- 1Graduate School of Science and Technology, Kumamoto University, Chuo-ku, Japan
- 2Department of Biomolecular Sciences, Graduate School of Life Sciences, Tohoku University, Aoba-ku, Japan
- 3RIKEN Center for Sustainable Resource Science, Yokohama, Japan
Numerous pathogenic or parasitic organisms attack plant roots to obtain nutrients, and the apoplast including the plant cell wall is where the plant cell meets such organisms. Root parasitic angiosperms and nematodes are two distinct types of plant root parasites but share some common features in their strategies for breaking into plant roots. Striga and Orobanche are obligate root parasitic angiosperms that cause devastating agricultural problems worldwide. Parasitic plants form an invasion organ called a haustorium, where plant cell wall degrading enzymes (PCWDEs) are highly expressed. Plant-parasitic nematodes are another type of agriculturally important plant root parasite. These nematodes breach the plant cell walls by protruding a sclerotized stylet from which PCWDEs are secreted. Responding to such parasitic invasion, host plants activate their own defense responses against parasites. Endoparasitic nematodes secrete apoplastic effectors to modulate host immune responses and to facilitate the formation of a feeding site. Apoplastic communication between hosts and parasitic plants also contributes to their interaction. Parasitic plant germination stimulants, strigolactones, are recently identified apoplastic signals that are transmitted over long distances from biosynthetic sites to functioning sites. Here, we discuss recent advances in understanding the importance of apoplastic signals and cell walls for plant–parasite interactions.
Introduction
The apoplast, including the plant cell wall, is a compartment outside of the plasma membrane. Cell walls consist of polysaccharides, such as cellulose, hemicellulose and pectin, and structural, catalytic or signaling proteins and function as structural support for cell shapes and also as a barrier against biotic and abiotic stresses (Yokoyama and Nishitani, 2004). Plant cells meet other pathogenic organisms at the apoplast and activate the immune systems against undesirable enemies (Hückelhoven, 2007; Hématy et al., 2009; Malinovsky et al., 2014).
Plant roots typically grow in soil and face diverse microbes and pathogens that are present in the rhizosphere. Roots have a centralized vascular cylinder that contains xylem and phloem cells surrounded by endodermis, cortex and the outermost epidermal cell layers. Primary cell wall impregnation at the endodermal cell layer is called the Casparian strip that acts as a diffusion barrier in the apoplastic space. Many, but not all plants, have hypodermal/exodermal cell layers below the epidermis that are highly lignified and suberized and also act similarly to the Casparian strip (Geldner, 2013). Parasitic plants and plant-parasitic nematodes are the two major root pathogens that parasitize important crops and cause huge economic losses in agriculture globally.
Some of the parasitic plants in the family Orobanchaceae, such as Striga and Orobanche, are recognized as noxious weeds. Striga spp. mainly grow in sub-Saharan Africa and parts of Asia, and their preferable hosts include important agricultural crops such as sorghum, pearl millet, rice, maize, and cowpea (Spallek et al., 2013). Broomrapes, Orobanche and Phelipanche spp., are native to the Mediterranean region and Western Asia and have extended their distribution to Asia, Africa, Australia, and North and South America (Parker, 2009). For host penetration, the primary root tip of an obligate root parasitic plant is transformed into a haustorium, an invasion organ (Yoshida and Shirasu, 2012). Each haustorium attaches to the host root surface and invades the host tissues. The tip of the haustorium eventually reaches the host’s stele, and a xylem connection, called a xylem bridge, is established (Heide-Jorgensen and Kuijt, 1995). Through the xylem bridge, parasites are able to acquire water and nutrients from the host plants.
Plant-parasitic nematodes parasitize a wide range of crop plants and greatly affect agriculture with an estimated loss of almost 100 billion USD per year (Abad et al., 2008; Nicol et al., 2011). Plant-parasitic nematodes are obligate parasites and are classified according to their feeding strategy. Sedentary endoparasites are the most evolutionarily advanced and the most damaging nematode group and, therefore, the molecular mechanisms underlying successful parasitism by this group have been extensively investigated. Two of the major agricultural pest nematodes are root-knot nematodes (Meloidogyne spp.) and cyst nematodes (Heterodera and Globadera spp.), both of which are sedentary endoparasites. Cyst and root-knot nematodes have developed stylets, hollow mouth spears that are used to pierce the epidermis of a host root. Esophageal gland cells consisting of two subventral and one dorsal gland cell are specialized for the production and secretion of secretory proteins, whereas the stylet punctures root cell walls, injects the secretory compounds produced in the gland cells and sucks host nutrients (Hussey, 1989; Mitchum et al., 2013). After invasion into the root, nematodes migrate to establish a feeding site.
Although these two categories of organisms are taxonomically very different, there are common features in their strategies for infecting plant cells (Figure 1). Both types of organisms are able to penetrate plant root tissues in an intra- or inter-cellular manner and grow or move toward plant vasculatures where the parasites can access the host nutrients.
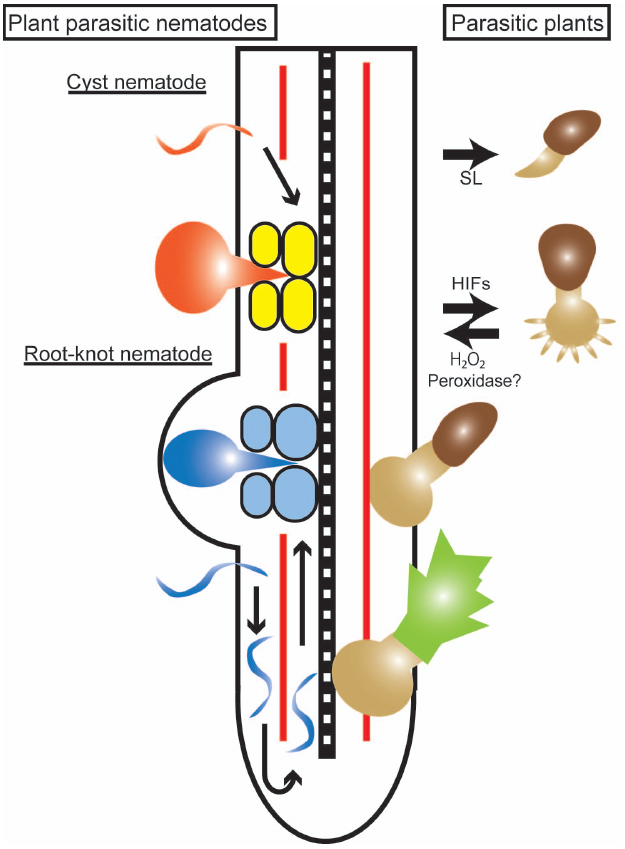
Figure 1. Infection processes of parasitic plants and plant-parasitic nematodes. An illustration of a host root with the right side showing an infection by a parasitic plant (Striga), and the left side showing infections by cyst and root-knot nematodes. The parasitic plant germinates in response to strigolactone (SL) and forms a haustorium in response to haustorium-inducing factors (HIFs) represented by 2,6-dimethoxy-p-benzoquinone. The parasite haustorium grows toward the host stele and, after reaching the host vasculature, a parasitic plant shoot begins to develop. A cyst nematode (orange) infects a host root, migrates through the cortical cells and creates a multinucleated syncytium (yellow) formed by cell fusion. A root-knot nematode (blue) infects a host root and migrates toward the root tip to avoid the Casparian strip (red line), turns and moves acropetally along the host vasculature. Giant cells (light blue) are formed by karyokinesis to provide nutrients to the nematodes.
In this review, we focus on the events occurring at the cell walls and the apoplastic spaces of host plant roots for these two different types of parasitic organisms.
Cell Wall Modifications During Parasite Invasion
Plant cells form two types of cell walls, i.e., primary and secondary cell walls. In general, primary cell walls are synthesized in growing cells and are composed predominantly of cellulose, pectin, and hemicelluloses such as xyloglucans. In grass species, however, the hemicellulosic materials of primary cell walls are arabinoxylans and mixed-linkage glucans. Secondary cell walls are formed in mature cells, are laid down on the inside of the primary wall (Cosgrove, 2005) and are typically composed of cellulose, xylans and lignin, providing cellular rigidity and strength (King et al., 2011). In addition, the middle lamella, a pectin layer, fills the space between the adjacent cells and firmly adheres them (Jarvis et al., 2003). During the establishment of a parasitic infection, the cell wall barrier is broken, and apoplastic signals may function to communicate between the host and parasites (Hamann, 2014; Hofte, 2014). For breaking into plant tissues to acquire nutrients, plant cell wall degrading enzymes (PCWDEs) play important roles in pathogenic fungi, oomycetes, and bacteria (Toth and Birch, 2005; Kämper et al., 2006). Pectin degrading enzymes, including pectin methylesterase (PME), pectin lyases, and polygalacturonases, were identified in cultures of the necrotic pathogen Botrytis cinerea (Shah et al., 2009), and pectate lyases (PL), endoglucanases, pectin esterases, and polygalacturonases were identified in the Phytophthora plurivora secretome (Severino et al., 2014). Degradation of pectin layers increases the accessibility of other enzymes such as cellulases and xylanases to break down the hemicellulosic chains (Hématy et al., 2009). Large-scale activity profiling of plant pathogenic and non-pathogenic fungi revealed that the cell wall hydrolytic enzymes secreted from pathogenic fungi reflect the monocot or dicot host preferences of the tested pathogens (King et al., 2011). This result implies that fine-tuned secretion of PCWDEs contributes to fungal adaptation to the host. Against that, arrays of inhibitor proteins for fungal PCWDEs were identified from various plants and some of them are indeed involved in immune responses (Juge, 2006).
Cell Wall Modifications by Parasitic Plants
Similar to microbe pathogens, parasitic plants are likely using PCWDEs during host root invasion. Parasitic plants form a haustorium, a unique multicellular invasion organ common to all parasitic plants. In the Orobanchaceae root parasites, globular-shaped haustoria invade host roots and form direct vascular connections with host plants, which likely enables nutrient transfer (Hibberd and Jeschke, 2001; Yoshida and Shirasu, 2012). Haustoria are formed either on the lateral side of primary or lateral roots (lateral haustoria) or at the tip of primary roots (terminal haustoria; Musselman, 1980). Haustorium development includes cell expansion and cell division of the cortical, epidermal, and pericycle cell layers (Baird and Riopel, 1984; Yoder, 2001; Ishida et al., 2011).
Electron microscopic analysis revealed that intrusive cells in the haustoria of Orobanche and Striga push their way through the host epidermis and cortex to reach the host stele (Neumann et al., 1999). Biochemical and histological studies have confirmed the activities of PCWDEs and compositional changes in the host and parasite cell walls (Figure 2). For instance, cellulase, polygalacturonase and, to a lesser extent, xylanase activities were detected from Phelipanche aegyptiaca shoots, roots and tubers (Singh and Singh, 1993), and pectinolytic activities, such as PME and polygalacturonase, as well as peroxidase were detected in Orobanche seedlings (Veronesi et al., 2005, 2007; Gonzalez-Verdejo et al., 2006). Several recent transcriptome analyses confirmed expression of PCWDEs in haustoria. Highly expressed genes in Triphysaria versicolor haustoria analyzed by laser microdissection included homologs of pectinesterase and polygalacturonase (Honaas et al., 2013). Comparative transcriptomics of three Orobanchaceae species identified a core set of genes expressed in haustoria, including pectate lyase and cellulase (Yang et al., 2015). Immunogold labeling of PME showed that this enzyme accumulates at the cell wall and Golgi apparatus of Orobanche cumana intrusive cells (Losner-Goshen et al., 1998). PME may have a crucial role in parasitism since application of catechin, a PME inhibitor, results in reduced attachment of the facultative root parasite Castilleja indivisa to its host and has a similar effect on the stem parasite Cuscuta pentagona on Arabidopsis (Lewis et al., 2010, 2008). Pectin de-esterification by PME is a prerequisite for pectin degradation by polygalacturonase and pectate lyase. Therefore, PME may modify the host cell wall pectin to become more accessible to other pectinolytic enzymes. Indeed, a correlation between pectinolytic enzyme activity and parasite virulence was reported in O. cumana, highlighting the importance of enzymatic activity in parasite virulence (Veronesi et al., 2005).
