- 1Department of Plant Breeding, Institute for Sustainable Agriculture, Spanish National Research Council, Córdoba, Spain
- 2Department of Plant-Microbe Interactions, Max Planck Institute of Molecular Plant Physiology, Potsdam, Germany
Resistance to powdery mildew has been studied in a number of plant species, yet the molecular mechanisms remain largely unknown. Transcription factors (TFs) play a critical role in the plant defense response by regulating the transcriptional machinery which coordinates the expression of a large group of genes involved in plant defense. Using high-throughput quantitative real-time PCR (qPCR) technology more than 1000 Medicago truncatula TFs were screened in a pair of susceptible and resistant genotypes of M. truncatula after 4 h of Erysiphe pisi infection. Seventy nine TF genes, belonging to 33 families showed a significant transcriptional change in response to E. pisi infection. Forty eight TF genes were differentially expressed in the resistant genotypes compared to the susceptible one in response to E. pisi infection, including pathogenesis-related transcriptional factors, AP2/EREBP (APETALA2/ETHYLENE-RESPONSIVE ELEMENT BINDING FACTORS), WRKY (highly conserved WRKYGQK amino-acid sequence), MYB (Myeloblastoma), homeodomain (HD) and zinc finger C2C2 (CYS2-CYS2), C2H2, (CYS2-HIS2), LIM (Lin-11, Isl-1, Mec-3) gene families, which are involved in known defense responses. Our results suggest that these TF genes are among the E. pisi responsive genes in resistant M. truncatula that may constitute a regulatory network which controls the transcriptional changes in defense genes involved in resistance to E. pisi.
Introduction
Plants grown in the natural environment are confronted by a variety of pathogens. Remaining healthy depends on their ability to recognize pathogens and to activate defense mechanisms against them. The plant defense responses are regulated by a broad number of signaling pathways. Transcription factors (TFs) control the transfer of genetic information from DNA to RNA by activation or repression of transcription, playing important roles in plant development and defense by regulating different signaling pathways (Singh et al., 2002; Udvardi et al., 2007). Data from several plant genome projects suggest that more than five percent of the plant genome encodes TF sequences (around 2000 TFs) (Riechmann and Ratcliffe, 2000). Therefore, many biologic processes, including responses to pathogens, are controlled by multiple genes managed by TFs (Singh et al., 2002). Several analyses have shown their differential expression in plants as responses to interactions with biotic and abiotic effectors (Udvardi et al., 2007). In spite of the importance of legumes as sources of protein and oil, and in the symbiotic nitrogen fixation, less than one percent of the transcript-specific regulation roles of TFs have been characterized in legumes (Udvardi et al., 2007). Among legumes, Medicago truncatula is a model with key attributes such as self-fertility, rapid generation time, and a small diploid genome (Singh et al., 2007) that have facilitated the use of molecular and genetic tools (Rose, 2008).
Powdery mildews are biotrophic plant pathogens that seriously constrain crop production worldwide (Bélanger et al., 2002). Erysiphe spp. cause considerable losses in various important legume crops (Sillero et al., 2006). This fungus has been classified into three physiologically specialized forms, f.sp. pisi specialized on Pisum, f.sp. medicaginis specialized on Medicago, and f.sp. vicia sativa, specialized on Vicia (Falloon and Viljanen-Rollinson, 2001). Breeding for powdery mildew resistance is the most desirable strategy to control this disease by means of resistant cultivars (Fondevilla and Rubiales, 2012; Rubiales et al., 2015). Consequently, several genes involved in resistance to powdery mildew have been reported in different plant species (Fondevilla et al., 2007; Yang et al., 2013; Barilli et al., 2014; Curto et al., 2015; Iglesias-García et al., 2015). High-throughput methods have resulted in identification of genes potentially associated with specific processes and characterization of the regulatory networks that control their expression (Czechowski et al., 2004; Caldana et al., 2007). Among them, DNA microarrays have been used successfully to characterize global gene expression patterns in M. truncatula (Foster-Hartnett et al., 2007; Samac et al., 2011; Zhang et al., 2014; Curto et al., 2015; Song et al., 2015) providing detailed information of metabolic pathways involved in the analyzed systems. Previous studies have analyzed the E. pisi/M. truncatula pathosystem (Foster-Hartnett et al., 2007; Samac et al., 2011; Curto et al., 2015) using different genotypes and microarray platforms, such as Mt16kOLI1, Mt16kOLI1plus (Küster et al., 2004, 2007), and Affymetrix GeneChip® (http://www.affymetrix.com). These studies have increased the knowledge of mechanisms involved in E. pisi resistance in M. truncatula, which are agreement that a wide variety of mechanisms and pathways are involved in E. pisi resistance including pathogenesis-related genes (i.e., PR10, Pprg2), as well as other genes involved in signal transduction, cell wall metabolism (i.e., Glucan endo-1,3-beta-D-glucosidase, Pectinase) and abiotic stress, such as Heat shock protein 17.7, UVB-resistance protein BudCAR5, and Dehydration-responsive protein (RD22). Although DNA microarrays have been shown to be five times less sensitive than qPCR (Czechowski et al., 2004), due to its high cost qPCR remains a technique used for low- to middle-scale studies. Several large-scale TF profiling approaches have employed the M. truncatula qPCR-based platform available (Kakar et al., 2008) in various studies (Verdier et al., 2008; Gao et al., 2010; Madrid et al., 2010; Villegas-Fernández et al., 2014). In this study, we screened the TF transcriptome of M. truncatula for altered expression during E. pisi infection using qPCR. Previous histological assessments showed that the resistance mechanisms carried out by the resistant genotype SA1306 is mainly related to hampering spore germination and further colony establishment by epidermal cell death as a hypersensitive response to E. pisi germlings that develop appressoria (Curto et al., 2015). Several mechanisms capable of monitoring changes in the plant cell wall are carried out by cellular signaling responses (Ringli, 2010; Cheung and Wu, 2011). The present study has allowed us to identify the transcription factor-encoding genes involved in the E. pisi/M. truncatula pathosystem, which are candidates for further functional studies. In addition, this approach provides a model for the regulatory network controlling the expression of TF genes in this pathosystem.
Materials and Methods
Plant Material, Growth Conditions, and Inoculation
The study was performed through an analysis of two genotypes of M. truncatula, the commercial cultivar M. truncatula Gaertn. v. Parabinga and the accession SA1306, shown to be susceptible and resistant, respectively to E. pisi f.sp. Medicaginis (Curto et al., 2015).
The seeds of M. truncatula were pre-soaked in filter paper, kept in dark conditions at 4°C for 24 h, and germinated in the dark for 48 h in a growth chamber at 65% relative humidity and 20°C. The seedlings were placed in pots (125 ml) containing a 1:1 mixture of perlite and sand substrate, fertilized with half-strength Hoagland's solution (Hoagland and Arnon, 1950) 3 times a week, and grown (25°C, 12 h photoperiod, 250 μmol/m2 light intensity, 80% relative humidity) for 4 weeks before pathogen inoculation.
As pathogen, we used a monosporic isolate of E. pisi f.sp. medicaginis strain CO05, derived from a mildew population collected on M. truncatula plants at Córdoba (Prats et al., 2007), which was maintained and propagated by infecting Parabinga plants. One day before inoculation the highly infected plants were shaken to remove old conidia in order to produce an inoculum with vigorous young spores. M. truncatula plants of both lines were inoculated when the fourth trifoliate leaf was completely expanded (4-week-old-plant). Inoculation was carried out using a setting tower to give an inoculum density of 5 conidia mm−2 (Prats et al., 2007). Five plants of each M. truncatula genotype were inoculated per triplicate, at the same time keeping five non-infected plants as a control, in total 60 M. truncatula plants. Thus, three independent biological replicates, five plants per condition (control and infected) and per genotype (Parabinga and SA1306), were performed with leaflets of control and E. pisi infected plants of both M. truncatula genotypes that were harvested 4 h after E. pisi inoculation. The samples were immediately washed with water, blot dried with filter paper, frozen in liquid nitrogen, and stored at −80°C until RNA extraction.
