- 1Department of Life Science, National Taiwan Normal University, Taipei, Taiwan
- 2Department of Entomology, National Taiwan University, Taipei, Taiwan
- 3Division of Silviculture, Taiwan Forestry Research Institute, Taipei, Taiwan
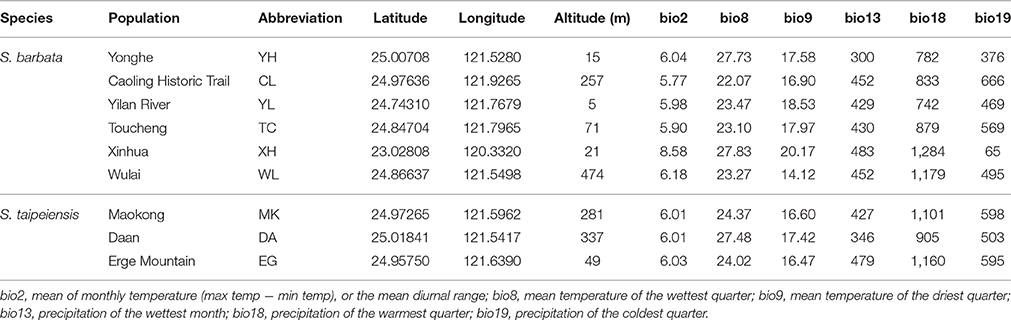
Spatial climate heterogeneity may not only affect adaptive gene frequencies but could also indirectly shape the genetic structure of neutral loci by impacting demographic dynamics. In this study, the effect of local climate on population genetic variation was tested in two phylogenetically close Scutellaria species in Taiwan. Scutellaria taipeiensis, which was originally assumed to be an endemic species of Taiwan Island, is shown to be part of the widespread species S. barbata based on the overlapping ranges of genetic variation and climatic niches as well as their morphological similarity. Rejection of the scenario of “early divergence with secondary contact” and the support for multiple origins of populations of S. taipeiensis from S. barbata provide strong evolutionary evidence for a taxonomic revision of the species combination. Further tests of a climatic effect on genetic variation were conducted. Regression analyses show nonlinear correlations among any pair of geographic, climatic, and genetic distances. However, significantly, the bioclimatic variables that represent the precipitation from late summer to early autumn explain roughly 13% of the genetic variation of our sampled populations. These results indicate that spatial differences of precipitation in the typhoon season may influence the regeneration rate and colonization rate of local populations. The periodic typhoon episodes explain the significant but nonlinear influence of climatic variables on population genetic differentiation. Although, the climatic difference does not lead to species divergence, the local climate variability indeed impacts the spatial genetic distribution at the population level.
Introduction
Species delimitation is a subjective judgment of the level of divergence between organisms despite using objective evidences, while exploring the mechanisms causing genetic divergence is a more objective approach to describing the process of species divergence. In the genic species concept (Wu, 2001), introgression should be heterogeneous across the genome during the speciation process. As time goes by, the segregation of genes will become more and more obvious, and whenever the balancing selection that maintains the shared adaptive polymorphisms in both species is relaxed, random genetic drift may cause decreasing numbers of highly introgressed genes (cf. de Lafontaine et al., 2015). Under this process, an allopatric distribution is not a necessary criterion for speciation, and the proportion of the permeable loci could therefore be an indicator for evaluating the completeness of speciation (Wu, 2001; de Lafontaine et al., 2015).
Interspecific gene flow between two genetically distinct species could be the result of two contrasting evolutionary hypotheses. The first hypothesis is ecologically based speciation with continuous gene flow during the speciation process. When species occur in sympatry, ecological selection should act to maintain their divergence. An alternative hypothesis addresses the introgression following secondary contact after the completion of speciation. Such ecologically divergent species might be able to interbreed with each other during the onset of divergence and secondary contact (Pinho and Hey, 2010), and recent secondary contact might also reduce the number of adaptive divergent loci and result in high genetic convergence (Strasburg et al., 2012). When the introgression occurs after secondary contact, neutral loci can be more easily co-vary to reflect the history of differentiation than the selected loci (i.e., the genetic—environment association) (Bierne et al., 2013). Under both hypothesis, niche of two recently divergent species might be either bear some similarity from ancestor to descendant through time due to endogenous factor constraint (Losos, 2008), or be constrained through the mechanism of phylogenetic niche conservatism (Ackerly, 2003; Wiens, 2004; Pyron et al., 2015). Consequently, the ancestral fundamental niche characteristics will be retained in species with different ecological environments.
In addition to its effect on species divergence, environmental heterogeneity could also accelerate genetic change and divergence among populations if they lack sufficient compensation for gene flow (cf. Fountain et al., 2016). Temperature and water availability could be the main factors influencing the distribution range of plant species (Jump and Penuelas, 2005). Differential responses in genetic variation to the climate could be at a much finer geographic scale than might be expected (Owuor et al., 1997; Li et al., 1999; Huang et al., 2002). The local climate is a complicated set of different environmental elements. Temperature and precipitation are the two main dimensions of local climate change, and have also been suggested as the main niche characteristics related to the occurrence, abundance, and distribution of species (Grinnell, 1917; Gavin and Hu, 2006; Dormann, 2007). Identifying the specific climatic variables that affect the spatial and temporal genetic distribution is important for understanding how plants adapt and respond to global climate change (Carta et al., 2016; Huang C. L. et al., 2016; Vidigal et al., 2016).
In Taiwan, the short distance from continental Asia (180 km on average) and the recency of island formation (< 5 Mya; Chi et al., 1981; Teng, 1996; Wang et al., 2002) together with its ragged terrain have created a large number of recently speciated endemic taxa. For instance, six of the eight Scutellaria species are endemic to Taiwan Island. The high endemism in this group of species is suggested to be a consequence of both multiple origins and in situ diversification (Chiang et al., 2012a). Scutellaria indica, one of the two non-endemic species in Taiwan, is suggested to have colonized Taiwan from China through the north, and subsequently to have split off S. tashiroi, S. austrotaiwanensis, and S. playfairii, which are genetically (Chiang et al., 2012a) and morphologically (Yamazaki, 1992; Hsieh and Huang, 1995, 1997) similar to each other, via a sequence of episodes of dispersal and fragmentation corresponding to the interglacial–glacial cycles (Chiang et al., 2012a). Another endemic species, S. taiwanensis, is phylogenetically distinct from the others and has a restricted distribution in southern Taiwan (Chiang et al., 2012a). A recently discovered endemic species, S. hsiehii, is largely divergent from the other Taiwanese species in morphology and phenology and was only found on a logging trail in central Taiwan (Hsieh, 2013). Scutellaria hsiehii is another phylogenetic lineage distinct from any other Taiwanese species inferred by a neutral nuclear gene CHACLONE SYNTHASE (Huang B.-H. et al., 2016).
Scutellaria barbata and S. taipeiensis (hereinafter referred to as bar and tpe, respectively) are two morphologically similar species with overlapping distributional ranges (Figure 1 and Supplementary Figure 1). Scutellaria barbata is a widespread Asian species while tpe is endemic to northern Taiwan (Huang et al., 2003). They can be recognized as distinct species under the morphological species concept due to slight but constant morphological differences (Huang et al., 2003). However, such slight morphological differences with overlapping geographical distribution imply similar niches attributing to convergent selective pressures. The chloroplast and nuclear DNA sequences reveal that these two species diverged very recently but are distinct from other Taiwanese species (Chiang et al., 2012a). In contrast with the eastern and southern distribution of other Taiwanese Scutellaria species, bar and tpe are sparsely distributed in western and northern Taiwan. This, together with their similar genetic composition and overlapping geographic distribution, suggests the lineage of bar and tpe is an independent evolutionary branch (Chiang et al., 2012a).
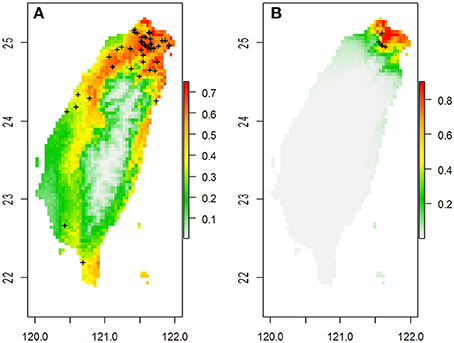
Figure 1. The predicted spatial distribution of two Scutellaria species in Taiwan based on 19 bioclimatic variables in (A) S. barbata and (B) S. taipeiensis. Crosses on the maps are the distribution of specimen records.
Accordingly, we propose two hypotheses to explain the divergence of these two species with their high morphological similarity and distributional overlap. As the first hypothesis, we posit that these two species have been diverged for a long time, but similar selective pressures have led to their convergent morphologies (e.g., Yeaman et al., 2016). Their morphological differentiation is the relictual evidence of species divergence. In addition, its relatively small distributional range could also imply a bottleneck event for tpe. The second hypothesis is that the island endemic tpe is recently derived from the widespread species bar. This means that its relative small distributional range is the consequence of a founder event. The insufficient time for divergence has led to only slight morphological differences between them, except in certain minor characters, such as leaf shape and seed coat pattern (Huang et al., 2003).
In this study, the genetic variation and genetic differentiation between bar and tpe were assessed by multilocus markers and the plastid DNA sequences. We examined the genetic components for some small but stable morphological differences and the climatic niches occupied by these two species to assess the current stage of the species' divergence. The following speciation scenarios of island species were examined: early speciation with a subsequent bottleneck and secondary contact events, or recent founder speciation. In a further evolutionary scenario, tests were conducted to evaluate the single or multiple derivation of tpe in northern Taiwan, which provides an explanation for the phenomenon of interspecific gene flow and describes the process of genetic differentiation during the early stage of speciation (i.e., when the speciation process is not completed yet). In addition, the climate's effect on genetic divergence at the population level was further examined to understand the local adaption of these two allied species. Based on our genetic evidence, we suggest that the amount of genetic divergence between these two morphologically divergent organisms may not be sufficient to grant them the level of species.
