- 1Department of Molecular Biology and Genetic Engineering, College of Basic Sciences and Humanities, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, India
- 2International Rice Research Institute Division, International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India
- 3Indian Council of Agricultural Research-Vivekananda Institute of Hill Agriculture, Almora, India
Calcium is an essential macronutrient for plants and animals and plays an indispensable role in structure and signaling. Low dietary intake of calcium in humans has been epidemiologically linked to various diseases which can have serious health consequences over time. Major staple food-grains are poor source of calcium, however, finger millet [Eleusine coracana (L.) Gaertn.], an orphan crop has an immense potential as a nutritional security crop due to its exceptionally high calcium content. Understanding the existing genetic variation as well as molecular mechanisms underlying the uptake, transport, accumulation of calcium ions (Ca2+) in grains is of utmost importance for development of calcium bio-fortified crops. In this review, we have discussed molecular mechanisms involved in calcium accumulation and transport thoroughly, emphasized the role of molecular breeding, functional genomics and transgenic approaches to understand the intricate mechanism of calcium nutrition in finger millet. The objective is to provide a comprehensive up to date account of molecular mechanisms regulating calcium nutrition and highlight the significance of bio-fortification through identification of potential candidate genes and regulatory elements from finger millet to alleviate calcium malnutrition. Hence, finger millet could be used as a model system for explaining the mechanism of elevated calcium (Ca2+) accumulation in its grains and could pave way for development of nutraceuticals or designer crops.
Mineral malnutrition is affecting one half of the world’s population. People have no access to a variety of minerals such as iron, calcium, zinc, magnesium, and copper. Most of the staple food crops such as rice, wheat and maize which constitute the major part of the diet of people are often deficient in these macro/micronutrients, thus insufficient to meet the daily needs (Hirschi, 2009). Deficiency of these minerals leads to an increasing risk of diseases such as rickets, osteoporosis, anemia, hypertension etc. It has been estimated by the Food and Agriculture Organization (FAO) that the world’s population will reach 9.1 billion by 2050 (Food and Agriculture Organization [FAO] of the United Nations, 2009). Hence, to meet the food demands of such a large population the quantity as well as the quality of food needs to be improved in terms of their nutritional value.
Calcium plays a very important role in the development of bones and teeth. Besides, it is required for a number of basic regulatory functions such as: contraction and relaxation of muscles, transmission of nerve impulses, coagulation of blood, activation of enzymatic reactions, stimulation of hormonal secretion and many other processes including synaptic plasticity, cell proliferation and cell death (Pravina et al., 2013). Calcium deficiency is a serious health problem which prevails in developed as well as in the developing world. Insufficient intake of calcium in human diets has been linked to diseases such as rickets (Heaney, 1993; Chan et al., 2007) and osteoporosis (Bhatia, 2008; Pettifor, 2008), both of which are the underlying cause for low bone density and poor bone growth. Eighty percent of the people who suffer from osteoporosis are women. Intake of calcium (Ca2+) in pregnant women is predominantly important for fetal skeleton development, increasing birth weight prenatal hypertension and avoidance of pre-clamsia (Chan et al., 2006). The human dietary intake of calcium in the majority of cases is below the recommended daily intake (RDI) of 800–1,300 mg per capita, and this is believed to result in widespread health and economic costs (Kranz et al., 2007).
Various approaches used to improve the calcium (Ca2+) content in grains/edible portion of crop plants include conventional breeding methodologies, marker assisted selection, transgenic technology and biofortification. Conventional plant breeding which aims in improving the target traits mainly by introgression from donor lines through hybridization has number of drawbacks such as the time required for breeding of cultivars, linkage drag, and absence of sufficient genetic variability in the primary gene pool with respect to a particular trait (Collard and Mackill, 2008). Molecular markers are considered best for indirect selection of traits and to avoid linkage drag. The association of markers with genes/QTLs controlling the traits of economic importance has been used for indirect marker assisted selection. However, genetic markers spread across the whole genome allow not only identification of individual genes associated with complex traits by QTL analysis but also the exploration of genetic diversity with regard to natural variation and can be extended for identification of major genes for nutritional traits. The genome wide markers saturating the whole genome are being used for genomic selection and results are encouraging in maize, wheat and barley (Piepho, 2009; Crossa et al., 2010; Guo et al., 2010; Heffner et al., 2011; Zhao et al., 2014). Another approach used for calcium biofortification is transgenics, by manipulating Ca2+ transporter genes. Several attempts have been made to increase calcium content in plant tissues and up to 300% increase of calcium content is recorded in plants (Park et al., 2005a; Kumar et al., 2014).
Hence, molecular marker assisted selection and transgenic technology are the two widely used approaches for biofortification to overcome the problem of “Hidden hunger,” which is the dietary insufficiency of one or more micronutrients such as calcium. However, for successful accomplishment of biofortification, high throughput ‘omics’ technologies provide useful insights and opportunities for development of third generation molecular markers, and identification of candidate genes for development of transgenics for ensuring nutritional security. The techniques of functional genomics along with the help of bioinformatics tools help in isolation and characterization of genes of nutritional importance. Genomics has also given us the information of complete genomes of a large number of food plants (Varshney et al., 2006). One of the main pillars of genomic studies is the development of high-throughput DNA sequencing technologies, collectively known as Next Generation Sequencing (NGS) methods, which can readily provide Single Nucleotide Polymorphism (SNPs) information of important nutrient rich genes (Govindaraj et al., 2015). Transcriptome sequencing gives information about the functional genes expressed in specific tissues of an organism at any given time in response to nutritional changes or other changes in the growth conditions. Transcriptome data is highly useful not only to know the gene content and transcriptional status in various tissues but also helps in identifying SSRs and SNPs in the genic regions, which can be converted to gene-based markers (Narina et al., 2011). Further, proteomics has helped researchers understand the effects of proteins on plant mineral homeostasis. It helps in monitoring the changes in proteins under different developmental and environmental conditions, as guided by the genome and signaled by the transcriptome.
This review comprehensively describes the three pronged molecular approaches for augmenting grain calcium content in finger millet: Molecular breeding, Functional Genomics and Transgenics. It also explains the challenges faced in a successful biofortification program and strategies for biofortification of calcium in plants for improved nutrition and development of functional foods. Further, it describes the mechanism of calcium uptake and transport from source to sink organs in plants and the molecular players involved in it.
Biofortification: Major Challenges and Issues
Biofortification is the development of nutrient-dense staple crops using the best conventional breeding practices and modern biotechnology, without sacrificing agronomic performance and important consumer-preferred traits (Nestel et al., 2006). Plants are the ultimate source of nutrients in human diet. However, majority of the essential vitamins and minerals are lacking in all our staple food crops. Although, a balanced diet provides sufficient nutrients but most of the human population, particularly in developing countries depends upon staple cereals, such as rice or maize, which fail to provide the full complement of essential nutrients. Malnutrition has become a significant public health issue in most of the developing world (Müller and Krawinkel, 2005). One way to tackle this problem is through the enrichment of staple crops to increase their essential nutrient content. Several different tactics for biofortification have been adopted including addition of the appropriate mineral as an organic compound to the fertilizer, improving the nutritional content of plants by conventional breeding in combination with mutagenesis and the use of marker- assisted selection to introgress such traits into widely cultivated, adapted genotypes. Although breeding-based strategies for biofortification are unproven as yet, they have the potential to become sustainable, cost effective and reach remote rural populations (Bouis, 2003; Genc et al., 2005). It is argued that once mineral-dense lines have been developed, there will be little additional cost in incorporating them into on-going breeding programs (Welch and Graham, 2002; Bouis, 2003; Timmer, 2003), and it has been reported that seed of mineral dense crops produce more vigorous seedlings on infertile soils (Rengel and Graham, 1995). To implement successful biofortification programs through plant breeding there is need for a comprehensive exploration of potential genetic resources in the form of land races, wild species and an in-depth understanding of the physiological and genetic basis of mineral nutrients accumulation in staple food crop. It therefore becomes necessary to understand the genes and processes involved in grain mineral accumulation in order to couple the information with marker/genomics assisted selection, for efficient enhancement of grain mineral content.
A breeding program aiming at development of new genotypes with high Ca2+ concentration first requires existence of useful genetic variation for Ca2+ accumulation in grain. Little information is, however, available about the genetic control and molecular physiological mechanisms contributing to high accumulation of Ca2+ and other micronutrients in grain of different genetic materials.
Bioavailability: A Complex Determining Factor
Plant foods contain substances (i.e., antinutrients) that interfere with the absorption or utilization of these nutrients in humans (Welch and Graham, 1999). Thus, efforts should be made toward increasing the concentrations of “promoter substances” (stimulating the absorption of essential mineral elements) and reducing the concentrations of “antinutrients” (interfering with their absorption) of the biofortified crops (White and Broadley, 2005). The best described promoter substances are certain vitamins, inulins and cysteine amino acid. Vitamin E, D, choline, niacin and provitamin A, help in the absorption of Se, Ca, P, Fe, and Zn (Carvalho and Vasconcelos, 2013). Finger millet contains both water soluble and liposoluble vitamins: thiamin, riboflavin, niacin, and tocopherols (Obilana and Manyasa, 2002), which could act as potential promoter substances for crop biofortification. Utilization of the maximum nutrient potential of the millets is limited by the presence of phytates, phenols, tannins and enzyme inhibitors. Among millets, finger millet has been reported to contain high amounts of tannins ranging from 0.04 to 3.74% of catechin equivalents (Rao et al., 1994; Antony and Chandra, 1999). Phytate content in finger millet as observed by various authors has been found to be in range 0.679–0.693 g/100 mg (Antony and Chandra, 1999). It is the main phosphorous store in mature seeds, has a strong binding capacity and readily forms complexes with multivalent cations and proteins (Haug and Lantzsch, 1983). Finger millet has been found to contain 41% phytic phosphorus as percentage of total phosphorus (Deosthale, 2002). The dietary phytic acid binds not only with the seed derived minerals but also with other endogenous minerals encountered in the digestive tract (Raboy, 2000). Another group of anti-nutritional compound is polyphenols, which contains more than one phenol unit or building block per molecule (Carvalho and Vasconcelos, 2013). The level of polyphenols in cereal seeds can be reduced by incubation with polyphenol oxidase which, when combined with a phytase-mediated phytate reduction, shows significant increase in the availability of iron (Matuschek et al., 2001). On an average, finger millet genotypes contain 0.04–3.47% polyphenols (Chethan and Malleshi, 2007). Rao and Muralikrishna (2002) found proto-catechuic acid (45.0 mg/100 g) as the major free phenolic acid in finger millet grains. Among bound phenloic acids, ferulic and p-coumaric acid are the major fractions and account for 64–96 and 50–99% of total ferulic and p-coumaric acid content of finger millet grains, respectively, (Devi et al., 2014).
Numerous complexities pervade the determination of bioavailability of micronutrients in plant foods to humans. Determining the bioavailability of a particular micronutrient to an individual eating a mixed diet in a given environment is actually governed by the interaction of a multitude of factors (Fairweather-Tait and Hurrell, 1996; House, 1999; Van Campen and Glahn, 1999; Graham et al., 2001). Two approaches of conventional and genetic biofortification as given in Figure 1 are being currently practiced. Through plant breeding approaches one could select genotypes with low concentration of anti-nutrients or alternatively molecular biologists alter genes in staple crops so as to reduce or completely eliminate these anti-nutrients. However, doing so is associated with many risk factors and should be done with utmost care because many of the anti-nutrients are major plant metabolites that might play important roles in plant abiotic stress resistance, plant metabolism and in resistance of plant to crop pests or pathogens (Graham et al., 2001). Moreover, some of the anti-nutrients such as phytate and polyphenols might play key beneficial roles in human diets by acting as anti-carcinogens or by promoting health in other ways such as decreasing the risk of heart diseases or diabetes (Zhou and Erdman, 1995; Anonymous, 1996; Saied and Shamsuddin, 1998; Shamsuddin, 1999). Therefore, molecular biologists and plant breeders must consider the possible negative consequences of altering the concentration of these anti-nutrients in staple food crops.
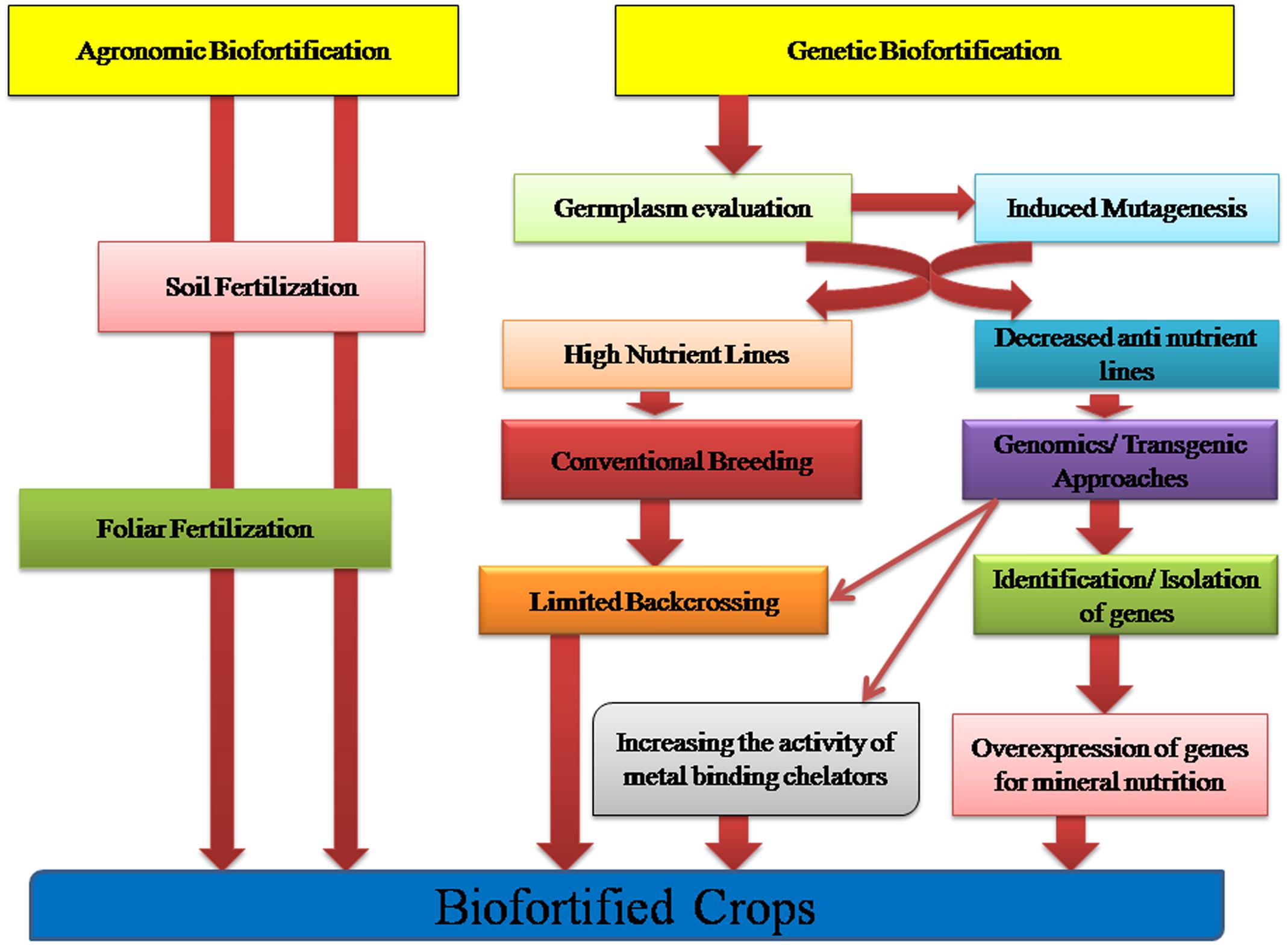
FIGURE 1. Crop Biofortification approaches: Agronomic and Genetic Biofortification strategies aiming to increase the bioavailability and accumulation of micronutrients in the edible tissues of crops.
