- Department of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI, USA
The specification of distinct cell types in multicellular organisms is accomplished via establishment of differential gene expression. A major question is the nature of the mechanisms that establish this differential expression in time and space. In plants, the formation of the hair and non-hair cell types in the root epidermis has been used as a model to understand regulation of cell specification. Recent findings show surprising complexity in the number and the types of regulatory interactions between the multiple transcription factor genes/proteins influencing root epidermis cell fate. Here, we describe this regulatory network and the importance of the multiple feedback loops for its establishment and maintenance.
Epidermal Cell Patterning in the Arabidopsis Root
The specification of root hair cells and non-hair cells in the Arabidopsis root is a well-studied model for understanding cell fate decisions in plants (Schiefelbein et al., 2009; Tominaga-Wada et al., 2011; Grebe, 2012). Newly formed epidermal cells located outside the cleft separating two adjacent underlying cortical cells (the “H” cell position) differentiate into root-hair cells, whereas epidermal cells not located over the cleft (the “N” cell position) develop into non-hair cells, due to differential cell-type-specific gene expression (Figure 1) (Cormack, 1962; Berger et al., 1998; Bruex et al., 2012). Genetic and molecular studies over the past 20 years have now provided a fairly clear picture of the transcriptional regulators responsible for establishing this differential cell-type gene expression. What has been surprising is the large number of regulatory mechanisms and interactions by these transcription factors in the process of root epidermal cell specification. In this mini-review, we describe the basic transcription factor components and then we outline the many categories of regulatory mechanisms and their roles in establishing the epidermal cell fates.
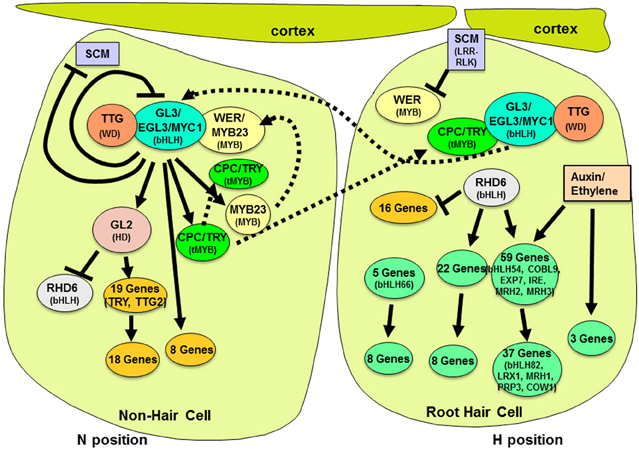
Figure 1. Molecular genetic regulation of cell fate specification in the Arabidopsis root epidermis. Root-hair specific gene expression occurs in differentiating epidermal cells located in a cleft between adjacent cortical cells (right-most cell). Gene regulatory activity is indicated by solid lines with arrows (positive transcriptional effect) or bars (negative transcriptional effect). Dotted lines represent the movement of proteins within or between cells. The downstream genes shown here are taken from Bruex et al. (2012) and defined as hair or non-hair genes based on their accumulation in the root epidermis in either cpc try (non-hair) mutants vs. ttg1, wer myb23, or gl3 egl3 (hairy) mutants.
The Basic Components of the Network
At its core, cell fate in the root epidermis is dependent on the relative abundance of a transcription factor complex consisting of three types of proteins: the Myb-domain protein WEREWOLF (WER) (Lee and Schiefelbein, 1999), two redundantly acting bHLHs GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) (Payne et al., 2000; Bernhardt et al., 2003, 2005; Simon et al., 2013), and the WD-repeat TRANSPARENT TESTA GLABRA (TTG1) (Galway et al., 1994). Differentiating epidermal cells that generate a significant amount of this WER-bHLH-TTG complex express the downstream HD-ZIP gene GLABRA2 (GL2), which represses transcription of the hair-cell promoting bHLH ROOT HAIR DEFECTIVE 6 (RHD6) (Masucci and Schiefelbein, 1994; Menand et al., 2007), leading to the expression of dozens of non-hair-cell-specific genes and the non-hair cell fate (Figure 1) (Masucci et al., 1996; Bruex et al., 2012). Differentiating cells that do not accumulate a significant amount of the WER-bHLH-TTG complex are able to express RHD6, and as a result, initiate transcription of hundreds of root-hair-cell-specific genes (Cvrckova et al., 2010; Bruex et al., 2012). Given the key role of the WER-bHLH-TTG transcriptional complex for the cell fate decision, there has been great interest in defining and understanding the mechanisms that regulate its accumulation in the two cell types. Recent research has uncovered an array of intra- and intercellular mechanisms responsible for controlling the abundance of this key complex.
