattel, S. (2011). Atrial fibrillation pathophysiology: implications for management. Circulation 124, 2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893
Jackman, S. L., and Regehr, W. G. (2017). The mechanisms and functions of synaptic facilitation. Neuron 94, 447–464. doi: 10.1016/j.neuron.2017.02.047
Jayakar, S. S., Pugh, P. C., Dale, Z., Starr, E. R., Cole, S., and Margiotta, J. F. (2014). PACAP induces plasticity at autonomic synapses by nAChR-dependent NOS1 activation and AKAP-mediated PKA targeting. Mol. Cell. Neurosci. 63, 1–12. doi: 10.1016/j.mcn.2014.08.007
Jungen, C., Scherschel, K., Eickholt, C., Kuklik, P., Klatt, N., Bork, N., et al. (2017). Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat Commun. 8:14155. doi: 10.1038/ncomms14155
Katritsis, D. G., Giazitzoglou, E., Zografos, T., Pokushalov, E., Po, S. S., and Camm, A. J. (2011). Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 8, 672–678. doi: 10.1016/j.hrthm.2010.12.047
Kawashima, T. (2005). The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embriol. 209, 425–438. doi: 10.1007/s00429-005-0462-1
Klemm, M. F., Wallace, D. J., and Hirst, G. D. S. (1997). Distribution of synaptic boutons around identified neurones lying in the cardiac plexus of the guinea-pig. J. Auton. Nerv. Syst. 66, 201–207. doi: 10.1016/S0165-1838(97)00084-2
Kneller, J., Zou, R., Vigmond, E. J., Wang, Z., Leon, L. J., Nattel, S., et al. (2002). Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ. Res. 90, E73–E87. doi: 10.1161/01.RES.0000019783.88094.BA
Kron, J., Kasirajan, V., Wood, M. A., Kowalski, M., Han, F. T., and Ellenbogen, K. A. (2010). Management of recurrent atrial arrhythmias after minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. Heart Rhythm. 7, 445–451. doi: 10.1016/j.hrthm.2009.12.008
Kuyumcu, M. S., Ozeke, O., Cay, S., Ozcan, F., Bayraktar, M. F., Kara, M., et al. (2017). The short-term impact of the catheter ablation on noninvasive autonomic nervous system parameters in patients with paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 40, 1193–1199. doi: 10.1111/pace.13179
Lafuente-Lafuente, C., Valembois, L., Bergmann, J. F., and Belmin, J. (2015). Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 28:CD005049. doi: 10.1002/14651858.CD005049.pub4
Lancaster, T. S., Melby, S. J., and Damiano, R. J. Jr. (2016). Minimally invasive surgery for atrial fibrillation. Trends Cardiovasc. Med. 26, 268–277. doi: 10.1016/j.tcm.2015.07.004
Landis, S. C. (1976). Rat sympathetic neurons and cardiac myocytes developing in microcultures: correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc. Nat. Acad. Sci. U.S.A. 73, 4220–4224. doi: 10.1073/pnas.73.11.4220
Larsen, H. E., Bardsley, E. N., Lefkimmiatis, K., and Paterson, D. J. (2016a). Dysregulation of neuronal Ca2+ channel linked to heightened sympathetic phenotype in prohypertensive states. J. Neurosci. 36, 8562–8573. doi: 10.1523/JNEUROSCI.1059-16.2016
Larsen, H. E., Lefkimmiatis, K., and Paterson, D. J. (2016b). Sympathetic neurons are a powerful driver of myocyte function in cardiovascular disease. Sci. Rep. 6:38898. doi: 10.1038/srep38898
Lee, K. J., Rhyu, I. J., and Pak, D. T. (2014). Synapses need coordination to learn motor skills. Rev. Neurosci. 25, 223–230. doi: 10.1515/revneuro-2013-0068