Plants: The Power House of Calcium
It’s common knowledge that we need calcium for strong bones, teeth and besides this for a number of regulatory functions in our body. But, the best source of dietary and supplemental calcium is not understood yet. Following dairy products, plant products prove to be the largest potential contributor to Ca2+ intake (Weaver and Plawecki, 1994; Weaver et al., 1999; Lanham-New, 2008). Ever since the beginning of mankind, plant based foods constitute one of the potential nutrient sources in human diet (Carvalho and Vasconcelos, 2013). For proper calcium (Ca2+) absorption we need to consume food sources that contain types of calcium that are easily digested, assimilated and absorbed. Because of the calcium-magnesium ratio in dairy products, our body is not capable of absorbing the calcium (Bennett and Sammartano, 2012). However, calcium is naturally bound with other minerals, vitamins, proteins, and phyto-nutrients in the plants. Plant foods provide our body with calcium which is safe to absorb and directs our body to store it in our bones. Most of the staple foods, however, (e.g., Rice, wheat, and maize) have very low amount of calcium (Jeong and Guerinot, 2008). Plant based calcium sources includes green leafy vegetables, nuts, fruits, seeds, sea vegetables which help us to gain more calcium.
To implement a successful biofortification program for increasing calcium content in plants, comprehensive exploration of potential genetic resources in the form of land races and wild species is required. Also there is a need to have an in depth knowledge of physiological and genetic basis of calcium accumulation in staple food crops. It is therefore necessary to understand the genes and processes involved in grain calcium accumulation so as to blend this information with marker assisted selection for enhancing grain calcium concentration.
Finger Millet: A High Calcium Accumulating Crop
Finger millet is a potential staple crop cultivated mostly in Eastern and Central Africa and India. It ranks fourth in importance among millets in the world after sorghum, pearl millet, foxtail millet and commonly referred as ragi, mandua, bird’s foot millet, caracan millet and African millet (Upadhyaya et al., 2007). Nutritionally, finger millet is an excellent sources of nutrients especially calcium, other minerals and dietary fiber. The mineral composition of finger millet grains is highly variable. The mineral content of food grains is affected by the presence of genetic factors and environmental conditions prevailing in particular growing region (Singh and Raghuvanshi, 2012; Singh et al., 2014). Finger millet contains a fair amount of protein (7.3%) (Malleshi and Klopfenstein, 1998), dietary fiber (15–20%) (Chethan and Malleshi, 2007), and a rich source of calcium (344 mg/ 100 g) (Gopalan et al., 1999; Bhatt et al., 2003) and iron (3.7–6.8 mg/ 100 g) (Barbeau and Hilu, 1993). Ravindran (1991) estimated the protein content of finger millet to be 9.8%, that of calcium, oxalate and phytic acid to be 0.24, 0.44, and 0.48%, respectively. In several studies on estimation of calcium content in different genotypes of finger millet, high calcium values have been reported. Calcium content varied from 162 to 487 mg/100 g with a mean value of 320.8 mg/100 g grain in 36 genotypes of finger millet (Vadivoo et al., 1998), 293–390 mg/100 g in six varieties of finger millet (Babu et al., 1987); 50–300 mg/100 g in another set of six varieties (Admassu et al., 2009). Furthermore, very high calcium content, 450 mg/100 g (Panwar et al., 2010) and 489 mg/100 g (Upadhyaya et al., 2011) has been reported in few finger millet genotypes. It has also been found that the average calcium content (329 mg/100 g grain) in white genotypes was considerably higher than the brown (296 mg/100 grain) genotypes (Seetharam, 2001). Besides high nutritional value, finger millet has high grain yield potential in the range of 4-5 tons/ha (Food and Agriculture Organization [FAO] of the United Nations, 2008). Thus, finger millet is up-coming as an important food crop due to its exceptionally high calcium (Ca2+) content. It is not only an excellent source of dietary calcium (Ca2+) but also an excellent model to explore the genetic control and molecular mechanisms contributing to high grain calcium (Ca2+) content.
The millet is also well known for its health benefits such as hypocholesterolemic, hypoglycaemic and anti-ulcerative properties (Chethan and Malleshi, 2007). Further, the crop is productive in wide range of environments and growing conditions from Karnataka in South India to foothills and middle hills of Himalayas in North India and Nepal, and throughout the middle-elevation areas of Eastern and Southern Africa (Kumar et al., 2016). Finger millet being a promising source of micronutrients and protein (Malleshi and Klopfenstein, 1998), can play a significant role in alleviation of micronutrient and protein malnutrition, which affects more than one half of the world’s population, especially women and preschool children in most countries of South-east Asia and Africa (Underwood, 2000).
Molecular Players Involved in Calcium Transport
Source to Sink Transport in Root, Stem, Leaves and Developing Spikes
Calcium (Ca2+) is normally acquired by root from the soil solution in free ionic form. The outer layers of root, the epidermal cells and their elongated projection (root hairs) get in touch with the soil solution where Ca2+ are present in milli-molar range. Through a variety of Ca2+ permeable channels, which includes cyclic nucleotide gated channels (CNGCs), glutamate receptor like (GLR) proteins, two-pore channels (TPCs) and mechano-sensitive Ca-permeable channels (MSCCs) calcium (Ca2+) enters inside the root epidermal cells, where it moves both apoplastically and symplastically to the cortex and then to the stele. Further, it may reach xylem either apoplastically through extracellular spaces or symplastically, by entering root cells and moving from cell to cell through plasmodesmata.
When the root is young, metaxylem and protoxylem are the main routes of Ca2+ transport while in later stage when the vessels are fully conductive, central xylem becomes the major sink. After being released from root, Ca2+ are transported in the apoplast and xylem vessels of the shoot where the cation exchange capacity (CEC) of xylem walls and intensity of transpiration is the major factor for Ca2+ transportation (McLaughlin and Wimmer, 1999). Ca2+ present in the xylem sap exchanges with bound Ca2+ from the xylem walls; the movement of an individual Ca2+ will be in a series of jumps between exchange sites (Atkinson et al., 1992). Trunk xylem tissues deliver Ca2+ inside the leaf tissue via vein extensions. The calcium (Ca2+) delivery and distribution in leaves is mostly dependent on the pathway of water flow to and through the leaves (Gilliham et al., 2011). After separation of mineral cations from water, Ca2+ moves slower in apoplast. This could be due to the fact that Ca2+ transport within the leaf is not only by mass flow but also via an extracellular pathway (Canny, 1993). Growing part of leaf tissue requires high Ca2+ concentration, hence providing a sink for Ca2+ movement.
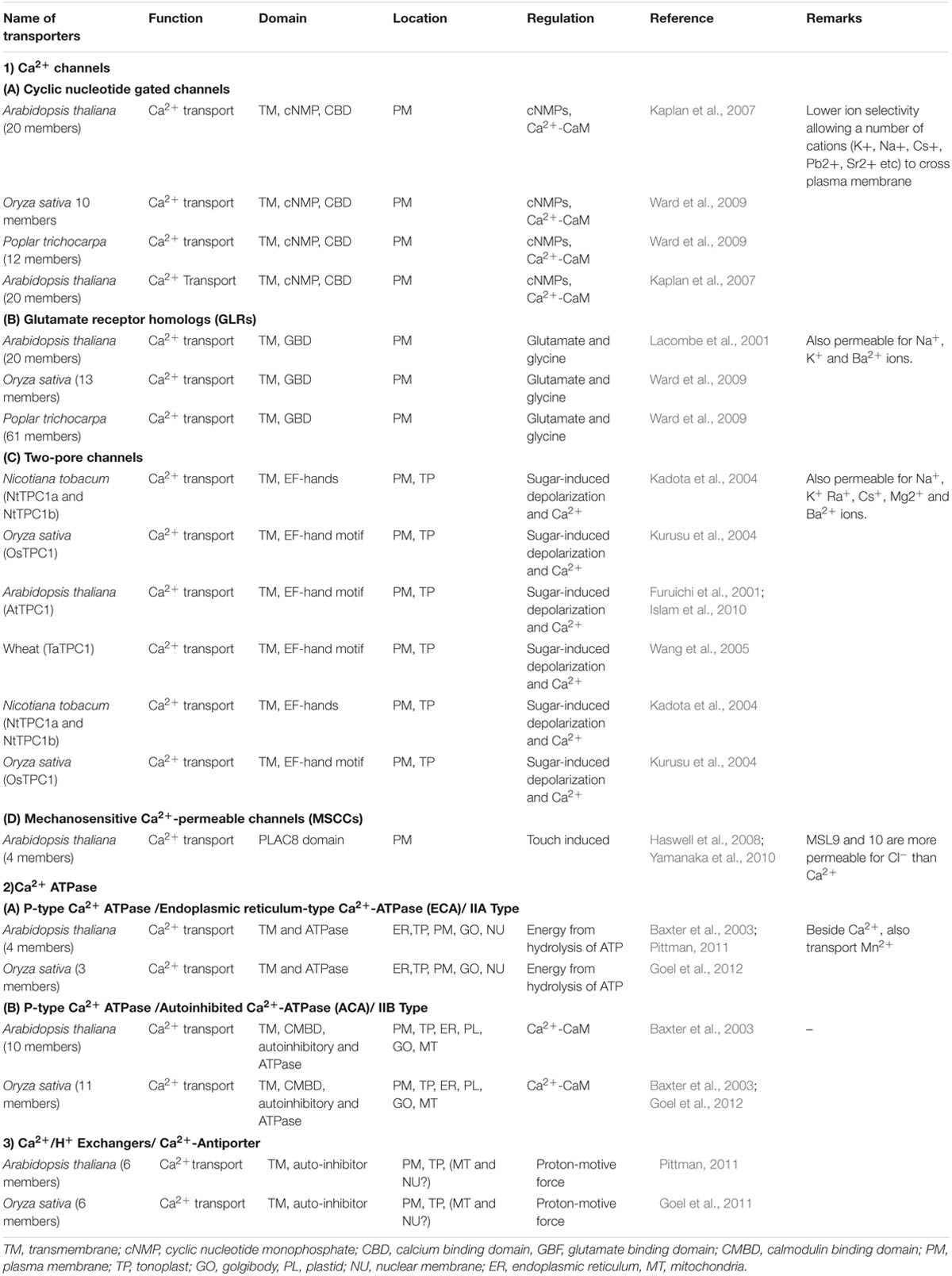
Members of calcium signaling and transport genes are reported to be involved in calcium uptake, transport and accumulation at cellular levels in plants. These include various types of calcium sensors viz., calcium dependent protein kinases (CDPKs), Calcinurin- B like protein kinases (CIPKs), calmodulin dependent protein kinases (CaMKs), calcium/calmodulin dependent protein kinases (CCaMKs) and transporters genes viz., Ca2+ -ATPases, Calcium/cation exchangers and calcium channels (Singh et al., 2015). Calcium transporters are actively involved in the uptake and transport of calcium in the cells, while calcium sensors are involved in the regulation of these transporters (Table 1). Understanding the role of these genes and the availability of the calcium in rhizosphere might help in development of calcium biofortified plants. Mirza et al. (2014) analyzed the expression of calcium transporter and their regulator genes in different tissues at different growth stages in two finger millet genotypes differing in calcium content and reported higher expression of CAX1 gene in roots of high calcium genotype GPHCPB45 in addition to two more genes (TPC1 and ATPase) in most of the tissues at vegetative growth and developing spikes except flag leaf. Higher expression of CAX1 in roots suggests that calcium uptake is taking place at high rate probably under the regulation of calmodulin independent pathway as the expression of calmodulin is invariably low in all vegetative tissues (Mirza et al., 2014). The expression of CAX1 was observed to be low in root tissue as compared to those in other tissues as reported earlier (Carter et al., 2004; Cocozza et al., 2008; Conn and Gilliham, 2010). This suggests that although calcium uptake is taking place at high rate, it is not getting stored in the vacuole rather it is effluxed in the root apoplast and is trafficked to the cells by means of the water transpiration stream. The calcium content in the leaves was higher than those in the root and stem tissue with higher content in GPHCPB45 leaves as compared to GPHCPB1. This indicates that higher transpiration might attribute to higher calcium (Ca2+) accumulation in GPHCPB45 genotype. Interestingly, the expression pattern of 14-3-3 gene was observed to be similar to CAX1 gene in leaf tissue indicating that 14-3-3 might interact with the CAX1 for calcium content regulation in leaves. Further in the developing spike (S1 – S4 stage), all the transporters exhibited an increased expression with higher expression in GPHCPB45 and hence correspond to the higher calcium (Ca2+) content (Mirza et al., 2014). Higher expression of TPC1 and CAX1 genes in GPHCPB45 indicates higher uptake and accumulation of calcium (Ca2+) in comparison to GPHCPB1 in the developing spikes. All the regulatory proteins also exhibited expression patterns similar to the transporter genes in developing spike with generally a higher expression in GPHCPB45 genotype. CaM exhibited a similar expression pattern as Ca2+ ATPase gene indicating that this CaM isoform might be specific to developing spike and activating the Ca2+ ATPase through binding to the CBD domain (Amtmann and Blatt, 2009). CAM1 was strongly expressed during developing spikes of high grain calcium genotype along with CAX1. Insilico analysis showed that EcCAM interacts with aquaporin indicating calcium is probably delivered to developing spikes via mass flow of water (Kumar et al., 2014). The results indicate that calmodulin independent and dependent pathways cause greater stimulation of transport machinery operative in vegetative and spike tissues leading to higher accumulation of calcium from source to sink. A CAX–CaM dual gene construct may be designed for targeted co-expression under grain endosperm cell-specific promoters to fortify cereals with bioavailable calcium. In flag leaf, the expression of all the transporter and regulatory genes was found to be down regulated, however, the calcium content in flag leaf was highest among all the tissues in both the genotypes. This indicates that the calcium accumulation in flag leaf is largely dependent on the transpirational pull and isoforms.
