- Institute of Plant and Microbial Biology – Academia Sinica, Taipei, Taiwan
Cytochrome b559 (Cyt b559) is one of the essential components of the Photosystem II reaction center (PSII). Despite recent accomplishments in understanding the structure and function of PSII, the exact physiological function of Cyt b559 remains unclear. Cyt b559 is not involved in the primary electron transfer pathway in PSII but may participate in secondary electron transfer pathways that protect PSII against photoinhibition. Site-directed mutagenesis studies combined with spectroscopic and functional analysis have been used to characterize Cyt b559 mutant strains and their mutant PSII complex in higher plants, green algae, and cyanobacteria. These integrated studies have provided important in vivo evidence for possible physiological roles of Cyt b559 in the assembly and stability of PSII, protecting PSII against photoinhibition, and modulating photosynthetic light harvesting. This mini-review presents an overview of recent important progress in site-directed mutagenesis studies of Cyt b559 and implications for revealing the physiological functions of Cyt b559 in PSII.
Introduction
Cytochrome b559 is one of the essential components of Photosystem II in all oxygenic photosynthetic organisms (Whitmarsh and Pakrasi, 1996; Stewart and Brudvig, 1998; Guskov et al., 2009; Umena et al., 2011). Cyt b559 is a heme-bridged heterodimer protein comprising one α- and one β- subunit (encoded by the psbE and psbF genes) of 9 and 4 kDa, respectively (see Figure 1). Each subunit provides a His ligand (His-22 residue of the α- or β-subunit of Cyt b559 in Synechocystis sp. PCC 6803, corresponding to His-23 residue of α- or His-24 residue of the β-subunit of Cyt b559 in Thermosynechococcus elongatus) for the non-covalently bound heme, which is located near the stromal side of PSII. In addition, Cyt b559 has different redox potential forms depending on the type of PSII preparations and treatments: a HP form with a midpoint redox potential of about +400 mV, an IP form of about +200 mV, and a LP form with a midpoint redox potential of about 0–80 mV (Stewart and Brudvig, 1998; Roncel et al., 2001 and references therein). In intact PSII preparations, Cyt b559 is mostly in the reduced HP form under ambient conditions. In inactive or less intact PSII preparations, Cyt b559 is typically in the LP or IP form and mostly oxidized (presumably by molecular oxygen) under ambient conditions (Barber and De Las Rivas, 1993; Poulson et al., 1995; Pospíšil et al., 2006).
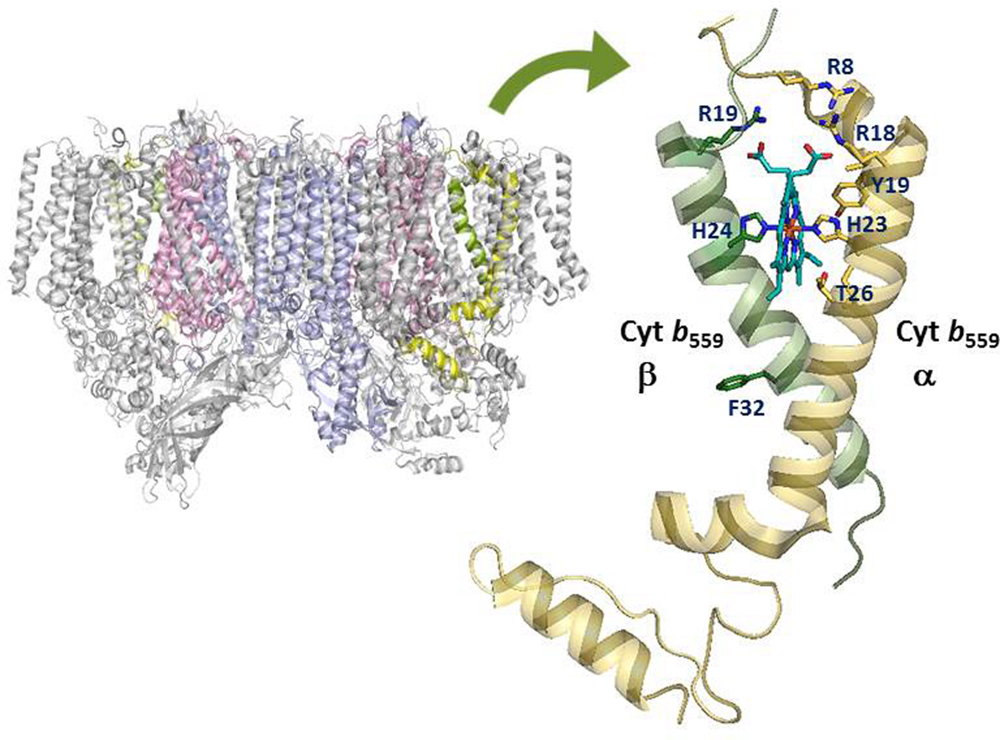
FIGURE 1. Structural model of the cytochrome b559 in Photosystem II. Amino acid residues of cytochrome b559 targeted by recent site-directed mutagenesis work are labeled by using amino acid sequences of cytochrome b559 from Thermosynechococcus elongatus. The figure was created using PyMol and the PDB file 4UB8.
