- 1State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University, Hangzhou, China
- 2Department of Traditional Chinese Medicine, Zhejiang A & F University, Hangzhou, China
- 3Key Laboratory of Plant Secondary Metabolism and Regulation of Zhejiang Province, Hangzhou, China
- 4Flower Research Institute, Jinhua Academy of Agricultural Sciences, Jinhua, China
The aim of this study was to investigate the effects of light quality on the morphological traits, leaf anatomical characteristics, antioxidant enzyme (superoxide dismutase, catalase, and peroxidase) activities, photosynthetic pigments content, and bioactive compounds (phenols, flavonoids, and polysaccharides) content in Anoectochilus roxburghii. Plants of A. roxburghii were grown under light filtered through four differently colored films for 8 months. The four treatments were red film (RF), blue film (BF), yellow film (YF), and colorless plastic film (control, CK). Compared with the A. roxburghii plants in CK, those in the BF treatment showed significantly greater stem diameter, fresh weight, leaf area, stomatal frequency, chlorophyll content (Chl a, Chl b, Chl a+b), antioxidant enzyme activities, and active compound (polysaccharides, flavones) content. The plants in the RF treatment showed the greatest plant height and phenolics contents. These results show that growing A. roxburghii plants under blue film is a useful technique to improve quality. This technique is conducive to achieving large-scale sustainable production of high-quality plant materials.
Introduction
Anoectochilus roxburghii (Orchidaceae) is a valued plant species in many Asian countries, where it is used for ornamental, culinary, and medicinal purposes. It is a widely used and popular functional food with several beneficial effects, such as its notable curative effects of clearing heat and cooling the blood, eliminating dampness, and detoxification. Various health products and foods can be produced from A. roxburghii, for example, health beverages, snacks, and soups. Because A. roxburghii is rich in polysaccharides, amino acids, alkaloids, flavonoids, and organic acids, it has been used to prevent and treat diabetes, hyperliposis, hepatitis, and tumors. Nevertheless, wild A. roxburghii resources are dwindling as a result of its specific growth conditions, slow growth rate, low seed germination rate, and long-term excavation (Lv et al., 2015). Thus, to meet the growing demands for this plant from herbal and functional food industries, and to avoid overexploitation of the wild resource, it is now cultivated instead of being harvested from wild populations.
Light is the most important factor affecting plant growth, with changes in irradiance affecting plant growth, morphology, various aspects of physiology, and plant productivity. In A. roxburghii, chloroplast ultrastructure in leaves developed better under 30% irradiance. It suggested that A. roxburghii increased levels of chloroplasts, grana, and grana lamellae, and higher POD and SOD activities to adapt shade conditions (Shao et al., 2014). Nevertheless, the effects of light quality are more complex. Plant species differ in their responses to light quality, but red and blue light generally have the strongest effects on the plant growth. The plant height of Tagetes erecta L. and Salvia miltiorrhiza Bunge was greater under blue light treatment than under red and fluorescent white-light treatments (Heo et al., 2002). Whereas, red light had the strongest stimulatory effect on the weight and height of Rehmannia glutinosa (Gaertn.) DC. (Manivannan et al., 2015). However, the effects of light quality on A. roxburghii have not been systematically studied and analyzed. Thus, the hypothesis of this study was that A. roxburghii plants would grow better under monochrome film compared to colorless plastic film (CLF). The objectives of this study were to determine the effects of light quality on the physiology, photosynthetic pigments content, enzyme activities, and bioactive compounds content in A. roxburghii, and to identify which light color was optimum for plant growth. The final goal of the research was to give an optimal suggestion of light quality to make growers obtain maximum economic benefits through regulating greenhouse light environment, as well as achieve large-scale continuous production.
Materials and Methods
Plant Materials and Growth Conditions
Anoectochilus roxburghii plants were collected in November 2015, and were maintained in a greenhouse at the Baicaoyuan test site of Zhejiang Agriculture and Forestry University, China (30°15′N, 119°43′E). The relative humidity in the greenhouse was 75%, and the temperature is in the range of 22–28°C. Plants were subjected to four different light quality treatments for 8 months. The treatments consisted of light filtered through red film (RF), blue film (BF), yellow film (YF), or CLF (Guofeilong trade co., LTD, Shenzhen, China) as the control (CK). Transmittance of the filters showed in Figure 1. Each treatment consisted of 10 pots with three replications. All plants were kept well-irrigated and were protected from bacterial pathogens and weeds.
