- 1State Key Laboratory of Dao-Di Herbs, National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 2School of Public Health, Ningxia Medical University, Yinchuan, China
- 3School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, China
The poor quality and low productivity of cultivated liquorice (Glycyrrhiza uralensis) continues to put pressure on wild plant populations. As arbuscular mycorrhizal fungi are known to support plant growth and in some cases even to enhance the accumulation of valuable molecules in the plant, the effect of Glomus mosseae on the growth and active ingredient contents was evaluated in liquorice plants grown under nutrient deficiency. We created a nutrient-deficient environment by mixing paddy soil, washed river sand, and pumice at a ratio of 1:5:1. Our results showed that the inoculation of pot-grown liquorice plants with G. mosseae significantly increased the shoot and root biomass (by 25- and 17-folds, respectively) and the contents of glycyrrhizic acid, liquiritin, isoliquiritin, and isoliquiritigenin in the main root (by 1.6-, 4.8-, 6.5-, and 4.4-folds, respectively). Both isoliquiritin and isoliquiritigenin were detectable in the lateral roots of the plants inoculated with G. mosseae, but not in plants without G. mosseae inoculation. G. mosseae inoculation improved the features of the root system and increased photosynthetic efficiency of liquorice. The uptake of P and K by liquorice increased when G. mosseae was inoculated, leading to the depletion of these macronutrients in the soil; G. mosseae also improved the availability of Mg, Cu, Zn, and Mn. Based on these results, we concluded that the inoculation of liquorice plants with G. mosseae is beneficial, particularly for those grown in nutrient-deficient soil, and such positive effect is related to the improvement of the root system and an increased photosynthetic efficiency.
Introduction
Liquorice (Glycyrrhiza uralensis Fisch.) is a leguminous species grown widely in the northern, north-eastern, and north-western regions of China1. As a hardy plant well adapted to soils of low fertility, liquorice has been shown to restore degraded soils in arid and semi-arid regions of the country (Pan et al., 2006; Liu et al., 2007). The major value of liquorice, however, lies in the valuable compounds found in its roots, some of which are associated with positive health effects and have substantial economic and pharmacological values (Hayashi and Sudo, 2009). The increasing demand for liquorice has caused much pressure on its wild populations (Wang et al., 2003; Hodge et al., 2009), and over the past 30 years, attempts have been made to cultivate it as a crop out of both economic and ecological considerations, but both the yield and quality of cultivated liquorice has remained insufficient (Wei et al., 2003).
Liquorice cultivation is targeted at nutrient-poor soils. In order to obtain a satisfactory yield and quality, intensive fertilization is indispensable (Fu et al., 2013; Fan et al., 2016). But massive and long-term application of chemical fertilizer will lead to deterioration of soil physical and chemical properties and environmental pollution (Zhang et al., 2010). Arbuscular mycorrhizal fungi (AMF) as a biofertilizer provide us with a substitute solution. Already known advantages of symbiosis with AMF include: the promotion of vegetative growth (Huang et al., 2010; Khabou et al., 2014), secondary metabolite content (Sarkar et al., 2015; Urcoviche et al., 2015), and nutrient acquisition of plant (Weisany et al., 2016); the improvement of soil conditions for the host plants by improving the soil structure and soil aggregate stability (Piotrowski et al., 2004; Van der Heijden et al., 2006); and the contribution to the ecosystem stability (Feddermann et al., 2010).
It was reported that inoculation of Glomus mosseae (=Funneliformis mosseae) (Schüßler and Walker, 2010) and G. versiforme, alone or in combination, improved G. uralensis plant growth during early and late growth stages in comparison with the control; In addition, mycorrhiza formation enhanced the glycyrrhizic acid concentration in roots, but resulted in a considerable reduction of the root oxidase activity (Liu et al., 2007). Similarly, G. mosseae and G. intraradices could stimulate accumulation of glycyrrhizic acid in plantlets of another Glycyrrhiza species, G. glabra; and G. mosseae was more effective (Orujeia et al., 2013).
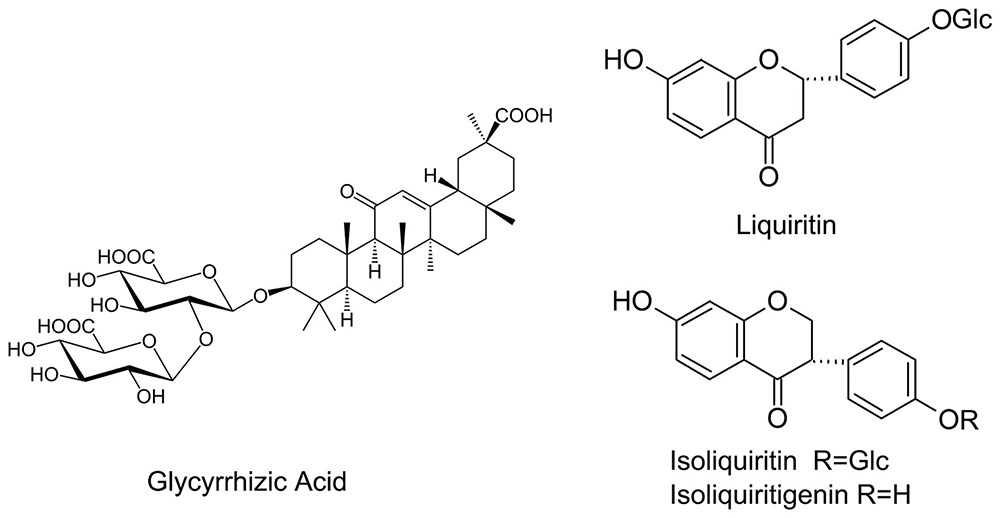
Flavonoids is another another group of bioactive constituents in G. uralensis, of which liquiritin, liquiritgenin, isoliquirtin, isoliquiritigenin, and licochalcone (Figure 1) represent the major ones (Asl and Hosseinzadeh, 2008; Fujii et al., 2014). Effects of AMF on the flavonoids in liquorice under nutrient deficiency have not been thoroughly explored. Nor is it clear whether the presence of AMF has any influence on important factors affecting plant growth status such as photosynthetic efficiency and root architecture of the host plant.
In this study, we created a nutrient-deficient environment for liquorice growth by mixing paddy soil, washed river sand, and pumice, and aimed to evaluate the effects of inoculating G. uralensis with the AMF species G. mosseae on the accumulation of active ingredients, both glycyrrhizic acid and the flavonoids, in root of the plant under such a nutrient stress. We also examined the effect of the treatment on detailed morphological changes of the host plant roots, its utilization of mineral nutrients in soil, and photosynthetic efficiency of the aerial parts.