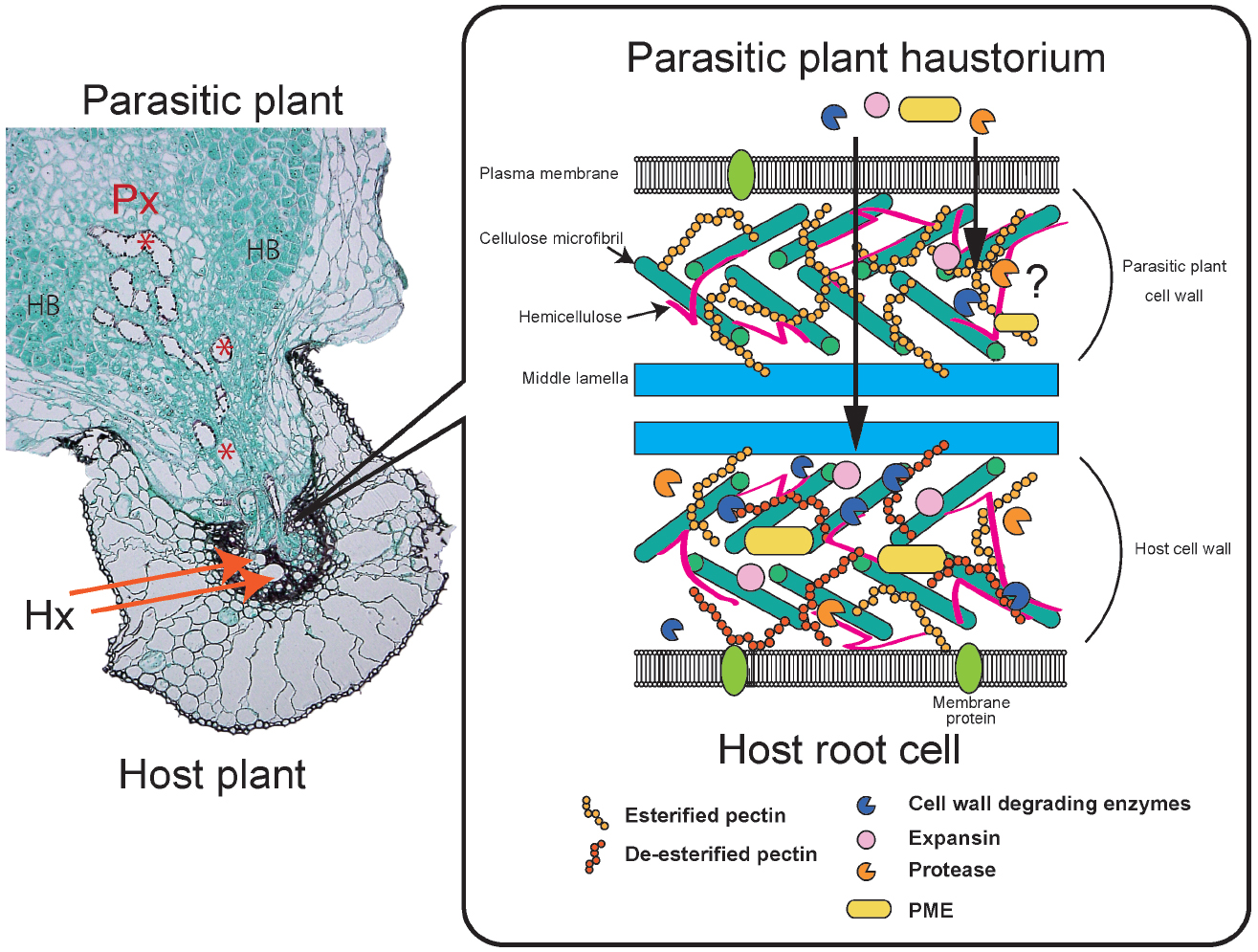
Figure 2. Parasitic plant interactions with a host plant. Striga infecting a rice root (A). A parasitic plant haustorium penetrates a host root and connects to the vasculatures. Parasite xylem cells (Px) are indicated by red asterisks. Host xylem cells (Hx) are indicated by orange arrows. Hyaline bodies (HB) are rich in AGPs. A schematic model of parasite and host cell walls and plant cell wall degrading enzymes (PCWDEs; B). Parasites secrete cell wall degrading enzymes, expansins and proteases. Pectin methylesterase (PME) de-esterifies the host cell wall pectin, thereby making the pectin more accessible to polygalacturonases and pectate lyase. Host cell wall pectin is often de-esterified. PCWDEs also include xylanases targeting cell wall xylan and cellulases targeting cellulose. It is still uncertain whether parasitic plant PCWDEs affect their own cell walls.
Changes in cell wall compositions are also observed during the penetration process. Immunofluorescence labeling revealed that the level of pectin esterification differs between Orobanche spp. cell walls and host cell walls during the interaction of these two organisms (Losner-Goshen et al., 1998; Figure 2). High- and low-esterified pectins were found in the parasites, whereas only low-esterified pectins were detected in host tissues close to the interface (Losner-Goshen et al., 1998). Similarly, during stem parasite dodder penetration, antibody-based comprehensive profiles of cell wall epitopes and PCWDEs revealed the presence of highly de-esterified homogalacturonans in susceptible host cell walls coincident with high pectinolytic activity in Cuscuta haustoria (Johnsen et al., 2015). In the interaction between Striga hermonthica and sorghum, often collapsed and necrotic host cells appear at the lateral site of the invading haustorium and cytoplasmic degradation products are detected, whereas none of these changes occur at the tip of the penetrating haustorium (Neumann et al., 1999). These observations indicate that the tips of penetrating haustoria are an active site for invasion, where parasites make their way while maintaining adhesive interaction with the host cells, undergoing continuous cell division and expansion of the haustorial internal cells, and creating physical pressure on the side of the haustorium to compress and eventually collapse the host root cells. In cowpeas infected with Striga gesnerioides, host cells surrounding the Striga intrusive cells were compressed and the middle lamella was degraded (Reiss and Bailey, 1998). Although not frequent, outgrowths of haustorial cells into host root cells are observed, suggesting that the host cell walls in the interface between host and parasite are weakened (Reiss and Bailey, 1998).
High levels of expression of expansins, cell wall-loosening proteins with no known enzymatic activity, were confirmed in the haustoria of a facultative parasite, T. versicolor, by tissue-specific expression analysis (Honaas et al., 2013). Interestingly, β-expansin that specifically loosens monocot cell walls was expressed higher in haustoria infecting Zea mays roots than in infections of Medicago truncatula, but the expression levels of α-expansin that targets both dicot and monocot cell walls were similar in both interactions (Li et al., 2003; Honaas et al., 2013). Therefore, the generalist parasites may use distinct sets of genes for different host interactions; this hypothesis supports the model that expansins may directly act on host cell walls.
Proteases likely play roles in host plant penetration by parasitic plants. Expression of cysteine protease-encoding genes was confirmed in haustoria of Phelipanche aegyptiaca as well as in the stem parasite Cuscuta reflexa (Rehker et al., 2012). Expression of inhibitor peptides in their hosts reduces parasite infection, suggesting that the proteases function in host tissues (Rehker et al., 2012). Apart from root parasites, the transcriptome of the stem parasite Cuscuta revealed that abundant transcripts in the prehaustorial stage are enriched with mRNAs encoding proteins with hydrolase activity or are associated with plant cell wall structure or function (Ranjan et al., 2014).
Cell wall modifying enzymes can be secreted to degrade host cell walls; alternatively, these enzymes may also affect parasite cell walls. Hyaline bodies occupy the central region of haustoria, an observation characteristic of cells with dense cytoplasm and extracellular deposits (Visser et al., 1984; Figure 2). Development of hyaline bodies is well correlated with host compatibility, but the physiological roles of these cells still remain obscure. The cell wall of hyaline bodies has a characteristic composition enriched with arabinogalactan proteins (AGPs) and reduced de-esterified pectins (Pielach et al., 2014). Furthermore, intercellular deposits and globular ergastic bodies composed of pectins, xyloglucans, extensins and AGPs were found in the facultative parasite Rhinanthus (Pielach et al., 2014). Therefore, the cell wall modifying and degrading enzymes may also act to develop hyaline bodies inside the haustorium.
Host Cell Wall Modification by Plant-Parasitic Nematodes
Plant-parasitic nematodes invade host root tissues to gain access to vasculatures. Patterns of migration and feeding site formation of cyst nematodes and root-knot nematodes are slightly different (Lambert and Bekal, 2002). Root-knot nematodes migrate intercellularly along the cortex toward the root tip and then turn around at the elongation zone to avoid the Casparian strip where the cell walls are highly lignified. The root-knot nematodes migrate up in the vascular cylinder until they reach the differentiation zone and colonialize this region (Figure 1). In contrast, cyst nematodes enter a root, migrate through cortical cells to the vascular cylinder and fuse multiple cells to form a multinucleated syncytium that functions as a feeding site (Figure 1). Root-knot nematodes modulate host cells to form multinucleated giant cells to serve as feeding sites by inducing karyokinesis without cell division. The function of the host root is significantly impaired by the intra- or inter-cellular migration of nematodes and the formation of a syncytium or giant cells that results in the destruction of root tissue structures (Rasmann et al., 2012).
Plant-parasitic nematodes secrete various PCWDEs when they penetrate and migrate inside host roots (Figure 3). Genes encoding β-1,4-endoglucanase, an enzyme that degrades cellulose, were identified from two species of cyst nematodes, marking the first confirmation of PCWDEs originating from animals (Smant et al., 1998). These endoglucanases were classified as glycosyl hydrolase (GH) family five cellulases. GH5 endoglucanases were found not only in the endoparasitic genera of Globodera, Heterodera, and Meloidogyne (Smant et al., 1998; Béra-Maillet et al., 2000; Goellner et al., 2000; Gao et al., 2002; Abad et al., 2008) but also in many other plant-parasitic nematode genera (Danchin et al., 2010; Haegeman et al., 2011b, 2012). In the cyst nematode Heterodera, endoglucanases are expressed in the subventral glands in second-stage juveniles (J2) before penetration and during migration, indicating that these cellulases play an early role in the parasitism process (Smant et al., 1998; Davis et al., 2004).
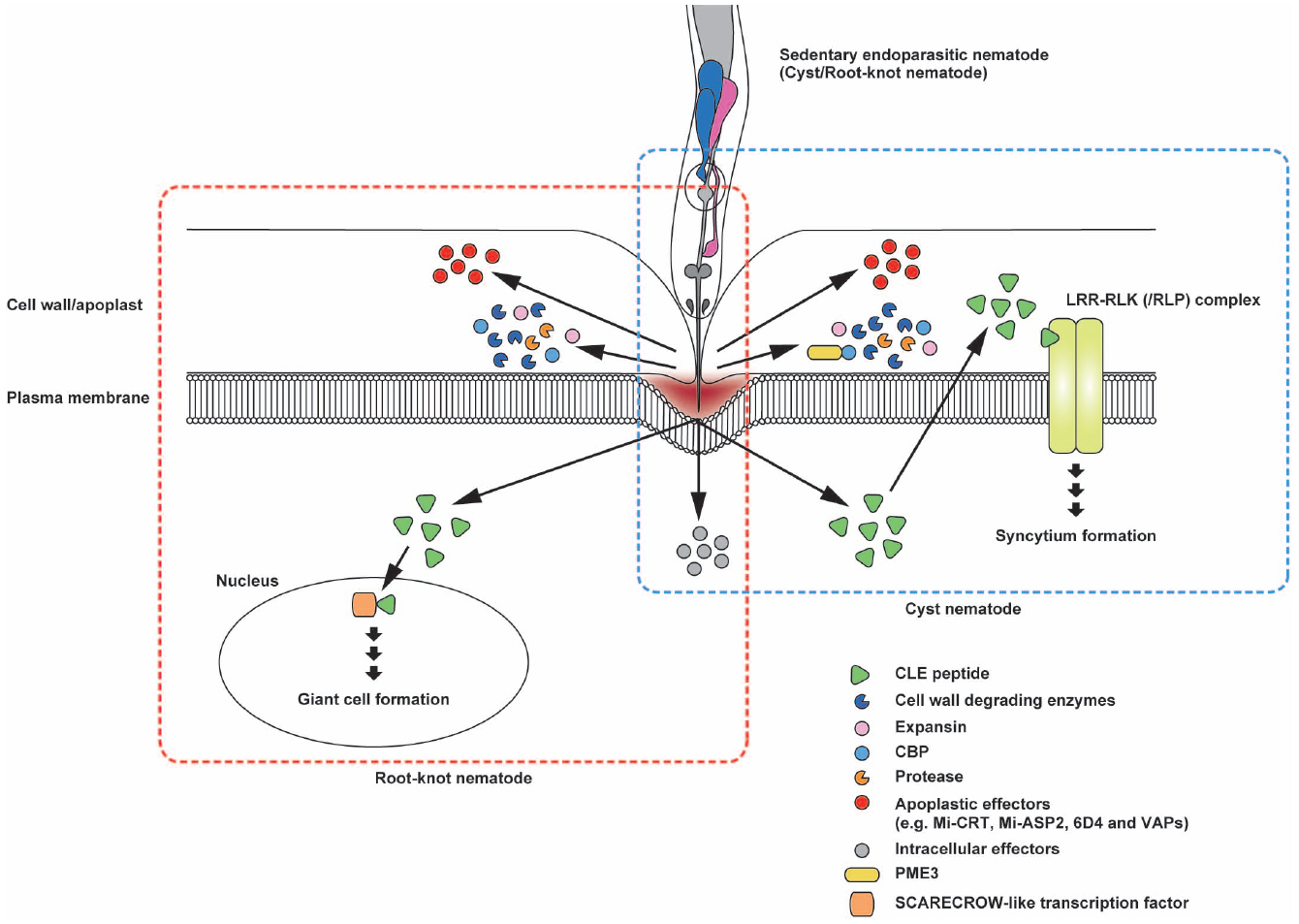
Figure 3. Schematic representation of the potential interactions of secreted effector proteins with nematode feeding cells. Sedentary endoparasites pierce the plant cell wall with their sclerotized stylets and release effector proteins from the stylet tip. Cell wall degrading enzymes, expansin and cellulose-binding protein (CBP) are beneficial for loosening the cell wall structure to facilitate nematode migration after invasion. Cyst nematode CBPs interact with plant pectin methylesterase 3 (PME3). In cyst nematodes, CLE-like peptides appear to mimic plant CLE signaling that probably promotes syncytium formation. The CLE-like peptide 16D10 from root-knot nematodes binds to two SCARECROW-like transcription factors in the host plant. Mi-CRT is involved in the inhibition of defense responses. In addition, Mi-ASP2, 6D4, and VAPs in root-knot nematodes and cyst nematodes function as apoplastic effector proteins. Illustrations framed with boxes of blue- and red-dashed lines show effector proteins and their topology for cyst nematodes and root-knot nematodes, respectively.
In addition to cellulase activity, hemicellulolytic and pectolytic enzyme activities are detected in the secretions of plant-parasitic nematodes. Heterodera GH5 endoglucanases degrade hemicellulose (Gao et al., 2004). A root-knot nematode, Meloidogyne incognita, produces xylanases from the GH5 and GH30 families (Mitreva-Dautova et al., 2006; Abad et al., 2008; Danchin et al., 2010). Polygalacturonases from family GH28 and PL from family PL3, two types of pectolytic enzymes, were identified in M. incognita (Abad et al., 2008; Danchin et al., 2010; Haegeman et al., 2011a). Genes encoding pectate lyase from family PL3 were also identified in Heterodera and Globodera cyst nematodes (Popeijus et al., 2000; De Boer et al., 2002; Gao et al., 2003; Vanholme et al., 2007; Danchin et al., 2010), and these genes are expressed in the subventral esophageal glands of pre-parasitic and parasitic J2-stage nematodes. Putative arabinanases from family GH43 were also identified in Globodera, Heterodera, and Meloidogyne (Danchin et al., 2010). Arabinan, the substrate of arabinanase, is a main component of pectin side chains; thus, hydrolysis of arabinan may result in easier access to pectin backbones for polygalacturonases and PL (Danchin et al., 2010).