RNA Extraction, cDNA Synthesis, and qPCR Assays
RNA was purified from collected samples using the Nucleospin RNA II kit (MACHEREY-NAGEL, Bethlehem, PA) following the manufacturer's procedure. The integrity of total RNA was assessed on 1% agarose gels (samples were denatured in formaldehyde/formamide buffer), as well as for quantity and purity by using a NanoDrop ND-100 spectrophotometer (NanoDrop Technologies, Wilmington, DE) to measure the optical density. RNA samples were digested with RNase-free DNase1 (Ambion Inc., Houston, TX), according to the manufacturer's protocol. The absence of genomic DNA was checked by PCR analysis using primers designed on the M. truncatula ubiquitin gene intron sequence (Kakar et al., 2008).
Synthesis of first-strand cDNA was carried out with oligo-dT12–18 (Qiagen, Hilden, Germany) using SuperScript III reverse transcriptase (Invitrogen GmbH, Karlsruhe, Germany). The efficiency of cDNA synthesis was evaluated by real-time quantitative PCR (qPCR) amplification of 5′ and 3′ regions of two reference genes, GAPDH (Glyceraldehyde 3 phosphate dehydrogenase) and Ubiquitin (Kakar et al., 2008). A single peak in the dissociation curve at the end of the PCR reaction allowed confirmation of the specificity of the amplified products.
A M. truncatula transcription factor platform composed of more than 1000 M. truncatula TFs gene-specific primers was used to carry out the qPCR experiments (Kakar et al., 2008). The qPCR reactions were carried out in triplicate in an optical 384-well plate with an ABI PRISM® 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA) as described previously (Kakar et al., 2008).
Normalization and Data Analysis
SDS software ver. 2.3 (Applied Biosystems) was used to analyze fluorescent signals and calculate the quantification cycle (Cq) (Bustin et al., 2009). The baseline data were collected from the fluorescence signal between cycles 3 and 15, and used to correct the fluorescence signal of the samples. The PCR efficiencies (E) and correlation coefficients (R2) from linear regression analysis were calculated for each performed PCR reaction by the software LinRegPCR ver. 7.5 as described previously (Kakar et al., 2008) (Table S1). The amplification reactions with R2 < 0.99 that showed efficiencies lower than 1.8 were excluded for further analysis (24.4% of reactions). TF genes were considered detected if they were expressed in at least two biological replicates with a Cq < 40.
Eight reference genes encoding Pentatricopeptide repeat protein (PPRrep; TC96273), Protein phosphatase 2A subunit A3 (PDF 2; TC107161), Polypyrimidine tract-binding protein homolog (PTB; TC111751), Helicase (CB892427), Ubiquitin (TC102473), Ubiquitin-protein ligase 7 (UPL7; TC111218), Ubiquitin-conjugating enzyme E2 (UBC; AW686873), and Ubiquitin-conjugating enzyme E2 9 (UBC9; TC106312) (Kakar et al., 2008) were studied in order to determine the best suited reference genes for transcript normalization. The expression stability of the eight reference genes was analyzed by the geNorm software (Vandesompele et al., 2002; Hellemans et al., 2007) for each cDNA sample under study. In addition, pair-wise comparison analysis allowed determining the optimal number of reference genes in this assay (Vandesompele et al., 2002).
Expression values were calculated from ECq of each individual plot. To normalize the gene expression of each PCR reaction, ratios of the geometric mean of the selected reference genes to the different biological conditions were used using the Equation (1):
The relative induction/repression of TFs from E. pisi infected samples compared to untreated samples was calculated using the Equation (2):
Non-parametric Levene's test and Spearman's correlation coefficient were used to verify the equality of variances in the samples and to study the similarity between TF gene expression profiles, respectively. TF genes showing statistically significant differences (P < 0.05) were clustered using a hierarchical cluster analysis by complete linkage. A model for the regulatory network controlling the expression of regulated genes induced by E. pisi in both M. truncatula genotypes studied was built using NodeXL (http://nodexl.codeplex.com).
Results
Evaluation of Resistance in Medicago truncatula Genotypes
Differences in the response to E. pisi between the two genotypes were not yet visible at the time the leaves were sampled for RNA extraction. Powdery mildew infection was macroscopically visible on remaining leaflets 2 weeks after inoculation, with profuse sporulation in the susceptible Parabinga genotype and absence of symptoms in the resistant SA1306 genotype (Figure S1). Thus, previous study described that colony formation was much higher in Parabinga than in SA1306, as well as the hypersensitive response associated with epidermal cell death was negligible in Parabinga, but marked in SA1306 (Curto et al., 2015). Former studies unveiled that at early E. pisi infection times, such as 4 h (Curto et al., 2015) and 12 h (Samac et al., 2011), M. truncatula plants induce a highly number of metabolic pathways in response to E. pisi infection. Hence, we choose to analyze the TF transcriptome of M. truncatula during E. pisi infection at 4 h after pathogen infection.
Selection of Reference Genes
Eight reference genes were studied to determine those best suited for transcript normalization. Transcripts levels of all reference genes were calculated, in each cDNA sample, using the average expression stability (M) calculated by geNorm software (Figure 1A). All reference genes showed high average expression stability (M < 0.66) among them the UBC9, Helicase, PTB, and UPL7 reference genes showed the lower average expression stability (M) indicating a greater transcript stability (Figure 1A). Pair-wise variation (V) was also calculated as described by Vandesompele et al. (2002) allowing determining the optimal number of stable reference genes. The results indicated that the inclusion of a third gene (V3/4) or more genes (V4/5, V5/6,V6/7, and V7/8) has no significant effect (Figure 1B). Therefore, we selected UBC9 and Helicase as the best reference genes for this experiment, which were used for transcript normalization of the analyzed TF genes.
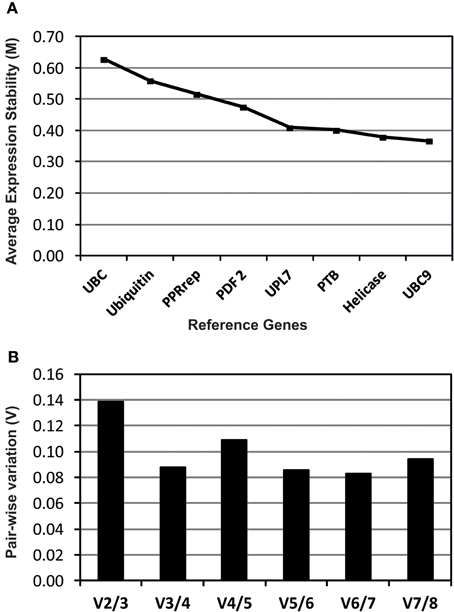
Figure 1. Evaluation of candidate reference genes analyzed using geNorm software. Expression stability (A) and pair-wise variation (B) plots for the eight reference genes studied. A lower M value indicates a more stable expression. The pair-wise variation (V) values indicate the optimal number of reference genes.
Expression Patterns of TF Genes in M. truncatula Following E. pisi Infection
We analyzed and compared the expression patterns of TF genes in the susceptible cv. Parabinga and the resistant SA1306 genotypes at 4 h after E. pisi inoculation (Figure S1). A total of 623 genes of the qPCR TF platform (59.6%) were considered detected (Cq < 40; n ≥ 2) and 95 showed statistically significant differences (P < 0.05) upon E. pisi infection in SA1306 and Parabinga genotypes. The relative gene expression ratios (m), log2expression ratios inoculated/control after E. pisi inoculation, were calculated for all TF genes. TF genes were considered to be differentially up- or down-regulated in response to E. pisi infection, if they met the prerequisites p ≤ 0.05 and m ≥ 0.7 or m ≤ −0.7, respectively. We studied the expression pattern of these genes that showed statistically significant differences through a hierarchical clustering analysis based on gene expression profiles (Figure 2, Table 1).
