- 1Plant and Environmental Sciences, Clemson University, Clemson, SC, USA
- 2Natural Resources Canada, Pacific Forestry Centre, Victoria, BC, Canada
- 3Department of Forestry and Natural Resources, Purdue University, West Lafayette, IN, USA
- 4Department of Biological Sciences, Purdue University, West Lafayette, IN, USA
- 5Department of Biology, University of Massachusetts Boston, Boston, MA, USA
Environmental stresses not only influence production of plant metabolites but could also modify their resorption during leaf senescence. The production-resorption dynamics of polyphenolic tannins, a class of defense compound whose ecological role extends beyond tissue senescence, could amplify the influence of climate on ecosystem processes. We studied the quantity, chemical composition, and tissue-association of tannins in green and freshly-senesced leaves of Quercus rubra exposed to different temperature (Warming and No Warming) and precipitation treatments (Dry, Ambient, Wet) at the Boston-Area Climate Experiment (BACE) in Massachusetts, USA. Climate influenced not only the quantity of tannins, but also their molecular composition and cell-wall associations. Irrespective of climatic treatments, tannin composition in Q. rubra was dominated by condensed tannins (CTs, proanthocyanidins). When exposed to Dry and Ambient*Warm conditions, Q. rubra produced higher quantities of tannins that were less polymerized. In contrast, under favorable conditions (Wet), tannins were produced in lower quantities, but the CTs were more polymerized. Further, even as the overall tissue tannin content declined, the content of hydrolysable tannins (HTs) increased under Wet treatments. The molecular composition of tannins influenced their content in senesced litter. Compared to the green leaves, the content of HTs decreased in senesced leaves across treatments, whereas the CT content was similar between green and senesced leaves in Wet treatments that produced more polymerized tannins. The content of total tannins in senesced leaves was higher in Warming treatments under both dry and ambient precipitation treatments. Our results suggest that, though climate directly influenced the production of tannins in green tissues (and similar patterns were observed in the senesced tissue), the influence of climate on tannin content of senesced tissue was partly mediated by the effect on the chemical composition of tannins. These different climatic impacts on leaves over the course of a growing season may alter forest dynamics, not only in decomposition and nutrient cycling dynamics, but also in herbivory dynamics.
Introduction
Global changes, through their widespread influence on all biological processes, can significantly impact plant life cycles and functions (Norby and Luo, 2004). Elevated levels of CO2 and ozone, increased temperatures, as well as higher drought frequency, all alter the content and composition of plant metabolites by disrupting the physiological stoichiometry in plants (Kuokkanen et al., 2001; Xu and Zhou, 2006; Prior et al., 2011; Xu et al., 2014, 2015), thus influencing plant survival and distribution. Furthermore, by influencing the chemical composition of senesced litter that fuels the metabolism of soil heterotrophs, the climate-induced physiological alterations in plants could also influence soil nutrient cycling and ecosystem productivity. For example, climate-induced changes in the composition of senescing leaves (Top and Filley, 2014; Suseela et al., 2015) could affect the subsequent detritivory (Currano et al., 2008; Couture et al., 2012) that regulates the cycling of soil carbon and mineral nutrients (Aerts, 1997; Liu et al., 2009; Suseela et al., 2013), which in turn could influence ecosystem productivity. These protracted influences of environmental stress on ecosystem performance could be mediated in part by climatic regulation of defense compounds that retain their biological-inhibitory properties even after tissue senescence.
Polyphenols, specifically tannins, are ecologically relevant, multifaceted carbon-based secondary metabolites that are common in most plant species (Kraus et al., 2003a). These compounds perform multiple protective functions and facilitate the interactions of plants with their biotic and abiotic environments during the active growth of tissues and after tissue senescence. In green tissues, as protective compounds, tannins play a critical role in plant—herbivore and plant-to-plant interactions (Salminen and Lempa, 2002; Kraus et al., 2003a; Barbehenn and Constabel, 2011), and also serve as a photo-protectant (Close and Mcarthur, 2002). Tannins in senescent leaves can account for up to 25% leaf dry weight (Kraus et al., 2003b) and thus can be a major form of carbon reaching the belowground ecosystems. Tannins in senescent tissues also retain their ability to complex with proteins (Hagerman et al., 1998) and inactivate soil enzymes (Triebwasser et al., 2012), which could hinder soil N mineralization (Hättenschwiler and Vitousek, 2000; Kraus et al., 2003a; Adamczyk et al., 2013; Tharayil et al., 2013). Thus, environmental conditions that exist during plant growth, by regulating the production of tannins in green tissues, can influence carbon and nitrogen cycling in soils.
Tannins can be broadly classified into condensed tannins (CTs) and hydrolysable tannins (HTs; Kraus et al., 2003a), with gymnosperms and monocots producing primarily CTs, and dicots able to produce either HTs or CTs alone or as mixtures (Bate-Smith, 1977; Haslam, 1988; Kraus et al., 2003a; Triebwasser et al., 2012). Condensed tannins, also known as proanthocyanidins, are polymers of flavan-3-ols such as catechin and gallocatechin (Xie and Dixon, 2005). Hydrolysable tannins are composed of gallic acid units linked via an ester linkage to a glucose functional group, and they can be further classified as gallotannins and ellagitannis by the respective presence or absence of C–C linked galloyl groups (Hartzfeld et al., 2002). In plants, tannin production is genetically (Scioneaux et al., 2011), as well as environmentally, controlled (Tharayil et al., 2011). Environmental conditions that affect the production of tannins include photoperiod, soil pH, moisture and nutrient availability, herbivory, and atmospheric CO2 and O3 (Herms and Mattson, 1992; Bussotti et al., 1998; Kraus et al., 2003a; Cohen and Kennedy, 2010; Jaakola and Hohtola, 2010; Lindroth, 2010; Malisch et al., 2016). Along with their total quantity, the biological reactivity of tannins is closely regulated by the chemistry of the polymer, viz. identity of monomers, substitution pattern of the phenolic ring, and the degree of polymerization (Kraus et al., 2003b). Although tannin quantities have been shown to respond to environmental changes, little is known about the influence of climate on the chemical composition of tannins. Further, less is known about the changes in the composition of tannins between green and senesced tissues, which are primarily influenced by the overall resorption metabolism during tissue senescence.
We investigated the influence of warming and altered precipitation on tannin production in Quercus rubra, an important species in many forested ecosystems of eastern North America (Prasad et al., 2007-ongoing). Specifically, we sought to determine the interactive effects of temperature and precipitation on (1) the content and molecular composition of tannins, (2) the association of tannins within different tissue fractions (operationally defined as extractable and non-extractable tannins) and (3) whether climatic effects on content and chemical composition of tannins differ between green and senescent leaves. We hypothesized that the climate stresses we imposed (drought, increased temperature) would induce the production of tannins that are quantitatively and compositionally different, and that the climate-induced changes in composition of tannins in green leaves would also influence the content of tannins that are retained in the senescent leaves.
