- 1Department of Silviculture and Genetics, Forest Research Institute in Poland, Sekocin Stary, Poland
- 2Department of Ecology and Biogeography, Faculty of Biology and Environmental Protection, Nicolaus Copernicus University, Toruń, Poland
- 3Biometry Division, Department of Econometrics and Statistics, Faculty of Applied Informatics and Mathematics, Warsaw University of Life Sciences, Warsaw, Poland
- 4Department of Life Sciences, Centre for Functional Ecology, University of Coimbra, Coimbra, Portugal
Intra-annual density fluctuations (IADFs) can imprint environmental conditions within the growing season and most of the research on IADFs has been focused on their climatic signal. However, to our knowledge, the genetic influence on the frequency and type of IADFs has not been evaluated. To understand if the genotype can affect the formation of IADFs we have used a common garden experiment using eight families of Larix decidua established in two neighboring forest stands in northern Poland. Four types of IADFs were identified using X-ray density profiles: latewood-like cells within earlywood (IADF-type E), latewood-like cells in the transition from early- to latewood (IADF type E+), earlywood-like cells within latewood (IADF-type L), and earlywood-like cells in the border zone between the previous and present annual ring (IADF-type L+). The influence of explanatory variables i.e., families, sites, and years on identified density fluctuations was analyzed using generalized estimating equations (GEE). We hypothesized that trees from different families will differ in terms of frequency and type of IADFs because each family will react to precipitation and temperature in a different way, depending on the origin of those trees. The most frequent fluctuation was E+ and L types on both sites. The most important factors in the formation of IADFs were the site and year, the last one reflecting the variable climatic conditions, with no significant effect of the family. However, the relation between the formation of IADFs and selected climate parameters was different between families. Although, our results did not give a significant effect of the genotype on the formation of IADFs, the different sensitivity to climatic parameters among different families indicate that there is a genetic influence.
Introduction
Radial growth of trees reflects the interactions between external (environmental) and internal factors (physiological processes determined in relation to genotype; Savva et al., 2002). The influence of environment (mainly climatic) has been very well-documented in numerous studies (Wimmer et al., 2000; Rigling et al., 2001; Masiokas and Villalba, 2004). Much work has also addressed the influence that biotic and abiotic environmental variables exert in generating anomalies in the course of xylogenesis (Dmuchowski et al., 1997; Eilmann et al., 2013; Vieira et al., 2015).
Trees' plasticity expressed in terms of stress-induced growth reactions is known to differ in line with the expression of the genotype determining the physiological processes (López-Maury et al., 2008). Differences in the radial reaction are most probably dependent on trees' degrees of relatedness at the level of the species, provenance, or genotype. The few long-term provenance experiments that have been carried out confirm inter-population variability of the radial reaction as expressed in terms of tree ring width (McLane et al., 2011; Kalliokoski et al., 2012; Wilczyński and Kulej, 2013).
The temperature increase and precipitation anomalies observed in recent years, assuming the form of droughts, heatwaves, and heavy rainfall (Lindner et al., 2014), also finds reflection in the anatomical structure of annual rings (Masiokas and Villalba, 2004; Rozas et al., 2011; Seo et al., 2011). The typical anatomical structure, with a clear distinction between early- and latewood, can be modified in a growing season with climatic conditions considered non-typical (Wimmer, 2002). In this sense, latewood-like cells can appear within earlywood, accounted for as a physiological reaction of plants to stress associated with a shortage of water, to prevent risks of cell embolism (Battipaglia et al., 2014). On the other hand, at the end of a growing season, which has featured a water shortage, the arrival of precipitation can lead to the appearance of modifications in the anatomical structure of latewood cells, with the formation of earlywood-like cells (Vieira et al., 2015). These types of modifications are classified as intra-annual density fluctuations (IADFs). Campelo et al. (2013) proposed a classification of four types of IADFs based on their location within the tree-ring: IADF-type E, with latewood-like cells within earlywood; IADF-type E+, representing a smooth transition between early- and latewood cells; IADF-type L, with earlywood-like cells within latewood; and IADFs-type L+, with earlywood-like cells between the end of the latewood and the earlywood of the next tree ring. This classification for wood density fluctuations has been used for the purposes of the work described here.
Previous attempts to explain differences in trees' reactions to climatic anomalies, as manifested in the development of density fluctuations in wood, have concentrated on the aspect of the variability characterizing trees differing in terms of size or age (Vieira et al., 2009; Campelo et al., 2013, 2015; Novak et al., 2013), or aspects reflecting intraspecific competition (biosocial position or growth rate; Copenheaver et al., 2006). Differences in the radial-growth reaction relating to soil-moisture conditions have also been investigated (Battipaglia et al., 2010; de Luis et al., 2011). Schweingruber (1980) emphasized that wide rings have more fluctuations than narrow ones. According to this, we can assume that climate conditions favoring growth can also affect the frequency of density fluctuations. The use of IADFs for environmental studies in different parts of Europe were published recently by Battipaglia et al. (2016). However, few works have taken into account the genotype effect on the formation of IADFs. It is known that the wood density of annual rings and its component elements (i.e., density of earlywood and latewood) are strongly determined genetically, with a high degree of heritability (Pâques, 2004; Fujimoto et al., 2008; Klisz, 2011). In conditions extremely unfavorable to growth (a water shortage induced by drought and high temperatures), it is possible to observe a high level of genetic control of the density profiles of annual rings, including the location of peak density within earlywood or the transition zone (Rozenberg et al., 2002). This findings support the idea that the frequency of occurrence of the different types of density fluctuation in wood might also be determined by a genetic factor.
The aim of this work was to determine the influence of the origin of trees on the frequency of different types of IADFs. We have used a common garden experiment (seed orchard) using half-sib families of Larix decidua established in two neighboring forest stands in northern Poland since 1985. We hypothesized that trees from different families will differ in terms of frequency and type of IADFs because each family will react to precipitation and temperature in a different way, depending on the origin of those trees.
Materials and Methods
Study Site and Samples
Two study areas (even-aged seed orchards) were established in 1985 in northern Poland, within the Gdańsk Coast (Pobrzeże Gdańskie) Macroregion, under similar site conditions within the Forest Districts of Młynary (fresh broadleaved forest; N: 54°13′E: 19°54′; 55 m a.s.l.) and Zaporowo (fresh mixed/broadleaved forest; N: 54°24′E: 19°51′; 10 m a.s.l.). The climate is temperate with a mean temperature of 7.6°C and annual precipitation of 742 mm, mostly distributed in June–November [climate data from European Climate Assessment & Dataset (ECA&D) project, weather station in Elblag and Kaliningrad, reference period 1948–2014; (Klein Tank et al., 2002)]. Site condition characterized sandy clay soil in Mlynary and brown earth soil in Zaporowo. Both of the seed orchards were laid out in line with a randomized complete block design represented by the progeny of 33 plus trees of European larch. The 33 families in turn represented four seed zones for the European larch in northern Poland. i.e., nos. 103, 106, 157, and 205 (Figure 1). Twenty-five families of European larch were represented by at least 20 specimens at the two experimental sites. Based on the microdensity profiles of these 25 families, eight wood density classes were established. Afterwards, a selection of eight families was made to study the frequency of the different types of IADFs (Klisz, 2011). The criteria to select eight families among the 25 were based on the representation of each wood density class. At each experimental site (seed orchard), at least 20 sample trees representative of each of the eight families of European larch were selected. A total of 188 trees were selected at the Młynary site, and 201 at Zaporowo. In 2007, by means of Pressler borer, two cores of 5 mm diameter were taken from each tree at a height of 1.3 m above the ground, from the eastern and southern directions.