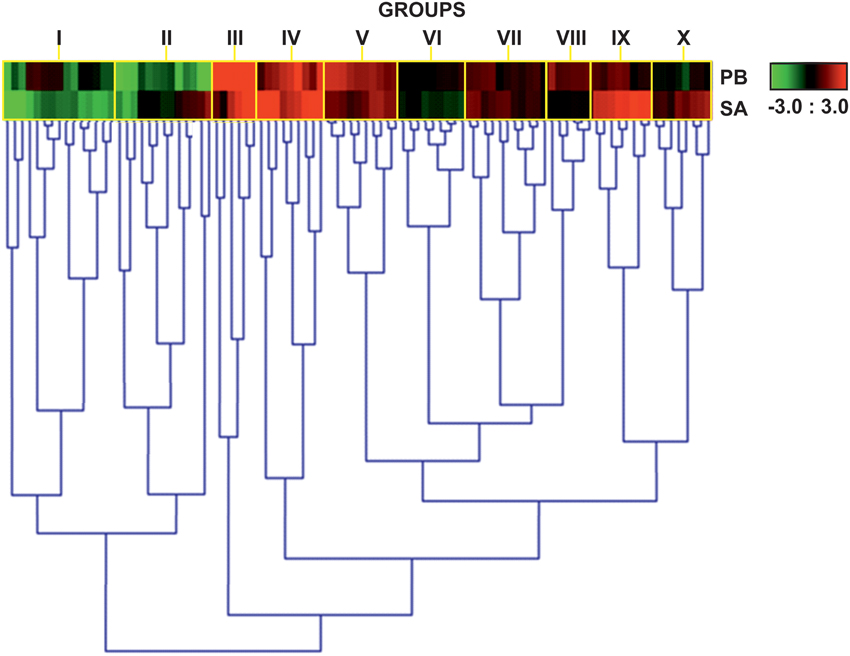
Figure 2. Heat map expression profiles of TF genes. Heat map showing expression profiles of 95 genes that were differentially expressed in Parabinga (PB) and SA1306 (SA) M. truncatula genotypes in response to E. Pisi infection. Genes were considered differentially expressed if they met the prerequisites p ≤ 0.05 and m ≤ −0.7 or m ≥ 0.7. Up-regulation (m ≥ 0.7) is indicated in red; down-regulation (m ≤ −0.7) in green; black indicates no differential expression (−0.7 ≤ m ≤ 0.7). The heat map expression profiles are grouped by yellow rectangles (I–X). Additional information is available in Table 1.
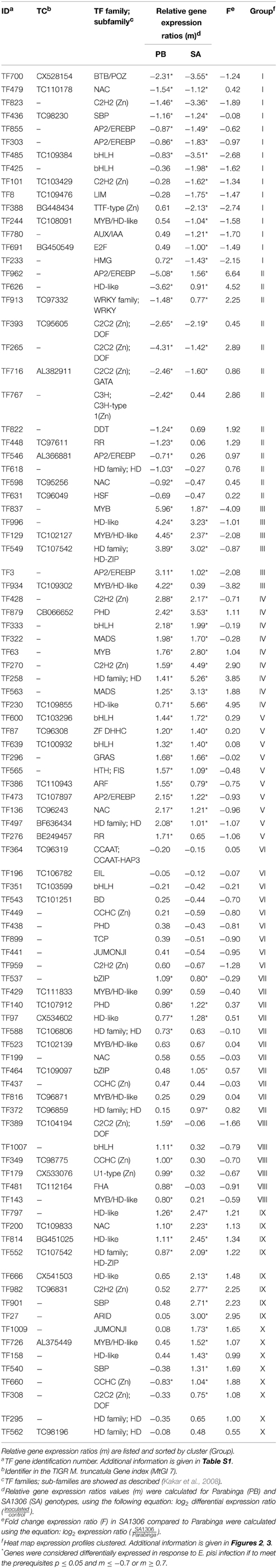
Table 1. Details of regulated TF genes clustered in groups among Medicago truncatula genotypes analyzed, SA1306 (SA), and Parabinga (PB), in response to Erysiphe pisi infection.
Transcription Factor Regulatory Network Induced by E. pisi Infection in M. truncatula
The qPCR platform allowed identification of TF genes differentially expressed in the two M. truncatula genotypes in response to E. pisi infection. Genes regulated in response to E. pisi infection (p ≤ 0.05 and m ≥ 0.7 or m ≤ −0.7) were clustered into 10 groups with different expression patterns (Figure 3). The first group (GI) includes 15 genes that were down-regulated in SA1306. In Parabinga eight of the genes were differentially expressed, one was up-regulated and seven TFs were repressed. These genes belong to 12 TF families, including Zn-Finger members (C2H2 (CYS2-HIS2), TTF-type (THYROID TRANSCRIPTION FACTOR), LIM (Lin-11, Isl-1, Mec-3), AP2/EREBP (APETALA2/ETHYLENE-RESPONSIVE ELEMENT BINDING FACTORS), AUX/IAA (AUXIN/INDOLE ACETIC ACID), bHLH (BASIC-HELIX-LOOP-HELIX), BTB/POZ (BROAD COMPLEX, TRAMTRACK, BRIC-A-BRAC/POX VIRUS, AND ZINC FINGER), E2F (E2 FACTOR), HMG (HIGH-MOBILITY GROUP), MYB/HD-like (MYELOBLASTOSIS/ HOMEODOMAIN-LIKE), NAC (NAM/ATAF/CUC), and SBP (SQUAMOSA-PROMOTER BINDING PROTEIN) (Table 1). The second group (GII) includes 13 genes which were down-regulated in Parabinga, except for one gene that was not differentially expressed. In the resistant SA1306 genotype, three were up-regulated and other three repressed belong to Zn-finger families, such as C2C2 (CYS2-CYS2) and C3H-type I (CYS3-HIS1) family. The remaining genes belong to AP2/EREBP, DDT (DNA BINDING HOMEOBOX, AND DIFFERENT TRANSCRIPTION FACTOR), HD, HD-like, HSF (HEAT STRESS TRANSCRIPTION FACTOR), NAC, RR (RESPONSE REGULATOR RECEIVER), and WRKY TF families.
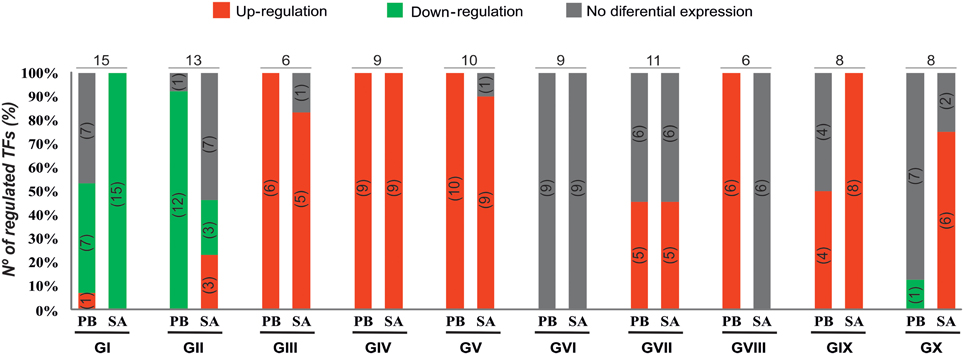
Figure 3. Expression profiles of TF genes. Percentages and numbers (into the brackets) of up-regulated (in red), down-regulated (in green), and no differential expressed (in gray) genes detected in Parabinga (PB) and SA1306 (SA) for each of the 10 clusters (groups GI–GX). Top on each cluster the total number of TF regulated genes is indicated. Additional information is available in Table 1.
Groups III, IV, and V include genes that were induced in both genotypes. Group III contains six genes that showed lower transcription levels in SA1306 than in Parabinga. These genes belong to AP2/EREBP, HD, HD-like, MYB, and MYB/HD-like TF families. Group IV includes nine genes that showed stronger transcription activation in the resistant SA1306 genotype. These genes belong to bHLH, C2H2 (Zn), HD, HD-like, MADS (MADS box), MYB, and PHD (PLANT HOMEODOMAIN MOTIF) TF families. Group V contains 10 genes that showed similar up-regulation expression patterns in both susceptible and resistant genotypes. Genes of this fifth group encode proteins belonging to AP2/EREBP, ARF (AUXIN-RESPONSE FACTOR), bHLH, GRAS (GAI, RGA, SCR), HD, HTH (HELIX-TURN-HELIX), NAC, RR, and DHHC (ASP-HIS-HIS-CYS) (Zn) TF families.
