- 1Department of Horticulture, Zhejiang University, Hangzhou, China
- 2Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Hangzhou, China
Calcium-dependent protein kinases (CDPKs) play critical roles in regulating growth, development and stress response in plants. Information about CDPKs in tomato, however, remains obscure although it is one of the most important model crops in the world. In this study, we performed a bioinformatics analysis of the entire tomato genome and identified 29 CDPK genes. These CDPK genes are found to be located in 12 chromosomes, and could be divided into four groups. Analysis of the gene structure and splicing site reflected high structure conservation within different CDPK gene groups both in the exon-intron pattern and mRNA splicing. Transcripts of most CDPK genes varied with plant organs and developmental stages and their transcripts could be differentially induced by abscisic acid (ABA), brassinosteroids (BRs), methyl jasmonate (MeJA), and salicylic acid (SA), as well as after exposure to heat, cold, and drought, respectively. To our knowledge, this is the first report about the genome-wide analysis of the CDPK gene family in tomato, and the findings obtained offer a clue to the elaborated regulatory role of CDPKs in plant growth, development and stress response in tomato.
Introduction
Plants have evolved different strategies to acclimatize themselves to various challenging environments, including biotic and abiotic stresses. Hereinto calcium (Ca2+) participates in miscellaneous signal transduction pathways as the second messenger (Boudsocq et al., 2012). Calcium sensors or calcium-binding proteins can recognize transient calcium concentration variations, and in turn alter transcript of downstream genes, proteins phosphorylation or enzyme activity (Sanders et al., 1999; Harmon et al., 2000). To date, three classes of calcium sensors have been characterized in plants: calmodulin (CaM) and CaM-like protein (CaML), calcineurin B-like protein (CBL), and calcium-dependent protein kinases (CDPKs). Among these calcium sensors, only CDPKs, depending on their special structure, can directly sense, respond, and translate Ca2+ signals into downstream protein phosphorylation without protein partner conformational change (Cheng et al., 2002; Ludwig et al., 2004; Harper and Harmon, 2005; Poovaiah et al., 2013).
The basal architecture of the CDPK family shows highly conserved in land plant, even in bryophyte and pteridophyte (Hamel et al., 2014). They display a conserved molecular structure with four characterized domains, N-terminal domain, Ser/Thr kinase domain, autoinhibitory junction domain, and calmodulin-like domain (Hrabak et al., 2003). Different species, even different CDPK variants in the same species, have distinguished length of N-terminal domain (Ito et al., 2010), which is associated to protein localization and in vivo phosphorylation (Schulz et al., 2013). The Ser/Thr kinase domain is the catalytic domain with ATP binding site, which plays a central role for the function of CDPK. The intracellular calcium concentrations could significantly modulate CDPK activity (Harper et al., 2004; Boudsocq et al., 2012), sub-cellular localization (Raichaudhuri et al., 2006), and the interactions with other proteins (Ishida et al., 2008; Zou et al., 2010). The autoinhibitory junction domain found in CDPK at CDPK-SnRK superfamily is implied as an autoinhibitor to maintain CDPK inactive or to be activated by stimulation through typical cytosolic calcium signals, which leads CDPK activation as a relieving autoinhibition mechanism (Yang and Poovaiah, 2003; Klimecka and Muszynska, 2007). The C-terminal calmodulin-like domain of CDPKs holds up to four elongation factor (EF) hands, a 29 aa helix-loop-helix structure, which owns 13 conserved residues to sense calcium signals and bind to the free calcium ion (Harmon, 2003). Taken CDPK autoinhibition mechanism as a consideration, a truncated CDPK form, without the autoinhibitory junction domain and calmodulin-like domain exhibits constitutive activity in the absence of calcium (Harper et al., 1994).
Except for calcium signal, other regulatory components, like 14-3-3 proteins (Camoni et al., 1998; Lachaud et al., 2013), phospholipids (Dixit and Jayabaskaran, 2012), and sometimes reversible phosphorylation and/or autophosphorylation (Cheng et al., 2002; Chehab et al., 2004; Hegeman et al., 2006) can also regulate CDPKs activity. On the other hand, CDPKs could interact with and modified downstream targets involved in abiotic tolerance, anti-pathogens response, plant immunity, hormonal signal transduction, plant growth and development, pollen germination and seed development (Boudsocq and Sheen, 2013; Schulz et al., 2013). For example, AtCPK10 is likely to regulate the stomata movement in response to drought through interacting with HSP1 in Arabidopsis (Zou et al., 2010). In Arabidopsis guard cells, AtCPK6/21/23 interacted with AtSLAC1 and participated in abscisic acid (ABA)-induced stomatal closure (Geiger et al., 2010; Brandt et al., 2012). In tomato, LeCDPK2 could modulate the wounding signaling by phosphorylating LeACS2 for controlling the ethylene production (Kamiyoshihara et al., 2010). Recent research has revealed reduplicate roles of CDPKs in nucleotide-binding domain leucine-rich repeat (NLR) immune signaling. AtCPK4/11 could directly phosphorylate AtWRKY8/28/48 in response to pathogen infection while AtCPK4/11 could phosphorylate AtRBOHD and AtRBOHF in the generation of reactive oxygen species (ROS; Gao et al., 2013). Though the big family of CDPK genes showed high functional redundancy and little morphological diversity in single mutants, the anatomical observation of Atcpk28 mutant proved AtCPK28 is involved in plant stem and petiole elongation, and vascular development (Matschi et al., 2013). Recently, AtCPK28 was found to be involved in the regulation of plant immunity through phosphorylating BIK1 and regulating its turnover (Monaghan et al., 2014). Besides, AtCPK17/34 were found to be the pivotal regulators in pollen tube tip growth (Myers et al., 2009). AtCPK12 boosted seeding germination as a negative factor in ABA-signaling, while overexpression of OsCDPK2 in rice plants resulted in disruption of seed development (Morello et al., 2000; Zhao et al., 2011).
As the plant special protein kinases, CDPKs consist of multigene family. There are 34 CDPK genes in Arabidopsis (Arabidopsis thaliana; Cheng et al., 2002; Hrabak et al., 2003), 29 CDPK genes in rice (Oryza sativa; Asano et al., 2005), 20 CDPK genes in wheat (Triticum aestivum; Li et al., 2008), 40 CDPK genes in maize (Zea mays; Kong et al., 2013), 30 CDPK genes in poplar (Populus trichocarpa; Zuo et al., 2013), 17 CDPK genes in grapevine (Vitis vinifera; Chen et al., 2013), 41 CDPK genes in diploid cotton (Gossypium raimondii; Liu et al., 2014), respectively. Based on long time evolution view, CDPK family exhibited highly conserved architecture from bryophytes to higher species (Hamel et al., 2014). Gene duplication is the main reason for the expansion of the CDPK family. Notably evidence shown in Arabidopsis, CPK21/22/23/27/31 are tandem duplicated in Chromosome IV (Cheng et al., 2002). Besides, some duplication specifically occurs in certain species, like AtCPK18 and OsCPK4 verse CPK39/40 in maize (Kong et al., 2013). All land plant CDPK gene family members could be clustered into four groups based on their phylogenetic relationship. Among them, Group IV has smallest amount of CDPK gene members (Cheng et al., 2002; Hrabak et al., 2003; Asano et al., 2005; Li et al., 2008; Chen et al., 2013; Kong et al., 2013; Zuo et al., 2013; Liu et al., 2014). Meanwhile, Group IV is more close to ancient algae CDPK genes as compared to Group I, II, and III (Hamel et al., 2014).
Until now, no systematic genome-wide identification and expression analysis of CDPK family genes have been carried out in vegetable crops and so far, only four CDPK genes, LeCDPK1 (same to SlCDPK18 in this article), LeCPK1 (same to SlCDPK16 in this article), LeCDPK2 (same to SlCDPK4 in this article) and LeCPK2 (same to SlCDPK29 in this article), have been characterized in tomato. These CDPK genes were found to be responsive to wounding, heat stress, and hormones, respectively (Chico et al., 2002; Rutschmann et al., 2002; Chang et al., 2009; Kamiyoshihara et al., 2010). In this regard, the completion of tomato genome sequencing (Sato et al., 2012) will greatly aid us to explore their role in plant growth, development and stress response in tomato.
In this study, we performed bioinformatics analysis of the whole tomato genome and identified 29 CDPK genes. These tomato CDPKs were grouped based on their phylogenetic relationships and were anchored to specific chromosomes. Furthermore, we analyzed the transcript of 28 CDPK genes in various organs at different developmental stages and their response to plant hormones, like ABA, brassinosteroids (BRs), methyl jasmonate (Me-JA), and salicylic acid (SA) as well as to several typical abiotic stresses heat, cold, and drought.
Materials and Methods
Identification of Tomato CDPK Genes
To comprehensively annotate CDPK genes in tomato, all reported 34 Arabidopsis and 29 rice CDPK protein sequences were retrieved from The Arabidopsis Information Resource1 and Rice Genome Annotation Database2. The 63 sequences from Arabidopsis and rice were blasted against the Sol Genomics Network3 and Tomato genome sequencing project4 databases to obtain putative CDPK sequences in tomato. The putative CDPK candidate genes were analyzed with ScanProsite5, Conserved Domain Database6, and Pfam7 for conserved CDPK domains and structure.