Materials and Methods
Plant and AMF Materials and Cultivation
Liquorice seeds were purchased from a retailer in Anguo Market of Chinese Traditional and Herbal Drugs, which is located at Hebei Province in North China. After germination, the seedlings were identified as G. uralensis by the authors. G. mosseae was obtained from Professor Honggang Wang (Beijing Chinese Academy of Agricultural Sciences, China). The fungi were propagated using sorghum (Sorghum bicolor) as the host, and the infected roots, hyphae, spores, and substrates were collected. The pot-based plant culture involved growing liquorice plant in a 1:5:1 mixture of paddy soil, washed river sand, and pumice. The culture medium contained organic matter (0.49 g/kg), total N (3.85 g/kg), total P (8.43 g/kg), available P (2.27mg/kg), total K (28.43 g/kg), available K (8.71 mg/kg), available Zn (0.07 mg/kg), available Mn (0.74 mg/kg), available Fe (1.6mg/kg), and available Cu (0.13mg/kg), with a pH value of 8.7. The medium was passed through a 2-mm mesh sieve and autoclaved for 2 h. Each pot was filled with 800 g medium plus 40 g (equivalent to ∼500 spores) of either live or heat-killed (autoclaved at 121°C for 30 min) inoculum. Prior to autoclaving, the inoculum was washed and filtered through a 20 μm mesh twice to remove AMF propagules. This filtrate was added to all CK pots to create a uniform microbial community in all treatments (Karasawa et al., 2012). Liquorice seeds were surface-sterilized by immersion in 0.5% (V/V) NaClO for 10 min, followed by a wash in tap water for 30 min.
Experimental Design
The experiment consisted of 6 pots containing live inoculum (GM) and 6 containing heat-killed inoculum (CK) randomly arranged in a greenhouse (Liu et al., 2007; Karasawa et al., 2012; Orujeia et al., 2013). The liquorice seeds were sown directly into the pot and thinned to two seedlings per pot 1 week after seedling emergence. The pots were weighed three times per week and watered to maintain a field capacity of the medium at around 80%. After 6 months, seedlings were fertilized with 20% Hoagland’s solution which do not contain P nutrient (Hoagland and Arnon, 1950). The plants were harvested 1 year after sowing, at which point the fresh weights of the shoots and roots were recorded, and root system measurement was done. Some fresh root samples were used to visualize AMF colonization. Macro- and micronutrients in the shoots and roots were then determined. After that, the contents of glycyrrhizic acid and flavonoids were determined by main roots and lateral roots separately. Chlorophyll fluorescence parameters were measured before plants were harvested.
AMF Root Colonization
To determine the extent of AMF colonization, the roots of liquorice were cut into small blocks (about 1 cm3 in dimension), and stained with Trypan Blue following a modification of the procedure described by Phillips and Hayman (Koske and Gemma, 1989). AMF colonization in the root of liquorice was determined using the method described by Giovannetti and Mosse (1980).
Chlorophyll Fluorescence Measurement
Chlorophyll fluorescence parameters of the two uppermost leaves of liquorice were measured at room temperature (25°C) using a dual-PAM-100 device (Heinz Walz, Effeltrich, Germany) following the protocols described by Ritchie and Bunthawin (2010). Prior to the measurement, the plants were kept in the dark for a minimum of 30 min, after which the minimal fluorescence in the dark-adapted state (F0) was recorded. A saturating pulse of irradiation (2 mmol m-2 s-1) for 3 s was then administered to measure the maximal fluorescence in the dark-adapted state (Fm) (Gong et al., 2013). The leaves were then placed under actinic light (300 μmol m-2 s-1) to determine the maximal fluorescence (Fm′), the minimal fluorescence in the light-adapted state (F0′) and the steady-state fluorescence (Fs). We calculated chlorophyll fluorescence parameters (Fv/Fm), Y(II), Y(NO), and Y(NPQ) following the description by Zai et al. (2012) and Tao (2014).
Root System Measurement
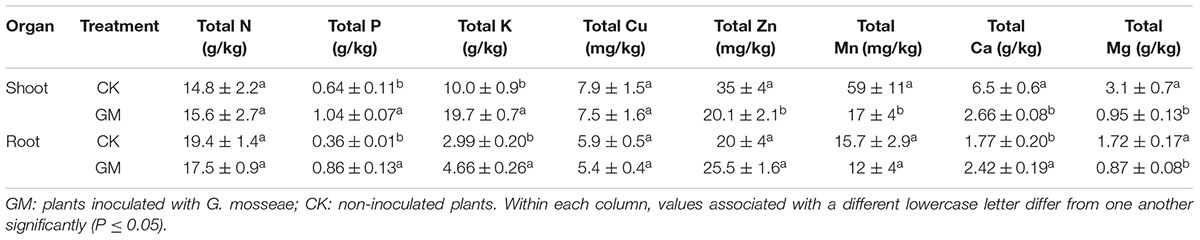
The roots of cultivated liquorice were scanned with an Epson Expression/STD 4800 scanner (Seiko Epson Corporation, Nagano, Japan), and WinRHIZO image analysis software (Regent Instruments Inc., Quebec, QC, Canada) was used to derive the root length, surface area, volume, average diameter, the numbers of tips and forks, and the distribution of root lengths.
Quantification of Bioactive Compounds in Root
Root materials were dried at 40°C for 2 days. An aliquot (0.1 g) of powdered root material (40 mesh) was extracted in 10 mL methanol/water (70:30) for 30 min in an ultrasonic bath at room temperature. The extract solution was cooled to room temperature and filtered through a 0.45 μm filter. A 10 μL aliquot of the filtrate was subjected to separation by high-performance liquid chromatography (HPLC) through a reverse phase C18 Symmetry® column (4.0 mm × 250 mm, pore size of 3 μm; Waters Corp., Milford, MA, United States). The mobile phase comprised a gradient of deionized water: phosphoric acid (100: 0.020, V/V) and acetonitrile. The separation was operated in the gradient elution mode (Supplementary Table S1) at 25°C with a flow rate of 1.0 mL/min. Eluted compounds were detected spectrophotometrically at 276, 360, 248, and 370 nm using a 996 PDA photodiode array detector (Waters Corp., Milford, MA, United States). Glycyrrhizic acid, liquiritin, isoliquiritin, isoliquiritigenin, and licochalcone were purchased from China National Institutes for Food and Drug Control. Stock solutions were diluted with 70% aqueous methanol to appropriate concentrations for calibration purposes. (Zhang et al., 2013)
Mineral Content Analysis of Soil and Plant Tissues
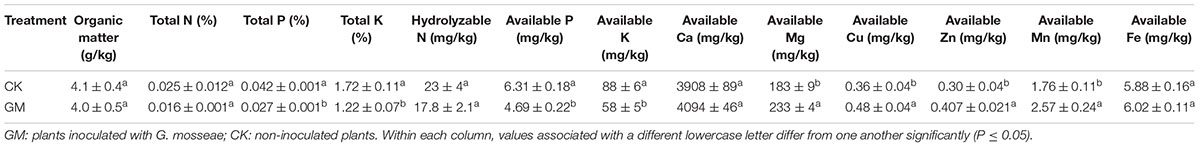
The dried plant tissue or soil samples (0.2 g) were digested in 10 mL mixture of perchloric acid (12.7 mol/L), sulfuric acid (18mol/L), and water (10:1:2) using the Mars 6 microwave reaction system (CEM Corporation, Matthews, NC, United States) until a clear liquid was obtained. The contents of total N, P, K in the samples were routinely analyzed, i.e., Kjeldahl method for total N, vanadium molybdate blue colorimetric method for total P, flame photometry for total K (Bao, 2000). The contents of Ca, Mg, Zn, Cu, Fe, and Mn were quantified by ICP-MS (Thermo Fisher Scientific Inc., Milford, MA, United States) (Gorecka et al., 2006).