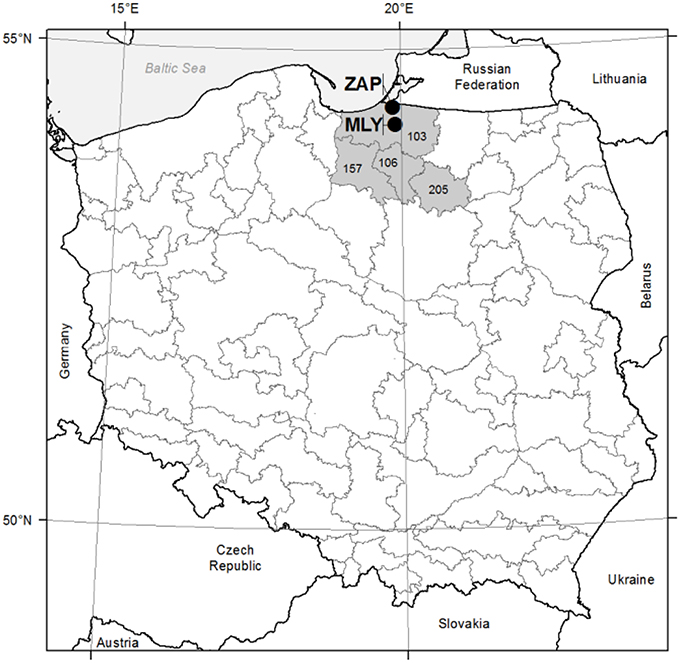
Figure 1. Study sites location. MLY—Młynary experimental site; ZAP—Zaporowo experimental site; 103, 106, 157, 205—seed regions. Gray line—seed regions boarders.
Wood Properties
Each wood sample was subject to the standard preparatory procedure for X-ray scanning. Uniformity of wood-sample width was achieved by way of lengthwise cuts using two circular saws (Larsson et al., 1994). Resin extraction was carried out in distilled water (Grabner et al., 2005), while stabilization of sample humidity levels at 15% was achieved in the Itrax Scanner density scanner. To obtain each of the required microdensity profiles, samples of wood were X-ray scanned using the Itrax Scanner at constant parameters: voltage 35 V, exposure 50 ms and resolution 25 μm. The exact procedure for X-ray scanning of wood samples using an Itrax Density Scanner (Cox Analytical Systems) is described in Lindeberg (2004), Bergsten et al. (2001), and Fries and Ericsson (2006). The images obtained were analyzed using the WinDENDRO 2009b program to establish the density profiles. To define the limits of earlywood and latewood, a constant value of 70% of the maximum density of wood in the given annual ring was adopted. This was the value used in comparisons with sample material—for European larch, and with samples of Scots pine (constant value for 50% of maximum wood density in a given annual ring), as analyzed using the same method at the same laboratory, by Fries and Ericsson (2009). The tree-rings analyzed covered the period from 1990 to 2006. All sample trees had the same cambial age.
Determination of Different Types of IADF
In line with the locations of density fluctuations in annual rings, Campelo et al. (2013) identified four types of fluctuations. Our study improves on this classification, incorporating measured values for wood density. As a limit value allowing the occurrence of fluctuations to be identified, we adopted a microdensity local maximum >0.1 g/cm3 of the average early or latewood (Figure 2). The classification was then into E—high values for density in earlywood; E+—high values for wood density in the transition zone between earlywood and latewood; L—low values for density in latewood; and L+—low values for density in the latewood adjacent to the next annual ring. Determination of IADF occurrence was done for each core separately, and then the frequency value (present, absence) for each pair of cores was verified in order to avoid data duplication.
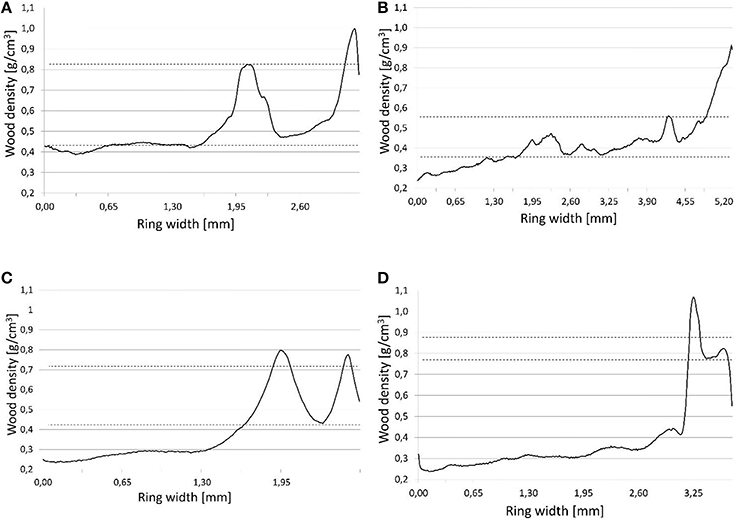
Figure 2. IADFs types determination based on microdensity profiles. (A) IADF E, (B) IADF E+, (C) IADF L, (D) IADF L+. Graphs (A) and (B): Upper dashed line—IADFs maximum density, lower dashed line—average earlywood density. Graphs (C) and (D): Upper dashed line—average latewood density, lower dashed line—IADFs minimum density.
Statistical Analysis
The Impact of the Environment and Genotype on IADF Frequency
To determine the influence of provenances of the studied trees, site conditions, and years (the quality variables) on the presence of different types of IADF (the binary presence-absence dependent variable) we have used generalized estimating equations methodology (GEE). Proposed by Liang and Zeger (1986) as an extension of GzLM generalized linear models (Nelder and Weddenburn, 1972), this allows for the analysis of correlated data (Stokes et al., 2000). Use is made of a model assuming the form:
where g(μijk) is the logit link function, μijk is the mean for the ijth site × family combination in the kth year, Si is the effect of the ith site (i = 1, 2), Fj is the effect of the jth family (j = 1, …, 8), S × Fij is site × family interaction effect, and Yk is the kth repeated measures year effect (k = 1, …, 17). The significance of model effects was tested using Wald statistics for the type 3 analysis. Decomposition of variance (DOV) was performed to further quantify how much variance in the predicted IADF can be attributed to site, family, site × family interaction, and year effects (Huang et al., 2014). The contrast analysis was in turn applied for comprehensive comparisons of significant model effects. Differences between tested families in the IADFs frequencies were shown on diffograms (mean-mean scatter plot). The analyses were carried out using SAS 9.3 software (SAS Institute Inc, 2011), and the GENMOD procedure (Stokes et al., 2000) was followed.
Relationships between Climate and IADF
To investigate climate-IADF relationships, a GzLM model was applied using the MASS package (Author: Brian Ripley) from R (R Development Core Team, 2007). Climate data from two meteorological stations (Elblag and Kaliningrad) were obtained from the European Climate Assessment & Dataset (ECA&D) project (Klein Tank et al., 2002). For the purposes of the study, a dependent relationship between earlywood and climate data from March to June was assumed, as well as between latewood and the climate between May and September. The most parsimonious model according to AIC criterion was applied to the different IADF types, for each family and site (Akaike, 1974). A chi-square test for the type 3 analyses was applied to check for the significance of relationships. On the basis of Wald estimates a percent change in the odds of the occurrence of IADF for each 1-unit increase of climatic variables was determined (Allison, 2012).
Results
IADF Frequency
At the Młynary and Zaporowo site, 9.1 and 11.1% of all rings showed the occurrence of IADFs, respectively (Table 1). Taking into account all tree-rings with IADFs, the dominant type at both experimental sites was type E+ accounting for 44.8% in the case of Młynary, and 42.6% in the case of Zaporowo. The second most frequently identified fluctuation at both sites was type L (35.8% in Młynary and 31.8% in Zaporowo). Taken together, the remaining two types of IADFs (E and L+) accounted for just 19.2% of fluctuations in trees from Młynary, and 26% of those recorded in trees from Zaporowo (Table 1).