Chromosomal Location, Gene Structure, Splicing Sites and Phylogenetic Tree of CDPKs
Calcium-dependent protein kinases from Arabidopsis, rice, and tomato were aligned by Clustal X 2.01 program in default settings (Larkin et al., 2007). Phylogenetic tree was built under MEGA5.03 program using the maximum-like hood method (Tamura et al., 2011). Chromosomal location of CDPKs was determined by the information achieved from Tomato genome sequencing project. Based on the tomato genome information, exon/intron organizations of 29 individual CDPK genes were depicted with the help of Gene Structure Display Server8. Splicing sites were depicted into CDPK protein structure provided by ScanProsite and Pfam according to exon/intron end sites.
Forecast of the Ratios of Ka/Ks in Tomato CDPK Paralogous Gene Pair
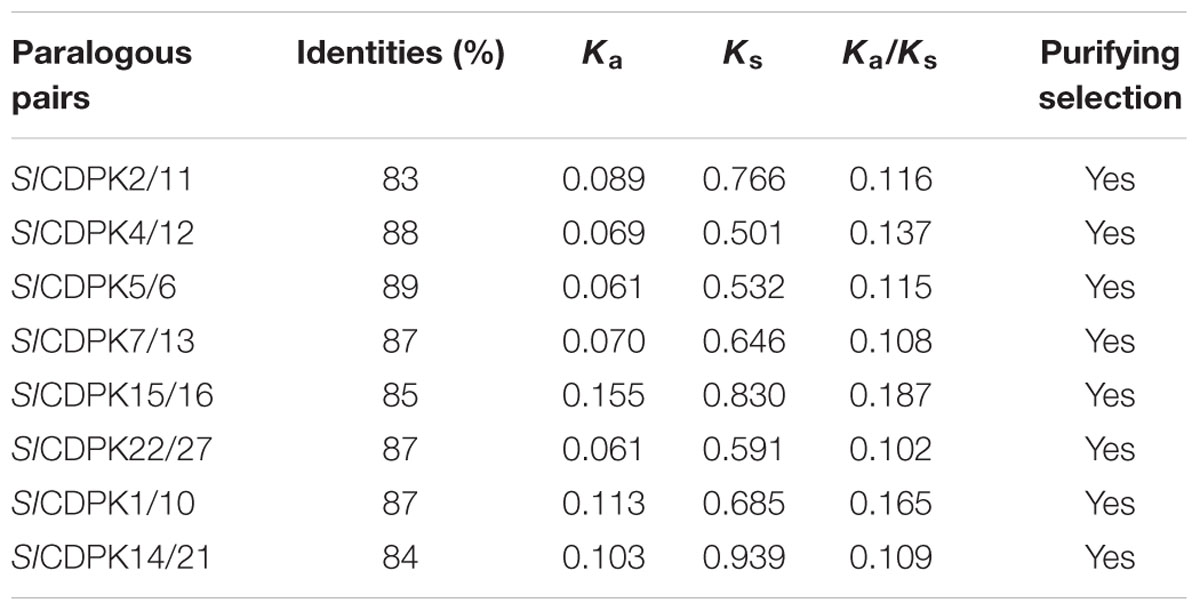
A total of eight pairs of CDPK paralogous genes were selected based on the query coverage and identity values over 80%. Only the tightly linked genes were qualified for one duplication event. The paralogous genes were aligned based on coding sequence with the Clustal W program in MEGA 5.03. The means of DnaSP v5.0 software, non-synonymous substitutions per non-synonymous site (Ka) to the number of synonymous substitutions per synonymous site (Ks) were calculated accordingly (Librado and Rozas, 2009).
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum L. cv. MicroTom) plants were cultured in growth chambers, which were maintained at a 16 h light (200 μmol m-2 s-1) at 25°C and an 8 h dark at 19°C, respectively. For the analysis of the transcripts of CDPK genes, 3-month-old tomato plants were used. Roots, stems, leaves, flowers, and fruits at different developmental stages were collected for RNA extraction.
To analyze the response of CDPK genes to different phytohormones at the transcriptional level, leaves of 8-week-old tomato plants were sprayed with ABA at 100 μM, 24-epibrassinolite (EBR) at 200 nM, Me-JA at 100 μM, SA at 2 mM, and water, respectively. Leaves samples were collected for RNA extraction at 0, 0.5, 1, 3, and 9 h, respectively. Leaves with water treatment at 0 h were used as the control.
To analyze the response of CDPK genes to different abiotic stresses at the transcriptional level, 8-week-old tomato plants were exposed to 45°C (high temperature), 4°C (low temperature), or drought by water withdrawing, respectively. Plants grown at 25°C with well irrigated were used as control. For the high temperature and low temperature treatments, leaves were sampled at 1, 3, and 9 h, respectively, after the treatment. For the drought treatment, leaf samples were taken at 1, 3, and 6 days, respectively.
Total RNA Extraction and Gene Expression by Real-Time Quantitative RCR
Total RNA was isolated from tomato leaves using TRIZOL reagent (Sangon, China) according to the instructions supplied by the manufacturer. After extraction, total RNA was dissolved in diethyl pyrocarbonate-treated water. The cDNA template for real time RT-PCR was synthesized using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan) from 2 μg total RNA.
For quantitative RT-PCR analysis, we amplified PCR products in triplicate using iQ SYBR Green SuperMix (Bio-Rad, Hercules, CA, USA) in 25 μL qRT-PCR reactions. PCR was performed using the iCycleriQ 96-well real-time PCR Detection System (Bio-Rad) and cycling conditions consisted of denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s and extension at 72°C for 30 s. The tomato actin gene was used as an internal control. Gene-specific primers were designed and used for amplification as described in Supplementary Table S1. Relative gene expression was calculated as described by Livak and Schmittgen (2001).
Results and Discussion
Genome-Wide Identification of CDPK Gene Family in Tomato
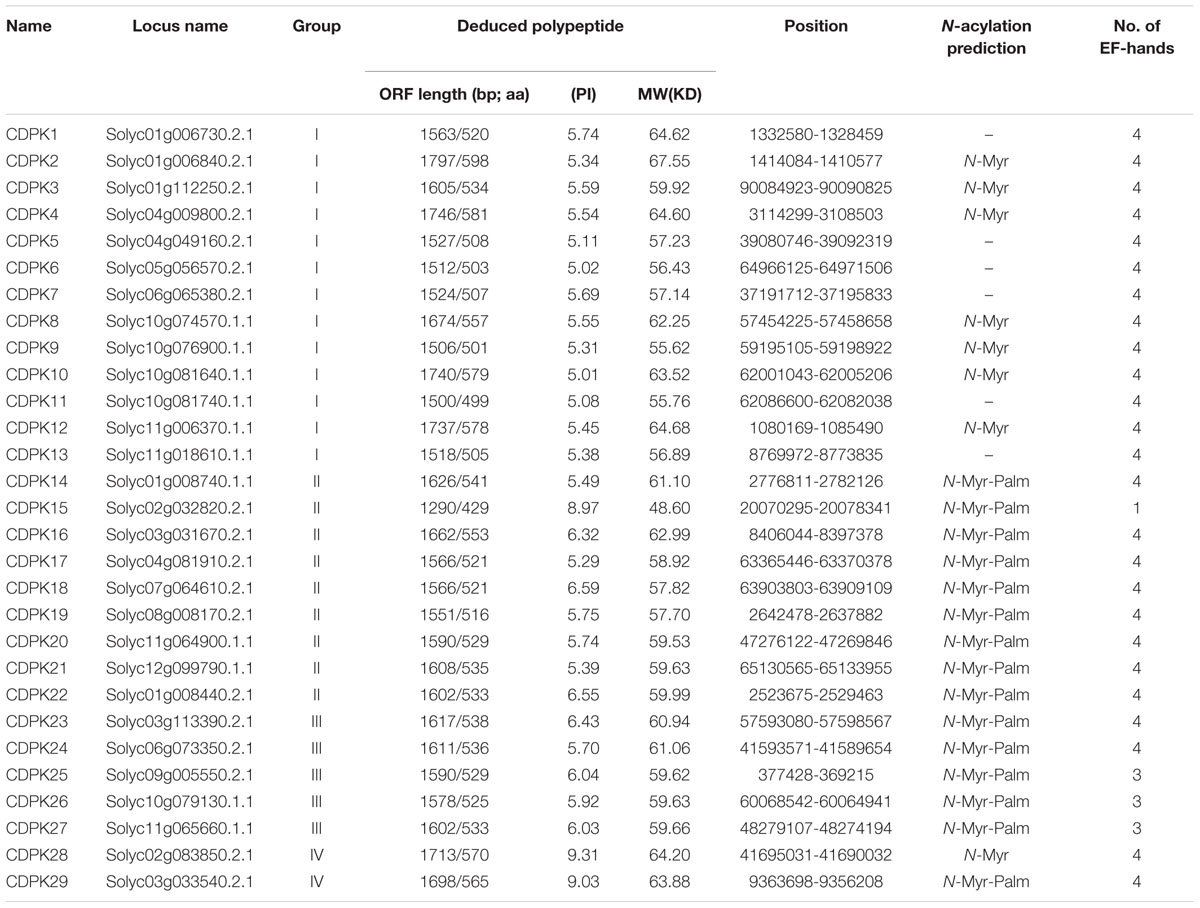
BLAST searches of CDPKs in tomato genome (Tomato Genome Sequencing Project, 2012) were performed against the Arabidopsis, rice and maize CDPK gene sequences. This allowed us to identify 29 putative CDPK genes, designated as SlCDPK1-SlCDPK29 following the proposed nomenclature for CDPK genes (Table 1). All the 29 CDPKs had conserved CDPK domains, a variable N-terminal domain, a Ser/Thr kinase domain, an autoinhibitory junction domain, and a calmodulin-like domain. A variable N-terminal domain was present in all of 29 SlCDPK genes with their open reading frame (ORF) length ranging from 1290 (SlCDPK15) to 1749 (SlCDPK2), which encoded polypeptides in the range of 429 to 598 aa, with predicted protein molecular mass from 48.06 to 67.55 kDa, respectively (Table 1). Interestingly, the isoelectric point of all SlCDPKs tended to be acidic with the exception of 3-EF-hands deficient SlCDPK15 and SlCDPK28/29, which showed isoelectric point alkalinity. Meanwhile, 23 of the 29 SlCDPKs contained predicted myristoylation site and 15 SlCDPKs had putative palmitoylation site (Table 1). Although the N-terminal sequences varied with CDPKs, SlCDPK23-SlCDPK29 all had the myristoylation site, which was similar to those in Arabidopsis and maize (Boudsocq and Sheen, 2013; Kong et al., 2013). As N-myristoylation promotes protein-membrane and protein–protein interaction, while as a second lipid modification, N-palmitoylation can stabilize the interaction between protein and membrane (Martin and Busconi, 2000), these CDPKs may function in many physiological processes by membrane association in plants.