Several studies have proposed that Cyt b559 participates in secondary electron transfer pathways that protect PSII against photoinhibition (see Figure 2; Heber et al., 1979; Falkowski et al., 1986; Thompson and Brudvig, 1988; Barber and De Las Rivas, 1993; Poulson et al., 1995; Magnuson et al., 1999; Faller et al., 2001; Tracewell and Brudvig, 2008 and references therein). In these models, the HP form of Cyt b559 is thought to donate its electron, via a β-carotene molecule (CarD2), to reduce highly oxidized chlorophyll radicals in PSII under donor-side photoinhibitory conditions (e.g., the oxygen-evolving complex is impaired or under assembly). Oxidized Cyt b559 may accept an electron from the acceptor side of PSII (QB−, or reduced PQH2 from the pool), thus forming a cyclic pathway of electron transfer within PSII. On the other hand, when the electron transfer on the acceptor side of PSII is inhibited (e.g., under high-light conditions), the oxidized Cyt b559 might accept an electron from the acceptor side of PSII to prevent the formation of damaging singlet oxygen species (Nedbal et al., 1992; Vass et al., 1992; Barber and De Las Rivas, 1993; Bondarava et al., 2010). In addition, several different enzymatic functions of Cyt b559 have been proposed, such as superoxide dismutase (Ananyev et al., 1994) and PQH2 oxidase in intact PSII (Kruk and Strzałka, 1999, Kruk and Strzalka, 2001; Bondarava et al., 2003, 2010) and superoxide oxidase and reductase in tris-washed PSII (Tiwari and Pospísil, 2009; Pospíšil, 2011). Moreover, a novel quinone-binding site (QC) was identified close (about 15 Å) to the heme of Cyt b559 in the 2.9-Å PSII crystal structure from T. elongatus (Guskov et al., 2009). The occupancy of this QC site with PQ (or PQH2) has been proposed to modulate the redox equilibration between Cyt b559 and the PQ pool (Kaminskaya et al., 2006, 2007; Kaminskaya and Shuvalov, 2013) or be involved in the exchange of PQ on the QB site from the pool (Guskov et al., 2009). However, the QC site was not detected in the more recent 1.9-Å PSII crystal structure (Umena et al., 2011). Despite the recent remarkable progress in understanding the structure and function of PSII, the exact function of Cyt b559 in PSII remains unclear.
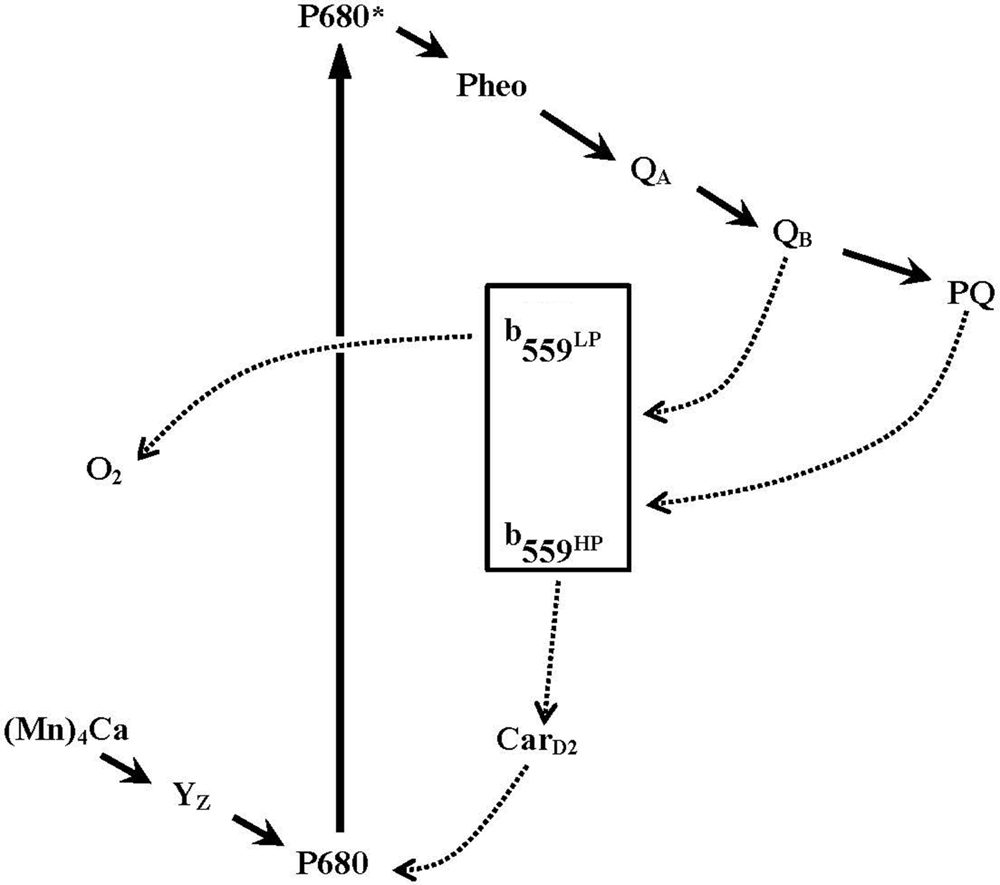
FIGURE 2. A simplified scheme for electron transfer pathways within Photosystem II reaction centers. Primary electron transfer pathway (bold solid lines with arrows) and possible secondary electron transfer pathways involving cytochrome b559 (dotted line with arrows) are shown. The scheme is modified from Whitmarsh and Pakrasi (1996).
This mini-review gives an overview of important progress in recent site-directed mutagenesis studies performed to reveal the physiological function(s) of Cyt b559 in PSII. More comprehensive reviews on the structure and functions of Cyt b559 are available (Whitmarsh and Pakrasi, 1996; Stewart and Brudvig, 1998; Faller et al., 2005; Pospíšil, 2011; Shinopoulos and Brudvig, 2012).
