- 1Department of Environmental and Biological Chemistry, Chungbuk National University, Cheongju, South Korea
- 2Department of Agricultural Microbiology, Agricultural College and Research Institute, Tamil Nadu Agricultural University, Madurai, India
- 3Horticultural and Herbal Crop Environment Division, National Institute of Horticultural and Herbal Science, Rural Development Administration, Wanju, South Korea
Soil salinity, being a part of natural ecosystems, is an increasing problem in agricultural soils throughout the world. Pseudomonas frederiksbergensis OS261 has already been proved to be an effective bio-inoculant for enhancing cold stress tolerance in plants, however, its effect on salt stress tolerance is unknown. The main aim of the present study was to elucidate P. frederiksbergensis OS261 mediated salt stress tolerance in red pepper. The plants were exposed to a salt stress using NaCl at the concentrations of 50, 100, and 150 mM after 12 days of transplantation, while plant growth and enzyme activity were estimated 50 days after sowing. The height in P. frederiksbergensis OS261 inoculated plants was significantly increased by 19.05, 34.35, 57.25, and 61.07% compared to un-inoculated controls at 0, 50, 100, and 150 mM of NaCl concentrations, respectively, under greenhouse conditions. The dry biomass of the plants increased by 31.97, 37.47, 62.67, and 67.84% under 0, 50, 100, and 150 mM of NaCl concentrations, respectively. A high emission of ethylene was observed in un-inoculated red pepper plants under salinity stress. P. frederiksbergensis OS261 inoculation significantly reduced ethylene emission by 20.03, 18.01, and 20.07% at 50, 100, and 150 mM of NaCl concentrations, respectively. Furthermore, the activity of antioxidant enzymes (ascorbate peroxidase, superoxide dismutase, and catalase) also varied in the inoculated red pepper plants. Salt stress resistance in the bacterized plants was evident from the improved antioxidant activity in leaf tissues and the decreased hydrogen ion concentration. Thus, we conclude that P. frederiksbergensis OS261 possesses stress mitigating property which can enhance plant growth under high soil salinity by reducing the emission of ethylene and regulating antioxidant enzymes.
Introduction
Abiotic stresses exert a serious impact on crop productivity throughout the world. Soil salinity is an alarming stress, which limits plant growth and affects crop production to a large extent (Allakhverdiev et al., 2000; Bano and Fatima, 2009; Bacilio et al., 2016). The high amount of salt in soil disturbs the ionic balance in plant cells and decreases water uptake capacity in plants (Cheng et al., 2007; Singh and Jha, 2016; Stavridou et al., 2016; Yaish et al., 2016). In principle, high amounts of potassium or calcium ions are needed for the proper functioning of plant metabolism, whereas increased uptake of sodium ion due to high salinity reduces the ionic uptake of potassium and calcium ions (Cheng et al., 2007), thereby negatively affecting the growth of plants. It disturbs plant cell division and elongation and reduces the rate of photosynthesis (Munns and Tester, 2008; Forieri et al., 2016). This finally induces the generation of reactive oxygen species (ROS). The generated ROS negatively affect plant growth and development (Bojórquez-Quintal et al., 2014). Thus developing salt stress tolerant plants has been a highly desirable goal but there has been a little success to date; as only a few major genetic determinants of salt stress have been identified (Munns and Tester, 2008; Schubert et al., 2009). The alternate strategy is to introduce salt-tolerant microbes into plants under saline conditions. Beneficial microbes, such as plant growth promoting rhizobacteria (PGPR), which live in and around the root zone (rhizosphere), have been investigated as the means to overcome salinity stress. Most of the bacterial genera, such as Agrobacterium, Azospirillum, Bacillus, and Rhizobium, have already been reported to increase salt tolerance in plants (Hamdia et al., 2004; Ahmad et al., 2012; Bharti et al., 2013). They are known to stimulate plant growth through a variety of mechanisms such as the production of phytohormones, ACC deaminase (Siddikee et al., 2015), exopolysaccharide (EPS) (Siddikee et al., 2011), fixation of atmospheric nitrogen (Islam et al., 2013), and by solubilizing phosphate (Tank and Saraf, 2010). The stress usually induces ethylene emission (Morgan and Drew, 1997) and PGPR play a major role in reducing ethylene in plants via the action of ACC deaminase that cleaves ACC to α-ketobutyrate and ammonia (Glick et al., 1998). An abiotic stress induces ROS as a result of the stress signal within the plant. Mostly, H2O2, O2-, and OH- are generated, which damage biological macromolecules: DNA, RNA, and proteins. ROS not only affect plants at a molecular level but also hamper main physiological activities. There are some antioxidant enzymes that can control the production of ROS under stressful conditions (Munne-Bosch and Pinto-Marijuan, 2016). Superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) are the main enzymes that play a major role in the self-defense in plants (Chojak-Koźniewska et al., 2017). SOD primarily converts superoxide radicals into hydrogen peroxide, thus defending the plants from oxidative damage. APX mainly works in peroxisomes where it helps in the breakdown of H2O2. CAT lowers the hydrogen peroxide level by converting it into water and oxygen (Habib et al., 2016). The microbes provide plants resistance to stress by enhancing the activity of the antioxidant enzymes and other non-enzymatic antioxidants (Gururani et al., 2013).
Pepper (Capsicum annuum L. family Solanaceae) is widely grown in the different parts of the world and is one of the most popular vegetables in South Korea. It is an important ingredient of many traditional Korean foods, including Kimchi (Sang et al., 2008; Patra et al., 2016). Pepper is being cultivated in greenhouses as well as in fields since a long time. The repeated cultivation of pepper can increase soil salinity through the accumulation of organic fertilizers and pesticides, and as a result, an agricultural land can become highly saline, unsuitable for agriculture. High soil salinity causes major economic losses by reducing plant yield. In South Korea, a low temperature which is unfavorable for plant growth dominates 6 months of the year making productive agriculture possible only in summer. In addition to that, the crop production in the coastal regions of South Korea is also limited due to high salinity. This leads to an economically unfavorable situation. Recently, various approaches have been suggested to control salinity stress, one of them is the use of beneficial PGPR, which can enhance plant growth and productivity by eliciting salt-stress tolerance to host plants (Yang et al., 2009; Siddikee et al., 2010; Dodd and Pérez-Alfocea, 2012). In order to maximize the crop yield and make it economically favorable, it would be convenient to use a bacterial strain capable of mitigating both low temperature and salinity stress. P. frederiksbergensis OS261, a PGPR, and member of the group Gammaproteobacteria, has been proved to increase the fitness of tomato plants under cold stress conditions (Subramanian et al., 2016) and also displayed its ability to colonize in plant parts of red pepper (Sandipan et al., 2016) and tomato (Subramanian et al., 2015). Even though P. frederiksbergensis OS261 has been proved to be an effective bio-inoculant for enhancing cold stress tolerance in plants (Subramanian et al., 2016), its effect on increasing salt stress tolerance still remains largely unknown. This work was aimed to evaluate P. frederiksbergensis OS261 as an effective inoculant for enhancing salt stress tolerance in plants through the production of ACC deaminase, reduction of stress ethylene level, and induction of antioxidant enzymes.
Materials and Methods
Bacterial Strain and Culture Conditions
Pseudomonas frederiksbergensis OS261 (GenBank Accession no. KF424313, 16S ribosomal RNA gene) was previously isolated from the experimental fields of Chungbuk Agricultural Research and Extension Services, Ochang-eup, South Korea (36°43′ N; 127°27′E). This strain was selected on the basis of plant growth promoting effects and ACC deaminase activity (Subramanian et al., 2016). For inoculum preparation, a single colony of P. frederiksbergensis OS261 grown on a nutrient agar (NA) plate was transferred to 5 mL nutrient broth (NB) and incubated at 30°C on a shaker (150 rpm) for 24 h. After the incubation, 1.0 mL of the culture (∼1.0 × 108 CFU mL-1) was transferred to 50 mL fresh NB and allowed to grow for further 24 h. The culture was centrifuged at 5000 rpm for 5 min at 4°C, and the pellet was washed twice with 30 mM MgSO4. The pellet was resuspended in 30 mM MgSO4 to an optical density of 0.8 at 600 nm (cell count ∼1.0 × 108 CFU mL-1).
