- 1Centre for Resources, Environment and Food Security, Department of Plant Nutrition, Key Laboratory of Plant-Soil Interactions, Ministry of Education, China Agricultural University, Beijing, China
- 2College of Life Science, Hebei University, Baoding, China
- 3Soil Science and Plant Nutrition, School of Earth and Environment, The UWA Institute of Agriculture, The University of Western Australia, Crawley, WA, Australia
- 4Rothamsted Research, Harpenden, UK
The relationship between root morphological and physiological responses to variable P supply in different plant species is poorly understood. We compared root morphological and physiological responses to P supply in seven crop species (Zea mays, Triticum aestivum, Brassica napus, Lupinus albus, Glycine max, Vicia faba, Cicer arietinum) treated with or without 100 mg P kg-1 in two soils (acidic and calcareous). Phosphorus deficiency decreased root length more in fibrous root species (Zea mays, Triticum aestivum, Brassica napus) than legumes. Zea mays and Triticum aestivum had higher root/shoot biomass ratio and Brassica napus had higher specific root length compared to legumes, whereas legumes (except soybean) had higher carboxylate exudation than fibrous root species. Lupinus albus exhibited the highest P-acquisition efficiency due to high exudation of carboxylates and acid phosphatases. Lupinus albus and Cicer arietinum depended mostly on root exudation (i.e., physiological response) to enhance P acquisition, whereas Zea mays, Triticum aestivum and Brassica napus had higher root morphology dependence, with Glycine max and Vicia faba in between. Principal component analysis using six morphological and six physiological responses identified root size and diameter as the most important morphological traits, whereas important physiological responses included carboxylate exudation, and P-acquisition and P-utilization efficiency followed by rhizosphere soil pH and acid phosphatase activity. In conclusion, plant species can be grouped on the basis of their response to soil P being primarily via root architectural or exudation plasticity, suggesting a potential benefit of crop-specific root-trait-based management to cope with variable soil P supply in sustainable grain production.
Introduction
Phosphorus (P) is an essential macronutrient for plant growth and metabolism. It is a structural element in nucleic acids and membrane phospholipids. However, P nutrition is a major limiting factor for crop production in many soils due to relatively low P availability because P can be readily adsorbed or fixed by free lime present in some calcareous soils (CS) and by aluminium (Al) and iron (Fe) in acid soils (AS) (Hinsinger, 2001; Shen et al., 2011). Around 70–90% of P applied as fertilizer may become unavailable to plants (Holford, 1997), exacerbating economic losses from fertilizer-P overuse in intensive agriculture (Shen et al., 2011). On the other hand, P is a limited and non-renewable resource; current estimates suggest that economic P supply may be severely depleted over the next 300 years, although some research indicates that estimates of P reserves have increased by more than threefold recently (Oelkers and Valsami-Jones, 2008; Cordell et al., 2011; Elser and Bennett, 2011; Cordell and White, 2013). Improvement of P-acquisition efficiency through mobilizing the residual P accumulated in soil, as well as enhancing root absorbing surface and acquisition capacity for P applied to soil, is critical for sustainable P management and food production (Shen et al., 2011, 2013; Li et al., 2014).
In response to low concentration of available P in the rhizosphere, plants have developed highly specialized root morphological (e.g., increases in root growth rate, specific root length (SRL), lateral density and elongation, and density and length of root hairs) and physiological (e.g., carboxylate exudation, proton release, and phosphatase secretion) adaptations to increase P acquisition from soil (Neumann et al., 1999; Hinsinger, 2001; Lambers et al., 2006; Shen et al., 2011). The ultimate consequence of these modifications is increased P availability and acquisition via (1) increasing a rhizosphere soil volume exploited by an enlarged root length/surface area to improve soil P spatial availability, and (2) enhancing P availability by mobilizing P in the rhizosphere via root exudation (Raghothama, 1999; Rengel and Marschner, 2005; Richardson et al., 2009; Shen et al., 2011).
Many studies showed a range of adaptive strategies (especially in terms of root growth and rhizosphere processes) evolved to cope with limited P availability and allow efficient P acquisition by different plant species (Lambers et al., 2006; Zhang et al., 2010; Shen et al., 2011, 2013). For example, P-deficient Brassica napus had high P influx rates, whereas P-deficient Triticum aestivum had high root/shoot ratios to enhance P-acquisition efficiency (Föhse et al., 1988). Under low P supply, the root/shoot ratio increased in Zea mays and Triticum aestivum significantly, and Triticum aestivum had increased SRL (Nuruzzaman et al., 2005; Pearse et al., 2006a, 2007; Calderón-Vázquez et al., 2009). In contrast, there was no difference in root carboxylate exudation by Zea mays or Triticum aestivum between P-sufficient and P-deficient plants (Pearse et al., 2006b, 2007; Carvalhais et al., 2011). However, for Brassica napus, P deficiency increased the root hair density and length and enhanced exudation of protons and carboxylates (Foehse and Jungk, 1983; Moorby et al., 1988; Hoffland et al., 1989a,b). The exudation of acid phosphatase by Brassica napus increased with increasing P supply (Marschner et al., 2006, 2007; Solaiman et al., 2007; Zhang et al., 2009).
Legume plants enhance rhizosphere chemical processes more than cereal crops to mobilize sparingly soluble soil P by rhizosphere acidification and enhanced exudation of carboxylates and phosphatases (Houlton et al., 2008; Shen et al., 2013; Li et al., 2014). For example, Lupinus albus could respond to P deficiency stress by forming cluster roots (Gardner et al., 1982) accompanied by high exudation of carboxylates, protons and acid phosphatase from such roots, which greatly enhanced P acquisition from soil (Tadano et al., 1993; Neumann et al., 1999; Yan et al., 2002; Shen et al., 2003; Vance et al., 2003; Lambers et al., 2006; Wang et al., 2007; Cheng et al., 2014). In addition, P uptake by Cicer arietinum exhibited a positive correlation with rate of carboxylate exudation into the rhizosphere (Veneklaas et al., 2003; Wouterlood et al., 2004; Rose et al., 2010) as well as with the activity of acid phosphatase (APase) extruded by roots (Li et al., 2004; Pearse et al., 2006b, 2007). Phosphorus deficiency strongly increased proton release from roots of tomato, chickpea, and white lupin, but only small effects were observed in wheat (Neumann and Römheld, 1999). Compared with white lupin, root exudation of carboxylates under P deficiency was lower in tomato, wheat and chickpea (Neumann and Römheld, 1999).
Soil acid phosphatase activity was higher in the rhizosphere of Cicer arietinum than Zea mays regardless of P sources (Li et al., 2004). While root morphological traits in Cicer arietinum had a minor contribution to potentially enhancing P uptake in the low-P environments, the concentration of carboxylates in the rhizosphere increased 10-fold (Veneklaas et al., 2003). Under P-deficient conditions, roots of Glycine max exuded more carboxylates than Zea mays, but much less than Cicer arietinum, Vicia faba, and Lupinus albus (Ohwaki and Hirata, 1992; Watt and Evans, 2003; Li et al., 2007). Vicia faba released smaller amounts of protons and carboxylates into rhizosphere than Cicer arietinum, but much greater than Triticum aestivum and Zea mays (Zhou et al., 2009; Li et al., 2010; Rose et al., 2010). In Maltais-Landry’s (2015) study, legumes (Vicia faba and Pisum sativum) had higher organic acid concentration and phosphatase activity in the rhizosphere compared with cereals crops (Secale cereale, Avena sativa, and Triticum aestivum).
To reiterate, plants can enhance phosphorus (P) acquisition from soil via modifying root morphological and physiological traits. We assumed that some plant species were mainly dependent on the enhanced root growth and spatial distribution of roots, whereas others could dominantly rely on increased root exudation to mobilize soil P; a strategy combining both types of responses is also possible. Understanding the complexity of the relationships between root morphological and physiological responses across different plant species is critical for improved manipulation of the root and rhizosphere processes to increase P-acquisition efficiency for a given plant species.
In this study, we hypothesized an existence of different combinations of strategies related to root morphological and physiological adaptations to cope with variable P supply in seven plant species with contrasting root systems. We intended to determine if plant species could be grouped based on their response to soil P being predominantly via root architectural plasticity (morphological response) or exudation (physiological response). We chose seven plant species widely grown in agriculture, having either fibrous (Zea mays, Triticum aestivum, and Brassica napus) or tap-rooted root systems (Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum) to test the above hypothesis. Our objective was to characterize the relationship between root morphological and physiological responses to P supply in different plant species with contrasting root systems in AS or CS.
Materials and Methods
Plant and Soil Materials
Seven plant species were tested in the study, including those with fibrous roots (Zea mays L. cv. NE15 (Zm), Triticum aestivum L. cv. KN9204 (Ta), Brassica napus L. cv. LY5 (Bn)) and tap-rooted legumes (Lupinus albus L. Kiev mutant (La), Glycine max (L.) Merr. cv. HX1 (Gm), Vicia faba L. cv. LC5 (Vf) and Cicer arietinum L. cv. LY1 (Ca)). In this study, Brassica napus was factitiously regarded as a fibrous root species due to its fine root system based on our previous study (Solaiman et al., 2007).
Acid soil and CS with low P availability were collected from the top 20 cm of unfertilized native vegetation sites: AS in Guangdong, South China (23° 27′ 21″ N, 114° 31′ 39″ E), and CS in Beijing area, North China (40° 8’ 5? N, 116° 11’ 3? E). AS had the following properties (in parentheses: CS): pH 5.5 (8.2) in 1:5 soil:CaCl2, organic carbon 14.6 (11.5) g kg-1, total N 0.37 (0.72) g kg-1, Olsen-P 7.1 (2.6) mg kg-1, and NH4Ac-extractable K 44 (32) mg kg-1. The soils were air-dried and sieved to 2 mm prior to potting. Field capacity moisture contents were determined to be 35% w/w for AS and 32% w/w for CS according to Rose et al. (2010).
Plant Growth
The experiment was conducted in a glasshouse at China Agricultural University, Beijing (40° 1′ 46″ N, 116° 17′ 11″ E). Plants were grown in 240-mm-wide and 190-mm-deep plastic pots containing 3 kg soil each. Phosphorus was added to soils as KH2PO4 at a rate of 100 (P-sufficient) or 0 (P-deficient) mg P kg-1 soil. All other basal nutrients were provided as follows (mg per pot): Ca(NO3)2⋅4H2O 5060; K2SO4 400; CaCl2 377; MgSO4⋅7H2O 130; EDTA-Fe 16.5; MnSO4⋅H2O 20; ZnSO4⋅7H2O 30; CuSO4⋅5H2O 6; H3BO3 2; and (NH4)6Mo7O24⋅4H2O 0.365. There were four replicates in each treatment. Soils were irrigated to approximately 80% field capacity with deionized water prior to sowing.
Seeds of all species were surface-sterilized by 10% v/v H2O2 for 30 min, rinsed with water and placed in a dish containing aerated saturated solution of CaSO4 at 26°C in the dark until a radicle emerged. Six germinated seeds of uniform size were sown in each pot. Seedlings were thinned to four plants per pot 5 days after germination. Each pot was watered daily to 80% field capacity as measured by weight. Temperature ranged from a minimum of 22°C at night to a maximum of 30°C during the day.