At the C-terminal CaM-like domain, a typical CDPK embraces up to 4 EF hand motifs. A detailed analysis revealed that SlCDPK22/25/26/27 had three EF hand motifs, and others had common four EF hand motifs. However, SlCDPK15 only had one EF hand like AtCPK25, OsCPK6, ZmCPK7, and PtCDPK15. EF hands of CDPKs act as a calcium sensor in calcium-binding affinities (Harper et al., 2004; Harper and Harmon, 2005; Liese and Romeis, 2013). In agreement with this, AtCPK25 was calcium independent owing to lacking of the functional EF hands (Boudsocq et al., 2012). Therefore, these CDPKs like SlCDPK15 might be insensitive to the changes in cellular calcium in plants.
CDPK Gene Chromosomal Distribution and Phylogenetic Analysis in Tomato
The 34 and 29 CDPK genes in Arabidopsis and rice, respectively, were distributed among all 5 and 12 chromosomes of their respective genomes (Cheng et al., 2002; Kong et al., 2013; Zuo et al., 2013; Liu et al., 2014). In silico chromosomal localization of CDPKs revealed that 29 SlCDPK genes were anchored in all of the 12 chromosomes, which implied multigene family functions in tomato plants (Figure 1). Chromosome I and chromosome X of tomato both had up to 5 SlCDPK genes but only one unique SlCDPK gene was distributed in each of chromosome V, chromosome VII, chromosome VIII, chromosome IX, and chromosome XII, respectively. Besides, SlCDPK8/9/26/10/11 and SlCDPK1/2/22/14 were separately clustered on the edge of chromosome I and chromosome X. Interestingly, eight paralogous gene pairs were dispersedly distributed in two different chromosomes. However, no paralogous gene pairs were found in chromosome VII/VIII/IX. All these results suggested that CDPK genes are widely distributed in tomato genomes.
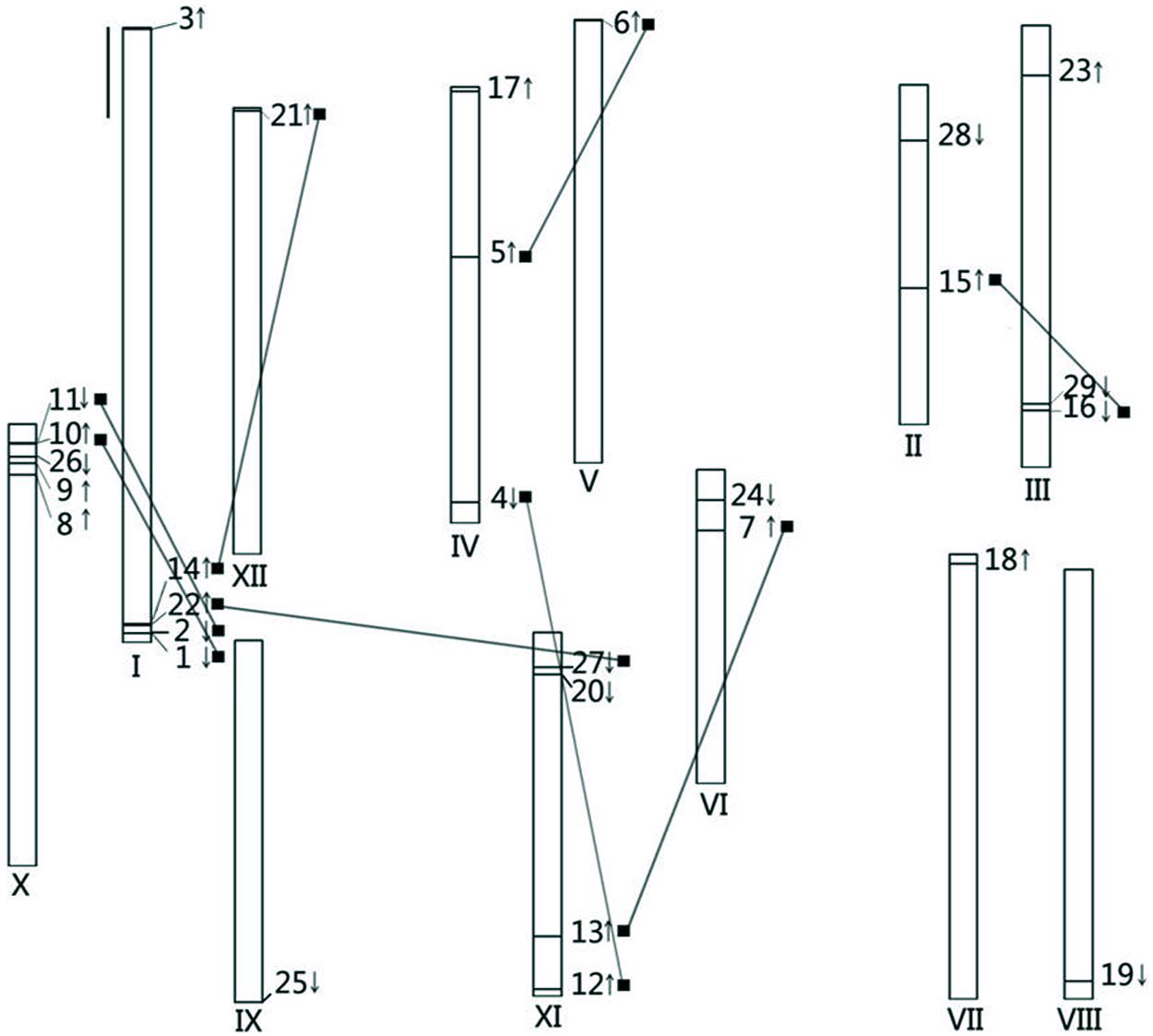
FIGURE 1. Chromosomal distributions of calcium-dependent protein kinases (CDPK) genes in the tomato genome. The roman numerals under each chromosome represent the nomber of the chromosomes. The arrows accompanied with each CDPK gene stand for the gene encoding direction. Each of paralogous gene pair is linked by the black line. The black bar on the left up concern means the length of 10 Mb.
To dissect the evolutionary relationships of CDPK family members, the dicot model plant Arabidopsis, the monocot model plant rice, and tomato CDPK full-length amino acid sequences were used to construct an unrooted tree (Figure 2). The phylogenetic analysis clearly showed all 29 SlCDPKs could be divided into four groups (Group I–Group IV), like that in Arabidopsis and rice CDPKs. Up to 13 tomato CDPKs together with 11 rice CDPKs and 10 Arabidopsis CDPKs belonged to Group I, which made it to be the largest group in tomato CDPK family. Group II contained 7 CDPK members from tomato, 8 from rice, and 13 from Arabidopsis. Meanwhile, 6 SlCDPKs, 8 OsCDPKs, and 8 AtCDPKs were within the family of Group III, respectively. Finally, Group IV was the smallest family, and it only contained 2 tomato CDPKs (SlCDPK28/29), 3 Arabidopsis CDPKs (AtCDPK16, AtCDPK18, and AtCDPK28), and 2 rice CDPKs (OsCDPK4 and OsCDPK18).
