- 1Laboratory of Plant Physiology, Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, Netherlands
- 2Botanical Institute and Cluster of Excellence on Plant Sciences, Cologne Biocenter, University of Cologne, Cologne, Germany
- 3Plant Biology and Crop Science Department, Rothamsted Research, Harpenden, UK
The impact of sulfate deprivation and atmospheric H2S and SO2 nutrition on the content and composition of glucosinolates was studied in Brassica juncea and B. rapa. Both species contained a number of aliphatic, aromatic and indolic glucosinolates. The total glucosinolate content was more than 5.5-fold higher in B. juncea than in B. rapa, which could solely be attributed to the presence of high levels of sinigrin, which was absent in the latter species. Sulfate deprivation resulted in a strong decrease in the content and an altered composition of the glucosinolates of both species. Despite the differences in patterns in foliarly uptake and metabolism, their exposure hardly affected the glucosinolate composition of the shoot, both at sulfate-sufficient and sulfate-deprived conditions. This indicated that the glucosinolate composition in the shoot was hardly affected by differences in sulfur source (viz., sulfate, sulfite and sulfide). Upon sulfate deprivation, where foliarly absorbed H2S and SO2 were the sole sulfur source for growth, the glucosinolate composition of roots differed from sulfate-sufficient B. juncea and B. rapa, notably the fraction of the indolic glucosinolates was lower than that observed in sulfur-sufficient roots.
Introduction
Brassicaceae are nutritionally important crops containing relatively high levels of sulfur-containing secondary metabolites, viz., glucosinolates, which are not only responsible for the flavor of these species but also may be of great significance as phytopharmaceuticals considering their potential anti-carcinogenic properties (Fahey et al., 2001; Wittstock and Halkier, 2002; Jahangir et al., 2009). The glucosinolate content varies strongly between Brassica species and in seedlings may account for 10–30 % of the organic sulfur fraction (Castro et al., 2004; Aghajanzadeh et al., 2014). Glucosinolates contain 2-3 S groups per molecule and have a common core structure of a β-D-thioglucose group linked to a sulfonated aldoxime moiety and a variable side chain derived from an amino acid (Wittstock and Halkier, 2002). Cysteine, the end product of the sulfate reduction pathway in the chloroplasts (and plastids in the root), functions as the reduced sulfur donor for the synthesis of glucosinolates. Moreover, 3′-phosphoadenosine 5′-phosphosulfate (PAPS), which is synthetized from adenosine 5′-phosphosulfate (APS), the first intermediate in the sulfate reduction pathway by APS kinase, is essential for the synthesis of the sulfated moiety of glucosinolates (Schnug, 1990, 1993; Halkier and Gershenzon, 2006; Falk et al., 2007; Kopriva et al., 2012). Brassica species contain a wide variety of glucosinolates, which on the basis of amino acid precursors, side chain elongation and further modification are classified in aliphatic, indolic, and aromatic glucosinolates (Wittstock and Halkier, 2002; Halkier and Gershenzon, 2006).
The content and composition of the glucosinolate pool in Brassicaceae depends on the developmental stage of the plant (Falk et al., 2007; Agneta et al., 2014). For instance, the highest glucosinolate contents were found in seeds, siliques and young leaves, while intermediate contents were found in leaves, stems and roots (Petersen et al., 2002; Brown et al., 2003). The composition of the glucosinolate pools in plant tissue appears be the consequence of in situ synthesis and/or their redistribution via long-distance transport (Matsuda et al., 2010; Andersen et al., 2013). Glucosinolates may be transported in plants via the xylem (Sattelmacher, 2001) and the phloem (Lucas et al., 2013) and recently two glucosinolate-specific transporters, GTR1 and GTR2, have been identified, which are involved in the long-distance inter-organ transport (Andersen et al., 2013).
It has been presumed that glucosinolates would have significance in the storage of sulfur and that at sulfur-deprived conditions these compounds would be degraded by myrosinase in order to enable the re-distribution of sulfur in plants (Schnug, 1990; Hirai et al., 2004, 2005; Bloem et al., 2007; Falk et al., 2007). However, SO2 and H2S exposure did not affect the glucosinolate content of Brassica juncea and B. rapa, which are characterized by a relatively high and low glucosinolate content, respectively, showing that these sulfur-containing secondary metabolites did not form a sink for excessive atmospheric sulfur supply (Aghajanzadeh et al., 2014). Whilst sulfate deprivation resulted in a decrease in the glucosinolate content of Brassica seedlings, the proportion in the organic sulfur fraction was higher than that of sulfate-sufficient plants, even upon SO2 and H2S exposure, indicating that in Brassica seedlings glucosinolates had hardly any significance in the re-distribution of sulfur upon sulfate deprivation (Aghajanzadeh et al., 2014).
In the current paper, the impact of the plants’ sulfur source for growth, viz., sulfate taken up by the root, and/or SO2 and H2S taken up by the shoot, on the content and composition of glucosinolates was studied in detail in shoots and roots of B. juncea and B. rapa.
Materials and Methods
Plant Material and H2S and SO2 Exposure
Seeds of B. juncea cv. Rugosa and B. rapa cv. Komatsuna (Van der Wal, Hoogeveen, The Netherlands) were germinated in vermiculite in a climate-controlled room. Day and night temperatures were 22 and 18°C (±1°C), respectively, relative humidity was 60–70%. The photoperiod was 14 h at a photon fluence rate of 300 ± 20 μmol m-2 s-1 (400–700 nm) at plant height, supplied by Philips GreenPower LED (deep red/white 120) production modules. 10 day-old seedlings were transferred to an aerated 25% Hoagland nutrient solution at 0.5 mM sulfate for 3 days and subsequently transferred to fresh Hoagland nutrient solution at 0 mM sulfate (-S, sulfate-deprived) or 0.5 mM sulfate (+S, sulfate-sufficient) in 13 l stainless steel containers (30 plants per container). Plants were exposed to 0.25 μl l-1 H2S or SO2 for 7 days in 150 l cylindrical stainless steel cabinets (0.6 m diameter) with a polymethyl methacrylate top. Sealing of the lid of the containers and plant sets prevented absorption of atmospheric H2S or SO2 by the solution. Day and night temperatures were 24 and 20°C (±2°C), respectively, and relative humidity was 40–50%. The photoperiod was 14 h at a photon fluence rate of 300 ± 20 μmol m-2 s-1 (400–700 nm) at plant height, supplied by Philips GreenPower LED (deep red/white 120) production modules. The temperature inside the cabinets was controlled by adjusting the cabinet wall temperature. See for further details Aghajanzadeh et al. (2014). Plants were harvested 3 h after the onset of the light period and the roots were rinsed in ice-cold demineralized water (for 3 s × 20 s). Roots were separated from the shoots, weighed, and for glucosinolate analyses, plant material was frozen immediately in liquid N2 and stored at -80°C.