Briefly, the differential expression of genes shows differential spatial and temporal accumulation of calcium (Ca2+) in the two genotypes of finger millet. Studies on finger millet CAX1 and ATPase might offer further insights in understanding their auto-inhibition and strategic regulation. However, higher transcript abundance does not always corroborate with higher gene product abundance and activity. There are many post translational modifications and other bottle necks that may alter the fate of transcriptional activity. The results of transcriptome needs further validation using knock in and knock out approaches. Thus, finger millet plant might as well befall as a model system for better understanding of the underlying genetic control and molecular physiological mechanisms contributing to high grain calcium. Figure 2 depicts the role of potential transporters and regulatory genes involved in calcium (Ca2+) transport from source to sink in finger millet.
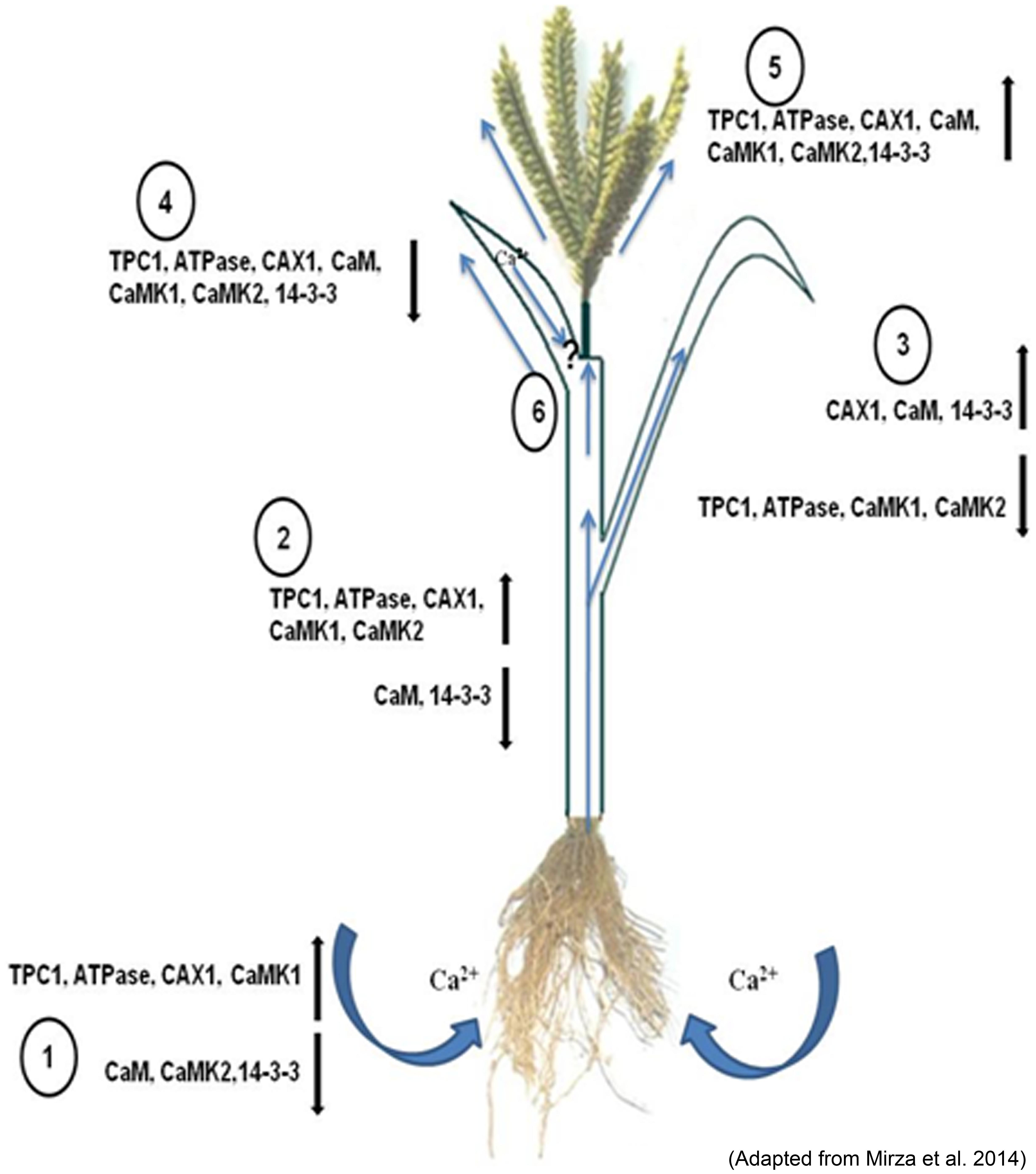
FIGURE 2. Depiction of the role of the potential transporters and their regulatory genes during translocation of calcium (Ca2+) from the rhizosphere by the (1) root and root hair and translocation. Along with the xylem stream through (2) stem, distribution to the (3) leaves and phloem loading for movement into the (4) flag leaf and (5) developing spike (6). The up (↑) and down (↓) regulation of these genes are given in the sketch diagram (Adapted from Mirza et al., 2014).
Calcium Accumulation in Seed
Seed consists of filial tissues (aleurone and endosperm cell) and maternal tissues (seed coat and nucellar cell) with no direct vascular connection between them (Wolswinkel, 1992). Generally, differentiated vascular systems are restricted to seed tissues and are primarily comprised of phloem with no or limited xylem (Patrick, 1997). Phloem immobility of Ca2+ forces it to take different route for delivery in seed. The possibilities include the non-vascular symplastic pathway from funiculas, viz., xylem transfer cell, pigment strands, nucellar cell, or apoplastic (cell wall) routes (Van Bel, 1990). The calcium concentration in phloem-fed tissues, such as fruits, seeds and tubers is in general low in many crops (Karley and White, 2009), but finger millet seeds have been found to be accumulating very high calcium varying from 100 to 450 mg/100 g (Panwar et al., 2010). Therefore, it becomes an important target crop toward elucidating the genetic and epigenetic basis of calcium uptake, transport and accumulation in plant. The information generated through this could be utilized for biofortification of staple crops through transgenic approaches which relies on improving the uptake, translocation and accumulation in edible tissues or through adequate availability in their roots.
Based on the existing knowledge about calcium (Ca2+) transport and accumulation, a hypothetical pathway of seed calcium accumulation is proposed in Figure 3. In this model, it is proposed that Ca2+ from xylem passes through the pedicel tissues (Xylem transfer cell and pigmented strand) and then enters the maternal tissue (seed coat) with the help of transporters. It gets pumped toward the apoplast from seed coat, where it is absorbed by aleurone and then in endodermal cell. So, prior to uptake by the embryo, Ca2+ are deposited in the seed-coat apoplastic space abutting the surface of the embryo (Patrick, 1990). The seed coat is major Ca2+ storage tissue followed by aleurone layer and the endosperm (Nath et al., 2012). The contrasting distribution pattern of Ca2+ in different tissues of seed during seed development indicates the role of physiological and molecular mechanisms of their accumulation. The presence of prominent, insoluble calcium oxalate crystals (especially in seed coat) and calcium phytates in the embryo of mature seed has been reported to be an important factor for high grain calcium in plants (Barnabas and Arnott, 1990; Ilarslan et al., 2001).
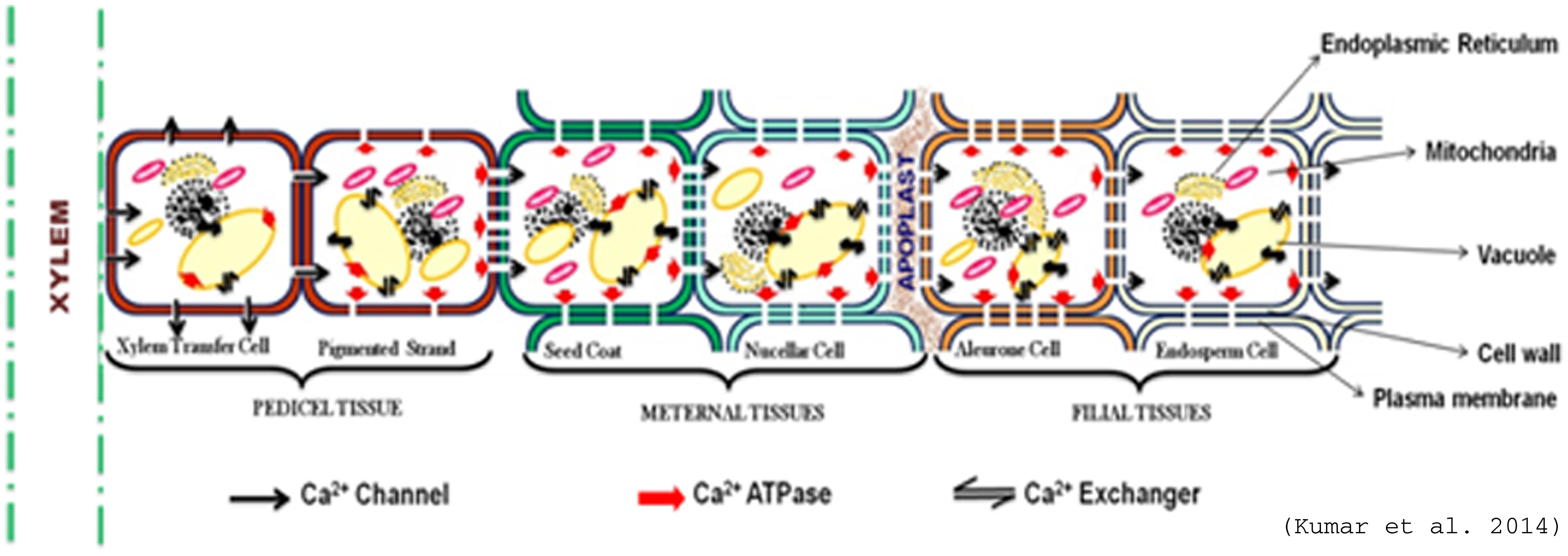
FIGURE 3. Hypothetical model of Ca2+ transport in seed (Adapted from Kumar et al., 2014).
Spatial distribution of calcium transporters within the cell provides the path for storage and movement of calcium (Ca2+). There is good evidence that Ca uptake from the apoplast is mediated by activity of Ca channels, which would be the most energetically efficient mechanisms because they utilize the large inward directed electrochemical potential gradient for Ca (Franceschi and Nakata, 2005). The occurrence of abundant Ca2+ channels in the cell membrane toward the xylem (funiculus) and Ca2+-ATPases toward endosperm might be responsible to pump Ca2+ in the seed (Figure 2). Once Ca2+ enters inside the seed it might get stored inside cell organelles viz., vacuole, ER etc. Vacuole is reported as the major reservoir of Ca2+ and up to 80% or more of the cellular volume of plant cells is occupied by vacuole (Pittman, 2011). The abundance of Ca2+ transporters especially antiporters on tonoplast contribute toward high calcium (Ca2+) in seeds (Conn et al., 2011; Punshon et al., 2012). Higher expression of Ca2+ transporters and calcium binding proteins (CaBPs) in the tonoplast membrane has been reported for the uptake and storage of Ca2+ in vacuole of leaf cell (Blumwald and Poole, 1986; Wang et al., 1995).
The mechanism of Ca transport inside seed is still not known, as Ca is phloem immobile and seed is fed mainly by phloem. Our preliminary study based on rice Ca sensor and transporter data suggests that Ca transporters (especially Ca Exchanger) are the main Ca transporting proteins that pump Ca inside seed. Based on the structural and expression analysis of the calcium transporter genes and available data in literature, a hypothetical model for accumulation of calcium in the cereal grains was proposed (Goel et al., 2011, 2012). The nine calcium transporter genes identified through the analysis of rice MPSS and microarray data were tested and one calcium ATPase and one calcium exchanger was found to be highly expressed in high seed calcium finger millet genotype. In finger millet, out of the five genes encoding calcium transporters and sensors (Ca ATPase-01, CAX-01, TPC-01, CaMK-02 and one gene of 14.3.3) isolated from finger millet, CAX1 showed strong expression in the later stages of spike development indicating its role in accumulating high amounts of calcium in seeds (Mirza et al., 2014). Therefore, it can be speculated that the same mechanism of grain calcium accumulation exists in finger millet as is found in rice. However, the model is probabilistic and experimental evidence like gain or loss of function studies are required to validate this model. Figure 4 depicts a hypothetical model showing the possible mechanism of calcium accumulation in various stages of cereal grains development.