Besides PCWDEs, nematodes secrete cell wall modifying proteins. Gr-EXP, an expansin isolated from Globodera rostochiensis, exerts cell wall loosening activity similar to the plant expansins (Qin et al., 2004; Kudla et al., 2005). In M. incognita, genes encoding expansin-like proteins were identified (Abad et al., 2008; Danchin et al., 2010). Expansins may promote the accessibility of cell wall degrading enzymes to their substrates by disrupting non-covalent bonds between polysaccharide chains (Haegeman et al., 2012). Interestingly, homologous genes encoding PCWDE and expansins have not been found in free-living nematodes. Amino acid sequences of these proteins are similar to those of bacteria or fungi, indicating that these nematode genes could have been acquired from bacteria or fungi by horizontal gene transfer (Danchin et al., 2010; Haegeman et al., 2011a, 2012). Additionally, nematodes secrete a cellulose-binding protein (CBP) that contains a cellulose recognition domain and binds to cellulose but has no hydrolytic activity. CBP from the sugar beet cyst nematode Heterodera schachtii (Hs CBP) interacts with Arabidopsis pectin methyl esterase 3 (PME3) to facilitate cyst nematode parasitism (Hewezi et al., 2008). Hs CBP expression peaks at the parasitic J3 stage, suggesting a role during the early phases of syncytium formation (Hewezi et al., 2008). PMEs catalyze the demethylesterification of pectin in the cell wall and modify the stiffness of the cell wall (Sasidharan et al., 2011). Overexpression of Hs CBP slightly, but statistically significantly, increased the PME activity of Arabidopsis, indicating that a reduction in the methylesterification level in the cell wall pectin through Hs CBP-mediated PME3 activity induces a cell wall modification required for syncytium development (Hewezi et al., 2008). Plant-parasitic nematodes also secrete proteases. Proteases could be used to loosen the cell wall structure, thereby facilitating the migration of nematodes. In addition, proteases may degrade plant defense proteins or digest host proteins in the giant cells (Haegeman et al., 2012).
Plant Immunity and Cell Wall Integrity
Cell walls are the physical barriers against pathogen attacks. Cell wall reinforcement is one of the primary responses against pathogen infection. The deposition of callose, a 1,3-β-glucan, is commonly observed in plant leaves and roots upon pathogen challenge (Millet et al., 2010; Voigt, 2014). In addition, alteration of cell wall integrity sensed by the host plant also is a signal to activate innate defense responses (Hématy et al., 2009). Various mutants with altered cell wall composition have a pathogen-resistant phenotype (Nühse, 2012; Malinovsky et al., 2014). For example, the Arabidopsis cellulose synthase mutant cesa3 is more resistant toward powdery mildew, and defects in secondary wall-forming CESAs provide resistance against necrotrophic pathogens (Malinovsky et al., 2014). The Arabidopsis powdery-mildew-resistant (pmr) mutants also link cell wall composition and plant immunity (Nühse, 2012). The pmr5 and pmr6 have increased levels of unesterified pectin and enhanced powdery-mildew resistance independent of the salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) pathways (Vogel et al., 2004). The disruption and overexpression of PMR4, a callose synthase, resulted in a resistant phenotype against powdery mildew likely with a different mechanism, indicating the involvement of PMR4 in establishing a physical barrier and in defense signaling (Nishimura et al., 2003; Ellinger et al., 2013). In the interaction of plants and microbial pathogens, the presence of pathogens is recognized by pathogen (or microbe) associated molecular patterns (PAMPs/MAMPs), which are widely conserved molecules among microbes, through cell surface localized pattern recognition receptors. Cell wall fragments, such as oligogalacturonides (OGs), also act as damage-associated molecular patterns (DAMPs) that induce basal plant defenses (Boller and Felix, 2009; Ferrari et al., 2013). OGs are oligomers of α-1,4-linked galacturonosyl residues released from homogalacturonan, a major cell wall pectin component. Therefore, recognition of OGs through membrane-localized receptor WALL-ASSOCIATED-KINASES (WAKs) is considered a system for monitoring pectin integrity that induces a set of basal defense responses, including accumulation of reactive oxygen species and pathogenesis-related proteins (Brutus et al., 2010; Ferrari et al., 2013).
In the theory of the co-evolution of plant defense systems and pathogen infection strategies, pathogens evolved to overcome basal plant immunity by generating effectors (Jones and Dangl, 2006). Effectors are typically small proteins or peptides delivered into plant cells or the apoplast (Win et al., 2012; Stotz et al., 2014). Plants recognize the effectors directly or indirectly through resistance (R) proteins, typically nuclear-binding site (NBS) and leucine-rich repeat (LRR) motif-containing proteins, and induce effector-triggered immunity (ETI) that often results in race-specific resistance. The sequential evolution of the innate immunity systems and breakdown processes is referred to as the “zigzag” model (Jones and Dangl, 2006).
Although the above theory was based on work conducted with leaf-infecting pathogens, accumulating evidence suggests that root immune systems are, at least partially, similar to those in leaves. Arabidopsis roots respond to MAMP signals by promoter activation of defense-related genes and epidermal callose deposition (Millet et al., 2010). As is known in leaves, coronation, a phytotoxin and JA-Ile mimic produced by Pseudomonas syringae pathovars, suppresses the defense responses. This suppression relies on JA-signaling genes such as COI1 and MYC2, but not on JA-SA antagonism (Millet et al., 2010). The importance of root defense is indirectly supported by the observation that MAMP responses are suppressed upon colonization by beneficial microbes (Jacobs et al., 2011; Nakagawa et al., 2011; Brotman et al., 2013). However, transcriptome analysis of roots infected with the wilt fungus Fusarium oxysporum revealed that gene expression patterns are distinct from those in leaves infected by the same pathogen, indicating the presence of a root-specific defense system (Chen et al., 2014). Interestingly, six quantitative trait loci (QTL) responsible for RESISTANCE TO FUSARIUM OXYSPORUM (RFO) were identified, and RFO1 encodes a dominant allele of the WALL-ASSOCIATED KINASE-LIKE KINASE 22 (WAKL22) gene, a receptor kinase similar to WAK (Diener and Ausubel, 2005). Collectively, these results suggest that a membrane-bound receptor kinase is involved in root defense systems.
Host Immune Responses Against Parasitic Plants
Cell wall reinforcement is likely involved in the resistance against parasite intrusion. In some incompatible or non-host interactions with parasitic plants, “mechanical barrier” formation is reported, in which deposition of phenolic compounds at the host-parasitic plant interface was observed (Maiti et al., 1984; Goldwasser et al., 1999; Haussmann et al., 2004; Yoshida and Shirasu, 2009). Deposition of callose is recognized in resistant hosts although the physiological significance of callose deposition remains to be investigated (Lozano-Baena et al., 2007; Yoder and Scholes, 2010). In the resistant pea and Orobanche crenata interaction, parasite intrusion stopped at the host cortex cell layers before reaching the central cylinder and accompanied the accumulation of peroxidase, H2O2 and callose (Pérez-de-Luque et al., 2006). Protein cross-linking in the host cell walls may also play a role to block parasite invasion.
Although no molecular patterns associated with parasitic plants have been identified yet, the innate immune system is provoked by parasitic plant intrusion. Whole genome microarray of resistant (Nipponbare) and susceptible (IAC165) rice cultivars revealed that resistant interactions against S. hermonthica are characterized by upregulation of pathogenesis-related (PR) genes including many SA-responsive genes (Swarbrick et al., 2008). Proteome analyses identified defense-related proteins that are induced in resistant cultivars of pea and M. truncatula against O. crenata (Castillejo et al., 2004, 2009). Similarly, defense-related genes are enriched among the late-responsive genes in resistant interactions of cowpea and S. gesnerioides, whereas defense genes are suppressed in a compatible interaction (Huang et al., 2012a). These findings indicate the possible suppression of defense by parasitic plants in compatible interactions.
The defense-related plant hormones JA and SA likely contribute to parasitic plant resistance. Exogenous application of SA on red clover reduces O. minor infection with lignification of cell layers in the host endodermis (Kusumoto et al., 2007). Foliar application of benzo-1,2,3-thiadiazole-7-carbothioic acid S-methyl ester (BTH), a SA analog, or methyl jasmonate (MeJA) on rice induced increased resistance against S. hermonthica (Mutuku et al., 2015), indicating that SA and JA induce a systemic defense against parasitic plants. Indeed, endogenous SA and JA accumulate in rice after S. hermonthica infection. Transgenic rice containing a silencing construct for WRKY45, a key transcriptional factor in the SA signaling pathway, showed increased susceptibility against S. hermonthica. However, SA-deficient transgenic rice that was transformed with NahG, a gene encoding salicylate hydroxylase, did not show increased susceptibility, suggesting that endogenous SA is not required for S. hermonthica resistance. WRKY45 regulates the JA biosynthesis genes, and application of MeJA complements S. hermonthica susceptibility in transgenic rice. These findings suggest synergistic effects of SA and JA on S. hermonthica resistance in rice (Mutuku et al., 2015).
Race-specific incompatibilities in parasitic plants and their hosts have been reported in several species (Huang et al., 2012b). Cowpea cultivar B301 contains the RSG3-301 gene for resistance to S. gesnerioides race SG3 but is susceptible to race SG4z (Li and Timko, 2009). The RSG3-301 gene encodes a typical R protein, consisting of a coiled-coil, NBS and LRR motifs. Knock-down of this gene results in a susceptible interaction with S. gesnerioides race SG3 (Li and Timko, 2009), indicating that this R protein has a crucial role in this race-specific resistance. Therefore, it is possible that a virulent S. gesnerioides strain may possess effectors that suppress plant immunity.
Host Defenses Against Plant-Parasitic Nematode Infection
Endoparasitic nematodes migrate inside root tissues. Thus, physical and physiological changes within the root concomitant with nematode invasion and migration provoke defense responses in the host plant. Callose is deposited within plasmodesmata in young syncytia, and reduced callose degradation in an Arabidopsis β-1,3-glucanase insertion line results in smaller sizes of syncytia. Both of these observations indicate that callose deposition restricts syncytial growth in cyst nematodes (Hofmann et al., 2010).
Microarray analyses using tomato and root-knot nematodes revealed that JA- and SA-responsive genes are upregulated to different extents during nematode infection either in compatible or incompatible interactions (Bhattarai et al., 2008). Application of the SA analog BTH resulted in a slight increase in the expression of defense-related genes and resistance against nematodes (Nahar et al., 2011, 2012). SA-deficient tomato and rice overexpressing NahG had higher levels of nematode infection, suggesting that SA is involved in nematode resistance (Nahar et al., 2011, 2012). However, the effects of SA on nematode resistance are still controversial because others have reported that the NahG transgene had only partial or no effects on the NBR-LRR resistance gene Mi-1 in mediating resistance as well as for basal resistance in tomato (Branch et al., 2004; Bhattarai et al., 2008). Foliar application of MeJA induces systemic defense responses in rice, and the rice JA biosynthesis mutants were more susceptible to Mycosphaerella graminicola (Nahar et al., 2011). Similarly, application of MeJA and defects in JA biosynthesis in tomato result in enhanced resistance and susceptibility to plant-parasitic nematodes, respectively (Cooper et al., 2005; Fan et al., 2014). These findings indicate that JA contributes to nematode resistance. In addition, ET and ABA also have positive and negative roles in nematode resistance, respectively (Nahar et al., 2012). The crosstalk among these hormonal signals may regulate host defenses against nematode infection.
Apoplastic Effectors Secreted From Plant-Parasitic Nematodes
The expression of defense-related genes is suppressed during giant cell development upon root-knot nematode infection, suggesting that the host defense is shut down by nematode infection (Jammes et al., 2005; Barcala et al., 2010). Furthermore, several R-proteins have been identified for resistance against cyst- and root-knot nematodes from various plants, and this aspect is extensively reviewed elsewhere (Williamson and Kumar, 2006; Kaloshian et al., 2011). These observations suggest that the interaction between plants and plant-parasitic nematodes follows the zig-zag model (Figure 4).
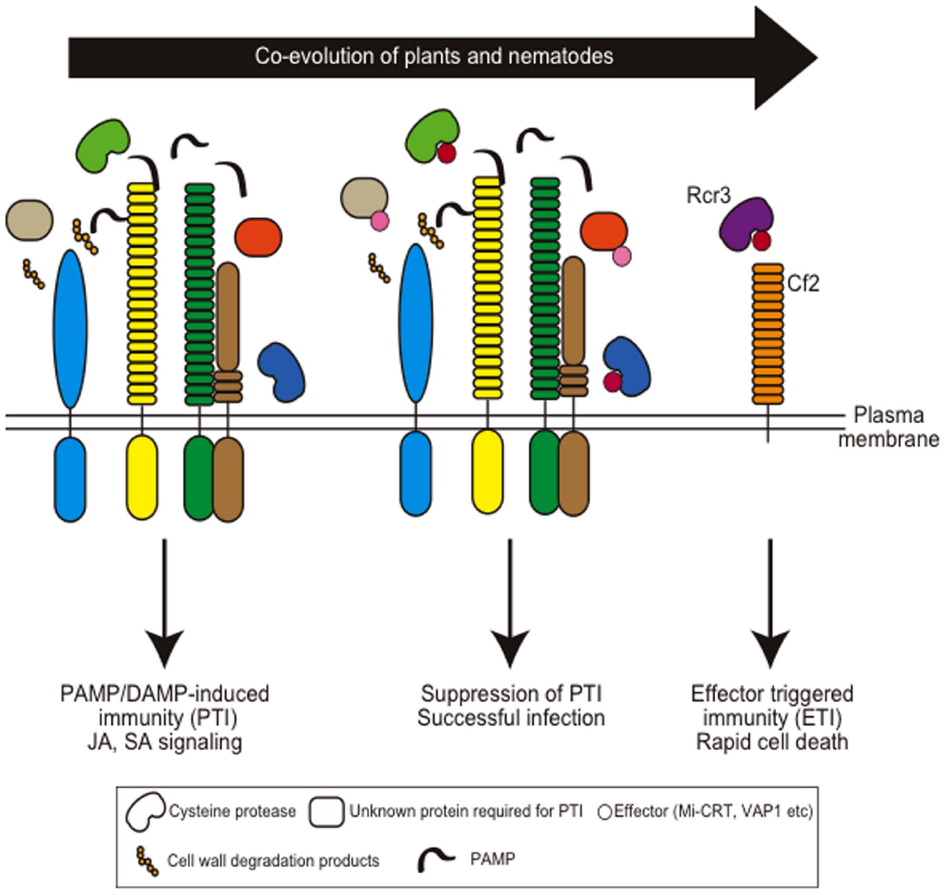
Figure 4. Coevolution of host plant immunity and effectors of plant-parasitic nematodes. Hypothetical nematode PAMPs or DAMPs are recognized by membrane-localized PAMP receptors to induce PAMP-induced immunity (PTI). Nematodes secrete apoplastic effectors that target PTI components and suppress PTI. Mi-CRT suppresses EFR-mediated defense responses when this effector is overexpressed in Arabidopsis. Papain-like cysteine proteases are required for nematode resistance in Arabidopsis and VAP effectors target these proteases. Gr-VAP1 interacts with Rcr3pim in tomato and provokes Cf2-mediated defense. Note that so far no PAMP receptors have been identified that are involved in nematode resistance.