Genes clustered in groups VI and VII were mainly not expressed differentially in either genotype. None of the genes included in group VI were regulated whereas seven of the 11 genes of group VII were regulated. Three genes were induced in both genotypes and they are members of HD-like, PHD, and bZIP (BASIC LEUCINE ZIPPER) TF families, respectively. The remaining four regulated genes of group VII were specifically up-regulated in Parabinga and SA1306, which were included in HD, bZIP, and MYB/HD-like TF families.
Group VIII is comprised of six genes which were induced in Parabinga and not differentially expressed in SA1306. These genes belong to bHLH, FHA (FORKHEAD-ASSOCIATED), MYB/HD-like and three Zn-finger TF families (C2C2, CCHC (CYS-X8-CYS-X5-CYS-X3-HIS) and U1-type). The eight genes included in group IX were induced in SA1306, whereas in Parabinga half of them were up-regulated and the remaining genes were not differentially expressed. Genes of group IX are members of ARID (AT-RICH INTERACTION DOMAIN), C2H2 (Zn), HD, HD-like, NAC, and SBP TF families. Finally, group X includes eight genes which were mostly up-regulated in SA1306 and not differentially expressed in Parabinga. All genes belonging to this group were induced in the resistant genotype, except for two genes that were not differentially expressed. Only one repressed gene was detected in Parabinga. The genes of this last cluster belong to Zn finger families C2C2, CCHC, HD, HD-like, JUMONJI (JmjC domain), MYB/HD-like, and SBP TF families.
Around 80% of TF genes (79/95 genes) that showed statistically significant differences (P < 0.05) had at least a 1.6-fold change in transcript accumulation (−0.7 ≥ m ≥ 0.7) (Table S2). To study the regulatory network controlling the expression and interactions of these 79 genes during E. pisi infection, we further analyzed their expression in the susceptible Parabinga and the resistant SA1306 genotypes (Figure 4).
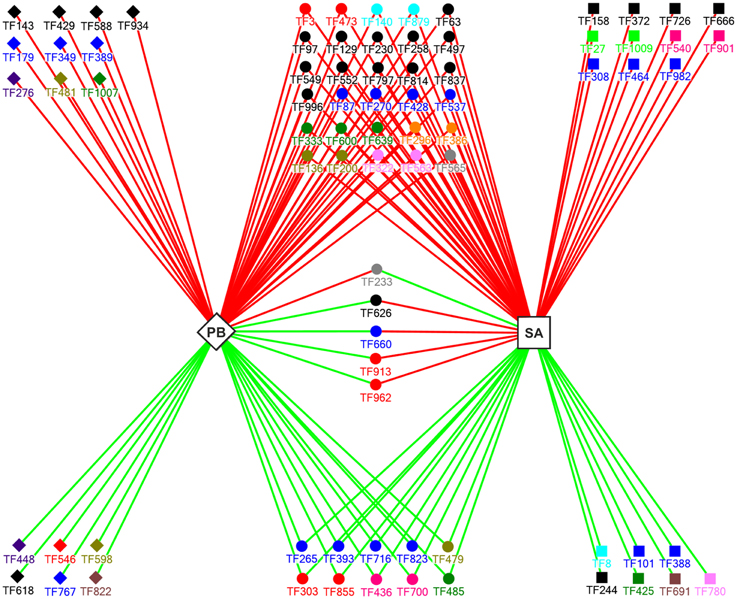
Figure 4. A model for the regulatory network that controls the expression of E. Pisi-induced TF genes (p = 0.05; −0.7 ≥ m ≥ 0.7) in Parabinga (PB; diamond) and SA1306 (SA; square) M. truncatula genotypes. Solid diamonds and solid squares indicate the TF genes regulated in Parabinga and SA1306, respectively. The TF genes that were regulated in both genotypes are indicated by solid spheres. Up- and down-regulation are indicated by red and green lines, respectively. The colors of the solid diamonds, solid squares, and solid spheres indicate TF families: Green (bHLH); black (HD family; HD-Like; MYB; MYB/HD-like); orange (ARF; GRAS); red (AP2/ERBP; WRKY); blue (Zn-fingers TF families; bZIP); olive green (FHA; NAC); violet (RR); pink (SBP; BTB/POZ); gray (HMG; HTH); brown (E2F; DDT); sky-blue (LIM; PHD); pea green (JUMONJI; ARID); dark pink (AUX/IAA; MADS). A detailed description of these genes is shown in Table S2.
Our analysis revealed that 16 and 18 of the 79 TF genes were specifically regulated in Parabinga and in SA1306, respectively. The remaining 45 genes were regulated in both genotypes (Figure 4, Table S2). In the susceptible Parabinga genotype 10 of the 16 specifically regulated genes were induced and the remaining six genes were repressed. Most of the genes specifically induced in Parabinga are members of MYB/HD-like, HD, C2C2, CCHC, RR, FHA, bHLH, and U1-type Zn finger families. Meanwhile, the six genes specifically down-regulated in Parabinga are included in RR, AP2/EREBP, NAC, HD-family, C3H- type 1 (Zn), and DDT TF families. Moreover, the resistant SA1306 genotype showed 11 and seven genes specifically up- and down-regulated, respectively. Genes specifically induced in SA1306 are members of HD/HD-like, MYB/HD-like, SBP, C2C2 (Zn), C2H2 (Zn), bZIP, ARID, and JUMONJI TF families. The genes down-regulated in SA1306 belong to LIM, C2H2 (Zn), TTF-type (Zn), MYB/HD-like, bHLH, E2F, and AUX/IAA TF families.
On the other hand, the genes regulated in both genotypes were mainly up-regulated; 30 induced and 10 repressed genes. Most of these 30 induced genes are in the HD-like, HD, and MYB TF families (Figure 4, Table S2). Moreover, the 10 repressed genes are included mainly in Zn finger families, C2C2 and C2H2. Interestingly, four of five common regulated genes were induced in SA1306 and repressed in Parabinga, and belong to the HD-like (TF626), CCHC (Zn) (TF660), WRKY (TF913), and AP2/EREBP (TF962) TF families. The fifth gene, TF233 (HMG), was up-regulated in Parabinga and repressed in SA1306.
Interestingly, 48 genes were differentially expressed in SA1306 compared to Parabinga (P < 0.05; −0.7 > F > 0.7) in response to E. pisi infection, and they belong to 25 TF families (Figure 5, Table S3). Among them, the most represented TF families are HD-like, C2H2 (Zn), AP2/EREBP, MYB/HD-like, and HD/HD, which comprise approximately half of the differentially expressed genes. The bHLH, C2C2/DOF (CYS2-CYS2/DNA-BINDING ONE ZINC FINGER) (Zn), HD-ZIP (HD-ZINC-REGULATED TRANSPORTER), MYB, NAC, and SBP TF families were also well represented (≈24%) and the rest of TF families were poorly represented (Figure 5). Eleven of 25 families include genes with greater transcript accumulation (F > 0.7) in SA1306 compared to Parabinga (Figure 5). Among them, the genes belonging to the ARID, WRKY family/WRKY, C2C2 (Zn)/DOF, SBP, HD-like, MADS, and CCHC (Zn) families were up-regulated around two fold in SA1306 compared to Parabinga. Meanwhile only three families, TTF-type (Zn), bHLH, and HMG, include genes that were down-regulated (F ≤ −2) in SA1306 compared to Parabinga (Figure 5). Generally, families HD-ZIP and NAC include genes that were induced in both genotypes. However, the AP2/ERBP family was lightly up-regulated in SA1306 and almost not regulated in Parabinga (Table S3).