Function in Assembly and Stability of PSII Reaction Centers
Prior mutagenesis studies with Synechocystis sp. PCC 6803, Chlamydomonas reinhardtii or Nicotiana tabacum showed no stable PSII reaction centers assembled in the absence of either Cyt b559 subunit (Pakrasi et al., 1988, 1989, 1990; Morais et al., 1998; Swiatek et al., 2003; Suorsa et al., 2004). Several authors proposed that Cyt b559 plays an important structural role, such as being a nucleating factor, during the early stage of PSII assembly (Pakrasi et al., 1988; Morais et al., 1998; Komenda et al., 2004).
In addition, mutagenesis studies with Synechocystis sp. PCC 6803 showed that substituting either of the heme axial ligands (His22 of the α-subunit or His22 of the β-subunit of Cyt b559 in Synechocystis sp. PCC 6803) with Leu, Met, Glu, Gln, Tyr, Lys, Arg, or Cys abolished the photoautotrophic growth and severely diminished the assembly or stability of PSII in the mutant cells, except for H22Kα mutant cells, which were able to grow photoautotrophically and accumulated stable PSII reaction centers (∼81% as compared with wild-type cells; Pakrasi et al., 1991; Hung et al., 2007, 2010). Electron paramagnetic resonance results indicated the displacement of one of the two axial ligands to the heme of Cyt b559 in H22Kα mutant reaction centers, at least in isolated reaction centers (Hung et al., 2010). In addition, H22Kα and Y18Sα (corresponding to Y19Sα in T. elongatus) in mutant PSII core complexes contained predominately the LP form of Cyt b559. The findings support the concept that the redox properties of Cyt b559 are strongly influenced by the hydrophobicity and ligation environment of the heme (Krishtalik et al., 1993; Gadjieva et al., 1999; Roncel et al., 2001; Pospíšil and Tiwari, 2010).
Spectroscopic and functional characterizations of the cyanobacterium Synechocystis sp. PCC 6803 with mutation of charged residues on the cytoplasmic side of Cyt b559 in PSII have been reported (Chiu et al., 2013). All mutant cells grew photoautotrophically and assembled stable PSII. However, R7Eα, R17Eα, and R17Lβ mutant cells grew significantly slower and were more susceptible to photoinhibition as compared with wild-type cells. In addition, the PSII core complexes from R7Eα and R17Lβ cells contained predominantly the LP form of Cyt b559. Electron paramagnetic resonance results indicated the displacement of one of the two axial ligands to the heme of Cyt b559 in the reaction centers of the R7Eα and R17Lβ mutants. In recent PSII crystal structural models (Guskov et al., 2009; Umena et al., 2011), the side chains of these Arg residues of Cyt b559 (corresponding to Arg8 and Arg18 residues of the α-subunit and Arg19 residue of the β-subunit of Cyt b559 in T. elongatus) are in close contact with the heme propionates of Cyt b559 (see Figure 1). Thus, the electrostatic interactions between these Arg residues and the heme propionates of Cyt b559 may affect the ligation structure and redox properties of the heme in Cyt b559 (Chiu et al., 2013).
Furthermore, mutagenesis studies of C. reinhardtii showed that the H23Yα, H23Mα, and H23Cα mutant cells were unable to grow photoautotrophically, were sensitive to photoinhibition, accumulated 10–20% of the PSII (compared to wild-type cells), and contained a disrupted heme pocket while still retaining significant O2 evolution activity (Morais et al., 2001; Hamilton et al., 2014). Thus, the heme of Cyt b559 was not required for photosynthetic water oxidation by PSII (Morais et al., 2001). A recent study also presented evidence to ascribe the photoinhibition phenotype of H23Cα mutant cells to a faster rate of photodamage and an impaired PSII repair cycle (Hamilton et al., 2014). Hence, Cyt b559 may play important roles in the assembly, repair and maintenance of the PSII complex in vivo.
In the other recent mutant study of T. elongatus that took advantage of the robustness of the PSII variant with PsbA3 as the D1 subunit, the four constructed Cyt b559 mutants (H23Aα, H23Mα, Y19Fα, and T26Pα) grew photoautotrophically (T. elongatus is an obligate photoautotroph; Sugiura et al., 2015). Although the H23Aα and H23Mα mutants assembled only an apo-Cyt b559, the steady-state level of active PSII was comparable to that in the wild-type control. The results suggest that the heme has no structural role in the assembly of PSII in the presence of α- and β-subunits of Cyt b559. This finding is in strong contrast to the Synechocystis sp. PCC 6803 mutant showing that proper coordination of the heme cofactor in Cyt b559 is important to the assembly or stability of PSII (Pakrasi et al., 1991; Hung et al., 2007). In addition, Cyt b559 mutant cells of T. elongatus showed no correlation between the rate of photoinhibition and the redox potential of the heme. However, the recovery of the oxygen-evolving activity of PSII after photoinhibition was significantly slower in these mutant cells. PsbA3 is the D1 isoform expressed in T. elongatus under high-light conditions (Nakamura et al., 2002; Kós et al., 2008). The high-light D1 isoform in cyanobacteria has a Glu instead of a Gln residue (for the low-light D1 isoform) at position 130 in the D1 protein sequence (for a review, see Mulo et al., 2009) and this Glu residue forms hydrogen-bonding interactions with pheophytinD1 (Dorlet et al., 2001; Shibuya et al., 2010). Cyanobacterial PSIIs with the high-light D1 isoform showed increased photo-tolerance and accelerated non-radiative charge recombination (Tichy et al., 2003). This phototolerant property has been attributed to a photoprotection mechanism involving the redox potential of pheophytinD1, which enhances the probability for non-radiative recombination of the singlet radical pair and prevents the formation of potentially damaging 3P680 and singlet oxygen species (Vass and Cser, 2009; Sugiura et al., 2014). Further investigation could determine whether PsbA3 may compensate the photoprotective function of Cyt b559 in the assembly and stability of PSII in these Cyt b559 mutant cells.