Indeed, cyst and root-knot nematodes produce effector proteins and inject them into the target cells during a successful infection. Effector proteins act to modify the host–plant signaling pathway to syncytium or giant cell formation and to suppress plant defense responses that are activated by disruption of cell structure and secreted components from nematodes. In several cases, targets of effector proteins and sensors of exogenous nematode-derived substances are known to be present in the apoplast of host roots (Figure 3). Two M. incognita secreted proteins, Mi-ASP2, an aspartic protease-like protein, and 6D4 protein, are secreted into the apoplast along the giant cells during the migratory pre-parasitic J2 early sedentary juvenile stages, although their function during the early stage of parasitism has not been characterized (Vieira et al., 2011). Calreticulin, a Ca2+-binding chaperone from the root-knot nematode M. incognita (Mi-CRT), localizes to the apoplastic space of the giant cells (Jaubert et al., 2005). Mi-CRT is produced in the subventral and the dorsal esophageal gland cells during the migratory and sedentary stages, respectively. After secretion, Mi-CRT localizes outside of the nematode stylet tip and accumulates along the cell walls of the giant cells (Jaubert et al., 2005). Ectopic overexpression of Mi-CRT in Arabidopsis thaliana increases susceptibility to M. incognita in terms of gall formation, as well as to the pathogenic oomycete Phytophthora parasitica. Mi-CRT overexpression suppresses the expression of defense marker genes and callose deposition upon treatment with elf18 peptide, a well-known PAMP signal derived from acetylated, elongation factor Tu (EF-Tu), suggesting that Mi-CRT suppresses PAMP-mediated plant defense responses (Jaouannet et al., 2013). The expression of an enhanced green fluorescence protein (eGFP)-fusion construct in tobacco leaves confirmed that the overexpressed Mi-CRT localizes mainly to apoplasts, but it also localizes to the endoplasmic reticulum (ER) and Golgi network. Therefore, it remains possible that ectopic expression of Mi-CRT in the ER rather than in the apoplast suppresses elf18-induced defense responses as the elf18 receptor EFR-mediated pathway is highly sensitive to ER protein dysfunction (Tintor and Saijo, 2014).
Apoplastic venom allergen-like proteins (VAPs) are secreted proteins uniquely conserved among plant- and animal-parasitic nematodes. The expression of VAP from the cyst nematode G. rostochiensis (Gr-VAP1) was detected in the subventral esophageal glands at the infective juvenile stage (Lozano-Torres et al., 2012). Recent analyses revealed that suppression of Gr-VAP1 expression by dsRNA in G. rostochiensis results in reduced infection, and ectopic overexpression of Gr-VAP1 and VAPs from the Arabidopsis-infective cyst nematode H. schachtii (Hs-VAP1 and Hs-VAP2) enhanced infection by H. schachtii (Lozano-Torres et al., 2014). Moreover, Arabidopsis plants expressing the Hs-VAPs had enhanced susceptibility to multiple fungal and oomycete pathogens, suggesting that VAPs are effectors that modulate basal immunity in host plants. The delivery of Gr-VAP1 coincides with the enzymatic breakdown of plant cell walls by migratory nematodes (Lozano-Torres et al., 2014). Thus, these effectors may suppress host defenses activated by cell wall breakdown products during nematode infection and migration. Interestingly, in tomato, Gr-VAP1 targets the apoplastic cysteine protease Rcr3pim from Solanum pimpinellifolium and provokes receptor-like protein Cf2-mediated defense responses by perturbing Rcr3pim function (Lozano-Torres et al., 2012). This strategy is similar to the effector protein Avr2 secreted from the fungal pathogen Cladosporium fulvum that also induces a Cf2-mediated defense response by targeting Rcr3pim (Lozano-Torres et al., 2012). This is one example of an effector that suppresses PAMP-triggered immunity and that has evolved to induce ETI in a particular host species (Figure 4).
Last year, a novel group of hyper-variable extracellular effectors (HYP) from Globodera pallida was discovered (Eves-van den Akker et al., 2014). Unlike general nematode effectors, the Gp-HYP genes are expressed in the amphid sheath cells of parasitic females rather than in the esophageal gland cells. Gp-HYP effector proteins secreted from the amphids were detected in the apoplast adjacent to the feeding site. In planta RNAi-mediated knock down of all members of the effector family reduced the level of successful parasitism, although the functional role and the significance of the remarkable variability of the HYP gene family is under discussion.
CLAVATA3/ESR-Related (CLE)-Like Peptides as Effectors of Plant-Parasitic Nematodes
Both cyst and root-knot nematodes produce CLAVATA3/ESR-related (CLE)-like peptides as effector proteins (Figure 3). CLE peptides act like peptide hormones in plants by interacting with the apoplastic side of a complex of LRR receptor-like kinases (LRR-RLKs) and LRR-receptor-like protein (RLP) that regulate apical and cambial meristem maintenance (Betsuyaku et al., 2011). Unlike cell wall modifying enzymes, nematode CLE-like peptides are thought not to be acquired by horizontal gene transfer but to have evolved as mimics of the host peptides by convergent evolution (Haegeman et al., 2011a). CLE peptides from the soybean cyst nematode Heterodera glycines are transported from the cytoplasm to the host cell apoplast to function as plant CLE mimics (Wang et al., 2010). Thus, nematode CLEs mimic plant CLEs by interacting on the apoplastic side of the LRR-RLKs complex. The ectopic expression of HgSYV46, a gene encoding the H. glycines CLE peptide, in an Arabidopsis clavata (clv)-3 mutant partially or fully rescues the mutant phenotype. Additionally, HgSYV46 transgenic plants mimic the wuschel (wus)-like phenotype and the short-root phenotype in shoot apices and roots, respectively, similar to the phenotype reported in plants overexpressing AtCLV3 (Wang et al., 2005). Localization of HgSYV46 in syncytia was demonstrated using an affinity-purified anti-HgCLE peptide antibody (Wang et al., 2010). The number of female nematodes are significantly reduced in Glycine max inoculated with HgSYV46-knockdown H. glycines, and in A. thaliana expressing the Hssyv46 (a Hgsyv46 gene homolog in Heterodera schachtii) RNAi construct inoculated with H. schachtii (Bakhetia et al., 2007; Patel et al., 2008). Similar to plant intrinsic CLV3 peptide signaling, nematode CLE signaling genetically requires CLV2 and CORYNE (CRN)/SUPPRESSOR OF LLP1 2 (SOL2; Replogle et al., 2011). These results indicate that Heterodera CLEs are functionally similar to the CLV3 peptide and may play a role in the formation of syncytia. The CLE-like genes of a potato cyst nematode, G. rostochiensis, encode secreted proteins containing multiple CLE motifs (Lu et al., 2009). Ectopic GrCLE gene expression rescues the Arabidopsis clv3-2 mutant phenotype. Furthermore, overexpression of GrCLE genes as well as exogenous application of synthetic GrCLE peptides result in a short-root phenotype in Arabidopsis roots and potato hairy roots, suggesting that GrCLE peptides also have an activity similar to plant and Heterodera CLE peptides. Therefore, the evolution of multiple CLE motifs may allow the generation of functional diversity in nematode CLE proteins, thereby facilitating parasitism (Lu et al., 2009). In M. incognita, the 16D10 gene encodes a CLE-like peptide precursor protein that is processed to a mature peptide consisting of 13 amino acids. Silencing of 16D10 expression in nematodes by dsRNA ingestion or in an A. thaliana host expressing dsRNA as a transgene resulted in reduced nematode infectivity (Huang et al., 2006). Overexpression of 16D10 stimulated root growth but could not rescue the clv3-1 phenotype in Arabidopsis. The 16D10 was found to interact with two SCARECROW-like (SCL) transcription factors that are members of the GRAS family implicated in root development (Huang et al., 2006). The 16D10 peptide seems to regulate root cell differentiation to form feeding cells, but its detailed function has not been determined yet. CLE peptides are strong effector candidates that modulate root development. Further investigations will reveal the similarity and differences of CLE peptide signaling between cyst nematodes and root-knot nematodes.
Apoplastic Signals for Parasites
In the interaction between host plants and microbes, host-secreted compounds act as chemical cues to establish the interaction. For example, in the symbiotic interaction between legume plants and nitrogen-fixing rhizobia, root-released flavonoids activate the production of rhizobial nod factors, lipochitin oligosaccharides, that are perceived by plants to start symbiotic programs (Hassan and Mathesius, 2012). Parasitic plants recognize host-secreted germination stimulants, strigolactones (SLs) and related compounds, that have recently attracted attention as apoplastic signaling molecules. Haustorium formation by parasitic plants also is provoked by cell-wall related compounds.
SLs as Apoplastic Signals in Plants and the Rhizosphere
The germination stimulants, SLs, belong to a class of terpenoid lactones with the tricyclic lactone part (ABC-ring) connected to another lactone (D-ring) through an enol ether bridge (Figure 5). SLs were first reported in 1966; strigol, a natural SL, was isolated from cotton root exudates as a compound that stimulates seed germination of the obligate parasite Striga lutea (Cook et al., 1966). After this initial discovery of strigol, a variety of related compounds were identified from various plant species, and these compounds are collectively called SLs (Yoneyama et al., 2010). Later, SLs were rediscovered as inducers for the hyphal branching of arbuscular mycorrhizal (AM) fungi, thereby facilitating the uptake of inorganic nutrients to the host plant (Akiyama et al., 2005), and as a novel class of plant hormone that inhibits shoot branching. The more axillary growth (max) mutants in Arabidopsis, the ramosus (rms) mutants in pea and a few of the dwarf (d) mutants in rice have a greater degree of shoot branching and contain mutations in either the SL biosynthesis genes or the SL signaling genes (Gomez-Roldan et al., 2008; Umehara et al., 2008). More recently, SLs were demonstrated to have multiple hormonal roles in diverse developmental processes (Seto et al., 2012; Brewer et al., 2013; Ruyter-Spira et al., 2013; Waldie et al., 2014). Thus, SL functions can be classified into two groups: rhizosphere signals (allelochemical) to communicate with symbionts and parasites and endogenous hormones to regulate various plant developmental processes.
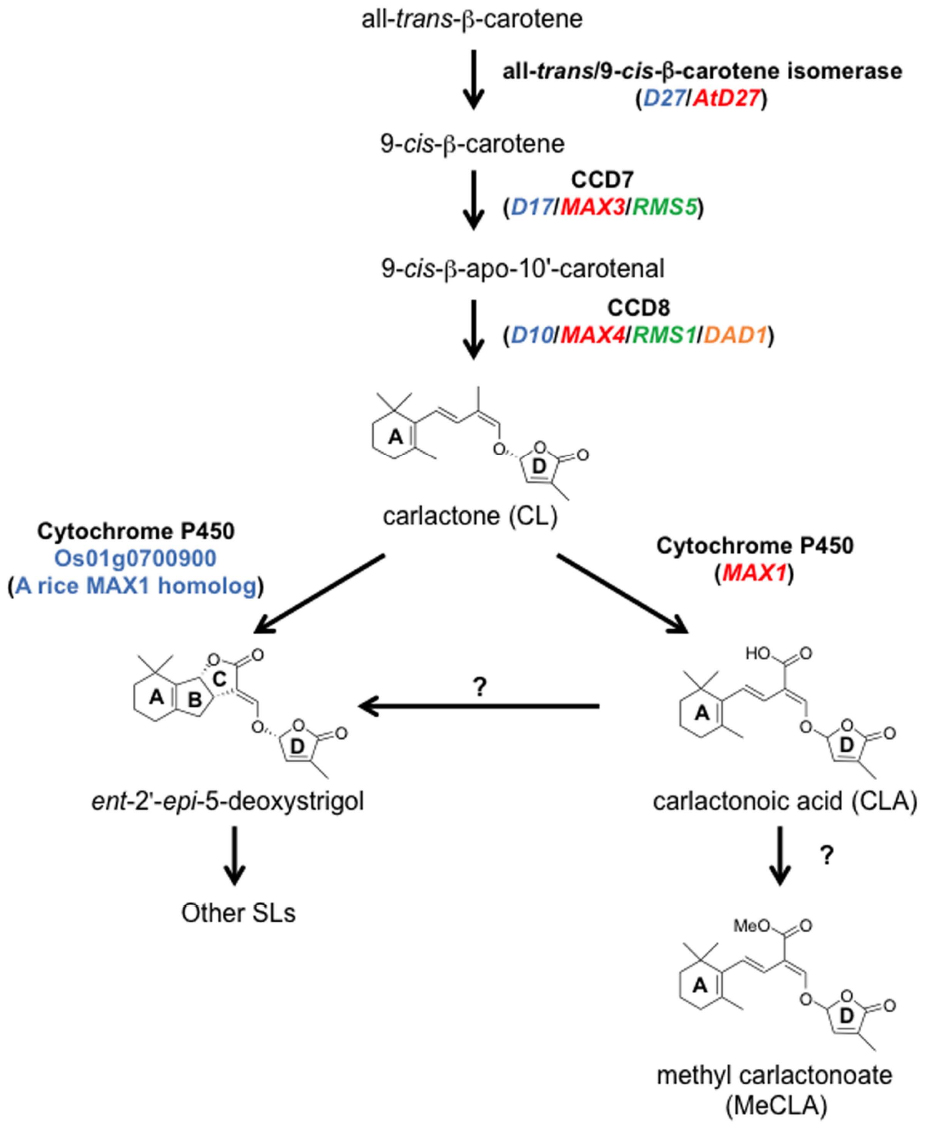
Figure 5. The SL biosynthetic pathway. CL is produced from all-trans-β-carotene by three enzymes, D27, CCD7, and CCD8. CL is oxidized by MAX1 into CLA, and then CLA is converted to the methyl ester derivative, MeCLA, in Arabidopsis. CLA is converted to strigolactones such as ent-2′-epi-5-deoxystrigol in rice. In addition, one of the MAX1 homologs in rice, Os01g0700900, can catalyze the conversion from CL to ent-2′-epi-5-deoxystrigol by a single enzymatic reaction. Ent-2′-epi-5-deoxystrigol might be converted into other SLs. Blue, red, green, and orange letters indicate genes of rice, Arabidopsis, pea, and petunia, respectively.