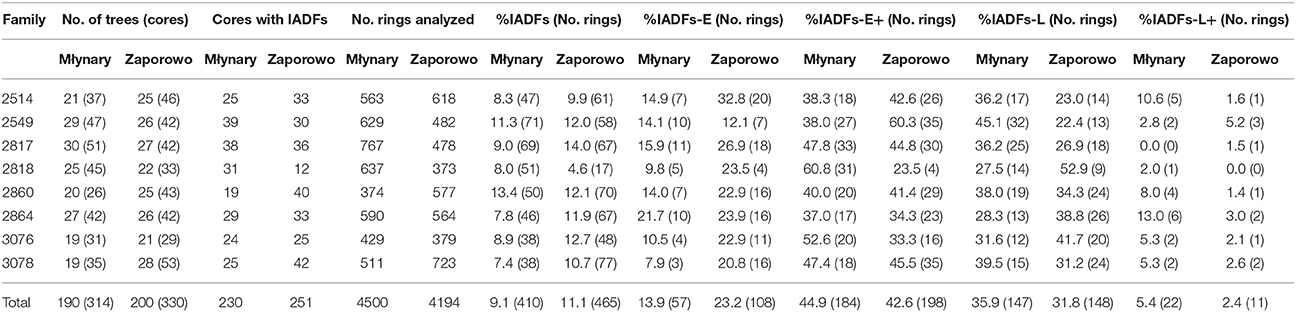
Table 1. Descriptive statistic of the intra-annual density fluctuations of Larix decidua from different families and growing in two sites in Northern Poland.
From 1990 to 2006, trees at the Młynary site showed IADFs every year, while in Zaporowo no IADFs were observed in 1992 (Figure 3). At the Młynary site, the highest frequencies occurred in 1996, 1998, and 2000, with a high percentage of IADF type E+, accounting for 59.6, 68.9, and 38.9%, respectively (Figure 3). In turn, the highest frequency of IADFs observed in Zaporowo occurred in 1997 and 2003 dominated by IADF type L and type E+, respectively (Figure 3). At Młynary the highest frequencies of IADFs type L occurred in the years 1991, 1992, 1995, 2002, 2005, and 2006, while at Zaporowo, occurred in 1994, 1996, 1997, 2002, and 2004. The IADFs type L+ occurred in 1992 and 1994 at the Młynary, and in 1994 at Zaporowo.
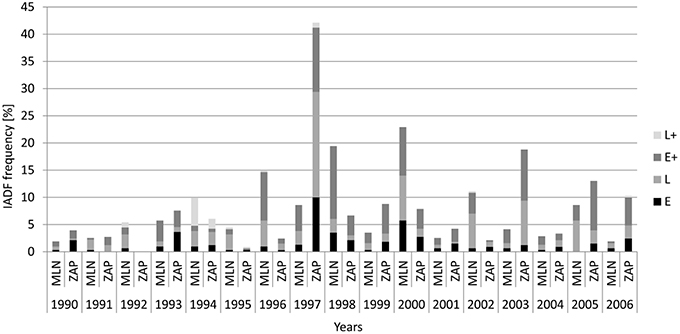
Figure 3. Frequency of the different types of IADFs in relation to calendar years in the Młynary (MLN) and Zaporowo (ZAP) Forest Districts.
IADFs vs. Site, Family, and Year
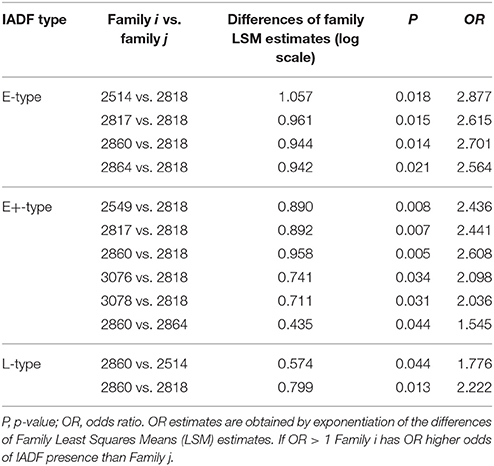
GEE analysis allowed the identification of the significant factors (site, family, year, and their interactions) determining the frequency of the different types of IADFs. The most significant factors determining the formation of IADFs type E were the site and year, with P = 0.001 and P < 0.001, respectively (Table 2). Although, the families did not have a significant impact in the formation of IADFs type E (P = 0.099), it was possible to identify pairs of families that differed significantly in the frequency of IADFs type E. Family 2818 was found to differ significantly from families 2514, 2817, 2860, and 2864 (P = 0.018, 0.015, 0.014, and 0.021, respectively; Table 3, Figure 4).
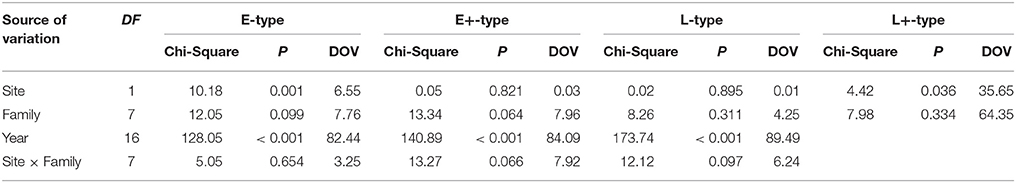
Table 2. Wald statistic for type 3 GEE analysis, the significance of the models' effects and the decomposition of variance DOV (%) for different IADFs' types.
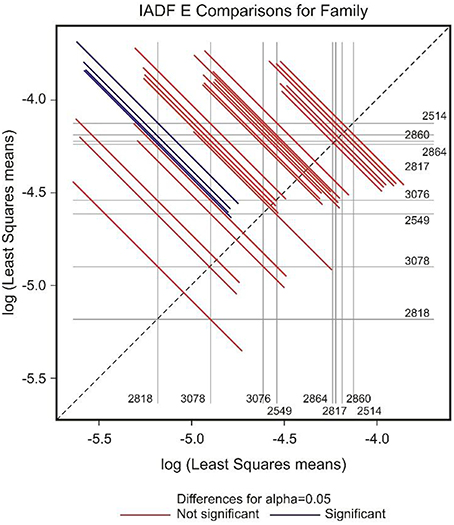
Figure 4. Diffogram of differences in the IADF-type E frequencies between the European larch families. The point of intersection of the horizontal and vertical line emanating from both axes for the two families represents the value of their difference (in log scale) and segments on both sides of the crossing point show the confidence interval for this difference. The difference between pair of families is significant when lower and upper endpoints of the confidence interval are either positive or negative i.e., they both are above or below the dotted line of equality.
In the case of IADFs type E+, the GEE analysis revealed that the only significant factor was associated with the calendar year (P < 0.001; Table 2). Nonetheless it was possible to distinguish pairs of families differing significantly in the frequency of IADFs type E+. Family 2818 showed a lower frequency of IADFs type E+ compared with 2549, 2817, 2860, 3076, and 3078 (Table 3, Figure 5). Additionally, family 2860 showed a higher frequency of IADFs type E+ compared with family 2864.
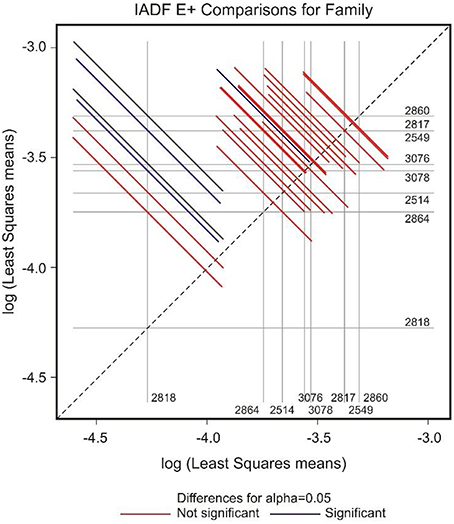
Figure 5. Diffogram of differences in the IADF-type E+ frequencies between the European larch families. The point of intersection of the horizontal and vertical line emanating from both axes for the two families represents the value of their difference (in log scale) and segments on both sides of the crossing point show the confidence interval for this difference. The difference between pair of families is significant when lower and upper endpoints of the confidence interval are either positive or negative i.e., they both are above or below the dotted line of equality.
