- 1Inner Mongolia Research Center for Prataculture, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China
- 2Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences, Hohhot, China
- 3University of Chinese Academy of Sciences, Beijing, China
The formation of fertility islands by shrubs increases soil resources heterogeneity in thicketization-grasslands. Clonal plants, especially rhizomatous or stoloniferous clonal plants, can form large clonal networks and use heterogeneously distributed resources effectively. In addition, shrubs, especially spiny shrubs, may also provide herbaceous plants with protection from herbivores, acting as ‘shelters’. The interaction between pre-dominated clonal herbaceous plants and encroaching shrubs remains unclear in thicketization-grassland under grazing pressure. We hypothesized that clonal herbaceous plants can be facilitated by encroached shrubs as a ‘shelter from herbivores’ and/or as an ‘increased soil resources’ under grazing pressure. To test this hypothesis, a total of 60 quadrats were chosen in a thicket-grassland in northern China that was previously dominated by Leymus chinensis and was encroached upon by the spiny leguminous plant Caragana intermedia. The soil and plant traits beneath and outside the shrub canopies were sampled, investigated and contrasted with an enclosure. The soil organic matter, soil total nitrogen and soil water content were significantly higher in the soil beneath the shrub canopies than in the soil outside the canopies. L. chinensis beneath the shrub canopies had significantly higher plant height, single shoot biomass, leaf length and width than outside the shrub canopies. There were no significantly differences between plant growth in enclosure and outside the shrub canopies. These results suggested that under grazing pressure in a grassland undergoing thicketization, the growth of the rhizomatous clonal herbaceous plant L. chinensis was facilitated by the spiny shrub C. intermedia as a ‘shelter from herbivores’ more than through ‘increased soil resources’. We propose that future studies should focus on the community- and ecosystem-level impacts of plant clonality.
Introduction
The encroachment of woody plants into grasslands, or the thicketization of grasslands, occurs worldwide (Archer, 1995; van Auken, 2000; Asner et al., 2003; Cabral et al., 2003; Laiolo et al., 2004; Brook and Bowman, 2006; Wigley et al., 2010; Soliveres et al., 2014; Masson et al., 2015). Possible drivers of these changes in the vegetation structure include climate change (Fensham et al., 2005; IPCC, 2013), livestock grazing (Brown and Archer, 1999; Sharpe and Bowman, 2004), altered fire regimes (Brook and Bowman, 2006; Srinivasan, 2012), and elevated carbon dioxide (Bond and Midgley, 2000). Through an extensive shift in the plant community structure, the thicketization of grasslands has strong potential to alter key ecosystem processes (Zavaleta and Kettley, 2006). It may lead to a decline in biodiversity (Báez and Collins, 2008), a reduction in ecosystem functioning and resilience (Caldeira et al., 2015), a loss of ecosystem carbon (Jackson et al., 2002), an increase in the soil quality (Mills and Fey, 2004; Liao et al., 2006), an increase in the soil microbial biomass (Liao and Boutton, 2008) or the enhancement of soil animal activity (Hobbs and Mooney, 1986; Fabricius et al., 2003). Soliveres et al. (2014) found that plant diversity and multifunctionality peaked at intermediate levels of woody cover. However, another 15-year study showed that the shrub invasion in an undisturbed wetland had few community-level effects (Mills et al., 2009). McKinley et al. (2008) also reported that the conversion of grasslands to coniferous woodland has a limited effect on soil nitrogen cycle processes. Anyway, it is commonly recognized that the thicketization of grassland may increase spatial heterogeneity in soil resources and enhance soil nutrient levels on small spatial scales due to the formation of fertility islands by shrubs in arid, semi-arid, and semi-humid areas (Rong et al., 2016).
Soil resources are enriched under shrub canopies, forming so-called ‘fertility islands’ (Schlesinger et al., 1996), which might affect seedling establishment (Maestre et al., 2003), plant–plant interactions (Aguiar and Sala, 1999), species distribution (Pan et al., 1998), the diversity and productivity of plant communities (Mou et al., 1995; Anderson et al., 2004), microbial activity/diversity (Molina-Montenegro et al., 2016), and the abundance and diversity of mycorrhizal fungi (Casanova-Katny et al., 2011). Many studies have documented higher nutrient levels in fertility island soil (Ludwig and Tongway, 1997; Bochet et al., 1999; Hirobe et al., 2001; Li et al., 2007, 2008). Higher nutrient levels and higher competition from shrub common affects the growth of grass beneath shrub canopies, while lower nutrient levels and lower competition from shrub affects the growth of grass outside shrub canopies. The ultimate effects (positive or negative) of fertility islands on the growth of grass were species-specific (Zhang et al., 2016).
Clonal plants, especially rhizomatous or stoloniferous clonal plants, occupy relatively large habitats (Liu et al., 2007), dominate many ecosystems (Song et al., 2002), and play a key role in thicketization-grassland (Formica et al., 2014). Clonal plants can efficiently use heterogeneously distributed resources through their unique features, such as clonal plasticity (Gough et al., 2012), clonal integration (Roiloa and Hutchings, 2013; Liu et al., 2016), the intra-clonal division of labor (de Kroon and Hutchings, 1995), and clonal foraging (Yan et al., 2013), although there are trade-offs between different clonal growth forms (Ye et al., 2006, 2015). Clonal plants have the potential to decrease the differences in resource supplies between different parts of the clones (Eriksson and Jerling, 1990), and then may have powerful effects on resource heterogeneity (Magyar et al., 2004; Cornelissen et al., 2014). Rhizomatous or stoloniferous clonal plants always create a clonal network consisting of a large number of interconnected ramets (Charpentier et al., 2012; Song et al., 2013), some of which are distributed beneath shrub canopies in thicketization-grassland. A large body of evidence has demonstrated that ramets growing in high-resource patches translocate resources to the interconnected ramets growing in low-resource patches through horizontal structures such as rhizomes, stolons, or roots (Atkinson and Else, 2012; Roiloa and Hutchings, 2013; Pinno and Wilson, 2014; Roiloa et al., 2014). That observation indicates that the rich resources in the soil of fertility islands might be translocated by ramets that are distributed beneath shrub canopies to ramets that are distributed in the interspace between shrubs to promote the growth of ramets outside the canopy, resulting the feedback of soil nutrient outside the canopy through plant decomposition (Magyar et al., 2004) and/or resources releasing (Ye et al., 2016). Thus, when shrubs encroach into an ecosystem dominated by rhizomatous clonal plants, the effects of shrub fertility islands may then diminish via the influence of clonal plants.
Shrubs with poor palatability, especially spiny shrubs, may provide herbaceous plants with protection from herbivores by reducing access to plants living underneath their canopies (Rousset and Lepart, 1999). Under grazing pressure, clonal herbaceous plants may make the shrub canopies as ‘shelter from herbivores’ and selectively distribute more resources (higher plant biomass and/or higher ramets number) beneath the canopies. Additionally, the decaying biomass of clonal plants feeds nutrients back into the soil (Magyar et al., 2004). Thus, clonal plants can intensify the effects of shrub fertility islands.
The aim of the present study is to investigate the effects of a spiny shrub, Caragana intermedia, on a rhizomatous herbaceous plant, Leymus chinensis, in the thicketization-grassland of Inner Mongolia, China. We hypothesized that clonal plants can be facilitated by encroached shrubs as a ‘shelter from herbivores’ and/or as an ‘increased soil resources’ under grazing pressure. Specifically, does a clonal plant network of L. chinensis diminish or intensify the effects of the fertility islands formed by C. intermedia shrubs? Alternatively, does the clonal herbaceous zone treat the presence of the shrub canopy as a ‘increased soil resources’ more than a ‘shelter from herbivores’?