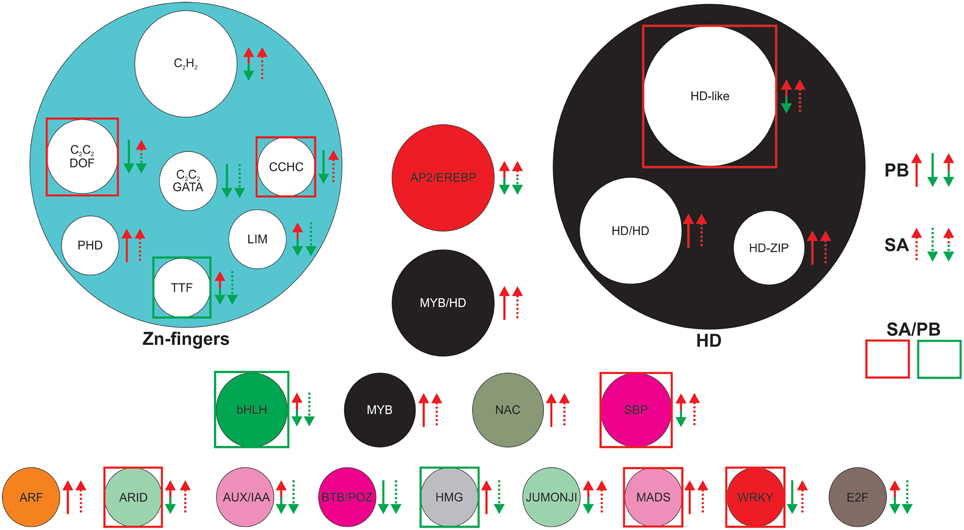
Figure 5. Most differentially expressed TF families in SA1306 compared to Parabinga (−0.7 > F > 0.7) in response to Erysiphe pisi infection. The TF families are represented by circles whose size is proportional to the number of genes in the family. Up-regulation (m ≥ 0.7) is indicated by red arrows; down-regulation (m ≤ −0.7) by green arrows; no differential expression (−0.7 ≤ m ≤ 0.7) are indicate by double red/green arrows; solid lines represent Parabinga (PB), and dotted lines SA1306 (SA). TF families highly up-regulated (F ≥ 1.8) and down-regulated (F ≤ −1.8) are indicated by red and green boxes, respectively. For expanded information, see Table S3.
Discussion
Thanks to the high-throughput methods genes, potentially associated with specific processes and characterization of the regulatory networks that control their expression, have been identified (Czechowski et al., 2004; Caldana et al., 2007). DNA microarrays have been successfully applied to characterize global gene expression patterns in M. truncatula (Foster-Hartnett et al., 2007; Samac et al., 2011; Zhang et al., 2014; Curto et al., 2015; Song et al., 2015). Previous DNA microarray studies have analyzed the E. pisi/M. truncatula pathosystem (Foster-Hartnett et al., 2007; Samac et al., 2011; Curto et al., 2015) using different genotypes and microarray platforms, such as Mt16kOLI1, Mt16kOLI1plus, and Affymetrix GeneChip® (http://www.affymetrix.com), which have increased the knowledge of mechanisms involved in E. pisi resistance in M. truncatula. Several large-scale TF profiling approaches have employed the M. truncatula qPCR-based platform available (Kakar et al., 2008) in various studies (Verdier et al., 2008; Gao et al., 2010; Madrid et al., 2010; Villegas-Fernández et al., 2014; Noguero et al., 2015). In spite of the progress in characterizing TFs, those involved in the expression of stress-related genes in plants remain undiscovered (Singh et al., 2002). Particularly, the TFs involved in the defense mechanisms against E. pisi need to be clarified in order to completely understand the mechanisms involved in the plant's defense against this pathogen.
In our study we found that 95 of the TF genes analyzed (15%) were expressed differentially. These results agree with similar approaches carried out in response to infection by Uromyces striatus (≈13%) (Madrid et al., 2010) and Botrytis spp. (≈20%) (Villegas-Fernández et al., 2014). A subset of these genes belong to 25 TF families (Figure 5, Table S3), including AUX/IAA, bHLH, E2F, HD, JUMONJI, MYB, SBP, and zinc finger families (C2C2, C2H2, LIM), were specifically regulated in the resistant SA1306 genotype suggesting that they act as major regulators of transcription throughout E. pisi defense responses.
Zinc finger and HD families represent the most of these genes specifically regulated in the resistant SA1306 genotype, and are members of the C2H2 (Zn), C2C2/DOF, LIM, HD-like, HD/HD, and HD-ZIP TF families, which are agree with recent studies that used the same qPCR TF platform (Villegas-Fernández et al., 2014). The C2H2 (Zn) family playing a critical role as key transcriptional repressors involved in the defense response of plants to stress (Brayer and Segal, 2008; Ciftci-Yilmaz and Mittler, 2008; Kiełbowicz-Matuk, 2012) and M. truncatula to biotrophic and necrotrophic pathogens. Nevertheless, previous studies have reported the relationship between SBP genes and plant disease resistance, such as programmed cell death in Arabidopsis (Stone et al., 2005) and these studies are in agreement that programmed cell death, frequently associated to host cell death, is a common plant defense mechanisms against E. pisi (Curto et al., 2006; Barilli et al., 2014). Thus, a member of the SPB TF family (VpSBP5) has been reported to be induced by powdery mildew (E. necator) (Hou et al., 2013), suggesting that this TF is involved in the resistance to powdery mildew by inducing salicylic acid and methyl jasmonate molecular signals. The C2C2 (Zn)/DOF family member have divergent physiological roles (Yanagisawa, 2002) including defense gene expression in response to salicylic acid and oxidative stress signals (Chen et al., 1996; Yanagisawa, 2002) and phytohormone-regulated expression (De Paolis et al., 1996; Yanagisawa, 2002). Recent studies have shown that E. pisi infection induces several enzymes, such as psCHS1 and PEAPAL2, involved in phenylpropanoid biosynthesis leading to biosynthesis of phytoalexin (Barilli et al., 2014), reinforcing the role of phenylpropanoid pathway in the elicited defense. Our findings are in agreement with these previous studies, suggesting the important role of these TF families in the resistance to E. pisi in M. truncatula.
Moreover, the LIM zinc TF family has also been found to be involved in the resistance mechanism against pathogens. Thus, several studies have revealed that this family has the capacity to regulate the expression of some lignin biosynthetic genes (Rogers and Campbell, 2004). In addition, previous studies have reported the role of lignification processes into the M. truncatula defense responses against to E. pisi infection (Prats et al., 2007). Our findings are in agreement with these previous studies and recent studies (Barilli et al., 2014), in which enzymes involved in ROIs stress, such as peroxidase Prx7, are regulated in pea after E. pisi inoculation. Other studies have found that monolignol biosynthesis plays a critical role in cell wall apposition mediated defense against powdery mildew fungus penetration (Bhuiyan et al., 2009). Reactive oxygen intremediates (ROIs) are associated with the hypersensitive response (Torres et al., 2005), which has been related to programmed cell death (hypersensitive response) that plays a critical role in resistance to E. pisi (Barilli et al., 2014).
Our results also reveal a subset of TF genes that encode TFs with a HD (homeodomain) protein domain, which showed a different expression pattern in the resistant SA1306 compared to the susceptible Parabinga genotype in response to E. pisi infection. These genes belong to the HD-like and HD family/HD. Members of this family may play a role in the defense response against necrotrophic fungal pathogens regulated by jasmonic acid (Korfhage et al., 1994; Coego et al., 2005; Villegas-Fernández et al., 2014). In addition, a recent study has revealed that several homeodomain-like TF families are involved in the defense responses in M. truncatula when confronted with the necrotrophic pathogens, Botrytis fabae, and B. cinerea (Villegas-Fernández et al., 2014), supporting that these TF families may play an important role in the defensive mechanism of M. truncatula to fungal pathogens. Nevertheless, our results show that the Auxin/Indole-3-acetic acid (AUX/IAA) family was repressed in the resistant SA1306 genotype in response to E. pisi infection, which suggests that this family may play a role in the resistance mechanism against E. pisi. Earlier studies are agree with our results and have described that down regulation of auxin signaling contribute to plant induced immune responses in Arabidopsis (Navarro et al., 2006). The bHLH TFs were also induced in the resistant genotype in response to E. pisi infection, which are in agreement with the results obtained by Villegas-Fernández et al. (2014). The bHLH TFs up-regulated by E. pisi key TF regulating the expressions of jasmonic acid responsive genes (Fernández-Calvo et al., 2011), which mediate the transcriptional reprogramming associated with the plant immune response.