Plant Experiments
Seed Sterilization and Bacterization
Red pepper (C. annuum L. cv. Bulmat) seeds (Syngenta seeds, Seoul, Republic of Korea) were surface sterilized with 70% ethanol for 30 s and 2% sodium hypochlorite for 1 min, followed by rinsing for several times with sterile deionized water. The bacterial cultures were grown in NB media at 30°C for 24 h with 150 rpm. The grown culture was harvested by centrifuging at 5000 rpm for 5 min at 4°C and the cell pellets were resuspended in 30 mM MgSO4 to obtain an optical density of 0.8 at 600 nm (cell count ∼1.0 × 108 CFU mL-1). The bacterization was performed by soaking the seeds in bacterial suspension for 4 h whereas the control seeds were soaked only in MgSO4.
Gnotobiotic Pouch Experiments
The red pepper seeds were surface sterilized and bacterized as mentioned above. After decanting the suspension, the seeds were transferred to growth pouches (six seeds per pouch), with six replications (i.e., six pouches) per treatment. The pouches were then kept in a growth chamber (DS 54 GLP, DASOL Scientific Co., Ltd., Korea) at 25°C with 12 h day-night photoperiod for 7 days (relative humidity 70% and light intensity 18 μmol m-2 s-1). After 7 days of plant growth, 0, 50, 100, and 150 mM of sodium chloride solutions prepared in 1X Hoagland solution were used to induce salt stress. The effect of inoculation on early growth promotion was determined by measuring the root and shoot length after 14 days of sowing (DAS). Total dry matter accumulation was measured after drying the seedlings in an oven at 70°C for 48 h.
Determination of Ethylene Emission
The ethylene emission from the red pepper seedlings was measured following the protocol of Mayak et al. (2004) with some modifications. The seed bacterization was performed as mentioned above. Following the bacterization, 30 seeds were imbibed in either a bacterial suspension or 30 mM MgSO4 for 2 h, the suspension or buffer was drained, the seeds were placed inside 120 mL narrow neck bottles on a piece of filter paper and 2 mL of de-ionized water was added to each bottle. After 8 days, the excess liquid was drained and 2 mL of respective salt solution (50, 100, and 150 mM) was added. The seeds those were imbibed in buffer and were not treated with salt served as un-inoculated control. After 4 h of salt addition, the narrow neck bottles were closed for 2 h with a rubber septum and 1 mL sample from the headspace was injected into a gas chromatograph (dsCHROM 6200, Donam Instruments Inc., Republic of Korea) packed with a Porapak-Q column and equipped with a flame ionization detector. The oven, injection, and detection temperatures were set at 40, 150, and 250°C, respectively. Six replicates were used for each treatment.
Greenhouse Experiments
The bacterized and non-bacterized red pepper seeds were sown in seedling trays containing 40 g of nursery soil (Nongwoo-Bio Co., Ltd., Yeoju-gun, Gyeonggi-do, Republic of Korea; composition: 58.8% coco peat, 17% peat moss, 10% perlite, 10% vermiculate, 4% zeolite, 0.004% pyroligneous acid and 0.01% wetting agent) and incubated in the greenhouse at a temperature range of 25–30°C with 50–70% relative humidity and a 15–9 h day/night period. Seven days old red pepper seedlings were transplanted into plastic pots (16 cm × 16 cm) containing 500 g of nursery soil. After transplanting, the plants were allowed to grow under normal conditions in the greenhouse. The bacterial inoculum was prepared as mentioned above and the inoculation was carried out in the soil at 14 DAS by adding 10 mL of the bacterial suspension (cell count ∼1.0 × 108 CFU mL-1) near the root zone. Five days after applying the bacterial suspension, salt stress was induced gradually by applying 25 mM of sodium chloride solution to each pot on alternative days to avoid osmotic shock; and the desired salt concentrations of 50, 100, and 150 mM were achieved after 2, 6, and 10 days, respectively. Parallel controls were maintained by irrigating with tap water. The leaching of water from the pots was prevented by retaining the soil water to a level below water holding capacity. The soil electrical conductivity of 0, 50, 100, and 150 mM treatments were 1.31 ± 0.04, 6.23 ± 0.07, 10.23 ± 0.35, and 16.23 ± 0.21 dS/m, respectively, at the time of harvest. Fifty days after the sowing, the plants were uprooted and the length of the plants was measured. Additionally, the number of leaves per plant was also recorded. The total dry biomass of the plants was determined after drying the plants in an oven at 70°C for 72 h.
Determination of Antioxidant Enzymatic Activity
Antioxidant enzymes were measured from a crude enzyme extract derived from red pepper leaves (approximately ∼1 g) obtained from the pot culture plants that were harvested at 50 DAS. Briefly, the fresh leaves were ground using liquid nitrogen and the ground leaf samples were stored at –80°C. The ground leaf samples (0.5 g) were homogenized in ice using 10 mL of 50 mM of potassium phosphate buffer, 1% (w/v) polyvinylpyrrolidone (pH 7.8) then incubated for 10 min at 4°C. The homogenate was filtered using Advantech Qualitative Filter Papers (110 mm) and centrifuged (VS–24SMTi, High Speed Refrigerated Centrifuge, Vision Scientific Co. Ltd., Korea) at 4000 × g for 15 min at 4°C. The supernatant was considered as the enzyme extract and was used for the determination of enzyme activities. The CAT activity (EC 1.11.1.6) was determined by a hydrogen peroxide assay based on the formation of its stable complex with ammonium molybdate (Goth, 1991). Briefly, 0.2 mL of the enzyme extract was incubated in 1 mL reaction mixture containing 65 mM hydrogen peroxide in 60 mM sodium phosphate buffer at room temperature for 4 min. The reaction was terminated using 1 mL of 32.4 mM of ammonium molybdate and the concentration of the yellow complex formed during the reaction was measured at 405 nm. SOD (EC 1.15.1.1) and APX (EC 1.11.1.11) activities were measured according to Ding et al. (2011). In the case of SOD, the assay mixture contained 50 mM phosphate buffer (pH 7.8), 9.9 mM L-methionine, 57 μM NBT, 0.025% (w/v) Triton X-100, 0.0044% (w/v) riboflavin and the enzyme extract. The SOD activity was measured at 560nm by following the decrease in the absorbance due to the photochemical reduction of nitro-blue tetrazolium (NBT). The APX activity was evaluated by the oxidative conversion of ascorbic acid to dehydroascorbate with regard to the decrease in the absorbance. It was calculated by the means of extinction coefficient 2.8 mM-1 cm-1 at 290 nm. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.3 mM ascorbic acid, 0.1 mM H2O2, 0.1 mM EDTA and 50 μL of the enzyme extract. Hydrogen peroxide content in the leaves was evaluated according to the protocol of Theocharis et al. (2012).
Statistical Analysis
In this study, a completely randomized block design was employed for the early growth and greenhouse experiments. The data from experimental results were subjected to a two-way analysis of variance (ANOVA). The percentage data and the significant differences between the means were determined by Duncan’s Multiple Range Test (DMRT) at P < 0.05 using SAS package, Version 9.4.