Materials and Methods
Study Site
This study was conducted in Siziwang Banner Research Station, affiliated with the Inner Mongolia Academy of Agriculture and Animal Husbandry Sciences. The station (41°47.28′ N, 111°53.77′ E; 1450 m, a. s. l.) is located in western Inner Mongolia, China, and it has a temperate continental climate that is characterized by a short growing season and a long, cold winter. The mean annual temperature ranges from 5.0 to 8.5°C, with a minimum mean month temperature of -15.1°C (January) and a maximum mean month temperature of 19.6°C (July). The mean annual precipitation is 280 mm, with most precipitation occurring between June and September.
Desert grassland is the dominant ecosystem in this area, and it is dominated by Stipa breviflora Griseb. (Gramineae), Artemisia frigida Willd. (Asteraceae), and Cleistogenes songorica (Roshev.) Ohwi (Gramineae), accompanied by Convolvulus ammannii Desr. (Convolvulaceae), Heteropappus altaicus (Willd.) Novopokr. (Asteraceae), C. stenophylla Pojark. (Fabaceae), C. intermedia Kuang et H. C. Fu (Fabaceae), and L. chinensis (Trin.) Tzvelev (Gramineae). However, the thicketization of grassland occurs frequently due to the long-term overgrazing (about 1 sheep per hectare in summer) in this area (Zhao et al., 2014; Supplementary Figure 1). In our study site, the encroaching spiny shrub C. intermedia has poor palatability, and the rhizomatous clonal plant L. chinensis has strong clonal growth and dominates the thicketization-grassland. L. chinensis always form a large rhizomatous network which distributes in the 5–15 cm soil layer, and the rhizomatous between ramets can survive for approximately 4 years (Bai et al., 2009).
Field Sampling and Laboratory Analysis
In September 2014, a 200 × 200 m C. intermedia–L. chinensis plant community near the station was chosen. Forty-eight shrub canopies of C. intermedia with different size (from 60 cm × 50 cm to 500 cm × 358 cm) was chose and the shrub crown width (both the long and narrow sides) was measured. A 50 cm × 50 cm quadrats was established in the center of each shrub canopy for field investigation and sampling. For contrast, six 50 cm × 50 cm quadrats outside the C. intermedia canopies in the community and six 50 cm × 50 cm quadrats in an enclosure (an area of 4 ha, southwest 600 m of investigated community) were also chosen for field investigation and sampling. This enclosure was dominated by L. chinensis but without shrub encroachment, and in which grazing had been prohibited for more than 10 years. For each quadrat, the coverage and abundance of L. chinensis were measured, and five plants (that had not been damaged by animals) were chosen for measuring plant height; five intact leaves per plant were chosen for measuring the leaf length and width. All the living shoot biomass of L. chinensis was harvested and oven-dried at 75°C for ≥24 h to a constant mass before being weighed. A soil core (5 cm in diameter) was taken and divided into two strata from the 0–10 cm and 10–20 cm depths. Half of each soil sample was weighed to obtain the fresh weight, followed by oven-drying at 150°C for ≥48 h and weighing to obtain the dry weight, and then calculated soil water content (SWC). The other half was used for chemical analysis. For each quadrat beneath the C. intermedia canopies, the coverage, plant height and branch density of C. intermedia were also measured.
The soil total nitrogen (STN) and soil total phosphorus (STP) were analyzed according to the micro-Kjeldahl method (Kjeltec 2200 Auto Distillation Unit, FOSS, Sweden), the soil organic matter (SOM) was analyzed using an elemental analyzer (Vario EL III, CHNOS Elemental Analyzer, Elementar Analysensysteme GmbH, Germany), and the pH was measured by soil acidometer titration (Sartorius PB-10, Sartorius, Germany).
Data Analysis
We calculated the single shoot biomass of L. chinensis by taking the total shoot biomass divided by the number of individuals. Statistical analyses were performed using SPSS18 (SPSS Inc., United States, 2009). A One-way analysis of variance (ANOVA) was used to test the difference in plant and soil traits among different sites (beneath C. intermedia canopies under grazing, outside C. intermedia canopies under grazing, and under grazing prohibition), followed by Tukey’s HSD tests for multiple comparisons. We used two-way ANOVAs to analyze the effects of different soil layers and different sites on soil traits. And a two-tailed Pearson’s correlation test was used to analyze the relationship between each L. chinensis and C. intermedia trait. We also used linear regressing to analysis the relationship between plant cover of C. intermedia and plant cover, plant density and plant biomass of L. chinensis beneath the shrub canopies. The data for the L. chinensis coverage, soil pH and STN were log10 (x+1)-transformed to satisfy the requirements for normality and homogeneity of variance.
Results
All the soil traits showed statistically significant differences among the different types of sites (beneath C. intermedia canopies under grazing, outside C. intermedia canopies under grazing, and under grazing prohibition), except for STP at the 0–10 cm depth (Table 1). Soil outside the C. intermedia canopies had the lowest SOM, STN, and SWC at both the 0–10 cm and 10–20 cm depths, and the lowest STP at the 10–20 cm depth (Figure 1). In comparison with the soil beneath the C. intermedia canopies, the soil outside the canopies had significantly lower SOM (Tukey’s test, P = 0.046) and marginally significantly lower SWC (Tukey’s test, P = 0.050) at the 0–10 depth and lower SOM (Tukey’s test, P = 0.019), SWC (Tukey’s test, P = 0.047), and STN (Tukey’s test, P = 0.042) at the 10–20 cm depth (Figure 1). Compared with the soil under grazing prohibition, the soil outside the C. intermedia canopies under grazing had significantly lower STN (Tukey’s test, P = 0.003), SOM (Tukey’s test, P = 0.020), and SWC (Tukey’s test, P = 0.023) at the 0–10 cm depth and lower STN (Tukey’s test, P = 0.031), SWC (Tukey’s test, P = 0.013), and STP (Tukey’s test, P = 0.018) at the 10–20 cm depth (Figure 1). There were no statistically significant differences in the soil traits between the soil beneath the canopies and the soil under grazing prohibition (Figure 1).
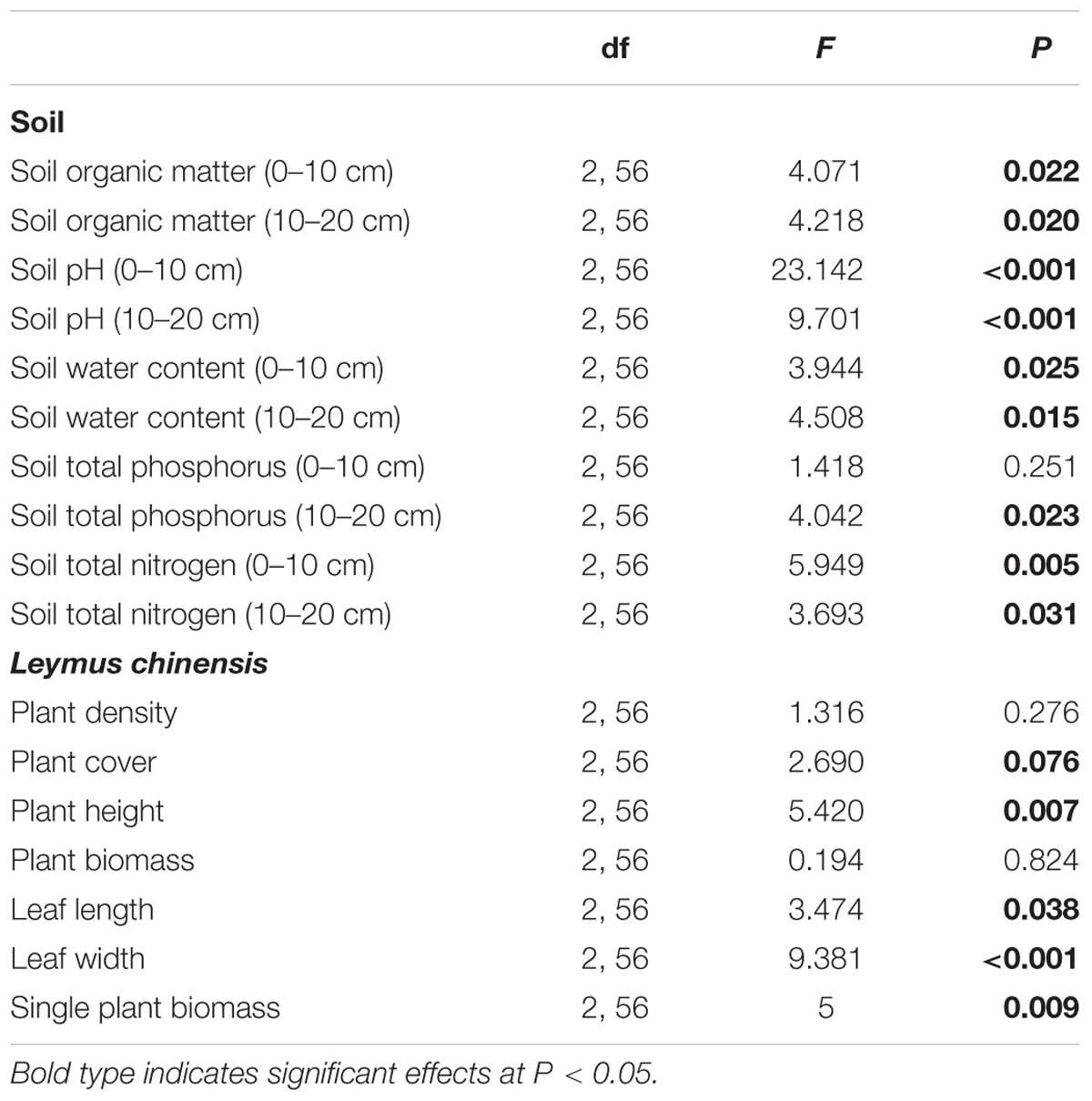
TABLE 1. Effects of the sample sites (enclosure, beneath the shrub canopies, and outside the canopies) on soil characteristics and the clonal herbaceous plant Leymus chinensis.