Specifically, hydrolyzable N, available P, available K in soil was quantified using alkaline hydrolysis-diffusion method, ammonium acetate extraction-flame photometry, sodium bicarbonate extraction-vanadium molybdate blue colorimetric method, respectively. Available Ca, Mg were extracted with ammonium acetate; available Zn, Cu, Fe, and Mn were extracted with diethylenetriamine pentaacetic acid (Bao, 2000).
Statistical Analysis
All the data were analyzed using the Statistical Package for Social Sciences (SPSS, version 18.0). Independent two-sample t-test was used to determine the statistical significance between the results of inoculated plants (GM) and non-inoculation control (CK; alpha = 0.05).
Results
Effect of G. mosseae Inoculation on Plant Growth
Among the liquorice plants treated with GM, 85% ± 3% were successfully colonized by G. mosseae as shown by the presence of hyphae, arbuscules, and vesicles (data not shown). No AMF fungal structures were observed in the roots of the plants with CK treatment. G. mosseae improve liquorice plant growth (Figure 2) and significantly increased the shoot biomass, root∗ biomass, plant height, leaf number, branch number, and root diameter by 25-, 17-, 5-, 6-, 2.5-, and 3-folds, respectively (Figure 3). The presence of AMF increased the projected area of the root system and the root surface area by 66.0 and 22.6%, respectively. The root volume and the overall length and the numbers of tips and forks were increased by 8.8-, 9.2-, 5.9-, and 26.8-folds, respectively (Table 1). The correlation coefficients of shoot biomass with the root system’s projected area, the roots’ surface area, mean diameter, volume, and the number of tips and forks were 0.76, 0.64, 0.78, 0.71, 0.64, and 0.76, respectively; the correlation coefficients of root biomass with these root parameters were 0.57, 0.57, 0.67, 0.75, 0.55, and 0.64, respectively.
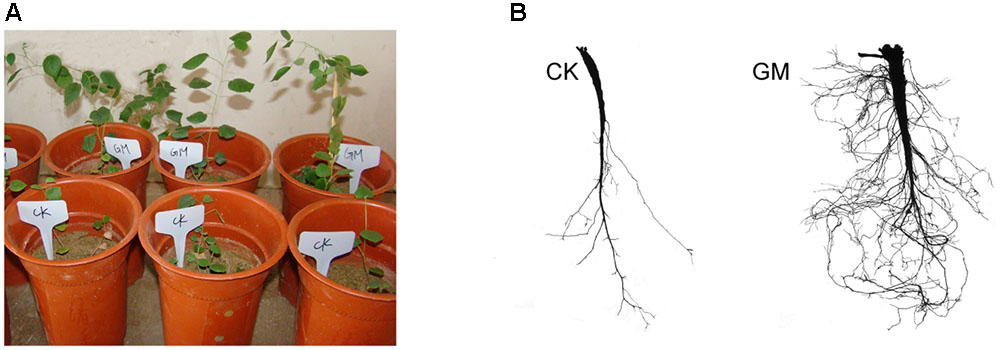
FIGURE 2. Glomus mosseae inoculated (GM) and non-inoculated liquorice plants (CK) in nutrients-deficient medium and their root system (A) plants (B) root system.
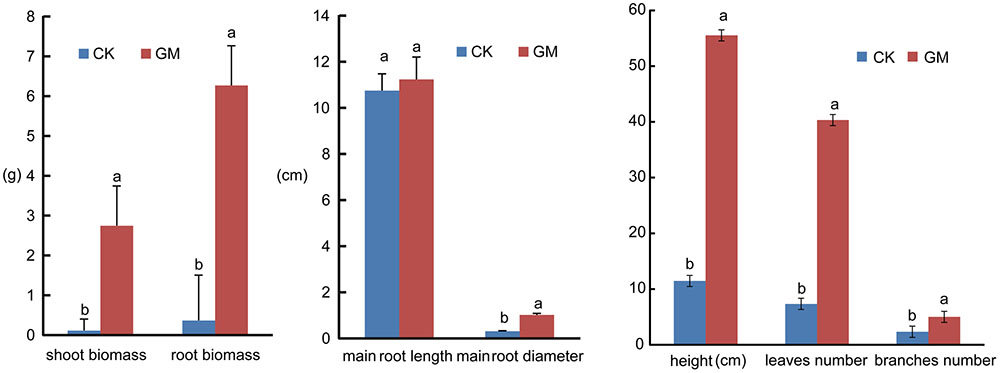
FIGURE 3. Shoot and root biomass accumulation, plant height, leaf number, branch number and root diameter of inoculated and non-inoculated liquorice plants. GM: plants inoculated with G. mosseae; CK: non-inoculated plants. Bars labeled with a different lowercase letter indicate a significantly different level of performance (P ≤ 0.05).
Effects of G. mosseae on Chlorophyll Fluorescence
The presence of G. mosseae decreased the photosynthesis-related parameter Y(NPQ) by 44%, while increased Fv/Fm,∗ ETR, ∗and∗ Y(II) by 13.4, 34.7, and 31.3%, respectively (Table 2). There was no difference in Y(NO) between GM and CK treatment groups.