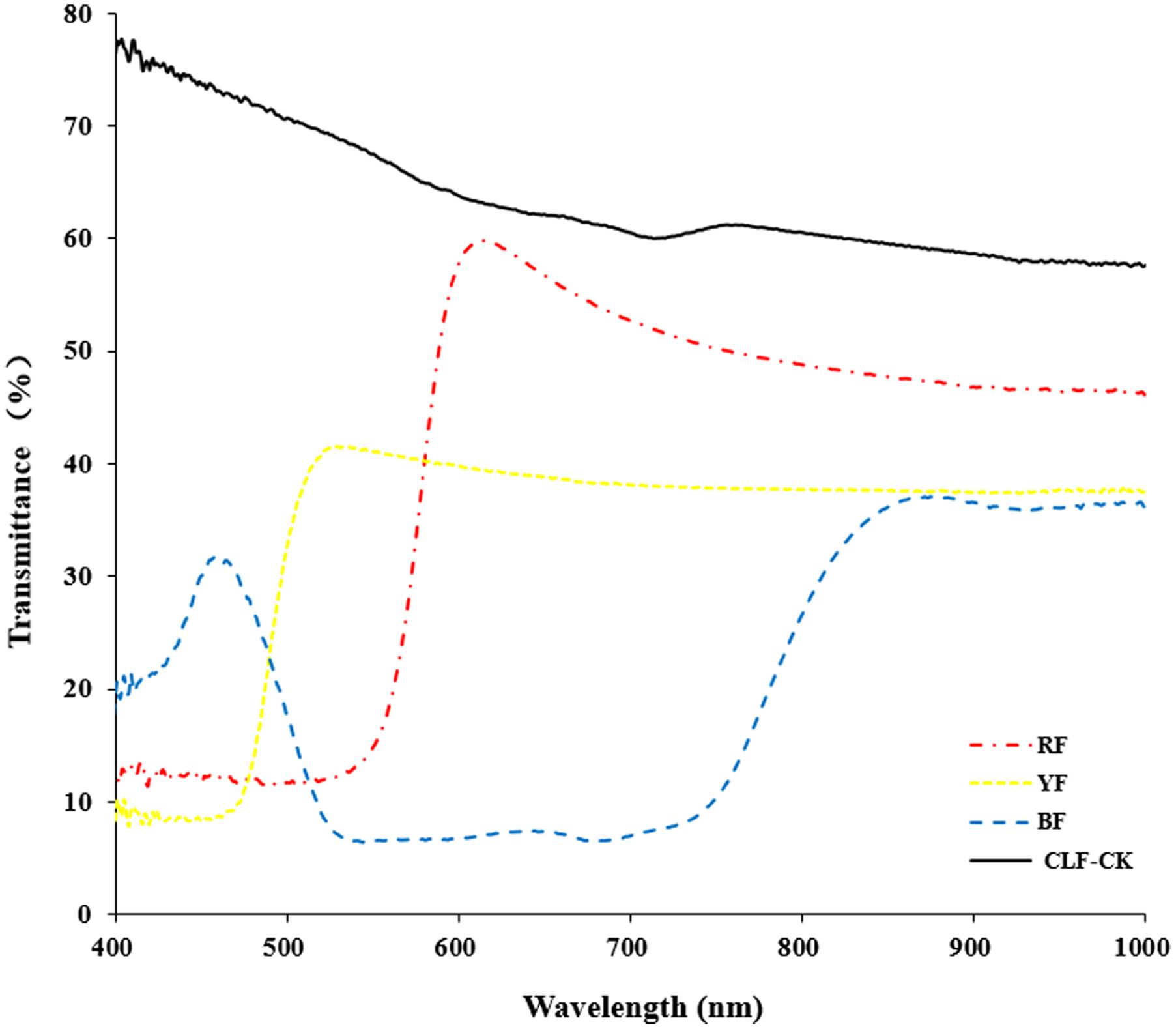
FIGURE 1. Transmittance of the filters (RF: red film, YF: yellow film, BF: blue film, CLF-CK: colorless film).
Morphological Observations
For measurements of morphological features, 15 plants were randomly selected from each treatment and the fresh weight, plant height, stem diameter, and leaf area were measured. Values shown in Table 1 are the mean of 10 replicates.
Leaf Anatomical Characteristics
Anoectochilus roxburghii leaf stomata were examined under a scanning electron microscope (SEM). Small leaf samples (ca. 2 mm × 5 mm) were collected and immediately immersed in cold 3% (v/v) glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2), and then fixed in 1% (v/v) osmium acid. The fixed samples were examined under a KYKY-EM3200 SEM (KYKY Technology Development Ltd., Beijing, China) (Yu et al., 2016).
Photosynthetic Pigment Contents
Mature leaves were collected for determination of chlorophyll content (Chl a, Chl b, Chl a+b, Chl a/b). Chlorophylls were extracted by grinding leaves in 80% acetone in the dark at room temperature. The concentrations of chlorophylls were calculated based on the equations described by Porra and are expressed as mg g-1 FW (Porra, 2002).