Methods and Materials
Site Description
Samples were collected from the Boston-Area Climate Experiment (BACE), located in Waltham, Massachusetts, USA. The experiment is located in an old-field ecosystem, and the soil is a Mesic Typic Dystrudept (Haven series) with loamy topsoil (0–30 cm; sand:silt:clay ratios of 45:46:9) over a gravelly sandy loam subsoil. The BACE has four temperature treatments and three precipitation treatments and is divided into three replicate blocks with 12 plots (2 × 2 m, with 1 m spacing between plots) within each block. Lateral movement of water is prevented by lining each plot with polyethylene sheets to a depth of 0.6 m. Each block has ambient (i.e., control, unmanipulated), wet (150% of ambient rainfall during the growing season) and dry (50% of ambient rainfall year-round) precipitation zones, which are administered through a passive removal and active redistribution system mounted above the plots on otherwise open greenhouse frames. Dry plots are located under portions of the greenhouse frames that are covered in evenly spaced, clear, six-inch polycarbonate slats (15 cm slats arranged 15 cm apart) that exclude 50% of incoming precipitation. Frames over the Ambient and Wet treatments are covered in deer netting that provides similar shading, reducing photosynthetically active radiation by ~6% but letting all precipitation in. The precipitation collected from the dry plots was immediately reapplied to the Wet plots via electric pumps and an overhead sprinkler system (Hoeppner and Dukes, 2012). Within each precipitation zone are plots designated for one of four levels of warming: no warming, low (+c. 1.0°C), medium (+c. 2.7°C) and high (+c. 4.0°C) (Tharayil et al., 2011; Hoeppner and Dukes, 2012; Suseela and Dukes, 2013). The warming treatments were applied, starting in 2008, with infrared heaters that were mounted 1 m above each corner of the plot. Temperature at the middle of the plots was measured every 10 s. The difference in temperature between the plant canopies of the warmest and ambient plots within each group of four plots was used to achieve feedback control (Hoeppner and Dukes, 2012). Three bare-root plants (30–45 cm) of different tree species such as Q. rubra, Betula lenta, Ulmus americanus, and Betula populifolia were planted along the margins of each plot during April 2012. For this study, mature, non-shaded, leaves were collected from three Q. rubra trees per plot in 2013 during the first week of September (green leaves) and in the second week of October (freshly senesced leaves). Leaves of similar size were collected from 2 to 3 apical whorls per tree during each harvest, resulting in at least 12 leaves per plot per harvest. Leaves from trees within a plot were pooled together to obtain a composite sample. Leaves were flash frozen between slabs of dry ice immediately after harvest, and the samples were maintained below −20°C during all analyses. The Q. rubra leaves used for this study were collected from the unwarmed (No Warm) and high temperature (Warm) treatments exposed to Dry, Ambient, and Wet precipitation treatments. Three treatment replicates were maintained for each treatment in all analyses.
Extraction of Tissues
Leaf petioles were removed, and frozen leaf samples were finely ground in dry ice using mortar and pestle. Approximately 100 mg of the ground tissue was extracted twice with 10 ml of 100% MeOH. The methanol extracted tissues were further extracted with 10 ml of 75% acetone, three more times. During each extraction step the tissues were sonicated for 2 min, and then shaken for 2 h. The supernatant from the five extractions was pooled to obtain a composite extractable-fraction. The extracts were stored at −20°C and the residual litter (non-extractable) was washed with 2 ml of 80% methanol and dried overnight at 40°C.
Condensed Tannin Quantity
The total content of condensed tannins (CTs) in the pooled extracts and in the litter residue was quantified using the acid-butanol assay modified from Porter et al. (1986). For the extractable tannins, subsamples (2 ml) of the pooled extract were dried down under N2 gas at 40°C before adding 6 ml of the butanol:HCl (95:5) reagent. For the analysis of the non-extractable tannins, approximately 20 mg of the residual litter was weighed into glass tubes and combined with 6 ml of the butanol:HCl reagent. Samples were then placed in a water bath at 90–95°C for an hour and then cooled on ice. The amount of depolymerized anthocyanidin in the samples was quantified spectrophotometrically by measuring the absorbance at 550 nm (Jasco V-550 UV/VIS spectrophotometer, Jasco, Analytical Instruments, Easton, MD, USA) with the amount of tannins quantified from a standard curve derived from purified tannins of Q. rubra collected from the same experimental plot as described in Tharayil et al. (2011). Since Quercus tannins are a mixture of CT and HTs, the weight percent of CTs in the purified tannins were determined during the depolymerizing of the purified tannins in the presence of excess phloroglucinol as described below. The CT concentration in the standard curve was adjusted accordingly to avoid the potential overestimation of tannins.
Mean Degree of Polymerization and Monomer Identity of Condensed Tannin
Mass spectrometric analysis of intact polymeric tannins is challenging, especially when using electrospray as the ionization interface. This is because as the degree of polymerization increases the ionization efficiency of proanthocyanidins decreases (Karonen et al., 2006; Mouls et al., 2011). Also, stability of deprotonated proanthocyanidins and their fragments decreases with the increase in degree of polymerization of the molecule (Gu et al., 2003). Combined, this would result in an under sampling of the polymers with a higher chain-length, thus underestimating the degree of polymerization of proanthocyanidins in a sample. In order to further elucidate CT chemistry, subunit composition and mean degree of polymerization were determined by depolymerizing CTs in the presence of excess phloroglucinol (Kennedy and Jones, 2001; Karonen et al., 2006). Phloroglucinol reagent was prepared by dissolving 2.5 g phloroglucinol in 55 ml methanol containing 400 μl of concentrated HCl. The reagent was sparged with argon for ~30 min until the methanol volume was reduced to 50 ml. Two milliliters of the reagent were added to glass tubes containing ~150 mg of finely ground leaf tissues that were not subjected to prior solvent extraction. Samples in the tubes were then sparged with argon for another 2 min, immediately closed with Teflon-lined caps and incubated on a heating block at 50°C for 130 min. The tubes were then cooled on ice, centrifuged (1,000 g, 5 min), and 1.5 ml supernatant was transferred to centrifuge tubes. Saturated MgSO4 solution (~45 g in 100 ml water) was added to the supernatant and samples were cooled on ice again to facilitate the subsequent phase separation. For liquid-liquid extraction, 1.5 ml of ethyl ether was added and the tubes were shaken end to end on a rotatory shaker for 5 min. The tubes were centrifuged (1000 g, 5 min) and 40 μl of the top ethyl ether fraction was transferred to 100 μl glass inserts and completely dried under N2 gas. The samples were reconstituted in 50% methanol and analyzed using a liquid chromatograph coupled to a triple quadrupole mass spectrometer. Detailed chromatography and mass spectrometry parameters are given in supporting information.
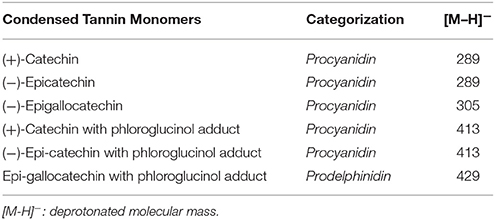
Peak identities were determined based on authentic standards of catechin and gallocatechin as well as literature values reported for phloroglucinol adducts and procyanidin fragments (Table 1). Mass spectral patterns relative to un-adducted parent monomers were also compared. Concentrations of extractable catechin monomers were subtracted from the result of the phloroglucinol depolymerization assay. Relative mass responses (Kennedy and Jones, 2001) were used to normalize the difference in the ESI responses among the terminal units and phloroglucinol adducts. Procyanidin content was determined using the catechin and epicatechin monomers as well as fragments. Prodelphinidin content was defined as the gallocatechin/epi-gallocatechin pair (Table 1). Mean degree of polymerization of proanthocyanidins, was determined by dividing the sum of the normalized peak areas of extender units (phloroglucinol adducts of catechin, gallocatechin) by the sum of the peak areas of the terminal units (catechin and gallocatechin).
Hydrolysable Tannin Analysis
For the quantification of hydrolysable tannins (HTs), methanolysis was carried out with a subsample of the methanol extract or 40 mg of methanol-extracted residual litter in methanolic H2SO4 at 85°C as described by Hartzfeld et al. (2002). The concentrations of methyl gallate were then quantified using high-pressure liquid chromatography (HPLC; see supporting information). Ellagic acid (ellagitannins), and methyl gallate (gallotannins), were identified and quantified in samples using authentic standards. Efficiency of methylation was monitored by measuring percent methylation of gallic acid standard under similar conditions as described above.
Calculations and Statistical Analysis
The total concentration of tannins in the leaf tissue was calculated by summing CT and HT concentrations. A subsample of leaves collected at the same time was used for determining specific leaf area, which was similar between the green and freshly-senesced for all treatments meaning that trends observed on a mass basis would be similar if based on area (See Figure S1 in Suseela et al., 2015).
To test the main and interactive effects of warming and altered precipitation on CTs and HTs, a mixed model restricted maximum likelihood estimation (REML) with time of sampling (green or senesced) as repeated measures was used in SAS 9.2 (SAS Institute, Inc., Cary, North Carolina, USA). Degrees of freedom were calculated using the Kenward-Rogers method. Warming and precipitation treatments were fixed effects and blocks were treated as the random effects. Tukey's HSD multiple comparison test was used to identify differences among treatments. Significance was set at α = 0.05.
