- 1Key Laboratory of Plant Cell Engineering and Germplasm Innovation, Ministry of Education, School of Life Science, Shandong University, Jinan, China
- 2Donald Danforth Plant Science Center, St. Louis, MO, United States
- 3College of Life Science, Jiangsu Normal University, Xuzhou, China
Guard cells shrink in response to drought and abscisic acid (ABA), which is caused by efflux of ions that in turn reduces stomatal aperture and improves the plant’s ability to retain moisture. Cytosolic free calcium is an essential secondary messenger in guard cell ABA signaling, but the details of this regulatory pathway remain sketchy. Here, the calmodulin-like protein CML20, which has four EF-hand domains and calcium-binding activity in vitro, was found to be a negative regulator of ABA-induced stomatal movement in Arabidopsis. The guard cells of cml20 loss-of-function mutant plants were hypersensitive to both ABA-activated S-type anion currents, and ABA inhibited inward K+ currents than those of wild type. Additional, due to smaller stomatal aperture, cml20 showed less water loss from the leaves than wild type. These phenotypes of CML20 overexpressing plants contrasted with wild type in the opposite direction. In the cml20 mutant, the transcripts of stress responsive genes, such as MYB2, RAB18, ERD10, COR47, and RD29A were up-regulated in response to drought and ABA, while down-regulated of APX2 transcription and higher reactive oxygen species (ROS) accumulation. These observations support the CML20, a functional Ca2+ sensor, is a negative regulator in guard cell ABA signaling.
Introduction
Drought stress represents one of the most important constraints on crop productivity. Stomatal closure is a key early plant response to drought stress (Verslues et al., 2006), a process which is controlled by the turgor of the pair of guard cells surrounding each stoma (Kwak et al., 2008). Osmotically driven influx of water is required to expand the guard cell. Drought stress promotes the tissue content of the phytohormone abscisic acid (ABA), which acts as a prominent regulator of stomatal movement through its effect on ion channel activity (Schroeder et al., 2001). ABA inhibits the inward K+ channel and activates the anion channel (Schroeder et al., 1987; Pandey et al., 2007; Vahisalu et al., 2008; Li et al., 2014). The major transducer of ABA signaling is free calcium ion (Ca2+) concentration alterations in the cytoplasm (McAinsh et al., 1990, 1997). The supply of exogenous Ca2+ not only can induce stomatal closure but also can oscillate the concentration of cytosolic Ca2+ (McAinsh et al., 1995; Pei et al., 2000; Siegel et al., 2009). Certain stimuli that induce stomatal opening may also act to enhance the concentration of cytosolic Ca2+ (Kenichiro et al., 1992; Cousson and Vavasseur, 1998).
Drought stress (along with other abiotic stresses) alters the plant’s cellular redox balance, often promotes the accumulation of reactive oxygen species (ROS) (Rentel and Knight, 2004). The most prominent ROS is H2O2; its prolonged half-life and high permeability allow it to activate Ca2+ permeable channels (Rentel and Knight, 2004). As mentioned above, stomatal movements are also associated with guard cell calcium concentration changes (Pei et al., 2000; Thor and Peiter, 2014). Ca2+ is required for oxidative burst by activating NADPH oxidase (Xing et al., 1997) and for the activity of NAD kinase, which generates the NADP that subsequently used as a cofactor for NADPH oxidase (Karita et al., 2004; Turner et al., 2004). Many proteins involved in ROS signaling was regulated by Ca2+ signaling (Gong et al., 1997; Yang and Poovaiah, 2002; Takahashi et al., 2011; Ma et al., 2012; Wang et al., 2015). Both, Ca2+ and ROS serve as important signaling molecules in plants, are inter-regulated by feedback loops to keep their homeostasis in plant cells (Sierla et al., 2016).
Ca2+ plays important signaling roles in response to abiotic stress and many Ca2+ sensors are targets of Ca2+ in the signaling pathways, and kinds of Ca2+ sensors have been classified into four distinct groups, namely the calmodulins (CaMs), the CaM-like proteins (CMLs), the Ca2+ dependent protein kinase (CDPKs), and the calcineurin B-like proteins (CBLs). All of these proteins contain EF-hand motifs, which allow the binding of Ca2+ (David and Anthony, 2000; Oliver and Jorg, 2012). Transcriptomic analyses have shown that some CMLs members are induced by stress (McCormack et al., 2005). Previously reports have shown that CML9 (Tzahi et al., 1999; Helen and Marc, 2002; Hongtao et al., 2003; Fabienne et al., 2008), CML18, CaM15, and CML24 (Delk et al., 2005; Yamaguchi et al., 2005) are involved in abiotic stress signaling. The function of other CMLs, which represent the largest of these four protein families, remains unclear. To understand more details about the roles of CMLs in abiotic stress signaling, we have screened and found that the mutant cml20 is more tolerant to drought stress. As CML20 an expected Ca2+ sensor, we also analyzed its molecular roles in drought stress and ABA signaling pathway, especially in stomatal movement. CML20 like-proteins have been (a.k.a. CEN1) found to be present in the animal centrosome, where its role implicated in microtubule organization (Juliette et al., 2008). Here, we study the molecular role of CML20 in Arabidopsis in response to drought stress, and its contribution to stomatal movement, ROS production, and ion channel activity in response to ABA.
Materials and Methods
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) and the T-DNA insertion mutant cml20 (SALK_079974c) generated in a Col-0 background were obtained from the ABRC1. The zygosity of the mutant individuals was validated by PCR using the cml20-specific primer pair cml20-LP/-RP and the T-DNA left border primer LBb1.32. The relevant primer sequences are given in Table 1. To grow plants, seeds were surface-sterilized by immersing in 75% v/v ethanol for 3 min, followed by 95% v/v ethanol for 1 min, then the ethanol was allowed to evaporate. The seeds were then plated on solidified (0.7% w/v agar) half strength Murashige and Skoog (1962) medium (1/2 MS) and held first for 2 days at 4°C and then kept in a growth chamber under long-day conditions (16 h light/8 h darkness, 22 ± 1/16 ± 4°C) with illumination by 100 μmol m-2s-1 light); the relative humidity was maintained at ∼70%.
Heterologous Expression of CML20 and Ca2+-Binding Assay
The coding region of CML20 (At3g50360) was amplified from Col-0 cDNA by using the primer pair CML20-HisF/-HisR (Table 1), while CaM7 (At3g43810) was amplified by using CaM7-HisF/-HisR (Table 1) as positive control. The amplicons and the prokaryotic expression vector pET30a (+) were both digested with NdeI and XhoI, and then ligated using T4 DNA ligase (TransGen Biotech, China). For heterologous expression of CML20, E. coli BL21 (DE3) transformants harboring recombinant plasmid CML20 were induced with 0.4 mM isopropyl β-D-1-thiogalactopyranoside (IPTG); the recombinant protein was thereafter purified using the Ni-NTA Purification System (Invitrogen, Uinted States). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) mobility shift assay for Ca2+ binding was performed by exposing denatured CML20 to either 15 mM CaCl2 or 15 mM ethylene glycol tetraacetic acid (EGTA), as described by Garrigos et al. (1991). The structure of CML20 was predicted using InterPro software3.
Construction of Plant Expression Vector and Generation of Transgenic Arabidopsis thaliana
To engineer plant expression vector for CML20 over-expression, the gene’s open reading frame was amplified using the primer pair CML20-OEF/-OER (Table 1), and the resulting amplicon was digested with XbaI and SacI, then ligated into the XbaI and SacI cloning sites of pSTART (+) which generated the p35S::CML20 construct. The CML20 open reading frame was amplified using the primer pair CML20-CF/-CR (Table 1) cloned into pDonor 221 vector and then introduced into pB2GW7.0 vector through gateway reaction (Invitrogen, United States) to generate pB2GW7.0-CML20. cml20 mutant plants were transformed with pB2GW7.0-CML20 to generate complemented lines, C-1 and C-2. The CML20 promoter sequence was amplified using the primer pair CML20-PF/-PR (Table 1), then ligated into the HindIII/BamHI cloning site of pCambia::UBI-GUS to generate the construct pCML20::GUS. All the constructs were transferred into Agrobacterium tumefaciens strain GV3101 and then introduced into Arabidopsis using the floral dip method (Clough and Bent, 1998). T1 transgenic seedlings were selected by growing on 1/2 MS containing 30 mg/L kanamycin (p35S::CML20 lines), or hygromycin (pCML20::GUS lines). The zygosity of T3 lines was deduced from the transgene’s segregation behavior, and the abundance of the transgene transcript was assessed by using PCR or quantitative real time PCR (qRT-PCR), and the selected seeds from T3 homozygous lines were used for further analysis.
Subcellular Localization of CML20
A p35S::CML20-GFP construct was generated by inserting the CML20 open reading frame (amplified using CML20-GFPF/-GFPR, see Table 1) into the BamHI/SalI cloning sites of a modified GFP expression vector (Lin et al., 2009). Both the p35S::CML20-GFP and the empty p35S::GFP plasmids were purified using a NucleoBond® Xtra Midi Kit4 (Macherey–Nagel) before introducing into Arabidopsis mesophyll protoplasts following Sheen (2001). After 16 h incubation at 23°C in the dark, protoplasts were examined for GFP signal using confocal laser scanning microscopy (performed at The Microscopy Characterization Facility, Shandong University).