Enzyme Activities
Leaf samples (approximately 0.5 g) were collected from each treatment. The activities of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) were determined as described elsewhere (Mahdavikia et al., 2017).
Bioactive Compounds Content
Powdered samples (1 g) were accurately weighed and then extracted for 8 h in a Soxhlet extractor with ethanol–water solvent (85%, v/v) until the samples became colorless. The extract solution was concentrated and centrifuged.
Total Phenolics
A modified version of the Folin–Ciocalteu method (Costa et al., 2016) was used to quantify total phenolics, with gallic acid (Aladdin, Shanghai, China) as the standard. The absorbance of the solution at 760 nm was measured using a spectrophotometer. The yield of phenols was calculated. All samples were analyzed in triplicate.
Total Flavonoids
The flavonoids content was determined by the NaNO2-Al(NO3)3-NaOH method (Zhu et al., 2009). Rutin (Aladdin) was used as the standard. The absorbance of the solution was determined by visible spectrophotometry at 510 nm. The yield of flavonoids was calculated. All samples were analyzed in triplicate.
Total Polysaccharides
Total polysaccharides were extracted from A. roxburghii plants using the ethanol subsiding method (Liu et al., 2014). The polysaccharide concentration was determined using the phenol-sulfuric acid method. The absorbance of the solution was measured at 488 nm, and d-glucose (Aladdin) was used as the standard. The yield of polysaccharide was calculated. Samples were analyzed in triplicate.
Statistical Analysis
Mean values of treatments were compared by one-way ANOVA using SPSS 22.0 software (SPSS, Chicago, IL, United States). The least significant difference test (LSD) was used to detect differences between means (P < 0.05). In figures and tables, values shown are mean ± standard error (SE).
Results
Morphological Traits
The different light qualities affected the morphological traits of A. roxburghii. Stem diameter, fresh weight, and leaf area were significantly higher in the BF treatment than in CK (Table 1). The largest stem diameter was in the BF treatment (3.89 mm), and the mean stem diameter differed significantly among BF, YF, RF, and CK. The highest fresh weight (2.84 g) was in the BF treatment, and the lowest fresh weights were in the RF and YF treatments. The leaf area was greater in the BF treatment (6.29 cm2) than in other treatments, but there was no significant difference in leaf area between the RF and YF treatments. These results indicate that BF enhanced the photosynthesis rate, reduced plant height, and increased the fresh weight of A. roxburghii.
Leaf Anatomical Characteristics
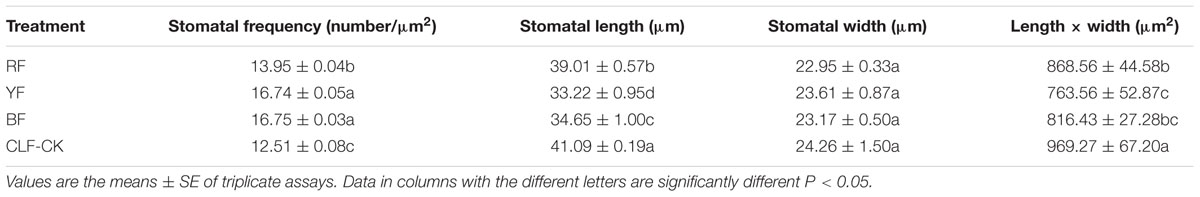
Cells were longer and narrower in plants grown under RF and YF than in those in CK. However, the cell shape was the same in BF and CK (Figure 2). Overall, stomatal frequency was significantly higher in the BF and YF treatments than in the RF treatment and CK, but it did not differ significantly between the BF and YF treatments (Table 2). Stomatal length was smaller in the RF, BF, and YF treatments than in CK. However, stomatal width did not differ significantly among the treatments. The stomatal area (stomatal length × width; in μm2) under the different color film treatments was as follows: 969.27 (CK), 868.56 (RF), 816.43 (BF), and 763.56 (YF).