Results
Effect of P. frederiksbergensis OS261 on the Growth of Red Pepper Seedlings under Gnotobiotic Conditions
The effects of inoculation of P. frederiksbergensis OS261 on growth of red pepper under gnotobiotic conditions are shown in Figure 1. The plants inoculated with the bacterium developed longer roots (Figure 1A) compared to the un-inoculated controls after a period of 14 days under salinity stress. The inoculation increased the root length by 13.93, 18.62, 28.26, and 22.22% under 0, 50, 100, and 150 mM of NaCl treatment, respectively, compared to the controls. On the other hand, the changes in shoot length (Figure 1B) were also noticeable. The bacterial inoculation increased the shoot length by 11.76, 34.48, 37.03, and 45.09% under 0, 50, 100, and 150 mM of salt treatment, respectively, compared to the controls. Similarly, the inoculated red pepper plants accumulated higher plant biomass (Figure 1C) compared to the un-inoculated controls under salt-stressed conditions with a significant increase in the salt stress induced by 50 mM NaCl.
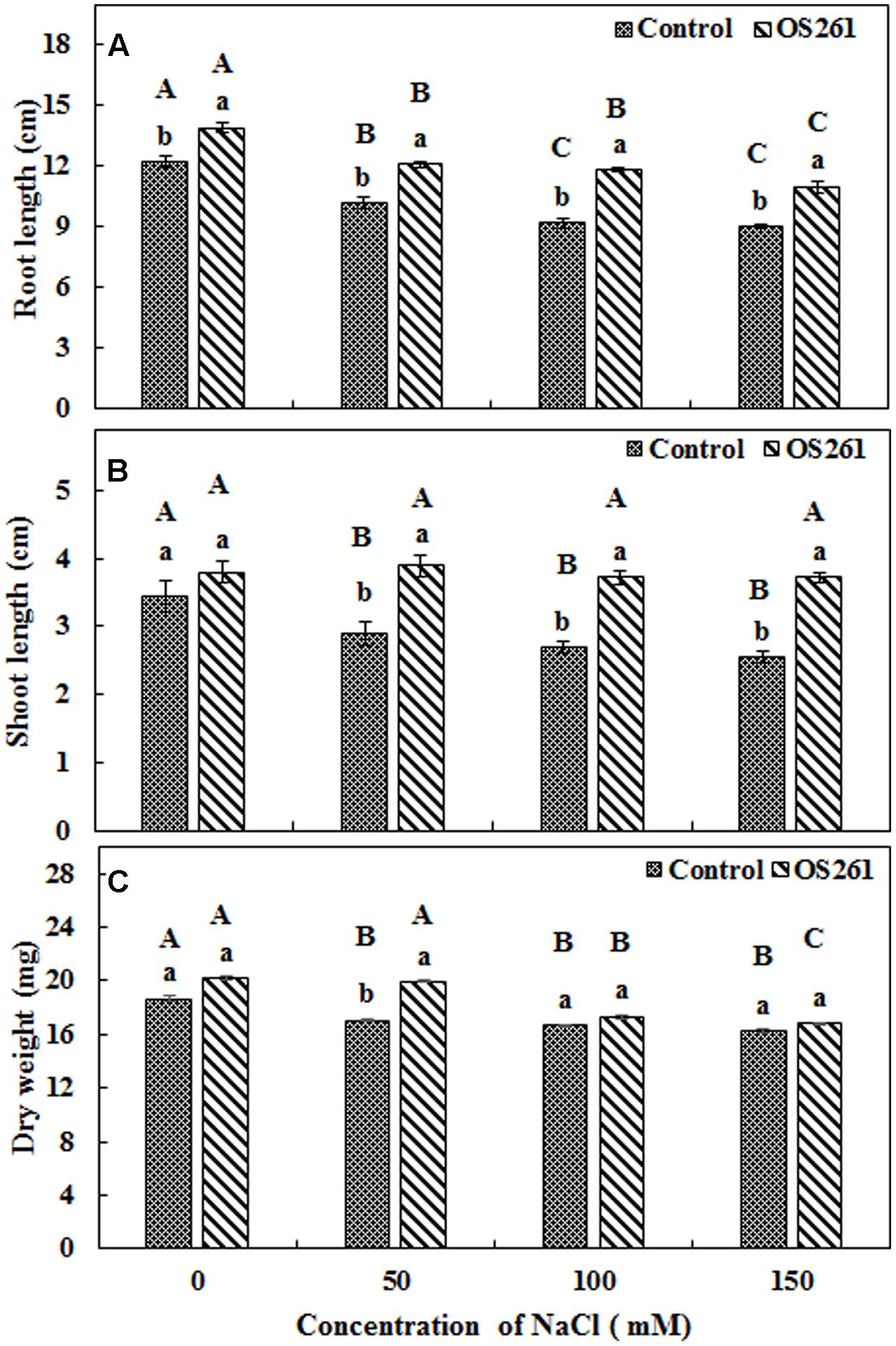
FIGURE 1. Effect of inoculation of Pseudomonas frederiksbergensis OS261 on red pepper seedlings under gnotobiotic conditions. (A) Root length, (B) Shoot length, (C) Dry weight. Different letters indicate significant differences P < 0.05 among the treatments at each salt levels (a, b) or among salt levels for each treatment: Control (A, B, C, D), OS261 (A, B, C, D). Each value represents the mean of six replicates ± standard error (SE).
Determination of Ethylene Emission from Red Pepper Seedlings with and without Inoculation
A higher amount of ethylene emission was observed from un-inoculated red pepper seedlings under 50, 100, and 150 mM of salt stress (Figure 2), but inoculation of P. frederiksbergensis OS261 reduced ethylene emission. It was observed that ethylene production increased by 20, 18, and 20.07% in un-inoculated red pepper seedlings at 50, 100, and 150 mM NaCl, respectively, compared to the inoculated red pepper seedlings.
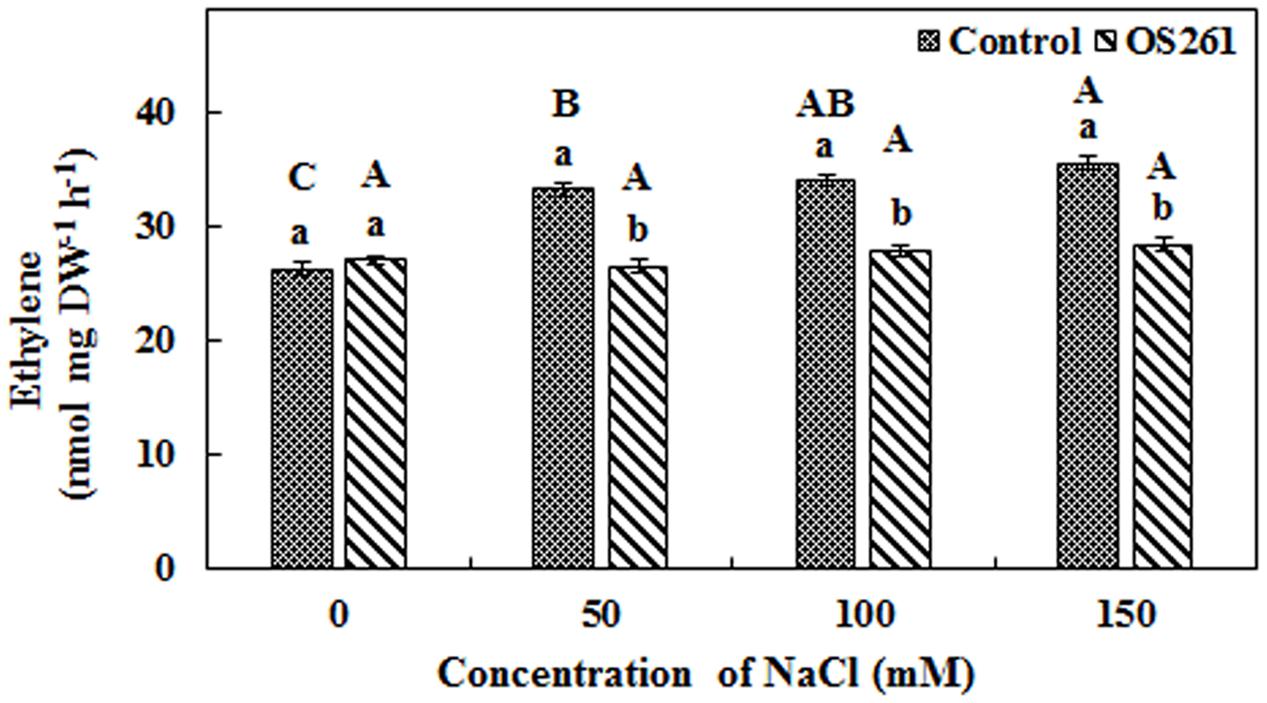
FIGURE 2. Effect of P. frederiksbergensis OS261 on ethylene production by red pepper seedlings under 0, 50, 100, and 150 mM salinity stress. Different letters indicate significant differences P < 0.05 among the treatments at each salt levels (a, b) or among salt levels for each treatment: Control (A, B, C, D), OS261 (A, B, C, D). Each value represents the mean of six replicates ± standard error (SE).