Plant Harvest and Root Sampling
Plants were harvested 40 days after germination; at this time visual differences in growth between P treatments or soil types could be observed (Figure 1). The method for rhizosphere exudate collection was modified from Pearse et al. (2007). The pots were squeezed gently to allow dislodgement of the soil column and loosening of soil around roots. Plants were then gently lifted from soil and shaken lightly to remove bulk soil from the root systems. The root system was then transferred into a 200-mL vial containing 50 mL of 0.2 mmol L-1 CaCl2. Roots were gently dunked for 60 s to remove as much rhizosphere soil as possible; care was taken to minimize root damage and thus leakage of solutes from damaged cells. After removing roots, the containers were shaken by hand, and 0.5 mL of soil suspension was transferred into a 2-mL centrifuge tube for measurement of acid phosphatase (APase) activity (Alvey et al., 2001), representing secretory acid phosphatase (Neumann et al., 1999; Shen et al., 2005). A sub-sample of 10 mL supernatant from soil suspension was kept in a vial [with addition of microbial inhibitor Micropur (Sicheres Trinkwasser, Germany) at 0.01 g L-1 and also three drops of concentrated phosphoric acid] at –20°C until analysis of carboxylates by HPLC that was done after passing the supernatant through a 0.22-μm filter according to the method developed in our lab (Shen et al., 2003; Wang et al., 2007).
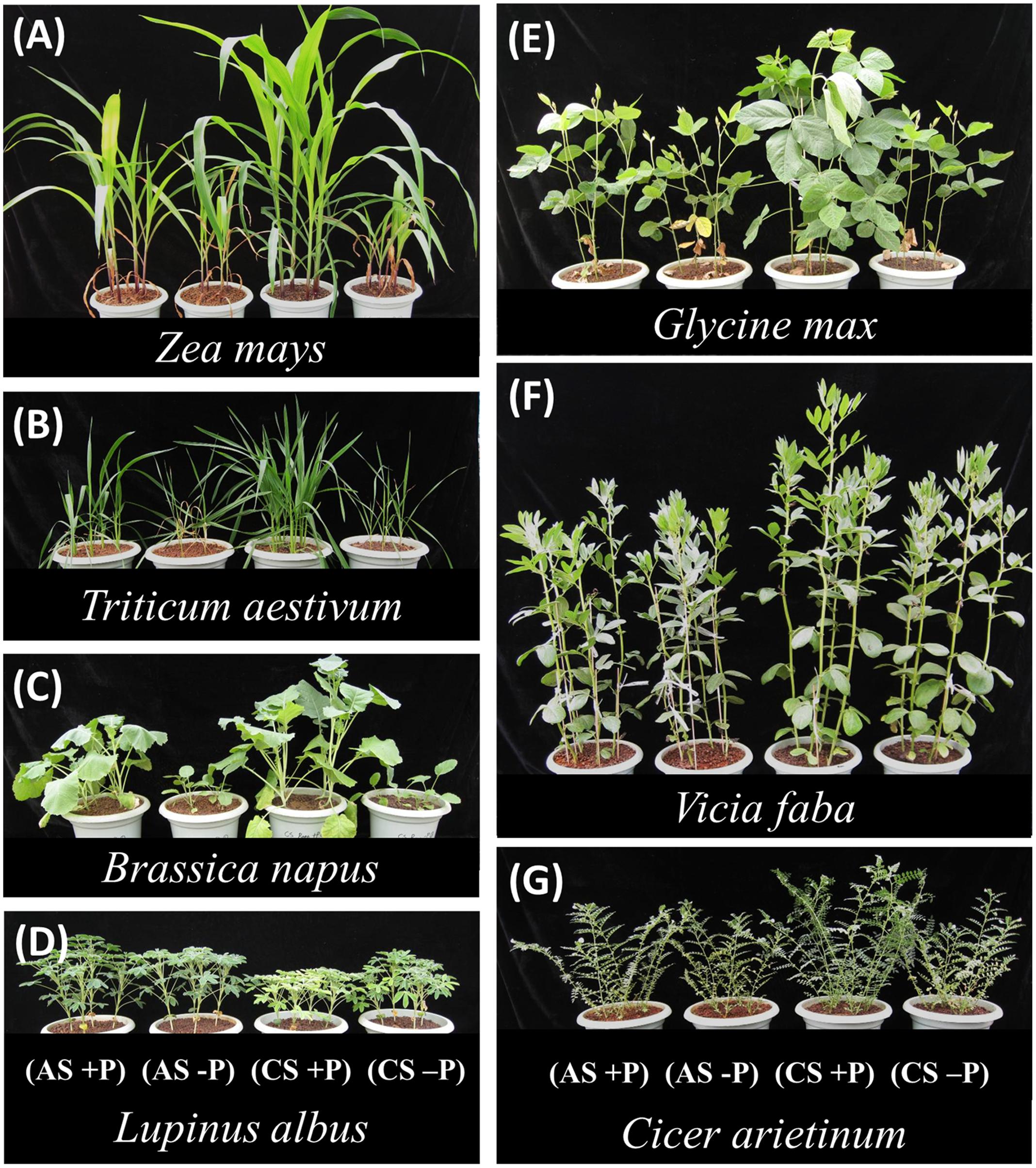
FIGURE 1. Shoot growth of seven species after 40 days (just before harvest). For each species, the treatments are (from left to right): acid soil (AS) with P addition (100 mg P kg-1 soil) (AS+P), AS without P addition (AS-P), calcareous soil (CS) with P addition (100 mg P kg-1 soil) (CS+P) and CS without P addition (CS-P).
Root Parameter Measurement
The roots from each pot were washed out of soil with water and evenly spread apart on a transparent tray (25 cm × 19 cm) to get images at a resolution of 600 dpi (dots per inch) with an Epson Perfection V700 dual lens scanning system. Root images were analyzed for total root length and total root surface area using WinRHIZO software (Pro 2009b, Regent Instruments Inc., Quebec, QC, Canada).
Plant Biomass and Phosphorus Uptake
Shoots and roots were oven-dried at 70°C for 3 days until constant weight to measure biomass. Shoots and roots were ground into powder, and then weighed and digested with a mixture of concentrated H2SO4 and H2O2 (modified from Thomas et al., 1967). Phosphorus content was determined using the vanado-molybdate method (Westerman, 1990).
Phosphorus-acquisition efficiency refers to the ability of plants to acquire P from soils, and P-utilization efficiency is the capacity to produce biomass or yield using the P taken up (Hammond et al., 2009; Wang X.R. et al., 2010). In this study, P-acquisition efficiency was calculated by dividing the P content of whole plant (shoots + roots) by total root length; P-utilization efficiency was calculated by dividing the total plant biomass by whole-plant P content.
Acid Phosphatase Activity and Carboxylate Analysis
Acid phosphatase activity in the rhizosphere was measured using a spectrophotometric method based on the measurement of p-nitrophenol (PNP) absorbance at 405 nm (Alvey et al., 2001). Carboxylates in the rhizosphere soil were analyzed using a reversed phase high-performance liquid chromatography (HPLC) system according to a previous report (modified from Shen et al., 2003; Wang et al., 2007; Wang B.L. et al., 2010). The chromatographic separation was conducted on a 250 mm × 4.6 mm reversed-phase column (Alltima C18, 5 μm; Alltech Associates, Inc., Deerfield, IL, USA). The mobile phase was 25 mmol L-1 KH2PO4 (pH 2.25) with a flow rate of 1 mL min-1 at 31°C, and detection of carboxylates was carried out at 214 nm.
Statistical Analyses
Analysis of variance was conducted using the SAS statistical software (SAS 2001, Version 6.1, SAS Institute Inc., USA). The LSD multiple range comparisons were performed at the 5, 1, and 0.1% probability level (0.01 < P ≤ 0.05, 0.001 < P ≤ 0.01, and P ≤ 0.001).
Principal component analysis (PCA) was used to evaluate the relative responses of root morphological and physiological traits to P deficiency in two soil types based on our previous method (Tang et al., 2013b). Six root morphological variables (total root surface area, root biomass, total root length, root/shoot ratio, SRL, and specific root surface area) and six root physiological variables (malate exudation, citrate exudation, P-acquisition efficiency, acid phosphatase activity, rhizosphere pH, P-utilization efficiency) were analyzed. We did not use the root clusters as a morphological parameter in this PCA analysis because only one (Lupinus albus) out of seven species tested had these special root structures (Supplementary Figure S1). The first three principal components were used to describe the relative responses of seven plant species to P deficiency in two soil types and to calculate the total scores for root morphological or physiological response variables.
Results
Biomass Accumulation and Distribution
The ANOVA analysis showed that shoot dry weight varied among species depending on differential P supply and soil types (Supplementary Table S1). Phosphorus supply significantly stimulated shoot growth of all species, except Lupinus albus in CS (Figures 2A,B). The magnitude of shoot dry weight response to P application in AS followed the order Brassica napus (+598%) > Triticum aestivum (+410%) > Zea mays (+325%) > Cicer arietinum (+63%) > Glycine max (+44%) > Lupinus albus (+17%) > Vicia faba (+11%), and the order in the CS was Brassica napus (+1093%) > Zea mays (+749%) > Triticum aestivum (+704%) > Glycine max (+155%) > Cicer arietinum (+131%) > Vicia faba (+27%) > Lupinus albus (+2%). The fibrous root species Zea mays, Triticum aestivum, and Brassica napus exhibited greater shoot growth responses to P supply than the tap-rooted legumes Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum regardless of soil type. However, the general response of shoot dry weight to P application was greater in CS than AS (P < 0.01), despite a variation among different plant species. In AS, the species accumulated shoot biomass in the order Vicia faba > Zea mays > Glycine max > Lupinus albus > Cicer arietinum > Brassica napus > Triticum aestivum. In CS, the order was Zea mays > Vicia faba > Glycine max > Cicer arietinum > Brassica napus > Triticum aestivum > Lupinus albus.