GUS Staining
T3 homozygous transgenic plants harboring pCML20::GUS were assayed for GUS activity as described by Jefferson et al. (1987) with slight modification. Briefly, transgenic plant tissues were soaked in a GUS staining solution (2 mM X-Gluc, 2 mM K3Fe(CN)6, 2 mM K4Fe(CN)6, 0.1% Triton X-100, 10 mM EDTA in 50 mM sodium phosphate buffer, pH7.2) and incubated overnight at 37°C. After staining, the samples were washed in 50, 70, and 100% ethanol for 5 min consecutively, and then shook in 100% ethanol for about 5 h to remove chlorophyll. Subsequently, the samples were examined under an optical microscope.
Drought Stress and Water Loss Experiments
To apply drought stress, the protocol described by Sakamoto et al. (2004) was followed with minor modifications. Seedlings were grown under well-watered conditions for four weeks, and then deprived of water for 2–3 weeks. Then, the plants were re-watered for over three days and photographed. For water-loss assays, rosette leaves were collected from 4-week-old plants as test samples. The samples were weighed immediately on a piece of paper, and then placed on the laboratory bench (relative humidity: 40%–50%; 22°C–23°C). The weight lost by each sample at a set of pre-assigned time points was recorded.
Stomatal Movement
Arabidopsis thaliana plants were grown under a 10 h photoperiod (100 μmol m-2s-1 light), where the light and dark period temperatures were 22 and 20°C, respectively. The stomatal aperture assay was performed using fully expanded young leaves of 4-week-old plants. To characterize stomatal opening, detached rosette leaves were first floated adaxial side up for 2.5 h in the dark in 10 mM KCl, 7.5 mM iminodiacetic acid, 10 mM Mes-KOH (pH 6.15). Subsequently, either 50 μM ABA or solvent control (ethanol) was added, then the treated leaves were illuminated for 2.5 h. For the stomatal closure assay, initially leaves were floated in the solution containing 20 mM KCl, 1 mM CaCl2, 5 mM Mes-KOH (pH 6.15) for 2.5 h in the light, then either ABA (1, 10, or 50 μM) or solvent control (ethanol) was added. In both cases, abaxial epidermal strips were peeled off after 2.5 h incubation (with ABA/solvent control ethanol) and photographed immediately. Stomatal aperture widths were estimated from the captured digital images using ImageJ v1.475 software.
Electrophysiology
Arabidopsis thaliana guard cell protoplasts were isolated following the Zhang et al. (2008) method. K+ currents were recorded following Li et al. (2016) with slight modifications. The bath solution contained 4 mM MgCl2, 10 mM MES-Tris (pH 5.6), 1 mM CaCl2, 10 mM K-Glu, and the osmolarity was adjusted to 500 mOSM with sorbitol for measuring whole-cell channel K+ currents. The pipette solution contained 20 mM KCl, 10 mM HEPES-Tris (pH 7.8), and 80 mM K-Glu, and the osmolarity was adjusted to 550 mOSM, and also fresh ATP (5 mM Mg-ATP) was added before use. Axopatch-200B amplifier (Axon Instruments, United States) connected to a computer via an interface (TL-1 DMA Interface; Axon Instruments) was used to achieve whole-cell configuration, and the holding potential was set as –60 mV. The whole-cell currents were recorded 5 min later. The test voltage steps were from –200 mV to –40 mV with +20 mV increments, and each test voltage has 4.9-s duration. For the ABA treatment, 50 μM ABA was added to the bath solution after the whole-cell configuration was achieved. The whole-cell currents were recorded 10 min later. Guard cell anion currents were recorded as described by (Acharya et al., 2013). Briefly, the bath solutions contained 2 mM MgCl2, 30 mM CsCl, 10 mM MES-Tris (pH 5.6), and 1 mM CaCl2. The pipette solutions contained 150 mM CsCl, 2 mM MgCl2, 6.7 mM EDTA, 3.35 mM CaCl2, and 10 mM HEPES-Tris (pH 7.5). Osmolarity of the solutions was adjusted to 480 and 500 mOSM for bath and pipette solutions, respectively, with sorbitol. ATP (10 mM Mg-ATP) and GTP (10 mM) were added to the pipette solution before use. The holding potential was +30 mV. The voltage steps were applied from -145 to +35 mV with +30 mV increments, and each test voltage has a 60-s duration. For the ABA treatment, guard cell protoplasts were treated with 50 μM ABA for at least 1 h before recording.
RT-PCR and qRT-PCR
Total RNA was isolated using the TRIzol reagent (Roche, Switzerland), treated with RNase-free DNase I (Invitrogen, United States) and reverse transcribed using oligo-dT primers and SuperScriptTM III Reverse Transcriptase (Invitrogen, United States). The resulting cDNA was used as template in both RT- and qRT-PCRs. The former employed the CML20-specific primer pair CML20-RTF/-RTR (Table 1) and reference reactions were primed by ACTIN2-RTF/-RTR (Table 1), to amplify the gene ACTIN2 (At3g18780). For the qRT-PCRs, leaves and epidermal strips derived from four-week old plants were exposed to 50 μM ABA for 6 h. All reactions were performed in triplicates using FastStart Universal SYBR Green master mix (Roche, Switzerland), and ACTIN2 was used as the reference. The CML20-specific primer pair CML20-QF/-QR and the ACTIN2 pair ACTIN2-QF/-QR (Table 1) were used for the qRT-PCR assays. The abundance of transcript of a set of known stress-responsive genes was also assayed by qRT-PCR using cDNA synthesized from seedlings (two-week old) that were harvested 6 h after exposure to 50 μM ABA. The gene/primer pair combinations were RAB18 (At5g66400)/RAB18F/R, COR47 (At1g20440)/ COR47F/R, MYB2 (At2g47190)/MYB2F/R, ERD10 (At1g20450)/ERD10F/R, KIN1 (At5g15960)/KIN1F/R, RD29A (At5g52310)/RD29AF/R, KIN2 (At5g15970)/ KIN2F/R, and APX2 (At3g09640)/APX2F/R. All primer sequences are given in Table 1.
Fluorescent Imaging of ROS Production
Reactive oxygen species production in guard cells was detected by loading abaxial epidermal strips with H2DCF-DA (Zhang et al., 2011). Epidermal strips were peeled from detached leaves from plants, and floated in 10 mM KCl, 7.5 mM iminodiacetic acid, 10 mM Mes-KOH (pH 6.15) for 2 h to induce stomatal opening. Then, the epidermal strips were transferred to 10 mM Tris HCl, 50 mM KCl (pH 7.2) containing 50 mM H2DCF-DA, and held in the dark for 10 min. Unincorporated H2DCF-DA was removed by rinsing three times in double distilled water. The guard cells were then subjected to laser scanning confocal microscopy, and treated with either 50 μM ABA or solvent control before data collection. The fluorescence of H2DCF was captured by imaging at 2.5 min intervals over 25 min. ZEN software6 (2012, blue edition) was used to quantify the data. The change in fluorescence intensity at each time point was calculated in the form of a percentage of its initial intensity.
Results
CML20 Encodes a CAM-Like Protein with the Ability to Bind Ca2+
The predicted CML20 product was a CAM-like protein consisting of 169 aa residues. The structure of CML20, according to InterPro7, contains four EF-hand domains (Figure 1B), as seen in numerous Ca2+ binding proteins (Figure 1A) such as CAM2, CAM7 and CML9 (Fabienne et al., 2008; Garrigos et al., 1991; McCormack et al., 2005). The SDS-PAGE mobility shift assay revealed that CML20 migrated faster in the presence of free Ca2+ than in the presence of Ca2+ chelator EGTA (Figure 1C). Like CML20, we also observed similar result for CAM7 (Garrigos et al., 1991). Our findings suggested that CML20 could bind Ca2+ in vitro and thus potentially acts as a Ca2+ sensor in plant.