Although SLs are known to be carotenoid-derived compounds (Matusova et al., 2005), their biosynthetic pathway has been a long-standing question until recently. Analyses of shoot-branching mutants from several plant species identified four key enzymes in the SL biosynthetic pathway: DWARF27 (D27), a carotenoid cleavage dioxygenase7 (CCD7/MAX3), a carotenoid cleavage dioxygenase8 (CCD8/MAX4) and a cytochrome P450 (CYP711A1/MAX1; Booker et al., 2004; Arite et al., 2007; Lin et al., 2009; Crawford et al., 2010). Alder et al. (2012) demonstrated the biochemical functions of three of these enzymes, D27, CCD7, and CCD8, using in vitro biochemical reactions (Alder et al., 2012). As a result, carlactone (CL), a key biosynthetic intermediate with an SL-like carbon skeleton including the D-ring, was identified as a product of the sequential reaction of these three enzymes with all-trans-β-carotene (Figure 5). CL was further demonstrated to be an endogenous biosynthetic precursor of SL (Seto et al., 2014). In a series of grafting experiments using the Arabidopsis max mutants, the shoot branching phenotype of the max4 (ccd8) mutant was restored by grafting onto the max1 (cyp711A1) mutant rootstock (Booker et al., 2005), suggesting that MAX1 is a downstream component of CCD8. Interestingly, the Arabidopsis max1 mutants accumulate extremely high levels of endogenous CL. Thus, we predicted that CL is the substrate of the MAX1 enzyme. Indeed, MAX1 was experimentally shown to convert CL into its C-19 oxidative product, carlactonoic acid (CLA) through three consecutive oxidation steps (Abe et al., 2014; Figure 6). Furthermore, among the five copies of the CYP711A1 family of genes in rice, one gene product catalyzes several multi-step reactions converting CL into ent-2′-epi-5-deoxystrigol, a bioactive SL (Zhang et al., 2014; Figure 6).
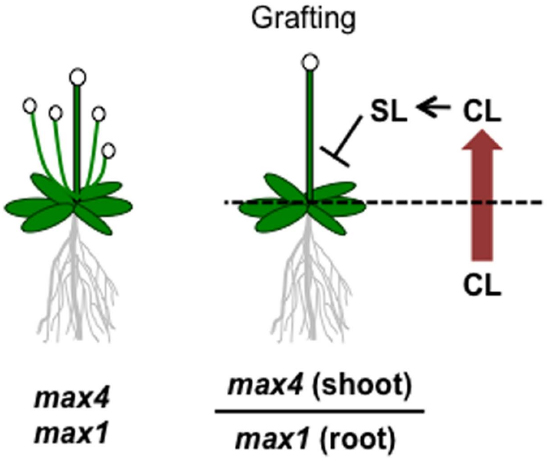
Figure 6. Graphical summary of a grafting experiment using the Arabidopsis max4 and max1 mutants. The shoot-branching phenotype of max4 was restored by grafting onto max1 roots. The model proposes that CL biosynthesized in the max1 mutant roots is transported to the shoots, converted to SL, and inhibits shoot branching.
As a rhizosphere signal, root-synthesized SL should be secreted into the rhizosphere through the plant apoplast. Experiments in which shoots of SL-biosynthesis mutants were grafted on wild type rootstocks and recovered the shoot branching phenotype demonstrated that this endogenous hormonal signal is transmissible long distances from roots to shoots (Booker et al., 2005; Ongaro and Leyser, 2008). This result strongly suggests that root-synthesized SL or related compounds can be transported to aerial tissues to control shoot branching. In fact, SLs have been detected in the xylem sap of Arabidopsis and tomato (Kohlen et al., 2011, 2012). Moreover, the results of the max1-shoot and max4-root grafting experiment described above raises another important issue; that is, the SL biosynthetic intermediate in the pathway between MAX4 and MAX1 is the transmitted form of the long-distance SL signal (Booker et al., 2005). Considering the recent discovery of CL as a product of CCD8 and a substrate of MAX1, CL is hypothesized to be the transmissible intermediate that moves from roots to shoots (Figure 5), although experimental confirmation is still necessary. On the other hand, CL is biologically inactive for shoot branching inhibition because the CL over-accumulating max1 mutant still has the branched phenotype, and CL rescues the phenotype of the max4 mutant but not that of the max1 mutant (Scaffidi et al., 2013; Seto et al., 2014). These results indicate that CL is converted into an active compound after transport to the aerial parts of the plant (Figure 5). D27, CCD7, and CCD8 localize in plastids and are expressed in root tips, the root cortex, and hypocotyls (Sorefan et al., 2003; Booker et al., 2004; Auldridge et al., 2006; Arite et al., 2007; Lin et al., 2009; Waters et al., 2012a), whereas MAX1 is expressed in vascular-associated tissues such as the cambial region and xylem-associated parenchyma in shoots and roots (Booker et al., 2005). These different expression patterns imply that MAX1 oxidizes CL during loading or unloading from the xylem (Waldie et al., 2014). In addition to CL and CLA, the methyl ester derivative of CLA (MeCLA) was also identified from Arabidopsis root extracts (Figure 5; Abe et al., 2014; Seto et al., 2014). Interestingly, these CL-related chemicals are able to stimulate the germination of parasitic plant seeds (Abe et al., 2014). Recently, heliolactone, a derivative of MeCLA, was identified from sunflower root exudates as a germination stimulant for seeds of root parasitic plants (Ueno et al., 2014). These results indicate that the germination of parasitic plant seeds is stimulated by chemicals secreted from host plant roots that include not only typical SLs but also CL-related compounds of more diverse structure.
The ATP-binding cassette (ABC) type transporter, PDR1, characterized as an SL-exporter in petunia, provided a clue for understanding SL transport (Kretzschmar et al., 2012). In the pdr1 mutant, exudation of SL from roots is highly reduced compared with that from wild type roots, resulting in reduced symbiotic interactions with AM fungi and reduced germination rates of the parasitic plant Phelipanche ramosa. Moreover, the pdr1 mutant has an increased branching phenotype, possibly due to a decrease in SL transported from roots to shoots. These results strongly imply that controlling SL exudation can be a powerful tool for regulating parasitic plant infections, AM fungal symbioses and plant architecture. Identification of SL transporters from various plant species and detailed substrate-specificity analyses will be required to reveal the SL secretion mechanism in plants.
Although how parasitic plants recognize SL is still unknown, a receptor candidate protein and its partner proteins for the shoot branching inhibition pathway were identified from SL-insensitive mutants. MAX2/D3/RMS4 encodes an F-box protein, the substrate recognition subunit of the ubiquitin E3 ligase, Skp, Cullin, and F-box containing complex (SCF complex; Stirnberg et al., 2002; Ishikawa et al., 2005; Johnson et al., 2006). D14 encodes an α/β-fold hydrolase family protein (Arite et al., 2009). Through biochemical and biological analyses, D14 is proposed to be an SL receptor protein (Arite et al., 2009; Hamiaux et al., 2012; Kagiyama et al., 2013; Zhao et al., 2013). D14 was originally identified from the rice dwarf14 mutant (Arite et al., 2009), and later its orthologous genes were identified in Arabidopsis (AtD14) and petunia (DAD2, Hamiaux et al., 2012; Waters et al., 2012b). D14/AtD14/DAD2 has conserved catalytic triad residues that are important for catalyzing the hydrolase reaction; in fact, these enzymes can hydrolyze a synthetic SL analog, GR24 (Hamiaux et al., 2012; Kagiyama et al., 2013; Zhao et al., 2013). Additionally, D14 can interact with D53, a recently characterized repressor protein in the SL pathway, in an SL-dependent manner (Jiang et al., 2013; Zhou et al., 2013). Furthermore, D53 protein is degraded through the 26S proteasome pathway in a manner requiring the SCFD3 F-box protein (Jiang et al., 2013; Zhou et al., 2013). Interestingly, MeCLA interacts directly with AtD14 protein in vitro, whereas CL and CLA do not (Abe et al., 2014). These results suggest that, in addition to SL, MeCLA may act as an active hormone in the shoot branch inhibition pathway.
A database search of the S. hermonthica ESTs showed that this species has at least one D14 homologous gene and multiple copies of a closely related gene, HTL/KAI2 (Toh et al., 2014). Possibly these proteins are involved in the SL perception step in parasitic plant seeds. In addition to the D14-related genes, S. hermonthica has a D3/MAX2/RMS4 homologous gene, ShMAX2. The expression of ShMAX2 in the Arabidopsis max2 mutant complements its branching and hypocotyl elongation phenotypes (Liu et al., 2014). These results suggest that components of the SL perception system in non-parasitic plants are conserved in parasitic plants. However, functional confirmation is necessary to clarify whether parasitic plant seeds recognize SL for their germination through these SL perception systems. The presence of SL biosynthetic genes such as CCD7 and CCD8 in the S. hermonthica EST was reported, and germination assay experiments using in vitro cultured Striga extracts suggested the presence of endogenous SL in Striga (Liu et al., 2014). If Striga plants also produce SL, it will be very interesting to know how endogenous and exogenous SLs are distinguished.
Haustorium-Inducing Signals in Parasitic Plants
Haustorium formation in Orobanchaceae parasitic plants are also medicated by apoplastic compounds, commonly called haustorium-inducing factors (HIFs), which are likely derived from cell wall degradation. The first identified HIF, 2,6-dimethoxy-p-benzoquinone (DMBQ), was characterized from sorghum root extracts to induce haustorium formation in a facultative parasite Agalinis purpurea and an obligate parasite Striga asiatica (Chang and Lynn, 1986). Later, several structurally related quinones, phenolic acids and flavonoids were found to have HIF activity, including p-coumaric acid, syringic acid and vanillic acid, major components of lignin, a cell wall polymer. Furthermore, flavonoids and quinones within a certain redox range were shown to have HIF activities in S. asiatica (Smith et al., 1996; Albrecht et al., 1999; Figure 1). Catalase, an enzyme that catalyzes the decomposition of H2O2, terminates the HIF activity of syringic acid but not DMBQ. Thus, a hypothesis was proposed that host-released cell wall phenolics are oxidized and converted into a signal for invasion. Indeed, S. asiatica seedlings accumulate H2O2, and apoplastic peroxidases identified from the parasite are able to convert syringic acid to DMBQ in vitro, although these peroxidases may not be specific to parasitic plants (Kim et al., 1998; Keyes et al., 2000, 2007). However, two fundamental questions are still unsolved. How are the host cell wall components released to the rhizosphere? And why do parasitic plants not respond to their own cell walls? PCWDEs secreted from parasitic plants may activate the release of cell wall phenolics from host roots and provoke haustorium development, although molecular evidence is still lacking. Finding that quinone oxidoreductase (TvQR1) from the facultative parasite, T. versicolor, is important for haustorium formation supports the hypothesis that redox regulation of quinones is a key step for haustorium induction (Bandaranayake et al., 2010). High rates of natural polymorphisms in TvQR1 may contribute to the responses to diverse quinones that exist in various host plants (Ngo et al., 2013).
Conclusions and Future Perspectives
Parasitic plants and plant-parasitic nematodes are both root invaders and share some commonality in their invasion systems. Notably, breaking the cell wall barrier and perceiving the preceding apoplastic signals are the most crucial steps for infection. In obligate parasitic plants in the Orobanchaceae family, the start of their life cycle is already under the control of the host-derived apoplastic/rhizosphere signal, SL and related compounds. Furthermore, parasitic plants also recognize apoplastic signals likely derived from host cell walls for haustorium formation. However, the role of cell wall degradation in producing HIFs remains to be discovered. In plant-parasitic nematodes, the rhizosphere signals required for host root recognition remain to be identified with difficulty, though chemotaxis has been suggested to be the primary means by which plant-parasitic nematodes locate host roots (Zhao et al., 2000; Reynolds et al., 2011; Dong et al., 2014). Both parasitic plants and plant-parasitic nematodes use PCWDEs to penetrate and reach host plant steles. Pectinolytic enzymes, including PME, polygalacturonase and pectate lyase are commonly used by parasitic plants and plant-parasitic nematodes to degrade primary cell walls and middle lamella, suggesting that these enzymes may contribute to the intercellular invasion by the parasites. Plant-parasitic nematodes acquired PCWDEs by horizontal gene transfer. In parasitic plants, horizontal gene transfer from host plants can occur (Yoshida et al., 2010). Cell wall degradation from parasitic invasion may act as a defense-activating signal. The identification of cyst nematode effectors that suppress the surface-localized receptor-mediated defense response implies that the effectors may have evolved to overcome DAMP-induced immunity. Several nematode effector proteins were found to mimic or inhibit the elaborate signaling networks of host cells to suppress host immune responses or facilitate reconstruction of their feeding cells, but this pathway is still far from being completely identified. Detailed analysis of nematode effector functions and their target sites will aid efforts to confer nematode resistance to host crops. Effector proteins have not been reported in parasitic plants, but the accumulating evidence for race-specific relationships between hosts and parasites suggests the existence of parasitic plant effectors to suppress host defenses. The identification of more effectors and secreted proteins, including PCWDEs, will reveal how parasites successfully invade plants and how host plants defend themselves. This approach will help to determine how such sophisticated interaction systems are established using the coevolution processes of host and parasites. Furthermore, more detailed characterization of the parasite invasion and host defense systems will contribute to the development of new control methods for these noxious agricultural pathogens.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by KAKENHI (No.15H01246, 25711019 and 25128716 to SY and No. 25891021 to KM) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Japan Society for the Promotion of Science (JSPS). We thank Drs. Yasunori Ichihashi and Yasuhiro Kadota for critically reading the manuscript.