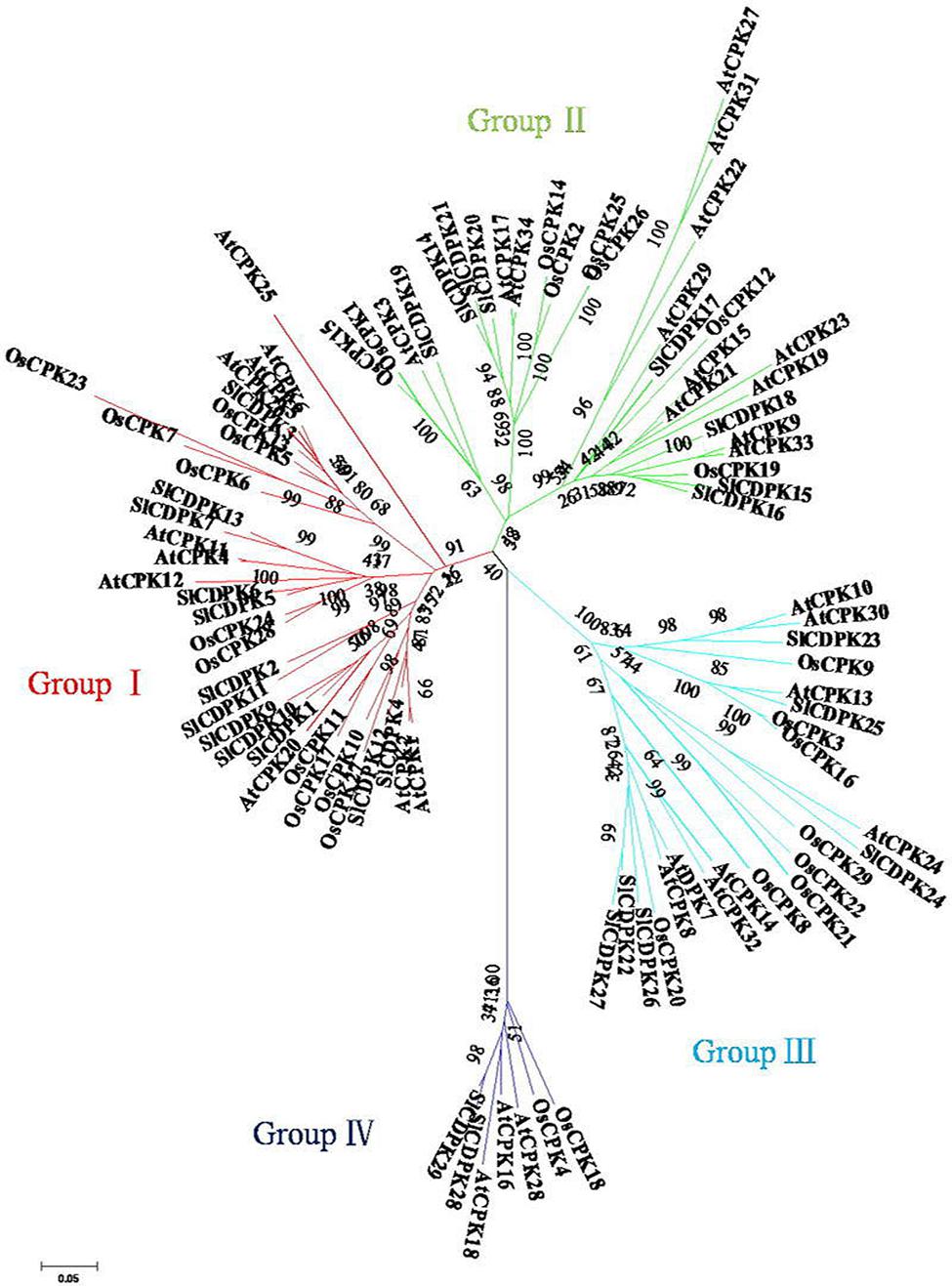
FIGURE 2. Phylogeny and distribution of CDPK proteins from Arabidopsis, rice, and tomato. Methods follow neighbor-joining with 1000 bootstrap in MEGA 5.0 according to full length amino acid sequences of 29 tomato, 34 Arabidopsis, and 29 rice CDPKs.
Based on the phylogenetic map, a total of eight pairs SlCDPK genes were presented as paralogous gene pairs, and all of these paralogous gene pairs showed higher than 80% similarity in nucleotide sequence, indicating an overlap of these CDPKs in function (Table 2). As showed in CDPK chromosomal distribution (Figure 1), all of these eight paralogous gene pair belonged to segmental duplications rather than tandem duplications, suggesting that segmental duplication caused SlCDPK gene family expansion. Furthermore, by calculating the ratio of Ka/Ks, an indicator for the selection history of these paralogous gene pairs (Yang et al., 2006), we found that the Ka/Ks of eight paralogous gene pairs was lower than 0.2, suggesting that these gene pairs had strong purifying selection stress, and made the function of these gene pairs trend to be relative similar.
CDPK Genes Structure and Splicing Sites in Tomato
As the most abundant serine/threonine kinases in plants, CDPK gene family presented high hereditary conservation (Sheen, 1996). The exon-intron structure and splicing site mapping are essential approaches for discovering family gene structure diversity and evolutionary divergence. Based on coding DNA sequence of SlCDPK genes and completed tomato genome sequence, maps of tomato CDPK exon-intron structure (Figure 3) and splicing sites were integratively depicted (Figure 4). In general, the total intron numbers varied from 4 (SlCDPK15) to 11 (SlCDPK28/29) in 29 SlCDPK genes, as observed in rice, maize, and Arabidopsis. Majority of SlCDPK genes from Group I to Group III had six introns in their unsplicing sequences, and even showed the same intron phase. Other extra introns were inserted mainly in four different ways. Firstly, an extra intron was inserted in N-terminal domain of SlCDPK21/24. Secondly, different subarea of kinase domain inSlCDPK1/24, SlCDPK9, and SlCDPK22/26/27 exist another intron. Thirdly, almost all SlCDPK genes from Group II had the extra intron in third EF hand motif expect for SlCDPK15. Finally, SlCDPK7/11 showed a unique intron in other part of CaM-like domain. In spite of the diverse length of introns and different numbers of exons, almost all the SlCDPK genes from Group I to Group III owned six basic exons, which utilized the identical splicing sites at almost the same point, except for structure-deficient SlCDPK15 which lacked three EF-hands in CaM-like domain. Like SlCDPK15, SlCDPK9 was another particular member because of its incomplete kinase domain. Meanwhile, SlCDPK28/29 had totally different gene splicing structures (Figure 4), and the gene structure of Group IV has been reported more similar to CDPK-related kinases (CRK; Zuo et al., 2013), suggesting a high conservation of this family through evolution.
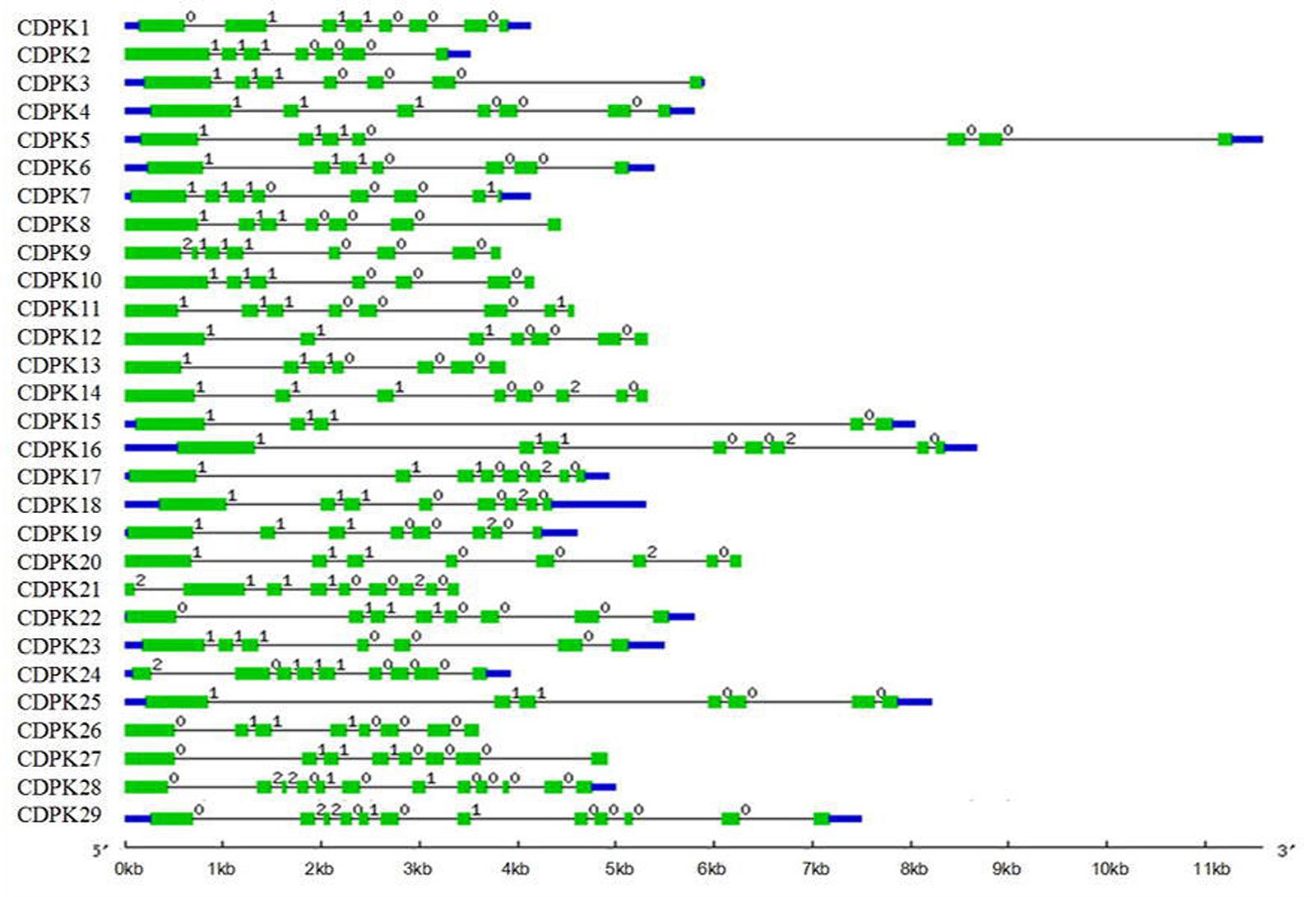
FIGURE 3. Gene structure in tomato CDPK gene family. Blue bars stand for untranslated regions (UTR). One green bars stand for an exon and one black string stand for an intron. The number (0, 1, and 2) above the black strings stand for intron phase.
