- 1Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture, The Chinese Academy of Sciences, Changsha, China
- 2Dongting Lake Station for Wetland Ecosystem Research, Institute of Subtropical Agriculture, The Chinese Academy of Sciences, Changsha, China
Despite the predominant role of bud banks in the regeneration of clonal macrophyte populations, few studies have examined the way in which clonal macrophytes adjust the demographic features of bud banks to regulate population dynamics in response to defoliation in wetlands. We investigated the density and composition of bud banks under repeated defoliation in the wetland sedge Carex brevicuspis C. B. Clarke in the Dongting Lake wetlands, China. The density and biomass of rhizome buds and shoots did not decrease significantly in response to repeated defoliation over two consecutive years. The composition of bud banks, which consisted of long and short rhizome buds, also did not change significantly in response to repeated defoliation. Nevertheless, the ramet height and the shoot, root, and rhizome mass of C. brevicuspis declined significantly under repeated defoliation. Our findings suggest that bud banks are a conservative reproductive strategy that enables C. brevicuspis to tolerate a certain amount of defoliation. The maintenance of large bud banks after repeated defoliation may enable C. brevicuspis populations to regenerate and persist in disturbed habitats. However, bud bank density of C. brevicuspis might decline in the long term because the amount of carbon stored in rhizome buds and plants is reduced by frequent defoliation.
Introduction
Defoliation by herbivores or mowing is a common disturbance in ecosystems dominated by perennials, such as grasslands and wetlands (Zhao et al., 2008; Terer et al., 2012). In these ecosystems, clonal plants reproduce predominantly from a belowground population of meristems, the “bud bank” (Harper, 1977; Benson et al., 2004; Benson and Hartnett, 2006; Sosnová et al., 2010; Ott and Hartnett, 2012; Deng et al., 2015). Tillering from bud banks is one of the major mechanisms conferring plant resilience to herbivory (Tuomi et al., 1994; Strauss and Agrawal, 1999; Tiffin, 2000). Therefore, the population dynamics of clonal species in response to defoliation may be determined by the demographic features of bud banks, such as the number of buds available for tiller recruitment and their emergence rate (Tuomi et al., 1994; Huhat et al., 2000; Lehtilä, 2000; Dalgleish and Hartnett, 2009).
The effect of defoliation on bud bank demography differs among plant guilds (Vesk, 2006; Dalgleish and Hartnett, 2009; VanderWeide and Hartnett, 2015). For example, defoliation by grazers increases grass bud banks but decreases forb bud banks in tallgrass prairies (Dalgleish and Hartnett, 2009). In addition, the number of buds available for tiller recruitment fluctuates over the year (Benson et al., 2004; Dalgleish and Hartnett, 2006; Zhang et al., 2009; Chen et al., 2014, 2015b,c). Therefore, the effect of defoliation on bud bank demography may also vary among seasons.
Buds in the bud bank may be classified according to their size, developmental stage, location, and level of protection (Vesk and Westoby, 2004; Deng et al., 2013a; Qian et al., 2014; Chen et al., 2015a). Defoliation may affect types of buds differently depending on their positioning and activation sensitivity (Huhat et al., 2000; N’Guessan and Hartnett, 2011). The release of apical dominance following decapitation may stimulate lateral bud outgrowth along the axis of a tiller (Cline, 1997). In Schizachyrium scoparium, an increase in defoliation frequency is associated with a significant increase in the proportion of extravaginal buds and a decrease in the proportion of intravaginal buds, resulting in a more spread-out, prostrate growth form (N’Guessan and Hartnett, 2011). Therefore, changes in the bud bank composition may contribute to changes in clonal growth strategies among perennial grasses in response to defoliation (Qian et al., 2014).
Previous studies on bud banks under defoliation have focused on the bud banks at the community or plant guild level in terrestrial grasslands (Dalgleish and Hartnett, 2009; Qian et al., 2014; VanderWeide and Hartnett, 2015). Clonal macrophytes, which are a common feature of wetland habitats, are grazed by herbivorous waterfowl and domestic livestock (Smith et al., 2012; Mesa et al., 2015). The way in which clonal macrophytes adjust the demographic features of bud banks, such as bud density and composition, to regulate population dynamics in response to defoliation has not been studied in wetlands.
In the present study, we investigated the effects of repeated defoliation on density, composition, and biomass of bud banks in the wetland sedge Carex brevicuspis C. B. Clarke, an important forage species for cattle and migratory birds, in the Dongting Lake wetlands, China. Belowground bud banks contribute almost 100% of the aboveground shoot recruitment in mature populations of C. brevicuspis (Deng et al., 2015). The plant produces two types of rhizome buds: short rhizome buds (SRB), which form clumping ramets, and long rhizome buds (LRB), which form spreading ramets (Chen et al., 2011, 2014; Deng et al., 2013b), resulting in a combined growth form. Our hypotheses were (1) that repeated defoliation would result in a decrease in the density and biomass of rhizome buds in C. brevicuspis and (2) that repeated defoliation in C. brevicuspis would produce a higher proportion of LRB and a lower proportion of SRB to promote a more spread-out growth form in order to avoid grazers. To test these hypotheses, we investigated the temporal dynamics of the shoot population and bud banks by sampling the aboveground shoot populations and the belowground bud banks for three defoliation frequencies (none, monthly, and bimonthly) over two consecutive years in the Dongting Lake wetlands.
Materials and Methods
Study Site
Dongting Lake (28°30′–30°20′N, 111°40′–113°10′E), the second largest freshwater lake in China, is located in the northern part of Hunan Province. It lies in a basin south of the Yangtze River and is connected to it by distributary channels. The surrounding wetlands are characterized by large seasonal fluctuations in the water level (up to 15 m) and are completely flooded June–October and exposed November–May. The mean annual temperature is 16.8°C, with hot summers (June–August, 27.3°C) and cold winters (December–February, 5.8°C) (Huang et al., 2013). The annual precipitation is 1382 mm, more than 60% of which falls between April and August. Our study site was located in the fence-enclosed monitoring plot (112°47′11.6″E, 29°29′14.3″N) of the Dongting Lake Station for Wetland Ecosystem Research from the Chinese Academy of Sciences. The number of days submerged in 2012 and 2013 were 171 and 167 days, respectively, and mean flooding depths were 2.60 ± 1.25 and 2.91 ± 1.15 m (mean ± SE) respectively, at our study site.
Study species
Carex brevicuspis (Cyperaceae) is a perennial rhizomatous sedge found in eastern mainland China and Taiwan (Dai et al., 2010). The pseudoculm of the plant, consisting of a series of overlapping leaf sheaths, is usually 20–55 cm high. In the Dongting Lake wetlands, this species forms mono-dominant communities or is co-dominant with other Carex species. During the flood season (June–October), the Carex vegetation is completely submerged and the aboveground shoots senesce. C. brevicuspis shoots emerge immediately after flooding (November), growing to a standing crop before January (Chen et al., 2014). In January, the plants are relatively dormant and the shoots partially wither because of the low temperatures. New ramets sprout in March, after which the plants grow rapidly, flowering and fruiting from March to May, but producing only a few seedlings in the field (Chen et al., 2015c; Deng et al., 2015). C. brevicuspis populations outside natural reserves are grazed frequently by cattle, whereas those within natural reserves are grazed less frequently in the Dongting Lake wetlands.