Function in Protecting PSII Against Photoinhibition
Numerous site-directed mutagenesis studies have investigated the role of Cyt b559 in protecting PSII against photoinhibition under high light. In the photoprotective models, oxidized Cyt b559 may accept an electron from the acceptor side of PSII (QB−, QC, or reduced PQH2 from the pool). Previous mutant studies of dark-adapted leaves of the F26Sβ Cyt b559 tobacco mutant showed a greatly reduced PQ pool, conversion of the redox-potential form of Cyt b559 to the LP form, and photosynthetic activities sensitive to high light (Bondarava et al., 2003, 2010). In addition, R7Eα and R17Lβ Cyt b559 mutant cells of Synechocystis sp. PCC 6803 and R18Sα Cyt b559 mutant cells of T. elongatus showed markedly reduced PQ pools, altered redox-potential forms of Cyt b559, and high susceptibility to light stress (Chiu et al., 2013; Guerrero et al., 2014). A defect in PQH2 oxidase activity of Cyt b559 due to altered redox-potential forms of Cyt b559 in these mutant strains could explain their high susceptibility to strong light and greatly reduced PQ pools. Therefore, Cyt b559 may function as a PQH2 oxidase to keep the PQ pool and the acceptor side of PSII oxidized in the dark, thereby preventing PSII from acceptor-side photoinhibition (Kruk and Strzałka, 1999, Kruk and Strzalka, 2001; Bondarava et al., 2003, 2010). However, one recent study reported no defect in PQH2 oxidation in the dark in H23Cα mutant cells of C. reinhardtii, even though H23Cα mutant cells contained a disrupted heme-binding pocket of Cyt b559 and were sensitive to photoinhibition (Hamilton et al., 2014). Further studies are required to clarify this discrepancy. In addition, study of the 2.9-Å resolution PSII crystal structure reported the binding of QC at a hydrophobic cavity near Cyt b559 (Guskov et al., 2009). Several spectroscopic studies have provided evidence that the occupancy of the QC site by PQ (or PQH2) may modulate the redox potential of Cyt b559 and mediate the redox equilibration between Cyt b559 and the PQ pool (Kaminskaya et al., 2006, 2007; Kaminskaya and Shuvalov, 2013). A recent study provided evidence of a possible one-electron oxidation of PQH2 by Cyt b559 at the QC site involved in the formation of a superoxide anion radical (Yadav et al., 2014). The above results are consistent with Cyt b559 possibly accepting an electron from PQH2 via the QC site in PSII. However, the QC site was not present in the more recent 1.9-Å PSII crystal structure (Umena et al., 2011). Further investigations are needed to solve this important issue.
Spectroscopic and functional characterization of the H22Kα and Y18Sα Cyt b559 mutant cells of Synechocystis sp. PCC 6803 showed that both mutants have functional PSII and exhibited the normal period-four oscillation in oxygen yield (Hung et al., 2010). However, both mutants were more susceptible to photoinhibition than the wild type under high-light conditions. In addition, PSII core complexes from the H22Kα and Y18Sα mutants predominantly contained the oxidized LP form of Cyt b559 (∼79 and 86%, respectively). A defect in the photoprotective function of Cyt b559 in H22Kα and Y18Sα mutants could explain their high susceptibility to strong light. Furthermore, H22Kα and Y18Sα Cyt b559 mutants in a D1-D170A genetic background that prevented assembly of the Mn cluster showed almost completely abolished accumulation of PSII even under normal-growth-light conditions. The data support an important redox role of Cyt b559 in protecting PSII under donor-side photoinhibition conditions (Hung et al., 2010).
Furthermore, under low light, the H23Cα Cyt b559 mutant showed more rapid assembly of the Mn4CaO5 cluster than the wild-type control in C. reinhardtii (Hamilton et al., 2014). However, the photoactivation of oxygen-evolving PSII in the H23Cα mutant was inhibited under high light. The results suggest that reduction of P680+ via cyclic electron flow within PSII (via Cyt b559 and CarD2, Figure 2) may compete with the photoactivation process and provides important in vivo evidence for a photoprotective role of Cyt b559 in photo-assembly of the Mn4CaO5 cluster in PSII (Hamilton et al., 2014).
A recent mutant study involving T. elongatus showed that the midpoint redox potential of the HP form of Cyt b559 was significantly destabilized (converted to the IP form) in mutant PSII core complexes of Cyt b559 mutant strains (I14Aα, I14Sα, R18Sα, I27Aα, I27Tα, and F32Yβ; Guerrero et al., 2014). When the oxygen-evolving complex was inactive, the yield of dark-reduction of Cyt b559 was lower and the kinetics was slower in the R18Sα mutant than in wild-type cells. The results support the concept that the HP form of Cyt b559 may function as a PQH2 oxidase to keep the PQ pool oxidized and also as an electron reservoir for the cyclic electron flow within PSII when the donor-side of PSII is impaired (Guerrero et al., 2014).