Glucosinolate Content and Composition
The glucosinolates contents were analyzed with reverse phase HPLC and UV detection method, as described by Burow et al. (2006). The glucosinolates were extracted at 70°C from 50 mg freeze-dried plant material in 70% methanol (v:v) for 45 min. The extract was centrifuged for 5 min at 13,000 g. After centrifugation, 1 ml of the supernatant was loaded on a DEAE Sephadex A-25 column. The column was washed with 2 ml × 0.5 ml water and 20 mM sodium acetate. After washing, the column was treated overnight with sulfatase to convert the glucosinolates to their desulfated derivatives. The desulfated glucosinolates were eluted off the column with 2 ml × 0.5 ml water and were separated by reverse-phase HPLC using an ODS2 column (Waters) and a gradient of acetonitrile in water (5–30% in 8 min, 30–50% in 7 min). The desulfo-glucosinolates were detected and quantified by UV absorption at 229 nm relative to sinigrin used either as internal standard (for B. rapa) or as standard curve (for B. juncea) using response factors. The individual desulfo-glucosinolates were identified based on retention times and known profiles.
Statistical Analysis
Data from different experimental sets ware analyzed for statistical significance using an unpaired two-tailed Student’s t-test (P < 0.01).
Results
Impact of Sulfur Nutrition on Glucosinolate Content
Similarly to previous observations (Aghajanzadeh et al., 2014), the glucosinolate content was substantially higher in shoots of B. juncea than B. rapa seedlings (5.5-fold), but it was not affected upon a 7-day exposure to 0.25 μl l-1 H2S or 0.25 μl l-1 SO2 (Figure 1). Sulfate-deprivation resulted in strongly decreased glucosinolate content, both in shoots and roots of B. juncea and B. rapa (Aghajanzadeh et al., 2014; Figure 1). The glucosinolate content of both shoot and root of sulfate-deprived plants was substantially enhanced upon H2S or SO2 exposure, although was still lower than that observed in sulfate-sufficient plants (Aghajanzadeh et al., 2014; Figure 1).
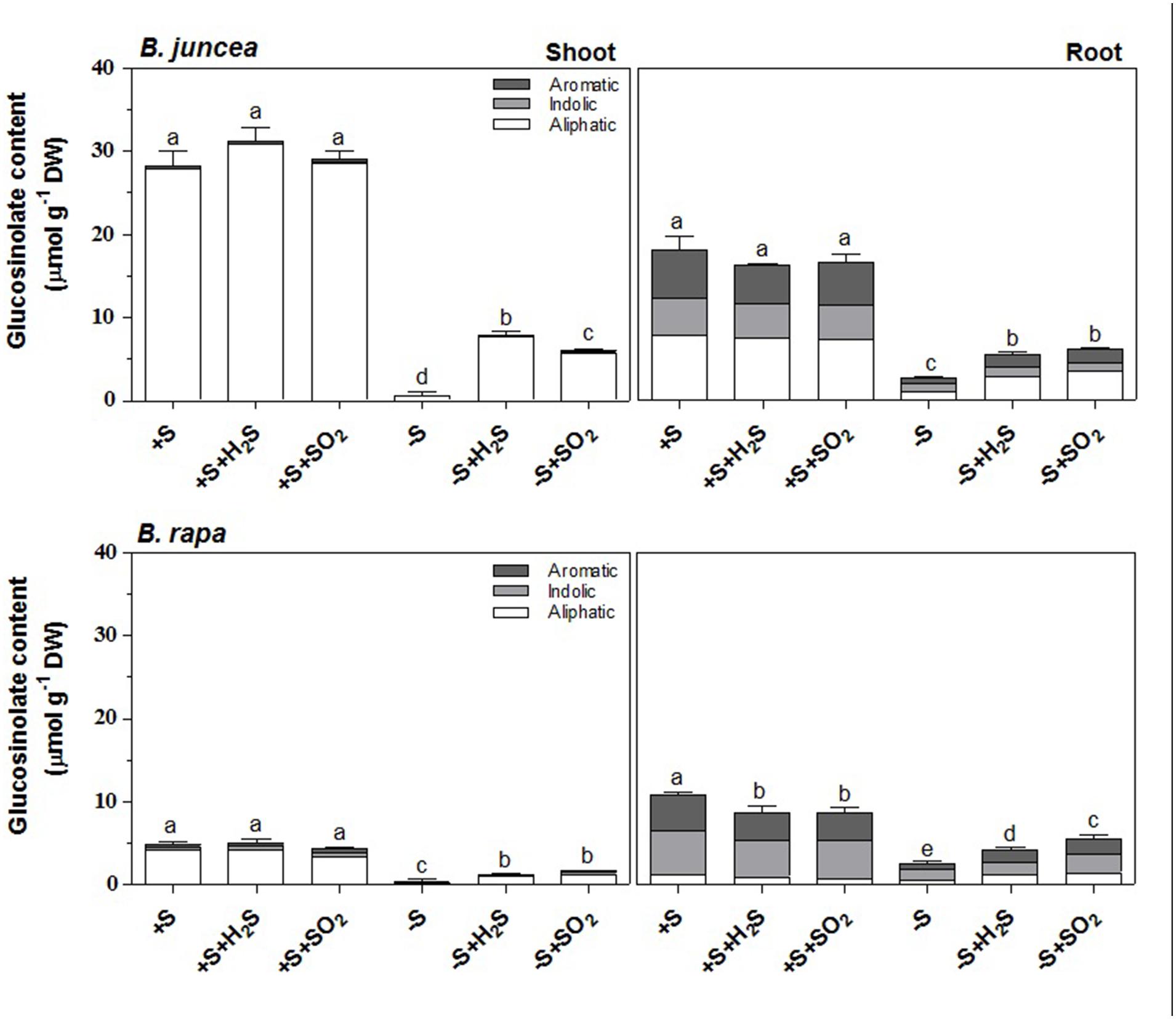
FIGURE 1. Impact of H2S and SO2 and sulfate deprivation on the content and composition of glucosinolates in shoots and roots of Brassica juncea and B. rapa. 10 day-old seedlings were grown on a 25 % Hoagland solution containing 0.5 mM sulfate for 3 days and subsequently transferred to fresh 25% Hoagland solution at 0 (-S) or 0.5 mM sulfate (+S) and exposed to 0.25 μl l-1 H2S or SO2 for 7 days. The content of the aliphatic (white bars; μmol g-1 DW), indolic glucosinolates (gray bars; μmol g-1 DW) and aromatic (dark gray bars; μmol g-1 DW) represent the mean of three measurements with nine plants in each (±SD). Different letters indicate significant differences between treatments (P < 0.01, Student’s t-test).