Regulatory Mechanisms in the Network
Lateral Inhibition
The activity of the WER-bHLH-TTG complex is inhibited by a set of small, one-repeat Myb proteins, which includes CAPRICE (CPC), TRIPTYCHON (TRY), and ENHANCER OF TRY AND CPC1 (ETC1) (Wada et al., 1997; Schellmann et al., 2002; Kirik et al., 2004; Simon et al., 2007). These proteins are able to bind to the GL3/EGL3 bHLHs, competitively inhibiting WER binding and generating a non-functional complex (Lee and Schiefelbein, 2002). Accordingly, these proteins accumulate in the H-position cells, where they promote the hair cell fate (Kurata et al., 2005; Kang et al., 2013). Unexpectedly, the transcription of these one-repeat Mybs was found to occur predominately in the N cells, due to positive regulation by the WER-bHLH-TTG complex itself, and the proteins appear to move through plasmodesmata to accumulate in the H cells (Kurata et al., 2005; Kang et al., 2013). The ability of cells adopting the non-hair fate (accumulating WER-bHLH-TTG) to generate diffusible molecules (CPC/TRY/ETC1) that prevent adjacent cells from adopting the same fate (via inhibition of the WER-bHLH-TTG action) effectively represents a kind of lateral inhibition mechanism, a general strategy widely employed by multicellular organisms to establish distinct cell identities in an initially equivalent field of cells (Meinhardt and Gierer, 2000). What is unusual about the lateral inhibition used here is its direct nature; the molecule produced by the inhibiting cell is used as both the signal and the inhibitor of the recipient cell.
Feedback at Multiple Developmental Times
Although the CPC, TRY, and ETC1 genes are all positively regulated by the WER-bHLH-TTG complex, the effect on TRY is indirect because it is downstream of the N-cell regulator GL2 (Figure 1) (Simon et al., 2007). This means that TRY production will be developmentally delayed, relative to CPC and ETC1. Since the CPC/ETC1 and TRY proteins are members of different subtypes and appear to vary in their properties (Pesch and Hulskamp, 2011), this regulatory organization may generate different ratios of subtypes during epidermis development important for pattern establishment.
Positive Feedback
The MYB23 protein is the Arabidopsis MYB most closely related to WER, and MYB23 is capable of substituting for WER in root hair development (Kang et al., 2009). Further, the MYB23 gene is under the positive transcriptional regulatory control of the WER-bHLH-TTG complex. Taken together, these data indicate that the MYB23 gene participates in a positive feedback loop in the N cells (Figure 1), which apparently is used to maintain relatively high levels of the complex, due to MYB23's presumed participation in the complex (Kang et al., 2009). The identification of a positive feedback loop affecting the WER-bHLH-TTG complex was satisfying, because theoretical models of lateral inhibition in pattern formation typically require such self-promoting loops to create stable peaks of activator accumulation even in the presence of high inhibitor levels (Meinhardt and Gierer, 1974, 2000). Accordingly, in the root epidermal system, mutants lacking MYB23 function are less able to adopt appropriate cell fate decisions (Kang et al., 2009).
Mutual Reinforcing Loops
The GL3 and EGL3 bHLH genes were found to be preferentially transcribed, and to have their transcripts preferentially accumulate, in the H cells rather than the N cells of the developing root epidermis, due to negative transcriptional regulation of these genes by the WER-bHLH-TTG complex (Bernhardt et al., 2005). This was an unexpected finding, since the GL3 and EGL3 proteins are required for the proper function and differentiation of the N cells. Based on the use of GFP-tagged proteins, the GL3/EGL3 gene products were found to preferentially accumulate in the N cells, implying movement of these proteins from H to N cells (Figure 1). The opposite movement of the hair-promoting CPC/TRY/ETC1 (N to H cells) vs. the non-hair-promoting GL3/EGL3 (H to N cells) has been proposed to represent an intercellular mutual reinforcing loop that exists to provide robustness to the patterning system (Figure 1) and this view has received support from theoretical modeling studies (Savage et al., 2008; Benitez and Alvarez-Buylla, 2010).