Results
Type of Tannins
The total tannin concentration in green leaves varied with temperature and precipitation treatments (Figures 1A,B; Table S1). The green leaves formed in the Dry*Warm and Ambient*Warm treatment had the highest concentrations of total tannins (121.2 and 128.8 mg g−1 tissue, respectively; Figure 1A), while the Ambient*No Warm treatment had the lowest total tannin concentration (73.5 mg g−1 tissue; Figure 1A). Only the Ambient treatment exhibited an increase in green leaf tannin concentrations with an increase in temperature (P < 0.001). This pattern was also observed in senesced tissues, where the tannin concentration of senesced leaves also increased with higher temperature in both Dry (34% increase; P < 0.005) and Ambient (53% increase; P < 0.004) treatments (Figure 1B, Table S1). Within the Warm treatment, the senesced leaves in the drier treatments had more tannins (P < 0.05; Figure 1B). Dry*Warm had the highest concentration (110.5 mg g−1 tissue) and Wet*Warm had the lowest concentration (62.1 mg g−1 tissue) of total tannins (Figure 1B). The same trend was evident for senesced leaves exposed to Dry, Ambient, and Wet conditions in the NoWarm treatments. Decreases between the green and senesced tissue were observed in total tannins for the Wet treatments (P < 0.05) and the Ambient*No Warm treatment (P < 0.05).
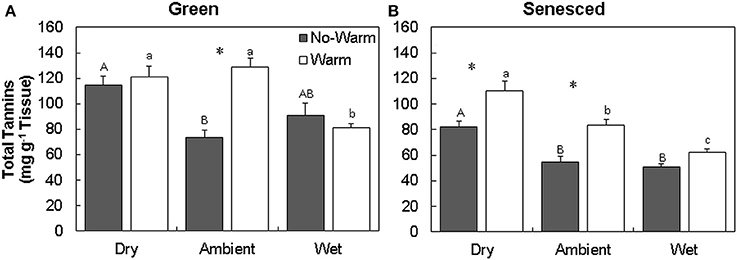
Figure 1. Total average (±SE) tannin content for green (A) and senesced (B) leaf tissue. Asterisks (*) above a set of columns indicate significant (P < 0.05) differences between No Warm and Warm treatments within each precipitation treatment. Uppercase letters indicate significant differences (P < 0.05) between precipitation treatments within the No Warm temperature regime. Lowercase letters indicate significant differences (P < 0.05) between precipitation treatments within the Warm temperature regime.
When separating total tannins into CTs and HTs, CTs made up between 29 and 89% of the green leaf tissue and 53–88% of the senesced leaf tissue. In the green tissue of the Wet*No Warm treatment, HTs made up the larger percentage of total tannins (~69%) (Table 2). In green leaf tissue, warming did not alter HT concentrations in the Dry, Ambient (P = 0.06), or the Wet (P = 0.12) treatments (Table 2). The Wet precipitation treatment had the highest percentages of HTs in green leaf tissue for both the No Warm (69.1%) and the Warm (50.0%) treatments (Table 2). In senesced leaf tissue, warming increased (P < 0.001) the percent of HTs in the Ambient treatment, but decreased HTs (P < 0.001) in the Wet treatment (Table 2). In unwarmed plots, leaves in the Ambient treatment had the lowest percentage of HTs and those in the Wet treatment had the highest percentage (Table 2). Senesced leaf tissue had lower (P < 0.05) in HT percentages than green tissue in the all the Wet treatments and the Ambient*Warm treatment.
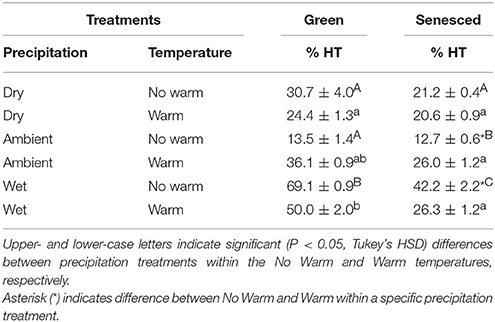
Table 2. Percentages of hydrolysable tannins calculated from total tannins (±SE) for both green and senesced leaf tissue.
The responses of CT to climate treatments were similar to those of total tannins (Figures 2A,B). Warm treatments did not increase the total CTs in the green leaves in any of the precipitation treatments Dry (P = 0.41), Ambient (P = 0.14), or Wet (P = 0.42; Figure 2A). Warm treatments increased the total CTs in the senesced leaves for the Dry (P < 0.02), but not for the Ambient (P = 0.15) or the Wet (P = 0.082; Figure 2B). While total content of CTs did not change between the green and senesced leaf tissue, the content that CTs represented of the total proportion of tannins significantly (P < 0.05) increased for the Wet*No Warm and the Ambient*Warm and Wet*Warm.
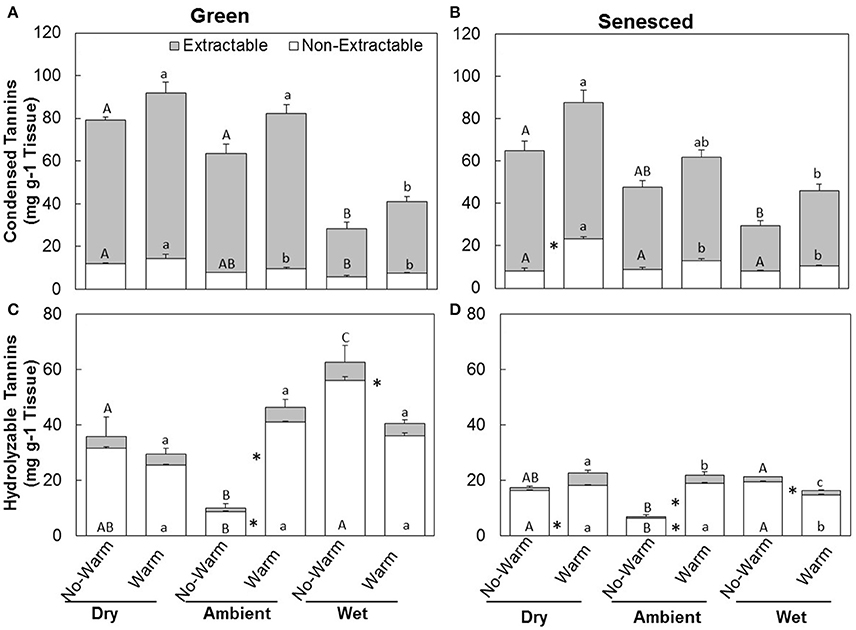
Figure 2. Total average (±SE) condensed (A,B) and hydrolysable (C,D) tannin content for the extractable and non-extractable portions of both green (A,C) and senesced (B,D) leaf tissue. Upper asterisks (*) indicate significant differences (P < 0.05) between the No Warm and Warm treatments within each precipitation treatment for extractable condensed tannins and lower asterisks indicate significant effects of warming within each precipitation treatment for non-extractable condensed tannins. Different upper case letters indicate significant differences (P < 0.05) between precipitation treatments within the No Warm temperature regime. Different lowercase letters indicate significant differences between precipitation treatments within the Warm treatment.
Climatic treatments influenced the chemistry of CTs in leaf tissues (Table 3). The Wet treatment had CTs with longer subunit chains than in the Dry and Ambient treatments (Table 3). The proportion of epi/catechin to epi/gallocatechin or procyanidin (PC) to prodelphinidin (PD) differed only by precipitation treatment; warming did not change the percentage of CT monomers that were PD or PC. The Ambient treatment had the highest percentage of PDs (~27%) and Wet had the lowest, at <6.8%, with the Dry treatment intermediate (~10–14%; Table 3).
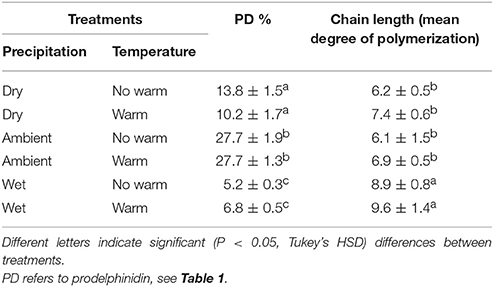
Table 3. Percentage (±SE) of type of condensed tannins and average (±SE) chain length of condensed tannin polymers.