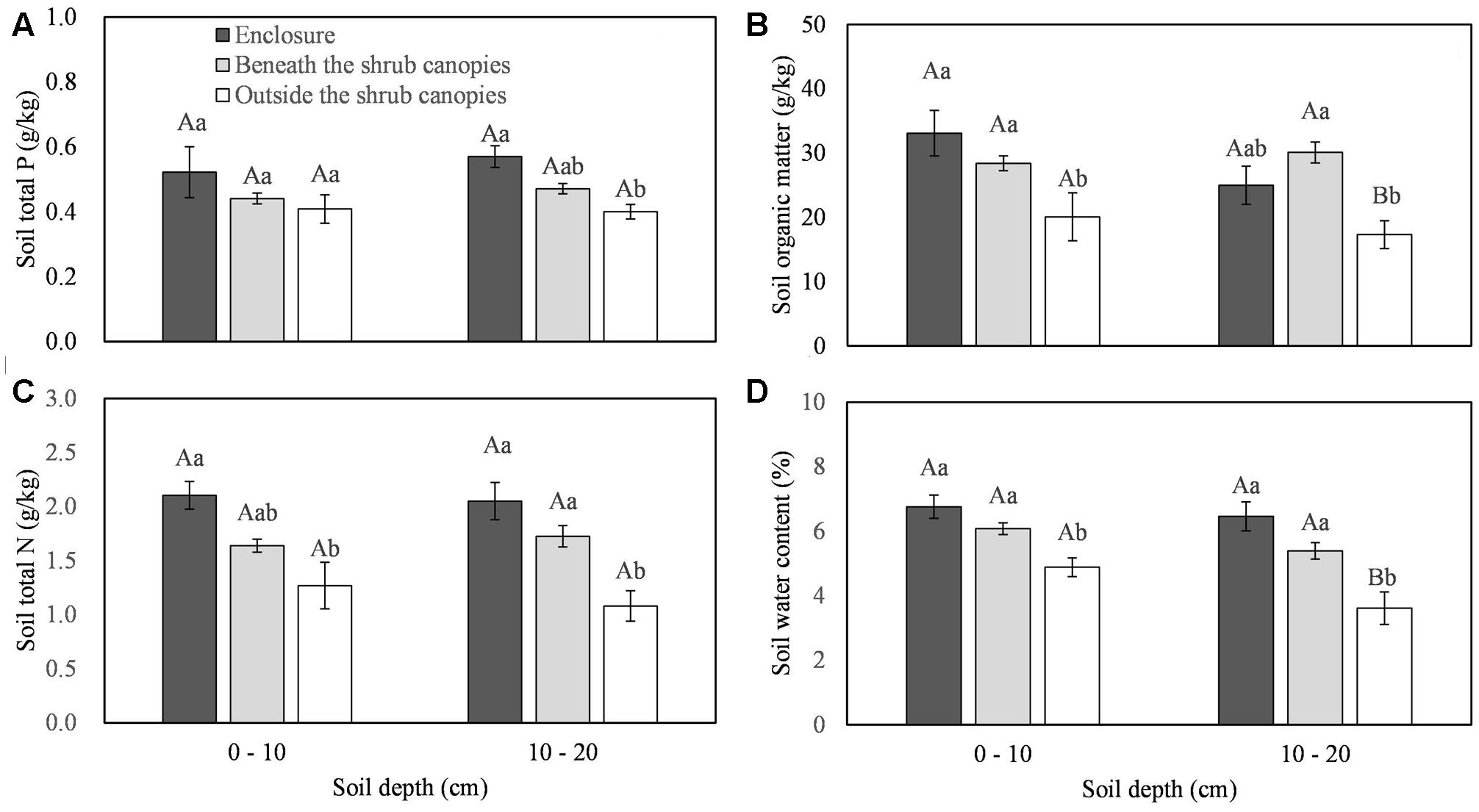
FIGURE 1. Effects of the sampling sites (enclosure, beneath the shrub canopies, and outside the shrub canopies) on the soil total P (A), soil organic matter (B), soil total N (C) and soil water content (D) in different soil layers. Different capital letters indicate a significant difference between the 0–10 cm and 10–20 cm soil layers, and different lowercase letters indicate a significant difference among the three sampling sites at P < 0.05.
The site significantly affected the plant cover, plant height, leaf length and width, and single-plant biomass of L. chinensis. However, there was no significant effect of the site on the plant density and plant biomass (Table 1). The L. chinensis beneath the C. intermedia canopies had the highest values for plant height, single shoot biomass, and leaf length and width, and showed similar plant cover and shoot biomass among the three sampling sites (Figure 2). There was no significant difference in the plant traits of L. chinensis between the area under grazing prohibition and outside the shrub canopies under grazing (Figure 2).