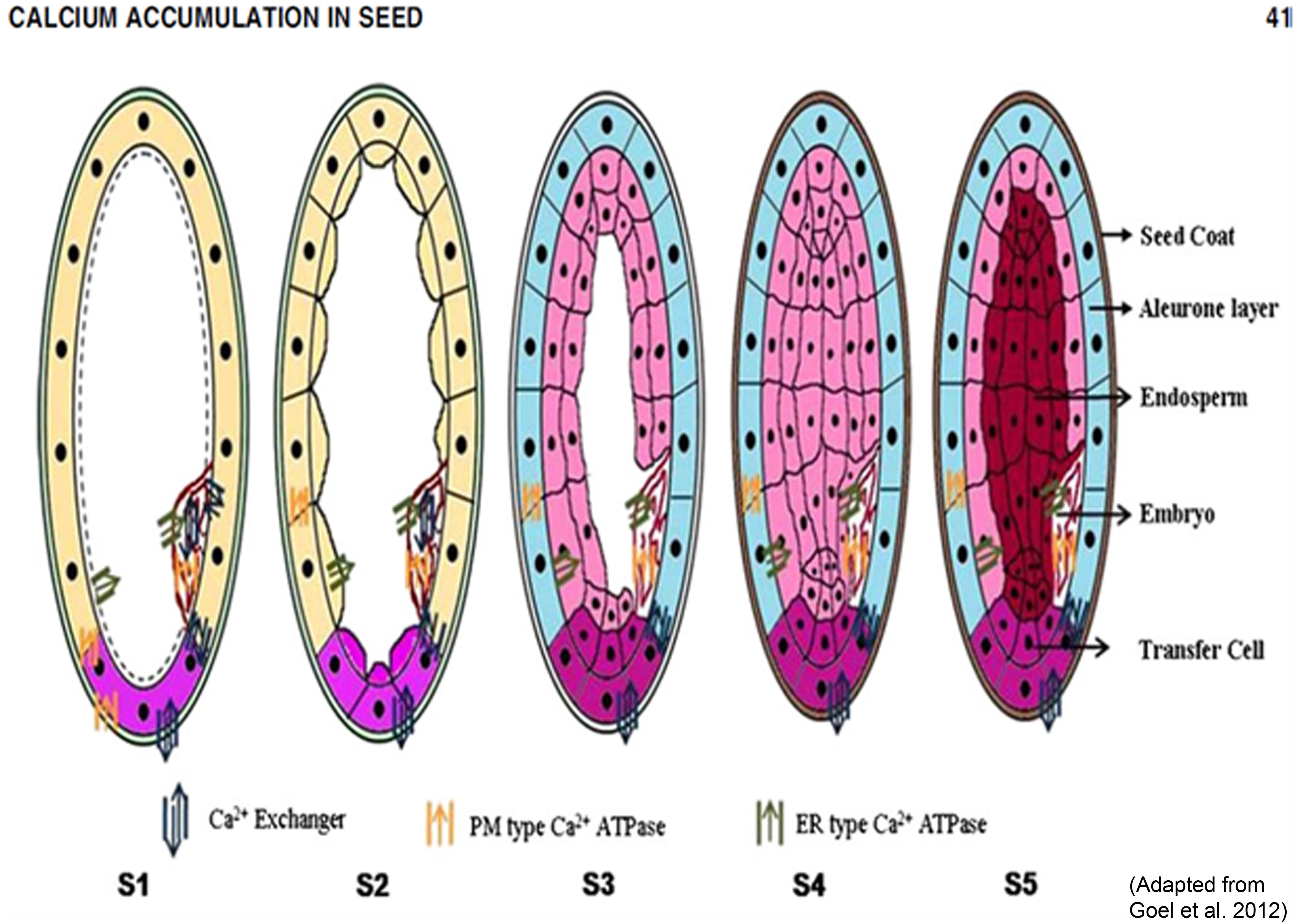
FIGURE 4. A hypothetical model showing the possible mechanism of calcium accumulation in various stages of cereal grains development. S1 (booting), S2 (spike emergence), S3 (pollination), S4 (Grain filling or milky) and S5 (seed maturation). (Adapted from Goel et al., 2012).
Transcriptome sequence data of finger millet showed high expression of Ca2+ transporters including CAX-1, CAX-3 and calcium sensor protein such as CIPK-24 and CaM in high seed calcium finger millet genotype (GPHCPB45). Transcript profiling (qPCR) study of such identified candidate genes indicated that Ca2+ transporters including CAX-1, CAX-3 and calcium sensor proteins such as CIPK-8 and CaM are highly expressed in high seed calcium finger millet genotype (GPHCPB45) than GPHCPB1. Based on expression analysis and insilico interaction studies a speculative model is shown in Figure 5 which shows tri-partite interaction of calcium transport and sensor genes leading to activation of transport machinery that pumps calcium from cytosol to vacuole in developing seeds in finger millet. It is suggested that SOS3/CBL4 and SOS2/CBL10 strongly interact with CIPK-24 in either vegetative or developing spikes stage. Such SOS/CBL complexes bind and activate CIPK-24 kinase activity by relieving self-inhibitory folding within the regulatory and kinase domain of the CIPK-24 protein (Halfter et al., 2000) and thus modulating the efficiency of CAX1 and CAX3 channel transporters. Ion transporters present at the tonoplast, including an H+ pump and Ca2+/H+ exchanger are the targets of CIPK-24 for their activation (Verslues et al., 2007). The calcium transport machinery regulated by calmodulin dependent and calmodulin independent pathways operative in seed tissues is leading to not only differential accumulation of calcium in seeds but also varied accumulation of calcium in different seed tissues viz. higher accumulation in aleurone layer, followed by seed coat and least in endosperm as reported by SEM-DEX analysis (Nath et al., 2012).
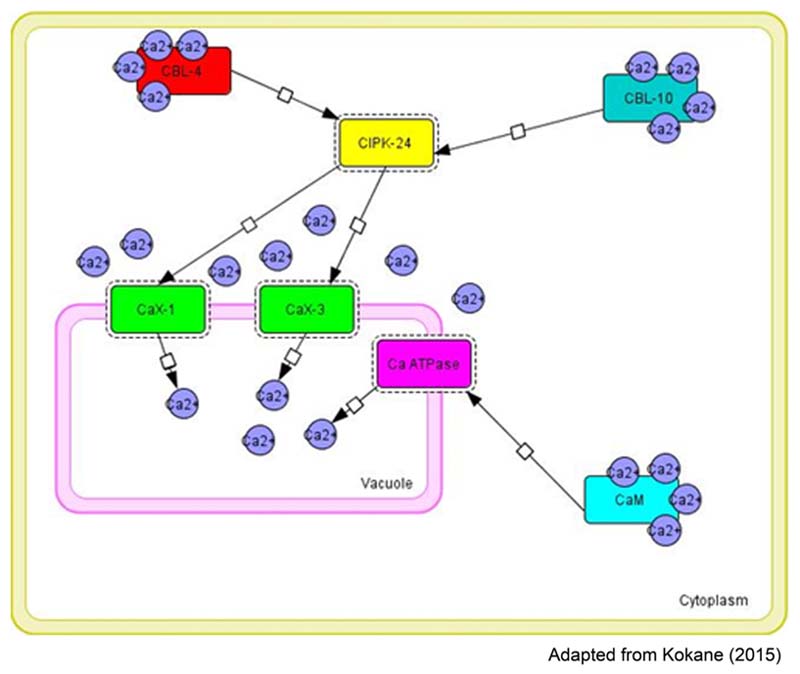
FIGURE 5. Tripartite molecular interactions map using system biology graphical notation (SBGN) of the calcium exchangers and sensors in different tissues [CBL-4-CIPK24_CaX1 (Vegetative tissue), CBL-10-CIPK24-CaX1; CBL10-CIPK24-CaX3 (Vegetative tissue or developing spikes)] involved in the regulation of calcium transport and accumulation in finger millet (constructed by CellDesigner4.4).
Molecular Approaches for Calcium Biofortification
Marker Discovery and Omics Approaches to Study the Molecular Basis of Calcium Accumulation
Fr From the last 20 years, molecular biology has revolutionized conventional breeding techniques in all areas. Biochemical and molecular techniques have shortened the duration of breeding programs from years to months or eliminated the need for them all together. The use of molecular markers in conventional breeding techniques have also improved the accuracy of crosses and allowed breeders to produce genotypes with combined traits that were very difficult before the advent of DNA technology. DNA markers can be generated in large numbers and can prove to be very useful for a variety of purposes relevant to crop improvement. Figure 6 gives a schematic representation of genomics and molecular breeding approaches for developing calcium biofortified finger millet.
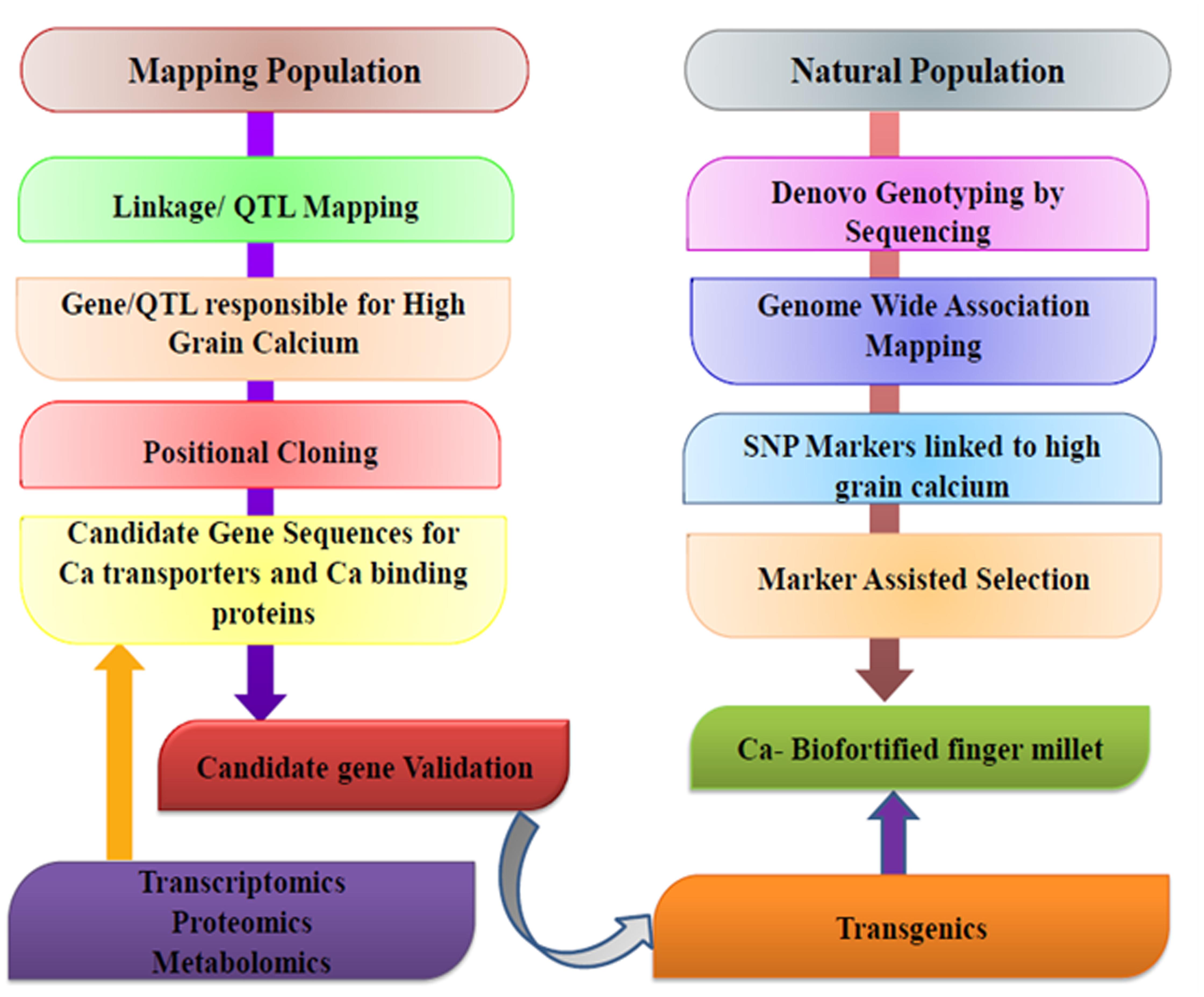
FIGURE 6. Schematic representation of genomics and molecular breeding approaches for developing calcium biofortified finger millet.
Low level of polymorphism has been reported in most of the studies on diversity analysis using molecular markers in cultivated finger millet (Muza et al., 1995). For the first time Dida et al. (2007) developed genomic SSRs by isolating di- and trinucleotide SSRs from random genomic HindIII, PstI and SalI libraries of finger millet. They developed first genetic map of finger millet with 31 genomic SSRs as well as RFLP, AFLP and EST markers. The sudden increase in the volume of sequence data generated from EST projects in several plant species facilitated the identification of genic SSRs in large numbers. Since the genomes of minor grasses like finger millet is yet to be sequenced, markers in minor grasses can be fetched from the major cereals. Comparative genetic mapping of cereal crops has shown that both gene contents and/or gene orders are largely conserved over the evolutionary history of the grasses (Moore et al., 1995) to the extent that grass genomes represent a ‘single genetic system’ (Bennetzen and Freeling, 1997).