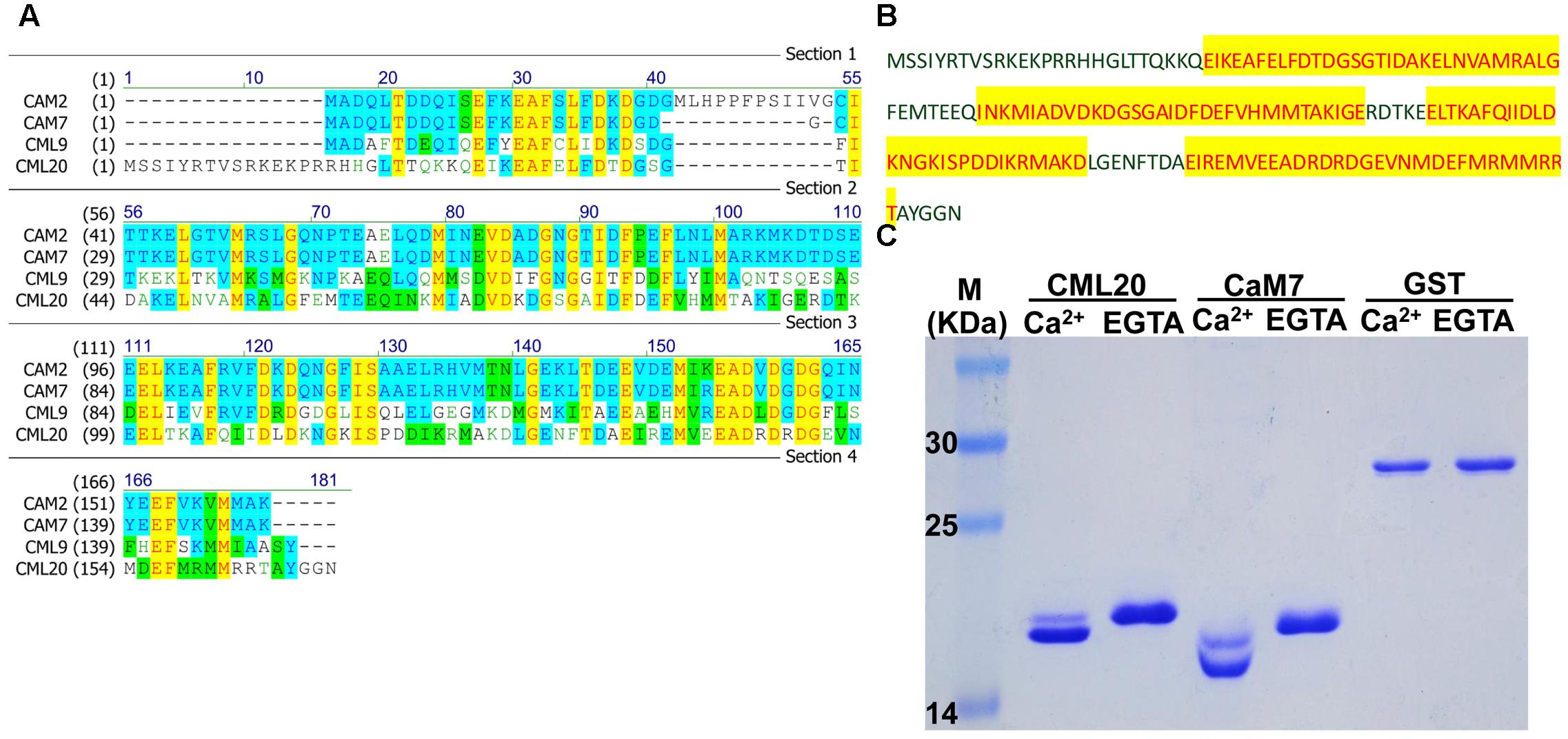
FIGURE 1. CML20, a calmodulin-like protein, is able to bind Ca2+. (A) Amino acid sequence alignment between CML20 and its homologs CAM2, CAM7, and CML9. (B) The amino acid sequence of CML20. The Ca2+-binding EF-hand motifs are highlighted in yellow. (C) The SDS-PAGE mobility shift assay showing that CML20 and CaM7 bind to Ca2+. GST was used as a negative control. The left-hand lane contains a protein size marker.
Expression Profiling of CML20
As deduced from the sites of GUS production in transgenic plants harboring pCML20::GUS, expression of CML20 was observed in the root, leaf, inflorescence and silique (Figures 2A–G). CML20 expression was also induced in the guard cells of plants that were exposed to ABA (Figures 2F,G). qRT-PCR result also supported that CML20 was induced in response to ABA treatment in the leaf and epidermis, which contains a large number of guard cells (Figure 2H). All of these findings suggested the possible involvement of CML20 in the ABA-mediated regulation of stomatal movement. When mesophyll protoplasts were transformed with p35S::CML20-GFP, GFP signal was detected exclusively in the cytoplasm (Figure 2I), but mesophyll protoplasts transformed with p35S::GFP empty vector control showed GFP signal dispersed throughout the protoplast (Figure 2J). These findings indicate that CML20 is a cytosol-localized protein.
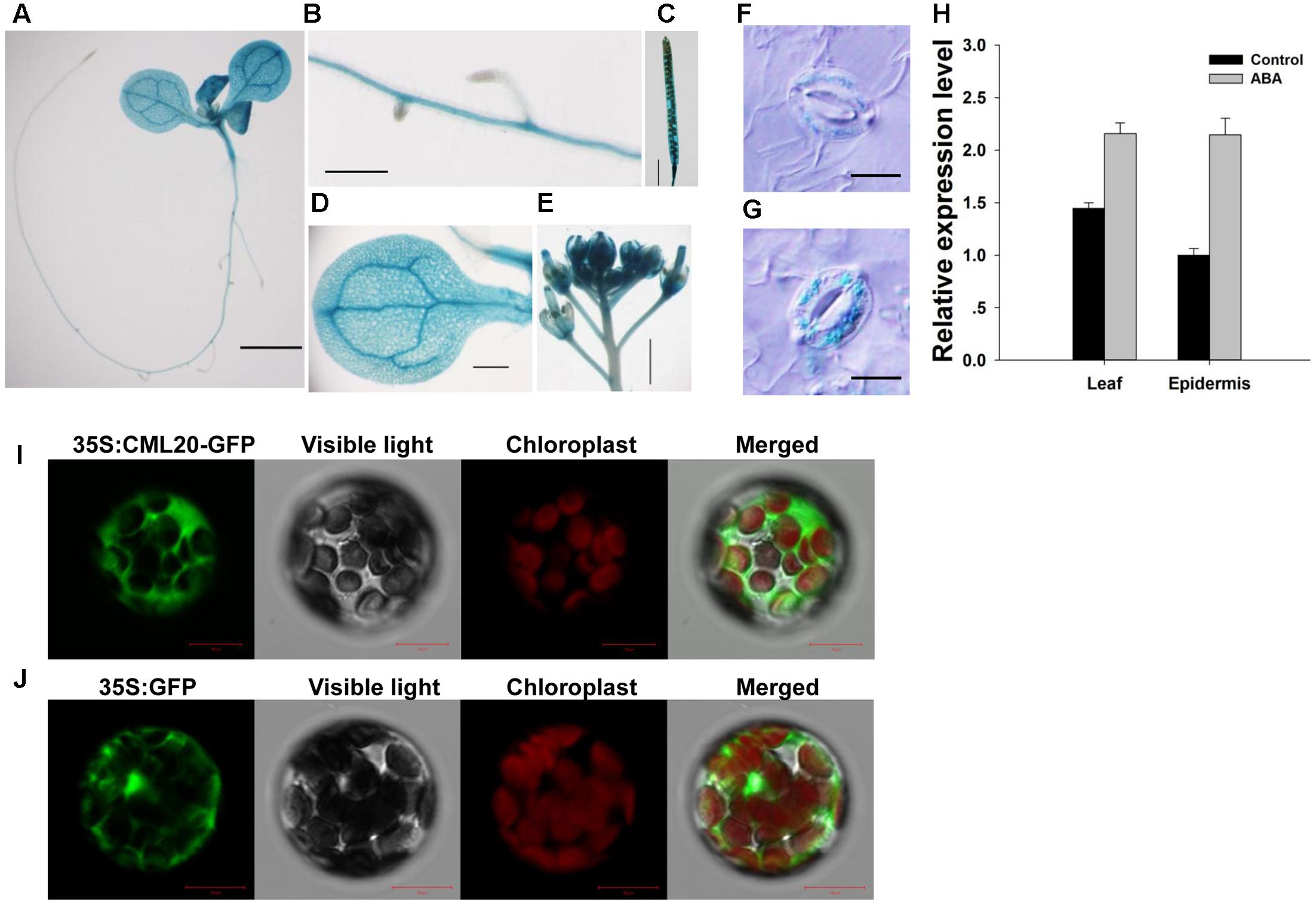
FIGURE 2. The site of expression of CML20. (A–G) GUS staining of various tissues in A. thaliana plants harboring pCML20::GUS. (A) The whole seedling. Bar: 2 mm, (B) the root. Bar: 0.5 mm, (C) the silique. Bar: 3 mm, (D) the leaf. Bar: 1.5 mm, (E) the Inflorescence. Bar: 1.5 mm, all without abscisic acid (ABA) treatment, (F) a guard cell in plants not exposed to ABA treatment. Bar: 10 μm, (G) a guard cell in plants 6 h after exposure to 50 μM ABA treatment. Bar: 10 μm. (H) Transcript levels of CML20 in leaves and epidermis exposed to 50 μM ABA treatment for 6 h or solvent control. Error bars show the SE (n = 3). (I,J) Transient expression of GFP and CML20-GFP in mesophyll protoplasts showing their sub-cellular localization (I) p35S::CML20-GFP and (J) p35S::GFP. Bar: 10 μm.
cml20 Mutant Plants Were Hypersensitive to ABA-Regulated Stomatal Movement and Show a Greater Tolerance to Drought Stress
Transcriptional analysis of the cml20 mutant (Figure 3A) was carried out using both RT- PCR and qRT-PCR which confirmed the absence of CML20 transcript (Figures 3B,C). Given that CML20 was clearly up-regulated by ABA in the guard cells, the response of cml20 guard cells was of interest to study its role in ABA signaling. Stomatal aperture was indistinguishable between the mutant and WT when plants were not treated with ABA, but in the presence of ABA (1, 10, or 50 μM), the mutant stomata showed smaller aperture than the WT (Figures 3D,E). The results indicated that cml20 stomata were hypersensitive to both ABA-promoted stomatal closure as well as the ABA-inhibited stomatal opening, and thus to minimize the water loss to increase the plant drought tolerance. This hypothesis was supported by the observed results of both the detached leaf assay and the whole plant response to drought stress as well (Figures 3F,G).