Materials and Methods
Current Distribution and Sampling
Scutellaria barbata is distributed in northern Taiwan. Some populations were recorded from western and northeastern Taiwan. This species is usually scattered in the sallow hills, on farmland ridges and along roadsides, and is usually distributed sporadically, making it hard to form a dense population. Scutellaria taipeiensis is very rare and restricted to the Taipei Basin of northern Taiwan. It grows attached to bare rock or the surrounding soil, usually in sunny environments along trails (Huang et al., 2003). Therefore, each population size of these two species is quite limited and uneven through their distribution range. We collected samples from populations of both bar and tpe distributed in the Taipei Basin, and the surrounding areas. One population of bar was sampled in southwestern Taiwan to test the effect of long-distance geographic and climatic differences. In total, six and three populations of bar and tpe were collected, respectively (Table 1). Because of their morphological similarity, we first sampled in the MK population, which is the type location of tpe; the collected samples were subsequently checked and confirmed by one of the original authors of S. taipeiensis (Mr. Arthur Hsiao). Identification of other tpe populations was based on the macromorphology and growth habit of the MK population. In total, 80 and 74 individuals of bar and tpe were sampled, respectively, for genotyping and sequencing. The seed coat patterns of all populations were further checked by Scanning Electron Microscopy (SEM).
Scanning Electron Microscopy for Seed Coat Pattern
Seeds were collected from all studied populations for SEM observation. An accelerating voltage of 15 kV was applied in order to obtain high image resolution signals. SEM was conducted using the tabletop SEM TM3000 (Hitachi, Tokyo, Japan).
Sequencing and Genotyping
Chloroplast DNA fragments ndhF-rpl32 and rpl32-trnL were chosen for sequencing. The PCR amplification and sequencing conditions followed Chiang et al.'s (2012a) protocol. According to Chiang et al.'s (2012b) primer test, 14 microsatellite primers were amplifiable for both bar and tpe. Herein these 14 primer sets were used to test for polymorphism at the population level; three monomorphic loci were discarded in the further analyses. The amplification and genotyping conditions followed Chiang et al.'s (2012b) protocol. All sequences were deposited in GenBank (Supplementary Table 1).
Neutrality Tests and Genetic Diversity
After sequence alignment and trimming the 5′ and 3′ ends to equal lengths, neutrality tests were conducted by Tajima's (1989) D-test with coalescent simulations. For the neutrality test of microsatellite loci, we used the Fdist (Beaumont and Nichols, 1996) and Bayesian-based outlier analysis of the multinomial-Dirichlet model (BayeScan analysis) to assess the FST distribution among all loci. The genetic diversity of chloroplast DNA sequences was then estimated by DnaSP 5.10 (Librado and Rozas, 2009). The genetic diversity of microsatellite loci was estimated using Arlequin 3.5 (Excoffier and Lischer, 2010) and GenAlEx 6.5 (Peakall and Smouse, 2012).
Analysis of Molecular Variance
The analysis of molecular variance (AMOVA) was used for testing the degrees of genetic variation between species, among, and within populations. We conducted the AMOVA using Arlequin 3.5 (Excoffier and Lischer, 2010).
Bayesian Clustering Analysis for Examining the Genetic Components of Populations
Bayesian clustering analysis (BCA) was used to examine the similarity and divergence of genetic components among populations. The BCA was performed using STRUCTURE 2.3.4 (Hubisz et al., 2009). A non-admixture model was applied to STRUCTURE with a priori sample localities. The posterior probability of grouping number (K = 1–10) was estimated by 10 independent runs using one-million steps Markov chain Monte Carlo (MCMC) replicates after a 100,000-step burn-in for each run to evaluate consistency. The best grouping number was evaluated by ΔK (Evanno et al., 2005) in STRUCTURE HARVESTER v. 0.6.94 (Earl and Vonholdt, 2012).
Discriminant Analysis of Principal Components for Discriminating Species and Populations
The discriminant analysis of principal components (DAPC) was used for distinguishing bar and tpe and the populations that were subjectively identified. Properties of the “without a priori” using partial synthetic variables to minimize variation within groups (Manel et al., 2005; Jombart et al., 2010) could help to evaluate the artificial classification objectively. The DAPC was conducted using the R (R Core Team, 2015) package adegenet (Jombart, 2008). Kruskal–Wallis tests for the first two principal components (PCs) and the first two linear discriminants (LDs) of the DAPC were conducted to test the genetic divergence between species and between populations.
Speciation Scenarios for the Approximate Bayesian Computation
Based on the estimated genetic variation and genetic structure and current geographic distributions, two evolutionary scenarios were proposed: the first is the demographic bottleneck of both bar and tpe under common environmental pressures with recent interspecific gene flow (secondary contact) after completed speciation (Figure 2A); the second scenario illustrates the recent founder speciation of tpe derived from the progenitor species bar with continuous gene flow (i.e., incomplete speciation, Figure 2B). We further tested two extended scenarios of the recent founder speciation scenario, namely that tpe originated multiple times (Figure 2C) or in a single origin from bar (Figure 2D). Testing these two extended scenarios helps clarifying whether the species-determining morphological traits are derived from the common ancestor or are the consequence of convergence. An approximate Bayesian computation (ABC) was used to test these evolutionary scenarios. First, package simcoal2 of the ABCtoolbox was used to generate one million pseudodata for simulation (Wegmann et al., 2010). We used arlsumstat to estimate diversity parameters (e.g., number of alleles, allele frequency, fixation indices FST, FIS, FIT, etc.) of the pseudodata and transform them by the pls. To validate the best scenario, the general linear model (GLM) post-sampling regression adjustments on the best 5,000 simulated parameters were retained for obtaining the marginal density of models by the ABCestimator. The best evolutionary scenario was selected according to the Bayes factor and the ratio of marginal densities.
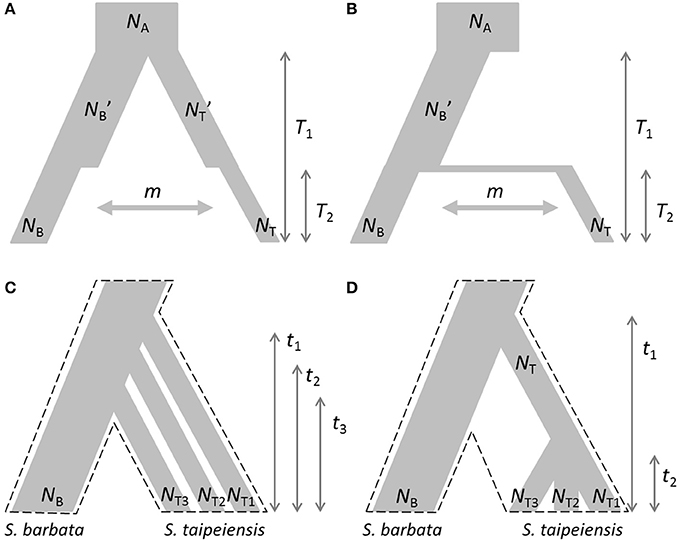
Figure 2. Evolutionary scenarios of the divergence of S. barbata (bar) and S. taipeiensis (tpe) for approximate Bayesian computation (ABC). Contrasting scenarios illustrate (A) the bottleneck events after species divergence with recent gene flow (abbreviation: the secondary-contact scenario) and (B) the recent founder speciation of tpe derived from bar with continuous gene flow (abbreviation: the founder scenario). Two different contrasting extended scenarios of the founder scenario were further tested: (C) multiple origins of tpe from bar and (D) a single origin of tpe from bar. In scenarios (C,D), migration rate was set as a free parameter and is not illustrated. The dashed frames in (C,D) indicate the pattern of species divergence.
Distinguishing the Grinnellian Niches (Temperature and Precipitations) between bar and tpe
Grinnellian niches are defined as the scenopoetic environmental variables a species requires to survive, such as the temperature and precipitation, etc. (Grinnell, 1917). Nineteen bioclimatic variables (Bioclim) that represent the Grinnellian niches were extracted from the WorldClim website (http://www.worldclim.org/bioclim). Principal component analysis (PCA) of independent climatic variables to reduce the dimensionality defining the niche space allowed comparison of the integrity of Grinnellian niches between bar and tpe. Firstly, the variables with a high variance inflation factor (VIF) were removed to reduce the multicollinearity. This procedure was repeated until the VIF values of all remaining variables were < 10 and finally six bioclimatic variables were retained for the further analyses. Species occurrence data were collected from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org), herbarium records, public records available on the web, and our collecting sites. Unclear records and wrong identifications were ruled out. Finally, 56 records of bar and five records of tpe were used for the PCA. We further used multinomial logistic regression (MLGR) to test the effect of bioclimatic variables on the prediction of species occurrence. Because the simpler model of no correlation among predictors (bioclimatic variables) could not be rejected by the model of correlated predictors (likelihood ratio statistic = 12.214, df = 37, P = 0.99996), we used the model with independent predictors for MLGR analysis. The significance of each predictor was tested by the type-II analysis of variance (ANOVA) Wald χ2 test.
Predicting Potential Distributions by Ecological Niche Modeling
To predict the potential habitats of both bar and tpe, species distribution models were built under the maximum entropy model implemented in MaxEnt (Phillips and Dudík, 2008) and the R package dismo (Hijmans et al., 2013). The occurrence data of both species were obtained from our sampling sites, herbarium data, and GBIF. The bioclimatic variables were also downloaded from WorldClim with a resolution of 30 arc-sec (about 1 × 1 km). A maximum of 2,000 iterations of each species were conducted, and species occurrence data were randomly divided (10%) to train the model. One regularization multiplier and 10,000 background points were set to create models for each species set. The logistic output, consisting of a grid map with a suitability value range from 0 to 1, was generated and visualized with the R package maptools (Lewin-Koh et al., 2011).
Mantel Test for the Isolation-By-Distance and Isolation-By-Environment Tests
To test how the geographic distance and environmental differences affect the genetic composition, the Mantel test of Spearman correlation was performed between genetic, geographic, and environmental (climatic) distances. Pairwise FST was calculated between populations based on Nei's (1977) approach and then Rousset's (1997) estimate FST/(1 − FST) was used as the genetic distance metric. Geographic distance was estimated using the Euclidean distance according to three dimensional factors (latitudes, longitudes, and altitudes). Environmental distance was also calculated using the Euclidean distance with six retained Grinnellian niches variables. A partial Mantel test between genetic and environmental distances controlled for the geographic distance was also done. A multiple matrix regression with randomization (MMRR) was further performed to test whether the genetic distance responds to the variation of geographic and/or environmental distances. We first examined the correlation between the genetic distance and geographic distance and between genetic distance and environmental distance. Autocorrelation between the geographic and environmental distances was further examined. Finally, the joint effect of both geographic and environmental distances on genetic distance was examined. Regression coefficients of Mantel test (ρ) and MMRR (β) and their significance were determined based on 9,999 random permutations.