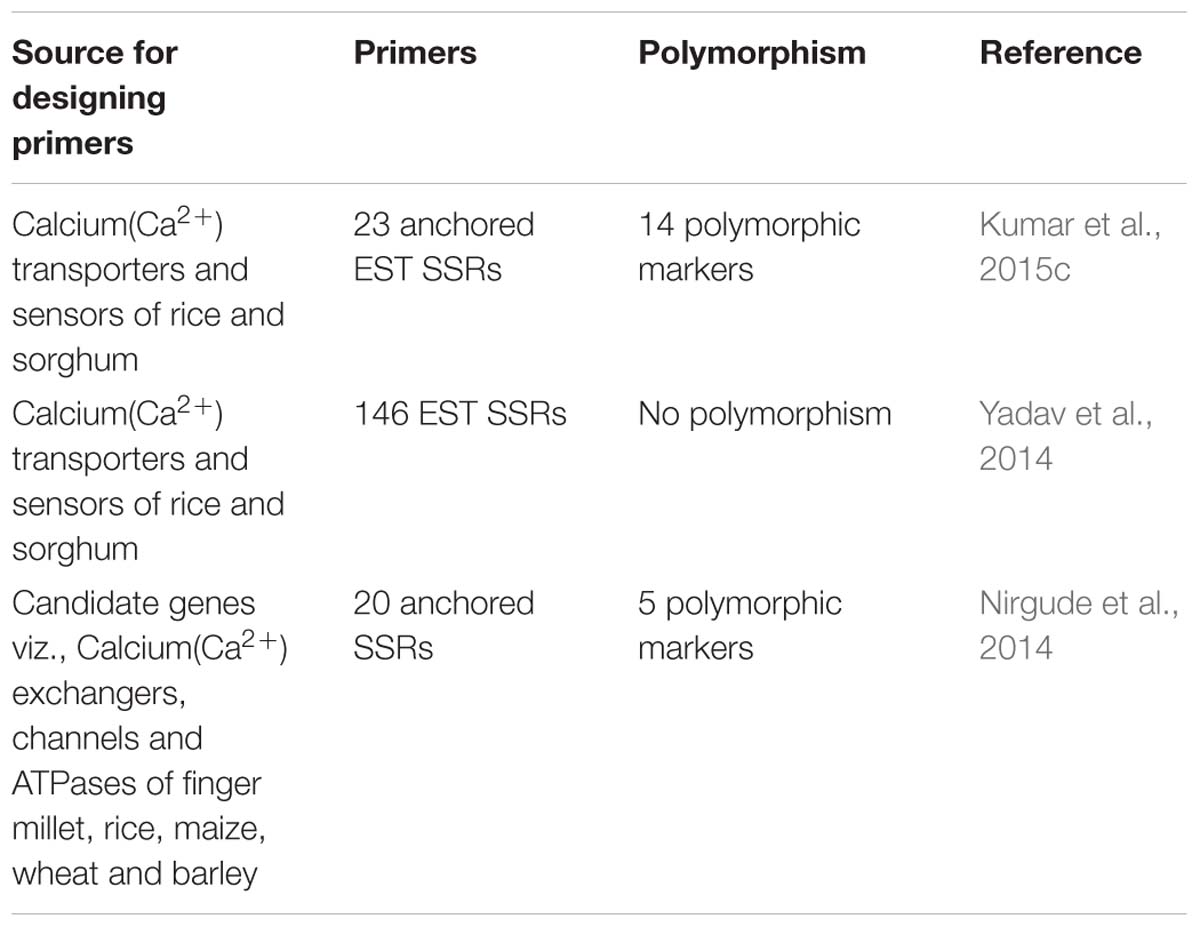
Assessment of genetic diversity in finger millet revealed important information that South Indian and the African genotypes are close together and genetically distinct from North Indian genotypes including Uttarakhand (Panwar et al., 2010). Some of these genotypes of this crop possess very high grain calcium (Ca2+) content (450 mg/100 g) which is 10–30 times higher than wheat and rice (Panwar et al., 2010). In a study of Uttarakhand finger millet genotypes, molecular marker analysis differentiated the genotypes into three distinct clusters according to calcium (Ca2+) content indicating that variation in calcium content is also genetically controlled (Panwar et al., 2010). Markers have been utilized extensively for marker assisted selection, based on their association with genes/QTLs controlling grain calcium trait (Table 2). In order to identify the markers associated with high grain calcium trait, 146 genic SSR markers were assessed for cross species transferability across a diverse panel of grass species. The average transferability of genic SSR markers from sorghum to other grasses was highest (73.2%) followed by rice (63.4%) with an overall average of 68.3% which establishes the importance of these major crops as a useful resource of genomic information for minor crops. The genic SSR primers (69.7%) failed to detect variations across the finger millet germplasm, indicating that the mineral transport and storage machinery remain conserved in plants and even SSR variations in them remain suppressed during the course of evolution (Yadav et al., 2014). Development and molecular characterization of genic molecular markers for grain protein and calcium content has also been done (Nirgude et al., 2014). Of the 86 SSRs used in linkage mapping study, only 6 primers were polymorphic among the two parents PRM 801 (low calcium genotype) and GE 86 (high calcium genotype). Further, 20 polymorphic primers used across the association mapping panel of 238 genotypes led to the identification of 5 SSR markers viz. ugep67, ugep24, ugep77, ugep12 and ugep 10, which were significantly associated with calcium trait. For identifying QTLs for calcium content marker trait associations have been explored through association mapping studies and two minor QTLs associated with grain calcium (Ca2+) content on linkage group 3 and 8 respectively have been identified (Yadav et al., 2014). Linkage group 8 has been found to harbor a minor QTL for the trait and high levels of conserved co-linearity between rice and finger millet genomes (Srinivasachary et al., 2007) reveals that finger millet chromosome 8 might also contain genes/regions responsible for effective mineral accumulation. Similarly, finger millet LG 3 shares co-linearity with rice chromosome 3 and Ca2+ QTLs have also been mapped on chromosome 3 of rice. Furthermore, these results indicate that broad genome-wide search will be required to identify all the genes that control this complex trait and variation in a population.
The putative QTLs for calcium accumulation and increased calcium uptake have been explored in several plant species by different workers (Guzmán-Maldonado et al., 2003; Lexer et al., 2003; Zhang et al., 2009). In rice, QTLs for calcium accumulation have been identified and mapped on chromosomes 1, 3, 4, 5, 9, 10, 11, and 12 with phenotypic variation ranging from 5 to 18% in rice (Garcia-Oliveira et al., 2009; Du et al., 2013). Owing to sufficient variation for calcium content in finger millet germplasm, putative QTLs could be located using comparative genomics approach due to high co-linearity between rice and finger millet genomes.
One of the greatest challenges of biology in the post genomic era understands the functional connections between genes, transcripts, proteins, metabolites and nutrients (Kumar et al., 2015a). This understanding will highly beneficial for the plant biofortification processes. Storage of nutrients in different plant organs such as leaves, roots or seeds is governed by a specific subset of genes which ultimately control whole plant concentrations for numerous elements. The field of genomics has significantly contributed to this information, as it has helped in identifying the genes and genomes of several food plants. As it is already known that some genes are responsible for the uptake and transport of more than one mineral, thus, affecting the mineral homeostasis in a coordinated way. Therefore, alternate tools for expediting the prioritization of candidate genes that control mineral accumulation in plants are desirable (Conn et al., 2012). Therefore, the field of high throughput sequencing/transcriptomics has been fundamental in accelerating gene discovery.
Since, the finger millet crop is valued for its high calcium content, studies have been focused to characterize calcium (Ca2+) sensing, transport and accumulation mechanisms across genotypes differing in their grain calcium (Ca2+) content with the use of high-throughput transcriptome sequencing (Kumar et al., 2014; Singh et al., 2014). To generate the nucleotide sequence information resource and gain deep insights about the developing grain transcriptome and identify the genes responsible or involved in the process of high grain calcium (Ca2+) accumulation, Kumar et al. (2015b) used Illumina HiSeq-2000 high-throughput RNA sequencing technology to identify differentially expressed targets for calcium (Ca2+) content variation in two contrasting finger millet genotypes differing in grain calcium (Ca2+) content (GPHCPB45 – a high calcium genotype and GPHCPB1 – a low calcium genotype). The differential expression analysis on the basis of FPKM value resulted in 24 genes highly expressed in GPHCPB45 genotype and 11 were highly expressed in GPHCPB1 genes (Kumar et al., 2015b). The results obtained from transcriptome analysis were validated on spikes of finger millet grown on different concentration of calcium (Ca2+) (0.1, 5.0, 10, and 20 mM) at four stages of spike development. Significant correlation between the expression of selected Ca2+ sensor genes and amount of exogenous Ca2+ supply was observed (Singh et al., 2014).
Through peptide mass finger printing, two Ca2+ binding protein viz., ‘Calcineurin-B’ and ‘Calreticulin’ were identified in finger millet seeds (Singh et al., 2016). Following the information about the involvement of calcineurin and calreticulin proteins in binding calcium (Ca2+) in seed, expressions of 5 each calcineurin and calreticulin genes of finger millet were studied during grain filling. EcCRT1, which shows high expression in the S1 stage of seed development, was partially cloned, sequenced and its full length cloning is under progress (Singh et al., 2014). The mechanism of how Ca2+ are moving/transporting inside seed is still not known, as Ca2+ in phloem is immobile and seed is mainly fed by phloem. The preliminary results based on rice Ca2+ sensor and transporter data suggest that Ca2+ transporters (especially Ca Exchanger) are the main Ca2+ transporting proteins that pump Ca2+ inside seed (Li, 2006). The activities of these Ca2+ transporters are governed by Ca2+ sensor proteins such as CaM. Southern hybridization results showed the presence of at least four copies of the previously isolated CaM, that is located on different regions of the finger millet genome. Immuno-detection using mono-specific polyclonal anti-EcCaM antibodies revealed that EcCaM is localized in the embryo, close to the aleurone layer and accumulates in higher amounts in the high grain calcium genotype. Abundance of CaM around aleurone layer in high grain Ca2+ genotype suggests that CaM might be responsible for high grain calcium accumulation (Kumar et al., 2014). However, all studies on Ca2+ sensor and transporter genes were based on cloning of gene by designing primers from conserved region of related species/genus that may miss important gene of major effects. A detailed transcriptome wide study of calcium (Ca2+) sensors and transporters was carried out to identify the differentially expressed genes during developing spikes of finger millet and can serve as potential candidate genes having role in seed calcium accumulation (Singh et al., 2014, 2016). Transgenics for Ca2+ Exchanger (CAX), an important Ca2+ transporter that pump Ca2+ inside vacuoles were developed, which resulted in significant Ca2+ increase but deleterious effects like mineral imbalance, stunted plant growth and other structural changes were also seen (Kumar et al., 2015a). GPHCPB45 was found to be more efficient calcium (Ca2+) accumulator at low external calcium (Ca2+) concentrations, whereas GPHCPB1 was found to be more responsive to increased external calcium (Ca2+) concentration (Singh et al., 2014). Comprehensive sequencing efforts and analysis of gene function in the developing spikes transcriptome of finger millet (E. coracana) represents the most extensive expressed sequence resource available for finger millet to date (Kumar et al., 2014). Analysis of transcriptome sequence data of finger millet has indicated that Ca2+ transporter genes including CAX-1, CAX-3 and calcium (Ca2+) sensor protein such as CIPK-24 and CaM are highly expressed in high seed Ca2+ finger millet genotype. In order to understand the role and interrelationship expression of these genes in high seed calcium (Ca2+) accumulation, comparative tissue wide transcript profiling study of potential candidate genes, viz. CAX-1, CAX-3, CIPK-24 and CaM at vegetative stage and developing spikes stage have shown mostly higher expression in high grain calcium (Ca2+) genotype. The up-regulation of CAX-1 in vegetative tissues and developing spikes and CAX-3 in developing spikes provide most plausible clue explaining the accumulation of high calcium (Ca2+) in finger millet. The genetic information generated from these studies can be deployed in calcium (Ca2+) biofortification program by using genetic engineering or marker assisted selection strategies. Breeding for high grain Ca2+ in finger millet can be accelerated by using the closely linked markers to identified genes (EcCIPKs). This is just beginning, and it needs further studies in cloning and validation of the above genes using a set of different contrasting genotypes.
Recently, an effort has been also made to study the genetic make of diverse world finger millet collection and population structure analysis of 113 finger millet accessions using genotyping by sequencing (GBS) (Kumar et al., 2016). This resulted in a genome wide set of 23000 Single Nucleotide Polymorphisms (SNPs) segregating across the entire collection and several thousand SNPs segregating within every accession. It will provide a better opportunity to dissect complex traits and identification of superior alleles/ genes contributing to the target traits.
Considering the examination of mineral/micronutrients and their complex networks, ionomics or ionome profiling has emerged as a striking area that facilitates the genome-scale understanding of dynamics of elemental accumulation in the living systems (Baxter, 2010). In the context to ionomics, several techniques driven by the electronic or nuclear property of the element are currently being used to investigate elemental composition in different organisms (Singh et al., 2013). These techniques include AAS, ion beam analysis (IBA), X-ray fluorescence spectroscopy (XRF), ICP mass spectroscopy (ICP-MS) and neutron activation analysis (NAA) (Pfeiffer and McClafferty, 2007; Djingove et al., 2013; Singh et al., 2013).
Metabolomics is another area to better explore the chemical components which are present in food grains, how they are synthesized and the genetic and environmental factors that are involved in determining food composition and stability. Metabolomics is much more than just a diagnostics tool (Schauer and Fernie, 2006) and can help us greatly to improve our understanding of the complexity of metabolic regulation and explain how minor perturbations can have a myriad of biochemical end-points. This creates the potential to provide us with the knowledge necessary to facilitate a more targeted approach to crop improvement, specifically in terms of biochemical composition and nutritional value (Robert and Jennifer, 2010).
Transgenic Technology
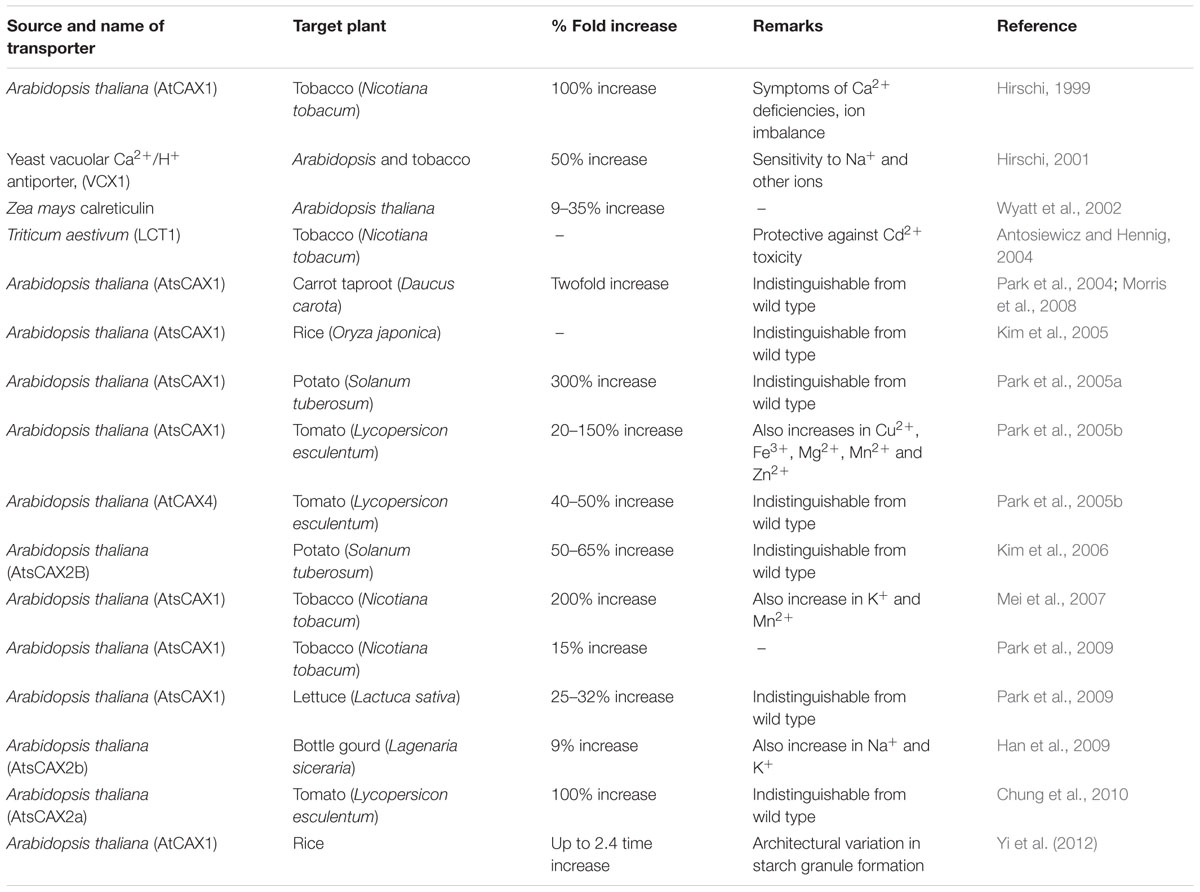
Transgenic may provide an alternative approach for biofortification of major food to enhance the concentration of calcium (Ca2+) content. The major aim of calcium biofortification strategy is to increase calcium (Ca2+) content in edible parts of crops without adversely affecting plant growth or increasing the plant requirement for additional inputs, i.e., calcium (Ca2+) fertilization or increased water use (Dayod et al., 2010). In plant three transgenic approaches can be used to increase the concentration of calcium (Ca2+) in edible part. The first approach is to enhance calcium (Ca2+) storage capacity through expression of calcium (Ca2+) transporter proteins viz., Ca2+ ATPase and Ca2+/H+ antiporters. Ca2+ ATPase requires energy for calcium (Ca2+) transport in the form of ATP while Ca2+/H+ antiporters is activated by proton motive force. Lower energy demands of transport through Ca2+/H+ antiporters make them a good candidate for Ca2+ increase (Conn and Gilliham, 2010). Arabidopsis thaliana CAX1 (for CALCIUM EXCHANGER 1) gene was first over expressed in tobacco plant under the control of cauliflower mosaic virus 35S promoter and 100% increase in calcium content was observed (Hirschi, 1999). Hirschi and colleagues expressed Yeast vacuolar Ca2+/H+ antiporter, (VCX1) in Arabidopsis and tobacco (Hirschi, 2001). VCX1-expressing plants demonstrated increased tonoplast-enriched Ca2+/H+ antiport activity as well as increased Ca2+ accumulation (upto 50%). Later several transgenic event were attempted by expression of different CAX proteins viz., AtsCAX1 in carrot (Park et al., 2004; Morris et al., 2008), rice (Kim et al., 2006; Yi et al., 2012), potato (Park et al., 2005a), tomato (Park et al., 2005b), tobacco (Mei et al., 2007; Park et al., 2009) lettuce (Park et al., 2009); AtCAX4 in tomato (Park et al., 2005b), AtsCAX2B in potato (Kim et al., 2006); AtsCAX2a in tomato (Chung et al., 2010); AtsCAX2b in bottle guard (Han et al., 2009) and upto 300% calcium (Ca2+) increase were reported. However, tissue Ca2+ increase was negatively co-related with Ca2+ deficiency symptoms. Interestingly, over expression of AtCAX4 or modified AtsCAX2B have shown weaker Ca2+ transport and fewer deficiency symptoms (Park et al., 2005b; Chung et al., 2010). This indicates that AtsCAX1 is too strong in pulling cytosolic Ca2+ away from cytoplasm and makes it unavailable for plant cellular processes than weaker alleles like AtCAX4, AtsCAX2B. Increasing bioavailable Ca2+, CAX transporters are excellent candidates but measure has to taken to avoid Ca2+ deficiency symptoms. Moreover, over expression of some CAX have shown little accumulation of other ions like Cd (cadmium), Mn (magnese) modification of the region could be used to increase specificity (Shigaki et al., 2003).