Effects of Inoculation with P. frederiksbergensis OS261 on the Growth of Red Pepper Plants under Salt Stress Conditions in the Greenhouse
In order to validate the results obtained in gnotobiotic conditions, similar experiments were performed under greenhouse conditions and the effect of inoculation on plant growth was checked by the means of plant height, dry biomass, and the number of leaves (Figure 3). The inoculated plants showed 37.79% increase in root length, 19.05% increase in shoot length and 31.97% increase in dry weight under normal conditions. Among the different levels of salt stress (50, 100, and 150 mM), the trends were similar, where inoculation increased root length (Figure 3A) by 36.44, 84.44, and 86.63%, respectively, as well shoot length (Figure 3B) by 34.35, 57.25, and 61.07%, respectively, compared to the un-inoculated controls. The number of leaves in each plant (Figure 3C) also increased significantly, contributing to the increase in dry biomass (Figure 3D), by 37.47, 62.67, and 67.84% under 50, 100, 150 mM of NaCl treatments in comparison to the un-inoculated plants. Overall, the inoculation resulted in a significant improvement in plant growth under both normal and salt-stressed conditions.
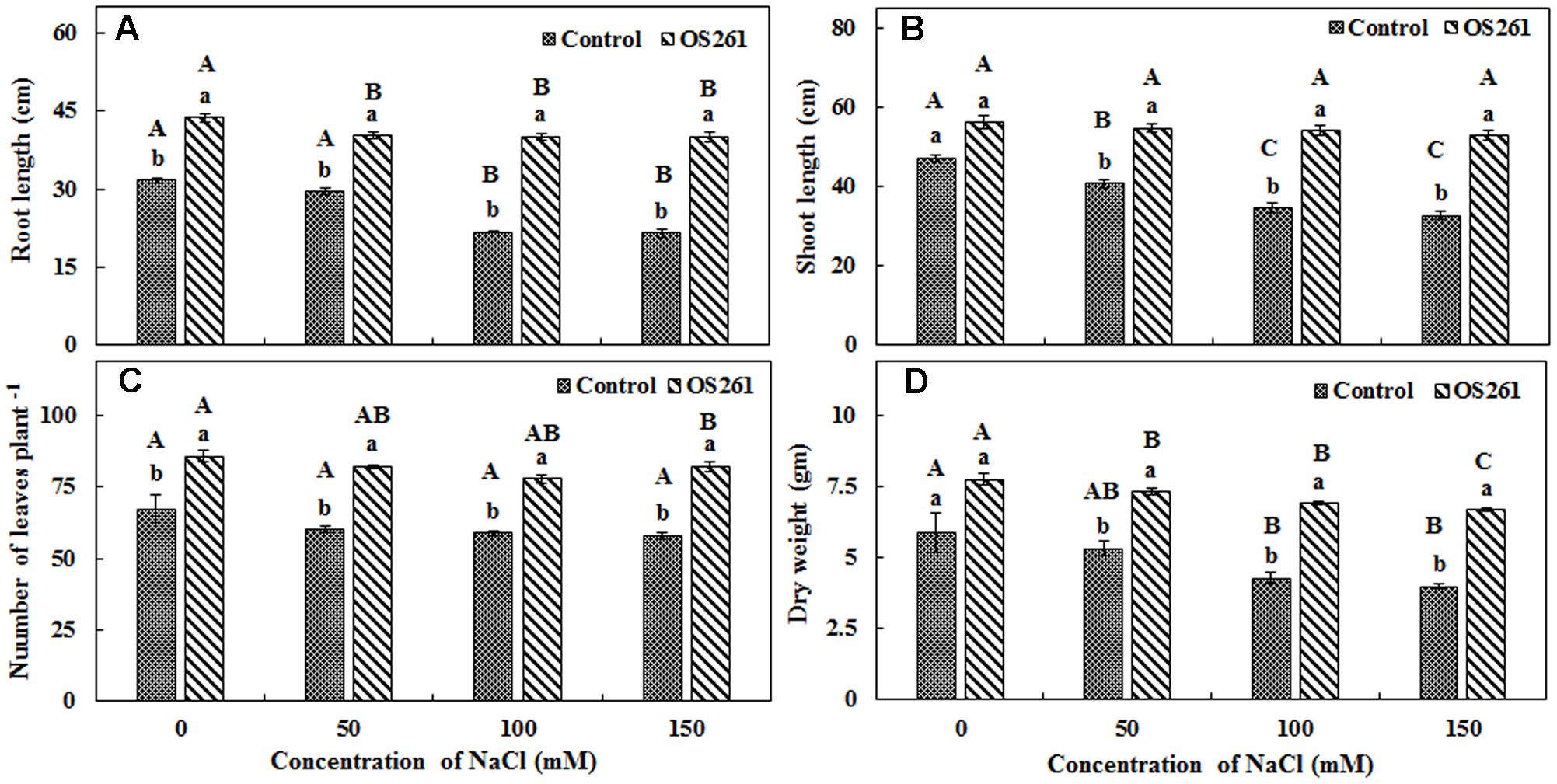
FIGURE 3. Effect of inoculation of P. frederiksbergensis OS261 on red pepper plants under greenhouse conditions. (A) Root length, (B) Shoot length, (C) Number of leaves per plant, (D) Dry weight. Different letters indicate significant differences P < 0.05 among the treatments at each salt levels (a, b) or among salt levels for each treatment: Control (A, B, C, D), OS261 (A, B, C, D). Each value represents the mean of three replicates ± standard error (SE).
Antioxidant Enzyme Activities and Hydrogen Peroxide Content in Red Pepper Plants Inoculated with P. frederiksbergensis OS261
The activity of three antioxidant enzymes (CAT, SOD, and APX) varied under salt stress condition compared to the normal conditions. The CAT activity in the plants seemed to increase significantly after the treatment with the bacterium under the different levels of salt concentrations (Figure 4A). The inoculated plants showed 5.37, 5.62, 15.96, and 45.45% increase in CAT activity at 0, 50, 100, and 150 mM of the salt treatments. In the inoculated plants, the SOD activity showed no significant changes upon the inoculation at 0 and 50 mM salt concentration but was significantly reduced at 100 and 150 mM salt concentrations in comparison to the un-inoculated control plants (Figure 4B). Similarly, APX activity (Figure 4C) showed a similar pattern with SOD where bacterial inoculation significantly reduced the activity under 50, 100, and 150 mM of salt concentrations with no significant changes at 0 mM compared to the un-inoculated plants. Hydrogen peroxide content increased considerably in the un-inoculated control plants with an increase in the soil salinity (Figure 5). Interestingly, the bacterial inoculation reduced the peroxide content significantly, which may help the plant cope up with the effect of stress.