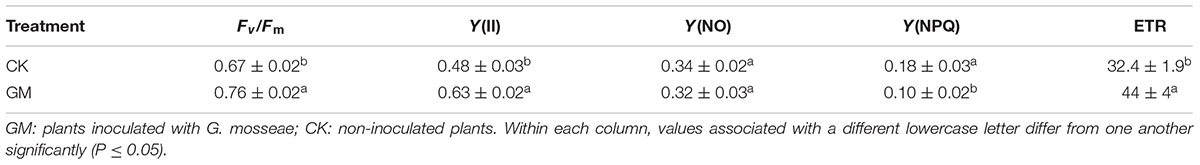
TABLE 2. Effects of G. mosseae Inoculation on chlorophyll fluorescence parameters of liquorice seedlings.
Effect of G. mosseae Inoculation on Active Ingredients Contents in Roots
Glycyrrhizic acid was the most prominent compound in liquorice root, whereas licochalcone was not detectable. G. mosseae inoculation increased the contents of glycyrrhizic acid, liquiritin, isoliquiritin, and isoliquiritigenin in the main root by 1.6-, 4.8-, 6.5-, and 4.4-folds, respectively, and increased glycyrrhizic acid and liquiritin contents in the lateral roots by 1.8- and 4.5-folds, respectively, as compared with those in CK-treated plants. Both isoliquiritin and isoliquiritigenin were detected in the lateral roots of GM-treated plants, but neither of these compounds was found in the lateral roots of CK-treated plants. The contents of glycyrrhizic acid, liquiritin, isoliquiritin, and isoliquiritigenin were all higher in the main root than in the lateral roots irrespective of the treatment of the plants (Table 3 and Figure 4).
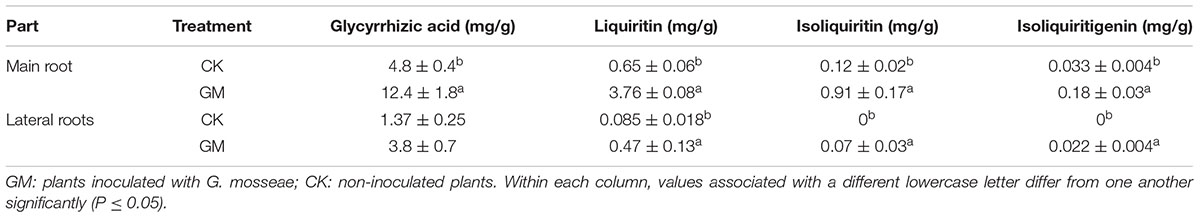
TABLE 3. Effects of G. mosseae inoculation on the contents of four bioactive constituents in the root system.
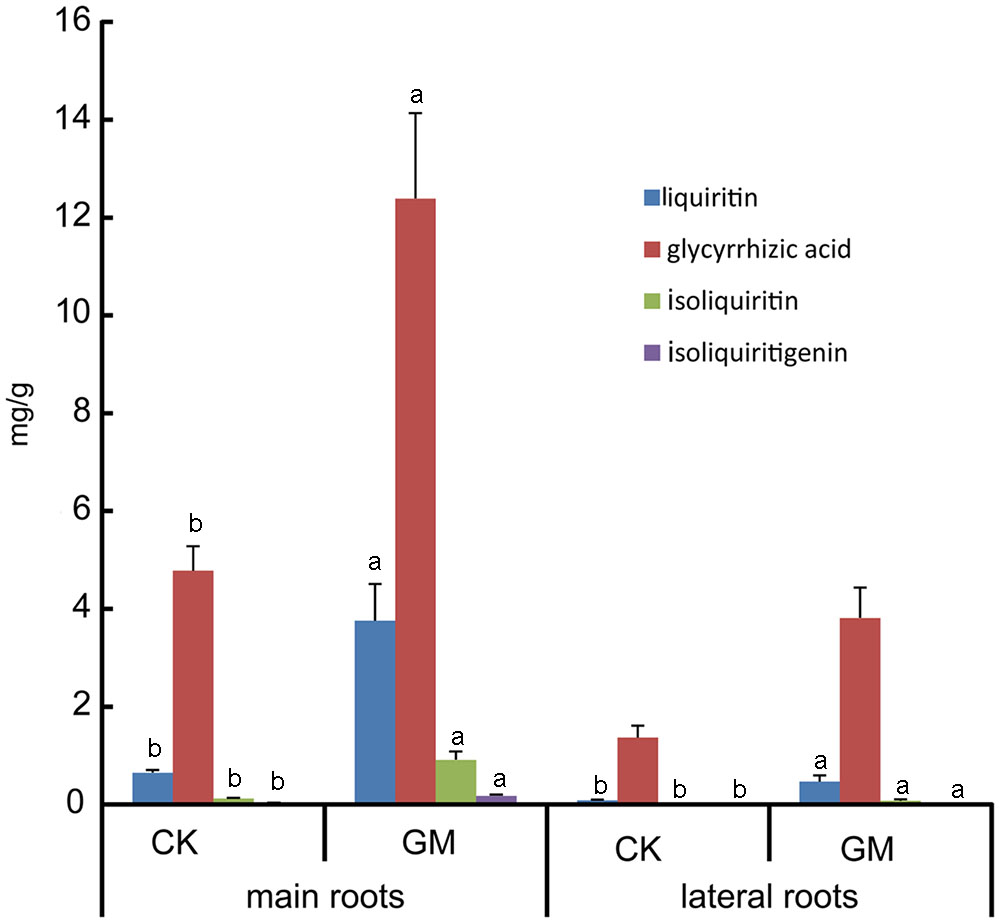
FIGURE 4. Bioactive constituents accumulation in inoculated and non-inoculated liquorice plants. GM: plants inoculated with G. mosseae; CK: non-inoculated plants. Bars labeled with a different lowercase letter indicate a significantly different level of performance (P ≤ 0.05).
Effect of G. mosseae Inoculation on Plant and Soil Mineral Nutrient Status
In the plants with AMF treatment, the shoot and root P concentrations were raised by 62.5 and 138.9% and K concentration by 96.8 and 55.9%, respectively. The roots of GM-treated plants also showed a 36.7% increase in Ca content. The concentration of Mg in both the roots and shoots of the plant was reduced, while Zn, Mn, and Ca concentrations were lowered in the shoots. GM treatment induced no significant changes in root or shoot N and Cu contents or in root Zn and Mn contents of the plants, although their total accumulative quantities were higher in GM group (Table 4).
Inoculation with G. mosseae decreased the overall level in the rhizosphere soil of P and K as well as the levels of available P and K, but increased the levels of available Mg, Cu, Zn and Mn. In the soil samples, the presence of AMF decreased the contents of the total and available P contents by 53.5 and 34.5% and K contents by 41.0 and 51.2%, respectively, and increased Mg, Cu, Zn, and Mn contents by 27.3, 25.0, 34.9, and 46.0%, respectively (Table 5).