The frequency of IADFs type L was significantly associated with the year (P < 0.001; Table 2). In the pairwise comparison of the different families, the family 2860 showed a higher frequency of IADFs type L compared with family 2818, and between family 2514 and 2860, it was marginally significant (Table 3, Figure 6).
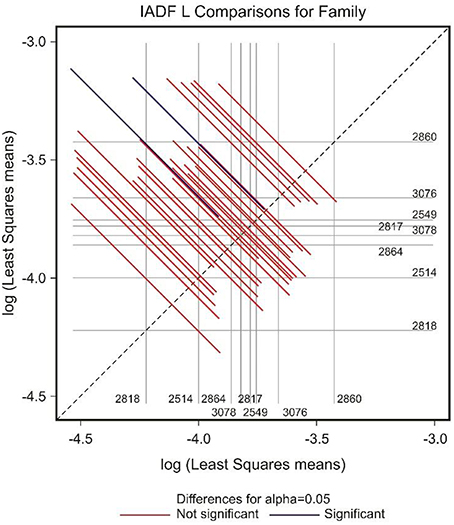
Figure 6. Diffogram of differences in the IADF-type L frequencies between the European larch families. The point of intersection of the horizontal and vertical line emanating from both axes for the two families represents the value of their difference (in log scale) and segments on both sides of the crossing point show the confidence interval for this difference. The difference between pair of families is significant when lower and upper endpoints of the confidence interval are either positive or negative i.e., they both are above or below the dotted line of equality.
The overall variation in IADFs frequency was strongly explained by the year effect, as suggested by a higher percentage of DOV, namely 82.4, 84.1, and 89.5% for IADFs type E, E+, and L, respectively. The site effect represents 6.55, 0.03, and 0.01%, and the family effect represents 7.76, 7.96, and 4.25% of the variation in the frequency of IADFs type E, E+, and L, respectively. Although, IADFs type L+ occurred in quite low frequencies, the overall variation in their frequency was explained by the family (64.4%) and site effect (35.7%).
The low number of observations of IADFs type L+ did not allow the factor year to be included in the model for the statistical analysis (Table 2). The site showed a marginal significance in the frequency of IADFs type L+. In the pairwise comparison of families, family 2864 showed a higher frequency when compared with family 2817 (Figure 7).
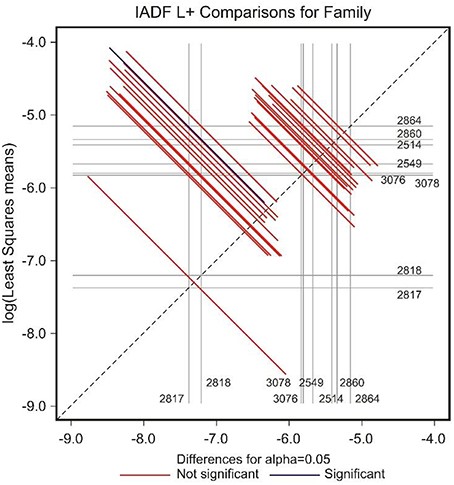
Figure 7. Diffogram of differences in the IADF-type L+ frequencies between the European larch families. The point of intersection of the horizontal and vertical line emanating from both axes for the two families represents the value of their difference (in log scale) and segments on both sides of the crossing point show the confidence interval for this difference. The difference between pair of families is significant when lower and upper endpoints of the confidence interval are either positive or negative i.e., they both are above or below the dotted line of equality.
IADFs and Climate
In general, IADFs type E showed an increase with high precipitation in May and temperature in April, with more significant relationships occurring in Młynary, compared with Zaporowo (Table 4). IADFs type E of the family 2860 showed no climatic signal, in both sites. Concerning IADFs type E+ in general the most prominent significant relationships with climatic conditions were an increase of type E+ occurrence with high precipitation in March, April, and May (Table 5). IADFs type E+ also showed, in general, a decrease with temperature in March and an increase with temperature in April and May (Table 5). All families showed significant relationships with some of the climatic parameters. Interestingly, families 2864, 3076, 3078, 2860, and 2514 seem to respond earlier to climatic conditions, with an increase with precipitation in March and a decrease with the temperature also in March (Table 5). IADFs type E+ of the family 2860 growing in Zaporowo showed no significant climatic relationships.
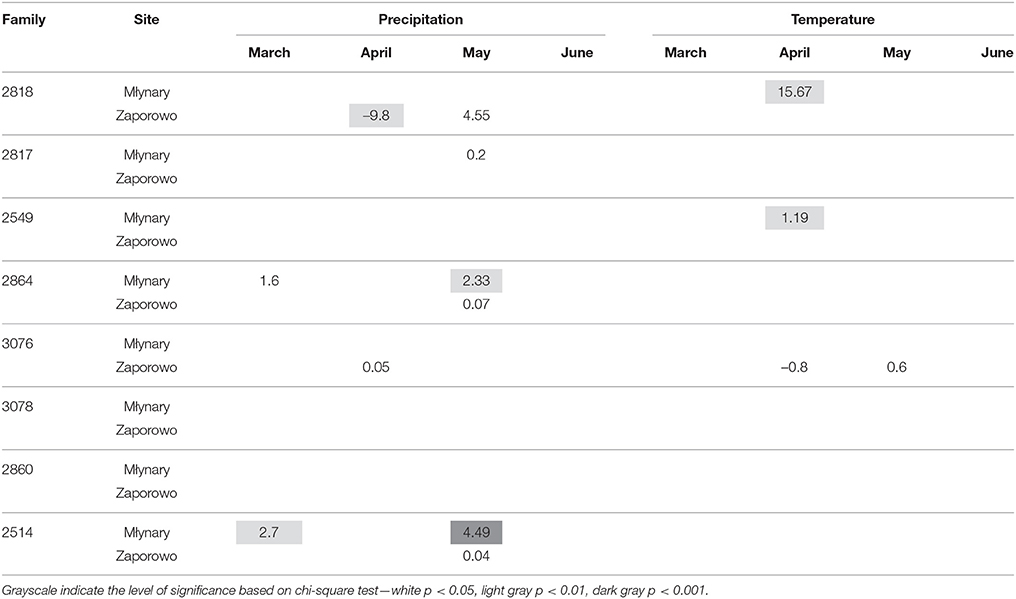
Table 4. Percent change (positive value indicates increase; negative value indicates decrease) in the odds of the occurrence of IADF type E for each 1-unit increase in monthly climatic data (mean temperature and total precipitation) from March to June, for the period 1990–2006.