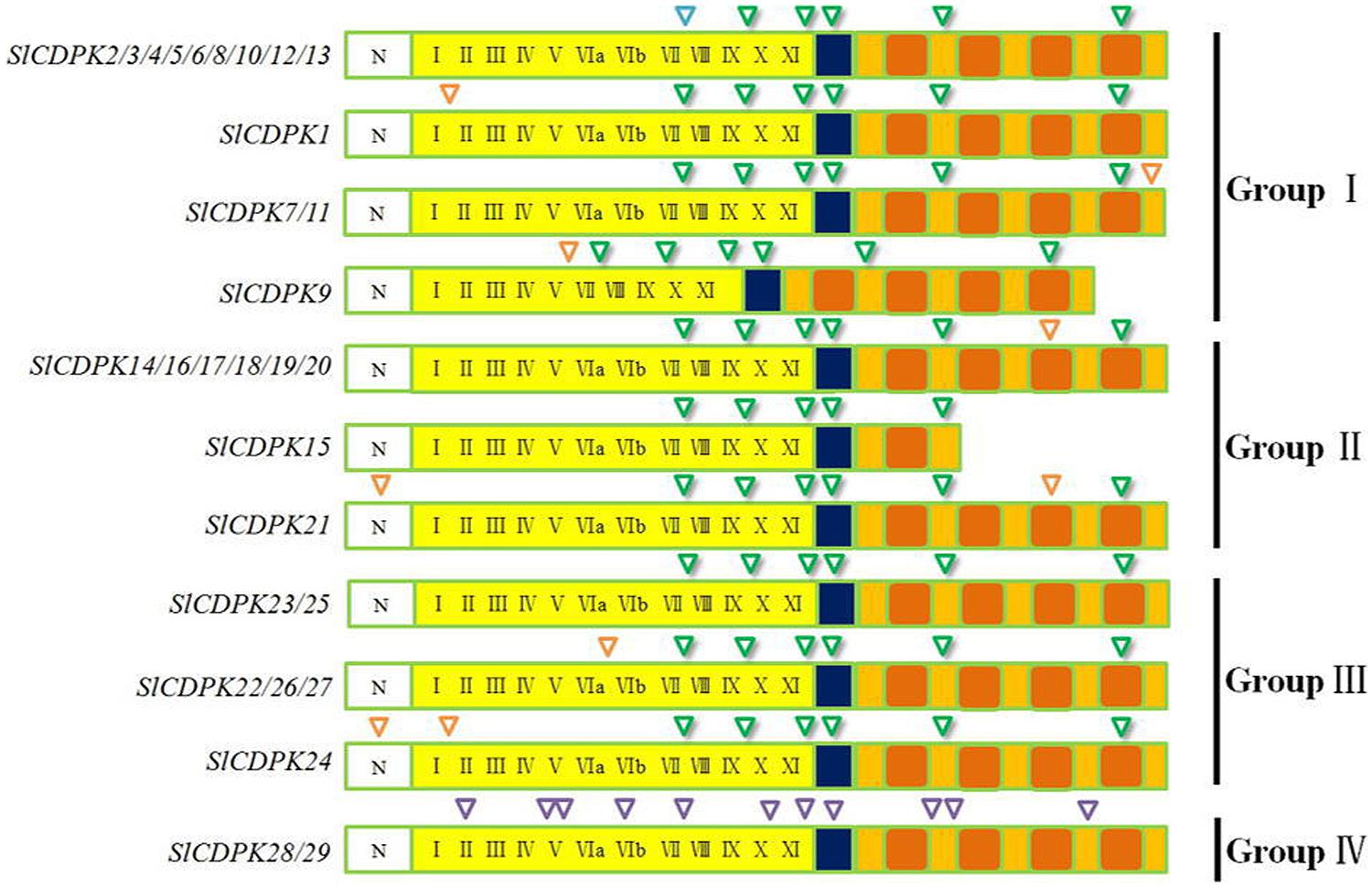
FIGURE 4. Splicing sites of CDPK genes in tomato. Green inverted triangles represent the basic splicing sites in CDPK genes. Orange inverted triangles represent the special splicing sites in CDPK genes. Purples inverted triangles represent the unique splicing sites in Group IV CDPK genes.
Expression of Tomato CDPK Genes in Different Plant Tissues
To characterize CDPK transcript patterns in different organs of tomato plants, we synthesized 28 corresponding primer pairs for 29 tomato CDPK genes except for CDPK20, which was not accurately found (Figure 5). As found in Figure 5, the transcript of most CDPK genes was organ-dependent. In agreement with those observed in poplar and maize (Kong et al., 2013; Zuo et al., 2013), transcript for most CDPK genes was highest in stems, followed by those in red fruits and flowers, respectively. In comparison, senescence leaves, roots and green fruits showed decreased transcripts of most CDPKs as compared to those in mature leaves. It is interesting to note that transcripts of most CDPK genes were down-regulated in senescence leaves as compared to mature leaves whilst transcripts of most these genes were up-regulated in the red fruits. As observed in earlier study, we also found high transcriptional level of SlCDPK29 in flower (Chang et al., 2009).
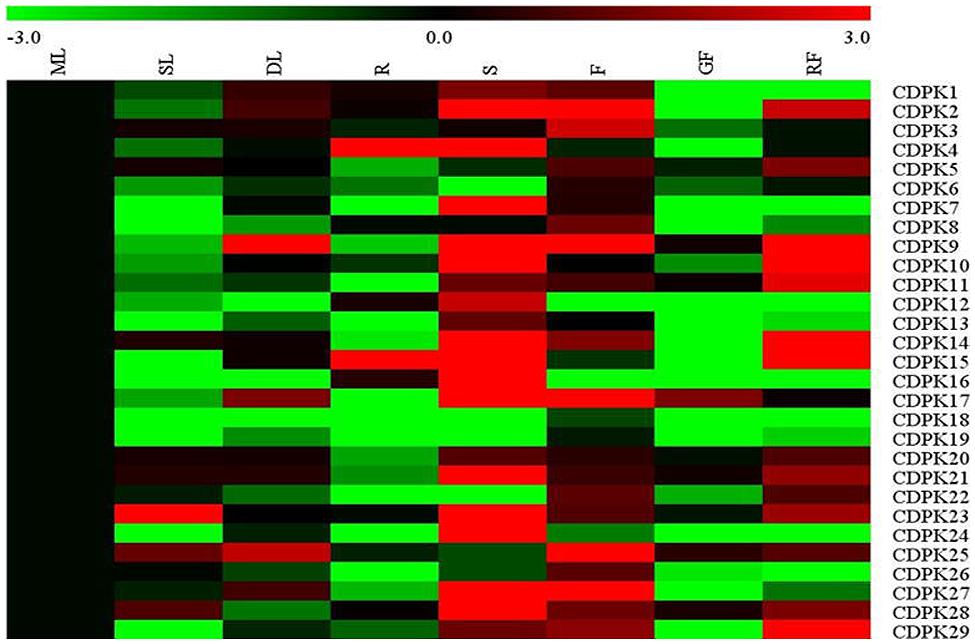
FIGURE 5. Expression profiles of CDPK genes across different tissues in tomato. ML, mature leave; SL, senescence leave; DL, developing leave; R, root; S, stem; F, flower; GF, green fruit; RF, red fruit. The color scale stands for the relative signal intensity values.
CDPK Transcript in Response to Exogenous Phytohormones
Increasing studies have shown the crosstalk between CDPK and phytohormones in plant defense and development processes (Yang and Komatsu, 2000; Ludwig et al., 2005; Zhao et al., 2011; Yang et al., 2012; Ding et al., 2013). Here we found that the transcript of these SlCDPK genes responded differentially to exogenous phytohormones (Figure 6). In response to exogenous ABA, transcripts of an overwhelming majority of SlCDPK genes were up-regulated within 0.5 h and lasted until to 3 h, eventually dropped to the control level in 9 h. It seems likely that transcripts of SlCDPK genes from Group II were more significantly up-regulated in response to ABA which is in agreement with the changes in AtCPK3/23 in Group II of Arabidopsis (Mori et al., 2006; Geiger et al., 2010). However, transcript of SlCDPK7, which is a homolog gene ZmCPK14 functioning as an ABA negative regulator (Kong et al., 2013) in maize, was down-regulated by ABA, at 1 and 3 h. In addition, SlCDPK5/6 kept at a low transcript level after ABA treatment, and their homologous AtCPK12 is known to negatively modulate ABA signaling in seeding germination and growth (Zhao et al., 2011). In Arabidopsis, it has been reported CDPKs were involved in ABA signal pathways by two ABA-associated factors. One of them is ABA-responsive element binding factors (ABFs), such like ABF1 and ABF4, which could be phosphorylated by certain CDPKs, as CPK4 and CPK11, positively participated in CDPK-induced ABA pathways (Zhu et al., 2007). The other showed that suppressed PP2C-type phosphatases like ABI1 and ABI2 protein could activate certain CDPKs-induced stomatal closure after ABA treatment (Geiger et al., 2010). However, the role of these SlCDPKs in ABA signaling remains to be studied in tomato.