Testing the Effect of Climate on Population Genetic Components
To understand whether the climatic variables (Grinnellian niches) explain the genetic distribution of populations of bar and tpe, partial distance-based redundancy analyses (partial dbRDA) were performed. The first five principal components (PC1–PC5) were used to represent the genetic components, and six retained climatic variables were used as predictors conditioning on the geographic distribution (latitude and longitude). Analysis of variance was further used to test the significance of each predictor. The partial dbRDA was conducted using the R package vegan. We further analyzed the distribution of climatic variables along ordination axes using the GLM. We also used the MLGR to test the effect of bioclimatic variables on populations. The type-II ANOVA Wald χ2 test was used to test the significance of each bioclimatic factor.
Results
Morphological Similarity of the Nutlet Coat of Mature Seeds
Because the characters of vegetative morphology that are the most distinctive between bar and tpe are variable and can be affected by growth and nutrient conditions, we specifically checked the seed coat patterns, another distinguishing trait in the reproductive organs, to confirm the stability of this character for species delimitation. Based on our observations, there is slight variation in the seed size among individuals but no obvious difference between species (Figure 3). The SEM also shows that the nutlet coats of mature seeds in all populations of bar and tpe are of the rounded concentric type (i.e., type 3 described in Huang et al., 2003, Figure 3). The radiated umbrella-like nutlet coat (type 2) described by Hsieh and Huang (1995) was only seen in immature seeds (the bottom-left picture of Figure 3). After repeatedly comparing the nutlet-coat patterns, we believe that the differences between these two character states (i.e., rounded-concentric vs. radiated umbrella-like) reflect differences in seed maturity rather than differences between species.
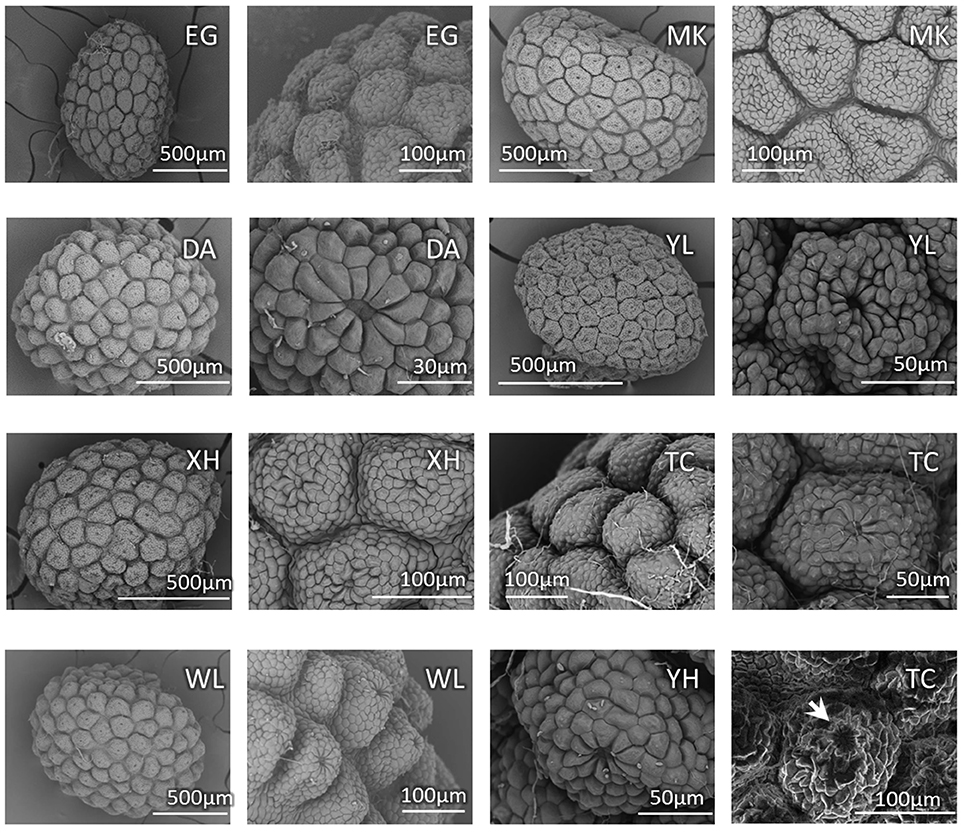
Figure 3. Seed coat pattern of sampled populations (except CL) in this study. The SEM pictures show similarities with slight size variations in the nutlet coats of mature seeds among populations but no obvious difference between species. The bottom-right picture shows the nutlet coat of an immature seed of population TC (S. barbata), in which the “radiated umbrella-like” structure can be observed (indicated by arrow). Population MK is the type location of S. taipeiensis.
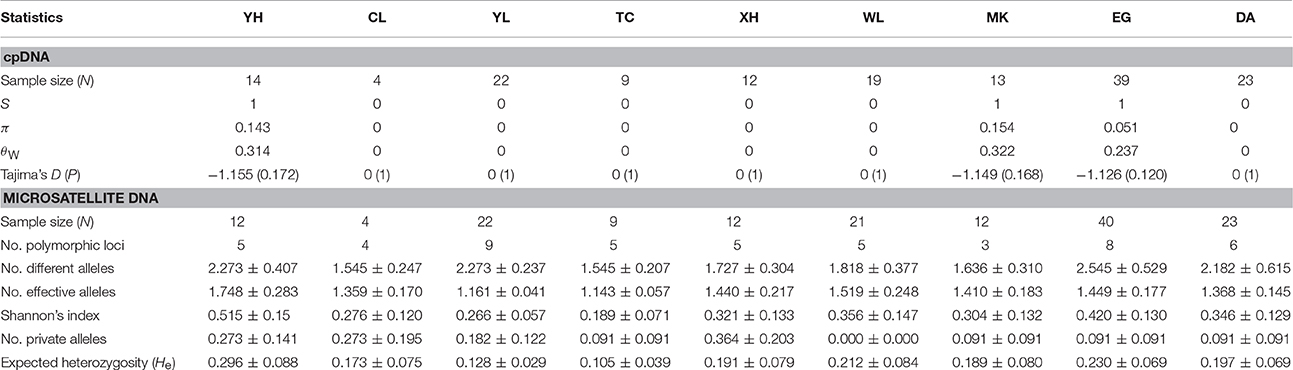
Neutrality Tests and Genetic Diversity
Before estimating genetic diversity, we performed neutrality tests by Tajima's D-test for cpDNA sequences and by Fdist and the multinomial-Dirichlet model (BayeScan) for the outlier analysis for the microsatellite loci. No deviation from zero in Tajima's D (D = 1.315, P > 0.10, coalescent simulation D = −0.066, 95% confidence interval −1.550–2.112, P = 0.911) and no positive or negative outliers of microsatellite loci (Supplementary Figure 2) suggest that the genetic markers used in this study all evolve neutrally and are appropriate for further genetic population assessment and speciation model tests.
In cpDNA, only the population YH of bar and populations MK and EG of tpe revealed nucleotide polymorphisms. The genetic diversity index Π of these three populations (YH, MK, and EG) is 0.143, 0.154, and 0.051, respectively, and the index θW is 0.314, 0.322, and 0.237, respectively. In total, when considering the indels six haplotypes were obtained, and the haplotype frequencies, distributions, and relationships (i.e., the minimum spanning tree) are illustrated in Figure 4. Genetic diversity assessed by microsatellite markers revealed varying degrees of genetic diversity among populations, ranging from 0.105 ± 0.039 to 0.296 ± 0.088 in expected heterozygosity (He) in bar and from 0.189 ± 0.080 to 0.230 ± 0.069 in He in tpe (Table 2). Among the 11 loci examined, roughly half are polymorphic (5.556 ± 1.878 polymorphic loci) in every population and the high proportions of private alleles per locus (ranges from 0 to 0.364 ± 0.203, Table 2) indicate a genetic fixation phenomenon in small populations by the drift effect. The drift effect on small populations is reflected in the significantly high genetic differentiation among populations (F statistic = 0.681, P < 0.00001) with a relatively small proportion of genetic variation within populations (35.94% variation, F = 0.641, P < 0.00001; Table 3). However, genetic variation does not contribute to species divergence, i.e., genetic differentiation between species is lacking (F < 0, P = 0.820, Table 3).
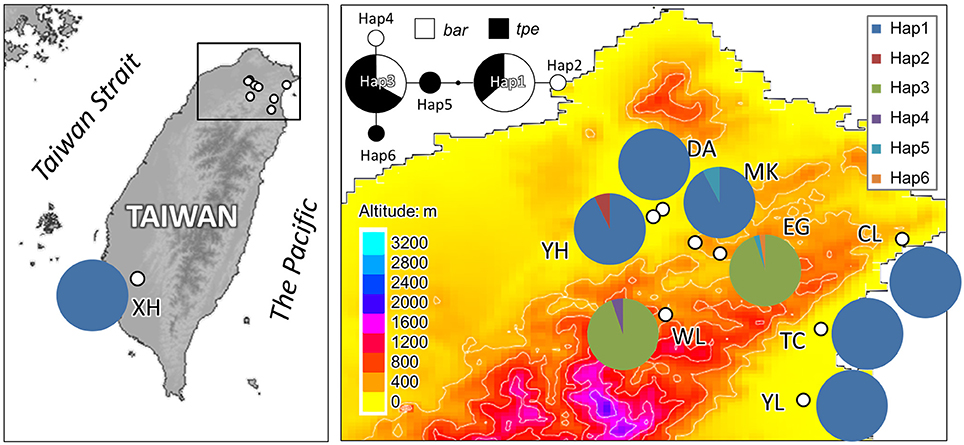
Figure 4. Sampling sites and chloroplast haplotype distribution of S. barbata and S. taipeiensis. The minimum spanning network of haplotypes is also shown. Populations DA, MK, and EG are S. taipeiensis; populations XH, YH, WL, CL, TC, and YL are S. barbata.