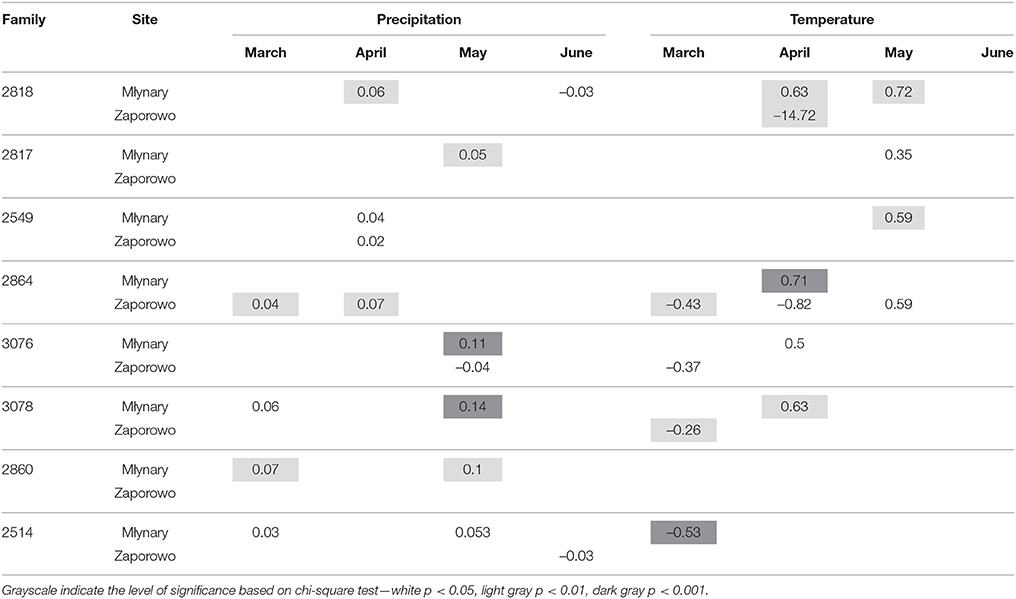
Table 5. Percent change (positive value indicates increase; negative value indicates decrease) in the odds of the occurrence of IADF type E+ for each 1-unit increase in monthly climatic data (mean temperature and total precipitation), from March to June, for the period 1990–2006.
Generally, IADFs type L also showed an increase with precipitation in May and a decrease with precipitation in June and September (Table 6). IADFs type L also occurs more often with temperature in May. The families 2864, 3076, 3078, 2860, and 2514 present more significant relationships with climatic parameters, compared with families 2818, 2817, and 2549, as observed for IADFs type E+. Interestingly, an increase of IADFs type L occurrence with temperature in May was only found in Zaporowo (Table 6).
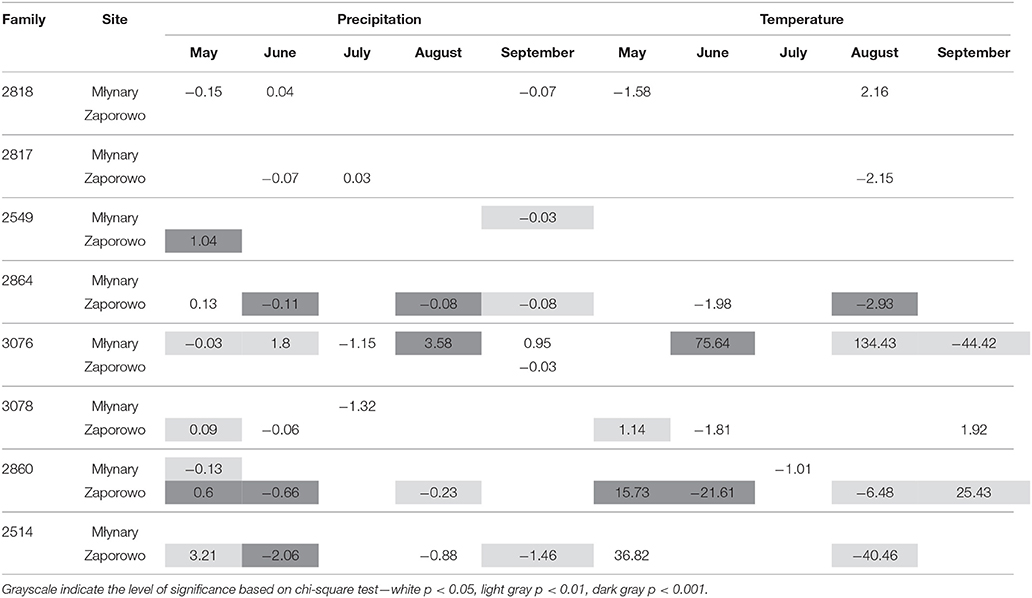
Table 6. Percent change (positive value indicates increase; negative value indicates decrease) in the odds of the occurrence of IADF type L for each 1-unit increase in monthly climatic data (mean temperature and total precipitation) from May to September, for the period 1990–2006.
Given the limited frequency of the L+ type, no significant relationships were found.
Discussion
Our results showed that the IADF frequency was largely dependent on the climatic conditions, with IADF type E also showing a significant influence of the site. However, our results did not find a significant effect of the genotype on the formation of the different types of IADFs. Nonetheless, different families appear to show different sensitivities to climatic conditions, reflected in the different correlations between IADFs and climate.
IADFs and Genotypes
The presence of IADFs in the earlywood indicates the capacity of trees to adapt to stress conditions, lowering the risk of xylem cells embolism through a reduction in the lumen area and an increase in cell-wall thickness (Hacke et al., 2001). The presence of density fluctuations within earlywood seems to be influenced by both tree genotype and environmental conditions (Rozenberg et al., 2002). Experiments with clones of Norway spruce showed a strong genetic effect on the occurrence of IADF type E as a reaction to water shortages (Rozenberg et al., 2002). The use of specimens with a high degree of relatedness increases the chances to observe the influence of the genotype on certain traits (Rozenberg and Cahalan, 1997). In our study we have used half-sib families of European larch and this may explain why we could not see a strong effect of the genotype on the occurrence of IADFs type E and E+. This indirectly points out a high level of heritability of the wood density components of the European larch families (Klisz, 2011), has also observed in other tree species (Hylen, 1999; Louzada and Fonseca, 2002; Louzada, 2003). Nonetheless, some of the families of the European larch significantly differ in terms of the frequency of IADFs type E and E+ (Table 3).
The heritability of the latewood density in conifers would predict that the occurrence of IADF type L and L+ was determined by the trees genotypes (Hylen, 1999; Hannrup et al., 2004). However, the frequency of density fluctuations in latewood was similar among the analyzed families of European larch. While some authors point to a higher heritability of earlywood as opposed to latewood (Rozenberg and Cahalan, 1997; Louzada and Fonseca, 2002), others have observed the reverse trend (Goggans, 1964; Hylen, 1999; Hannrup et al., 2004). In our study, the occurrence of IADFs type L was much more dependent on climatic conditions, namely humidity conditions at the end of the growing season. Trees at both experimental sites were characterized by very low frequencies of IADF L+, probably linked to an absence of an autumn growing season that favor the generation of this type of fluctuation (Campelo et al., 2006). Vieira et al. (2009) suggests that this kind of fluctuation is due to the occurrence of climatic conditions unfavorable for the complete maturation of xylem cells.
IADFs and Climate
The frequency of IADFs in annual rings is associated, inter alia with the type of climate shaping the course of the processes by which wood cells arise, grow, and differentiate. The low frequency of IADFs in the tree rings of European larch growing in northern Poland, with 9.1 and 11.1% of the annual rings showing IADFs, at the Młynary and Zaporowo sites, respectively, is within the range observed by other authors under temperate and boreal climates (Wimmer et al., 2000; Rigling et al., 2001; Franceschini et al., 2013). Relatively high frequencies of such fluctuations are observed in conifers growing under Mediterranean or Atlantic climate (15–32 and 30–52%, respectively; Campelo et al., 2006; Bogino and Bravo, 2009; Vieira et al., 2009; Rozas et al., 2011). This is probably related with a wider length of the growing season under Mediterranean or Atlantic climate, increasing the probability of environmental variations affecting wood development processes, with the consequent formation of IADFs (Campelo et al., 2015). Nonetheless, the frequency of IADFs in European larch was quite high in specific years, namely in 1998 and 2000, with more than 15% of the trees showing IADFs in Młynary, and in 1997 with more than 40% of the trees showing IADFs at Zaporowo. This could be related with very specific climatic conditions of those years that induced the formation of IADFs in more trees. However, because Młynary and Zaporowo are close by and under similar climatic conditions, and if the formation of IADFs is mainly controlled by climatic conditions, then we would expect that years with a higher frequency of IADFs would be the same among the sites, which was not observed in the mentioned years above. Thus, other site differences (e.g., microclimatic conditions, soil differences) could explain the observed year-lag of IADFs frequency among sites.