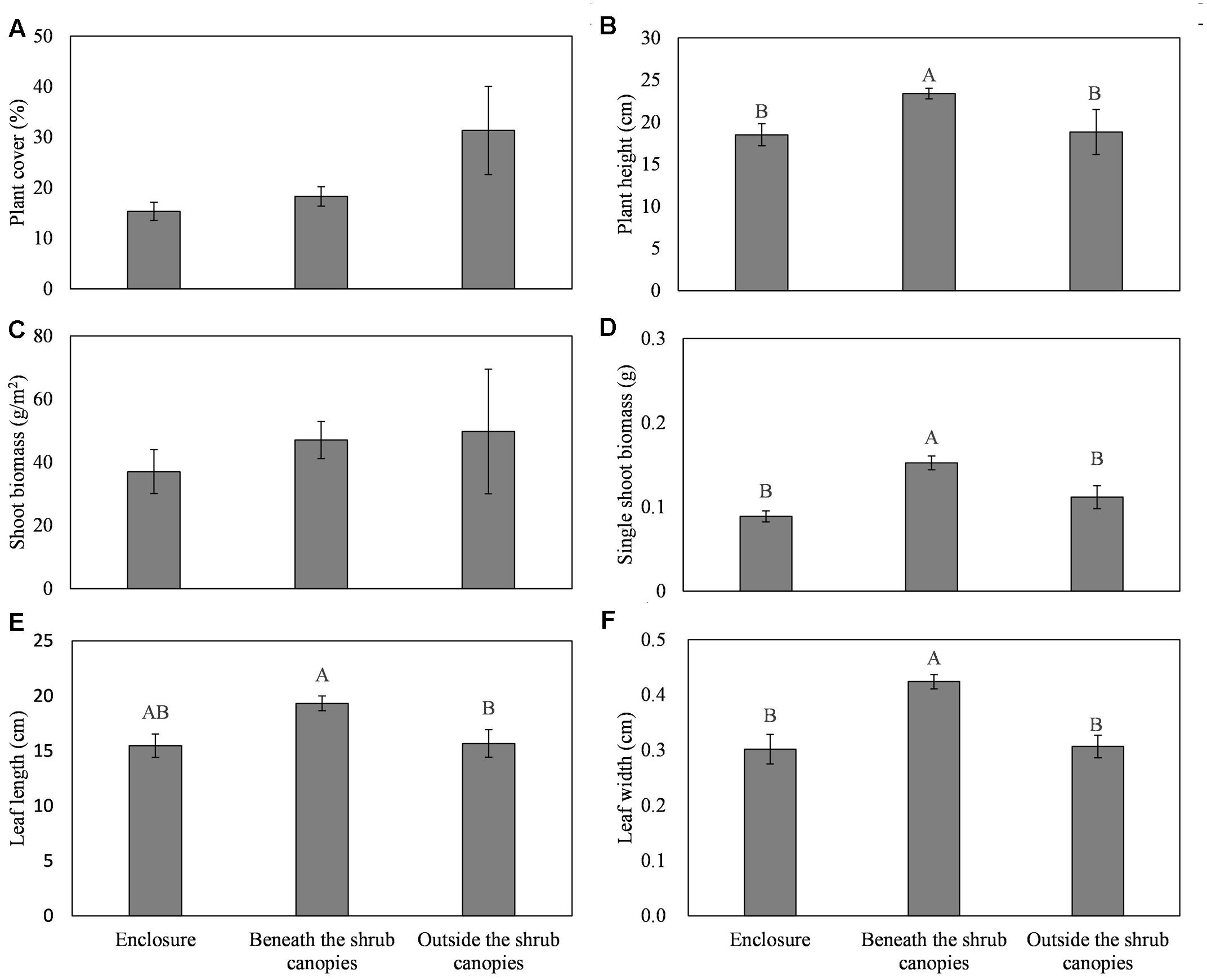
FIGURE 2. Effects of the sampling sites (enclosure, beneath the shrub canopies, and outside the shrub canopies) on the plant cover (A), height (B), shoot biomass (C), single shoot biomass (D), leaf length (E), and leaf width (F) of the clonal herbaceous plant L. chinensis. Different letters represent significant differences among the three sampling sites, while the same letters and/or no letters indicate no significant differences between any treatments at P < 0.05.
Beneath the C. intermedia canopies, the plant density, cover and biomass of L. chinensis showed significantly negative relationships with the plant cover of C. intermedia, and the leaf length of L. chinensis had a significantly positive relationship with the crown width of C. intermedia (Table 2 and Figure 3). There were no significant relationships between other L. chinensis and C. intermedia traits (Table 2).
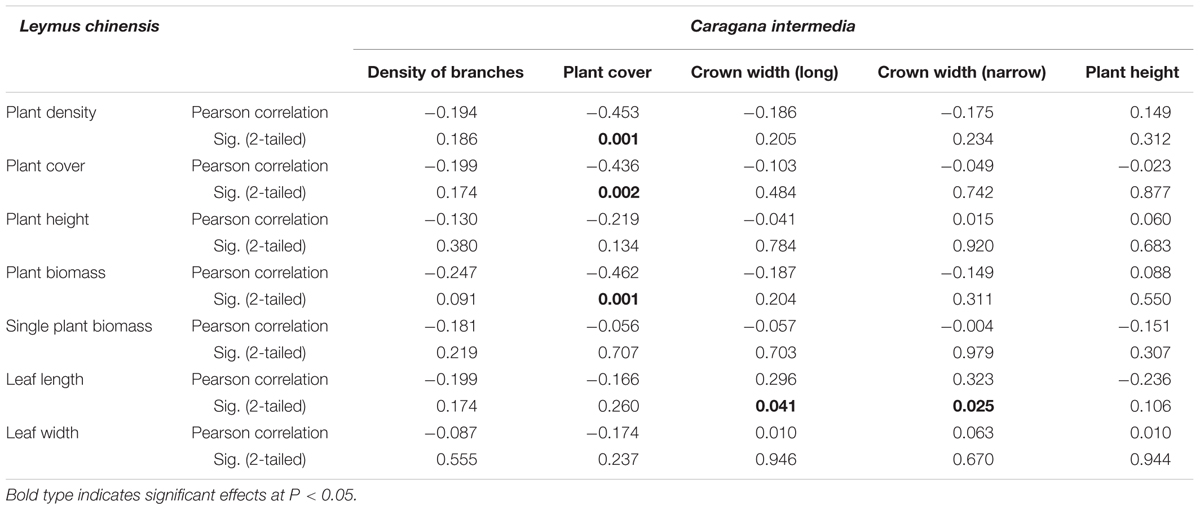
TABLE 2. The relationships between characteristics of the shrub species Caragana intermedia and the clonal herbaceous plant Leymus chinensis.
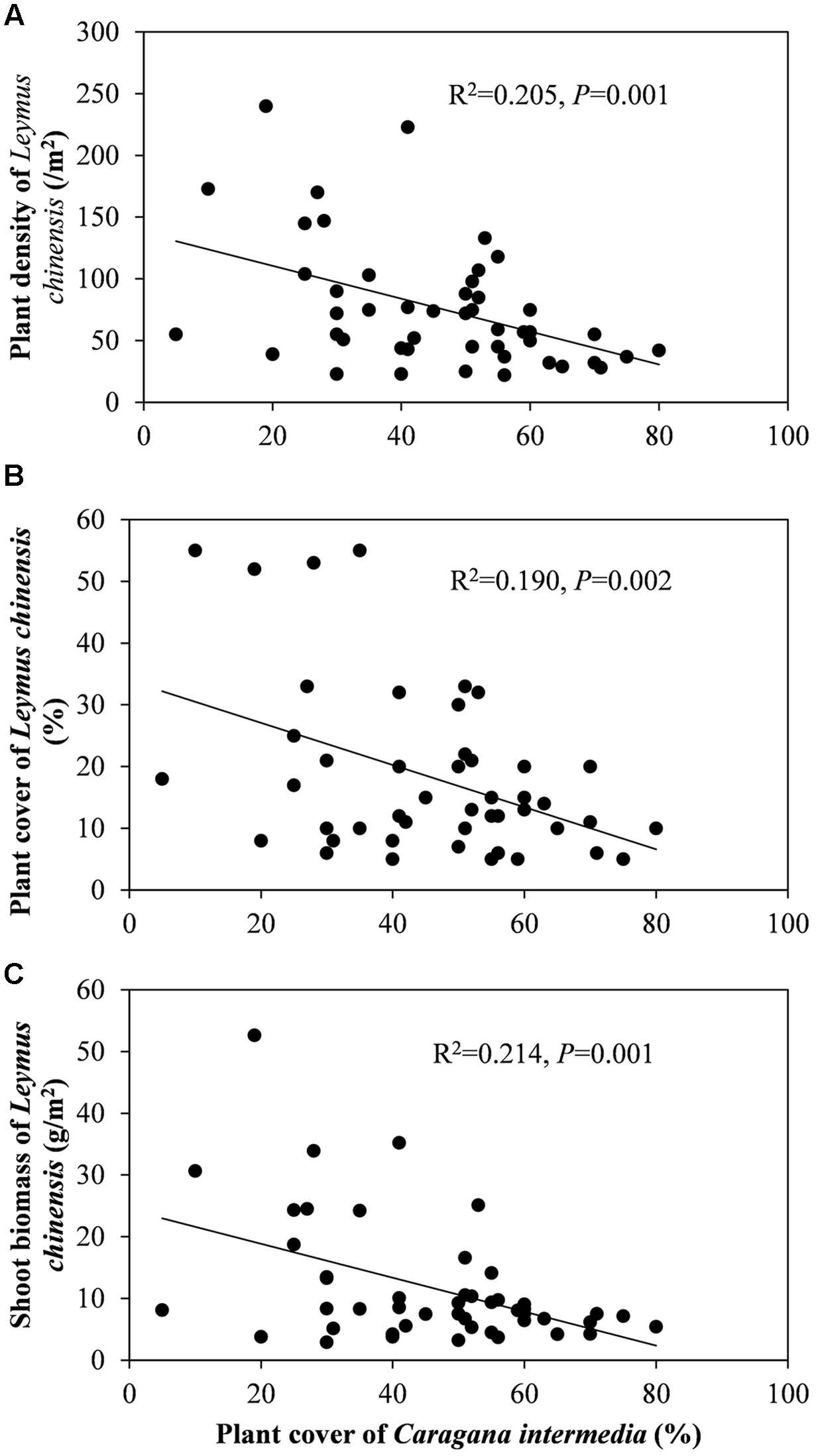
FIGURE 3. Relationship between plant cover of Caragana intermedia and plant density (A), plant cover (B) and plant shoot biomass (C) of Leymus chinensis beneath the canopies of C. intermedia.