Molecular Trapping
The observed preferential accumulation of CPC (and presumably TRY and ETC1) in the H cells is believed to be necessary for robust pattern formation, though the mechanism responsible for causing these mobile factors to accumulate in H cells has long been a mystery. A possible explanation has recently been provided by the finding that this accumulation is EGL3 dependent (Kang et al., 2013). A CPC-GFP fusion protein, expressed under control of the CPC or SHORTROOT (SHR) promoter, lacked preferential H-cell accumulation in the gl3 egl3 mutant and exhibited reduced movement in GL3/EGL3 overexpression lines (Kang et al., 2013). One possibility is that EGL3 serves to “trap” the CPC inside the H cells by relatively strong binding (Kang et al., 2013). This is at least superficially similar to the proposed nuclear trapping of TTG1 by GL3 in the trichome specification system (Balkunde et al., 2011).
Feedback on Positional Signaling
The position-dependent pattern of hair and non-hair cells is dependent on signaling through the SCRAMBLED (SCM) receptor-like kinase (Kwak and Schiefelbein, 2007). SCM action leads to reduced WER transcription, and since this appears to preferentially occur in the H cell position, it explains how SCM signaling helps generate the cell-type pattern. Interestingly, the preferential SCM action in the H cells is likely due to differential accumulation of SCM. This differential SCM accumulation is achieved by a negative feedback loop between the WER-bLHLH-TTG complex and the SCM gene expression, ensuring reduced SCM signaling in the N position (Kwak and Schiefelbein, 2008). It is proposed that this mechanism helps to “lock in” the cell fate decision, by amplifying the differential SCM signaling ability of the two cell types.
Regulation by Hormones
Root hair development is affected by several plant hormones, most commonly reported for auxin and ethylene (Masucci and Schiefelbein, 1996; Pitts et al., 1998). In general, the hormones appear to promote root hair formation, because addition of exogenous hormone typically leads to increased hair length or number whereas inhibition of hormone production/action tends to reduce hair length or number. Indeed, transcriptome assays show that a majority of the downstream root hair genes, but not the non-hair genes, are responsive to addition of auxin (IAA) or ethylene (ACC) (Bruex et al., 2012). Thus, the accumulation and activity of hormone pathway elements provide the opportunity for regulation of root-hair pattern at a relatively late stage, perhaps allowing for environmental influence of root hair formation.
Regulation by Histone Modification
The expression of the patterning genes and the arrangement of the root cell types are influenced by histone acetylation. Treatment of roots with trichostatin A (histone deacetylase inhibitor) or mutations in the histone deacetylase gene HDA18 cause N-position cells to become root hair cells (Xu et al., 2005). Because the HDA18 protein does not directly bind to the patterning gene promoters (Liu et al., 2013), there may be an intermediate set of histone-regulated genes responsible for this level of control.
Thoughts on the Complexity of the Network
In this minireview, we have highlighted the multitude of regulatory mechanisms that are employed to control the relative abundance of the critical transcription factors in epidermal cell specification. Considering these many components and interactions (Figure 1), it is appropriate to wonder why this system has evolved such complexity to control a seemingly simple case of cell fate specification. One possible explanation is that the complex regulatory interactions reflect a requirement for robustness; to ensure that once a cell fate decision is made, that this decision is fully adopted and is not allowed to be altered at any step (Barkai and Leibler, 1997; Benitez and Alvarez-Buylla, 2010). Another possibility for the existence of multiple regulatory mechanisms may be that they provide opportunities for the modification/adjustment of the cell fate decision at many points in the process, perhaps enabling it to respond to the many known internal and external factors that influence root hair development (Forde and Lorenzo, 2001; Datta et al., 2011). Future studies on the control of root epidermal cell fate in Arabidopsis and other species will likely yield additional insight into the importance of the many components and interactions in this complex regulatory network.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank members of our research laboratory for helpful discussions. We apologize to those whose work could not be cited due to space constraints. Research in our group is supported by grants from the National Science Foundation (IOS-0723493 and IOS-1121602).