Experimental Design
Five sections of the lake shoreline dominated by C. brevicuspis were selected as study sites. The distance between each section was at least 200 m. In each section, three permanent quadrats (each 5 m × 5 m) were established parallel to the lake shoreline. The corners of each quadrat were marked by hammering durable plastic tubes into the soil. The distance between each quadrat was 5 m. One of three treatments (monthly defoliation, bimonthly defoliation, and no defoliation) was randomly assigned to each quadrat, with five replications of each treatment.
Above- and Belowground Sampling
The experiment started on November 13, 2012 (after flooding). On that day, all ramets in the monthly and bimonthly quadrats were clipped to a height of 5 cm, to simulate cattle grazing. Thereafter, the plants in each quadrat were clipped according to the designed frequency (monthly, bimonthly, or not at all) during the non-flooding season.
Above- and belowground sampling occurred 2 months after the last bimonthly clipping and before the next clipping during the non–flooding season, January 2013–March 2014: in mid-November (1 week after flooding), mid-January (the coldest month), and mid-March (after spring sprouting). During each sampling, one square (50 cm × 50 cm) was randomly selected in each quadrat, for a total of 15 squares per sampling. In each square, all living (>50% green, potentially photosynthetically active) aboveground shoots were counted, clipped, and placed in plastic bags. Undisturbed soil within the squares was excavated to a depth of 15 cm using a shovel and stored in plastic bags (Chen et al., 2014, 2015b).
Sample Processing
Belowground tissue samples were carefully washed to remove the soil while protecting the integrity of the rhizome buds. For each sampled square, the roots, LRB, SRB, and spacers (connections between ramets) were separated. The LRB were defined as the rhizome buds that grew horizontally further than 1 cm from the parent shoot (Bernard, 1990; Chen et al., 2011), whereas the SRB grew vertically and clumped around the parent shoot (Chen et al., 2014). As axillary buds in the rhizome nodes were inconspicuous (usually less than 1 mm in length), especially in short rhizomes, and contribute little to shoot populations in Carex species (Bernard, 1990; Deng et al., 2013b), only apical rhizome buds, which have the potential to sprout into ramets, were classified and counted (Chen et al., 2015c). The total rhizome bud (TRB) density was calculated as the sum of the SRB and LRB per m2. Aboveground shoots, roots, spacers, LRB, and SRB were dried separately in an oven at 80°C for 48 h before the dry weight was measured. The LRB or SRB biomass included, in each case, the apical bud and the attached rhizome. The total plant biomass was defined as the total dry weight of the shoots, roots, spacers, LRB, and SRB per m2. Total biomass per ramet was calculated as the total plant biomass divided by ramet density in each square. Biomass per TRB was calculated as the TRB mass divided by TRB density in each square.
Data Analysis
The significance of differences in the height, density, and biomass of ramets, density, and biomass of rhizome buds, and proportion of SRB to TRB between defoliation treatments and sampling periods were evaluated by repeated analysis of variance (ANOVA), using defoliation frequency as a main factor and the sampling period as a repeated measure. Because some squares did not produce rhizome buds in March (i.e., the TRB density was zero), we did not analyze the differences in the proportion of SRB to TRB density between defoliation treatments for that month. Multiple comparisons of the means of plant traits under three defoliation frequencies at each sampling period were performed using Tukey’s honest significant difference (HSD) test at a 0.05 significance level. If necessary, the data were square root- or log10-transformed to reduce the variance heterogeneity, and the homogeneity was tested using Levene’s test. The data were expressed as the mean ± standard error (SE) and p < 0.05 was considered significant. All statistical analyses were performed using the statistical software SPSS V15.0 (SPSS Inc., USA).
Results
Ramet Height and Density
The ramet height was significantly affected by the defoliation frequency and sampling time, with significant interactions between both factors (Table 1). The monthly and bimonthly defoliation treatments significantly decreased the ramet height for all samplings January 2013–March 2014, with the exception of a non-significant decrease in January and November 2013 for the bimonthly defoliation treatment (Figure 1A). The ramet density was significantly affected by the sampling time but not by the defoliation frequency (Table 1; Figure 1B).
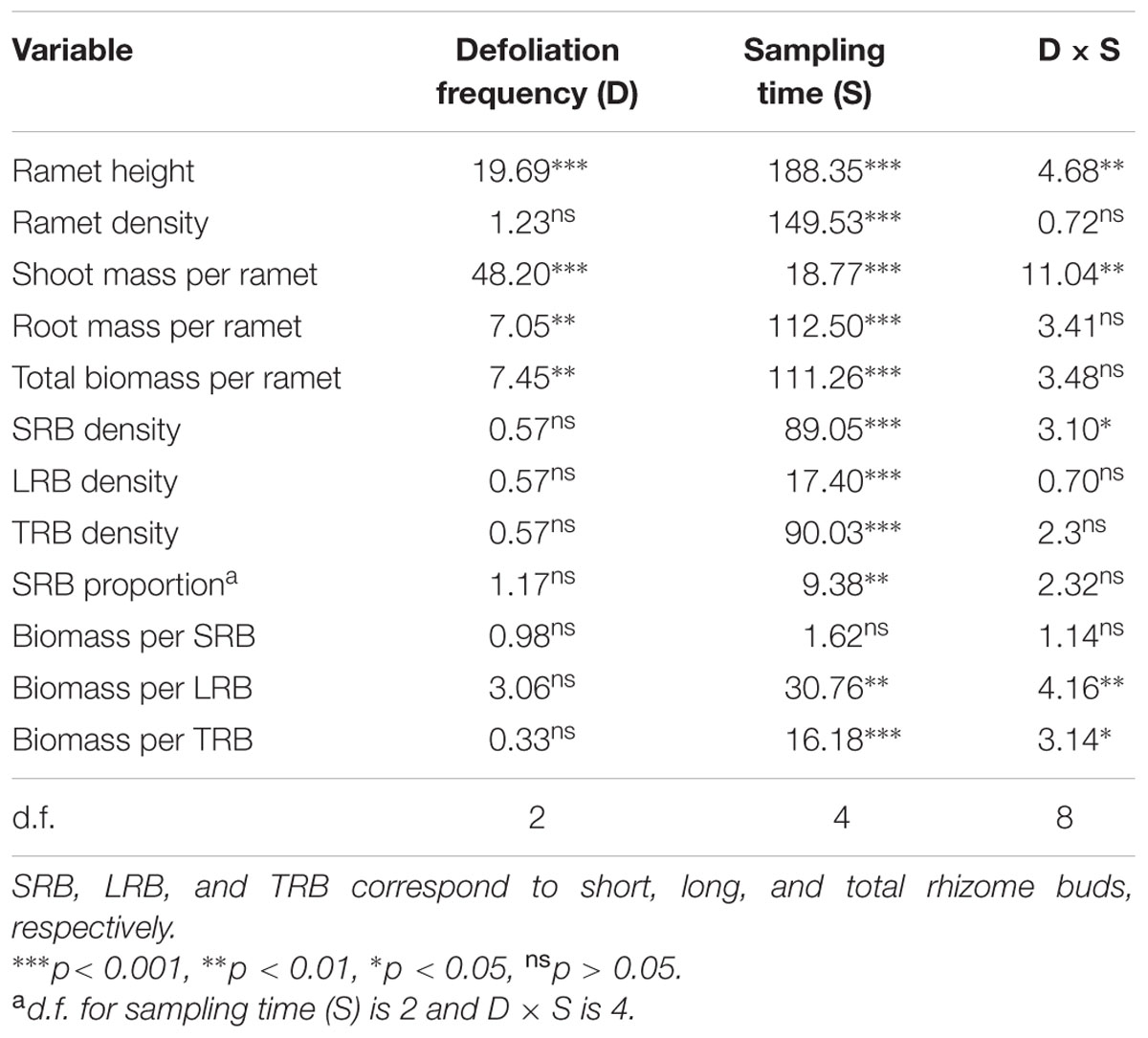
TABLE 1. Summary of repeated ANOVAs on plant traits in Carex brevicuspis populations for three defoliation frequencies January 2013–March 2014 (F and P-values).