Leighton, A. H., and Lohmann, C. (2016). The wiring of developing sensory circuits-from patterned spontaneous activity to synaptic plasticity mechanisms. Front. Neural Circuits 10:71. doi: 10.3389/fncir.2016.00071
Lim, P. B., Malcolme-Lawes, L. C., Stuber, T., Kojodjojo, P., Wright, I. J., Francis, D. P., et al. (2011). Stimulation of the intrinsic cardiac autonomic nervous system results in a gradient of fibrillatory cycle length shortening across the atria during atrial fibrillation in humans. J. Cardiovasc. Electrophysiol. 22, 1224–1231. doi: 10.1111/j.1540-8167.2011.02097.x
Lin, Y. Q., Brain, K. L., and Bennett, M. R. (1998). Calcium in sympathetic boutons of rat superior cervical ganglion during facilitation, augmentation and potentiation. J. Autonom. Nerv. Sys. 73, 26–37. doi: 10.1016/S0165-1838(98)00108-8
Linz, D., Ukena, C., Mahfoud, F., Neuberger, H. R., and Böhm, M. (2014). Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J. Am. Coll. Cardiol. 63, 215–224. doi: 10.1016/j.jacc.2013.09.020
Liu, D. M., Cuevas, J., and Adams, D. J. (2000). VIP and PACAP potentiation of nicotinic ACh-evoked currents in rat parasympathetic neurons is mediated by G-protein activation. Eur. J. Neurosci. 12, 2243–2251. doi: 10.1046/j.1460-9568.2000.00116.x
Magee, J. C., and Schofield, G. G. (1992). Neurotransmission through sympathetic ganglia of spontaneously hypertensive rats. Hypertension 20, 367–373. doi: 10.1161/01.HYP.20.3.367
Magee, J. C., and Schofield, G. G. (1994). Alterations of synaptic transmission in sympathetic ganglia of spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 267, R1397–R1407. doi: 10.1152/ajpregu.1994.267.5.R1397
Mawe, G. M., Talmage, E. K., Lee, K. P., and Parsons, R. L. (1996). Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tiss. Res. 285, 281–286. doi: 10.1007/s004410050645
McAllen, R. M., Salo, L. M., Paton, J. F. R., and Pickering, A. E. (2011). Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J. Physiol. 589, 5801–5818. doi: 10.1113/jphysiol.2011.214320
McGrattan, P. A., Brown, J. H., and Brown, O. M. (1987). Parasympathetic effects on in vivo rat heart can be regulated through an alpha 1-adrenergic receptor. Circ. Res. 60, 465–471. doi: 10.1161/01.RES.60.4.465
Mellor, J., Nicoll, R. A., and Schmitz, D. (2002). Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science 295, 143–147. doi: 10.1126/science.1064285
Montgomery, J. M., Pavlidis, P., and Madison, D. V. (2001). Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of LTP. Neuron 29, 691–701. doi: 10.1016/S0896-6273(01)00244-6
Morris, R. G., Anderson, E., Lynch, G. S., and Baudry, M. (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776. doi: 10.1038/319774a0
Nabavi, S., Fox, R., Proulx, C. D., Lin, J. Y., Tsien, R. Y., and Malinow, R. (2014). Engineering a memory with LTD and LTP. Nature 511, 348–352. doi: 10.1038/nature13294
Ng, G. A., Brack, K. E., Patel, V. H., and Coote, J. H. (2007). Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc. Res. 73, 750–760. doi: 10.1016/j.cardiores.2006.12.001
Osman, F., Kundu, S., Tuan, J., Jeilan, M., Stafford, P. J., and Ng, G. A. (2010). Ganglionic plexus ablation during pulmonary vein isolation–predisposing to ventricular arrhythmias? Indian Pacing Electrophysiol. J. 10, 104–107. www.ncbi.nlm.nih.gov/pubmed/20126597