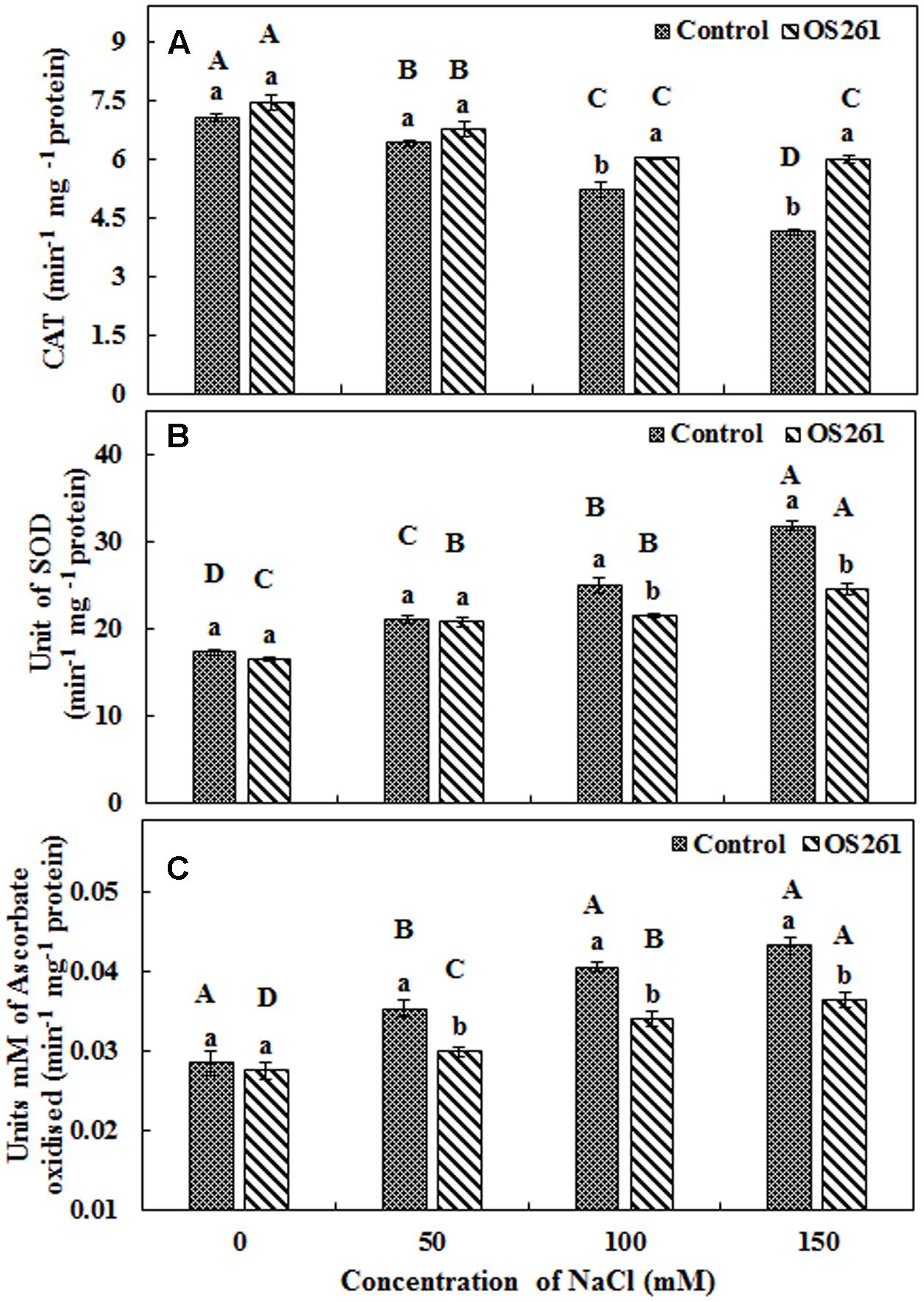
FIGURE 4. Antioxidant enzymatic activities (A) Catalase, (B) Superoxide dismutase, (C) Ascorbate peroxidase. Three replicates were used for each treatment. Different letters indicate significant differences P < 0.05 among the treatments at each salt levels (a, b) or among salt levels for each treatment: Control (A, B, C, D), OS261 (A, B, C, D). Each value represents the mean of three replicates ± standard error (SE).
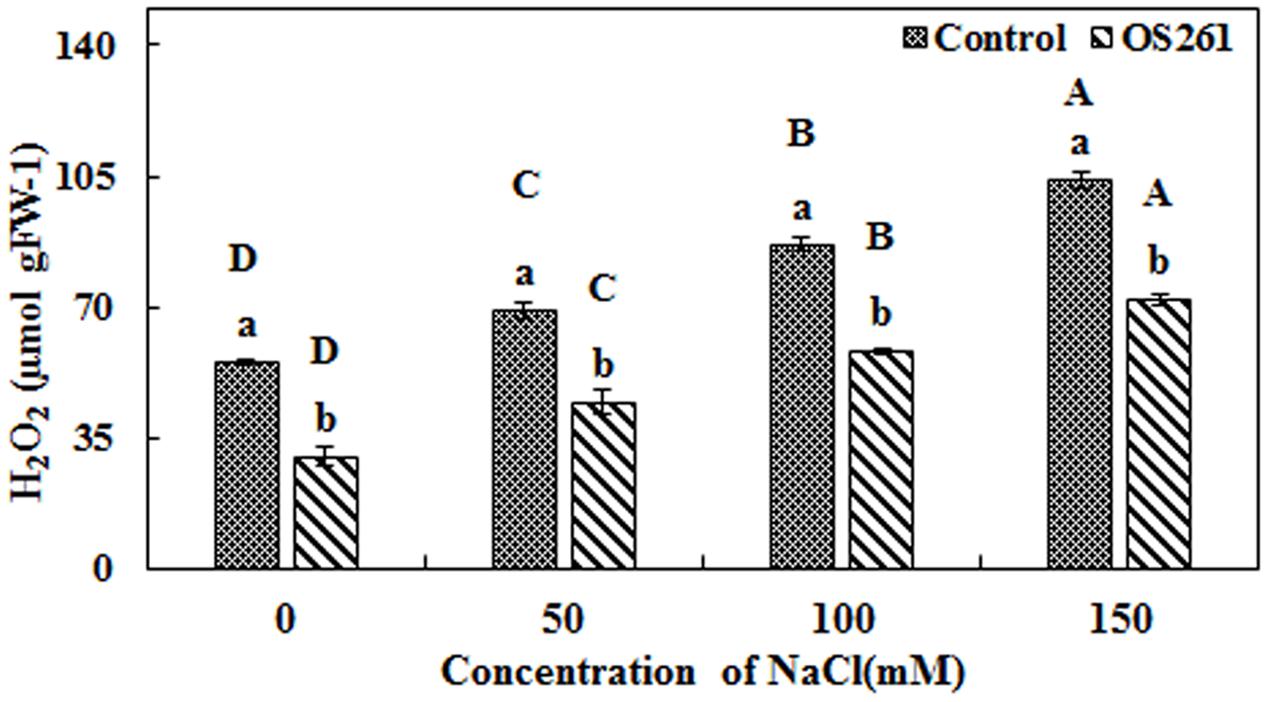
FIGURE 5. Effect of P. frederiksbergensis OS261 inoculation on hydrogen peroxide content in red pepper plants under salinity stress. Different letters indicate significant differences P < 0.05 among the treatments at each salt levels (a, b) or among salt levels for each treatment: Control (A, B, C, D), OS261 (A, B, C, D). Each value represents the mean of three replicates ± standard error (SE).