Moreover, a previous spectroscopic study showed that different spectral forms of Car were oxidized in PSII samples containing different redox forms of Cyt b559 (Tracewell and Brudvig, 2008). The authors proposed that the quenching properties of PSII may be controlled by the redox form of Cyt b559 by modulating the different type of oxidized Car species (radical cation or neutral radical) formed in PSII. Future study could investigate the quenching properties of PSII in the wild type versus Cyt b559 mutant strains of cyanobacteria with different redox forms of Cyt b559 to validate this proposal.
Effects on Photosynthetic Light Harvesting
Recent mutant studies revealed a novel role of Cyt b559 in modulating photosynthetic light harvesting in PSII reaction centers. A spontaneously generated mutant from Synechocystis sp. PCC 6803 wild-type cells grown in BG-11 agar plates containing 5 mM Glu and 10 μM DCMU carried an Arg7 to Leu mutation on the alpha-subunit of Cyt b559 in PSII (Chiu et al., 2009). Results of 77-K fluorescence and room-temperature chlorophyll a fluorescence spectra indicated that the energy transfer from phycobilisomes to PSII reaction centers was partially inhibited or uncoupled in this mutant. In addition, the cytoplasmic side of Cyt b559 is located within the predicted contact sites in PSII for the APC core complex of the phycobilisome (Barber et al., 2003). The Arg7 to Leu mutation of Cyt b559 may alter the interaction between the APC core complex and PSII reaction centers, thereby reducing energy delivery from the antenna to the reaction center and protecting mutant cells against DCMU-induced photo-oxidative stress (Rutherford and Krieger-Liszkay, 2001).
Many cyanobacteria including Synechocystis sp. PCC 6803 have a novel blue-green light-induced NPQ mechanism to protect PSII reaction centers against photodamage under high-light stress (Kirilovsky and Kerfeld, 2012). Under high-light conditions, a soluble orange carotenoid protein is able to absorb blue–green light and undergoes photo-conversion into the active red form, which interacts with the APC core of the phycobilisome and dissipates excess excitation energy from the phycobilisome as heat. Interestingly, several Synechocystis sp. PCC 6803 mutant cells (e.g., R7Lα and R17Lβ) with mutations on the cytoplasmic side of Cyt b559 in PSII showed significant inhibition of the effects of blue–green light-induced NPQ and apparent acceleration on its recovery (Chiu et al., 2013). These results are consistent with the proposal that the mutations on Cyt b559 may alter the interaction between the phycobilisome and PSII reaction centers, thereby affecting the regulation of photosynthetic light harvesting in Synechocystis sp. PCC 6803.
Conclusion and Perspectives
Site-directed mutagenesis studies combined with spectroscopic and functional characterization have revealed multiple roles of Cyt b559 in the assembly and photoprotection of PSII reaction centers. The findings provide convincing evidence for the physiological role(s) of Cyt b559 in a photoprotective secondary electron transfer pathway within PSII reaction centers, as was suggested from earlier studies of isolated PSII complexes (reviews in Whitmarsh and Pakrasi, 1996; Stewart and Brudvig, 1998; Shinopoulos and Brudvig, 2012). In the near future, site-directed mutagenesis studies combined with advanced high-resolution protein crystallography and spectroscopic and functional analysis will provide further new insights (e.g., structure and function relationships for different redox forms of Cyt b559) and possibly the final proof of the molecular mechanisms of Cyt b559 in PSII.
Author Contributions
H-AC wrote the major part of the manuscript. Y-FC wrote the minor part of the manuscript, contributed the Figure 1 and edited the references.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work is supported by the Ministry of Science and Technology in Taiwan (104-2311-B-001-035 and 104-2627-M-001-001) and Academia Sinica to H-AC.
Abbreviations
APC, allophycocyanin; Car, β-carotene; Cyt b559, cytochrome b559; HP, high potential; IP, intermediate potential; LP, low potential; NPQ, non-photochemical fluorescences quenching; PQ, plastoquinone; PQH2, plastoquinol; PSII, Photosystem II; QA, primary quinone electron acceptor in PSII; QB, the secondary quinone electron acceptor in PSII; QC, the third plastoquinone-binding site in PSII.
