- 1College of Crop Science, Fujian Agriculture and Forestry University, Fuzhou, China
- 2Department of Botany, Government College University, Faisalabad, Pakistan
- 3State Key Laboratory of Grassland Agro-Ecosystems, School of Life Science, Lanzhou University, Lanzhou, China
- 4Department of Botany, Government College Women University Sialkot, Sialkot, Pakistan
- 5Department of Botany, University of Agriculture, Faisalabad, Pakistan
- 6National Education Minister, Key Laboratory of Plant Genetic Improvement and Comprehensive Utilization, Fujian Agriculture and Forestry University, Fuzhou, China
Developing new ornamental cultivars with improved floral attributes is a major goal in floriculture. Biotechnological approach together with classical breeding methods has been used to modify floral color, appearance as well as for increasing disease resistance. Transgenic strategies possess immense potential to produce novel flower phenotypes that are not found in nature. Adoption of Genetic engineering has supported the idea of floral trait modification. Ornamental plant attributes like floral color, fragrance, disease resistance, and vase life can be improved by means of genetic manipulation. Therefore, we witness transgenic plant varieties of high aesthetic and commercial value. This review focuses on biotechnological advancements in manipulating key floral traits that contribute in development of diverse ornamental plant lines. Data clearly reveals that regulation of biosynthetic pathways related to characteristics like pigment production, flower morphology and fragrance is both possible and predictable. In spite of their great significance, small number of genetically engineered varieties of ornamental plants has been field tested. Today, novel flower colors production is regarded as chief commercial benefit obtained from transgenic plants. But certain other floral traits are much more important and have high commercial potential. Other than achievements such as novel architecture, modified flower color, etc., very few reports are available regarding successful transformation of other valuable horticultural characteristics. Our review also summarized biotechnological efforts related to enhancement of fragrance and induction of early flowering along with changes in floral anatomy and morphology.
Introduction
Unequivocally, horticultural industry has been revolutionized due to contribution by ornamental plants. Now a day, diverse ornamental plants are being widely used in home gardening, professional landscaping, and cut flowers as well (Dobres, 2011). Ornamental plant products are globally traded commodities. Due to rising needs, ornamental plant industry requires new plant varieties with elite traits such as improved anatomical attributes, floral color, pigments, stress tolerance, and disease resistance (Chandler and Sanchez, 2012; Azadi et al., 2016). Although we witness extensive employment of classical breeding strategies for developing new plant lines yet limitations and draw backs are also evident i.e., degree of heterozygosity (Shibata, 2008; Da Silva et al., 2011). Since inception of last decade, techniques like genetic engineering (GE), genome editing has been broadly adopted as more feasible methods to deal with intrinsic obstacles of classical techniques (Noman et al., 2016a). Global GM crop cultivation has touched its acme during the last few years (Noman et al., 2016b). Situation can be imagined from the in hand data that reveals GM crop cultivation reached 181.5 Mha in 2014 (Azadi et al., 2016).
Interest as well as contribution of private and government sector toward biotechnology and genetic engineering is increasing day by day. Over the years the main targets were food and feed along with improvements in herbicide and pesticide tolerance. Recently, scientists have also focused on improvement and enhancement of quality attributes for industry (Noman et al., 2016b, 2017; Parisi et al., 2016). The prime benefit in adopting GE is that gene from other species gene pool can be introduced in ornamental plants (Li and Pei, 2006; Chandler and Brugliera, 2011). So it is very possible to introduce genes for disease resistance and stress tolerance in ornamental plant species (Auer, 2008; Kamthan et al., 2016). Similarly, plant characteristics like floral architecture, color, fragrance, resistance to abiotic stress and post-harvest life can be addressed through GE.
This century is considered as the era of bio-economy lead by bioscience and biotechnology. This bio-economy is directly linked to sustainable developments in core areas of agriculture, environment and economy (Huang, 2011). Today, work is going on to produce GM flowers with broad color range and other attributes. Transgenic ornamental plants may become prospective benefit to growers and consumers due to their changed floral appearance, novel colors and improved fragrance (Chandler and Sanchez, 2012). Instead of their immense value, small number of GM ornamental plant varieties have been field tested and released. So far ornamental varieties released in market are mostly the color variant plant varieties e. g., rose (Tanaka and Brugliera, 2013). Therefore, in this review we have highlighted recent advancements in application of GE and biotechnology upon ornamental plants. We have tried to point out developments and requisite attention for far reaching benefits for sustainability of technology and society.
Why Floral Traits Modifications are of Special Concern?
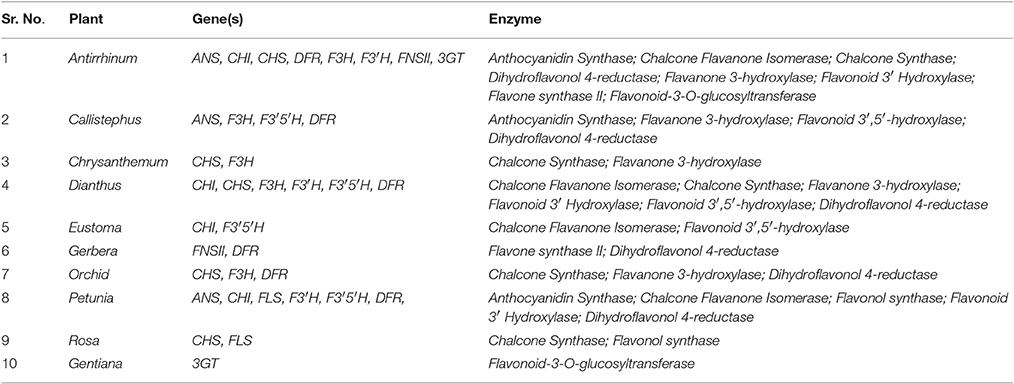
Ornamental plants generally possess extraordinarily beautiful and eye catching flowers. Other than aesthetic value, floral traits are crucial for plant survival. These characteristics like shape, shape, fragrance and color have their individual, and collaborative significance. Besides ornamental value, these flowers are utilized in pharmaceutical and other industries. For example, rose plants possess valuable secondary metabolites that are used in production of cosmetics, perfume etc. (Feng et al., 2010). Therefore, floral characteristics have unique importance for plant genetic engineers. Transgenic strategies possess prospective potential for producing innovative flower phenotypes that are not found in nature (Table 1). The genome based modifications for flowers have potential to yield utmost benefits in different aspects. Today, work is going on to produce GM flowers with multiple colors and rest of attributes. Normally, ornamental plants face problems because of troublesome sexual hybridization. This is mainly due to high heterozygosity, high chromosome number, inadequate gene pool, and elevated sterility (Van der Salm et al., 1998; Kim et al., 2006). For example, being alloploid chrysanthemum has chromosome number 36–75 rather than basic chromosome number 9. Anthurium sp. have life cycle of about 3 years. Therefore, developing new cultivar may require long time span of about 8–10 years (Azadi et al., 2016). Similarly, most of the carnation lines are self-fertile and unable to produce seeds (Nontaswatsri and Fukai, 2010). Huge genome size in ornamental plants e.g., lilly is a hurdle in mining genomic information (Du et al., 2015). Moreover, other than genome issues, presence or absence of certain metabolites leads to floral changes. One of the reported issues is precocious petal pigmentation and increased sepals pigmentation in Lisianthus sp. (Schwinn et al., 2014). Now we will evaluate role of biotechnology and GE for modifications in diverse floral characteristics.
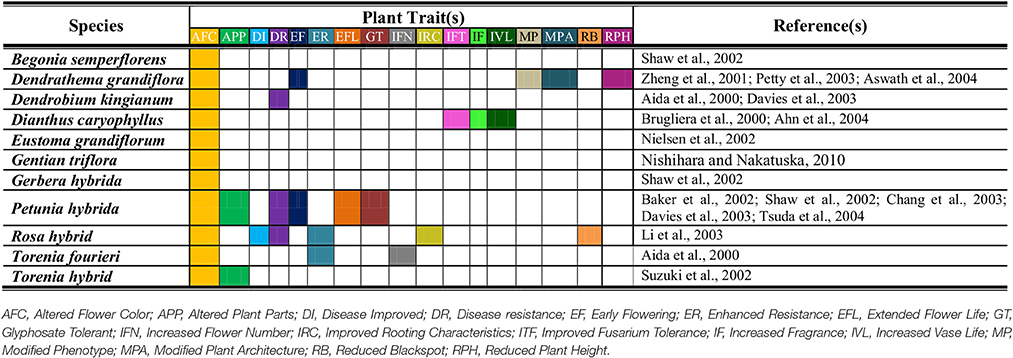
Table 1. Successful adoption of biotechnology for modification(s) in various attributes of ornamental plants.
Plant Pigments and Flower Color
Generally, traditional plant breeding strategies are applied to perk up attractiveness as well as effectiveness of ornamental plants. But these strategies face limitations in terms of genes pool and some other characteristics reported in sexually resembling species (Da Silva et al., 2011). During last 20 years, biotechnology has produced innovative and exclusive characters in ornamentals by adopting genes from different plant species (Li and Pei, 2006). Floriculturists and related entrepreneur are always eager to introduce innovations in flower colors. The major pigments responsible for attractiveness of flower colors are anthocyanins, flavonoids, carotenoids, and betalains. Several kinds of anthocyanins are on record (Veitch and Grayer, 2008). These pigments are primarily based upon six anthocyanidins types i.e., cyanidin, delphinidin, peonidin, petunidin, malvidin, and pelargonidin. Three of the described anthocyanidins i.e., delphinidin, cyanidin and pelargonidin are regarded as major types (Figure 1, Table 2). Blue flowers tend to have high level of delphinidin and derivatives while intense red flower color is due to pelargonidin working as anthocyanidin base (Tanaka et al., 2009).
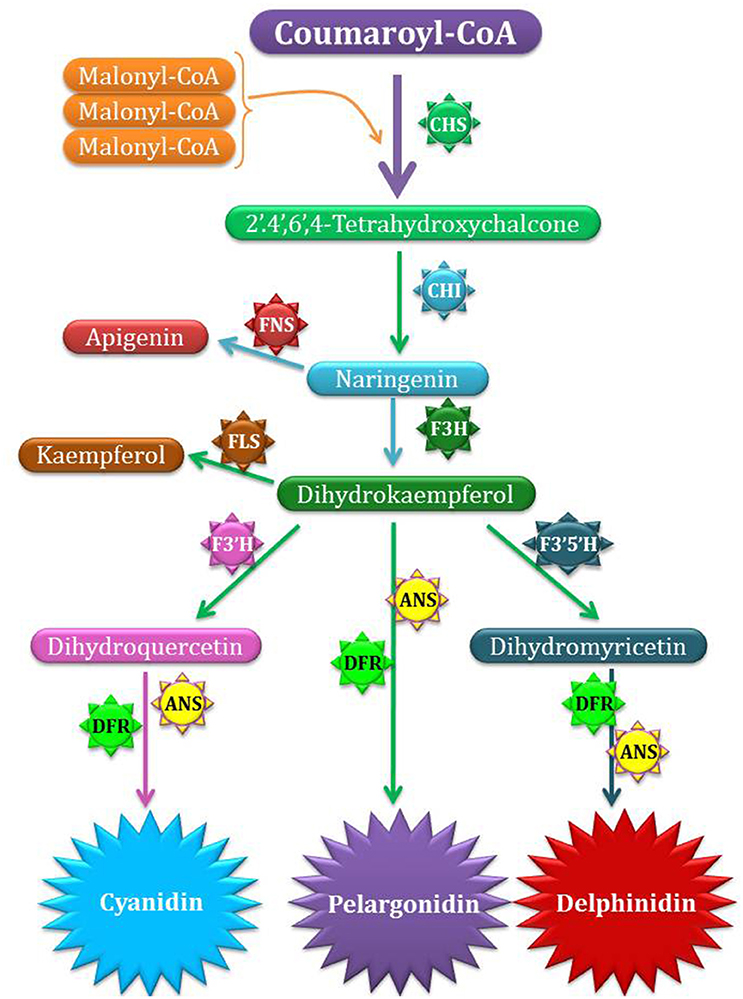
Figure 1. Biosynthesis of anthocyanidin. CHS catalyze the formation of Tetrahydroxy calchone. Later on, different enzymes such as CHI, F3H, DFR, ANS catalyze other steps of pigment production. The methyl groups are only added to anthocyanins not to anthocyanidins. The actual pigment color production is not solely dependent upon the enzyme catalyzing reactions but also depends upon other factors. CHS, chalcone synthase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase; MT, methyltransferase, GT, glucosyltransferase; AT, acyltransferase; FNS, flavone synthase; FLS, flavonol synthase.