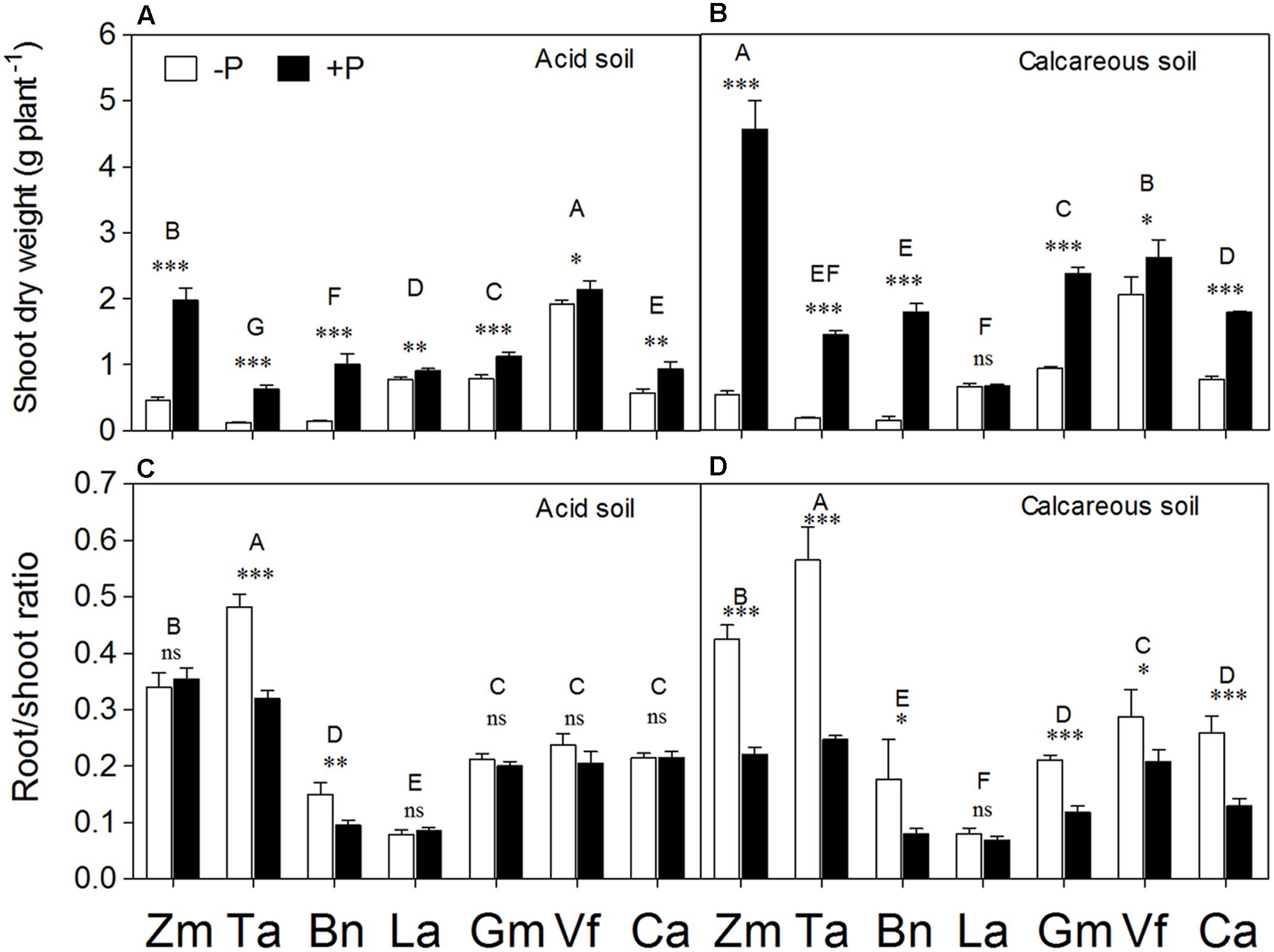
FIGURE 2. Shoot dry weight (A,B) and root/shoot ratio (C,D) of Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 (open bars) or 100 mg P kg-1 soil (closed bars) in AS (A,C) or CS (B,D). Each value is the mean (+SE) of four replicates. Different letters denote significant differences among seven plant species (P ≤ 0.05). For a given species, asterisks indicate significant difference between the P treatments: ns (non-significant), ∗(0.01 < P ≤ 0.05),∗∗(0.001 < P ≤ 0.01), and ∗∗∗(P ≤ 0.001).
Zea mays and Triticum aestivum had higher root/shoot ratio relative to other species in both soils (Figures 2C,D). Lupinus albus had the lowest root/shoot ratio among all the species, with a relatively weak response to differential P supply in both soils. In CS, P deficiency significantly enhanced the root/shoot ratio of plant species, except in Lupinus albus (Figures 2C,D). The fibrous root species Zea mays, Triticum aestivum, and Brassica napus growing in CS exhibited more evident effects of P deficiency on increasing root/shoot ratio compared with the tap-rooted legume species Vicia faba, Glycine max, Lupinus albus, and Cicer arietinum. In contrast, in AS, the low-P conditions significantly enhanced root/shoot ratio only in Triticum aestivum and Brassica napus. Nevertheless, there were no significant differences in root/shoot ratio due to the soil factor (Supplementary Table S1).
Root Morphology
The fibrous root species Zea mays, Triticum aestivum, and Brassica napus exhibited greater responses of total root length to P supply compared with the tap-rooted legumes Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum in both soils (Figures 3A,B). In AS, total root length per plant was in the order of Zea mays > Triticum aestivum > Vicia faba > Glycine max > Brassica napus > Cicer arietinum > Lupinus albus. In CS, the order was Triticum aestivum > Vicia faba > Zea mays = Brassica napus > Glycine max = Cicer arietinum > Lupinus albus. Phosphorus application significantly enhanced total root length of Zea mays, Triticum aestivum, Brassica napus, Glycine max and Cicer arietinum, but not of Lupinus albus and Vicia faba in the two soil types.
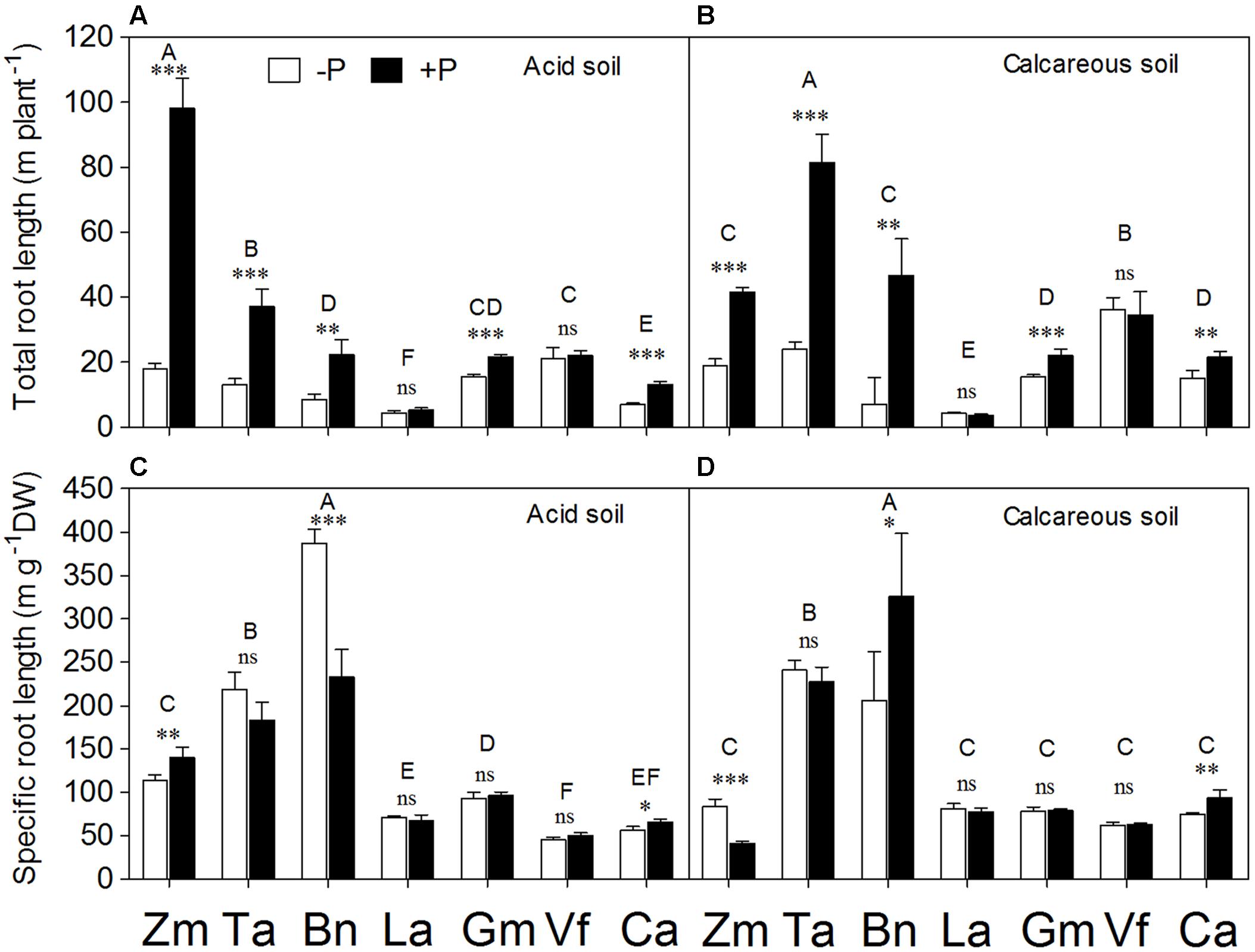
FIGURE 3. Total root length (A,B) and specific root length (SRL) (C,D) of Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 (open bars) or 100 mg P kg-1 soil (closed bars) in AS (A,C) or CS (B,D). Each value is the mean (+SE) of four replicates. Different letters denote significant differences among seven plant species (P ≤ 0.05). For a given species, asterisks indicate significant difference between the P treatments: ns (non-significant), ∗(0.01 < P ≤ 0.05),∗∗(0.001 < P ≤ 0.01), and ∗∗∗(P ≤ 0.001).
Specific root length (ratio of total root length to root biomass) showed significant differences among species, but not between soil types, or P levels (Supplementary Table S1). In both soils, Brassica napus had the highest SRL, followed by Triticum aestivum (Figures 3C,D). Phosphorus deficiency significantly increased SRL of Brassica napus in AS, but a reverse occurred in CS. In contrast, P deficiency significantly increased SRL of Zea mays in CS, but decreased it in AS. Cicer arietinum exhibited a higher SRL in the +P than –P treatments in both soils. There was no significant difference in SRL between the P treatments in Triticum aestivum, Lupinus albus, Glycine max, and Vicia faba regardless of the soil.
Acid Phosphatase Activity
Lupinus albus had the highest APase activity among plant species (on average 3.5 and 7.6 times higher in AS and CS, respectively), but there was no evident response of Lupinus albus to P application (Figures 4A,B). In AS, P deficiency did not change the activity of APase in the rhizosphere of any of the species except Glycine max in which an increase occurred with P application. In CS, Triticum aestivum, Glycine max, Vicia faba, and Cicer arietinum exhibited significantly higher activity of APase under P deficiency relative to the P-sufficient treatment, but Zea mays showed an opposite response.