The second approach is to enhance calcium (Ca2+) accumulation by over expression of calcium (Ca2+) channel proteins. Calcium (Ca2+) channels in plasma membrane have been reported in all known cell types (Véry and Sentenac, 2002) but there are few reports on the genes that encode these proteins. GLR Proteins (Gilliham et al., 2006) and cyclic nucleotide gated channels (Demidchik and Maathuis, 2007) are a few among the candidate genes. Influx of calcium (Ca2+) across the PM is passive (i.e., down the Ca2+ electrochemical gradient) and is driven by Ca2+ channels (White and Broadley, 2003). The wheat low-affinity cation transporter gene (LCT1) was expressed in tobacco under the control of CaMV 35S promoter. LCT1-transformed plants expressed a phenotype distinct from controls only under conditions of low calcium, they grew significantly better and had slightly higher shoot calcium concentration (Antosiewicz and Hennig, 2004).
The third approach to enhance calcium (Ca2+) content in plant was by over expression of calcium (Ca2+) binding protein. The use of calcium (Ca2+) binding proteins might be very efficient way to increase calcium (Ca2+) accumulation in tissues. The over expression of Zea mays calreticulin (an ER-localized calcium binding protein) in Arabidopsis resulted in bioavailable calcium (Ca2+) increase in plant (Wyatt et al., 2002). Upto 9–35% increase of calcium (Ca2+) content was measured for induced transformants compared to controls. The use of calcium binding protein as candidate for Ca2+ biofortification is good but compared to CAX transporter very less increase of Ca2+ has been reported (Wyatt et al., 2002).
The fourth approach was through mutagenesis. Model forage crop Medicago truncatula was mutagenised with EMS and mutant with no (cod5) or reduced (cod6) calcium oxalate crystals were identified. In this report 10% reduction in calcium(Ca2+) content but 23% increase in calcium(Ca2+) absorbed by mice in feeding trial was observed (Morris et al., 2008). Out of four transgenic approach for calcium(Ca2+) biofortification in plants, maximum calcium(Ca2+) increase (up to 300% time) has been reported in potato over expressing modified Arabidopsis AtsCAX1 protein but the bioavailability of calcium(Ca2+) was found with calreticulin gene (Table 3).
Conclusion
Hi- throughput technologies of genomics, transcriptomics, proteomics, metabolomics and ionomics have shifted the focus from single gene research to a holistic understanding of gene function. None of the methods used in isolation provides enough information to infer function of an unknown gene, instead combined data from different functional genomics tools bring us close to this goal. Potential genes involved in high grain calcium (Ca2+) accumulation in finger millet once identified through these approaches can be validated by over expressing them through transgenics and subsequently the candidate genes may possibly be used to genetically modify crops and help them increase their grain calcium content. Genome wide variant detection in crops like finger millet is a preliminary step toward linking genotypic variation and phenotypes. The conversion of these genetic variants (the most prevalent of these being SNPs) into genetic markers is particularly important in agronomically valuable crops to allow for effective marker assisted selection strategies, map based gene cloning, whole genome fingerprinting, association studies and population based analyses. Toward these goals, in the absence of the genome sequence of finger millet an increasing number of large scale genetic variant discovery initiatives are being taken in conjunction with NGS platforms, allowing for drastically quicker and cheaper variant discovery, and leading toward a far more comprehensive view of the genome or transcriptome.
Future Prospects
Genomics information has not only helped in efficient understanding of structural and functional aspects of many plant genomes but also has provided a feasible platform for manipulation of genomes for crop improvement. The near-future completion of genome sequencing project of finger millet will help in determining the function of every gene and ultimately how genes interact to form the basis of complex traits such as calcium nutrition. Finger millet is hence, no more called as a coarse cereal rather referred to as a nutri-cereal or nutraceutical. The properties on the whole make finger millet an ideal model for studying genomics and an implausible source for gene mining for complex traits such as grain calcium (Ca2+) content. Potential candidate genes responsible for high grain calcium (Ca2+) accumulation isolated from finger millet can help in improving other cereal crops through functional genomics and molecular breeding approaches and pave way for the development of designer crops.
Author Contributions
AK conceptualized the manuscript. DS, GJ, and US wrote the manuscript. DS and SS assisted, edited and updated the manuscript. AK contributed critically in revising the draft and updating the manuscript for publication.
Funding
The work is published under the research program funded by Department of Biotechnology, Govt. of India in the form of Program Support for research and development in Agricultural Biotechnology at G. B. Pant University of Agriculture and Technology, Pantnagar, India (Grant No. BT/PR7849/AGR/02/2006).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer TB and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
References
Admassu, S., Teamir, M., and Alemu, D. (2009). Chemical composition of local and improved finger millet (Eleusine coracana (L.) Gaetrtin) varieties grown in Ethiopia. Ethiop. J. Health Sci. 19, 1–8.
Amtmann, A., and Blatt, M. R. (2009). Regulation of macronutrient transport. New Phytol. 81, 35–52. doi: 10.1111/j.1469-8137.2008.02666.x
Antony, U., and Chandra, T. S. (1999). Enzymatic treatment and use of starters for the nutrient enhancement in fermented flour of red and white varieties of finger millet (Eleusine coracana). J. Agric. Food Chem. 47, 2016–2019. doi: 10.1021/jf980564a
Antosiewicz, D. M., and Hennig, J. (2004). Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ. Pollut. 129, 237–245. doi: 10.1016/j.envpol.2003.10.025
Atkinson, C. J., Ruiz, L. P., and Mansfield, T. A. (1992). Calcium in xylem sap and the regulation of its delivery to the shoot. J. Exp. Bot. 43, 1315–1324. doi: 10.1093/jxb/43.10.1315
Babu, B. V., Ramana, T., and Radhakrishnan, T. M. (1987). Chemical composition and protein content in hybrid varieties of finger millet. Indian J. Agric. Sci. 57, 520–522.
Barbeau, W. E., and Hilu, K. W. (1993). Protein, calcium, iron, and amino acid content of selected wild and domesticated cultivars of finger millet. Plant Foods Hum. Nutr. 43, 97–104. doi: 10.1007/BF01087914
Barnabas, A. B., and Arnott, H. J. (1990). Calcium oxalate crystal formation in the bean (Phaseolus vulgaris L.) seed coat. Bot. Gaz. 151, 331–341. doi: 10.1086/337833
Baxter, I. (2010). Ionomics: the functional genomics of elements. Brief. Funct. Genomics 9, 149–156. doi: 10.1093/bfgp/elp055
Baxter, I., Tchieu, J., Sussman, M. R., Boutry, M., Palmgren, M. G., Gribskov, M., et al. (2003). Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 132, 618–628. doi: 10.1104/pp.103.021923
Bennett, B., and Sammartano, R. (2012). The Complete Idiot’s Guide to Vegan Living, Second Edn. Sittingbourne: Health and Fitness.
Bennetzen, J. L., and Freeling, M. (1997). The unified grass genome: synergy in synteny. Genome Res. 7, 301–306.
Bhatt, A., Singh, V., Shrotria, P. K., and Baskheti, D. C. (2003). Coarse grains of uttaranchal: ensuring sustainable food and nutritional security. Indian Farmer Digest 7, 34–38.
Blumwald, E., and Poole, R. J. (1986). Kinetics of Ca2+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 80, 727–731. doi: 10.1104/pp.80.3.727
Bouis, H. E. (2003). Micronutrient fortification of plants through plant breeding: can it improve nutrition in man at low cost? Proc. Nutr. Soc. 62, 403–411. doi: 10.1079/PNS2003262
Canny, M. J. (1993). The transpiration stream in the leaf apoplast-water and solutes. Philos. Trans. R. Soc. B 341, 87–100. doi: 10.1098/rstb.1993.0094
Carter, C., Pan, S., Zouhar, J., Avila, E. L., Girke, T., and Raikhel, N. V. (2004). The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16, 3285–3303. doi: 10.1105/tpc.104.027078
Carvalho, S. M., and Vasconcelos, M. W. (2013). Producing more with less: strategies and novel technologies for plant-based food biofortification. Food Res. Int. 54, 961–971. doi: 10.1016/j.foodres.2012.12.021
Chan, B. K., Marshall, L. M., Winters, K. M., Faulkner, K. A., Schwartz, A. V., and Orwoll, E. S. (2007). Incident fall risk and physical activity and physical performance among older men the osteoporotic fractures in men study. Am. J. Epidemiol. 165, 696–703. doi: 10.1093/aje/kwk050
Chan, G. M., McElligott, K., McNaught, T., and Gill, G. (2006). Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet. Gynecol. 108, 565–571. doi: 10.1097/01.AOG.0000231721.42823.9e
Chethan, S., and Malleshi, N. G. (2007). Finger millet polyphenols: characterization and their nutraceutical potential. Am. J. Food Technol. 2, 582–592. doi: 10.3923/ajft.2007.582.592
Chung, M. Y., Han, J. S., Giovannoni, J., Liu, Y., Kim, C. K., Lim, K. B., et al. (2010). Modest calcium increase in tomatoes expressing a variant of Arabidopsis cation/H+ antiporter. Plant Biotech Rep. 4, 15–21. doi: 10.1007/s11816-009-0112-9
Cocozza, C., Minnocci, A., Tognetti, R., Iori, V., Zacchini, M., and Scarascia Mugnozza, G. (2008). Distribution and concentration of cadmium in root tissue of Populus alba determined by scanning electron microscopy and energy-dispersive x-ray microanalysis. iFor. Biogeosci. For. 1, 96–103. doi: 10.3832/ifor0458-0010096
Collard, B. C., and Mackill, D. J. (2008). Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 12 363, 557–572. doi: 10.1098/rstb.2007.2170
Conn, S., and Gilliham, M. (2010). Comparative physiology of elemental distributions in plants. Ann. Bot. 105, 1081–1102. doi: 10.1093/aob/mcq027
Conn, S. J., Berninger, P., Broadley, M. R., and Gilliham, M. (2012). Exploiting natural variation to uncover candidate genes that control element accumulation in Arabidopsis thaliana. New Phytol. 193, 859–866. doi: 10.1111/j.1469-8137.2011.03977.x
Conn, S. J., Gilliham, M., and Athman, A. (2011). Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23, 240–257. doi: 10.1105/tpc.109.072769
Crossa, J., De los Campos, G., Perez, P., Gianola, D., Burgueno, J., and Araus, J. L. (2010). Prediction of genetic values of quantitative traits in plant breeding using pedigree and molecular markers. Genetics 186, 713–724. doi: 10.1534/genetics.110.118521
Dayod, M., Tyerman, S. D., Leigh, R. A., and Gilliham, M. (2010). Calcium storage in plants and the implications for calcium biofortification. Protoplasma 247, 215–231. doi: 10.1007/s00709-010-0182-0
Demidchik, V., and Maathuis, F. J. (2007). Physiological roles of non selective cation channels in plants: from salt stress to signalling and development. New Phytol. 175, 387–404. doi: 10.1111/j.1469-8137.2007.02128.x
Deosthale, Y. G. (2002). The Nutritive Value of Foods and the Significance of some Household Processes. Available at: http://www.unu.edu. p. 6
Devi, P. B., Vijayabharathi, R., Sathyabama, S., Malleshi, N. G., and Priyadarisini, V. B. (2014). Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J. Food Sci. Technol. 51, 1021–1040. doi: 10.1007/s13197-011-0584-9
Dida, M. M., Ramakrishnan, S., Bennetzen, J. L., Gale, M. D., and Devos, K. M. (2007). The genetic map of finger millet, Eleusine coracana. Theor. Appl. Genet. 114, 321–332. doi: 10.1007/s00122-006-0435-7
Djingove, R., Mihaylova, V., Lyubomirova, V., and Tsalev, D. L. (2013). Multi element analytical spectroscopy in plant ionomics research. Appl. Spectrosc. Rev. 48, 384–424. doi: 10.1080/05704928.2012.703153
Du, J., Zeng, D., Wang, B., Qian, Q., Zheng, S., and Ling, H. Q. (2013). Environmental effects on mineral accumulation in rice grains and identification of ecological specific QTLs. Environ. Geochem. Health 35, 161–170. doi: 10.1007/s10653-012-9473-z
Fairweather-Tait, S., and Hurrell, R. F. (1996). Bioavailability of minerals and trace elements. Nutr. Res. Rev. 9, 295–324. doi: 10.1079/NRR19960016
Food and Agriculture Organization [FAO] of the United Nations (2008). Food Insecurity and Vulnerability Information and Mapping Systems (FIVMS). Rome: FAO.