Discussion
Positive interactions among plants, or facilitation, can occur when the presence of one plant enhances the growth, survival, or reproduction of a neighbor. Canopy facilitation plays a key role in the interaction between trees or shrubs and grass in thicketization-grassland, which is very common in semiarid and desert grasslands (Scholes and Archer, 1997; van Auken, 2000; Callaway, 2007). In northern China, livestock grazing has been demonstrated as a primary factor in the thicketization of grassland (Zhao et al., 2014). Our results showed that under grazing pressure, the rhizomatous grass L. chinensis growth was facilitated by C. intermedia shrub in northern China, with the highest plant performance occurring beneath the C. intermedia canopy (Table 1 and Figure 2). This canopy facilitation may be due to direct mechanisms such as fertility islands and/or to indirect mechanisms such as herbivore-mediated control (Callaway, 2007 as a review).
Higher levels of nutrients, in forms such as higher SOM and/or STN, were found in the soil beneath the shrub canopy in sandy grassland (Hirobe et al., 2001; Li et al., 2008), typical steppe (Peng et al., 2014), desert steppe (Pan et al., 2015), desert (Schlesinger et al., 1996; Li et al., 2007) and savanna (Mills and Fey, 2004), due to the effects of fertility islands (Schlesinger et al., 1996). A meta-analysis showed that shrub encroachment would increase the soil organic carbon content in semi-arid and humid regions, and there was a greater rate of increase in grassland that was encroached upon by leguminous shrubs than in grassland encroached upon by non-legumes (Li et al., 2016). In our study, significantly higher SOM, STN, and marginally significantly higher SWC were found in the soil beneath shrub canopies than in the soil outside the canopies (Table 1 and Figure 1), indicating that fertility islands were actually formed during the thicketization of grassland that was previously dominated by the rhizomatous clonal plant L. chinensis. If we do not consider the clonal plant traits, it seems that L. chinensis growth was facilitated by the effects of C. intermedia fertility islands in our study.
Rhizomatous clonal plants, such as L. chinensis in our study, always create large clonal networks consisting of a large number of interconnected ramets (Zhou et al., 2015), and they may cover a number of shrub canopies in the thicketization-grassland. These plants can flexibly regulate their biomass allocation to cope with changing resource availability (Xie et al., 2016). Considering the greater soil resources and lower light conditions beneath the shrub canopies, clonal plants may selectively distribute more ramets beneath the shrub canopies, and they may allocate more biomass to the roots of ramets beneath the canopies to absorb the ‘rich’ soil resources beneath shrub canopies effectively and then transport these resources to ramets outside the canopies through clonal integration (Ye et al., 2015). These resources can be used by clonal plants themselves, or they can even be redistributed into the soil and used by other plants outside the shrub canopies (Cornelissen et al., 2014; Ye et al., 2016). Therefore, one hypothesis is that the L. chinensis plants beneath the shrub canopies may have a higher plant density and lower shoot biomass/higher root biomass, and the difference in soil quality between the areas beneath and outside the C. intermedia canopies may be diminished in the context of our experiment. However, this hypothesis was obviously inconsistent with our results. Our results showed that L. chinensis allocated more resources to the shoots of the ramets beneath the shrub canopies to enhance their competitive ability and to then insure the growth of the whole clonal network under grazing pressure. In our study, clonal plant L. chinensis seems to benefit from C. intermedia shrub as a ‘shelter from herbivores’ more than as a source of ‘increased soil resources.’
Grazing is one of the primary causes for the scarcity of native herbs between the bushes and the relative abundance beneath the bushes (Jaksic and Fuentes, 1980). Under grazing pressure, shrubs may provide the herbaceous plants beneath their canopies with protection from herbivores (Rousset and Lepart, 1999), an even more important factor than mitigating abiotic stress (Louthan et al., 2014). Clonal plants may place their ramets in different microhabitats to spread the mortality of the organism and to engage in so-called risk-spreading (Eriksson and Jerling, 1990; Dong, 1996). To spread the risk from herbivores, L. chinensis in our experiment distributed some ramets beneath the C. intermedia canopies. L. chinensis ramets beneath the shrub canopies showed higher shoot biomass and larger leaves, allowing them to adapt to the lower light and higher inter-species competition (Figures 1, 2). The decaying biomass of clonal plants feeds nutrients back into the soil (Magyar et al., 2004) and then enhances the effects of shrub fertility islands, to some extent, leading to higher SOM, STN, and SWC in our study.
The interactions between shrubs and herbaceous plants are complex, including bidirectional positive and negative effects, and the ratios between positive and negative effects can shift (Holzapfel and Mahall, 1999). Facelli and Temby (2002) found that shrubs can have simultaneously facilitative and inhibitory effects on the annual plants through different mechanisms, and more importantly, different shrub species have different effects. We found that C. intermedia provided L. chinensis with facilitation (positive effects) relating to ‘shelter from herbivores’ and/or ‘increased soil resources.’ However, we also found a significantly negative relationship between the C. intermedia plant cover and plant density and the cover and biomass of L. chinensis, indicating some level of competition (negative effects) between these two species (Table 2 and Figure 3). The ‘stress gradient hypothesis’ predicts that competition should predominate in low-stress environments, with facilitation increasing in strength and/or frequency in high-stress areas (Bertness and Callaway, 1994; Callaway et al., 2002). This model indicates that if the grazing pressure is weakened to less than the competition pressure from the shrubs, or even eliminated, then the relationship between shrubs and clonal plants may change. Without grazing pressure, the clonal plants may allocate more biomass to the roots of the ramets beneath the shrub canopy to absorb the ‘rich’ soil resources and then diminish the effect of the fertility islands formed by shrubs. A previous study showed that the changes in the direction and magnitude of the effects of shrub encroachment on soil varied with abiotic (climate and soil) and biotic (shrub species) factors (Li et al., 2016). Clonal integration may be one of the factors affecting the direction and magnitude of the variation in soil traits during the encroachment of shrubs into grassland since this integration can diminish or enhance the effect of the fertility islands formed by shrubs.