Ornamentals like petunia and torenia are considered as more suitable plants for studying floral color modifications produced by genetic engineering. Changes in gene expression were sought out as a base line for production of altered floral colors. Initially, pelargonidin derived anthocyanin production was successfully achieved by maize Dfr expression in petunia deficient in F3′5′H and F3′H (Forkmann and Ruhnau, 1987). We are well aware that varied gene expression for different biosynthetic pathways is leading strategy to achieve flower color variations. For example, variations in genes for flavonoid biosynthesis may generate new colors. In 2007, Katsumoto et al. reported down regulation of Dfr gene in hybrid rose and over-expression of the same gene in Iris hollandica responsible for delphinidin accumulation in petals. Rose has been specifically emphasized for search of suitable host to achieve blue flower (Yoshida et al., 2009). It has been found that over-expression of voila gene F3′5′H resulted in high delphinidin accumulation and production of blue flower color (Ogata et al., 2005; Katsumoto et al., 2007). Strong role of anthocyanin related genes for rose petals color variation was suggested by Fukuchi-Mizutani et al. (2011). RhUF3GT2 catalyze flavonol 3-glucosylation in petals which results in accumulation of anthocyanidin 3-glucoside. Zvi et al. (2012) attributed higher production of anthocyanin to introduction of Arabidopsis PAP1 transcription factors in transgenic rose. They also confirmed enhanced isoprenoid production in transgenic plants as compared to control plants. Similarly, Chen et al. (2012) informed that white Chrysanthemum cultivars e.g., Keikai and Jinba possess major genes for anthocyanins pathways i.e., Chs, Chi, and F3′H.
We can analyze consequences of cross and mutation breeding in ornamental plants. Analysis reveals ultimate consequence as an array of flower colors such as orange, yellow, red, white, and pink. These color changes are directly related to regulation of targeted genes controlling synthesis of pigment precursors. White flowers from different transgenic plants have been obtained by down regulation of genes for anthocyanin production (Tanaka and Ohmiya, 2008). For finding the most suitable promoter for chrysanthemum gene expression, EF1α promoter (elongation factor 1α protein) was combined with GUS gene for introduction into C. morifolium cv. Ramat. Transgenic chrysanthemum plants exhibited high GUS expression and petal based transgene expression driven by the 35S CaMV promoter (Aida et al., 2005).
Diverse colors in chrysanthemum are largely resultants of carotenoids and/or red malonylated cyanidin glucosides (Kishimoto et al., 2004). A gene CmCCD4a is exclusively expressed in the white chrysanthemum ray petals. This is single dominant gene responsible for inhibition of formation/accumulation of carotenoids in petals (Ohmiya et al., 2009; Yoshioka et al., 2012). So, we may infer from the findings that due to suppression of CmCCD4a in white flowers, synthesized carotenoids break down into colorless compound (Ohmiya et al., 2006). Suppression technology like RNAi, co-suppression or antisense mediated silencing have been noticed more helpful in studying flower color variations (Tanaka et al., 2010).
Absence of delphinidin-based anthocyanins in chrysanthemum is chiefly due to deficiency of flavonoid 3′,5-hydroxylase (F3′5′H; Brugliera et al., 2013). That's why we do not observe violet/blue chrysanthemum flowers. Introduction of F3′5′H genes under different promoters control remained successful. Increase in delphinidin accumulation was very prominent under rose chalcone synthase promoter. Success was achieved by observing bluish petals in transgenic plants with higher anthocyanidin content characterized by delphinidin (Yoshida et al., 2009). Silencing of endogenous F3′H gene with hairpin RNA interference and over-expression of F3′5′H led to production of bluish petal with ~80% delphinidin derived anthocyanins (Brugliera et al., 2013). Combination of various promoters and F3′5′H gene proved enhanced delphinidin-based anthocyanins in transgenic plants.
Anthocyanins, carotenoids or both pigments can be noticed in Lilly sepals (Yamagishi et al., 2012). Azadi et al. (2010) confirmed numerous pigments in the transgenic lilly calli and leaves after transferring some key genes for carotenoid biosynthesis pathway under 35S CaMV. Callus and leaves presented orange color. This is due to presence of keto-carotenoids. In the variety Lilium oriental =Sorbonne', transient petal transformation by particle bombardment was conducted using three constructs (Chs pro, HyDfr, petunia DifF using 35S CaMV) possessing Ph F3′5′H (Phalaenopsis F3′5′H gene). Over-expression of Ph F3′5′H resulted in color change from pink to pale purple. A dark purple color was generated by synchronized expression of Ph F3′5′H and HyDfr contrary to expression alone. This propose potential role of HyDfr gene in facilitating delphinidin production (Qi et al., 2013, 2014). The GMYB10 over-expression in transgenic gerbera plants considerably improved pigment accumulation and induce cyanidin biogenesis (Laitinen et al., 2008). Non-existence of delphinidin derived anthocyanins in gerbera has produced interest in blue flower production by using genetic engineering. Unfortunately we do not have successful reports for of blue gerbera production yet.
Plants express some genes in cyclical style during the day. This cyclical expression lets them to initiate photoassimilation during sunlight or release scents in the evening when pollinating agents are active. For example development of Petunia circadia will facilitate this internal circadian clock to control flower color leading to flower color changing over every 12 h approximately. Similarly, genome modification by means of zinc finger nucleases, CRISPR-Cas systems can help us to attain firmly changed phenotypes (Noman et al., 2016a). For redirecting pigment biosynthesis for achieving desirable color change, not only over-expression of a particular gene taking part in key enzyme synthesis is essential but a selection of appropriate host with suitable genetic background is also mandatory. This selection will be very helpful in reducing antagonism of indigenous pathways with the introduced enzyme or to permit down-regulation of another competing pathway.
Induction of Early Flowering
Together with synthetic biology, flowers with changing color characteristic have been engineered. Along with other related benefits, the color changing flower is a substantial and striking outcome of biotechnology (Figure 2). It is reflection of ability to outline our world with extensive appeal to all. Flowering time is reckoned as chief determinant for successful commercial plants (Jung and Müller, 2009). Induction of early flowering help plants to cater human needs by bearing more flowers and fruits ultimately. Hence, great economic benefit from ornamental plants can be obtained from establishment of practical floral regulation system. Fundamental studies on flowering time in model plants have described regulatory system of floral transition pathways (Blázquez, 2000). Genetic alterations by means of transforming key flowering associated genes appears to offer valuable advancements for operating floral attributes (Srikanth and Schmid, 2011).
Figure 2. Benefits from floral traits modification are substantial evidence of successful application of biotechnology. Advances in genome engineering provide innovation in ornamental plants and crops. These have the potential to circumvent the regulatory concerns raised about GMOs. The competent techniques such as ZFNs, TALENs, and CRISPR-Cas9 enable precise genome engineering by introducing DSBs and NHEJ. The techniques have ability to produce non-transgenic plants for next-generation plant breeding.
The reduction in flowering time by developing early flowering cultivars or plants able to produce flowers during long days are considerable breeding objectives in ornamental plant breeding (Shulga et al., 2011). The low cost of production make them highly feasible for growers and customers i.e., Chrysanthemum. Reports are in hand that comprehensively describe successful gene introduction to produce flowers in comparatively short time. One example is member of MADS box gene family e.g., AP1 involved in flowering (Litt and Irish, 2003). MADS box genes are indispensable for floral development because they control flowering time as well as floral organ development (Thiruvengadam and Yang, 2009). In transgenic Chrysanthemum, AP1 over-expression during short-days can start bud initiation 14 days prior to non-transgenic plants. Interestingly, the inserted gene did not exert any change in plant development under long days. The differential gene over-expression during short days is seemingly linked to the plant biochemical changes. Moreover, transgenic flowers displayed prior color configuration and complete inflorescence opening in comparison with non-transgenic plants (Shulga et al., 2011).
Transformation mediated by Agrobacterium in Siningia sp. supported that exogenous LFY over-expression promotes early flowering (Zheng et al., 2001). Transgenic Gloxinia plants with over-expressing or suppressed miRNA159, led to late or early flower appearance respectively. Meanwhile, varied miR159a expression levels resulted in SsGAMYB up or down-regulation during flower development. Thus, in transgenic lines transcript level of endogenous Lfy as well as MADS-box genes is changed. So it was established that miR159-mediated GAMYB expression also play crucial role in controlling flowering time period in ornamentals (Li et al., 2013). This will be helpful in establishing a practical regulation system that will facilitate flowering to be accelerated or delayed proportional to the demand. Fu et al. (2012) have demonstrated that rice miR156 over-expression amplify biomass and increment number of tillers in Panicum. Hence, it appears to be a competent strategy for boosting or curtailing target genes activity via transformation of conserved miRNA in different plant species.
In Chrysanthemum seticuspe, CsFTL3 gene (Flowering Locus T like paralog) has been found to work as photoperiodic flowering regulator. Over-expression of this gene can induce flowering under long day conditions in Chrysanthemum (Oda et al., 2012). RNA sequencing has been adopted to illustrate quantitative effects of bulb vernalization on flowering and facilitates gene expression in the meristems. Investigations involving lilly bulbs subjected to cold treatment revealed many feasible candidate genes involved in vernalization (Huang et al., 2014; Villacorta-Martin et al., 2015).
In Glebra, transformation of GSQUA2 gene accelerated flowering (Ruokolainen et al., 2010). In 2011, Ruokolainen et al. detected three paralogs of At-SOC1. Over-expressing Gh-SOC1 resulted in fractional loss of floral distinctiveness. This over-expression caused decrease in epidermal cell size in ray flower petals along with shape modification but flowering time remained uninfluenced. Conversely, Gh-SOC1 down regulation failed to produce any considerable phenotypic alteration in transgenic plants (Ruokolainen et al., 2011). In Lisianthus sp. transformation of OMADS1 showed significant reduction in flowering time as well as increased no. of flowers in comparison to non-transformed ones.