Discussion
The use of ACC deaminase producing PGPR in soil resulted in an enhanced growth of red pepper plants under salt stress. These positive effects were exerted by maintaining the low levels of ethylene and modulating the expression of ROS-scavenging enzymes. Ethylene is a plant growth regulator, which is involved in several physiological responses (Abeles et al., 1992). In addition to that, it is also a stress hormone as it is synthesized rapidly under stress (Stearns and Glick, 2003). Several studies (Belimov et al., 2001; Saravanakumar and Samiyappan, 2007) have reported that ethylene reduces seed germination rate and root development during stress, which eventually affects plant growth. Microorganisms synthesizing the ACC deaminase enzyme can cleave ACC to α-ketobutyrate and ammonia, thereby decreasing ethylene stress in plants (Glick, 2005; Sun et al., 2009; Siddikee et al., 2011; Rashid et al., 2012). In this study, a PGPR strain P. frederiksbergensis OS261 which possesses ACC deaminase activity demonstrated its effectiveness in inducing salt tolerance and consequent improvement in the growth of pepper plants under salt stress.
Pepper (Capsicum spp.) along with tomato and potato is an economically important genus of Solanaceae family. Owing to its ability to adapt to different climates and being available in varieties of shapes, sizes and color, pepper plants are cultivated all over the world (Qin et al., 2014). Various biotic stresses such as viruses and abiotic stresses especially drought and salinity are known to affect this economically important family. Aktas et al. (2006) reported that pepper plants are highly susceptible to salinity stress. In plant growth experiments, pepper plants exhibited a decrease in plant height with an increase in the salinity in the un-inoculated controls. Salinity stress is one of the main obstacles that limit higher crop production. It increases the osmotic potential of the growth medium resulting the seeds to require more energy for water absorption, which leads to poor germination (Jamil and Rha, 2004). It decreases the rate of photosynthesis by restricting the photosynthetic electron transport, which in turn paralyzes the whole plant mechanism for survival and development (Hameed et al., 2015). In the present study, in contrast to the un-inoculated plants, the inoculated or bacterized plants showed enhancement in the growth despite increasing salinity. It indicates the ACC deaminase producing isolate P. frederiksbergensis OS261 is able to ameliorate the effect of salt on growth medium. Our results were consistent with the other findings (Mayak et al., 2004; Madhaiyan et al., 2006; Zahir et al., 2009; Ahmad et al., 2012; Cheng et al., 2012), where ACC deaminase-producing bacteria enhanced the growth of red pepper plants compared to un-inoculated control. Additionally, the stimulation of different crops by PGPR has been verified in both laboratory and field trials. P. putida and P. fluorescens have been found to play a considerable role in root and shoot elongation in canola, lettuce, and tomato (Kloepper et al., 1988; Hall et al., 1996) as well as crop yields in potato, radish, rice, sugar beet, tomato, lettuce, apple, citrus, beans, some ornamental plants, and wheat (Kloepper et al., 1988; Lemanceau, 1992; Kloepper, 1994). Interestingly, a study by Li et al. (2000) and Madhaiyan et al. (2006) observed that PGPR with ACC deaminase mutants could not modulate plant growth whereas ACC deaminase transgenic canola plants showed improved growth under salinity stress (Sergeeva et al., 2006). On the basis of the earlier studies, it can be suggested that ACC deaminase producing bacteria can promote plant growth and increase dry mass under salinity stress by cleaving ACC, which consequently reduces ethylene levels.
Abiotic stress such as salinity leads to ROS formation especially superoxide, hydroxyl ion, singlet oxygen and hydrogen peroxide, which results in the oxidation of cell membranes thereby causing damage to membrane and cell structures. These processes are called as oxidative stress (Apel and Hirt, 2004; Hussain et al., 2013). In plants, a defensive system consisting of ROS scavenging enzymes namely SOD, peroxidase (POD), glutathione reductase (GR), mono-hydroascorbate reductase (MDHAR), APX, CAT and redox ions work in a concerted way to carry out ROS detoxification (Mittler, 2002; Abogadallah, 2011; Abbas et al., 2013).
The pepper plants inoculated with ACC deaminase-containing PGPR exhibited a significant elevation of the antioxidant enzyme CAT compared to the un-inoculated plants suggesting that they were adapted to saline conditions by eliminating ROS. Our results were consistent with Gururani et al. (2013), where the CAT activity was enhanced in the inoculated potato plants under salt stress and also Subramanian et al. (2015), where the enzyme activity was improved in the inoculated tomato plants under cold stress. In the bacterium inoculated plants, the SOD activity showed a significant decrease in higher salinity levels suggesting neutralization of potentially harmful molecules leading to alleviation of salt stress (Cavalcanti et al., 2004). Similarly, the APX activity showed a pattern similar to SOD, where the bacterial inoculation significantly reduced the enzyme activity under salt stress (Han and Lee, 2005; Upadhyay et al., 2012). The reason behind the decrease may be the lack of hydrogen peroxide production in the plants inoculated with bacteria, where PGPR played a major role in maintaining the normal homeostasis of plant system (Gururani et al., 2013). The antioxidant enzyme activities have been studied extensively, but still the significance of enzymes in salt tolerance is not clear, as high antioxidant activities are linked to both salt tolerance and sensitivity (Abogadallah, 2010). Many studies (Shalata and Tal, 1998; Shalata et al., 2001; Mittova et al., 2003; Sumithra et al., 2006; Hediye Sekmen et al., 2007) have related the salt tolerance to an increase in the activity of antioxidant enzymes, whereas on the contrary, few studies (Cavalcanti et al., 2004; Kim et al., 2004) have shown that a higher salt tolerance does not depend always on a higher antioxidant activity.
Conclusion
The present study illustrated that P. frederiksbergensis OS261 can promote salt stress tolerance in red pepper by regulating ethylene biosynthesis and increasing CAT enzyme activity. A further investigation on the genes responsible for CAT enzyme may reveal the exact mechanism by which P. frederiksbergensis OS261 enhances salt stress tolerance. This strain has already been well studied and proved to enhance plant cold stress tolerance. The use of this strain as a bio-inoculant can also enhance plant growth under other stress conditions. Therefore, it may be recommended to the farmer as a stress mitigator for the regions with high soil salinity after assessing its field level performance.
Author Contributions
PC, SS, and TS: conception and design of the work. PC, SS, and YK: performed the work. PC, SS, YK and KK: acquisition of data. PC, SS, GS and KK: analyzed the data. PC, SS, GS, RA and TS: critical revision of manuscript. PC, SS, RA and TS: wrote the paper.
Funding
This work was supported by the Strategic Initiative for Microbiomes in Agriculture and Food, Ministry of Agriculture (914004-4), Food and Rural Affairs, Republic of Korea.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbas, T., Pervez, M. A., Ayyub, C. M., and Ahmad, R. (2013). Assessment of morphological, antioxidant, biochemical and ionic responses of salt-tolerant and salt-sensitive okra (Abelmoschus esculentus) under saline regime. Pakistan J. Life Soc. Sci. 11, 147–153.
Abeles, F. B., Morgan, P. W., and Saltveit, M. E. Jr. (1992). Ethylene in Plant Biology, 2nd Edn. San Diego, CA: Academic Press Inc.