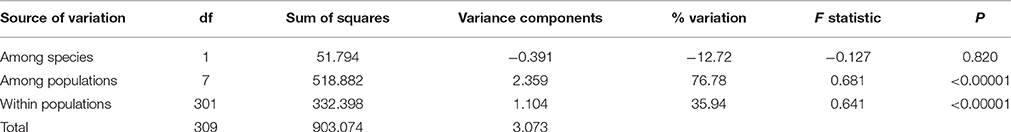
Table 3. Analysis of molecular variance (AMOVA) of populations of two Scutellaria species in Taiwan in microsatellite loci.
Population Structure of bar and tpe
To understand the pattern of genetic distribution of these two species, we performed a BCA in STRUCTURE and ordination analysis by DAPC. The STRUCTURE result suggested the best grouping number (K) is 3 and the second-best K is 2 based on the ΔK (Figure 5A). When K = 2, population EG of tpe and population WL of bar were inferred to be composed of identical genetic components, and population YL of bar is composed of both genetic components (Figure 5B). When K = 3, DA of tpe and YL of bar were inferred to be two further components different from EG and WL, and the other populations were comprised of both genetic components of DA and YL (Figure 5C). These two inferences are similar and consistently indicate that the populations WL and EG are divergent from the other populations, and such divergence is inconsistent with the species delimitation.
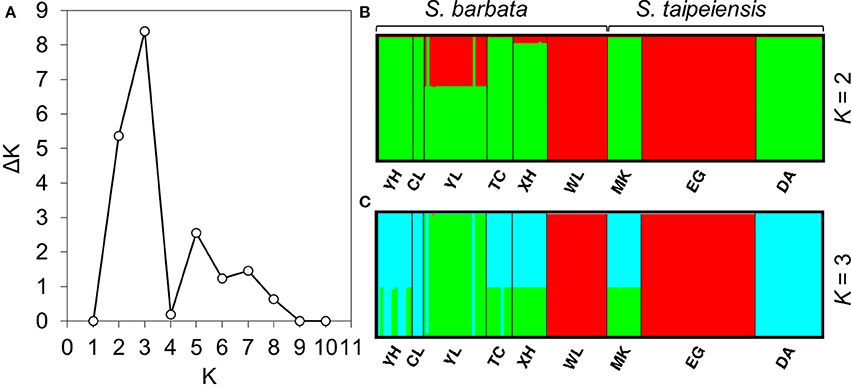
Figure 5. Results of the Bayesian clustering analysis conducted using STRUCTURE. (A) The ΔK plot shows that K = 3 gets the highest ΔK value and K = 2 gets the second-best ΔK value, meaning that the most probable grouping number could be three or two. The clustering patterns of genetic components by (B) two groups, (C) three groups.
In the DAPC analysis, which uses one discriminant function to distinguish five principal components (PCs), a broader range of genetic variation was detected in bar than in tpe, and the range of genetic variation of tpe is skewed but within the range of bar at the species level (Figure 6A). A further DAPC analysis on populations shows a similar result, i.e., that the range of genetic variation of three populations of tpe was clustered within the range of bar. Two main clusters were distinguished by two discriminant functions for five PCs: one is the cluster of the WL of bar and EG of tpe, the other populations belonging to another cluster (Figure 6B). The clustering pattern inferred by STRUCTURE and DAPC is congruent.
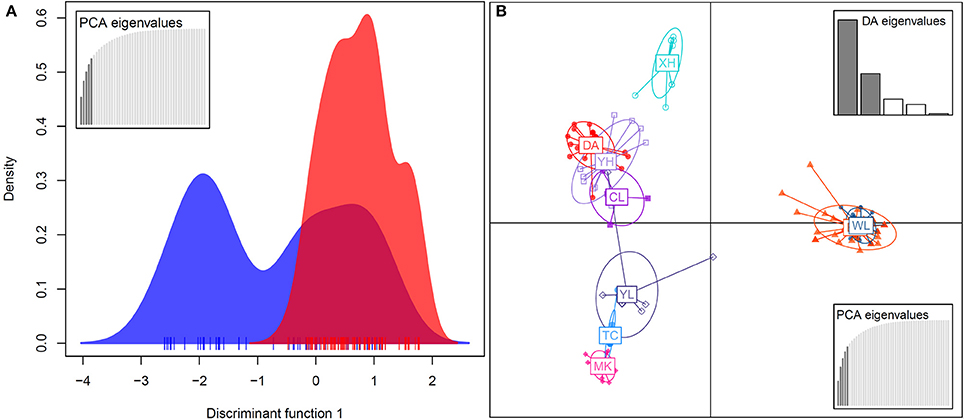
Figure 6. Population structure revealed by the discriminant analysis of principal components (DAPC). (A) The DAPC for separating the species S. barbata (bar, blue) and S. taipeiensis (tpe, red). (B) The DAPC for separating populations of bar and tpe. The red series belongs to tpe and the blue-color series are bar.
We further tested the differences in genetic variation according to the transformed variables of the first two principal components PC1 and PC2 of PCA and the first two linear discriminants LD1 and LD2 of DAPC at both the species level and the population level. Only the transformed variable PC2 (Kruskal–Wallis test, χ2 = 32.33, df = 1, P = 1.301e–08) revealed species divergence between bar and tpe but no species-level differences were found in PC1 (P = 0.872), LD1 (P = 0.810), or LD2 (P = 0.864; Figure 7). At the population level, all four transformed vectors showed significant differences in genetic variation among populations (Kruskal–Wallis test, P < 2.2e–16, Figure 7). Taken together, these results suggest obvious population divergence but no or less genetic differentiation between species.
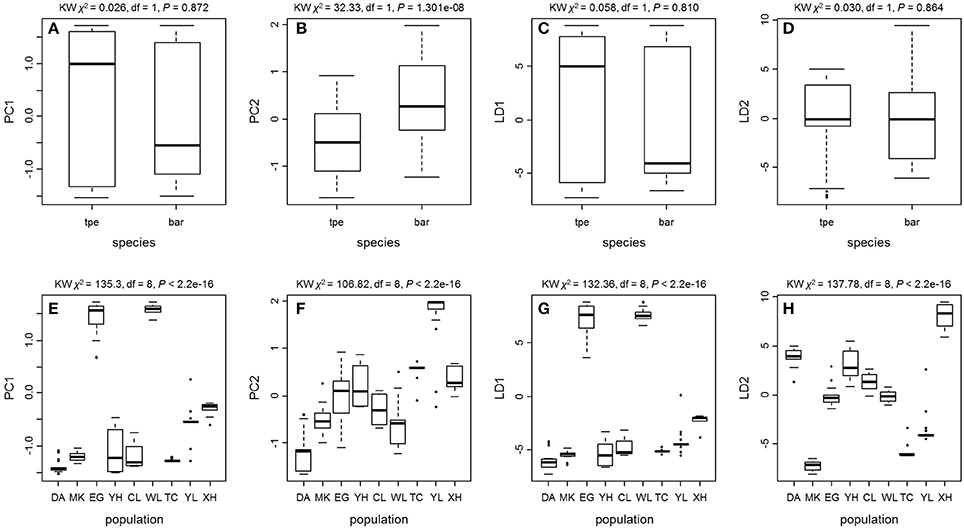
Figure 7. The Kruskal–Wallis test of the first two principal components and the first two linear discriminants of the genetic variation at microsatellite loci reveals no or little genetic divergence between species but significant population differentiation. (A–D) Comparisons between species. (E–H) Comparisons among populations.
Multiple Origins with Very Recent Divergence Explain the Current Situation of No Genetic Differentiation between Species
The non-significant genetic differentiation between species may suggest frequent gene flow geographically after secondary contact, or alternatively, the sharing of large amounts of ancestral polymorphism. Following these two possibilities, evolutionary scenarios of early divergence with secondary contact (Figure 2A) and recent founder speciation with incomplete divergence (Figure 2B) were tested using ABC. The former scenario (secondary contact) scored a marginal density of 9.24 × 10−13 (0.199%), much lower than the founder-speciation scenario (marginal density: 4.63 × 10−10, 99.801%). The estimated ancestral population size of bar is suggested to have decreased under the founder-speciation scenario (NA = 597.487, 95% CI: 164.557–8172.980; NB' = 199.497, 95% CI: 126.479–4701.65, Figure 8A). However, a recovery of the current population size was estimated (NB = 683.417, 95% CI: 235.953–958.165), but the founder populations of tpe have a very small effective population size (NT < 100, Figure 8B). The estimated time since the founder speciation of tpe is less than 100 generations (Figure 8D), indicating the poor inference of species divergence despite no or little interspecific gene flow (m < 0.01, Figure 8C) between bar and tpe. The estimated mutation rate (μ) of each locus per generation in secondary contact scenario has been estimated (μb = 6.965 × 10−4, 95% CI: 2.238-9.703 × 10−4, Figure 8H).