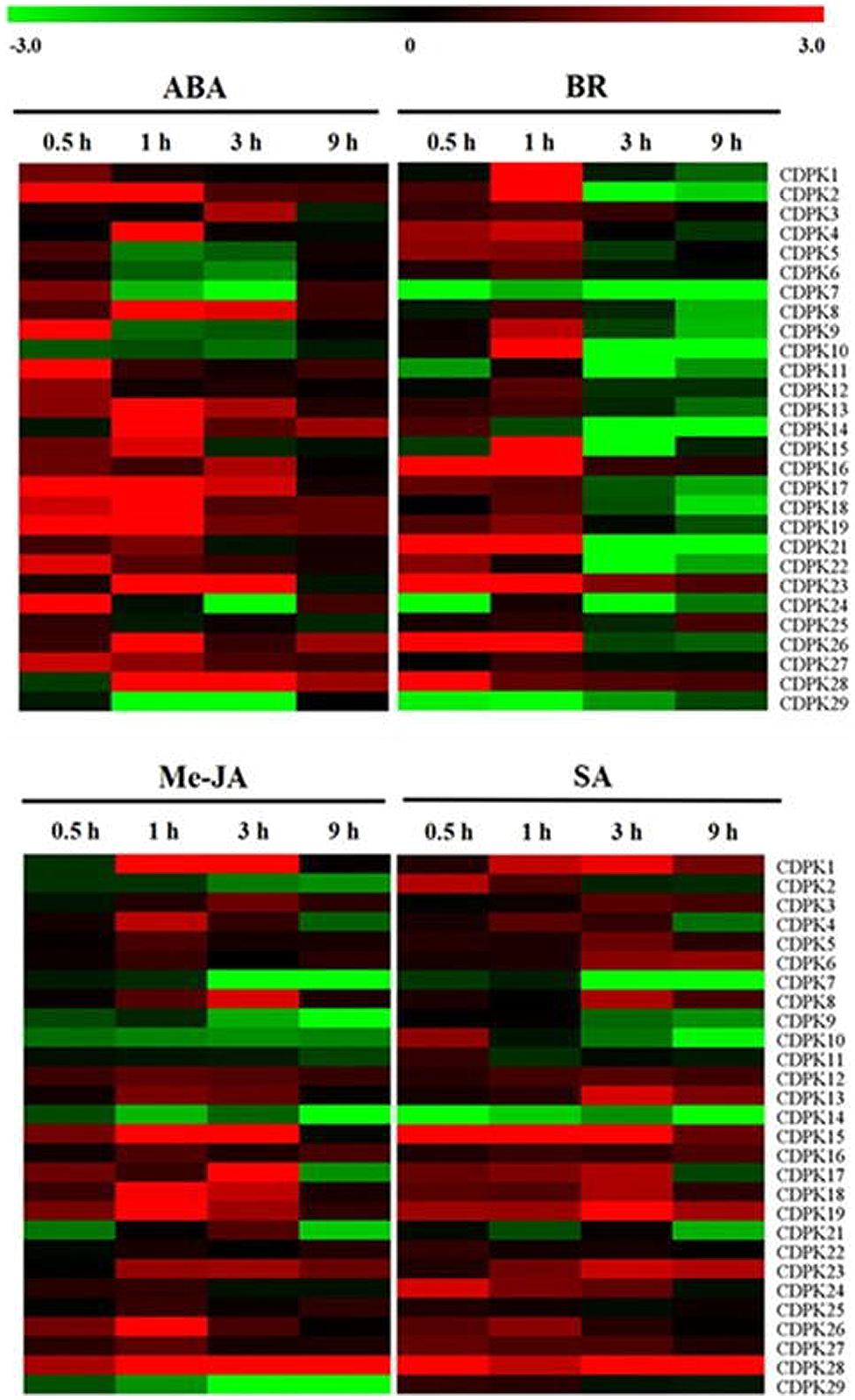
FIGURE 6. Expression analysis of 28 CDPK genes in leaves of tomato in response to phytohormones. Leaves were exposed to ABA at 100 μM, EBR at 200 nM, MeJA at100 μM and SA at 2 mM and sampled at various times for the quantitative real-time RT-PCR analysis. The color scale stands for the relative signal intensity values.
Brassinosteroids (BRs) are noted for having a broad-spectrum of physiological functions; meanwhile crosstalk and interactions of BRs and other hormones seemed to be pervasive in stress response (Choudhary et al., 2012). In our study, lots of SlCDPK genes were stimulated at transcript levels only within 1 h, but suppressed then after foliar application of EBR and this is especially apparent in SlCDPK2, SlCDPK10, SlCDPK15, and SlCDPK21. However, transcripts of SlCDPK6 and SlCDPK27 showed null response to exogenous EBR whist transcript of SlCDPK7 was down-regulated by EBR throughout the times. Until now, little is known about the CDPK in BR signaling although a few studies demonstrated that some CDPKs may play a role as downstream components of the BRs receptor in rice (Yang and Komatsu, 2000; Sharma et al., 2001).
Jasmonate (JA)- and SA-mediated defense pathways were two important defense mechanisms for plant against microorganisms and herbivores, including bacteria, oomycetes, fungi, and insects (Dong, 1998). There are evidences that CDPKs are involved in these responses (Boudsocq and Sheen, 2013). In our study, transcripts of most CDPK genes showed similar response to exogenous SA and JA and both SA and JA induced transcripts of a majority of SlCDPK genes (Figure 6). In rice, overexpression of OsCPK10 or OsCPK20 induced the transcript of SA- and JA-related genes and resistance against Pseudomonas syringae pv. tomato and Magnaporthe grisea (Fu et al., 2013, 2014). Recently, it has been reported Arabidopsis cpk28 mutant regulated development by inducing JA (Matschi et al., 2015). Nevertheless, SlCDPK genes of Group I seemed to be less sensitive to SA and JA as indicated by less induction in their transcript at 1 h as compared to those in other groups. Interestingly, transcripts of SlCDPK7 and SlCDPK14 were downregulated by both SA and JA while transcript of SlCDPK29 was downregulated by SA but not altered by JA. Similarly, transcript of SlCDPK2 was downregulated by SA but induced as soon as 1 h after SA application. The different response of the transcripts of these SlCDPK genes to JA and SA suggested their potential role in the resistance against biotic stress.
Expression Profiles of Tomato CDPK Genes under Abiotic Stress
The role of CDPKs, plant-specific calcium sensor protein kinases, is well known for their involvement in different aspects of plant response to abiotic stresses. Here, transcripts of these SlCDPK genes were analyzed after exposure to heat, cold, and drought stresses, respectively (Figure 7). We found that 13, 11, and 11 SlCDPK genes were induced at the transcriptional level after exposure to heat, cold, and drought stresses, respectively. Significantly, the transcript response of these genes to drought stress was largely similar to their response to ABA treatment, which highly implied the involvement of CDPK-mediated ABA signal pathways in drought response in tomato (Supplementary Figure S1). Among them, SlCDPK21 was the gene with most highly induced by heat and cold treatments while SlCDPK23 was the gene with most highly induced by drought treatment, respectively. Other paralogous genes such as SlCDPK5/6, SlCDPK22/27 were highly induced under high temperature. Interestingly, SlCDPK5/6, presented similar expression pattern under high temperature stress and drought stress, but quite different pattern at low temperature. These results implied that SlCDPK paralogous genes could play a differential role under certain conditions. Thitherto, function of several orthologous CDPK genes in other plant species has been studied. For instance, AtCPK1, orthologous to SlCDPK4/12, could modulate cold tolerance by altering the phosphoproteome in Arabidopsis (Bohmer and Romeis, 2007). Overexpression OsCPK7 in rice and AtCPK6 in Arabidopsis, respectively, which were homologous with drought-induced SlCDPK3 from Group I, resulted in enhanced drought tolerance (Saijo et al., 2000; Xu et al., 2010). There are also evidences that OsCPK9 and AtCPK10, which were clustered as an orthologous of highly drought induced SlCDPK23, participated in ABA-responsive drought tolerance (Zou et al., 2010; Wei et al., 2014). Function elucidation of these CDPK genes in plant growth, development and stress response in tomato is highly desirable in the future.
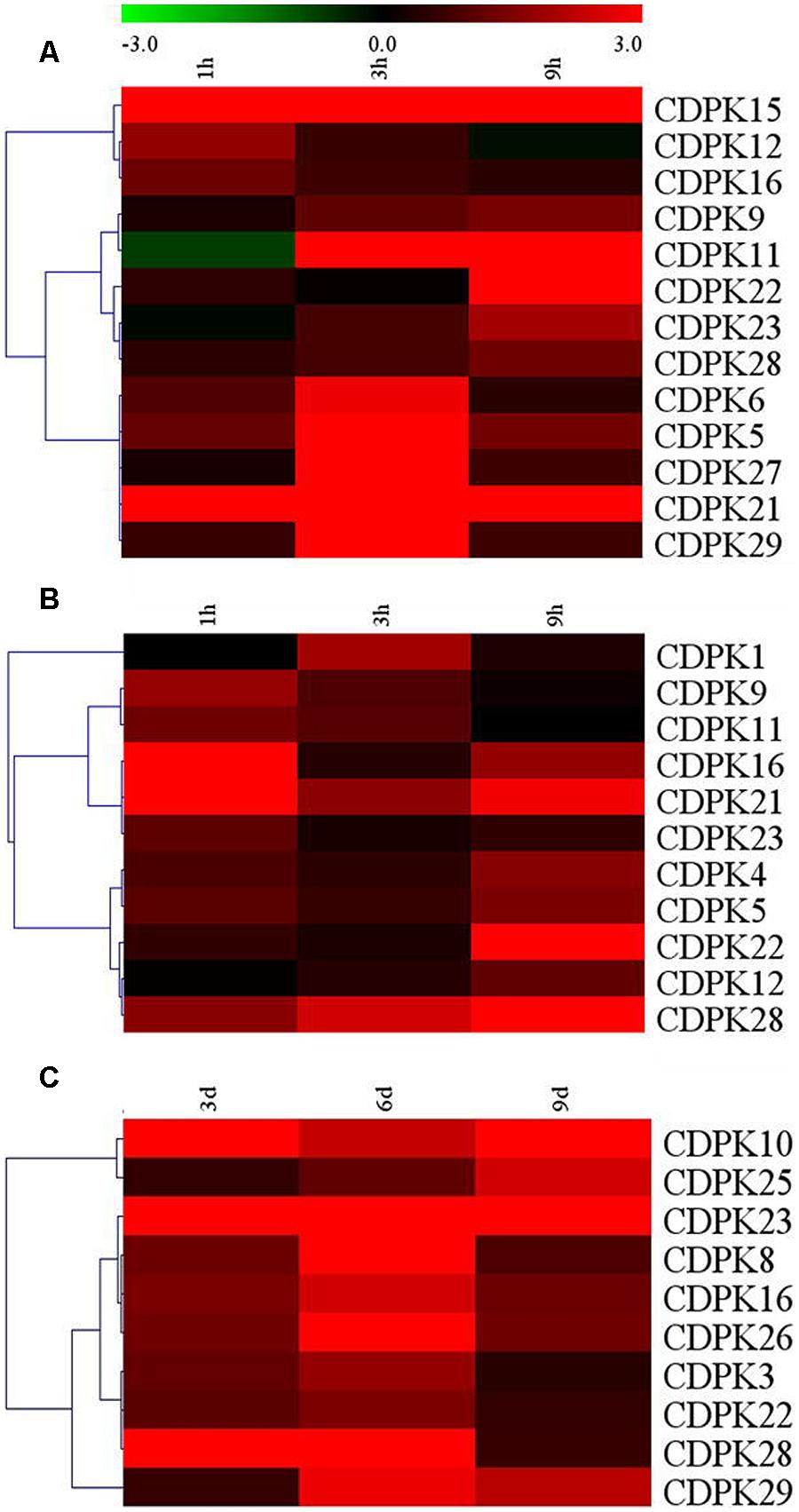
FIGURE 7. Up-regulated expression of selected CDPK genes under different abiotic stress conditions. (A) Upregulated CDPK genes under 45°C; (B) Upregulated CDPK genes under 4°C; (C) Upregulated CDPK genes under drought. The color scale stands for the relative signal intensity values. Hierarchical clustering was deepened on the data analysis.