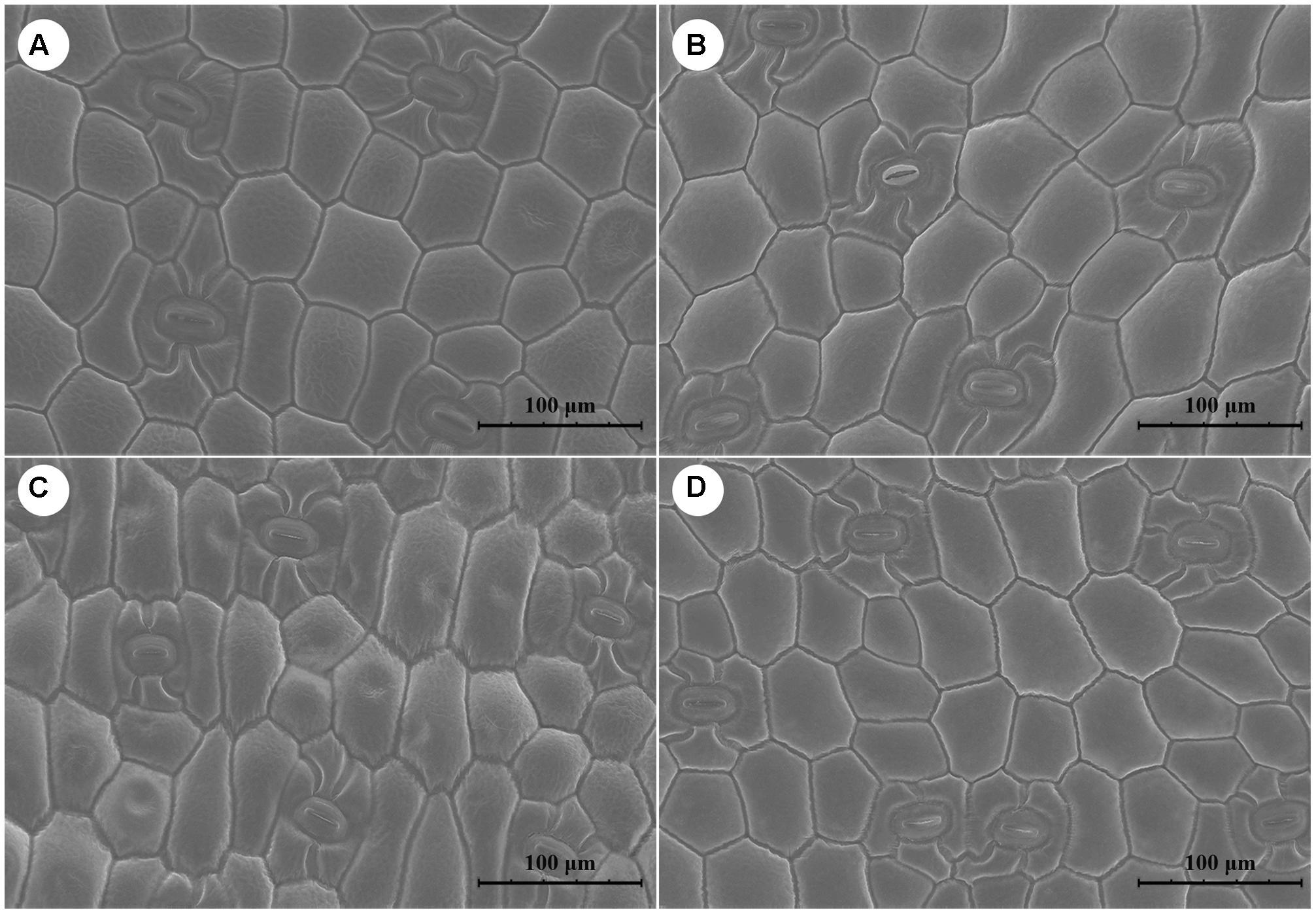
FIGURE 2. Scanning electron microscope (SEM) figure of leave surfaces of A. roxburghii plants. (A) A. roxburghii in CLF-CK treatment; (B) A. roxburghii in RF treatment; (C) A. roxburghii in YF treatment; (D) A. roxburghii in BF treatment.
Photosynthetic Pigment Content
Different light qualities significantly affected the chlorophyll content (Table 3). The Chl a, Chl b, and Chl a+b contents were higher in the BF treatment than in the RF and YF treatments, as compared with CK. The chlorophyll concentrations in the BF treatment were as follows: 1.48 (Chl a), 0.75 (Chl b), 2.23 (Chl a+b) mg⋅g-1 FW. This result indicated that BF strongly affected the photosynthetic system of A. roxburghii. The highest Chl a/b value (2.26 mg⋅g-1 FW) was in the YF treatment, and the lowest was in the BF treatment.

TABLE 3. Effects of different color films on photosynthetic pigments content of A. roxburghii leaves.
Protective Enzyme System Activity
After the 8-month treatment, the SOD, POD, and CAT activities were significantly lower in the RF treatment than in the other treatments. The activities of these enzymes were significantly higher in the BF treatment than in CK (Figure 3). The highest SOD activity (85.83 U⋅mg-1 protein) was in the BF treatment. The activity of POD differed significantly among the four treatments, and ranged from 8.73 (RF) to 16.45 U⋅mg-1 protein (BF). The highest CAT activity was 33.12 U⋅mg-1 protein (BF), and CAT activity did not differ significantly between the YF treatment and CK.