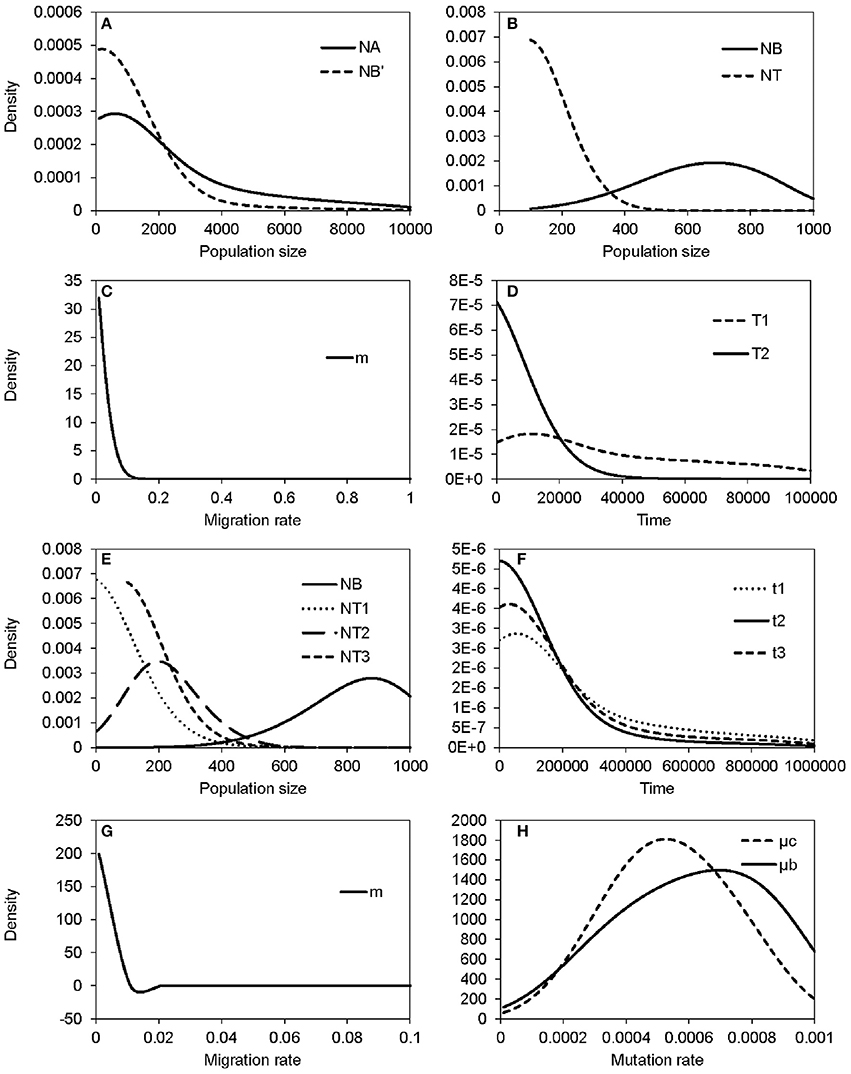
Figure 8. Summary results of the ABC analyses. (A–D) display the distribution of estimated parameters of Figure 2B: (A) The estimated population sizes of ancestral populations NA and NB′; (B) the estimated population sizes of current populations NB and NT; (C) the migration rate; (D) the coalescent time of S. barbata (bar, T1) and the time of founder speciation of S. taipeiensis (tpe, T2). (E–G) display the distribution of estimated parameters of Figure 2C: (E) The population sizes of bar (NB) and three populations of tpe (NT1–NT3); (f) colonization times of three populations of tpe; (G) the migration rate; (H) the distribution of the estimated mutation rates of scenario Figure 2B (μb) and Figure 2C (μc).
Since the divergence time is extremely short, we wondered whether tpe is a good species or not. If it is, populations of tpe are expected to be a monophyletic group. Two evolutionary scenarios were further tested following the founder speciation scenario: multiple origins (i.e., polyphyly) of tpe (Figure 2C) and a single origin followed by divergence (i.e., monophyly) in tpe (Figure 2D). The multiple origins scenario has a higher marginal density (5.577 × 10−49, 99.836%) than the single origin (marginal density: 9.167 × 10−52, 0.164%), indicating a rejection of the monophyly of tpe. Similar to the estimation above, the effective population size of populations of tpe is smaller than that of bar (Figure 8E), while the divergence times have wide ranges from < 100 generations to 55,370 generations (95% CI: 6,814–861,759 generations; Figure 8F). The estimated mutation rate (μ) of each locus in multiple origins scenario has also been estimated (μc: 5.274 × 10−4, 95% CI: 1.999-8.9 × 10−4, Figure 8H). Such results of a wide range of divergence times of tpe populations from bar reflect (1) recent divergence and (2) sharing common ancestral polymorphisms that results in long coalescence times. This result also suggests that the divergence time is insufficient for complete speciation. Therefore, populations of both bar and tpe are combined into a single taxonomic group for the further analysis of local climatic adaptation. However, the very small migration rate on average (m < 0.001, Figure 8G) indicates that the gene flow seems to have ceased after population colonization.
The Overlapping Grinnellian Niches in bar and tpe
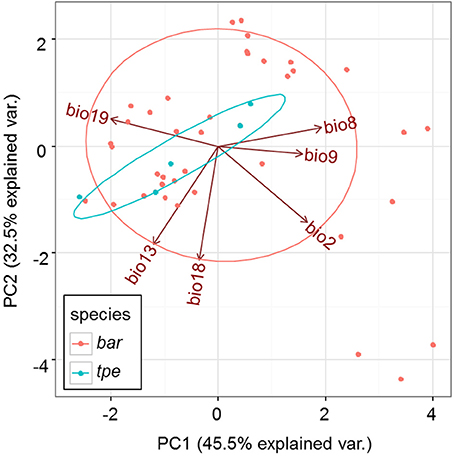
After removing 13 bioclimative variables of collinearity, the remaining six bioclimatic variables are: bio2 [mean of monthly (max temp − min temp), named the Mean Diurnal Range], bio8 (mean temperature of wettest quarter), bio9 (mean temperature of driest quarter), bio13 (precipitation of wettest month), bio18 (precipitation of warmest quarter), bio19 (precipitation of coldest quarter). Among these six bioclimatic variables, the first three (bio2, bio8, and bio9) belong to the temperature dimension, while the last three (bio13, bio18, and bio19) are the precipitation dimension.
Although multicollinearity was prevented by removing variables with VIF > 10, certain variables were still partially intercorrelated. For example, the temperature variable bio2 was negatively correlated with the precipitation variable bio19, and two precipitation variables, bio13 and bio18, are negatively correlated (Supplementary Figure 3). The partial correlation of bioclimatic variables shown by PCA also revealed that the explanatory direction for the Grinnellian niche distribution is opposite for bio2 and bio19, and that bio13 and bio18 have similar explanatory intensity and directions (Figure 9). The PCA plot drawn on the first two axes explains 45.5 and 32.5% of the variation in the Grinnellian niche, but this biaxial PC distribution cannot separate bar and tpe well (Figure 9). When solely considering the temperature dimension (bio2, bio8, and bio9), or solely considering the precipitation dimension (bio13, bio18, and bio19), similar results of indistinguishable clustering between bar and tpe were revealed (Supplementary Figure 4). It is worth noting that the explanatory percentage of the first two PCs of the precipitation dimension (95.2%, Supplementary Figure 4B) was estimated to be higher than that of the temperature dimension (84.2%, Supplementary Figure 4A), suggesting that the precipitation could be more relevant in explaining the geographic distribution of these two species. Among these six non-collinear bioclimatic variables, only bio8 (mean temperature of wettest quarter) was shown by the MLGR analysis to be significant for predicting the occurrence of species (P = 0.036, Supplementary Table 2).
Spatial Distribution Predicted By Ecological Niche Modeling
Ecological niche modeling (ENM) was further used to predict the suitable distributions of both bar and tpe as well as to examine the key climatic variables in the prediction. Distribution models of both species showed high discrimination performance. The cross-validation area under the curve (AUC) value for all models is 0.999, indicating that 99.9% of the records were correctly predicted. The climatic suitability of each species is proportional to similar climatic variables. Bio18 (precipitation of warmest quarter) and bio2 (mean diurnal range) are the first two contributors to the species distribution model in bar (57.9 and 28%, respectively) and in tpe (27.7 and 47.6%, respectively).
The ranges of both species predicted by the six bioclimatic variables (bio2, bio3, bio8, bio13, bio18, and bio19) are roughly consistent with the currently known distributions, except for a certain probability (< 50%) of suitable habitats predicted in the Hengchun Peninsula of southern Taiwan for the tpe (Supplementary Figure 1). In accordance with the specimen records, northern Taiwan is suggested as the most suitable habitat for both species by ENM. In contrast to the smaller ranges of tpe, the predicted distribution of bar is broader, including the most southern area and the northeastern and northwestern areas of Taiwan (Figure 1 and Supplementary Figure 1). The central area of Taiwan, where the Central Mountain Range is, is not suitable for either species distribution.
Fitness of Isolation-By-Distance and Isolation-By-Environment Models
To assess whether the geographic or the environmental difference drives the genetic divergence among populations, isolation-by-distance and isolation-by-environment tests were conducted using the Mantel test. The Spearman correlation shows no significant correlation between geographic and environmental distances (ρ = 0.2888, P = 0.0872), between genetic and geographic distances (ρ = 0.2454, P = 0.0659), or between genetic and environmental distances (ρ = 0.1105, P = 0.3606; Table 4). The partial Mantel test did not detect significant correlation between genetic and environmental distance when conditioning on the geographic effect either (ρ = 0.2743, P = 0.0814, Table 4). Similar results of no autocorrelation between geographic and environmental distances (r2 = 0.0152, β = 0.1201, P = 0.501) and non-significant effects of geographic (r2 = 0.0373, β = 0.1418, P = 0.281) and environmental distances (r2 = 0.0063, β = 0.0600, P = 0.685) on the change of genetic distance were obtained by MMRR analyses (Table 4). The joint effect of both geographic and environmental distances also did not affect the genetic distance significantly (r2 = 0.0404, βgeo = 0.1367, P = 0.345, βenv = 0.0426, P = 0.797, Table 4). These results indicate that the population genetic differentiation (or gene flow) is not linearly correlated with the geographic and climatic differentiation.
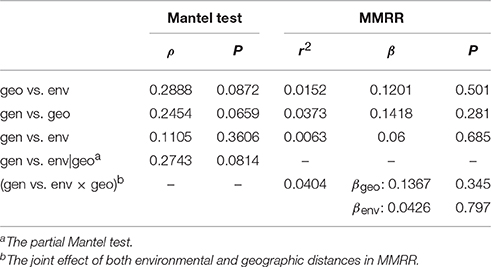
Table 4. Summary of the Mantel test and multiple matrix regression with randomization (MMRR) between the genetic (gen), geographic (geo), and environmental (env) distances.
Explanation of the Population Genetic Variations by Environmental Factors
Despite nonlinear correlation between the climatic differentiation and genetic distance, we still wondered whether the local climatic heterogeneity explains the genetic variation of populations. When conditioning on the geographic distribution, we found that 59.5% of the variation is explained by climatic variables (Table 5). The ANOVA further indicates that all six predictors (bio2, bio8, bio9, bio13, bio18, and bio19) can significantly explain population genetic components (P < 0.0001, Table 5), and the bio9 and bio2 have the highest explanatory proportions (23.0 and 11.7%, respectively) for predicting the population genetic variation (Table 5). The distribution of climatic variables along the ordination axis was further examined by GLM. Four bioclimatic variables including two variables (bio8 and bio9) of the temperature dimension and two variables (bio13 and bio18) of the precipitation dimension have significant F statistics (adjusted R2 = 0.041, 0.104, 0.216, and 0.178, P < 0.05, Table 5), but only the precipitation variables bio13 and bio18 correlate significantly with the ordination axis1 of dbRDA, while all four variables are significantly correlated with axis2 (Table 5). However, the adjusted R2 of bio8 could be too small (roughly explaining 4% of the variation on axis2) to be meaningful. If only the geographic clusters are considered (i.e., locations of populations) without genetic information, all the bioclimatic variables cannot predict the occurrence of populations by MLGR independently. Nevertheless, the joint effect of the four factors, bio8 × bio13 × bio18 × bio19, can predict the populations by MLGR significantly (χ2 = 26.540, df = 8, P = 0.0008).