Of the reported genes dealing with flowering pathways, FT (Flowering locus T) is chief integrator of several flowering genes that respond to different signals such as light, temperature etc. (Fornara et al., 2010). FT supports flowering by up-regulating flowering genes such as AP1, Lfy, and SOC1. Over-expression of VcFT-Aurora in the transgenic blueberry supported early and continuous flowering in both in vitro shoots and greenhouse plants. Additionally, all transgenic VcFT-Aurora plants exhibited dwarf phenotype (Gao et al., 2016). The end result of VcFT-OX is differential expression of 110 pathway genes for five phytohormones. This connection between phytohormones pathway genes and VcFT implies a probable role of phytohormones as signals regulating plant growth and development.
Mostly, the knowledge regarding flowering time regulation is applied by means of gene over-expression or suppression of gene activity in crops (Jung and Müller, 2009). However, the research has not met full commercial execution. Due to multiple reasons, we are expecting to see wide range transgenic flowers in market. Less advancement in ornamentals can be observed in case of targeted genetic change for flowering time. The restricted access is seemingly because of relative unidentified genomic information in most of the commercial plant species. Hence, it is mandatory to search an appropriate approach for generating fresh varieties with changed flowering conduct in commercial plants. A substantial sum of try and error is certainly required to attain an agreeable final result. Analysis of in hand data pinpoints genetic modifications in ornamental plants as realistic and flourishing scheme both in scientific and commercial perspective.
Floral Anatomy and Morphology
Customers demand for purposeful and distinctive horticultural plants urge scientists for development of novel cultivars, their large scale production and circulation as well. Ornamental plants are cultivated with rationale of beautifying, embellishing, or improving human environments irrespective of indoor or outdoor. Novel floral figure in ornamental plants is essential for high market value. Up till now, molecular events for developing flower pattern in the meristem have remained clandestine. Primarily this is due to significant inflorescence variations among plant species. Majority of these plant types are not cross-compatible with each other. Therefore, we agree upon the decision that conventional cross-breeding strategy cannot be applicable to point out the genes responsible for flowering patterns.
The diverse gene behaviors and their differential expressions reflect diversity in plant responses. In the start of this century, tobacco phytochrome b1 gene over-expression in chrysanthemum led to production of small sized plants but with larger branch angles (Zheng et al., 2001). Later, decrease in chrysanthemum plant height was achieved by introducing Arabidopsis GA insensitive gene (Petty et al., 2003). Furthermore, Aida et al. (2008) demonstrated transfer of CAG gene in antisense orientation into C. morifolium. They revealed that CAG gene suppression alters gynoecium and androecium to corolla-like tissues. However, the alteration pace was very small and simply changed the phenotype for flower-shape. It has been reported that Ls-like antisense gene expression in transgenic Dendranthema grandiflorum result in drop of axillary branching (Han et al., 2007). PttKN1ectopic expression in D. caryophyllus L. resulted in pleiotropic morphological wavering inclusive of phyllotaxis modifications (Meng et al., 2009).
GRAS TF restrains lateral branch production. Transformation of DgLsL gene with vector pCAMBIA1301-sense and antisense DgLsL was attributed to abundant branches in chrysanthemum. Conversely, branching was lessened in antisense transformants (Jiang et al., 2010). Collective silencing of DmCPD and DmGA20ox genes is a feasible strategy to create dwarf chrysanthemum varieties (Xie et al., 2015). It has also been proved that D27 gene mutants (gene involved in strigolactone biosynthesis) reveal high tillering with dwarf phenotypic appearance (Lin et al., 2009). Cloned DgD27 from D. grandiflorum expression in Arabidopsis put forward a new approach for development of chrysanthemum cultivars with reduced number of tillers (Wen et al., 2016). Similarly, GSQUA2 over-expressing transgenic Gerbera plants presented vivid vegetative alterations like elongation of vegetative axis. However, these plants were found to be infection susceptible with very limited root formation and tiny inflorescence (Ruokolainen et al., 2010). Thiruvengadam and Yang (2009) demonstrated that transgenic plants with 35S::L MADS1-M gene from lily produced more flowers from leafy branches in comparison to wild types. Moreover, transgenic Lisianthus flowers displayed a change of floral structure. Transgenic plants were characterized with conversion of petal second whorl into sepal like structures and deformation of third whorl stamens. Of note, transgenic carnations harboring rol C gene with promoter 35S CaMV demonstrated higher stem cutting yield and flowering stems and root organogenesis (Zuker et al., 2001; Casanova et al., 2004). So we can attribute such gene expression as solid evidence of their function for endorsing changes in floral shape and structure Conclusively, molecular breeding methods allow critical assessment of biological processes for floral changes and help scientific community as well as public to put GE of ornamental plants as deep insight regarding its effectiveness in plant breeding.
Fragrance Engineering
Floral scents have vital function in plants reproductive process and possess substantial economic significance. It essentially improves aesthetic characteristics of ornamental plants. Many floral scent volatiles fall into the category of terpenoid, phenylpropanoid/benzenoid, and aromatic amino acid (Oliva et al., 2015). Flowers produce a great range of specific metabolites like fragrant volatiles to attract pollinators, hormones to stimulate or repress signaling cascades and fragrant volatiles for protection against herbivores or pathogens (Baldwin, 2010; Dudareva et al., 2013). The array of particular metabolites synthesized by flowers of different plants is wide-ranging (Muhlemann et al., 2012; Zvi et al., 2012). On the other hand, different flower specific metabolites are actually produced in low amounts. Thus, their detection and characterization is cumbersome task. Therefore, enhanced production of floral specific metabolites may contribute in detection, isolation, and identification of compounds and improvement of flower properties like fragrance and pigmentation. Although the floral scent biochemistry is relatively new field yet during last decade researchers identified several scent controlling genes. Not all but a number of scent genes encode enzymes that directly catalyze the formation of volatile compounds. The manipulation of scent genes through genetic engineering has revealed success in adoption of this technology for amplifying floral scent potential.
For induction of fragrance in petals, C. breweri BEAT gene (benzyl alcohol acetyl transferase for benzyl acetate production) was introduced in Lisianthus (Aranovich et al., 2007). Recorded observations affirmed volatile compounds production including benzyl acetate among transgenic leaves and flowers upon feeding with alcoholic substrate. From the results, we can infer that alcoholic substrates are mandatory for fragrance induction by GE in lisianthus cut flowers. The innovative transcriptomic profiling offer substantial source for plant functional genomics and provide us deep insight in biological processes for petals development in H. coronarium. These data helps to elucidate the molecular mechanism of floral scent genesis and its regulation in monocotyledons (Yue et al., 2015).
Production of specialized metabolites for scent biosynthesis is not solely dependent upon enzymatic actions and greatly relies upon several TFs. In recent years, transcriptional regulation of fragrance biosynthesis pathways has been deeply studied (Muhlemann et al., 2012) that implies crucial roles of different transcriptional factors in controlling scent emission (Colquhoun and Clark, 2011). In spite of immense value, few TFs involved in fragrance emission regulation have been identified. From petunia petals, exclusively expressed ODORNT1 (ODO1) has been found to regulate shikimate pathway (Verdonk et al., 2005). ODO1 was also reportedly involved in promoter activation of plasma membrane based ABC transporter of unknown function (Van Moerkercke et al., 2012). Petunia EOBI (emission of benezoids 1) is R2-R3 type flower specific transcription factor that acts upstream of ODO1 and downstream of EOBII. Silencing of this EOBI expression caused down regulation of several shikimate pathway and fragrance related genes (Spitzer-Rimon et al., 2012).
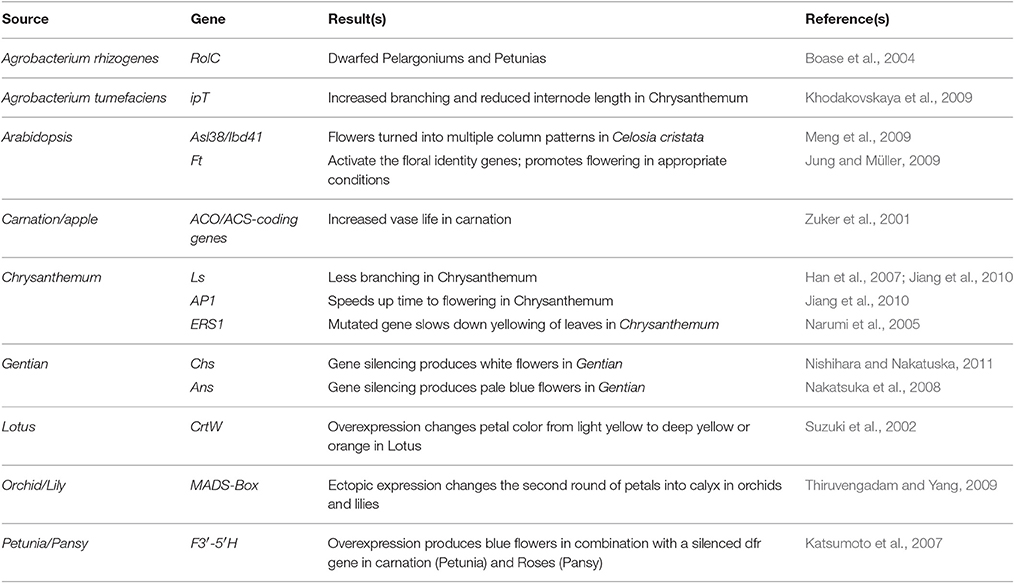
TFs regulating phenyl propanoid/benezoid pathways have been detected and characterized but terpenoids pathway transcriptional regulation is still obscure. Few years back, in Arabidopsis inflorescence, MYC2 helix loop helix TF was detected for expression of two TPS11 and TPS21sesquiterpene synthase genes (Hong et al., 2012). Other than identification and characterization of different TFs, master regulators which control diverse volatile compound production and different associated metabolic pathways are yet to be discovered. Recent progress in identifying the genes and their product enzymes taking part in volatile compounds biosynthesis have declared this metabolic engineering extremely practicable. Noteworthy successes are on record in improving plant defense and enhancing scent and aroma value of flowers and fruits (Table 3). The result of discussed research indicates that the GE for changing flower scents has significant potential. But, research outcomes also expose the drawbacks resulting from our insufficient knowledge of the scents metabolic processes and their regulation.