Food and Agriculture Organization [FAO] of the United Nations (2009). 2050: A Third more Mouths to Feed. Rome: FAO.
Franceschi, V. R., and Nakata, P. A. (2005). Calcium oxalate in plants: formation and function. Annu. Rev. Plant Biol. 56, 41–71. doi: 10.1146/annurev.arplant.56.032604.144106
Furuichi, T., Cunningham, K. W., and Muto, S. (2001). A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 42, 900–905. doi: 10.1093/pcp/pce145
Garcia-Oliveira, A. L., Tan, L., Fu, Y., and Sun, C. (2009). Genetic identification of quantitative trait loci for contents of mineral nutrients in rice grain. J. Integr. Plant Biol. 51, 84–92. doi: 10.1111/j.1744-7909.2008.00730.x
Genc, Y., Humphries, J. M., Lyons, G. H., and Graham, R. D. (2005). Exploiting genotypic variation in plant nutrient accumulation to alleviate micronutrient deficiency in populations. J. Trace Elem. Med. Biol. 18, 319–324. doi: 10.1016/j.jtemb.2005.02.005
Gilliham, M., Campbell, M., Dubos, C., Becker, D., and Davenport, R. (2006). “The Arabidopsis thaliana glutamate-like receptor family (AtGLR),” in Communication in Plants, eds F. Baluška, S. Mancuso, and D. Volkmann (Berlin: Springer), 187–204.
Gilliham, M., Dayod, M., Hocking, B. J., Xu, B., Conn, S. J., Kaiser, B. N., et al. (2011). Calcium delivery and storage in plant leaves: exploring the link with waterflow. J. Exp. Bot. 62, 2233–2250. doi: 10.1093/jxb/err111
Goel, A., Gaur, V. S., Arora, S., Gupta, S., and Kumar, A. (2012). In silico analysis of expression data for identification of genes involved in spatial accumulation of calcium in developing seeds of rice. Omics 16, 402–413. doi: 10.1089/omi.2012.0004
Goel, A., Taj, G., Pandey, D., Gupta, S., and Kumar, A. (2011). Genome-wide comparative in silico analysis of calcium transporters of rice and sorghum. Genomics Proteomics Bioinformatics 9, 138–150. doi: 10.1016/S1672-0229(11)60017-X
Gopalan, C., Ramshashtri, B. V., and Balasubramanian, S. C. (1999). Nutritive Value of Indian Foods, eds B. S. NarasingaRao, Y. G. Deosthale and K. C. Pant. Hyderabad: National Institute of Nutrition, 156.
Govindaraj, M., Vetriventhan, M., and Srinivasan, M. (2015). Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genet. Res. Int. 2015:431487. doi: 10.1155/2015/431487
Graham, R. D., Welch, R. M., and Bouis, H. E. (2001). Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv. Agron. 70, 77–142. doi: 10.1016/S0065-2113(01)70004-1
Guo, Z. A., Song, Y. X., Zhou, R. H., Ren, Z. L., and Jia, J. Z. (2010). Discovery, evaluation and distribution of haplotypes of the wheat Ppd-D1 gene. New Phytol. 185, 841–851. doi: 10.1111/j.1469-8137.2009.03099.x
Guzmán-Maldonado, S. H., Martinez, O., Acosta-Gallegos, J. A., Guevara-Lara, F., and Paredes-Lopez, O. (2003). Putative quantitative trait loci for physical and chemical components of common bean. Crop Sci. 43, 1029–1035. doi: 10.2135/cropsci2003.1029
Halfter, U. M., Ishitani, M., and Zhu, J. K. (2000). The arabidopsis SOS2protein kinase physically interacts with and activated by the calcium binding protein SOS3. Proc. Natl. Acad. Sci. U.S.A. 97, 3735–3740. doi: 10.1073/pnas.97.7.3735
Han, J. C., Yang, X. D., Zhang, T., Li, H., Li, W. L., Zhang, Z. Y., et al. (2009). Effects of 1alpha-hydroxycholecalciferol on growth performance, parameters of tibia and plasma, meat quality, and type IIb sodium phosphate co transporter gene expression of one- to twenty-one-day-old broilers. Poult. Sci. 88, 323–329. doi: 10.3382/ps.2008-00252
Haswell, E. S., Peyronnet, R., Barbier-Brygoo, H., Meyerowitz, E. M., and Frachisse, J. M. (2008). Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 18, 730–734. doi: 10.1016/j.cub.2008.04.039
Haug, W., and Lantzsch, H. J. (1983). Sensitive method for the rapid determination of the phytate in cereals and cereal products. J. Sci. Food Agric. 34, 1423–1426. doi: 10.1002/jsfa.2740341217
Heaney, R. P. (1993). Nutritional factors in osteoporosis. Annu. Rev. Nutr. 13, 287–316. doi: 10.1146/annurev.nu.13.070193.001443
Heffner, E. L., Jannink, J. L., and Sorrells, M. E. (2011). Genomic selection accuracy using multifamily prediction models in a wheat breeding program. Plant Genome 4, 65–75. doi: 10.3835/plantgenome.2010.12.0029
Hirschi, K. D. (1999). Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11, 2113–2122. doi: 10.2307/3871013
Hirschi, K. D. (2001). Vacuolar H+/Ca2+ transport: who’s directing the traffic? Trends Plant Sci. 6, 100–104. doi: 10.1016/S1360-1385(00)01863-X
Hirschi, K. D. (2009). Nutrient biofortification of food crops. Annu. Rev. Nutr. 21, 401–421. doi: 10.1146/annurev-nutr-080508-141143
House, W. A. (1999). Trace element bioavailability as exemplified by iron and zinc. Field Crops Res. 60, 115–141. doi: 10.1016/S0378-4290(98)00136-1
Ilarslan, H., Palmer, R. G., and Horner, H. T. (2001). Calcium oxalate crystals in developing seeds of soybean. Ann. Bot. 88, 243–257. doi: 10.1006/anbo.2001.1453
Islam, M. M., Munemasa, S., Hossain, M. A., Nakamura, Y., Mori, I. C., and Murata, Y. (2010). Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol. 51, 302–311. doi: 10.1093/pcp/pcq001
Jeong, J., and Guerinot, M. L. (2008). Biofortified and bioavailable: the gold standard for plant-based diets. Proc. Natl. Acad. Sci. U.S.A. 105, 1777–1778. doi: 10.1073/pnas.0712330105
Kadota, Y., Furuichi, T., Ogasawara, Y., Goh, T., Higashi, K., Muto, S., et al. (2004). Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem. Biophys. Res. Commun. 317, 823–830. doi: 10.1016/j.bbrc.2004.03.114
Kaplan, B., Sherman, T., and Fromm, H. (2007). Cyclic nucleotide-gated channels in plants. FEBS Lett. 581, 2237–2246. doi: 10.1016/j.febslet.2007.02.017
Karley, A. J., and White, P. J. (2009). Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Curr. Opin. Plant Biol. 12, 291–298. doi: 10.1016/j.pbi.2009.04.013
Kim, C. K., Han, J. S., Lee, H. S., Oh, J. Y., Shigaki, T., Park, S. H., et al. (2006). Expression of an Arabidopsis CAX2 variant in potato tubers increases calcium levels with no accumulation of manganese. Plant Cell Rep. 25, 1226–1232. doi: 10.1007/s00299-006-0214-6
Kim, K. M., Park, Y. H., Kim, C. K., Hirschi, K., and Sohn, J. K. (2005). Development of transgenic rice plants overexpressing the Arabidopsis H+/Ca2+ antiporter CAX1 gene. Plant Cell Rep. 23, 678–682. doi: 10.1007/s00299-004-0861-4
Kokane, S. (2015). Tissue Wide Expression Analysis of Potential Candidate Genes Involved in Calcium Signaling and Transport in Finger Millet (Eleusine coracana) Genotypes Differing in Grain Calcium Content. Master’s thesis, G. B Pant University of Agriculture and Technology, Pantnagar.
Kranz, S., Lin, P. J., and Wagstaff, D. A. (2007). Children’s dairy intake in the United States: too little, too fat? J. Paediatr. 151, 642–646. doi: 10.1016/j.jpeds.2007.04.067
Kumar, A., Gaur, V. S., Goel, A., and Gupta, A. K. (2015b). De novo assembly and characterization of developing spikes transcriptome of finger millet (Eleusine coracana): a minor crop having nutraceutical properties. Plant Mol. Biol. Rep. 33, 905–922. doi: 10.1007/s11105-014-0802-5
Kumar, A., Mirza, N., Charan, T., Sharma, N., and Gaur, V. S. (2014). Isolation, characterization and immunolocalization of a seed dominant CaM from finger millet (Eleusinecoracana L. Gartn.) for studying its functional role in differential accumulation of calcium in developing grains. Appl. Biochem. Biotechnol. 172, 2955–2973. doi: 10.1007/s12010-013-0714-0
Kumar, A., Pathak, R. K., Gupta, S. M., Gaur, V. S., and Pandey, D. (2015a). Systems biology for smart crops and agricultural innovation: filling the gaps between genotype and phenotype for complex traits linked with robust agricultural productivity and sustainability. OMICS 19, 581–601. doi: 10.1089/omi.2015.0106
Kumar, A., Sharma, D., Tiwari, A., Jaiswal, J. P., Singh, N. K., and Sood, S. (2016). Genotyping-by-sequencing analysis for determining population structure of finger millet germplasm of diverse origins. Plant Genome 9, doi: 10.3835/plantgenome2015.07.0058
Kumar, A., Yadav, S., Panwar, P., Gaur, V. S., and Sood, S. (2015c). Identification of anchored simple sequence repeat markers associated with calcium content in finger millet (Eleusine coracana). Proc. Natl. Acad. Sci. India B Biol. Sci. 85, 311–317. doi: 10.1007/s40011-013-0296-1
Kurusu, T., Sakurai, Y., Miyao, A., Hirochika, H., and Kuchitsu, K. (2004). Identification of a putative voltage-gated Ca2+-permeable channel (OsTPC1) involved in Ca2+ influx and regulation of growth and development in rice. Plant Cell Physiol. 45, 693–702. doi: 10.1093/pcp/pch082
Lacombe, B., Becker, D., Hedrich, R., DeSalle, R., Hollmann, M., Kwak, J. M., et al. (2001). The identity of plant glutamate receptors. Science 292, 1486–1487. doi: 10.1126/science.292.5521.1486b
Lanham-New, S. A. (2008). Importance of calcium, vitamin D and vitamin K for osteoporosis prevention and treatment. Proc. Nutr. Soc. 67, 163–176. doi: 10.1017/S0029665108007003
Lexer, C., Welch, M. E., Durphy, J. L., and Rieseberg, L. H. (2003). Natural selection for salt tolerance quantitative trait loci (QTLs) in wild sunflower hybrids: implications for the origin of Helianthus paradoxus, a diploid hybrid species. Mol. Ecol. 12, 1225–1235. doi: 10.1046/j.1365-294X.2003.01803.x
Li, X. (2006). The Importance of Sorting Calcium in Plant Cells: Uncovering the Roles of A Sarcoplasmic/Endoplasmic Reticulum-Like Calcium ATPase. Ph.D. thesis, University of Maryland College Park, Maryland, MD.
Malleshi, N. G., and Klopfenstein, C. F. (1998). Nutrient composition, amino acid and vitamin contents of malted sorghum, pearl millet, finger millet and their rootlets. Int. J. Food Sci. Nutr. 49, 415–422. doi: 10.3109/09637489809086420
Matuschek, E., Towo, E., and Svanberg, U. (2001). Oxidation of polyphenols in phytate-reduced high-tannin cereals: effect on different phenolic groups and on in vitro accessible iron. J. Agric. Food Chem. 49, 5630–5638. doi: 10.1021/jf0108157
McLaughlin, S. B., and Wimmer, R. (1999). Calcium physiology and terrestrial ecosystem processes. New Phytol. 142, 373–417. doi: 10.1046/j.1469-8137.1999.00420.x
Mei, Y., Yang, H., Sun, J., Yin, X., Guo, X., Wang, Z., et al. (2007). Effects of different dust particles on calcium current in the neurons of dorsal root ganglia of rats. J. Life Sci. 4, 10–14.
Mirza, N., Taj, G., Arora, S., and Kumar, A. (2014). Transcriptional expression analysis of genes involved in regulation of calcium translocation and storage in finger millet (Eleusine coracana L.Gartn.). Gene 550, 171–179. doi: 10.1016/j.gene.2014.08.005
Moore, G., Devos, K. M., Wang, Z., and Gale, M. D. (1995). Cereal genome evolution: grasses, line up and form a circle. Curr. Biol. 5, 737–739. doi: 10.1016/S0960-9822(95)00148-5
Morris, J., Hawthorne, K. M., Hotze, T., Abrams, S. A., and Hirschi, K. D. (2008). Nutritional impact of elevated calcium transport activity in carrots. Proc. Natl. Acad. Sci. U.S.A. 105, 1431–1435. doi: 10.1073/pnas.0709005105
Müller, O., and Krawinkel, M. (2005). Malnutrition and health in developing countries. Can. Med. Assoc. J. 173, 279–286. doi: 10.1503/cmaj.050342
Muza, F. R., Lee, D. J., Andrews, D. J., and Gupta, S. C. (1995). Mitochondrial DNA variation in finger millet (Eleusinecoracana L. Gaertn). Euphytica 81, 199–205. doi: 10.1007/BF00025434
Narina, S. S., Buyyarapu, R., Kottapalli, K. R., Sartie, A. M., Ali, M. I., Robert, A., et al. (2011). Generation and analysis of expressed sequence tags (ESTs) for marker development in yam (Dioscoreaa lata L.). BMC Genomics. 12:1. doi: 10.1186/1471-2164-12-100
Nath, M., Partha, R., Shukla, A., and Kumar, A. (2012). Spatial distribution and accumulation of calcium in different tissues, developing spikes and seeds of finger millet (Eleusine coracana) genotype. J. Plant Nutr. 36, 539–550. doi: 10.1080/01904167.2012.748072
Nestel, P., Bouis, H. E., Meenakshi, J. V., and Pfeiffer, W. (2006). Biofortification of staple food crops. J. Nutr. 1, 1064–1067.