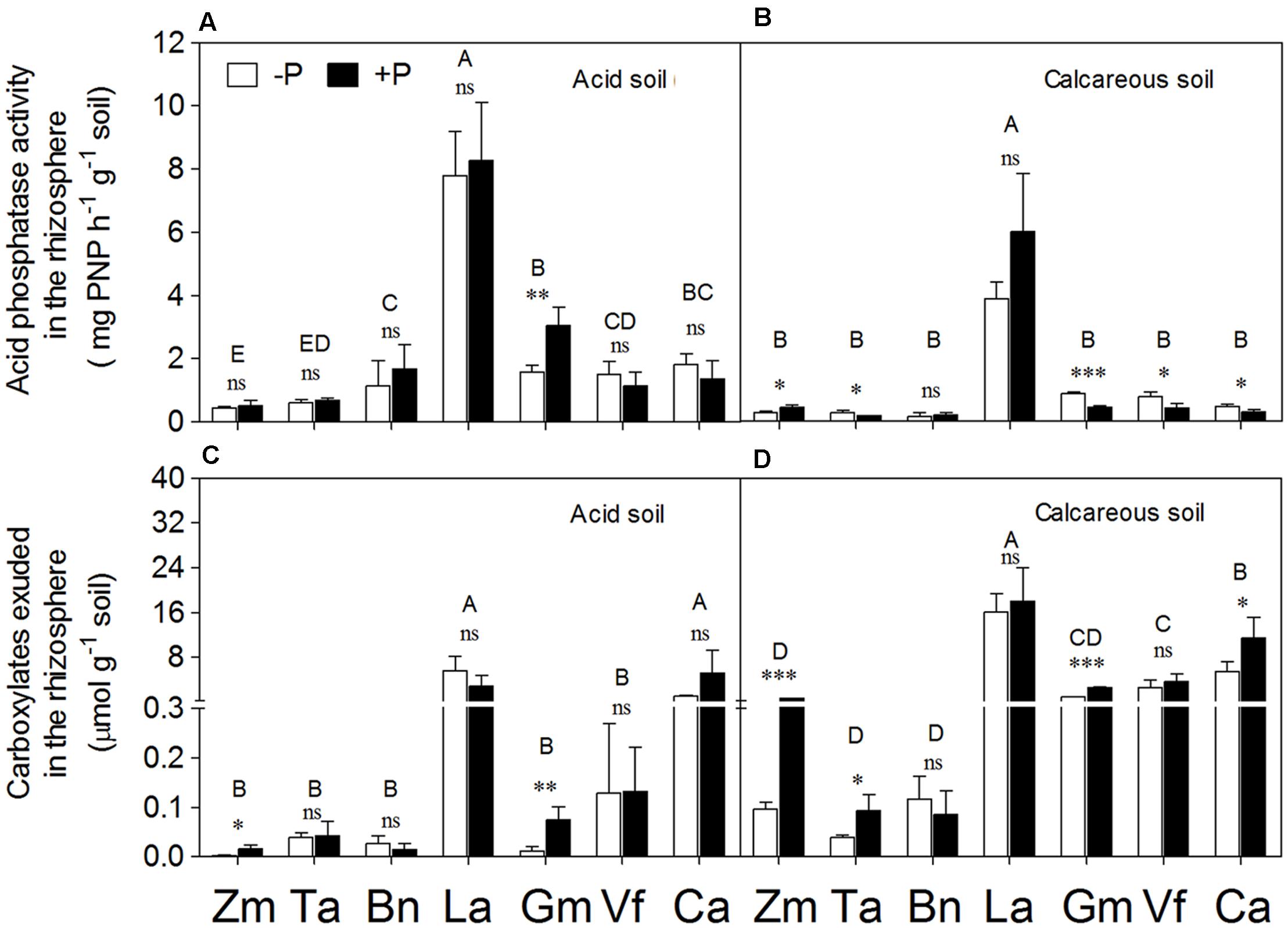
FIGURE 4. Acid phosphatase activity (A,B) and total carboxylate concentration (C,D) in the rhizosphere of Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 (open bars) or 100 mg P kg-1 soil (closed bars) in AS (A,C) or CS (B,D). Each value is the mean (+SE) of four replicates. Different letters denote significant differences among seven plant species (P ≤ 0.05). For a given species, asterisks indicate significant difference between the P treatments: ns (non-significant), ∗(0.01 < P ≤ 0.05),∗∗(0.001 < P ≤ 0.01), and ∗∗∗(P ≤ 0.001).
Carboxylate Exudation into the Rhizosphere
Significantly higher amounts of carboxylates were measured in the rhizosphere of Lupinus albus and Cicer arietinum relative to the other five species in AS (Figures 4C,D). A similar trend was found in CS, with the tap-rooted legumes Lupinus albus, Vicia faba, and Cicer arietinum releasing a larger amount of carboxylates to the rhizosphere than the fibrous-root species Zea mays, Triticum aestivum, and Brassica napus.
Compared to the P-sufficiency treatment, P-deficiency stress did not increase carboxylate exudation into the rhizosphere in either soil type. On the contrary, P application stimulated carboxylate exudation in Zea mays and Glycine max in both soils and in Triticum aestivum and Cicer arietinum in CS.
Carboxylate composition varied among species. Malate and citrate were the two major carboxylates in the rhizosphere of all the species except Zea mays (Figure 5). In CS, Zea mays was the only species that not just exuded trans-aconitate, but exuded it as the major carboxylate (on average, 88% of total). When Zea mays was grown in AS, trans-aconitate was not found in the -P treatment, but represented 33% of all carboxylates in the +P treatment, whereas fumarate was 83% of all carboxylates in the P-deficient treatment and almost 50% in the P-sufficient treatment. Triticum aestivum, Brassica napus, Lupinus albus, Glycine max, and Vicia faba increased the percentage of citrate in root exudates under P deficiency compared with the +P treatment in both soil types. On the contrary, in AS, Cicer arietinum decreased the proportion of citrate exuded under P deficiency, but increased malate exudation compared with CS.
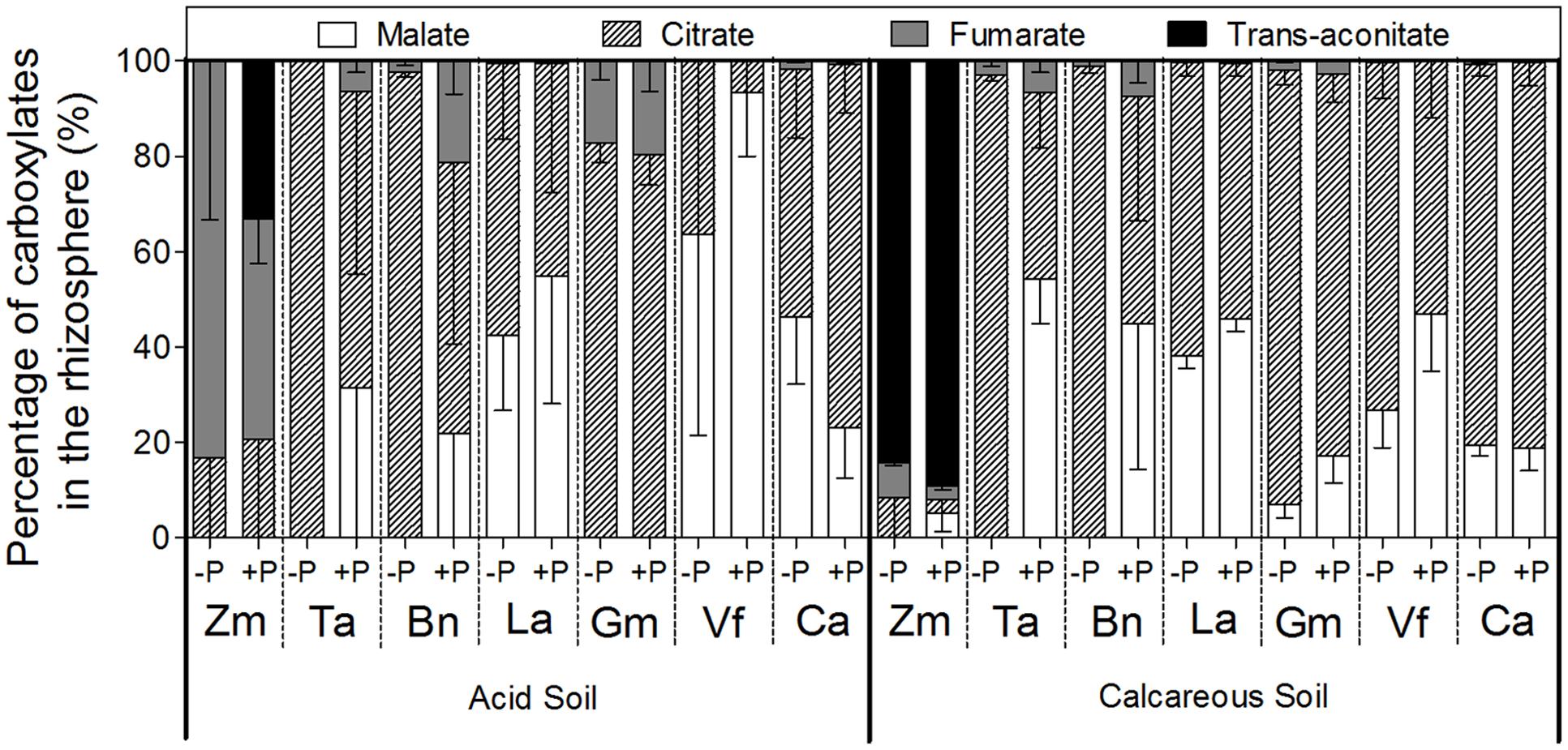
FIGURE 5. Proportion of various carboxylates in the rhizosphere soil of Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 (–P) or 100 mg P kg-1 soil (+P) in AS or CS. Each value is the mean (+SE) of four replicates.
Shoot P Concentration and Content
Phosphorus application significantly increased shoot P concentration in all species × soil combinations, except Zea mays, Lupinus albus, and Cicer arietinum in AS (Figures 6A,B). The effect of P supply on increased shoot P concentration became more evident in calcareous than AS. Similar to shoot P concentration, the +P treatment significantly increased shoot P content in all species in two soils, except Lupinus albus in AS (Figures 6C,D). In particular, huge differences in shoot P content were noted in CS, with 23–24 times greater P content in shoots of Zea mays and Triticum aestivum in the +P than –P treatments.