Plant clonality plays a key role in the thicketization of grassland, and many studies have focused on the encroachment of clonal shrubs into grassland (Lett and Knapp, 2005; Smit et al., 2010). Formica et al. (2014) found that clonal growth (78%) accounts for more woody plant expansion than seed dispersal (22%). However, there have been few studies on the encroachment of woody plants into grassland dominated by clonal plants (Kesting et al., 2015; Zhang et al., 2016). Our study provides novel and firm evidence to support the idea that the growth of the rhizomatous grass L. chinensis was facilitated by the canopy of the C. intermedia shrub, and this facilitation may be due to ‘shelter from herbivores’ more than to ‘increased soil resources’ under grazing pressure in northern China. These results can help with understanding the process and ecological consequences of woody plant encroachment into grasslands that are dominated by clonal plants.
We believe that the process and ecological consequences of woody plant encroachment into grasslands that are dominated by clonal plants may be different from those in grasslands dominated by non-clonal plants, due to the unique features of clonal plants. We also acknowledge that the present study was based on a rough field investigation, and it may not completely explain the effects of clonal plants during the thicketization of grassland. More precision and empirical experiments are needed to confirm the interactions between clonal plants and shrubs, and long-term experiments are also needed to focus on the community- and ecosystem-level impacts of plant clonality, with or without disturbances such as grazing.
Conclusion
The formation of fertility islands by the leguminous shrub C. intermedia increased the soil resources beneath the shrub canopies and provided the clonal herbaceous L. chinensis beneath the shrub canopies with protection from herbivores in thicketization-grassland in northern China. Under grazing pressure, the growth of the clonal plant L. chinensis beneath the shrub canopy was facilitated by the spiny shrub C. intermedia as a ‘shelter from herbivores’ more than as an ‘increased soil resources’ in a thicketization-grassland that was previously dominated by the rhizomatous clonal plant L. chinensis. These results can help to understand the process and ecological consequences of woody plant encroachment into grasslands dominated by clonal plants. We propose that future studies should focus on the community- and ecosystem-level impacts of plant clonality.
Author Contributions
XHY directed, coordinated, and funded this study with intellectual input from ZH. S, DY, XHY, GL, and XJY carried out the fieldwork and lab analyses. XHY, S, and SZ did the data analysis and wrote the first manuscript draft. All authors commented on the manuscript and consent with the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (31470032), and the Initiative Projects of Inner Mongolia Research Center for Prataculture, Chinese Academy of Sciences.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Many thanks to Xiaojuan Wang, Fenyang Yi, Ruhan Ye, and Yuanyuan Zhang for their help with our field work.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00809/full#supplementary-material
References
Aguiar, M. R., and Sala, O. E. (1999). Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends Ecol. Evol. 14, 273–277. doi: 10.1016/S0169-5347(99)01612-2
Anderson, T. M., McNaughton, S. J., and Ritchie, M. E. (2004). Scale-dependent relationships between the spatial distribution of a limiting resource and plant species diversity in an African grassland ecosystem. Oecologia 139, 277–287. doi: 10.1007/s00442-004-1499-1
Archer, S. (1995). Tree-grass dynamics in a Prosopis-thornscrub savanna parkland, reconstructing the past and predicting the future. Ecoscience 2, 83–99. doi: 10.1080/11956860.1995.11682272
Asner, G. P., Archer, S., Hughes, R. F., Ansley, R. J., and Wessman, C. A. (2003). Net changes in regional woody vegetation cover and carbon storage in Texas drylands, 1937-1999. Global Change Biol. 9, 316–355. doi: 10.1046/j.1365-2486.2003.00594.x
Atkinson, C. J., and Else, M. A. (2012). Hydraulic conductivity and PAT determine hierarchical resource partitioning and ramet development along Fragaria stolons. J. Exp. Bot. 63, 5093–5104. doi: 10.1093/jxb/ers155
Báez, S., and Collins, S. L. (2008). Shrub invasion decreases diversity and alters community stability in Northern Chihuahuan Desert plant communities. PLoS ONE 3:e2332. doi: 10.1371/journal.pone.0002332
Bai, W. M., Sun, X. Q., Wang, Z. W., and Li, L. H. (2009). Nitrogen addition and rhizome severing modify clonal growth and reproductive modes of Leymux chinensis population. Plant Ecol. 205, 13–21. doi: 10.1007/s11258-009-9595-2
Bertness, M. D., and Callaway, R. M. (1994). Positive interactions in communities. Trends Ecol. Evol. 9, 191–193. doi: 10.1016/0169-5347(94)90088-4
Bochet, E., Rubio, J. L., and Poesen, J. (1999). Modified topsoil islands within patchy Mediterranean vegetation in SE Spain. Catena 38, 23–44. doi: 10.1016/S0341-8162(99)00056-9
Bond, W. J., and Midgley, G. F. (2000). A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Change Biol. 6, 865–869. doi: 10.1046/j.1365-2486.2000.00365.x
Brook, B. W., and Bowman, M. J. S. (2006). Postcards from the past, charting the landscape-scale conversion of tropical Australian savanna to closed forest during the 20th century. Landscape Ecol. 21, 1253–1266. doi: 10.1007/s10980-006-0018-7
Brown, J. R., and Archer, S. (1999). Shrub invasion of grassland, Recruitment is continuous and not regulated by herbaceous biomass or density. Ecology 80, 2385–2396. doi: 10.1890/0012-9658(1999)080[2385:SIOGRI]2.0.CO;2
Cabral, A., de Miguel, J., Rescia, A., Schmitz, M., and Pineda, F. (2003). Shrub encroachment in Argentinean savannas. J. Veg. Sci. 14, 145–152. doi: 10.1111/j.1654-1103.2003.tb02139.x
Caldeira, M. C., Lecomte, X., David, T. S., Pinto, J. G., Bugalho, M. N., and Werner, C. (2015). Synergy of extreme drought and shrub invasion reduce ecosystem functioning and resilience in water-limited climates. Sci. Rep. 5, 15110. doi: 10.1038/srep15110
Callaway, R. M. (2007). Positive Interactions and Interdependence in Plant Communities. Dordrecht: Springer.
Callaway, R. M., Brooker, R. W., Choler, P., Kikvidze, Z., Lortie, C. J., Michalet, R., et al. (2002). Positive interactions among alpine plants increase with stress. Nature 417, 844–848. doi: 10.1038/nature00812
Casanova-Katny, M. A., Torres-Mellado, G. A., Palfner, G., and Cavieres, L. A. (2011). The best for the guest: high Andean nurse cushions of Azorella madreporica enhance arbuscular mycorrhizal status in associated plant species. Mycorrhiza 21, 613–622. doi: 10.1007/s00572-011-0367-1
Charpentier, A., Anand, M., and Bauch, C. T. (2012). Variable offspring size as an adaptation to environmental heterogeneity in a clonal plant species, integrating experimental and modelling approaches. J. Ecol. 100, 184–195. doi: 10.1111/j.1365-2745.2011.01899.x
Cornelissen, J. H. C., Song, Y. B., Yu, F. H., and Dong, M. (2014). Plant traits and ecosystemeffects of clonality, a new research agenda. Ann. Bot. 114, 369–376. doi: 10.1093/aob/mcu113
de Kroon, H., and Hutchings, M. J. (1995). Morphological plasticity in clonal plants–the foraging concept reconsidered. J. Ecol. 83, 143–152. doi: 10.2307/2261158
Dong, M. (1996). Plant clonal growth in heterogeneous habitats, risk-spreading. Act. Phytoecol. Sin. 20, 543–548.
Eriksson, O., and Jerling, L. (1990). “Hierarchical selection and risk spreading in clonal plants,” in Clonal Growth in Plants, Regulation and Function, eds L. van Groenendael and H. de Kroon (The Hague: SPB Academic Publishing), 79–94.