Glucosinolate Composition
Seven different glucosinolates could be identified in shoots and roots of B. juncea; three were short-chain (C3–C4) aliphatic glucosinolates (sinigrin, gluconapin and progoitrin), one long-chain (C5) aliphatic glucosinolate (glucobrassicanapin), two indolic glucosinolates (glucobrassicin, neoglucobrassicin) and the aromatic glucosinolate gluconasturtiin; Table 1, Figure 2). All of these glucosinolates, except sinigrin were also detected in the shoots and roots of B. rapa (Table 1 and Figure 2). Roots and shoots of B. rapa also contained glucoerucin (an aliphatic glucosinolate) and 4-hydroxy-glucobrassicin (an indolic glucosinolate; Figure 2). The aliphatic glucosinolates were the predominant secondary sulfur compounds present in the shoots of both B. juncea and B. rapa, and they accounted for more than 98 and 84% of the total glucosinolates, respectively (Figure 1).
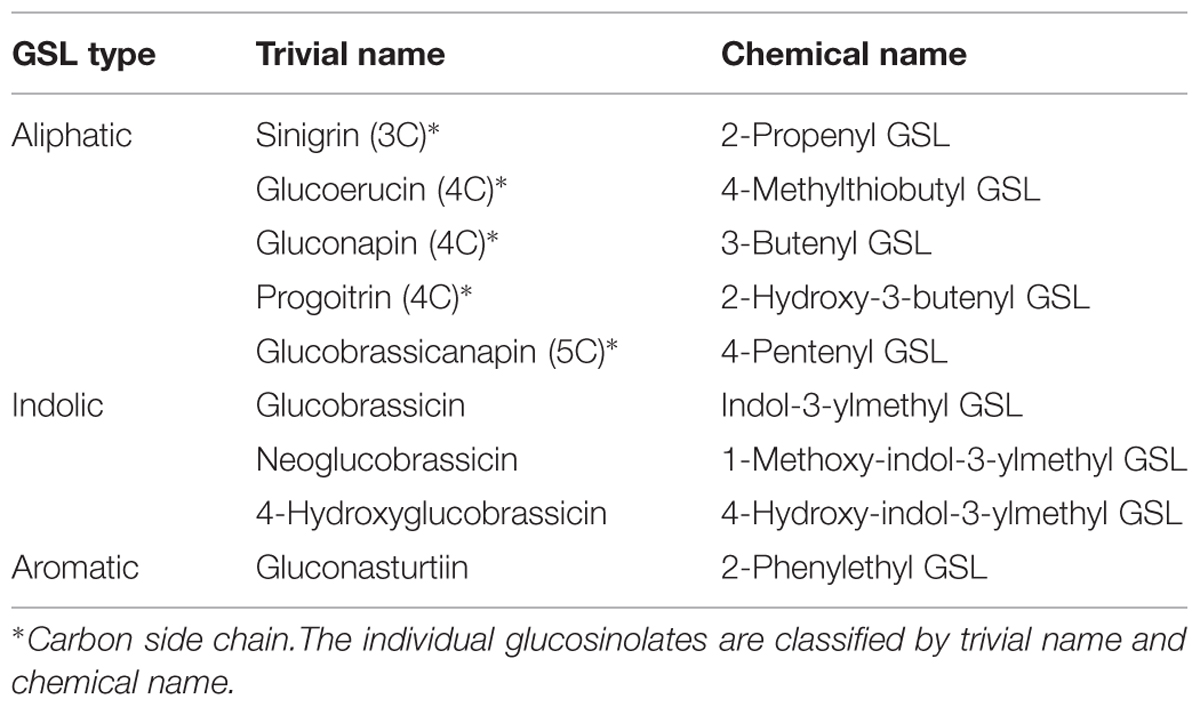
TABLE 1. Nomenclature of the individual glucosinolates identified in shoots and roots of Brassica juncea and B. rapa.
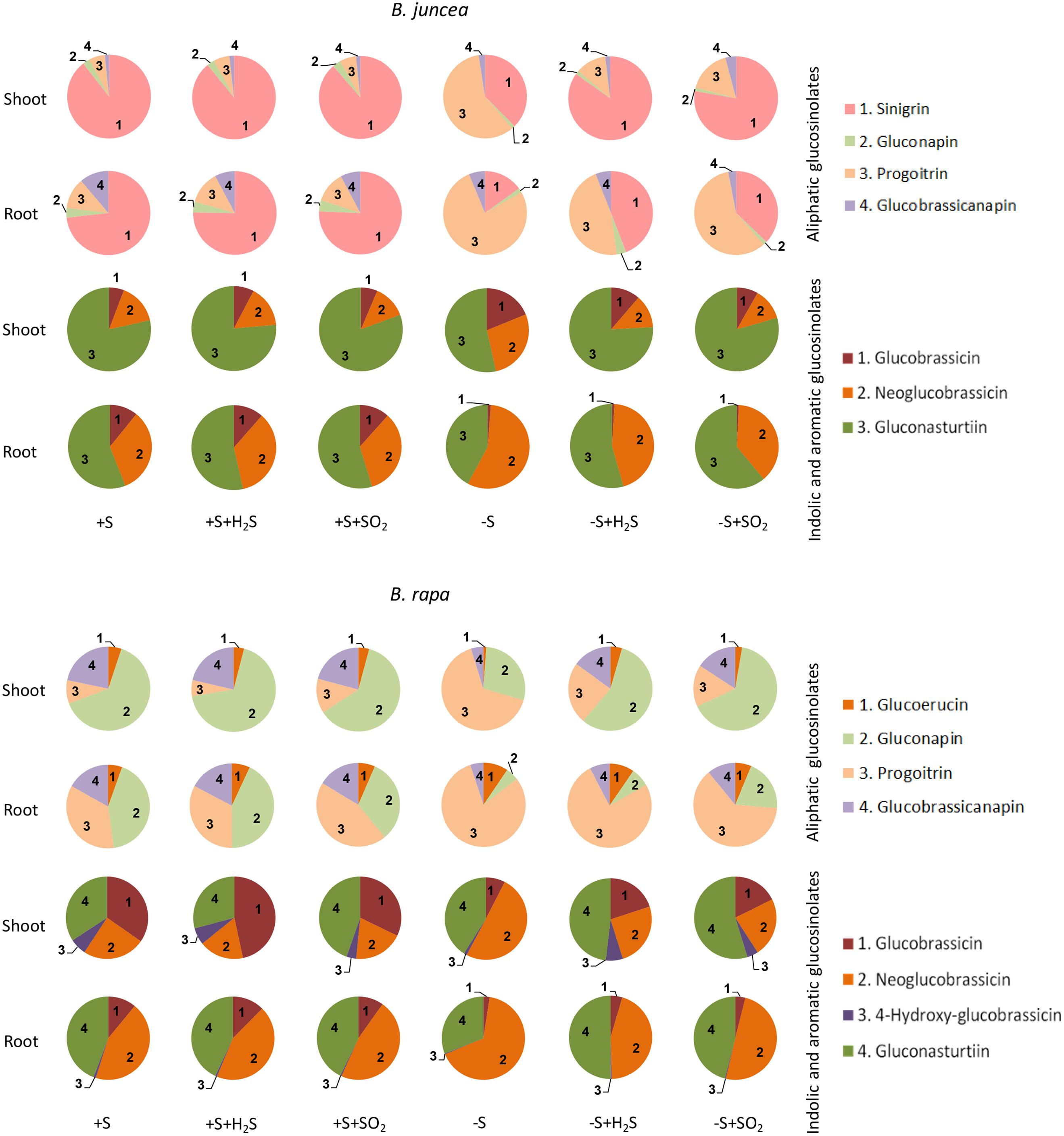
FIGURE 2. Impact of H2S and SO2 and sulfate deprivation on the composition of glucosinolates in shoots and roots of B. juncea and B. rapa. For experimental details, see legends of Figure 1. The pie graphs represent the composition of the glucosinolates (for details on their nomenclature see Table 1), the absolute data are presented in Figures 3 and 4.