Abogadallah, G. M. (2010). Insights into the significance of antioxidative defense under salt stress. Plant Signal. Behav. 5, 369–374. doi: 10.4161/psb.5.4.10873
Abogadallah, G. M. (2011). Differential regulation of photorespiratory gene expression by moderate and severe salt and drought stress in relation to oxidative stress. Plant Sci. 180, 540–547. doi: 10.1016/j.plantsci.2010.12.004
Ahmad, M., Zahir, Z. A., Asghar, H. N., and Arshad, M. (2012). The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vigna radiata L.) under salt-stressed conditions. Ann. Microbiol. 62, 1321–1330. doi: 10.1007/s13213-011-0380-9
Aktas, H., Abak, K., and Cakmak, I. (2006). Genotypic variation in the response of pepper to salinity. Sci. Hortic. 110, 260–266. doi: 10.1016/j.scienta.2006.07.017
Allakhverdiev, S. I., Sakamoto, A., Nishiyama, Y., Inaba, M., and Murata, N. (2000). Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 123, 1047–1056. doi: 10.1104/pp.123.3.1047
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Bacilio, M., Moreno, M., and Bashan, Y. (2016). Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl. Soil Ecol. 107, 394–404. doi: 10.1016/j.apsoil.2016.04.012
Bano, A., and Fatima, M. (2009). Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 45, 405–413. doi: 10.1007/s00374-008-0344-9
Belimov, A. A., Safronova, V. I., Sergeyeva, T. A., Egorova, T. N., Matveyeva, V. A., Tsyganov, V. E., et al. (2001). Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 47, 642–652. doi: 10.1139/w01-062
Bharti, N., Yadav, D., Barnawal, D., Maji, D., and Kalra, A. (2013). Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J. Microbiol. Biotechnol. 29, 379–387. doi: 10.1007/s11274-012-1192-1
Bojórquez-Quintal, E., Velarde-Buendía, A., Ku-González, A., Carillo-Pech, M., Ortega-Camacho, D., Echevarría-Machado, I., et al. (2014). Mechanisms of salt tolerance in habanero pepper plants (Capsicum chinense Jacq.): proline accumulation, ions dynamics and sodium root-shoot partition and compartmentation. Front. Plant Sci. 5:605. doi: 10.3389/fpls.2014.00605
Cavalcanti, F. R., Oliveira, J. T. A., Martins-Miranda, A. S., Viégas, R. A., and Silveira, J. A. G. (2004). Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 163, 563–571. doi: 10.1111/j.1469-8137.2004.01139.x
Cheng, Z., Park, E., and Glick, B. R. (2007). 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 53, 912–918. doi: 10.1139/W07-050
Cheng, Z., Woody, O. Z., McConkey, B. J., and Glick, B. R. (2012). Combined effects of the plant growth-promoting bacterium Pseudomonas putida UW4 and salinity stress on the Brassica napus proteome. Appl. Soil Ecol. 61, 255–263. doi: 10.1016/j.apsoil.2011.10.006
Chojak-Koźniewska, J., Linkiewicz, A., Sowa, S., Radzioch, M. A., and Kuźniak, E. (2017). Interactive effects of salt stress and Pseudomonas syringae pv. lachrymans infection in cucumber: involvement of antioxidant enzymes, abscisic acid and salicylic acid. Environ. Exp. Bot. 136, 9–20. doi: 10.1016/j.envexpbot.2017.01.004
Ding, S., Huang, C. L., Sheng, H. M., Song, C. L., Li, Y. B., and An, L. Z. (2011). Effect of inoculation with the endophyte Clavibacter sp. strain Enf12 on chilling tolerance in Chorispora bungeana. Physiol. Plant. 141, 141–151. doi: 10.1111/j.1399-3054.2010.01428.x
Dodd, I. C., and Pérez-Alfocea, F. (2012). Microbial amelioration of crop salinity stress. J. Exp. Bot. 63, 3415–3428. doi: 10.1093/jxb/ers033
Forieri, I., Hildebrandt, U., and Rostás, M. (2016). Salinity stress effects on direct and indirect defence metabolites in maize. Environ. Exp. Bot. 122, 68–77. doi: 10.1016/j.envexpbot.2015.09.007
Glick, B., Penrose, D., and Li, J. (1998). A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 190, 63–68. doi: 10.1006/jtbi.1997.0532
Glick, B. R. (2005). Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 251, 1–7. doi: 10.1016/j.femsle.2005.07.030
Goth, L. (1991). A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 196, 143–151. doi: 10.1016/0009-8981(91)90067-M
Gururani, M. A., Upadhyaya, C. P., Baskar, V., Venkatesh, J., Nookaraju, A., and Park, S. W. (2013). Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 32, 245–258. doi: 10.1007/s00344-012-9292-6
Habib, S. H., Kausar, H., and Saud, H. M. (2016). Plant growth-promoting rhizobacteria enhance salinity stress tolerance in Okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016:6284547. doi: 10.1155/2016/6284547
Hall, J. A., Peirson, D., Ghosh, S., and Glick, B. R. (1996). Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12–2. Isr. J. Plant Sci. 44, 37–42. doi: 10.1080/07929978.1996.10676631
Hamdia, M. A., Shaddad, M. A. K., and Doaa, M. M. (2004). Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 44, 165–174. doi: 10.1007/s10725-004-3131-0
Hameed, A., Gulzar, S., Aziz, I., Hussain, T., Gul, B., and Khan, M. A. (2015). Effects of salinity and ascorbic acid on growth, water status and antioxidant system in a perennial halophyte. AoB Plants 7:lv004. doi: 10.1093/aobpla/plv004
Han, H. S., and Lee, K. D. (2005). Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 1, 210–215.
Hediye Sekmen, A., Türkan, I., and Takio, S. (2007). Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol. Plant. 131, 399–411. doi: 10.1111/j.1399-3054.2007.00970.x
Hussain, S., Khaliq, A., Matloob, A., Wahid, M. A., and Afzal, I. (2013). Germination and growth response of three wheat cultivars to NaCl salinity. Soil Environ. 32, 36–43.
Islam, M. R., Sultana, T., Joe, M. M., Yim, W., Cho, J. C., and Sa, T. (2013). Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper. J. Basic Microbiol. 53, 1004–1015. doi: 10.1002/jobm.201200141
Jamil, M., and Rha, E. S. (2004). The effect of salinity (NaCl) on the germination and seedling of sugar beet (Beta vulgaris L.) and cabbage (Brassica oleracea L.). Plant Resour. 7, 226–232.
Kim, Y., Arihara, J., Nakayama, T., Nakayama, N., Shimada, S., and Usui, K. (2004). Antioxidative responses and their relation to salt tolerance in Echinochloa oryzicola vasing and Setaria virdis (L.) Beauv. Plant Growth Regul. 44, 87–92. doi: 10.1007/s10725-004-2746-5
Kloepper, J. W. (1994). “Plant growth-promoting rhizobacteria (other systems),” in Azospirillum/Plant Associations, ed. Y. Okon (Boca Raton, FL: CRC Press), 137–166.