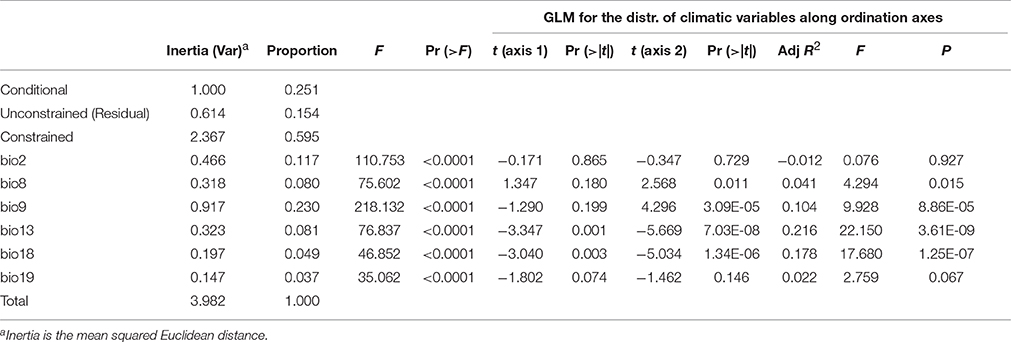
Table 5. Summary of partial dbRDA, showing the significance of climatic variables (constrained factors) for explaining the variation in the genetic components.
Discussion
Indistinguishable Morphological Characters
Scutellaria taipeiensis is a species morphologically alike to bar. It was named in 2003 based on its leaf shape (length-to-width ratio < 2 and triangular-ovate to broadly ovate shape) and the rounded concentric tuberculae type of nutlet coat that distinguished it from bar (Huang et al., 2003). However, in our SEM observation, we found that the nutlet coat pattern is not different between species and the “radiated umbrella-like” character (Hsieh and Huang, 1995) may disappear when seeds mature (Figure 3). In other words, the morphological differences of nutlet-coat patterns described by Hsieh and Huang (1995) and Huang et al. (2003) may result from the comparison of seeds at different developmental stages. In addition, the leaf size and shape is usually plastic in response to environmental changes (Rozendaal et al., 2006; Royer et al., 2009), and the range of leaf shape is wider and more varied in bar, covering the range of tpe (Supplementary Table 3). Morphological similarity in both vegetative and reproductive organs implies that tpe could merely be an ecotype or form of bar instead of a distinct species. To confirm this speculation, a comprehensive study of the genetic and ecological properties was carried out to complement the morphological evidence (Camargo et al., 2012).
Lack of Genetic and Climatic Niche Differentiation between Species Implies an Incomplete Speciation Process
The genetic variation of tpe is essentially a subset of that found in bar (Figure 6A), which matches the geographic distribution of these two species (Figure 1 and Supplementary Figure 1). In addition, both genetic and niche components are non-significantly different between species (Figures 7A–D, 9, Supplementary Figure 4). These analyses suggest that the speciation is incomplete, or at least that species divergence is not reflected in the overall genetic differentiation and niche preference. Our result resembles the common phenomenon that the progenitor species is paraphyletic to its derived species in plants because the ongoing gene flow increases the time for the derived species to achieve monophyly (Rieseberg and Brouillet, 1994). However, in this case, the daughter species tpe does not form a monophyletic group but fits the model of multiple founder speciation events (Figure 2B).
In fact, the estimated time of founder speciation of tpe is quite short (< 100 generations) in contrast to the long coalescent time of the Taiwanese populations of bar (11,145 generations ago, 95% CI: 1,505–92,795 generations ago, Figure 8D). If a generation is taken as 1 year, the coalescent time of the Taiwanese population of bar is roughly at the end of the Last Glacial Maximum (LGM), the time that Taiwan Island was most recently connected to continental Asia (Nino and Emery, 1961; Voris, 2000), which is probably the time when bar colonized Taiwan. However, the extremely short divergence time of tpe supports the hypothesis that tpe is not a good species but could be a subset of bar, or alternatively, that the loci that contribute to the population's differentiation are not involved in the species' divergence (Table 3). Shared polymorphisms across closely related species appear to be common in island species (Lopez-Sepulveda et al., 2013; Takayama et al., 2015), which might result from introgression following secondary contact (Minder and Widmer, 2008) or from maintaining ancestral polymorphisms due to incomplete lineage sorting within recently diverged species (Nagl et al., 1998). The ABC analysis suggested that sharing ancestral polymorphism could better explain the genetic structure of bar and tpe than secondary contact did. Furthermore, the best-fit scenario of multiple origins suggests insufficient time for lineage sorting in neutral genes after the divergence of speciation genes or speciation traits (i.e., the genes or traits involved in speciation). Multiple origins from a single progenitor usually are seen as an evolutionary mechanism for the recent and rapid radiation of island species rather than the mature radiation that sometimes leads to a high diversity of continental species (cf. Linder, 2008). However, such an evolutionary mechanism of multiple origins could be unstable to fusion and convergence to monophyly.
Climatic Variables Are Not Associated with Species Delimitation
The Grinnellian niche that is comprised of temperature and precipitation is important and related to plant speciation, in particular in those budding speciation cases with peripheral ranges or distributional overlaps (Martinez-Cabrera et al., 2012; Grossenbacher et al., 2014). Although no positively selected loci was detected (Supplementary Figure 2), variation of neutral genes could still be affected due to demographic changes (e.g., population size decline, Figures 8A,B) under strong environmental pressures (Schoville et al., 2012). Bio8 is the only bioclimatic variable that has a significant effect on the prediction of groups of species. Bio8 is the mean temperature of the wettest quarter. However, a non-significant difference in bio8 was found between bar (24.72 ± 2.273°C) and tpe (24.66 ± 2.947°C) in means (independent two-group Mann–Whitney U-test, W = 132.5, P = 0.854) and variance (Kruskal–Wallis test, χ2 = 0.039, df = 1, P = 0.844). This is probably because the overlapping distribution ranges of bar populations and tpe populations result in a similar climatic situation, thus causing a false positive for species prediction by the climate factor.
Despite the lack of species divergence in genetics and niches, local climatic heterogeneity has driven the genetic differentiation among populations within the island. This phenomenon is very like the case of Aeonium davidbramwelii and A. nobile on the island of La Palma in the Canarian archipelago: the former is an ecological generalist while the latter is an ecological specialist. There is a lack of species divergence but an obvious population structure exists (Harter et al., 2015). However, in our case, although the niche space of tpe is smaller than that of bar, their spatial distribution predicted by ENM on the basis of six bioclimatic variables is almost overlapping (Supplementary Figure 1). The only certain difference with the real distribution of tpe in the Taipei Basin is that the spatial model predicts a moderate probability for the potential distribution of tpe in southern Taiwan (Supplementary Figure 1). The dispersal mechanism of Scutellaria species is suggested to be attributable to rivers (Williams, 1992; Middleton, 2000; Chiang et al., 2012a). The lack of rivers running north to south in Taiwan decreases the probability of a southward long-distance dispersal of tpe.
Climate Heterogeneity Leads to Local Adaptation
Considering all genetic, evolutionary, ENM, and morphological evidence, we conclude that no species delimitation can be made between bar and tpe. Therefore, all populations are taken as one species. A climatic effect, in particular the precipitation dimension, leads to local population differentiation. Although, geographic and environmental distances do not matter in the genetic differentiation among populations (Table 4), we still propose that the climatic variables determine the genetic components of populations inferred by both partial dbRDA (Table 5) and MLGR significantly. These results suggest that climatic heterogeneity is not linearly correlated with the genetic composition, but could affect genetic structure independently and locally, i.e., climate leading to local adaptation.
The environmental heterogeneity, which causes significant genetic divergence (Figures 7E–H) and contributes a high proportion of population genetic variation (76.78%, Table 3), could be a major driver leading to postzygotic isolation (Martin and Willis, 2007; Anacker and Strauss, 2014). According to the AMOVA, roughly 3/4 of the variation can be attributed to population divergence (Table 3), and the partial dbRDA indicates that ~60% of the variation among populations can be explained by constrained variables (i.e., climatic factors, Table 5). This means that roughly half the genetic variation can be attributed to local climatic heterogeneity. Environmental heterogeneity could increase the genetic or genomic barriers among populations, and thus result in local adaption (Kawecki and Ebert, 2004; Savolainen et al., 2007).
Precipitation from Late Summer to Early Autumn Is the Key Climatic Factor Responsible for the Current Genetic Distribution
In the analyses that did not take the genetics into account, the joint effect of bio8 × bio13 × bio18 × bio19 inferred by MLGR and of bio2/bio18 inferred by ENM are suggested as the main climatic variables affecting population clustering and distribution; if the genetic factor is considered, the bio13/bio18 on axis1 and bio9/bio13/bio18 on axis2 of the dbRDA are suggested as significant explanations for the genetic variation among populations. The climatic variables bio18 and bio13 are two key variables inferred from different statistical analyses (except bio13 by the ENM). These two variables belong to the precipitation dimension, standing for the precipitation of the warmest quarter and the precipitation of the wettest month, respectively (Supplementary Table 4). The significant effect of bio13 and bio18 was revealed in the multiple hills (clusters) of the ordisurf plots of bio13 and bio18 in partial dbRDA (Supplementary Figures 5D,E) in contrast to the other bioclimatic factors (Supplementary Figures 5A–C,F), and their high correlation is also revealed in the almost overlapping coordinate axes (i.e., arrows) of the original bioclimatic variables bio13 and bio18 (Supplementary Figure 5).