Sinigrin was the major aliphatic glucosinolate present in the roots and shoots of B. juncea. In the shoots it even accounted for more than 90% of the aliphatic and more that 80% of the total glucosinolates (Figures 2 and 3). The observed 5.5-fold higher glucosinolate content in the shoots of B. juncea was for the greater part attributed to the high sinigrin content, a compound which was not detected in the shoots and roots of B. rapa (Figures 2 and 3). Gluconapin was the major aliphatic glucosinolate present in roots and shoots of B. rapa. In the shoot its content accounted for 65% of the aliphatic glucosinolates and 57% of the total glucosinolates (Figures 2 and 3). The roots of both species, however, contained high contents of aromatic glucosinolates, which in accounted for 32 and 40% and indolic glucosinolates, which content accounted for 56 and 50% of the total glucosinolates in roots of B. juncea and B. rapa, respectively (Figure 1). Gluconasturtiin was the major glucosinolate in roots of B. juncea and B. rapa (Figures 2 and 4).
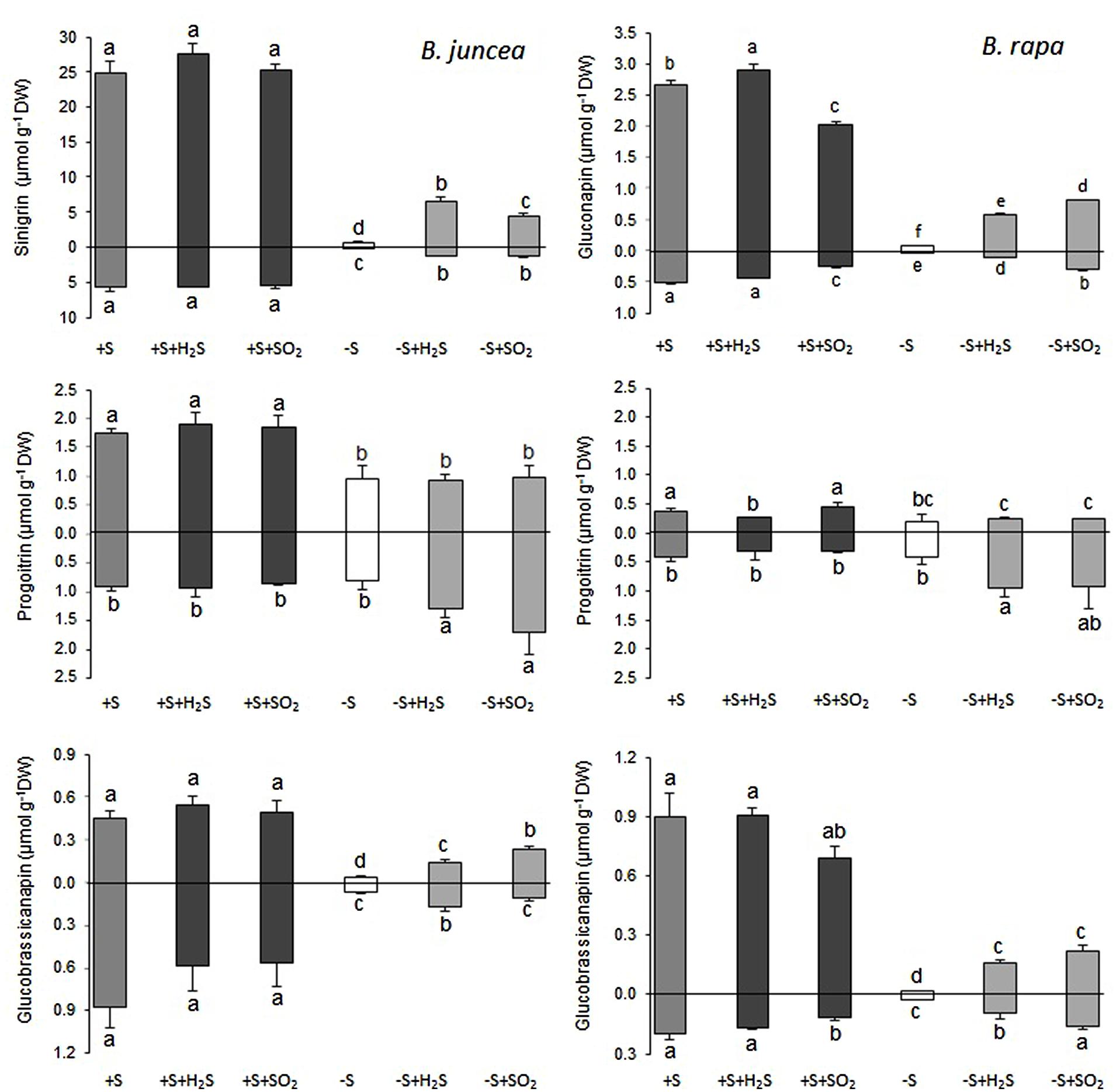
FIGURE 3. Impact of H2S and SO2 and sulfate deprivation on the content of the aliphatic glucosinolates in shoots (above x axis) and roots (below x axis) of B. juncea and B. rapa. For experimental details, see legends of Figure 1; for details on their nomenclature see Table 1. Data on glucosinolate content (μmol g-1 DW) represent the mean of three measurements with nine plants in each (±SD). Different letters indicate significant differences between treatments (P < 0.01, Student’s t-test).