Our findings also indicate that E2F and JUMONJI TF families are involved in the defense response against E. pisi. The E2F transcription factor family has been found to induce transcription of genes required for cell cycle progression and DNA replication (Vandepoele et al., 2005). In addition, more than 100 E2F target genes have been identified, including genes involved in several defense responses and signaling (Ramirez-Parra et al., 2003). The members if the family JUMONJI play a role in the histone methylation process (Li et al., 2013). Dimethylated or trimethylated histone H3 lysine 27 (H3K27me2/3) marks silent or repressed genes involved in stress responses in plants. Li et al. (2013) studied the jumonji C protein gene JMJ705 that is induced by stress signals during pathogen infection, and is involved in methyl jasmonate–induced dynamic removal of H3K27me3 and in gene activation increasing their basal and induced expression during pathogen infection (Balciunas and Ronne, 2000; Li et al., 2013). Our results are in agreement with the results obtained by Villegas-Fernández et al. (2014), which It is that have reported that the JUMONJI TF family may be involved in the defense response to a fungal pathogen, reinforcing the role of this TF family in defense responses against fungal pathogens.
Moreover, an important number of TF genes were regulated in both genotypes, which mainly belonged to AP2/EREBP (Singh et al., 2002; Gutterson and Reuber, 2004; Dietz et al., 2010; Villegas-Fernández et al., 2014), C2H2 (Zn) (Ciftci-Yilmaz and Mittler, 2008; Villegas-Fernández et al., 2014), MYB (Singh et al., 2002; Villegas-Fernández et al., 2014), HD (Coego et al., 2005; Villegas-Fernández et al., 2014), MYB/HD-like (Singh et al., 2002; Coego et al., 2005), NAC (Dangl and Jones, 2001; Villegas-Fernández et al., 2014), and PHD (Libault et al., 2007; Villegas-Fernández et al., 2014) TF families. Interestingly, the susceptible and resistant genotypes showed different expression patterns in five of these TF genes in response to E. pisi infection (Figure 4). Four of these five genes (TF626, TF660, TF913, and TF962) encode TFs belonging to known defense system pathways, which were induced in SA1306 and repressed in Parabinga, respectively. TF962 encodes a member of the AP2/EREBP family known to be linked to response to abiotic and biotic stresses (Dietz et al., 2010), as well as involved in response to a chitin elicitor and in metabolism of the plant hormone methyl jasmonate (McGrath et al., 2005; Libault et al., 2007). Results obtained were similar to those generated in of a study of M. truncatula of responses to Botrytis infection, in which AP2/EREBP was shown to be a key regulator of defense responses (Villegas-Fernández et al., 2014). TF913 encodes a WRKY protein, whose family members have been shown to be key components in the regulation of plant disease resistance (Eulgem and Somssich, 2007). Previous studies have shown that WRKY TF family members are involved in the regulation of R gene-mediated disease resistance as well as in the regulation of transcriptional reprogramming associated with plant immune responses (Eulgem and Somssich, 2007; Buscaill and Rivas, 2014). Several genes encoding WRKY proteins (AtWRKY18, AtWRKY40) have been identified that confer resistance against powdery mildew (Shen et al., 2007). In addition, AtWRKY18 has been characterized to act as a positive regulator required for full SAR (Wang et al., 2006), whose transcriptional expression may be is linked to AtWRKY70, which modulates the cross-talk between signaling pathways regulating salicylic acid (SA)-dependent and jasmonic acid-dependent responses (Eulgem and Somssich, 2007). These results are in accord with results of recent studies in M. truncatula that WRKY TFs involved in the defensive reaction of M. truncatula to Uromyces and Botrytis (Madrid et al., 2010; Villegas-Fernández et al., 2014), supporting the critical role of this TF family in the plant defense responses against fungal pathogens.
The Zn finger family CCHC (TF660) was also detected as induced in SA1306 and repressed in Parabinga. Previous studies (Mangeon et al., 2010; Villegas-Fernández et al., 2014) are in agreement with our results, suggesting the important role of this TF family, A member of HD-like (TF626) was also induced in the resistant genotype and highly repressed in Parabinga. This family has been found to be involved in defense responses against fungal necrotroph pathogens regulated by jasmonic acid (Korfhage et al., 1994; Coego et al., 2005; Villegas-Fernández et al., 2014). We suggest that the 10 TFs that were repressed in both genotypes may play a role suppressing genes involved in photosynthetic metabolism leading to a reduction in the photosynthetic rate, as previously suggested (Swarbrick et al., 2006; Bolton, 2009).
Conclusion
We have screened more than 1000 TFs genes of M. truncatula for altered expression during E. pisi infection using qPCR platform. Forty eight TFs from them showed significant differences in the resistant SA1306 genotype compared to the susceptible Parabinga. These TF genes belong mainly to AP2/EREBP, WRKY, MYB, HD, and zinc finger families (C2C2, C2H2, LIM) gene families, which are involved in known defense responses. In addition, we suggest a regulatory network that controls the expression in M. truncatula of genes involved in resistance to E. pisi. These results will help to systematically decipher the functional roles of TF genes and to develop new strategies against powdery mildew.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the European Commission FP6 Framework Programme Grain Legume Integrated Project (FOOD-CT-2004-506223) and by Spanish project AGL2014-52871-R co-financed by FEDER. We are grateful to Udvardi research Group (The Samuel Roberts Noble Foundation, Okla, USA) for advice and support and to Carolina Johnstone for grammatical review. This research work is lovingly dedicated to my mother, who has fought cancer two times.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00517
Figure S1. Macroscopic disease symptoms in the susceptible cultivar Parabinga (A) and in the resistant SA1306 accession (B).
Table S1. Complete list of TF genes. The accession numbers (AC), the TIGR Medicago gene accession numbers (TC) if available, as well as the transcription factor family and the subfamily names are shown as described Kakar et al. (Kakar et al., 2008). The quantification cycle (Cq), PCR efficiencies (PCReff) and correlation coefficients for the qPCR reactions performed on both Medicago truncatula genotypes, Parabinga and SA1306, in three biological replicates are indicated.
Table S2. Details of regulated TF genes among Medicago truncatula genotypes analyzed, SA1306 (SA) and Parabinga (PB), in response to Erysiphe pisi infection (p ≤ 0.05; −0.7 ≥ m ≥ 0.7). Relative gene expression ratios (m) are listed and sorted by “Class” and “m” values. ID: Identification number of TF gene. TF family: Transcription factor family. TF subfamily: Subfamily names. m: Average log2 differential expression ratios (inoculated/control) for Parabinga (PB) and SA1306 (SA) genotypes. F: Fold change expression ratios of differentially expressed genes in SA1306 compared to Parabinga, log2 expression ratio SA1306/Parabinga. Class: Genes that were specifically regulated in SA1306 (SA), Parabinga (PB) and both genotypes (Common) (Figure 4).
Table S3. Most differentially expressed TF families in SA1306 compared to Parabinga (−0.7>F>0.7) in response to Erysiphe pisi infection.
References
Balciunas, D., and Ronne, H. (2000). Evidence of domain swapping within the jumonji family of transcription factors. Trends Biochem. Sci. 25, 274–276. doi: 10.1016/S0968-0004(00)01593-0
Barilli, E., Rubiales, D., Gjetting, T., and Lyngkjaer, M. F. (2014). Differential gene transcript accumulation in peas in response to powdery mildew (Erysiphe pisi) attack. Euphytica 195, 1–16. doi: 10.1007/s10681-014-1062-z
Bélanger, R. R., Bushnell, W. R., Dik, A. J., and Carver, T. L. W. (2002). The Powdery Mildews: A Comprehensive Treatise. St. Paul, MN: APS Press.