Conifers growing in temperate climatic conditions usually present a higher frequency of earlywood IADFs (Rozenberg et al., 2002; Hoffer and Tardif, 2009), while under boreal or alpine climate, latewood fluctuations tend to prevail (accounting for 69–100% of the total; Rigling et al., 2001) as well as under Mediterranean climate (Campelo et al., 2006; Vieira et al., 2009; Nabais et al., 2014). Our results showed that the dominant types of wood density fluctuations in European larch growing in the North of Poland were E+ and L, accounting for 42.6–44.9 and 31.8–35.9% of all reported fluctuations, respectively. This intermediate behavior might be related to the fact that the study sites were located in the transition area from temperate to boreal climate.
In general, all the main types of IADFs found in European larch (type E, E+, and L) showed a significant positive correlation with the precipitation in May. Thus, it seems that higher precipitation levels in May increases the probability of IADFs formation of any type. High water availability during the growing season can increase the division rate of cambial cells (Schuppler et al., 1998; Rossi et al., 2009). Cells must go through a process of growth and maturation, and an increase in the amount of cells means that more cells are available to integrate variations in the environmental conditions, increasing the probability of the occurrence of an IADF (Carvalho et al., 2015). In fact, precipitation in May–July stimulates the growth of larch (Oleksyn and Fritts, 1991; Koprowski, 2012) and as a result wide rings are present. Previous studies have also shown that density fluctuations are more common in wider rings than in narrow ones (Schweingruber, 1980).
The formation of IADFs type E is usually determined by water shortages during the growing season, with the formation of latewood-like cells within earlywood (Rozas et al., 2011). The relation between IADFs type E and climatic conditions among sites and families was quite scattered and thus with no clear pattern. Even so, there was a positive relationship of IADFs type E with the temperature in April and precipitation in May. Although, high precipitation in May does not indicate water stress, high temperatures in April could indicate a potential increase in evapotranspiration and thus water stress. Higher temperatures during the growing season could also increase the respiratory rate, reducing the hexose pool in cambium and xylem (Deslauriers et al., 2016). Hexose is produced to increase cell osmotic potential to generate turgor pressure for cell expansion (Koch, 2004). If there is a lower availability of carbon for growth, particularly for cell enlargement (Deslauriers et al., 2016), then an IADF type E could be formed. Cuny et al. (2014) have also showed that cell enlargement duration contributed to 75% of changes in cell diameter, while the amount of wall material per cell was quite constant. Thus, the thicker walls of an IADF type E do not represent a higher amount of carbon for cell wall thickening but is just a consequence of a lower lumen diameter. Interestingly the site significantly affected the formation of this type of IADF, with a higher frequency in Zaporowo compared to Młynary. A potential environmental factor modeling the reaction of the cambium to drought-associated stress conditions may be the capacity of the soil to retain water (Rozenberg et al., 2002). Soils with a high water storage capacity, mainly determined by soil type and depth, may weaken the impact of shortfalls in precipitation in the first half of the growing season, thereby reducing the frequency of earlywood density fluctuations (Rozenberg et al., 2002). The soil-types were different between the two sites, with a sandy clay soil in Młynary, and a brown earth soil in Zaporowo. It is known that sandy soils retain less water but under water stress conditions plants retrieve water more easily, compared with soils richer in organic matter, like the brown earth soils. Thus, the soil-type might explain the higher frequency of IADFs-type E in Zaporowo, compared with Młynary.
IADFs type E+ can be understood as a smooth transition between early- to latewood, i.e., a smooth decreasing of lumen diameter and increase in cell wall width. Although, there were no significant effects of the family on the formation of IADFs type E+ (P = 0.064), the correlations with climatic conditions seems to separate two groups of families, with one group showing positive correlations with precipitation in March and May, and a negative correlation with temperatures in March, and the other group showing positive correlations with precipitation in April and temperatures in April and May. This could indicate a different sensitivity of these two groups of families toward climatic conditions. However, further research is necessary to understand if these dissimilarities are due to genotypic differences.
The mechanism behind the formation of IADFs type L, commonly found in trees from the Mediterranean region, is associated with the cambium reactivation following a summer drought (Vieira et al., 2010; Novak et al., 2013; Nabais et al., 2014). The fact that this kind of fluctuation is prevalent under the circumstances of a Mediterranean climate is related with the bimodal growth pattern present among trees growing in this climatic zone (Cherubini et al., 2003). While the generation of IADF type E is interpreted as a reaction to unfavorable growth conditions in spring, the formation of an IADF-type L is indicative of better conditions for growth at the end of the growing season (Campelo et al., 2007; Nabais et al., 2014). In the case of the European larch growing in Poland, the formation of IADFs type L is generally associated with a positive correlation with precipitation and temperature in May. As observed for IADFs type E+, it appears that some of the families are more sensitive to climatic conditions at the end of the growing season, as indirectly revealed by the occurrence of more significant correlations with climatic conditions and IADFs-type L. Although, there was no significant effect of the site, at least some families growing in Zaporowo showed a negative correlation between IADFs-type L and the precipitation in June and the temperature in August. While the negative correlation with temperature in August makes sense from the point of view of the formation of IADFs type L, the negative correlation with June precipitation is counter-intuitive. To further understand how and when climatic conditions can be imprinted in wood anatomy, studies of xylogenesis of European larch are necessary to see if the timings of the different phases of wood formation differ among families and/or sites.
Conclusion
The frequency of IADFs under temperate climates is quite low when compared with Mediterranean or Atlantic climates. This is probably related to differences in the length of the growing season. Most of the IADFs found in the European Larch growing under a temperate climate in Northern Poland were of the type E+ and type L. Genetic determination underpinning the adaptation of the radial growth process to anomalies arising cyclically in a temperate climate should mainly concern earlywood and the transition zone with latewood, with a focus on related individuals. Although, we did not find a clear effect of genotype on the frequency of the IADFs, differences occurred in the pairwise comparison of the different families. The influence of a tree's genotype on its predisposition to generate density fluctuations within the annual rings would seem to be dependent on the degree of relatedness. In the European larch, anomalies in the anatomical structure of earlywood and wood of the transition zone are expressed most clearly in clones (i.e., trees that are identical genetically) and weakly among the families has in our study. Besides a general positive correlation of May precipitation and the frequency of IADFs, no clear pattern of the climatic signal of the different types of IADFs could be found. This can be due to the fact that we have used different families weakening a clearer climatic signal. Nonetheless, two groups of families could be distinguished based on their different sensitivity to climatic parameters, although further studies are necessary to confirm this observation.
Author Contributions
MK, MKo, JU, and CN gave a substantial contribution to the conception and design of the study, MK performed x-ray density analyses, MK and JU were in charge of genetic analyses, MKo and JU performed climatic analyses, MK wrote the first draft of the manuscript, MK, MKo, JU, and CN contributed to writing specific sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was performed under the Forest Research Institute statutory aid No. 24.12.17 of the Ministry of Science and Higher Education in Poland. This research is linked to activities conducted within the COST FP1106 “STReESS” network.
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723. doi: 10.1109/TAC.1974.1100705
Allison, P. D. (2012). Logistic Regression using SAS: Theory and Application, 2nd Edn. Cary, NC: SAS Institute Inc.