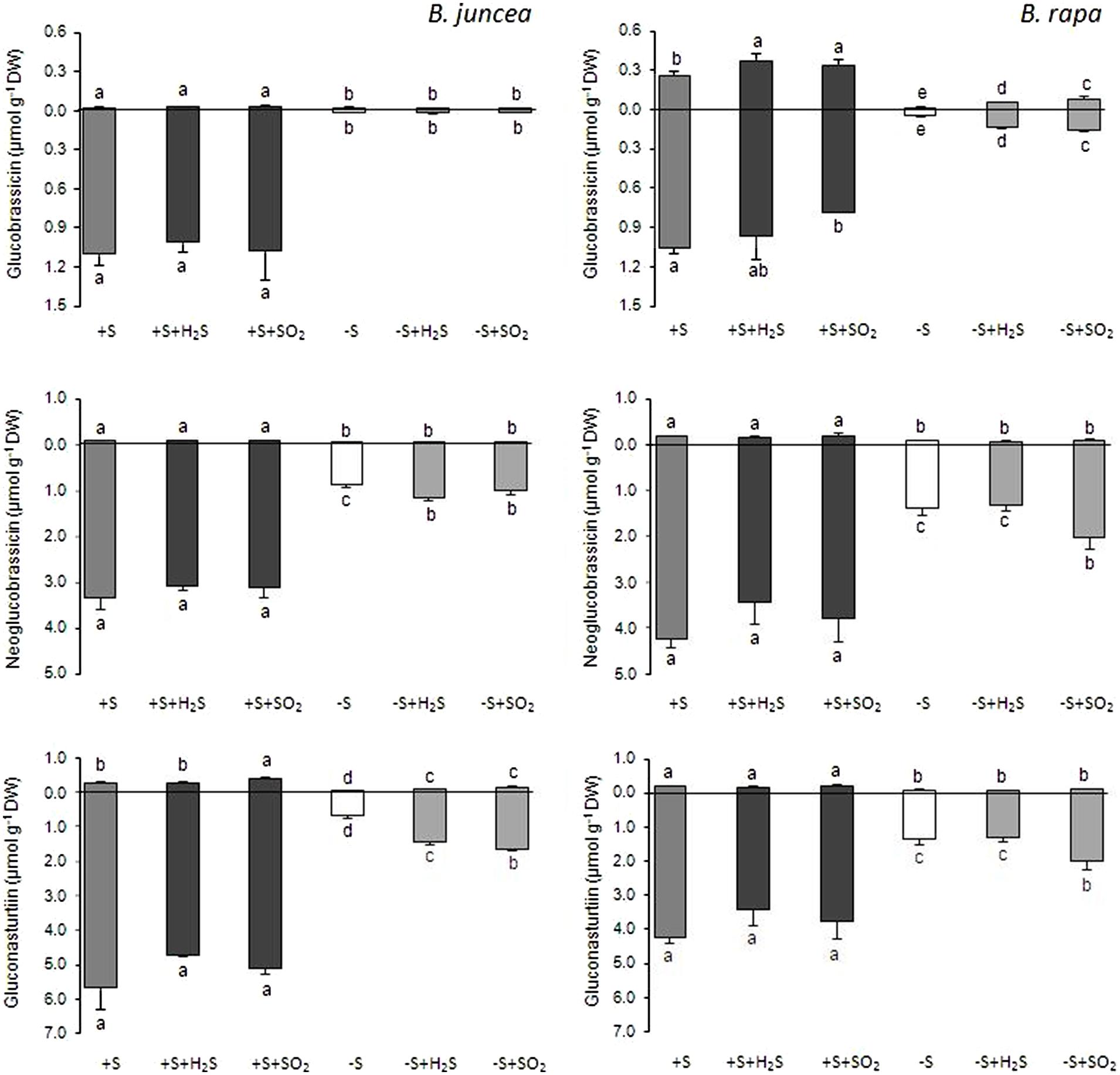
FIGURE 4. Impact of H2S and SO2 and sulfate deprivation on the content of the indolic and aromatic glucosinolates in shoots (above x axis) and roots (below x axis) of B. juncea and B. rapa. For experimental details, see legends of Figure 1; for details on their nomenclature see Table 1. Data on glucosinolate content (μmol g-1 DW) represent the mean of three measurements with nine plants in each (±SD). Different letters indicate significant differences between treatments (P < 0.01, Student’s t-test).
Impact of Sulfur Nutrition on Glucosinolate Composition
H2S and SO2 exposure did not affect the total content, and only slightly affected the glucosinolate composition in the shoots and roots of sulfate-sufficient B. rapa seedlings (Figures 1–4). H2S exposure resulted in a slight increase in the gluconapin and glucobrassicin content and slight decrease in the progoitrin in the shoots of B. rapa, whereas it did not affect the aliphatic and indolic glucosinolate composition in the roots (Figure 2–4). SO2 exposure resulted in a slight increase in the glucobrassicin content and a decrease in the gluconapin and glucobrassicanapin content of the shoots of B. rapa.
A 7-day sulfate deprivation resulted in 96 and 85% decreases in total glucosinolate content in shoots and roots of B. juncea and 90 and 76% decreases in shoots and roots of B. rapa seedlings, respectively (Figure 1). Sulfate deprivation also strongly affected the glucosinolate composition and resulted in a 94, 74, and 82% decrease in the content of the aliphatic, indolic, and aromatic glucosinolates in the shoot and a 84, 85, and 88% decrease of these glucosinolates in the roots of B. juncea, respectively (Figure 1). In B. rapa, the content of aliphatic, indolic, and aromatic glucosinolates decreased by 93, 79, and 97% in the shoots, and by 55, 73, and 84% in the roots, respectively, upon sulfate deprivation (Figure 1). Evidently, the content of progoitrin and neoglucobrassicin in the shoots and roots of B. juncea and B. rapa were less affected by sulfate deprivation than that of the other glucosinolates (Figures 3 and 4).