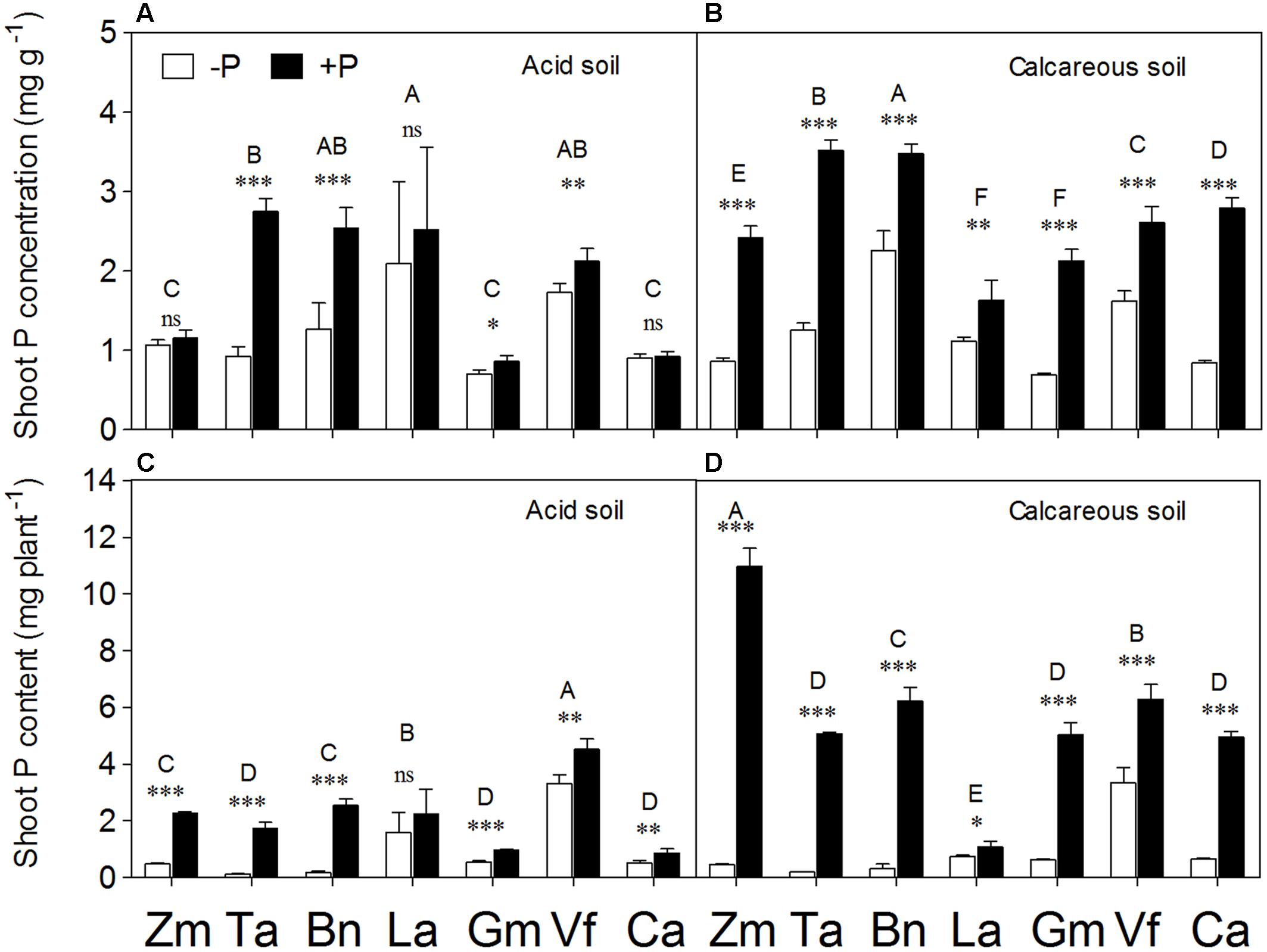
FIGURE 6. Phosphorus concentration (A,B) and content (C,D) in the shoot tissues of Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 (open bars) or 100 mg P kg-1 soil (closed bars) in AS (A,C) or CS (B,D). Each value is the mean (+SE) of four replicates. Different letters denote significant differences among seven plant species (P ≤ 0.05). For a given species, asterisks indicate significant difference between the P treatments: ns (non-significant), ∗(0.01 < P ≤ 0.05),∗∗(0.001 < P ≤ 0.01), and ∗∗∗(P ≤ 0.001).
P-Acquisition and P-Utilization Efficiency
Plant species and P supply had the significant effects on P-acquisition and P-utilization efficiency (Supplementary Table S1). Lupinus albus had the highest P-acquisition efficiency among all species in both soil types (Figures 7A,B). In general, all species had a significant increase in P-acquisition efficiency in the +P relative to P-deficiency treatment, except Lupinus albus and Cicer arietinum in AS and Brassica napus in CS. In contrast, P deficiency significantly increased P-utilization efficiency in all species compared with the +P treatment, except Zea mays, Lupinus albus, and Cicer arietinum in AS.
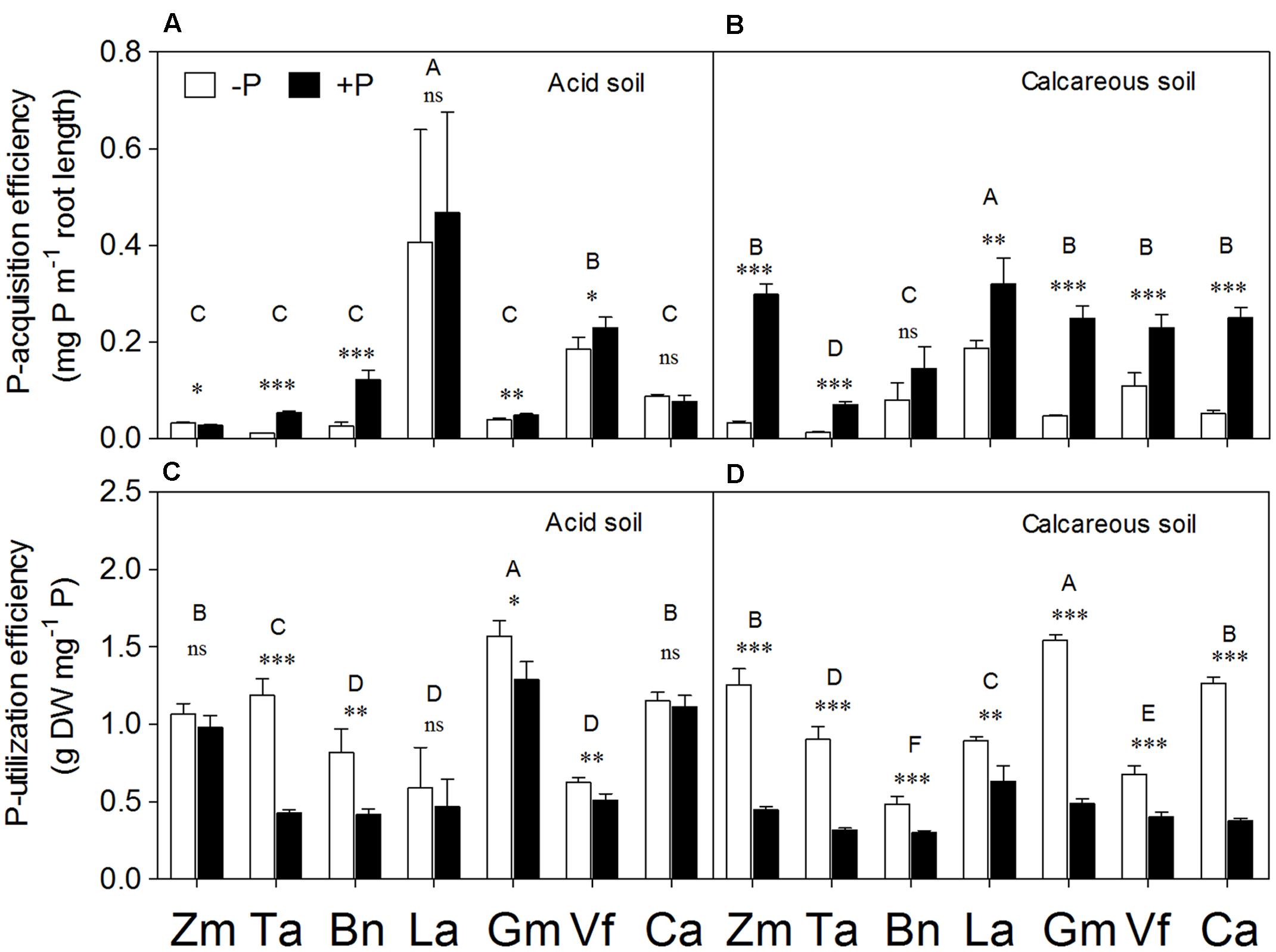
FIGURE 7. Phosphorus-acquisition efficiency (A,B) and P-utilization efficiency (C,D) of Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 (open bars) or 100 mg P kg-1 soil (closed bars) in AS (A,C) or CS (B,D). Each value is the mean (+SE) of four replicates. Different letters denote significant differences among seven plant species(P ≤ 0.05). For a given species, asterisks indicate significant difference between the P treatments: ns (non-significant), ∗(0.01 < P ≤ 0.05),∗∗(0.001 < P ≤ 0.01), and ∗∗∗ (P ≤ 0.001).
Root Morphological and Physiological Responses
For the root morphological responses, the first principal component (PC1) was related to root size and accounted for 45% of the total variance. Principal component 2 was root diameter (root fineness), explaining 33% of the total variance. Principal component 3 was root/shoot ratio, accounting for 15% of total variance. For the root physiological responses, the PC 1 was related to carboxylate exudation into the rhizosphere, explaining 35% of the total variance. The PC 2 represented P-acquisition and P-utilization efficiency, accounting for 28% of the total variance. The PC 3 comprised rhizosphere soil pH and acid phosphatase activity, accounting for 22% of total variance (Supplementary Table S2).
All plant species showed a wide variation in morphological and physiological responses to variable soil P supply (Figure 8). Zea mays grown in both soils had the root morphology scores significantly increased with P addition, whereas root physiology scores did not show differences. A similar trend was also found in Triticum aestivum and Brassica napus. In contrast to the fibrous-root species, Lupinus albus and Cicer arietinum had significant differences in root physiology scores and nearly no differences in root morphology scores with differential P supply. Glycine max and Vicia faba did not show significant differences in either morphological or physiological scores with differential P supply.
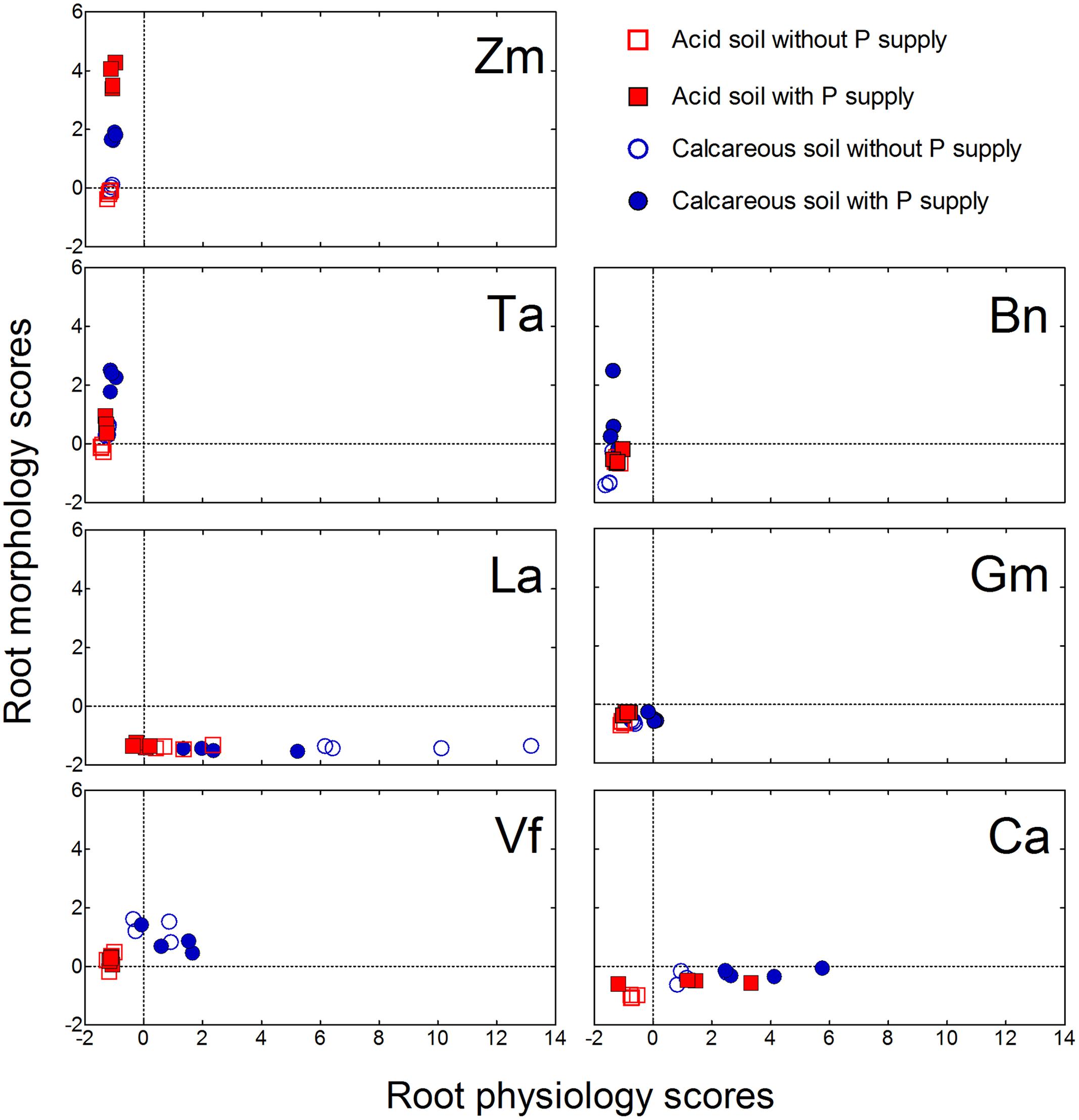
FIGURE 8. Principal components analysis of root morphological and physiological responses to variable phosphorus supply and soil types in Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca). Each symbol represents one replicate.