Battipaglia, G., Campelo, F., Vieira, J., Grabner, M., De Micco, V., Nabais, C., et al. (2016). Structure and function of intra–annual density fluctuations: mind the gaps. Front. Plant Sci. 7:595. doi: 10.3389/fpls.2016.00595
Battipaglia, G., De Micco, V., Brand, W. A., Linke, P., Aronne, G., and Cherubini, P. (2010). Variations of vessel diameter and d 13 C in false rings of Arbutus unedo L. reflect different environmental conditions. New Phytol. 188, 1099–1112. doi: 10.1111/j.1469-8137.2010.03443.x
Battipaglia, G., De Micco, V., Brand, W. A., Saurer, M., Aronne, G., Linke, P., et al. (2014). Drought impact on water use efficiency and intra-annual density fluctuations in Erica arborea on Elba (Italy). Plant Cell Environ. 37, 382–391. doi: 10.1111/pce.12160
Bergsten, U., Lindeberg, J., Rindby, A., and Evans, R. (2001). Batch measurements of wood density on intact or prepared drill cores using x-ray microdensitometry. Wood Sci. Technol. 35, 435–452. doi: 10.1007/s002260100106
Bogino, S., and Bravo, F. (2009). Climate and intraannual density fluctuations in Pinus pinaster subsp. mesogeensis in Spanish woodlands. Can. J. For. Res. 39, 1557–1565. doi: 10.1139/X09-074
Campelo, F., Gutiérrez, E., Ribas, M., Nabais, C., and Freitas, H. (2007). Relationships between climate and double rings in Quercus ilex from northeast Spain. Can. J. For. Res. 37, 1915–1923. doi: 10.1139/X07-050
Campelo, F., Nabais, C., Freitas, H., and Gutiérrez, E. (2006). Climatic significance of tree-ring width and intra-annual density fluctuations in Pinus pinea from a dry Mediterranean area in Portugal. Ann. For. Sci. 64, 229–238. doi: 10.1051/forest:2006107
Campelo, F., Vieira, J., Battipaglia, G., de Luis, M., Nabais, C., Freitas, H., et al. (2015). Which matters most for the formation of intra-annual density fluctuations in Pinus pinaster: age or size? Trees 29, 237–245. doi: 10.1007/s00468-014-1108-9
Campelo, F., Vieira, J., and Nabais, C. (2013). Tree-ring growth and intra-annual density fluctuations of Pinus pinaster responses to climate: does size matter ? Trees 27, 763–772. doi: 10.1007/s00468-012-0831-3
Carvalho, A., Nabais, C., Vieira, J., Rossi, S., and Campelo, F. (2015). Plastic responses of tracheids in Pinus pinaster in a water-limited environment: adjusting lumen size instead of wall thickness. PLoS ONE 10:e0136305. doi: 10.1371/journal.pone.0136305
Cherubini, P., Gartner, B. L., Tognetti, R., Braker, O. U., Schoch, W., and Innes, J. L. (2003). Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol. Rev. 78, 119–148. doi: 10.1017/S1464793102006000
Copenheaver, C. A., Pokorski, E. A., Currie, J. E., and Abrams, M. D. (2006). Causation of false ring formation in Pinus banksiana: a comparison of age, canopy class, climate and growth rate. For. Ecol. Manage. 236, 348–355. doi: 10.1016/j.foreco.2006.09.020
Cuny, H. E., Rathgeber, C. B. K., Frank, D., Fonti, P., and Fournier, M. (2014). Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 203, 1231–1241. doi: 10.1111/nph.12871
de Luis, M., Novak, K., Raventós, J., Gričar, J., Prislan, P., and Čufar, K. (2011). Cambial activity, wood formation and sapling survival of Pinus halepensis exposed to different irrigation regimes. For. Ecol. Manage. 262, 1630–1638. doi: 10.1016/j.foreco.2011.07.013
Deslauriers, A., Huang, J.-G., Balducci, L., Beaulieu, M., and Rossi, S. (2016). The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiol. 170, 01525.2015. doi: 10.1104/pp.15.01525
Dmuchowski, W., Bytnerowicz, A., Kurczyńska, E. U., and Włoch, W. (1997). The influence of air pollutants on needles and stems of Scots pine (Pinus sylvestris L.) trees. Environ. Pollut. 98, 325–334. doi: 10.1016/S0269-7491(97)00141-3
Eilmann, B., de Vries, S. M. G., den Ouden, J., Mohren, G. M. J., Sauren, P., and Sass-Klaassen, U. (2013). Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. For. Ecol. Manage. 302, 133–143. doi: 10.1016/j.foreco.2013.03.031
Franceschini, T., Longuetaud, F., Bontemps, J.-D., Bouriaud, O., Caritey, B.-D., and Leban, J.-M. (2013). Effect of ring width, cambial age, and climatic variables on the within-ring wood density profile of Norway spruce Picea abies (L.) Karst. Trees 27, 913–925. doi: 10.1007/s00468-013-0844-6
Fries, A., and Ericsson, T. (2006). Estimating genetic parameters for wood density of Scots pine (Pinus sylvestris L.). Silvae Genet. 55, 84–92.
Fries, A., and Ericsson, T. (2009). Genetic parameters for earlywood and latewood densities and development with increasing age in Scots pine. Ann. For. Sci. 66, 404. doi: 10.1051/forest/2009019
Fujimoto, T., Kita, K., and Kuromaru, M. (2008). Genetic control of intra-ring wood density variation in hybrid larch (Larix gmelinii var. japonica × L. kaempferi) F1. Wood Sci. Technol. 42, 227–240. doi: 10.1007/s00226-007-0171-4
Goggans, J. F. (1964). Correlation and heritability of certain wood properties in Loblolly pine (Pinus taeda L.). Tappi J. 47, 318–322.
Grabner, M., Wimmer, R., Gierlinger, N., Evans, R., and Downes, G. M. (2005). Heartwood extractives in larch and effects on X-ray densitometry. Can. J. For. Res. 35, 2781–2786. doi: 10.1139/x05-196
Hacke, U. G., Sperry, J. S., Pockman, W. T., Davis, S. D., and McCulloh, K. A. (2001). Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126, 457–461. doi: 10.1007/s004420100628
Hannrup, B., Cahalan, C., Chantre, G., Grabner, M., Karlsson, B., Bayon, I., et al. (2004). Genetic parameters of growth and wood quality traits in Picea abies. Scand. J. For. Res. 19, 14–29. doi: 10.1080/02827580310019536
Hoffer, M., and Tardif, J. C. (2009). False rings in jack pine and black spruce trees from eastern Manitoba as indicators of dry summers. Can. J. For. Res. 39, 1722–1736. doi: 10.1139/X09-088
Huang, J. G., Deslauriers, A., and Rossi, S. (2014). Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytol. 203, 831–841. doi: 10.1111/nph.12859
Hylen, G. (1999). Age trends in genetic parameters of wood density in young Norway spruce. Can. J. For. Res. 29, 135–143. doi: 10.1139/x98-170
Kalliokoski, T., Reza, M., Jyske, T., Mäkinen, H., and Nöjd, P. (2012). Intra-annual tracheid formation of Norway spruce provenances in southern Finland. Trees 26, 543–555. doi: 10.1007/s00468-011-0616-0
Klein Tank, A. M. G., Wijngaard, J. B., Können, G. P., Böhm, R., Demarée, G., Gocheva, A., et al. (2002). Daily dataset of 20th-century surface air temperature and precipitation series for the European climate assessment. Int. J. Climatol. 22, 1441–1453. doi: 10.1002/joc.773
Klisz, M. (2011). Genetic Aspects of Wood Properties of European Larch (Larix decidua Mill.). Dissertation/Doctoral thesis, Forest Research Institute, Sȩkocin Stary, PL (in Polish).
Koch, K. (2004). Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 7, 235–246. doi: 10.1016/j.pbi.2004.03.014
Koprowski, M. (2012). Long-term increase of March temperature has no negative impact on tree rings of European larch (Larix decidua) in lowland Poland. Trees 26, 1895–1903. doi: 10.1007/s00468-012-0758-8
Larsson, B., Pernestål, K., and Jonsson, B. (1994). A Wood Sample Preparation Method for Direct Scanning X-Ray Microdensitometry. Umeå: Swedish University of Life Sciences.