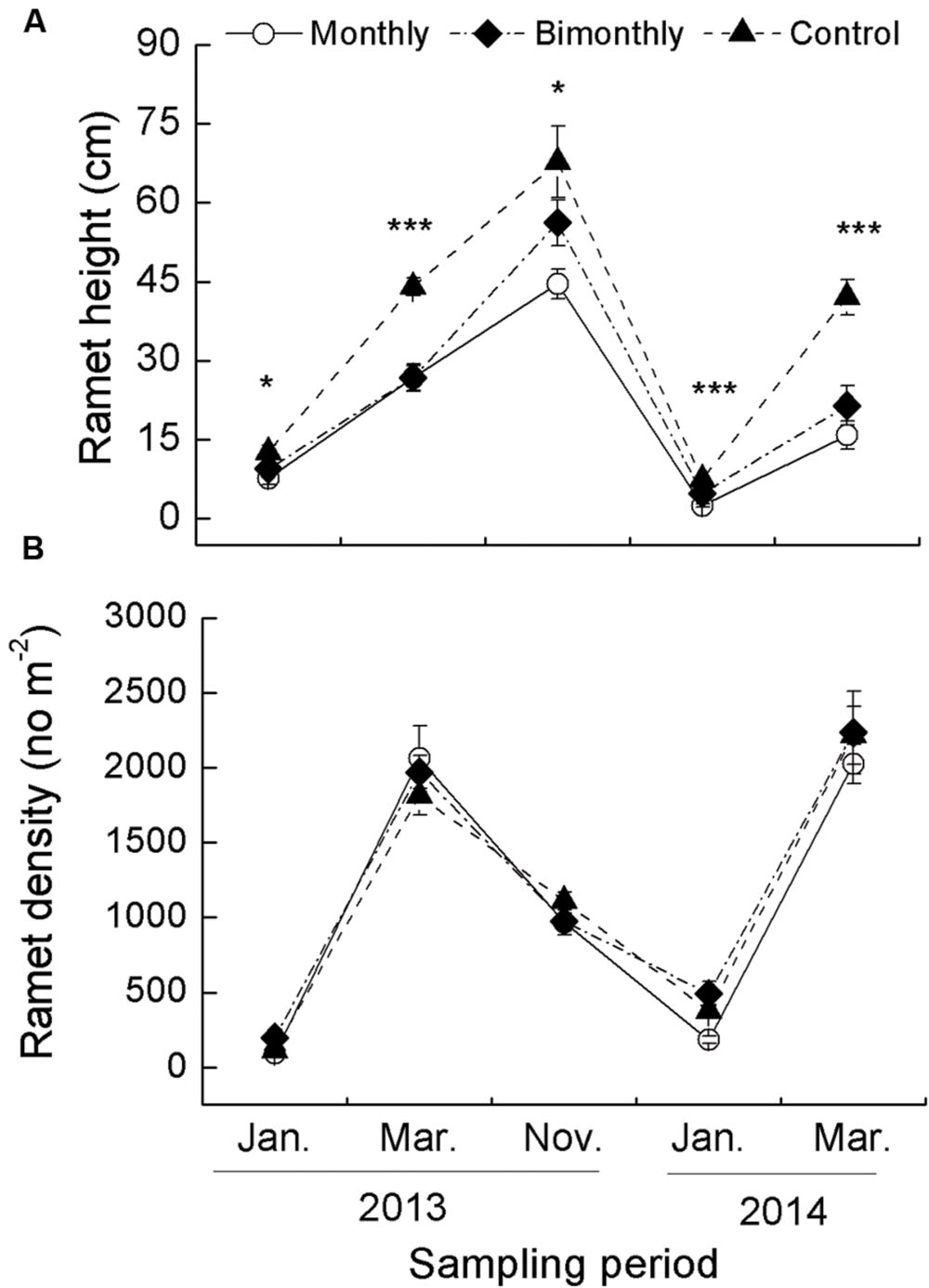
FIGURE 1. Ramet height (A) and density (B) of Carex brevicuspis populations for three defoliation frequencies, January 2013–March 2014. The data are expressed as the mean ± standard error (SE). ∗p < 0.05, ∗∗∗p < 0.001.
Shoot, Root, and Total Biomass Per Ramet
Shoot mass per ramet was significantly affected by defoliation frequency and sampling time, with significant interactions between both factors (Table 1). Monthly defoliation significantly decreased the shoot mass per ramet for all sampling times, except for a non-significant reduction in March 2013 (Figure 2A). Bimonthly defoliation significantly decreased the shoot mass per ramet for all sampling times, except for non-significant reductions in March and November 2013 (Figure 2A).