References
Ananyev, G., Renger, G., Wacker, U., and Klimov, V. (1994). The photoproduction of superoxide radicals and the superoxide dismutase activity of Photosystem II. The possible involvement of cytochrome b559. Photosynth. Res. 41, 327–338. doi: 10.1007/BF00019410
Barber, J., and De Las Rivas, J. (1993). A functional model for the role of cytochrome b559 in the protection against donor and acceptor side photoinhibition. Proc. Natl. Acad. Sci. U.S.A. 90, 10942–10946. doi: 10.1073/pnas.90.23.10942
Barber, J., Morris, E. P., and da Fonseca, P. C. A. (2003). Interaction of the allophycocyanin core complex with Photosystem II. Photochem. Photobiol. Sci. 2, 536–541. doi: 10.1039/b300063j
Bondarava, N., De Pascalis, L., Al-Babili, S., Goussias, C., Golecki, J. R., Beyer, P., et al. (2003). Evidence that cytochrome b559 mediates the oxidation of reduced plastoquinone in the dark. J. Biol. Chem. 278, 13554–13560. doi: 10.1074/jbc.M212842200
Bondarava, N., Gross, C. M., Mubarakshina, M., Golecki, J. R., Johnson, G. N., and Krieger-Liszkay, A. (2010). Putative function of cytochrome b559 as a plastoquinol oxidase. Physiol. Plant. 138, 463–473. doi: 10.1111/j.1399-3054.2009.01312.x
Chiu, Y.-F., Chen, Y.-H., Roncel, M., Dilbeck, P. L., Huang, J.-Y., Ke, S.-C., et al. (2013). Spectroscopic and functional characterization of cyanobacterium Synechocystis PCC 6803 mutants on the cytoplasmic-side of cytochrome b559 in Photosystem II. Biochim. Biophys. Acta 1827, 507–519. doi: 10.1016/j.bbabio.2013.01.016
Chiu, Y.-F., Lin, W.-C., Wu, C.-M., Chen, Y.-H., Hung, C.-H., Ke, S.-C., et al. (2009). Identification and characterization of a cytochrome b559 Synechocystis 6803 mutant spontaneously generated from DCMU-inhibited photoheterotrophical growth conditions. Biochim. Biophys. Acta 1787, 1179–1188. doi: 10.1016/j.bbabio.2009.05.007
Dorlet, P., Xiong, L., Sayre, R. T., and Un, S. (2001). High field EPR study of the pheophytin anion radical in wild type and D1-E130 mutants of Photosystem II in Chlamydomonas reinhardtii. J. Biol. Chem. 276, 22313–22316. doi: 10.1074/jbc.M102475200
Falkowski, P. G., Fujita, Y., Ley, A., and Mauzerall, D. (1986). Evidence for cyclic electron flow around Photosystem II in Chlorella pyrenoidosa. Plant Physiol. 81, 310–312. doi: 10.1104/pp.81.1.310
Faller, P., Fufezan, C., and Rutherford, A. W. (2005). “Side-path electron donors: cytochrome b559, chlorophyll Z and β-Carotene,” in Photosystem II, eds T. J. Wydrzynski and K. Satoh (Dordrecht: Springer), 347–365.
Faller, P., Pascal, A., and Rutherford, A. W. (2001). Beta-carotene redox reactions in Photosystem II: electron transfer pathway. Biochemistry 40, 6431–6440. doi: 10.1021/bi0026021
Gadjieva, R., Mamedov, F., Renger, G., and Styring, S. (1999). Interconversion of low- and high-potential forms of cytochrome b559 in tris-washed Photosystem II membranes under aerobic and anaerobic conditions. Biochemistry 38, 10578–10584. doi: 10.1021/bi9904656
Guerrero, F., Zurita, J. L., Roncel, M., Kirilovsky, D., and Ortega, J. M. (2014). The role of the high potential form of the cytochrome b559: study of Thermosynechococcus elongatus mutants. Biochim. Biophys. Acta 1837, 908–919. doi: 10.1016/j.bbabio.2014.02.024
Guskov, A., Kern, J., Gabdulkhakov, A., Broser, M., Zouni, A., and Saenger, W. (2009). Cyanobacterial Photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 16, 334–342. doi: 10.1038/nsmb.1559
Hamilton, M. L., Franco, E., Deák, Z., Schlodder, E., Vass, I., and Nixon, P. J. (2014). Investigating the photoprotective role of cytochrome b559 in Photosystem II in a mutant with altered ligation of the haem. Plant Cell Physiol. 55, 1276–1285. doi: 10.1093/pcp/pcu070
Heber, U., Kirk, M. R., and Boardman, N. K. (1979). M.R. Kirk a, N.K. BoardmanPhotoreactions of cytochrome b559 and cyclic electron flow in Photosystem II of intact chloroplasts. Biochim. Biophys. Acta 546, 292–306. doi: 10.1016/0005-2728(79)90047-1
Hung, C.-H., Huang, J.-Y., Chiu, Y.-F., and Chu, H.-A. (2007). Site-directed mutagenesis on the heme axial-ligands of cytochrome b559 in Photosystem II by using cyanobacteria Synechocystis PCC 6803. Biochim. Biophys. Acta 1767, 686–693. doi: 10.1016/j.bbabio.2007.02.016
Hung, C.-H., Hwang, H.-J., Chen, Y.-H., Chiu, Y.-F., Ke, S.-C., Burnap, R. L., et al. (2010). Spectroscopic and functional characterizations of cyanobacterium Synechocystis PCC 6803 mutants on and near the heme axial ligand of cytochrome b559 in Photosystem II. J. Biol. Chem. 285, 5653–5663. doi: 10.1074/jbc.M109.044719