Liang, K. Y., and Zeger, S. L. (1986). Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22. doi: 10.1093/biomet/73.1.13
Lindner, M., Fitzgerald, J. B., Zimmermann, N. E., Reyer, C., Delzon, S., Maaten, E., et al. (2014). Climate change and European forests: what do we know, what are the uncertainties, and what are the implications for forest management ? J. Environ. Manage. 146, 69–83. doi: 10.1016/j.jenvman.2014.07.030
López-Maury, L., Marguerat, S., and Bähler, J. (2008). Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat. Rev. Genet. 9, 583–593. doi: 10.1038/nrg2398
Louzada, J. L. P. C. (2003). Genetic correlations between wood density components in Pinus pinaster Ait. Ann. For. Sci. 60, 285–294. doi: 10.1051/forest:2003020
Louzada, J. L. P. C., and Fonseca, F. M. A. (2002). The heritability of wood density components in Pinus pinaster Ait and the implications for tree breeding. Ann. For. Sci. 59, 867–873. doi: 10.1051/forest:2002085
Masiokas, M., and Villalba, R. (2004). Climatic significance of intra-annual bands in the wood of Nothofagus pumilio in southern Patagonia. Trees 18, 696–704. doi: 10.1007/s00468-004-0355-6
McLane, S. C., Daniels, L. D., and Aitken, S. N. (2011). Climate impacts on lodgepole pine (Pinus contorta) radial growth in a provenance experiment. For. Ecol. Manage. 262, 115–123. doi: 10.1016/j.foreco.2011.03.007
Nabais, C., Campelo, F., Vieira, J., and Cherubini, P. (2014). Climatic signals of tree-ring width and intra-annual density fluctuations in Pinus pinaster and Pinus pinea along a latitudinal gradient in Portugal. Forestry 87, 598–605. doi: 10.1093/forestry/cpu021
Nelder, J. A., and Weddenburn, R. W. M. (1972). Generalized linear models. J. R. Statist. Soc. A 135, 370–384. doi: 10.2307/2344614
Novak, K., Čufar, K., de Luis, M., Sánchez, M. A. S., and Raventós, J. (2013). Age, climate and intra-annual density fluctuations in Pinus halepensis in Spain. IAWA J. 34, 459–474. doi: 10.1163/22941932-00000037
Oleksyn, J., and Fritts, H. C. (1991). Influence of climatic factors upon tree rings of Larix decidua and L. decidua × L. kaempferi from Pulawy, Poland. Trees 5, 75–82. doi: 10.1007/BF00227488
Pâques, L. E. (2004). Roles of European and Japanese larch in the genetic control of growth, architecture and wood quality traits in interspecific hybrids (Larix × eurolepis Henry). Ann. For. Sci. 61, 25–33. doi: 10.1051/forest,:2003081
R Development Core Team (2007). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org.
Rigling, A., Waldner, P. O., Forster, T., Bräker, O. U., and Pouttu, A. (2001). Ecological interpretation of tree-ring width and intraannual density fluctuations in Pinus sylvestris on dry sites in the central Alps and Siberia. Can. J. For. Res. 31, 18–31. doi: 10.1139/x00-126
Rossi, S., Simard, S., Rathgeber, C. B. K., Deslauriers, A., and De Zan, C. (2009). Effects of a 20-day-long dry period on cambial and apical meristem growth in Abies balsamea seedlings. Trees 23, 85–93. doi: 10.1007/s00468-008-0257-0
Rozas, V., Garcia-Gonzalez, I., and Zas, R. (2011). Climatic control of intra-annual wood density fluctuations of Pinus pinaster in NW Spain. Trees 25, 443–453. doi: 10.1007/s00468-010-0519-5
Rozenberg, P., and Cahalan, C. (1997). Spruce and wood quality: genetic aspects (A review). Silvae Genet. 46, 270–279.
Rozenberg, P., Loo, J., Van Hannrup, B., and Grabner, M. (2002). Clonal variation of wood density record of cambium reaction to water deficit in Picea abies (L.) Karst. Ann. For. Sci. 59, 533–540. doi: 10.1051/forest:2002038
Savva, Y., Schweingruber, F., Milyutin, L., and Vaganov, E. (2002). Genetic and environmental signals in tree rings from different provenances of Pinus sylvestris L. planted in the southern taiga, central Siberia. Trees 16, 313–324. doi: 10.1007/s00468-001-0136-4
Schuppler, U., He, P. H., John, P. C., and Munns, R. (1998). Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves. Plant Physiol. 117, 667–678. doi: 10.1104/pp.117.2.667
Schweingruber, F. (1980). Dichtenschwankungen in Jahrringen von Nadelhölzern in Beziehung zu Klimatisch-Ökologischen Faktoren, Oder das Problem der Falschen Jahrringen. Eidgenössische Anstalt für das Forstliche Versuchswesen. Berichte 213: 35. Birmensdorf.
Seo, J.-W., Eckstein, D., Jalkanen, R., and Schmitt, U. (2011). Climatic control of intra- and inter-annual wood-formation dynamics of Scots pine in northern Finland. Environ. Exp. Bot. 72, 422–431. doi: 10.1016/j.envexpbot.2011.01.003
Stokes, M. E., Davies, C. S., and Koch, G. G. (2000). Categorical Data Analysis using the SASSystem, 2nd Edn. Cary, NC SAS: Institute Inc.
Vieira, J., Campelo, F., and Nabais, C. (2009). Age-dependent responses of tree-ring growth and intra-annual density fluctuations of Pinus pinaster to Mediterranean climate. Trees 23, 257–265. doi: 10.1007/s00468-008-0273-0
Vieira, J., Campelo, F., and Nabais, C. (2010). Intra-annual density fluctuations of Pinus pinaster are a record of climatic changes in the western Mediterranean region. Can. J. For. Res. 40, 1567–1575. doi: 10.1139/X10-096
Vieira, J., Campelo, F., Rossi, S., Carvalho, A., Freitas, H., and Nabais, C. (2015). Adjustment capacity of maritime pine cambial activity in drought-prone environments. PLoS ONE 10:e0126223. doi: 10.1371/journal.pone.0126223
Wilczyński, S. B., and Kulej, M. (2013). The influence of climate on the radial increment of larches of different provenances on the basis of the experiment in the Carpathian Mountains in Southern Poland. Eur. J. For. Res. 132, 919–929. doi: 10.1007/s10342-013-0731-0
Wimmer, R. (2002). Wood anatomical features in tree-rings as indicators of environmental change. Dendrochronologia 20, 21–36. doi: 10.1078/1125-7865-00005
Keywords: IADF, genetics, G × E, European larch, generalized estimating equations, wood density
Citation: Klisz M, Koprowski M, Ukalska J and Nabais C (2016) Does the Genotype Have a Significant Effect on the Formation of Intra-Annual Density Fluctuations? A Case Study Using Larix decidua from Northern Poland. Front. Plant Sci. 7:691. doi: 10.3389/fpls.2016.00691
Received: 25 January 2016; Accepted: 05 May 2016;
Published: 20 May 2016.
Edited by:
Jian-Guo Huang, Chinese Academy of Sciences, ChinaReviewed by:
Rubén Retuerto, Universidad de Santiago de Compostela, SpainPatrick R. N. Lenz, Natural Resources Canada, Canada
Copyright © 2016 Klisz, Koprowski, Ukalska and Nabais. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcin Klisz, m.klisz@ibles.waw.pl
 Marcin Klisz
Marcin Klisz Marcin Koprowski
Marcin Koprowski Joanna Ukalska
Joanna Ukalska Cristina Nabais
Cristina Nabais