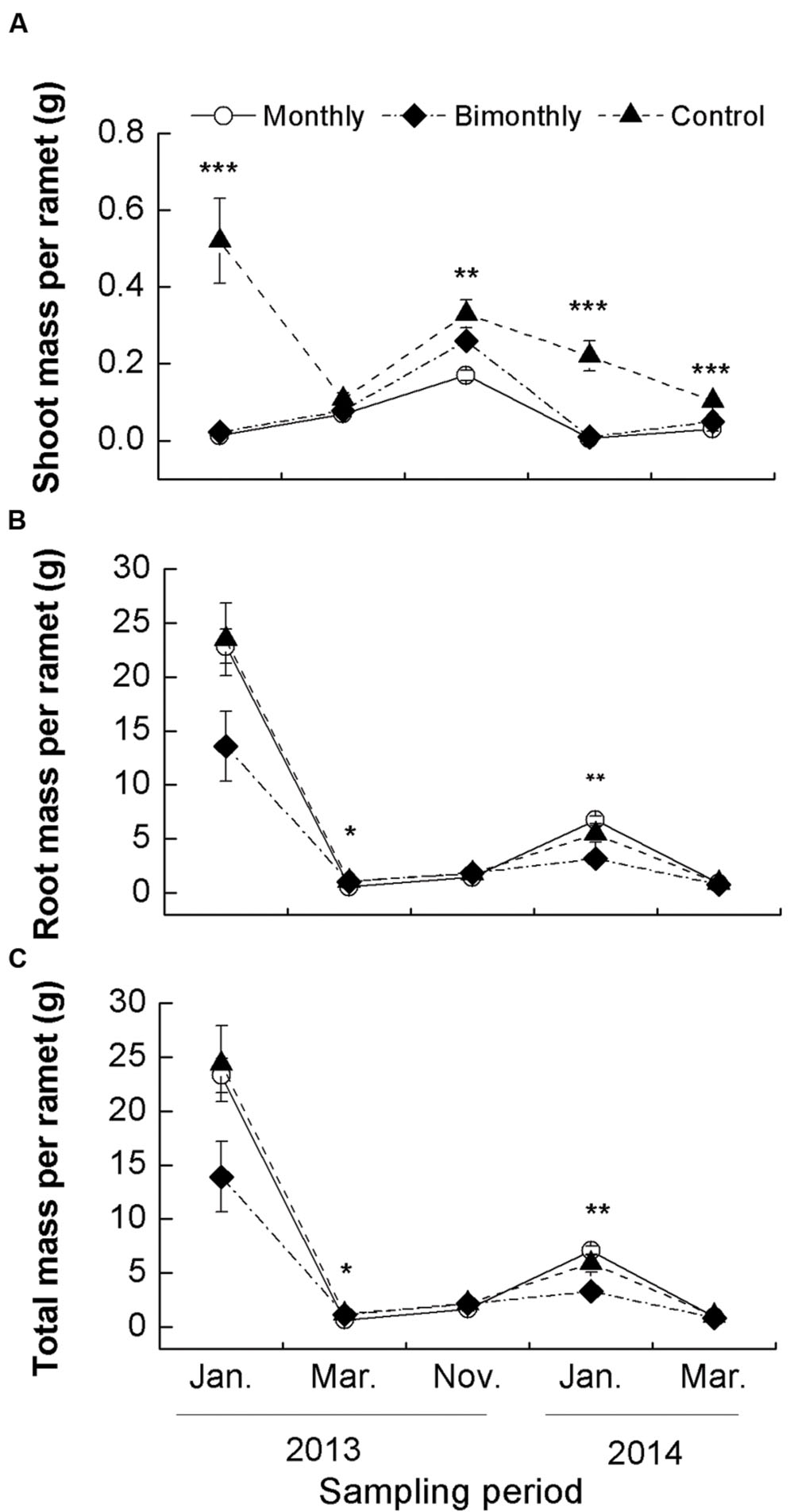
FIGURE 2. Shoot (A), root (B), and total (C) mass per ramet of C. brevicuspis populations for three defoliation frequencies, January 2013–March 2014. The data are expressed as the mean ± standard error (SE). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
The root and total biomass per ramet were also significantly affected by the defoliation frequency and sampling time (Table 1). In March 2013, monthly defoliation decreased the root and total biomass per ramet, while bimonthly defoliation did not reveal a significant reduction (Figures 2B,C). In January 2014, bimonthly defoliation significantly reduced the root and total biomass per ramet (Figures 2B, C).
SRB, LRB, and TRB Density
The SRB density was significantly affected by the sampling time, with significant interactions between the sampling time and defoliation frequency (Table 1). The monthly defoliation increased the SRB density in November 2013 (Figure 3A). The LRB and TRB density were significantly affected by the sampling time but not by the defoliation frequency (Figures 3B,C). The seasonal changes in the SRB, LRB, and TRB densities displayed similar trends for all treatments, peaking in January and dramatically decreasing in March (Figures 3A–C).
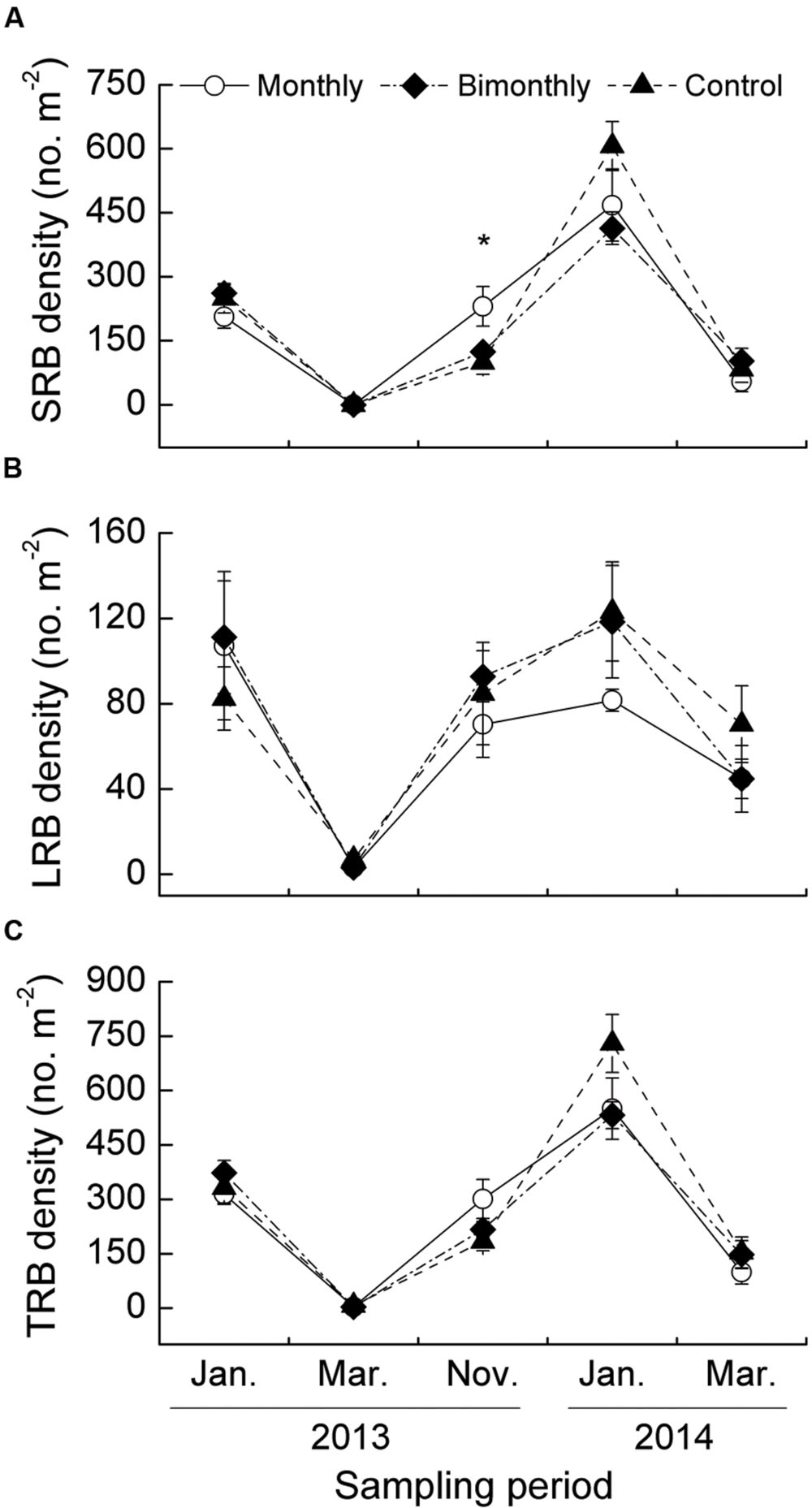
FIGURE 3. Short rhizome bud (SRB, A), long rhizome bud (LRB, B), and total rhizome bud (TRB, C) density of C. brevicuspis populations for three defoliation frequencies, January 2013–March 2014. Different scales are used on the y-axis. The data are expressed as the mean ± standard error (SE). ∗p < 0.05.