References
Abad, P., Gouzy, J., Aury, J. M., Castagnone-Sereno, P., Danchin, E. G., Deleury, E., et al. (2008). Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 26, 909–915. doi: 10.1038/nbt.1482
Abe, S., Sado, A., Tanaka, K., Kisugi, T., Asami, K., Ota, S., et al. (2014). Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. U.S.A. 111, 18084–18089. doi: 10.1073/pnas.1410801111
Akiyama, K., Matsuzaki, K., and Hayashi, H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. doi: 10.1038/nature03608
Albrecht, H., Yoder, J. I., and Phillips, D. A. (1999). Flavonoids promote haustoria formation in the root parasite Triphysaria versicolor. Plant Physiol. 119, 585–592. doi: 10.1104/pp.119.2.585
Alder, A., Jamil, M., Marzorati, M., Bruno, M., Vermathen, M., Bigler, P., et al. (2012). The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335, 1348–1351. doi: 10.1126/science.1218094
Arite, T., Iwata, H., Ohshima, K., Maekawa, M., Nakajima, M., Kojima, M., et al. (2007). DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 51, 1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x
Arite, T., Umehara, M., Ishikawa, S., Hanada, A., Maekawa, M., Yamaguchi, S., et al. (2009). D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 50, 1416–1424. doi: 10.1093/pcp/pcp091
Auldridge, M. E., Block, A., Vogel, J. T., Dabney-Smith, C., Mila, I., Bouzayen, M., et al. (2006). Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 45, 982–993. doi: 10.1111/j.1365-313X.2006.02666.x
Baird, W. M. V., and Riopel, J. L. (1984). Experimental studies of haustorium initiation and early development in Agalinis purpurea (L.) RAF (Scrophulariaceae). Am. J. Bot. 71, 803–814. doi: 10.2307/2443471
Bakhetia, M., Urwin, P. E., and Atkinson, H. J. (2007). QPCR analysis and RNAi define pharyngeal gland cell-expressed genes of Heterodera glycines required for initial interactions with the host. Mol. Plant Microbe. Interact. 20, 306–312. doi: 10.1094/MPMI-20-3-0306
Bandaranayake, P. C. G., Filappova, T., Tomilov, A., Tomilova, N. B., Jamison-McClung, D., Ngo, Q., et al. (2010). A single-electron reducing quinone oxidoreductase is necessary to induce haustorium development in the root parasitic plant Triphysaria. Plant Cell 22, 1404–1419. doi: 10.1105/tpc.110.074831
Barcala, M., García, A., Cabrera, J., Casson, S., Lindsey, K., Favery, B., et al. (2010). Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J. 61, 698–712. doi: 10.1111/j.1365-313X.2009.04098.x
Béra-Maillet, C., Arthaud, L., Abad, P., and Rosso, M.-N. (2000). Biochemical characterization of MI-ENG1, a family 5 endoglucanase secreted by the root-knot nematode Meloidogyne incognita. Eur. J. Biochem. 267, 3255–3263. doi: 10.1046/j.1432-1327.2000.01356.x
Betsuyaku, S., Sawa, S., and Yamada, M. (2011). The function of the CLE peptides in plant development and plant–microbe interactions. Arabidopsis Book 9, e0149. doi: 10.1199/tab.0149
Bhattarai, K. K., Xie, Q.-G., Mantelin, S., Bishnoi, U., Girke, T., Navarre, D. A., and Kaloshian, I. (2008). Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant Microbe. Interact. 21, 1205–1214. doi: 10.1094/MPMI-21-9-1205
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Booker, J., Auldridge, M., Wills, S., McCarty, D., Klee, H., and Leyser, O. (2004). MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 14, 1232–1238. doi: 10.1016/j.cub.2004.06.061
Booker, J., Sieberer, T., Wright, W., Williamson, L., Willett, B., Stirnberg, P., et al. (2005). MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 8, 443–449. doi: 10.1016/j.devcel.2005.01.009
Branch, C., Hwang, C., Navarre, D. A., and Williamson, V. M. (2004). Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol. Plant Microbe Interact. 17, 351–356. doi: 10.1094/MPMI.2004.17.4.351
Brewer, P. B., Koltai, H., and Beveridge, C. A. (2013). Diverse roles of strigolactones in plant development. Mol. Plant 6, 18–28. doi: 10.1093/mp/sss130
Brotman, Y., Landau, U., Cuadros-Inostroza, Á., Takayuki, T., Fernie, A. R., Chet, I., et al. (2013). Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 9:e1003221. doi: 10.1371/journal.ppat.1003221
Brutus, A., Sicilia, F., Macone, A., Cervone, F., and De Lorenzo, G. (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. U.S.A. 107, 9452–9457. doi: 10.1073/pnas.1000675107
Castillejo, A. M., Amiour, N., Dumas-Gaudot, E., Rubiales, D., and Jorrin, J. V. (2004). A proteomic approach to studying plant response to crenate broomrape (Orobanche crenata) in pea (Pisum sativum). Phytochemistry 65, 1817–1828. doi: 10.1016/j.phytochem.2004.03.029
Castillejo, M. A., Maldonado, A. M., Dumas-Gaudot, E., Fernández-Aparicio, M., Susín, R., Diego, R., et al. (2009). Differential expression proteomics to investigate responses and resistance to Orobanche crenata in Medicago truncatula. BMC Genomics 10:294. doi: 10.1186/1471-2164-10-294
Chang, M., and Lynn, D. G. (1986). The haustorium and the chemistry of host recognition in parasitic angiosperms. J. Chem. Ecol. 12, 561–579. doi: 10.1007/BF01020572
Chen, Y. C., Wong, C. L., Muzzi, F., Vlaardingerbroek, I., Kidd, B. N., and Schenk, P. M. (2014). Root defense analysis against Fusarium oxysporum reveals new regulators to confer resistance. Sci. Rep. 4, 5584. doi: 10.1038/srep05584
Cook, C. E., Whichard, L. P., Turner, B., and Wall, M. E. (1966). Germination of witchweed (Striga lutea Lour)—isolation and properties of a potent stimulant. Science 154, 1189–1190. doi: 10.1126/science.154.3753.1189
Cooper, W. R., Jia, L., and Goggin, L. (2005). Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. J. Chem. Ecol. 31, 1953–1967. doi: 10.1007/s10886-005-6070-y
Cosgrove, D. J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861. doi: 10.1038/nrm1746
Crawford, S., Shinohara, N., Sieberer, T., Williamson, L., George, G., Hepworth, J., et al. (2010). Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137, 2905–2913. doi: 10.1242/dev.051987
Danchin, E. G., Rosso, M. N., Vieira, P., de Almeida-Engler, J., Coutinho, P. M., Henrissat, B., et al. (2010). Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc. Natl. Acad. Sci. U.S.A. 107, 17651–17656. doi: 10.1073/pnas.1008486107
Davis, E. L., Hussey, R. S., and Baum, T. J. (2004). Getting to the roots of parasitism by nematodes. Trends Parasitol. 20, 134–141. doi: 10.1016/j.pt.2004.01.005
De Boer, J. M., Davis, E. L., Hussey, R. S., Popeijus, H., Smant, G., and Baum, T. J. (2002). Cloning of a putative pectate lyase gene expressed in the subventral esophageal glands of Heterodera glycines. J. Nematol. 34, 9–11.
Diener, A. C., and Ausubel, F. M. (2005). RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics 171, 305–321. doi: 10.1534/genetics.105.042218
Dong, L., Li, X., Huang, L., Gao, Y., Zhong, L., Zheng, Y., et al. (2014). Lauric acid in crown daisy root exudate potently regulates root-knot nematode chemotaxis and disrupts Mi-flp-18 expression to block infection. J. Exp. Bot. 65, 131–141. doi: 10.1093/jxb/ert356
Ellinger, D., Naumann, M., Falter, C., Zwikowics, C., Jamrow, T., Manisseri, C., et al. (2013). Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 161, 1433–1444. doi: 10.1104/pp.112.211011
Eves-van den Akker, S., Lilley, C. J., Jones, J. T., and Urwin, P. E. (2014). Identification and characterisation of a hyper-variable apoplastic effector gene family of the potato cyst nematodes. PLoS Pathog. 10:e1004391. doi: 10.1371/journal.ppat.1004391
Fan, J. W., Hu, C. L., Zhang, L. N., Li, Z. L., Zhao, F. K., and Wang, S. H. (2014). Jasmonic acid mediates tomato’s response to root knot nematodes. J. Plant Growth Regul. 34, 196–205. doi: 10.1007/s00344-014-9457-6
Ferrari, S., Savatin, D. V., Sicilia, F., Gramegna, G., Cervone, F., and De Lorenzo, G. (2013). Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4:49. doi: 10.3389/fpls.2013.00049
Gao, B., Allen, R., Davis, E. L., Baum, T. J., and Hussey, R. S. (2004). Developmental expression and biochemical properties of a β-1,4-endoglucanase family in the soybean cyst nematode, Heterodera glycines. Mol. Plant Pathol. 5, 93–104. doi: 10.1111/j.1364-3703.2004.00209.x
Gao, B., Allen, R., Maier, T., Davis, E. L., Baum, T. J., and Hussey, R. S. (2002). Identification of a new ss-1,4-endoglucanase gene expressed in the esophageal subventral gland cells of Heterodera glycines. J. Nematol. 34, 12–15.
Gao, B., Allen, R., Maier, T., Davis, E. L., Baum, T. J., and Hussey, R. S. (2003). The parasitome of the phytonematode Heterodera glycines. Mol. Plant Microbe Interact. 16, 720–726. doi: 10.1094/MPMI.2003.16.8.720
Geldner, N. (2013). The endodermis. Annu. Rev. Plant Biol. 64, 531–558. doi: 10.1146/annurev-arplant-050312-120050
Goellner, M., Smant, G., De Boer, J. M., Baum, T. J., and Davis, E. L. (2000). Isolation of β-1,4-endoglucanase genes from Globodera tabacum and their expression during parasitism. J. Nematol. 32, 154–165.
Goldwasser, Y., Hershenhorn, J., Plakhine, D., Kleifeld, Y., and Rubin, B. (1999). Biochemical factors involved in vetch resistance to Orobanche aegyptiaca. Physiol. Mol. Plant Pathol. 54, 87–96. doi: 10.1006/pmpp.1998.0191
Gomez-Roldan, V., Fermas, S., Brewer, P. B., Puech-Pages, V., Dun, E. A., Pillot, J. P., et al. (2008). Strigolactone inhibition of shoot branching. Nature 455, 189–194. doi: 10.1038/nature07271
Gonzalez-Verdejo, C. I., Barandiaran, X., Moreno, M. T., Cubero, J. I., and Di Pietro, A. (2006). A peroxidase gene expressed during early developmental stages of the parasitic plant Orobanche ramosa. J. Exp. Bot. 57, 185–192. doi: 10.1093/jxb/erj024
Haegeman, A., Jones, J. T., and Danchin, E. G. (2011a). Horizontal gene transfer in nematodes: a catalyst for plant parasitism? Mol. Plant. Microbe Interact. 24, 879–887. doi: 10.1094/MPMI-03-11-0055
Haegeman, A., Joseph, S., and Gheysen, G. (2011b). Analysis of the transcriptome of the root lesion nematode Pratylenchus coffeae generated by 454 sequencing technology. Mol. Biochem. Parasitol. 178, 7–14. doi: 10.1016/j.molbiopara.2011.04.001
Haegeman, A., Mantelin, S., Jones, J. T., and Gheysen, G. (2012). Functional roles of effectors of plant-parasitic nematodes. Gene 492, 19–31. doi: 10.1016/j.gene.2011.10.040
Hamann, T. (2014). The plant cell wall integrity maintenance mechanism—concepts for organization and mode of action. Plant Cell Physiol. 56, 215–223. doi: 10.1093/pcp/pcu164
Hamiaux, C., Drummond, R. S. M., Janssen, B. J., Ledger, S. E., Cooney, J. M., Newcomb, R. D., et al. (2012). DAD2 Is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22, 2032–2036. doi: 10.1016/j.cub.2012.08.007
Hassan, S., and Mathesius, U. (2012). The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 63, 3429–3444. doi: 10.1093/jxb/err430
Haussmann, B. I., Hess, D. E., Omanya, G. O., Folkertsma, R. T., Reddy, B. V., Kayentao, M., et al. (2004). Genomic regions influencing resistance to the parasitic weed Striga hermonthica in two recombinant inbred populations of sorghum. Theor. Appl. Genet. 109, 1005–1016. doi: 10.1007/s00122-004-1706-9
Heide-Jorgensen, H. S., and Kuijt, J. (1995). The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. Am. J. Bot. 82, 782–797. doi: 10.2307/2445619
Hématy, K., Cherk, C., and Somerville, S. (2009). Host-pathogen warfare at the plant cell wall. Curr. Opin. Plant Biol. 12, 406–413. doi: 10.1016/j.pbi.2009.06.007
Hewezi, T., Howe, P., Maier, T. R., Hussey, R. S., Mitchum, M. G., Davis, E. L., et al. (2008). Cellulose binding protein from the parasitic nematode Heterodera schachtii interacts with Arabidopsis pectin methylesterase: cooperative cell wall modification during parasitism. Plant Cell 20, 3080–3093. doi: 10.1105/tpc.108.063065
Hibberd, J. M., and Jeschke, W. D. (2001). Solute flux into parasitic plants. J. Exp. Bot. 52, 2043–2049. doi: 10.1093/jexbot/52.363.2043
Hofmann, J., Youssef-Banora, M., de Almeida-Engler, J., and Grundler, F. M. W. (2010). The role of callose deposition along plasmodesmata in nematode feeding sites. Mol. Plant Microbe. Interact. 23, 549–557. doi: 10.1094/MPMI-23-5-0549
Hofte, H. (2014). The yin and yang of cell wall integrity control: brassinosteroid and FERONIA signaling. Plant Cell Physiol. 56, 224–2231. doi: 10.1093/pcp/pcu182
Honaas, L. A., Wafula, E. K., Yang, Z., Der, J. P., Wickett, N. J., Altman, N. S., et al. (2013). Functional genomics of a generalist parasitic plant: laser microdissection of host-parasite interface reveals host-specific patterns of parasite gene expression. BMC Plant Biol. 13:9. doi: 10.1186/1471-2229-13-9
Huang, G., Allen, R., Davis, E. L., Baum, T. J., and Hussey, R. S. (2006). Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. U.S.A. 103, 14302–14306. doi: 10.1073/pnas.0604698103
Huang, K., Mellor, K. E., Paul, S. N., Lawson, M. J., Mackey, A. J., and Timko, M. P. (2012a). Global changes in gene expression during compatible and incompatible interactions of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. BMC Genomics 13:402. doi: 10.1186/1471-2164-13-402
Huang, K., Whitlock, R., Press, M. C., and Scholes, J. D. (2012b). Variation for host range within and among populations of the parasitic plant Striga hermonthica. Heredity (Edinb.) 108, 96–104. doi: 10.1038/hdy.2011.52
Hückelhoven, R. (2007). Cell wall-associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127. doi: 10.1146/annurev.phyto.45.062806.094325
Hussey, R. S. (1989). Disease-inducing secretions of plant-parasitic nematodes. Annu. Rev. Phytopathol. 27, 123–141. doi: 10.1146/annurev.py.27.090189.001011
Ishida, J. K., Yoshida, S., Ito, M., Namba, S., and Shirasu, K. (2011). Agrobacterium rhizogenes-mediated transformation of the parasitic plant Phtheirospermum japonicum. PLoS ONE 6:e25802. doi: 10.1371/journal.pone.0025802
Ishikawa, S., Maekawa, M., Arite, T., Onishi, K., Takamure, I., and Kyozuka, J. (2005). Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 46, 79–86. doi: 10.1093/pcp/pci022
Jacobs, S., Zechmann, B., Molitor, A., Trujillo, M., Petutschnig, E., Likpa, V., et al. (2011). Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol. 156, 726–740. doi: 10.1104/pp.111.176446
Jammes, F., Lecomte, P., de Almeida-Engler, J., Bitton, F., Martin-Magniette, M.-L., Renou, J. P., et al. (2005). Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J. 44, 447–458. doi: 10.1111/j.1365-313X.2005.02532.x
Jaouannet, M., Magliano, M., Arguel, M. J., Gourgues, M., Evangelisti, E., Abad, P., et al. (2013). The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol. Plant Microbe Interact. 26, 97–105. doi: 10.1094/MPMI-05-12-0130-R
Jarvis, M. C., Briggs, S. P. H., and Knox, J. P. (2003). Intercellular adhesion and cell separation in plants. Plant Cell Environ. 26, 977–989. doi: 10.1046/j.1365-3040.2003.01034.x
Jaubert, S., Milac, A. L., Petrescu, A. J., de Almeida-Engler, J., Abad, P., and Rosso, M. N. (2005). In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol. Plant Microbe Interact. 18, 1277–1284. doi: 10.1094/MPMI-18-1277
Jiang, L., Liu, X., Xiong, G., Liu, H., Chen, F., Wang, L., et al. (2013). DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504, 401–5. doi: 10.1038/nature12870
Johnsen, H. R., Striberny, B., Olsen, S., Vidal-melgosa, S., Fangel, J. U., Willats, W. G. T., et al. (2015). Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: a priori differences and induced changes. New Phytol. 207, 805–816. doi: 10.1111/nph.13378
Johnson, X., Brcich, T., Dun, E. A., Goussot, M., Haurogne, K., Beveridge, C. A., et al. (2006). Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 142, 1014–1026. doi: 10.1104/pp.106.087676
Jones, J. D. G., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Juge, N. (2006). Plant protein inhibitors of cell wall degrading enzymes. Trends Plant Sci. 11, 359–367. doi: 10.1016/j.tplants.2006.05.006
Kagiyama, M., Hirano, Y., Mori, T., Kim, S. Y., Kyozuka, J., Seto, Y., et al. (2013). Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18, 147–160. doi: 10.1111/gtc.12025
Kaloshian, I., Desmond, O. J., and Atamian, H. S. (2011). “Disease resistance-genes and defense responses during incompatible interactions,” in Genomics and Molecular Genetics of Plant-Nematode Interactions, eds J. Jones, G. Gheysen, and C. Fenoll (Dordrechit: Springer Science & Business Media), 309–323.