Exposure of sulfate-deprived B. juncea seedlings to 0.25 μl l-1 H2S or SO2 partly alleviated the decrease in the glucosinolate content in both shoots and roots, but was always lower than that observed in sulfate-sufficient plants (Figure 1). Moreover, exposure of sulfate-deprived B. rapa seedlings to 0.25 μl l-1 H2S and SO2 partly alleviated the decrease in the glucosinolate content in the roots, whereas that of the shoots hardly changed (Figure 1). Again, sinigrin was the most abundant glucosinolate in the shoots of B. juncea and the overall glucosinolate composition in the shoot of this species was quite similar to that observed in sulfate-sufficient plants both in presence and absence of H2S and SO2 (Figures 2 and 3). Gluconapin was the most abundant glucosinolate present in the shoots of sulfate-deprived B. rapa upon H2S and SO2 exposure, and the overall glucosinolate composition was somewhat altered to that observed in sulfate-sufficient plants in presence and absence of H2S and SO2, due to the relatively higher content of this glucosinolate (Figures 2 and 3). In both sulfate-deprived H2S- and SO2-exposed B. juncea and B. rapa plants, the short-chain (C3) and (C4) aliphatic glucosinolates were predominant glucosinolates present in the shoots (Figure 3). Exposure of sulfate-deprived B. juncea to H2S and SO2 resulted in a substantial enhancement in the sinigrin content of the root, whereas the content of the other glucosinolates were less affected (Figures 2 and 3). As a consequence, the overall composition of the glucosinolates in H2S and SO2 exposed sulfate-deprived B. juncea roots was somewhat different than that observed in sulfate-sufficient plants (Figure 2). The composition of the glucosinolates of sulfate-deprived roots of B. rapa upon H2S and SO2 exposure was also different to that observed in sulfate-sufficient plants (Figure 2). The latter was mainly due to the overall higher proportion of progoitrin in the roots (Figures 2 and 3). H2S and SO2 exposure largely alleviated the decrease in short-chain (C4) aliphatic glucosinolates in roots of both B. juncea and B. rapa upon sulfate-deprivation.
Discussion
Brassicaceae are able to utilize foliarly absorbed atmospheric H2S or SO2 as sulfur sources for growth which may replace sulfate taken up by the root as the sulfur source for growth (Stuiver and De Kok, 2001; Buchner et al., 2004; Yang et al., 2006; De Kok et al., 2007; Koralewska et al., 2008; Shahbaz et al., 2013; Aghajanzadeh et al., 2014). It was evident, that an atmospheric concentration of 0.25 μl l-1 of these sulfur gasses was sufficient to cover the sulfur requirements for growth. At an ample sulfate supply, the total sulfur contents of the plants were hardly affected, indicating a good coordination between uptake and assimilation of these sulfur gasses in the shoot and uptake of sulfate by the root and exposure of Brassicaceae to atmospheric sulfur resulted to a down-regulation of the sulfate uptake by the root and its reduction in the shoot (Westerman et al., 2000, 2001; Buchner et al., 2004; De Kok et al., 2007; Durenkamp et al., 2007; Koralewska et al., 2008; Shahbaz et al., 2013).
Despite the differences in the uptake of H2S and SO2 in the shoots (the uptake of H2S is determined by the rate of metabolism into cysteine, whereas that of SO2 by dissociation in the aqueous phase of the mesophyll), their impact at an atmospheric concentration of 0.25 μl l-1 on the total sulfur content of B. juncea and B. rapa seedlings was quite similar for both sulfate-sufficient and sulfate-deprived conditions (Aghajanzadeh et al., 2014). Neither H2S nor SO2 exposure affected the glucosinolate contents of shoots and roots of these species, demonstrating that these sulfur compounds did not form a sink for the storage of excessive sulfur, at least at a seedling stage (Aghajanzadeh et al., 2014). From the present study it was again evident that the shoots of B. rapa contained considerably lower levels of glucosinolates compared to B. juncea, although the differences in contents were mainly due to the high levels of sinigrin, which was absent in B. rapa. Moreover, the transcript level of APS kinase, a key enzyme in the synthesis of the sulfate moiety of the glucosinolates, was also substantially higher in shoots and roots of B. juncea (data not shown). However, H2S and SO2 exposure also hardly affected the composition of the glucosinolates.
Sulfate-deprivation of B. juncea and B. rapa for a week resulted in stunted growth and diminished sulfur and glucosinolate contents (Aghajanzadeh et al., 2014). The exposure of sulfate-deprived plants to 0.25 μl l-1 H2S or SO2 fully alleviated the decrease in biomass production, demonstrating that plants to utilize these atmospheric sulfur gasses as sole sulfur sources for growth. Again, despite the differences in patterns of uptake and metabolism between H2S and SO2, their impact on sulfur and glucosinolate contents in shoots and roots of B. juncea and B. rapa were quite similar. Their contents were lower than that observed in sulfur-sufficient plants in absence or presence of H2S and SO2 (Aghajanzadeh et al., 2014), although the proportion of the glucosinolates in the organic sulfur fraction was higher than that of sulfate-sufficient plants (Aghajanzadeh et al., 2014). From the current results it was evident that sulfate deprivation not only decreased the total glucosinolates contents but also strongly affected the composition. However, if sulfate-deprived plants were exposed to H2S and SO2, the composition of the glucosinolates in the shoots of both B. juncea and B. rapa hardly differed from that of sulfate-sufficient plants. Remarkably, the pattern of synthesis of the different aliphatic and indolic glucosinolates was hardly affected by the differences in oxidation state of the supplied sulfur sources in the shoots. Apparently, upon absorption and subsequent metabolism of H2S and SO2 in the shoots, not only sufficient cysteine, which functions as the reduced sulfur donor for the synthesis of glucosinolates, but also sufficient sulfate was formed/available for the synthesis of the sulfate moiety of glucosinolates via the ATP sulfurylase/APS kinase/sulfotransferase pathway. Evidently, foliarly absorbed SO2 may, after its reaction with water and dissociation in the mesophyll apoplast and symplast, be either non-enzymatically and/or enzymatically oxidized to sulfate or reduced in the chloroplast, and subsequently assimilated into cysteine (De Kok, 1990; De Kok and Tausz, 2001; De Kok et al., 2007). The direct metabolism of the foliarly absorbed H2S by O-acetyl(thiol)lyase, the rate limiting step for the uptake of these gas by the shoot, should provide ample cysteine as reduced sulfur donor for the synthesis of glucosinolates (De Kok, 1990; De Kok and Tausz, 2001; Stuiver and De Kok, 2001; De Kok et al., 2007). However, the source of sulfate moiety under these conditions needs further to be evaluated. The pattern of H2S oxidation in plant tissues is rather obscure; the presence of superoxide may catalyze the oxidation of sulfide, though it is still unclear to what extent elemental sulfur or sulfate is formed (De Kok et al., 1983). It has been observed that degradation in cysteine in cells may result in the formation of sulfate, although here the pathway also needs further to be investigated (Harrington and Smith, 1980).