Pappone, C., Santinelli, V., Manguso, F., Vicedomini, G., Gugliotta, F., Augello, G., et al. (2004). Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 109, 327–334. doi: 10.1161/01.CIR.0000112641.16340.C7
Pardini, B. J., Lund, D. D., and Schmid, P. G. (1989). Organization of the sympathetic postganglionic innervation of the rat heart. J. Auton. Nerv. Syst. 28, 193–201. doi: 10.1016/0165-1838(89)90146-X
Pauza, D. H., Skripka, V., Pauziene, N., and Stropus, R. (2000). Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 259, 353–82. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R
Pauziene, N., and Pauza, D. H. (2003). Electron microscopic study of intrinsic cardiac ganglia in the adult human. Ann. Anat. 185, 135–148. doi: 10.1016/S0940-9602(03)80077-8
Pokushalov, E., Romanov, A., Shugayev, P., Artyomenko, S., Shirokova, N., Turov, A., et al. (2009). Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 6, 1257–1264. doi: 10.1016/j.hrthm.2009.05.018
Pugh, P. C., Jayakar, S. S., and Margiotta, J. F. (2010). PACAP/PAC1R signaling modulates acetylcholine release at neuronal nicotinic synapses. Mol. Cell. Neurosci. 43, 244–257. doi: 10.1016/j.mcn.2009.11.007
Rajendran, P. S., Nakamura, K., Ajijola, O. A., Vaseghi, M., Armour, J. A., Ardell, J. L., et al. (2016). Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J. Physiol. 594, 321–341. doi: 10.1113/JP271165
Richardson, R. J., Grkovic, I., and Andreson, C. R. (2003). Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res. 314, 337–350. doi: 10.1007/s00441-003-0805-2
Rimmer, K., and Harper, A. A. (2006). Developmental changes in electrophysiological properties and synaptic transmission in rat intracardiac ganglion neurons. J. Neurophysiol. 95, 3543–3552. doi: 10.1152/jn.01220.2005
Scherlag, B. J., Patterson, E., and Po, S. S. (2006). The neural basis of atrial fibrillation. J. Electrocardiol. 39, S180–S183. doi: 10.1016/j.jelectrocard.2006.05.021
Schotten, U., Verheule, S., Kirchhof, P., and Goette, A. (2011). Pathophysiological mechanisms of atrial fibrillation : a translational appraisal. Physiol. Rev. 91, 265–325. doi: 10.1152/physrev.00031.2009
Shanks, J., Manou-Stathopoulou, S., Lu, C. J., Li, D., Paterson, D. J., and Herring, N. (2013). Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. Am. J. Physiol. Heart Circ. Physiol. 305, H980–H986. doi: 10.1152/ajpheart.00255.2013
Shcherbakova, O. G., Hurt, C. M., Xiang, Y., Dell'Acqua, M. L., Zhang, Q., Tsien, R. W., et al. (2007). Organization of b-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J. Cell Biol. 176, 521–533. doi: 10.1083/jcb.200604167
Shen, M. J., Choi, E.-K., Tan, A. Y., Lin, S.-F., Fishbein, M. C., Chen, L. S., et al. (2012). Neural mechanisms of atrial arrhythmias. Nat. Rev. Cardiol. 9, 30–39. doi: 10.1038/nrcardio.2011.139
Shi, S., Liu, T., Li, Y., Qin, M., Tang, Y., Shen, J. Y., et al. (2014). Chronic N-methyl-d-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing. Clin. Electrophysiol. 37, 1367–1377. doi: 10.1111/pace.12430
Shi, S., Liu, T., Wang, D., Zhang, Y., Liang, J., Hu, D., et al. (2017). Activation of N-methyl-d-aspartate receptors reduces heart rate variability and facilitates atrial fibrillation in rats. EP. Europace. 19, 1237–1243. doi: 10.1093/europace/euw086
Shvalev, V. N., and Sosunov, A. A. (1985). A light and electron microscopic study of cardiac ganglia in mammals. Z. Mikrosk Anat. Forsch. 99, 676–694.