References
Balkunde, R., Bouyer, D., and Hulskamp, M. (2011). Nuclear trapping by GL3 controls intercellular transport and redistribution of TTG1 protein in Arabidopsis. Development 138, 5039–5048. doi: 10.1242/dev.072454
Barkai, N., and Leibler, S. (1997). Robustness in simple biochemical networks. Nature 387, 913–917. doi: 10.1038/43199
Benitez, M., and Alvarez-Buylla, E. R. (2010). Dynamic-module redundancy confers robustness to the gene regulatory network involved in hair patterning of Arabidopsis epidermis. Biosystems 102, 11–15. doi: 10.1016/j.biosystems.2010.07.007
Berger, F., Haseloff, J., Schiefelbein, J., and Dolan, L. (1998). Positional information in root epidermis is defined during embryogenesis and acts in domains with strict boundaries. Curr. Biol. 8, 421–430. doi: 10.1016/S0960-9822(98)70176-9
Bernhardt, C., Lee, M. M., Gonzalez, A., Zhang, F., Lloyd, A., and Schiefelbein, J. (2003). The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130, 6431–6439. doi: 10.1242/dev.00880
Bernhardt, C., Zhao, M., Gonzalez, A., Lloyd, A., and Schiefelbein, J. (2005). The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132, 291–298. doi: 10.1242/dev.01565
Bruex, A., Kainkaryam, R. M., Wieckowski, Y., Kang, Y. H., Bernhardt, C., Xia, Y., et al. (2012). A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet. 8:e1002446. doi: 10.1371/journal.pgen.1002446
Cormack, R. G. H. (1962). Development of root hairs in angiosperms. II. Bot. Rev. 28, 446–464. doi: 10.1007/BF02868690
Cvrckova, F., Bezvoda, R., and Zarsky, V. (2010). Computational identification of root hair-specific genes in Arabidopsis. Plant Signal. Behav. 5, 1407–1418. doi: 10.4161/psb.5.11.13358
Datta, S., Kim, C. M., and Pernas, M. (2011). Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil 346, 1–14. doi: 10.1007/s11104-011-0845-4
Forde, B., and Lorenzo, H. (2001). The nutritional control of root development. Plant Soil 232, 51–68. doi: 10.1023/A:1010329902165
Galway, M. E., Masucci, J. D., Lloyd, A. M., Walbot, V., Davis, R. W., and Schiefelbein, J. W. (1994). The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev. Biol. 166, 740–754. doi: 10.1006/dbio.1994.1352
Grebe, M. (2012). The patterning of epidermal hairs in Arabidopsis–updated. Curr. Opin. Plant Biol. 15, 31–37. doi: 10.1016/j.pbi.2011.10.010
Kang, Y. H., Kirik, V., Hulskamp, M., Nam, K. H., Hagely, K., Lee, M. M., et al. (2009). The MYB23 gene provides a positive feedback loop for cell fate specification in the Arabidopsis root epidermis. Plant Cell 21, 1080–1094. doi: 10.1105/tpc.108.063180
Kang, Y. H., Song, S. K., Schiefelbein, J., and Lee, M. M. (2013). Nuclear trapping controls the position-dependent localization of CAPRICE in the root epidermis of Arabidopsis. Plant Physiol. 163, 193–204. doi: 10.1104/pp.113.221028
Kirik, V., Simon, M., Huelskamp, M., and Schiefelbein, J. (2004). The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev. Biol. 268, 506–513. doi: 10.1016/j.ydbio.2003.12.037
Kurata, T., Ishida, T., Kawabata-Awai, C., Noguchi, M., Hattori, S., Sano, R., et al. (2005). Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132, 5387–5398. doi: 10.1242/dev.02139
Kwak, S. H., and Schiefelbein, J. (2007). The role of the SCRAMBLED receptor-like kinase in patterning the Arabidopsis root epidermis. Dev. Biol. 302, 118–131. doi: 10.1016/j.ydbio.2006.09.009
Kwak, S. H., and Schiefelbein, J. (2008). A feedback mechanism controlling SCRAMBLED receptor accumulation and cell-type pattern in Arabidopsis. Curr. Biol. 18, 1949–1954. doi: 10.1016/j.cub.2008.10.064
Lee, M. M., and Schiefelbein, J. (1999). WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99, 473–483. doi: 10.1016/S0092-8674(00)81536-6
Lee, M. M., and Schiefelbein, J. (2002). Cell pattern in the Arabidopsis root epidermis determined by lateral inhibition with feedback. Plant Cell 14, 611–618. doi: 10.1105/tpc.010434
Liu, C., Li, L. C., Chen, W. Q., Chen, X., Xu, Z. H., and Bai, S. N. (2013). HDA18 affects cell fate in Arabidopsis root epidermis via histone acetylation at four kinase genes. Plant Cell 25, 257–269. doi: 10.1105/tpc.112.107045
Masucci, J. D., Rerie, W. G., Foreman, D. R., Zhang, M., Galway, M. E., Marks, M. D., et al. (1996). The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122, 1253–1260.