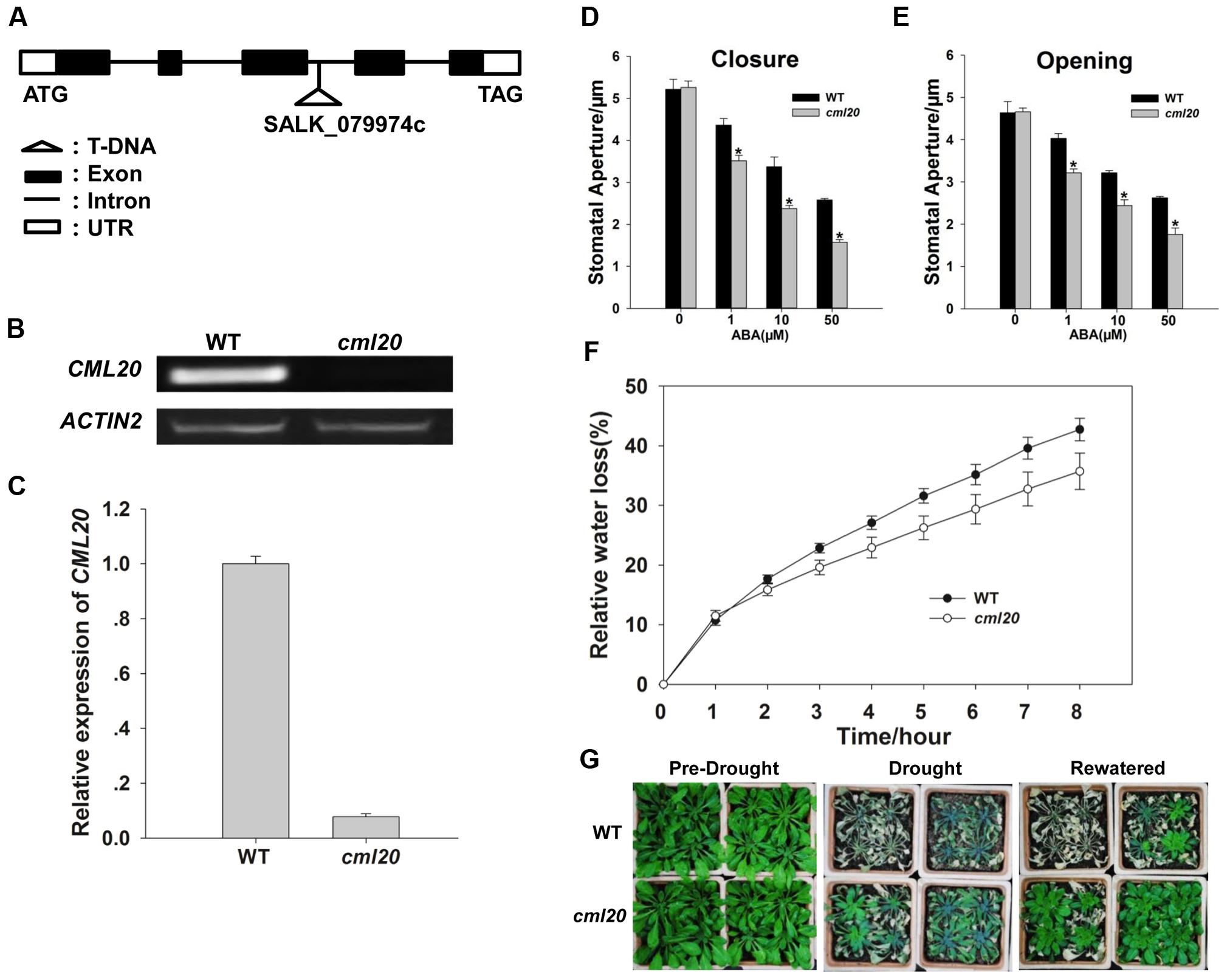
FIGURE 3. Stomatal movement of the cml20 mutant is hypersensitive to ABA treatment, making the plant more tolerant to drought stress. (A) The T-DNA insertion site in the third intron of CML20. (B,C) CML20 is not transcribed in the cml20 mutant, as shown by (B) RT-PCR analysis and (C) qRT-PCR analysis. Error bars represent the SE (n = 3). The ACTIN2 was used as an internal control. (D,E) The stomatal movement assay: (D) ABA induction of stomatal closure, (E) ABA inhibition of stomatal opening. At least 60 stomata were measured for each genotype per replication. ∗means differ significantly (P < 0.05). Error bars represent the SE (n = 3) from three independent experiments. (F) The rate of water loss from detached leaves of WT and the cml20 mutant. Error bars represent the SE (n = 3). (G) The cml20 mutant was more tolerant to drought stress than WT. The experiment was repeated three times with similar results.
CML20 Over-Expression Reduces the Sensitivity of Stomatal Movement to ABA and Has a Negative Effect on Drought Stress Tolerance
qRT-PCR result revealed that CML20 over-expressing lines, OE-1 and -2 both produced more CML20 transcript than WT (Figure 4A). Stomatal movement in these lines was less affected by ABA than in the WT (Figures 4B,C). As predicted, the rate of water loss from detached leaves of the two OE lines was higher than WT leaves (Figure 4D), and the OE-1 and OE-2 plants were more sensitive to drought stress (Figure 4E). In addition, cml20/CML20 complementation lines (C-1 and C-2) showed similar phenotype for both ABA regulation of stomatal movement and drought stress resistance compared to WT (Supplementary Figure S1). These findings provided additional support that CML20 functions as a negative regulatory signaling component in response to both ABA and drought stress.
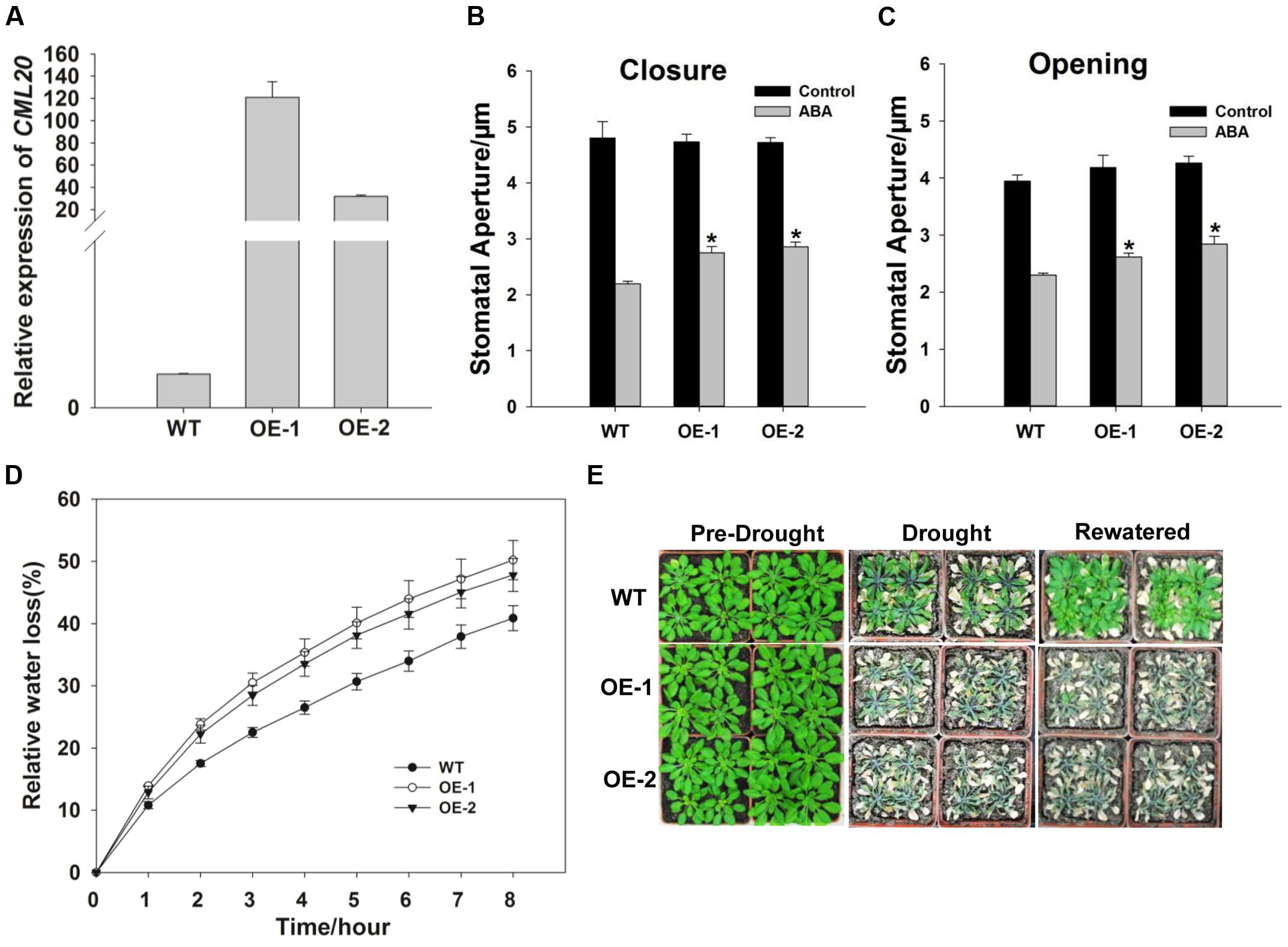
FIGURE 4. The CML20 over-expression lines OE-1 and -2 show hypersensitivity to drought stress. (A) CML20 transcript abundance in WT and the two over-expression lines (qRT-PCR assay). ACTIN2 was used as the internal control. Error bars represent the SE (n = 3). (B,C) Stomatal movement in WT, OE-1 and OE-2 under control (without ABA) and 50 μM ABA conditions. At least 60 stomata were measured for each genotype per replication ∗: means differ significantly (P < 0.05), Error bars represent the SE (n = 3) from three independent experiments. (D) The rate of water loss from detached leaves of WT and the CML20 over-expression lines. Each data point represents the mean Error bars represent the SE (n = 3). (E) The appearance of WT and the CML20 over-expression lines grown under drought stress. The experiment was repeated three times with similar results.