Bhuiyan, N. H., Selvaraj, G., Wei, Y., and King, J. (2009). Role of lignification in plant defense. Plant. Signal. Behav. 4, 158–159. doi: 10.4161/psb.4.2.7688
Bolton, M. D. (2009). Primary metabolism and plant defense-fuel for the fire. Mol. Plant Microbe Interact. 22, 487–497. doi: 10.1094/MPMI-22-5-0487
Brayer, K. J., and Segal, D. J. (2008). Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem. Biophys. 50, 111–131. doi: 10.1007/s12013-008-9008-5
Buscaill, P., and Rivas, S. (2014). Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 20, 35–46. doi: 10.1016/j.pbi.2014.04.004
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. doi: 10.1373/clinchem.2008.112797
Caldana, C., Scheible, W.-R., Mueller-Roeber, B., and Ruzicic, S. (2007). A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods 3:7. doi: 10.1186/1746-4811-3-7
Chen, W., Chao, G., and Singh, K. B. (1996). The promoter of a H2O2-inducible, Arabidopsis glutathione S-transferase gene contains closely linked OBF- and OBP1-binding sites. Plant J. 10, 955–966. doi: 10.1046/j.1365-313X.1996.10060955.x
Cheung, A. Y., and Wu, H.-M. (2011). THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 14, 632–641. doi: 10.1016/j.pbi.2011.09.001
Ciftci-Yilmaz, S., and Mittler, R. (2008). The zinc finger network of plants. Cell Mol. Life Sci. 65, 1150–1160. doi: 10.1007/s00018-007-7473-4
Coego, A., Ramirez, V., Gil, M. J., Flors, V., Mauch-Mani, B., and Vera, P. (2005). An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OF CATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. Plant Cell 17, 2123–2137. doi: 10.1105/tpc.105.032375
Curto, M., Camafeita, E., Lopez, J. A., Maldonado, A. M., Rubiales, D., and Jorrín, J. V. (2006). A proteomic approach to study pea (Pisum sativum) responses to powdery mildew (Erysiphe pisi). Proteomics 6, S163–S174. doi: 10.1002/pmic.200500396
Curto, M., Krajinski, F., Küster, H., and Rubiales, D. (2015). Plant defense responses in Medicago truncatula unveiled by microarray analysis. Plant Mol. Biol. Rep. 33, 569–583. doi: 10.1007/s11105-014-0770-9
Czechowski, T., Bari, R. P., Stitt, M., Scheible, W.-R., and Udvardi, M. K. (2004). Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J. 38, 366–379. doi: 10.1111/j.1365-313X.2004.02051.x
Dangl, J. L., and Jones, J. D. G. (2001). Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. doi: 10.1038/35081161
De Paolis, A., Sabatini, S., De Pascalis, L., Costantino, P., and Capone, I. (1996). A rolB regulatory factor belongs to a new class of single zinc finger plant proteins. Plant J. 10, 215–223. doi: 10.1046/j.1365-313X.1996.10020215.x
Dietz, K.-J., Vogel, M., and Viehhauser, A. (2010). AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma 245, 3–14. doi: 10.1007/s00709-010-0142-8
Eulgem, T., and Somssich, I. E. (2007). Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 10, 366–371. doi: 10.1016/j.pbi.2007.04.020
Falloon, R. E., and Viljanen-Rollinson, S. L. H. (2001). “Powdery mildew,” in Compendium of Pea Diseases and Pests, 2nd Edn., eds J. M. Karaft and F. L. Pfleger (St. Paul, MN: APS Press), 28–29.
Fernández-Calvo, P., Chini, A., Fernández-Barbero, G., Chico, J.-M., Gimenez-Ibanez, S., Geerinck, J., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23, 701–715. doi: 10.1105/tpc.110.080788
Fondevilla, S., and Rubiales, D. (2012). Powdery mildew control in pea. A review. Agron. Sustain. Dev. 32, 401–409. doi: 10.1007/s13593-011-0033-1
Fondevilla, S., Torres, A. M., Moreno, M. T., and Rubiales, D. (2007). Identification of a new gene for resistance to powdery mildew in Pisum fulvum, a wild relative of pea. Breed. Sci. 57, 181–184. doi: 10.1270/jsbbs.57.181
Foster-Hartnett, D., Danesh, D., Peñuela, S., Sharopova, N., Endre, G., Vandenbosch, K. A., et al. (2007). Molecular and cytological responses of Medicago truncatula to Erysiphe pisi. Mol. Plant Pathol. 8, 307–319. doi: 10.1111/j.1364-3703.2007.00395.x
Gao, L.-L., Kamphuis, L. G., Kakar, K., Edwards, O. R., Udvardi, M. K., and Singh, K. B. (2010). Identification of potential early regulators of aphid resistance in Medicago truncatula via transcription factor expression profiling. New Phytol. 186, 980–994. doi: 10.1111/j.1469-8137.2010.03229.x
Gutterson, N., and Reuber, T. L. (2004). Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr. Opin. Plant Biol. 7, 465–471. doi: 10.1016/j.pbi.2004.04.007
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F., and Vandesompele, J. (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8:R19. doi: 10.1186/gb-2007-8-2-r19
Hoagland, D. R., and Arnon, D. I. (1950). The water-culture method of growing plants without soil. Calif. Agric. Exp. Stn. Circ. 347, 1–32.
Hou, H., Yan, Q., Wang, X., and Xu, H. (2013). A SBP-Box gene VpSBP5 from chinese wild Vitis Species Responds to Erysiphe necator and defense signaling molecules. Plant Mol. Biol. Rep. 31, 1261–1270. doi: 10.1007/s11105-013-0591-2
Iglesias-García, R., Rubiales, D., and Fondevilla, S. (2015). Penetration resistance to Erysiphe pisi in pea mediated by er1 gene is associated with protein cross-linking but not with callose apposition or hypersensitive response. Euphytica 201, 381–387. doi: 10.1007/s10681-014-1221-2
Kakar, K., Wandrey, M., Czechowski, T., Gaertner, T., Scheible, W.-R., Stitt, M., et al. (2008). A community resource for high-throughput quantitative RT-PCR analysis of transcription factor gene expression in Medicago truncatula. Plant Methods 4:18. doi: 10.1186/1746-4811-4-18
Kiełbowicz-Matuk, A. (2012). Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci. 185–186, 78–85. doi: 10.1016/j.plantsci.2011.11.015
Korfhage, U., Trezzini, G. F., Meier, I., Hahlbrock, K., and Somssich, I. E. (1994). Plant homeodomain protein involved in transcriptional regulation of a pathogen defense-related gene. Plant Cell 6, 695–708. doi: 10.1105/tpc.6.5.695
Küster, H., Becker, A., Firnhaber, C., Hohnjec, N., Manthey, K., Perlick, A. M., et al. (2007). Development of bioinformatic tools to support EST-sequencing, in silico- and microarray-based transcriptome profiling in mycorrhizal symbioses. Phytochemistry 68, 19–32. doi: 10.1016/j.phytochem.2006.09.026
Küster, H., Hohnjec, N., Krajinski, F., El Yahyaoui, F., Manthey, K., Gouzy, J., et al. (2004). Construction and validation of cDNA-based Mt6k-RIT macro- and microarrays to explore root endosymbioses in the model legume Medicago truncatula. J. Biotechnol. 108, 95–113. doi: 10.1016/j.jbiotec.2003.11.011
Li, T., Chen, X., Zhong, X., Zhao, Y., Liu, X., Zhou, S., et al. (2013). Jumonji C domain protein JMJ705-mediated removal of histone H3 lysine 27 trimethylation is involved in defense-related gene activation in rice. Plant Cell 25, 4725–4736. doi: 10.1105/tpc.113.118802
Libault, M., Wan, J., Czechowski, T., Udvardi, M., and Stacey, G. (2007). Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol. Plant Microbe Interact. 20, 900–911. doi: 10.1094/MPMI-20-8-0900