Abiotic and Biotic Stress Tolerance
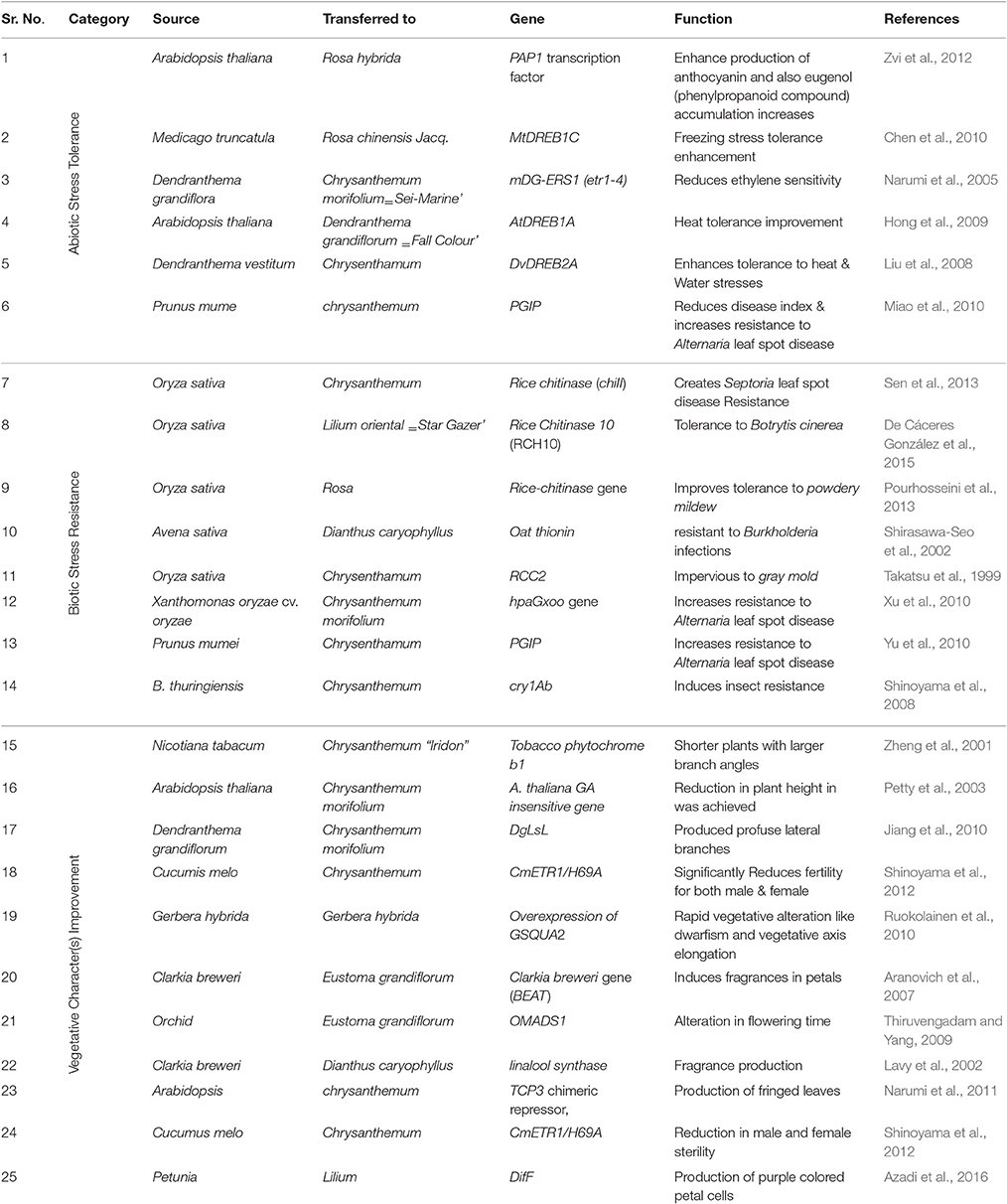
Plant growth and productivity are severely affected by abiotic and biotic stresses (Ali et al., 2016; Islam et al., 2016; Noman and Aqeel, 2017). Due to inadequate number of resistance genes, breeding for stress resistance/tolerance in ornamental plants is difficult (Azadi et al., 2016). During last few years, use of biotechnological strategies for conferring resistance against abiotic and biotic stresses e.g., drought, pathogen attack have gained attention (Table 4). The critical appraisal of transgenic ornamental plants indicates resistance to biotic stresses in comparison to the wild plants (Chandler and Sanchez, 2012; Teixeira da Silva et al., 2013). Fungal, viral, or bacterial pathogens sternly affect plants by decreasing plant growth and yields (Islam et al., 2016, 2017) inclusive of reduced quality of ornamental products. In 2002, Dohm et al., 2002 reported transgenic roses possessing antifungal genes i.e., class II chitinase and type I RIP (ribosome inhibiting protein). In rose, resistance to powdery mildew disease was improved by introduction of rice-chitinase gene (Pourhosseini et al., 2013). Three N-methyltransferase genes e.g., CaXMT1, CaMXMT1, and CaDXMT1 were introduced in D. grandiflorum cv. Shinba. The observations confirmed high resistance to Botrytis cinerea (Kim et al., 2011). In chrysanthemum, transfer of PGIP gene (polygalacturonase-inhibiting protein) from Prunus mumei reduced disease index and occurrence of Alternaria leaf spot (Miao et al., 2010). Similarly, chiII endorsed improved resistance to septoria leaf spot disease in transgenic chrysanthemum (Sen et al., 2013). De Cáceres González et al. (2015) have produced transgenic L. longifolium cv. Star Gazer with high resistance against B. cinerea. The ectopic over-expression of the RCH10 gene (Rice Chitinase 10) under 35S CaMV promoter was found positively correlated with high resistance to Botrytis. Recently, several SNP markers have been identified for additional linkage mapping along with other transcripts that might have involvement in Botrytis resistance pathways (Fu et al., 2016).
Environmental conditions play very important role in survival of plants. Presence of pathogens and their infection dissemination is directly proportional to prevailing environmental conditions. In 2008, Clarke et al. (Clarke et al., 2008) developed GM Euphorbia pulcherrima with enhanced resistance to virus. Different genes from various sources have been validated for their crucial role in plant life during stress conditions. Targets of these biotic or abiotic stress-related genes also function in various cellular responses and metabolic processes e.g., transcriptional regulation, cell proliferation which indicate the variety of gene functions to tackle abiotic stress. For example, in transgenic chrysanthemum, over-expressing hpaGxoo gene increased resistance to Alternaria tenuissima (Xu et al., 2011). Agrobacterium mediated transformation of coat protein gene (cp) inducted resistance against CMV (Cucumber mosaic virus). Integration of cp gene in C. morifolium genome enhanced acclimatization percentage of transgenic plants displaying no or least CMV symptoms (Kumar et al., 2012). Transgenic Grebra plants harboring Nucleoprotein gene (N-gene) did not display TSWV (Tomato spotted wilt virus) symptoms (Korbin, 2006). Similar to this, transformation of glagiolus plants with sense or antisense orientation CP gene survived well under BYMV attack (bean yellow mosaic virus; Kamo et al., 2005).
Review of literature affirmed that expression of cry1Ab gene in chrysanthemum is strongly related to insect resistance i.e., Spodoptera litura (Shinoyama et al., 2012). Agrobacterium mediated transformation of LLA gene (Lycoris Longituba agglutinin) incremented resistance to aphids in transgenic chrysanthemum plants (He et al., 2009). Stable expression of different genes in ornamental plants like lilies has shown promising results in outdoor or green house conditions. Genes such as bar, nptII has been reported highly expressed in transgenic Lily plants under varying environmental conditions (Kamo, 2014). Transformation of OcIΔD86 into Lily cv. Nellie White exhibited comparatively high resistant to an endoparasite, Pratylenchus penetrans causing RLN (root lesion disease). OcIΔD86 expression inhibited parasitic effects of Pratylenchus by 75% in transgenic plants as compared to their wild relatives (Vieira et al., 2015; Table 4).
Saline soil, nutrient acidity, nutrients imbalance, water scarcity are major constraints that severely cut off plant yield, and other attributes (Noman et al., 2015; Zafar et al., 2016). Different strategies such as conventional plant breeding and genetic engineering are in use for developing abiotic stress tolerant varieties (Noman et al., 2016b). Several transcription factors e.g., ZIP, WRKY, NAC, and their products are crucial in plant response to stress conditions like drought, high temperature etc. (Vinocur and Altman, 2005). Ornamental plants also exhibit different responses upon exposure to abiotic stresses. For example, Rosa sp. is very susceptible to cold stress. R. chinensis Jacq. was transformed with Medicago gene MtDREB1C. Transgenic plants performed well under freezing temperature and hence proved improvement in tolerance to low temperature (Chen et al., 2010). It was proposed that frost tolerance in petunia may be enhanced by transforming AtCBF3 gene (Warner, 2011). Hong et al. (2006a,b) developed low temperature resistant chrysanthemum lines by transformation of AtDREB1A gene. They noticed great increase in transgenic seedling and plant growth in winter. Two constructs i.e., 35S:DREB1A and rd29A:DREB1A were integrated into chrysanthemum genome for enhancing tolerance to salinity and water deficiency. The rd29A:DREB1A transgenic plants showed more tolerance in comparison with 35S:DREB1A transgenic plants (Hong et al., 2006a). Furthermore, AtDREB1A over-expression in D. grandiflorum produced sturdy heat tolerance. Upon exposure to 45°C for 36 h, wild and transgenic plants exhibited a marked difference in survival rate e.g., 20 and 70%, respectively (Hong et al., 2009). Salt stress tolerance of chrysanthemum cv. Jinba was enhanced with CcSOS1constitutive expression encoding plasma Na+/H+ antiporter (An et al., 2014; Song et al., 2014). Ascorbic acid (AsA) has very important role in abiotic stress tolerance (Noman et al., 2015). GLDH (L-galactono-1, 4-lactone dehydrogenase) catalyzes the oxidation of L-galactose to AsA (Agius et al., 2003). pCAMBIA vector harboring GLDH from apple was used for transformation of L. davidii var. unicolor. The results highlighted that GLDH over expression in transgenic plants, considerably amplified AsA production and endorsed resistance against abiotic stresses (Shi et al., 2012). In the light of above quoted examples, we are convinced that genetic engineering offers unprecedented arena for plant trait improvement. Particularly in ornamental plants, attributes like environmental stress tolerance, disease resistance can be retained in transgenic cultivars coupled with improved product range.
Shelf Life Enhancement
One of the daunting challenges for researchers is to increase shelf life of ornamental plant products coupled with maintained characteristics such as aroma, taste, etc. (Kamthan et al., 2016). Transgenic ornamental plants have potential to enhance leaf and flower longevity. Generally, cut flowers are treated with different kinds of chemicals for increasing their shelf life (Teixeira da Silva et al., 2013). With special reference to cut flowers, improved shelf life is decisive attribute and is of particular significance for breeders. To achieve the target of enhanced vase life, different biotechnological techniques have been used (Matas et al., 2009). Decreased autocatalytic ethylene synthesis by suppression of genes for ethylene production pathway such as ACO (ACC oxidase) or ACS (1-aminocyclopropane-1-carboxylicacid synthase) has been found effective to improve fruit vase life (Hamilton and Baulcombe, 1999). Transgenic carnation plants exhibiting delayed petal senescence have been developed by silencing the ACO gene that down regulate ethylene synthesis (Savin et al., 1995). Bovy et al. (1999) developed transgenic carnations harboring Arabidopsis etr1-1 gene. Vase life of transgenic flowers was incremented 3 times in comparison with control plant.
Increased shelf life can be achieved by maintaining resistance to ethylene or by the inhibition of ethylene biosynthesis genes. Success has been witnessed in Oncidium and Odontoglossum by mutating ethylene receptor gene (Raffeiner et al., 2009). Different research groups have conducted experiments involving ACS or ACO genes in antisense fashion for increasing the shelf life of ornamental plant products (Bapat et al., 2010; Litz and Padilla, 2012). Transcriptional regulators such as ERFs (Ethylene response factors) modulate ethylene-induced fruit ripening. In transgenic Lycopersicum esculentum, antisense LeERF1 fruits exhibited more vase-life contrary to the wild-type plants (Li et al., 2007). Vase life of transgenic pot plants has been maintained by developing ethylene sensitive pot plant species with lessened ethylene sensitivity (Sanikhani et al., 2008; Milbus et al., 2009). Agrobacterium mediated transformation of PSAG12-ipt in Rose cultivar “Linda” augmented the ipt expression level in transgenic plants upon exposure to darkness and high level of exogenous ethylene. Moreover, chlorophyll amount was considerably increased (Zakizadeh et al., 2013).