The total content of HTs in green tissues increased with warming under Ambient (P < 0.01) precipitation, but decreased with warming in the Wet treatment (P < 0.02; Figure 2C). In senesced tissue, HTs generally made up a lower percentage of the total tannin concentration than they did in green tissue (Figure 2D). Similar trends between Warm and No Warm treatments also appeared in the senesced tissue, except in the senesced leaf tissue the differences between Warm and No Warm were significant for all the precipitation treatments. Warming increased HTs in the Dry (P < 0.01) and the Ambient (P < 0.001) treatments, but decreased them in the Wet treatment (P < 0.005; Figure 2C). Total HT content significantly decreased from green to senesced leaf tissue in the Wet*No Warm treatment and all the Warm treatments.
The HTs can be further differentiated into two HT structural compounds in the Q. rubra leaves: ellagitannins and gallotannins (Tables 2, 4). Both green and senesced tissues generally had higher proportions of ellagitannins, although total contents were higher in the green tissue compared to the senesced tissue (Table 4). In the green tissue both gallotannins and ellagitannins displayed the same relationship, where the Ambient*No Warm treatment had the least (P < 0.05; Table 4). A similar relationship also existed in the senesced leaf tissue (Table 4). Over the course of the growing season, ellagitannin content decreased in green to senesced leaf tissue, but only in the Wet treatments (No Warm, P < 0.05; Warm, P < 0.001). Total gallotannin content also decreased (P < 0.05) between green and senesced leaf tissue in all the treatments except the Ambient*No Warm control.
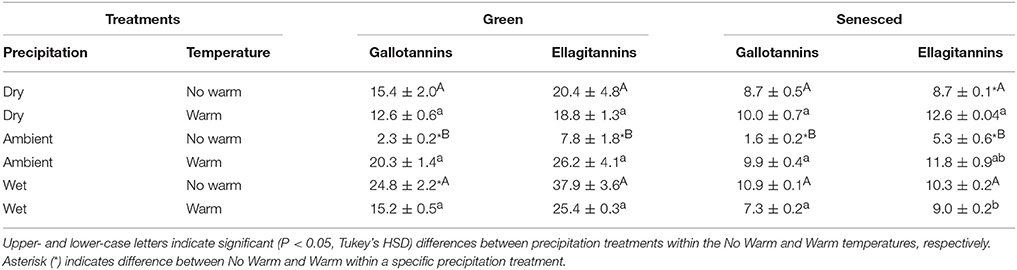
Table 4. Average concentrations, mg g−1 tissue, (±SE) of structural types of hydrolysable tannins for both green and senesced leaf tissue.
Tannin Fractions
Total tannins, when separated into extractable and non-extractable fractions, showed similar patterns in both the green and senesced leaf tissue (Table 5). For the extractable fractions, the lowest concentration of tannins was in the Wet precipitation treatment, with no warming effect (Table 5). Warming increased the non-extractable fraction in green leaf tissue (P < 0.05) in the Ambient treatment (Table 5), and did not affect the non-extractable fraction in senesced tissue. In both the green and senesced leaf tissues, the lowest non-extractable tannin concentration existed in the Ambient*No Warm treatment (Table 5). Between seasons (green and senesced) there was a decrease (5.2%) in the Ambient*No Warm control and an increase (13.2%) in the Wet*Warm treatment in the percentage of tannins that were extractable.
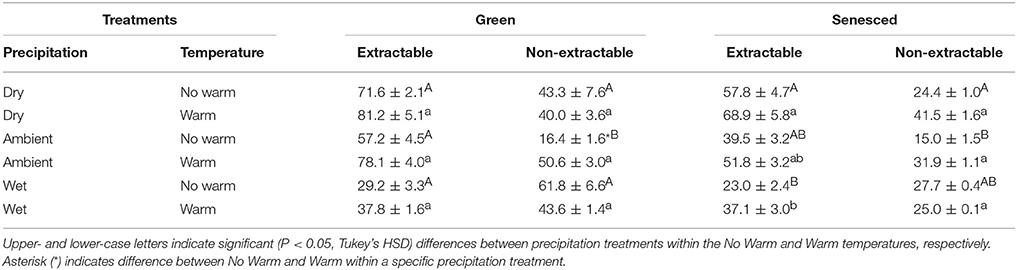
Table 5. Average concentrations, mg g−1 tissue, (±SE) of total extractable and non-extractable tannins for both green and senesced leaf tissue.
The majority of CTs were located in the extractable fraction (Figures 2A,B). Warming did not affect the total content of CTs in each fraction in the green leaf tissue (Figure 2). The percentage of non-extractable CTs in the green tissue was higher in the Wet*No Warm (P < 0.005), but not the Wet*Warm (P = 0.09; Figure 4A). The Ambient treatments had the lowest percentage of non-extractable CTs (No Warm = 12.2%, Warm = 11.7%; Figure 4A). In the senesced tissue, warming had an effect only in the Dry treatment, where it increased (P < 0.001) the content of CTs in the non-extractable fraction (Figure 2B). The percentage of non-extractable CTs tended to increase with warming only in the Dry treatment, and then only marginally (P < 0.1; Figure 3B). Within the No Warm temperature treatment, the Wet had the highest percentage (28.4%) of non-extractable CTs (Figure 3B). The percentage of non-extractable CTs significantly increased (by 8.5, 11.4, and 9.3%) from green leaves to senesced leaves for the Wet*No Warm, Dry*Warm, and Ambient*Warm, respectively.
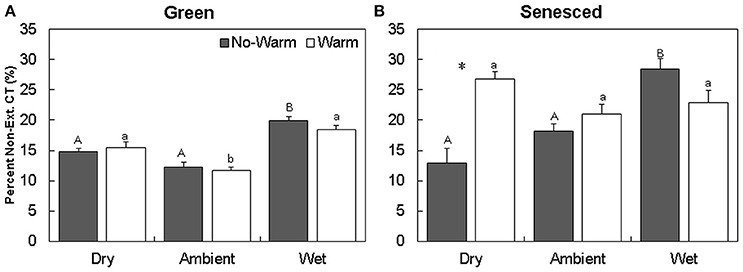
Figure 3. Average (±SE) percent of non-extractable condensed tannins for green (A) and senesced (B) leaf tissue. Asterisks (*) above a set of columns indicate significant (P < 0.05) differences between the No Warm and Warm treatments within each precipitation treatment. Different uppercase letters indicate significant differences between precipitation treatments within the No Warm temperature regime. Different lowercase letters indicate significant differences between precipitation treatments within the Warm temperature regime.
The relative majority of HTs were located in the non-extractable fraction (Figures 2C,D) for both the green and senesced tissue. In green leaf tissue, warming increased the proportions of HTs in both the extractable (P < 0.03) and non-extractable (P < 0.005) fractions of the Ambient treatment (Figure 2C). Warming also decreased (P < 0.03) HTs in the non-extractable fraction in the Wet treatment (Figure 2C). Similar patterns occurred in the senesced tissue as well (Figure 2D), where HTs also increased with warming in the Dry treatment (Figure 2D).
For individual HTs, the percent of gallotannins increased the most in the Ambient treatment, with Ambient*No Warm having the lowest percentages of gallotannins (Figure 4). In the Ambient treatment, warming increased gallotannins in the extractable fraction in both the green and senesced leaf tissue (Figures 4B,D). In senesced tissue, warming in the Ambient treatment also increased the gallotannin percentage of the non-extractable fraction (this pattern did not occur in the green leaf tissue; P = 0.09, Figures 4A,C). In the Wet treatment, senesced leaves had more gallotannins (11.8%, No Warm; 7.7% Warm) in the non-extractable fraction than green leaf tissue.