The majority of buds among all treatments throughout the growing season were SRB (53.3–83.8%, Figure 4). The proportion of SRB to TRB was significantly affected by the sampling time but not by the defoliation frequency (Table 1; Figure 4).
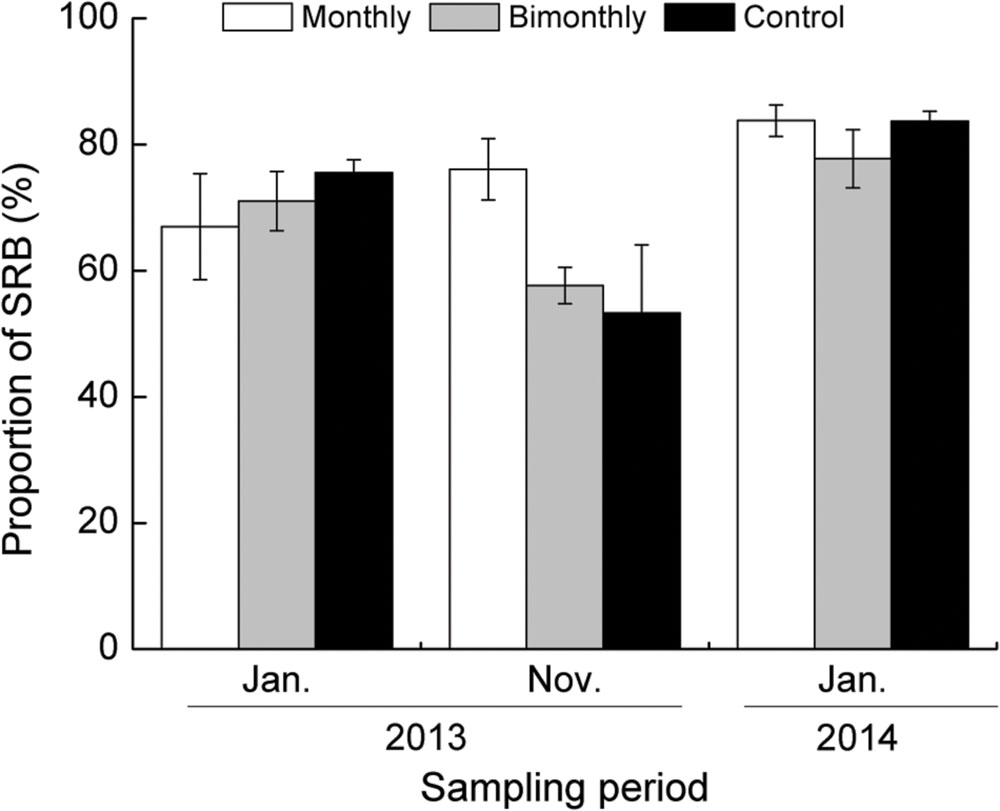
FIGURE 4. Proportion of short rhizome buds (SRB) to total rhizome buds (TRB) of C. brevicuspis populations for three defoliation frequencies in January and November 2013 and January 2014. The data are expressed as the mean ± standard error (SE). ∗p < 0.05.
Biomass Per SRB, LRB, and TRB
The biomass per SRB was not significantly affected by defoliation frequency and sampling time (Table 1, Figure 5A). The biomass per LRB and TRB were significantly affected by the sampling time, with significant interactions between the sampling time and defoliation frequency (Table 1). Monthly defoliation decreased the biomass per LRB and TRB in November 2013 and January 2014, but bimonthly defoliation was not associated with a significant reduction (Figures 5B,C).