Normally, leaves of cut flowers show yellowness, even before the start of senescence. On the other hand, exposure to ethylene can also speeds up yellowing. This yellowing of leaves is a feature of senescence. This destroys flower attractiveness, diminishes quality and shortens vase life. Consequently, transgenic plants with reduced ethylene sensitivity i.e., chrysanthemum is anticipated to possess improved vase life (Satoh et al., 2006). In transgenic D. grandiflorum, reduced leaf senescence has been proved very useful (Satoh et al., 2008). Similarly, transformation of etr1-1 gene impeded senescence in petunia. But unfortunately this senescence reduction was coupled with curtailed rooting of cuttings that is unacceptable in market (Gubrium et al., 2000). This deficiency highlights need for a more tissue specific expression gene of genes regulating some crucial processes (Chandler and Sanchez, 2012). Additionally, the endogenous production and distribution of plant growth regulators such as IAA, iPA, and ABA during the course of flower induction and initiation may facilitate us to better plan the harvest timing for maximum flower uniformity and the quality of ornamentals (Jiang et al., 2010; Teixeira da Silva et al., 2013).
Concluding Remarks
Worldwide increase in economic worth of ornamental plants is somehow a result of promising prospects of gene transformation. Novel transgenic ornamental plants may therefore provide prospective benefits to growers and consumers by generating diverse floral appearance, novel colors, and improved fragrance. Taking aid from in hand data, we recommend that genetic modification of ornamental plants is very pragmatic and successful scheme both in scientific and commercial perspective. We should not be worried about acceptance of transgenic flowers in the market. Very luckily, consumers have shown their satisfaction over genetically modified flowers e.g., rose, carnation. Transgenic ornamental varieties have been proved successful during vegetative propagation and have not shown any undesired effect on environment or on the health of handler. Several exploitable traits of ornamental plants can be particularly associated with secondary metabolites production such as phytoceuticals. Development of molecular markers and complete genome sequences of ornamental plants will lead to discovery of new genes and related pathways. This will momentously contribute in production of new transgenic varieties of ornamental plants. Furthermore, application of novel techniques like ZFNs, TALENs, and CRISPR has substantial potential to facilitate floriculture industry by targeted genomic modifications. Despite sound scientific prospects of transgenic flowers, economic and regulatory barriers have hampered the expansion in commercialization of GM ornamentals. Time has come to address regulatory obstacles for commercial release of GM plants including flowers. In absence of internationally apt and approved process for regulation of GM products, release of ornamental products will remain very difficult due to expenditures and capability needed for commercial development. To alleviate such inconvenience the regulatory requisites for non-food plants like ornamentals ought to be decreased.
Author Contributions
AN has collected research data and compiled manuscript. MA has made all figures and tables. NK and TS have compiled table and corrected the references as well as DOI. JD and HS have critically read this manuscript and suggested for improvement.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agius, F., González-Lamonthe, R., Caballero, J. L., Muñoz-Blanco, J., Botella, M. A., and Valpuesta, V. (2003). Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid. Nat. Biotechnol. 21, 177–181. doi: 10.1038/nbt777
Ahn, B. J., Shin, H. Y., Hwang, K. H., Min, B. H., and Joung, H. Y. (2004). Transformation of carnations with jasmonate methyl transferase gene for fusarium tolerance. In Vitro Cell. Dev. Biol. 40:45-A.
Aida, R., Kishimoto, S., Tanaka, Y., and Shibata, M. (2000). Modification of flower color in torenia (Torenia fournieri Lind.) by genetic transformation. Plant Sci. 153, 33–42. doi: 10.1016/S0168-9452(99)00239-3
Aida, R., Komano, M., Saito, M., Nakase, K., and Murai, K. (2008). Chrysanthemum flower shape modification by suppression of chrysanthemum-AGAMOUS gene. Plant Biotechnol. 25, 55–59. doi: 10.5511/plantbiotechnology.25.55
Aida, R., Nagaya, S., Yoshida, K., Kishimoto, S., Shibata, M., and Ohmiya, A. (2005). Efficient transgene expression in Chrysanthemum morifolium Ramat with the promoter of a gene for tobacco elongation factor 1 α protein. JARQ 39, 269–274. doi: 10.6090/jarq.39.269
Ali, Q., Haider, M. Z., Iftikhar, W., Jamil, S., Javed, M. T., Noman, A., et al. (2016). Drought tolerance potential of Vigna mungo L. lines as deciphered by modulated growth, antioxidant defense, and nutrient acquisition patterns. Braz. J. Bot. 39, 801–812. doi: 10.1007/s40415-016-0282-y
An, J., Song, A., Guan, Z., Jiang, J., Chen, F., Lou, W., et al. (2014). The over-expression of Chrysanthemum crassum CcSOS1 improves the salinity tolerance of chrysanthemum. Mol. Biol. Rep. 41, 4155–4162. doi: 10.1007/s11033-014-3287-2
Aranovich, D., Lewinsohn, E., and Zaccai, M. (2007). Post-harvest enhancement of aroma in transgenic lisianthus (Eustoma grandiflorum) using the Clarkia breweri benzyl alcohol acetyl transferase (BEAT) gene. Postharvest Biol. Biotechnol. 43, 255–260. doi: 10.1016/j.postharvbio.2006.09.001
Aswath, C. R., Mo, S. Y., Kim, S. H., and Kim, D. H. (2004). IbMADS4 regulates the vegetative shoot development in transgenic chrysanthemum (Dendranthema grandiflora (Ramat.) Kitamura). Plant Sci. 166, 847–854. doi: 10.1016/j.plantsci.2003.11.030
Auer, C. (2008). Ecological risk assessment and regulation for genetically-modified ornamental plants. Crit. Rev. Plant Sci. 27, 255–271. doi: 10.1080/07352680802237162
Azadi, P., Bagheri, H., Nalousi, A. M., Nazari, F., and Chandler, S. F. (2016). Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol. Adv. 34, 1073–1090. doi: 10.1016/j.biotechadv.2016.06.00
Azadi, P., Otang, N. V., Chin, D. P., Nakamura, I., Fujisawa, M., Harada, H., et al. (2010). Metabolic engineering of Lilium × formolongiusing multiple genes of the carotenoid biosynthesis pathway. Plant Biotechnol. Rep. 4, 269–280. doi: 10.1007/s11816-010-0147-y
Baker, C., Zhang, H., Hall, G., Scrocki, D., Medina, A., and Dobres, M. S. (2002). The use of the GAI and CO genes to create novel ornamental plants. In Vitro Cell. Dev. Biol. 38:105-A.
Bapat, V. A., Trivedi, P. K., Ghosh, A., Sane, V. A., Ganapathi, T. R., and Nath, P. (2010). Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnol. Adv. 28, 94–107. doi: 10.1016/j.biotechadv.2009.10.002
Boase, M. R., Winefield, C. S., Lill, T. A., and Bendall, M. J. (2004). Transgenic regal pelargoniums that express the rolC gene from Agrobacterium rhizogenes exhibit a dwarf floral and vegetative phenotype. In Vitro Cell Dev. Biol. Plant 40, 46–50. doi: 10.1079/IVP2003476
Bovy, A. G., Angenent, G. C., Dons, H. J., and van Altvorst, A. C. (1999). Heterologous expression of the Arabidopsis etr1-1 allele inhibits the senescence of carnation flowers. Mol. Breed. 5, 301–308. doi: 10.1023/A:1009617804359
Brugliera, F., Kalc-Wright, G., Hyland, C., Webb, L., Herbert, S., Sheehan, B., et al. (2000). Improvement of Fusarium wilt tolerance in carnations expressing chitinase. Int. Plant Mol. Biol. Rep. 18, 522–529.
Brugliera, F., Tao, G. Q., Tems, U., Kalc, G., Mouradova, E., Price, K., et al. (2013). Violet/blue chrysanthemums metabolic engineering of the anthocyanin biosynthetic pathway results in novel petal colors. Plant Cell Physiol. 54, 1696–1710. doi: 10.1093/pcp/pct110
Casanova, E., Valdes, A. E., Zuker, A., Fernandez, B., Vainstein, A., Trillas, M. I., et al. (2004). rolC-transgenic carnation plants: adventitious organogenesis and levels of endogenous auxin and cytokinins. Plant 167, 551–560. doi: 10.1016/j.plantsci.2004.04.029
Chandler, S. F., and Brugliera, F. (2011). Genetic modification in floriculture. Biotechnol. Lett. 33, 207–214. doi: 10.1007/s10529-010-0424-4
Chandler, S. F., and Sanchez, C. (2012). Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotech. J. 10, 891–903. doi: 10.1111/j.1467-7652.2012.00693.x
Chang, H., Jones, M. L., Nanowetz, G. M., and Clark, D. G. (2003). Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delay corolla senescence and decrease sensitivity to ethylene. Plant Physiol. 132, 2174–2183. doi: 10.1104/pp.103.023945
Chen, J. R., Lu, J. J., Liu, R., Xiong, X. Y., Wang, T. X., Chen, S. Y., et al. (2010). DREB1C from Medicago truncatula enhances freezing tolerance in transgenic M. truncatula and China Rose (Rosa chinensis Jacq.). Plant Growth Regul. 60, 199–211. doi: 10.1007/s10725-009-9434-4
Chen, S. M., Li, C. H., Zhu, X. R., Deng, Y. M., Sun, W., Wang, L. S., et al. (2012). The identification of flavonoids and the expression of genes of anthocyanin biosynthesis in the chrysanthemum flowers. Biol. Plant. 56, 458–464. doi: 10.1007/s10535-012-0069-3
Clarke, J. L., Spetz, C., Haugslien, S., Xing, S., Dees, M. W., Moe, R., et al. (2008). Agrobacterium tumefaciens-mediated transformation of poinsettia, Euphorbia pulcherrima, with virus-derived hairpin RNA constructs confers resistance to Poinsettia mosaic virus. Plant Cell Rep. 27, 1027–1038. doi: 10.1007/s00299-008-0526-9
Colquhoun, T. A., and Clark, D. G. (2011). Unraveling the regulation of floral fragrance biosynthesis. Plant Signal Behav. 6, 378–381. doi: 10.4161/psb.6.3.14339
Da Silva, J. A. T., Chin, D. P., Van, P. T., and Mii, M. (2011). Transgenic orchids. Sci. Hort. 130, 673–680. doi: 10.1016/j.scienta.2011.08.025
Davies, K. M., Schwinn, K. E., Deroles, S. C., Manson, D. G., Lewis, D. H., Bloor, S. J., et al. (2003). Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 131, 259–268. doi: 10.1023/A:1024018729349
De Cáceres González, F. F. N., Davey, M. R., Sanchez, E. C., and Wilson, Z. A. (2015). Conferred resistance to Botrytis cinerea in Lilium by overexpression of the RCH10 chitinase gene. Plant Cell Rep. 34, 1201–1209. doi: 10.1007/s00299-015-1778-9
Dobres, M. S. (2011). “Prospects for commercialisation of transgenic ornamentals,” in Transgenic Horticultural Crops Challenges and Opportunities, eds B. Mou and R. Scorza (Boca Raton, FL: CRC Press), 305–316.