CML20 Is Involved in the ABA Regulation of Guard Cell Ion Channels
Stomatal closure relies on anion efflux via channels that are activated by ABA, which also simultaneously inhibits the influx of K+. The patch clamp assay was conducted to test whether ABA-modulated currents depended on CML20. Without ABA treatment, we did not observe any difference in either the K+ or the anion currents between WT, cml20 and the two OE lines. In contrast, in response to ABA treatment, the mutant’s guard cells showed hypersensitivity to ABA inhibition of K+ currents and ABA activation of anion currents, while the over-expression of CML20 impaired them both (Figures 5A–D). These results coincided with the performance of stomatal movement in response to ABA treatment. The conclusion was that CML20 functioned as a negative regulatory signaling component for ABA regulation of stomatal movement, partly via its regulatory effect on ion channels activity in guard cells.
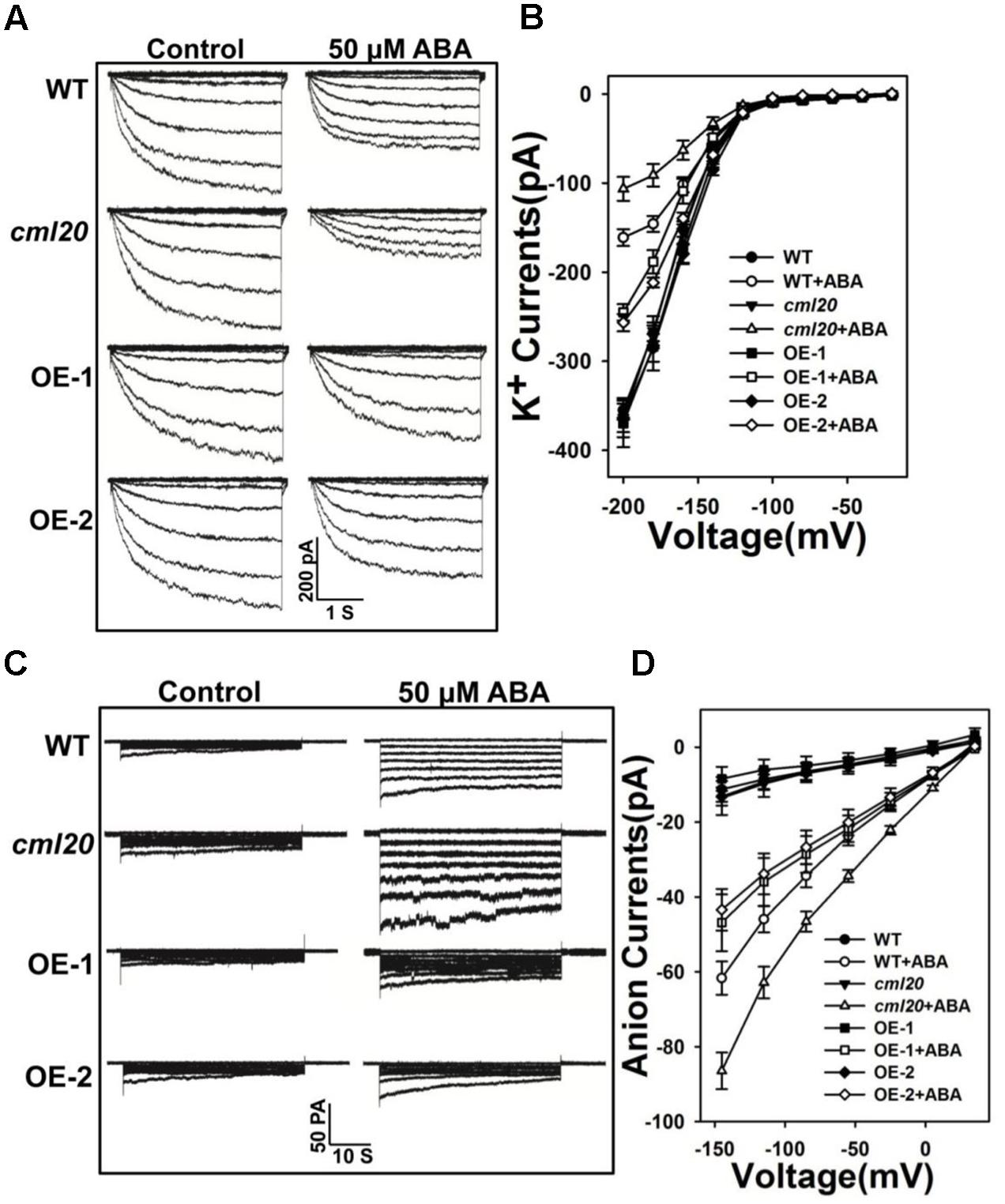
FIGURE 5. CML20 is involved in the ABA regulation of inward K+ and slow-type anion channels. (A) Patch-clamp whole cell recordings of the inward K+ current in guard cell protoplasts isolated from WT, cml20 and the CML20 over-expression lines OE-1 and -2, either in the presence or absence of 50 μM ABA. (B) Current/voltage relationships of whole cell K+ currents. The number of guard cells measured were: WT (8), WT+ABA (9), cml20 (7), cml20+ABA (8), OE-1 (7), OE-1+ABA (11), OE-2 (11), OE-2+ABA (12). Error bars represent the SE. (C) ABA (50 μM) activation of slow anion currents in guard cell protoplasts of WT, cml20, OE-1, and OE-2. (D) Current/voltage relationships of whole cell slow anion currents. The number of guard cells measured were: WT (9), WT+ABA (7), cml20 (9), cml20+ABA (11), OE-1 (6), OE-1+ABA (7), OE-2 (11), OE-2+ABA (10). Error bars represent the SE.
Loss of Function of CML20 Affects the Transcription of Stress-Responsive Genes
Transcriptional (qRT-PCR) profiling showed that in cml20, certain ABA-inducible genes (ERD10, RAB18, COR47, and MYB2) were up-regulated by exposure to ABA for 6 h (Figure 6). The imposition of drought stress also induced the transcription of an ABA-independent gene RD29A in the cml20 mutant (Figure 6). The aforementioned genes are up-regulated in response to abiotic stresses also their transcript level could be correlated with sensitivity to a specific stress. These findings not only implicate that CML20 negatively regulates transcription of these above indicated genes but also suggest that CML20 is also a regulator of the ABA and drought stress signaling pathways.
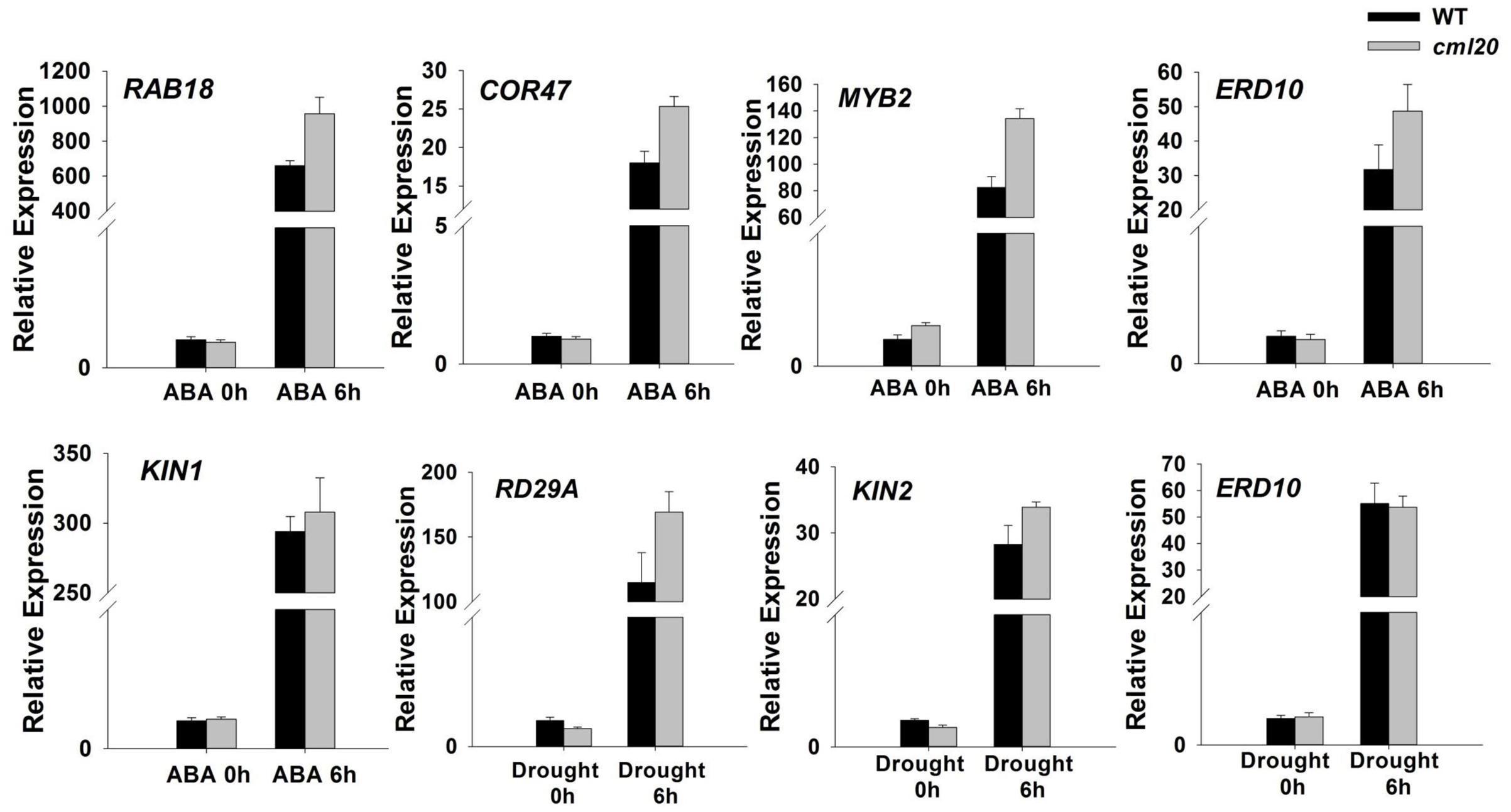
FIGURE 6. The transcription of the indicated stress-responsive genes was altered in the cml20 mutant. RAB18, COR47, MYB2 and ERD10 were up-regulated by exposure to 50 μM ABA for 6 h. RD29A and KIN2 were up-regulated by exposure to drought stress for 6 h. ACTIN2 was used as the internal control. Error bars represent the SE (n = 3).