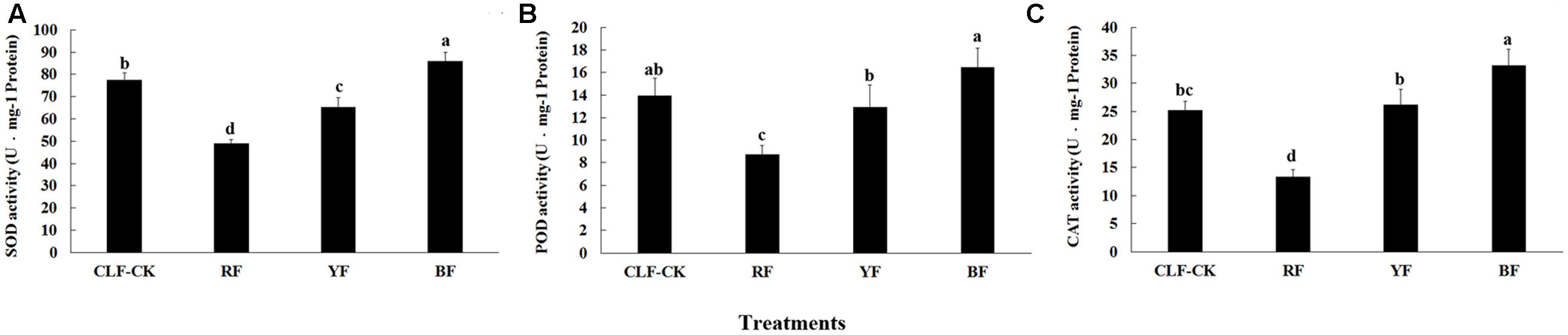
FIGURE 3. Effects of different color films on (A) SOD, (B) POD, and (C) CAT activity of A. roxburghii. Values are the means ± SE of triplicate assays. Data in columns with the different letters are significantly different P < 0.05.
Bioactive Compounds Content
The total flavonoid content was significantly higher in the BF treatment than in the other treatments, and ranged from 0.017 (YF) to 0.021 g⋅g-1 DW (BF) (Table 4). The total flavonoid content did not differ significantly between the RF and YF treatments. The total phenolics content was significantly higher in the RF treatment (0.018 g⋅g-1 DW) than in the other treatments, and differed significantly among the four treatments (Table 2). The total polysaccharides content in A. roxburghii plants differed among the treatments and ranged from 0.061 to 0.118 g⋅g-1 DW. The highest total polysaccharide content (0.118 g⋅g-1 DW) was in the BF treatment. These results showed that light quality strongly affected the accumulation of active compounds in A. roxburghii.
Discussion
Light plays an important role in plant growth. In the last decade, many studies have focused on the effect of light quality on plant growth. Plants grow faster in low-light or dark conditions, and more slowly in bright light. This phenomenon reflects the ability of plants to respond to changes in the light environment to complete their life cycle and improve their biological yield. In rice, OsHAL3 mediates light-controlled development, and its mechanism differs from that of other photoreceptors. The protein encoded by OsHAL3 must form a trimer to function (Su et al., 2016). Light, especially blue light, leads to the disintegration of the trimer, resulting in protein inactivation, and light also inhibits the expression of its encoding gene. This dual inhibition by light slows cell division and eventually slows the growth of rice plants.
Plant species differ in their responses to light quality, but red and blue light generally have the strongest effects on the plant growth. Heo et al. (2002) reported that the plant height of Tagetes erecta L. and Salvia miltiorrhiza Bunge was greater under blue light treatment than under red and fluorescent white-light treatments. Su et al. (2014) reported that red light had an inhibitory effect on plant height, leaf area, and fresh weight of cucumber seedlings. Among several light treatments, blue light resulted in the largest leaf area of Alternanthera brasiliana (L.) Kuntze (Macedo et al., 2011). Red light had the strongest stimulatory effect on the weight and height of Taraxacum officinale (L.) Weber ex F.H.Wigg. (Ryu et al., 2012) and Rehmannia glutinosa (Gaertn.) DC. (Manivannan et al., 2015). Stevia plantlets had shorter stems and roots under blue light (Simlat et al., 2016). In our study, the morphological characteristics of A. roxburghii grown for 8 months were strongly influenced by light quality. Plants of A. roxburghii grown under BF had shorter, thicker stems, and the greatest total fresh weight and leaf area among all the treatments. The opposite trends were observed in the RF treatment.