Fabricius, C., Burger, M., and Hockey, P. A. R. (2003). Comparing biodiversity between protected areas and adjacent rangeland in xeric succulent thicket, South Africa: arthropods and reptiles. J. Appl. Ecol. 40, 392–403. doi: 10.1046/j.1365-2664.2003.00793.x
Facelli, J. M., and Temby, A. M. (2002). Multiple effects of shrubs on annual plant communities in arid lands of South Australia. Austral Ecol. 27, 422–432. doi: 10.1046/j.1442-9993.2002.01196.x
Fensham, R. J., Fairfax, R. J., and Archer, S. R. (2005). Rainfall, land use and woody vegetation cover change in semiarid Australian savanna. J. Ecol. 93, 596–606. doi: 10.1111/j.1365-2745.2005.00998.x
Formica, A., Farrer, E. C., Ashton, I. W., and Suding, K. N. (2014). Shrub expansion over the past 62 years in Rocky Mountain alpine tudra: possible causes and consequences. Arct. Antarct. Alp. Res. 46, 616–631. doi: 10.1657/1938-4246-46.3.616
Gough, L., Gross, K. L., Cleland, E. E., Clark, C. M., Collins, S. L., Fargione, J. E., et al. (2012). Incorporating clonal growth form clarifies the role ofplant height in response to nitrogen addition. Oecologia 169, 1053–1062. doi: 10.1007/s00442-012-2264-5
Hirobe, M., Hhte, N., Karasawa, N., Zhang, G. S., Wang, L. H., and Yoshikawa, K. (2001). Plant species effect on the spatial patterns of soil properties in the Mu-us desert ecosystem, Inner Mongolia, China. Plant Soil 234, 195–205. doi: 10.1023/A:1017943030924
Hobbs, R. J., and Mooney, H. A. (1986). Community changes following shrub invasion of grassland. Oecologia 70, 508–513. doi: 10.1007/BF00379896
Holzapfel, C., and Mahall, B. E. (1999). Bidirectional facilitation and interference between shrubs and annuals in the Mojave Desert. Ecology 80, 1747–1761. doi: 10.1890/0012-9658(1999)080[1747:BFAIBS]2.0.CO;2
IPCC (2013). Summary for Policymakers of Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jackson, R. B., Banner, J. L., Jobbagy, E. G., Pockman, W. T., and Wall, D. H. (2002). Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418, 623–626. doi: 10.1038/nature00910
Jaksic, F. M., and Fuentes, E. R. (1980). Why are native herbs in the Chilean matorral more abundant beneath bushes, microclimate or grazing? J. Ecol. 68, 665–669. doi: 10.2307/2259427
Kesting, S., Petersen, U., and Isselstein, J. (2015). Humped-back shaped response of plant species richness to increasing shrub encroachment in calcareous grasslands. Community Ecol. 16, 189–195. doi: 10.1556/168.2015.16.2.6
Laiolo, P., Dondero, F., Ciliento, E., and Rolando, A. (2004). Consequences of pastoral abandonment for the structure and diversity of the alpine avifauna. J. Appl. Ecol. 41, 294–304. doi: 10.1111/j.0021-8901.2004.00893.x
Lett, M. S., and Knapp, A. K. (2005). Woody plant encroachment and removal in mesic grassland: production and composition responses of herbaceous vegetation. Am. Midl. Nat. 153, 217–231. doi: 10.1674/0003-0031(2005)153[0217:WPEARI]2.0.CO;2
Li, H., Shen, H. H., Chen, L. Y., Liu, T. Y., Hu, H. F., Zhao, X., et al. (2016). Effects of shrub encroachment on soil organic carbon in global grasslands. Sci. Rep. 6:28974. doi: 10.1038/srep28974
Li, J., Zhao, C., Zhu, H., and Wang, F. (2007). Effect of plant species on shrub fertile island at an oasis-desert ecotone in the South Junggar Basin, China. J. Arid. Environ. 71, 350–361. doi: 10.1016/j.jaridenv.2007.03.015
Li, P. X., Wang, N., He, W. M., Krusi, B. O., Gao, S. Q., Zhang, S. M., et al. (2008). Fertile islands under Artemisia ordosica in inland dunes of northern China, Effects of habitats and plant developmental stages. J. Arid. Environ. 72, 953–963. doi: 10.1016/j.jaridenv.2007.11.004
Liao, J. D., and Boutton, T. W. (2008). Soil microbial biomass response to woody plant invasion of grassland. Soil Biol. Biochem. 40, 1207–1216. doi: 10.1016/j.soilbio.2007.12.018
Liao, J. D., Boutton, T. W., and Jastrow, J. D. (2006). Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland. Soil Biol. Biochem. 38, 3184–3196. doi: 10.1016/j.soilbio.2006.04.003
Liu, F. H., Liu, J., and Dong, M. (2016). Ecological consequences of clonal integration in plants. Front. Plant Sci. 7:770. doi: 10.3389/fpls.2016.00770
Liu, F. H., Yu, F. H., Liu, W. S., Kruesi, B. O., Cai, X. H., Schneller, J. J., et al. (2007). Large clones on cliff faces, expanding by rhizomes through crevices. Ann. Bot. 100, 51–54. doi: 10.1093/aob/mcm086
Louthan, A. M., Doak, D. F., Goheen, J. R., Palmer, T. M., and Pringle, R. M. (2014). Mechanisms of plant-plant interactions: concealment from herbivores is more important than abiotic-stress mediation in an African savannah. Proc. R. Soc. B 281:20132647. doi: 10.1098/rspb.2013.2647
Ludwig, J. A., and Tongway, D. (1997). “The conservation of water and nutrients within landscapes,” in Landscape Ecology Function and Management, Principles from Australia’s Rangelands, eds J. A. Ludwig, D. Tongway, D. Freudenberger, J. Noble, and K. Hodgkinson (Clayton, VIC: CSIRO Publishing).
Maestre, F. T., Cortina, J., Bautista, S., Bellot, J., and Vallejo, R. (2003). Small-scale environmental heterogeneity and spatiotemporal dynamics of seedling survival in a degraded semiarid ecosystem. Ecosystems 6, 630–643. doi: 10.1007/s10021-002-0222-5
Magyar, G., Kertesz, M., and Oborny, B. (2004). Resource transport between ramets alters soil resource pattern, a simulation study on clonal growth. Evol. Ecol. 18, 469–492. doi: 10.1007/s10682-004-5140-x
Masson, S., Mesleard, F., and Dutoit, T. (2015). Using shrub clearing, draining, and herbivory to control bramble invasion in Mediterranean Dry Grasslands. Environ. Manage. 56, 933–945. doi: 10.1007/s00267-015-0541-x
McKinley, D. C., Rice, C. W., and Blair, J. M. (2008). Conversion of grassland to coniferous woodland has limited effects on soil nitrogen cycle processes. Soil Biol. Biochem. 40, 2627–2633. doi: 10.1016/j.soilbio.2008.07.005
Mills, A., and Fey, M. (2004). Transformation of thicket to savanna reduces soil quality in the Eastern Cape, South Africa. Plant Soil 265, 153–163. doi: 10.1007/s11104-005-0534-2
Mills, J. E., Reinartz, J. A., Meyer, G. A., and Young, E. B. (2009). Exotic shrub invasion in an undisturbed wetland has little community-level effect over a 15-year period. Biol. Invasions 11, 1803–1820. doi: 10.1007/s10530-008-9359-2
Molina-Montenegro, M. A., Oses, R., Atala, C., Torres-Diaz, C., Bolados, G., and Leon-Lobos, P. (2016). Nurse effect and soil microorganisms are key to improve the establishment of native plants in a semiarid community. J. Arid. Environ. 126, 54–61. doi: 10.1016/j.jaridenv.2015.10.016
Mou, P., Jones, R. H., Mitchell, R. J., and Zutter, B. (1995). Spatial distribution of roots in Swetgum and Lobolly Pine monocultures and relations with above-ground biomass and soil nutrients. Funct. Ecol. 9, 689–699. doi: 10.2307/2390162
Pan, D. Y., Bouchard, A., Legendre, P., and Domon, G. (1998). Influence of edaphic factors on the spatial structure of inland halophytic communities, a case study in China. J. Veg. Sci. 9, 797–804. doi: 10.2307/3237045
Pan, J., An, C. P., Wu, X. D., Zhou, J., Mi, N., and Song, N. P. (2015). Distribution pattern of soil nutrients in the thicketization of 2 types of Caragana in desert steppe. J. Soil Water Conser. 29, 131–136.