ROS Production in cml20 Guard Cells Is Enhanced by Exposure to ABA
The ABA treatment resulted in a greater increase of ROS production in the guard cells of cml20 than in those of WT (Figures 7A,B). A qRT-PCR based assay of the transcription of a gene known to be involved in ROS elimination showed that APX2 (encoding an H2O2 scavenger) was down-regulated in cml20 whether or not the plants were exposed to ABA (Figure 7C). These above findings suggest that CML20 may play a positive role in guard cell ROS removal; in its absence, the down-regulation of APX2 could have compromised the plant’s ability to control the accumulation of ROS.
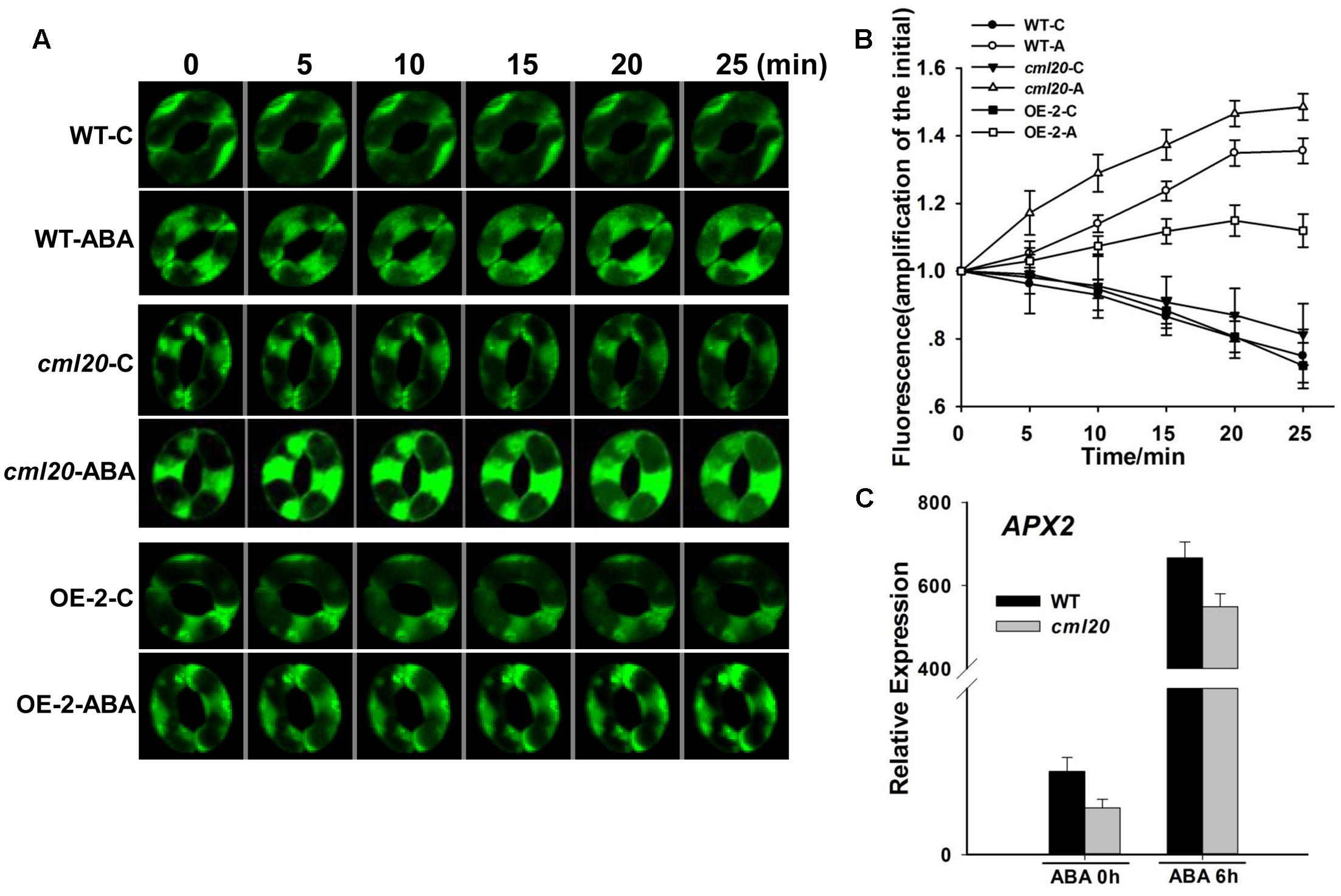
FIGURE 7. Reactive oxygen species (ROS) production is elevated in the guard cells of cml20 plants exposed to ABA. (A) H2DCF fluorescence in WT, cml20 and OE-2 imaged at 5 min intervals over 25 min. At least ten guard cells were measured. (B) H2DCF-DA staining revealed a higher level of ROS production in cml20 than in WT, and a lower level in OE-2. Error bars represent the SE. (C) The transcription of APX2 was down-regulated in cml20. Error bars represent the SE (n = 3).
Discussion
As shown by the SDS-PAGE mobility shift assay (Figure 1), CML20 demonstrated an ability to bind Ca2+ in vitro. Many Ca2+ sensors have been implicated as signaling components in the abiotic stress response. An example is the rice gene OsCaM1-1, which is induced by exposure to high temperature; its product enhances thermo tolerance when the gene is constitutively expressed in A. thaliana (Wu et al., 2012). Transcription factors such as AtCAMTA1, -A2 and -A3 are known to participate in response to low temperature stress (Kim et al., 2013). The product of AtCAMTA1 is also involved in the drought stress response (Pandey et al., 2013). Despite being one of the largest Ca2+ sensor families, few CMLs have been identified yet that participate in the abiotic stress response: these comprise the A. thaliana proteins CML9, 10, 18, 24, and 42, as well as the rice protein OsMSR and ShCML44 from Solanum habrochaites (Delk et al., 2005; Yamaguchi et al., 2005; Fabienne et al., 2008; Xu et al., 2011; Vadassery et al., 2012; Cho et al., 2015; Munir et al., 2016). Here, the current work demonstrated that AtCML20 is negatively involved in guard cell ABA signaling during plant drought response.
A consistent implication emerged from the present set of experiments was that CML20 acted as a negative regulator of ABA and drought stress responses in plants. Stomatal movement was more sensitive to the ABA treatment in the cml20 mutant than in the WT (Figure 3). In addition, OE lines with higher expression of CML20 showed less sensitivity to ABA-mediated stomatal movement, which might have caused OE plants to be lower ability of drought tolerant. In plants, stomatal closure represents an early response to drought stress, and aids for the maintenance of the plant’s internal osmotic environment (Verslues et al., 2006). Thus, the action of CML20 to inhibit stomatal movement has a negative effect on the plant’s tolerance to drought stress. Nevertheless, WT plants treated with ABA showed 100% increase of CML20 transcript (Figure 2H). A possibility is that the function of CML20 during an episode of drought stress is to enforce stomata to remain in a partial open state, a physiological condition that would allow the plant to exchange gas.
Interestingly, we further found that CML20 had roles in ABA regulating ion channels. The results from patch clamp assay indicated that both K+in channels and anion channels were influenced in loss-of-function mutant and OE lines in response to ABA (Figure 5). Some CDPKs, as Ca2+ sensors like CMLs, have been reported to regulate guard cell SLAC1 channel’s activity (Geiger et al., 2010). We also explored the molecular mechanism to define the function of CML20 in ABA signaling.
ABA is a critical regulator of the abiotic stress response, which acts via complex signaling networks. From the qRT-PCR analysis, it was obvious that the loss-of-function of CML20 affected the transcription of a number of ABA-inducible genes in mutant plants treated with ABA (Figure 6), which in turn may have been responsible for the mutant’s enhanced level of drought stress tolerance. By acting as a negative regulator, CML20 likely limits the extent of the responsiveness of these genes to ABA; it also negatively regulates the transcription of RD29A, a gene which responds to drought stress, in an ABA- independent manner (Yamaguchi-shinozaki and Shinozaki, 2006).
Fluorescent probes demonstrated that the cml20 mutant generated more ROS than WT under ABA treatment (Figures 7A,B), consistent with the negative role of CML20 in stomatal movements and drought response. ROS is generated by NADPH oxidases (Respiratory Burst Oxidase Homologs, RBOHs), and the enzyme AtRBOHD and AtRBOHF are mainly expressed in guard cells and could be up-regulated by ABA. In the ABA signal pathway, OST1 is released by ABA from PP2C to activate AtRBOHF through directly phosphorylation (Kwak et al., 2003; Sirichandra et al., 2009; Shang et al., 2016). In another way, the elimination of ROS is achieved by a range of antioxidants and enzymes. The gene APX2, which encodes a cytosolic ascorbate peroxidase (a ROS scavenger), had a lower expression level in cml20 than WT whether or not the plants were exposed to ABA (Figure 7C). A reduced presence of the ROS scavenging enzyme APX2 in ABA-treated cml20 mutant plants was predicted from the lowered abundance of APX transcript in cml20 compared to that in WT, may have allowed for a greater accumulation of ROS, which in turn could have enhanced the transduction of the ABA signal.