According to the WorldClim database, the wettest month is September (340.4 mm on average) and the warmest quarter of all recorded populations is July to September (29.63°C on average; Supplementary Figure 6). In fact, bio13 and bio18 are highly correlated (Spearman's rank correlation, ρ = 0.757, P = 1.75e–12). According to the common characteristics of bio13 and bio18 as well as their significant correlation, we suggest that the main determinant governing the genetic structure of populations is actually the precipitation from late summer to early autumn. This period is during the typhoon season (late summer and early autumn) in Taiwan and just after the flowering and fruiting season (tpe: Mar–Jun; bar: Oct–May; Hsieh and Huang, 1995; Huang et al., 2003) with subsequent germination and growth. This is the most important period for population regeneration in this group of species (i.e., bar and tpe). Precipitation during seed germination and growth could affect the demographic size and could also influence the success of seed colonization. In addition, since the seed dispersal of Scutellaria species is thought to be spread by water (Williams, 1992; Cruzan, 2001), the abundant rainfall in typhoon season may also contribute to the long-distance dispersal, and further affects the population genetic structure.
Conclusions
Spatial climatic heterogeneity is usually suggested as an important driver leading to population differentiation, even accelerating speciation. Taxonomic splitters sometimes name a species for slight morphological differences and link these characters to conjectural environmental differences. Here we provide integrated evidence including morphology, genetics, climatic niche modeling, and evolutionary tests to validate the taxonomic treatment of S. taipeiensis. When all these aspects are considered, all sampled populations should be regarded as the same species, i.e., S. barbata. We further find that the precipitation in the typhoon season is an important climatic agent that influences the population genetic structure significantly. Such periodic episodes of climatic events rather than the long-term constant climatic variation explain the nonlinear relationship between genetic and climatic differences. By taking into account the small-scale spatial effect of climate heterogeneity and its impact on genetic diversity, our study indicates the importance of local climatic episodes in governing the genetic diversity of plants.
Author Contributions
PL conceived the study; HH, BH, and JC conducted the molecular experiments. YH sampled and identified species. HH and BH analyzed the data. PL wrote the paper. HH, BH, CH, and PL critically reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Funding
This research was financially supported by the Ministry of Science and Technology (MOST) in Taiwan (MOST 102-2621-B-003-005-MY3) and also subsidized by the National Taiwan Normal University (NTNU), Taiwan.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Shih-Ying Hwang for his statistical suggestions, Hubert Turner for English editing and Arthur Hsiao for assisting with the sampling and classification of Scutellaria taipeiensis. We also thank the National Center for Genome Medicine of the National Core Facility Program for Biotechnology, the MOST, Taiwan, for technical and bioinformatics support.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00159/full#supplementary-material
Supplementary Table 1. Accession number of each haplotype.
Supplementary Table 2. Multinomial logistic regression analysis and the significance test for the bioclimatic effect on predicting species cluster by type-II ANOVA.
Supplementary Table 3. Leaf shape of Scutellaria barbata and S. taipeiensis.
Supplementary Table 4. Codes for the bioclimatic variables used in this study.
Supplementary Figure 1. The predicted spatial distribution of two Scutellaria species in Taiwan based on six bioclimatic variables (bio2, bio3, bio8, bio13, bio18, and bio19) in (A) S. barbata and (B) S. taipeiensis. Crosses on the maps are the distribution of specimen records.
Supplementary Figure 2. Neutrality tests for examining the FST distribution of 11 microsatellite loci by (A) the Fdist approach and (B) the BayeScan approach. Both analyses show that none of the examined loci have extremely high (positive outlier) or low (negative outlier) FST, suggesting all loci used in identifying species bar and tpe are neutral.
Supplementary Figure 3. Correlation of six climatic variables. The upper and lower panels are the magnitude of the correlation and scatterplots with the confidence ellipse and smoothed line, respectively. Blue and red colors encode the sign of positive and negative correlation, respectively. bio2, mean of monthly (max temp − min temp), or the mean diurnal range; bio8, mean temperature of wettest quarter; bio9: mean temperature of driest quarter; bio13, precipitation of wettest month; bio18, precipitation of warmest quarter; bio19, precipitation of coldest quarter.
Supplementary Figure 4. Principal component analysis of (A) the temperature factors (bio2, bio8, and bio9) and (B) the precipitation factors (bio13, bio18, and bio19) for S. barbata and S. taipeiensis. Bioclimatic variables were extracted from the WorldClim website (http://www.worldclim.org/bioclim).
Supplementary Figure 5. Scatter and ordisurf plots of the partial dbRDA for six bioclimatic variables. (A) bio2, (B) bio8, (C) bio9, (D) bio13, (E) bio18, (F) bio19.
Supplementary Figure 6. The average temperature of every quarter (three months) (A) and the average precipitation of every month (B) of the records of the distribution of bar and tpe.
References
Ackerly, D. D. (2003). Community assembly, niche conservatism, and adaptive evolution in changing environments. Int. J. Plant Sci. 164, S165–S184. doi: 10.1086/368401
Anacker, B. L., and Strauss, S. Y. (2014). The geography and ecology of plant speciation: range overlap and niche divergence in sister species. Proc. R. Soc. B Biol. Sci. 281:20132980. doi: 10.1098/rspb.2013.2980
Beaumont, M. A., and Nichols, R. A. (1996). Evaluating loci for use in the genetic analysis of population structure. Proc. R. Soc. Lond. B Biol. Sci. 263, 1619–1626. doi: 10.1098/rspb.1996.0237
Bierne, N., Gagnaire, P. A., and David, P. (2013). The geography of introgression in a patchy environment and the thorn in the side of ecological speciation. Curr. Zool. 59, 72–86. doi: 10.1093/czoolo/59.1.72
Camargo, A., Morando, M., Avila, L. J., and Sites, J. W. (2012). Species delimitation with ABC and other coalescent-based methods: a test of accuracy with simulations and an empirical example with lizards of the Liolaemus darwinii complex (Squamata: Liolaemidae). Evolution 66, 2834–2849. doi: 10.1111/j.1558-5646.2012.01640.x
Carta, A., Hanson, S., and Muller, J. V. (2016). Plant regeneration from seeds responds to phylogenetic relatedness and local adaptation in Mediterranean Romulea (Iridaceae) species. Ecol. Evol. 6, 4166–4178. doi: 10.1002/ece3.2150
Chi, W.-R., Namson, J., and Suppe, J. (1981). Stratigraphic record of plate interactions in the Coastal Range of eastern Taiwan. Mem. Geol. Soc. China 4, 155–194.
Chiang, Y. C., Huang, B. H., and Liao, P. C. (2012a). Diversification, biogeographic pattern, and demographic history of Taiwanese Scutellaria species inferred from nuclear and chloroplast DNA. PLoS ONE 7:e50844. doi: 10.1371/journal.pone.0050844
Chiang, Y. C., Huang, B. H., Shih, H. C., Hsu, T. W., Chang, C. W., and Liao, P. C. (2012b). Characterization of 24 transferable microsatellite loci in four skullcaps (Scutellaria, Labiatae). Am. J. Bot. 99, E24–E27. doi: 10.3732/ajb.1100279
Cruzan, M. B. (2001). Population size and fragmentation thresholds for the maintenance of genetic diversity in the herbaceous endemic Scutellaria montana (Lamiaceae). Evolution 55, 1569–1580. doi: 10.1111/j.0014-3820.2001.tb00676.x
de Lafontaine, G., Prunier, J., Gérardi, S., and Bousquet, J. (2015). Tracking the progression of speciation: variable patterns of introgression across the genome provide insights on the species delimitation between progenitor-derivative spruces (Picea mariana x P. rubens). Mol. Ecol. 24, 5229–5247. doi: 10.1111/mec.13377
Dormann, C. F. (2007). Promising the future? Global change projections of species distributions. Basic Appl. Ecol. 8, 387–397. doi: 10.1016/j.baae.2006.11.001
Earl, D. A., and Vonholdt, B. M. (2012). STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361. doi: 10.1007/s12686-011-9548-7
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x
Excoffier, L., and Lischer, H. E. L. (2010). Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. doi: 10.1111/j.1755-0998.2010.02847.x
Fountain, T., Nieminen, M., Siren, J., Wong, S. C., and Hanski, I. (2016). Predictable allele frequency changes due to habitat fragmentation in the Glanville fritillary butterfly. Proc. Natl. Acad. Sci. U.S.A. 113, 2678–2683. doi: 10.1073/pnas.1600951113
Gavin, D. G., and Hu, F. S. (2006). Spatial variation of climatic and non-climatic controls on species distribution: the range limit of Tsuga heterophylla. J. Biogeogr. 33, 1384–1396. doi: 10.1111/j.1365-2699.2006.01509.x
Grinnell, J. (1917). The niche relationships of the California thrasher. Auk 34, 427–433. doi: 10.2307/4072271
Grossenbacher, D. L., Veloz, S. D., and Sexton, J. P. (2014). Niche and range size patterns suggest that speciation begins in small, ecologically diverged populations in North American monkeyflowers (Mimulus spp.). Evolution 68, 1270–1280. doi: 10.1111/evo.12355
Harter, D. E. V., Thiv, M., Weig, A., Jentsch, A., and Beierkuhnlein, C. (2015). Spatial and ecological population genetic structures within two island-endemic Aeonium species of different niche width. Ecol. Evol. 5, 4327–4344. doi: 10.1002/ece3.1682
Hijmans, R. J., Phillips, S., Leathwick, J., and Elith, J. (2013). Dismo: Species Distribution Modeling. R Package Version 0.8–17. Available online at: http://CRAN.R-project.org/package=dismo
Hsieh, T.-H. (2013). Scutellaria hsiehii (Lamiaceae), a new species from Taiwan. Taiwania 58, 242–245. doi: 10.6165/tai.2013.58.242
Hsieh, T. H., and Huang, T. C. (1995). Notes on the flora of Taiwan (20)-Scutellaria (Lamiaceae) in Taiwan. Taiwania 40, 35–56.
Hsieh, T.-H., and Huang, T.-C. (1997). Notes on the flora of Taiwan (29)-Scutellaria austrotaiwanensis Hsieh & Huang sp. nov. (Lamiaceae) from Taiwan. Taiwania 42, 109–116.