Conclusion
Based on the tomato genome database, a total 29 SlCDPK genes were identified and categorized into four groups. Each of tomato 12 chromosomes was located with more than one SlCDPK variants, which manifested the conservative of SlCDPK family in long evolution. All SlCDPK genes shared the common protein motifs and the exon-intron structures of each SlCDPK genes group showed highly similarity. In addition to the differential transcript levels in different organs, these SlCDPK genes showed quite different response to phytohormones and environmental stimuli. Like drought, ABA upregulated a dozen of SlCDPK genes in transcript level whilst BRs seemed to have only modest effects on the transcript of these CDPKs. In comparison, SA and JA had similar effects on the transcript of these CDPKs. All these results suggested their diverse roles in growth, development, and stress response. The results presented here would be helpful for the better understanding of the evolutionary relationship of this gene family and their biological functions in plant growth, development, and stress response in tomato species.
Author Contributions
JY and YZ conceived and designed the experiment. ZH and XL performed the experiments. ZH, XX, JZ, and KS carried out the analysis. JY and YZ helped to revise the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was supported by the State Key Program of National Natural Science Foundation of China (31430076), the National Natural Science Foundation of China (31372109, 31272155).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00469
Footnotes
- ^ http://www.arabidopsis.org/
- ^ http://rice.plantbiology.msu.edu/
- ^ http://www.solgenomics.net
- ^ http://mips.helmholtz-muenchen.de/plant/tomato/index.jsp
- ^ http://prosite.expasy.org/scanprosite/
- ^ http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi
- ^ http://pfam.sanger.ac.uk/search
- ^ http://gsds.cbi.pku.edu.cn/
References
Asano, T., Tanaka, N., Yang, G. X., Hayashi, N., and Komatsu, S. (2005). Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 46, 356–366. doi: 10.1093/Pcp/Pci035
Bohmer, M., and Romeis, T. (2007). A chemical-genetic approach to elucidate protein kinase function in planta. Plant Mol. Biol. 65, 817–827. doi: 10.1007/s11103-007-9245-9
Boudsocq, M., Droillard, M. J., Regad, L., and Lauriere, C. (2012). Characterization of Arabidopsis calcium-dependent protein kinases: activated or not by calcium? Biochem. J. 447, 291–299. doi: 10.1042/Bj20112072
Boudsocq, M., and Sheen, J. (2013). CDPKs in immune and stress signaling. Trends Plant Sci. 18, 30–40. doi: 10.1016/j.tplants.2012.08.008
Brandt, B., Brodsky, D. E., Xue, S. W., Negi, J., Iba, K., Kangasjarvi, J., et al. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. U.S.A. 109, 10593–10598. doi: 10.1073/pnas.1116590109
Camoni, L., Harper, J. F., and Palmgren, M. G. (1998). 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett. 430, 381–384. doi: 10.1016/S0014-5793(98)00696-6
Chang, W. J., Su, H. S., Li, W. J., and Zhang, Z. L. (2009). Expression profiling of a novel calcium-dependent protein kinase gene, LeCPK2, from tomato (Solanum lycopersicum) under heat and pathogen-related hormones. Biosci. Biotechnol. Biochem. 73, 2427–2431. doi: 10.1271/Bbb.90385
Chehab, E. W., Patharkar, O. R., Hegeman, A. D., Taybi, T., and Cushman, J. C. (2004). Autophosphorylation and subcellular localization dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiol. 135, 1430–1446. doi: 10.1104/pp.103.035238
Chen, F., Fasoli, M., Tornielli, G. B., Dal Santo, S., Pezzotti, M., Zhang, L., et al. (2013). The evolutionary history and diverse physiological roles of the grapevine calcium-dependent protein kinase gene family. PLoS ONE 8:e80818. doi: 10.1371/journal.pone.0080818
Cheng, S. H., Willmann, M. R., Chen, H. C., and Sheen, J. (2002). Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 129, 469–485. doi: 10.1104/Pp.005645
Chico, J. M., Raices, M., Tellez-Inon, M. T., and Ulloa, R. M. (2002). A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol. 128, 256–270. doi: 10.1104/Pp.010649
Choudhary, S. P., Yu, J. Q., Yamaguchi-Shinozaki, K., Shinozaki, K., and Tran, L. S. P. (2012). Benefits of brassinosteroid crosstalk. Trends Plant Sci. 17, 594–605. doi: 10.1016/j.tplants.2012.05.012
Ding, Y. F., Cao, J. M., Ni, L., Zhu, Y., Zhang, A. Y., Tan, M. P., et al. (2013). ZmCPK11 is involved in abscisic acid-induced antioxidant defence and functions upstream of ZmMPK5 in abscisic acid signalling in maize. J. Exp. Bot. 64, 871–884. doi: 10.1093/Jxb/Ers366
Dixit, A. K., and Jayabaskaran, C. (2012). Phospholipid mediated activation of calcium dependent protein kinase 1 (CaCDPK1) from chickpea: a new paradigm of regulation. PLoS ONE 7:e51591. doi: 10.1371/journal.pone.0051591
Dong, X. (1998). SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1, 316–323. doi: 10.1016/1369-5266(88)80053-0
Fu, L. W., Yu, X. C., and An, C. C. (2013). Overexpression of constitutively active OsCPK10 increases Arabidopsis resistance against Pseudomonas syringae pv. tomato and rice resistance against Magnaporthe grisea. Plant Physiol. Biochem. 73, 202–210. doi: 10.1016/j.plaphy.2013.10.004
Fu, L. W., Yu, X. C., and An, C. C. (2014). OsCPK20 positively regulates Arabidopsis resistance against Pseudomonas syringae pv. tomato and rice resistance against Magnaporthe grisea. Acta Physiol. Plant. 36, 273–282. doi: 10.1007/s11738-013-1408-5
Gao, X. Q., Chen, X., Lin, W. W., Chen, S. X., Lu, D. P., Niu, Y. J., et al. (2013). Bifurcation of arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog. 9:e1003127. doi: 10.1371/journal.ppat.1003127
Geiger, D., Scherzer, S., Mumm, P., Marten, I., Ache, P., Matschi, S., et al. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. U.S.A. 107, 8023–8028. doi: 10.1073/pnas.0912030107
Hamel, L. P., Sheen, J., and Sequin, A. (2014). Ancient signals: comparative genomics of green plant CDPKs. Trends Plant Sci. 19, 79–89. doi: 10.1016/j.tplants.2013.10.009
Harmon, A. C. (2003). Calcium-regulated protein kinases of plants. Gravit. Space Biol. Bull. 16, 83–90.