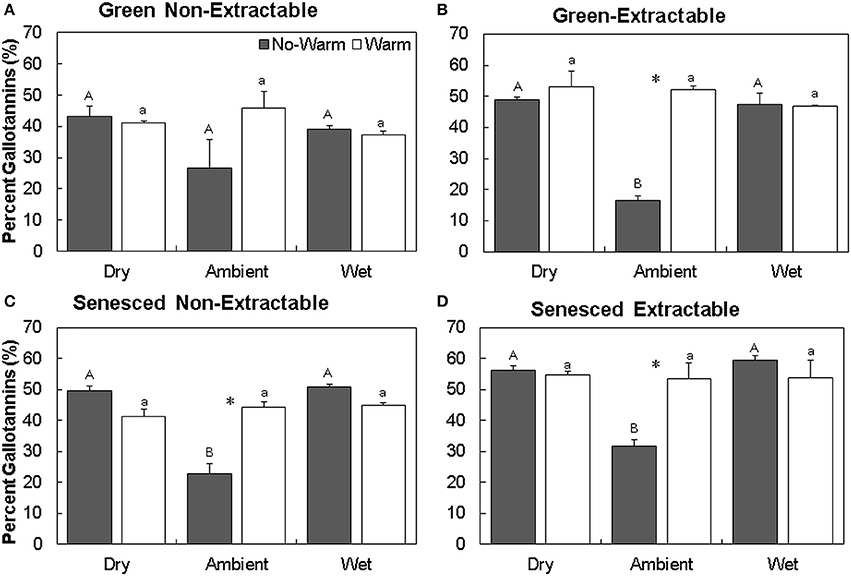
Figure 4. Average (±SE) percent gallotannins in non-extractable and extractable hydrolysable tannins for both green (A,B) and senesced (C,D) leaf tissue. Asterisks (*) above a set of columns indicate significant (P < 0.05) differences between No Warm and Warm treatments within each precipitation treatment. Uppercase letters indicate significant differences between precipitation treatments within the No Warm temperature regime. Lowercase letters indicate significant differences between precipitation treatments within the Warm temperature regime.
Discussion
Climatic stresses that disrupt cellular functioning in plants initiate readjustments in metabolic pathways, which in turn facilitate the acclimation of plants to their new environment. The resulting reprogramming of important metabolic pathways, including the phenylpropanoid pathway, citric acid cycle, glycolysis, and urea cycle, results in the upregulation of several metabolite classes such as amino acids, phenolic acids, sugars, organic acids, sugar alcohols, polyamines, and polyols (Bohnert et al., 1995; Peñuelas et al., 2013; Suseela et al., 2015). Drought and warmer temperatures have been shown to influence many different morphological (yield, growth) and physiological (rate of photosynthesis, stomatal conductance, leaf pigmentation, water potential, protein concentrations, etc.) responses in plants (Benjamin and Nielsen, 2006; Rennenberg et al., 2006; Praba et al., 2009; Anjum et al., 2011). However, the influence of environmental stressors on the content and composition of polymeric defense compounds across green and senesced plant tissues is less well-known.
In this study, climate-induced changes in leaf tannin composition influenced not only the quantity of tannins, but also the proportion of HTs to CTs, monomer composition and mean degree of polymerization of CTs, composition of HTs, the association of tannins within the cells (extractable and non-extractable), and the composition of the tannins remaining in the senescent leaves. In general, plants growing in favorable conditions (Ambient*No Warm, Wet) produced less tannins per unit leaf mass than those growing in more stressful conditions (Dry, Ambient*Warm). Increase in tissue content of tannins with increasing environmental stress has been frequently reported (Bussotti et al., 1998; Cohen and Kennedy, 2010). Reallocation of nutrients in plants based on the favorability of growth and nutrient conditions is expected under two major hypotheses: the carbon/nutrient balance and the growth/differentiation balance (Herms and Mattson, 1992). The increase in tannin production under Dry*Warm conditions could be a defense strategy in plants to protect the resources that they have already acquired (Herms and Mattson, 1992; Wright et al., 2010; Massad et al., 2014). At the same time, in the Wet treatments, because growth conditions were presumably closer to optimal, the decrease in tannin production could be a result of the plant preferentially allocating C for growth instead of defense (Bryant et al., 1983; Tuomi, 1992). Also, a greater concentration of tannins binding with cellulose and lignin matrices (Zucker, 1983; Bussotti et al., 1998) could potentially create a physical impedance to cell expansion. A similar allocation pattern of photosynthates for growth, rather than for defense compounds, has been previously reported in other tree species (Donaldson et al., 2006), where the plant growth was negatively correlated with phenolics and CTs. In a previous study conducted at BACE, the tannin content in senesced leaves of Acer rubrum was greater in Dry than in Ambient treatments, while the senesced leaves from Wet and the Ambient treatments had similar tannin contents (Tharayil et al., 2011). This contrasting pattern could be attributed to a difference in the physiological responses of the two species to stress, and also to differences in precipitation patterns between the two growing seasons. In the 2009 growing season (April to October; Tharayil et al., 2011), 720 mm of rain fell, as opposed to 606 mm during the 2013 growing season. Further, only half as much rain fell in the last 4 months of the 2013 growing season (July to October) as fell during that period in 2009 (210 mm and 428 mm, respectively). The larger amount of precipitation received by A. rubrum during the 2009 growing season would have led to a similar physiological water status between Ambient and Wet treatments, which could have resulted in similar tannin content between these two precipitation treatments (Tharayil et al., 2011). In the present study, the scarcer and less evenly distributed precipitation of 2013 likely exposed Q. rubra in Ambient to a greater physiological water stress, which could explain the observed similarity in tannin content between Ambient and Dry treatments.
Along with the total quantity of tannins, climate influenced both the monomer composition and mean degree of polymerization of tannins, the two parameters that are seldom investigated despite their regulatory influence on the potential biological reactivity of tannins (Zucker, 1983; Kraus et al., 2003b; Tharayil et al., 2011; Triebwasser et al., 2012). Reactivity of CT is a function of the hydroxylation pattern of the B-ring, and tannins with trihydroxy B-rings (PD) are more reactive than those with dihydroxy B-ring (PC). Both Wet and Dry treatments, despite their contrasting influences on total content of CTs, reduced the relative proportion of PD units. Compared to PCs, the PD units are formed only during later parts of cell development (Stafford et al., 1989). Thus, the lower accumulation of the PDs in wet climates could indicate a metabolic modification in CT biosynthesis. Under the metabolic stress in Dry conditions the triphenolic vicinal hydroxyls predispose PDs to oxidation and polymerization reactions (Close and Mcarthur, 2002; Aron and Kennedy, 2008) resulting in a lower proportion of PDs. This could partly explain the lower proportion of PDs in Dry treatments despite a higher production of CTs. Compared to Ambient and Dry treatments, the proportion of PD units and the mean degree of polymerization were higher under Wet treatments. The biological reactivity of tannins is a function of their degree of polymerization, where tannins with greater chain length have a higher capacity to complex with and precipitate proteins. Thus, though the leaves that were formed under ambient growing conditions and Wet treatments had a lower tannin quantity, these tannins would have a greater capacity to defend herbivory due to their higher biological reactivity contributed by a greater polymerization and higher proportion of PD units. Environmental conditions have been shown to influence the degree of polymerization of tannins (Cohen and Kennedy, 2010). A lower polymerization of tannins exposed to non-optimal growing conditions has been reported before (Tharayil et al., 2011) in senesced leaves of Acer rubrum from the same site. In Vitis vinifera, environmental stressors, especially moisture deficit, have been reported to increase the mean degree of polymerization of tannins (Kennedy et al., 2002; Cohen and Kennedy, 2010). In Onobrychis viciifolia the influence of drought on the mean degree of polymerization of CTs was dependent on otogeny of the plant (Malisch et al., 2016). These results indicate that the influence of environment on molecular composition of tannins could be regulated by the physiological stress perceived by the plant (as opposed to the magnitude of the treatment applied), the identity of the species, as well as their growth stage.