Madrid, E., Gil, J., Rubiales, D., Krajinski, F., Schlereth, A., and Millán, T. (2010). Transcription factor profiling leading to the identification of putative transcription factors involved in the Medicago truncatula–Uromyces striatus interaction. Theor. Appl. Genet. 121, 1311–1321. doi: 10.1007/s00122-010-1390-x
Mangeon, A., Junqueira, R. M., and Sachetto-Martins, G. (2010). Functional diversity of the plant glycine-rich proteins superfamily. Plant Signal. Behav. 5, 99–104. doi: 10.4161/psb.5.2.10336
McGrath, K. C., Dombrecht, B., Manners, J. M., Schenk, P. M., Edgar, C. I., Maclean, D. J., et al. (2005). Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol. 139, 949–959. doi: 10.1104/pp.105.068544
Navarro, L., Dunoyer, P., Jay, F., Arnold, B., Dharmasiri, N., Estelle, M., et al. (2006). A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312, 436–439. doi: 10.1126/science.1126088
Noguero, M., Le Signor, C., Vernoud, V., Bandyopadhyay, K., Sanchez, M., Fu, C., et al. (2015). DASH transcription factor impacts Medicago truncatula seed size by its action on embryo morphogenesis and auxin homeostasis. Plant J. 81, 453–466. doi: 10.1111/tpj.12742
Prats, E., Llamas, M. J., and Rubiales, D. (2007). Characterization of resistance mechanisms to Erysiphe pisi in Medicago truncatula. Phytopathology 97, 1049–1053. doi: 10.1094/PHYTO-97-9-1049
Ramirez-Parra, E., Fründt, C., and Gutierrez, C. (2003). A genome-wide identification of E2F-regulated genes in Arabidopsis. Plant J. 33, 801–811. doi: 10.1046/j.1365-313X.2003.01662.x
Riechmann, J. L., and Ratcliffe, O. J. (2000). A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 3, 423–434. doi: 10.1016/S1369-5266(00)00107-2
Ringli, C. (2010). Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 153, 1445–1452. doi: 10.1104/pp.110.154518
Rogers, L. A., and Campbell, M. M. (2004). The genetic control of lignin deposition during plant growth and development. New Phytol. 164, 17–30. doi: 10.1111/j.1469-8137.2004.01143.x
Rose, R. J. (2008). Medicago truncatula as a model for understanding plant interactions with other organisms, plant development and stress biology: past, present and future. Funct. Plant Biol. 35, 253–264. doi: 10.1071/FP07297
Rubiales, D., Fondevilla, S., Chen, W., Gentzbittel, L., Higgins, T. J. V., Castillejo, M. A., et al. (2015). Achievements and challenges in legume breeding for pest and disease resistance. Crit. Rev. Plant Sci. 34, 195–236. doi: 10.1080/07352689.2014.898445
Samac, D. A., Peñuela, S., Schnurr, J. A., Hunt, E. N., Foster-Hartnett, D., Vandenbosch, K. A., et al. (2011). Expression of coordinately regulated defence response genes and analysis of their role in disease resistance in Medicago truncatula. Mol. Plant Pathol. 12, 786–798. doi: 10.1111/j.1364-3703.2011.00712.x
Shen, Q.-H., Saijo, Y., Mauch, S., Biskup, C., Bieri, S., Keller, B., et al. (2007). Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315, 1098–1103. doi: 10.1126/science.1136372
Sillero, J., Fondevilla, S., Davidson, J., Patto, M., Warkentin, T., Thomas, J., et al. (2006). Screening techniques and sources of resistance to rusts and mildews in grain legumes. Euphytica 147, 255–272. doi: 10.1007/s10681-006-6544-1
Singh, K. B., Foley, R. C., and Oñate-Sánchez, L. (2002). Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 5, 430–436. doi: 10.1016/S1369-5266(02)00289-3
Singh, R. J., Chung, G. H., and Nelson, R. L. (2007). Landmark research in legumes. Genome 50, 525–537. doi: 10.1139/G07-037
Song, J., Wang, Y., Li, H., Li, B., Zhou, Z., Gao, S., et al. (2015). The F-box family genes as key elements in response to salt, heavy mental, and drought stresses in Medicago truncatula. Funct. Integr. Genomics 1–13. doi: 10.1007/s10142-015-0438-z
Stone, J. M., Liang, X., Nekl, E. R., and Stiers, J. J. (2005). Arabidopsis AtSPL14, a plant-specific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1. Plant J. 41, 744–754. doi: 10.1111/j.1365-313X.2005.02334.x
Swarbrick, P. J., Schulze-Lefert, P., and Scholes, J. D. (2006). Metabolic consequences of susceptibility and resistance (race-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ. 29, 1061–1076. doi: 10.1111/j.1365-3040.2005.01472.x
Torres, M. A., Jones, J. D. G., and Dangl, J. L. (2005). Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37, 1130–1134. doi: 10.1038/ng1639
Udvardi, M. K., Kakar, K., Wandrey, M., Montanari, O., Murray, J., Andriankaja, A., et al. (2007). Legume transcription factors: global regulators of plant development and response to the environment. Plant Physiol. 144, 538–549. doi: 10.1104/pp.107.098061
Vandepoele, K., Vlieghe, K., Florquin, K., Hennig, L., Beemster, G. T. S., Gruissem, W., et al. (2005). Genome-wide identification of potential plant E2F target genes. Plant Physiol. 139, 316–328. doi: 10.1104/pp.105.066290
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: research0034.0031. doi: 10.1186/gb-2002-3-7-research0034
Verdier, J., Kakar, K., Gallardo, K., Le Signor, C., Aubert, G., Schlereth, A., et al. (2008). Gene expression profiling of M. truncatula transcription factors identifies putative regulators of grain legume seed filling. Plant Mol. Biol. 67, 567–580. doi: 10.1007/s11103-008-9320-x
Villegas-Fernández, A. M., Krajinski, F., Schlereth, A., Madrid, E., and Rubiales, D. (2014). Characterization of transcription factor following expression profiling of Medicago truncatula – Botrytis spp interactions. Plant Mol. Biol. Rep. 32, 1030–1040. doi: 10.1007/s11105-014-0710-8
Wang, D., Amornsiripanitch, N., and Dong, X. (2006). A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2:e123. doi: 10.1371/journal.ppat.0020123
Yanagisawa, S. (2002). The Dof family of plant transcription factors. Trends Plant. Sci. 7, 555–560. doi: 10.1016/S1360-1385(02)02362-2
Yang, S., Tang, F., Caixetab, E. T., and Zhu, H. (2013). Epigenetic regulation of a powdery mildew resistance gene in Medicago truncatula. Mol. Plant. 6, 2000–2003. doi: 10.1093/mp/sst106
Keywords: Erysiphe pisi, legumes, Medicago truncatula, transcription factors, qPCR
Citation: Curto M, Krajinski F, Schlereth A and Rubiales D (2015) Transcriptional profiling of Medicago truncatula during Erysiphe pisi infection. Front. Plant Sci. 6:517. doi: 10.3389/fpls.2015.00517
Received: 05 May 2015; Accepted: 26 June 2015;
Published: 09 July 2015.
Edited by:
Antonio M. De Ron, National Spanish Research Council, SpainReviewed by:
Ana M. Gonzalez, Misión Biológica de Galicia-Spanish National Research Council, SpainTom Warkentin, University of Saskatchewan, Canada
Copyright © 2015 Curto, Krajinski, Schlereth and Rubiales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel Curto, Department of Plant Breeding, Institute for Sustainable Agriculture, Spanish National Research Council, Apdo. 4084, E-14080 Córdoba, Spain, b72curum@uco.es
 Miguel Curto
Miguel Curto Franziska Krajinski
Franziska Krajinski Armin Schlereth
Armin Schlereth Diego Rubiales
Diego Rubiales