Singh, S., Johnson, P. I., Javed, A., Gray, T. S., Lonchyna, V. A., and Wurster, R. D. (1999). Monoamine- and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation 99, 411–419. doi: 10.1161/01.CIR.99.3.411
Slavíková, J., Kuncová, J., Reischig, J., and Dvoráková, M. (2003). Catecholaminergic neurons in the rat intrinsic cardiac nervous system. Neurochem. Res. 28, 593–598. doi: 10.1023/A:1022837810357
Smith, F. M., Vermeulen, M., and Cardinal, R. (2016). Long-term spinal cord stimulation modifies canine intrinsic cardiac neuronal properties and ganglionic transmission during high-frequency repetitive activation. Physiol. Rep. 4:e12855. doi: 10.14814/phy2.12855
Spyer, K. M. (2011). Vagal preganglionic neurons innervating the heart. Compr. Physiol. 2011, 213–239. doi: 10.1002/cphy.cp020105
Staerk, L., Sherer, J. A., Ko, D., Benjamin, E. J., and Helm, R. H. (2017). Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ. Res. 120, 1501–1517. doi: 10.1161/CIRCRESAHA.117.309732
Tan, A. Y., Li, H., Wachsmann-Hogiu, S., Chen, L. S., Chen, P. S., and Fishbein, M. C. (2006). Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J. Am. Coll. of Cardiol. 48, 132–143. doi: 10.1016/j.jacc.2006.02.054
Tan, A. Y., Zhou, S., Ogawa, M., Song, J., Chu, M., Li, H., et al. (2008). Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 118, 916–925. doi: 10.1161/CIRCULATIONAHA.108.776203
Tang, A. H., Chen, H., Li, T. P., Metzbower, S. R., MacGillvary, H. D., and Blanpied, T. A. (2016). A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214. doi: 10.1038/nature19058
Tompkins, J. D., Ardell, J. L., Hoover, D. B., and Parsons, R. L. (2007). Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J. Physiol. 582, 87–93. doi: 10.1113/jphysiol.2007.134965
Tompkins, J. D., Lawrence, Y. T., Parsons, R. L., Dyavanapalli, J., Rimmer, K., and Harper, A. A. (2009). Enhancement of Ih , but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R52–R59. doi: 10.1152/ajpregu.00039.2009
Tompkins, J. D., Merriam, L. A., Girard, B. M., May, V., and Parsons, R. L. (2015). Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am. J. Physiol. Cell Physiol. 308, C857–C866. doi: 10.1152/ajpcell.00403.2014
Vargas, R., Cifuentes, F., and Morales, M. A. (2011). Role of presynaptic and postsynaptic IP3-dependent intracellular calcium release in long-term potentiation in sympathetic ganglion of the rat. Synapse. 65, 441–448. doi: 10.1002/syn.20862
Volk, L., Chiu, S. L., Sharma, K., and Huganir, R. L. (2015). Glutamate synapses in human cognitive disorders. Ann. Rev. Neurosci. 38, 127–149. doi: 10.1146/annurev-neuro-071714-033821
Wake, E., and Brack, K. (2016). Characterization of the intrinsic cardiac nervous system. Auton. Neurosci. 199, 3–16. doi: 10.1016/j.autneu.2016.08.006
Weerasooriya, R., Khairy, P., Litalien, J., Macle, L., Hocini, M., Sacher, F., et al. (2011). Catheter ablation for atrial fibrillation: are results maintained at 5 years follow up? J. Am. Coll. of Cardiol. 57, 160–166. doi: 10.1016/j.jacc.2010.05.061
Wijffels, M. C., Kirchhof, C. J., Dorland, R., and Allessie, M. A. (1995). Atrial fibrillation begets atrial fibrillation. a study in awake chronically instrumented goats. Circulation 92, 1954–1968. doi: 10.1161/01.CIR.92.7.1954
Yarowsky, P., and Weinreich, D. (1985). Loss of accommodation in sympathetic neurons from spontaneously hypertensive rats. Hypertension 7, 268–276. doi: 10.1161/01.HYP.7.2.268
Keywords: atria, innervation, ganglionated plexi, synapse plasticity, atrial fibrillation, LTP
Citation: Ashton JL, Burton RAB, Bub G, Smaill BH and Montgomery JM (2018) Synaptic Plasticity in Cardiac Innervation and Its Potential Role in Atrial Fibrillation. Front. Physiol. 9:240. doi: 10.3389/fphys.2018.00240
Received: 20 October 2017; Accepted: 06 March 2018;
Published: 20 March 2018.
Edited by:
Tijana Bojić, Vinča Nuclear Institute, University of Belgrade, SerbiaReviewed by:
Keith L. Brain, University of Birmingham, United KingdomG. Andre Ng, University of Leicester, United Kingdom
Copyright © 2018 Ashton, Burton, Bub, Smaill and Montgomery. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johanna M. Montgomery, jm.montgomery@auckland.ac.nz
 Jesse L. Ashton
Jesse L. Ashton Rebecca A. B. Burton2
Rebecca A. B. Burton2 Bruce H. Smaill
Bruce H. Smaill Johanna M. Montgomery
Johanna M. Montgomery