Stomata regulate gas exchange and water loss in plants. Their opening and closing is influenced by many environmental factors, including light, CO2, and temperature. Among all these factors, light is the main environmental signal that controls stomatal movement. Usually, stomata are open in the light and closed in the dark. Blue light induces the guard cells to swell by activating their osmotic potential, leading to stomatal opening. The blue-light-induced stomatal opening response depends on the activation of a plasma membrane H+-ATP enzyme, the proton pump that produces the cell membrane potential. The blue light signal causes the voltage-dependent plasma membrane K+ channels to open, which enhances K+ and water flow into the guard cells, and finally forces the stomata to open (Schroeder et al., 2001; Shimazaki et al., 2007). Blue light resulted in reduced numbers of stomata in A. brasiliana (Macedo et al., 2011) and Salvia splendens Sellow ex Schult.. However, blue light led to more stomata in T. erecta (Heo et al., 2002) and all of the grape genotypes tested (Poudel et al., 2008). However, stomatal size in the grape genotypes did not differ significantly among the different light treatments. Simlat et al. (2016) reported similar results for Stevia plantlets. In our research, we observed that blue light led to the highest number of stomata in A. roxburghii among all of the light treatments. The stomatal length also differed significantly among the four light treatments, with the longest stomata in CK. However, stomatal width did not differ significantly among the four light quality treatments in this study.
Chlorophyll is one of the most important pigments in higher plants. It is the pigment responsible for capturing light for photosynthesis, which converts light energy into the chemical energy needed for plant growth. Therefore, chlorophyll is the key player in the interaction with light during the entire life cycle of plants. Light quality directly affects photosynthesis because of changes in chlorophyll content and composition. Xu et al. (2004) reported that in strawberry plants grown under different colors of plastic film, the treatments were ranked, based on highest chlorophyll content to lowest, as follows: RF > white film > YF > green film > BF. The chlorophyll a/b ratio was negatively correlated with the ratio of red to blue light. Galdiano et al. (2012) showed that red light strongly promoted chlorophyll b synthesis in Cattleya loddigesii Lindl.. Similar results were also reported for R. glutinosa and Triticum aestivum L. (Dong et al., 2014; Manivannan et al., 2015). However, Kobayashi et al. (2013) reported that the chlorophyll content in lettuce leaves was higher under blue light than under red light. Similar results were reported for Toona sinensis (Juss.) M.Roem. (Zhang et al., 2010). In this study, blue light had the strongest effect to stimulate chlorophyll accumulation in A. roxburghii. The markedly higher leaf chlorophyll content in the BF treatment than in the other treatments illustrated that A. roxburghii plants are able to maximize their light harvesting capacity under blue light.
In cells, CAT, SOD, and POD scavenge harmful free radicals. The O2- produced in plants is removed by SOD and CAT, which protect plant cells against damage caused by free radicals and their derivatives. The activity of POD directly affects the metabolism and distribution of auxin (indole acetic acid, IAA), which controls plant growth and development. Strong POD activity enhances the oxidative decomposition of endogenous IAA, resulting in growth inhibition and a dwarf phenotype. Kim et al. (2013) reported that CAT and SOD activities in tomato leaves were higher under blue light than under a red light. Similar results were reported for R. glutinosa (Manivannan et al., 2015). In Stevia plantlets, CAT and POD activities were higher under blue light than under red light (Simlat et al., 2016), consistent with our results. These findings further confirmed that BF treatment benefits the growth and quality of A. roxburghii plants.
Light strongly affects the primary metabolism of plants, but it also affects the accumulation of secondary metabolites. Blue light promotes the accumulation of polysaccharides. This is achieved by increasing the Ca2+-CaM signal’s control of the photosynthetic apparatus or glucose metabolism (Lin, 2015). In Dendrobium catenatum Lindl., red light was shown to promote the accumulation of carbohydrates, thereby increasing the polysaccharide content (Lin and Lai, 2015). In contrast, blue light promoted polysaccharide accumulation in Astragalus membranaceus (Fisch.) Bunge, to a level 23.9% higher than that in the control (Ren et al., 2014). Our results were consistent with that finding, as blue light had the strongest stimulatory effect on polysaccharide accumulation in A. roxburghii among all of the treatments. In Arabidopsis thaliana, the blue light receptor cryptochromes (CRY1 and CRY2) and PhyA mediate responses to blue light to promote flavonoid biosynthesis and accumulation (Lin, 2009). In A. membranaceus, a blue light treatment resulted in the highest flavonoids content leaves, a level 51% higher than that in the control (Ren et al., 2014). In A. roxburghii, the flavonoids content was higher in the BF treatment than in the other treatments. Light promotes the accumulation of phenolic compounds, via increased production of malonyl CoA and coumaroyl CoA that serve as substrates for phenolics biosynthesis (Kim et al., 2006). Johkan et al. (2010) reported that blue light promoted the accumulation of phenolic compounds in Lactuca sativa L. seedlings. In sweet basil, the total phenolics content was lower in a blue light treatment than in a white light treatment (Shoji et al., 2011). In our study, the total phenolics content in A. roxburghii was higher under red light than under blue light.