Meanwhile the prominent ROS species H2O2, which is induced by ABA in the guard cells, can activate Ca2+ channels and regulate stomatal movement (Pei et al., 2000; Mustilli et al., 2002), inversely, Ca2+ could also bind and activate RBOH for ROS generation (Ogasawara et al., 2008; Kimura et al., 2012). Both Ca2+ and ROS are important signal integrators in plants (Murata et al., 2015). Given its proven Ca2+ binding ability, CML20 may play roles both in Ca2+ and ROS signal pathways to regulate stomatal aperture in plants either exposed to exogenous ABA or subjected to drought stress.
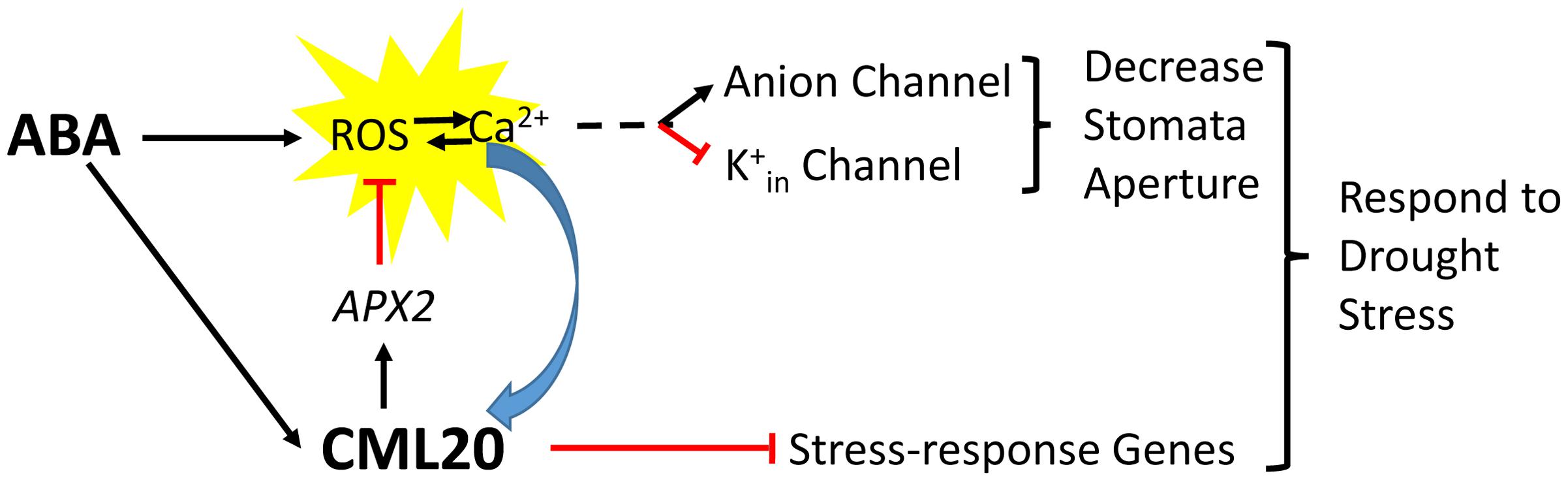
The CML20 may serve as a fine regulator for cytosol ROS homeostasis in guard cell ABA signaling. Previous studies have demonstrated that the guard cell anion channels (SLAC1) and K+in channels are regulated by ROS (Köhler et al., 2003; Joo et al., 2005; Sierla et al., 2016), and the APX2 serves as a ROS scavenger to reduce ROS concentration in cytosol (Fryer et al., 2003). Here we show that, the cml20 mutants were with lower APX2 transcripts and higher ROS (Figure 7), so the CML20 may serve as opposite factor with ABA to avoid too much ROS accumulation, and to keep appropriate stomatal aperture under finely balanced control. In addition, CML20 could also be the negative regulator of ABA and drought via other plant abiotic stress responsive gene mediated pathways (Figure 8). Besides ROS, Ca2+ and ion channels, there may be other factors along with CML20 are involved in the signaling cascades that play critical roles in response to drought stress and ABA treatment. Follow-up research will attempt to identify the targets of CML20, so as to further elucidate the signaling pathways used by plants that are exposed to abiotic stress.
Author Contributions
WZ designed the experiments; XW, CL, and ZQ performed the experiments with assistance from HL; WZ, XW, CL, ZQ, and BA analyzed and discussed the results; WZ, XW, CL, and ZQ wrote the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (31271506, 31500211), the key special project “Breeding and Cultivation of Novel GM varieties” (2014ZX08009-022B), the Program for New Century Excellent Talents in University (NCET-13-0354) and the Shandong Science Fund for Distinguished Young Scholars (2014JQE27047).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GM and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgment
We thank the Arabidopsis Biological Resource Center (ABRC) for providing T-DNA insertion seeds SALK_079974c.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00824/full#supplementary-material
Footnotes
- ^ http://abrc.osu.edu/
- ^ http://signal.salk.edu/tdnaprimers.2.html
- ^ http://www.ebi.ac.uk/interpro/
- ^ http://www.mn-net.com
- ^ https://imagej.nih.gov/ij/
- ^ http://www.zeiss.com.cn/microscopy/zh_cn/downloads/zen.html
- ^ http://www.ebi.ac.uk/interpro/
References
Acharya, B. R., Jeon, B. W., Zhang, W., and Assmann, S. M. (2013). Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New phytol. 200, 1049–1063. doi: 10.1111/nph.12469
Cho, K. M., Nguyen, H. T., Kim, S. Y., Shin, J. S., Cho, D. H., Hong, S. B., et al. (2015). CML10, a variant of calmodulin, modulates ascorbic acid synthesis. New Phytol. 209, 664–678. doi: 10.1111/nph.13612
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Cousson, A., and Vavasseur, A. (1998). Putative involvement of cytosolic Ca < sup > 2+ < /sup > and GTP-binding proteins in cyclic-GMP-mediated induction of stomatal opening by auxin in Commelina communis L. Planta 206, 308–314. doi: 10.1007/s004250050405
David, C., and Anthony, R. M. (2000). Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10, 322–328. doi: 10.1016/S0962-8924(00)01800-6
Delk, N. A., Johnson, K. A., Chowdhury, N. I., and Braam, J. (2005). CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 139, 240–253. doi: 10.1104/pp.105.062612
Fabienne, M., Benoit, R., Martine, C., Bruno, S., Jeanphilippe, G., and Didier, A. (2008). Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 56, 575–589. doi: 10.1111/j.1365-313X.2008.03622.x
Fryer, M. J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P. M., and Baker, N. R. (2003). Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 33, 691–705. doi: 10.1046/j.1365-313X.2003.01656.x
Garrigos, M., Deschamps, S., Viel, A., Lund, S., Champeil, P., Moller, J. V., et al. (1991). Detection of Ca2+-binding proteins by electrophoretic migration in the presence of Ca2+ combined with 45Ca2+ overlay of protein blots. Anal. Biochem. 194, 82–88. doi: 10.1016/0003-2697(91)90154-L
Geiger, D., Scherzer, S., Mumm, P., Marten, I., Ache, P., Matschi, S., et al. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. U.S.A. 107, 8023–8028. doi: 10.1073/pnas.0912030107
Gong, M., Chen, S., Song, Y., and Li, Z. (1997). Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Funct. Plant Biol. 24, 371–379. doi: 10.1071/pp96118
Helen, T., and Marc, R. K. (2002). Calmodulin as a potential negative regulator of Arabidopsis COR Gene Expression. Plant Physiol. 128, 1169–1172. doi: 10.1104/pp.010814
Hongtao, L., Bing, L., Zhonglin, S., Li, X., Ruiling, M., Daye, S., et al. (2003). Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 132, 1186–1195. doi: 10.1104/pp.102.018564
Jefferson, R. A., Kavanagh, T. A., and Bevan, M. W. (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907.