Kaminskaya, O., Shuvalov, V. A., and Renger, G. (2006). Evidence for a novel quinone-binding site in the Photosystem II (PS II) complex that regulates the redox potential of cytochrome b559. Biochemistry 46, 1091–1105. doi: 10.1021/bi0613022
Kaminskaya, O., Shuvalov, V. A., and Renger, G. (2007). Two reaction pathways for transformation of high potential cytochrome b559 of PS II into the intermediate potential form. Biochim. Biophys. Acta 1767, 550–558. doi: 10.1016/j.bbabio.2007.02.005
Kaminskaya, O. P., and Shuvalov, V. A. (2013). Biphasic reduction of cytochrome b559 by plastoquinol in Photosystem II membrane fragments: evidence for two types of cytochrome b559/plastoquinone redox equilibria. Biochim. Biophys. Acta 1827, 471–483. doi: 10.1016/j.bbabio.2013.01.007
Kirilovsky, D., and Kerfeld, C. A. (2012). The orange carotenoid protein in photoprotection of Photosystem II in cyanobacteria. Biochim. Biophys. Acta 1817, 158–166. doi: 10.1016/j.bbabio.2011.04.013
Komenda, J., Reisinger, V., Muller, B. C., Dobakova, M., Granvogl, B., and Eichacker, L. A. (2004). Accumulation of the D2 protein is a key regulatory step for assembly of the Photosystem II reaction center complex in Synechocystis PCC 6803. J. Biol. Chem. 279, 48620–48629. doi: 10.1074/jbc.M405725200
Kós, P. B., Deák, Z., Cheregi, O., and Vass, I. (2008). Differential regulation of psbA and psbD gene expression, and the role of the different D1 protein copies in the cyanobacterium Thermosynechococcus elongatus BP-1. Biochim. Biophys. Acta 1777, 74–83. doi: 10.1016/j.bbabio.2012.04.010
Krishtalik, L. I., Tae, G.-S., Cherepanov, D. A., and Cramer, W. A. (1993). The redox properties of cytochromes b imposed by the membrane electrostatic environment. Biophys. J. 65, 184–195. doi: 10.1016/S0006-3495(93)81050-6
Kruk, J., and Strzałka, K. (1999). Dark reoxidation of the plastoquinone-pool is mediated by the low-potential form of cytochrome b559 in spinach thylakoids. Photosynth. Res. 62, 273–279. doi: 10.1023/a:1006374319191
Kruk, J., and Strzalka, K. (2001). Redox changes of cytochrome b559 in the presence of plastoquinones. J. Biol. Chem. 276, 86–91. doi: 10.1074/jbc.M003602200
Magnuson, A., Rova, M., Mamedov, F., Fredriksson, P.-O., and Styring, S. (1999). The role of cytochrome b559 and tyrosineD in protection against photoinhibition during in vivo photoactivation of Photosystem II. Biochim. Biophys. Acta 1411, 180–191. doi: 10.1016/s0005-2728(99)00044-4
Morais, F., Barber, J., and Nixon, P. J. (1998). The chloroplast-encoded α subunit of cytochrome b559 is required for assembly of the Photosystem Two complex in both the light and the dark in Chlamydomonas reinhardtii. J. Biol. Chem. 273, 29315–29320. doi: 10.1074/jbc.273.45.29315
Morais, F., Kuhn, K., Stewart, D. H., Barber, J., Brudvig, G. W., and Nixon, P. J. (2001). Photosynthetic water oxidation in cytochrome b559 mutants containing a disrupted heme-binding pocket. J. Biol. Chem. 276, 31986–31993. doi: 10.1074/jbc.M103935200
Mulo, P., Sicora, C., and Aro, E.-M. (2009). Cyanobacterial psbA gene family: optimization of oxygenic photosynthesis. Cell. Mol. Life Sci. 66, 3697–3710. doi: 10.1007/s00018-009-0103-6
Nakamura, Y., Kaneko, T., Sato, S., Ikeuchi, M., Katoh, H., Sasamoto, S., et al. (2002). Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 9, 123–130. doi: 10.1093/dnares/9.4.123
Nedbal, L., Samson, G., and Whitmarsh, J. (1992). Redox state of a one-electron component controls the rate of photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. U.S.A. 89, 7929–7933. doi: 10.1073/pnas.89.17.7929
Pakrasi, H. B., Ciechi, P. D., and Whitmarsh, J. (1991). Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the Photosystem II complex. EMBO J 10, 1619–1627.
Pakrasi, H. B., Diner, B. A., Williams, J. G. K., and Arntzen, C. J. (1989). Deletion mutagenesis of the cytochrome b559 protein inactivates the reaction center of Photosystem II. Plant Cell 1, 591–597. doi: 10.2307/3868946
Pakrasi, H. B., Nyhus, K. J., and Granok, H. (1990). Targeted deletion mutagenesis of the beta subunit of cytochrome b559 protein destabilizes the reaction center of Photosystem II. Z. Naturforsch. C 45, 423–429.
Pakrasi, H. B., Williams, J. G., and Arntzen, C. J. (1988). Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in Photosystem II. EMBO J. 7, 325–332.