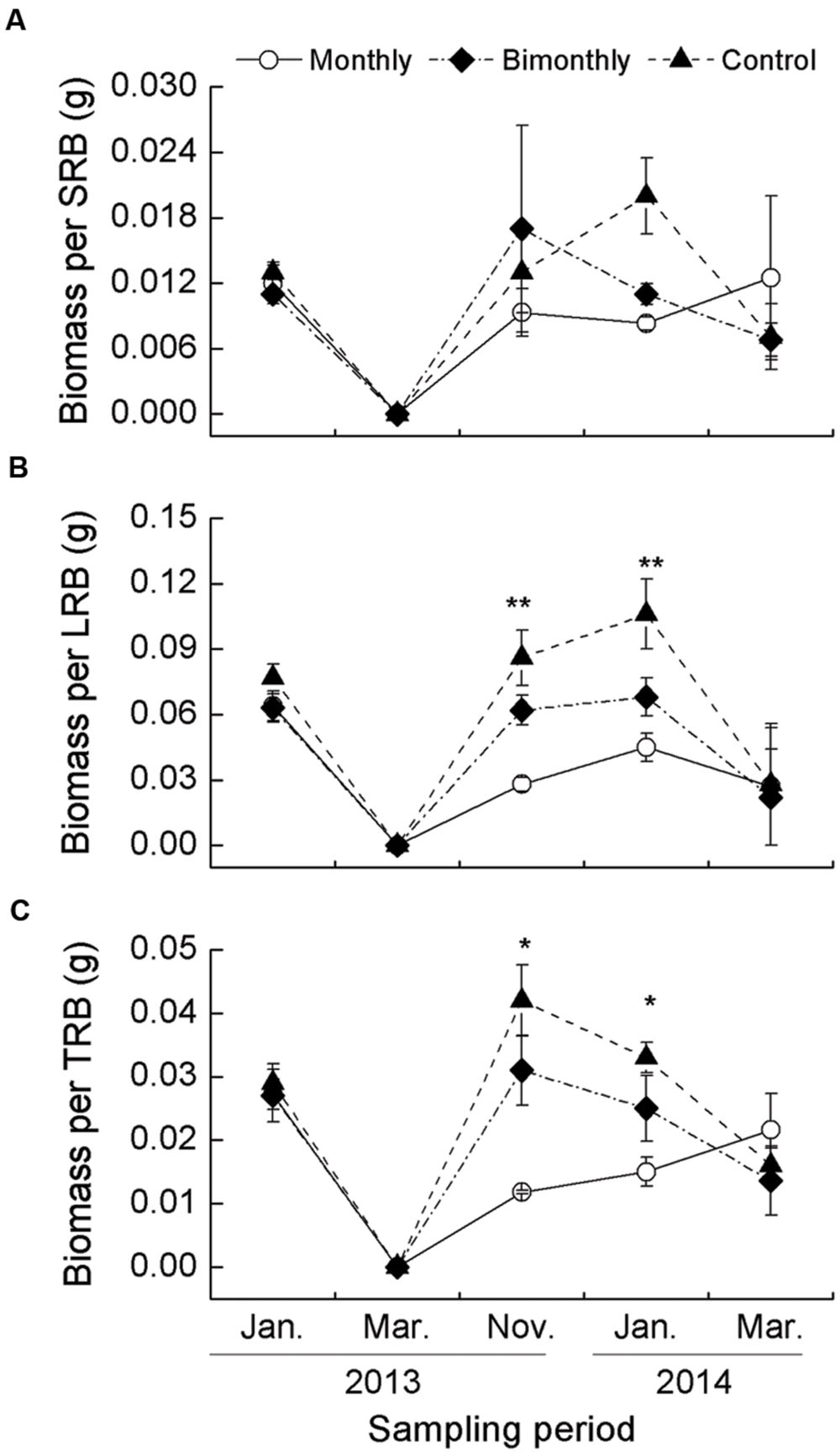
FIGURE 5. Biomass per short rhizome bud (SRB, A), long rhizome bud (LRB, B), and total rhizome bud (TRB, C) C. brevicuspis populations for three defoliation frequencies, January 2013–March 2014. The data are expressed as the mean ± standard error (SE). ∗p < 0.05, ∗∗p < 0.01
Discussion
Repeated defoliation did not change the seasonal dynamics of the bud bank density of C. brevicuspis, which peaked in January and was lowest in March for all treatments. In addition, the TRB density of C. brevicuspis did not decrease significantly under repeated defoliation for 2 years (a total of 10 times for the monthly defoliation treatment). Therefore, our first hypothesis—that repeated defoliation would result in a decrease of the bud bank density—was proven false.
As a large number of buds sprout to replace the shoot population after defoliation, the number of dormant buds in the bud bank should decline after defoliation (Chen et al., 2015b). For example, grass bud banks decrease when the grass stem densities increase after grazing (Dalgleish and Hartnett, 2009). However, in the case of C. brevicuspis, which is a non-stem species, apical meristems were able to survive defoliation, as they are close to the ground (Chen et al., 2014). Defoliated ramets usually add buds as they regrow new leaves (Williams and Briske, 1991; Ott and Hartnett, 2011). Therefore, there were no significant differences in ramet or TRB density between defoliation treatments.
Nevertheless, the ramet height and the shoot, root, and rhizome bud biomass of C. brevicuspis decreased significantly under repeated defoliation. The results indicated that C. brevicuspis produces small-sized ramets and rhizome buds in response to repeated defoliation. Intense defoliation, which removes most of the photosynthetic tissues, usually reduces the plant’s growth and results in a smaller amount of carbon being stored (Ferraro and Oesterheld, 2002; Li et al., 2004; Zhao et al., 2008; Esmaeili et al., 2009; Liu and Li, 2010). The capacity of the plants to resprout from buds and grow after disturbances might be closely related to the carbon reserves in the perennial organs (Deng et al., 2013a), with bud production incurring significant opportunity and carbon allocation costs (Vesk and Westoby, 2004). Therefore, due to limited carbon storage after defoliation, C. brevicuspis might produce smaller individual ramet and rhizome buds.
Although the monthly defoliation treatment increased the density of short rhizomes in November 2013, the proportion of SRB to TRB did not change significantly during the study period. Therefore, our second hypothesis—that defoliation would promote a higher proportion of LRB and a lower proportion of SRB, creating a more spread-out growth form—was invalidated.
In S. scoparium, repeated defoliation was associated with a shift from vertical to more prostrate growth through changes in bud position, resulting in a greater proportion of tissue being inaccessible to herbivores (N’Guessan and Hartnett, 2011). In response to sedimentation or competitive stress, C. brevicuspis demonstrated a change from phalanx to guerrilla growth by producing a higher proportion of LRB and a lower proportion of SRB (Chen et al., 2011; Li et al., 2015). Previous studies indicated that long rhizomes enable tillers to escape from stressful microsites in a spatially heterogeneous habitat (de Kroon and Hutchings, 1995; Cheplick, 1997). However, the risk of being grazed may be equal for phalanx and guerrilla tillers, meaning that the grazing pressure may be homogenous for the C. brevicuspis tiller population. Furthermore, more energy may be required for the production of long rhizomes than for the production of short rhizomes (Cheplick, 1997). After defoliation, plants that allocate energy to produce long connections may be less competitive than plants that produce a dense population of ramets with short connections (Benot et al., 2009).
The maintenance of a large bud bank after repeated defoliation may contribute to the regeneration and persistence of C. brevicuspis populations in disturbed habitats. In C. brevicuspis, a large bud bank may confer greater ability to recover from severe damage than a small bud bank (Tuomi et al., 1994; Klimešová and Klimeš, 2007), potentially increasing the rates of shoot population recovery after disturbance (Dalgleish and Hartnett, 2009; Chen et al., 2015b). In addition, a large bud bank may increase the ability of clonal plants to respond to resource pulses such as increased precipitation or nutrient concentrations after disturbances (Dalgleish and Hartnett, 2006).
However, persistent grazing for many years may gradually deplete the amount of carbon stored and the bud banks, reducing the species’ capacity for recovery (N’Guessan and Hartnett, 2011; Qian et al., 2014). The present study also indicated that monthly defoliation reduced the biomass of rhizome buds and total biomass per ramet of C. brevicuspis, potentially affecting bud density in the long-term. Furthermore, small ramets and rhizomes of a C. brevicuspis population may be susceptible to invasion and replacement by exotic species (Dalgleish and Hartnett, 2009). The SRB and TRB density of C. brevicuspis were higher in January 2014 than in January 2013, especially in the control treatment, indicating inter-annual variation in bud bank density. Bud bank demography may be influenced by environmental factors such as soil water status and precipitation (Dalgleish and Hartnett, 2006; Deng et al., 2013b). The C. brevicuspis population that we studied was located in a natural reserve that experienced less herbivory than populations outside natural reserves. Tolerance to defoliation could differ among populations that have different histories of exposure to herbivores (Lu and Ding, 2012). Further investigation should include populations outside natural reserves and clarify the long-term effects of defoliation on bud banks of C. brevicuspis.