The glucosinolate composition of sulfate-deprived roots after H2S and SO2 exposure was different from that observed in sulfate-sufficient B. juncea and B. rapa. In sulfate-deprived plants exposed to H2S or SO2, the roots fully depend on the sulfur supplied by the shoots. Roots of B. juncea and B. rapa express all enzymes of the sulfate reduction pathway and APS kinase (data not shown) which is essential for the synthesis of the sulfate moiety of the glucosinolates, indicating that the roots have the capacity to synthesize glucosinolates, despite the observation that they might also be transported in plant tissue (Andersen et al., 2013). It is widely accepted that in the majority of plant species glutathione is most important form of reduced sulfur transported from source (viz., shoot) to sink (root; Rennenberg et al., 1979; Herschbach et al., 2000). However, the pathway of degradation of glutathione, which, e.g., would be necessary for the synthesis of the sulfated moiety (via the APS/PAPS pathway) of glucosinolates in the roots, is still not well understood (Ohkama-Ohtsu et al., 2008).
Conclusion
The glucosinolate composition in the shoot was hardly affected by differences in sulfur source (viz., sulfate, sulfite and sulfide) for growth, whereas that in the root was substantially altered. The latter indicated that the presence of sulfate in the root environment is essential for the synthesis of some of the glucosinolates.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Rothamsted Research is supported via the 20:20 Wheat® Programme by the UK Biotechnology and Biological Sciences Research Council. Research in SK’s lab is supported by Deutsche Forschungsgemeinschaft (EXC 1028).
References
Aghajanzadeh, T., Hawkesford, M. J., and De Kok, L. J. (2014). The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant Sci. 5:704. doi: 10.3389/fpls.2014.00704
Agneta, R., Lelario, F., De Maria, S., Möllers, C., Bufo, S. A., and Rivelli, A. R. (2014). Glucosinolate profile and distribution among plant tissues and phenological stages of field-grown horseradish. Phytochemistry 106, 178–187. doi: 10.1016/j.phytochem.2014.06.019
Andersen, T. G., Nour-Eldin, H. H., Louise Fuller, V., Olsen, C. E., Burow, M., and Halkiera, B. A. (2013). Integration of biosynthesis and long-distance transport establish organ-specific glucosinolate profiles in vegetative Arabidopsis. Plant Cell 25, 3133–3145. doi: 10.1105/tpc.113.110890
Bloem, E., Haneklaus, S., and Schnug, E. (2007). Changes in the sulphur and glucosinolate content of developing pods and seeds of oilseed rape (Brassica napus L.) in relation to different cultivars. Landbauforsch. Völk. 57, 297–306.
Brown, P. D., Tokuhisa, J. G., Reichelt, M., and Gershenzon, J. (2003). Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62, 471–481. doi: 10.1016/S0031-9422(02)00549-6
Buchner, P., Stuiver, C. E. E., Westerman, S., Wirtz, M., Hell, R., Hawkesford, M. J., et al. (2004). Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiol. 136, 3396–3408. doi: 10.1104/pp.104.046441
Burow, M., Müller, R., Gershenzon, J., and Wittstock, U. (2006). Altered glucosinolate hydrolysis in genetically engineered Arabidopsis thaliana and its influence on the larval development of Spodoptera littoralis. J. Chem. Ecol. 32, 2333–2349. doi: 10.1007/s10886-006-9149-1
Castro, A., Aires, A., Rosa, E., Bloem, E., and Stulen, I. (2004). Distribution of glucosinolates in Brassica oleracea cultivars. Phyton 44, 133–143.
De Kok, L. J. (1990). “Sulfur metabolism in plants in exposed to atmospheric sulfur,” in Sulfur Nurtition and Sulfur Assimilation in Higher Plants; Fundamental, Environmental and Agricultural Aspects, eds H. Rennenberg, C. Brunold, L. J. De Kok, and I. Stulen (The Hague: SPB Academic Publishing), 111–130.
De Kok, L. J., Durenkamp, M., Yang, L., and Stulen, I. (2007). “Atmospheric sulfur,” in Sulfur in Plants: An Ecological Perspective, eds M. J. Hawkesford and L. J. De Kok (Berlin: Springer), 91–106.
De Kok, L. J., and Tausz, M. (2001). “The role of glutathione in plant reaction and adaptation to air pollutants,” in Significance of Glutathione to Plant Adaptation to the Environment, eds D. Grill, M. Tausz, and L. J. De Kok (Dordrecht: Kluwer Academic Publishers), 185–201.