There were large differences in average root morphology and physiology scores among the plant species. Zea mays had the highest root morphology scores, followed by Triticum aestivum = Vicia faba > Brassica napus = Glycine max = Cicer arietinum > Lupinus albus. In contrast, Lupinus albus had the highest root physiology scores, followed by Cicer arietinum > Vicia faba = Glycine max > Zea mays > Triticum aestivum = Brassica napus (Figure 9).
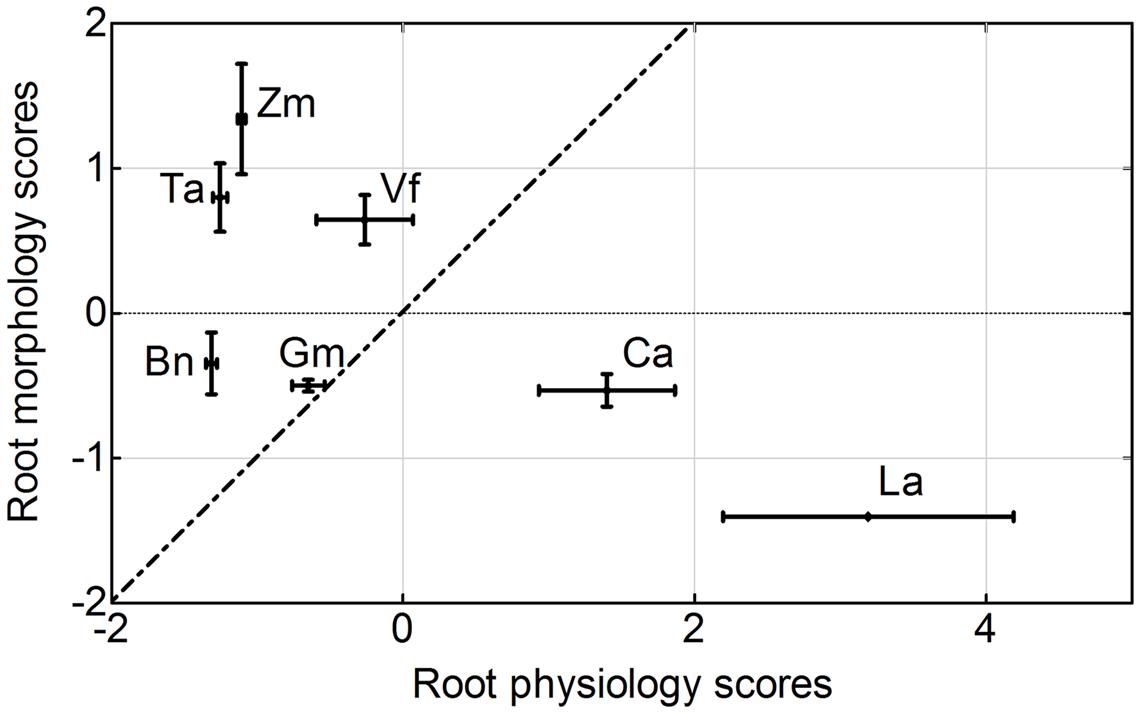
FIGURE 9. Principal components analysis of root morphological and physiological responses to variable phosphorus supply by Zea mays (Zm), Triticum aestivum (Ta), Brassica napus (Bn), Lupinus albus (La), Glycine max (Gm), Vicia faba (Vf), and Cicer arietinum (Ca) supplied with 0 or 100 mg P kg-1 soil in AS or CS. Each value is the mean (±SE in each direction) of 16 replicates for each plant species. A dashed line represents root morphology scores equal to root physiology scores.
Discussion
Plant Growth, Biomass Distribution and Root Morphological Responses to Variable P Supply
This study revealed that the plant growth responses to P supply under variable soil conditions (AS vs. CS) varied among plant species with contrasting root properties (Figure 1). The fibrous-root species Zea mays, Triticum aestivum and Brassica napus had greater shoot growth responses to P supply than the tap-rooted legumes Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum in both soil types (Figure 2). The results suggested that the fibrous-root species had strong dependence on external P application, but low dependence on inherent soil residual P with relatively low availability; in contrast, the tap-rooted legumes could acquire substantial amounts of residual P from soil, relying relatively little on fertilizer P application. A similar pattern was also found for shoot P content in the present study: the fibrous-root species Zea mays, Triticum aestivum, and Brassica napus had greater responses to P supply than the tap-rooted legumes Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum in both soil types. Clearly, the responses of shoot dry weight, root/shoot ratio (except Zea mays in AS, Figure 2), and shoot P content (Figure 6) to P supply showed consistent results, compared with plant growth performance (Figure 1, Supplementary Table S1). Hence, plant growth responses to P supply under given soil conditions vary among plant species with contrasting root traits, indicating a potential strategy for modification of specific root traits to improve access to different P resources and thus enhance P acquisition by a given species (Shen et al., 2011, 2013).
The general response of shoot dry weight to P application was greater in CS than AS (P < 0.01), despite a variation among different plant species (Table S1). Phosphorus supply increased shoot biomass of all plant species except Lupinus albus in CS because this species is sensitive to CS as shown by leaf chlorosis (Figure 1, see also Kerley, 2000; Kerley and Huyghe, 2001). All other species (especially Zea mays, Triticum aestivum, Brassica napus, and Cicer arietinum) accumulated more shoot biomass in calcareous than AS (Figures 2A,B; see also Nuruzzaman et al., 2005; Pearse et al., 2007; Li et al., 2010; Rose et al., 2010) because plants could take up P from Ca complexes easier than from Fe and Al oxide complexes (Hinsinger, 2001; Veneklaas et al., 2003; Pearse et al., 2007). These effects could partially account for the increased biomass of plants (Zea mays, Triticum aestivum, Brassica napus, and Cicer arietinum) in CS compared with AS.
Many species distributed a greater proportion of total dry matter to root growth under P deficiency (Hill et al., 2006; Richardson et al., 2009). In the present study, cereals (Zea mays and Triticum aestivum) had higher root/shoot ratio than the legume species (Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum), suggesting the former species allocated proportionally more biomass to roots in P-deficient soil. Also, the species with fibrous roots (particularly Triticum aestivum and Brassica napus) had relatively higher SRL compared with legume species (Figures 3C,D), indicating a smaller root diameter for fibrous root species. This means the fibrous-root species (Zea mays, Triticum aestivum, and Brassica napus, root diameter = 0.12 ± 0.04 mm, n = 48) have significantly thinner roots compared with the legume species (especially Lupinus albus, Vicia faba, and Cicer arietinum, root diameter = 0.24 ± 0.01 mm, n = 48) (data not shown).
Phosphorus deficiency significantly increased the SRL of Zea mays in CS and Brassica napus (but not of legume species) in AS (Figure 3). Even though an increase in SRL is not always a universal response to low P supply (Schroeder and Janos, 2005; Pang et al., 2010), increased production of relatively fine roots to create a large surface area of contact between roots and soil would be expected to enhance acquisition of P (see also Barber et al., 1963; Nuruzzaman et al., 2005; Pearse et al., 2006a, 2007; Calderón-Vázquez et al., 2009; Shen et al., 2011; Liu et al., 2016). The roots with small diameter were considered an efficient and economical means of increasing P acquisition (Eissenstat, 1992; Lynch, 2011, 2013). The reason for the increased SRL of Zea mays in AS and of Brassica napus in CS under P addition could be mainly due to low available P in soil, which appeared to have been quite severe in P-deficiency stress with low shoot P concentrations in this study (Figure 6). Hence, adding P alleviated the P stress, allowing plants to respond by increasing total root length and SRL.
Root/shoot ratio of legume species did not show a significant variation in response to P application, especially in AS, and quite a similar pattern was found for total root length and SRL. In particular, Lupinus albus had the smallest root/shoot ratio and total root length among all the species (Figures 2 and 3). These relatively small morphological responses of Lupinus albus to P application suggested that effective mobilization of rhizosphere P (physiological response) is more resource-efficient than extending roots (morphological response) to get P from far away (Shen et al., 2011). Indeed, cluster roots (Supplementary Figure S1) enhanced root exudation, thus enhancing the root physiological responses (Shen et al., 2005; Tang et al., 2013b).
In conclusion, the fibrous-root species (Zea mays, Triticum aestivum, and Brassica napus) showed larger root morphological plasticity as a way of increasing P acquisition to cope with variable P supply than the tap-rooted legumes (Lupinus albus, Glycine max, Vicia faba, and Cicer arietinum).
Root Physiological Responses
The physiological and biochemical responses involved decreased shoot P concentration and content (Figure 6), enhanced internal P utilization and increased root exudation into the rhizosphere. Root exudation of acid phosphatase (APase) and carboxylates are considered important for P mobilization and acquisition (Raghothama, 1999; Vance et al., 2003; Lambers et al., 2006; Richardson et al., 2009). In the present study, APase activity and carboxylate content in the rhizosphere of Lupinus albus were significantly higher than in the other plant species, but Lupinus albus showed no significant difference between the P treatments in either soil (Figure 4). The reason could be related to cluster root formation in both P treatments, and there was no difference in the proportion of cluster root dry weight with respect to the whole root system (data not shown) in either P treatment, possibly because of a relatively small soil volume restricting the total amount of P present.
In the present study, the Lupinus albus shoot P concentration was <2.5 mg g-1 in AS and <1.5 mg g-1 in CS regardless of P treatments, which is lower than the critical level at or below which cluster root formation and citrate exudation would be significantly up-regulated according to our previous study (Li et al., 2008). This could partly account for the strong cluster root formation, citrate exudation and APase activity in the rhizosphere, which are regulated by low shoot P status as an internal systemic signal (Shen et al., 2005; Li et al., 2008; Shen et al., 2013; Tang et al., 2013a). Indeed, in the present study Lupinus albus efficiently acquired soil P through high exudation of carboxylates and acid phosphatases that modified rhizosphere chemistry, but this species exhibited a relatively low response to the applied fertilizer P; in contrast, the fibrous-root species with low capacity for root exudation of carboxylates and acid phosphatases showed a strong response to the applied P through altering the root morphological traits.