Dohm, A., Ludwig, C., Schilling, D., and Debener, T. (2002). Transformation of roses with genes for antifungal proteins to reduce their susceptibility to fungal diseases. Acta Hortic. 572, 105–111. doi: 10.17660/actahortic.2002.572.12
Du, F., Wu, Y., Zhang, L., Li, X. W., Zhao, X. Y., Wang, W. H., et al. (2015). De novo assembled transcriptome analysis and SSR marker development of a mixture of six tissues from Lilium Oriental hybrid-Sorbonne. Plant Mol. Biol. Rep. 33, 281–293. doi: 10.1007/s11105-014-0746-9
Dudareva, N., Klempien, A., Muhlemann, J. K., and Kaplan, I. (2013). Biosynthesis, function and metabolic engineering of plant volatile organic compounds. N. Phytol. 198, 16–32. doi: 10.1111/nph.12145
Feng, L. G., Chen, C., Sheng, L. X., Liu, P., Tao, J., Su, J. L., et al. (2010). Comparative analysis of headspace volatiles of Chinese Rosa rugosa. Molecules 15, 8390–8399. doi: 10.3390/molecules15118390
Forkmann, G., and Ruhnau, B. (1987). Distinct substrate specificity of dihydroflavonol 4-reductase from flowers of Petunia Hybrida. Z. Naturforsch 42, 1146–1148. doi: 10.1515/znc-1987-9-1026
Fornara, F., de Montaigu, A., and Coupland, G. (2010). SnapShot: control of flowering in Arabidopsis. Cell 141, 550–550. doi: 10.1016/j.cell.2010.04.024
Fu, C., Sunkar, R., and Zhou, C. (2012). Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotechnol. J. 10, 443–452. doi: 10.1111/j.1467-7652.2011.00677.x
Fu, Y., Esselink, G. D., Visser, R. G., Van Tuyl, J. M., and Arens, P. (2016). Transcriptome analysis of Gerbera hybrida including in silico confirmation of defense genes found. Front. Plant Sci. 7:247. doi: 10.3389/fpls.2016.00247
Fukuchi-Mizutani, M., Akagi, M., Ishiguro, K., Katsumoto, Y., Fukui, Y., Togami, J., et al. (2011). Biochemical and molecular characterization of anthocyanidin/flavonol 3-glucosylation pathways in Rosa × hybrida. Plant Biotechnol. 28, 239–244. doi: 10.5511/plantbiotechnology.10.1220a
Gao, X., Walworth, A. E., Mackie, C., and Song, G. (2016). Overexpression of blueberry FLOWERING LOCUS T is associated with changes in the expression of phytohormone-related genes in blueberry plants. Horti. Res. 3, 1605–1615. doi: 10.1038/hortres.2016.53
Gubrium, E. K., Clevenger, D. J., Clark, D. G., Barrett, J. E., and Nell, T. A. (2000). Reproduction and horticultural performance of transgenic ethylene in sensitive petunias. J. Am. Soc. Hort Sci. 125, 277–281.
Hamilton, A. J., and Baulcombe, D. C. (1999). A species of small antisense RNA in post transcriptional gene silencing in plants. Science 286, 950–952. doi: 10.1126/science.286.5441.950
Han, B. H., Suh, E. J., Lee, S. Y., Shin, H. K., and Lim, Y. P. (2007). Selection of non-branching lines induced by introducing Ls-like cDNA into Chrysanthemum (Dendranthema × grandiflorum (Ramat.) Kitamura) Shuho-no-chikara. Sci. Hortic. 115, 70–75. doi: 10.1016/j.scienta.2007.07.012
He, J. P., Chen, F. D., Chen, S. M., Fang, W. M., Miao, H. B., and Luo, H. L. (2009). Transformation of Lycoris longituba agglutinin gene to cut chrysanthemum and identification of aphid resistance in the transgenic plants. Acta Bot. Boreal. Occident. Sin. 29, 2318–2325.
Hong, B., Ma, C., Yang, Y., Wang, T., Yamaguchi-Shinozaki, K., and Gao, J. (2009). Over-expression of AtDREB1A in chrysanthemum enhances tolerance to heat stress. Plant Mol. Biol. 70, 231–240. doi: 10.1007/s11103-009-9468-z
Hong, B., Tong, Z., Li, Q. H., Ma, C., Kasuga, M., Yamaguchi-Shinozaki, K., and Gao, J. (2006b). Regeneration and transformation through somatic embryogenesis, and determination of cold stress tolerance in ground cover Chrysanthemum cv Fall color. Sci. Agric. Sin. 39, 1443–1450.
Hong, B., Tong, Z., Ma, C., Kasuga, M., Yamaguchi-Shinozaki, K., and Gao, J. (2006a). Heterologous expression of the AtDREB1A gene in transgenic chrysanthemum enhances tolerance to low temperature. PLoS ONE 49:436. doi: 10.1007/s11427-006-2014-1
Hong, G. J., Xue, X. Y., Mao, Y. B., Wang, L. J., and Chen, X. Y. (2012). Arabidopsis MYC2 850 interacts with DELLA proteins in regulating sesquiterpene synthase gene 851 expression. Plant Cell 24, 2635–2648. doi: 10.1105/tpc.112.098749
Huang, H. (2011). Plant diversity and conservation in China: planning a strategic bioresource for a sustainable future. Botanical. J. Linnean Soc. 166, 282–300. doi: 10.1111/j.1095-8339.2011.01157.x
Huang, J., Liu, X., Wang, J., and Lü, Y. (2014). Transcriptomic analysis of Asiatic lily in the process of vernalization via RNA-seq. Mol. Biol. Rep. 41, 3839–3852. doi: 10.1007/s11033-014-3250-2
Islam, W., Awais, M., Noman, A., and Jian, W. U. (2016). Success of bio Products against bacterial leaf blight disease of rice caused by Xanthomonas oryzae cv. Oryzae PSm Microbio. 1, 50–55.
Islam, W., Zhang, J., Adnan, M., Noman, A., Zainab, M., and Jian, W. (2017). Plant virus ecology: a glimpse of recent accomplishments. Appl. Econ. Environ. Res. 15, 691–705. doi: 10.15666/aeer/1501_691705
Jiang, B., Miao, H., Chen, S., Zhang, S., Chen, F., and Fang, W. (2010). The lateral suppressor-like gene,DgLsL, alternated the axillary branching in transgenic chrysanthemum (Chrysanthemum × morifolium) by modulating IAA and GA content. Plant Mol. Biol. Rep. 28, 144–151. doi: 10.1007/s11105-009-0130-3
Jung, C., and Müller, A. E. (2009). Flowering time control and applications in plant breeding. Trends Plant Sci. 14, 563–573. doi: 10.1016/j.tplants.2009.07.005
Kamo, K. (2014). Long term, transgene expression in Lilium longiflorum cv Nellie white grown outdoors and in the greenhouse. Sci. Hortic. 167, 158–163. doi: 10.1016/j.scienta.2013.12.011
Kamo, K., Gera, A., Cohen, J., Hammond, J., Blowers, A., Smith, F., et al. (2005). Transgenic gladiolus plants transformed with the bean yellow mosaic virus coat-protein gene in either sense or antisense orientation. Plant Cell Rep. 23, 654–663. doi: 10.1007/s00299-004-0888-6
Kamthan, A., Chaudari, A., Kamthan, M., and Datta, A. (2016). Genetically modified (GM) crops: milestones and new advances in crop improvement. Theo. Appl. Genet. 29, 1639–1655. doi: 10.1007/s00122-016-2747-6
Katsumoto, Y., Fukuchi-Mizutani, M., Fukui, Y., Brugliera, F., Holton, T. A., Karan, M., et al. (2007). Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 48, 1589–1600. doi: 10.1093/pcp/pcm131
Khodakovskaya, M., Vanková, R., Malbeck, J., Li, A., Li, Y., and McAvoy, R. (2009). Enhancement of flowering and branching phenotype in chrysanthemum by expression of ipt under the control of a 0.821kb fragment of the LEACO1 gene promoter. Plant Cell Rep. 28, 1351–1362. doi: 10.1007/s00299-009-0735-x
Kim, J. B., Raemakers, C. J. J. M., Jacobsen, E., and Visser, R. G. F. (2006). Efficient somatic embryogenesis in Alstroemeria. Plant Cell Tissue Organ Cult. 86, 233–238. doi: 10.1007/s11240-006-9110-6
Kim, Y. S., Lim, S., Yoda, H., Choi, Y. E., and Sano, H. (2011). Simultaneous activation of salicylate production and fungal resistance in transgenic chrysanthemum producing caffeine. Plant Signal. Behav. 6, 409–412. doi: 10.4161/psb.6.3.14353
Kishimoto, S., Maoka, T., Nakayama, M., and Ohmiya, A. (2004). Carotenoid composition in petals of chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura). Phytochemistry 65, 2781–2787. doi: 10.1016/j.phytochem.2004.08.038
Korbin, M. (2006). Assessment of gerbera plants genetically modified with TSWV nucleocapsid gene. J. Fruit Ornam. Plant Res. 14, 85–93.
Kumar, S., Raj, S. K., Sharma, A. K., and Varma, H. N. (2012). Genetic transformation and development of cucumber mosaic virus resistant transgenic plants of Chrysanthemum morifolium cv Kundan'. Sci. Hortic. 134, 40–45. doi: 10.1016/j.scienta.2011.10.019
Laitinen, R. A., Ainasoja, M., Broholm, S. K., Teeri, T. H., and Elomaa, P. (2008). Identification of target genes for a MYB-type anthocyanin regulator in Gerbera hybrida. J. Exp. Bot. 59, 3691–3703. doi: 10.1093/jxb/ern216
Lavy, M., Zuker, A., Lewinsohn, E., Larkov, O., Ravid, U., Vainstein, A., et al. (2002). Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool synthase gene. Mol. Breed. 9, 103–111. doi: 10.1023/A:1026755414773
Li, X., Bian, H., Song, D., Ma, S., Han, N., Wang, J., et al. (2013). Flowering time control in ornamental gloxinia (Sinningia speciosa) by manipulation of miR159 expression. Ann. Bot. 111, 791–799. doi: 10.1093/aob/mct034
Li, X., Gasic, K., Cammue, B., Broekaert, W., and Korban, S. S. (2003). Transgenic rose lines harboring an antimicrobial protein gene, Ace-AMP1, demonstrate enhanced resistance to powdery mildew (Sphaerotheca pannosa). Planta 218, 226–232. doi: 10.1007/s00425-003-1093-5
Li, Y., and Pei, Y. (2006). Plant Biotechnology in Ornamental Horticulture. New York, NY: Haworth Food and Agricultural Products Press.
Li, Y., Zhu, B., Xu, W., Zhu, H., Chen, A., Xie, Y., et al. (2007). LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Rep. 26, 1999–2008. doi: 10.1007/s00299-007-0394-8
Lin, H., Wang, R., Qian, Q., Yan, M., Meng, X., Fu, Z., et al. (2009). DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21, 1512–1525. doi: 10.1105/tpc.109.065987
Litt, A., and Irish, V. F. (2003). Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165, 821–833. doi: 10.3410/f.1017498.204267
Litz, R. E., and Padilla, G. (2012). “Genetic transformation of fruit trees,” in Genomics of Tree Crops, eds P. M. Priyadarshan and R. J. Schnell (Berlin: Springer), 117–153.