De Kok, L. J., Thompson, C. R., Mudd, J. B., and Kats, G. (1983). Effect of H2S fumigation of water-soluble sulfhydryl compounds in shoots of crop plants. Z. Pflanzenphysiol. 111, 85–89. doi: 10.1016/S0044-328X(83)80076-2
Durenkamp, M., De Kok, L. J., and Kopriva, S. (2007). Adenosine 5’-phosphosulphate reductase is regulated differently in Allium cepa L. and Brassica oleracea L. upon exposure to H2S. J. Exp. Bot. 58, 1571–1579. doi: 10.1093/jxb/erm031
Fahey, J. W., Zalcmann, A. T., and Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56, 5–51. doi: 10.1016/S0031-9422(00)00316-2
Falk, K. L., Tokuhisa, J. G., and Gershenzon, J. (2007). The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biol. 9, 573–581. doi: 10.1055/s-2007-965431
Halkier, B. A., and Gershenzon, J. (2006). Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57, 303–333. doi: 10.1146/annurev.arplant.57.032905.105228
Harrington, H. M., and Smith, I. K. (1980). Cysteine metabolism in cultured tobacco cells. Plant Physiol. 65, 151–155. doi: 10.1104/pp.65.1.151
Herschbach, C., van der Zalm, E., Schneider, A., Jouanin, L., De Kok, L. J., and Rennenberg, H. (2000). Regulation of sulfur nutrition in wild-type and transgenic poplar over-expressing gamma-glutamylcysteinesynthetase in the cytosol as affected by atmospheric H2S. Plant Physiol. 124, 461–474. doi: 10.1104/pp.124.1.461
Hirai, M. Y., Klein, M., Fujikawa, Y., Yano, M., Goodenowe, D. B., Yamazaki, Y., et al. (2005). Elucidation of gene-to-gene and metabolite-to-gene networks in Arabidopsis by integration of metabolomics and transcriptomics. J. Biol. Chem. 280, 25590–25595. doi: 10.1074/jbc.M502332200
Hirai, M. Y., Yano, M., Goodenowe, D. B., Kanaya, S., Kimura, T., Awazuhara, M., et al. (2004). Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 101, 10205–10210. doi: 10.1073/pnas.0403218101
Jahangir, M., Bdel-Farid, I. B., Kim, H. K., Choi, Y. H., and Verpoorte, R. (2009). Healthy and unhealthy plants: the effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 67, 23–33. doi: 10.1016/j.envexpbot.2009.06.007
Kopriva, S., Mugford, S. J., Baraniecka, P., Lee, B., Matthewman, C. A., and Koprivova, A. (2012). Control of sulfur partitioning between primary and secondary metabolism in Arabidopsis. Front. Plant Sci. 3:163. doi: 10.3389/fpls.2012.00163
Koralewska, A., Stuiver, C. E. E., Posthumus, F. S., Kopriva, S., Hawkesford, M. J., and De Kok, L. J. (2008). Regulation of sulfate uptake, expression of the sulfate transporters Sultr1;1 and Sultr1;2, and APS reductase in Chinese cabbage (Brassica pekinensis) as affected by atmospheric H2S nutrition and sulfate deprivation. Funct. Plant Biol. 35, 318–327. doi: 10.1071/FP07283
Lucas, W. J., Groover, A., Lichtenberger, R., Furuta, K., Yadav, S. R., Helariutta, Y., et al. (2013). The plant vascular system: evolution, development and functions. J. Integr. Plant Biol. 55, 294–388. doi: 10.1111/jipb.12041
Matsuda, F., Hirai, M. Y., Sasaki, E., Akiyama, K., Yonekura-Sakakibara, K., Provart, N. J., et al. (2010). AtMetExpress development: a phytochemical atlas of Arabidopsis development. Plant Physiol. 152, 566–578. doi: 10.1104/pp.109.148031
Ohkama-Ohtsu, N., Oikawa, A., Zhao, P., Xiang, C., Saito, K., and Oliver, D. J. (2008). A gamma-glutamyl transpeptidase-independent pathway of glutathione catabolism to glutamate via 5-oxoproline in Arabidopsis. Plant Physiol. 148, 1603–1613. doi: 10.1104/pp.108.125716
Petersen, B., Chen, S., Hansen, C., Olsen, C., and Halkier, B. (2002). Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 214, 562–571. doi: 10.1007/s004250100659
Rennenberg, H., Schmitz, K., and Bergmann, L. (1979). Long-distance transport of sulfur in Nicotiana tabacum. Planta 147, 57–62. doi: 10.1007/BF00384591
Sattelmacher, B. (2001). The apoplast and its significance for plant mineral nutrition. New Phytol. 149, 167–192. doi: 10.1046/j.1469-8137.2001.00034.x
Schnug, E. (1990). “Glucosinolates – fundamental, environmental and agricultural aspects,” in Sulfur Nutrition and Sulfur Assimilation in Higher Plant; Fundamental, Environmental and Agricultural Aspects, eds H. Rennenberg, C. Brunold, L. J. De Kok, and I. Stulen (The Hague: SPB Academic Publishing), 97–106.
Schnug, E. (1993). “Physiological functions and environmental relevance of sulfur-containing secondary metabolites,” in Sulfur Nutrition and Sulfur Assimilation in Higher Plant; Regulatory Agricultural and Environmental Aspects, eds L. J. De Kok, I. Stulen, H. Rennenberg, C. Brunold, and W. E. Rauser (The Hague: SPB Academic Publishing), 179–190.
Shahbaz, M., Parmar, S., Stuiver, C. E. E., Hawkesford, M. J., and De Kok, L. J. (2013). Copper toxicity and sulfur metabolism in Chinese cabbage are affected by UV radiation. Environ. Exp. Bot. 88, 60–70. doi: 10.1016/j.envexpbot.2012.04.007
Stuiver, C. E. E., and De Kok, L. J. (2001). Atmospheric H2S as sulfur source for plant growth: kinetics of H2S uptake and activity of O-acetylserine (thiol)lyase as affected by sulfur nutrition. Environ. Exp. Bot. 46, 29–36. doi: 10.1016/S0098-8472(01)00080-6
Westerman, S., De Kok, L. J., and Stulen, I. (2000). Interaction between metabolism of atmospheric H2S in the shoot and sulfate uptake by the roots of curly kale (Brassica oleracea L). Physiol. Plant. 109, 443–449. doi: 10.1034/j.1399-3054.2000.100411.x
Westerman, S., Stulen, I., Suter, M., Brunold, C., and De Kok, L. J. (2001). Atmospheric H2S as sulfur source for Brassica oleracea: consequences for the activity of the enzymes of the assimilatory sulfate reduction pathway. Plant Physiol. Biochem. 39, 425–432. doi: 10.1016/S0981-9428(01)01258-X
Wittstock, U., and Halkier, B. A. (2002). Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 7, 263–270. doi: 10.1016/S1360-1385(02)02273-2
Keywords: atmospheric sulfur nutrition, Brassica juncea, Brassica rapa, glucosinolates, hydrogen sulfide, sulfate nutrition, sulfate deprivation, sulfur dioxide
Citation: Aghajanzadeh T, Kopriva S, Hawkesford MJ, Koprivova A and De Kok LJ (2015) Atmospheric H2S and SO2 as sulfur source for Brassica juncea and Brassica rapa: impact on the glucosinolate composition. Front. Plant Sci. 6:924. doi: 10.3389/fpls.2015.00924
Received: 06 August 2015; Accepted: 12 October 2015;
Published: 28 October 2015.
Edited by:
Richard Sayre, New Mexico Consortium – Los Alamos National Laboratory, USACopyright © 2015 Aghajanzadeh, Kopriva, Hawkesford, Koprivova and De Kok. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luit J. De Kok, l.j.de.kok@rug.nl
 Tahereh Aghajanzadeh
Tahereh Aghajanzadeh Stanislav Kopriva
Stanislav Kopriva Malcolm J. Hawkesford
Malcolm J. Hawkesford Anna Koprivova
Anna Koprivova Luit J. De Kok
Luit J. De Kok