Other legume species (Glycine max, Vicia faba, and Cicer arietinum) showed higher or slightly higher activity of APase in the rhizosphere soil compared with the cereal species in the present study (Figures 4A,B) as well as in other studies (Tadano et al., 1993; Duff et al., 1994; Li et al., 2004; Nuruzzaman et al., 2006; Wang et al., 2008). Similarly, exudation of carboxylates was greater in the legume species, especially Lupinus albus and Cicer arietinum, compared with the fibrous-root species (Figures 4C,D; see also Ryan et al., 2001; Veneklaas et al., 2003; Li et al., 2007, 2010; Rose et al., 2010).
Phosphorus-utilization efficiency of different plant species was directly related to P concentration in plant tissues (Barker and Pilbeam, 2007); P starvation increased P-utilization efficiency as reported before (Rose et al., 2011). Therefore, the fibrous-root species (Zea mays, Triticum aestivum) coped with variable P supply through expanding the root absorption surface area to enhance spatial availability of P, but were less dependent on root exudation, compared with the legume species with intensive root exudation (Eissenstat, 1992; Ryan et al., 2001; Veneklaas et al., 2003; Pearse et al., 2006a, 2007; Rose et al., 2010). The results indicated a strategic variation in how different plant species enhance P acquisition by changing the coordination between root morphological and physiological traits.
Root Morphological and Physiological Responses to Variable P Supply
Numerous studies compared the responses of plant species to various external P supply conditions. However, the conclusions of all these studies mainly focused on independent changes in either root morphological or physiological parameters, such as shoot biomass or carboxylate exudation (Hoffland et al., 1989a,b; Tadano et al., 1993; Neumann and Römheld, 1999; Nuruzzaman et al., 2005; Li et al., 2010; Rose et al., 2010; Vu et al., 2010; Maltais-Landry, 2015), or some correlation between these parameters (Shen et al., 2003; Watt and Evans, 2003; George et al., 2006; Pearse et al., 2007; Zhang et al., 2009). In this study, the PCA method was used as reported before (Tang et al., 2013b) to calculate the relative contribution of the root morphological or physiological response parameter scores to P acquisition, and then quantitatively evaluate the relationship between root morphological and physiological traits in response to P supply and soil types (Figures 8 and 9). Zea mays and Triticum aestivum had higher root morphology scores (strong response in root/shoot ratio, total root length and SRL; Figures 2 and 3) and low physiology scores (low exudation of APase and carboxylates and consequently low P-acquisition efficiency; Figures 4 and 7), exhibiting greater changes in the root morphology scores to P supply in the two soil types compared with the legume species.
Brassica napus had the lowest physiology scores, but exhibited large changes in the root morphology scores. Previous studies indicated that Brassica napus had a strong physiological response to acquiring P (Foehse and Jungk, 1983; Moorby et al., 1988; Hoffland et al., 1989a,b), but most of these studies were conducted at an early growth stage of Brassica napus. In the present study, plants were harvested at relatively mature stage, and the results showed Brassica napus mainly relied on the root morphological changes in response to variable P supply; indeed, at this growth stage some previous studies also reported low root exudation and high SRL in Brassica napus (Marschner et al., 2007; Pearse et al., 2007; Solaiman et al., 2007; Zhang et al., 2009).
Lupinus albus and Cicer arietinum showed the highest root physiology scores and the lowest root morphology scores among all plant species, probably related to strong root exudation and low root length despite formation of cluster roots in Lupinus albus (Figures 3 and 4). Furthermore, these two species also had strong variation in the root physiological scores to cope with P deficiency under different soil conditions. Lupinus albus had higher physiology scores in the –P compared with the +P treatment; Cicer arietinum had the opposite result. These patterns were related to higher exudation of carboxylates and acid phosphatase from cluster roots of Lupinus albus in the P-deficient treatment, and higher exudation of carboxylates by Cicer arietinum in the P-sufficient conditions (Figure 4, see also Tadano et al., 1993; Neumann et al., 1999; Yan et al., 2002; Shen et al., 2003; Vance et al., 2003; Veneklaas et al., 2003; Wouterlood et al., 2004; Lambers et al., 2006; Wang et al., 2007; Rose et al., 2010; Cheng et al., 2014). These results indicated that Lupinus albus and Cicer arietinum depended mainly on the physiological responses to variable P supply in AS and CS, emphasizing an exudation component of cluster roots. In contrast, Glycine max and Vicia faba roots showed changes in neither morphological nor physiological response to P supply. Taken together, the Lupinus albus and Cicer arietinum response to P deficiency mainly depended on the root physiological traits, whereas Zea mays, Triticum aestivum, and Brassica napus had the strong root morphological responses. Glycine max and Vicia faba showed a combination of root morphological and physiological responses. This knowledge is critical for manipulating root morphology and rhizosphere processes in a given species to enhance plant growth and P-acquisition efficiency.
Conclusion
This study provided novel evidence of variable coordination and balance between root morphological and physiological responses to cope with P supply changes in different plant species, suggesting that the specific strategy could be developed to modify root morphological or physiological traits for a given plant species to increase P-acquisition and P-utilization efficiency. Further work on other morphological and physiological traits (e.g., root angles, root hairs, mycorrhizal colonization, P uptake) as well as on other plant species to underpin phylogenetic extrapolation is needed.
Author Contributions
YL, HL, FZ, and JS designed the experiments; YL performed the experiments; YL, HT, and JS carried out data analysis and wrote the manuscript; ZR and WW contributed to the interpretation of data analyses and manuscript writing.
Funding
The study was supported by the National Natural Science Foundation of China (NSFC) (Nos. 31330070, 31210103906, 30925024), the National Basic Research Program (973-2015CB150405) and the Innovative Group Grant of the National Science Foundation of China (31421092), and the Program of Introducing International Advanced Agricultural Science and Technology of the Ministry of Agriculture of China (No. 2011-G18). ZR is supported by Australian Research Council (DP160104434). At Rothamsted Research, WW is supported via the 20:20 Wheat® Programme by the UK Biotechnology and Biological Sciences Research Council.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01939/full#supplementary-material
References
Alvey, S., Bagayoko, M., Neumann, G., and Buerkert, A. (2001). Cereal/legume rotations affect chemical properties and biological activities in two West African soils. Plant Soil 231, 45–54. doi: 10.1023/A:1010386800937
Barber, S. A., Walker, J. M., and Vasey, E. H. (1963). Mechanisms for movement of plant nutrients from soil and fertilizer to plant root. J. Agric. Food Chem. 11, 204–207. doi: 10.1021/jf60127a017
Calderón-Vázquez, C., Alatorre-Cobos, F., Simpson-Williamson, J., and Herrera-Estrella, L. (2009). “Maize under phosphate limitation,” in Handbook of Maize: Its Biology, eds J. L. Bennetzen and S. C. Hake (New York, NY: Springer), 381–404.
Carvalhais, L. C., Dennis, P. G., Fedoseyenko, D., Hajirezaei, M., Borriss, R., and von Wirén, N. (2011). Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 174, 3–11. doi: 10.1002/jpln.201000085
Cheng, L., Tang, X., Vance, C. P., White, P. J., Zhang, F., and Shen, J. (2014). Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.). J. Exp. Bot. 65, 2995–3003. doi: 10.1093/jxb/eru135
Cordell, D., Rosemarin, A., Schröder, J. J., and Smit, A. L. (2011). Towards global phosphorus security: a systems framework for phosphorus recovery and reuse options. Chemosphere 84, 747–758. doi: 10.1016/j.chemosphere.2011.02.032
Cordell, D., and White, S. (2013). Sustainable phosphorus measures: strategies and technologies for achieving phosphorus security. Agronomy 3, 86–116. doi: 10.3390/agronomy3010086
Duff, S. M., Sarath, G., and Plaxton, W. C. (1994). The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 90, 791–800. doi: 10.1111/j.1399-3054.1994.tb02539.x
Eissenstat, D. M. (1992). Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 15, 763–782. doi: 10.1080/01904169209364361
Elser, J., and Bennett, E. (2011). Phosphorus cycle: a broken biogeochemical cycle. Nature 478, 29–31. doi: 10.1038/478029a
Foehse, D., and Jungk, A. (1983). Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 74, 359–368. doi: 10.1007/BF02181353
Föhse, D., Claassen, N., and Jungk, A. (1988). Phosphorus efficiency of plants. I. external and internal p requirement and p uptake efficiency of different plant species. Plant Soil 110, 101–109.