Distributions of tannins within leaves are highly species-specific (Kraus et al., 2003a), and within a species the cellular localization of tannins, herein operationally defined as solvent extractable and non-extractable tannins, can change based on environmental conditions (Gagné et al., 2006). In green leaves, CTs are usually stored in the cell vacuoles (Stafford, 1988; Bussotti et al., 1998; Marles et al., 2003), and thus are sequestered away from potential interactions with the structural matrix and plant metabolic compounds. During leaf maturation the tannins penetrate the cell walls (Bussotti et al., 1998), which may result in an increase in the fiber-bound proportion of tannins. These complexes can protect the cell wall or organelle against microbial attack, and can also delay the decomposition after senescence of the tissue (Zucker, 1983). Irrespective of the warming treatment, the leaves from the Wet treatment had a greater proportion of total CT that was non-extractable during the sequential solvent extraction. While we cannot be certain of the exact complexation of the tannins, some of them may be in association with cellulose and pectin (Le Bourvellec et al., 2009; Padayachee et al., 2012a,b; Jakobek, 2015) in the cell walls and middle lamellae. A high affinity of PCs binding to pectin has been observed (Le Bourvellec et al., 2009; Watrelot et al., 2013; Jakobek, 2015), meaning that tannins in the Wet treatment with the highest percentage of PCs and greater chain-length could have a greater complexation capacity with cell wall components. Alternatively, the greater chain-length and higher proportion of PC units would have resulted in lower extractability of tannins from the leaf tissues, resulting in the observed lower content of tannins in Wet treatments. Also, the Wet treatments, despite a lower content of CTs, have a higher percentage of HTs, which have a higher enzyme inhibition capacity compared to CTs (Triebwasser et al., 2012). Thus, overall, the leaves formed under Wet treatments would have a similar herbivore deterrence contributed by tannins, despite a lower content of CTs in the tissue.
Climate influenced the proportion of HTs and CTs. Across most treatments, the dominant type of tannin in Q. rubra was CT, with lower content of HTs. A similar, lower proportion of HT in red oak species has been reported before (Barbehenn et al., 2005). Compared to the Ambient*NoWarm control, all climate treatments had higher percentages of HTs, which may reflect the ease of maintenance, mobility, and greater responsiveness of HTs relative to CTs (Zucker, 1983; Shure et al., 1998). Because HTs are less associated with the structural matrix of the plants than CTs, they offer less resistance to normal cell growth and expansion, making them a preferable type of defense compound over CTs in environments that favor active plant growth (Zucker, 1983), as evidenced by the higher percentages of HTs in the Wet treatments. HTs are also thought to be metabolically cheaper; the metabolic cost for the formation of proanthocyanidin (monomers of CT) is 0.395 ATP equivalent g−1, where as that of HTs is 0.27 equivalent g−1 (Lewis and Yamamoto, 1989).
Both gallotannins and ellagitannins also exhibit antioxidant properties (Barbehenn et al., 2006), and thus may play a role in the inhibition of higher radical formation in leaves, especially under climatic stress. Ellagitannins can also be highly variable in structure (Zucker, 1983). This could have specific relevance inhibiting digestive enzymes in herbivore digestive tracts (Zucker, 1983), which would be an effective defense mechanism against herbivory for the plant. In most climates in this study, the green leaves exhibited higher content of ellagitannins, however, the Ambient*No Warm control had the highest percentage of ellagitannins compared to all other treatments, suggesting that the alteration of climate in any way can significantly alter the proportion of certain types of tannins present, either by production rates or utilization of certain hydrolysable tannin structural components, such as the glucose molecule (Zucker, 1983).
During tissue senescence, following the disruption of membrane-bound vesicles, CTs can form insoluble complexes with proteins and cell wall carbohydrates (Kraus et al., 2003a; Marles et al., 2003), which could contribute to an increase in the percent of non-extractable CTs in the senesced tissue. Differences in the content of tannins in green leaves compared to senesced leaves observed in this study may be the result of different overall resorption strategies of the plants. Considering their protein complexation capacity, polymeric nature, and the C-C linkages, the CT would less amenable to enzyme mediated catalysis and subsequent resorption. Due to their lower complexity and the presence of more labile ester bonds, the gallotannins would be more amenable to resorption during tissue senescence than CTs. Compared to the green tissues, the greater mobilization of HTs during senescence is reflected in the lower proportion of HTs in the senesced tissues across the treatments. The CT content of the Wet treatments were similar in both green and senesced tissues, which could be primarily attributed to the greater degree of polymerization and a greater PC content of tannins produced in this treatment, which would have resulted in a greater association of these tannins with the cell wall.
Tannins can complex with carbohydrates and proteins and this complexation can persist after the leaf senesces, potentially delaying decomposition. With the increase in CTs and non-extractable CTs for some of the treatments, this might mean that those leaves would break down more slowly, lose less material to leaching, and possibly provide more sustained nutrient input into the soil. The higher tannin content of foliage observed under less favorable growing conditions could be partly countered by the changes in allocation patterns at the tree level, since trees exposed to climatic stress often produce less leaf biomass. However, the reduced carbon input through litterfall in such ecosystems, coupled with higher content of phenolic compounds in these tissues, might impose a greater constraint on nutrient cycling in these soils. Extractable phenolics and tannins are lost rapidly from the leaves, suggesting that leaching is a primary route for loss of tannins from leaves (Benner et al., 1988; Schofield et al., 1998; Hernes et al., 2001), and lower molecular weight tannins are lost more rapidly than higher molecular weight tannins (Schofield et al., 1998). Thus, the Wet treatments with their higher length CTs could better retain some of the tannin-related defense capabilities which could interfere with decomposition processes. However, the overall herbivory and decomposition processes are influenced not only by the content of anti-nutrients in the tissues, but also by their overall nutrient content (Suseela et al., 2013; Almuzini et al., 2017), which in turn is also regulated by environmental stressors.
Overall, our study elucidates multiple factors that regulate the production and seasonal change of tannins in Q. rubra. In agreement with the nutrient-balance hypothesis, the tannin concentrations in tissues formed under favorable climates were lower, but the tannins produced were more complex and potentially more protective due to higher chain lengths of CTs and greater proportions of HTs. When exposed to climatic stress, Q. rubra responded by producing a greater quantity of tannins that were of shorter chain length. The differential influence of the environment on the production dynamics of plant metabolites is important in the context of global changes and carbon and nitrogen cycle feedbacks. Although over longer time scales climate modifies ecosystem processes through shifts in species composition, over shorter time scales the physiological adaptations of plants to changing climate could influence soil C and nutrient cycling through changes in chemical composition of biomass. These changes in tannin content and composition may alter forest dynamics, not only by influencing decomposition and nutrient cycling dynamics, but also by regulating herbivore dynamics.
Author Contributions
ST and NT contributed to the conceptualization of the project and design of the experiment, JD provided the experimental framework, ST collected and analyzed the data, CP and NT guided the analyses, ST drafted the article, all authors contributed to the critical revision of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Vidya Suseela two reviewers for their critiques and thoughtful remarks on earlier drafts of this manuscript. We also thank Carol Goranson for maintaining the BACE field site, and Vidya Suseela for reoptimizing the tannin extraction protocols for this experiment. This research was supported by the National Science Foundation Grant (DEB-1145993) to NT and JD. The BACE infrastructure was supported by grants to JSD from NSF (grant DEB-0546670) and the U.S. Department of Energy's Office of Science (BER), through the Northeastern Regional Center of the National Institute for Climatic Change Research and the Terrestrial Ecosystem Sciences program. This is Technical Contribution No. 6549 of the Clemson University Experiment Station and is Purdue Climate Change Research Center Publication number 1709. This material is based upon work supported by NIFA/USDA, under project number SC-1700538.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00423/full#supplementary-material
References
Adamczyk, B., Kitunen, V., and Smolander, A. (2013). Response of soil C and N transformations to condensed tannins and different organic N-condensed tannin complexes. Agric. Ecosyst. Environ. Appl. Soil Ecol. 64, 163–170. doi: 10.1016/j.apsoil.2012.12.003
Aerts, R. (1997). Climate, leaf litter chemistry, and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79, 439–449.