Harmon, A. C., Gribskov, M., and Harper, J. F. (2000). CDPKs - a kinase for every Ca2+ signal? Trends Plant Sci. 5, 154–159. doi: 10.1016/S1360-1385(00)01577-6
Harper, J. E., Breton, G., and Harmon, A. (2004). Decoding Ca2+ signals through plant protein kinases. Ann. Rev. Plant Biol. 55, 263–288. doi: 10.1146/annurev.arplant.55.031903.141627
Harper, J. F., and Harmon, A. (2005). Plants, symbiosis and parasites: a calcium signalling connection. Nat. Rev. Mol. Cell Biol. 6, 555–566. doi: 10.1038/Nrm1679
Harper, J. F., Huang, J. F., and Lloyd, S. J. (1994). Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33, 7267–7277. doi: 10.1021/bi00189a031
Hegeman, A. D., Rodriguez, M., Han, B. W., Uno, Y., Phillips, G. N., Hrabak, E. M., et al. (2006). A phyloproteomic characterization of in vitro autophosphorylation in calcium-dependent protein kinases. Proteomics 6, 3649–3664. doi: 10.1002/pmic.200500926
Hrabak, E. M., Chan, C. W. M., Gribskov, M., Harper, J. F., Choi, J. H., Halford, N., et al. (2003). The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 132, 666–680. doi: 10.1104/pp.102.011999
Ishida, S., Yuasa, T., Nakata, M., and Takahashi, Y. (2008). A tobacco calcium-dependent protein kinase, CDPK1, regulates the transcription factor REPRESSION OF SHOOT GROWTH in response to gibberellins. Plant Cell 20, 3273–3288. doi: 10.1105/tpc.107.057489
Ito, T., Nakata, M., Fukazawa, J., Ishida, S., and Takahashi, Y. (2010). Alteration of substrate specificity: the variable N-terminal domain of tobacco Ca2+-dependent protein kinase is important for substrate recognition. Plant Cell 22, 1592–1604. doi: 10.1105/tpc.109.073577
Kamiyoshihara, Y., Iwata, M., Fukaya, T., Tatsuki, M., and Mori, H. (2010). Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 64, 140–150. doi: 10.1111/j.1365-313X.2010.04316.x
Klimecka, M., and Muszynska, G. (2007). Structure and functions of plant calcium-dependent protein kinases. Acta Biochim. Pol. 54, 219–233.
Kong, X. P., Lv, W., Jiang, S. S., Zhang, D., Cai, G. H., Pan, J. W., et al. (2013). Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genomics 14:433. doi: 10.1186/1471-2164-14-433
Lachaud, C., Prigent, E., Thuleau, P., Grat, S., Da Silva, D., Briere, C., et al. (2013). 14-3-3-Regulated Ca2+-dependent protein kinase CPK3 is required for sphingolipid-induced cell death in Arabidopsis. Cell Death. Dif. 20, 209–217. doi: 10.1038/Cdd.2012.114
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. doi: 10.1093/bioinformatics/btm404
Li, A. L., Zhu, Y. F., Tan, X. M., Wang, X., Wei, B., Guo, H. Z., et al. (2008). Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Mol. Biol. 66, 429–443. doi: 10.1007/s11103-007-9281-5
Librado, P., and Rozas, J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. doi: 10.1093/bioinformatics/btp187
Liese, A., and Romeis, T. (2013). Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophy. Acta 1833, 1582–1589. doi: 10.1016/j.bbamcr.2012.10.024
Liu, W., Li, W., He, Q., Daud, M. K., Chen, J., and Zhu, S. (2014). Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS ONE 9:e98189. doi: 10.1371/journal.pone.0098189
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ludwig, A. A., Romeis, T., and Jones, J. D. G. (2004). CDPK-mediated signalling pathways: specificity and cross-talk. J. Exp. Bot. 55, 181–188. doi: 10.1093/Jxb/Erh008
Ludwig, A. A., Saitoh, H., Felix, G., Freymark, G., Miersch, O., Wasternack, C., et al. (2005). Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. U.S.A. 102, 10736–10741. doi: 10.1073/pnas.0502954102
Martin, M. L., and Busconi, L. (2000). Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 24, 429–435. doi: 10.1046/j.1365-313x.2000.00889.x
Matschi, S., Hake, K., Herde, M., Hause, B., and Romeis, T. (2015). The calcium-dependent protein kinase CPK28 regulates development by inducing growth phase-specific, spatially restricted alterations in jasmonic acid levels independent of defense responses in Arabidopsis. Plant Cell 27, 591–606. doi: 10.1105/tpc.15.00024
Matschi, S., Werner, S., Schulze, W. X., Legen, J., Hilger, H. H., and Romeis, T. (2013). Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development. Plant J. 73, 883–896. doi: 10.1111/tpj.12090
Monaghan, J., Matschi, S., Shorinola, O., Rovenich, H., Matei, A., Segonzac, C., et al. (2014). The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16, 605–615. doi: 10.1016/j.chom.2014.10.007
Morello, L., Frattini, M., Giani, S., Christou, P., and Breviario, D. (2000). Overexpression of the calcium-dependent protein kinase OsCDPK2 in transgenic rice is repressed by light in leaves and disrupts seed development. Transgenic Res. 9, 453–462. doi: 10.1023/A:1026555021606
Mori, I. C., Murata, Y., Yang, Y., Munemasa, S., Wang, Y. F., Andreoli, S., et al. (2006). CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4:e327. doi: 10.1371/journal.pbio.0040327
Myers, C., Romanowsky, S. M., Barron, Y. D., Garg, S., Azuse, C. L., Curran, A., et al. (2009). Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 59, 528–539. doi: 10.1111/j.1365-313X.2009.03894.x
Poovaiah, B. W., Du, L. Q., Wang, H. Z., and Yang, T. B. (2013). Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant Physiol. 163, 531–542. doi: 10.1104/pp.113.220780
Raichaudhuri, A., Bhattacharyya, R., Chaudhuri, S., Chakrabarti, P., and DasGupta, M. (2006). Domain analysis of a groundnut calcium-dependent protein kinase - Nuclear localization sequence in the junction domain is coupled with nonconsensus calcium binding domains. J. Biol. Chem. 281, 10399–10409. doi: 10.1074/jbc.M511001200
Rutschmann, F., Stalder, U., Piotrowski, M., Oecking, C., and Schaller, A. (2002). LeCPK1, a calcium-dependent protein kinase from tomato. Plasma membrane targeting and biochemical characterization. Plant Physiol. 129, 156–168. doi: 10.1104/Pp.000869
Saijo, Y., Hata, S., Kyozuka, J., Shimamoto, K., and Izui, K. (2000). Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 23, 319–327. doi: 10.1046/j.1365-313x.2000.00787.x
Sanders, D., Brownlee, C., and Harper, J. F. (1999). Communicating with calcium. Plant Cell 11, 691–706. doi: 10.1105/tpc.11.4.691
Sato, S., Tabata, S., Hirakawa, H., Asamizu, E., Shirasawa, K., Isobe, S., et al. (2012). The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641. doi: 10.1038/Nature11119
Schulz, P., Herde, M., and Romeis, T. (2013). Calcium-dependent protein kinases: hubs in plant stress signaling and development. Plant Physiol. 163, 523–530. doi: 10.1104/pp.113.222539
Sharma, A., Matsuoka, M., Tanaka, H., and Komatsu, S. (2001). Antisense inhibition of a BRI1 receptor reveals additional protein kinase signaling components downstream to the perception of brassinosteroids in rice. FEBS Lett. 507, 346–350. doi: 10.1016/S0014-5793(01)03001-0
Sheen, J. (1996). Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274, 1900–1902. doi: 10.1126/science.274.5294.1900
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. doi: 10.1093/molbev/msr121
Wei, S. Y., Hu, W., Deng, X. M., Zhang, Y. Y., Liu, X. D., Zhao, X. D., et al. (2014). A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 14:133. doi: 10.1186/1471-2229-14-133
Xu, J., Tian, Y. S., Peng, R. H., Xiong, A. S., Zhu, B., Jin, X. F., et al. (2010). AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 231, 1251–1260. doi: 10.1007/s00425-010-1122-0
Yang, D. H., Hettenhausen, C., Baldwin, I. T., and Wu, J. Q. (2012). Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound- and herbivory-induced jasmonic acid accumulations. Plant Physiol. 159, 1591–1607. doi: 10.1104/pp.112.199018
Yang, G. X., and Komatsu, S. (2000). Involvement of calcium-dependent protein kinase in rice (Oryza sativa L.) lamina inclination caused by brassinolide. Plant Cell Physiol. 41, 1243–1250. doi: 10.1093/Pcp/Pcd050
Yang, T. B., and Poovaiah, B. W. (2003). Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 8, 505–512. doi: 10.1016/j.tplants.2003.09.004
Yang, X., Tuskan, G. A., and Cheng, M. Z. (2006). Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant Physiol. 142, 820–830. doi: 10.1104/pp.106.083642
Zhao, R., Sun, H. L., Mei, C., Wang, X. J., Yan, L., Liu, R., et al. (2011). The Arabidopsis Ca2+-dependent protein kinase CPK12 negatively regulates abscisic acid signaling in seed germination and post-germination growth. New Phytol. 192, 61–73. doi: 10.1111/j.1469-8137.2011.03793.x
Zhu, S. Y., Yu, X. C., Wang, X. J., Zhao, R., Li, Y., Fan, R. C., et al. (2007). Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19, 3019–3036. doi: 10.1105/tpc.107.050666
Zou, J. J., Wei, F. J., Wang, C., Wu, J. J., Ratnasekera, D., Liu, W. X., et al. (2010). Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 154, 1232–1243. doi: 10.1104/pp.110.157545
Keywords: CDPK, environmental stimuli, gene family, phytohormone, Solanum lycopersicum, transcript
Citation: Hu Z, Lv X, Xia X, Zhou J, Shi K, Yu J and Zhou Y (2016) Genome-Wide Identification and Expression Analysis of Calcium-dependent Protein Kinase in Tomato. Front. Plant Sci. 7:469. doi: 10.3389/fpls.2016.00469
Received: 21 December 2015; Accepted: 24 March 2016;
Published: 08 April 2016.
Edited by:
Shabir Hussain Wani, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, IndiaReviewed by:
Panagiotis Kalaitzis, Mediterranean Agronomic Institute of Chania, GreeceYing Li, Nanjing Agricultural University, China
Copyright © 2016 Hu, Lv, Xia, Zhou, Shi, Yu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhong Zhou, yanhongzhou@zju.edu.cn
 Zhangjian Hu1
Zhangjian Hu1 Jie Zhou
Jie Zhou Kai Shi
Kai Shi Jingquan Yu
Jingquan Yu Yanhong Zhou
Yanhong Zhou