Nirgude, M., Babu, B. K., Shambhavi, Y., Singh, U. M., Upadhyaya, H. D., and Kumar, A. (2014). Development and molecular characterization of genic molecular markers for grain protein and calcium content in finger millet (Eleusine coracana (L.) Gaertn.). Mol. Biol. Rep. 41, 1189–1200. doi: 10.1007/s11033-013-2825-7
Obilana, A. B., and Manyasa, E. (2002). “Millets,” in Pseudocereals and Less Common Cereals. Grain Properties and Utilization Potential, eds P. S. Belton and J. R. N. Taylor (Berlin: Springer-Verlag), 176–217.
Panwar, P., Nath, M., Kumar, V., and Kumar, A. (2010). Comparative evaluation of genetic diversity using RAPD, SSR and cytochrome P450 gene based markers with respect to calcium content in finger millet (Eleusine coracana L. Gaertn.). J. Genet. 89, 121–133. doi: 10.1007/s12041-010-0052-8
Park, S., Cheng, N. H., Pittman, J. K., Yoo, K. S., Park, J., Smith, R. H., et al. (2005a). Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol. 139, 1194–1206. doi: 10.1104/pp.105.066266
Park, S., Elless, M. P., Park, J., Jenkins, A., Lim, W., Chambers, E., et al. (2009). Sensory analysis of calcium-biofortified lettuce. Plant Biotechnol. J. 7, 106–111. doi: 10.1111/j.1467-7652.2008.00379.x
Park, S., Kang, T. S., Kim, C. K., Han, J. S., Kim, S., Smith, R. H., et al. (2005b). Genetic manipulation for enhancing calcium content in potato tuber. J. Agric. Food Chem. 53, 5598–5603. doi: 10.1021/jf050531c
Park, S., Kim, C. K., Pike, L. M., Smith, R. H., and Hirschi, K. D. (2004). Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol. Breed. 14, 275–282. doi: 10.1023/B:MOLB.0000047773.20175.ae
Patrick, J. W. (1990). Sieve element unloading: cellular pathway, mechanism and control. Physiol. Plant. 78, 298–308. doi: 10.1034/j.1399-3054.1990.780220.x
Patrick, J. W. (1997). Phloem unloading: sieve element unloading and post-sieve element transport. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 191–222. doi: 10.1146/annurev.arplant.48.1.191
Pettifor, J. M. (2008). Vitamin D &/or calcium deficiency rickets in infants & children: a global perspective. Indian J. Med. Res. 127, 245.
Pfeiffer, W. H., and McClafferty, B. (2007). Harvest plus: breeding crops for better nutrition. Crop Sci. 47, 88–105. doi: 10.2135/cropsci2007.09.0020IPBS
Piepho, H. P. (2009). Ridge regression and extensions for genomewide selection in maize. Crop Sci. 49, 1165–1176. doi: 10.2135/cropsci2008.10.0595
Pittman, J. K. (2011). Vacuolar Ca2+ uptake. Cell Calcium 50, 139–146. doi: 10.1016/j.ceca.2011.01.004
Pravina, P., Sayaji, D., and Avinash, M. (2013). Calcium and its role in human body. Int. J. Res. Pharm. Biomed. Sci. 4, 659–668.
Punshon, T., Hirschi, K., Yang, J., Lanzirotti, A., Lai, B., and Guerinot, M. L. (2012). The role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed. Plant Physiol. 158, 352–362. doi: 10.1104/pp.111.184812
Raboy, V. (2000). Low phytic acid grains. Food Nutr. Bull. 21, 423–427. doi: 10.1177/156482650002100416
Rao, A. S., Prabhu, U. H., Sampath, S. R., and Schiere, J. B. (1994). The effect of level of allowance on the intake and digestibility of finger millet (Eleusine coracana) straw in crossbred heifers. Anim. Feed Sci. Technol. 49, 37–41. doi: 10.1016/0377-8401(94)90079-5
Rao, M. V. S. S. T. S., and Muralikrishna, G. (2002). Evaluation of the antioxidant properties of free and bound phenolic acids from native and malted finger millet (ragi, Eleusine coracana Indaf-15). J. Agric. Food Chem. 50, 889–892. doi: 10.1021/jf011210d
Ravindran, G. (1991). Studies on millets: proximate composition, mineral composition, phytate, and oxalate contents. Food Chem. 39, 99–107. doi: 10.1016/0308-8146(91)90088-6
Rengel, Z., and Graham, R. D. (1995). Importance of seed zinc content for wheat growth on zinc-deficient soil. I. Vegetative growth. Plant Soil 173, 259–266. doi: 10.1007/BF00011463
Robert, M. Y., and Jennifer, K. (2010). Systems mapping of consumer acceptance of agri food nanotechnology. J. Consum. Policy. 33, 299–322. doi: 10.1007/s10603-010-9134-5
Saied, H. T., and Shamsuddin, A. M. (1998). Up-regulation of the tumour suppressor gene p53 and WAF1 gene expression by IP6 in HT-29 human colon carcinoma cell line. Anticancer Res. 18, 1479–1484.
Schauer, N., and Fernie, A. R. (2006). Plant metabolomics: towards biological function and mechanism. Trends Plant Sci. 11, 508–516. doi: 10.1016/j.tplants.2006.08.007
Seetharam, A. (2001). Annual Report: All India Coordinated Small Millets Improvement Project. Bangalore: University of Agricultural Sciences, 1–28.
Shamsuddin, A. M. (1999). Metabolism and cellular functions of IP6: a review. Anticancer Res. 19, 3733–3736.
Shigaki, T., Pittman, J. K., and Hirschi, K. D. (2003). Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J. Biol. Chem. 278, 6610–6617. doi: 10.1074/jbc.M209952200
Singh, M., Metwal, M., Kumar, V. A., and Kumar, A. (2016). Identification and molecular characterization of 48 kDa calcium binding protein as calreticulin from finger millet (Eleusine coracana) using peptide mass fingerprinting and transcript profiling. J. Sci. Food Agric. 96, 672–679. doi: 10.1002/jsfa.7139
Singh, P., and Raghuvanshi, R. S. (2012). Finger millet for food and nutritional security. Afr. J. Food Sci. 6, 77–84.
Singh, U. M., Metwal, M., Singh, M., Taj, G., and Kumar, A. (2015). Identification and characterization of calcium transporter gene family in finger millet in relation to grain calcium content. Gene 566, 37–46. doi: 10.1016/j.gene.2015.04.021
Singh, U. M., Pandey, D., and Kumar, A. (2014). Determination of calcium responsiveness towards exogenous application in two genotypes of Eleusine coracana L. differing in their grain calcium content. Acta Physiol. Plantarum. 36, 2521–2529. doi: 10.1007/s11738-014-1625-6
Singh, U. M., Sareen, P., Sengar, R. S., and Kumar, A. (2013). Plant ionomics: a newer approach to study mineral transport and its regulation. Acta Physiol. Plant. 35, 2641–2653. doi: 10.1007/s11738-013-1316-8
Srinivasachary, Dida, M. M., Gale, M. D., and Devos, K. M. (2007). Comparative analyses reveal high levels of conserved colinearity between the finger millet and rice genomes. Theor. Appl. Genet. 115, 489–499. doi: 10.1007/s00122-007-0582-5
Timmer, C. P. (2003). Biotechnology and food systems in developing countries. J. Nutr. 133, 3319–3322.
Underwood, R. A. (2000). Overcoming micronutrient deficiencies in developing countries: is there a role for agriculture? Food Nutr. Bull. 21, 356–360. doi: 10.1177/156482650002100403
Upadhyaya, H. D., Gowda, C. L. L., and Reddy, V. G. (2007). Morphological diversity in finger millet germplasm introduced from Southern and Eastern Africa. J. SAT Agric. Res. 3, 1–3.
Upadhyaya, H. D., Ramesh, S., Sharma, S., Singh, S. K., Varshney, S. K., Sarma, N. D. R. K., et al. (2011). Genetic diversity for grain nutrients contents in a core collection of finger millet (Eleusine coracana (L.) Gaertn.)germplasm. Field Crops Res. 121, 42–52. doi: 10.1016/j.fcr.2010.11.017
Vadivoo, A. S., Joseph, R., and Ganesan, N. M. (1998). Genetic variability and diversity for protein and calcium contents in finger millet (Eleusine coracana (L.)Gaertn) in relation to grain color. Plant Foods Hum. Nutr. 52, 353–364. doi: 10.1023/A:1008074002390
Van Bel, A. J. E. (1990). Xylem-phloem exchange via the rays: the undervalued route of transport. J. Exp. Bot. 41, 631–644. doi: 10.1093/jxb/41.6.631
Van Campen, D. R., and Glahn, R. P. (1999). Micronutrient bioavailability techniques: accuracy, problems and limitations. Field Crops Res. 60, 93–113. doi: 10.1016/S0378-4290(98)00135-X
Varshney, R. K., Hoisington, D. A., and Tyagi, A. K. (2006). Advances in cereal genomics and applications in crop breeding. Trends Biotechnol. 24, 490–499. doi: 10.1016/j.tibtech.2006.08.006
Verslues, P. E., Batelli, G., Grillo, S., Agius, F., Kim, Y. S., and Zhu, J. (2007). Interaction of SOS2 with nucleoside diphosphate kinase 2 and catalase reveals a point of connection between salt sress and H2O2 signaling in arabidopsis thaliana. Mol. Cell. Biol. 27, 7771–7780. doi: 10.1128/MCB.00429-07
Véry, A. A., and Sentenac, H. (2002). Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 7, 168–175. doi: 10.1016/S1360-1385(02)02262-8
Ward, J. M., Mäser, P., and Schroeder, J. I. (2009). Plant ion channels: gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 71, 59–82. doi: 10.1146/annurev.physiol.010908.163204
Wang, X. D., Harrington, G., Patrick, J. W., Offler, C. E., and Fieuw, S. (1995). Cellular pathway of photosynthate transport in coats of developing seed of Viciafaba L. and Phaseolus vulgaris L. II.Principal cellular site(s) of efflux. J. Exp. Bot. 46, 49–63. doi: 10.1093/jxb/46.1.49
Wang, Y. J., Yu, J. N., Chen, T., Zhang, Z. G., Hao, Y. J., Zhang, J. S., et al. (2005). Functional analysis of a putative Ca2+ channel gene TaTPC1 from wheat. J. Exp. Bot. 56, 3051–3060. doi: 10.1093/jxb/eri302
Weaver, C. M., and Plawecki, K. L. (1994). Dietary calcium: adequacy of a vegetarian diet. Am. J. Clin. Nutr 59, 1238S–1241S.
Weaver, C. M., Proulx, W. R., and Heaney, R. (1999). Choices for achieving adequate dietary calcium with a vegetarian diet. Am. J. Clin. Nutr. 70, 543s–548s.
Welch, R. M., and Graham, R. D. (1999). A new paradigm for world agriculture: meeting human needs: productive, sustainable nutritious. Field Crops Res. 60, 1–10. doi: 10.1016/S0378-4290(98)00129-4
Welch, R. M., and Graham, R. D. (2002). Breeding crops for enhanced micronutrient content. Plant Soil 245, 205–214. doi: 10.1023/A:1020668100330
White, P. J., and Broadley, M. R. (2003). Calcium in plants. Ann. Bot. 92, 487–511. doi: 10.1093/aob/mcg164
White, P. J., and Broadley, M. R. (2005). Biofortifying crops with essential mineral elements. Trends Plant Sci. 10, 586–593. doi: 10.1016/j.tplants.2005.10.001
Wolswinkel, P. (1992). Transport of nutrients into developing seeds: a review of physiological mechanisms. Seed Sci. Res. 2, 59–73. doi: 10.1017/S096025850000115X
Wyatt, S. E., Tsou, P. L., and Robertson, D. (2002). Expression of the high capacity calcium binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res. 11, 1–10. doi: 10.1023/A:1013917701701
Yadav, S., Gaur, V. S., Jaiswal, J. P., and Kumar, A. (2014). Simple sequence repeat (SSR) analysis in relation to calcium transport and signaling genes reveals transferability among grasses and a conserved behaviour within finger millet genotypes. Plant Syst. Evol. 300, 1561–1568. doi: 10.1007/s00606-014-0982-3
Yamanaka, T., Nakagawa, Y., Mori, K., Nakano, M., Imamura, T., Kataoka, H., et al. (2010). MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 152, 1284–1296. doi: 10.1104/pp.109.147371
Yi, H., Juergens, M., and Jez, J. M. (2012). Structure of soybean β-cyanoalanine synthase and the molecular basis for cyanide detoxification in plants. Plant Cell 24, 2696–2706. doi: 10.1105/tpc.112.098954
Zhang, B., Chen, P., Shi, A., Hou, A., Ishibashi, T., and Wang, D. (2009). Putative quantitative trait loci associated with calcium content in soybean seed. J. Heredity 100, 263–269. doi: 10.1093/jhered/esn096
Zhao, Y., Mette, M. F., Gowda, M., Longin, C. F. H., and Reif, J. C. (2014). Bridging the gap between marker-assisted and genomic selection of heading time and plant height in hybrid wheat. Heredity 112, 638–645. doi: 10.1038/hdy.2014.1
Keywords: calcium, biofortification, bioavailability, finger millet, molecular breeding, functional genomics, transgenics
Citation: Sharma D, Jamra G, Singh UM, Sood S and Kumar A (2017) Calcium Biofortification: Three Pronged Molecular Approaches for Dissecting Complex Trait of Calcium Nutrition in Finger Millet (Eleusine coracana) for Devising Strategies of Enrichment of Food Crops. Front. Plant Sci. 7:2028. doi: 10.3389/fpls.2016.02028
Received: 01 September 2016; Accepted: 19 December 2016;
Published: 17 January 2017.
Edited by:
Manoj Prasad, National Institute of Plant Genome Research, IndiaReviewed by:
Shanker Lal Kothari, Amity University, Jaipur, IndiaTirthankar Bandyopadhyay, National Institute of Plant Genome Research, India
Copyright © 2017 Sharma, Jamra, Singh, Sood and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anil Kumar, anilkumar.mbge@gmail.com
 Divya Sharma
Divya Sharma Gautam Jamra1
Gautam Jamra1 Uma M. Singh
Uma M. Singh Salej Sood
Salej Sood Anil Kumar
Anil Kumar