Kloepper, J. W., Lifshitz, R., and Schroth, M. N. (1988). Pseudomonas inoculants to benefit plant production. ISI Atlas Sci. Anim. Plant Sci. 1, 60–64. doi: 10.1007/s00253-009-2092-7
Lemanceau, P. (1992). Effects bénefiqués de rhizobactéries sur les plants: example des Pseudomonas spp. fluorescents. Agronomie 12, 413–437. doi: 10.1051/agro:19920601
Li, J., Ovakim, D. H., Charles, T. C., and Glick, B. R. (2000). An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr. Microbiol. 41, 101–105. doi: 10.1007/s002840010101
Madhaiyan, M., Poonguzhali, S., Ryu, J., and Sa, T. (2006). Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta 224, 268–278. doi: 10.1007/s00425-005-0211-y
Mayak, S., Tirosh, T., and Glick, B. R. (2004). Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 42, 565–572. doi: 10.1016/j.plaphy.2004.05.009
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Mittova, V., Tal, M., Volokita, M., and Guy, M. (2003). Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 26, 845–856. doi: 10.1046/j.1365-3040.2003.01016.x
Morgan, P. W., and Drew, M. C. (1997). Ethylene and plant responses to stress. Physiol. Plant. 100, 620–630. doi: 10.1034/j.1399-3054.1997.1000325.x
Munne-Bosch, S., and Pinto-Marijuan, M. (2016). Free radicals, oxidative stress and antioxidants. Encycl. Appl. Plant Sci. 2, 16–19. doi: 10.1016/b978-0-12-394807-6.00077-0
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Patra, J. K., Das, G., Paramithiotis, S., and Shin, H. S. (2016). Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front. Microbiol. 7:1493. doi: 10.3389/fmicb.2016.01493
Qin, C., Yu, C., Shen, Y., Fang, X., Chen, L., Min, J., et al. (2014). Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. U.S.A. 111, 5135–5140. doi: 10.1073/pnas.1400975111
Rashid, S., Charles, T. C., and Glick, B. R. (2012). Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 61, 217–224. doi: 10.1016/j.apsoil.2011.09.011
Sandipan, S., Parthiban, S., Melvin Jeo, M., Chanratana, M., Poulami, C., and Sa, T. (2016). “Plant growth promoting characteristics of Pseudomonas frederiksbergensis OS261 and its effect on early growth of red pepper plants under greenhouse conditions,” in Proceedings of the Korea Society of Soil Science and Fertilizer Day 2016 Celebrates the Spring Conference of the Republic of Korea Soil, Seoul, 61–62.
Sang, M. K., Chun, S. C., and Kim, K. D. (2008). Biological control of Phytophthora blight of pepper by antagonistic rhizobacteria selected from a sequential screening procedure. Biol. Control 46, 424–433. doi: 10.1016/j.biocontrol.2008.03.017
Saravanakumar, D., and Samiyappan, R. (2007). ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 102, 1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x
Schubert, S., Neubert, A., Schierholt, A., Sümer, A., and Zörb, C. (2009). Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Sci. 177, 196–202. doi: 10.1016/j.plantsci.2009.05.011
Sergeeva, E., Shah, S., and Glick, B. R. (2006). Growth of transgenic canola (Brassica napus cv. Westar) expressing a bacterial 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene on high concentrations of salt. World J. Microbiol. Biotechnol. 22, 277–282. doi: 10.1007/s11274-005-9032-1
Shalata, A., Mittova, V., Volokita, M., Guy, M., and Tal, M. (2001). Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol. Plant. 112, 487–494. doi: 10.1034/j.1399-3054.2001.1120405.x
Shalata, A., and Tal, M. (1998). The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant. 104, 169–174. doi: 10.1034/j.1399-3054.1998.1040204.x
Siddikee, M. A., Chauhan, P. S., Anandham, R., Han, G. H., and Sa, T. (2010). Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 20, 1577–1584. doi: 10.4014/jmb.1007.07011
Siddikee, M. A., Glick, B. R., Chauhan, P. S., Yim, W. J., and Sa, T. (2011). Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 49, 427–434. doi: 10.1016/j.plaphy.2011.01.015
Siddikee, M. A., Sundaram, S., Chandrasekaran, M., Kim, K., Selvakumar, G., and Sa, T. (2015). Halotolerant bacteria with ACC deaminase activity alleviate salt stress effect in canola seed germination. J. Korean Soc. Appl. Biol. Chem. 58, 237–241. doi: 10.1007/s13765-015-0025-y
Singh, R. P., and Jha, P. N. (2016). Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp. SL- 12 isolated from a salt lake. Symbiosis 69, 101–111. doi: 10.1007/s13199-016-0387-x
Stavridou, E., Hastings, A., Webster, R. J., and Robson, P. R. H. (2016). The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. GCB Bioenergy 9, 92–104. doi: 10.1111/gcbb.12351
Stearns, J. C., and Glick, B. R. (2003). Transgenic plants with altered ethylene biosynthesis or perception. Biotechnol. Adv. 21, 193–210. doi: 10.1016/S0734-9750(03)00024-7
Subramanian, P., Kim, K., Krishnamoorthy, R., Mageswari, A., Selvakumar, G., and Sa, T. (2016). Cold stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. PLoS ONE 11:e0161592. doi: 10.1371/journal.pone.0161592
Subramanian, P., Mageswari, A., Kim, K., Lee, Y., and Sa, T. (2015). Psychrotolerant endophytic Pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum Mill.) by activation of their antioxidant capacity. Mol. Plant Microbe Interact. 28, 1073–1081. doi: 10.1094/MPMI-01-15-0021-R
Sumithra, K., Jutur, P. P., Carmel, B. D., and Reddy, A. R. (2006). Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul. 50, 11–22. doi: 10.1007/s10725-006-9121-7
Sun, Y., Cheng, Z., and Glick, B. R. (2009). The presence of a1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol. Lett. 296, 131–136. doi: 10.1111/j.1574-6968.2009.01625.x
Tank, N., and Saraf, M. (2010). Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 5, 51–58. doi: 10.1080/17429140903125848
Theocharis, A., Bordiec, S., Fernandez, O., Paquis, S., Dhondt-Cordelier, S., Baillieul, F., et al. (2012). Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. Mol. Plant Microbe Interact. 25, 241–249. doi: 10.1094/MPMI-05-11-0124
Upadhyay, S. K., Singh, J. S., Saxena, A. K., and Singh, D. P. (2012). Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 14, 605–611. doi: 10.1111/j.1438-8677.2011.00533.x
Yaish, M. W., Al-Lawati, A., Jana, G. A., Patankar, H. V., and Glick, B. R. (2016). Impact of soil salinity on the structure of the bacterial endophytic community identified from the roots of caliph medic (Medicago truncatula). PLoS ONE 11:e0159007. doi: 10.1371/journal.pone.0159007
Yang, J., Kloepper, J. W., and Ryu, C. M. (2009). Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14, 1–4. doi: 10.1016/j.tplants.2008.10.004
Zahir, Z. A., Ghani, U., Naveed, M., Nadeem, S. M., and Asghar, H. N. (2009). Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 191, 415–424. doi: 10.1007/s00203-009-0466-y
Keywords: plant growth promotion, salt stress, Pseudomonas frederiksbergensis, antioxidant enzyme, bio-fertilizer
Citation: Chatterjee P, Samaddar S, Anandham R, Kang Y, Kim K, Selvakumar G and Sa T (2017) Beneficial Soil Bacterium Pseudomonas frederiksbergensis OS261 Augments Salt Tolerance and Promotes Red Pepper Plant Growth. Front. Plant Sci. 8:705. doi: 10.3389/fpls.2017.00705
Received: 17 January 2017; Accepted: 18 April 2017;
Published: 04 May 2017.
Edited by:
Jesús Mercado-Blanco, Consejo Superior de Investigaciones Científicas, SpainReviewed by:
Khaled Abbas El-Tarabily, United Arab Emirates University, United Arab EmiratesDilfuza Egamberdieva, Leibniz-Zentrum für Agrarlandschaftsforschung, Germany
László Kredics, University of Szeged, Hungary
Copyright © 2017 Chatterjee, Samaddar, Anandham, Kang, Kim, Selvakumar and Sa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongmin Sa, tomsa@chungbuk.ac.kr
†These authors have contributed equally to this work.
 Poulami Chatterjee
Poulami Chatterjee Sandipan Samaddar
Sandipan Samaddar Rangasamy Anandham
Rangasamy Anandham Yeongyeong Kang1
Yeongyeong Kang1 Gopal Selvakumar
Gopal Selvakumar Tongmin Sa
Tongmin Sa