Liu, L., Zhu, K., Yang, Y., Wu, J., Chen, F., and Yu, D. (2008). Molecular cloning, expression profiling and trans-activation property studies of a DREB2-like gene from chrysanthemum (Dendranthema vestitum). J. Plant Res. 121, 215–226. doi: 10.1007/s10265-007-0140-x
Matas, A. J., Gapper, N. E., Chung, M. Y., Giovannoni, J. J., and Rose, J. K. (2009). Biology and genetic engineering of fruit maturation for enhanced quality and shelf-life. Curr. Opin. Biotechnol. 20, 197–203. doi: 10.1016/j.copbio.2009.02.015
Meng, L. S., Song, J. P., Sun, S. B., and Wang, C. Y. (2009). The ectopic expression of PttKN1 gene causes pleiotropic alternation of morphology in transgenic carnation (Dianthus caryophyllus L.). Acta Physiol. Plant. 31, 1155–1164. doi: 10.1007/s11738-009-0334-z
Miao, H., Jiang, B., Chen, S., Zhang, S., Chen, F., Fang, W., et al. (2010). Isolation of a gibberellin 20-oxidasec DNA from and characterizationof its expression in chrysanthemum. Plant Breed. 129, 707–714. doi: 10.1111/j.1439-0523.2009.01736.x
Milbus, H., Sriskandarajah, S., and Serek, M. (2009). Genetically modified flowering potted plants with reduced ethylene sensitivity. Acta Hortic. 847, 75–80. doi: 10.17660/ActaHortic.2009.847.8
Muhlemann, J. K., Maeda, H., Chang, C. Y., Miguel, P. S., Baxter, I., Cooper, B., et al. (2012). Developmental changes in the metabolic network of snapdragon flowers. PLoS ONE 7:e40381. doi: 10.1371/journal.pone.0040381
Nakatsuka, T., Mishiba, K., Abe, Y., Kubota, A., Kakizaki, Y., Yamamura, S., et al. (2008). Flower color modification of gentian plants by RNAi-mediated gene silencing. Plant Biotechnol. 25, 61–68. doi: 10.5511/plantbiotechnology.25.61
Narumi, T., Aida, R., Koyama, T., Yamaguchi, H., Sasaki, K., Shikata, M., et al. (2011). Arabidopsis chimeric TCP3 repressor produces novel floral traits in Torenia fournieri and Chrysanthemum morifolium. Plant Biotechnol. 28, 131–140. doi: 10.5511/plantbiotechnology.11.0121a
Narumi, T., Aida, R., Ohmiya, A., and Satoh, S. (2005). Transformation of chrysanthemum with mutated ethylene receptor genes: mDG-ERS1 transgenes conferring reduced ethylene sensitivity and characterization of the transformants Postharvest Biol. Technol. 37, 101–110. doi: 10.1016/j.postharvbio.2005.04.008
Nielsen, K., Deroles, S. C., Markham, K. R., Bradley, M. J., Podivinsky, E., and Manson, D. (2002). Antisense flavonol synthase alters copigmentation and flower color in lisianthus. Mol. Breed. 9, 217–229. doi: 10.1023/A:1020320809654
Nishihara, M., and Nakatsuka, T. (2010). Genetic engineering of novel flower colors in floricultural plants: recent advances via transgenic approaches. Methods Mol. Biol. 589, 325–347. doi: 10.1007/978-1-60327-114-1_29
Nishihara, M., and Nakatuska, T. (2011). Genetic engineering of flavonoid pigments to modify flower color in floricultural plants. Biotechnol. Lett. 33, 433–441. doi: 10.1007/s10529-010-0461-z
Noman, A., Ali, Q., Naheed, F., Rizwan, M., Ali, S., and Irshad, K. (2015). Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch. Agron. Soil Sci. 61, 1659–1672. doi: 10.1080/03650340.2015.1028379
Noman, A., and Aqeel, M. (2017). miRNA-based heavy metal homeostasis and plant growth. Environ. Sci. Pollut. Res. doi: 10.1007/s11356-017-8593-5. [Epub ahead of print].
Noman, A., Aqeel, M., and He, S. (2016a). Crispr-cas9: tool for qualitative and quantitative plant genome editing. Front. Plant Sci. 7:1740. doi: 10.3389/fpls.2016.01740
Noman, A., Bashir, R., Aqeel, M., Anwer, S., Iftikhar, W., Zainab, M., et al. (2016b). Success of transgenic cotton (Gossypiumhirsutum, L.): fiction or reality? Cogent Food Agric. 2:1207844. doi: 10.1080/23311932.2016.1207844
Noman, A., Fahad, S., Aqeel, M., Ali, U., Amanullah, Anwer, S., et al. (2017). miRNAs: major modulators for crop growth and development under abiotic stresses. Biotechnol Lett. doi: 10.1007/s10529-017-2302-9. [Epub ahead of print].
Nontaswatsri, C., and Fukai, S. (2010). “Genetic transformation of carnation (Dianthus caryophylus L.),” in Protocols for In Vitro Propagation of Ornamental Plants, eds S. M. Mohan and S. J. Ochatt (Amsterdam: Springer), 87–96. doi: 10.1007/978-1-60327-114-1_9
Oda, A., Narumi, T., Li, T., Kando, T., Higuchi, Y., Sumitomo, K., et al. (2012). CsFTL3, a chrysanthemum FLOWERING LOCUS T-like gene, is a key regulator of photoperiodic flowering in chrysanthemums. J. Exp. Bot. 63, 1461–1477. doi: 10.1093/jxb/err387
Ogata, J., Kanno, Y., Itoh, Y., Tsugawa, H., and Suzuki, M. (2005). Plant biochemistry: anthocyanin biosynthesis in roses. Nature 435, 757–758. doi: 10.1038/nature435757a
Ohmiya, A., Kishimoto, S., Aida, R., Yoshioka, S., and Sumitomo, K. (2006). Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol. 142, 1193–1201. doi: 10.1104/pp.106.087130
Ohmiya, A., Sumitomo, K., and Aida, R. (2009). “Yellow Jimba”: suppression of carotenoid cleavage dioxygenase (CmCCD4a) expression turns white chrysanthemum petals yellow. J. Jpn. Soc. 78, 450–455. doi: 10.2503/jjshs1.78.450
Oliva, M., Ovadia, R., Perl, A., Bar, E., Lewinsohn, E., Galili, G., et al. (2015). Enhanced formation of aromatic amino acids increases fragrance without affecting flower longevity or pigmentation in Petunia × hybrida. Plant Biotechnol. J. 13, 125–136. doi: 10.1111/pbi.12253
Parisi, C., Tillie, P., and Rodríguez-Cerezo, E. (2016). The global pipeline of GM crops out to 2020. Nat. Biotechnol. 34, 31–36. doi: 10.1038/nbt.3449
Petty, L. M., Harberd, N. P., Carre, I. A., Thomas, B., and Jackson, S. D. (2003). Expression of the Arabidopsis gaigene under its own promoter causes a reduction in plant height in chrysanthemum by attenuation ofthe gibberellin response. Plant Sci. 164, 175–182. doi: 10.1016/S0168-9452(02)00380-1
Pourhosseini, L., Kermani, M. J., Habashi, A. A., and Khalighi, A. (2013). Efficiency of direct and indirect shoot organogenesis in different genotypes of Rosa hybrida. Plant Cell Tissue Organ Cult. 112, 101–108. doi: 10.1007/s11240-012-0210-1
Qi, Y., Du, L., Quan, Y., Tian, F., Liu, Y., and and Wang, Y. (2014). Agrobacterium-mediated transformation of embryogenic cell suspension cultures and plant regeneration in Liliumtenuifolium oriental × trumpet cv. Robina'. Acta Physiol. 36, 2047–2057. doi: 10.1007/s11738-014-1582-0
Qi, Y., Lou, Q., Quan, Y., Liu, Y., and Wang, Y. (2013). Flower-specific expression of the Phalaenopsis flavonoid 3′, 5′-hydoxylase modifies flower color pigmentation in Petunia and Lilium. Plant Cell Tissue Organ Cult. 115, 263–273. doi: 10.1007/s11240-013-0359-2
Raffeiner, B., Serek, M., and Winkelmann, T. (2009). Agrobacterium tumefaciens mediated transformation of Oncidium and Odontoglossum orchid species with the ethylene receptor mutant gene etr1-1. Plant Cell Tissue Organ Cult. 98, 125–134. doi: 10.1007/s11240-009-9545-7
Ruokolainen, S., Ng, Y. P., Albert, V. A., Elomaa, P., and Teeri, T. H. (2010). Large scale interaction analysis predicts that the Gerbera hybrida floral E function is provided both by general and specialized proteins. BMC Plant Biol. 10:129. doi: 10.1186/1471-2229-10-129
Ruokolainen, S., Ng, Y. P., Albert, V. A., Elomaa, P., and Teeri, T. H. (2011). Over-expression of the Gerbera hybrida At-SOC1-like1 gene Gh-SOC1 leads to floral organ identity deterioration. Ann. Bot. 107, 1491–1499. doi: 10.1093/aob/mcr112
Sanikhani, M., Mibus, H., Stummann, B. M., and Serek, M. (2008). Kalanchoe blossfeldiana plants expressing the Arabidopsis etr1-1 allele show reduced ethylene sensitivity. Plant Cell Rep. 27, 729–737. doi: 10.1007/s00299-007-0493-6
Satoh, S., Kosugi, Y., Iwazaki, Y., Narumi, T., and Kinouchi, T. (2006). “Genetic engineering of ethylene production and perception in carnation and chrysanthemum,” in Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, ed J. A. Teixeira da Silva (Isleworth: Global Science Books, Ltd.), 140–163.
Satoh, S., Watanabe, M., Chisaka, K., and Narumi, T. (2008). Suppressed leaf senescence in Chrysanthemum transformed with a mutated ethylene receptor gene mDG-ERS1 (etr1-4). J. Plant Biol. 51, 424–427. doi: 10.1007/BF03036064
Savin, K. W., Baudinette, S. C., Graham, M. W., Michael, M. Z., Nugent, G. D., Lu, C. Y., et al. (1995). Antisense ACC oxidase RNA delays carnation petal senescence. HortScience 30, 970–972.