Pospíšil, P. (2011). Enzymatic function of cytochrome b559 in Photosystem II. J. Photochem. Photobiol. B 104, 341–347. doi: 10.1016/j.jphotobiol.2011.02.013
Pospíšil, P., Šnyrychová, I., Kruk, J., Strzałka, K., and Nauš, J. (2006). Evidence that cytochrome b559 is involved in superoxide production in Photosystem II: effect of synthetic short-chain plastoquinones in a cytochrome b559 tobacco mutant. Biochem. J. 397, 321–327. doi: 10.1042/BJ20060068
Pospíšil, P., and Tiwari, A. (2010). Differential mechanism of light-induced and oxygen-dependent restoration of the high-potential form of cytochrome b559 in Tris-treated Photosystem II membranes. Biochim. Biophys. Acta 1797, 451–456. doi: 10.1016/j.bbabio.2009.12.023
Poulson, M., Samson, G., and Whitmarsh, J. (1995). Evidence that cytochrome b559 protects Photosystem II against photoinhibition. Biochemistry 34, 10932–10938. doi: 10.1021/bi00034a027
Roncel, M., Ortega, J. M., and Losada, M. (2001). Factors determining the special redox properties of photosynthetic cytochrome b559. Eur. J. Biochem. 268, 4961–4968. doi: 10.1046/j.0014-2956.2001.02427.x
Rutherford, A. W., and Krieger-Liszkay, A. (2001). Herbicide-induced oxidative stress in Photosystem II. Trends Biochem. Sci. 26, 648–653. doi: 10.1016/S0968-0004(01)01953-3
Shibuya, Y., Takahashi, R., Okubo, T., Suzuki, H., Sugiura, M., and Noguchi, T. (2010). Hydrogen bond interaction of the pheophytin electron acceptor and its radical anion in Photosystem II as revealed by Fourier transform infrared difference spectroscopy. Biochemistry 49, 493–501. doi: 10.1021/bi9018829
Shinopoulos, K. E., and Brudvig, G. W. (2012). Cytochrome b559 and cyclic electron transfer within Photosystem II. Biochim. Biophys. Acta 1817, 66–75. doi: 10.1016/j.bbabio.2011.08.002
Stewart, D. H., and Brudvig, G. W. (1998). Cytochrome b559 of Photosystem II. Biochim. Biophys. Acta 1367, 63–87. doi: 10.1016/s0005-2728(98)00139-x
Sugiura, M., Azami, C., Koyama, K., Rutherford, A. W., Rappaport, F., and Boussac, A. (2014). Modification of the pheophytin redox potential in Thermosynechococcus elongatus Photosystem II with PsbA3 as D1. Biochim. Biophys. Acta 1837, 139–148. doi: 10.1016/j.bbabio.2013.09.009
Sugiura, M., Nakamura, M., Koyama, K., and Boussac, A. (2015). Assembly of oxygen-evolving Photosystem II efficiently occurs with the apo-Cyt b559 but the holo-Cyt b559 accelerates the recovery of a functional enzyme upon photoinhibition. Biochim. Biophys. Acta 1847, 276–285. doi: 10.1016/j.bbabio.2014.11.009
Suorsa, M., Regel, R. E., Paakkarinen, V., Battchikova, N., Herrmann, R. G., and Aro, E.-M. (2004). Protein assembly of Photosystem II and accumulation of subcomplexes in the absence of low molecular mass subunits PsbL and PsbJ. Eur. J. Biochem. 271, 96–107. doi: 10.1046/j.1432-1033.2003.03906.x
Swiatek, M., Regel, R. E., Meurer, J., Wanner, G., Pakrasi, H. B., Ohad, I., et al. (2003). Effects of selective inactivation of individual genes for low-molecular-mass subunits on the assembly of Photosystem II, as revealed by chloroplast transformation: the psbEFLJ operon in Nicotiana tabacum. Mol. Genet. Genomics 268, 699–710. doi: 10.1007/s00438-002-0791-1
Thompson, L. K., and Brudvig, G. W. (1988). Cytochrome b559 may function to protect Photosystem II from photoinhibition. Biochemistry 27, 6653–6658. doi: 10.1021/bi00418a002
Tichy, M., Lupinkova, L., Sicora, C., Vass, I., Kuvikova, S., Prasil, O., et al. (2003). Synechocystis 6803 mutants expressing distinct forms of the Photosystem II D1 protein from Synechococcus 7942: relationship between the psbA coding region and sensitivity to visible and UV-B radiation. Biochim. Biophys. Acta 1605, 55–66. doi: 10.1016/S0005-2728(03)00064-1
Tiwari, A., and Pospísil, P. (2009). Superoxide oxidase and reductase activity of cytochrome b559 in Photosystem II. Biochim. Biophys. Acta 1787, 985–994. doi: 10.1016/j.bbabio.2009.03.017
Tracewell, C. A., and Brudvig, G. W. (2008). Characterization of the secondary electron-transfer pathway intermediates of Photosystem II containing low-potential cytochrome b559. Photosynth. Res. 98, 189–197. doi: 10.1007/s11120-008-9360-8
Umena, Y., Kawakami, K., Shen, J. R., and Kamiya, N. (2011). Crystal structure of oxygen-evolving Photosystem II at a resolution of 1.9 Å. Nature 473, 55–60. doi: 10.1038/nature09913
Vass, I., and Cser, K. (2009). Janus-faced charge recombinations in Photosystem II photoinhibition. Trends Plant Sci. 14, 200–205. doi: 10.1016/j.tplants.2009.01.009
Vass, I., Styring, S., Hundal, T., Koivuniemi, A., Aro, E., and Andersson, B. (1992). Reversible and irreversible intermediates during photoinhibition of Photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc. Natl. Acad. Sci. U.S.A. 89, 1408–1412. doi: 10.1073/pnas.89.4.1408
Whitmarsh, J., and Pakrasi, H. (1996). “Form and function of cytochrome b559,” in Oxygenic Photosynthesis: The Light Reactions, eds D. Ort and C. Yocum (Dordrecht: Springer), 249–264.
Keywords: photosynthesis, photosystem II, cytochrome b559, site-directed mutagenesis, photoprotection, photoinhibition
Citation: Chu H-A and Chiu Y-F (2016) The Roles of Cytochrome b559 in Assembly and Photoprotection of Photosystem II Revealed by Site-Directed Mutagenesis Studies. Front. Plant Sci. 6:1261. doi: 10.3389/fpls.2015.01261
Received: 03 November 2015; Accepted: 24 December 2015;
Published: 12 January 2016.
Edited by:
Julian Eaton-Rye, University of Otago, New ZealandCopyright © 2016 Chu and Chiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hsiu-An Chu, chuha@gate.sinica.edu.tw
 Hsiu-An Chu
Hsiu-An Chu Yi-Fang Chiu
Yi-Fang Chiu