Discussion
It is well established that AMFs can act as symbionts to promote the growth and productivity of the host plant (Shahabivand et al., 2012; Colla et al., 2015; Palencia et al., 2015; Yang et al., 2015). In our experiment, we prepared a nutrient deficient medium for liquorice growth by mixing paddy soil with river sand and pumice. All nutrients in the medium were in severe deficiency (Tian and Gao, 2005; Wang et al., 2013). According to a previous research, for 1000 Kg biomass accumulation of liquorice whole plant, there’s a need of 20.9 Kg N, 11.5 Kg P2O5 and 7.2 Kg K2O; and the P absorption increases significantly at the later growth stage (Cheng et al., 2005). The medium we prepared couldn’t meet these needs. Thus, the growth of liquorice plants in the experiment was under an obvious nutrient stress, especially P.
The major benefit of AMF derives from their effect on the host root system (Gutjahr et al., 2009; Vos et al., 2013). Inoculated seedlings produce longer, larger roots, which are thus able to exploit a larger volume of soil (Gutjahr et al., 2009). Mycorrhizal alterations of the root system architecture were AMF species-dependent. In the case of peach (Prunus persica), inoculation with G. mosseae and G. versiforme markedly increased the root length, root projected area, root surface area and root volume (Wu et al., 2011). Our results showed that the provision of G. mosseae effectively enhanced the biomass accumulation of liquorice plants, and markedly improved the host’s root system in terms of the projected area, the roots’ surface area, mean diameter, length and volume, and the number of tips and forks, which was consistent with previous reports (Atkinson et al., 2003; Liu et al., 2007; Orfanoudakis et al., 2010; Orujeia et al., 2013). The root system∗ architecture is critical in determining the capacity of plants to efficiently explore the soil (de Dorlodot et al., 2007). Here we demonstrate a positive correlation between the extensiveness of the root system and the accumulation of biomass. The plants provided with AMF (GM treatment) grew more rapidly than CK-treated plants with a more extensive root system, which facilitates nutrient uptake from the soil (Koske and Gemma, 1989; Olesniewicz and Thomas, 1999).
The presence of AMF has been shown to enhance the availability of certain soil micronutrients (Subramanian et al., 2013). We found that inoculation of G. mosseae increased the contents of available Mg, Fe, Cu, and Zn in the soil to promote their uptake by the plant. Kaya et al. (2009) reported that mycorrhizae helped to acidify the rhizosphere and solubilize certain tightly bound residual forms of Zn to allow more efficient transport of such metal ions via the symbionts’ hyphae to the host root. Available P and K in the soil were depleted in the pots with G. mosseae inoculation, probably due to their markedly enhanced uptake by the host plant, a phenomenon observed by previous researchers as well (Liu et al., 2007; Orujeia et al., 2013). As a result of the higher root and shoot biomass induced by G. mosseae inoculation, the concentration of Mg in both the roots and shoots of the plant was reduced, and for the same reason, Zn, Mn, and Ca concentrations were lowered in the shoots.
Biomass accumulation is strongly related to photosynthetic activity of the plant, and chlorophyll fluorescence parameters have been established as accurate predictors of photosynthetic ability and energy conversion efficiency (Dumlao et al., 2012). Photosynthesis is commonly reduced by nutrient deficiency (Kirschbaum and Tompkins, 1990; Turnbull et al., 2007). AMF symbiosis can reverse the unfavorable growth status of host plants under nutrient stresses through improving photosynthesis. According to Abdel-Fattah et al. (2014) the presence of Glomus symbionts improves growth and nutrition of the soybean plant through increasing photosynthesis in leaves, particularly at low P in soil. G. mosseae inoculation appeared to protect the photosystem II in beach plum (P. maritima) individuals subjected to salinity stress, enhance the efficiency of primary light energy conversion, and improve the response of photosynthesis (Zai et al., 2012). In Medicago truncatula, the provision of AMF is associated with an increase in leaf surface area, thereby raising the plant’s photosynthetic capacity without increasing the photosynthetic activity per unit leaf area (Adolfsson et al., 2015). In this study, inoculation with G. mosseae suppressed the photosynthesis-related parameter Y(NPQ), but promoted Fv/Fm, ETR and Y(II). The plants grown in the absence of AMF showed an Fv/Fm of only 0.70 (Table 2), which was lower than the normal level, suggesting that photosynthesis in these plants was not operating at its full capacity (Klamkowski et al., 2009). In the presence of G. mosseae, Fv/Fm was raised by 13%, which resulted in an about 35% increase in ETR. As the energy captured by chlorophyll can either be assimilated or dissipated as heat or re-emitted light, we presume that inoculation with G. mosseae bolstered energy assimilation [as measured by the parameter Y(II)] while reducing energy dissipation [Y(NO) and Y(NPQ)].
In the plants with AMF treatment, the shoot and root P and K concentrations were significantly raised. K is the most abundant univalent cation in plant cells and plays a significant part in regulating stomatal function (MacRobbie, 1998); its deficiency reduces photosynthesis by decreasing stomatal conductance (Peaslee and Moss, 1968; Terry and Ulrich, 1973). Increased K uptake accompanied with G. mosseae inoculation provided sufficient K supply for normal stomatal function, and thus might enhance photosynthesis of liquorice plant. In a recent study on cotton, it was found that K application significantly promoted the net photosynthetic rate of unit chlorophyll and increased significantly the Fv/Fm, ΦPSII and qP (Hu et al., 2016). The increased uptake of P might also contribute to the improvement of photosynthesis. Similar phenomenon had been observed by Warren that higher concentrations of P in the nutrient solution led to significantly faster maximum net photosynthetic rate in Eucalyptus globulus (Warren, 2011).
Inoculation with AMF has been demonstrated to affect the production of the secondary metabolites, predominantly phenolics and terpenes (Pedone-Bonfim et al., 2015). In this study, we found that the provision of G. mosseae enhanced the capacity of liquorice root to produce flavonoids (with an increase by 4.4- to 4.8-folds) but to a lesser extent to enhance triterpenoid saponin content (1.6-folds). Both isoliquiritin and isoliquiritigenin were detected in the lateral roots of inoculated plants but not in those of non-inoculated plants. The increased glycyrrhizic acid accumulation in the roots of licorice plantlets inoculated with mycorrhizal fungi was proposed to be mainly due to the induction of plant defense system (Orujeia et al., 2013). In many cases of fungi colonization, flavonoids are specifically induced by symbionts and pathogens, and respond to purified signaling molecules from these organisms. The flavonoid pathway to synthesize specifically certain products has been suggested as an avenue to improve root-rhizosphere interactions (Hassan and Mathesius, 2012). The release of flavonoids into the rhizosphere can help to protect the host against a number of pests and diseases (Ndakidemi and Dakora, 2003) and regulate root growth and functions (Buer et al., 2010).