Conclusion
Filtering light through BF resulted in high-quality plants of A. roxburghii with the highest fresh weight, the most robust stem, the largest leaf area, and the highest stomatal frequency, as well as the highest photosynthetic pigment concentration and activities of antioxidant enzymes (CAT, SOD, and POD). The plants grown under BF also showed higher bioactive compounds contents, compared with the plants in other light treatments. Growing A. roxburghii plants under BF is a useful technique to improve quality. In terms of economic significance, this technique has the advantage of being low-cost for large-scale cultivation. Further studies are needed to explore the mechanism and interaction between bioactive compounds and light signal transduction pathways.
Author Contributions
SY is responsible for the whole process of experimenting and writing the paper. QS provides experimental guidance. MW mainly assisted in the cultivation of experimental samples. MX, SL, XT, LS assisted in the main part of the experiment.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81303167, 81673531), Major Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province (2016C02058) and the Open Funds of Key Laboratory of Plant Secondary Metabolism and Regulation of Zhejiang Province.
References
Costa, G., Grangeia, H., Figueirinha, A., Figueiredo, I. V., and Batista, M. T. (2016). Influence of harvest date and material quality on polyphenolic content and antioxidant activity of Cymbopogon citratus infusion. Ind. Crops Prod. 83, 738–745. doi: 10.1016/j.indcrop.2015.12.008
Dong, C., Fu, Y., Liu, G., and Liu, H. (2014). Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivum L.) exposed to LED light sources with different spectra combinations. J. Agron. Crop Sci. 200, 219–230. doi: 10.1111/jac.12059
Galdiano, R. F. Jr., Mantovani, C., Pivetta, K. F. L., and Lemos, E. G. D. M. (2012). In vitro growth and acclimatization of Cattleya loddigesii Lindley (Orchidaceae) with actived charcoal in two light spectra. Ciên. Rural 42, 801–807. doi: 10.1590/S0103-84782012005000019
Heo, J., Lee, C., Chakrabarty, D., and Paek, K. (2002). Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a Light-Emitting Diode (LED). Plant Growth Regul. 38, 225–230. doi: 10.1023/A:1021523832488
Johkan, M., Shoji, K., Goto, F., Hashida, S., and Yoshihara, T. (2010). Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortscience 45, 414–415.
Kim, E. H., Kim, S. H., Chung, J. I., Chi, H. Y., Kim, J. A., and Chung, I. M. (2006). Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol. 222, 201–208. doi: 10.1007/s00217-005-0153-4
Kim, K., Kook, H. S., Jang, Y. J., Lee, W. H., Kamala-Kannan, S., Chae, J. C., et al. (2013). The effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. J. Plant Pathol. Mocrobiol. 4:49.
Kobayashi, K., Amore, T., and Lazaro, M. (2013). Light-emitting diodes (LEDs) for miniature hydroponic lettuce. Opt. Photon. J. 3, 74–77. doi: 10.4236/opj.2013.31012
Lin, J. Z. (2009). Functional Analysis of Arabidopsis 4CL3 Gene in Flavonoid Biosynthesis. Ph.D. thesis, Hunan University, Changsha.
Lin, X. (2015). Influence of Calcium Signal on the Growth and Polysaccharide Accumulation of Dendrobium Officinale Protocorm under Photoinduction. M.S. thesis, Sichuan Agricultural University, Sichuan.
Lin, X. P., and Lai, Z. X. (2015). Effect light quality on the proliferation of protocorm and active ingredient contents of Dendrobium officinale. Chin. J. Trop. Crops 36, 1796–1801.