Schwinn, K. E., Boase, M. R., Bradley, J. M., Lewis, D. H., Deroles, S. C., Martin, C. R., et al. (2014). MYB and bHLH transcription factor transgenes increase anthocyanin pigmentation in petunia and lisianthus plants, and the petunia phenotypes are strongly enhanced under field conditions. Front. Plant Sci. 5:603. doi: 10.3389/fpls.2014.00603
Sen, S., Kumar, S., Ghani, M., and Thakur, M. (2013). Agrobacterium mediated genetic transformation of chrysanthemum (Dendranthema grandiflora Tzvelev) with rice chitinase gene for improved resistance against Septoria obesa. Plant Pathol. J. 12, 1–10. doi: 10.3923/ppj.2013.1.10
Shaw, J., Chen, H., Tsai, M., Kuom, C. I., and Huangm, L. C. (2002). Extended flower longevity of Petunia hybrida plants transformed with boers,a mutated ERS gene of Brassica oleracea. Mol. Breed. 9, 211–216. doi: 10.1023/A:1019703627019
Shi, S., Ma, F., Li, Y., Feng, F., and Shang, Z. (2012). Overexpression of l-galactono-1, 4-lactone dehydrogenase (GLDH) in Lanzhou lily (Lilium davidii var. unicolor) via particle bombardment-mediated transformation. In Vitro Cell. Dev. Biol. 48, 1–6. doi: 10.1007/s11627-011-9383-2
Shibata, M. (2008). Importance of genetic transformation in ornamental plant breeding. Plant Biotechnol. 25, 3–8. doi: 10.5511/plantbiotechnology.25.3
Shinoyama, H., Mochizuki, A., Nomura, Y., and Kamada, H. (2008). Environmental risk assessment of genetically modified chrysanthemums containing a modified cry1Ab gene from Bacillus thuringiensis. Plant Biotechnol. 25, 17–29. doi: 10.5511/plantbiotechnology.25.17
Shinoyama, H., Sano, T., Saito, M., Ezura, H., Aida, R., Nomura, Y., et al. (2012). Induction of male sterility in transgenic chrysanthemums (Chrysanthemum morifolium Ramat.) by expression of a mutated ethylene receptor gene, Cm-ETR1/H69A, and the stability of this sterility at varying growth temperatures. Mol. Breed. 29, 285–295. doi: 10.1007/s11032-010-9546-6
Shirasawa-Seo, N., Nakamura, S., Ukai, N., Honkura, R., Iwai, T., and Ohashi, Y. (2002). Ectopic expression of an oat thionin gene in carnation plants confers enhanced resistance to bacterial wilt disease. Plant Biotechnol. 19, 311–317. doi: 10.5511/plantbiotechnology.19.311
Shulga, O. A., Mitiouchkina, T. Y., Shchennikova, A. V., Skryabin, K. G., and Dolgov, S. V. (2011). Overexpression of AP1-like genes from Asteraceae induces early-flowering in transgenic Chrysanthemum plants. In Vitro Cell. Dev. Biol. 47, 553–560. doi: 10.1007/s11627-011-9393-0
Song, A., An, J., Guan, Z., Jiang, J., Chen, F., Lou, W., et al. (2014). The constitutive expression of a two transgene construct enhances the abiotic stress tolerance of Chrysanthemum. Plant Physiol. Biochem. 80, 114–120. doi: 10.1016/j.plaphy.2014.03.030
Spitzer-Rimon, B., Farhi, M., Albo, B., Cnaani, A., Ben Zvi, M. M., Masci, T., et al. (2012). The R2R3-MYB-like regulatory factor EOBI, acting downstream of EOBII, regulates scent production by activating ODO1 and structural acent-related genes in Petunia. Plant Cell 24, 5089–5105. doi: 10.1105/tpc.112.105247
Srikanth, A., and Schmid, M. (2011). Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 68, 2013–2037. doi: 10.1007/s00018-011-0673-y
Suzuki, K., Mizutani, M., Fukui, Y., Ueyama, Y., Katsumoto, Y., Miyazaki, K., et al. (2002). Flower color modification of Torenia by engineering gene expression of cytochromes P450 involved in flavonoid biosynthesis. In Vitro Cell. Dev. Biol. 38:109-A.
Takatsu, Y., Nishizawa, Y., Hibi, T., and Akutsu, K. (1999). Transgenic chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura) expressing a rice chitinase gene shows enhanced resistance to gray mold (Botrytis cinerea). Sci. Hortic. 82, 113–123. doi: 10.1016/S0304-4238(99)00034-5
Tanaka, Y., and Brugliera, F. (2013). Flower colour and cytochromes P450. Philos. Trans. R. Soc. 368:20120432. doi: 10.1098/rstb.2012.0432
Tanaka, Y., Brugliera, F., and Chandler, S. (2009). Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 10, 5350–5369. doi: 10.3390/ijms10125350
Tanaka, Y., Brugliera, F., Kalc, G., Senior, M., Dyson, B., Nakamura, N., et al. (2010). Flower color modification by engineering of the flavonoid biosynthetic pathway: practical perspectives. Biosci. Biotechnol. Biochem. 74, 1760–1769. doi: 10.1271/bbb.100358
Tanaka, Y., and Ohmiya, A. (2008). Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 19, 190–197. doi: 10.1016/j.copbio.2008.02.015
Teixeira da Silva, J. A., Shinoyama, H., Aida, R., Matsushita, Y., Raj, S. K., and Chen, F. (2013). Chrysanthemum biotechnology: quo vadis? Crit. Rev. Plant Sci. 32, 21–52. doi: 10.1080/07352689.2012.696461
Thiruvengadam, M., and Yang, C. H. (2009). Ectopic expression of two MADS box genes from orchid (Oncidium Gower Ramsey) and lily (Liliumlongiflorum) alters flower transition and formation in Eustoma grandiflorum. Plant Cell Rep. 28, 1463–1473. doi: 10.1007/s00299-009-0746-7
Tsuda, S., Fukui, Y., Makamura, N., Katsumoto, Y., Yonekura-Sakakibara, K., Fukuchi-Mizutani, M., et al. (2004). Flower color modification of Petunia hybrida commercial varieties by metabolic engineering. Plant Biotechnol. 21, 377–386. doi: 10.5511/plantbiotechnology.21.377
Van der Salm, T. P. M., Bouwer, R. V., Van Dijk, A. J., Keizer, L. C. P., Ten Cate, C. H. H., Van Der Plas, L. H. W., et al. (1998). Stimulation of scion bud release by rolgene transformed rootstocks of Rosa hybrida L. J. Exp. Bot. 49, 847–852. doi: 10.1093/jxb/49.322.847
Van Moerkercke, A., Galvan-Ampudia, C. S., Verdonk, J. C., Haring, M. A., and Schuurink, R. C. (2012). Regulators of floral fragrance production and their target genes in 1122 petunia are not exclusively active in the epidermal cells of petals. J. Exp. Bot. 63, 3157–3171. doi: 10.1093/jxb/ers034
Veitch, N. C., and Grayer, R. J. (2008). Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 25, 555–611. doi: 10.1039/b718040n
Verdonk, J. C., Haring, M. A., van Tunen, A. J., and Schuurink, R. C. (2005). ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 17, 1612–1624. doi: 10.1105/tpc.104.028837
Vieira, P., Wantoch, S., Lilley, C. J., Chitwood, D. J., Atkinson, H. J., and Kamo, K. (2015). Expression of a cystatin transgene can confer resistance to root lesion nematodes in Lilium longiflorum Nellie White'. Transgenic Res. 24, 421–432. doi: 10.1007/s11248-014-9848-2
Villacorta-Martin, C., Núñez de Cáceres González, F. F., de Haan, J., Huijben, K., Passarinho, P., Hamo, M. L. B., et al. (2015). Whole transcriptome profiling of the vernalization process in Lilium longiflorum (cultivar White Heaven) bulbs. BMC Genomics 16:1. doi: 10.1186/s12864-015-1675-1
Vinocur, B., and Altman, A. (2005). Recent advances in engineering plant tolerance to abiotic stress, achievements and limitations. Curr. Opin. Biotechnol. 16, 123–132. doi: 10.1016/j.copbio.2005.02.001
Warner, R. (2011). Genetic Approaches to Improve Cold Tolerance of Petunia Funding Industry Solutions Through Research and Education. Available online at: https://endowment.org/wp-content/uploads/2014/03/306Warner.pdf
Wen, C., Zhao, Q., Nie, J., Liu, G., Shen, L., Cheng, C., et al. (2016). Physiological controls of chrysanthemum DgD27 gene expression in regulation of shoot branching. Plant Cell Rep. 35, 1053–1070. doi: 10.1007/s00299-016-1938-6
Xie, Q., Chen, G., Liu, Q., Zhu, Z., and Hu, Z. (2015). Dual silencing of DmCPD and DmGA20ox genes generates a novel miniature and delayed-flowering Dendranthema morifolium variety. Mol. Breed. 35, 1–13. doi: 10.1007/s11032-015-0239-z
Xu, G., Chen, S., and Chen, F. (2010). Transgenic chrysanthemum plants expressing a harpinXoo gene demonstrate induced resistance to Alternaria leaf spot and accelerated development. Russ. J. Plant Physiol. 57, 548–553. doi: 10.1134/S1021443710040138
Xu, G., Liu, Y., Chen, S., and Chen, F. (2011). Potential structural and biochemical mechanisms of compositae wild species resistance to Alternaria tenuissima. Russ. J. Plant Physiol. 58, 491–497. doi: 10.1134/S1021443711030216
Yamagishi, M., Yoshida, Y., and Nakayama, M. (2012). The transcription factor LhMYB12 determines anthocyanin pigmentation in the tepals of Asiatic hybrid lilies (Lilium spp.) and regulates pigment quantity. Mol. Breed. 30, 913–925. doi: 10.1007/s11032-011-9675-6
Yoshida, K., Mori, M., and Kondo, T. (2009). Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat. Prod. Rep. 26, 884–915. doi: 10.1039/b800165k
Yoshioka, S., Aida, R., Yamamizo, C., Shibata, M., and Ohmiya, A. (2012). The carotenoid cleavage dioxygenase 4 (CmCCD4a) gene family encodes a key regulator of petal color mutation in chrysanthemum. Euphytica 184, 377–387. doi: 10.1007/s10681-011-0602-z
Yu, M., Liu, Z. L., Chen, S. M., and Chen, F. D. (2010). Expression of Prunus mume PGIP gene in transgenic Dendranthema morifolium increased tolerance to disease resistance. Acta Bot. Boreal. Occident. Sin. 30, 1111–1116.
Yue, Y., Yu, R., and Fan, Y. (2015). Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genomics 16:470. doi: 10.1186/s12864-015-1653-7
Zafar, S., Ashraf, M. Y., Anwar, S., Ali, Q., and Noman, A. (2016). Yield enhancement in wheat by soil and foliar fertilization of K and Zn under saline environment. Soil Environ. 35, 46–55.
Zakizadeh, H., Lütken, H., Sriskandarajah, S., Serek, M., and Müller, R. (2013). Transformation of miniature potted rose (Rosa hybrida cv Linda') with P SAG12-ipt gene delays leaf senescence and enhances resistance to exogenous ethylene. Plant Cell Rep. 32, 195–205. doi: 10.1007/s00299-012-1354-5
Zheng, Z. L., Yang, Z., Jang, J. C., and Metzger, J. D. (2001). Modification of plant architecture in chrysanthemum by ectopic expression of the tobacco phytochrome B1 gene. J. Am. Soc. Hortic. Sci. 126, 19–26.
Zuker, A., Tzfira, T., Scovel, G., Ovadis, M., Shklarman, E., Itzhaki, H., et al. (2001). RolC transgenic carnation with improved horticultural traits: quantitative and qualitative analyses of greenhouse-grown plants. J. Am. Soc. Hortic. Sci. 126, 13–18.
Keywords: biotechnology, commercial resource, environment, flower characteristics, horticulture, transgenic plants
Citation: Noman A, Aqeel M, Deng J, Khalid N, Sanaullah T and Shuilin H (2017) Biotechnological Advancements for Improving Floral Attributes in Ornamental Plants. Front. Plant Sci. 8:530. doi: 10.3389/fpls.2017.00530
Received: 16 December 2016; Accepted: 24 March 2017;
Published: 20 April 2017.
Edited by:
Nokwanda Makunga, Stellenbosch University, South AfricaReviewed by:
Sailendra Nath Sarkar, University of Calcutta, IndiaTaras P. Pasternak, Albert Ludwig University of Freiburg, Germany
Copyright © 2017 Noman, Aqeel, Deng, Khalid, Sanaullah and Shuilin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Aqeel, aqeelbutt99@gmail.com
He Shuilin, shlhe201304@aliyun.com
 Ali Noman
Ali Noman Muhammad Aqeel
Muhammad Aqeel Jianming Deng
Jianming Deng Noreen Khalid
Noreen Khalid Tayyaba Sanaullah
Tayyaba Sanaullah He Shuilin
He Shuilin