Kämper, J., Kahmann, R., Bölker, M., Ma, L.-J., Brefort, T., Saville, B. J., et al. (2006). Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444, 97–101. doi: 10.1038/nature05248
Keyes, W. J., O’Malley, R. C., Kim, D., and Lynn, D. G. (2000). Signaling organogenesis in parasitic angiosperms: xenognosin generation, perception, and response. J. Plant Growth Regul. 19, 217–231.
Keyes, W. J., Palmer, A. G., Erbil, W. K., Taylor, J. V., Apkarian, R. P., Weeks, E. R., et al. (2007). Semagenesis and the parasitic angiosperm Striga asiatica. Plant J. 51, 707–716. doi: 10.1111/j.1365-313X.2007.03171.x
Kim, D. J., Kocz, R., Boone, L., Keyes, W. J., and Lynn, D. G. (1998). On becoming a parasite: evaluating the role of wall oxidases in parasitic plant development. Chem. Biol. 5, 103–117. doi: 10.1016/S1074-5521(98)90144-2
King, B. C., Waxman, K. D., Nenni, N. V., Walker, L. P., Bergstrom, G. C., and Gibson, D. M. (2011). Arsenal of plant cell wall degrading enzymes reflects host preference among plant pathogenic fungi. Biotechnol. Biofuels 4, 4. doi: 10.1186/1754-6834-4-4
Kohlen, W., Charnikhova, T., Lammers, M., Pollina, T., Toth, P., Haider, I., et al. (2012). The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 196, 535–547. doi: 10.1111/j.1469-8137.2012.04265.x
Kohlen, W., Charnikhova, T., Liu, Q., Bours, R., Domagalska, M. A., Beguerie, S., et al. (2011). Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 155, 974–987. doi: 10.1104/pp.110.164640
Kretzschmar, T., Kohlen, W., Sasse, J., Borghi, L., Schlegel, M., Bachelier, J. B., et al. (2012). A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483, 341–344. doi: 10.1038/nature10873
Kudla, U., Qin, L., Milac, A., Kielak, A., Maissen, C., Overmars, H., et al. (2005). Origin, distribution and 3D-modeling of Gr-EXPB1, an expansin from the potato cyst nematode Globodera rostochiensis. FEBS Lett. 579, 2451–2457. doi: 10.1016/j.febslet.2005.03.047
Kusumoto, D., Goldwasser, Y., Xie, X., Yoneyama, K., Takeuchi, Y., and Yoneyama, K. (2007). Resistance of red clover (Trifolium pratense) to the root parasitic plant Orobanche minor is activated by salicylate but not by jasmonate. Ann. Bot. 100, 537–544. doi: 10.1093/aob/mcm148
Lambert, K., and Bekal, S. (2002). Introduction to plant-parasitic nematodes. Plant Health Instruct. doi: 10.1094/PHI-I-2002-1218-01
Lewis, K., Alers-Garcia, J., and Wright, L. (2010). Green tea catechins applied to susceptible hosts inhibit parasitic plant attachment success. Crop Sci. 50, 253–264. doi: 10.2135/cropsci2008.12.0720
Lewis, K. C., Selzer, T., Shahar, C., Udi, Y., Tworowski, D., and Sagi, I. (2008). Inhibition of pectin methyl esterase activity by green tea catechins. Phytochemistry 69, 2586–2592. doi: 10.1016/j.phytochem.2008.08.012
Li, J., and Timko, M. P. (2009). Gene-for-gene resistance in Striga-cowpea associations. Science 325, 1094. doi: 10.1126/science.1174754
Li, Y., Jones, L., and McQueen-Mason, S. (2003). Expansins and cell growth. Curr. Opin. Plant Biol. 6, 603–610. doi: 10.1016/j.pbi.2003.09.003
Lin, H., Wang, R., Qian, Q., Yan, M., Meng, X., Fu, Z., et al. (2009). DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21, 1512–1525. doi: 10.1105/tpc.109.065987
Liu, Q., Zhang, Y., Matusova, R., Charnikhova, T., Amini, M., Jamil, M., et al. (2014). Striga hermonthica MAX2 restores branching but not the very low fluence response in the Arabidopsis thaliana max2 mutant. New Phytol. 202, 531–541. doi: 10.1111/nph.12692
Losner-Goshen, D., Portnoy, V. H., Mayer, A. M., and Joel, D. M. (1998). Pectolytic activity by the haustorium of the parasitic PlantOrobancheL. (Orobanchaceae) in host roots. Ann. Bot. 81, 319–326. doi: 10.1006/anbo.1997.0563
Lozano-Baena, M. D., Prats, E., Moreno, M. T., Rubiales, D., and Pérez-de-Luque, A. (2007). Medicago truncatula as a model for nonhost resistance in legume-parasitic plant interactions. Plant Physiol. 145, 437–449. doi: 10.1104/pp.107.097089
Lozano-Torres, J. L., Wilbers, R. H. P., Gawronski, P., Boshoven, J. C., Finkers-Tomczak, A., Cordewener, J. H. G., et al. (2012). Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc. Natl. Acad. Sci. U.S.A. 109, 10119–10124. doi: 10.1073/pnas.1202867109
Lozano-Torres, J. L., Wilbers, R. H. P., Warmerdam, S., Finkers-Tomczak, A., Diaz-Granados, A., van Schaik, C. C., et al. (2014). Apoplastic venom allergen-like proteins of cyst nematodes modulate the activation of basal plant innate immunity by cell surface receptors. PLoS Pathog. 10:e1004569. doi: 10.1371/journal.ppat.1004569
Lu, S. W., Chen, S., Wang, J., Yu, H., Chronis, D., Mitchum, M. G., et al. (2009). Structural and functional diversity of CLAVATA3/ESR (CLE)-like genes from the potato cyst nematode Globodera rostochiensis. Mol. Plant Microbe Interact. 22, 1128–1142. doi: 10.1094/MPMI-22-9-1128
Maiti, R. K., Ramaiah, K. V., Bisen, S. S., and Chidley, V. L. (1984). A comparative-study of the haustorial development of Striga asiatica (L) Kuntze on sorghum cultivars. Ann. Bot. 54, 447–457.
Malinovsky, F. G., Fangel, J. U., and Willats, W. G. T. (2014). The role of the cell wall in plant immunity. Front. Plant Sci. 5:178. doi: 10.3389/fpls.2014.00178
Matusova, R., Rani, K., Verstappen, F. W., Franssen, M. C., Beale, M. H., and Bouwmeester, H. J. (2005). The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 139, 920–9934. doi: 10.1104/pp.105.061382
Millet, Y. A., Danna, C. H., Clay, N. K., Songnuan, W., Simon, M. D., Werck-Reichhart, D., et al. (2010). Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22, 973–990. doi: 10.1105/tpc.109.069658
Mitchum, M. G., Hussey, R. S., Baum, T. J., Wang, X., Elling, A. A., Wubben, M., et al. (2013). Nematode effector proteins: an emerging paradigm of parasitism. New Phytol. 199, 879–894. doi: 10.1111/nph.12323
Mitreva-Dautova, M., Roze, E., Overmars, H., de Graaff, L., Schots, A., Helder, J., et al. (2006). A symbiont-independent endo-1,4-β-xylanase from the plant-parasitic nematode Meloidogyne incognita. Mol. Plant Microbe Interact. 19, 521–529. doi: 10.1094/MPMI-19-0521
Musselman, L. J. (1980). The biology of Striga, Orobanche, and other root-parasitic weeds. Annu. Rev. Phytopathol. 18, 463–489. doi: 10.1146/annurev.py.18.090180.002335
Mutuku, J. M., Yoshida, S., Shimizu, T., Ichihashi, Y., Wakatake, T., Seo, M., et al. (2015). The WRKY45-dependent signaling pathway is required for resistance against Striga parasitism. Plant Physiol. 168, 1152–1163. doi: 10.1104/pp.114.256404
Nahar, K., Kyndt, T., De Vleesschauwer, D., Höfte, M., and Gheysen, G. (2011). The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol. 157, 305–316. doi: 10.1104/pp.111.177576
Nahar, K., Kyndt, T., Nzogela, Y. B., and Gheysen, G. (2012). Abscisic acid interacts antagonistically with classical defense pathways in rice-migratory nematode interaction. New Phytol. 196, 901–913. doi: 10.1111/j.1469-8137.2012.04310.x
Nakagawa, T., Kaku, H., Shimoda, Y., Sugiyama, A., Shimamura, M., Takanashi, K., et al. (2011). From defense to symbiosis: limited alterations in the kinase domain of LysM receptor-like kinases are crucial for evolution of legume-Rhizobium symbiosis. Plant J. 65, 169–180. doi: 10.1111/j.1365-313X.2010.04411.x
Neumann, U., Vian, B., Weber, H. C., and Salle, G. (1999). Interface between haustoria of parasitic members of the Scrophulariaceae and their hosts: a histochemical and immunocytochemical approach. Protoplasma 207, 84–97. doi: 10.1007/BF01294716
Ngo, Q. A., Albrecht, H., Tsuchimatsu, T., and Grossniklaus, U. (2013). The differentially regulated genes TvQR1 and TvPirin of the parasitic plant Triphysaria exhibit distinctive natural allelic diversity. BMC Plant Biol. 13:28. doi: 10.1186/1471-2229-13-28
Nicol, J. M., Turner, S. J., Coyne, D. L., den Nijs, L., Hockland, S., and Tahna Maffi, Z. (2011). “Current nematode threats to world agriculture,” in Genomics and Molecular Genetics of Plant-Nematode Interactions, eds J. Jones, G. Gheysen, and C. Fenoll (Dordrecht: Springer Netherlands), 21–43.
Nishimura, M., Stein, M., Hou, B., Vogel, J., Edwards, H., and Somerville, S. (2003). Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301, 969–972. doi: 10.1126/science.1086716
Nühse, T. S. (2012). Cell wall integrity signaling and innate immunity in plants. Front. Plant Sci. 3:280. doi: 10.3389/fpls.2012.00280
Ongaro, V., and Leyser, O. (2008). Hormonal control of shoot branching. J. Exp. Bot. 59, 67–74. doi: 10.1093/jxb/erm134
Parker, C. (2009). Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 65, 453–459. doi: 10.1002/ps.1713
Patel, N., Hamamouch, N., Li, C., Hussey, R., Mitchum, M., Baum, T., et al. (2008). Similarity and functional analyses of expressed parasitism genes in Heterodera schachtii and Heterodera glycines. J. Nematol. 40, 299–310.