Flavonoids exudation can also affect nutrient availability through soil chemical changes, such as N, P, Fe, Mn, Cu, etc. (Cesco et al., 2010). This may partly be the reason we found increased P, K in the plant and available Fe, Mn, Cu in the soil. In turn, the increased nutrient absorption seems to improve the flavonoids accumulation. For example, in American skullcap (Scutellaria lateriflora), the yield of scutellarein, baicalin, baicalein, and chrysin increased with addition of P, and a linear response to K fertilization was observed for scutellarein concentration (Shiwakoti et al., 2016). Similarly, K and P application significantly increased liquirtin and glycyrrhizic acid content in the root of liquorice cultivated for 2 or 3 years in the fields (Fu et al., 2013). The increase of bioactive compounds accumulation and the increase of P and K uptake in G. mosseae inoculated liquorice might be of mutual promotion.
The contents of four major flavonoid constituents investigated in the main root were all higher than those in the lateral roots (Table 3); it was consistent with the finding that the secondary metabolites in liquorice were not evenly distributed throughout the plant (Guo et al., 2014). The failure of isoliquiritin and isoliquiritigenin detection in lateral roots of non-inoculated liquorice may due to its poor growth, of which the lateral roots were thin and small. Isoliquiritin and isoliquiritigenin might not be synthesized by the roots in such a state, or the amount is too little to be detected.
Conclusion
The inoculation of liquorice plants with G. mosseae is beneficial, particularly for plants in nutrient-deficient soils. G. mosseae inoculation can facilitate the absorption of nutrients by improving the structure of root system, so as to raise photosynthesis efficiency, promote the growth of plants and increase the bioactive components.
Author Contributions
GY, PL, YS, and XZ carried out experiments; MC and HQ analyzed data; MC and ZC completed the manuscript. MC, ZC, and LH designed the experiments.
Funding
This work was supported by Special Fund for TCM supported by State Administration of Traditional Chinese Medicine of China (No. 201407005).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00931/full#supplementary-material
Footnotes
References
Abdel-Fattah, G. M., Asrar, A. A., Al-Amri, S. M., and Abdel-Salam, E. M. (2014). Influence of arbuscular mycorrhiza and phosphorus fertilization on the gas exchange, growth and phosphatase activity of soybean (Glycine max L.) plants. Photosynthetica 52, 581–588. doi: 10.1007/s11099-014-0067-0
Adolfsson, L., Solymosi, K., Andersson, M. X., Keresztes, A., Uddling, J., Schoefs, B., et al. (2015). Mycorrhiza symbiosis increases the surface for sunlight capture in Medicago truncatula for better photosynthetic production. PLoS ONE 10:e0115314. doi: 10.1371/journal.pone.0115314
Asl, M. N., and Hosseinzadeh, H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 22, 709–724. doi: 10.1002/ptr.2362
Atkinson, D., Black, K. E., Forbes, P. J., Hooker, J. E., Baddeley, J. A., and Watson, C. A. (2003). The influence of arbuscular mycorrhizal colonization and environment on root development in soil. Eur. J. Soil Sci. 54, 751–757. doi: 10.1046/j.1351-0754.2003.0565.x
Buer, C. S., Imin, N., and Djordjevic, M. A. (2010). Flavonoids: new roles for old molecules. J. Integr. Plant Biol. 52, 98–111. doi: 10.1111/j.1744-7909.2010.00905.x
Cesco, S., Neumann, G., Tomasi, N., Pinton, R., and Weisskopf, L. (2010). Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329, 1–25. doi: 10.1007/s11104-009-0266-9
Cheng, B., Zhang, Q., Yang, Z. P., Zhao, R. F., and Liu, P. (2005). Nutrient properties and fertilizer requirement regularity of Glycyrrhiza uralensis Fisch. Chin. J. Eco Agric. 13, 128–130.
Colla, G., Rouphael, Y., Di Mattia, E., El-Nakhel, C., and Cardarelli, M. (2015). Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 95, 1706–1715. doi: 10.1002/jsfa.6875
de Dorlodot, S., Forster, B., Pagès, L., Price, A., Tuberosa, R., and Draye, X. (2007). Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 12, 474–481. doi: 10.1016/j.tplants.2007.08.012
Dumlao, M. R., Darehshouri, A., Cohu, C. M., Muller, O., Mathias, J., Adams, W. W. III. et al. (2012). Low temperature acclimation of photosynthetic capacity and leaf morphology in the context of phloem loading type. Photosynth. Res. 113, 181–189. doi: 10.1007/s11120-012-9762-5
Fan, M., Cao, A. N., Jin, X. J., Jin, L. J., Zhang, H., and Tan, S. (2016). Fertilization on yield and quality of Glycyrrhiza in semiarid regions of middle of Gansu province. Mod. Chin. Med. 18, 608–615. doi: 10.13313/j.issn.1673-4890.2016.5.01
Feddermann, N., Finlay, R., Boller, T., and Elfstrand, M. (2010). Functional diversity in arbuscular mycorrhiza – the role of gene expression, phosphorous nutrition and symbiotic efficiency. Fungal Ecol. 3, 1–8. doi: 10.1016/j.funeco.2009.07.003
Fu, X. Y., Ma, L., Zhang, X. H., Zhao, Y. S., Yang, X. Y., and Jiang, Q. (2013). Effects of different ratios of nitrogen, phosphorus and potassium fertilizer on the content of main active components in Glycyrrhiza uralensis Fisch. J. Chin. Med. Mater. 36, 1735–1739. doi: 10.13863/j.issn1001-4454.2013.11.006
Fujii, S., Morinaga, O., Uto, T., Nomura, S., and Shoyama, Y. (2014). Development of a monoclonal antibody-based immunochemical assay for liquiritin and its application to the quality control of licorice products. J. Agric. Food Chem. 62, 3377–3383. doi: 10.1021/jf404731z
Giovannetti, M., and Mosse, B. (1980). An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84, 489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x
Gong, M., Tang, M., Chen, H., Zhang, Q., and Feng, X. (2013). Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 44, 399–408. doi: 10.1007/s11056-012-9349-1
Gorecka, H., Chojnacka, K., and Gorecki, H. (2006). The application of ICP-MS and ICP-OES in determination of micronutrients in wood ashes used as soil conditioners. Talanta 70, 950–956. doi: 10.1016/j.talanta.2006.05.061
Guo, Z. Z., Wu, Y. L., Wang, R. F., Wang, W. Q., Liu, Y., Zhang, X. Q., et al. (2014). Distribution patterns of the contents of five active components in taproot and stolon of Glycyrrhiza uralensis. Biol. Pharm. Bull. 37, 1253–1258. doi: 10.1248/bpb.b14-00173
Gutjahr, C., Casieri, L., and Paszkowski, U. (2009). Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol. 182, 829–837. doi: 10.1111/j.1469-8137.2009.02839.x
Hassan, S., and Mathesius, U. (2012). The role of flavonoids in root-rhizosphere signaling: opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 63, 3429–3444. doi: 10.1093/jxb/err430
Hayashi, H., and Sudo, H. (2009). Economic importance of licorice. Plant Biotechnol. 26, 101–104. doi: 10.5511/plantbiotechnology.26.101
Hoagland, D. R., and Arnon, D. I. (1950). The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 347, 1–32.