Joo, J., Wang, S., Chen, J., Jones, A. M., and Fedoroff, N. V. (2005). Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17, 957–970. doi: 10.1105/tpc.104.029603
Juliette, A., Philippe, N., Anna, C., Stephanie, D., Christine, C., Nardjis, A., et al. (2008). Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 20, 2146–2159. doi: 10.1105/tpc.107.056812
Karita, E., Yamakawa, H., Mitsuhara, I., Kuchitsu, K., and Ohashi, Y. (2004). Three types of tobacco calmodulins characteristically activate plant NAD kinase at different Ca2+ concentrations and pHs. Plant Cell Physiol. 45, 1371–1379. doi: 10.1093/pcp/pch158
Kenichiro, S., Toshinori, K., and Mitsuo, N. (1992). Involvement of calmodulin and calmodulin-dependent myosin light chain kinase in blue light-dependent H+ pumping by guard cell protoplasts from Vicia faba L. Plant Physiol. 99, 1416–1421. doi: 10.1104/pp.99.4.1416
Kim, Y., Park, S., Gilmour, S. J., and Thomashow, M. F. (2013). Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 75, 364. doi: 10.1111/tpj.12205
Kimura, S., Kaya, H., Kawarazaki, T., Hiraoka, G., Senzaki, E., Michikawa, M., et al. (2012). Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Biophys. Acta 1823, 398–405. doi: 10.1016/j.bbamcr.2011.09.011
Köhler, B., Hills, A., and Blatt, M. R. (2003). Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol. 131, 385–388. doi: 10.1104/pp.016014
Kwak, J., Maser, P., and Schroeder, J. (2008). The clickable guard cell, version II: interactive model of guard cell signal transduction mechanisms and pathways. Arabidopsis Book 6:e0114. doi: 10.1199/tab.0114
Kwak, J., Mori, I., Pei, Z., Leonhardt, N., Torres, M., Dang, J., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. doi: 10.1093/emboj/cdg277
Li, C. L., Wang, M., Ma, X. Y., and Zhang, W. (2014). NRGA1, a putative mitochondrial pyruvate carrier, mediates ABA regulation of guard cell ion channels and drought stress responses in Arabidopsis. Mol. Plant 7, 1508–1521. doi: 10.1093/mp/ssu061
Li, C. L., Wang, M., Wu, X. M., Chen, D. H., Lv, H. J., Shen, J. L., et al. (2016). THI1,a thiamine thiazole synthase, interacts with Ca2+-dependent protein kinase CPK33 and modulates the S-Type anion channels and stomatal closure in Arabidopsis. Plant Physiol. 170, 1090–1104. doi: 10.1104/pp.15.01649
Lin, H., Wang, R., Qian, Q., Yan, M., Meng, X., Fu, Z., et al. (2009). DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21, 1512–1525. doi: 10.1105/tpc.109.065987
Ma, F., Lu, R., Liu, H., Shi, B., Zhang, J., Tan, M., et al. (2012). Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. J. Exp. Bot. 63, 4835–4847. doi: 10.1093/jxb/ers161
McAinsh, M. R., Brownlee, C., and Hetherington, A. M. (1990). Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343, 186–188. doi: 10.1038/343186a0
McAinsh, M. R., Brownlee, C., and Hetherington, A. M. (1997). Calcium ions as second messengers in guard cell signal transduction. Physiol. Plant. 100, 16–29. doi: 10.1111/j.1399-3054.1997.tb03451.x
McAinsh, M. R., Webb, A. A. R., Taylor, J. E., and Hetherington, A. M. (1995). Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7, 1207–1219. doi: 10.1105/tpc.7.8.1207
McCormack, E., Tsai, Y.-C., and Braam, J. (2005). Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 10, 383–389. doi: 10.1016/j.tplants.2005.07.001
Munir, S., Liu, H., Xing, Y. Z., Hussain, S., Ouyang, B., Zhang, Y., et al. (2016). Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci Rep. 6:31772. doi: 10.1038/srep31772
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Murata, Y., Mori, I. C., and Munemasa, S. (2015). Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 66, 369–392. doi: 10.1146/annurev-arplant-043014-114707
Mustilli, A., Merlot, S., Vavasseur, A., Fenzi, F., and Giraudat, J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14, 3089–3099. doi: 10.1105/tpc.007906
Ogasawara, Y., Kaya, H., Hiraoka, G., Yumoto, F., Kimura, S., Kadota, Y., et al. (2008). Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 283, 8885–8892. doi: 10.1074/jbc.M708106200
Oliver, B., and Jorg, K. (2012). Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta 1820, 1283–1293. doi: 10.1016/j.bbagen.2011.10.012
Pandey, N., Ranjan, A., Pant, P., Tripathi, R. K., Ateek, F., Pandey, H. P., et al. (2013). CAMTA1 regulates drought responses in Arabidopsis thaliana. BMC Genomics 14:216. doi: 10.1186/1471-2164-14-216
Pandey, S., Zhang, W., and Assmann, S. M. (2007). Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 581, 2325–2336. doi: 10.1016/j.febslet.2007.04.008
Pei, Z. M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. doi: 10.1038/35021067
Rentel, M. C., and Knight, M. R. (2004). Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 135, 1471–1479. doi: 10.1104/pp.104.042663
Sakamoto, H., Maruyama, K., Sakuma, Y., Meshi, T., Iwabuchi, M., Shinozaki, K., et al. (2004). Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 136, 2734–2746. doi: 10.1104/pp.104.046599
Schroeder, J., Kwak, J., and Allen, G. (2001). Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330. doi: 10.1038/35066500
Schroeder, J. I., Raschke, K., and Neher, E. (1987). Voltage dependence of K+ channels in guard-cell protoplasts. Proc. Natl. Acad. Sci. U.S.A. 84, 4108–4112. doi: 10.1073/pnas.84.12.4108
Shang, Y., Dai, C., Lee, M. M., Kwak, J. M., and Nam, K. H. (2016). BRI1-associated receptor kinase 1 regulates guard cell ABA signaling mediated by open stomata 1 in Arabidopsis. Mol. Plant 9, 447–460. doi: 10.1016/j.molp.2015.12.014
Sheen, J. (2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 127, 1466–1475. doi: 10.1104/pp.010820
Siegel, R. S., Xue, S., Murata, Y., Yang, Y., Nishimura, N., Wang, A., et al. (2009). Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. Plant J. 59, 207–220. doi: 10.1111/j.1365-313X.2009.03872.x
Sierla, M., Waszczak, C., Vahisalu, T., and Kangasjarvi, J. (2016). Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171, 1569–1580. doi: 10.1104/pp.16.00328
Sirichandra, C., Gu, D., Hu, H., Davanture, M., Lee, S., Djaoui, M., et al. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583, 2982–2986. doi: 10.1016/j.febslet.2009.08.033
Takahashi, F., Mizoguchi, T., Yoshida, R., Ichimura, K., and Shinozaki, K. (2011). Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell. 41, 649–660. doi: 10.1016/j.molcel.2011.02.029
Thor, K., and Peiter, E. (2014). Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 204, 873–881. doi: 10.1111/nph.13064
Turner, W. L., Waller, J. C., Vanderbeld, B., and Snedden, W. A. (2004). Cloning and characterization of two NAD kinases from Arabidopsis. Identification of a calmodulin binding isoform. Plant Physiol. 135, 1243–1255. doi: 10.1104/pp.104.040428
Tzahi, A., Ramanjulu, S., Boaz, K., and Hillel, F. (1999). A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 20, 171–182. doi: 10.1046/j.1365-313x.1999.00588.x
Vadassery, J., Reichelt, M., Hause, B., Gershenzon, J., Boland, W., and Mithofer, A. (2012). CML42-mediated calcium signaling coordinates responses to spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 159, 1159–1175. doi: 10.1104/pp.112.198150
Vahisalu, T., Kollist, H., Wang, Y. F., Nishimura, N., Chan, W. Y., Valerio, G., et al. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452, 487–491. doi: 10.1038/nature06608
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J., and Zhu, J. K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45, 523–539. doi: 10.1111/j.1365-313X.2005.02593.x
Wang, G., Zeng, H., Hu, X., Zhu, Y., Chen, Y., Shen, C., et al. (2015). Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant Soil 386, 205–221. doi: 10.1007/s11104-014-2267-6
Wu, H., Luo, D., Vignols, F., and Jinn, T. (2012). Heat shock-induced biphasic Ca2+ signature and OsCaM1-1 nuclear localization mediate downstream signalling in acquisition of thermotolerance in rice (Oryza sativa L.). Plant Cell Environ. 35, 1543–1557. doi: 10.1111/j.1365-3040.2012.02508.x
Xing, T., Higgins, V. J., and Blumwald, E. (1997). Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell 9, 249–259. doi: 10.1105/tpc.9.2.249
Xu, G., Rocha, P. S. C. F., Wang, M., Xu, M., Cui, Y., Li, L., et al. (2011). A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234, 47–59. doi: 10.1007/s00425-011-1386-z
Yamaguchi, T., Aharon, G. S., Sottosanto, J. B., and Blumwald, E. (2005). Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 102, 16107–16112. doi: 10.1073/pnas.0504437102
Yamaguchi-shinozaki, K., and Shinozaki, K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803. doi: 10.1146/annurev.arplant.57.032905.105444
Yang, T., and Poovaiah, B. W. (2002). Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. U.S.A. 99, 4097–4102. doi: 10.1073/pnas.052564899
Zhang, W., Jeon, B. W., and Assmann, S. M. (2011). Heterotrimeric G-protein regulation of ROS signalling and calcium currents in Arabidopsis guard cells. J. Exp. Bot. 62, 2371–2379. doi: 10.1093/jxb/erq424
Keywords: CML20, drought stress, guard cell, stomatal movement, abscisic acid
Citation: Wu X, Qiao Z, Liu H, Acharya BR, Li C and Zhang W (2017) CML20, an Arabidopsis Calmodulin-like Protein, Negatively Regulates Guard Cell ABA Signaling and Drought Stress Tolerance. Front. Plant Sci. 8:824. doi: 10.3389/fpls.2017.00824
Received: 27 January 2017; Accepted: 02 May 2017;
Published: 23 May 2017.
Edited by:
Girdhar Kumar Pandey, University of Delhi, IndiaReviewed by:
Girish Mishra, University of Delhi, IndiaKai Shu, Sichuan Agricultural University, China
Copyright © 2017 Wu, Qiao, Liu, Acharya, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlong Li, lichunlong216@126.com Wei Zhang, weizhang@sdu.edu.cn
†These authors have contributed equally to this work.
 Xiaomeng Wu
Xiaomeng Wu Zhu Qiao
Zhu Qiao Huiping Liu1
Huiping Liu1 Biswa R. Acharya
Biswa R. Acharya Wei Zhang
Wei Zhang