Almuzini, M., Decker, C., Wang, D., Gerard, P., and Tharayil, N. (2017). Nutrient supply and simulated herbivory differentially alter the metabolite pools and the efficacy of the glucosinolate-based defense system in Brassica Species. J. Chem. Ecol. 43, 129–142. doi: 10.1007/s10886-016-0811-y
Anjum, S., Xie, X., and Wang, L. (2011). Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 6, 2026–2032. doi: 10.5897/AJAR10.027
Aron, P. M., and Kennedy, J. (2008). Flavan-3-ols: nature, occurrence and biological activity. Mol. Nutr. Food Res. 52, 79–104. doi: 10.1002/mnfr.200700137
Barbehenn, R., Cheek, S., Gasperut, A., Lister, E., and Maben, R. (2005). Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midgut fluids of Malacosoma disstria and Orgyia leucostigma caterpillars. J. Chem. Ecol. 31, 969–988. doi: 10.1007/s10886-005-4242-4
Barbehenn, R. V, and Constabel, C. P. (2011). Tannins in plant – herbivore interactions. Phytochemistry 72, 1551–1565. doi: 10.1016/j.phytochem.2011.01.040
Barbehenn, R. V., Jones, C. P., Hagerman, A. E., Karonen, M., and Salminen, J.-P. (2006). Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: potential impact on caterpillars. J. Chem. Ecol. 32, 2253–2267. doi: 10.1007/s10886-006-9143-7
Bate-Smith, E. C. (1977). Astringent tannins of Acer species. Phytochemistry 16, 1421–1426. doi: 10.1016/S0031-9422(00)88795-6
Benjamin, J. G., and Nielsen, D. C. (2006). Water deficit effects on root distribution of soybean, field pea and chickpea. Field. Crop. Res. 97, 248–253. doi: 10.1016/j.fcr.2005.10.005
Benner, R., Kirchmann, D., and Hodson, R. E. (1988). Bacterial abundance and production on mangrove leaves during initial stages of leaching and biodegradation. Arch. Hydrobiol. Beih. 31, 19–26.
Bohnert, H. J., Nelson, D. E., and Jensen, R. G. (1995). Adaptations to environmental stresses. Plant Cell 7, 1099–1111. doi: 10.1105/tpc.7.7.1099
Bryant, J. P., Chapin, F. S., and Klein, D. R. (1983). Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40, 357–368.
Bussotti, F., Gravano, E., Grossoni, P., and Tani, C. (1998). Occurrence of tannins in leaves of beech trees (Fagus sylvatica) along an ecological gradient, detected by histochemical and ultrastructural analyses. New Phytol. 138, 469–479. doi: 10.1046/j.1469-8137.1998.00121.x
Close, D. C., and Mcarthur, C. (2002). Rethinking the role of many plant phenolics: protection from photodamage not herbivores? Oikos 99, 166–172. doi: 10.1034/j.1600-0706.2002.990117.x
Cohen, S. D., and Kennedy, J. A. (2010). Plant metabolism and the environment: implications for managing phenolics. Crit. Rev. Food Sci. Nutr. 50, 620–643. doi: 10.1080/10408390802603441
Couture, J. J., Meehan, T. D., and Lindroth, R. L. (2012). Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia 168, 863–876. doi: 10.1007/s00442-011-2139-1
Currano, E. D., Wilf, P., Wing, S. L., Labandeira, C. C., Lovelock, E. C., and Royer, D. L. (2008). Sharply increased insect herbivory during the Paleocene-Eocene Thermal Maximum. Proc. Natl. Acad. Sci. U.S.A. 105, 1960–1964. doi: 10.1073/pnas.0708646105
Donaldson, J. R., Kruger, E. L., and Lindroth, R. L. (2006). Competition- and resource-mediated tradeoffs between growth and defensive chemistry in trembling aspen (Populus tremuloides). New Phytol. 169, 561–570. doi: 10.1111/j.1469-8137.2005.01613.x
Gagné, S., Saucier, C., and Gény, L. (2006). Composition and cellular localization of tannins in Cabernet Sauvignon skins during growth. J. Agric. Food Chem. 54, 9465–9471. doi: 10.1021/jf061946g
Gu, L., Kelm, M. A., Hammerstone, J. F., Zhang, Z., Beecher, G., Holden, J., et al. (2003). Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J. Mass Spectrom. 38, 1272–1280. doi: 10.1002/jms.541
Hagerman, A. E., Riedl, K. M., Jones, G. A., Sovik, K. N., Ritchard, N. T., Hartzfeld, P. W., et al. (1998). High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 46, 1887–1892.
Hartzfeld, P. W., Forkner, R., Hunter, M. D., and Hagerman, A. E. (2002). Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 50, 1785–1790. doi: 10.1021/jf0111155
Haslam, E. (1988). Plant polyphenols (syn. vegetable tannins) and chemical defense-A reappraisal. J. Chem. Ecol. 14, 1789–1805. doi: 10.1007/BF01013477
Hättenschwiler, S., and Vitousek, P. M. (2000). The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 15, 238–243. doi: 10.1016/S0169-5347(00)01861-9
Herms, D. A., and Mattson, W. J. (1992). The dilemma of plants: Grow or defend hypothesis. Q. Rev. Biol. 67, 283–335.
Hernes, P. J., Benner, R., Cowie, G. L., Goñi, M. A., Bergamaschi, B. A., and Hedges, J. I. (2001). Tannin diagenesis in mangrove leaves from a tropical estuary: a novel molecular approach. Geochim. Cosmochim. Acta 65, 3109–3122. doi: 10.1016/S0016-7037(01)00641-X
Hoeppner, S. S., and Dukes, J. S. (2012). Interactive responses of old-field plant growth and composition to warming and precipitation. Glob. Chang. Biol. 18, 1754–1768. doi: 10.1111/j.1365-2486.2011.02626.x
Jaakola, L., and Hohtola, A. (2010). Effect of latitude on flavonoid biosynthesis in plants. Plant. Cell Environ. 33, 1239–1247. doi: 10.1111/j.1365-3040.2010.02154.x
Jakobek, L. (2015). Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 175, 556–567. doi: 10.1016/j.foodchem.2014.12.013
Karonen, M., Ossipov, V., Sinkkonen, J., Loponen, J., Haukioja, E., and Pihlaja, K. (2006). Quantitative analysis of polymeric proanthocyanidins in birch leaves with normal-phase HPLC. Phytochem. Anal. 17, 149–156. doi: 10.1002/pca.898
Kennedy, J., and Jones, G. P. (2001). Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 49, 1740–1746. doi: 10.1021/jf001030o
Kennedy, J. A., Matthews, M. A., and Waterhouse, A. L. (2002). Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Vitic. 53, 268–274.
Kraus, T. E. C., Dahlgren, R. A., and Zasoski, R. J. (2003a). Tannins in nutrient dynamics of forest ecosystems - a review. Plant Soil 256, 41–66. doi: 10.1023/A:1026206511084
Kraus, T. E. C., Yu, Z., Preston, C. M., Dahlgren, R. A., and Zasoski, R. J. (2003b). Linking chemical reactivity and protein precipitation to structural characteristics of foliar tannins. J. Chem. Ecol. 29, 703–730. doi: 10.1023/A:1022876804925
Kuokkanen, K., Julkunen-Tiitto, R., Keinänen, M., Niemelä, P., and Tahvanainen, J. (2001). The effect of elevated CO2 and temperature on the secondary chemistry of Betula pendula seedlings. Trees 15, 378–384. doi: 10.1007/s004680100108
Le Bourvellec, C., Guyot, S., and Renard, C. M. G. C. (2009). Interactions between apple (Malus x domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydr. Polym. 75, 251–261. doi: 10.1016/j.carbpol.2008.07.010
Lewis, N. G., and Yamamoto, E. (1989). “Tannins—their place in plant metabolism,” in Chemistry and Significance of Condensed Tannins, eds R. W. Hemingway and J. J. Karchesy (New York, NY: Plenum Press), 23–46.