Masucci, J. D., and Schiefelbein, J. W. (1994). The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 106, 1335–1346.
Masucci, J. D., and Schiefelbein, J. W. (1996). Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell 8, 1505–1517.
Meinhardt, H., and Gierer, A. (1974). Applications of a theory of biological pattern formation based on lateral inhibition. J. Cell Sci. 15, 321–346.
Meinhardt, H., and Gierer, A. (2000). Pattern formation by local self-activation and lateral inhibition. Bioessays 22, 753–760. doi: 10.1002/1521-1878(200008)22:8%3C753::AID-BIES9%3E3.3.CO;2-Q
Menand, B., Yi, K., Jouannic, S., Hoffmann, L., Ryan, E., Linstead, P., et al. (2007). An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316, 1477–1480. doi: 10.1126/science.1142618
Payne, C. T., Zhang, F., and Lloyd, A. M. (2000). GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics 156, 1349–1362.
Pesch, M., and Hulskamp, M. (2011). Role of TRIPTYCHON in trichome patterning in Arabidopsis. BMC Plant Biol. 11:130. doi: 10.1186/1471-2229-11-130
Pitts, R. J., Cernac, A., and Estelle, M. (1998). Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 16, 553–560. doi: 10.1046/j.1365-313x.1998.00321.x
Savage, N. S., Walker, T., Wieckowski, Y., Schiefelbein, J., Dolan, L., and Monk, N. A. (2008). A mutual support mechanism through intercellular movement of CAPRICE and GLABRA3 can pattern the Arabidopsis root epidermis. PLoS Biol. 6:e235. doi: 10.1371/journal.pbio.0060235
Schellmann, S., Schnittger, A., Kirik, V., Wada, T., Okada, K., Beermann, A., et al. (2002). TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 21, 5036–5046. doi: 10.1093/emboj/cdf524
Schiefelbein, J., Kwak, S. H., Wieckowski, Y., Barron, C., and Bruex, A. (2009). The gene regulatory network for root epidermal cell-type pattern formation in Arabidopsis. J. Exp. Bot. 60, 1515–1521. doi: 10.1093/jxb/ern339
Simon, M., Bruex, A., Kainkaryam, R. M., Zheng, X., Huang, L., Woolf, P., et al. (2013). Tissue-specific profiling reveals transcriptome alterations in Arabidopsis mutants lacking morphological phenotypes. Plant Cell 25, 3175–3185. doi: 10.1105/tpc.113.115121
Simon, M., Lee, M. M., Lin, Y., Gish, L., and Schiefelbein, J. (2007). Distinct and overlapping roles of single-repeat MYB genes in root epidermal patterning. Dev. Biol. 311, 566–578. doi: 10.1016/j.ydbio.2007.09.001
Tominaga-Wada, R., Ishida, T., and Wada, T. (2011). New insights into the mechanism of development of Arabidopsis root hairs and trichomes. Int. Rev. Cell Mol. Biol. 286, 67–106. doi: 10.1016/B978-0-12-385859-7.00002-1
Wada, T., Tachibana, T., Shimura, Y., and Okada, K. (1997). Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277, 1113–1116. doi: 10.1126/science.277.5329.1113
Keywords: root hairs, transcription factors, pattern formation, feedback loops, Arabidopsis thaliana
Citation: Schiefelbein J, Huang L and Zheng X (2014) Regulation of epidermal cell fate in Arabidopsis roots: the importance of multiple feedback loops. Front. Plant Sci. 5:47. doi: 10.3389/fpls.2014.00047
Received: 26 December 2013; Paper pending published: 14 January 2014;
Accepted: 30 January 2014; Published online: 17 February 2014.
Edited by:
Shucai Wang, Northeast Normal University, ChinaReviewed by:
Shu-Nong Bai, Peking University, ChinaRoss Sozzani, North Carolina State Unversity, USA
Copyright © 2014 Schiefelbein, Huang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Schiefelbein, Department of Molecular, Cellular, and Developmental Biology, University of Michigan, 830 North University Avenue, Ann Arbor, MI 48109, USA e-mail: schiefel@umich.edu
 John Schiefelbein
John Schiefelbein Ling Huang
Ling Huang