Hodge, A., Berta, G., Doussan, C., Merchan, F., and Crespi, M. (2009). Plant root growth, architecture and function. Plant Soil 321, 153–187. doi: 10.1007/s11104-009-9929-9
Hu, W., Jiang, N., Yang, J. H., Meng, Y. L., Wang, Y. H., Chen, B. L., et al. (2016). Potassium (K) supply affects K accumulation and photosynthetic physiology in two cotton (Gossypium hirsutum, L.) cultivars with different K sensitivities. Field Crops Res. 196, 51–63. doi: 10.1016/j.fcr.2016.06.005
Huang, M. J., Wang, W. Q., and Wei, S. L. (2010). Investigation on medicinal plant resources of Glycyrrhiza uralensis in China and chemical assessment of its underground part. China J. Chin. Mater. Med. 35, 947–952. doi: 10.4268/cjcmm20100802
Karasawa, T., Hodge, A., and Fitter, A. H. (2012). Growth, respiration and nutrient acquisition by the arbuscular mycorrhizal fungus Glomus mosseae and its host plant Plantago lanceolata in cooled soil. Plant Cell Environ. 35, 819–828. doi: 10.1111/j.1365-3040.2011.02455.x
Kaya, M., Kucukyumuk, Z., and Erdal, I. (2009). Phytase activity, phytic acid, zinc, phosphorous and protein contents in different chickpea genotypes in relation to nitrogen and zinc fertilization. Afr. J. Biotechnol. 15, 4508–4513. doi: 10.5897/AJB09.983
Khabou, W., Hajji, B., Zouari, M., Rigane, H., and Ben Abdallah, F. (2014). Arbuscular mycorrhizal fungi improve growth and mineral uptake of olive tree under gypsum substrate. Ecol. Eng. 73, 290–296. doi: 10.1016/j.ecoleng.2014.09.054
Kirschbaum, M. U. F., and Tompkins, D. (1990). Photosynthetic responses to phosphorus nutrition in Eucalyptus grandis seedlings. Aust. J. Plant Physiol. 17, 527–535.
Klamkowski, K., Borkowska, B., Treder, W., Tryngiel-Gac, A., and Krzewinska, D. (2009). Effect of mycorrhizal inoculation on photosynthetic activity and vegetative growth of cranberry plants grown under different water regimes. Acta Hortic. 838, 109–113. doi: 10.17660/ActaHortic.2009.838.18
Koske, R. E., and Gemma, J. N. (1989). A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 92, 486–488. doi: 10.1016/S0953-7562(89)80195-9
Liu, J., Wu, L., Wei, S., Xiao, X., Su, C., Jiang, P., et al. (2007). Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizic acid production of licorice (Glycyrrhiza uralensis Fisch.). Plant Growth Regul. 52, 29–39. doi: 10.1007/s10725-007-9174-2
MacRobbie, E. A. (1998). Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1475–1488. doi: 10.1098/rstb.1998.0303
Ndakidemi, P. A., and Dakora, F. D. (2003). Legume seed flavonoids and nitrogenous metabolites as signals and protectants in early seedling development. Funct. Plant Biol. 30, 729–745. doi: 10.1071/FP03042
Olesniewicz, K. S., and Thomas, R. B. (1999). Effects of mycorrhizal colonization on biomass production and nitrogen fixation of black locust (Robinia pseudoacacia) seedlings grown under elevated atmospheric carbon dioxide. New Phytol. 142, 133–140. doi: 10.1046/j.1469-8137.1999.00372.x
Orfanoudakis, M., Wheeler, C. T., and Hooker, J. E. (2010). Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. Mycorrhiza 20, 117–126. doi: 10.1007/s00572-009-0271-0
Orujeia, Y., Shabanib, L., and Sharifi-Tehrania, M. (2013). Induction of glycyrrhizic acid and total phenolic compound production in licorice by using arbuscular mycorrhizal fungi. Russ. J. Plant Physiol. 60, 855–860. doi: 10.1134/S1021443713050129
Palencia, P., Martinez, F., Pestana, M., Oliveira, J. A., and Correia, P. J. (2015). Effect of Bacillus velezensis and Glomus intraradices on fruit quality and growth parameters in strawberry soilless growing system. Hortic. J. 84, 122–130. doi: 10.2503/hortj.MI-002
Pan, Y., Wu, L. J., and Yu, Z. L. (2006). Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch.). Plant Growth Regul. 49, 157–165. doi: 10.1007/s10725-006-9101-y
Peaslee, D. E., and Moss, D. N. (1968). Stomatal conductivities in K-deficient leaves of maize (Zea mays L.). Crop Sci. 8, 427–430. doi: 10.2135/cropsci1968.0011183X000800040010x
Pedone-Bonfim, M. V. L., da Silva, F. S. B., and Maia, L. C. (2015). Production of secondary metabolites by mycorrhizal plants with medicinal or nutritional potential. Acta Physiol. Plant. 37, 27. doi: 10.1007/s11738-015-1781-3
Piotrowski, J. S., Denich, T., Klironomos, J. N., Graham, J. M., and Rillig, M. C. (2004). The effects of arbuscular mycorrhizas on soil aggregation depend on the interaction between plant and fungal species. New Phytol. 164, 365–373. doi: 10.1111/j.1469-8137.2004.01181.x
Ritchie, R. J., and Bunthawin, S. (2010). The use of pulse amplitude modulation (PAM) fluormetry to measure photosynthesis in a CAM orchid, Dendrobium spp. (D. cv. Viravuth Pink). Int. J. Plant Sci. 171, 575–585. doi: 10.1086/653131
Sarkar, A., Asaeda, T., Wang, Q., and Rashid, M. H. (2015). Arbuscular mycorrhizal influences on growth, nutrient uptake, and use efficiency of Miscanthus sacchariflorus growing on nutrient-deficient river bank soil. Flora 212, 46–54. doi: 10.1016/j.flora.2015.01.005
Schüßler, A., and Walker, C. (2010). The Glomeromycota: A Species List with New Families and New Genera. Edinburgh: The Royal Botanic Garden Edinburgh.