Peng, H. Y., Li, X. Y., and Tong, S. Y. (2014). Effects of shrub (Caragana microphylla Lam.) encroachment on water redistribution and utilization in the typical stepper of Inner Mongolia. Act. Ecol. Sin. 34, 2256–2265.
Pinno, B. D., and Wilson, S. D. (2014). Nitrogen translocation between clonal mother and daughter trees at a grassland-forest boundary. Plant Ecol. 215, 347–354. doi: 10.1007/s11258-014-0305-3
Roiloa, S. R., Antelo, B., and Retuerto, R. (2014). Physiological integration modifies δ15N in the clonal plant Fragaria vesca, suggesting preferential transport of nitrogen to water-stressed offspring. Ann. Bot. 114, 399–411. doi: 10.1093/aob/mcu064
Roiloa, S. R., and Hutchings, M. J. (2013). The effects of physiological integration on biomass partitioning in plant modules, an experimental study with the stoloniferous herb Glechoma hederacea. Plant Ecol. 214, 521–530. doi: 10.1007/s11258-013-0186-x
Rong, Q. Q., Liu, J. T., Cai, Y. P., Lu, Z. H., Zhao, Z. Z., Yue, W. C., et al. (2016). ”Fertile island” effects of Tamarix chinensis Lour. on soil N and P stoichiometry in the coastal wetland of Laizhou Bay, China. J. Soil Sediment 16, 864–877. doi: 10.1007/s11368-015-1296-y
Rousset, O., and Lepart, J. (1999). Shrub facilitation of Quercus humilis regeneration in succession in calcareous grasslands. J. Veg. Sci. 10, 493–502. doi: 10.2307/3237184
Schlesinger, W. H., Raikes, J. A., Hartley, A. E., and Cross, A. H. (1996). On the spatial pattern of soil nutrients in desert ecosystem. Ecology 77, 364–374. doi: 10.2307/2265615
Scholes, R., and Archer, S. (1997). Tree-grass interactions in savannas I. Annu. Rev. Ecol. Syst. 28, 517–544. doi: 10.1146/annurev.ecolsys.28.1.517
Sharpe, B. R., and Bowman, D. M. J. S. (2004). Patterns of long-term woody vegetation change in a sandstone-plateau savanna woodland, Northern Territory, Australia. J. Trop. Ecol. 20, 259–270. doi: 10.1017/S0266467403001238
Smit, C., Bakker, E. S., Apol, M. E. F., and Olff, H. (2010). Effects of cattle and rabbit grazing on clonal expansion of spiny shrubs in wood-pastures. Basic Appl. Ecol. 11, 685–692. doi: 10.1016/j.baae.2010.08.010
Soliveres, S., Maestre, F. T., Eldridge, D. J., Delgado-Baquerizo, M., Quero, J. L., Bowker, M. A., et al. (2014). Plant diversity and ecosystem multifunctionality peak at intermediate levels of woody cover in global drylands. Glob. Ecol. Biogeogr. 23, 1408–1416. doi: 10.1111/geb.12215
Song, M. H., Dong, M., and Jian, G. M. (2002). Importance of clonal plants and plant species diversity in the Northeast China Transect. Ecol. Res. 17, 705–716. doi: 10.1046/j.1440-1703.2002.00527.x
Song, Y. B., Yu, F. H., Keser, L. H., Dawson, W., Fischer, M., Dong, M., et al. (2013). United we stand, divided we fall, a meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 171, 317–327. doi: 10.1007/s00442-012-2430-9
Srinivasan, M. P. (2012). Exotic shrub invasion in a montane grassland, the role of fire as a potential restoration tool. Biol. Invasions 14, 1009–1028. doi: 10.1007/s10530-011-0136-2
van Auken, O. W. (2000). Shrub invasions of North American semiarid grasslands. Annu. Rev. Ecol. Syst. 31, 197–215. doi: 10.1146/annurev.ecolsys.31.1.197
Wigley, B. J., Bond, W. J., and Hoffman, M. T. (2010). Thicket expansion in a South African savanna under divergent land use, local vs. global drivers?. Glob. Change Biol. 16, 964–976. doi: 10.1111/j.1365-2486.2009.02030.x
Xie, X. F., Hu, Y. K., Pan, X., Liu, F. H., Song, Y. B., and Dong, M. (2016). Biomass allocation of stoloniferous and rhizomatous plant in response to resource availability: a phylogenetic meta-analysis. Front. Plant Sci. 7:603. doi: 10.3389/fpls.2016.00603
Yan, X., Wang, H. W., Wang, Q. F., and Rudstam, L. G. (2013). Risk spreading, habitat selection and division of biomass in a submerged clonal plant, Responses to heterogeneous copper pollution. Environ. Pollut. 174, 114–120. doi: 10.1016/j.envpol.2012.10.013
Ye, X. H., Gao, S. Q., Liu, Z. L., Zhang, Y. L., Huang, Z. Y., and Dong, M. (2015). Multiple adaptations to light and nutrient heterogeneity in the clonal plant Leymus secalinus with a combined growth form. Flora 213, 49–56. doi: 10.1016/j.flora.2015.04.006
Ye, X. H., Yu, F. H., and Dong, M. (2006). A trade-off between guerrilla and phalanx growth forms in Leymus secalinus under different nutrient supplies. Ann. Bot. 98, 187–191. doi: 10.1093/aob/mcl086
Ye, X. H., Zhang, Y. L., Liu, Z. L., Gao, S. Q., Song, Y. B., Liu, F. H., et al. (2016). Plant clonal integration mediates the horizontal redistribution of soil resources, benefiting neighbouring plants. Front. Plant Sci. 7:77. doi: 10.3389/fpls.2016.00077
Zavaleta, E. S., and Kettley, L. S. (2006). Ecosystem change along a woody invasion chronosequence in a California grassland. J. Arid Environ. 66, 290–306. doi: 10.1016/j.jaridenv.2005.11.008
Zhang, H. Y., Yu, Q., Lu, X. T., Trumbore, S. E., Yang, J. J., and Han, X. G. (2016). Impacts of leguminous shrub encroachment on neighboring grasses include transfer of fixed nutrigen. Oecologia 180, 1213–1222. doi: 10.1007/s00442-015-3538-5
Zhao, T. T., Zhao, N. X., and Gao, Y. B. (2014). Effects of grazing exclusion on the composition and structure of steppe communities dominated by Caragana microphylla. Act. Ecol. Sin. 34, 4280–4287.
Keywords: clonal plant, Caragana intermedia, environmental heterogeneity, fertility islands, Leymus chinensis, shelter from herbivores, thicketization of grassland
Citation: Saixiyala, Yang D, Zhang S, Liu G, Yang X, Huang Z and Ye X (2017) Facilitation by a Spiny Shrub on a Rhizomatous Clonal Herbaceous in Thicketization-Grassland in Northern China: Increased Soil Resources or Shelter from Herbivores. Front. Plant Sci. 8:809. doi: 10.3389/fpls.2017.00809
Received: 05 October 2016; Accepted: 30 April 2017;
Published: 16 May 2017.
Edited by:
Boris Rewald, University of Natural Resources and Life Sciences, Vienna, AustriaReviewed by:
Cristian Atala, Pontificia Universidad Católica de Valparaíso, ChileMerav Seifan, Ben-Gurion University of the Negev, Sede Boker Campus, Israel
Copyright © 2017 Saixiyala, Yang, Zhang, Liu, Yang, Huang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenying Huang, zhenying@ibcas.ac.cn Xuehua Ye, yexuehua@ibcas.ac.cn
 Saixiyala1,2
Saixiyala1,2 Shudong Zhang
Shudong Zhang Guofang Liu
Guofang Liu Xuejun Yang
Xuejun Yang Zhenying Huang
Zhenying Huang Xuehua Ye
Xuehua Ye