Conclusion
Our study demonstrated that the density, composition, and seasonal dynamics of bud banks did not change significantly in response to monthly or bimonthly defoliation for 2 years. Bud banks of C. brevicuspis appear to follow a conservative reproductive strategy and were tolerant to grazing. However, repeated defoliation significantly reduced the plant size and the amount of carbon stored in the rhizomes. Long-term, frequent defoliation could have a negative effect on bud bank density.
Author Contributions
X-SC and Y-HX wrote the manuscript and executed the technical assays and statistical analysis. X-SC and Y-HX designed the experiment and edited the manuscript text. Z-MD, FL, Z-YH, and CW contributed to data collection and interpretation. All authors reviewed the manuscript.
Funding
This work was supported by the Basic Work Program of the Ministry of Science and Technology of China (2013FY111800), the Knowledge Innovation Program of the Chinese Academy of Sciences (ISACX-LYQY-QN-1207), and the National Natural Science Foundation of China (31000143).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank X. Li, J. K. Gong, and Y. F. Li for assistance with the field investigation. We are especially grateful to two anonymous reviewers for providing helpful comments on an earlier version of the manuscript.
References
Benot, M., Mony, C., Puijalon, S., Mohammad-Esmaeili, M., Alphen, J., Bouzillé, J., et al. (2009). Responses of clonal architecture to experimental defoliation: a comparative study between ten grassland species. Plant Ecol. 201, 621–630. doi: 10.1007/s11258-008-9546-3
Benson, E. J., and Hartnett, D. C. (2006). The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecol. 187, 163–177. doi: 10.1007/s11258-005-0975-y
Benson, J., Hartnett, D. C., and Mann, K. H. (2004). Belowground bud banks and meristem limitation in tallgrass prairie plant populations. Am. J. Bot. 91, 416–421. doi: 10.3732/ajb.91.3.416
Bernard, J. M. (1990). Life history and vegetative reproduction in Carex. Can. J. Bot. 68, 1441–1448. doi: 10.1139/b90-182
Chen, X. S., Cao, C. S., Deng, Z. M., Xie, Y. H., Li, F., Hou, Z. Y., et al. (2015a). Assessment of regeneration potential in the clonal macrophyte Miscanthus sacchariflorus (Poaceae) after burial disturbance based on bud bank size and sprouting capacity. PLoS ONE 10:e012846. doi: 10.1371/journal.pone.0120846
Chen, X. S., Deng, Z. M., Xie, Y. H., Li, F., Hou, Z. Y., and Li, X. (2014). Demography of rhizome population of Carex brevicuspis (Cyperaceae): a wetland sedge produces both elongated and shortened rhizomes. Nord. J. Bot. 32, 51–256. doi: 10.1111/j.1756-1051.2013.00094.x
Chen, X. S., Deng, Z. M., Xie, Y. H., Li, F., Hou, Z. Y., and Li, X. (2015b). Belowground bud banks of four dominant macrophytes along a small-scale elevational gradient in Dongting Lake wetlands, China. Aquat. Bot. 122, 9–14. doi: 10.1016/j.aquabot.2014.12.006
Chen, X. S., Li, Y. F., Xie, Y. H., Deng, Z. M., Li, X., Li, F., et al. (2015c). Trade-off between allocation to reproductive ramets and rhizome buds in Carex brevicuspis populations along a small-scale elevational gradient. Sci. Rep. 5:e12688. doi: 10.1038/srep12688
Chen, X. S., Xie, Y. H., Deng, Z. M., Li, F., and Hou, Z. Y. (2011). A change from phalanx to guerrilla growth form is an effective strategy to acclimate to sedimentation in a wetland sedge species Carex brevicuspis (Cyperaceae). Flora 206, 347–350. doi: 10.1016/j.flora.2010.07.006
Cheplick, G. P. (1997). Responses to severe competitive stress in a clonal plant: differences between genotypes. Oikos 79, 581–591. doi: 10.2307/3546902
Cline, M. (1997). Concepts and terminology of apical dominance. Am. J. Bot. 84, 1064–1069. doi: 10.2307/2446149
Dai, L. K., Liang, S. Y., Zhang, S. R., Tang, Y. C., Koyama, T., Tucker, G. C., et al. (2010). Flora of China, Vol. 23 (Cyperaceae). St. Louis, MO: Missouri Botanical Garden Press.
Dalgleish, H. J., and Hartnett, D. (2006). Below-ground bud banks increase along a precipitation gradient of the North American great plains: a test of the meristem limitation hypothesis. New Phytol. 171, 81–89. doi: 10.1111/j.1469-8137.2006.01739.x
Dalgleish, H. J., and Hartnett, D. (2009). The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecol. 201, 411–420. doi: 10.1007/s11258-008-9562-3
de Kroon, H., and Hutchings, M. J. (1995). Morphological plasticity in clonal plants: the foraging concept reconsidered. J. Ecol. 83, 143–152. doi: 10.2307/2261158
Deng, Z. M., Chen, X. S., Xie, Y. H., Li, X., Pan, Y., and Li, F. (2013a). Effects of size and vertical distribution of buds on sprouting and plant growth of the clonal emergent macrophyte Miscanthus sacchariflorus (Poaceae). Aquat. Bot. 104, 121–126. doi: 10.1016/j.aquabot.2012.08.004
Deng, Z. M., Chen, X. S., Xie, Y. H., Pan, Y., Li, F., Hou, Z. Y., et al. (2013b). Plasticity of the clonal growth strategy of the wetland sedge Carex brevicuspis along an elevational gradient in Dongting Lake wetlands, China. Ann. Bot. Fenn. 50, 151–159. doi: 10.5735/085.050.0305
Deng, Z. M., Chen, X. S., Xie, Y. H., Xie, Y. J., Hou, Z. Y., and Li, F. (2015). The role of seedling recruitment from juvenile populations of Carex brevicuspis (Cyperaceae) at the Dongting Lake wetlands, China. Sci. Rep. 5:e8646. doi: 10.1038/srep08646
Esmaeili, M. M., Bonis, A., and Bouzillé, J. (2009). Consequence of ramet defoliation on plant clonal propagation and biomass allocation: example of five rhizomatous grassland species. Flora 204, 25–33. doi: 10.1016/j.flora.2007.11.008
Ferraro, D. O., and Oesterheld, M. (2002). Effects of defoliation on grass growth. A Quantitative Review. Oikos 98, 125–133. doi: 10.1034/j.1600-0706.2002.980113.x
Huang, J. M., Zou, Y. C., Cai, H. C., Qin, H., and Yu, Y. (2013). Change characteristics of the air temperature during the past 60a over Dongting Lake area. J. Meteorol. Sci. 33, 457–463.