Gardner, W. K., Parbery, D. G., and Barber, D. A. (1982). The acquisition of phosphorus by Lupinus albus L. I. Some characteristics of the soil/root interface. Plant Soil 68, 19–32. doi: 10.1007/BF02374724
George, T. S., Turner, B. L., Gregory, P. J., Cade-Menun, B. J., and Richardson, A. E. (2006). Depletion of organic phosphorus from oxisols in relation to phosphatase activities in the rhizosphere. Eur. J. Soil Sci. 57, 47–57. doi: 10.1111/j.1365-2389.2006.00767.x
Hammond, J. P., Broadley, M. R., White, P. J., King, G. J., Bowen, H. C., Haydenet, R., et al. (2009). Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J. Exp. Bot. 60, 1953–1968. doi: 10.1093/jxb/erp083
Hill, J. O., Simpson, R. J., Moore, A. D., and Chapman, D. F. (2006). Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286, 7–19. doi: 10.1007/s11104-006-0014-3
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237, 173–195. doi: 10.1023/A:1013351617532
Hoffland, E., Findenegg, G. R., and Nelemans, J. A. (1989a). Solubilization of rock phosphate by rape. I. Evaluation of the role of the nutrient uptake pattern. Plant Soil 113, 155–160. doi: 10.1007/BF02280175
Hoffland, E., Findenegg, G. R., and Nelemans, J. A. (1989b). Solubilization of rock phosphate by rape. II. Local root exudation of organic acids as a response to P-starvation. Plant Soil 113, 161–165. doi: 10.1007/BF02280176
Holford, I. C. R. (1997). Soil phosphorus: its measurement, and its uptake by plants. Aust. J. Soil Res. 35, 227–240. doi: 10.1071/S96047
Houlton, B. Z., Wang, Y. P., Vitousek, P. M., and Field, C. B. (2008). A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454, 327–330. doi: 10.1038/nature07028
Kerley, S. J. (2000). The effect of soil liming on shoot development, root growth, and cluster root activity of white lupin. Biol. Fert. Soils 32, 94–101. doi: 10.1007/s003740000222
Kerley, S. J., and Huyghe, C. (2001). Comparison of acid and alkaline soil and liquid culture growth systems for studies of shoot and root characteristics of white lupin (Lupinus albus L.) genotypes. Plant Soil 236, 275–286. doi: 10.1023/A:1012724821957
Lambers, H., Shane, M. W., Cramer, M. D., Pearse, S. J., and Veneklaas, E. J. (2006). Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann. Bot. 98, 693–713. doi: 10.1093/aob/mcl114
Li, H., Shen, J., Zhang, F., Marschner, P., Cawthray, G., and Rengel, Z. (2010). Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol. Fert. Soils 46, 79–91. doi: 10.1007/s00374-009-0411-x
Li, H., Shen, J., Zhang, F., Tang, C., and Lambers, H. (2008). Is there a critical level of shoot phosphorus concentration for cluster-root formation in Lupinus albus? Funct. Plant Biol. 35, 328–336. doi: 10.1071/FP07222
Li, L., Li, S. M., Sun, J. H., Zhou, L. L., Bao, X. G., Zhang, H. G., et al. (2007). Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. U.S.A. 104, 11192–11196. doi: 10.1073/pnas.0704591104
Li, L., Tilman, D., Lambers, H., and Zhang, F. S. (2014). Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol. 203, 63–69. doi: 10.1111/nph.12778
Li, S. M., Li, L., Zhang, F. S., and Tang, C. (2004). Acid phosphatase role in chickpea/maize intercropping. Ann. Bot. 94, 297–303. doi: 10.1093/aob/mch140
Liu, H., Tang, C., and Li, C. (2016). The effects of nitrogen form on root morphological and physiological adaptations of maize, white lupin and faba bean under phosphorus deficiency. AoB Plants 8, lw058. doi: 10.1093/aobpla/plw058
Lynch, J. P. (2011). Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 156, 1041–1049. doi: 10.1104/pp.111.175414
Lynch, J. P. (2013). Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 112, 347–357. doi: 10.1093/aob/mcs293
Maltais-Landry, G. (2015). Legumes have a greater effect on rhizosphere properties (pH, organic acids and enzyme activity) but a smaller impact on soil P compared to other cover crops. Plant Soil 394, 139–154. doi: 10.1007/s11104-015-2518-1
Marschner, P., Solaiman, Z., and Rengel, Z. (2006). Rhizosphere properties of Poaceae genotypes under P-limiting conditions. Plant Soil 283, 11–24. doi: 10.1007/s11104-005-8295-5
Marschner, P., Solaiman, Z., and Rengel, Z. (2007). Brassica genotypes differ in growth, phosphorus uptake and rhizosphere properties under P-limiting conditions. Soil Biol. Biochem. 39, 87–98. doi: 10.1016/j.soilbio.2006.06.014
Moorby, H., White, R. E., and Nye, P. H. (1988). The influence of phosphate nutrition on H ion efflux from the roots of young rape plants. Plant Soil 105, 247–256. doi: 10.1007/BF02376789
Neumann, G., Massonneau, A., Martinoia, E., and Römheld, V. (1999). Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208, 373–382. doi: 10.1007/s004250050572
Neumann, G., and Römheld, V. (1999). Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211, 121–130. doi: 10.1023/A:1004380832118
Nuruzzaman, M., Lambers, H., Bolland, M. D., and Veneklaas, E. J. (2005). Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 271, 175–187. doi: 10.1007/s11104-004-2386-6
Nuruzzaman, M., Lambers, H., Bolland, M. D., and Veneklaas, E. J. (2006). Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281, 109–120. doi: 10.1007/s11104-005-3936-2
Oelkers, E. H., and Valsami-Jones, E. (2008). Phosphate mineral reactivity and global sustainability. Elements 4, 83–87. doi: 10.2113/GSELEMENTS.4.2.83
Ohwaki, Y., and Hirata, H. (1992). Differences in carboxylic acid exudation among P-starved leguminous crops in relation to carboxylic acid contents in plant tissues and phospholipid level in roots. Soil Sci. Plant Nutr. 38, 235–243. doi: 10.1080/00380768.1992.10416486
Pang, J., Ryan, M. H., Tibbett, M., Cawthray, G. R., Siddique, K. H. M., Bolland, M. D. A., et al. (2010). Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil. 331, 241–255. doi: 10.1007/s11104-009-0249-x
Pearse, S. J., Veneklaas, E. J., Cawthray, G., Bolland, M. D. A., and Lambers, H. (2006a). Triticum aestivum shows a greater biomass response to a supply of aluminium phosphate than Lupinus albus, despite releasing fewer carboxylates into the rhizosphere. New Phytol. 169, 515–524. doi: 10.1111/j.1469-8137.2005.01614.x
Pearse, S. J., Veneklaas, E. J., Cawthray, G. R., Bolland, M. D. A., and Lambers, H. (2006b). Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288, 127–139. doi: 10.1007/s11104-006-9099-y
Pearse, S. J., Veneklaas, E. J., Cawthray, G., Bolland, M. D. A., and Lambers, H. (2007). Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol. 173, 181–190. doi: 10.1111/j.1469-8137.2006.01897.x
Raghothama, K. G. (1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 665–693. doi: 10.1146/annurev.arplant.50.1.665
Rengel, Z., and Marschner, P. (2005). Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol. 168, 305–312. doi: 10.1111/j.1469-8137.2005.01558.x
Richardson, A. E., Hocking, P. J., Simpson, R. J., and George, T. S. (2009). Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 60, 124–143. doi: 10.1071/CP07125
Rose, T. J., Hardiputra, B., and Rengel, Z. (2010). Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 326, 159–170. doi: 10.1007/s11104-009-9990-4
Rose, T. J., Rose, M. T., Pariasca-Tanaka, J., Heuer, S., and Wissuwa, M. (2011). The frustration with utilization: why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Sci. 2:73. doi: 10.3389/fpls.2011.00073
Ryan, P. R., Delhaize, E., and Jones, D. L. (2001). Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 527–560. doi: 10.1146/annurev.arplant.52.1.527
Schroeder, M. S., and Janos, D. P. (2005). Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 15, 203–216. doi: 10.1007/s00572-004-0324-3
Shen, J., Li, C., Mi, G., Li, L., Yuan, L., Jiang, R., et al. (2013). Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J. Exp. Bot. 64, 1181–1192. doi: 10.1093/jxb/ers342
Shen, J., Li, H., Neumann, G., and Zhang, F. (2005). Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Sci. 168, 837–845. doi: 10.1016/j.plantsci.2004.10.017
Shen, J., Rengel, Z., Tang, C., and Zhang, F. (2003). Role of phosphorus nutrition in development of cluster roots and release of carboxylates in soil-grown Lupinus albus. Plant Soil 248, 199–206. doi: 10.1023/A:1022375229625
Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., et al. (2011). Phosphorus dynamics: from soil to plant. Plant Physiol. 156, 997–1005. doi: 10.1104/pp.111.175232
Solaiman, Z., Marschner, P., Wang, D., and Rengel, Z. (2007). Growth, P uptake and rhizosphere properties of wheat and canola genotypes in an alkaline soil with low P availability. Biol. Fert. Soils 44, 143–153. doi: 10.1007/s00374-007-0188-8
Tadano, T., Ozawa, K., Sakai, H., Osaki, M., and Matsui, H. (1993). Secretion of acid phosphatase by the roots of crop plants under phosphorus-deficient conditions and some properties of the enzyme secreted by lupin roots. Plant Soil 155, 95–98. doi: 10.1007/BF00024992
Tang, H., Li, X., Zu, C., Zhang, F., and Shen, J. (2013a). Spatial distribution and expression of intracellular and extracellular acid phosphatases of cluster roots at different developmental stages in white lupin. J. Plant Physiol. 170, 1243–1250. doi: 10.1016/j.jplph.2013.04.015
Tang, H., Shen, J., Zhang, F., and Rengel, Z. (2013b). Interactive effects of phosphorus deficiency and exogenous auxin on root morphological and physiological traits in white lupin (Lupinus albus L.). Sci. China Life Sci. 56, 313–323. doi: 10.1007/s11427-013-4461-9
Thomas, R. L., Sheard, R. W., and Moyer, J. R. (1967). Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron. J. 59, 240–243. doi: 10.2134/agronj1967.00021962005900030010x
Vance, C. P., Uhde-Stone, C., and Allan, D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157, 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
Veneklaas, E. J., Stevens, J., Cawthray, G. R., Turner, S., Grigg, A. M., and Lambers, H. (2003). Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248, 187–197. doi: 10.1023/A:1022367312851
Vu, D. T., Armstrong, R. D., Sale, P. W. G., and Tang, C. (2010). Phosphorus availability for three crop species as a function of soil type and fertilizer history. Plant Soil 337, 497–510. doi: 10.1007/s11104-010-0545-5
Wang, B. L., Shen, J. B., Zhang, W. H., Zhang, F. S., and Neumann, G. (2007). Citrate exudation from white lupin induced by phosphorus deficiency differs from that induced by aluminum. New Phytol. 176, 581–589. doi: 10.1111/j.1469-8137.2007.02206.x
Wang, B. L., Tang, X. Y., Cheng, L. Y., Zhang, A. Z., Zhang, W. H., Zhang, F. S., et al. (2010). Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol. 187, 1112–1123. doi: 10.1111/j.1469-8137.2010.03323.x
Wang, X., Tang, C., Guppy, C. N., and Sale, P. W. G. (2008). Phosphorus acquisition characteristics of cotton (Gossypium hirsutum L.), wheat (Triticum aestivum L.) and white lupin (Lupinus albus L.) under P deficient conditions. Plant Soil 312, 117–128. doi: 10.1007/s11104-008-9589-1
Wang, X. R., Shen, J. B., and Liao, H. (2010). Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci. 179, 302–306. doi: 10.1016/j.plantsci.2010.06.007
Watt, M., and Evans, J. R. (2003). Phosphorus acquisition from soil by white lupin (Lupinus albus L.) and soybean (Glycine max L.), species with contrasting root development. Plant Soil 248, 271–283. doi: 10.1023/A:1022332700686
Westerman, R. L. (1990). Soil Testing and Plant Analysis, 3rd Edn. Madison, WI: Soil Science Society of America.
Wouterlood, M., Cawthray, G. R., Scanlon, T. T., Lambers, H., and Veneklaas, E. J. (2004). Carboxylate concentrations in the rhizosphere of lateral roots of chickpea (Cicer arietinum) increase during plant development, but are not correlated with phosphorus status of soil or plants. New Phytol. 162, 745–753. doi: 10.1111/j.1469-8137.2004.01070.x
Yan, F., Zhu, Y., Müller, C., Zörb, C., and Schubert, S. (2002). Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol. 129, 50–63. doi: 10.1104/pp.010869
Zhang, F., Shen, J., Zhang, J., Zuo, Y., Li, L., and Chen, X. (2010). Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: implications for China. Adv. Agron. 107, 1–32. doi: 10.2134/agronj14.0122
Zhang, H., Huang, Y., Ye, X., Shi, L., and Xu, F. (2009). Genotypic differences in phosphorus acquisition and the rhizosphere properties of Brassica napus in response to low phosphorus stress. Plant Soil 320, 91–102. doi: 10.1007/s11104-008-9873-0
Keywords: phosphorus uptake, fibrous root species, legume species, root morphological traits, root exudation, phosphorus supply
Citation: Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR and Shen J (2016) Major Crop Species Show Differential Balance between Root Morphological and Physiological Responses to Variable Phosphorus Supply. Front. Plant Sci. 7:1939. doi: 10.3389/fpls.2016.01939
Received: 06 October 2016; Accepted: 07 December 2016;
Published: 21 December 2016.
Edited by:
Karl H. Muehling, University of Kiel, GermanyReviewed by:
Uwe Ludewig, University of Hohenheim, GermanyCaixian Tang, La Trobe University, Australia
Copyright © 2016 Lyu, Tang, Li, Zhang, Rengel, Whalley, and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbo Shen, jbshen@cau.edu.cn
 Yang Lyu
Yang Lyu Hongliang Tang1,2
Hongliang Tang1,2 Haigang Li
Haigang Li Fusuo Zhang
Fusuo Zhang Zed Rengel
Zed Rengel William R. Whalley
William R. Whalley Jianbo Shen
Jianbo Shen