Liu, C., Liu, Q., Sun, J., Jiang, B., and Yan, J. (2014). Extraction of water-soluble polysaccharide and the antioxidant activity from Semen cassiae. J. Food Drug Anal. 22, 492–499. doi: 10.1016/j.jfda.2014.01.027
Lv, T., Teng, R., Shao, Q., Wang, H., Zhang, W., Li, M., et al. (2015). DNA barcodes for the identification of Anoectochilus roxburghii and its adulterants. Planta 242, 1167–1174. doi: 10.1007/s00425-015-2353-x
Macedo, A. F., Leal-Costa, M. V., Tavares, E. S., Lage, C. L. S., and Esquibel, M. A. (2011). The effect of light quality on leaf production and development of in vitrocultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 70, 43–50. doi: 10.1016/j.envexpbot.2010.05.012
Mahdavikia, F., Saharkhiz, M. J., and Karami, A. (2017). Defensive response of radish seedlings to the oxidative stress arising from phenolic compounds in the extract of peppermint (Mentha x piperita L.). Sci. Hortic. 214, 133–140. doi: 10.1016/j.scienta.2016.11.029
Manivannan, A., Soundararajan, P., Halimah, N., Ko, C. H., and Jeong, B. R. (2015). Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 56, 105–113. doi: 10.1007/s13580-015-0114-1
Porra, R. J. (2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73, 149–156. doi: 10.1023/A:1020470224740
Poudel, P. R., Kataoka, I., and Mochioka, R. (2008). Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 92, 147–153. doi: 10.1007/s11240-007-9317-1
Ren, J., Liang, J. P., Zhou, R., Feng, Q. J., Jia, M. L., Wang, X. X., et al. (2014). Effects of different light qualities on growth and accumulation of medicinal components in Astragalus membranaceus. J. Shanxi Agric. Sci. 42, 1078–1081.
Ryu, J. H., Seo, K. S., Choi, G. L., Rha, E. S., Lee, S. C., Choi, S. K., et al. (2012). Effects of LED light illumination on germination, growth and anthocyanin content of dandelion (Taraxacum officinale). Korea Sci. 25, 594–600. doi: 10.7732/kjpr.2012.25.6.731
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. doi: 10.1146/annurev.arplant.52.1.627
Shao, Q., Wang, H., Guo, H., Zhou, A., Huang, Y., Sun, Y., et al. (2014). Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS ONE 9:e85996. doi: 10.1371/journal.pone.0085996
Shimazaki, K., Doi, M., Assmann, S. M., and Kinoshita, T. (2007). Light regulation of stomatal movement. Plant Biol. 58, 219–247. doi: 10.1146/annurev.arplant.57.032905.105434
Shoji, K., Goto, E., Hashida, S., Goto, F., and Yoshihara, T. (2011). Effect of light quality on the polyphenol content and antioxidant activity of sweet basil (Ocimum basilicum L.). Acta Hortic. 907, 95–99. doi: 10.17660/ActaHortic.2011.907.10
Simlat, M., lêzak, P., Moś, M., Warcho, M., Skrzypek, E., and Ptak, A. (2016). The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 211, 295–304. doi: 10.1016/j.scienta.2016.09.009
Su, L., Shan, J. X., Gao, J. P., and Lin, H. X. (2016). OsHAL3, a blue light-responsive protein, interacts with the floral regulator Hd1 to activate flowering in rice. Mol. Plant 9, 233–244. doi: 10.1016/j.molp.2015.10.009
Su, N., Wu, Q., Shen, Z., Xia, K., and Cui, J. (2014). Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 73, 227–235. doi: 10.1007/s10725-013-9883-7
Xu, K., Guo, Y., Zhang, S., Zhang, L., and Zhang, L. (2004). Effect of light quality on photosynthesis and chlorophyll fluorescence in strawberry leaves. J. Integr. Agric. 3, 678–686.
Yu, W., Liu, Y., Song, L., Jacobs, D. F., Du, X., Ying, Y., et al. (2016). Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. J. Plant Growth Regul. 36, 148–160. doi: 10.1007/s00344-016-9625-y
Zhang, L., Liu, S., Zhang, Z., Yang, R., and Yang, X. (2010). Dynamic of different qualities on growth of Toona sinensis seedlings. Acta Agric. Boreali Occidentalis Sin. 19, 115–119.
Keywords: Anoectochilus roxburghii, light quality, morphology, enzyme activities, bioactive compounds
Citation: Ye S, Shao Q, Xu M, Li S, Wu M, Tan X and Su L (2017) Effects of Light Quality on Morphology, Enzyme Activities, and Bioactive Compound Contents in Anoectochilus roxburghii. Front. Plant Sci. 8:857. doi: 10.3389/fpls.2017.00857
Received: 14 March 2017; Accepted: 08 May 2017;
Published: 23 May 2017.
Edited by:
Barbara De Lucia, Università degli Studi di Bari Aldo Moro, ItalyReviewed by:
Karl-Johan Bergstrand, Swedish University of Agricultural Sciences, SwedenSergio Tombesi, Università Cattolica del Sacro Cuore, Italy
Copyright © 2017 Ye, Shao, Xu, Li, Wu, Tan and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingsong Shao, sqszjfc@126.com
 Shenyi Ye1,2
Shenyi Ye1,2