Shahabivand, S., Maivan, H. Z., Goltapeh, E. M., Sharifi, M., and Aliloo, A. A. (2012). The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Biochem. 60, 53–58. doi: 10.1016/j.plaphy.2012.07.018
Shiwakoti, S., Shannon, D. A., Wood, C. W., Joshee, N., Rimando, A., Lawrence, K. S., et al. (2016). Nitrogen, phosphorus, and potassium effects on biomass yield and flavonoid content of American skullcap (Scutellaria lateriflora). J. Plant Nutr. 39, 1240–1249. doi: 10.1080/01904167.2015.1050509
Subramanian, K. S., Balakrishnan, N., and Senthi, N. (2013). Mycorrhizal symbiosis to increase the grain micronutrient content in maize. Aust. J. Crop Sci. 7, 900–910.
Tao, Q. J. (2014). Mutagenic Effect of Irradiation to Grape Seeds and Identification of Early Selected Single Plants. Mater dissertation, Zhejiang University, Hangzhou.
Terry, N., and Ulrich, A. (1973). Effects of phosphorus deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 51, 43–47. doi: 10.1104/pp.51.1.43
Tian, Z. C., and Gao, X. S. (2005). Main nutrient conformation and plentiful-lack index in farming soil. Sci. Technol. Qinghai Agric. For. 45, 60–61.
Turnbull, T. L., Warren, C. R., and Adams, M. A. (2007). Novel mannose-sequestration technique reveals variation in subcellular orthophosphate pools do not explain the effects of phosphorus nutrition on photosynthesis in Eucalyptus globulus seedlings. New Phytol. 176, 849–861. doi: 10.1111/j.1469-8137.2007.02229.x
Urcoviche, R. C., Gazim, Z. C., Dragunski, D. C., Barcellos, F. G., and Alberton, O. (2015). Plant growth and essential oil content of Mentha crispa inoculated with arbuscular mycorrhizal fungi under different levels of phosphorus. Ind. Crop Prod. 67, 103–107. doi: 10.1016/j.indcrop.2015.01.016
Van der Heijden, M. G. A., Streitwolf-Engel, R., Riedl, R., Siegrist, S., Neudecker, A., Ineichen, K., et al. (2006). The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 172, 739–752. doi: 10.1111/j.1469-8137.2006.01862.x
Vos, C., Schouteden, N., van Tuinen, D., Chatagnier, O., Elsen, A., De Waele, D., et al. (2013). Mycorrhiza-induced resistance against the root-knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 60, 45–54. doi: 10.1016/j.soilbio.2013.01.013
Wang, J. Y., Liu, C. S., and Wang, W. Q. (2003). The investigation of the licorice resources in northeast China. China J. Chin. Mater. Med. 28, 308–312. PMID: 15139135
Wang, Y. Z., Chen, X., and Shi, Y. (2013). Phosphorus availability in cropland soils of China and related affecting factors. Chin. J. Appl. Ecol. 24, 260–268.
Warren, C. R. (2011). How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 31, 727–739. doi: 10.1093/treephys/tpr064
Wei, S. L., Wang, W. Q., and Wang, H. (2003). Study on licorice resources and their sustainable utilization in center and western area of China. China J. Chin. Mater. Med. 28, 202–206. PMID: 15015299
Weisany, W., Raei, Y., Salmasi, S. Z., Sohrabi, Y., and Ghassemi-Golezani, K. (2016). Arbuscular mycorrhizal fungi induced changes in rhizosphere, essential oil and mineral nutrients uptake in dill/common bean intercropping system. Ann. Appl. Biol. 169, 384–397. doi: 10.1111/aab.12309
Wu, Q. S., Li, G. H., and Zou, Y. N. (2011). Improvement of root system architecture in peach (Prunus persica) seedlings by arbuscular mycorrhizal fungi, related to allocation of glucose/sucrose to root. Not. Bot. Hortic. Agrobot. 39, 232–236.
Yang, H. S., Zhang, Q., Dai, Y. J., Liu, Q., Tang, J. J., Bian, X. M., et al. (2015). Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: a meta-analysis. Plant Soil 389, 361–374. doi: 10.1007/s11104-014-2370-8
Zai, X. M., Zhu, S. N., Qin, P., Wang, X. Y., Che, L., and Luo, F. X. (2012). Effect of Glomus mosseae on chlorophyll content, chlorophyll fluorescence parameters, and chloroplast ultrastructure of beach plum (Prunus maritima) under NaCl stress. Photosyntheica 50, 323–328. doi: 10.1007/s11099-012-0035-5
Zhang, B. Y., Chen, T. L., and Wang, B. (2010). Effects of long-term uses of chemical fertilizers on soil quality. Chin. Agr. Sci. Bull. 26, 182–187.
Keywords: Glycyrrhiza uralensis, arbuscular mycorrhizal fungus, nutrient, root system architecture, photosynthesis, flavonoids
Citation: Chen M, Yang G, Sheng Y, Li P, Qiu H, Zhou X, Huang L and Chao Z (2017) Glomus mosseae Inoculation Improves the Root System Architecture, Photosynthetic Efficiency and Flavonoids Accumulation of Liquorice under Nutrient Stress. Front. Plant Sci. 8:931. doi: 10.3389/fpls.2017.00931
Received: 13 February 2017; Accepted: 19 May 2017;
Published: 07 June 2017.
Edited by:
Angeles Calatayud, Instituto Valenciano de Investigaciones Agrarias, SpainReviewed by:
Marco Landi, University of Pisa, ItalyHua Qin, Zhejiang A&F University, China
Balasubramanian Natesan, Universidade Nova de Lisboa, Portugal
Copyright © 2017 Chen, Yang, Sheng, Li, Qiu, Zhou, Huang and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luqi Huang, huangluqi01@126.com Zhi Chao, chaozhi@smu.edu.cn
†These authors have contributed equally to this work.
 Meilan Chen
Meilan Chen Guang Yang
Guang Yang Ye Sheng
Ye Sheng Pengying Li
Pengying Li Hongyan Qiu
Hongyan Qiu Xiuteng Zhou
Xiuteng Zhou Luqi Huang
Luqi Huang Zhi Chao
Zhi Chao