Huhat, A., Lennartsson, T., Tuomi, J., Rautio, P., and Laine, K. (2000). Tolerance of Gentianella campestris in relation to damage intensity: an interplay between apical dominance and herbivory. Evol. Ecol. 14, 373–392. doi: 10.1023/A:1011028722860
Klimešová, J., and Klimeš, L. (2007). Bud banks and their role in vegetative regeneration–A literature review and proposal for simple classification and assessment. Perspect. Plant Ecol. Evol. Syst. 8, 115–129. doi: 10.1016/j.ppees.2006.10.002
Lehtilä, K. (2000). Modelling compensatory regrowth with bud dormancy and gradual activation of buds. Evol. Ecol. 14, 315–330. doi: 10.1023/A:1010869605855
Li, B., Shibuya, T., Yogo, Y., and Hara, T. (2004). Effects of ramet clipping and nutrient availability on growth and biomass allocation of yellow nutsedge. Ecol. Res. 19, 603–612. doi: 10.1111/j.1440-1703.2004.00685.x
Li, F., Xie, Y. H., Zhu, L. L., Jiang, L., Chen, X. S., Pan, B. H., et al. (2015). Changed clonal growth form induced by sand burial facilitates the acclimation of Carex brevicuspis to competition. PLoS ONE 10:e0121270. doi: 10.1371/journal.pone.0121270
Liu, Z. G., and Li, Z. Q. (2010). Effects of different grazing regimes on the morphological traits of Carex duriuscula on the Inner Mongolia steppe, China. N. Z. J. Agric. Res. 53, 5–12. doi: 10.1080/00288231003606047
Lu, X. M., and Ding, J. Q. (2012). History of exposure to herbivores increases the compensatory ability of an invasive plant. Biol. Invasions 14, 649–658. doi: 10.1007/s10530-011-0106-8
Mesa, L., Mayora, G., Saigo, M., and Giri, F. (2015). Nutrient dynamics in wetlands of the middle Paraná River subjected to rotational cattle management. Wetlands 35, 1117–1125. doi: 10.1007/s13157-015-0699-2
N’Guessan, M., and Hartnett, D. C. (2011). Differential responses to defoliation frequency in little bluestem (Schizachyrium scoparium) in tallgrass prairie: implications for herbivory tolerance and avoidance. Plant Ecol. 212, 1275–1285. doi: 10.1007/s11258-011-9904-4
Ott, J. P., and Hartnett, D. C. (2011). Bud production and dynamics of flowering and vegetative tillers in Andropogon gerardii (Poaceae): the role of developmental constraints. Am. J. Bot. 98, 1293–1298. doi: 10.3732/ajb.1000264
Ott, J. P., and Hartnett, D. C. (2012). Contrasting bud bank dynamics of two co-occurring grasses in tallgrass prairie: implications for grassland dynamics. Plant Ecol. 213, 1437–1448. doi: 10.1007/s11258-012-0102-9
Qian, J. Q., Wang, Z. W., Liu, Z. M., and Busso, C. A. (2014). Belowground bud bank responses to grazing intensity in the Inner-Mongolia steppe, China. Land Degrad. Develop. (in press). doi: 10.1002/ldr.2300
Smith, A. N., Vernes, K. A., and Ford, H. A. (2012). Grazing effects of black swans Cygnus atratus (Latham) on a seasonally flooded coastal wetland of eastern Australia. Hydrobiologia 697, 45–57. doi: 10.1007/s10750-012-1169-y
Sosnová, M., van Diggelen, R., and Klimešova, J. (2010). Distribution of clonal growth forms in wetlands. Aquat. Bot. 92, 33–39. doi: 10.1016/j.aquabot.2009.09.005
Strauss, S. Y., and Agrawal, A. A. (1999). The ecology and evolution of plant tolerance to herbivory. Tree 14, 179–185. doi: 10.1016/s0169-5347(98)01576-6
Terer, T., Triest, L., and Muasya, A. M. (2012). Effects of harvesting Cyperus papyrus in undisturbed wetland, Lake Naivasha, Kenya. Hydrobiologia 680, 135–148. doi: 10.1007/s10750-011-0910-2
Tiffin, P. (2000). Mechanisms of tolerance to herbivore damage: what do we know? Evol. Ecol. 14, 523–536. doi: 10.1023/A:1010881317261
Tuomi, J., Nilsson, P., and Astrom, M. (1994). Plant compensatory responses: bud dormancy as an adaptation to herbivory. Ecology 75, 1429–1436. doi: 10.2307/1937466
VanderWeide, B. L., and Hartnett, D. C. (2015). Belowground bud bank response to grazing under severe short-term drought. Oecologia 178, 795–806. doi: 10.1007/s00442-015-3249-y
Vesk, P. A. (2006). Plant size and resprouting ability: trading tolerance and avoidance of damage? J. Ecol. 94, 1027–1034. doi: 10.1111/j.1365-2745.2006.01154.x
Vesk, P. A., and Westoby, M. W. (2004). Funding the bud bank: a review of the costs of buds. Oikos 106, 200–208. doi: 10.1111/j.0030-1299.2004.13204.x
Williams, D. G., and Briske, D. D. (1991). The physiological individual in the bunchgrass Schizachyrium scoparium. Oikos 62, 41–47. doi: 10.2307/3545444
Zhang, J. T., Mu, C. S., Wang, D. L., Wang, J. F., and Chen, G. X. (2009). Shoot population recruitment from a bud bank over two seasons of undisturbed growth of Leymus chinensis. Botany 87, 1242–1249. doi: 10.1139/B09-080
Keywords: bud bank, clonal growth, clonal plant, disturbance, grazing, population regeneration
Citation: Chen X -S, Deng Z -M, Xie Y -H, Li F, Hou Z -Y and Wu C (2016) Consequences of Repeated Defoliation on Belowground Bud Banks of Carex brevicuspis (Cyperaceae) in the Dongting Lake Wetlands, China. Front. Plant Sci. 7:1119. doi: 10.3389/fpls.2016.01119
Received: 13 May 2016; Accepted: 13 July 2016;
Published: 29 July 2016.
Edited by:
Boris Rewald, University of Natural Resources and Life Sciences, Vienna, AustriaReviewed by:
Rubén Retuerto, University of Santiago de Compostela, SpainZhimin Liu, Institute of Applied Ecology, Chinese Academy of Sciences, China
Copyright © 2016 Chen, Deng, Xie, Li, Hou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Sheng Chen, xschen@isa.ac.cn Yong-Hong Xie, yonghongxie@163.com
 Xin-Sheng Chen
Xin-Sheng Chen Zheng-Miao Deng1,2
Zheng-Miao Deng1,2