Pérez-de-Luque, A., González-Verdejo, C. I., Lozano, M. D., Dita, M. A., Cubero, J. I., González-Melendi, P., et al. (2006). Protein cross-linking, peroxidase and β-1,3-endoglucanase involved in resistance of pea against Orobanche crenata. J. Exp. Bot. 57, 1461–1469. doi: 10.1093/jxb/erj127
Pielach, A., Leroux, O., Domozych, D. S., Knox, J. P., and Popper, Z. A. (2014). Arabinogalactan protein-rich cell walls, paramural deposits and ergastic globules define the hyaline bodies of rhinanthoid Orobanchaceae haustoria. Ann. Bot. 114, 1359–1373. doi: 10.1093/aob/mcu121
Popeijus, H., Overmars, H., Jones, J., Blok, V., Goverse, A., Helder, J., et al. (2000). Enzymology: degradation of plant cell walls by a nematode. Nature 406, 36–37. doi: 10.1038/35017641
Qin, L., Kudla, U., Roze, E. H., Goverse, A., Popeijus, H., Nieuwland, J., et al. (2004). Plant degradation: a nematode expansin acting on plants. Nature 427, 30. doi: 10.1038/427030a
Ranjan, A., Ichihashi, Y., Farhi, M., Zumstein, K., Townsley, B., David-Schwartz, R., et al. (2014). De novo assembly and characterization of the transcriptome of the parasitic weed Cuscuta pentagona identifies genes associated with plant parasitism. Plant Physiol. 166, 1186–1199. doi: 10.1104/pp.113.234864
Rasmann, S., Hiltpold, I., and Ali, J. (2012). “The role of root-produced volatile secondary metabolites in mediating soil interactions,” in Advances in Selected Plant Physiology Aspects, eds G. Montanaro and B. Dichio (Rijeka: InTech Open Access Publisher), 269–290. doi: 10.5772/34304
Rehker, J., Lachnit, M., and Kaldenhoff, R. (2012). Molecular convergence of the parasitic plant species Cuscuta reflexa and Phelipanche aegyptiaca. Planta 236, 557–566. doi: 10.1007/s00425-012-1626-x
Reiss, G. C., and Bailey, J. A. (1998). Striga gesnerioides parasitising cowpea: development of infection structures. Ann. Bot. 81, 431–440. doi: 10.1006/anbo.1997.0577
Replogle, A., Wang, J., Bleckmann, A., Hussey, R. S., Baum, T. J., Sawa, S., et al. (2011). Nematode CLE signaling in Arabidopsis requires CLAVATA2 and CORYNE. Plant J. 65, 430–440. doi: 10.1111/j.1365-313X.2010.04433.x
Reynolds, A. M., Dutta, T. K., Curtis, R. H. C., Powers, S. J., Gaur, H. S., and Kerry, B. R. (2011). Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes. J. R. Soc. Interface 8, 568–577. doi: 10.1098/rsif.2010.0417
Ruyter-Spira, C., Al-Babili, S., van der Krol, S., and Bouwmeester, H. (2013). The biology of strigolactones. Trends Plant Sci. 18, 72–83. doi: 10.1016/j.tplants.2012.10.003
Sasidharan, R., Voesenek, L. A. C. J., and Pierik, R. (2011). Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit. Rev. Plant Sci. 30, 548–562. doi: 10.1080/07352689.2011.615706
Scaffidi, A., Waters, M. T., Ghisalberti, E. L., Dixon, K. W., Flematti, G. R., and Smith, S. M. (2013). Carlactone-independent seedling morphogenesis in Arabidopsis. Plant J. 76, 1–9. doi: 10.1111/tpj.12265
Seto, Y., Kameoka, H., Yamaguchi, S., and Kyozuka, J. (2012). Recent advances in strigolactone research: chemical and biological aspects. Plant Cell Physiol. 53, 1843–1853. doi: 10.1093/pcp/pcs142
Seto, Y., Sado, A., Asami, K., Hanada, A., Umehara, M., Akiyama, K., et al. (2014). Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. U.S.A. 111, 1640–1645. doi: 10.1073/pnas.1314805111
Severino, V., Farina, A., Fleischmann, F., Dalio, R. J. D., Di Maro, A., Scognamiglio, M., et al. (2014). Molecular profiling of the Phytophthora plurivora secretome: a step towards understanding the cross-talk between plant pathogenic oomycetes and their hosts. PLoS ONE 9:e112317. doi: 10.1371/journal.pone.0112317
Shah, P., Gutierrez-Sanchez, G., Orlando, R., and Bergmann, C. (2009). A proteomic study of pectin-degrading enzymes secreted by Botrytis cinerea grown in liquid culture. Proteomics 9, 3126–3135. doi: 10.1002/pmic.200800933
Singh, A., and Singh, M. (1993). Cell wall degrading enzymes in Orobanche aegyptiaca and its host Brassica campestris. Physiol. Plant. 89, 177–181. doi: 10.1111/j.1399-3054.1993.tb01802.x
Smant, G., Stokkermans, J. P., Yan, Y., de Boer, J. M., Baum, T. J., Wang, X., et al. (1998). Endogenous cellulases in animals: isolation of β-1,4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc. Natl. Acad. Sci. U.S.A. 95, 4906–4911. doi: 10.1073/pnas.95.9.4906
Smith, C. E., Ruttledge, T., Zeng, Z., O’Malley, R. C., and Lynn, D. G. (1996). A mechanism for inducing plant development: the genesis of a specific inhibitor. Proc. Natl. Acad. Sci. U.S.A. 93, 6986–6991. doi: 10.1073/pnas.93.14.6986
Sorefan, K., Booker, J., Haurogné, K., Goussot, M., Bainbridge, K., Foo, E., et al. (2003). MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 17, 1469–1474. doi: 10.1101/gad.256603
Spallek, T., Mutuku, M., and Shirasu, K. (2013). The genus Striga: a witch profile. Mol. Plant Pathol. 14, 861–869. doi: 10.1111/mpp.12058
Stirnberg, P., van De Sande, K., and Leyser, H. M. O. (2002). MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129, 1131–1141.
Stotz, H. U., Mitrousia, G. K., de Wit, P. J. G. M., and Fitt, B. D. L. (2014). Effector-triggered defence against apoplastic fungal pathogens. Trends Plant Sci. 19, 491–500. doi: 10.1016/j.tplants.2014.04.009
Swarbrick, P. J., Huang, K., Liu, G., Slate, J., Press, M. C., and Scholes, J. D. (2008). Global patterns of gene expression in rice cultivars undergoing a susceptible or resistant interaction with the parasitic plant Striga hermonthica. New Phytol. 179, 515–529. doi: 10.1111/j.1469-8137.2008.02484.x
Tintor, N., and Saijo, Y. (2014). ER-mediated control for abundance, quality, and signaling of transmembrane immune receptors in plants. Front. Plant Sci. 5:65. doi: 10.3389/fpls.2014.00065
Toh, S., Holbrook-Smith, D., Stokes, M. E., Tsuchiya, Y., and McCourt, P. (2014). Detection of parasitic plant suicide germination compounds using a high-throughput Arabidopsis HTL/KAI2 strigolactone perception system. Chem. Biol. 21, 988–998. doi: 10.1016/j.chembiol.2014.07.005
Toth, I. K., and Birch, P. R. J. (2005). Rotting softly and stealthily. Curr. Opin. Plant Biol. 8, 424–429. doi: 10.1016/j.pbi.2005.04.001
Ueno, K., Furumoto, T., Umeda, S., Mizutani, M., Takikawa, H., Batchvarova, R., et al. (2014). Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower. Phytochemistry 108, 122–128. doi: 10.1016/j.phytochem.2014.09.018
Umehara, M., Hanada, A., Yoshida, S., Akiyama, K., Arite, T., Takeda-Kamiya, N., et al. (2008). Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. doi: 10.1038/nature07272
Vanholme, B., Van Thuyne, W., Vanhouteghem, K., De Meutter, J., Cannoot, B., and Gheysen, G. (2007). Molecular characterization and functional importance of pectate lyase secreted by the cyst nematode Heterodera schachtii. Mol. Plant Pathol. 8, 267–278. doi: 10.1111/j.1364-3703.2007.00392.x
Veronesi, C., Bonnin, E., Benharrat, H., Fer, A., and Thalouarn, P. (2005). Are pectinolytic activities of Orobanche cumana seedlings related to virulence towards sunflower? Isr. J. Plant Sci. 53, 19–27. doi: 10.1560/ETEL-C34X-Y6MG-YT0M
Veronesi, C., Bonnin, E., Calvez, S., Thalouarn, P., and Simier, P. (2007). Activity of secreted cell wall-modifying enzymes and expression of peroxidase-encoding gene following germination of Orobanche ramosa. 51, 391–394. doi: 10.1007/s10535-007-0084-y
Vieira, P., Danchin, E. G., Neveu, C., Crozat, C., Jaubert, S., Hussey, R. S., et al. (2011). The plant apoplasm is an important recipient compartment for nematode secreted proteins. J. Exp. Bot. 62, 1241–1253. doi: 10.1093/jxb/erq352
Visser, J. H., Dorr, I. N. E., and Kollmann, R. (1984). The “hyaline body” of the root parasite Alectra orobanchoides benth. (Scrophulariaceae) its anatomy, ultrastructure and histochemistry. Protoplasma 121, 146–156. doi: 10.1007/BF01279762
Vogel, J. P., Raab, T. K., Somerville, C. R., and Somerville, S. C. (2004). Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 40, 968–978. doi: 10.1111/j.1365-313X.2004.02264.x
Voigt, C. A. (2014). Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front. Plant Sci. 5:168. doi: 10.3389/fpls.2014.00168
Waldie, T., McCulloch, H., and Leyser, O. (2014). Strigolactones and the control of plant development: lessons from shoot branching. Plant J. 79, 607–622. doi: 10.1111/tpj.12488
Wang, J., Lee, C., Replogle, A., Joshi, S., Korkin, D., Hussey, R., et al. (2010). Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol. 187, 1003–1017. doi: 10.1111/j.1469-8137.2010.03300.x
Wang, X., Mitchum, M. G., Gao, B., Li, C., Diab, H., Baum, T. J., et al. (2005). A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR (CLE) of Arabidopsis thaliana. Mol. Plant Pathol. 6, 187–191. doi: 10.1111/j.1364-3703.2005.00270.x
Waters, M. T., Brewer, P. B., Bussell, J. D., Smith, S. M., and Beveridge, C. A. (2012a). The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in control of plant development by strigolactones. Plant Physiol. 159, 1073–1085. doi: 10.1104/pp.112.196253
Waters, M. T., Nelson, D. C., Scaffidi, A., Flematti, G. R., Sun, Y. K., Dixon, K. W., et al. (2012b). Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139, 1285–1295. doi: 10.1242/dev.074567
Williamson, V. M., and Kumar, A. (2006). Nematode resistance in plants: the battle underground. Trends Genet. 22, 396–403. doi: 10.1016/j.tig.2006.05.003
Win, J., Chaparro-Garcia, A., Belhaj, K., Saunders, D. G. O., Yoshida, K., Dong, S., et al. (2012). Effector biology of plant-associated organisms: concepts and perspectives. Cold Spring Harb. Symp. Quant. Biol. 77, 235–247. doi: 10.1101/sqb.2012.77.015933
Yang, Z., Wafula, E. K., Honaas, L. A., Zhang, H., Fernandez-aparicio, M., Huang, K., et al. (2015). Comparative transcriptome analyses reveal core parasitism genes and suggest gene duplication and repurposing as sources of structural novelty. Mol. Biol. Evol. 32, 767–790. doi: 10.1093/molbev/msu343
Yoder, J. I. (2001). Host-plant recognition by parasitic Scrophulariaceae. Curr. Opin. Plant Biol. 4, 359–365. doi: 10.1016/S1369-5266(00)00185-0
Yoder, J. I., and Scholes, J. D. (2010). Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr. Opin. Plant Biol. 13, 478–484. doi: 10.1016/j.pbi.2010.04.011
Yokoyama, R., and Nishitani, K. (2004). Genomic basis for cell-wall diversity in plants. A comparative approach to gene families in rice and Arabidopsis. Plant Cell Physiol. 45, 1111–1121. doi: 10.1093/pcp/pch151
Yoneyama, K., Awad, A. A., Xie, X., Yoneyama, K., and Takeuchi, Y. (2010). Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 51, 1095–1103. doi: 10.1093/pcp/pcq055
Yoshida, S., Maruyama, S., Nozaki, H., and Shirasu, K. (2010). Horizontal gene transfer by the parasitic plant Striga hermonthica. Science 328, 1128. doi: 10.1126/science.1187145
Yoshida, S., and Shirasu, K. (2009). Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol. 183, 180–189. doi: 10.1111/j.1469-8137.2009.02840.x
Yoshida, S., and Shirasu, K. (2012). Plants that attack plants: molecular elucidation of plant parasitism. Curr. Opin. Plant Biol 15, 708–713. doi: 10.1016/j.pbi.2012.07.004
Zhang, Y., Dijk, A. D. J., Van, Scaffidi, A., Flematti, G. R., Hofmann, M., Charnikhova, T., et al. (2014). Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 10, 1028–1033. doi: 10.1038/nchembio.1660
Zhao, L. H., Zhou, X. E., Wu, Z. S., Yi, W., Xu, Y., Li, S., et al. (2013). Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 23, 436–439. doi: 10.1038/cr.2013.19
Zhao, X., Schmitt, M., and Hawes, M. C. (2000). Species-dependent effects of border cell and root tip exudates on nematode behavior. Phytopathology 90, 1239–1245. doi: 10.1094/PHYTO.2000.90.11.1239
Keywords: parasitic plants, plant-parasitic nematodes, cell wall, plant cell wall degrading enzymes, strigolactone, effector
Citation: Mitsumasu K, Seto Y and Yoshida S (2015) Apoplastic interactions between plants and plant root intruders. Front. Plant Sci. 6:617. doi: 10.3389/fpls.2015.00617
Received: 29 January 2015; Accepted: 27 July 2015;
Published: 14 August 2015.
Edited by:
Masaru Fujimoto, The University of Tokyo, JapanReviewed by:
Yusuke Saijo, Max Planck Institute for Plant Breeding Research, GermanyKian Hématy, Institut National de la Recherche Agronomique, France
Copyright © 2015 Mitsumasu, Seto and Yoshida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoko Yoshida, RIKEN Center for Sustainable Resource Science, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan, satoko.yoshida@riken.jp
 Kanako Mitsumasu
Kanako Mitsumasu