Lindroth, R. L. (2010). Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 36, 2–21. doi: 10.1007/s10886-009-9731-4
Liu, L., King, J. S., Giardina, C. P., and Booker, F. L. (2009). The influence of chemistry, production and community composition on leaf litter decomposition under elevated atmospheric CO2 and tropospheric O3 in a northern hardwood ecosystem. Ecosystems 12, 401–416. doi: 10.1007/s10021-009-9231-y
Malisch, C. S., Salminen, J.-P., Kölliker, R., Engström, M., Suter, D., Studer, B., et al. (2016). Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant's ontogenetic stage. J. Agric. Food Chem. 64, 9307–9316. doi: 10.1021/acs.jafc.6b02342
Marles, M. A. S., Ray, H., and Gruber, M. Y. (2003). New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 64, 367–383. doi: 10.1016/S0031-9422(03)00377-7
Massad, T. J., Trumbore, S. E., Ganbat, G., Reichelt, M., Unsicker, S., Boeckler, A., et al. (2014). An optimal defense strategy for phenolic glycoside production in Populus trichocarpa- isotope labeling demonstrates secondary metabolite production in growing leaves. New Phytol. 203, 607–619. doi: 10.1111/nph.12811
Mouls, L., Mazauric, J. P., Sommerer, N., Fulcrand, H., and Mazerolles, G. (2011). Comprehensive study of condensed tannins by ESI mass spectrometry: average degree of polymerisation and polymer distribution determination from mass spectra. Anal. Bioanal. Chem. 400, 613–623. doi: 10.1007/s00216-011-4751-7
Norby, R. J., and Luo, Y. (2004). Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytol. 162, 281–293. doi: 10.1111/j.1469-8137.2004.01047.x
Padayachee, A., Netzel, G., Netzel, M., Day, L., Zabaras, D., Mikkelsen, D., et al. (2012a). Binding of polyphenols to plant cell wall analogues - Part 1: anthocyanins. Food Chem. 134, 155–161. doi: 10.1016/j.foodchem.2012.02.082
Padayachee, A., Netzel, G., Netzel, M., Day, L., Zabaras, D., Mikkelsen, D., et al. (2012b). Binding of polyphenols to plant cell wall analogues - Part 2: phenolic acids. Food Chem. 135, 2287–2292. doi: 10.1016/j.foodchem.2012.07.004
Peñuelas, J., Sardans, J., Estiarte, M., Ogaya, R., Carnicer, J., Coll, M., et al. (2013). Evidence of current impact of climate change on life: a walk from genes to the biosphere. Glob. Change Biol. 19, 2303–2338. doi: 10.1111/gcb.12143
Porter, L. J., Hrstich, L. N., and Chan, B. G. (1986). The conversion of procyanidins and prodelphinidins to cyanidin and dephinidin. Phytochemistry 25, 223–230.
Praba, M. L., Cairns, J. E., Babu, R. C., and Lafitte, H. R. (2009). Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J. Agron. Crop Sci. 195, 30–46. doi: 10.1111/j.1439-037X.2008.00341.x
Prasad, A. M., Iverson, L. R., Matthews, S., and Peters, M. (2007-ongoing). A Climate Change Atlas for 134 Forest Tree Species of the Eastern United States [database]. Delaware, OH: Northern Research Station, USDA Forest Service. Avialble online at: https://www.nrs.fs.fed.us/atlas/tree
Prior, S. A., Runion, G. B., Marble, S. C., Rogers, H. H., Gilliam, C. H., and Torbert, H. A. (2011). A review of elevated atmospheric CO2 effects on plant growth and water relations: Implications for horticulture. HortScience 46, 158–162.
Rennenberg, H., Loreto, F., Polle, A., Brilli, F., Fares, S., Beniwal, R. S., et al. (2006). Physiological responses of forest trees to heat and drought. Plant Biol. 8, 556–571. doi: 10.1055/s-2006-924084
Salminen, J., and Lempa, K. (2002). Effects of hydrolysable tannins on a herbivorous insect: fate of individual tannins in insect digestive tract. Chemoecology 12, 203–211. doi: 10.1007/PL00012670
Schofield, J. A., Hagerman, A. E., and Harold, A. (1998). Loss of tannins and other phenolics from willow leaf litter. J. Chem. Ecol. 24, 1409–1421. doi: 10.1023/A:1021287018787
Scioneaux, A. N., Schmidt, M., Moore, M., Lindroth, R. L., Wooley, S. C., and Hagerman, A. E. (2011). Qualitative variation in proanthocyanidin composition of Populus species and hybrids: genetics is the key. J. Chem. Ecol. 37, 57–70. doi: 10.1007/s10886-010-9887-y
Shure, D. J., Mooreside, P. D., and Ogle, S. M. (1998). Rainfall effects on plant-herbivore processes in an upland oak forest. Ecology 79, 604. doi: 10.2307/176957
Stafford, H. A., Smith, E. C., and Weider, R. M. (1989). The development of proanthocyanidins (condensed tannins) and other phenolics in bark of Pseudotsuga mensiesii. Can. J. Bot. 67, 1111–1118.
Suseela, V., and Dukes, J. S. (2013). The responses of soil and rhizosphere respiration to simulated climatic changes vary by season. Ecology 94, 403–413. doi: 10.1890/12-0150.1
Suseela, V., Tharayil, N., Xing, B., and Dukes, J. S. (2013). Labile compounds in plant litter reduce the sensitivity of decomposition to warming and altered precipitation. New Phytol. 200, 122–133. doi: 10.1111/nph.12376
Suseela, V., Tharayil, N., Xing, B., and Dukes, J. S. (2015). Warming and drought differentially influence the production and resorption of elemental and metabolic nitrogen pools in Quercus rubra. Glob. Chang. Biol. 21, 4177–4195. doi: 10.1111/gcb.13033
Tharayil, N., Alpert, P., Bhowmik, P., and Gerard, P. (2013). Phenolic inputs by invasive species could impart seasonal variations in nitrogen pools in the introduced soils: a case study with Polygonum cuspidatum. Soil Biol. Biochem. 57, 858–867. doi: 10.1016/j.soilbio.2012.09.016
Tharayil, N., Suseela, V., Triebwasser, D. J., Preston, C. M., Gerard, P. D., and Dukes, J. S. (2011). Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: climatic stress-induced tannins are more reactive. New Phytol. 191, 132–145. doi: 10.1111/j.1469-8137.2011.03667.x
Top, S. M., and Filley, T. R. (2014). Effects of elevated CO2 on the extractable amino acids of leaf litter and fine roots. New Phytol. 202, 1257–1266. doi: 10.1111/nph.12762
Triebwasser, D. J., Tharayil, N., Preston, C. M., and Gerard, P. D. (2012). The susceptibility of soil enzymes to inhibition by leaf litter tannins is dependent on the tannin chemistry, enzyme class and vegetation history. New Phytol. 196, 1122–1132. doi: 10.1111/j.1469-8137.2012.04346.x
Tuomi, J. (1992). Toward integration of plant defense theories. Trends Ecol. Evol. 7, 365–367. doi: 10.1016/0169-5347(92)90005-V
Watrelot, A. A., Le Bourvellec, C., Imberty, A., and Renard, C. M. G. C. (2013). Interactions between pectic compounds and procyanidins are influenced by methylation degree and chain length. Biomacromolecules 14, 709–718. doi: 10.1021/bm301796y
Wright, S. J., Kitajima, K., Kraft, N. J. B., Reich, P. B., Ian, J., Bunker, D. E., et al. (2010). Functional traits and the growth — mortality trade-off in tropical trees. Ecology 91, 3664–3674. doi: 10.1890/09-2335.1
Xie, D.-Y., and Dixon, R. (2005). Proanthocyanidin biosynthesis–still more questions than answers? Phytochemistry 66, 2127–2144. doi: 10.1016/j.phytochem.2005.01.008
Xu, Z., Jiang, Y., and Zhou, G. (2015). Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 6:701. doi: 10.3389/fpls.2015.00701
Xu, Z., Shimizu, H., Ito, S., Yagasaki, Y., Zou, C., Zhou, G., et al. (2014). Effects of elevated CO2 warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239, 421–435. doi: 10.1007/s00425-013-1987-9
Xu, Z. Z., and Zhou, G. S. (2006). Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224, 1080–1090. doi: 10.1007/s00425-006-0281-5
Keywords: hydrolysable tannins, condensed tannins, drought, warming, Proanthocyanidins, Quercus rubra
Citation: Top SM, Preston CM, Dukes JS and Tharayil N (2017) Climate Influences the Content and Chemical Composition of Foliar Tannins in Green and Senesced Tissues of Quercus rubra. Front. Plant Sci. 8:423. doi: 10.3389/fpls.2017.00423
Received: 03 December 2016; Accepted: 13 March 2017;
Published: 16 May 2017.
Edited by:
Raquel Esteban, University of the Basque Country, SpainReviewed by:
Zhenzhu Xu, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany (CAS), ChinaGerald Moser, University of Giessen, Germany
Copyright © 2017 Top, Preston, Dukes and Tharayil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara M. Top, saramarietop@gmail.com
Nishanth Tharayil, ntharay@clemson.edu
 Sara M. Top
Sara M. Top Caroline M. Preston
Caroline M. Preston Jeffrey S. Dukes
Jeffrey S. Dukes Nishanth Tharayil
Nishanth Tharayil