Huang, B.-H., Chen, Y.-W., Huang, C.-L., Gao, J., and Liao, P.-C. (2016). Diversifying selection of the anthocyanin biosynthetic downstream gene UFGT accelerates floral diversity of island Scutellaria species. BMC Evol. Biol. 16:191. doi: 10.1186/s12862-016-0759-0
Huang, C. L., Chen, J. H., Chang, C. T., Chung, J. D., Liao, P. C., Wang, J. C., et al. (2016). Disentangling the effects of isolation-by-distance and isolation-by-environment on genetic differentiation among Rhododendron lineages in the subgenus Tsutsusi. Tree Genet. Genom. 12, 53. doi: 10.1007/s11295-016-1010-2
Huang, Q. Y., Beharav, A., Youchun, U. C., Kirzhner, V., and Nevo, E. (2002). Mosaic microecological differential stress causes adaptive microsatellite divergence in wild barley, Hordeum spontaneum, at Neve Yaar, Israel. Genome 45, 1216–1229. doi: 10.1139/g02-073
Huang, T.-C., Hsiao, A., and Wu, M.-J. (2003). Notes on the Flora of Taiwan (35)-Scutellaria taipeiensis TC Huang, A. Hsiao et MJ Wu sp. nov. (Lamiaceae). Taiwania 48, 129–137. doi: 10.6165/tai.2003.48(2).129
Hubisz, M. J., Falush, D., Stephens, M., and Pritchard, J. K. (2009). Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 9, 1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x
Jombart, T. (2008). adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405. doi: 10.1093/bioinformatics/btn129
Jombart, T., Devillard, S., and Balloux, F. (2010). Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 11:94. doi: 10.1186/1471-2156-11-94
Jump, A. S., and Penuelas, J. (2005). Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x
Kawecki, T. J., and Ebert, D. (2004). Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. doi: 10.1111/j.1461-0248.2004.00684.x
Lewin-Koh, N. J., Bivand, R., Pebesma, E., Archer, E., Baddeley, A., Bibiko, H., et al (2011). maptools: Tools for Reading and Handling Spatial Objects. R Package Version 0.8-10. Available online at: http://CRAN.R-project.org/package=maptools
Li, Y. C., Fahima, T., Beiles, A., Korol, A. B., and Nevo, E. (1999). Microclimatic stress and adaptive DNA differentiation in wild emmer wheat, Triticum dicoccoides. Theor. Appl. Genet. 98, 873–883. doi: 10.1007/s001220051146
Librado, P., and Rozas, J. (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. doi: 10.1093/bioinformatics/btp187
Linder, H. P. (2008). Plant species radiations: where, when, why? Philos. Trans. R. Soc. B 363, 3097–3105. doi: 10.1098/rstb.2008.0075
Lopez-Sepulveda, P., Takayama, K., Greimler, J., Penailillo, P., Crawford, D. J., Baeza, M., et al. (2013). Genetic variation (AFLPs and nuclear microsatellites) in two anagenetically derived endemic species of Myrceugenia (Myrtaceae) on the Juan Fernández Islands, Chile. Am. J. Bot. 100, 722–734. doi: 10.3732/ajb.1200541
Losos, J. B. (2008). Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995–1003. doi: 10.1111/j.1461-0248.2008.01229.x
Manel, S., Gaggiotti, O. E., and Waples, R. S. (2005). Assignment methods: matching biological questions techniques with appropriate. Trends Ecol. Evol. 20, 136–142. doi: 10.1016/j.tree.2004.12.004
Martin, N. H., and Willis, J. H. (2007). Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61, 68–82. doi: 10.1111/j.1558-5646.2007.00006.x
Martinez-Cabrera, H. I., Schlichting, C. D., Silander, J. A., and Jones, C. S. (2012). Low levels of climate niche conservatism may explain clade diversity patterns in the South African genus Pelargonium (Geraniaceae). Am. J. Bot. 99, 954–960. doi: 10.3732/ajb.1100600
Middleton, B. (2000). Hydrochory, seed banks, and regeneration dynamics along the landscape boundaries of a forested wetland. Plant Ecol. 146, 169–184. doi: 10.1023/A:1009871404477
Minder, A. M., and Widmer, A. (2008). A population genomic analysis of species boundaries: neutral processes, adaptive divergence and introgression between two hybridizing plant species. Mol. Ecol. 17, 1552–1563. doi: 10.1111/j.1365-294X.2008.03709.x
Nagl, S., Tichy, H., Mayer, W. E., Takahata, N., and Klein, J. (1998). Persistence of neutral polymorphisms in Lake Victoria cichlid fish. Proc. Natl. Acad. Sci. U.S.A. 95, 14238–14243. doi: 10.1073/pnas.95.24.14238
Nei, M. (1977). F-statistics and analysis of gene diversity in subdivided populations. Ann. Hum. Genet. 41, 225–233. doi: 10.1111/j.1469-1809.1977.tb01918.x
Nino, H., and Emery, K. O. (1961). Sediments of shallow portions of East China Sea and South China Sea. Geol. Soc. Am. Bull. 72, 731–762. doi: 10.1130/0016-7606(1961)72[731:SOSPOE]2.0.CO;2
Owuor, E. D., Fahima, T., Beiles, A., Korol, A., and Nevo, E. (1997). Population genetic response to microsite ecological stress in wild barley, Hordeum spontaneum. Mol. Ecol. 6, 1177–1187. doi: 10.1046/j.1365-294X.1997.00296.x
Peakall, R., and Smouse, P. E. (2012). GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28, 2537–2539. doi: 10.1093/bioinformatics/bts460
Phillips, S. J., and Dudík, M. (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161–175. doi: 10.1111/j.0906-7590.2008.5203.x
Pinho, C., and Hey, J. (2010). Divergence with gene flow: models and data. Annu. Rev. Ecol. Evol. Syst. 41, 215–230. doi: 10.1146/annurev-ecolsys-102209-144644
Pyron, R. A., Costa, G. C., Patten, M. A., and Burbrink, F. T. (2015). Phylogenetic niche conservatism and the evolutionary basis of ecological speciation. Biol. Rev. 90, 1248–1262. doi: 10.1111/brv,.12154
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rieseberg, L. H., and Brouillet, L. (1994). Are many plant species paraphyletic. Taxon 43, 21–32. doi: 10.2307/1223457
Rousset, F. (1997). Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145, 1219–1228.
Royer, D. L., Meyerson, L. A., Robertson, K. M., and Adams, J. M. (2009). Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum. PLoS ONE 4:e7653. doi: 10.1371/journal.pone.0007653
Rozendaal, D. M. A., Hurtado, V. H., and Poorter, L. (2006). Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 20, 207–216. doi: 10.1111/j.1365-2435.2006.01105.x
Savolainen, O., Pyhajarvi, T., and Knurr, T. (2007). Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 38, 595–619. doi: 10.1146/annurev.ecolsys.38.091206.095646
Schoville, S. D., Bonin, A., Francois, O., Lobreaux, S., Melodelima, C., and Manel, S. (2012). Adaptive genetic variation on the landscape: methods and cases. Annu. Rev. Ecol. Evol. Syst. 43, 23–43. doi: 10.1146/annurev-ecolsys-110411-160248
Strasburg, J. L., Sherman, N. A., Wright, K. M., Moyle, L. C., Willis, J. H., and Rieseberg, L. H. (2012). What can patterns of differentiation across plant genomes tell us about adaptation and speciation? Philos. Trans. R. Soc. B 367, 364–373. doi: 10.1098/rstb.2011.0199
Tajima, F. (1989). Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595.
Takayama, K., Lopez-Sepulveda, P., Greimler, J., Crawford, D. J., Penailillo, P., Baeza, M., et al. (2015). Genetic consequences of cladogenetic vs. anagenetic speciation in endemic plants of oceanic islands. AoB Plants 7:plv102. doi: 10.1093/aobpla/plv102
Teng, L. S. (1996). Extensional collapse of the northern Taiwan mountain belt. Geology 24, 949–952. doi: 10.1130/0091-7613(1996)024<0949:ECOTNT>2.3.CO;2
Vidigal, D. S., Marques, A. C. S. S., Willems, L. A. J., Buijs, G., Mendez-Vigo, B., Hilhorst, H. M., et al. (2016). Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant. Cell Environ. 39, 1737–1748. doi: 10.1111/pce.12734
Voris, H. K. (2000). Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J. Biogeogr. 27, 1153–1167. doi: 10.1046/j.1365-2699.2000.00489.x
Wang, C. S., Huang, C. P., Ke, L. Y., Chien, W. J., Hsu, S. K., Shyu, C. T., et al. (2002). Formation of the Taiwan island as a solitary wave along the Eurasian continental plate margin: magnetic and seismological evidence. Terr. Atmos. Ocean. Sci. 13, 339–354. doi: 10.3319/TAO.2002.13.3.,339(CCE)
Wegmann, D., Leuenberger, C., Neuenschwander, S., and Excoffier, L. (2010). ABCtoolbox: a versatile toolkit for approximate Bayesian computations. BMC Bioinformatics 11:116. doi: 10.1186/1471-2105-11-116
Wiens, J. J. (2004). Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution 58, 193–197. doi: 10.1111/j.0014-3820.2004.tb01586.x
Williams, P. A. (1992). Ecology of the endangered herb Scutellaria novae-zelandiae. N.Z. J. Ecol. 16, 127–135.
Wu, C. I. (2001). The genic view of the process of speciation. J. Evol. Biol. 14, 851–865. doi: 10.1046/j.1420-9101.2001.00335.x
Keywords: climate heterogeneity, genetic variation, precipitation, Scutellaria, taxonomy, typhoon
Citation: Hsiung H-Y, Huang B-H, Chang J-T, Huang Y-M, Huang C-W and Liao P-C (2017) Local Climate Heterogeneity Shapes Population Genetic Structure of Two Undifferentiated Insular Scutellaria Species. Front. Plant Sci. 8:159. doi: 10.3389/fpls.2017.00159
Received: 23 December 2016; Accepted: 25 January 2017;
Published: 10 February 2017.
Edited by:
Tian Tang, Sun Yat-sen University, ChinaReviewed by:
Yongpeng Ma, Kunming Institute of Botany (CAS), ChinaYong Yang, Chinese Academy of Sciences, China
Copyright © 2017 Hsiung, Huang, Chang, Huang, Huang and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-Chun Liao, pcliao@ntnu.edu.tw
†These authors have contributed equally to this work.
 Huan-Yi Hsiung1†
Huan-Yi Hsiung1† Bing-Hong Huang
Bing-Hong Huang Jui-Tse Chang
Jui-Tse Chang Yao-Moan Huang
Yao-Moan Huang Chih-Wei Huang
Chih-Wei Huang Pei-Chun Liao
Pei-Chun Liao